Abstract
Sarcoidosis is a complex disease with heterogeneous clinical presentations that can affect virtually any organ. While the lung is typically the most common organ involved, combined pulmonary and cardiac sarcoidosis (CS) account for most of the morbidity and mortality associated with this disease. Pulmonary sarcoidosis can be asymptomatic or result in impairment in quality of life, end-stage severe and/or life-threatening disease. The latter outcome is seen almost exclusively in those with fibrotic pulmonary sarcoidosis, which accounts for 10–20 percent of pulmonary sarcoidosis patients. CS is problematic to diagnose and may cause significant morbidity and death from heart failure (HF) or ventricular arrhythmias. The diagnosis of CS usually requires surrogate cardiac imaging biomarkers as endomyocardial biopsy (EMBx) has relatively low yield, even with directed electrophysiologic mapping. Treatment of CS is often multifactorial involving a combination of anti-granulomatous therapy and pharmacotherapy for cardiac arrhythmias and/or HF in addition to device placement and cardiac transplantation.
Keywords: Cardiac Sarcoidosis, Pulmonary Sarcoidosis, Imaging, Biomarkers
Condensed Abstract:
Sarcoidosis is a complex disease with heterogeneous clinical presentations that can affect virtually any organ. While the lung is typically the most common organ involved, combined pulmonary and CS account for most of the morbidity and mortality associated with this disease. Pulmonary sarcoidosis can be asymptomatic or result in life-threatening disease. CS is problematic to diagnose and may cause significant morbidity/death from HF or ventricular arrhythmias. The diagnosis of CS usually requires surrogate cardiac imaging biomarkers as EMBx has relatively low yield. Treatment of CS is multifactorial involving a combination of anti-granulomatous therapy and pharmacotherapy for cardiac arrhythmias and HF.
Introduction
Since its first description by Caesar Boeck in the 19th century, the diagnosis of sarcoidosis has remained a clinical challenge. Often referred to as the great mimicker, sarcoidosis is a diagnosis of exclusion, with no reliable biomarker for establishing the diagnosis or disease monitoring. There are also a no validated and adequate pre-clinical, in-vitro or animal models of disease, nor is there any standardized approach to treatment. Although non-necrotizing granulomas are considered an essential feature of the disease, these pathologic findings are not specific to sarcoidosis, being also seen in numerous infectious/inflammatory diseases. This review provides an overview of the epidemiology and postulated pathogenesis of sarcoidosis, as well as discusses the clinical features and up-to date therapeutic recommendations for both pulmonary and CS, the former being the most common manifestation and the latter the one associated with arguably the greatest diagnostic/therapeutic challenges and frequent adverse outcomes. The overarching goal is to solicit an international, collaborative effort to create common pathways for the multidimensional phenotyping of this condition followed by the generation of a multicenter registry and databank aimed at the identification of specific biomarkers to use for diagnosis, monitoring or prognostic estimates (Central Illustration).
Central Illustration: Precision Sarcoidosis.
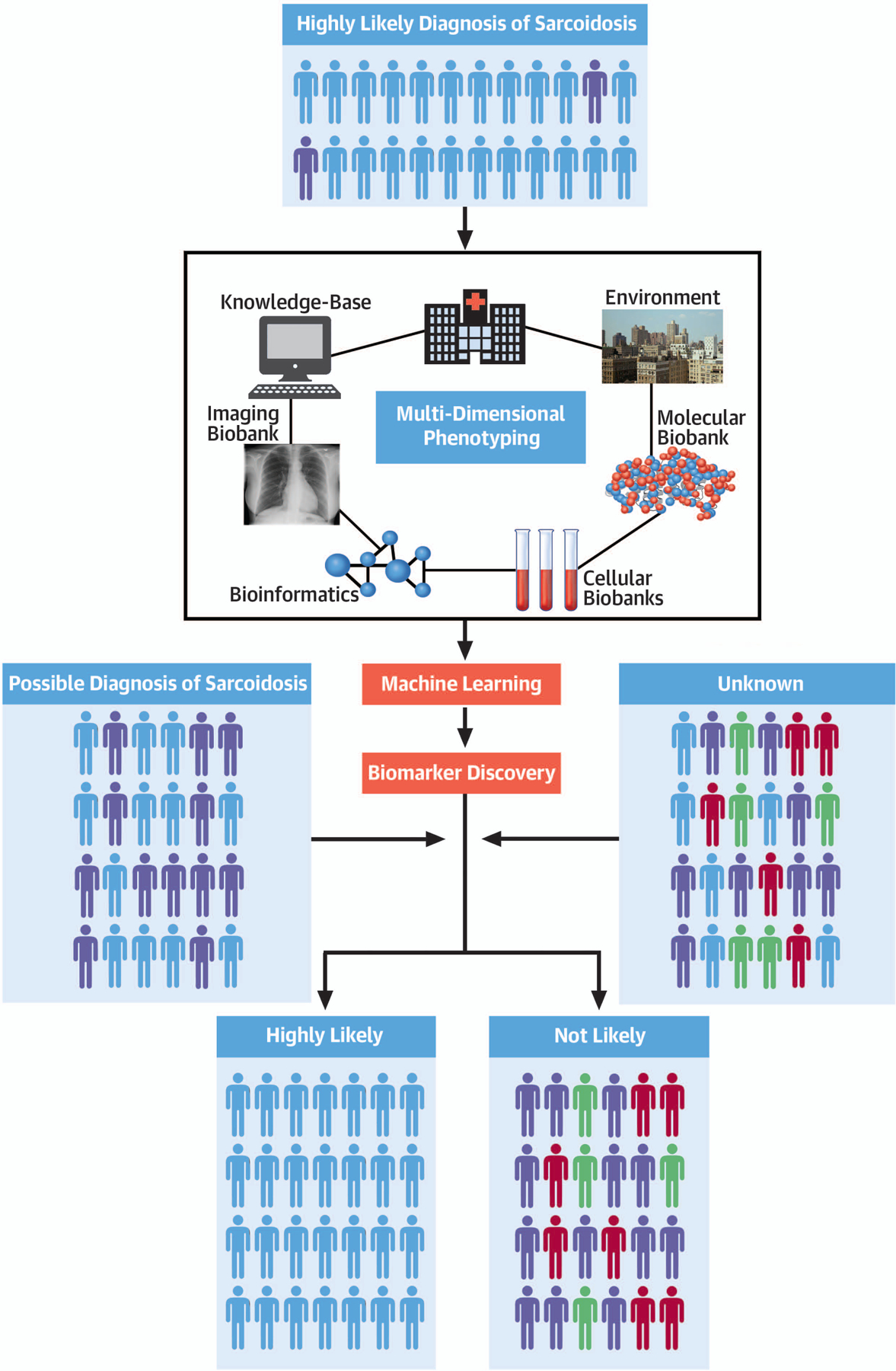
The integration of clinical, biological, omics and imaging data point from highly selected cases of sarcoidosis coupled with a standardized approach to patient phenotyping and machine learning algorithms is a promising pathway to enable the discovery of multidimensional disease specific biomarkers to use for accurate patient selection and targeted treatments.
Etiopathogenesis of Sarcoidosis: from Genes to Environmental Triggers.
Epidemiology
Sarcoidosis occurs worldwide and affects individuals of all gender, age and race. The exact burden of disease is problematic to estimate, mainly because of vague diagnostic criteria, variable methods of “case” ascertainment and variability in disease presentation; in addition, in a significant subgroup of cases, sarcoidosis is clinically silent and detected only on surveillance or incidental radiographic imaging. Sarcoidosis tends to be more common among African-Americans, particularly women, and residents of the northern latitudes, where the prevalence is estimated at 141–160/100,000, respectively (1,2). A lower prevalence has been reported in Japan, South-America and India (3). The pattern of organ involvement and disease severity also differ across ethnic and racial groups with sarcoidosis presenting in more organs, significantly earlier (by ~10 years) and more severely in African-Americans (2). Of the cutaneous manifestation of sarcoidosis, Lupus Pernio tends to be more common in Puerto Ricans and African-Americans, whereas Erythema-Nodosum is more prevalent in patients of European descent, particularly Scandinavians (4). According to the Swedish National Patient Register database, sarcoidosis (5) peaks at 30–50 years in males and 50–60 years in females, indicating a mean age at diagnosis higher than previously suggested (1).
Based on registry data, mortality from pulmonary sarcoidosis, as the primary cause of death, was 2.8/million (6) in the USA, with rates higher in women compared with men (3.3 versus 2.3/million) and in African-Americans versus Caucasians (16 versus 1.3/million). Akin to pulmonary disease, CS is also associated with significant morbidity and mortality. While clinically manifest cardiac involvement occurs in approximately 5% of sarcoidosis patients, CS is often subclinical and under-recognized (5). Recent studies have suggested an increase in CS prevalence. In Finland, the rate of diagnosis of CS was shown to have risen more than 20-fold between 1988 and 2012 (7). In the USA, the incidence of patients who underwent cardiac transplantation for a CS related cardiomyopathy (CMP) increased from 0.1% (1994–1997) to 0.5% (2010–2014) (5). Whether these trends are real or rather a reflection of increased awareness and improvements in detection remains unknown.
Pathogenesis
Genetic factors
Several lines of evidence support the existence of a genetic predisposition to develop sarcoidosis. These include 1) familial clustering (5–16% of cases)(8), 2) increased concordance in monozygotic twins, 3) large ethnicity-driven variations in disease frequency, clinical expression and outcomes and 4) genetic studies that have identified human leukocyte antigens (HLA) and non-HLA alleles as associated with sarcoidosis susceptibility, phenotype and prognosis (9). For example, the HLA-DRB1*03 allele is strongly associated with acute and self-limiting sarcoidosis (i.e., Löfgren’s syndrome), particularly in Scandinavians, whereas HLA-DRB1*14 and -DRB1*15 predispose to a chronic disease course (10). Additional genes associated with sarcoidosis risk include butyrophilin-like 2 gene (11) and annexin A11 (12). In combination, these findings suggest a heterogenous genetic profile composed of multiple gene variants each potentially contributing to disease susceptibility.
Environmental triggers
Organic and inorganic triggers combined with immune dysregulation are believed to play a critical role in the pathogenesis of sarcoidosis. Temporal and time-space clustering in the northern (Greece, United Kingdom, Spain, Norway, Japan and Finland) and southern hemisphere (New Zealand) provided early support for a connection between the environment and the incidence of sarcoidosis (1,13). Additionally, the observations that 1) sarcoidosis prevalence follows a rough north-south gradient and 2) that most sarcoidosis patients have pulmonary involvement, indicate that exposure to airborne agents in susceptible individuals might be causative of the disease. In further support of this hypothesis, the second most likely organ where sarcoidosis may occur is the skin, a tissue that is highly conducive to antigen capture and adaptive immune responses (14).
Among the organic environmental agents, Mycobacterium and Propionibacterium have both been implicated in sarcoidosis (15,16). Drake and colleagues performed polymerase chain reaction analysis for Mycobacterium species 16S-rRNA, rpoB and IS6110 sequences on tissue specimens in 25 sarcoidosis patients and 25 controls (15). Mycobacterial rRNA sequences were amplified in 60% of the sarcoidosis specimens but were not detected in any of the control tissues (15).Similarly, Song and colleagues detected mycobacterial catalase-peroxidase (mKatG) in 5/9 (55%) sarcoidosis specimens but in none of 14 control tissues (16). Propionibacterium acnes (P.acnes) and Propionibacterium granulosum (P. granulosum) have also been identified in sarcoidosis specimens. Eishi and co-workers found P.acnes or P.granulosum DNA in 106/108 (98%) sarcoidosis specimens, a percentage that was significantly higher than in tuberculosis and control samples (17). Lastly, meta-analyses of Mycobacterium and Propionibacterium species studies suggested involvement of these bacteria in sarcoidosis (18,19); no microbial pathogen has however been consistently isolated/cultured (20) nor a direct causation definitively established.
Other infectious agents that have been investigated with inconclusive or conflicting results include Chlamydia pneumoniae, Rickettsia helvetica, Borrelia burgdorferi, viruses, fungal infections, and Leishmania species.
Of the inorganic triggers, metal exposures to beryllium, zirconium, and aluminum, have traditionally been associated to the development of a sarcoidosis-like granulomatous disease(21). Granulomatous pulmonary disease with features of sarcoidosis also developed in the firefighters exposed to World Trade Center “dust” (22). Supplemental Table 1 summarizes the current infectious/environmental factors implicated in sarcoidosis.
Epigenetics
Epigenetics indicates heritable changes in gene expression that occur without a change in the underlying DNA sequence (23). The most extensively investigated mechanisms of epigenetic modulation of gene expression include DNA methylation, post-translational histone modifications and small non-coding RNAs, such as microRNA. These epigenetic mechanisms have emerged as important modulators of host defense and inflammatory response and may contribute to sarcoidosis risk in combination with genetic and environmental factors. Yang and coworkers identified DNA methylation and related gene expression changes in bronchoalveolar lavage (BAL) cells obtained from patients with chronic beryllium disease (CBD) and sarcoidosis (24). Notably, the authors observed an inverse relationship between methylation and expression of genes involved in T-helper cell type 1 differentiation, chemokines, chemokine receptors and other genes associated to immunity. Compared to subjects with CBD, DNA methylation changes in sarcoidosis were more subtle and variable, possibly because of disease heterogeneity and co-exposure to other pathogenic antigens (24).
Immunopathogenesis of granuloma formation
Non-necrotizing granulomas are the histological hallmark of sarcoidosis (Figure 1). They are discrete, well-circumscribed aggregates of epithelial cells, macrophages, multinucleated giant cells and CD4+ T lymphocytes, which tend to become confluent and form nodules in the mm size range. Granuloma formation requires a stepwise series of events, the first being the recruitment of monocytes to disease sites followed by their differentiation into antigen-presenting cells (APCs), such as macrophages or dendritic cells. APCs ingest the putative sarcoid antigen, which is processed and displayed on the surface of macrophages or similar APCs as small peptide fragments in the context of class I or II HLA molecules (25). In addition to T-cell receptor (TCR) binding to antigen-loaded HLA molecules, CD4+ T cells require several secondary signals to become activated and to orchestrate the immune response that culminates in granuloma formation (25). Compared to healthy controls, BAL fluid analysis from sarcoidosis patients reveals a marked increase in cellularity with a CD4+ T cells predominance (26). CD4+ T cells aided by regulatory T cells (Tregs) then initiate and amplify the immune response by releasing T-helper (Th)1-type cytokines such as 1) interleukin (IL)-2, which expands the activated lymphocyte population, 2) tumour necrosis factor (TNF)-α and 3) interferon (IFN)-γ. IFN-γ and TNF-α promote the accumulation and activation of macrophages, which in turn secrete a number of immuno-modulatory molecules such as TNF-α, IL-1, IL-6, IL-8, IL-12, IL-15, and IL-18 (27) (Figure 2). Sarcoidosis granuloma generally have a highly polarized Th1 cytokine response with some patients demonstrating a local shift from a Th1- to a Th2-type cytokine pattern that is speculated to stimulate the production of extracellular matrix components and hence the progression from granulomatous inflammation to fibrosis (28). The underlying mechanism of this differential pattern of activation remains however unclear and warrants further investigation.
