Abstract
Staphylococcus saprophyticus is the second most frequent community-acquired causative agent of acute urinary tract infection (UTI). Some strains of S. saprophyticus can create biofilms, increasing their virulence. Once biofilms have been produced, antibiotic resistance is exacerbated. Hence, the aims of the present study were the study of biofilm formation, structure and antibiotic resistance in S. saprophyticus strains causing UTIs in women in Ahvaz, Iran. Overall, 43 S. saprophyticus isolates were recovered from UTIs. Antibiotic resistance pattern and the biofilm production and structure were determined using phenotypic methods. Most S. saprophyticus isolates were resistant to erythromycin, but all isolates were sensitive to linezolid and vancomycin. Fifty-eight per cent of S. saprophyticus were multidrug resistant. Twenty-one per cent of S. saprophyticus isolates harbored the mecA gene. Biofilm formation was observed in 65% of S. saprophyticus isolates and most had polysaccharide matrix. Our data indicate high rates of antibiotic resistance and the capability of biofilm formation among S. saprophyticus isolates. The emergence of antibiotic resistance in the management of UTIs is a serious public health issue. The findings of this study could be used to improve treatment plans to control UTIs. Consequently, increased awareness of the mechanisms underlying biofilm formation and the development of drug resistance will allow UTIs to be more efficiently controlled and treated.
Keywords: Antibiotic resistance, antimicrobial, biofilm formation, Staphylococcus saprophyticus, urinary tract infection
Introduction
Urinary tract infections (UTIs) are the most common infections in a clinical setting and the second most prevalent infection after respiratory traction infections [1]. Worldwide, UTIs affect about 150 million people every year [2]. In most cases the infectious agents are Enterobacteriaceae, including Escherichia coli, Klebsiella sp., Enterobacter sp. and Proteus sp., and Gram-positive bacteria such as Enterococcus faecalis, Streptococcus agalactiae and Staphylococcus spp. Staphylococcus saprophyticus is a member of the coagulase-negative staphylococci, which are commonly responsible for 5%–10% of UTIs [3]. This microorganism is the second most frequent cause of uncomplicated UTIs, especially in sexually active women [4]. UTIs are more common in women than men because of their anatomical differences: the distance between the anus and the urethra and the shortness of the urinary tract [5]. Staphylococcus saprophyticus colonization occurs via several different types of adhesins such as hemagglutinins with autolytic and adhesive properties, as well as surface-associated lipase that forms fimbria-like surface appendages, helping the bacteria to maintain tight adherence to these surfaces. This high ability of S. saprophyticus to colonize within the tract is partly thanks to the adhesins permitting the microorganism to colonize the uroepithelium, at the side of the urease, giving rise to severe infections. [6]. S. saprophyticus can produce biofilms, which increases its virulence and shows a 100-fold to 1000-fold increase in its antibiotic tolerance in comparison with non-biofilm-producing isolates [7]. The biofilm matrix comprises extracellular material consisting of proteins, extracellular DNA and polysaccharides, which facilitate attachment to any surfaces. The biofilm confers antibiotic resistance through processes that include encoding antibiotic-resistant genes, restricting antibiotics, and even counteracting host immunity [8]. MecA-positive isolates of S. saprophyticus were first reported from Japan and have subsequently been described from different parts of the world [[9], [10], [11]]. Although these are predominantly reports of sporadic cases, the incidence of mecA among S. saprophyticus was found to be 7.9% [12]. In many developing countries, including Iran, the UTIs caused by S. saprophyticus, as well as the biofilm formation and structure of this species, are not well studied. To our knowledge, this is the first study of biofilm formation and structure in S. saprophyticus isolates. The present study aimed to study biofilm formation, structure and antibiotic resistance in S. saprophyticus strains that cause UTIs in women in Ahvaz, Iran.
Materials and methods
Ethical approval
The study was approved by the Research Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1399.465) Iran, and the necessary permission was granted for the work.
