Abstract
Context
The results of preclinical and observational studies support the beneficial effect of soy isoflavones on cognition.
Objective
This review aimed to evaluate the effects of soy isoflavones on cognition in adults.
Data Sources
The PUBMED, EMBASE, Ovid Medline, Cochrane Library, and clinicaltrials.gov databases were searched.
Study Selection
Two researchers independently screened 1955 records, using the PICOS criteria: participants were adults; intervention was dietary sources with soy isoflavones or isolated soy isoflavones; comparator was any comparator; outcome was cognitive function; study type was randomized controlled trials (RCTs). A third researcher was consulted to resolve any discrepancies. Sixteen RCTs were included and their quality assessed.
Data Extraction
Information on study design, characteristics of participants, and outcomes was extracted. PRISMA guidelines were followed.
Data Analysis
A random-effects meta-analysis was used to pool estimates across studies. In the 16 RCTs (1386 participants, mean age = 60 y), soy isoflavones were found to improve overall cognitive function (standardized mean difference [SMD], 0.19; 95% confidence interval [CI], 0.07–0.32) and memory (SMD, 0.15; 95%CI, 0.03–0.26).
Conclusion
The results showed that soy isoflavones may improve cognitive function in adults.
Systematic Review Registration
PROSPERO registration no. CRD42018082070.
Keywords: cognition, isoflavones, meta-analysis
INTRODUCTION
Dementia affects 5 million Americans.1 In the United States alone, total costs for patients with dementia were $259 billion in 2017, and are predicted to increase to $1.1 trillion by 2050.1 Effective prevention strategies for dementia is of critical importance given that a delayed onset of 5 years reduces the degree of dementia prevalence by 41%.2 Dietary intervention may significantly contribute to the prevention of dementia. Observational studies have shown the Dietary Approaches to Stop Hypertension (DASH) diet, Mediterranean diet (MedDiet), and similar dietary patterns to be associated with better cognitive function.3–6 A post-hoc analysis of the Prevención con Dieta Mediterránea (PREDIMED) study revealed that the MedDiet improved cognitive function among the older population.7,8 The National Academy of Medicine emphasizes the need to identify nutrients and food associated with cognitive benefits, which could be added to the DASH diet or the MedDiet to develop a comprehensive dietary intervention for cognitive decline and dementia.9
Soy isoflavones (ISFs) are phytoestrogens, which are thought to exert beneficial effects on cognition function through their estrogen-like activity. Preclinical studies highlight the importance of ISFs in several Alzheimer’s disease–like pathologies, including removing amyloid β10 and decreasing tau phosphorylation.11 Additionally, ISFs exert anti-inflammatory and anti-oxidative effects,12 and inhibit the mitochondrial apoptotic pathway, which leads to Alzheimer’s disease.13 It is unsurprising that animal studies have shown that ISFs improve cognitive function.10,11,13
In human research, some observational studies have highlighted the cognitive benefits of ISFs.14–18 Since the early 2000s, randomized controlled trials (RCTs) have been conducted to explore the effect of ISFs on cognition, with inconsistent results.19–25 Additionally, some systematic reviews have reported inconclusive results regarding the effect of ISFs on cognition.26–29 Cheng et al30 conducted a meta-analysis of the effects of ISFs in postmenopausal women, which indicated that ISFs improve cognitive function in this group. However, trials that included men or young women, some of which suggested positive effects of ISFs on cognition,32,34 were not included in their analysis,23,31–34 In addition, they failed to capture all of the trials involving postmenopausal women,22,35 and they included a study of red clover, which only contains 2% soy isoflavones.36
The primary aim of this systematic review and meta-analysis was to determine the effect of ISFs on overall and domain-specific cognitive functions by systematically and quantitatively summarizing the results of RCTs that have explored ISFs in adults (both men and women). The secondary aim was to evaluate any adverse effects of ISFs within these RCTs. With the above information, the potential role of ISFs in preventing cognitive decline and dementia was then further discussed.
MATERIALS AND METHODS
Literature search
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix S1 in the Supporting Information online) and the protocol was registered with PROSPERO (CRD42018082070).37 The databases PubMed, EMBASE through Embase.com, Ovid Medline, Cochrane Central Register of Controlled Trials, and clinicaltrials.gov were searched from inception through June 2018 by 2 investigators (C.C. and R.B.). One or more textual or MESH terms were used for isoflavones (isoflavone, genistein, daidzein, equol, soy, soya), cognitive factors (dementia, cognition, cognitive, memory, executive function, spatial, attention, brain, neuron, neuropsychological, Alzheimer’s disease), and RCTs (randomized, trial, human, placebo, epidemiological studies, intervention) (Table S1 in the Supporting Information online). The search terms were devised by 3 investigators (R.B., C.C., B.S., and A.S.) and confirmed by a librarian. Reference lists of included articles and related systematic reviews were reviewed to identify additional articles, and 2 ongoing and 2 unpublished trials were identified via clinicaltrials.gov (Table S2 in the Supporting Information online). The principal investigators of these 4 trials were contacted.
Study selection
A 2-stage screening process consisting of a title and abstract scan and a full-text review was used to determine the eligibility of articles. Both stages followed the same process. Each article was independently reviewed by 2 investigators (C.C. and R.B.). Discrepancies were discussed with another investigator (A.S.). Studies were selected in accordance with the PICOS (population, intervention, comparison, outcome, and study design) criteria shown in Table 1. The search was restricted to English-language articles only. The quality of included articles was graded by 2 investigators (C.C. and R.B.), using the Cochrane Risk of Bias tool from the Cochrane Handbook for Systematic Reviews of Interventions.38 Quality of trials was assessed in accordance with the following 7 domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.38 Study quality was evaluated in line with the Jadad scale.39,40 Jadad score calculation was based on random sequence generation, randomization, blinding, and incomplete outcome data. A Jadad score ≥4 indicated a high-quality study, and a score <4 indicated a lower-quality study. Discrepancies were discussed with another investigator (A.S.).