Figure 1: Non-necrotizing Granulomas.
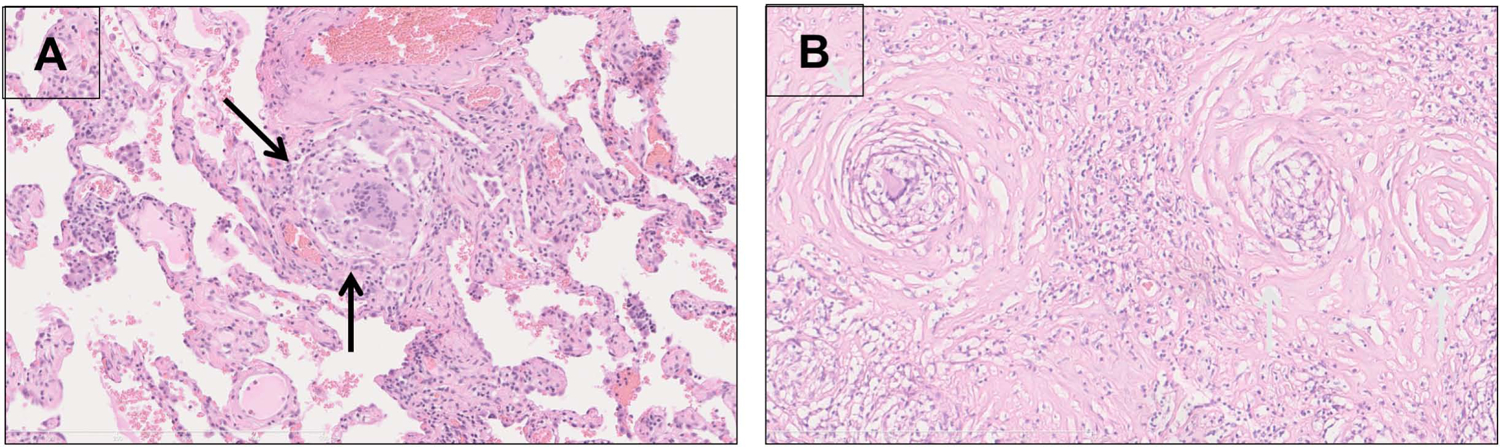
A: Early stage sarcoidosis (H&E stain, 20x) with a discrete perivascular granuloma (yellow arrows) comprised of epithelioid histiocytes, multinucleated giant cells, sparse lymphocytes and a lack of dense hyalinized collagen fibrosis. B: Late stage sarcoidosis (H&E stain, 20x) with a subpleural hyalinized nodule containing abundant dense eosinophilic hypocellular collagen fibrosis surrounding and bridging discrete granulomas (black arrows) with sparse lymphocytes. There prominent concentric lamellar fibrosis surrounding the granulomas, a finding characteristic of sarcoid granulomas association with scarring. Curtesy of Dr. M. A. Judson.
Figure 2: A hypothetical model of the immunopathogenesis of sarcoidosis.
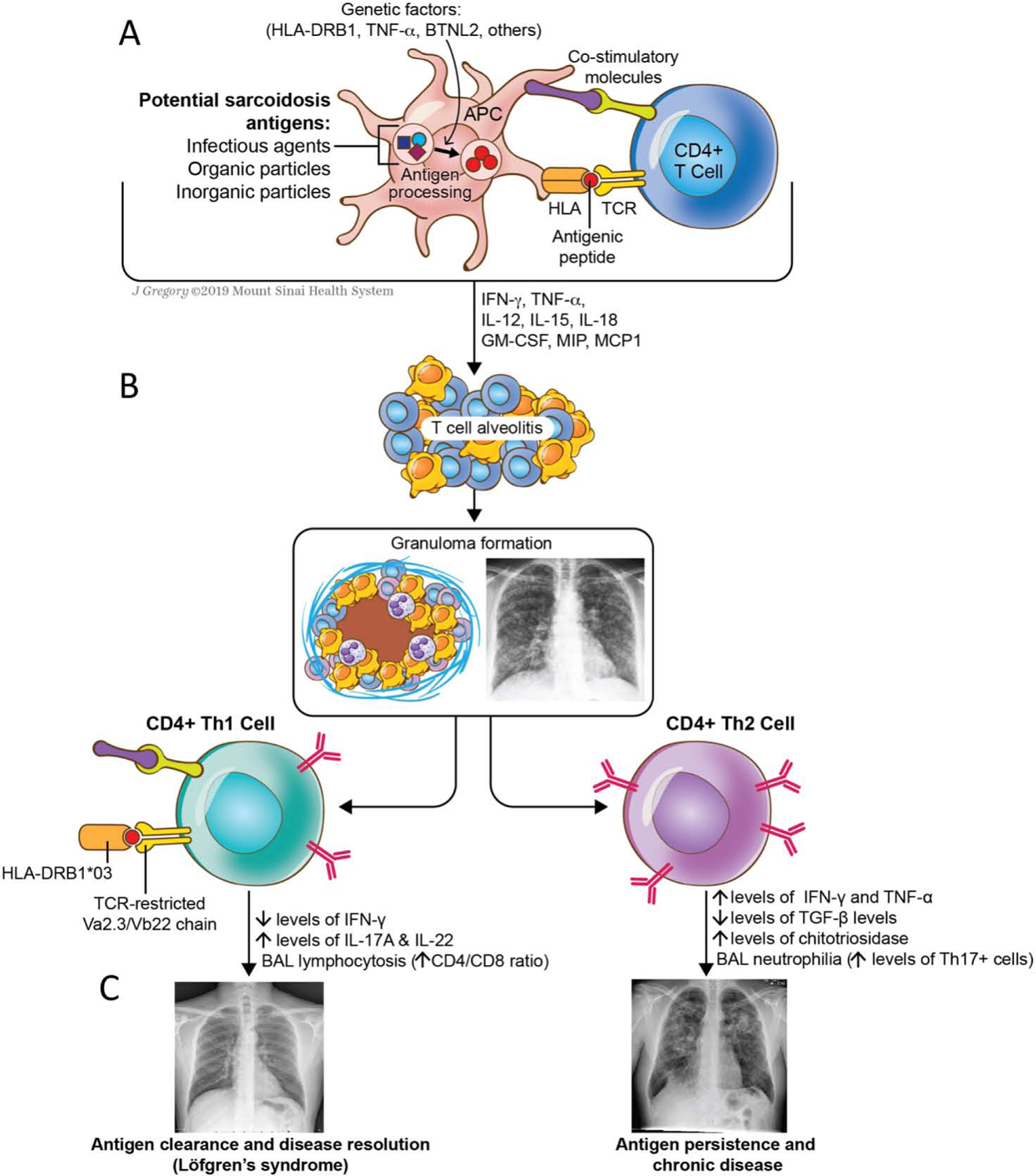
Granuloma formation requires activated T-cells and macrophages coupled with a milieu of cytokines released by the immune cells. (A) T-cells are activated by a specific antigen – either environmental, infectious, or an autoantigen - presented in the context of an HLA molecule and recognized by the T-cell receptor (TCR). (B) Once activated, APCs stimulate the helper Th1-promoting cytokines such as IL-2, IL-12, IL-18, TNF-α, and IFN-γ, which orchestrate the complex process of granuloma formation and inflammation. (C) The lungs are almost universally affected by the disease. Th1 response amplification may lead to antigen clearance, disease regression (as in Löfgren’s syndrome) and remission. Failure to remove the antigen along with the involvement of a different network of cells and/or cytokines may results in chronic inflammation and fibrosis.
Serum Biomarkers in Sarcoidosis
There is presently no reliable serum biomarker that has proven useful for diagnosis, monitoring of inflammatory activity or for “quantification” of disease severity. Several different biomarkers (Table 1) have been proposed but have failed to achieve the threshold for significance, being neither adequately specific nor sensitive to be used in isolation for clinical decision making. Among those, serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 and amyloid A have been evaluated for assessing disease activity. Interleukin-2 receptor and neopterin in particular appeared to predict fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) uptake, but the sensitivity was only modest (29). Similarly, elevation in high-sensitivity troponin has been reported in active CS with abnormal 18F-FDG uptake on PET/Computed Tomography (CT) (30) while cardiac troponin I elevation and urinary levels of 8-hydroxy-2’-deoxyguanosine appeared to predict fatal arrhythmias (31,32). Lastly, microRNAs (miRNAs) such as miRNA-126 and miRNA-223 were significantly increased in the blood of CS patients compared to healthy controls suggesting that these circulating miRNAs might be useful in diagnosing CS (33).
Table 1:
Serum biomarkers Investigated in Sarcoidosis and Their Potential Clinical Utility
| Biomarker | Significance | Sensitivity | Specificity | Diagnostic value | Prognosis | Treatment response | Comment |
|---|---|---|---|---|---|---|---|
| Serum Angiotensin-Converting Enzyme (ACE) | Marker of granulomatous inflammatory burden | 22%−83% | Low | Limited | Undefined | Undefined | Sensitivity varies based on disease manifestations and presence/absence of a 287 base-pair deletion within ACE gene. Serial measurements can be useful in disease follow-up. Interpretation of ACE levels may be confounded by use of drugs or immunosuppressants. Measurement of serum ACE is not useful in patients taking ACE inhibitors. |
| Serum soluble IL-2 receptor (sIL-2R) | Marker of T lymphocytes activation | ~80% (81%−98% for uveitis) | Low | Not fully established | + | Undefined | High diagnostic value in patients with uveitis. Potential useful for staging and/or marker of disease severity. Serial measurements can be useful in disease follow-up. High sIL-2R levels may predict relapse after infliximab therapy. Renal insufficiency may alter sIL-2R levels |
| Serum and urinary calcium | Marker of granulomatous inflammatory activity | Hypercalcemia: 3.6%−16% Hypercalciuria: 6.4%−33% |
Undefined | + | Undefined | Hypercalcemia and hypercalciuria are more common in men. Rare in patients with Löfgren’s syndrome. Hypercalcemia and hypercalciuria are associated with lower rates of spontaneous resolution and higher rate of relapses. | |
| Lysozyme | Marker of granulomas burden | 69%−80% | 60%−90.7% | Limited | Undefined | + | Serial measurements may be useful for monitoring response to systemic anti-inflammatory treatment. Elevated serum levels of lysozyme are also present in patients with tuberculosis, asbestosis and silicosis. Lysozyme levels should be interpreted with caution in patients with renal impairment. |
| Bronchoalveolar lavage (BAL) fluid lymphocytosis | CD4 activated T lymphocytes are the immunological hallmark of sarcoidosis | 71%−85% | 68%−93% | + | Uncertai n | Uncertain | Higher percentages of BAL lymphocytes may be associated with a more favorable disease course |
| BAL CD4+/CD8+ ratio | CD4+ T cell accumulation is the main feature of sarcoidosis granulomatous inflammation | 54%−80% | 59%−80% | + (highly specific when >3.5) | + | Uncertain | High CD4+/CD8+ ratios are associated with acute sarcoidosis and more favorable prognosis. Higher CD4+/CD8+ ratios are found in patients carrying the HLA-DRB1*03 genotype, which is associated with good prognosis. |
| BAL CD103+CD4+/CD4+ ratio | CD103+ cells are involved in fibrogenic inflammation | 63%−81% | 76%−78% | + (particularly if associated with a high CD4+/CD8+ ratio | Uncertain | Undefined | The CD103+CD4+/CD4+ ratio is typically reduced (<0.2) in sarcoidosis. Potentially useful as a marker of disease severity and prognosis, often seen in association with more advanced radiographic stages and worse outcome. |
| Additional, less established biomarkers | |||||||
| Chitotriosidase | Marker of disease activity | 89%−100% | About 90% | − | Uncertain | Uncertain | Potentially useful as a marker of severity and prognosis (highest levels are found in patients with persistent disease). Serial measurements can be useful in monitoring disease course. Interpretation of chitotriosidase levels may be confounded by corticosteroid treatment. |
| Th17 (CCR4+/CXCR3−) CD4+ T cells | Key players in many inflammatory disease | Undefined | Undefined | Undefined | + | Undefined | Higher BAL level of Th17+ cells have been found in patients developing chronic disease. |
| Serum Amyloid A (SSA) | SSA depositions are found in sarcoid granulomas | Undefined | Undefined | Uncertain | + | Undefined | Elevated serum SSA levels are found in patients with more active disease, severe lung function impairment and need for systemic therapy. |
| CXCL9 and CXCL10 | Stimulation of T cell migration to sites of inflammation | Undefined | Undefined | Uncertain | Uncertain | Undefined | Increased in BAL and serum from patients with sarcoidosis. Might predict disease outcome. Inversely correlated with lung function (FVC and DLCO). |
Table 1 summarizes “conventional” biomarkers as well as “potential” biomarkers for future use. Biomarkers could benefit sarcoidosis care in several ways: diagnosis, assessment of severity, prediction of disease behavior, prognosis, response to treatment and relapse. Unfortunately, none of the available biomarkers is sufficiently sensitive nor specific to be routinely used in clinical practice.
Diagnostic approach to Sarcoidosis-A general overview
The diagnosis of sarcoidosis is challenging because the disease may involve any organ, making the clinical presentations highly variable. As the cause of sarcoidosis is unknown, the diagnosis usually rests on a clinical presentation compatible with the diagnosis, the demonstration of non-necrotizing granulomatous inflammation in an involved tissue, exclusion of alternative granulomatous diseases based on patient demographics, occupational and environmental exposures (Supplemental Table 1)(34) and evidence of a “systemic” condition (35–37). There are however exceptions to this diagnostic approach. Indeed, some presentations of sarcoidosis are so specific that the diagnosis can be secured on clinical grounds alone without the need for a confirmatory biopsy. One such presentation is Löfgren’s syndrome that consists of a combination of Erythema-Nodosum, bilateral hilar adenopathy on chest radiograph, fever and ankle arthritis/periarthritis (13,38). Likewise, evidence of “systemic” involvement with a biopsy from a second organ showing granulomatous inflammation is generally not sought in “confirmed” pulmonary sarcoidosis cases (39) as suggested by the National Institute of Health sponsored “A Case Control Etiology of Sarcoidosis Study (ACCESS)” study where patients had evidence of sarcoidosis in only one organ, and the majority of these patients had pulmonary disease (39). Specifics challenges regarding the diagnosis of pulmonary and cardiac sarcoid are presented below.