Study design and sample collection
This cross-sectional research included 390 midstream urine samples from women who were inpatients with suspected UTIs and women who were referred to Razi teaching hospitals from January to December 2019. Women were aged from 18 to 60 years without any previous genitourinary anomalies, underlying diseases, or antibiotic usage. Urine samples were collected using the midstream method in toilet-trained women. The samples were transferred to the laboratory at the Department of Microbiology of Ahvaz Jundishapur University. Samples were inoculated on blood agar and eosin methylene blue agar plates (Merck KGaA, Darmstadt, Germany) and were incubated at 37°C for 24 hours. Then, the urine cultures were classified as negative, positive or contaminated. When polymorphic bacterial growth (two or more bacterial species growing on one plate) was observed, the samples were classified as contaminated (exclusion criteria). The urine cultures were considered negative when bacterial growth was <103 CFU/mL (exclusion criteria). When monomorphic bacterial growth was >105 CFU/mL, the culture was classified as positive (inclusion criteria). To confirm infection with S. saprophyticus, after culture on blood agar and mannitol salt agar, the colonies of suspected to S. saprophyticus were detected based on standard microbiological tests such as Gram stain, rapid tests for catalase, coagulase, and biochemical tests like maltose, sucrose, trehalose, xylose, novobiocin test, haemolysin, urease and nitrate reduction [13]. Finally, 43 isolates were suspected to be S. saprophyticus isolates but for definite identification, all 43 isolates were exposed to the specific primer by PCR assay. The S. saprophyticus (ATCC 15305) strain was used as a positive control.
Antimicrobial susceptibility testing
The resistance of the S. saprophyticus isolates to 13 specific antibiotics was investigated by the disc diffusion method according to CLSI (2019). The antibiotic discs represented 13 classes of antibiotics: The antibiotic discs including 13 classes of antibiotics: chloramphenicol (chloramphenicol); ciprofloxacin (fluorinated quinolones); clindamycin (lincosamides); gentamicin (aminoglycosides); erythromycin (macrolides); sulphamethoxazole/trimethoprim (sulphonamides); tetracycline (tetracyclines); vancomycin (glycopeptides); quinupristin-dalfopristin (streptogramins); cefoxitin (penicillinase-stable penicillins); nitrofurantoin (nitrofurantoin); rifampin (ansamycins) and linezolid (oxazolidinones). As per the standardized definition of multidrug-resistance (MDR), extensively drug-resistant, and pan-drug-resistant bacteria have been well studied. Multidrug-resistance was defined as acquired resistance to at least one agent in three or more antimicrobial classes. Extensively drug-resistant was defined as resistant to at least one agent in all but two or fewer antimicrobial classes (i.e. bacterial isolates remain susceptible to only one or two antimicrobial categories). Pan-drug-resistant was well-defined as resistant to all agents in all antimicrobial classes [14,15].
Determination of biofilm formation by microtitre plate method
The biofilm formation of S. saprophyticus isolates was performed using the microtitre plate method. First, the S. saprophyticus isolates were inoculated in brain–heart infusion agar at 37°C for 24 hours. Then, these isolates were adjusted to 0.5 McFarland. A 10-μL aliquot of each suspension was then diluted 1 : 200 in 190 μL of tryptic soy broth containing 1% glucose in 96-well microtitre plates. Following incubation at 37°C overnight, the plates were washed three times with phosphate-buffered saline (PBS). The adherent cells were fixed with methanol for 10 minutes and stained with 200 μL of 0.1% crystal violet (CV) for 20 minutes at room temperature. Again, the plates were washed with PBS and next, the unbound CV was removed by adding 200 μL of ethanol for 20 minutes, and the optical density at 570 nm (OD570) was measured using a UV-visible spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan). Staphylococcus epidermidis ATCC 35984 and tryptic soy broth broth were used as positive and negative controls (ODc) for the biofilm formation, respectively. The results were consistent with the criteria recommended by Zhang et al. [16]. The isolates were classified into several groups about the biofilm formation capacity: OD570 ≤ ODc = no bio-film producer; ODc < OD570 ≤ 2 × ODc = weak biofilm producer; 2 × ODc < OD570 ≤ 4 × ODc = moderate biofilm producer; and 4 × ODc < OD570 = strong biofilm producer, where ODc represents the positive control. All experiments were repeated three times.
Determination of biochemical characterization of biofilm structures
For the biochemical characterization of the biofilm structures, the 24-hour biofilms of S. saprophyticus isolates was grown in the 96-well microtitre plates and washed with PBS. The biofilms were treated for 1 hour at 37°C with (a) a solution of 10 mM sodium metaperiodate (NaIO4) in 50 mM sodium acetate buffer for the disruption of the extracellular polysaccharides, (b) 100 μg/mL of proteinase K for the disruption of the extracellular proteins, or (c) 100 μg/mL of DNAseI in 150 mM of NaCl and 1 mM CaCl2 for the disruption of the extracellular DNAs. After treatments, the biofilms were washed with PBS, stained with 0.1% CV, and the OD570 was measured, as described by Sheikh et al. [17].
DNA extraction
The boiling method was used to extract genomic DNA from S. saprophyticus isolates. A few bacterial colonies of S. saprophyticus strains grown overnight on nutrient agar (Merck, Germany) were resuspended in microtubes containing 500 μL of Tris–HCl–EDTA buffer, then the microtubes were placed in thermoblock (Denville Scientific, Metuchen, NJ, USA) for 5 min at 95°C, and centrifuged at 14 000 rpm for 10 min at 4°C. The supernatant was used as the DNA template in the PCR assays. UV absorbance ratios, A280/A260 were used to evaluate DNA extract purity using a Nanodrop instrument (Thermo Scientific, Waltham, MA, USA).
