Abstract
Background
Intravascular devices have significant potential for producing iatrogenic diseases resulting in catheter-related blood stream infections (CRBSIs). A study was undertaken to find the prevalence of CRBSI among patients in acute wards and to analyze the associated risk factors, causative pathogens with their antibiotic susceptibility (ABST) patterns.
Methods
Randomly ten days per month were chosen, for a period of two years. All the acute wards patients who were on indwelling blood catheters were identified. Those fulfilling the CRBSI criteria were further worked up for confirmation of diagnosis by differential time to positivity. The catheter tip was cultured by Maki's semiquantitative method. ABST of the isolates obtained was performed by Kirby–Bauer disk diffusion method.
Results
The prevalence of CRBSI was found to be 39.25% with the most common organism isolate being Serratia marcescens (23.81%). The immunocompromised status of the patients and catheterisation time were significant risk factors. Methicillin resistance was found to be 33.33% in coagulase-negative staphylococci. The resistance to vancomycin among the Entercoccus faecium isolates was found to be 33.33%. Among the gram negatives, resistance to aminoglycosides, fluoroquinolones and third-generation cephalosporins was high.
Conclusion
The study highlights the importance of regular surveillance programs, an efficient infection control program, strict adherence to antiseptic measures and use of a rational antibiotic policy for the early diagnosis and better management of CRBSI.
Keywords: Catheter related blood stream infections, Central venous catheter, Peripherally inserted central catheter, Nosocomial infections, Antibiotic susceptibility pattern
Introduction
Modern medical care is heavily dependent on a reliable and safe vascular access. Intravascular devices by providing a stable access have revolutionised the critical care. These devices, however, are also associated with a significant potential for producing iatrogenic diseases resulting in catheter-related bacteraemia or candidemia.1
Catheter-related blood stream infection (CRBSI), a nosocomial infection, is a significant clinical problem which is continuously evolving because of changes in the population at risk, changes in spectrum of available pathogens, and an increased use of broad-spectrum antibiotics.2, 3 According to the National Nosocomial Infections Surveillance (NNIS) system of the Centres for Disease Control and Prevention (CDC), the median rate of CRBSI in intensive care units (ICUs) of all types ranges from 1.8 to 5.2 per 1000 catheter-days with the rate of about 25.6%.4 However, these rates are highly variable, and can be expected to be much higher for the developing countries. There are limited Indian studies on CRBSI which have found the incidence of these infections to be around 27%.3 Each new episode of CRBSI increases the risk of septicaemia by 4–14% that of death by 12–25%, in addition to prolonging hospitalization and increasing healthcare costs by manifold.3, 5 Besides CRBSI, central venous catheters (CVCs) put a patient at risk of developing various local as well as systemic complications such as infection at the site of insertion, septic thrombophlebitis, endocarditis, metastatic infections and other serious complications such as bacteraemia, sepsis and death. It is, therefore, very important that such infections be recognized at the earliest and timely diagnosis be made by using a combination of clinical signs and quantitative culture techniques3 along with their antibiotic susceptibility (ABST) pattern which forms a very important tool in guiding the physician for starting the appropriate therapy.
With this backdrop, this study was undertaken with the objectives of studying the prevalence of CRBSI among the hospitalized patients admitted in the acute wards, the associated risk factors, causative pathogens and their respective ABST patterns.
Materials and methods
The present study was conducted in an urban tertiary care teaching hospital. Ten days were chosen at random, every month for a period of two years. During these ten days, all the patients admitted to the acute wards (surgical ICU, medical ICU, acute medical and acute surgical) of the hospital and who were put on indwelling blood catheters were identified. Patient inclusion criteria necessitated a hospital admission of equal to more than 48 h.
Exclusion criteria included neonatal and paediatric ICU, paediatric wards, obstetrics and gynaecology, chronic wards and patients undergoing treatment or surgery on the same day as the day of collection of data. A total of 2800 patients were hospitalised during the study period; of which, 1898 were on central or peripheral catheter. Among these patients on venous catheters (peripheral venous catheter or peripheral inserted central catheter [PICC]), suspected cases of CRBSI (based on definition, history and clinical findings) were 212. These 212 cases were then worked up for confirmation of the diagnosis.
Suspected cases of CRBSI were those patients on indwelling central catheter having symptoms of local phlebitis and inflammation, purulence or both at the insertion site along with fever, chills, hypotension and increased leucocyte count.6 The study was approved by the Institutional Ethical Committee. The findings were noted in a pro forma, which contained demographic details such as the ward the patient was admitted to, name, age, sex, date of admission, diagnosis, associated medical conditions, and so on.