Pulmonary Sarcoidosis
Clinical features of pulmonary sarcoidosis
The lung is the most common organ involved in sarcoidosis with a prevalence of 90% in most series (2,39). The presentations of pulmonary sarcoidosis can vary from asymptomatic state detected on a chest radiograph to a progressive, debilitating pulmonary disorder causing respiratory failure. The symptoms of pulmonary sarcoidosis are non-specific; dyspnea and cough are the most common, with the latter being reported in over 90% of patients with an acute pulmonary exacerbation of the disease (40). Chest radiographs are typically abnormal in > 90% of pulmonary sarcoidosis patients (41). Bilateral hilar adenopathy is observed in 50–85% and parenchymal opacities in 20–65% of cases. High resolution chest computed tomography scans (HRCTs) better identify parenchymal details so that sarcoidosis may often be distinguished from other diffuse lung diseases (41). The characteristic features of pulmonary sarcoidosis on HRCT include a) mediastinal (usually bilateral hilar) lymphadenopathy (41); b) nodular and micronodular opacities along the bronchovascular bundle, pleural and subpleural locations as well as the fissure lines (41); c) a confluence of micronodules into conglomerate larger masses. Pulmonary fibrosis may also occur with sarcoidosis, and it is also more easily detected on HRCT than the chest radiograph. Radiographic evidence of fibrosis from sarcoidosis includes fibrocystic disease, architectural distortion, hilar retraction, and traction bronchiectasis (41,42). These fibrotic changes are most common in the upper lobes.
Pulmonary function tests of pulmonary sarcoidosis patients generally reveals modest abnormalities (2,43,44), showing either restrictive, obstructive or mixed (obstructive/restrictive) ventilator defects (2,45).
The natural course of pulmonary sarcoidosis tends to follow three general paths. The first path is a benign course that presents in asymptomatic individuals undergoing a screening test: these patients tend to remain asymptomatic and usually require no therapeutic interventions. The second path is a non-fibrotic symptomatic course, where the patient presents with pulmonary symptoms, intra-parenchymal granulomatous inflammation on chest imaging and mild decrements in pulmonary function. These patients rarely experience progressive pulmonary deterioration. The disease can however last for months, years, or lifelong and might require immune-suppressive therapy(2,40). The third path is that of progressive fibrotic disease and it accounts for ~10–20% of sarcoidosis patients (2,46,47). The overwhelming majority of respiratory deaths occur in this subgroup of patients (47) from complications that includes bronchiectasis leading to severe lung infection (48), pulmonary hypertension (PH)(49) and mycetoma colonization triggering life-threatening hemoptysis (50)
The role of biomarkers in the diagnosis and assessment of pulmonary sarcoidosis
Currently available serum biomarkers for pulmonary sarcoidosis usually reflect total body granuloma burden and are not specific for lung involvement. Pulmonary specific biomarkers include BAL specimens, pulmonary function tests, and chest imaging studies. Although approximately 90% of pulmonary sarcoidosis patients demonstrate a BAL lymphocytes at the time of diagnosis (51), this finding is observed in several other lung diseases and, therefore, is not a useful diagnostic biomarker (52). The role of the BAL T-lymphocyte CD4/CD8 ratio remains also controversial, with some studies suggesting ratios greater than 3–4 have a > 95% specificity for sarcoidosis and others disputing it (51,53); regardless, it would be impractical to perform these tests serially to monitor pulmonary sarcoidosis activity. Pulmonary function tests and, specifically spirometry are often used to monitor pulmonary sarcoidosis clinically and in research trials (54,55)but the chest radiograph Scadding Staging System remains the oldest pulmonary sarcoidosis biomarkers (56). Although this system may distinguish the severity and prognosis of large pulmonary sarcoidosis cohorts, there is too much variability for it to be useful for individual patients. HRCT has vastly superior resolution compared to the chest radiograph and can more easily detect pulmonary lesions and areas of fibrosis. 18F-FDG PET scanning frequently demonstrates 18F-FDG uptake in active lung lesions (57). Parenchymal lung FDG-PET uptake has been found to correlate with improvement in pulmonary function in symptomatic pulmonary sarcoidosis patients who require therapy (58), including corticosteroid-refractory patients treated with infliximab (59). Gallium-67 scintigraphy was historically used to identify active pulmonary sarcoidosis but has fallen out of favor because of its lower sensitivity/specificity compared to 18F-FDG-PET (60), higher radiation exposure and lengthy acquisition protocols. Lastly, somatostatin receptor scintigraphy is a relatively new imaging technique to detect active sarcoid inflammatory lesions that has shown promise (61) in single center retrospective studies.
To conclude, no single pulmonary sarcoidosis biomarker appears to be adequate in isolation to make a diagnosis, assess disease activity or treatment responses, or to make prognostic estimates. Specifically, there is no biomarker or combination of biomarkers that can reliably predict the development of fibrosis (or more aggressive disease in CS) such that a decision can be made concerning early aggressive treatment versus withholding therapy with close observation. This deficiency is a critical unmet need in sarcoidosis and should be the focus of further investigations.
Cardiac Sarcoidosis
Clinical Features of Cardiac Sarcoidosis
Cardiac involvement in sarcoidosis has been reported in 25% to 58% of autopsy studies, with the highest prevalence described in Japanese cohorts. Granulomas in CS can be found anywhere in the heart, more commonly in the left ventricle (LV) and the interventricular septum (IVS). In the report by Tavora, 31.5±21.9% had sarcoid lesions in the IVS, 24.6±19% in the posterior wall, 18.0±9.9% in the anterior wall, 17.9±21.2% in the right ventricle (RV) and 14.1±17.9% in the lateral wall (62). Disease manifestations classically depend on location, extent, and stage of the inflammatory process, with greater involvement usually resulting in more symptoms. When symptomatic, CS typically manifests with conduction abnormalities, arrhythmias and less commonly HF. Right bundle branch block (RBBB) and atrioventricular block (AVB), ranging from first to third degree, are the most common manifestation (7,63); supraventricular arrhythmias such as paroxysmal atrial tachycardia, atrial ectopy, atrial flutter, and atrial fibrillation (AF) can occur as well (up to 32% of subjects), with AF being the most common (18%)(64). It is speculated that direct involvement of the atria with granulomas, as well as increased end-diastolic pressure from LV dysfunction and triggered automaticity from arrhythmic foci originating from the pulmonary veins contribute to the onset of atrial tachyarrhythmias (65). Ventricular tachyarrhythmias are the second most common CS manifestation (7); they are secondary to non-reentrant (i.e. increased automaticity and triggered activity) and reentrant mechanisms from the granulomatous scar. Because conduction abnormalities and ventricular arrhythmias are the most common presentations in CS, patients often have a history of palpitations, pre-syncope, and syncope. Both AV blocks and history of ventricular tachycardia (VT) elevate the risk for sudden cardiac death (SCD). Indeed, SCD can be the first and only manifestation of disease, accounting for as much as 65% of deaths in some studies (66,67). If myocardial involvement is extensive, CS can present with HF. In the studies by Kandolin, 9–27% of cases presented clinically with HF but LV dysfunction (EF<50%) was noted in 59%–82% of cases (7,68). Both HF with preserved EF (HFpEF) and HF with reduced EF (HFrEF) can occur in CS and are considered the most important cause of death from CS, accounting in some reports for 25% of CS-related mortality (68,69).
Akin to LV involvement, RV involvement is also quite common, ranging from 6 to 65% depending on the study(70), and is reflective of either direct granulomatous disease of the RV or the hemodynamic sequalae of longstanding LV CMP or PH. Because RV involvement usually parallels LV disease and predominant or isolated RV sarcoidosis is rare, the clinical and prognostic relevance of RV sarcoidosis “per se” is unknown. Nevertheless, the presence of RV involvement is generally considered a marker of poor outcome and RV late gadolinium enhancement (LGE) on Cardiac Magnetic Resonance (MR) was found to be associated with a heighten risk for ventricular tachyarrhythmias(71–74). RV dysfunction secondary to PH is typically seen in patients that have extensive lung fibrosis but can be observed in subjects with preserved lung architecture. Surveys in sarcoidosis clinics have identified PH in 5–20% of cases(75,76) with up to half of persistently dyspneic patients having sarcoidosis associated PH (SAPH)(37). In patients with more advanced lung disease listed for lung transplantation, the rate of SAPH was found to be as high as 70%(77); the majority of those patients have precapillary PH, but a significant proportion have elevated pressures due to left ventricular dysfunction (78,79). Clinical evaluation typically includes cardiac imaging such as MR and echocardiography as well as right heart catheterization.
Isolated CS (ICS), defined as CS in the absence of extracardiac evidence of the disease has a prevalence of 27–54%, based on patient cohort, diagnostic modality and definition of ICS (7,62,68). In the Finnish cohort, Kandolin et al. looked at 576 patients that underwent EMBx; biopsy proven CS was diagnosed in 52 of those patients. Of those, 33/52 were labeled as having ICS based on the absence of clinical and radiographic parameters suggestive of extracardiac disease (68). Nonetheless, 13 had prominent 18F-FDG uptake in the mediastinal lymph nodes, and 7 had uptake outside the chest. A recent case series that applied a stringent definition of ICS and used whole-body and cardiac 18F–FDG PET-CT to systematically exclude extra-cardiac disease identified ICS in 3.2% of the subjects (80). Compared to the prior retrospective review that suggested higher incidence of ICS (62,81), the study by the Ottawa group indicated an overall low incidence of ICS when extracardiac disease was systematically excluded. As such, standard definition and w/u for ICS appears critical for an accurate assessment of the true burden of ICS (see below).
The presence of clinically manifest cardiac involvement has traditionally been associated to a poor outcome. Indeed, Japanese studies identified CS as a leading cause of mortality in their sarcoid population, with 85% dying from cardiac complications (82). More recent reports have suggested a much better prognosis. Kandolin et al. reviewed the outcome of 110 Finnish patients with biopsy-proven CS and found that with the current available therapies, patients with CS did much better than anticipated; the 10-year likelihood of transplantation-free survival was 83% overall and 91% in patients receiving immunosuppressive treatment (7). Even with these encouraging outcome data in CS, determining prognosis remains challenging and is mostly based on “traditional” outcome metrics such as LV dysfunction, history of malignant arrhythmias or extent of LGE, with the latter showing superiority to standard prognostic parameter such as ejection fraction (EF) or cardiac volumes in predicting adverse outcome (74,83,84). Akin to pulmonary sarcoidosis, availability of a biomarker or cluster of biomarkers with diagnostic and prognostic power remains a missing piece in the CS puzzle.
Diagnostic approach to CS
EMBx is considered the gold standard to establish a diagnosis of CS but the reported yield of unguided myocardial biopsy is ~25%. Imaging (with PET/CT, MR) or voltage guided biopsy procedures were shown to increase the success rate to ~50% (68,85–88) and are recommended by consensus guideline(89) (Figure 3). In the absence of confirmatory tissue diagnosis, clinical guidelines combining proof of extracardiac disease with evidence of cardiac involvement have been proposed and are commonly used even if they lack prospective validation or supportive data (Table 2). The Japanese Ministry of Health and Welfare (JMHW) criteria were initially published in 1993 and subsequently modified in 2007 and 2017. They require either histologic confirmation of extracardiac sarcoid (histological-diagnosis group) or a combination of major and minor criteria (clinical-diagnosis group). The revised 2017 document from the Japanese Circulation Society Joint Working Group, includes PET and MR among the major diagnostic criteria for CS and establishes 4 specific criteria to diagnose ICS (Supplemental Table 2). Compared to the JMHW, the Heart Rhythm Society (HRS) criteria are thought to have greater sensitivity since they include cardiac PET and MR as well as responsiveness to steroid therapy (89). Lastly, the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) developed a sarcoidosis organ assessment instrument that requires histologic evidence of granulomatous inflammation (90). A recent study comparing the accuracy of those 3 main diagnostic tools, revealed high concordance between WASOG and HRS but low between JCS and the others, rising concerns about the specificity of the diagnosis of sarcoidosis in the absence of confirmatory biopsy (91).
Figure 3: Electrophysiological-guided biopsy of the RV.
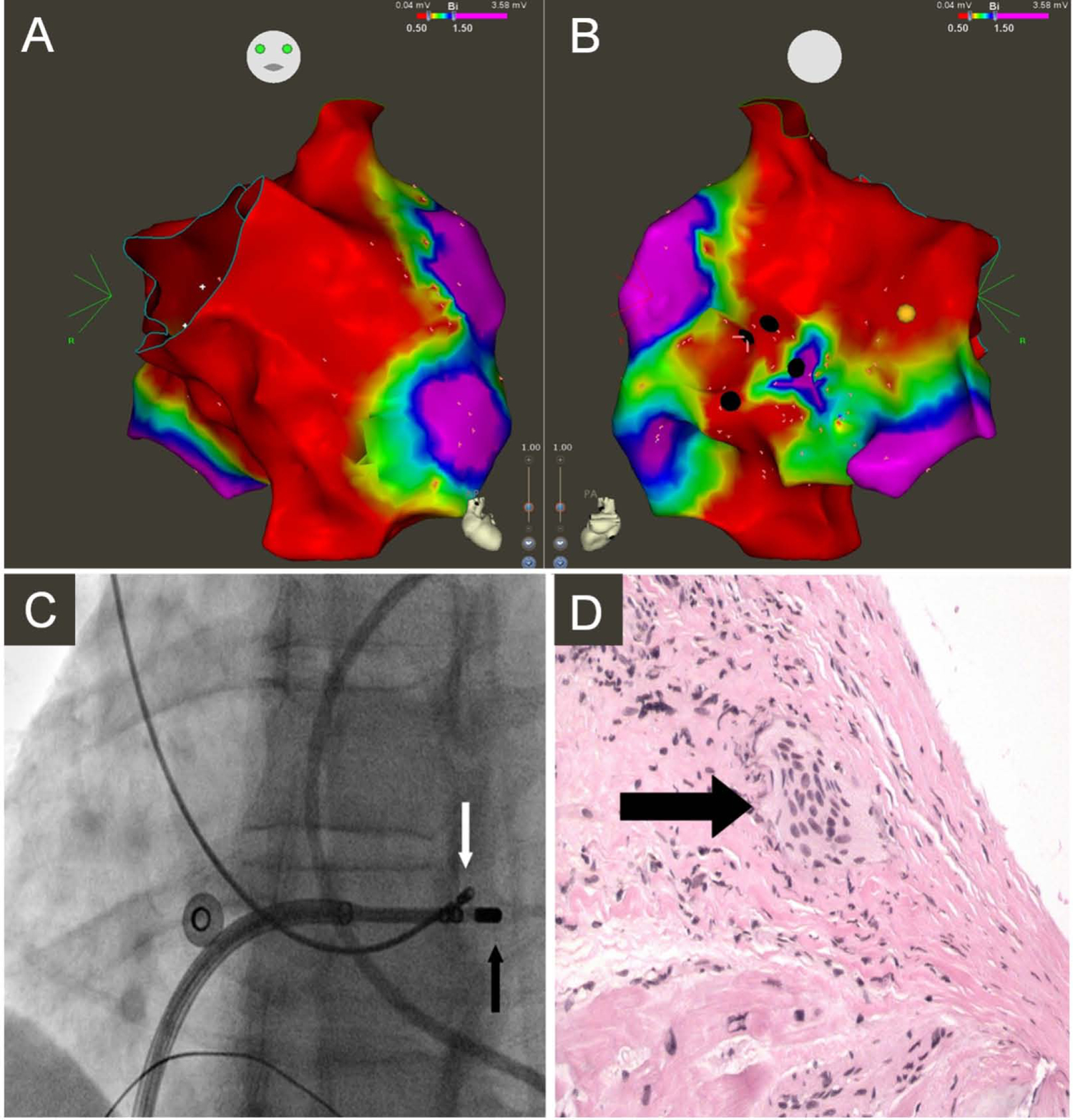
Electroanatomic bipolar voltage map of the RV displaying anterior (A) and posterior (B) views. Green, yellow, and red indicate low-voltage regions; purple denotes regions of normal voltage, defined as ≥ 1.5 mV. Black circles illustrate areas targeted for biopsy. Yellow circle illustrates location of right bundle. (C) Fluoroscopy images obtained in the left anterior oblique 25° projection showing bioptome (white arrow) targeting the low-voltage region in the RV septum, adjacent to mapping catheter (black arrow). (D) Microscopic view of an endomyocardial biopsy specimen obtained from the right ventricular septum showing noncaseating granuloma (arrow) (reproduced from(85)).
