Abstract
Auditory hallucinations (AH) are one of the core symptoms of schizophrenia (SZ) and constitute a significant source of suffering and disability. One third of SZ patients experience pharmacology-resistant AH, so an alternative/complementary treatment strategy is needed to alleviate this debilitating condition. In this study, real-time functional Magnetic Resonance Imaging neurofeedback (rt-fMRI NFB), a non-invasive technique, was used to teach 10 SZ patients with pharmacology-resistant AH to modulate their brain activity in the superior temporal gyrus (STG), a key area in the neurophysiology of AH. A functional task was designed in order to provide patients with a specific strategy to help them modify their brain activity in the desired direction. Specifically, they received neurofeedback from their own STG and were trained to upregulate it while listening to their own voice recording and downregulate it while ignoring a stranger's voice recording. This guided performance neurofeedback training resulted in a) a significant reduction in STG activation while ignoring a stranger's voice, and b) reductions in AH scores after the neurofeedback session. A single, 21-minute session of rt-fMRI NFB was enough to produce these effects, suggesting that this approach may be an efficient and clinically viable alternative for the treatment of pharmacology-resistant AH.
1. Introduction
1.1. Auditory hallucinations in schizophrenia and the study overview
Auditory hallucinations (AHs) are one of the five core symptoms of schizophrenia (SZ) (Dazzi et al., 2016). Affecting approximately 75% of patients with this disorder, AHs are often reported as one of its most distressing symptoms. Furthermore, in one third of patients experiencing AHs they are not responsive to pharmacology (Andreasen and Flaum, 1991; Copolov et al., 2004; Sartorius et al., 1986; Shergill et al., 1998). Alternative treatments for those who do not respond to pharmacology include psychotherapy to help cope with AH experience (Thomas et al., 2014) or, transcranial magnetic stimulation (TMS) (Slotema et al., 2011). However, both forms of therapy suffer from major shortcomings. Regarding psychotherapy, while often helpful, it does not impact the severity of AHs (Thomas et al., 2014), and TMS is not well tolerated by many patients and thus its efficacy may be limited (Slotema et al., 2011). Therefore, the development of new effective non-invasive treatments to reduce the severity and frequency of AHs is critically important.
One approach recently proposed to achieve this goal is the real-time functional Magnetic Resonance Imaging neurofeedback (rt-fMRI NFB) which gives individuals information about their own brain activity levels and thus provides an opportunity to change them as described below. In the paper that consists of Part I and Part II, we report on the preliminary evidence of using rt-fMRI NFB to reduce the severity and frequency of AH in chronic SZ patients whose AHs do not respond to pharmacology. Our overall hypothesis is that AHs are a complex phenomenon that arises from abnormalities in a network of brain regions that contribute to their clinical manifestations, as discussed in detail below. We further suggest that targeting different elements of this network will result in a similar clinical outcome, i.e., in AH reduction. This is the view that is shared not only by us but, increasing, by others (Scheinost et al., 2019). In Part I and Part II of the paper, we describe a rt-fMRI based neurofeedback approach that target different brain regions belonging to the AH network. In Part I, we focus on rt-fMRI NFB targeting the superior temporal gyrus (STG), while in Part II we focus on rt-fMRI NFB targeting the default mode network (DMN).
1.2. Real-time fMRI feedback as a tool to modulate brain function
Bio- and neuro-feedback relates to monitoring one's own biological indicators to gain a level of voluntary control. Recent technological advances allowed for direct control of specific brain region activation levels via feedback obtained during a functional MRI (fMRI) session (Bodurka, 2018; Stoeckel et al., 2014; Young et al., 2018a; Sitaram et al., 2017; Watanabe et al., 2017). While the first reports focused on demonstrating the ability to modulate the sensorimotor areas related to hand movement (deCharms et al., 2004; Yoo and Jolesz, 2002), subsequent studies demonstrated the feasibility of modulating activation in (a) subgenual anterior cingulate cortex, a region known to be implicated in depression (Hamilton et al., 2011) (b) auditory cortex (Hamilton et al., 2011; Yoo et al., 2007), (c) insula (Ruiz et al., 2013) and (d) inferior frontal gyrus (Rota et al., 2009; Ruiz et al., 2013).
The attractiveness of rt-fMRI NFB approach is that it can be used both as a tool of cognitive neuroscience to examine existing theories of different cognitive functions and, at the same time, as a clinical tool to mitigate a host of clinical symptoms. A neurofeedback intervention's success is measured by the extent to which a statistically significant change is observed in a target brain region in the comparison of pre-relative to post-rt-fMRI activation, and, for clinical applications, the demonstration of a relationship between the predicted, and obtained, brain activation changes and clinical symptoms changes. Evidence of clinically effective use of rt-fMRI has emerged for such conditions as chronic pain (deCharms et al., 2004), tinnitus (Haller et al., 2010), stroke (Sitaram et al., 2012; Wang et al., 2017), Parkinson's disease (Subramanian et al., 2011), autism (Ramot et al., 2017), depression (Linden et al., 2012; Young et al., 2018b), psychopathy (Sitaram et al., 2014, 2012), addiction (Hartwell et al., 2013;Li et al., 2013) anxiety (Hampson et al., 2012; Scheinost et al., 2014; Chiba et al., 2019), emotional face processing in SZ (Ruiz et al., 2013), and Tourette's syndrome (Hampson et al., 2011).
1.3. Theoretical models of auditory hallucinations
Neuroimaging studies have identified a network of brain regions that may underlie AHs in SZ, including the superior temporal gyrus (STG), middle temporal gyrus, inferior frontal gyrus (IFG), the medial prefrontal cortex (MPFC) and the posterior cingulate cortex (PCC) of the default mode network (DMN) as well as the parietal regions such as the temporo-parietal junction, angular gyrus, and inferior parietal lobule/gyrus (IPL/IPG) (Jardri et al., 2011; Alderson-Day et al., 2016; Thoma et al., 2016). Accordingly, several models of AHs, as described below, have been proposed.
Early models suggested that AHs are a result of source misattribution during self-generated thought and inner speech, where patients attributed the source to others instead of self (Frith et al., 1992; Frith and Done, 1988). More recent models have suggested a breakdown in the network of brain regions whereby the over-activated auditory perceptual regions in the temporal cortex, including the STG, are coupled with a lack of inhibitory control exhibited by the executive function regions in the frontal cortex (Hugdahl, 2015). In addition, there have been proposals that link AHs to abnormalities in the motor speech and language regions including STG and IFG (Allen et al., 2008; Jardri et al., 2011; Alonso-Solís et al., 2015; Northoff, 2014; Northoff and Qin 2011).