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Inclusion criterion |
|---|---|
| Participants | Adults ≥18 years |
| Intervention | Dietary-based (eg, soy) with isoflavones or isolated isoflavones |
| Comparison | Any |
| Outcome | Cognitive function (including global cognition, executive function, processing speed, attention, language, verbal memory, and visual memory) |
| Study design | Randomized controlled trial |
Data extraction and data synthesis
Two investigators (C.C. and R.B.) independently extracted the data from each article. Any disagreements were resolved through discussion with another investigator (A.S.).The data extracted included the following information: study design, duration of the trial, number of participants, age, participants’ characteristics (eg, postmenopausal), the source of isoflavones, intervention in control group, dose and constituents of the ISF, and any changes across the neuropsychological batteries. If the mean and standard deviation of the change from baseline were unavailable, the mean and standard deviation of the preintervention (b) and postintervention (a) performance were extracted. Then, the mean of change (c) was calculated by , and the standard deviation of change was calculated by .41 Next, was calculated according to the method described in the Cochrane Handbook,38 applying the data used in the study by Woo et al,35 since this was the largest trial to provide adequate data.41 The mean and standard deviation of the postintervention performance was used to represent the change when studies did not provide measurements of preintervention performance. The rationale was the assumption that the distributions of preintervention performance should not differ between the intervention and control groups owing to the randomization.
The standardized mean difference (SMD) was used to standardize the results, as represented by , where .41 Tests with lower scores, representing better performance (eg, time to complete the neuropsychological batteries), were multiplied by −1 so that positive values signified improvement.
Neuropsychological batteries were grouped by cognitive domains, ie, global cognition, executive function, psychomotor speed, attention/working memory, language, visuospatial reasoning, and memory (ie, learning, recall, and/or recognition of novel material), based primarily on traditional cognitive domain membership in the neuropsychological literature (Table S3 in the Supporting Information online).
The effect of ISFs on cognition was synthesized by calculating the overall and domain-specific SMD using the random-effects method. A summary SMD was calculated for each study and then added to a pooled SMD.30 The robustness of results was tested using the 3-level method to account for the dependence within studies and neuropsychological tests.42 For subgroup analysis, the SMD with 95% confidence interval (CI) was calculated for each stratum (ie, duration of trials [≥6 vs <6 mo], the dose of ISFs [≥100 vs <100 mg/d], age [≥60 vs <60 y], region [Asia vs other regions], and the study population [postmenopausal women only vs others)], and a Wald test was performed to compare the means. The adverse events were summarized. The heterogeneity was assessed by a χ2 test, and the corresponding I2 statistic was reported. A funnel plot was generated to identify potential publication bias. All tests were two-sided. A P value <0.05 was considered statistically significant. Subgroup analyses were assessed using the Bonferroni correction for multiple comparisons. The analysis was conducted using the metafor package in R.43
RESULTS
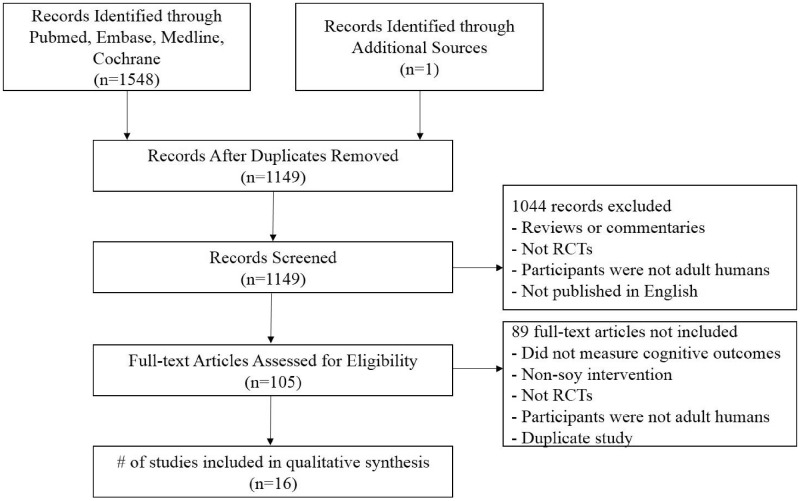
After the search of the databases, 1955 records were identified. Following the removal of duplicates, 1537 were retained. After excluding 1521 studies that did not meet the inclusion criteria (Figure 1), 16 remained. The risk of bias of the included RCTs is summarized in Table S4 in the Supporting Information online. All the RCTs used randomization, but most RCTs20–24,31,32,34,44 did not describe the methods used for the random sequence generation or allocation concealment in their reports. Regarding blinding, most RCTs used placebos that were similar to the interventions, and only some RCTs32,33,35,45 reported that they also performed blinding of the outcome assessors. In some RCTs,20,21,24,34,45,46 the loss-to-follow-up rate differed between the intervention group and control group. The study characteristics are summarized in Table 219–23,25,31–35,44–47. For the 16 studies selected, there were 1386 participants (mean age = 60 y, 21.8% Asians), of which 1252 were postmenopausal women, 27 were premenopausal women, and 107 were men. Two trials were conducted in Asian countries, and 14 trials in non-Asian regions. The median duration of intervention was 17 weeks (range 6–130 wk). The sample size varied from 27 to 313. Two trials recruited both men and women. Twelve trials were women-only, and 2 trials were men-only. Seven trials were conducted in individuals aged 60 years or older. The dose of ISFs ranged from 60 to 160 mg/d. The dose of daidzein and genistein ranged from 16 to 63 mg/d and from 12 to 64 mg/d, respectively.
Figure 1.
Flow diagram of the literature selection process. Abbreviation: RCT, randomized controlled trial.