Table 2:
HRS and Japanese Circulation Society Working Group criteria for the diagnosis of CS
|
1. Histological Diagnosis from Myocardial Tissue CS is diagnosed in the presence of non-caseating granuloma on histological examination of myocardial tissue with no alternative cause identified (including negative organismal stains if applicable) 2. Clinical Diagnosis from Invasive and Non-Invasive Studies: It is probable* that there is CS if: a) There is a histological diagnosis of extra-cardiac sarcoidosis and b) One or more of following is present
and c) Other causes for the cardiac manifestation(s) have been reasonably excluded *In general, ‘probable involvement’ is considered adequate to establish a clinical diagnosis of CS |
1. Histological diagnosis group (those with positive myocardial biopsy findings) 2. Clinical diagnosis group (those without a positive myocardial biopsy) Granulomas are found in organs other than the heart, and clinical findings are strongly suggestive of cardiac involvement; or clinical findings are strongly suggestive of pulmonary or ophthalmic sarcoidosis and at least two of the five characteristic laboratory# and clinical findings of sarcoidosis are strongly suggestive of CS Clinical findings that strongly suggest the presence of cardiac involvement. 1) Two or more of the five major criteria are satisfied. 2) One of the five major criteria and two or more of the three minor criteria are satisfied. 1. Major criteria (a) High-grade atrioventricular block or fatal ventricular arrhythmia (i.e VT or VF) (b) Basal thinning of the ventricular septum or abnormal ventricular wall anatomy (ventricular aneurysm, thinning of the middle or upper ventricular septum, regional ventricular wall thickening) (c) Left ventricular contractile dysfunction (left ventricular ejection fraction <50%) (d) Abnormalities of 67Ga citrate scintigraphy or 18F-FDG PET (e) Gadolinium-enhanced MRI with delayed contrast enhancement of the heart 2. Minor criteria (f) Abnormal ECG findings: Ventricular arrhythmias (NSVT, multifocal PVCs), bundle branch block, axis deviation, or abnormal Q waves (g) Perfusion defects on myocardial perfusion scintigraphy (SPECT) (h) Endomyocardial biopsy: Monocyte infiltration and myocardial fibrosis #1) Bilateral hilar lymphadenopathy; 2) Elevated ACE, serum lysozyme levels or soluble interleukin-2 receptor (sIL-2R) levels; 3) Abnormalities of 67Ga citrate scintigraphy or 18F-FDG PET; 4) A high percentage of lymphocytes with CD4/CD8 ratio of >3.5 in BAL. |
Imaging Biomarkers for the diagnosis of CS in subjects with Extracardiac Disease
The prevalence of cardiac involvement among patients with systemic sarcoidosis screened by advanced imaging has varied widely (3.7–54.9%), depending upon the techniques used and the population studied (89). If extracardiac sarcoid is confirmed by tissue diagnosis, localizing cardiac findings such as abnormal PET, MR or arrhythmias are enough to suggest the diagnosis is highly probable (Table 2). When screened with MR, nearly 20% of subjects with extracardiac sarcoidosis will have evidence of myocardial involvement with an estimated sensitivity and specificity of 75–100% and 76–78% respectively (92,93). The pattern of scar detected on MR with LGE imaging is classically described as non-ischemic and involves preferentially the subepicardium and midmyocardium. In patients with biopsy proven extracardiac sarcoid, a positive MR is generally indicative of probable CS, but a negative one cannot exclude early or subclinical disease. Multiparametric MR imaging with T2-mapping to evaluate for edema might prove valuable in this context as it might identify areas of edema/inflammation before there are detectable changes in EF or LGE; the diagnostic accuracy of T2-mapping in CS however remains uncertain and is undergoing investigation (94). PET/CT is also widely used to evaluate CS. It couples anatomical information from the CT with PET to identify mononuclear inflammatory cells avid of 18F–FDG localized to the granulomas. The combined sensitivity and specificity of PET/CT has been estimated at 89% and 78%, using the JMHW criteria as gold standard. PET/CT with 18F–FDG has also been used to monitor response to escalation of IS (95). Main limitations of PET/CT are the lack of concomitant tissue characterization and the high rate of false positive scans due to inadequate suppression of physiological myocardial 18F–FDG uptake (96), with the latter significantly affected by dietary preparation (97). Combining PET perfusion imaging with 13N- Ammonia or 82Rubidium or SPECT with Thallium or Technetium-99m Sestamibi to identify areas of resting hypoperfusion believed to be secondary to microvascular compression from inflammatory cells is routinely used to “normalize” PET-FDG data. This combined approach predicted outcome in a study of 118 patients referred for cardiac PET for either known or suspected CS; patients that had both myocardial perfusion defects and abnormal 18F-FDG had a 4-fold increase in the annual rate of VT and death (73). Additionally, patients with evidence of RV involvement and increased 18F–FDG uptake, albeit rare, had a 5-fold higher event rate compared to patients with normal perfusion and metabolism (73). However, when PET/CT was compared to MR, the latter was superior in predicting major adverse cardiac events (98).
The edema, granulomatous infiltration, and fibrosis associated to CS can result in ECG abnormalities (99). VT, premature ventricular complexes (PVCs), RBBB, abnormal axis and Q wave have classically been associated with sarcoidosis and evidence of these electrocardiographic changes is generally a trigger for further investigations (89). Transthoracic Echocardiography (TTE) can be useful in identifying subjects with known extracardiac sarcoidosis that might have cardiac involvement or for monitoring disease progression once CS is established; the positive predictive value of an abnormal TTE in this scenario is 92%. Common findings on echocardiography include regional wall motion abnormalities, thinning and aneurysm of the IVS; yet in isolation, TTE lacks specificity and sensitivity since a normal/abnormal echocardiogram cannot rule in or out the disease in the absence of confirmatory biopsy. Speckle tracking echocardiography to measure LV global longitudinal strain was recently investigated in 100 subjects with presumed CS based on histology or fulfillment of the JMHW criteria. In this cohort, strain abnormalities showed greater sensitivity compared to standard echocardiographic parameters and had prognostic power (100).
Compared to PET/CT and standalone MR, hybrid PET/MR imaging has multiparametric capabilities and might prove superior to PET and MR in isolation, owing to greater specificity/resolution (PET/MR co-localization) and sensitivity in the detection of disease prior to the development of functional and structural abnormalities (T2-mapping and PET)(101,102). Recent observations suggest that when the T2 values were considered in combination with the PET/MR interpretation, there was concordance between inflammation and failed myocardial suppression with high and low T2 values respectively, indicating how this parameter might increase the specificity of the scans (94)(103).
While screening for cardiac involvement should be pursued in patients with history of sarcoidosis and cardiac symptoms (Class IIa)(89), the approach to patients with a diagnosis of extra-cardiac disease but asymptomatic remains controversial as it is uncertain if subclinical disease should be treated, whether more than one subgroup exist within this population or if the prognosis is different compared to symptomatic patients (74,104). The HRS gave a Class III recommendation to screening for cardiac involvement in patients without symptoms or ECG/TTE (89) changes. Additional limitations of the current diagnostic work-up of CS include: 1) the qualitative interpretation of the PET scans (PET/CT and PET/MR) that are typically reported as “positive” or “negative” and do not accurately “grade” disease severity, 2) the uncertainty about the “warranty period” of a negative scan (i.e. the period during which patients remained at a low risk after a negative scan) or 3) the latency of a positive study once therapy has been instituted. Access to a quantitative serological and/or imaging biomarker that linearly correlates with both presence and severity of disease would potentially address all these limitations by identifying CS in the preclinical stage and enabling a more accurate evaluation of the impact of IS therapy on prognosis or to guide treatment duration.
Imaging Biomarkers for the diagnosis of CS in subjects without Extracardiac Disease
In the absence of extracardiac manifestations, the identification of CS remains a diagnostic dilemma. Of the available imaging biomarkers, changes on the ECG or TTE lack sensitivity and specificity and are unlikely to offer additional diagnostic benefit without a tissue diagnosis (105). Abnormal LGE patterns on MR imaging is usually a trigger to consider CS for an otherwise unspecified CMP. In fact, especially in tertiary centers, MR is routinely part of the diagnostic w/u of subjects with non-ischemic CMP, and not unfrequently uncovers areas of myocardial scar on LGE sequences that suggest CS. The differential diagnosis (DDx) would typically include arrhythmogenic right ventricular cardiomyopathy (ARVC), where compared to CS the RV is classically more involved, there is sparing of the septum and no history of conduction abnormalities (106). Similarly, myocarditis could mimic CS and present with LGE in the sub-epicardium/midwall and edema on T2-weighted images; compared to CS, patients with myocarditis have usually a more acute presentation with either a prodrome, if viral in etiology, or a more aggressive and frequently fatal course in giant cells myocarditis (Figure 4). Fabry disease, a lysosome storage disorder, is part of the DDx of CS but is rarer and classically associated to LV hypertrophy and midwall LGE of the basal inferolateral wall. Other mimicker of CS includes Hypertrophic CMP, iron overload disorders, Chagas and Tuberculosis, the latter two manifesting with aneurysm formation and ventricular arrhythmias.
Figure 4: Diagnosis of ICS.
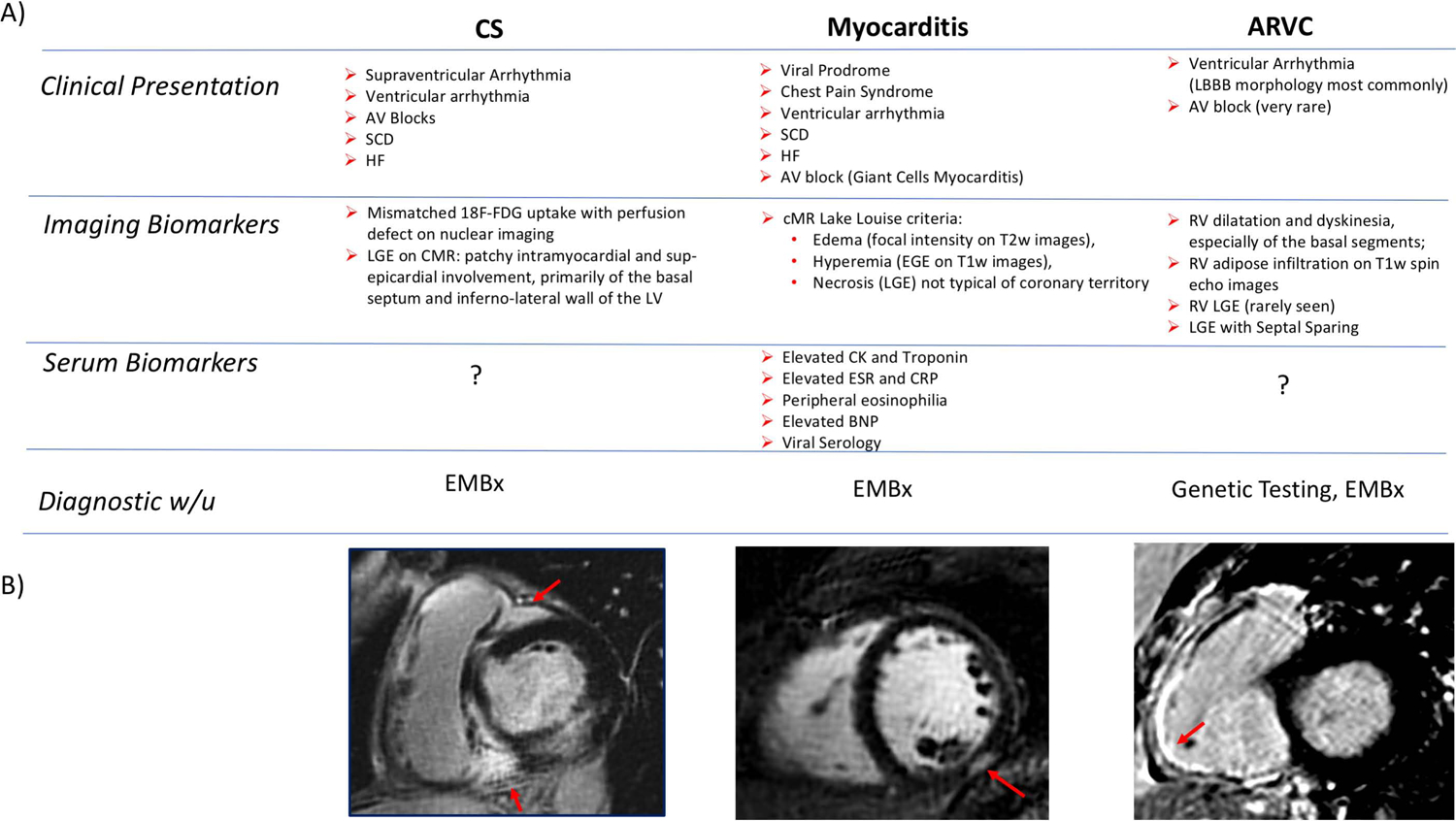
(A) Clinical, Imaging and Serological Biomarkers that might aid in the DDx of CS. (B) Short axis views of RV and LV showing classical patterns of LGE involving the 1) RV insertion point, basal septum as well as lateral wall in CS, 2) the sub-epicardium in myocarditis and 3) the RV free-wall in ARVC.
Simultaneous cardiac PET/MR detects both inflammation and scar in a single co-registered scan but does not have in isolation the discriminatory power to distinguish CS from other CMP. In our single center cohort of 180 subjects referred for the evaluation of CS either because of history of sarcoidosis or MR findings suggestive of CS, a discrete pattern of LGE and 18F–FDG uptake was identified in 3 subjects that appeared exclusive to cases of biopsy proven ICS (unpublished data, Figure 5). This pattern was characterized by significant RV involvement with LGE and 18F–FDG uptake along the RV and LV at the septal insertion points (“hug-signs”); more importantly, it appeared identical to the pattern of scar seen by Tavora et al. (62) on autopsy samples. Akin to LÖfgren’s syndrome for pulmonary sarcoidosis, this pattern might be the signature imaging biomarker of CS, and potentially sufficient to trigger institution of treatment even in the absence of a confirmatory biopsy. In keeping with this observation, a prior study had identified a predilection for the RV in subjects that presented with primary cardiac involvement, suggesting the presence of subgroups within the CS population that for unclear reason have selected tropisms for one of the myocardial chambers (80). The generation of a multicenter reference imaging library built upon similar case of “certain” CS with unique patterns will be useful in improving the diagnostic accuracy of CS, especially ICS; in addition it would offer insight in the pathogenesis of CS and promote a better understanding of the disease course as well as standardization of the therapeutic approach (Central Illustration).
