Abstract
Background & purpose
With the introduction of more conformal techniques for breast cancer radiation therapy (RT), motion management is becoming increasingly important. We studied the breast-shape variability during RT after breast-conserving surgery (BCS).
Materials & Methods
Planning computed tomography (CT) and follow-up cone-beam CT (CBCT) scans were available for 71 fractions of 17 patients undergoing RT after BCS. First, the CT and the CBCT scans were registered on bones. Subsequently, breast-contour data were generated. The CBCT contours were analyzed in 3D in terms of deviations (mean and standard deviation) relative to the contour of the CT scan for the upper medial, lower medial, upper lateral, and lower lateral breast quadrants, and the axilla.
Results
Regional systematic and random standard deviations of the breast quadrants varied between 1.5 and 2.1 mm and 1.0–1.6 mm, respectively, and were larger for the axilla (3.0 mm). An absolute average shape change of ≥4.0 mm in at least one region was present in 21/71 fractions (30%), predominantly in breast volumes > 800 cc (p = <0.01). Furthermore, seroma was associated with larger shape changes (p = 0.04).
Conclusions
Breast-shape variability varies between anatomic locations. Changes in the order of 4 mm are frequently observed during RT, especially for large breasts. This should be taken into account in the development of protocols for partial breast irradiation and boost treatment.
Keywords: Breast cancer, Breast-conserving surgery, Radiation therapy, Cone-beam computed tomography, Shape variability
1. Introduction
In recent years, more conformal radiation techniques have been introduced on a large scale in RT for breast cancer after breast-conserving therapy (BCT). Such techniques demand more attention to potential setup errors of the breast soft tissue. With the introduction of cone-beam computed tomography (CBCT) soft tissue evaluation of patient setup has become clinically feasible. At the same time, the delivered imaging dose throughout treatment became an issue of concern because of the nature of image-guidance protocols which often require frequent (up to daily) imaging of potential larger body volumes [1]. A recent study concluded that monitoring soft tissue motion should become the standard of care for patients at risk for large soft tissue variations [2]. However, available data on daily soft tissue motion and its correlation with clinical factors are limited.
RT after BCT typically consists of whole breast irradiation (WBI) combined with an additional boost to the original tumor position [3]. Boost irradiation can be planned and delivered separately from or integrated with WBI [4], [5]. Most of the local recurrences (70–80%) found after BCT are at or close to the original tumor position [6], [7], [8]. Therefore, in selected low-risk patients, WBI is unnecessary. Consequently, in low-risk patients partial breast irradiation (PBI) is upcoming, focusing on irradiation of the original tumor position only [9], [10].
One of the major challenges in RT boosting or PBI is the correct definition of the target volume and its localization during treatment. Geometrical uncertainties can be divided in three major categories: delineation uncertainties, patient setup, and organ motion, or in the case of the breast, shape changes. Accurate delineation of the original tumor position poses a major challenge [11], [12], [13]. Even when clips are implanted during surgery, variability in delineated volume is substantial [14]. Variation caused by day-to-day setup variation was found to be considerable, with central lung distance values (defined as the distance between the deep field edge and the interior chest wall at the central axis) ranging from 5.9 mm to 29.4 mm [15]. Little data have been published on inter-fractional shape changes of the breast. Significant changes and time trends on post-operative seroma volumes (i.e., fluid build-up in the excision cavity) have been reported prior to RT and during RT [16], [17], [18]. Further, breast surface deformation values, based on 3D surface data acquired with a video based surface imaging system, of <2.0 mm (2SD) were reported [19]. In a previous study, the robustness of dose distributions from three whole-breast RT techniques involving different levels of intensity modulation against patient setup inaccuracies and breast-shape changes was investigated [20]. Plan deterioration due to shape changes of the breast was primarily observed in planning techniques without glancing fields, demanding specific attention in PBI techniques.
If all geometric uncertainties are known, an evidence-based safety margin can be estimated by collating standard deviations of the uncertainties [21]. The purpose of this study was to thoroughly investigate the breast-shape changes during the course of RT after breast-conserving surgery (BCS). For this purpose, breast contours extracted from cone-beam computed tomography (CBCT) scans acquired during the course of treatment were analyzed in 3D in terms of deviations (mean and standard deviation) relative to the contour of the breast on the planning CT scan. Additionally, we divided the contour data in separate regions of interest (four breast quadrants and axilla) and investigated the shape changes for these regions separately. Furthermore, we investigated correlations between breast-shape changes and clinical parameters.