Confirmation of S. saprophyticus-specific PCR amplification primers
For definitive identification, all 43 suspected S. saprophyticus isolates were exposed to S. saprophyticus-specific PCR amplification primers [18]. The S. saprophyticus (ATCC 15305) strain was used as a positive control and Staphylococcus aureus ATCC 25923 strain was the negative control.
Detection of oxacillin resistance by disc diffusion method and mecA gene
All of the S. saprophyticus isolates were confirmed for resistance to oxacillin using cefoxitin (30 μg) (MAST Diagnostics, Bootle, UK) disc diffusion method and results were interpreted consistent with CLSI (2019). The S. aureus ATCC 33591 was used as the control strain [14]. Existence of the mecA gene was investigated using the PCR assay, as earlier defined by Moosavian et al. [19], The S. aureus ATCC 33591 strain was used as a positive control and S. aureus ATCC 25923 strain was the negative control.
Statistical analysis
All data were evaluated using SPSS version 23.0 software (IBM, Armonk, NY, USA). Two-tailed P value < 0.05 was considered statistically significant. Data normality of continuous variables was initially verified using the Shapiro–Wilk test. Fisher's exact test/χ2 and Mann–Whitney U test were used to determine the significant association between qualitative and continuous variables, respectively. Continuously distributed variables were described by reporting their mean.
Results
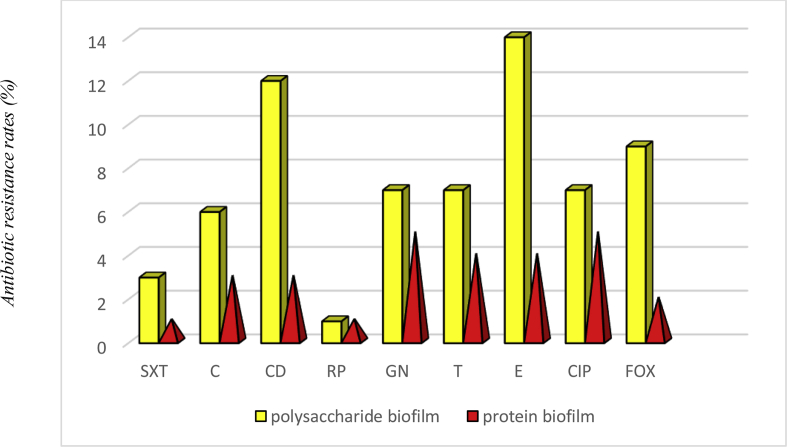
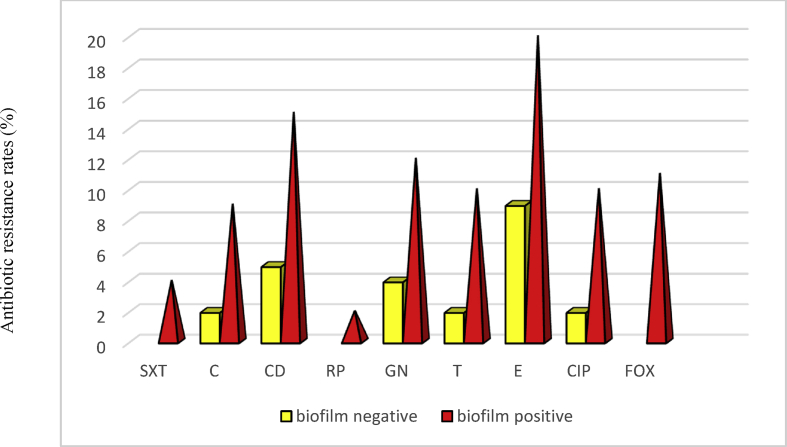
Overall, 43 clinical S. saprophyticus isolates were confirmed from UTIs based on culture, biochemical tests and PCR amplification. Details of molecular and phenotypic identification are shown in Table 1. The mean age of the study population was 35.53 years (standard deviation 8.01 years) (Table 2). Out of 43 isolates, 11 (25%) were resistant to the cefoxitin disc diffusion method and were considered as oxacillin-resistant. All oxacillin-resistant S. saprophyticus isolates harboured a mecA gene. Hence, the prevalence rates of oxacillin-resistant S. saprophyticus isolates and oxacillin-susceptible S. saprophyticus isolates were 11 (25%) and 32 (74%), respectively. According to antibiogram results, the maximum resistance was found with erythromycin (58%, 25/43), clindamycin (46%, 20/43), gentamicin (37%, 16/43), ciprofloxacin (34%, 15/43), tetracycline and chloramphenicol (25%, 11/43); and the minimum resistance was found with trimethoprim, sulfamethoxazole (9%, 4/43) and rifampin (4%, 2/43) (Table 3). The antibiotic resistance patterns of the 43 S. saprophyticus isolates from the UTIs are recorded in Table 4; 33 different patterns from 13 antibiotics in each combination. Out of the 43 S. saprophyticus isolates, none was resistant to all antibiotic classes. The present study also showed 25 (58%) S. saprophyticus isolates were comparatively more resistant to multiple antimicrobial agents and were MDR. None of our isolates were extensively drug-resistant or pan-drug-resistant (Table 2). All S. saprophyticus isolates were susceptible to vancomycin, linezolid, nitrofurantoin and quinupristin-dalfopristin (Table 3). Four S. saprophyticus isolates were sensitive to all antimicrobial agents used in this study. The biofilm formation of 43 S. saprophyticus isolates was performed using the microtitre plate method. The OD570 values of positive control and negative control were 0.410 ± 0.043 and 0.066 ± 0.006, respectively. The OD570 values for the S. saprophyticus isolates ranged from 0.137 ± 0.054 to 1.543 ± 0.050. Generally, 28 (63%) S. saprophyticus isolates were biofilm positive, and among them 10/28 (35%) isolates showed strong biofilm formation, 9/28 (32%) showed moderate biofilm formation, 