Another class of models conceptualized AHs as a disturbance in agency, where agency refers to a sense of self as contrasted with other agents or people (Blakemore et al., 2003; Brüne et al., 2008; Holt et al., 2011). The STG and medial prefrontal cortex (MPFC) have been shown to be a part of the self-referential network in both healthy (Jenkins and Mitchell, 2011; Kelley et al., 2002) and SZ individuals (Brent et al., 2014; Larivière et al., 2017). Finally, AHs have been associated with abnormal connectivity both within the DMN structures including MPFC, anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC), and between DMN structures and brain regions implicated in the experience of AH (Alonso-Solís et al., 2015; Northoff, 2014; Northoff and Qin 2011; Scheinost et al., 2019; Zweerings et al., 2019), the hypothesis which will be discussed in greater detail in Part II of our paper.
1.3.1. Theoretical AHs models as tested in rt-fMRI NFB approach
Recent theoretical papers advocated the use of rt-fMRI NFB as a potentially effective way to mitigate AHs (Fovet et al., 2016; McCarthy-Jones, 2012). The evidence for these claims is still limited. Three experimental papers reported on the use of rt-fMRI NFB to induce changes in brain regions implicated in AHs in order to reduce their severity. Dyck et al. (2016) preliminary report showed inconsistent activation in the ACC in three subjects, with modest AHs reductions achieved. The choice of ACC in this study was dictated by its involvement in AHs. Orlov et al. (2018) reported on a rt-fMRI NFB targeting the STG as one of the brain regions involved in AHs. Over the course of four rt-fMRI NFB sessions, using a strategy of their choice, the patients were able to significantly reduce the STG activation. Post-training, subjects increased functional connectivity between the STG and two language areas: IFG and IPG. This increase in the functional connectivity between the STG and IFG, but not the reduction in the targeted STG region, was associated with reductions in AH symptoms. Zweerings et al. (2019) adopted a similar framework and used rt-fMRI NFB targeting left IFG (lIFG) and left posterior STG (pSTG) to reduce AHs. Improved psychological wellbeing was associated with increased functional coupling between pooled lIFG and pSTG seed region and IPL.
1.3.2. Theoretical model adopted in the current study
In the study reported in Part I, we took a narrow theoretical view of AHs and focused on the role of the STG in their aetiology, given the central role of this area in several theories of AHs. We hypothesized that directly modulating activation in this region, and specifically, reducing the STG activation to non-self-voices, should result in reductions in AHs. We further hypothesized that reductions in AHs would be correlated with the reduction in the STG activation, post-rt-fMRI NFB. Our results suggested that providing a specific strategy for patients to modulate their brain activity in the STG can achieve both the expected brain activity and AH severity reduction in a single rt-fMRI NFB session. This approach is in contrast to the previous studies (Dyck et al., 2016; Orlov et al., 2018; Zweerings et al., 2019) that had participants choose their own strategy to regulate a target brain area.
2. Materials and methods
2.1. Participants
Ten patients (mean age = 43.3 (SD = 10.1); 1 female) diagnosed with SZ or schizoaffective disorder using DSM-5 criteria, and with AHs not responsive to pharmacology, as determined by chart review and a clinical interview. All participants reported having normal or corrected-to-normal vision and normal hearing (hearing was evaluated using the GS1 17 Audiometer and all participants were found to have hearing within normal range). Across patients, medications included: Aripiprizole, Clozapine, Chlondapine, Sertraline, Buspirone, Olanzopine, Citalopram, Risperidone, and Gabapentin. The exclusion criteria included neurologic illness or major head trauma, electroconvulsive therapy, alcohol or drug dependence, alcohol or drug abuse within the past five years, verbal IQ below 70, and absence of AHs not responsive to pharmacology as assessed with the SCID interview. All participants were native English speakers and right handed (Oldfield, 1971). The participants’ mean verbal IQ as assessed with WAIS was 102.6 (SD = 10.8) and their mean performance IQ was 93.6 (SD = 6.9). Demographic information for each patient is provided in detail in Table 1. All patients experienced AHs at least once daily within the two weeks prior to the assessment. Hallucinatory experience (AH score) was captured using the Auditory Hallucinations Rating Scale (AHRS) developed by Hoffman et al. (2005, 2003, 1999) and administered before the rt-fMRI NFB session and a week after the NFB session. The AHRS measures frequency, reality, loudness, number of voices, length, attentional salience, and distress on a 5–10 point scale for a possible range of 0–42 points All participants gave written consent obtained in accordance with the guidelines of Harvard Medical School, Veterans Affairs (VA) and MIT Committees on Human Subjects and they were compensated for their participation.
Table 1.
Patient demographics.
| Gender | Age | Dx | Medication | CPZ equivalent | IQ | AHRS score pre-Sess.2 | AHRS score post-Sess. 2 | AHRS score pre-Sess. 4 | AHRS score post-Sess. 4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 55 | PSz | Abilify | 166.7 | 105 | 20 | 0 | 0 | 0 |
| 2 | F | 33 | PSz | Clozapine | 250 | 91 | 26 | 23 | 24 | 28 |
| 3 | M | 51 | PSz | Abilify | 333.4 | 104 | 32 | 24 | 27 | 19 |
| 4 | M | 35 | SAD | Clozapine | 150 | 112 | 26 | 24 | 23 | 26 |
| 5 | M | 55 | PSz | Olanzapine | 300 | 91 | 11 | 17 | 26 | 17 |
| 6 | M | 30 | PSz | Clozapine | 200 | 90 | 30 | 25 | 33 | 31 |
| 7 | M | 38 | PSz | Risperdal | 200 | 91 | 17 | 0 | 27 | 27 |
| 8 | M | 50 | SAD | Clozapine | 150 | 106 | 19 | 0 | 29 | 9 |
| 9 | M | 35 | Sz | NA | NA | 89 | 21 | 1 | NA | NA |
| 10 | M | 55 | SAD | Ziprasodone | 900 | 75 | 10 | 0 | 0 | 0 |
PSz = Paranoid schizophrenia, SAD = Schizoaffective disorder, Sz = Schizophrenia.
Medication and dosage did not change for any patient throughout the duration of the study.