2. Materials and methods
This study included 17 female breast cancer patients who received RT after undergoing microscopically complete tumor excision. No study consent was needed for this retrospective analysis of clinically available data (waived by the Ethics Committee). To also enable investigation of the effect of different arm-support systems on breast-shape changes, we included patients treated in two periods (2006 and 2011). Characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of included female breast cancer patients.
| Characteristic (n = 17) | |
|---|---|
| Age year (median, range) | 60 (39–75) |
| Breast volume cc (median, range) | 927 (430–1312) |
| Tumor volume cc (median, range) | 15 (5–42) |
| Left-sided/right-sided n (%) | 6 (35%)/9 (65%) |
| Arm support + year of treatment (n) | |
| In-house developed type I (2006) | 8 |
| In-house developed type II (2006) | 1 |
| C-Qual Breastboard (2011) | 8 |
2.1. Treatment and imaging
The RT planning target volume included the whole breast (and the axilla if indicated by the physician). Prescribed dose and fractionation varied between patients. Six patients received 50 Gy in 25 fractions with a sequential photon boost of 16 Gy in 8 fractions. Two patients received 50 Gy (25 fractions) without a boost. For the other nine patients, different fractionation schedules >2 Gy/fraction were prescribed (seven patients with an integrated boost): 73.8 Gy in 31 fractions (n = 2), 64.4 Gy in 28 fractions (n = 1), 55.9 Gy in 21 fractions (n = 4), 42.6 Gy in 16 fractions with no boost (n = 2). Delineated organs at risk were the heart (for left-sided breast cancer) and the lungs.
For all but one patient treated in 2006 an in-house-developed arm-support system was used during RT whereas for the patients treated in 2011 an arm-support system developed by CIVCO Medical Solutions (C-Qual Breastboard, CIVCO Medical Solutions, Orange City, IA) was used. In both arm-support systems the positioning of the patients is similar with both arms brought above the shoulders out of the RT field. A difference between these two arm-support systems is that the C-Qual provides a more conformal support, enhancing the reproducibility of the patient arm setup. For one patient, with a limitation in arm function, a support system was used that allowed to position her arm next to the treatment table with the elbow flexed 90 degrees. During RT all patients were positioned on a breast board at a 10 degrees tilt. Further, a knee-support device (CIVCO Medical Solutions, Orange City, IA) was used for the patient’s comfort.
Free-breathing CT scans (Somatom Sensation Open, Siemens, Forchheim, Germany) made for RT planning were used. Immobilization during acquisition of the planning CT scan was identical to immobilization during treatment. At our institute, CBCT scans (Elekta Synergy, Elekta Oncology Systems, Crawley, West Sussex, UK) are acquired routinely for setup verification. A two-phase shrinking action level protocol was used with daily verification during the first phase and weekly verification during the second phase [22]. We selected for all patients CBCT scans that represented the entire treatment; CBCT scans with at least three days between acquisitions were included.
2.2. Contour extraction
First, the follow-up CBCT scans and planning CT scan were aligned by performing a rigid registration on the bony anatomy using a 3D box-shaped region of interest containing the sternum and ribs on the irradiated side, excluding the arm. A chamfer matching algorithm was used that only considers bony anatomy for registration [22]. Next, the CBCT data were pre-processed by use of a digital filtering technique (median filter with window size 5) to remove noise. After filtering, suitable thresholds, based on the ratio of the grey values of breast tissue and air, for segmentation of the patient contour in the CBCT data were manually assessed for the 2006 and 2011 patient groups separately. Further, a 3D contour was automatically extracted from the planning CT scan by means of thresh-holding and smoothing using in-house developed software [23]. Contours are a collection of 3D points in space that are connected into triangles (contour elements).