9/28 (32%) were weak biofilm producers and 15/28 (53%) could not form any detectable biofilm (Table 2). The biofilm structures in 18/28 (64%) S. saprophyticus isolates were composed of polysaccharide structures, in 10/28 (35%) they were composed of a combination of proteins, and none were isolated that did not dissolve with DNAase treatment. The composition of the biofilm structure of S. saprophyticus isolates is shown in Table 2. Consistent with our results, the antibiotic resistance of the S. saprophyticus polysaccharide biofilm structure was higher than that of the S. saprophyticus protein biofilm structure. The prevalence and rate of antibiotic resistance in the polysaccharide biofilm structure and protein biofilm structure in S. saprophyticus isolates are shown in Table 2 and Fig. 1. According to our results, antibiotic resistance in S. saprophyticus biofilm producers was higher than in S. saprophyticus non-biofilm producers (Fig. 2).
Table 1.
Results of Staphylococcus saprophyticus identification by phenotypic and genotypic tests
| IDNumber | Novobiocin test | Coagulase | Haemolysin | Urease production | Mannitol | Maltose | Trehalose | Sucrose | Xylose | Nitrate | Thioglycolate | Phenotypictests | Specific gene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 2 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 3 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 4 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 5 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 6 | R | — | — | — | — | + | + | — | — | + | S. saprophyticus | S. saprophyticus | |
| 7 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 8 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 9 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 10 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 11 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 12 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 13 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 14 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 15 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 16 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 17 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 18 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 19 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 20 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 21 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 22 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 23 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 24 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 25 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 26 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 27 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 28 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 29 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 30 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 31 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 32 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 33 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 34 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 35 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 36 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 37 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 38 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 39 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 40 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 41 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 42 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
| 43 | R | — | — | — | — | + | + | — | — | — | + | S. saprophyticus | S. saprophyticus |
Table 2.