2.2. Study structure
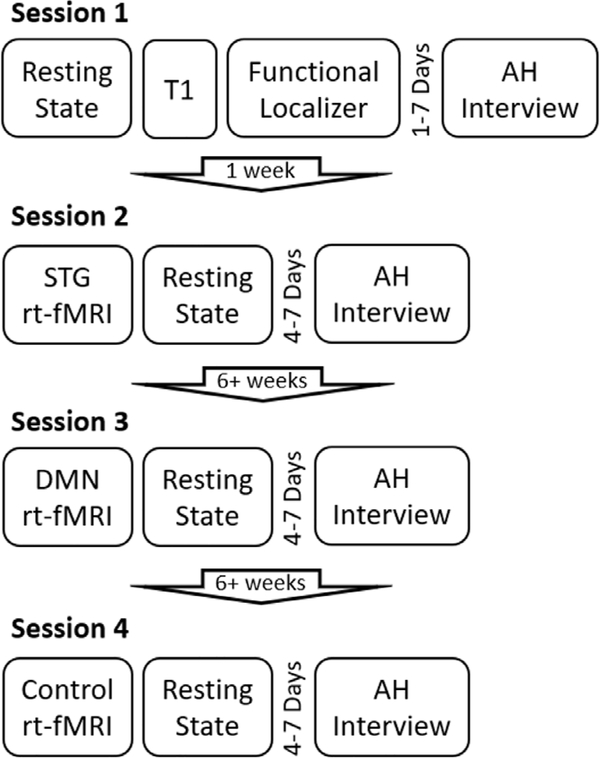
The overall structure of the study included four sessions. Data from session 1 and 2 are reported in Part I while data from session 3 are reported in Part II. Data from session 4, which includes a control condition, are discussed in both Part I and Part II. In the Session 1, participants completed a resting state scan, T1 weighted structural scan, and a functional localizer task. In the Session 2, participants completed an rt-fMRI NFB task targeting a superior temporal gyrus (STG) region (subject specific, see Section 2.5) (21 min) and resting state scan (11.8 min AP and PA). In Session 3, participants completed an rt-fMRI NFB task targeting the default mode network (DMN) and central executive network (15 min), and a resting state scan (12 min – AP and PA) (refer to Part 2 for details of Session 3). Session 4 was identical to Session 2 except the STG rt-fMRI NFB task was replaced with a control rt-fMRI NFB task targeting somatosensory cortex (SMC) (21 min). AHRS interviews were completed within one week prior to Sessions 2, and 4–7 days after Sessions 3 and 4. Sessions 2 and 3 were separated by a minimum of 6 weeks, and Sessions 3 and 4 were also separated by a minimum of 6 weeks. (See Fig. 1. for experiment structure).
Fig. 1.
Structure and timeline of the experiment. Please note that session 3 is described in Part 2 of the paper.
2.3. Audio recordings
Patients recorded 170 sentences to be used in the ‘self-voice’ condition on an Olympus, digital recorder, model VN-722PC. Before recording, subjects were asked to practice reading sentences aloud, without emotional intonation. After practice, recording was carried out in a sound-proof room. If patients made an error while reading a sentence, they were asked to repeat the sentence in its entirety. All sentences had a structure of subject + verb + adjective + object (e.g., “Jane liked chocolate-chip cookies”) with an average length of 6.65 words (SD = 1.13). All sentences were affect-neutral and written in third person. Recorded sentences were processed in 3 steps: 1. Sentences were imported into Praat software (http://www.fon.hum.uva.nl/praat/) and intensity was normalized using an in-house script. 2. The normalized sentences were then imported into the Audacity software (http://www.audacityteam.org) in order to remove background noise. 3. After background noise removal, sentences were reimported into Praat to segment into individual sentences. The same sentences were recorded by a male in his 40 s and edited in the same manner to be used in the ‘other’ condition. For use as ‘other’ stimuli for our female patient, we edited these recordings using Praat to mimic a female voice by changing the formant shift ratio to 1.2, to account for a shorter vocal tract, and pitch median to 220 Hz, to account for greater vocal fold tension in females. This resulted in an auditory experience of hearing a female voice.
2.4. fMRI acquisition
2.4.1. Scanning parameters
All scans were acquired using a 3T Trio MR System with a 32-channel, phased-array head coil (Siemens Healthcare, Erlangen, Germany). Structural scans were acquired using a three-dimensional T1-weighted MEMPRAGE pulse sequence with a voxel resolution of 1 mm3, flip angle (FA) = 7°, echo time (TE) = 1.61 ms, inversion time (TI) = 1200 ms, and repetition time (TR) = 2530 ms.
For functional images, the blood oxygen level dependant (BOLD) signal was measured using a gradient-echo, echo-planar imaging pulse sequence (EPI) with prospective acquisition correction (PACE) (Dale et al., 1999) for motion with imaging parameters: TR = 2 s, TE = 30 ms, FA = 90°, in-plane resolution of 3 × 3 mm2, number of slices = 32, slice thickness = 3.5, and slice gap = 10%, providing coverage around the STG.
2.4.2. Feedabck scans
During feedback scans, BOLD signal was measured using rt-fMRI analysis as described in Hinds et al. (2011) and implemented in murfi2, software that is freely available on Github (http://github.com/gablab/murfi2). During triggering, functional runs of incoming images from the scanner were analyzed in real-time to estimate mean activation levels from the subject specific regions of interest (ROIs) (see Section 2.5). To accomplish this, a voxel-wise incremental general linear model (GLM) fit was performed where the design matrix included a 30 s baseline and a 120 s active block to account for the mean voxel signal and linear trends. To discount components of the voxel signal due to nuisance sources (e.g., low-frequency signal drifts), the GLM reconstruction of the expected voxel intensity at time t was subtracted from the measured voxel intensity at time t, leaving a residual signal that has components due to two sources: BOLD signal fluctuations and unmodeled fMRI noise. This residual is scaled by an estimate of voxel reliability, which is computed as the average GLM residual over the first 15 functional images of the baseline. This analysis results in an estimate of the strength of activation at each voxel at time t in units of standard deviation (SD). Activations in target ROIs were computed as the median SD of the voxels in each ROI. To provide a feedback display for the participant, a signal was sent to the stimulus computer via a TCP/IP connection, where the stimulus program coded in PscychoPy (Peirce, 2008, 2007) received this signal and displayed real-time neurofeedback to the subject inside the scanner (see Section 2.5 for details on feedback task and visual display). The time delay between collection of a complete EPI volume and trial trigger was 0.5 s.
STG Feedback ROI definitions
Participant specific STGs were defined using a functional localizer task in which participants listened to pre-recorded sentences. Eighty unique sentences were presented: half of the sentences in the subject’s own voice (40 sentences) and the other half (40 sentences) in a stranger's voice. The task included four blocks each of the self-voice, other-voice, and rest blocks, each lasting 16 s. ‘Self-voice’ blocks included five unique sentences spoken in the subject’s voice; the ‘other’ voice blocks included five unique sentences spoken in a stranger's voice. The presentation of the sentences was counterbalanced across subjects such that the subject did not hear the same sentences twice spoken in his/her voice or in other person's voice but across subjects, the same sentences were spoken in self voice and in the other person's voice. The rest-blocks included a silent block where participants stared at a crosshair in the center of the screen. In order to maintain attention, after the ‘self’ and ‘other' –voice blocks, subjects were prompted with a ‘yes’ or ‘no’ question appearing on the screen regarding the content of the last sentence they had heard in the block. After the rest block, they answered a ‘yes’ or ‘no’ real-world question. These blocks were split evenly across two functional runs with each run containing 12 pseudo randomized blocks comprised of a total of 8 blocks of each condition across the two runs. Each run lasted four minutes 26 s. The pseudo randomization prevented the rest blocks from appearing back to back. In an off-line analysis, a participant’s specific target ROI was created from the cluster containing the maximum intensity voxels in bilateral STGs after thresholding for the contrast Self-block > Other-block. Statistical threshold was varied in order to keep the functionally defined ROI a similar size (Average = 190.1 voxels, SD = 35.94) across participants.
