Summary
Although high-performance carbon materials are widely used in surface engineering, with emphasis on carbon nanotubes (CNTs), the application of CNT nanocomposites on medical surfaces is poorly documented. In this study, we aimed to evaluate the antimicrobial and anti-adhesive properties of CNT-based surfaces. For this purpose, a PRISMA-oriented systematic review was conducted based on predefined criteria and 59 studies were selected for the qualitative analysis. Results from the analyzed studies suggest that surfaces containing modified CNTs, and specially CNTs conjugated with different polymers, exhibited strong antimicrobial and anti-adhesive activities. These composites seem to preserve the CNT toxicity to microorganisms and promote CNT-cell interactions, as well as to protect them from nonspecific protein adsorption. However, CNTs cannot yet compete with the conventional strategies to fight biofilms as their toxicity profile on the human body has not been thoroughly addressed. This review can be helpful for the development of new engineered medical surfaces.
Subject areas: Microfilms, Surface Science
Graphical abstract
Microfilms; Surface Science
Introduction
Carbon nanomaterials, such as carbon nanotubes (CNTs), graphene, fullerenes, and diamond-like carbon, are an emerging class of novel materials with proven antimicrobial properties. Graphene is a single-layer sheet of sp2-hybridized carbon atoms with a honeycomb structure. It is a two-dimensional material with outstanding electrochemical properties compared with CNTs (Elmekawy et al., 2017), being one of the strongest and thinnest materials available (Güler and Bağcı, 2020). This review focuses on CNTs, which were first fabricated in 1991 (Iijima, 1991) and are formed by rolling up a single graphene sheet (single-walled carbon nanotubes, SWCNTs) or a series of concentric graphene sheets (multi-walled carbon nanotubes, MWCNTs). CNTs have a diameter in the order of nanometers (depending on the number of walls) and a length of several microns (100 μm) extendable to up to a few millimeters (about 4 mm) (Upadhyayula and Gadhamshetty, 2010).
In general, CNTs are recognized for their exceptional mechanical strength (very high values of the Young modulus), high thermal conductivity, versatile applicability of electrical properties (armchair tubes are metallic, whereas the chiral tubes are semiconducting), high surface area, excellent photoluminescence properties, and high transparency, and structural stability (Al-Jumaili et al., 2017; Upadhyayula and Gadhamshetty, 2010). These unique properties make CNTs promising nanomaterials for applications in industrial, environmental, and medical fields (Teixeira-Santos et al., 2020).
Since the beginning of the twenty-first century, CNTs have been particularly used in pharmacy and medicine as a drug delivery system. This is possible due to their high surface area that is capable of absorbing or conjugating with several therapeutic molecules and transport them directly into cells without being metabolized by the human body (He et al., 2013). Antineoplastic and antibiotic drugs were first bound to CNTs for cancer (Zhang et al., 2011) and infection treatments (Rosen and Elman, 2009), respectively, and then other linkages of biomolecules (genes, proteins, DNA, antibodies, vaccines, biosensors, cells, etc.) have been also explored for gene therapy, immunotherapy, tissue regeneration, and diagnosis of different serious diseases (He et al., 2013; Bekyarova et al., 2005). CNTs may also be promising antioxidants for health-protective effects and disease prevention in the future (Galano, 2010). Besides these main applications of CNTs, they have been shown as a powerful tool for enantiomer separation of chiral drugs and chemicals in the pharmaceutical industry, as well as for the extraction of drugs and pollutants in different media by solid-phase extraction before analysis. Additionally, CNTs have high antimicrobial activity (Maas, 2016). It has been shown that they can inhibit bacterial adhesion and biofilm formation when anchored to a surface (Malek et al., 2016; Yick et al., 2015), in suspension (Dong and Yang, 2014), or embedded in polymeric matrices forming CNT-polymer composites (Goodwin et al., 2016; Vecitis et al., 2010; Ahmed et al., 2012; Vagos et al., 2020). This CNT property is particularly important given the alarming worldwide increase in morbidity and mortality due to multidrug-resistant bacterial infections. CNTs can be more effective and cost-efficient than traditional antibiotic therapies (Mocan et al., 2017) and appear to have high potential to solve the emergence of multidrug-resistant bacteria.
A significant part of human infections is associated with the establishment of biofilms by opportunistic pathogens. Due to the increasing number of untreatable biofilms by conventional antimicrobial therapies, there is a strong need for the development of novel strategies to prevent biofilm development on biomedical surfaces. Surfaces coated with CNTs succeed in preventing bacterial adhesion and the subsequent biofilm growth on medical devices and prosthetic implants (Aslan et al., 2010; Hirschfeld et al., 2017). However, there are some concerns about the possible toxicity of CNTs in both human and animal cells, and their hydrophobic nature and biocompatibility (Saliev, 2019; Upadhyayula and Gadhamshetty, 2010). These limitations of CNTs can be reduced with their functionalization and incorporation into polymeric matrices to enhance the use of these nanomaterials on antimicrobial and anti-adhesive surfaces for medical settings.