Characteristic of Staphylococcus saprophyticus isolated from women with urinary tract infections
| ID number | Age | Biofilm formation | Dnase | PK | SM | mecA | ARPs | MDR |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | Strong | — | — | D | — | C, CD, E | + |
| 2 | 33 | Strong | — | — | D | — | E, T | — |
| 3 | 39 | Weak | — | D | — | — | CIP, E, GN, T | + |
| 4 | 27 | Moderate | — | D | — | — | C, CIP, E, GN | + |
| 5 | 27 | Strong | — | D | — | + | CIP, CD, T, FOX | + |
| 6 | 28 | Strong | — | — | D | — | CD, E, GN | + |
| 7 | 45 | Moderate | — | — | D | + | CD, E, T, FOX | + |
| 8 | 35 | Weak | — | D | — | + | SXT, CD, T, FOX | + |
| 9 | 33 | Weak | — | D | — | — | CD, T | — |
| 10 | 42 | Strong | — | — | D | — | CIP, CD, E | + |
| 11 | 47 | — | — | — | — | — | CD, E, T | + |
| 12 | 43 | — | — | — | — | — | C, CD, E, GN, T | + |
| 13 | 22 | Strong | — | — | D | + | SXT, C, CIP, CD, T, FOX | + |
| 14 | 28 | Weak | — | — | D | — | CIP, CD, E, T | + |
| 15 | 32 | — | — | — | — | — | CD, E | — |
| 16 | 36 | Moderate | — | — | D | + | CIP, CD, FOX | + |
| 17 | 21 | — | — | — | — | — | CD, GN | — |
| 18 | 51 | Moderate | — | — | D | + | CIP, CD, GN, FOX | + |
| 19 | 53 | Strong | — | — | D | + | SXT, C, CD, GN, FOX | + |
| 20 | 36 | Weak | — | D | — | — | CIP, GN | — |
| 21 | 37 | Weak | — | D | — | + | C, RP, FOX | + |
| 22 | 40 | — | — | — | — | — | E | — |
| 23 | 34 | — | — | — | — | — | E | — |
| 24 | 22 | Moderate | — | — | D | — | C, CD | — |
| 25 | 26 | Moderate | — | — | D | + | SXT, CD, FOX | + |
| 26 | 39 | — | — | — | — | — | CIP, CD, E | + |
| 27 | 43 | Moderate | — | — | D | — | CIP, C, CD, E, GN | + |
| 28 | 27 | Strong | — | — | D | + | E, GN, RP, T, FOX | + |
| 29 | 41 | Weak | — | D | — | — | CIP, C, E, GN | + |
| 30 | 25 | — | — | — | — | — | E, GN | — |
| 31 | 36 | Moderate | — | — | D | + | E, T, FOX | + |
| 32 | 33 | — | — | — | — | — | CIP, GN | — |
| 33 | 30 | Moderate | — | — | D | — | E, GN, T | + |
| 34 | 30 | Strong | — | — | D | — | E, GN, FOX | + |
| 35 | 28 | Weak | — | D | — | — | — | — |
| 36 | 39 | Weak | — | D | — | — | E, GN | — |
| 37 | 47 | Strong | — | — | D | — | C, CIP, E | + |
| 38 | 46 | — | — | — | — | — | — | — |
| 39 | 45 | — | — | — | — | — | E | — |
| 40 | 35 | — | — | — | — | — | — | — |
| 41 | 42 | — | — | — | — | — | C, E | — |
| 42 | 39 | — | — | — | — | — | — | — |
| 43 | 37 | — | — | — | — | — | E | — |
Abbreviations: +, positive; –, negative; ARP,; C, Chloramphenicol; CD, clindamycin; CIP, ciprofloxacin; D, dissolve; E, erythromycin; FOX, cefoxitin; GN, gentamicin; MDR, multidrug resistance; mecA, methicillin-resistant gene; Nd, not done; PK, proteinase K; RP, rifampin; SM, sodium metaperiodate; SXT, sulfamethoxazole-trimethoprim; T, tetracycline.
Table 3.
Results of antimicrobial resistance tests by disc diffusion method
| Antimicrobial category | Antimicrobial agent | susceptible | resistant |
|---|---|---|---|
| Oxazolidinones | Linezolid | 43 (100%) | — |
| Folate pathway inhibitors | Trimethoprim-sulphamethoxazole | 39 (90%) | 4 (9%) |
| Phenicols | Chloramphenicol | 32 (74%) | 11 (25%) |
| Fluoroquinolones | Ciprofloxacin | 28 (65%) | 15 (34%) |
| Lincosamides | Clindamycin | 23 (53%) | 20 (46%) |
| Macrolides | Erythromycin | 18 (41%) | 25 (58%) |
| Aminoglycosides | Gentamycin | 27 (62%) | 16 (37%) |
| Ansamycins | Rifampin | 41 (95%) | 2 (4%) |
| Tetracyclines | Tetracycline | 32 (74%) | 11 (25%) |
| Glycopeptides | Vancomycin | 43 (100%) | — |
| Penicillinase-stable penicillins | Cefoxitin | 32 (74%) | 11 (25%) |
| Nitrofurantoins | Nitrofurantoin | 43 (100%) | — |
| Streptogramins | Quinupristin-dalfopristin | 43 (100%) | — |
Table 4.