Data were also collected pertaining to the type of catheter inserted, duration of catheterization, any past history of catheterization and any suspected infection due to the indwelling catheter. In addition, presence or absence of fever, total leucocyte count and any other relevant laboratory test reports were also noted. The criteria as laid down by the CDC were used to decide if a patient was immunocompetent or immunocompromised.7
From suspected cases of CRBSI, two blood samples were drawn. One was drawn from the catheter and the other from the peripheral site. The CVC used in our hospital were the two-lumen polyurethane latex-free catheters. Specimen was collected by the nurse in charge of the ward who was blinded for the study. Hands were thoroughly washed, and a new set of sterile gloves were worn. Any ongoing infusion was stopped by discontinuing the tubing from the injection cap and attaching a Luer lock. After a period of (1–3) min, the injection cap was scrubbed with 70% alcohol and allowed to dry. The catheter was then flushed using a 10-ml prefilled normal saline syringe. The first 3–5 mL of blood was removed and discarded. A blood specimen (5–10 mL) was then drawn to be inoculated into the 50-ml BACTEC bottles for further processing in a BD BACTEC 9120 instrument. For continuing the infusion, the Luer lock was removed and the catheter was unclamped. The hub was scrubbed with 70% alcohol. The administration set was attached into the injection cap, and the infusion was restarted.
For patients in whom the old catheter was to be replaced with a new one and on physician's discretion, the catheter was removed and the tip was cultured by the semiquantitative method as described by Maki et al. For removal of the catheter, hands were thoroughly washed and a new set of sterile gloves were worn. The dressing was removed. A dry 2 × 2 gauze pad was held over the insertion site, the pressure was slowly increased and the catheter withdrawn. A steady pressure was maintained over the site for (3–4) min, and a bandage was placed over the puncture site. A 5-cm distal segment of the catheter, which included the tip of the line, was sent in a sterile container to the laboratory for culture of microorganisms with an appropriate investigation form. The semiquantitative “Maki's roll”8 technique involved rolling the catheter over a blood agar plate containing 5% sheep blood. The plate was then incubated at 37 °C for 48 h, after which it was assessed for the growth of any microorganisms and the number of colonies obtained was counted.