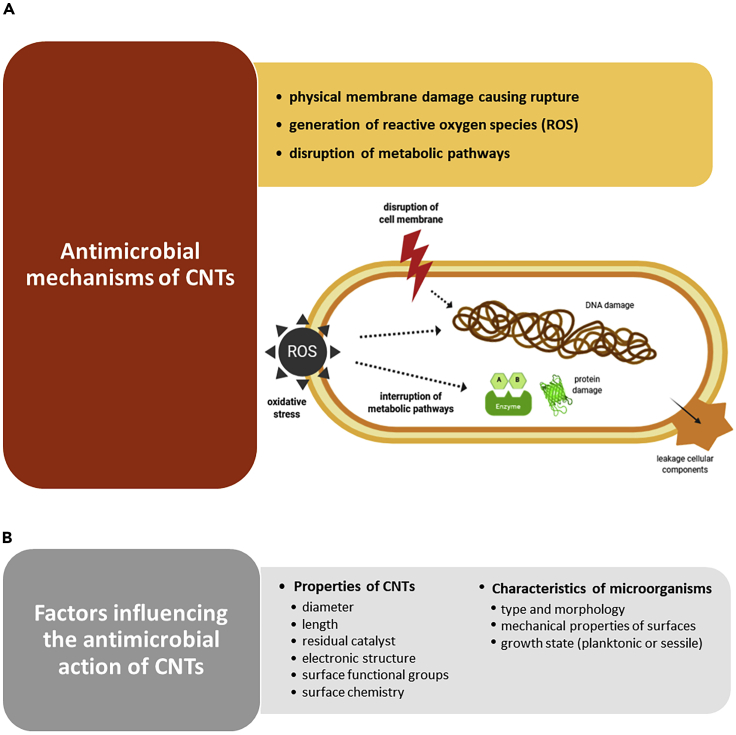
Kang et al. (2007) provided the first evidence that SWCNTs display strong antimicrobial activity and suggest that severe cell membrane damage by direct contact with SWCNTs is the likely mechanism responsible for the inactivation of Escherichia coli cells. Since then, a vast number of reports have shown that both SWCNTs and MWCNTs have powerful inhibitory effects against different microorganisms, even after short exposure times (Chen et al., 2013). Although several mechanisms have been proposed to explain the bactericidal effect of CNTs (Figure 1A), these are not fully understood. Different factors influencing the antimicrobial action of CNTs have been identified (Figure 1B), namely, the diameter, length, residual catalyst, electronic structure, surface functional group, surface chemistry, and type of CNT nanocomposites (Kang et al., 2008). In their follow-up study, Kang et al. (2008) showed that SWCNTs exhibit stronger antibacterial activity than MWCNTs, probably caused by their smaller size (diameter) that facilitates the partitioning and partial penetration into the cell wall, and by a larger surface area for contact and interaction with the bacterial surface. Besides, whereas the shorter length of CNTs may increase the chances for interaction between their open ends and a microorganism, leading to extra cell membrane damage (Aslan et al., 2010), other authors found that longer SWCNTs have stronger antimicrobial activity due to their improved aggregation with bacterial cells (Yang et al., 2010). CNTs produced not only mechanical damage and consequent cell disruption and release of intracellular content (a primary killing mechanism) but also generated oxidative stress (Kang et al., 2008). Another key factor regulating the antimicrobial efficacy of CNTs is their electronic structure (Vecitis et al., 2010). The greater bacterial cytotoxicity of metallic nanotubes compared with semiconducting nanotubes indicates that, after SWCNT-bacteria contact and physical perturbation of the cell membrane, the metallic tubes may induce the oxidation of intracellular components (Vecitis et al., 2010).
Figure 1.
Antimicrobial activity of CNTs
(A) List and schematic representation of the mechanisms of action of CNTs against bacteria. The main mechanism consists of physically piercing the microorganisms' outer membranes, leading to membrane damage and release of cellular components. Other modes of action include the generation of reactive oxygen species (ROS) and oxidative stress that can damage and destroy cellular components such as DNA and proteins, resulting in cell death.
(B) Factors that may affect the antimicrobial performance of CNTs associated with their inherent properties and the characteristics of the target microorganisms.