Antibiotic resistance patterns among Staphylococcus saprophyticus isolates
| No. of resistant antibiotics | Antibiotics | No.of species |
|---|---|---|
| 6 | SXT, C, CIP, CD, T, FOX | 1 |
| 5 | SXT, C, CD, GN, FOX | 1 |
| CIP, CD, GN, FOX | 1 | |
| E, GN, RP, T, FOX | 1 | |
| 4 | SXT, CD, T, FOX | 1 |
| CIP, CD, T, FOX | 1 | |
| CIP, C, CD, E, GN | 1 | |
| C, CD, E, GN, T | 1 | |
| CD, E, T, FOX | 1 | |
| C, CIP, E, GN | 1 | |
| CIP, C, E, GN | 1 | |
| E, GN, FOX | 1 | |
| CIP, E, GN, T | 1 | |
| 3 | SXT, CD, FOX | 1 |
| CIP, CD, FOX | 1 | |
| C, RP, FOX | 1 | |
| CIP, CD, E | 2 | |
| E, T, FOX | 1 | |
| CD, E, GN | 1 | |
| C, CIP, E | 1 | |
| E, GN, T | 1 | |
| CD, E, T | 1 | |
| C, CD, E | 1 | |
| 2 | CIP, GN | 1 |
| CD, GN | 1 | |
| CIP, GN | 1 | |
| E, GN | 2 | |
| C, CD | 1 | |
| CD, T | 1 | |
| CD, E | 1 | |
| E, T | 1 | |
| C, E | 1 | |
| 1 | E | 4 |
Antibiotic abbreviations: C, chloramphenicol; CD, clindamycin; CIP, ciprofloxacin; E, erythromycin; FOX, cefoxitin; GN, gentamicin; RP, rifampin; SXT, sulfamethoxazole-trimethoprim; T, tetracycline.
Fig. 1.
The frequency of antibiotic resistance in polysaccharide biofilm and protein biofilm.
Fig. 2.
The frequency of antibiotic resistance in biofilm positive and biofilm negative isolates.
Statistical analysis showed that the relationship between biofilm formation and antibiotic resistance among S. saprophyticus isolates was significant (p 0.0002). Moreover, our results indicated that biofilm formation in MDR S. saprophyticus isolates was significantly higher than that of non-MDR S. saprophyticus isolates (p 0.0003) (Table 2). Overall, 46.8% of MDR S. saprophyticus isolates had the ability to form a strong biofilm. However, no significant relationship was detected between biofilm formation intensity (strong, moderate and weak) and MDR S. saprophyticus isolates. Moreover, our results showed that all oxacillin-resistant S. saprophyticus isolates exhibited biofilm formation (p ≤ 0.05).
Discussion
Urinary tract infections are the most frequent types of hospital and community infections, and account for >30% of hospital infections [20]. Staphylococcus saprophyticus is one of the main pathogens of UTIs; however, little is known about antibiotic-resistant patterns and biofilm production in this species. The prevalence of S. saprophyticus in UTIs was 17%. This is higher than the reports by Onyemelukwe et al. and Magliano et al. [21,22]. In some studies, the term methicillin resistance is used, and in others the term oxacillin resistance is used. In both methods, a cefoxitin disc is used and the result is read according to the CLSI guideline. Therefore, resistance to methicillin or oxacillin both seem to be used for S. saprophyticus isolates [7,12]. Resistance to oxacillin was determined using a cefoxitin disc. Our results were confirmed using a mecA gene-based PCR method as a reference standard. The incidence of the mecA gene in S. saprophyticus isolates was 25%. All oxacillin-resistant S. saprophyticus isolates harbured the mecA gene. In previous studies, the prevalence of mecA among S. saprophyticus was 7.9% [12].
Unfortunately, UTIs are often treated with a broad-spectrum antibiotic without performing culture and sensitivity tests. This inappropriate usage of antibiotics has increased antibiotic resistance, leading to the development of MDR bacterial pathogens. Changing patterns of antibiotic resistance in the aetiological agents of urinary tract pathogens have been reported [23,24]. According to the CLSI, routine susceptibility testing of urinary S. saprophyticus isolates to choose antibiotics is not recommended as this microorganism is normally susceptible to trimethoprim/sulfamethoxazole [25]. However, in our study, 9% of the S. saprophyticus isolated from UTIs were resistant to sulfamethoxazole/trimethoprim. Similar to our results, 17.6% of the S. saprophyticus isolates were resistant to sulfamethoxazole/trimethoprim [26]. In addition, the S. saprophyticus isolates described in this study were susceptible to vancomycin, linezolid and quinupristin/dalfopristin [27]. In S. saprophyticus isolates, the maximum resistance has been observed against clindamycin (46%), followed by ciprofloxacin and gentamicin (37%). The results are comparable to studies conducted in other parts of the country [28,29]. Our results are alarming as they reveal the high rate of MDR S. saprophyticus in the majority of Ahvaz hospitals. The highest percentage of MDR in our study might be a result of the improper use of antibiotics. The virulence is connected with this species ability to form biofilms on host surfaces and its resistance to antibiotics. A similar result of high antimicrobial resistance in biofilm-forming bacterial isolates has been found in other studies [[30], [31], [32]]. Generally, 51% of MDR and 9% of non-MDR isolates can produce biofilms. The results of this study indicated that biofilm formation in MDR S. saprophyticus isolates was greater than in non-MDR S. saprophyticus isolates. However, our results are not supported by de Campos et al. [33]. In S. saprophyticus isolates that did not produce biofilm, less resistance was observed.