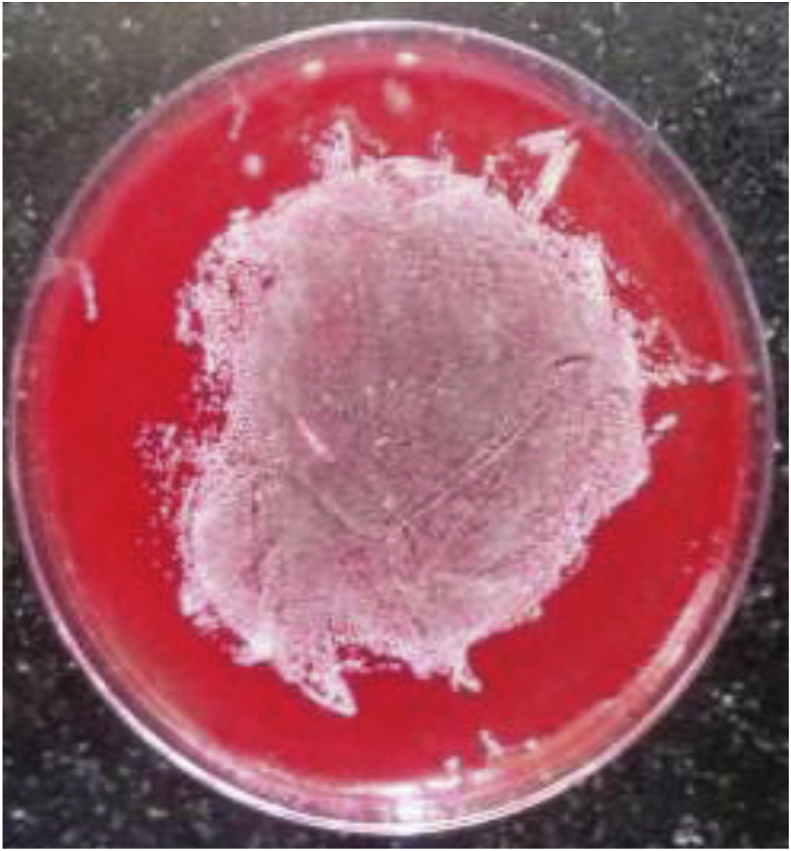
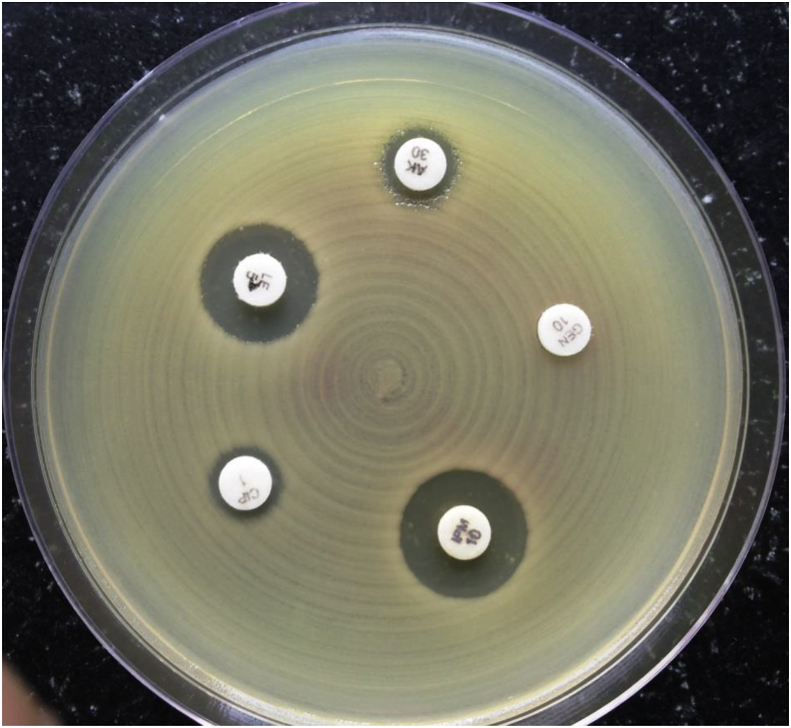
Catheter-tip colonization was defined as a positive semiquantitative tip culture of ≥15 CFU8 for the roll plate method (Fig. 1). Any number of yeast colonies growing on the agar plates was taken as being suggestive of a fungal line infection.9, 10 The BACTEC bottles being used in our laboratory were the BACTEC PLUS Aerobic/F Medium, BACTE Peds Plus/F Culture Vials and BACTEC MYCO/F LYTIC. The BACTEC bottles, that were flagged positive, were removed from the instrument. The contents of the bottle were mixed by inverting, and the bottle was placed in a biological biosafety cabinet. Maintaining universal safety precautions, a 2-ml needle was introduced through the rubber covering of the bottle, and an aliquot was removed for Gram staining and processing. A part of the aliquot was cultured on various culture medium plates. The plates were incubated at 37 °C for 24 h. After incubation, the plates were examined for any growth, and the colonies, if present, were subjected to a Gram stain, and identification was done using various biochemical tests. Candida isolates were identified by Gram staining and germ tube testing for identifying Candida albicans. The germ tube–negative strains were labelled as nonalbicans Candida spp. The patient was confirmed as a case of CRBSI, using the criteria of “differential time to positivity” which states that in CRBSI, blood samples drawn from the CVC usually give a positive signal at least 2 h earlier than the peripheral blood samples.6 Thus, a case was considered to be a CRBSI, if the sample from the catheter as well as from the peripheral site were found to be positive by BACTEC 9120 for the same organism, and the sample from catheter came out to be positive 2 h or more before the sample drawn from the peripheral site. All the isolates obtained from the cases of CRBSI were tested for their susceptibility to various antibiotics by Kirby–Bauer disk diffusion method using Clinical and Laboratory Standards Institute (CLSI) 201811 guidelines (Fig. 2).
Fig. 1.
Roll plate method for diagnosis of CRBSI showing >15 colonies on blood agar. CRBSI, catheter-related blood stream infection.
Fig. 2.
Antibiotic susceptibility testing done by Kirby–Bauer technique.
All the calculations were done using SPSS version 20 (IBM, Armonk, NY, USA). Group comparisons were performed by using unpaired t-test. Chi-square test was calculated, and a p value of <0.05 was considered, to indicate significant differences between groups.