2.3. Data analysis
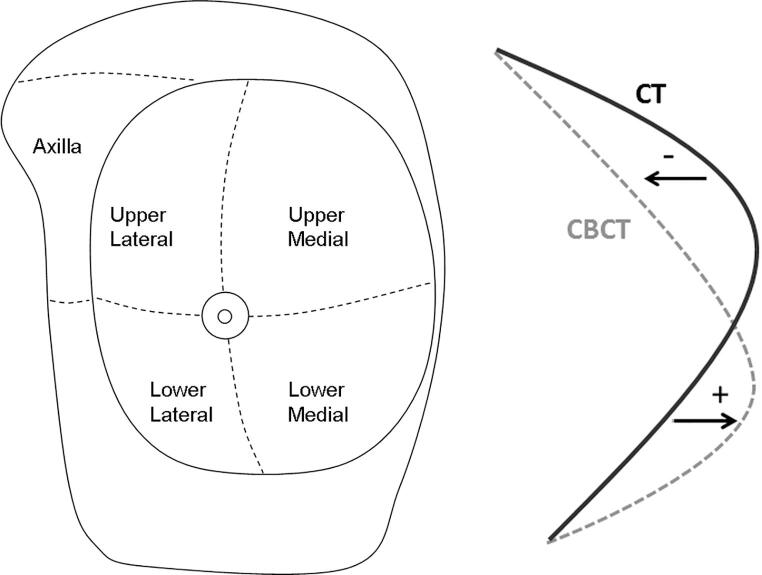
Different regions of interest (ROIs) were defined on the planning CT contour data: the upper lateral breast quadrant (UL), upper medial breast quadrant (UM), lower medial breast quadrant (LM), lower lateral breast quadrant (LL), and the axilla (Fig. 1-left).
Fig. 1.
Left: Illustration of the different regions of interest investigated in this study: the four breast quadrants and the axilla. The nipple area was excluded from the analysis. Right: Figure indicating how shape changes were assessed from the extracted CT and CBCT contours. The distance between each contour element in the planning CT contour and the nearest neighbor contour element in the CBCT contour was calculated. A negative distance value indicates that the corresponding CBCT contour element lies closer to the ribs than the CT contour element and a positive distance value indicates that the CBCT contour element lies further away from the ribs than the CT contour element.
For each contour element in the planning CT contour, the nearest neighbor contour element in the CBCT contour was determined and the distance between the two elements was calculated (Fig. 1-right).
For each included CBCT scan, we calculated, per ROI, the weighted mean distance and the standard deviation of the distances found for the contour elements. We also assessed these values for the whole breast by calculating the weighted average of the values found for the four breast quadrants. Further, based on the weighted mean values, group means (M), systematic error (Σ), and random errors (σ) were assessed [21].
Next, for each patient separately, for each contour element in the planning CT the mean and standard deviation of the contour-element distances computed for all included follow-up CBCT scans were assessed (inter-fractional shape changes). Then the proportion of contour area that exhibits a certain distance was assessed for each ROI separately as well as for the whole breast. These findings were averaged over all patients.
2.4. Statistical analysis
Correlations between breast-shape-change parameters and clinical parameters (seroma (y/n), breast volume, arm-support system, number of days from start treatment, tumor size, and original tumor position) were calculated (Spearman correlation). Statistical significance was set at p < 0.05. Differences in shape changes between subgroups were evaluated using a non-parametric test (Mann-Whitney) for continuous outcomes, and the Pearson Chi-square test for binomial and ordinal outcomes.
3. Results
3.1. Breast-shape changes
For 17 patients, data from 71 fractions were analyzed (range: 3–6 CBCT scans per patient distributed over week 1–5). Time since start treatment for the selected CBCTs varied between 1 and 38 days. Time between the acquisition of the planning CT scan and start treatment varied between 2 and 14 days (average of 8 days).