2.4.3. SMC control feedback roi definitions
Participant specific SMCs were defined using Resting State Scans and therefore this condition is explained in detail in Part II (Section 2.4.1.). This region was chosen to demonstrate that successful neurofeedback from a region not implicated in AHs will not result in AH symptom reduction. Motor cortex is not identified as belonging to the network of brain regions implicated in AHs (Alderson-Day et al., 2016; Allen et al., 2008; Fu et al., 2008; Scheinost et al., 2019). For this reason, we hypothesized that activation changes, post-rtfMRI NFB in motor regions associated with hand and finger movement will not impact the STG activation and will not contribute to AH reduction.
2.5. STG feedback task
All 10 participants completed the feedback task. Participants heard a new set of 80 unique sentences (average length = 6.64 words, SD = 1.31) presented over earphones in the scanner. Fourty sentences were in their own voice and another 40 in a stranger's voice. These sentences were not significantly different in their length from the 80 sentences heard in Session 1 (t(158) = 0.34, p = 0.73). There were six runs for this task, with the first and sixth run having no feedback (rt-transfer), while runs 2–5 provided real-time neurofeedback (rt-NFB) to the participants from the ROI extracted from the self-other voice task during fMRI session 1 (i.e. specific STG masks for each participant). Each run had four randomized blocks comprised of two listen blocks and two ignore blocks, each lasting 16 s.
Before each block, participants saw a prompt stating either “listen” to signal that they were asked to attend to the sentences played over the earphones (resulting in up-regulating STG) or “ignore”, to signal that they were asked to ignore the sentences along with any other envirnmental noises including the scanner noise (resulting in down-regulating STG). To successfully ignore the sentences and other noises, we trained the participants outside the scanner on a focused attention method, where they would attend to any sensation (other than hearing) that was most prominent to them at that moment. These sensations included seeing (i.e., seeing the crosshair on the screen), feeling (i.e., feeling the vibration of the scanner) or smelling (i.e., smelling the air around them). The training typically lasted about 15 min and all participants were able to grasp the concept within that time. The training was done shortly after the baseline clinical assessment and shortly before entering the MRI scanner.
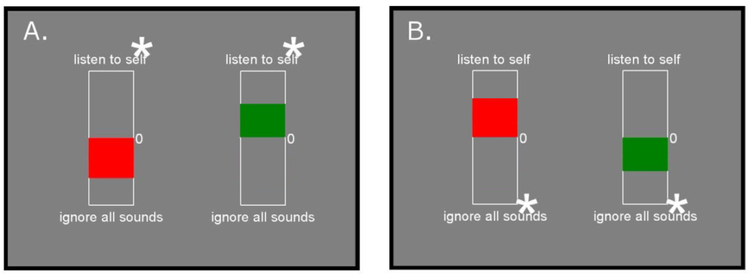
After each feedback block, participants saw a ‘thermometer’ showing their activation level in the STG (Fig. 2). The height of the ‘thermometer’ bar reflected a median activity level for a given block.
Fig. 2.
An example of feedback displays during “listen” and ‘ignore’ blocks seen by a participant after each block. The red bar provides feedback that a trial was unsuccessful, and the green bar provides feedback that a trial was successful. Panel A: Feedback in the “listen condition. Panel B: Feedback in the “ignore” condition. Asterisks indicate the direction in which the bar will display in a successful trial. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In addition, to gage the participant’s assessment of their own performance during the tasks, after each listen block, a prompt appeared to rate how well participants were able to attend to the voices on a scale of 1 (completely attended to) to 6 (could not attend at all); after each ignore block, a similar prompt appeared to rate how well they were able to ignore all sounds. Participants responded to these prompts with a button box in their right hand.
2.6. SMC control feedback task
Seven out of 10 participants completed the control feedback task. The control feedback task was structured identically to the feedback task, with the exception of the ‘listen’ and ‘ignore’ blocks being replaced by ‘right’ and ‘left’. Accordingly, participants initiated finger tapping with either their right hand or left hand index and middle fingers. There were six runs for this task, with the first and sixth run having no feedback (control-TRANSFER), while runs 2–5 provided real-time neural feedback (control-NFB) to the participants from the ROI extracted from their motor cortex. Each run had four randomized blocks comprised of two left finger blocks and two right finger blocks. Before each block, participants saw a prompt stating either “left”, so participants knew they had to tap their left fingers or “right”, in order to tap their right fingers against the scanner table. After each block, a prompt appeared to rate how vigorously they moved their fingers on a scale of 1 (very lightly) to 6 (very vigorous). In the feedback blocks, participants saw a thermometer showing their activation level in their motor cortex ROI
2.7. fMRI data analysis
Preprocessing and data analyses were completed using the following software packages: Nipype v0.7 (Gorgolewski et al., 2011) (http://nipy.org/nipype/), FSL v5.0 (Smith et al., 2004), Analysis of Functional Neuroimages (AFNI) software (Cox, 1996), FreeSurfer 5.1.0 (Dale et al., 1999), Advanced Normalization Tools (ANTS) (Avants et al., 2008), and artifact detection (ART) (Whitfield-Gabrieli, 2009). Surface reconstruction and subcortical segmentation were performed using FreeSurfer and verified via visual inspection. The ART toolbox was used to detect motion outliers. An image was defined as an outlier if head displacement deviated from the previous time point by more than 3 standard deviations. Participants averaged 9.9 (SD = 7.8) rejected time points across both runs of the task before the rt-NFB sessions, and 9.8 (SD = 8.13) after the rt-NFB sessions. There were no significant differences in the number of outliers removed before and after the rt-NFB sessions within subjects; paired two-tailed t-test; t(9) = 0.043, p = 0.97. Additionally, number of motion outliers did not correlate with AH scores before (r = 0.69, p = 0.07), or after (r = 0.15, p = 0.68) the rt-NFB sessions.
To determine within-group differences in STG hemodynamic activity during the rt-Transfer task before and after the four rt-NFB and four control-NFB sessions, we measured average percentage signal change, relative to a baseline of average signal intensity in one a priori region of interest (ROI). This STG ROI was defined by combining the left anterior STG, left posterior STG, right anterior STG, and right posterior STG from the fsl-harvard-oxford-cortical-lateralized-atlas (Desikan et al., 2006), in order to maximize the sensitivity of the analysis despite interindividual variability and to allow for future comparison across studies.