Results
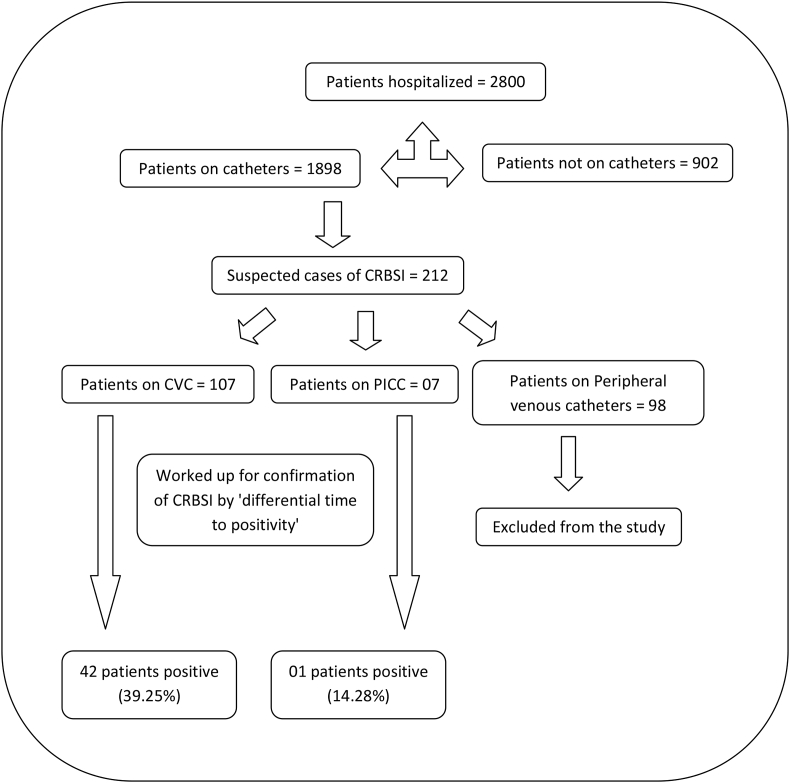
A total of 2800 patients were hospitalised during the study period; of which, 1898 were on central or peripheral catheter (Fig. 3). Among the 1898 on catheters, there were total 212 suspected cases of CRBSI. Of the 212 suspected cases, 107 were on CVC and 07 were on PICC. The remaining 98 cases were on peripheral venous catheters, and these were excluded from the study. Among the 107 suspected cases on CVC, 42 were positive by differential time to positivity (i.e. 39.25%), and from the 07 suspected cases on PICC, only one was positive by differential time to positivity (i.e. 14.28%). For the Maki's roll plate method, a total of 10 CVC's were removed according to the physician's discretion, while none of the PICC's could be removed. A total of 04 (40%) CVCs were culture positive by Maki's roll plate method, and of these, 03 (30%) were also positive by differential time to positivity. Those CVC negative by both differential time to positivity and Maki's roll plate method were 06 (60%).
Fig. 3.
Algorithm Showing Prevalence of CRBSI Cases in our Study. CRBSI, catheter-related blood stream infection.
Among the organisms isolated, the most common was Serratia marcescens with 10 isolates (23.81%) and followed by coagulase-negative staphylococci (CONS) with 09 isolates (21.43%) (Table 1). Of the total 07 patients on PICC, only one developed CRBSI, and the isolate was Acinetobacter baumannii.
Table 1.
The distribution of various isolates obtained from cases of CRBSI.
| Organism | Number | Percentage |
|---|---|---|
| Serratia marcescens | 10 | 23.81% |
| Coagulase negative Staphylococci | 09 | 21.43% |
| Acinetobacter baumannii | 05 | 11.90% |
| Enterococcus spp | 05 | 11.90% |
| Klebsiella spp | 04 | 9.52% |
| Stenotrophomonas maltophilia | 04 | 9.52% |
| Staphylococcus aureus | 02 | 4.76% |
| Pseudomonas aeruginosa | 01 | 2.38% |
| Escherichia coli | 01 | 2.38% |
| Achromobacter denitrificans | 01 | 2.38% |
| Total | 42 | 100% |
CRBSI, catheter-related blood stream infection.
The risk factors studied for the development of CRBSI were the immune status of the patient, the site of insertion of catheter, and the duration of catheterization. Of the 212 patients on venous catheters, 111 were immunocompromised, 60 were on CVC. Of 101 immunocompetent patients, 47 had CVC. All the 7 patients on PICC were immunocompetent. Of the total 212 patients on venous catheters, 63 developed CRBSI, and among these, 46 were immunocompromised. Among these 46 immunocompromised patients, 29 were on CVC. Among the remaining patients of CRBSI, 17 were immunocompetent; 13 of whom were on CVC, and 1 was on PICC. Of the total 212 patients on venous catheters, 149 did not develop CRBSI. Among those who did not develop CRBSI, 66 were immunocompromised, and among these, 32 were on CVC. The patients who did not develop CRBSI included 83 immunocompetent patients; of which, 33 were on CVC, and 6 were on PICC (Table 2). Using chi-square test to find the statistical significance, the p value came to be < 0.0001.
Table 2.
Immune status of patients as a risk factor for developing CRBSI.
| Catheter type | Total no of cases of CRBSI (n = 63) |
Total cases who did not develop CRBSI (n = 149) |
||
|---|---|---|---|---|
| Immunocompromised (46) | Immunocompetent (17) | Immunocompromised (66) | Immunocompetent (83) | |
| Central venous catheter (CVC) | 29 | 13 | 32 | 33 |
| Peripherally inserted central catheter (PICC) | 0 | 01 | 00 | 06 |
CRBSI, catheter-related blood stream infection.