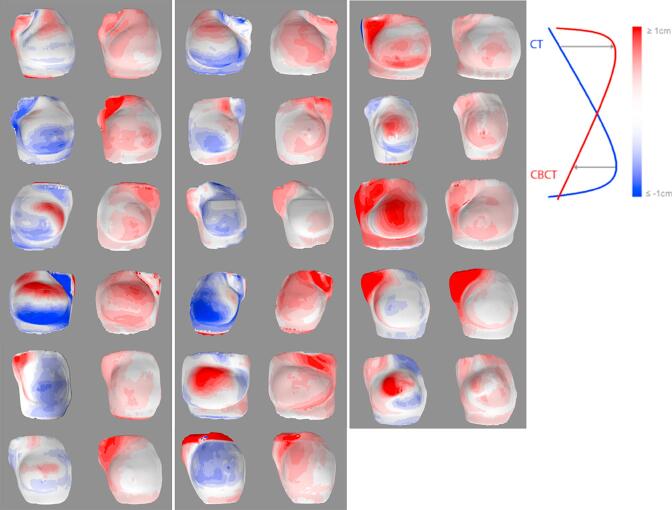
An overview of the calculated group mean, systematic error Σ, and random error σ found for the shape changes for the different ROIs are summarized in Table 2. In 11 patients, an absolute mean shape change of ≥4.0 mm in at least one breast quadrant was present in at least 1 fraction. The number of patients per quadrant with an absolute mean shape change ≥4.0 mm was: UL: n = 6, UM: n = 4, LM: n = 6, LL: n = 4. In total, an absolute mean shape change of ≥4.0 mm in at least one breast quadrant was observed in 30% (21/71) of the included fractions. Comparing shape changes (% with an absolute mean distance ≥4.0 mm) between ROIs (Table 2), the axilla showed more variability than the breast quadrants (p < 0.01). In Fig. 2, the inter-fractional shape changes (mean and sd) are illustrated by color-coded breast contours for each patient. Histograms indicating the mean and standard deviation of the shape changes over the course of treatment (i.e., inter-fractional shape changes) for the different ROIs are given in Supplementary 1 and 2, respectively. On average 14% of the breast contour had an average deviation ≥4.0 mm relative to the planning CT scan. Furthermore, the inter-fractional variation was between 1.5 and 2.5 mm for 30% and between 2.5 and 3.5 mm for 10% of the breast.
Table 2.
Group mean (M), systematic error (Σ), random error (σ) and % with an absolute mean distance ≥ 4.0 mm (17 patients, 71 fractions). The last 3 columns concern breast volumes (Vbreast) <800 cc (n = 8, 31 fractions) and > 800 cc (n = 9, 40 fractions) and the corresponding p value.
| ROI | M(mm) | Σ (mm) | σ (mm) | ROI |mean distance| ≥4.0 mm |
p |
||
|---|---|---|---|---|---|---|---|
| All fractions | Vbreast <800 cc | Vbreast >800 cc | |||||
| UL | 0.8 | 1.6 | 1.6 | 8/71 (11%) | 4/31 (13%) | 4/40 (10%) | NS |
| UM | 0.2 | 2.0 | 1.3 | 7/71 (10%) | 0/31 (0%) | 7/40 (18%) | 0.016 |
| LM | −1.4 | 2.1 | 1.1 | 9/71 (13%) | 1/31 (3%) | 8/40 (20%) | 0.07 |
| LL | −0.8 | 1.9 | 1.4 | 7/71 (10%) | 0/31 (0%) | 7/40 (18%) | 0.016 |
| WB | −0.3 | 1.5 | 1.0 | 2/71 (3%) | 0/31 (0%) | 2/40 (5%) | NS |
| Axilla | 0.6 | 3.0 | 2.9 | 17/71 (24%) | 5/31 (16%) | 12/40 (30%) | NS |
Abbreviations: ROI = region of interest, UL = upper lateral, UM = upper medial, LM = lower medial, LL = lower lateral, WB = whole breast, V = volume. NS = not significant (i.e., p value > 0.1).
Fig. 2.
The inter-fractional shape changes (per patient: left: mean, right: standard deviation) illustrated by color-coded breast contours for all study patients (n = 17).
3.2. Clinical parameters associated with breast-shape changes
Correlating the absolute mean distances of each fraction with time, we found significant correlations of time with the absolute distance (CBCT–CT) for the UM (r = 0.25, p = 0.03) and the UL (r = 0.25, p = 0.04). Evaluating patients with and without seroma separately, time was correlated with the absolute distance (CBCT – CT) for the UM (r = 0.44, p < 0.01) in patients without seroma and with the UL (r = 0.59, p < 0.01) and the LM (r = 0.49, p = 0.02) in patients with seroma. We identified clinical parameters (seroma, breast volume, and arm support) that correlated with breast-shape-change parameters, as summarized in Table 3. With respect to the breast-shape variability data of the patient with the limitation in arm function: these were well within the range of the other patients. The other tested clinical parameters (tumor size, original tumor position) did not correlate with any breast-shape-change parameter (p values ranging from 0.06 to 1.0).