Figure 5: Imaging features of CS.
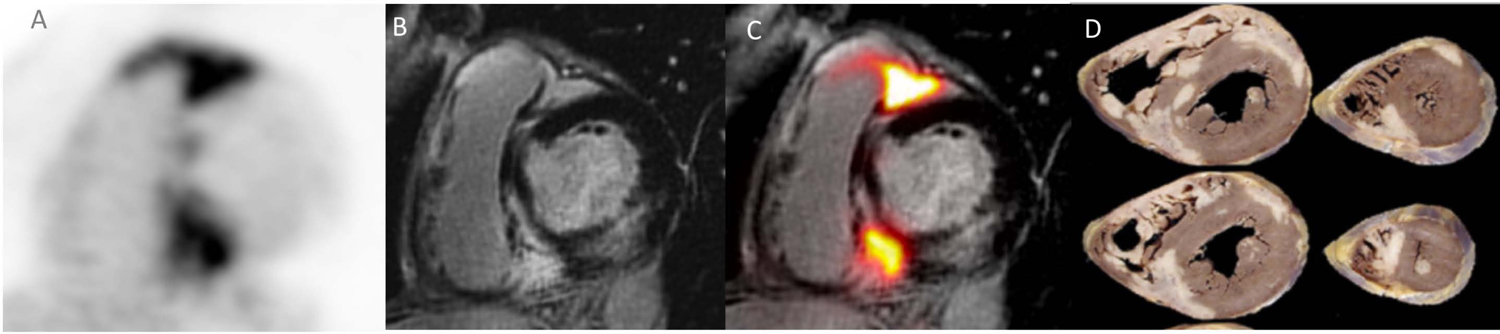
Short axis views of the RV/LV showing (A) areas of LGE in the RV, basal septum as well as RV lateral wall with matching increased 18F–FDG signal (B, C) perfectly co-localized to area of LGE (MR+PET+) on fused 18F–FDG-PET/MR images. This pattern of LGE and 18F–FDG uptake (“hug sign”) was solely observed in patients with biopsy proven CS and might constitute the imaging biomarker “signature” of the disease. This pattern faithfully replicated the one previously identified in explanted hearts from patients with ICS (D). Reproduced with permission (62).
Therapy
Pulmonary Sarcoidosis
The clinical course of pulmonary sarcoidosis may range from an asymptomatic state to a progressive fibrotic and potentially life-threatening disease. Because corticosteroids and other drugs used to treat pulmonary sarcoidosis are associated with significant drug toxicities, comorbidities(107) and quality of life impairment even after adjustment for disease severity (108), the outcome of treatment may be worse than withholding treatment in asymptomatic and mild cases (109). As such, therapy is not considered mandatory for all documented cases of pulmonary sarcoidosis (2).
The indications for treatment of pulmonary sarcoidosis can be distilled down to two: significant quality of life and/or functional impairment (more commonly) or a potentially dangerous forms of the disease that may result in severe organ dysfunction, disability, or death (110). Potentially dangerous scenarios of pulmonary sarcoidosis that would mandate therapy are listed in Supplemental Table 3 and they occur in less than 20% of pulmonary sarcoidosis patients. With the exception of progressive pulmonary fibrosis, these forms of pulmonary sarcoidosis are usually not treated with corticosteroids or anti-granulomatous therapy.
Although a consensus statement of international pulmonary and sarcoidosis societies has recommended a starting dose of 20 to 40mg of daily prednisone equivalent for the initial treatment of pulmonary sarcoidosis (35), 20mg appears to be near the top of the dose response curve for this form of sarcoidosis (40) with much less drug toxicity than higher doses. The prednisone dose can then be rapidly tapered to 10mg/day but relapses are very common (up to 70%) when prednisone is reduced to <10mg/day or discontinued (111). This reflects the opinion that anti-granulomatous therapy for sarcoidosis can transiently resolve granulomatous inflammation but may not affect the natural course of the disease (112). As sarcoidosis is often a chronic disease lasting for years, if not for a lifetime, chronic corticosteroids may be required and that may lead to cumulative toxicities. Therefore, corticosteroid-sparing/corticosteroid-replacing agents are often considered for the treatment of prolonged cases of pulmonary sarcoidosis. The drugs that are commonly used as corticosteroid sparing/replacing therapy of pulmonary sarcoidosis are listed in Table 4.
Table 4.
Common Corticosteroid Sparing/Replacing Agents for Treatment of Sarcoidosis
| Drugs | Usual dosage | Mechanism of action | Common Toxicities | Corticosteroid sparing potential | Corticosteroid replacing potential | Comments |
|---|---|---|---|---|---|---|
| Adalimumab | 40 units every week SC. Consider initial higher loading dosages. | TNF-α antagonism | Allergic reaction, increased risk of infection especially TB, CHF, possible increased risk of malignancy, injection site reaction | +++ | ++ | Less immunogenic than infliximab, screening for latent TB is required. |
| Azathioprine | 50–200 mg daily | As a purine analogue, inhibits purine synthesis necessary for T-, B- cell proliferation | Leukopenia, hepatotoxicity, risk of infection, skin cancer | +++ | + | Monitor CBC & LFT; check TPMT level at initiation (controversial)*. |
| Infliximab | 3–5 mg/kg initially & at 2 weeks and 6 weeks, then every 4–6 weeks IV | TNF-α antagonism | Allergic reaction, increased risk of infection especially TB, CHF, possible increased risk of malignancy | +++ | ++ | Autoantibodies may develop with chronic use that may reduce efficacy, screening for latent TB is required. |
| Leflunomide | 10–20 mg daily | Inhibits COX- 2 enzyme; DHODH inhibition affecting pyrimidine synthesis | Leukopenia, hepatotoxicity, risk of infection, skin rash, fatigue, pneumonitis, peripheral neuropathy | +++ | + | Monitor CBC & LFT; because of a long circulating half-life, leflunomide toxicity may require cholestyramine to quickly remove the drug and its metabolites. |
| Methotrexate | 10–15 mg weekly | Inhibits the metabolism of folic acid in purine and pyrimidine synthesis | GI intolerance, oral ulcers, hepatotoxicity, leukopenia, fatigue, risk of infection, pneumonitis | +++ | + | Teratogenic; folate supplementation is recommended; monitor CBC & LFT. |
| Mycophenolate | 3000–1500 daily | Inhibits de novo guanosine nucleotide synthesis and has a cytostatic effect on T and B cell proliferation | Leukopenia, risk of infection, lymphoproliferative disorders, skin cancer | +/− | +/− | Very limited data supporting both effectiveness and safety |
| Repository Corticotropin (RCI) | 40–80 unit SC twice weekly | Stimulates ACTH secretion | Mood change, elevated HBA1c, bruising | +++ | +++ | |
| Rituximab | 375 mg/m2 IV every 2 weeks | Monoclonal antibody against CD20 surface antigen of B-lymphocytes | Transfusion reaction, pancytopenia, opportunistic infection, fatigue, headache, neuropathy | + | + |
CBC: complete blood count; LFT: liver function test; DHODH: dihydroorotate dehydrogenase; COX 2: cyclooxygenase-2; TPMT: Thiopurine; IV: intravenous: SC: subcutaneous; TB: Tuberculosis; CHF: congestive heart failure; GI: gastrointestinal; +++: very good; ++: good; +: fair
Cardiac Sarcoidosis
The treatment of CS can be divided into two general categories: treatment of the inflammation or of its clinical sequelae, i.e. arrhythmias and ventricular dysfunction (Figure 6). In Figure 6 we have included the use of cardiac PET to assess for active cardiac inflammation as some authors have proposed that a positive PET scan be considered an indication for treatment; this is indeed an appealing concept, since a positive PET scan in the lung has proved to be an effective method to predict response to anti-inflammatory therapy (59,110,113). Because the use of PET to guide treatment has not yet been validated in clinical trials, the decision to use cardiac PET scan to guide therapy presently relies only on physician preference and center experience (Figures 7, 8) (5).
Figure 6: Proposed diagnostic and management approach for the assessment and treatment of CS.
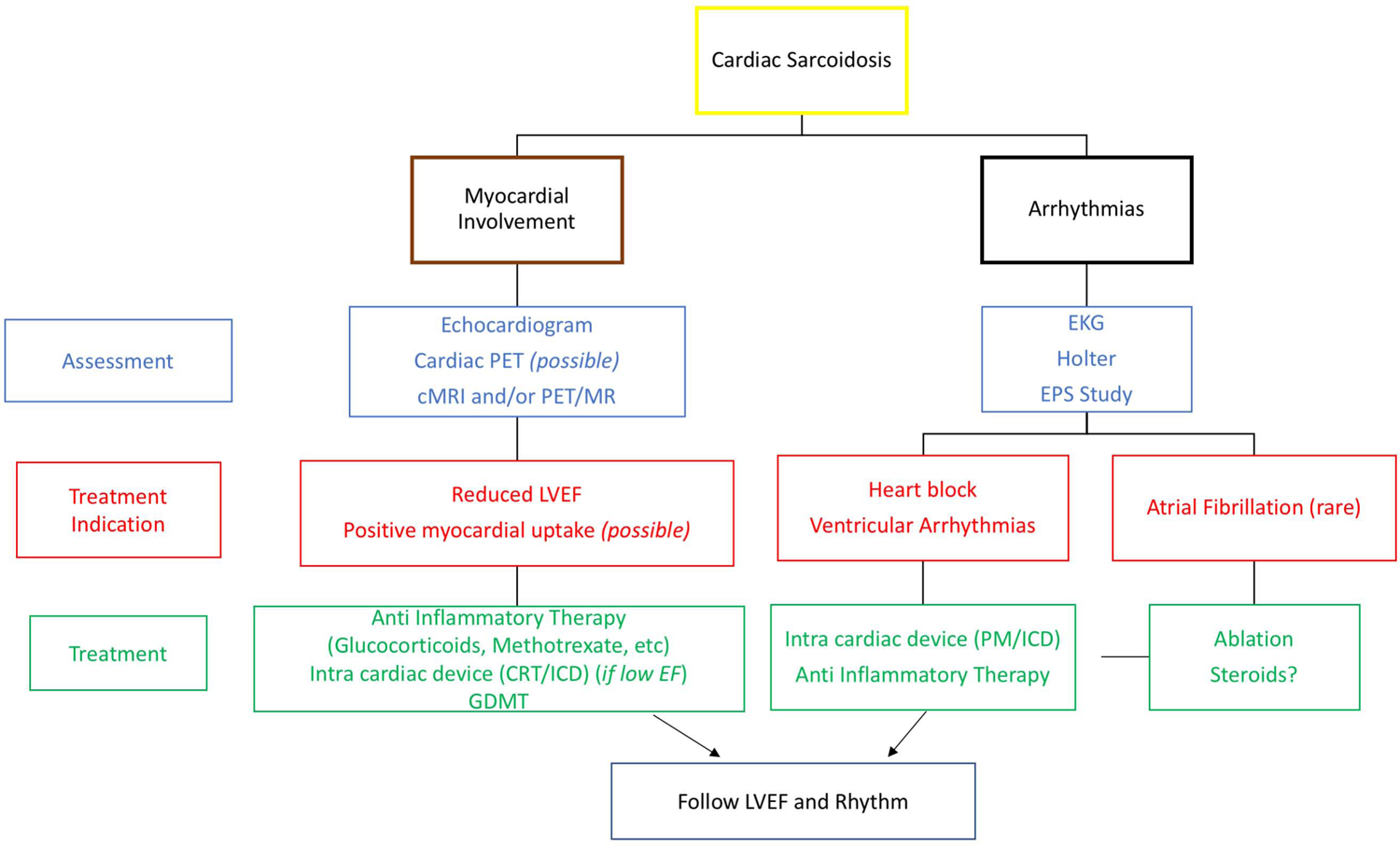
The treatment of CS can be divided into two general categories: treatment of the inflammation or of its clinical sequelae, i.e. arrhythmias and ventricular dysfunction. The figure summarizes the available imaging modalities, diagnostic and therapeutic options.
Figure 7: PET scan as a possible tool to predict response to anti-inflammatory therapy and to guide treatment decision.
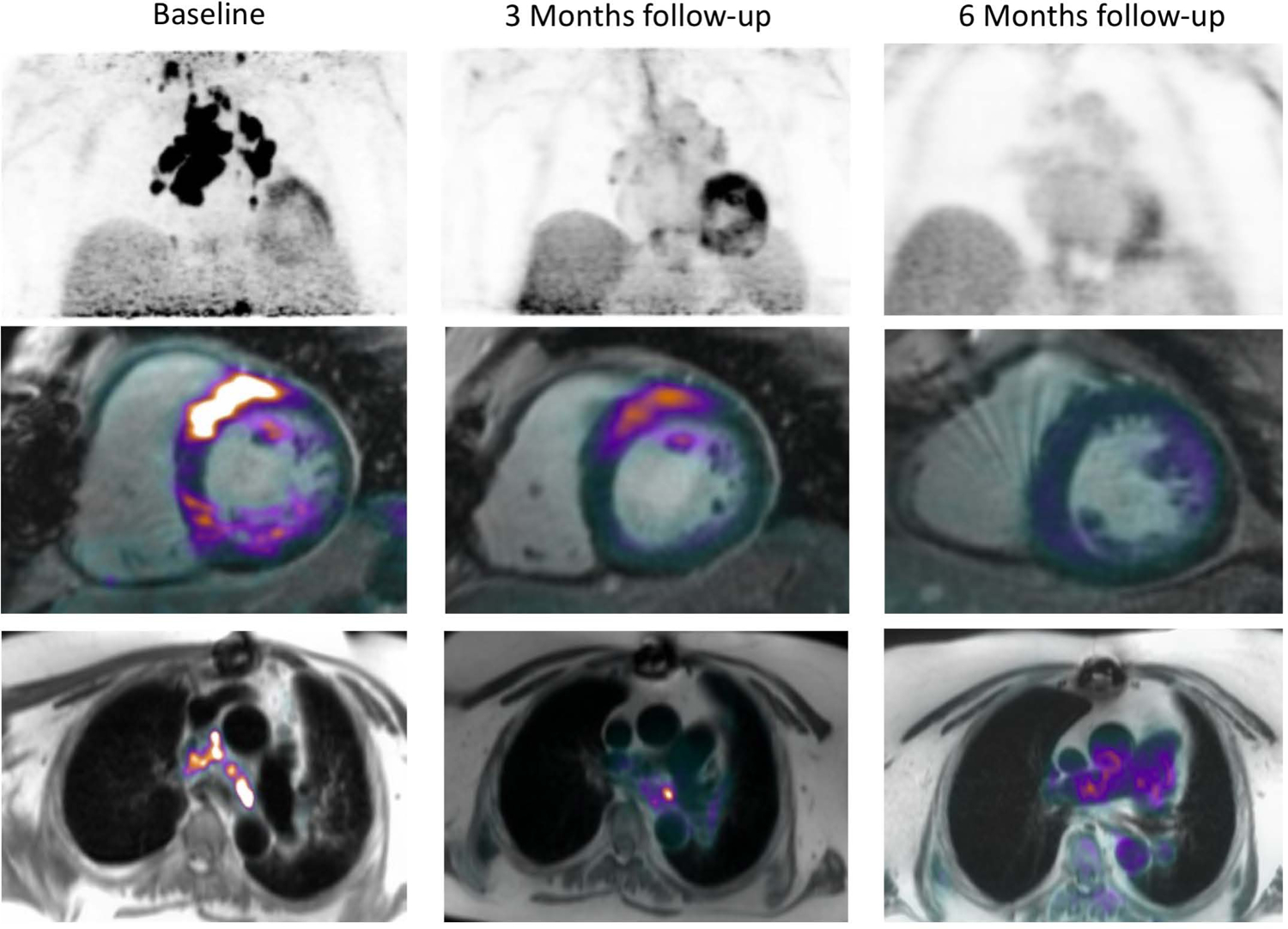
Cardiac PET/MR was performed in a patient with biopsy proven CS symptomatic with AF. The initial scan revealed 18F–FDG avid mediastinal LNDs as well as cardiac uptake in the basal segments of the antero-septum. IS therapy with steroids was instituted, with spontaneous cardioversion. Follow up scan at 3 and 6 months revealed progressive resolution of the inflammation.