Table 2.
| First author, y | #Participants (#F/#M) | Mean age, y | Participants’ characteristics | Country | Duration of trial, wk | Dose of ISFs, mg/d | Constituents of ISFs | Comparisons | Study design | Cognitive outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Basaria et al (2009)25 | 84 (84/0) | 56 | Postmenopausal | USA | 12 | 160 | 64 mg genistein, 63 mg daidzein, 34 mg glycitein | Whole-milk protein | DB, PC, P | Psychomotor speed; executive function; language; visuospatial reasoning |
| Casini et al (2006)22 | 77 (77/0) | 50 | Postmenopausal | Italy | 26 | 60 | 24 mg genistein, 24 mg daidzein, 12 mg glycitein | Placebo tablet | DB, PC, CO | Psychomotor speed; attention/working memory; visuospatial reasoning |
| Duffy et al (2003)21 | 33 (33/0) | 50–65 | Postmenopausal | UK | 12 | 60 | N/A | Lactose tablet | DB, PC, P | Executive function; attention/working memory; language |
| File et al (2001)34 | 27 (27/0) | 26 | Healthy | UK | 10 | 100 | N/A | Low-soy diet | P | Executive function; language |
| File et al (2005)20 | 50 (50/0) | 58 | Postmenopausal | UK | 6 | 60 | N/A | Placebo capsule | DB, PC, P | Memory; attention/working memory; language |
| Fournier et al (2007)24 | 79 (79/0) | 56 | Postmenopausal | USA | 16 | 70 |
|
Cow’s milk | DB, PC, P | Memory; attention/working memory; visuospatial reasoning |
| Gleason et al (2009)31 | 30 (15/15) | 74 | Postmenopausal women and men | USA | 26 | 100 | Glycitein | Placebo tablet | DB, PC, P | Psychomotor speed; memory; executive function; language; visuospatial reasoning |
| Gleason et al (2015)23 | 59 (34/25) | 76 | Alzheimer’s disease, postmenopausal women and men | USA | 26 | 100 | 85 mg daidzein and genistein, 15 mg others | Placebo capsule | DB, PC, P | Psychomotor speed; memory; global cognition; executive function; language; visuospatial reasoning |
| Henderson et al (2012)19 | 313 (313/0) | 61 | Postmenopausal | USA | 130 | 91 | 77 mg daidzein and genistein, 14 mg others | Milk protein–matched placebo | DB, PC, P | Psychomotor speed; memory; global cognition; executive function; attention/working memory; language; visuospatial reasoning |
| Ho et al (2007)45 | 176 (176/0) | 64 | Postmenopausal | China | 26 | 80 | 52 mg genistein, 36 mg daidzein, 3 mg glycitein | Placebo capsules | DB, PC, P | Psychomotor speed; memory; global cognition; executive function; language; visuospatial reasoning |
| Kreijkamp-Kaspers et al (2004)46 | 175 (175/0) | 67 | Postmenopausal | Netherlands | 52 | 99 | N/A | Whole-milk protein | DB, PC, P | Psychomotor speed; memory; global cognition; executive function; attention/working memory; language |
| Kritz-Silverstein et al (2003)47 | 53 (53/0) | 61 | Postmenopausal | USA | 26 | 110 | 52 mg genistein, 41 mg daidzein, 6 mg glycitein | Placebo pills | DB, PC, P | Psychomotor speed; memory; executive function; language |
| Santos-Galduroz et al (2010)44 | 36 (36/0) | 56 | Postmenopausal | Brazil | 17 | 80 | N/A | Placebo capsules | DB, PC, P | Psychomotor speed; memory; executive function; attention/working memory |
| Sharma et al (2009)33 | 33 (0/33) | 69 | Prostate cancer | USA | 12 | 160 | 60.8 mg genistein, 16 mg daidzein, 3.2 mg glycitein | Whole-milk protein | DB, PC, P | Psychomotor speed; memory; language; visuospatial reasoning |
| Thorp et al (2009)32 | 34 (0/34) | 40 | Healthy | Australia | 6 | 116 | 52 mg genistein, 36 mg daidzein, 3 mg glycitein | Placebo capsules | DB, PC, CO | Psychomotor speed; memory; executive function; attention/working memory; language; visuospatial reasoning |
| Woo et al (2003)35 | 127 (127/0) | 57 | Postmenopausal | China | 13 | 100 | 12 mg genistein, 68 mg daidzein, 36 mg glycitein | No treatment | DB, P | Psychomotor speed; memory; global cognition; executive function |
Abbreviations: CO, crossover design; DB, double-blind; P, parallel design; PC, placebo-controlled.