Table 3.
Spearman correlation coefficients (with corresponding p value) for breast-shape-change errors and clinical parameters (n = 17 patients).
| Error | Clinical parameter |
||
|---|---|---|---|
| Seroma y/n | Breast volume | Arm support | |
| UL sd (σ) | −0.24 NS |
−0.02 NS |
−0.03 NS |
| UM sd (σ) | −0.13 NS |
0.66 p < 0.01 |
0.38 NS |
| LM sd (σ) | −0.03 NS |
0.28 NS |
0.54 p = 0.03 |
| LL sd (σ) | 0.11 NS |
0.18 NS |
0.33 NS |
| Mean WB (M) | 0.55 p = 0.02 |
0.25 NS |
−0.46 p = 0.07 |
| WB sd (σ) | −0.08 NS |
0.53 p = 0.03 |
0.14 NS |
| Axilla sd (σ) | 0.00 NS |
−0.03 NS |
0.14 NS |
| % fractions absolute mean shape change ≥4.0 mm | −0.20 NS |
0.64 p < 0.01 |
0.37 NS |
Abbreviations: UL = upper lateral, UM = upper medial, LM = lower medial, LL = lower lateral, WB = whole breast, sd = standard deviation, σ = random error, M = group mean. NS = not significant (i.e., p value > 0.1).
In total, five patients developed post-operative seroma. The patient mean for the whole breast was significantly correlated with the presence of seroma (p = 0.02); patients with seroma (n = 5) had a larger group mean than patients without seroma (n = 12). The location of the seroma varied between patients (UL, LM, LL, UM, central).
The random error σ for the whole breast was correlated with breast volume (p = 0.03). Looking at the regional σ within each ROI, we found significant correlations between breast volume and the UM error. The % of fractions with an absolute mean distance ≥4.0 mm was correlated with breast volume (p = 0.006). Patients with breast volumes >800 cc had significantly more often clinically relevant shape changes (i.e., absolute mean distances ≥4.0 mm within at least one breast quadrant) compared to patients with breast volumes <800 cc, as indicated in Table 2. An absolute average shape change of ≥4.0 mm in at least one ROI was present in 17/40 fractions in the >800 cc subgroup (9 patients) versus 4/31 fractions in the <800 cc subgroup of 8 patients (p = 0.007). When we fitted the probability of a clinical relevant shape change in at least one breast quadrant as a function of breast volume, we obtained a probability that ranges from about 10% for a volume of 400 cc up to about 60% for volumes in the range of 1300 cc. Evaluating the ROIs separately, a larger breast volume was associated with significantly larger random errors (σ) for the whole breast and for the UM (Table 3). Furthermore, Table 3 indicates a significant correlation between breast volume (as a continuous variable) and the % of fractions (at patient level) with a mean breast-shape change of ≥4.0 mm.
Looking at the regional σ within each ROI, we found significant correlations between arm support and the LM error. We evaluated the clinically relevant shifts (within fractions) and its possible relationship with the applied arm support (the in-house developed type I versus the commercial support system). Overall, there were only small differences in absolute mean shape changes ≥4.0 mm for the axilla and the whole breast between the two arm-support systems. An absolute mean shape change ≥4.0 mm in ≥1 breast quadrant was present in 22% of the fractions with the commercial support and 56% of the fractions with the in-house developed support (p = 0.2). Looking at the breast quadrants separately, there tended to be more shifts ≥4.0 mm in the LM (18% vs 6% of the fractions, p > 0.1) and the LL (15% vs 3%, p = 0.09) for the in-house developed support whereas there tended to be more shifts ≥4.0 mm in the UL using the commercial arm support (19% vs 5%, p = 0.07).