In conclusion, it is important to take into consideration specific local resistance patterns when choosing appropriate antibiotic coverage. It seems that MDR S. saprophyticus strains have emerged and antimicrobial susceptibility testing of these strains is therefore necessary. The development of antibiotic resistance in UTIs is a serious issue, particularly in developing countries where in addition to a high level of poverty, poor hygienic practices are a serious concern. Our results can be used to improve treatment plans to control UTIs. Increased awareness of the mechanisms underlying biofilm formation and the development of drug resistance allow more efficient control and treatment of UTIs.
Authors' contributions
The concept and the design of the study were developed by AAZD and MH. The methodology was designed by MH. Data collection and the experimental work were carried out by AAZD and SH. Formal analyses and interpretation of data were carried out by FJ. The original draft was prepared by AAZD and reviewed by MH. All the authors have read and approved the final manuscript for submission.
Conflicts of interest
The authors report no conflicts of interest in this work.
Acknowledgements
The authors would like to thank the patients as they have agreed to participate in this study. This work was financially supported by Deputy Vice-Chancellor for research affair and Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.REC.1399.465).
References
- 1.Najar M.S., Saldanha C.L., Banday K.A. Approach to urinary tract infections. Ind J Nephrol. 2009;19:129. doi: 10.4103/0971-4065.59333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajdács M., Ábrók M., Lázár A., Burián K. Comparative epidemiology and resistance trends of common urinary pathogens in a tertiary-care hospital: a 10-year surveillance study. Medicina. 2019;55:356. doi: 10.3390/medicina55070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argemi X., Hansmann Y., Prola K., Prévost G. Coagulase-negative staphylococci pathogenomics. Int J Mol Sci. 2019;20:1215. doi: 10.3390/ijms20051215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers S., Merrill S.A. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2020 Jan. Staphylococcus Saprophyticus. [Updated 2020 Jun 28]https://www.ncbi.nlm.nih.gov/books/NBK482367/ Available from: [Google Scholar]
- 6.Latham R.H., Running K., Stamm W.E. Urinary tract infections in young adult women caused by Staphylococcus saprophyticus. JAMA. 1983;250:3063–3066. [PubMed] [Google Scholar]
- 7.Martins K.B., Ferreira A.M., Pereira V.C., Pinheiro L., Oliveira A.D., Cunha M.D. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus saprophyticus isolated from patients with urinary tract infections. Front Microbiol. 2019;10:40. doi: 10.3389/fmicb.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumaru R., Baral R., Shrestha L.B. Study of biofilm formation and antibiotic resistance pattern of gram-negative Bacilli among the clinical isolates at BPKIHS, Dharan. BMC Res Notes. 2019;12:38. doi: 10.1186/s13104-019-4084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami K., Minamide W., Wada K., Nakamura E., Teraoka H., Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain Z., Stoakes L., Massey V., Diagre D., Fitzgerald V., El Sayed S., Lannigan R. Correlation of oxacillin MIC with mecA gene carriage in Coagulase-negative staphylococci. J Clin Microbiol. 2000;38:752–754. doi: 10.1128/jcm.38.2.752-754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swenson J.M., Tenover F.C., Cefoxitin Disk Study Group Results of disk diffusion testing with cefoxitin correlate with presence of mecA in Staphylococcus spp. J Clin Microbiol. 2005;43:3818–3823. doi: 10.1128/JCM.43.8.3818-3823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashide M., Kuroda M., Ohkawa S., Ohta T. Evaluation of a cefoxitin disk diffusion test for the detection of mecA-positive methicillin-resistant Staphylococcus saprophyticus. Int J Antimicrob Agents. 2006;27:500–504. doi: 10.1016/j.ijantimicag.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Mahon C.R., Lehman D.C., Manuselis J.G. Elsevier Health Sciences; Amsterdam: 2014. Textbook of diagnostic Microbiology. [Google Scholar]
- 14.Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement. Clinical and Laboratory Standards Institute; Wayne, PA: 2016. [Google Scholar]