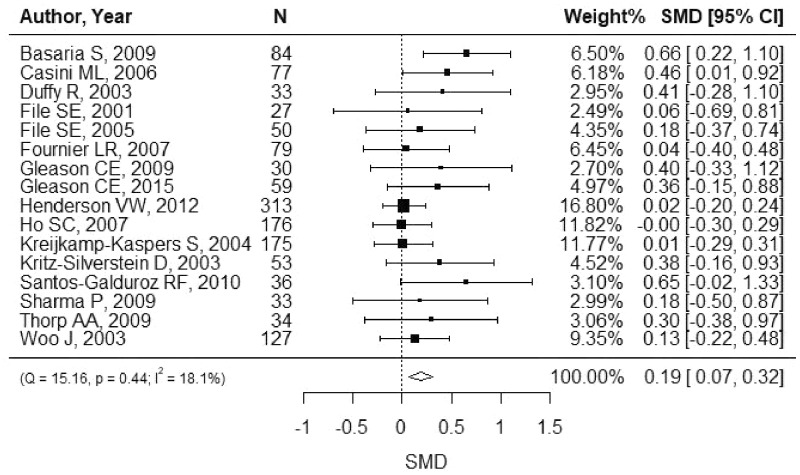
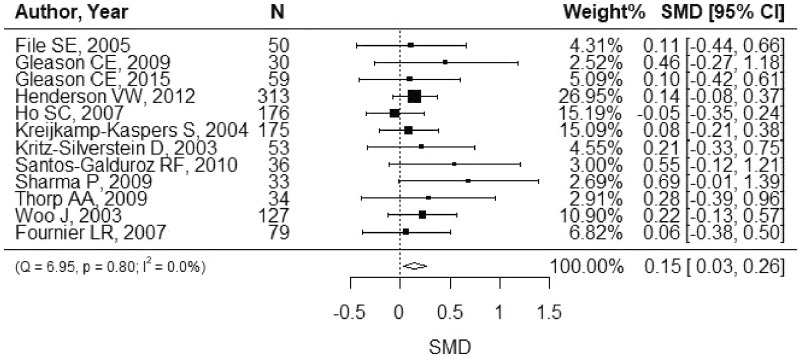
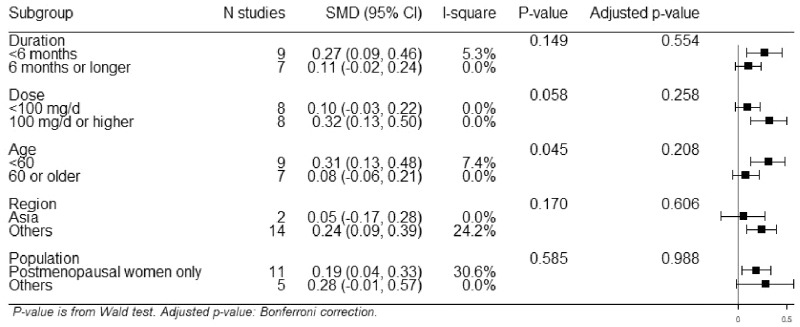
The summary effect for each cognitive domain is shown in Table 3. The overall SMD was 0.19 (Figure 2; 95%CI, 0.07–0.32). A statistically significant effect was observed for memory (Figure 3; SMD, 0.15; 95%CI, 0.03–0.26), but not for other domains (Figures S1–S6 in the Supporting Information online). Similar results were shown by the 3-level method (Table S5 in the Supporting Information online). Subgroup analysis showed no statistically significant difference by duration of intervention (<6 vs ≥6 mo), dose (<100 vs ≥100 mg/d), age (<60 vs ≥60 y), region (Asia vs others), or study population (postmenopausal vs others) with a consideration of the Bonferroni correction (Figure 4). I2 was 53.1% for the language domain, which indicated moderate heterogeneity. For other domains, I2 was smaller than 40%. There was no strong indication of publication bias (Figure S7 in the Supporting Information online).
Table 3.
Effect of soy isoflavones on cognition by domains
| Domain | No. of studies | Test type, n | SMD (95%CI) | P value | I 2 statistic (P value) |
|---|---|---|---|---|---|
| All domains a | 16 | 79 | 0.19 (0.07–0.32) | <0.01 | 18.1% (0.44) |
| Global cognition | 5 | 2 | 0.04 (−0.10 to 0.17) | 0.59 | 0.0% (0.64) |
| Executive function | 11 | 13 | 0.05 (−0.07 to 0.17) | 0.39 | 0.0% (0.89) |
| Psychomotor speed | 12 | 8 | 0.03 (−0.08 to 0.15) | 0.57 | 0.0% (0.94) |
| Attention/working memory | 8 | 9 | 0.08 (−0.11 to 0.27) | 0.41 | 33.9% (0.25) |
| Language | 12 | 11 | 0.10 (−0.10 to 0.29) | 0.33 | 53.1% (0.03) |
| Visuospatial reasoning | 8 | 8 | 0.05 (−0.08 to 0.19) | 0.45 | 0.0% (0.16) |
| Memory b | 12 | 28 | 0.15 (0.03–0.26) | 0.01 | 0.0% (0.80) |
An effect size >0 indicates that the intervention group exhibited more positive effects than the control group.
The difference in cognitive function between the control and intervention groups was found to be statistically significant at a P <0.05 level.
Abbreviations: SMD, standardized mean difference; CI, confidence interval.
Figure 2.
Overall standardized mean difference (SMD). The SMD with 95% confidence interval (CI) is displayed for each study. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD.
Figure 3.
Standardized mean difference (SMD) for memory. The SMD with 95% confidence interval (CI) is displayed for each study. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD.
Figure 4.
Subgroup analysis by duration, dose, age, region, and population. The SMD with 95% confidence interval (CI) was calculated for each subgroup. The P value was based on the Wald test.
The dropout rate and adverse events were summarized (Table S6 in the Supporting Information online). The dropout rate ranged from 0% to 24%. Adverse events were reported by 7 trials and missing in 9 trials. Two studies45,46 collected data on adverse events by different systems, such as the gastrointestinal system and gynecological system. No serious adverse events were reported, and other adverse events were similar, between the treatment group and placebo group. The Jadad scores were reported in each trial (Table S6 in the Supporting Information online). The detailed results of each trial are described in Tables S7 and S8 in the Supporting Information online.
DISCUSSION
The results of the present meta-analysis show that ISFs improve cognitive function in adults. This effect is pronounced in the domain of memory. According to the RCTs that reported adverse events in their study, no serious adverse effects were found.
Several systematic reviews summarized the findings from RCTs regarding the effects of ISFs on cognitive funtion.26–29 The recent reviews26,28,29 made more promising conclusions than the older reviews.27 One earlier meta-analysis indicated that ISFs improve cognitive function in postmenopausal women,30 despite the fact that some trials involving postmenopausal women were missed22,35 and a trial concerning red clover, whose major ingredients are non-soy isoflavones (ie, biochanin A and formononetin) was included.36 The present meta-analysis confirmed the findings of this earlier meta-analysis in relation to postmenopausal women and extended its results by including trials involving men and premenopausal women.23,31–34 An overall cognitive benefit of ISFs has been observed. The cognitive benefits were observed not only for postmenopausal women, but also for premenopausal women and men.