Paired T-tests were used to compare STG hemodynamic activity during the rt-Transfer task before and after the four rt-NFB blocks, as well as during control-Transfer task before and after the four control-NFB blocks. Analyses generated differential maps with MNI coordinates of voxels that showed family-wise error (FWE)-corrected group-wise differences by threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) using nonparametric statistics implemented in FSĽs randomise tool (http://www.fmrib.ox.ac.uk/fsl/randomise/) with variance smoothing of sigma 5 mm to improve the estimation of the variance due to the small sample size at a significance threshold of p < 0.05 (corrected for multiple comparisons) with 5000 permutations—for each voxel within the STG ROI.
Similarly, paired T-tests with continuous covariate interaction were used with AH score delta (pre minus post rt-NFB AH scores) as the covariate to determine the linear relationship between AH changes and STG hemodynamic activity changes in both the feedback and control feedback task.
3. Results
3.1. Neuroimaging results
3.1.1. STG feedback task
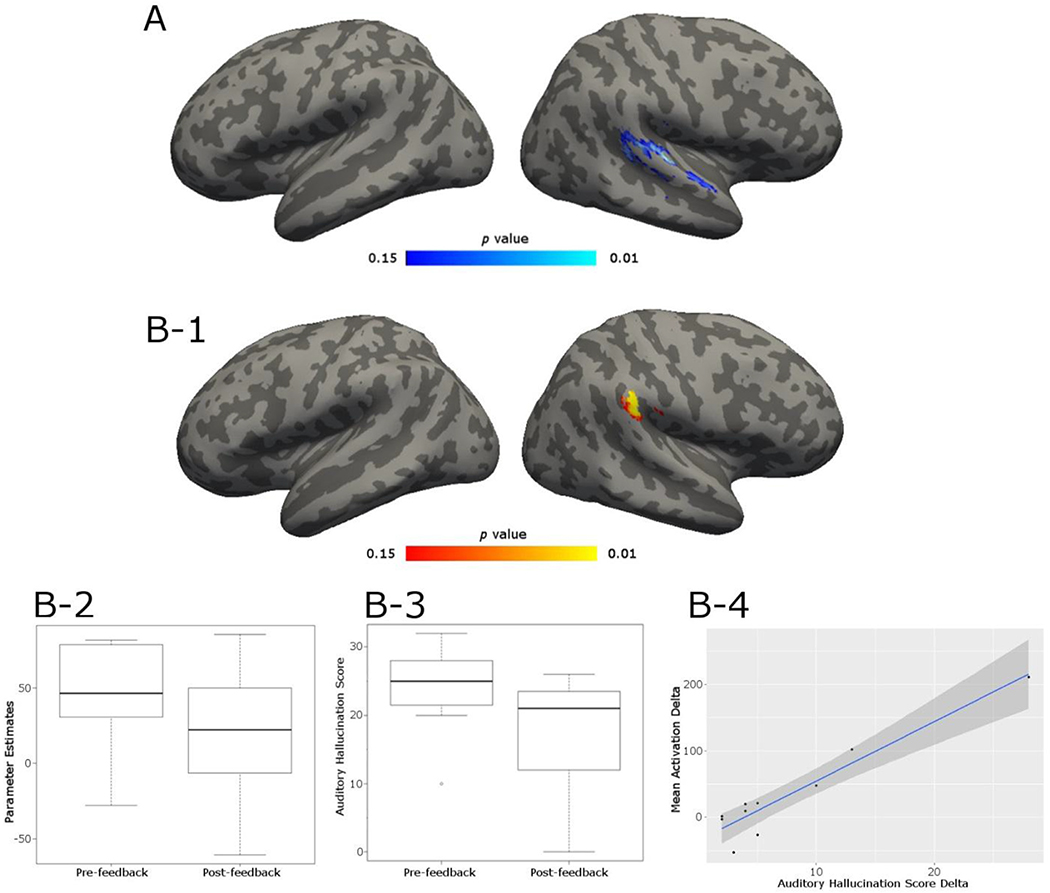
Paired t-test revealed a significant cluster in the right STG (MNI x, y, z = 60, -18, 10) where patients showed a decrease in activation post-neurofeedback compared to pre-STG feedback during the ignore blocks relative to baseline (tpeak = 2.76, KE = 114 voxels)(Fig. 3–A). At un-corrected threshold of p = 0.005, a cluster was also observed at the left STG.
Fig. 3.
A. Feedback transfer task results: highlighted cluster shows the area where activations during ignore block were greater in the pre-neurofeedback relative to post-neurofeedback (pre > post) run in a STG ROI analysis. Blue color denotes less activation in highlighted regions post neurofeedback. Statistics are non-parametric and FWE-TFCE corrected. B-1. Highlighted cluster shows area where the difference in activation during “ignore” blocks computed as activation preminus activation post- neurofeedback is significantly correlated with AH score delta score (pre – post). Statistics are non-parametric and FWE-TFCE corrected. B-2: Histogram of mean activation in the significant cluster (B-1) pre- and post-feedback. B-3: Histogram of mean auditory hallucination scores pre- and post- feedback B-4. Correlation (rs = 0.78) between mean activation delta values and AH delta scores extracted from significant cluster. The dark gray funnel depicts 95% confidence interval. Upon removal of the outlier in the far top right, the correlation remains high (rs = 0.70).
3.1.2. SMC control feedback task
In the SMC control feedback task, there were no significant differences in the STG for left or right finger movement blocks relative to baseline, nor left fingers relative to right fingers movement.
3.2. Behavioral results
3.2.1. Auditory hallucination scores
A paired-samples t-test was conducted to compare AH scores pre- and post- feedback sessions. We found a significant reduction in AH scores pre- and post- STG feedback task; t(9) = 3.01, p = 0.01 (Fig. 3–B3) but found no difference in AH scores pre- and post- control feedback task; t(6) = 1.00, p = 0.35.
3.2.2. STG localizer task
Participants correctly answered 97.08% (SD = 4.08%) of the comprehension questions. All participants’ accuracy was within two standard deviations from the mean and therefore all participants’ data were kept for further analysis.
3.2.3. STG feedback task
Using a five point scale displayed on the screen after each run, participants rated 1.93 (SD = 0.95) on how well they were able to attend to their own voice, and 2.51 (SD = 1.89) on how well they were able to ignore all sounds. A paired t-test revealed that participants found ignoring all sounds to be significantly more difficult than attending to their own voice (t (9) = 3.54, p < 0.01).
3.2.4. SMC control feedback task
Using a five point scale displayed on the screen after each run, on average, participants rated 2.79 (SD = 1.42) on how vigorously they moved their left fingers, and 2.60 (SD = 1.51) how vigorously they moved their right fingers. The two assessments were not significantly different (t (6) = 0.81, p = 0.45).
3.3. Correlational results
3.3.1. STG feedback task
A non-parametric correlational analysis between the change in AH scores and the STG activity (pre-to-post) identified a significant positive linear relationship (rs = 0.78, Fig. 3–B4) between the reduction in activity in the right STG (MNI x, y, z = 64, -30, 34) during ignore blocks relative to baseline (ignore > baseline) and the reduction in AH scores after feedback (tpeak = 4.78, KE = 68 voxels) (Fig. 3–B1). There were no significant linear relationships between AH score delta and activation differences during listen block relative to baseline or listen blocks relative to ignore blocks. There was no correlation between medication (CPZ equivalent values) and AH scores pre- or post- STG feedback, or STG activity pre- or post- STG feedback (all rs2s ≤ 0.15, all ps ≥ 0.29).