4. Discussion
In this work, breast-shape variability during the course of radiation therapy (RT) after breast-conserving surgery (BCS) was investigated in detail by investigating the whole breast as well as regions of interest separately (four breast quadrants and axilla). 3D analysis of follow-up cone-beam CT (CBCT) scans relative to the planning CT scan indicated that shifts in the order of 4 mm frequently occur; in 30% of the fractions in this data set. Clinical parameters associated with breast-shape-changes were large (>800 cc) breast volumes, the presence of seroma, and time since start of treatment.
Studies quantifying breast-shape changes in detail are scarce. One study [19] investigated breast-surface deformation in 15 patients with the whole breast as region of interest, and identified 10 patients with modest deformations versus 5 patients with higher deformation values, which was correlated with increasing breast volume, resection volume, planning target volume and height of the seroma above the chest wall. They reported absolute means and standard deviations in the range of 0.3–0.9 mm, which is smaller than the observed shape changes in our study for the whole breast. The patients that they identified as being at risk for larger deformations had an average breast volume of 939 cc versus 724 cc for the group not at risk. Large breasts are associated with a large breast separation (i.e., the distance between the entrance points of the medial and lateral tangential beams entering at the breast isocenter point plane) which may have a clinically relevant impact on dosimetry, especially with respect to the dose in the ipsilateral lung [24].
Different clinical setup verification strategies are currently applied in RT for breast cancer [2], [25], [26], [27]. A recent study concluded that for tangential breast RT there is no significant variation in the ability to detect setup errors with electronic portal imaging protocols versus CBCT imaging protocols [2]. Furthermore, clip-based setup verification based on CBCT has been reported as superior to bony anatomy-based setup verification during delivery of an RT boost. [25] Van der Salm et al. [28] compared online setup verification using surgical clips versus verification with skin markers in 35 patients, and found comparable setup corrections and target volume coverage. In our clinic, setup verification is based on rigid CBCT-CT registration of the bony anatomy (i.e. ribs and sternum). The evaluation of breast contours is usually not included in bony anatomy-based setup verification protocols, although several studies recognized the potential benefit of 3D surface imaging and/or contour registration [1], [2], [5], [19], [20], [23], [29].
In our study, the presence of seroma was found to be correlated with breast-shape changes. Similar observations have been reported by other [25], [30]. Harris et al. [25] reported significantly larger setup errors for patients with visible seroma, and argues for customized boost margins based on patient and treatment characteristics. Yeu et al. [30] proposed a seroma-specific marker-based approach to improve treatment accuracy for such patients. Our group previously reported on the dosimetric impact of post-operative seroma reduction during RT after BCS. A dosimetric advantage for these patients was demonstrated when a re-planning of the simultaneous integrated boost was done halfway treatment [5].
For the delivery of partial breast irradiation, safety margins are needed that include patient positioning errors as well as breast-shape changes. The impact of the observed shape changes on possible shifts of target volumes and locally delivered dose are currently not known. Furthermore, to what extent the surface is a good surrogate for the position of the tumor bed, will be different from patient to patient. It can be expected that tumors located relatively superficial are more at risk for geometrical shifts due to breast-shape changes than tumors located more at the site of the ribs. A setup verification protocol combining bony anatomy landmarks as well as surface information could be based on CBCT imaging alone as well as on a combination of CBCT and 3D surface imaging [29].
In conclusion, breast-shape changes are frequently observed during RT, especially for larger breasts. This should be taken into account in the development of planning- and setup verification protocols concerning partial breast irradiation and the delivery of breast boost treatment in whole breast irradiation.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank J.C. Duppen and J. Honnef (Department of Radiation Oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands) for their contributions to this study. Financial support of this work was provided by the Dutch Cancer Society (Grant No. KWF 2008-4024).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2018.05.006.
Contributor Information
Tanja Alderliesten, Email: t.alderliesten@amc.uva.nl.
Wilma D. Heemsbergen, Email: w.heemsbergen@erasmusmc.nl.
Anja Betgen, Email: a.betgen@nki.nl.
Paula H.M. Elkhuizen, Email: p.elkhuizen@nki.nl.
Corine van Vliet-Vroegindeweij, Email: c.v.vliet@nki.nl.
Peter Remeijer, Email: p.remeijer@nki.nl.