Preclinical studies indicate that ISFs have significant roles in important Alzheimer’s disease pathologies. ISFs can reduce the level of inflammation and oxidative stress, which alleviates cognitive impairment.48 In addition, ISFs promote clearance of amyloid β protein (Aβ)via peroxisome proliferator-activated receptor gamma (PPARγ)/apolipoprotein E (ApoE) activation.10 One ongoing RCT aimed to determine whether ISFs accelerate the degradation of Aβ.49 Furthermore, ISFs decrease tau phosphorylation, which causes the formation of neurofibrillary tangles.11 Besides this, ISFs suppress the mitochondrial apoptotic pathway.13 The mitochondrial apoptotic pathway leads to neuronal cell death, which contributes to Alzheimer’s disease.13 Through these mechanisms, ISFs can protect the brain and lower the risk of dementia.
The selective binding of ISFs to estrogen receptor beta (ERβ) contributes to the cognitive benefits of ISFs. Estrogen has pervasive effects on brains. However, the included RCTs failed to show the cognitive benefits of hormone replace therapy in postmenopausal women.50 Unlike estrogens, which bind to ER alpha (ERα) and ERβ, ISFs selectively bind to ERβ. ERβs are highly expressed in the brain, especially in the hippocampus and prefrontal cortex.51 In the current meta-analysis, the effect of ISFs was pronounced in the domain of memory. Memory is highly related to the hippocampus and prefrontal cortex. It suggests that ISFs benefit cognition via selective binding to ERβ.
The RCTs included in this review did not show any severe adverse effect. One previous concern about ISFs was that they might pose risks to cognitive function. This finding was highlighted by 3 particular observational studies, ie, the Honolulu-Asia Aging Study (HAAS)52 and studies in China53 and Indonesia,54 which revealed that the intake of tofu, which is made from soy and contains ISFs, is inversely associated with cognitive function. However, several points are worthy of note. HAAS was primarily focused on cardiovascular disease, and the findings concerning cognition were secondary. The baseline cognitive function was not measured, and the high-tofu-intake group had a less preferable profile regarding age, education, and ApoE4. The Chinese study was cross-sectional, and it may have been confounded by the participants’ overall health status and total energy intake.53 Compared to those with worse cognitive function, those with better cognitive function were much younger and more educated, and had a higher intake of both meat and vegetables. The Indonesian study was also cross-sectional. the authors of this study reported another soy product, tempeh, which was found to be associated with better memory.54 Moreover, their later analysis showed that tofu and tempeh consumption is associated with better recall.16 In fact, observational studies that aimed to evaluate the association between dietary intake of ISFs/soy and cognition showed no adverse effect, and rather, an improvement in cognitive function.14–18 Therefore, current evidence does not support the proposition that ISFs negatively affect cognition.
The other concern about ISFs is that their estrogen-like effects may increase the risk of cancer. However, epidemiological studies have shown that ISFs and soy do not increase the risk of breast, endometrial, or prostate cancer.55–57 Instead, soy intake is associated with decreased risk of breast cancer,58 and is beneficial to breast cancer survivors, especially estrogen receptor–negative breast cancer patients.59,60 The North American Menopause Society recommends that midlife women should increase their dietary intake of soy and isoflavone products.58 In the prostate, ISFs exhibit an anticarcinogenic effect through their antiproliferative, anti-invasive, and pro-apoptotic activities.57 Overall, current evidence shows no adverse effects in relation to ISFs.
Equol, a metabolite formed from ISFs by gut microbiota, may have greater cognitive benefit than ISFs themselves.61 This is because, compared to ISFs, equol possesses higher antioxidant properties, greater or similar affinity to ERβ, longer bioavailability, and the ability to increase mitochondrial activities.62–66 Equol cannot be produced unless one consumes ISFs, yet not all individuals can convert daidzein to equol. The ability to convert daidzein to equol is determined by the presence of specific bacteria in the gut,67 not by genetics.68,69 Interestingly, it is reported that 40%–70% of Asians can produce equol, in contrast to 20%–30% of westerners.65 A recent cross-sectional study of 152 elderly subjects in Japan reported that equol-producers had significantly better cognition and lower prevalence of mild cognitive impairment than non-producers.70 Two RCTs conducted in western countries that examined the effect of ISFs on cognition, also revealed that the equol-producers in these studies tended to have better cognitive outcomes in their post-hoc analyses.19,23 The results did not reach statistical significance, however, mainly due to the small percentages of equol-producers in these trials (ie, <25%). Since equol is now available as nutraceutical and pharmaceutical supplements, future research in this area is warranted to evaluate the cognitive effects of equol.
Some variances were observed across the included RCTs. First, the ISF dose varied across trials. The dose-response relationship has been reported in relation to the effect of ISFs on hot flashes71 and osteoporosis72 among postmenopausal women. An in-vivo study also showed the dose-effect relationship of ISFs in terms of reducing neuroinflammation in rats.73 In the present review, high-dose trials showed slightly better results than low-dose trials, suggesting a potential dose-response effect of ISFs. Second, the different constituents of ISFs may influence the effectiveness of the dose. Higher ratios of aglycones and glucosides (ie, two major chemical forms of ISFs) may increase the absorption of ISFs.74 Compared to daidzein, genistein has stronger digestibility and affinity for ERβ.75 Third, trials had various durations – a factor that may also influence the results. The subgroup analysis revealed no differences in the results of the short-duration vs long-duration trials. Yet, it is possible that the durations were too short to show a difference, as the longest duration of all the trials was 2 years. Fourth, study populations were different. The subgroup analysis suggested that younger individuals might gain more cognitive benefits from soy isoflavones than older individuals, although the results did not reach statistical significance after adjusting for multiple comparisons. All these variances should be considered while interpreting results.