3.3.2. SMC control feedback task
Aside from one patient who no longer experienced any AH after the STG feedback task, all other participants experienced similar AHs pre-STG feedback and pre-Control feedback task (t (6) = 0.73, p = 0.49). In the control feedback task, we found no significant linear relationships between AH score delta and right STG activation differences during left or right finger blocks relative to baseline, nor to left fingers relative to right fingers (rs = -0.34). There was no correlation between medication (CPZ equivalent values) and AH scores pre- or post- control feedback, or STG activity pre- or post- control feedback (all rs2s ≤ 0.02, all ps ≥ 0.67).
4. Discussion
Based on the existing theories of auditory hallucinations, and the principles of rt-fMRI NFB, we theorized that it is possible to reduce the severity and frequency of AHs in SZ patients with pharmacology resistant AH by targeting the STG, one of the brain regions consistently demonstrated to be involved in this clinical phenomenon. Using a novel approach of rt-fMRI-guided neurofeedback, we achieved the goal of reducing the STG activation as well as AH reduction, post neurofeedback, and demonstrated a statistically significant correlation between the STG activation reduction and AH reductions. We reached these goals with just one 21 min session of rt-fMRI NFB.
To show that reductions in AH were only achieved when feedback came from a brain region belonging to the network involved in auditory hallucinations, we implemented a control condition where the same participants received feedback from the motor cortex, a region not belonging to the targeted network. As predicted, no changes in AH were observed, post-neurofeedback session targeting the motor cortex.
Results showed that after four feedback runs, each lasting approximately three minutes, patients showed a significant reduction of activation in the STG when intentionally ignoring voices relative to baseline. This reduction was not observed in the activation when the subjects were deliberately attending to voices.
The reduction of STG activation was experienced by eight out of ten patients. Notably, the activation in the STG in each patient when ignoring voices relative to baseline was significantly correlated to the reduction in patients’ AH scores; i.e., less activation in the STG when ignoring voices was associated with greater reduction in AH scores. These findings suggest that the training aimed at the STG activation reduction can be effective in reducing the severity and frequency of AHs. Importantly, the same effect was not achieved when we gave the patients feedback from the motor cortex in our control feedback condition. This result is similar to the one achieved independently by Orlov et al. (2018). Both the Orlov study and the current investigation selected the STG as the target brain region and both studies succeeded in reducing the STG activation, post-neurofeedback as well as reducing the severity of AH. However, there are also notable differences between the two studies. In our study, right STG activation was significantly reduced, post-neurofeedback. We speculate that the right STG reduction may be related to the task we used in the rt-fMRI NFB session, which involved voice identity recognition. This is in accordance with the observation that right STG has been demonstrated to be preferentially involved in the voice identity recognition (Schelinski et al., 2016). Importantly, the right STG reduction was significantly correlated with AH reductions, post-neurofeedback.
Furthermore, the Orlov study used four sessions of rt-fMRI feedback to significantly reduce the left STG activation, while in our study a significant STG reduction was achieved with one rt-fMRI NFB session. In addition, the left STG reduction in the Orlov et al. study did not correlate with reductions in AHs directly but rather the AH reductions correlated with increased functional connectivity between the STG and IFG.
To our knowledge, the current paper is the first report of a significant activation reduction in a brain region involved in AHs (STG) achieved as a result of one session of rt-fMRI NFB. That, importantly, was coupled with significant reduction in the severity and frequency of AHs, with the two measures significantly associated with each other.
The feasibility of using rt-fMRI NFB to reduce AHs has been a hotly debated subject in recent publications (Fovet et al., 2016; McCarthy-Jones, 2012). Recent rt-fMRI NFB studies (Orlov et al., 2018; Zweerings et al., 2019) as well as our results clearly suggest that this approach is feasible. What is less well understood is what methodology within rt-fMRI NFB approach results in the most robust and sustainable neural response coupled with sustainable clinical improvement. Based on the results of the three papers (Dyck et al., 2016; Orlov et al., 2018; Zweerings et al., 2019) and the current study, it seems that selecting a specific brain target area that is involved in AH is critically important. It also appears that selecting a cognitive strategy for use by subjects in order to achieve a desired brain level change is more efficient than asking the subjects to work out a strategy that would lead to targeted brain region's and clinical changes. Future studies need to confirm the optimal method of inducing rt-fMRI guided neuro-behavioral changes. In addition, future studies also need to address the issue of the sustainability of achieved neurobehavioral changes. Given their pioneering character, none of the existing studies, including our own, were able to establish how many rt-fMRI NFB sessions are needed to introduce a permanent change in neuro-behavioral response.
The results of this study are also relevant for the theories of AHs. They confirmed a role of the STG in AH. The STG was targeted in this study as one of the hubs in the AH network. The results demonstrated that indeed the STG is a critically important region in mediating AH. Furthermore, our control condition results suggest that the successful neurofeedback relies on the engagement of brain regions belonging to the targeted network: the successful engagement of the brain region that is not known to be directly associated with the network of interest will not result in desired behavioral changes. In other words, the mere sense of successfully modulating any brain region does not lead to a desired clinical outcome, nor does it impact brain regions belonging to AHs network. While both the Orlov et al. (2018) and the Zweerings et al. (2019) studies suggest that changes in one element of the brain network will lead to changes in other elements of the network, our narrowly focused on the STG study cannot contribute to this discussion. To address the question of network wide consequences of rt-fMRI NFB, we designed the study that focused on a role of DMN in AHs which is discussed in Part II of the current paper.
5. Limitations
An important limitation of this study is the lack of a classic sham condition, in which feedback from another area of the brain not belonging to the targeted network is delivered while performing the same experimental task. These sham conditions often produce mostly inaccurate and misleading feedback, which can constitute a serious problem in the case of SZ patients who already feel a disconnection from their actions and thoughts. In order to prioritize the patient’s wellbeing, we chose an alternative strategy (i.e. the SMC control feedback task) and decided to perform first the STG feedback task in each session, so the patients could achieve the desired effect from the beginning and minimize any risk of frustration. For future studies, the team will work further on the development of other alternative strategies for sham conditions in rt-fMRI NFB that allows for an adequate experimental control while maximizing the patients’ welfare. Since this was a proof of concept study to demonstrate that rt-fMRI feedback can lead to changes in brain activation and reductions in AH, we also did not include healthy comparison group. In addition, as mentioned in the Methods section, we used Harvard atlas defined STG template to capture STG activation differences in pre- relative to post- neurofeedback contrasts rather than rely on indivually defined STG masks to maximize sensitivity in spite of individual variability given the low subject N. In future, well powered studies we will use individually defined STG in the analyses of the pre-post neurofeedback effects. Finally, we did not collect neuropsychological measures in our study group other than IQ measures; our future studies will address this issue.