Figure 8: Suggested treatment algorithm for patients with clinically manifest CS. The figure proposes an imaging guided approach to escalation and de-escalation of immunosuppressive therapy.
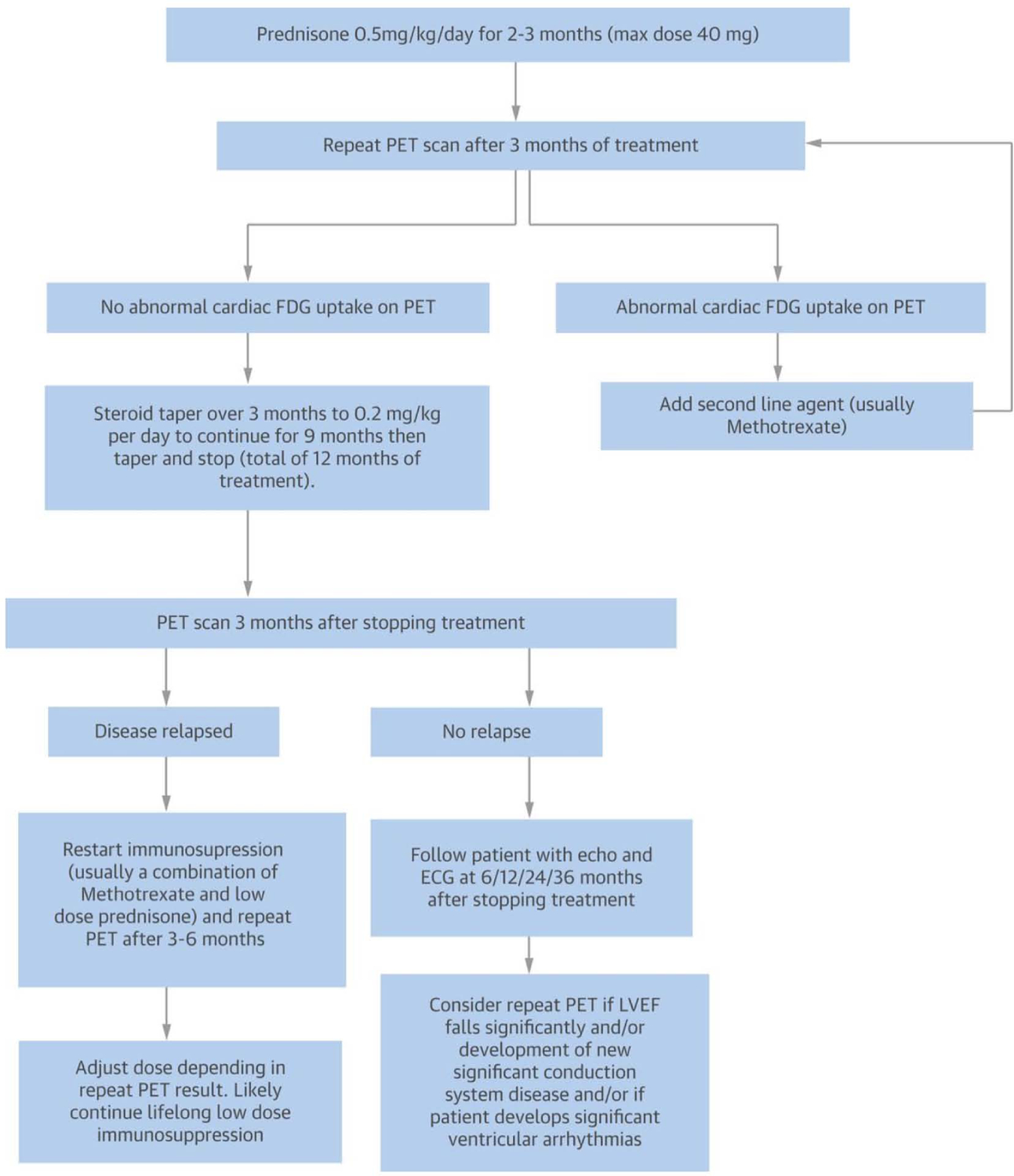
Reproduced with permission (5).
Figure 9 summarizes proposed, step wise approach to treating CS. Among the anti-inflammatory agents, most physicians would consider glucocorticoids for first line therapy (110), even though there is no randomized controlled study that has systematically evaluated their efficacy, ideal dosing and treatment duration, rendering the management of CS highly empiric and very heterogeneous. The decision to treat CS with an anti-inflammatory therapy is primarily based on clinical manifestations and it has been investigated in a handful of studies mostly focusing on glucocorticoids alone (114,115). In general, the earlier the treatment, the better the response. One retrospective study found that treating before the onset of reduced LVEF was associated with a significantly better outcome (116). A recent study found that patients treated with glucocorticoids for CS within the first six months were likely to have an improvement in LVEF, while those whose treatment was delayed for more than six months were unlikely to respond (114); interestingly, response to anti-inflammatory therapy was seen even in those with an LVEF of less than 30%. In terms of dosing, a retrospective study found no differences for those given more than 30mg of prednisone versus lower dose (116). Therefore, an initial dose of 30 to 40mg (117), is generally viewed as reasonable. If treatment with steroid is initiated, there are limited data to direct timing of dosage de-escalation and overall duration of therapy; a decision treatment algorithm was previously proposed and is often used in clinical practice (5) (Figure 8).
Figure 9: Stepwise approach to the therapy for CS.
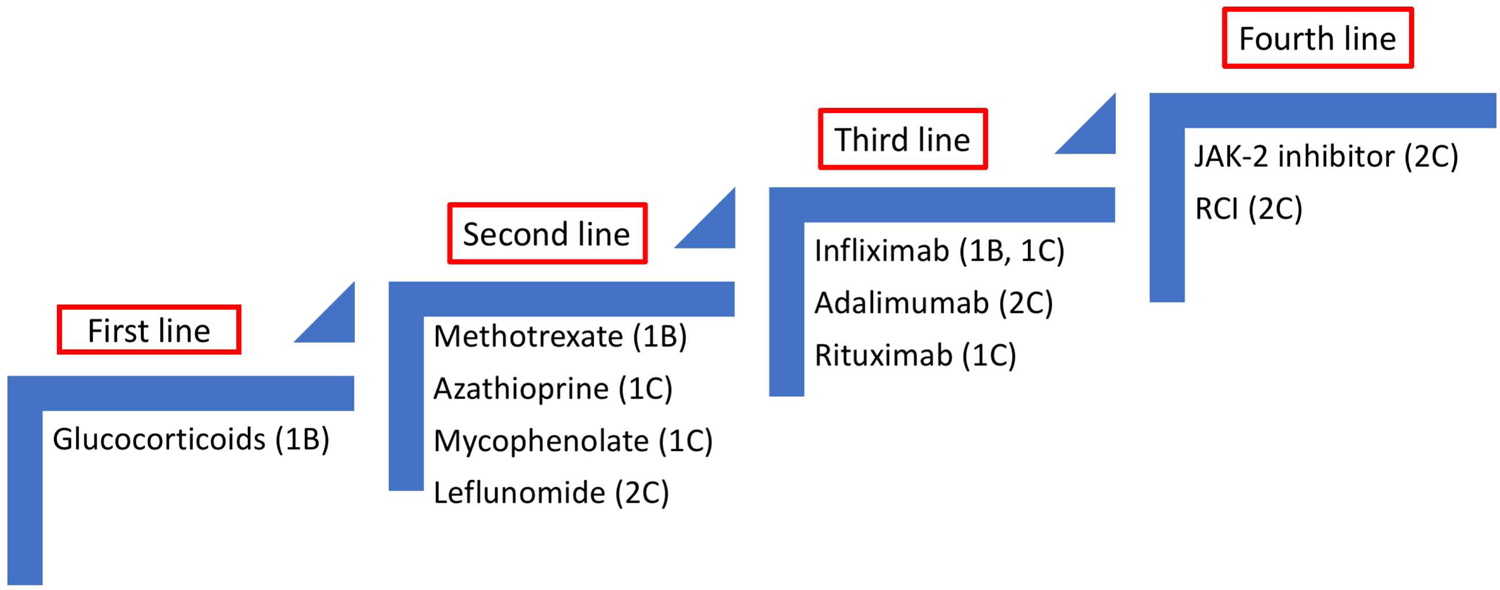
Treatments were scored based on reported effectiveness for cardiac (1) or extra-cardiac (2) sarcoidosis. Level of evidence: A for randomized trials, B for case series or C for case reports. There is presently no randomized trial in CS. Initiation and escalation of treatment is commonly guided by PET.
Steroid-sparing alternatives/anti-metabolites are considered second line/adjuvant agents in the management of patients with CS (118–120). Since CS patients often require years of therapy, the use of steroid sparing alternatives appears to be an obvious alternative to prolonged glucocorticoid use. Yet, most studies have been single centers reporting on a variety of agents (118,119). Ballul et al showed how combining immunosuppressive to glucocorticoids reduced the relapse rate from 46 to 17% (121). Nagai et al reported on the outcome of CS patient treated with prednisone alone versus low dose prednisone plus methotrexate (122). At one year, both regimens were associated to better outcomes with improvement in LVEF and reduction in BNP. However, by year five, the prednisone alone group’s LVEF had fallen and the BNP rose, while the prednisone plus methotrexate had a persistent beneficial effect (122). The CHASM CS-RCT (NCT03593759) is the first international study to be conducted in patients with CS that is tasked with the goal of obtaining high quality data for treating CS. The study plans to enroll subjects with clinically manifest CS and randomize them to standard prednisone for 6 months OR to combination of methotrexate and prednisone (123).
Of the steroid-sparing drugs listed in Figure 9 and Table 4, methotrexate has the most supportive evidence based on the Nagai study (122) as well as the larger experience with methotrexate for pulmonary sarcoidosis (59,124). Azathioprine and mycophenolate have been reported as effective in treating CS patients, but there is little to indicate that either of these drugs is superior to any other anti-metabolite (118,119,121). As such, the choice for an individual patient depends mostly on other factors, including health care provider experience.
Third line therapy with biologics are listed in Figure 9 and Table 4. Infliximab has proved to be an effective treatment for advanced pulmonary and neurologic sarcoidosis (54,125,126). Infliximab, rituximab, or corticotrophin have been used successfully in selected CS cases (127,128) and a recent large retrospective study from one institution supports that infliximab can be used safely and effectively in CS (129). However, because of the risk of increased mortality associated with use of anti-TNF therapy in patients with severe CMP (130), one must use with caution. Our own clinical practice is to consider anti-TNF therapy such as infliximab or adalimumab when first- and second-line therapies have failed, there is significant evidence of cardiac inflammation by PET scan, and no evidence for an alternative cause for the CMP.
Rituximab has been used in CS (131) as well; this agent has proven useful in other forms of sarcoidosis such as pulmonary (132) and ocular (133) and it does not have associated cardiotoxicity. Compared to other agents, has a longer latency to demonstrate a benefit and appears to be less effective than infliximab.
Figure 6 additionally highlights the importance of treating the underlying arrhythmias. Cardiac devices have become standard in symptomatic CS (89) and for patients with documented ventricular arrhythmias, especially VT, the placement of an implantable cardioverter defibrillator (ICD) can be lifesaving (117). Conversely, for the asymptomatic patients that lack classical triggers for ICD implantation, programmed electrical stimulation (PES) has been proposed for risk stratification based on a study by Metha where it was shown to predict event-free survival. As a caveat to the study, the majority of the PES-positive patients had LVEF <40, hence it’s uncertain if PES in isolation is more predictive than LVEF alone (89). In addition to ICD, treatment with anti-inflammatory drugs can further reduce the risk of arrhythmia and a number of studies have shown resolution of heart block and other arrhythmias after institution of anti-inflammatory therapy (96,133). In this particular scenario, the placement of an ICD can provide patients with a safety net while anti-inflammatory therapy is initiated, which is of critical importance since arrhythmias remain highly prevalent in the first few months of treatment. Indeed, in one large study of CS, over 1/3rd of patients received appropriate ICD therapy (134). Consistent with this observation, Kron et al found that 36% of patients that had an ICD implanted for primary (~62%) vs. secondary prevention (~37%) received appropriate ICD therapy over a mean follow-up of 4.2 year, with the majority having an EF of >35%(135), a percentage that is significantly higher than other CMPs. Table 3 and Figure 10 summarize the HRS suggested approach to arrhythmia management in patient with CS. Additional criteria for ICD therapy can be found in the AHA/ACC guidelines on SCD and Ventricular arrhythmias (136). Compared to the HRS criteria, the AHA guidelines give a class IIa recommendation to consider ICD implantation in patients that have EF>35%, scar on MR or abnormal PET.