- 15.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D., Xia J., Xu Y., Gong M., Zhou Y., Xie L. Biological features of biofilm-forming ability of Acinetobacter baumannii strains derived from 121 elderly patients with hospital-acquired pneumonia. Clin Exp Med. 2016;16:73–80. doi: 10.1007/s10238-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh A.F., Dezfuli A.A., Navidifar T., Fard S.S., Dehdashtian M. Association between biofilm formation, structure and antibiotic resistance in Staphylococcus epidermidis isolated from neonatal septicemia in southwest Iran. Infect Drug Resist. 2019;12:1771. doi: 10.2147/IDR.S204432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martineau F., Picard F.J., Ménard C., Roy P.H., Ouellette M., Bergeron M.G. Development of a rapid PCR assay specific for Staphylococcus saprophyticus and application to direct detection from urine samples. J Clin Microbiol. 2000;38:3280–3284. doi: 10.1128/jcm.38.9.3280-3284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moosavian M., Dehkordi P.B., Hashemzadeh M. Characterization of SCCmec, Spa types and multidrug resistant of methicillin-resistant Staphylococcus aureus isolates in Ahvaz. Iran Infect Drug Resist. 2020;13:1033. doi: 10.2147/IDR.S244896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC Methicillin-resistant Staphylococcus aureus (MRSA) https://www.cdc.gov/mrsa/index.html Available from:
- 21.Onyemelukwe N.F., Nwokocha A.R. Staphylococcus saprophyticus infection as a cause of UTI in female adolescents in Enugu area, Nigeria. IOSR J Dental Med Sci. 2013;11(5):37–40. [Google Scholar]
- 22.Lo D.S., Shieh H.H., Ragazzi S.L., Koch V.H., Martinez M.B., Gilio A.E. Community-acquired urinary tract infection: age and gender-dependent etiology. J Bras Nefrol. 2013 Jun;35(2):93–98. doi: 10.5935/0101-2800.20130016. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed S.S., Shariq A., Alsalloom A.A., Babikir I.H., Alhomoud B.N. Uropathogens and their antimicrobial resistance patterns: relationship with urinary tract infections. Int J Health Sci. 2019;13:48. [PMC free article] [PubMed] [Google Scholar]
- 24.Paul R. State of the globe: rising antimicrobial resistance of pathogens in urinary tract infection. J Glob Infect Dis. 2018;10:117. doi: 10.4103/jgid.jgid_104_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jancel T., Dudas V. Management of uncomplicated urinary tract infections. West J Med. 2002;176:51. doi: 10.1136/ewjm.176.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira A.M., Bonesso M.F., Mondelli A.L., Camargo C.H., Maria de Lourdes R.S. Oxacillin resistance and antimicrobial susceptibility profile of Staphylococcus saprophyticus and other staphylococci isolated from patients with urinary tract infection. Chemotherapy. 2012;58:482–491. doi: 10.1159/000346529. [DOI] [PubMed] [Google Scholar]
- 27.Tekin A., Dal T., Ö Deveci, Tekin R., Özcan N., Atmaca S. In vitro susceptibility to methicillin, vancomycin and linezolid of staphylococci isolated from bloodstream infections in eastern Turkey. Braz J Microbiol. 2014;45:829–833. doi: 10.1590/s1517-83822014000300010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoshbakht R., Salimi A., Shirzad H., Keshavarzi H. Antibiotic susceptibility of bacterial strains isolated from urinary tract infections in Karaj, Iran. Jundishapur J Microbiol. 2013;6:86–90. [Google Scholar]
- 29.Martins K.B., Ferreira A.M., Mondelli A.L., Rocchetti T.T., LR de S da Cunha M.D. Evaluation of MALDI-TOF VITEK® MS and VITEK® 2 system for the identification of Staphylococcus saprophyticus. Future Microbiol. 2018;13:1603–1609. doi: 10.2217/fmb-2018-0195. [DOI] [PubMed] [Google Scholar]
- 30.Qi L., Li H., Zhang C., Liang B., Li J., Wang L. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiol. 2016;7:483. doi: 10.3389/fmicb.2016.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurung J., Khyriem A.B., Banik A., Lyngdoh W.V., Choudhury B., Bhattacharyya P. Association of biofilm production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa from intensive care unit. Ind J Crit Care Med. 2013;17:214. doi: 10.4103/0972-5229.118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung J.Y. Molecular characterization and antimicrobial susceptibility of biofilm-forming Acinetobacter baumannii clinical isolates from Daejeon, Korea. Korean J Clin Lab Sci. 2018;50:100–109. [Google Scholar]
- 33.de Campos P.A., Royer S., da Fonseca Batistão D.W., Araújo B.F., Queiroz L.L., de Brito C.S. Multidrug resistance related to biofilm formation in Acinetobacter baumannii and Klebsiella pneumoniae clinical strains from different pulsotypes. Curr Microbiol. 2016;72:617–627. doi: 10.1007/s00284-016-0996-x. [DOI] [PubMed] [Google Scholar]