Acknowledgments
SSG was partially supported by R01EB020740. The realtime setup development and maintenance was supported by McGovern Institute Neurotechnology (MINT) program.
Footnotes
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Alderson-Day B, Diederen K, Fernyhough C, Ford JM, Horga G, Margulies DS, McCarthy-Jones S, Northoff G, Shine JM, Turner J, van de Ven V, van Lutterveld R, Waters F, Jardri R, 2016. Auditory hallucinations and the brain’s resting-state networks: findings and methodological observations. Schizophr. Bull. 10.1093/schbul/sbw078. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A, 2008. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 10.1016/j.neubiorev.2007.07.012. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Alonso-Solís A, Vives-Gilabert Y, Grasa E, Portella MJ, Rabella M, Sauras RB, Roldán A, Núñez-Marín F, Gómez-Ansón B, Pérez V, Alvarez E, Corripio I, 2015. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr. Res. 161, 261–268. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, 1991. Schizophrenia: the characteristic symptoms. Schizophr. Bull. 10.1093/schbul/17.1.27. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Avants B, Epstein C, Grossman M, Gee J, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J-J, Blakemore S, Oakley DA, Frith CD, 2003. Delusions of alien control in the normal brain. Neuropsychologia. 10.1016/s0028-3932(02)00313-5. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Bodurka J, 2018. 143. Amygdala emotional regulation training with real-time fMRI neurofeedback and concurrent eeg recordings. Biol. Psychiatry 10.1016/j.biopsych.2018.02.161. [DOI] [Google Scholar]
- Brent BK, Seidman LJ, Coombs G, Keshavan MS, Moran JM, Holt DJ, 2014. Neural responses during social reflection in relatives of schizophrenia patients: relationship to subclinical delusions. Schizophr. Res. 10.1016/j.schres.2014.05.033. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M, Lissek S, Fuchs N, Witthaus H, Peters S, Nicolas V, Juckel G, Tegenthoff M, 2008. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 10.1016/j.neuropsychologia.2008.01.023. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kanazawa T, Koizumi A, Ide K, Taschereau-Dumouchel V, Boku S, … Kawato M., 2019. Current status of neurofeedback for post-traumatic stress disorder: a systematic review and the possibility of decoded neurofeedback. Front. Hum. Neurosci. 13 10.3389/fnhum.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolov DL, Mackinnon A, Trauer T, 2004. Correlates of the affective impact of auditory hallucinations in psychotic disorders. Schizophr. Bull. 10.1093/oxfordjournals.schbul.a007060. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. Neuroimage. https://doi.org/10.1006/nimg.1998.0395. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Shafer A, Lauriola M, 2016. Meta-analysis of the brief psychiatric rating scale - expanded (BPRS-E) structure and arguments for a new version. J. Psychiatr. Res. 81, 140–151. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Christopher deCharms R, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JDE, 2004. Learned regulation of spatially localized brain activation using real-time fMRI. NeuroImage. 10.1016/j.neuroimage.2003.08.041. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ., 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31 (3), 968–980. 10.1016/j.neuroimage.2006.01.021. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Dyck MS, Mathiak KA, Bergert S, Sarkheil P, Koush Y, Alawi EM, Zvyagintsev M, Gaebler AJ, Shergill SS, Mathiak K, 2016. Targeting treatment-resistant auditory verbal hallucinations in schizophrenia with fMRI-Based neurofeedback exploring different cases of schizophrenia. Front. Psychiatry 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fovet T, Orlov N, Dyck M, Allen P, Mathiak K, Jardri R, 2016. Translating neurocognitive models of auditory-verbal hallucinations into therapy: using real-time fMRI-Neurofeedback to treat voices. Front. Psychiatry 10.3389/fpsyt.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Done DJ, 1988. Towards a neuropsychology of schizophrenia. Br. J. Psychiatry 153, 437–443. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RS, 1992. PET imaging and cognition in schizophrenia. J. R. Soc. Med. 85, 222–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Brammer MJ, Yágüez L, Allen P, Matsumoto K, Johns L, Weinstein S, Borgwardt S, Broome M, van Haren N, McGuire PK, 2008. Increased superior temporal activation associated with external misattributions of self-generated speech in schizophrenia. Schizophr. Res. 100, 361–363. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS, 2011. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R, 2010. Real-time fMRI feedback training may improve chronic tinnitus. Eur. Radiol. 10.1007/s00330-009-1595-z. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Paul Hamilton J, Glover GH, Hsu J-J, Johnson RF, Gotlib IH, 2011. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum. Brain Mapp. 10.1002/hbm.20997. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Scheinost D, Qiu M, Bhawnani J, Lacadie CM, Leckman JF, Constable RT, Papademetris X, 2011. Biofeedback of real-time functional magnetic resonance imaging data from the supplementary motor area reduces functional connectivity to subcortical regions. Brain Connect. 1, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Stoica T, Saksa J, Scheinost D, Qiu M, Bhawnani J, Pittenger C, Papademetris X, Constable T, 2012. Real-time fMRI biofeedback targeting the orbitofrontal cortex for contamination anxiety. J. Vis. Exp. 10.3791/3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Prisciandaro JJ, Borckardt J, Li X, George MS, Brady KT, 2013. Real-time fMRI in the treatment of nicotine dependence: a conceptual review and pilot studies. Psychol. Addict. Behav. 10.1037/a0028215. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O, Ghosh S, Thompson TW, Yoo JJ, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JDE, 2011. Computing moment-to-moment bold activation for real-time neurofeedback. NeuroImage. 10.1016/j.neuroimage.2010.07.060. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, Charney DS, 1999. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices.”. Biol. Psychiatry. 10.1016/s0006-3223(98)00358-8. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu Y-T, Carroll K, Krystal JH, 2005. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol. Psychiatry. 10.1016/j.biopsych.2005.03.041. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH, 2003. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch. Gen. Psychiatry. 10.1001/archpsyc.60.1.49. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM, 2011. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol. Psychiatry 69, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P, 2011. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP, 2011. Medial prefrontal cortex subserves diverse forms of self-reflection. Soc. Neurosci 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF, 2002. Finding the self? An event-related fMRI study. J. Cognit. Neurosci. 14, 785–794. [DOI] [PubMed] [Google Scholar]