There were several gaps in the current literature. First, the evidence on men is sparse. Observational studies showed an overall cognitive benefit of ISFs in both men and women.14,15,18 More trials are needed to confirm this observation. Second, trials of longer duration are critically needed. The longest duration trial was the Women's Isoflavone Soy Health (WISH) trial, which administered ISFs for 2.5 years.19 The evidence from one observational study supports the long-term effect by highlighting the cognitive benefits of ISFs with a follow-up of 15 years.15 Third, more observational studies are needed regarding the association between ISFs and dementia. One cohort showed that high-soy intake was associated with lower dementia risk,14 though further studies are needed to confirm this proposition.
The National Academy of Medicine recently reviewed strategies for preventing dementia. They highlighted dietary interventions, especially the MedDiet and DASH diet. Observational studies of the MedDiet and DASH diet revealed promising results for reducing dementia risk.6 The post-hoc analysis of the PREDIMED trial found that the MedDiet group had better performance in global cognition, memory, and psychomotor speed than the control group.7,8 The National Academy of Medicine also emphasized the need to identify components that should be included in dietary interventions for dementia. Many trials have been conducted with a focus on effective intervention components, yet the current knowledge remains limited. RCTs failed to show that marine omega-3 fatty acids improve cognitive function.76,77 For vitamin interventions, current evidence only noted some benefits of vitamin B12 plus folic acid, and did not show any beneficial effect for vitamins A, D, or E.78,79 Regarding plant extracts, the findings of a recent RCT of 40 participants indicated that curcumin improved cognitive function, and decreased amyloid and tau accumulation in the brain.80 The present study suggests that ISFs should be considered as an intervention for cognitive decline and dementia through a comprehensive evaluation of ISFs. ISFs are available as dietary supplements, which can be incorporated into the DASH diet and MedDiet. Overall, ISFs have the potential to improve cognitive function and reduce the risk of dementia as a component of dietary interventions.
The results should be interpreted in light of their strengths and weaknesses. The present study is the first systematic review and meta-analysis to quantitatively assess the cognitive effect of ISFs in both men and women. Besides this, the robustness of the results ws tested by conducting multiple subgroup analyses and using two methods of meta-analysis (traditional and 3-level methods). The present study is not without limitations. Most trials involved a small sample size. The subgroup analysis should be interpreted with caution on account of the small number of studies in certain strata; for example, only 2 trials were performed in Asia. More research is needed before these findings can be generalized to the Asian population. Nevertheless, the present study informs future dietary interventions aimed at reducing cognitive decline and dementia by comprehensively evaluating the cognitive benefits of ISFs and corresponding adverse events.
CONCLUSION
In adults, ISFs improve cognitive function. In the RCTs included in this review, ISF groups were associated with better performance in neuropsychological batteries than the control groups and no severe adverse effects were reported. These findings support the potential of ISFs to reduce the risk of cognitive decline and dementia. ISFs can be considered as a component of future dietary interventions for cognitive decline and dementia.
Supplementary Material
Acknowledgment
Author contributions. C.C., R.B., and A.S. conceptualized and planned the study, including production of the search strategy. C.C., R.B., and A.S. undertook the study selection, and assessed the quality of the studies. C.C., R.B., B.S., and A.S. performed the data extraction. C.C. performed the data analysis and synthesis. Data interpretation was discussed and executed by all authors. C.C. drafted the manuscript, which was critically reviewed by R.B., B.S., M.I., A.H., C.K., B.L., H.A., O.L., C.M., Y.M., L.K., and A.S. All authors read and approved the final manuscript.
Funding. This work was supported by RF1AG051615 from the National Institute on Aging (NIA). This funding source had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 Search strategies in EMBASE
Table S2 Ongoing or unpublished trials
Table S3 Cognitive tests in each cognitive domain
Table S4 Quality assessment of the included studies
Table S5 The effect of soy isoflavones on cognition by domains (using the 3-level method)
Table S6 Adverse events and Jadad scores reported by 16 studies
Table S7 The availability of results
Table S8 Detailed results of ISFs vs control provided by 16 studies
Figure S1 Standardized mean difference (SMD) in global cognition. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD
Figure S2 Standardized mean difference (SMD) in executive function. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD
Figure S3 Standardized mean difference (SMD) in psychomotor speed. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD
Figure S4 Standardized mean difference (SMD) in attention/working memory. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD
Figure S5 Standardized mean difference (SMD) in language. The weight was calculated using the inverse-variance weighting method. A random-effects model was used to calculate the summary SMD
Figure S6 Standardized mean difference (SMD) in visuospatial reasoning. The weight was calculated using the inverse-variance weighting method. The summary SMD was calculated using the 3-level random effects model
Figure S7 Funnel plot
Appendix S1 PRISMA checklist
References
- 1.Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- 2. Zissimopoulos J, Crimmins E, Clair PS.. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2014;18:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berendsen AM, Kang JH, Feskens EJM, et al. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J Nutr Health Aging. 2018;22:222–229. [DOI] [PubMed] [Google Scholar]
- 4. Berendsen AAM, Kang JH, van de Rest O, et al. The dietary approaches to stop hypertension diet, cognitive function, and cognitive decline in American older women. J Am Med Directors Assoc. 2017;18:427–432. [DOI] [PubMed] [Google Scholar]