Appendix A. Supplementary data
References
- 1.Ding G.X., Alaei P., Curran B., Flynn R., Gossman M., Mackie T.R. Image guidance doses delivered during radiotherapy: Quantification, management, and reduction: report of the AAPM Therapy Physics Committee Task Group. Med Phys. 2018;180 doi: 10.1002/mp.12824. doi: 10.1002/mp.12824. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Batumalai V., Phan P., Choong C., Holloway L., Delaney G.P. Comparison of setup accuracy of three different image assessment methods for tangential breast radiotherapy. J Med Radiat Sci. 2016;63:224–231. doi: 10.1002/jmrs.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartelink H., Horiot J.C., Poortmans P.M., Struikmans H., van den Bogaert W., Fourquet A. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881–10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 4.Hijal T., Fournier-Bidoz N., Castro-Pena P., Kirova Y.M., Zefkili S., Bollet M.A. Simultaneous integrated boost in breast conserving treatment of breast cancer: a dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol. 2010;94:300–306. doi: 10.1016/j.radonc.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 5.Alderliesten T., den Hollander S., Yang T.J., Elkhuizen P.H., van Mourik A.M., Hurkmans C. Dosimetric impact of post-operative seroma reduction during radiotherapy after breast-conserving surgery. Radiother Oncol. 2011;100:265–270. doi: 10.1016/j.radonc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Elkhuizen P.H., van de Vijver M.J., Hermans J., Zonderland H.M., van de Velde C.J., Leer J.W. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 7.Cajucom C.C., Tsangaris T.N., Nemoto T., Driscoll D., Penetrante R.B., Holyoke E.D. Results of salvage mastectomy for local recurrence after breast-conserving surgery without radiation therapy. Cancer. 1993;71:1774–1779. doi: 10.1002/1097-0142(19930301)71:5<1774::aid-cncr2820710511>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Osborne M.P., Borgen P.I., Wong G.Y., Rosen P.P., McCormick B. Salvage mastectomy for local and regional recurrence after breast-conserving operation and radiation therapy. Surg Gynecol Obstet. 1992;174:189–194. [PubMed] [Google Scholar]
- 9.Njeh C.F., Saunders M.W., Langton C.M. Accelerated partial breast irradiation using external beam conformal radiation therapy: a review. Crit Rev Oncol Hematol. 2012;81:1–20. doi: 10.1016/j.critrevonc.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Beitsch P.D., Shaitelman S.F., Vicini F.A. Accelerated partial breast irradiation. J Surg Oncol. 2011;103:362–368. doi: 10.1002/jso.21785. [DOI] [PubMed] [Google Scholar]
- 11.Petersen R.P., Truong P.T., Kader H.A., Berthelet E., Lee J.C., Hilts M.L. Target volume delineation for partial breast radiotherapy planning: clinical characteristics associated with low interobserver concordance. Int J Radiat Oncol Biol Phys. 2007;69:41–48. doi: 10.1016/j.ijrobp.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 12.Struikmans H., Wárlám-Rodenhuis C., Stam T., Stapper G., Tersteeg R.J., Bol G.H. Interobserver variability of clinical target volume delineation of glandular breast tissue and of boost volume in tangential breast irradiation. Radiother Oncol. 2005;76:293–299. doi: 10.1016/j.radonc.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 13.van Mourik A.M., Elkhuizen P.H., Minkema D., Duppen J.C. Dutch Young Boost Study Group, van Vliet-Vroegindeweij C. Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines. Radiother Oncol. 2010;94:286–291. doi: 10.1016/j.radonc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Coles C.E., Harris E.J., Donovan E.M., Bliss P., Evans P.M., Fairfoul J. Evaluation of implanted gold seeds for breast radiotherapy planning and on treatment verification: a feasibility study on behalf of the IMPORT trialists. Radiother Oncol. 2011;100:276–281. doi: 10.1016/j.radonc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Smith R.P., Bloch P., Harris E.E., McDonough J., Sarkar A., Kassaee A. Analysis of interfraction and intrafraction variation during tangential breast irradiation with an electronic portal imaging device. Int J Radiat Oncol Biol Phys. 2005;62:373–378. doi: 10.1016/j.ijrobp.