- Larivière S, Lavigne KM, Woodward TS, Gerretsen P, Graff-Guerrero A, Menon M, 2017. Altered functional connectivity in brain networks underlying self-referential processing in delusions of reference in schizophrenia. Psychiatry Res. 10.1016/j.pscychresns.2017.03.005. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R, 2012. Real-time self-regulation of emotion networks in patients with depression. PLoS ONE. 10.1371/journal.pone.0038115. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, Lematty T, Brady KT, George MS, 2013. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict. Biol. 18, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Jones S, 2012. Taking back the brain: could neurofeedback training be effective for relieving distressing auditory verbal hallucinations in patients with schizophrenia? Schizophr. Bull. 10.1093/schbul/sbs006. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, 2011. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr. Res. 127, 202–214. [DOI] [PubMed] [Google Scholar]
- Northoff G, 2014. Are auditory hallucinations related to the brain's resting state activity? A “Neurophenomenal resting state hypothesis. Clin. Psychopharmacol. Neurosci. 12, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 10.1016/0028-3932(71)90067-4. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Orlov ND, Giampietro V, O’Daly O, Lam S-L, Barker GJ, Rubia K, McGuire P, Shergill SS, Allen P, 2018. Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl. Psychiatry. 10.1038/s41398-017-0067-5. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW, 2008. Generating stimuli for neuroscience using psychopy. Front. Neuroinform. 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW, 2007. PsychoPy—psychophysics software in python. J. Neurosci. Methods. 10.1016/j.jneumeth.2006.11.017. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot M, Kimmich S, Gonzalez-Castillo J, Roopchansingh V, Popal H, White E, … & Martin A., 2017. Direct modulation of aberrant brain network connectivity through real-time neurofeedback. Elife 6, e28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N, 2009. Self-regulation of regional cortical activity using real-time fMRI: the right inferior frontal gyrus and linguistic processing. Hum. Brain Mapp. 30, 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Lee S, Soekadar SR, Caria A, Veit R, Kircher T, Birbaumer N, Sitaram R, 2013. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum. Brain Mapp. 34, 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius N, Jablensky A, Korten A, Ernberg G, Anker M, Cooper JE, Day R, 1986. Early manifestations and first-contact incidence of schizophrenia in different cultures: a preliminary report on the initial evaluation phase of the who collaborative study on determinants of outcome of severe mental disorders. Psychol. Med. 10.1017/s0033291700011910. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Stoica T, Wasylink S, Gruner P, Saksa J, Pittenger C, Hampson M, 2014. Resting state functional connectivity predicts neurofeedback response. Front. Behav. Neurosci. 8, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Tokoglu F, Hampson M, Hoffman R, Constable RT, 2019. Data-driven analysis of functional connectivity reveals a potential auditory verbal hallucination network. Schizophr. Bull. 45, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelinski S, Borowiak K, von Kriegstein K, 2016. Temporal voice areas exist in autism spectrum disorder but are dysfunctional for voice identity recognition. Soc. Cognit. Affect. Neurosci. 10.1093/scan/nsw089. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Murray RM, McGuire PK, 1998. Auditory hallucinations: a review of psychological treatments. Schizophr. Res. 32, 137–150. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Caria A, Veit R, Gaber T, Ruiz S, Birbaumer N, 2014. Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: a pilot study. Front. Behav. Neurosci. 8, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, … Sulzer J., 2017. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 18 (2), 86–100. 10.1038/nrn.2016.164. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Veit R, Stevens B, Caria A, Gerloff C, Birbaumer N, Hummel F, 2012. Acquired control of ventral premotor cortex activity by feedback training. Neurorehabil. Neural Repair. 10.1177/1545968311418345. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, de Weijer AD, Diederen KM, Goekoop R, Looijestijn J, Daalman K, Rijkaart A-M, Kahn RS, Hoek HW, Sommer IEC, 2011. Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol. Psychiatry. 10.1016/j.biopsych.2010.09.051. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith S, Nichols T, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 10.1016/j.neuroimage.2008.03.061. https://doi.org/. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Garrison KA, Ghosh SS, Wighton P, Hanlon CA, Gilman JM, Greer S, Turk-Browne NB, deBettencourt MT, Scheinost D, Craddock C, Thompson T, Calderon V, Bauer CC, George M, Breiter HC, Whitfield-Gabrieli S, Gabrieli JD, LaConte SM, Hirshberg L, Brewer JA, Hampson M, Van Der Kouwe A, Mackey S, Evins AE, 2014. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage. 10.1016/j.nicl.2014.07.002. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L, Hindle JV, Johnston S, Roberts MV, Husain M, Goebel R, Linden D, 2011. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson’s disease. J. Neurosci. 31 (45), 16309–16317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma RJ, Chaze C, Lewine JD, Calhoun VD, Clark VP, Bustillo J, Houck J, Ford J, Bigelow R, Wilhelmi C, Stephen JM, Turner JA, 2016. Functional mri evaluation of multiple neural networks underlying auditory verbal hallucinations in schizophrenia spectrum disorders. Front. Psychiatry 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N, Hayward M, Peters E, van der Gaag M, Bentall RP, Jenner J, Strauss C, Sommer IE, Johns LC, Varese F, Garcia-Montes JM, Waters F, Dodgson G, McCarthy-Jones S, 2014. Psychological therapies for auditory hallucinations (voices): current status and key directions for future research. Schizophr. Bull. 10.1093/schbul/sbu037. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Mantini D, Gillebert CR, 2017. The potential of real-time fMRI neurofeedback for stroke rehabilitation: a systematic review. Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sasaki Y, Shibata K, Kawato M, 2017. Advances in fMRI real-time neurofeedback. Trends Cognit. Sci. 21 (12), 997–1010. 10.1016/j.tics.2017.09.010. https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, 2009. Artifact Detection Tools (ART) [WWW Document] URL. https://www.nitrc.org/projects/artifact_detect.
- Yoo SS., Jolesz FA., 2002. Functional MRI for neurofeedback: feasibility studyon a hand motor task. NeuroReport. 10.1097/00001756-200208070-00005. [DOI] [PubMed] [Google Scholar]
- Yoo SS., Lee JH., OĽeary H., Lee V., Choo SE., Jolesz FA., 2007. Functional magnetic resonance imaging-mediated learning of increased activity in auditory areas. NeuroReport. 10.1097/wnr.0b013e3282f202ac. [DOI] [PubMed] [Google Scholar]
- Young KD, Siegle GJ, Misaki M, Zotev V, Phillips R, Drevets WC, Bodurka J, 2018a. Altered task-based and resting-state amygdala functional connectivity following real-time fMRI amygdala neurofeedback training in major depressive disorder. NeuroImage. 10.1016/j.nicl.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Zotev V, Phillips R, Misaki M, Drevets WC, Bodurka J, 2018b. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: a review. Psychiatry Clin. Neurosci. 72 (7), 466–481. 10.1111/pcn.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerings J, Hummel B, Keller M, Zvyagintsev M, Schneider F, Klasen M, Mathiak K, 2019. Neurofeedback of core language network nodes modulates connectivity with the default-mode network: a double-blind fMRI neurofeedback study on auditory verbal hallucinations. NeuroImage. 10.1016/j.neuroimage.2019.01.058. [DOI] [PubMed] [Google Scholar]