- 5. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175:1094–1103. [DOI] [PubMed] [Google Scholar]
- 8. Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318–1325. [DOI] [PubMed] [Google Scholar]
- 9.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division, Board on Health Sciences Policy, et al. Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: National Academies Press; (US) Copyright 2017 by the National Academy of Sciences. All rights reserved; 2017. [PubMed] [Google Scholar]
- 10. Bonet-Costa V, Herranz PV, Blanco GM, et al. Clearing amyloid-beta through PPARgamma/ApoE activation by genistein is a treatment of experimental Alzheimer’s disease. J Alzheimers Dis. 2016;51:701–711. [DOI] [PubMed] [Google Scholar]
- 11. Ye S, Wang TT, Cai B, et al. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer’s disease model rats. Neural Regen Res. 2017;12:1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganai AA, Farooqi H.. Bioactivity of genistein: a review of in vitro and in vivo studies. Biomed Pharmacother. 2015;76:30–38. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Cai B, Shao J, et al. Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease. Neural Regen Res. 2016;11:1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozawa M, Ninomiya T, Ohara T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr. 2013;97:1076–1082. [DOI] [PubMed] [Google Scholar]
- 15. Okubo H, Inagaki H, Gondo Y, et al. Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: a cross-sectional analysis of the SONIC study. Nutr J. 2017;16:56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogervorst E, Mursjid F, Priandini D, et al. Borobudur revisited: soy consumption may be associated with better recall in younger, but not in older, rural Indonesian elderly. Brain Res. 2011;1379:206–212. [DOI] [PubMed] [Google Scholar]
- 17. Huang MH, Luetters C, Buckwalter GJ, et al. Dietary genistein intake and cognitive performance in a multiethnic cohort of midlife women. Menopause (New York, NY). 2006;13:621–630. [DOI] [PubMed] [Google Scholar]
- 18. Nakamoto M, Otsuka R, Nishita Y, et al. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur J Clin Nutr. 2018;72:1458–1462. [DOI] [PubMed] [Google Scholar]
- 19. Henderson VW, St John JA, Hodis HN, et al. Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology 2012;78:1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. File SE, Hartley DE, Elsabagh S, et al. Cognitive improvement after 6 weeks of soy supplements in postmenopausal women is limited to frontal lobe function. Menopause (New York, NY). 2005;12:193–201. [DOI] [PubMed] [Google Scholar]
- 21. Duffy R, Wiseman H, File SE.. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. 2003;75:721–729. [DOI] [PubMed] [Google Scholar]
- 22. Casini ML, Marelli G, Papaleo E, et al. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85:972–978. [DOI] [PubMed] [Google Scholar]
- 23. Gleason CE, Fischer BL, Dowling NM, et al. Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J Alzheimers Dis. 2015;47:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fournier LR, Ryan Borchers TA, Robison LM, et al. The effects of soy milk and isoflavone supplements on cognitive performance in healthy, postmenopausal women. J Nutr Health Aging. 2007;11:155–164. [PubMed] [Google Scholar]
- 25. Basaria S, Wisniewski A, Dupree K, et al. Effect of high-dose isoflavones on cognition, quality of life, androgens, and lipoprotein in post-menopausal women. J Endocrinol Invest. 2009;32:150–155. [DOI] [PubMed] [Google Scholar]
- 26. Thaung Zaw JJ, Howe PRC, Wong R.. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann N Y Acad Sci. 2017;1403:150–163. [DOI] [PubMed] [Google Scholar]
- 27. Clement YN, Onakpoya I, Hung SK, et al. Effects of herbal and dietary supplements on cognition in menopause: a systematic review. Maturitas 2011;68:256–263. [DOI] [PubMed] [Google Scholar]
- 28. Soni M, White LR, Kridawati A, et al. Phytoestrogen consumption and risk for cognitive decline and dementia: with consideration of thyroid status and other possible mediators. J Steroid Biochem Mol Biol. 2016;160:67–77. [DOI] [PubMed] [Google Scholar]
- 29. Sumien N, Chaudhari K, Sidhu A, et al. Does phytoestrogen supplementation affect cognition differentially in males and females? Brain Res. 2013;1514:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng PF, Chen JJ, Zhou XY, et al. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause (New York, NY). 2015;22:198–206. [DOI] [PubMed] [Google Scholar]
- 31. Gleason CE, Carlsson CM, Barnet JH, et al. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age and Ageing. 2009;38:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorp AA, Sinn N, Buckley JD, et al. Soya isoflavone supplementation enhances spatial working memory in men. Br J Nutr. 2009;102:1348–1354. [DOI] [PubMed] [Google Scholar]
- 33. Sharma P, Wisniewski A, Braga-Basaria M, et al. Lack of an effect of high dose isoflavones in men with prostate cancer undergoing androgen deprivation therapy. J Urol. 2009;182:2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. File SE, Jarrett N, Fluck E, et al. Eating soya improves human memory. Psychopharmacology (Berlin). 2001;157:430–436. [DOI] [PubMed] [Google Scholar]
- 35. Woo J, Lau E, Ho SC, et al. Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause (New York, NY). 2003;10:352–361. [DOI] [PubMed] [Google Scholar]
- 36. Maki PM, Rubin LH, Fornelli D, et al. Effects of botanicals and combined hormone therapy on cognition in postmenopausal women. Menopause (New York, NY). 2009;16:1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yaneva-Sirakova T, Tarnovska-Kadreva R, Traykov L.. Pulse pressure and mild cognitive impairment. J Cardiovasc Med (Hagerstown, MD). 2012;13:735–740. [DOI] [PubMed] [Google Scholar]
- 39. Berger VW, Alperson SY.. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 41. Hughes TM, Wagenknecht LE, Craft S, et al. Arterial stiffness and dementia pathology: atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology. 2018;90:e1248–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van den Noortgate W, Lopez LJ, Marin MF, et al. Meta-analysis of multiple outcomes: a multilevel approach. Behav Res Methods. 2015;47:1274–1294. [DOI] [PubMed] [Google Scholar]
- 43. Srikanthan P, Horwich TB, Tseng CH.. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol. 2016;117:1355–1360. [DOI] [PubMed] [Google Scholar]