The sites of insertion of catheter, that is, jugular or subclavian, were assessed as a risk factor for CRBSI. The site of insertion of catheter in our institute comprised 74 jugular and 33 subclavian insertions. Of the jugular, 31 developed CRBSI, whereas 11 of subclavian insertions developed CRBSI (Table 3). Statistical assay was carried out by applying Chi square test, and p value was found to be < 0.402.
Table 3.
Site of insertion as a risk factor for development of CRBSI.
| Site of insertion of catheter | CRBSI |
Total | p value | |
|---|---|---|---|---|
| Yes | No | |||
| Internal jugular | 31 | 43 | 74 | <0.402 |
| Subclavian | 11 | 22 | 33 | |
| Total | 42 | 65 | 107 | |
CRBSI, catheter-related blood stream infection.
Of the total 42 patients on CVC who developed CRBSI, average duration of catheterization was 12.19 ± 1.89 days. In the remaining 65 patients on CVC who did not develop CRBSI, the average duration of catheterization was 8.43 ± 1.28. Using an unpaired t-test, the p value was <0.0001. ABST was carried out for all the isolates of CRBSI by Kirby–Bauer Disk diffusion test. Interpretation of the results was according to the CLSI guidelines 2014.
For the ease of interpretation, the isolates were divided into the following groups:
-
(a)
Nonfermenters: Pseudomonas aeruginosa, A. baumannii, Stenotrophomonas maltophilia and Achromobacter denitrificans.
-
(b)
Enterobacteriaceae: Escherichia coli, Klebsiella spp and S. marcescens
-
(c)
Nonalbicans candida
-
(d)
Gram-positive cocci: CONS, Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium
ABST pattern of the isolates obtained is shown in Table 4, Table 5. There were only two isolates of nonalbicans candida which showed susceptibility to all the antifungals tested.
Table 4.
ABST pattern of the nonfermenters and Enterobacteriaceae isolates obtained from CRBSI cases. CRBSI, catheter-related blood stream infection; ABST, antibiotic susceptibility.
Table 5.
ABST pattern of the gram positive isolates obtained from CRBSI cases. CRBSI, catheter-related blood stream infection; ABST, antibiotic susceptibility.
Discussion
Venous catheters are increasingly being used for administration of chemotherapeutic agents, blood products, parenteral nutrition and antibiotics, but they could prove to be “double-edged swords” and result in serious infections.12 According to studies, about 4–8% of all CVC eventually result in CRBSI.13, 14, 15 In a study carried out in England between 1997 and 2001, intravascular devices accounted for 43.3%–52.4% of nosocomial blood stream infections; of which, central lines were the most common source.15 These figures are much higher for the developing countries16, 17, 18, 19 where because of lack of surveillance, the true rates of infections may not be available.15 Some studies have even concluded that these rates may be as variable as 4.4%–88.8%.18, 19
In the Indian context, limited studies are available on the prevalence of CRBSI. In the present study, we found the prevalence of CRBSI due to CVC to be 39.25%, which is in close agreement to few other Indian studies, which have found a prevalence of 27–56%.2, 3, 20
The slightly higher prevalence in our study was because samples were from patients of acute wards where a large number of patients were on CVC, many were on multiple antibiotics with many comorbidities, all contributing to increased risk of CRBSI.
In the present study, the risk of developing CRBSI was found to be lower with PICC, with a prevalence of 14.28%. The PICC in various studies have been found to have a lower risk for CRBSI, with some studies estimating the risk of CRBSI being sixty-four times with CVC compared with PICC.21, 22 Some have, however, found no difference in the risk of CRBSI when both CVC and PICC were compared.23 The techniques of CRBSI diagnosis by the semiquantative roll plate method and the differential time to positivity have been compared by numerous studies. The results of these studies are quite varied, but most have concluded that the roll plate represents only the extraluminal pathogens or the colonisation of the catheter, whereas differential time to positivity indicates the intraluminal source of infection and hence true CRBSI.24, 25 The roll plate method in various studies has shown a positivity of 6%–57.5%.26, 27 It was 22.58% in the present study. In addition, simultaneous positivity by both the roll plate and blood culture varies in various studies from 13.7% to 52.32%.26, 27 However, in the present study, it was 19.35%. With reference to the causative organisms, CONS have been the most frequently isolated pathogens, accounting for about 28% of intravascular catheter–related infections as per the NNIS system.28, 29 It has also been recognized that CONS are potentially true nosocomial pathogens and not harmless culture contaminants,29 which has also been corroborated in the present study with CONS forming 23.81% of all isolates and with gram-positive organisms (CONS, S. aureus and Enterococcus spp) collectively comprising 38.04% of the isolates.