Table 3:
HRS Expert Consensus Statement on the Management of Arrhythmias Associated With Cardiac Sarcoidosis
| Conduction Abnormalities | |
|---|---|
| Class I | Pacing indication for Acquired Atrioventricular Block and Chronic Bifascicular Block should follow the American College of Cardiology/American Heart Association/ Heart Rhythm Society Guideline. |
| Class IIa | IS can be useful for patients with Mobitz II or third-degree heart block. |
| Class IIa | Device implantation can be useful when there is another indication for pacing even if the AV block reverses transiently. |
| Class IIa | Implantable cardioverter-defibrillator implantation can be useful in patients with another indication for permanent pacemaker implantation. |
| Atrial Arrhythmias | |
| Class I | Anticoagulation is recommended in patients with CS and AF if there is sufficiently high risk as determined by CHADS2 or CHA2DS2-VASc score. |
| Class IIa | An invasive electrophysiological study may be considered in patients with atrial arrhythmias other than AF to direct therapy. |
| Class III | Antiarrhythmic medication therapy with class I agents is not recommended for the treatment of arrhythmias associated with CS. |
| Ventricular Arrhythmias | |
| Class IIa | Assessment of myocardial inflammation with FDG-PET can be useful in CS patients with ventricular arrhythmias. |
| Class IIa | Immunosuppression can be useful in CS patients with frequent ventricular ectopy or non-sustained VT and evidence of myocardial inflammation. |
| Class IIa | Immunosuppression can be useful in CS patients with sustained ventricular arrhythmias and evidence of myocardial inflammation. |
| Class IIa | Antiarrhythmic medication therapy can be useful in patients with ventricular arrhythmias refractory to immunosuppressive therapy. |
| Class IIa | Catheter ablation can be useful in patients with CS and ventricular arrhythmias refractory to immunosuppressive and antiarrhythmic therapy. |
| Class IIa | Catheter ablation can be useful in patients with incessant ventricular arrhythmias. |
| Sudden Cardiac Death | |
| Class IIb | An electrophysiological study for the purpose of sudden death risk stratification may be considered in patients with LVEF435%, despite optimal medical therapy and a period of immunosuppression (if there is active inflammation). |
| Class IIa | CMR for the purpose of sudden death risk stratification may be considered in patients with CS. |
| ICD implantation | |
| Class I | ICD implantation is recommended in patients with CS and one or more of the following: 1. Spontaneous sustained ventricular arrhythmias, including prior cardiac; 2. LVEF <35%, despite optimal medical of immunosuppression (if there is active inflammation). |
| Class IIa | ICD implantation can be useful in patients with CS, independent of ventricular function, and one or more of the following: 1. An indication for permanent pacemaker implantation; 2. Unexplained syncope or near-syncope, felt to be arrhythmic in etiology; 3. Inducible sustained ventricular arrhythmias (430 seconds of monomorphic VT or polymorphic VT) or clinically relevant VF. |
| Class IIB | ICD implantation may be considered in patients with LVEF in the range of 36%−49% and/or an RV ejection fraction o40%, despite optimal medical therapy for heart failure and a period of immunosuppression (if there is active inflammation). |
| Class III | ICD implantation is not recommended in patients with no history of syncope, normal LVEF/RV ejection fraction, no LGE on CMR, a negative EP study, and no indication for permanent pacing. |
| Class III | ICD implantation is not recommended in patients with one or more of the following: 1. Incessant ventricular arrhythmias; 2. Severe New York Heart Association class IV heart failure. |
Figure 10: ICD Implantation for CS.
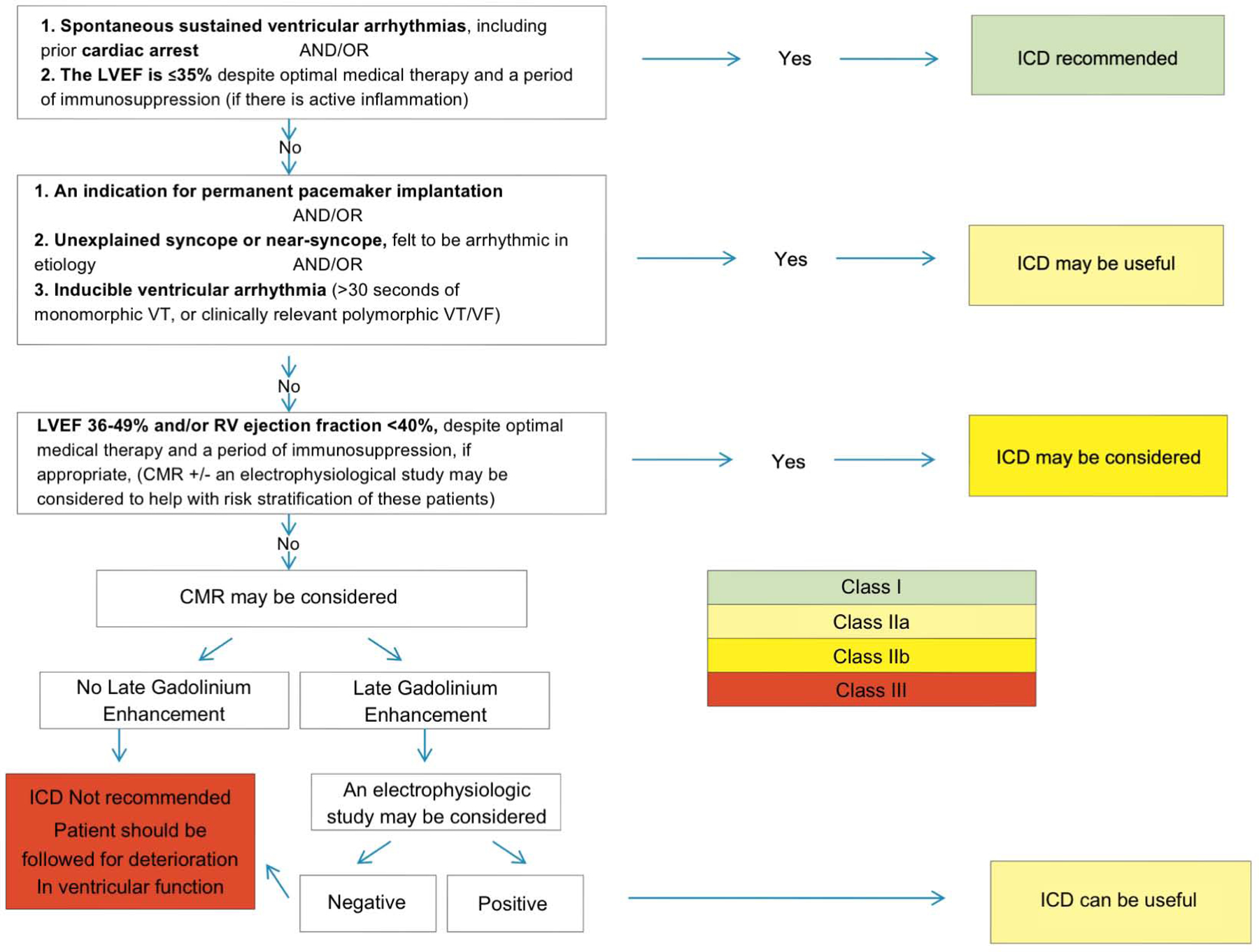
Consensus recommendations for ICD implantation in patients diagnosed with Cardiac Sarcoidosis. Reproduced from (89)
As final considerations, standard goal directed medical therapies (GDMT) for the management of HF are also indicated. While these therapies likely do not have any effect on sarcoid activity per se, they can have important beneficial effects in terms of preserving LVEF and improving HF-related outcomes. Treatment is also indicated for precapillary SAPH (134) Among the pulmonary vasodilatory agents, bosantan was shown to be beneficial after 16 weeks of treatment (135).
Conclusion and Future direction
The complexity of the disease that is likely genetic and/or environmental emphasizes the importance of centralizing care in “centers of excellence” but also creating international consortia with shared goals, protocols, imaging strategies, biobank and clinical repositories of predefined metrics of disease activity. These consortia will be able to collect enough multidimensional data points from cases of “certain” or “highly likely” sarcoidosis with varying degree of clinical activity to have the statistical power to effectively use novel techniques of genome/RNA sequencing, image processing and clinical phenotyping to uncover the biological signature of the disease (Central Illustration). This multidimensional “biomarker” signature will be the starting point for the discovery of specific molecular pathways, targeted/repurposed therapies as well as anti-signature approaches that will enable an improved understanding of the disease pathophysiology and treatment standardization.
A number of initiatives are already underway. Those include the Canadian /Japanese registry, with an imaging and biomarker core lab (NCT01477359); the US/European consortium with an imaging core lab as well as the Finnish (Myocardial Inflammatory Diseases in Finland)(7). Combining these efforts should be the next step for finding clarity in this puzzling disease.
Supplementary Material
Highlights.
Cardiac and pulmonary involvement is sarcoidosis is associated with greater morbidity and mortality than other manifestations of the disease.
Diagnosis of sarcoidosis should consider the clinical presentation, histologic evidence of granulomatous inflammation, and exclusion of alternative granulomatous diseases.
The lack of validated diagnostic tests or biomarkers of disease activity have hindered research into disease mechanisms, therapeutic opportunities or assessment of the response to treatment, and should be a focus of future investigations.
Acknowledgment:
The authors are grateful to Dr. Phil Robson, Dr. Ozioma Chioma, Dr. Alexander Maier and Dr. Vittoria Vergani for the editorial assistance and valuable comments.
Funding: This work was supported by National Institutes of Health (NIH) grants NIH/NHLBI R01HL071021 (to Dr. Fayad), R01 HL 117074 (to Dr. Drake) and KL2 TR001435 (to Dr. Trivieri).
Abbreviations and Acronyms:
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- APCs
antigen-presenting cells
- AF
Atrial Fibrillation
- AVB
atrioventricular block
- BAL
bronchoalveolar lavage
- CBD
chronic beryllium disease
- CMP
cardiomyopathy
- CS
cardiac sarcoidosis
- EF
ejection fraction
- ECG
Electrocardiogram
- EMBx
endomyocardial biopsy
- 18F-FDG
fluorodeoxyglucose
- FVC
forced vital capacity
- GDMT
goal directed medical therapy
- HF
heart failure
- HLA
human leukocyte antigens
- HRCT
high resolution chest computed tomography scans
- HRS
Heart Rhythm Society
- ICD
implantable cardioverter defibrillator
- ICS
isolated cardiac sarcoid
- IFN
interferon
- IL
interleukin
- IVS
interventricular septum
- JMHW
Japanese Ministry of Health and Welfare
- LGE
late gadolinium enhancement
- LV
left ventricle
- miRNAs
MicroRNAs
- PES
programmed electrical stimulation
- PET
positron emission tomography
- PET/CT
positron emission tomography coupled with X-ray computed tomography
- PET/MR
positron emission tomography coupled with cardiac magnetic resonance
- PH
pulmonary hypertension
- RBBB
right bundle branch block
- RV
right ventricle
- SAPH
sarcoidosis associated pulmonary hypertension
- SCD
sudden cardiac death
- TCR
T-cell receptor
- Th
T-helper cells
- TNF
tumour necrosis factor
- Tregs
regulatory T cells
- TTE
transthoracic echocardiography
- WASOG
World Association of Sarcoidosis and other Granulomatous Disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no relevant disclosures related to the content of this review.