- 44. Santos-Galduroz RF, Galduroz JC, Facco RL, et al. Effects of isoflavone on the learning and memory of women in menopause: a double-blind placebo-controlled study. Braz J Med Biol Res. 2010;43:1123–1126. [DOI] [PubMed] [Google Scholar]
- 45. Ho SC, Chan AS, Ho YP, et al. Effects of soy isoflavone supplementation on cognitive function in Chinese postmenopausal women: a double-blind, randomized, controlled trial. Menopause (New York, NY ).2007;14:489–499. [DOI] [PubMed] [Google Scholar]
- 46. Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. J Am Med Assoc. 2004;292:65–74. [DOI] [PubMed] [Google Scholar]
- 47. Kritz-Silverstein D, Von Muhlen D, Barrett-Connor E, et al. Isoflavones and cognitive function in older women: the SOy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause (New York, NY). 2003;10:196–202. [DOI] [PubMed] [Google Scholar]
- 48. Mirahmadi SM, Shahmohammadi A, Rousta AM, et al. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine. 2017;104:151–159. [DOI] [PubMed] [Google Scholar]
- 49. Tap L, van Opbroek A, Niessen WJ, et al. Aortic stiffness and brain integrity in elderly patients with cognitive and functional complaints. Clin Interv Aging. 2018;13:2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morito K, Hirose T, Kinjo J, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–356. [DOI] [PubMed] [Google Scholar]
- 52. White LR, Petrovitch H, Ross GW, et al. Brain aging and midlife tofu consumption. J Am Coll Nutr. 2000;19:242–255. [DOI] [PubMed] [Google Scholar]
- 53. Xu X, Xiao S, Rahardjo TB, et al. Tofu intake is associated with poor cognitive performance among community-dwelling elderly in China. J Alzheimers Dis. 2015;43:669–675. [DOI] [PubMed] [Google Scholar]
- 54. Hogervorst E, Sadjimim T, Yesufu A, et al. High tofu intake is associated with worse memory in elderly Indonesian men and women. Dement Geriatr Cogn Disord. 2008;26:50–57. [DOI] [PubMed] [Google Scholar]
- 55. Douglas CC, Johnson SA, Arjmandi BH.. Soy and its isoflavones: the truth behind the science in breast cancer. Anticancer Agents Med Chem. 2013;13:1178–1187. [DOI] [PubMed] [Google Scholar]
- 56. Zhang GQ, Chen JL, Liu Q, et al. Soy intake is associated with lower endometrial cancer risk: a systematic review and meta-analysis of observational studies. Medicine. 2015;94:e2281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Die MD, Bone KM, Williams SG, et al. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113:E119–E130. [DOI] [PubMed] [Google Scholar]
- 58. Shifren JL, Gass ML.. The North American Menopause Society recommendations for clinical care of midlife women. Menopause (New York, NY). 2014;21:1038–1062. [DOI] [PubMed] [Google Scholar]
- 59. Hilakivi-Clarke L, Andrade JE, Helferich W.. Is soy consumption good or bad for the breast? J Nutr. 2010;140:2326s–2334s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang FF, Haslam DE, Terry MB, et al. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: the Breast Cancer Family Registry. Cancer. 2017;123:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sekikawa A, Ihara M, Lopez O, et al. Effect of S-equol and soy isoflavones on heart and brain. Curr Cardiol Rev. 2019;15:114–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilkins HM, Mahnken JD, Welch P, et al. A mitochondrial biomarker-based study of s-equol in Alzheimer’s disease subjects: results of a single-arm, pilot trial. J Alzheimers Dis. 2017;59:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kelly GE, Joannou GE, Reeder AY, et al. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208:40–43. [DOI] [PubMed] [Google Scholar]
- 64. Setchell KDR, Brown NM, Lydeking-Olsen E.. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. [DOI] [PubMed] [Google Scholar]
- 65. Setchell KD, Clerici C.. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:1363s–1368s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Muthyala RS, Ju YH, Sheng SB, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorgan Med Chem. 2004;12:1559–1567. [DOI] [PubMed] [Google Scholar]
- 67. Setchell KD, Cassidy A.. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758s–767s. [DOI] [PubMed] [Google Scholar]
- 68. Nagata C, Ueno T, Uchiyama S, et al. Dietary and lifestyle correlates of urinary excretion status of equol in Japanese women. Nutr Cancer. 2008;60:49–54. [DOI] [PubMed] [Google Scholar]
- 69. Usui T, Tochiya M, Sasaki Y, et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol (Oxford). 2013;78:365–372. [DOI] [PubMed] [Google Scholar]
- 70. Igase M, Igase K, Tabara Y, et al. Cross-sectional study of equol producer status and cognitive impairment in older adults. Geriatr Gerontol Int. 2017;17:2103–2108. [DOI] [PubMed] [Google Scholar]
- 71. Crawford SL, Jackson EA, Churchill L, et al. Impact of dose, frequency of administration, and equol production on efficacy of isoflavones for menopausal hot flashes: a pilot randomized trial. Menopause. 2013;20:936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Taku K, Melby MK, Kurzer MS, et al. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: systematic review and meta-analysis of randomized controlled trials. Bone. 2010;47:413–423. [DOI] [PubMed] [Google Scholar]
- 73. Ahmad A, Ramasamy K, Jaafar SM, et al. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem Toxicol. 2014;65:120–128. [DOI] [PubMed] [Google Scholar]
- 74. Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–1699. [DOI] [PubMed] [Google Scholar]
- 75. Nielsen IL, Williamson G.. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57:1–10. [DOI] [PubMed] [Google Scholar]
- 76. Cooper RE, Tye C, Kuntsi J, et al. Omega-3 polyunsaturated fatty acid supplementation and cognition: a systematic review and meta-analysis. J Psychopharmacol. 2015;29:753–763. [DOI] [PubMed] [Google Scholar]
- 77. Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59:815–849. [DOI] [PubMed] [Google Scholar]
- 78. Kane RL, Butler M, Fink HA, et al. Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia (Comparative Effectiveness Reviews, No. 188). Rockville, MD: Agency for Healthcare Research and Quality (US); 2017. [PubMed]
- 79. Solfrizzi V, Agosti P, Lozupone M, et al. Nutritional intervention as a preventive approach for cognitive-related outcomes in cognitively healthy older adults: a systematic review. J Alzheimers Dis. 2018;64(suppl 1):S229–S254. [DOI] [PubMed] [Google Scholar]
- 80. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26:266–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.