The next predominant isolates found were from the Enterobacteriaceae family with S. marcescens being the predominant isolate. This organism has been reported as a cause of CRBSI in various studies, where its source was found to be contaminated infusates, tubings, lipid emulsions and even anaesthetic drugs.30 In the present study, isolates of S. marcescens were obtained from the surgical ICU, from patients who had undergone surgery in a particular time frame. However, the source could not be investigated as it was not a part of this study.
Nonfermenters formed the third most common group of the isolated organisms accounting for 12.7% of isolates. This has also been found in other studies which have commented on the increase in A. baumanii as a cause of CRBSI from 6.1% in 1981–1986 to 7.6% in 1999.31, 32
Candida infection resulted in only two cases of CRBSI in our study. This is in contradiction with other studies which have documented an increase in CRBSI due to Candida spp.5, 33 The reason for this could be that most of the studies which have reported an increase in Candida as a cause of CRBSI consisted of a study population of haematologic malignancies and transplant patients34, 35 and these formed a very small subset of patients in the present study.
Another interesting finding in the present study was the isolation of fewer isolates of Klebsiella spp, E. coli and P. aeruginosa. Similar findings are reported in other studies, which have found a decrease in nosocomial infections due to these pathogens while reporting an increase in CRBSI due to CONS and Candida.33 Among the risk factors, a statistically significant increase in CRBSI was associated with an immunocompromised state (p < 0.0001). This has been emphasized by various studies which have suggested that immunocompromised states such as malignancy, AIDS, immunodeficiency, severe burns and malnutrition predispose to higher rate of infection due to decreased functional ability of humoral and cell-mediated immunity.24, 25
Another risk factor assessed was the site of insertion of the CVC, that is, jugular versus the subclavian, which in the present study was not statistically significant. Studies are divided in their views on the site of insertion as a risk factor. Some have shown a higher rate of CRBSI for internal jugular when compared with Subclavian insertions,15, 22 whereas others have shown no significant difference.2, 3
Duration of catheterization was also found to be a significant risk factor in this study (p value of <0.0001). The average duration of catheterization for patients with CRBSI was 11.65 days and for those without CRBSI was 7.29 days. Similar trend is seen in other studies which suggest that a catheter stay of >10 days is associated with an intraluminal contamination and a definitive risk for CRBSI.25 In most of the studies on CRBSI, the most frequent isolates have been CONS, and especially methicillin-resistant CONS (MRCONS) strains. The formation of biofilm in these bacteria protects them from antibiotics as well as from the host immune system which enhances their virulence.36 In the present study, the methicillin resistance was found to be 33.33%. Studies have found the prevalence of MRCONS to be from 46.15% to about 71%.3, 29 But some have also found it to be as high as 64%–90.6%.33, 36 The isolates were uniformly sensitive to vancomycin, linezolid and teicoplanin, a finding elsewhere too.3
The incidence of vancomycin-resistant Enterococcus (VRE) as a cause of nosocomial infection has increased;33 the Morbidity and Mortality Weekly Report 2002 has also stated that the VRE isolates in ICU has increased from 0.5% to 25.9%. In the present study, the resistance to vancomycin among the E. faecium isolates was found to be 33.33%, whereas none of the E. faecalis isolate were found to be vancomycin resistant.
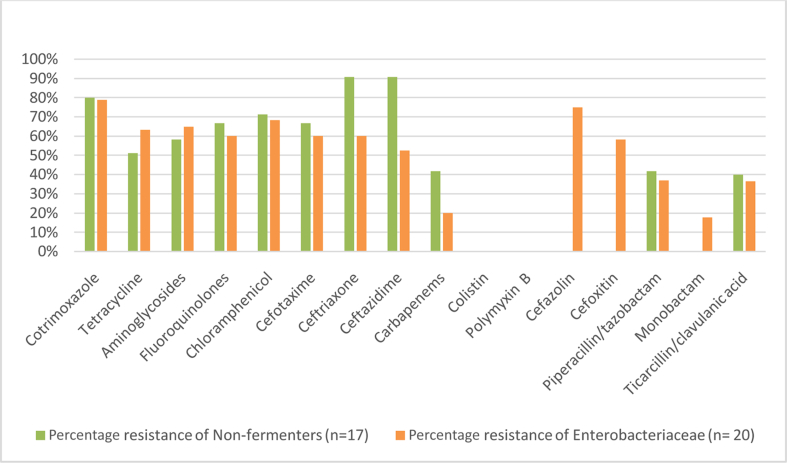
In our study, among the gram-negative organisms, resistance to the commonly used group of antibiotics, that is, aminoglycosides and fluoroquinolones, was high, with 65% each in Enterobacteriaceae and 58.33% and 66.67%, respectively, in nonfermenters. Nonfermenters also showed high resistance to third-generation cephalosporins ranging from 66.67% to 90.9%. Carbapenem resistance in Enterobacteriaceae was found to be 20% and 41.67% in nonfermenters. All isolates of Enterobacteriaceae and nonfermenters showed sensitivity to polymyxin B and colistin.