References
- 1.Arkema EV, Cozier YC. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis 2018;9:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:119–27. [PubMed] [Google Scholar]
- 3.Morimoto T, Azuma A et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 2008;31:372–9. [DOI] [PubMed] [Google Scholar]
- 4.Ohta H, Tazawa R et al. Acute-onset sarcoidosis with erythema nodosum and polyarthralgia (Lofgren’s syndrome) in Japan: a case report and a review of the literature. Intern Med 2006;45:659–62. [DOI] [PubMed] [Google Scholar]
- 5.Birnie DH, Nery PB et al. Cardiac Sarcoidosis. J Am Coll Cardiol 2016;68:411–21. [DOI] [PubMed] [Google Scholar]
- 6.Mirsaeidi M, Machado RF et al. Racial difference in sarcoidosis mortality in the United States. Chest 2015;147:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandolin R, Lehtonen J et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–32. [DOI] [PubMed] [Google Scholar]
- 8.Rybicki BA, Iannuzzi MC et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Respir Crit Care Med 2001;164:2085–91. [DOI] [PubMed] [Google Scholar]
- 9.Spagnolo P, Grunewald J. Recent advances in the genetics of sarcoidosis. J Med Genet 2013;50:290–7. [DOI] [PubMed] [Google Scholar]
- 10.Berlin M, Fogdell-Hahn A et al. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med 1997;156:1601–5. [DOI] [PubMed] [Google Scholar]
- 11.Valentonyte R, Hampe J et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet 2005;37:357–64. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann S, Franke A et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet 2008;40:1103–6. [DOI] [PubMed] [Google Scholar]
- 13.Grunewald J, Grutters JC, et al. Sarcoidosis. Nat Rev Dis Primers 2019;5:45. [DOI] [PubMed] [Google Scholar]
- 14.Bordignon M, Rottoli et al. Adaptive immune responses in primary cutaneous sarcoidosis. Clin Dev Immunol 2011;2011:235142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake WP, Pei Z et al. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerg Infect Dis 2002;8:1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Z, Marzilli L et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201:755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eishi Y, Suga M et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 2002;40:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta D, Agarwal R et al. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 2007;30:508–16. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Hu Y, Li H. Role of Propionibacterium Acnes in Sarcoidosis: A Meta-analysis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:262–7. [PubMed] [Google Scholar]
- 20.Chen ES, Moller DR. Etiologies of Sarcoidosis. Clin Rev Allergy Immunol 2015;49:6–18. [DOI] [PubMed] [Google Scholar]
- 21.Rosen Y Pathology of sarcoidosis. Semin Respir Crit Care Med 2007;28:36–52. [DOI] [PubMed] [Google Scholar]
- 22.Izbicki G, Chavko R et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest 2007;131:1414–23. [DOI] [PubMed] [Google Scholar]
- 23.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet 2007;8:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang IV, Konigsberg I et al. DNA Methylation Changes in Lung Immune Cells Are Associated with Granulomatous Lung Disease. Am J Respir Cell Mol Biol 2019;60:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moller DR, Chen ES. Genetic basis of remitting sarcoidosis: triumph of the trimolecular complex? Am J Respir Cell Mol Biol 2002;27:391–5. [DOI] [PubMed] [Google Scholar]
- 26.Forrester JM, Wang Y et al. TCR expression of activated T cell clones in the lungs of patients with pulmonary sarcoidosis. J Immunol 1994;153:4291–302. [PubMed] [Google Scholar]
- 27.Ziegenhagen MW, Muller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med 2003;253:18–30. [DOI] [PubMed] [Google Scholar]
- 28.Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol 2000;12:71–6. [DOI] [PubMed] [Google Scholar]
- 29.Mostard RL, Van. Kuijk SM et al. A predictive tool for an effective use of (18)F-FDG PET in assessing activity of sarcoidosis. BMC Pulm Med 2012;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandolin R, Lehtonen J et al. Usefulness of Cardiac Troponins as Markers of Early Treatment Response in Cardiac Sarcoidosis. Am J Cardiol 2015;116:960–4. [DOI] [PubMed] [Google Scholar]
- 31.Kiko T, Yoshihisa A et al. A Multiple Biomarker Approach in Patients with Cardiac Sarcoidosis. Int Heart J 2018;59:996–1001. [DOI] [PubMed] [Google Scholar]
- 32.Ishiguchi H, Kobayashi S et al. Urinary 8-Hydroxy-2’-Deoxyguanosine as a Myocardial Oxidative Stress Marker Is Associated With Ventricular Tachycardia in Patients With Active Cardiac Sarcoidosis. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 33.Fujiwara W, Kato Y et al. Serum microRNA-126 and −223 as new-generation biomarkers for sarcoidosis in patients with heart failure. J Cardiol 2018;72:452–457. [DOI] [PubMed] [Google Scholar]
- 34.Ganatra S, Sharma A et al. Mycobacterium Chimaera Mimicking Sarcoidosis. Methodist Debakey Cardiovasc J 2018;14:301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunninghake GW, Costabel U et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149–73. [PubMed] [Google Scholar]
- 36.Mathew S, Bauer KL et al. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol 2008;181:746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Judson MA. Lung transplantation for pulmonary sarcoidosis. Eur Respir J 1998;11:738–44. [PubMed] [Google Scholar]
- 38.Mana J, Gomez-Vaquero C et al. Lofgren’s syndrome revisited: a study of 186 patients. Am J Med 1999;107:240–5. [DOI] [PubMed] [Google Scholar]
- 39.Baughman RP, Teirstein AS et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885–9. [DOI] [PubMed] [Google Scholar]
- 40.McKinzie BP, Bullington WM et al. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am J Med Sci 2010;339:1–4. [DOI] [PubMed] [Google Scholar]
- 41.Lynch JP 3rd, Ma YL et al. Pulmonary sarcoidosis. Semin Respir Crit Care Med 2007;28:53–74. [DOI] [PubMed] [Google Scholar]
- 42.Abehsera M, Valeyre D et al. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am J Roentgenol 2000;174:1751–7. [DOI] [PubMed] [Google Scholar]
- 43.Judson MA, Baughman RP et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:204–11. [PubMed] [Google Scholar]
- 44.Bergin CJ, Bell DY et al. Sarcoidosis: correlation of pulmonary parenchymal pattern at CT with results of pulmonary function tests. Radiology 1989;171:619–24. [DOI] [PubMed] [Google Scholar]
- 45.Kalkanis A, Judson MA. Distinguishing asthma from sarcoidosis: an approach to a problem that is not always solvable. J Asthma 2013;50:1–6. [DOI] [PubMed] [Google Scholar]
- 46.Moller DR. Pulmonary fibrosis of sarcoidosis. New approaches, old ideas. Am J Respir Cell Mol Biol 2003;29:S37–41. [PubMed] [Google Scholar]
- 47.Nardi A, Brillet PY et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J 2011;38:1368–73. [DOI] [PubMed] [Google Scholar]
- 48.Lewis MM, Mortelliti MP et al. Clinical bronchiectasis complicating pulmonary sarcoidosis: case series of seven patients. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:154–9. [PubMed] [Google Scholar]
- 49.Handa T, Nagai S et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006;129:1246–52. [DOI] [PubMed] [Google Scholar]
- 50.Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J 2013;41:621–6. [DOI] [PubMed] [Google Scholar]
- 51.Costabel U, Bonella F et al. Diagnostic modalities in sarcoidosis: BAL, EBUS, and PET. Semin Respir Crit Care Med 2010;31:404–8. [DOI] [PubMed] [Google Scholar]
- 52.Nagai S, Izumi T. Bronchoalveolar lavage. Still useful in diagnosing sarcoidosis? Clin Chest Med 1997;18:787–97. [DOI] [PubMed] [Google Scholar]
- 53.Nagai S, Shigematsu M et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med 1999;5:293–8. [DOI] [PubMed] [Google Scholar]
- 54.Baughman RP, Drent M et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 55.Judson MA, Baughman RP et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J 2014;44:1296–307. [DOI] [PubMed] [Google Scholar]
- 56.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J 1961;2:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mostard RL, Voo S et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med 2011;105:1917–24. [DOI] [PubMed] [Google Scholar]
- 58.Keijsers RG, Verzijlbergen EJ et al. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2011;28:123–9. [PubMed] [Google Scholar]
- 59.Vorselaars AD, Crommelin HA et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J 2015;46:175–85. [DOI] [PubMed] [Google Scholar]
- 60.Malinowska E, Doboszynska A et al. The use of 67Ga scintigraphy in patients with sarcoidosis. Nucl Med Rev Cent East Eur 2018;21:59–65. [DOI] [PubMed] [Google Scholar]
- 61.Kamphuis LS, Kwekkeboom DJ et al. Somatostatin receptor scintigraphy patterns in patients with sarcoidosis. Clin Nucl Med 2015;40:925–9. [DOI] [PubMed] [Google Scholar]
- 62.Tavora F, Cresswell N et al. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 2009;104:571–7. [DOI] [PubMed] [Google Scholar]
- 63.Nery PB, Beanlands RS et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol 2014;25:875–881. [DOI] [PubMed] [Google Scholar]
- 64.Viles-Gonzalez JF, Pastori L et al. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest 2013;143:1085–1090. [DOI] [PubMed] [Google Scholar]
- 65.Mehta D, Willner JM, Akhrass PR. Atrial Fibrillation in Cardiac Sarcoidosis. J Atr Fibrillation 2015;8:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hulten E, Aslam S et al. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther 2016;6:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekstrom K, Lehtonen J et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J 2019;40:3121–3128. [DOI] [PubMed] [Google Scholar]
- 68.Kandolin R, Lehtonen J et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med 2011;270:461–8. [DOI] [PubMed] [Google Scholar]
- 69.Doughan AR, Williams BR. Cardiac sarcoidosis. Heart 2006;92:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smedema JP, van Geuns RJ et al. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Fail 2017;4:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crawford T, Mueller G et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014;7:1109–15. [DOI] [PubMed] [Google Scholar]
- 72.Murtagh G, Laffin LJ et al. Prognosis of Myocardial Damage in Sarcoidosis Patients With Preserved Left Ventricular Ejection Fraction: Risk Stratification Using Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging 2016;9:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blankstein R, Osborne M et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greulich S, Deluigi CC et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013;6:501–11. [DOI] [PubMed] [Google Scholar]
- 75.Tiosano S, Versini M et al. The long-term prognostic significance of sarcoidosis-associated pulmonary hypertension - A cohort study. Clin Immunol 2019;199:57–61. [DOI] [PubMed] [Google Scholar]
- 76.Keir GJ, Walsh SL et al. Treatment of sarcoidosis-associated pulmonary hypertension: A single centre retrospective experience using targeted therapies. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:82–90. [PubMed] [Google Scholar]
- 77.Shorr AF, Davies DB et al. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 2003;124:922–8. [PubMed] [Google Scholar]
- 78.Baughman RP, Engel PJ et al. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest 2010;138:1078–85. [DOI] [PubMed] [Google Scholar]
- 79.Baughman RP, Shlobin OA et al. Clinical features of sarcoidosis associated pulmonary hypertension: Results of a multi-national registry. Respir Med 2018;139:72–78. [DOI] [PubMed] [Google Scholar]
- 80.Juneau D, Nery P et al. How common is isolated cardiac sarcoidosis? Extra-cardiac and cardiac findings on clinical examination and whole-body (18)F-fluorodeoxyglucose positron emission tomography. Int J Cardiol 2018;253:189–193. [DOI] [PubMed] [Google Scholar]
- 81.Cheong BY, Muthupillai R et al. The utility of delayed-enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy-proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:39–46. [PubMed] [Google Scholar]
- 82.Lynch JP 3rd, Hwang J et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med 2014;35:372–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coleman GC, Shaw PW et al. Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2017;10:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kouranos V, Tzelepis GE et al. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2017;10:1437–1447. [DOI] [PubMed] [Google Scholar]
- 85.Nery PB, Keren A et al. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol 2013;29:1015 e1–3. [DOI] [PubMed] [Google Scholar]
- 86.Liang JJ, Hebl VB et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail 2014;2:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leone O, Veinot JP et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245–74. [DOI] [PubMed] [Google Scholar]
- 88.Ardehali H, Howard DL et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J 2005;150:459–63. [DOI] [PubMed] [Google Scholar]
- 89.Birnie DH, Sauer WH et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305–23. [DOI] [PubMed] [Google Scholar]
- 90.Judson MA, Costabel U et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:19–27. [PubMed] [Google Scholar]
- 91.Ribeiro Neto ML et al. Performance of diagnostic criteria in patients clinically judged to have cardiac sarcoidosis: Is it time to regroup? Am Heart J 2020;223:106–109. [DOI] [PubMed] [Google Scholar]
- 92.Ohira H, Tsujino I et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008;35:933–41. [DOI] [PubMed] [Google Scholar]
- 93.Smedema JP, Snoep G et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683–90. [DOI] [PubMed] [Google Scholar]
- 94.Trivieri MG, et al. Hybrid Magnetic Resonance Imaging and Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) Guided Cardiac Sarcoidosis Treatment. Circulation 2018;138:A16511 2018. [Google Scholar]
- 95.Osborne MT, Hulten EA et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014;21:166–74. [DOI] [PubMed] [Google Scholar]
- 96.Chareonthaitawee P, Beanlands RS et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol 2017;24:1741–1758. [DOI] [PubMed] [Google Scholar]
- 97.Tang R, Wang JT et al. Impact of Patient Preparation on the Diagnostic Performance of 18F-FDG PET in Cardiac Sarcoidosis: A Systematic Review and Meta-analysis. Clin Nucl Med 2016;41:e327–39. [DOI] [PubMed] [Google Scholar]
- 98.Bravo PE, Raghu G et al. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol 2017;241:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar S, Barbhaiya C et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol 2015;8:87–93. [DOI] [PubMed] [Google Scholar]
- 100.Joyce E, Ninaber MK et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail 2015;17:51–62. [DOI] [PubMed] [Google Scholar]
- 101.Wicks EC, Menezes LJ et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging 2018. [DOI] [PubMed] [Google Scholar]
- 102.Dweck MR, Abgral R et al. Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2018;11:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramirez R, Trivieri M et al. Advanced Imaging in Cardiac Sarcoidosis. J Nucl Med 2019;60:892–898. [DOI] [PubMed] [Google Scholar]
- 104.Patel MR, Cawley PJ et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Freeman AM, Curran-Everett D et al. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol 2013;112:280–5. [DOI] [PubMed] [Google Scholar]
- 106.Philips B, Madhavan S et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis: distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol 2014;7:230–6. [DOI] [PubMed] [Google Scholar]
- 107.Khan NA, Donatelli CV et al. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir Med 2017;132:9–14. [DOI] [PubMed] [Google Scholar]
- 108.Baughman RP, Field S et al. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc 2016;13:1244–52. [DOI] [PubMed] [Google Scholar]
- 109.Judson MA. Strategies for identifying pulmonary sarcoidosis patients at risk for severe or chronic disease. Expert Rev Respir Med 2017;11:111–118. [DOI] [PubMed] [Google Scholar]
- 110.Baughman RJMAW A The indications for the treatment of sarcoidosis: Wells Law. SARCOIDOSIS VASCULITIS AND DIFFUSE LUNG DISEASES 2017;34:280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gottlieb JE, Israel HL et al. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest 1997;111:623–31. [DOI] [PubMed] [Google Scholar]
- 112.Panselinas E, Judson MA. Acute pulmonary exacerbations of sarcoidosis. Chest 2012;142:827–836. [DOI] [PubMed] [Google Scholar]
- 113.Schimmelpennink MC, Vorselaars ADM et al. Efficacy and safety of infliximab biosimilar Inflectra((R)) in severe sarcoidosis. Respir Med 2018;138S:S7–S13. [DOI] [PubMed] [Google Scholar]
- 114.Padala SK, Peaslee S et al. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol 2017;227:565–570. [DOI] [PubMed] [Google Scholar]
- 115.Hiramitsu S, Morimoto S et al. National survey on status of steroid therapy for cardiac sarcoidosis in Japan. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:210–3. [PubMed] [Google Scholar]
- 116.Yazaki Y, Isobe M et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001;88:1006–10. [DOI] [PubMed] [Google Scholar]
- 117.Birnie D, Ha AC et al. Cardiac Sarcoidosis. Clin Chest Med 2015;36:657–68. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y, Lower EE et al. Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum 2017;47:143–148. [DOI] [PubMed] [Google Scholar]
- 119.Fussner LA, Karlstedt E et al. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail 2018;20:1713–1720. [DOI] [PubMed] [Google Scholar]
- 120.Chapelon-Abric C, de Zuttere D et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004;83:315–34. [DOI] [PubMed] [Google Scholar]
- 121.Ballul T, Borie R et al. Treatment of cardiac sarcoidosis: A comparative study of steroids and steroids plus immunosuppressive drugs. Int J Cardiol 2019;276:208–211. [DOI] [PubMed] [Google Scholar]
- 122.Nagai S, Yokomatsu T et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014;53:2761. [DOI] [PubMed] [Google Scholar]
- 123.Birnie D, Beanlands RSB et al. Cardiac Sarcoidosis multi-center randomized controlled trial (CHASM CS- RCT). Am Heart J 2020;220:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:60–6. [PubMed] [Google Scholar]
- 125.Jamilloux Y, Cohen-Aubart F et al. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin Arthritis Rheum 2017;47:288–294. [DOI] [PubMed] [Google Scholar]
- 126.Gelfand JM, Bradshaw MJ et al. Infliximab for the treatment of CNS sarcoidosis: A multi-institutional series. Neurology 2017;89:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crouser ED, Lozanski G et al. The CD4+ lymphopenic sarcoidosis phenotype is highly responsive to anti-tumor necrosis factor-{alpha} therapy. Chest 2010;137:1432–5. [DOI] [PubMed] [Google Scholar]
- 128.Barnabe C, McMeekin J et al. Successful treatment of cardiac sarcoidosis with infliximab. J Rheumatol 2008;35:1686–7. [PubMed] [Google Scholar]
- 129.Harper LJ, McCarthy M et al. Infliximab for Refractory Cardiac Sarcoidosis. Am J Cardiol 2019. [DOI] [PubMed] [Google Scholar]
- 130.Chung ES, Packer M et al. Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107:3133–40. [DOI] [PubMed] [Google Scholar]
- 131.Krause ML, Cooper LT et al. Successful use of rituximab in refractory cardiac sarcoidosis. Rheumatology (Oxford) 2016;55:189–91. [DOI] [PubMed] [Google Scholar]
- 132.Sweiss NJ, Lower EE et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 2014;43:1525–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol 2012;6:1613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shlobin OA, Baughman RP. Sarcoidosis-Associated Pulmonary Hypertension. Semin Respir Crit Care Med 2017;38:450–462. [DOI] [PubMed] [Google Scholar]
- 135.Baughman RP, Culver DA et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014;145:810–817. [DOI] [PubMed] [Google Scholar]
- 136.Terasaki F, Azuma A et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis- Digest Version. Circ J 2019;83:2329–2388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