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson G., Bets V., Smith B. Change in volume of lumpectomy cavity during external-beam irradiation of the intact breast. Int J Radiat Oncol Biol Phys. 2006;65:1161–1164. doi: 10.1016/j.ijrobp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Kader H.A., Truong P.T., Pai R., Panades M., Jones S., Ansbacher W. When is CT-based postoperative seroma most useful to plan partial breast radiotherapy? Evaluation of clinical factors affecting seroma volume and clarity. Int J Radiat Oncol Biol Phys. 2008;72:1064–1069. doi: 10.1016/j.ijrobp.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Yang T.J., Elkhuizen P.H., Minkema D., Heemsbergen W., van Mourik A.M., Cassee J. Clinical factors associated with seroma volume reduction in breast-conserving therapy for early stage breast cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2010;76:1325–1332. doi: 10.1016/j.ijrobp.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Riboldi M., Book L.N., Chen G.T.Y., Taghian A., Baroni G., Gierga D.P. Quantitative assessment of surface deformation in accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2007;69:S669. [Google Scholar]
- 20.van Mourik A., van Kranen S., den Hollander S., Sonke J.J., van Herk M., van Vliet-Vroegindeweij C. Effects of setup errors and shape changes on breast radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1557–1564. doi: 10.1016/j.ijrobp.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 21.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.van Herk M., de Munck J.C., Lebesque J.V., Muller S., Rasch C., Touw A. Automatic registration of pelvic computed tomography data and magnetic resonance scans including a full circle method for quantitative accuracy evaluation. Med Phys. 1998;25:2054–2067. doi: 10.1118/1.598393. [DOI] [PubMed] [Google Scholar]
- 23.Honnef J., Zijp L., Sonke J.-J., Duppen J., Nijkamp J., Betgen A. Automatic breast-surface segmentation of deep inspiration breath hold cone-beam CT scans. Radiother Oncol. 2010;96:S99. [Google Scholar]
- 24.Heineman T.E., Sabbas A., Delamerced M.S., Chiu Y.L., Smith M., Parashar B. Impact of a large breast separation on radiation dose delivery to the ipsilateral lung as result of respiratory motion quantified using free breathing and 4D CT-based planning in patients with locally advanced breast cancers: a potential for adverse clinical implications. J Can Res Ther. 2013;9:154–160. doi: 10.4103/0973-1482.110368. [DOI] [PubMed] [Google Scholar]
- 25.Harris E.J., Mukesh M.B., Donovan E.M., Kirby A.M., Haviland J.S., Jena R. Evans PM; IMPORT high trialists. A multicentre study of the evidence for customized margins in photon breast boost radiotherapy. Br J Radiol. 2016;89:20150603. doi: 10.1259/bjr.20150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batumalai V., Holloway L., Delaney G.P. A review of setup error in supine breast radiotherapy using cone-beam computed tomography. Med Dosim. 2016;41:225–229. doi: 10.1016/j.meddos.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Tsang Y., Ciurlionis L., Kirby A.M., Locke I., Venables K., Yarnold J.R. IMPORT trial management group. Clinical impact of IMPORT HIGH trial (CRUK/06/003) on breast radiotherapy practices in the United Kingdom. Br J Radiol. 2015;88:20150453. doi: 10.1259/bjr.20150453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Salm A., Murrer L., Steenbakkers I., Houben R., Boersma L.J. Actual target coverage after setup verification using surgical clips compared with external skin markers in postoperative breast cancer radiation therapy. Pract Radiat Oncol. 2017;7:e369–76. doi: 10.1016/j.prro.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Betgen A., Alderliesten T., Sonke J.-J., van Vliet-Vroegindeweij C., Bartelink H., Remeijer P. Assessment of set-up variability during deep inspiration breath hold radiotherapy for breast cancer patients by 3D-surface imaging. Radiother Oncol. 2013;106:225–230. doi: 10.1016/j.radonc.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Yue N.J., Goyal S., Zhou J., Khan A.J., Haffty B.G. Intrafractional target motions and uncertainties of treatment setup reference systems in accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:1549–1556. doi: 10.1016/j.ijrobp.2010.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.