The limited studies available on the ABST pattern of gram-negative bacteria in CRBSI have mostly concentrated on E. coli, A. baumannii and P. aeruginosa. Studies have demonstrated about 43% A. baumanii isolates and 13% of both P. aeruginosa and K. pneumoniae isolates to be resistant to fluoroquinolones and third-generation cephalosporins.5 In these studies, of the total isolates, about 30% were found to be resistant to carbapenem, fluoroquinolones and third-generation cephalosporins and only 35% of isolates were found to be susceptible to all three.5 Multiresistant gram-negative bacteria (E. coli, Acinetobacter spp., K. pneumonia) have been shown to cause intravascular catheter-related infections in several institutions.3, 33, 36 Also nonfermenters have shown an increasing resistance to third-generation cephalosporins and carbapenems in several studies.18, 19, 33, 36
In conclusion, the prevalence of CRBSI in this study was in keeping with the other studies. Most of the isolates obtained from the patients were found to be multidrug resistant, leaving the physician with only limited options. The study highlights the importance of regular surveillance programs, strict adherence to antiseptic measures (during device insertion and care), an efficient infection control program and use of a strict and rational antibiotic policy for the early diagnosis and better management of CRBSI.
The limitations of the study include inability to perform semiquantative culture of all the catheters from suspected cases of CRBSI. This is because they were removed only on physician's discretion and, in case, repeated access was not a problem. Also there are many other risk factors associated with development of CRBSI, but we studied only three. Thus, more studies would contribute towards better understanding of the topic.
Conflicts of interest
All authors have none to declare.
References
- 1.Safdar N., Maki D.G. The pathogenesis of catheter- related blood stream infection with noncuffed short- term central venous catheters. Intensive Care Med. 2004;30:62–67. doi: 10.1007/s00134-003-2045-z. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A., Sharma R.M., Jaideep C.N., Hazra N. Diagnosis of Central venous catheter-related bloodstream infection without catheter removal: a prospective observational study. Med J Armed Forces India. 2014;70:17–212. doi: 10.1016/j.mjafi.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil H.V., Patil V.C., Ramteerthkar M.N., Kulkarni R.D. Central venous catheter related bloodstream infections in the intensive care unit. Indian J Crit Care Med. 2011;15:213–223. doi: 10.4103/0972-5229.92074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magill S.S., Edwards J.R., Bamberg W. Multistate point- prevalence survey of health care- associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deliberato R.O., Marra A.R., Correa T.D. Catheter related bloodstream infection (CR-BSI) in ICU patients: making the decision to remove or not to remove the central venous catheter. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032687. e32687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathur P. 1st ed. Wolters Kluwer Pvt Ltd; New Delhi: 2010. Hospital Acquired Infections; pp. 338–373. Chapter 11, Catheter- Related Bloodstream Infections. [Google Scholar]
- 7.Centers for Disease Control and Prevention . 2018. Altered Immunocompetence Guidelines for Immunizations-CDC.http://www.cdc.gov/hcp/generalrecs/immunocompetence.html Retrieved from. [Google Scholar]
- 8.Maki D.G., Weise C.E., Sarafin H.W. A Semiquantative culture method for identifying intravenous – catheter- related infections. N Engl J Med. 1977;296:1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 9.Slobbe L., Barzouhi A., Boersma E., Rijnders B.J.A. Comparison of the roll plate method to the sonication method to diagnose catheter colonisation and bacteremia in patients with long-term tunnelled catheters: a randomized prospective study. J Clin Microbiol. 2009;47:885–888. doi: 10.1128/JCM.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouza E., Alvarado N., Alcala L. A prospective, randomised, and comparative study of 3 different methods for the diagnosis of intravascular catheter colonization. Clin Infect Dis. 2005;40:1096–1100. doi: 10.1086/428576. [DOI] [PubMed] [Google Scholar]
- 11.CLSI . 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2018. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. [Google Scholar]
- 12.Carratala J., Niubo J., Sevilla A.F. Randomised, double-blind trial of an antibiotic- lock technique for prevention of gram-positive central venous catheter- related infection in neutropenic patients with cancer. Antmicrob Agents Chemother. 1999;43:2200–2204. doi: 10.1128/aac.43.9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis R.E., Kontoyiannis D.P., Darouiche R.O., Raad, Prince R.A. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter–related bloodstream infection. Antimicrob Agents Chemother. 2002;46:3499–3505. doi: 10.1128/AAC.46.11.3499-3505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher S. Catheter- related bloodstream infection. Cont Educ Anaesth Crit Care Pain. 2005;5:49–51. [Google Scholar]
- 15.Johnston B.L., Conly J.M. What do central venous catheter-associated bloodstream infections have to do with bundles? Can J Infect Dis Med Microbiol. 2005;16:215–218. doi: 10.1155/2005/582156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal V.D., Maki D.G., Salomao R. Device- associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 17.Singhi S., Nallaswamy K. Catheter related blood stream infection in Indian PICUs: several Unanswered issues! Indian J Crit Care Med. 2013;17:127–128. doi: 10.4103/0972-5229.117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Joint Commission . Joint Commission Resources; Oak Brook, IL: May 2012. Preventing Central Line–Associated Bloodstream Infections: A Global Challenge, a Global Perspective.http://www.PreventingCLABSIs.pdf [Google Scholar]
- 19.Kilic A.U., Ahmed S.S., Alp E., Doganay M. Challenge of intensive care unitacquired infections and Acinetobacter baumannii in developing countires. OA Crit Care. 2013;1:1–5. [Google Scholar]
- 20.Singh S., Chaturvedi R., Garg S.M. Incidence of healthcare associated infection in the surgical ICU of a tertiary care hospital. Med J Armed Forces India. 2013;69:124–129. doi: 10.1016/j.mjafi.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicalini S., Palmieri F., Petrosillo N. Clinical review: new technologies for prevention of intravascular catheter-related infections. Crit Care. 2004;8:157–162. doi: 10.1186/cc2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki D.G., Kluger D.M., Crnich C.J. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 23.Safdar N., Maki D.G. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalised patients. Chest. 2005;128:489–495. doi: 10.1378/chest.128.2.489. [DOI] [PubMed] [Google Scholar]
- 24.Goldmann D.A., Pier G.B. Pathogenesis of infection related to intravascular catheterization. Clin Microbiol Rev. 1993;6:176–192. doi: 10.1128/cmr.6.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassil S.K., Crill C.M., Phelps S.J. Antimicrobial impregnated catheters in the prevention of catheter-related bloodstream infection in hospitalized patients. J Pediatr Pharmacol Ther. 2007;12:77–90. doi: 10.5863/1551-6776-12.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouza E., Alvarado N., Alcala L., Perez M.J., Rincon C., Munoz P. A randomised and prospective study of 3 procedures for the diagnosis of catheter- related bloodstream infection without catheter withdrawal. Clin Infect Dis. 2007;44:820–826. doi: 10.1086/511865. [DOI] [PubMed] [Google Scholar]
- 27.Sherertz R.J., Raad, Belani A. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grady N.P.O., Alexander M., Burns L.A. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nouwen J.L., Belkum A.V., Marie S. Clonal expansion of Staphylococcus epidermidis strains causing hickman catheter-related infections in an hemato- oncologic department. J Clin Microbiol. 1998;36:2696–2702. doi: 10.1128/jcm.36.9.2696-2702.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett S.N., McNeil M.M., Bland L.A. Postoperative infections traced to contamination of an intravenous anaesthetic, propofol. N Engl J Med. 2014;333:147–154. doi: 10.1056/NEJM199507203330303. [DOI] [PubMed] [Google Scholar]
- 31.Raad I., Hanna H., Maki D. Intravascular Catheter-related infections: advances in diagnosis, prevention, and management. Lancet. 2007;7:645–657. doi: 10.1016/S1473-3099(07)70235-9. [DOI] [PubMed] [Google Scholar]
- 32.Blot S.I., Depuydt P., Annemansh L. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591–1598. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh P.R., Chen M.L., Sun C.C. Antimicrobial drug resistance in pathogens causing nosocomial infections at a university hospital in taiwan, 1981-1999. Emerg Infect Dis. 2002;8:63–68. doi: 10.3201/eid0801.000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alangaden G.J. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin N Am. 2011;25:201–225. doi: 10.1016/j.idc.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Schaberg D.R., Culver D.H., Gaynes R.P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:3B–72S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 36.Paragioudaki M., Stamouli V., Kolonitsiou F., Anastassiou E.D., Dimitracopoulos G., Spilliopoulou Intravenous catheter infections associatedwith bacteraemia: a 2-year study in a University Hospital. Clin Microbiol Infect. 2004;10:431–435. doi: 10.1111/j.1469-0691.2004.00851.x. [DOI] [PubMed] [Google Scholar]