Apart from depending on their composition, geometry, surface modification, and intrinsic properties, the antibacterial activity of CNTs may also depend on the type and morphology of bacteria (Chen et al., 2013; Liu et al., 2009), the mechanical properties of cell surfaces (Liu et al., 2009), and the growth state (planktonic or sessile) (Rodrigues and Elimelech, 2010). The work of Chen et al. (2013) reinforced the hypothesis that CNTs cause cell death by acting mainly as “nano-darts” that pierce bacterial membranes, rather than inhibiting cell growth or oxidative stress. Bacteria with softer surfaces, including Gram-positive bacteria such as Bacillus subtilis and Staphylococcus aureus, are more vulnerable to SWCNT piercing, which results in higher cell death rates (Chen et al., 2013; Liu et al., 2009). Moreover, due to their shape, CNTs seem to have higher bactericidal action against spherical-shaped bacteria compared with rod-shaped ones (Chen et al., 2013). Regarding the growth state of bacteria, when microorganisms become protected within the structure of a biofilm, they are less susceptible to the effects of CNTs than free-floating cells. The extracellular polymeric substances (EPS) that are secreted by microbial cells in the biofilm play a key role in reducing the killing effects of CNTs (Rodrigues and Elimelech, 2010). It has been reported that the mobility associated with longer immobilized nanotubes created unstable substrates, thereby preventing bacterial settlement and biofilm growth (Malek et al., 2016). Also, vertically aligned arrays consisting of tubes much smaller than the usual size of a bacterial cell were demonstrated to reduce biofilm formation by preventing the penetration of microorganisms in between the nanotubes (Yick et al., 2015).
The overall mechanisms by which CNTs prevent biofilm development have not been widely addressed. More in-depth knowledge of how CNTs interact with biofilms is required to design robust and effective coating materials to resist biofilm formation. This study provides fundamental findings on the antimicrobial and anti-adhesive properties of CNTs and critically discusses the most important strategies and trends to guide researchers on the development of engineered surfaces containing modified CNTs for medical applications.
Results and discussion
Study selection and characterization
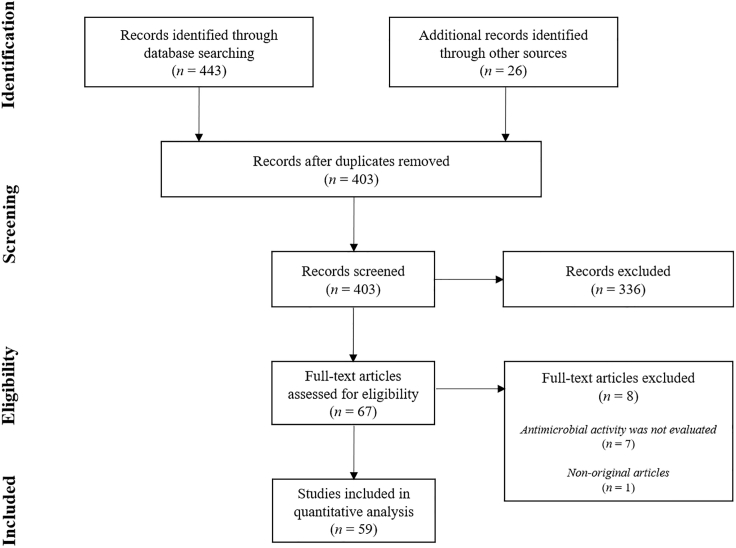
A total of 443 studies were found using the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) search methodology. After removal of duplicates, 377 studies were accepted for screening. This number was increased to 403 with the inclusion of 26 studies found by reference list searching. During the screening of titles and abstracts, 336 studies were excluded for not fulfilling the inclusion criteria and the remaining 67 studies were selected for full revision. Afterward, eight studies were excluded according to the following criteria: seven studies did not evaluate the antimicrobial activity of CNT-based films and one study was a non-original article. Therefore, 59 studies were included in the qualitative synthesis (Figure 2).
Figure 2.
Summary of the literature search based on the PRISMA flowchart (Moher et al., 2009)
CNTs found numerous applications in the medical field, having been used mainly for the development of drug delivery systems, antimicrobial therapy, and development of medical devices and implants. Because of their chemical stability, CNTs can bind with a broad diversity of therapeutic molecules, acting as vehicles for drug delivery (Nie et al., 2016; Mohamed et al., 2019). Likewise, the combination of CNTs and antimicrobial drugs revealed to be a promising strategy to combat antimicrobial resistance and develop new therapeutic options (Da Silva et al., 2019; Lee et al., 2018). Moreover, due to their intrinsic antimicrobial activity, CNTs have been widely used for the construction of medical devices and prosthetic implants (Sivaraj and Vijayalakshmi, 2018; Cho et al., 2019).
The increasing number of biofilm-related infections has motivated the development of effective strategies to prevent microbial adhesion and biofilm formation on medical surfaces (Malek et al., 2016). CNTs are attractive materials with proven antimicrobial and anti-adhesive activities against a broad range of Gram-positive and -negative bacteria, including drug-resistant microorganisms and fungi (Mohamed et al., 2019; Pangule et al., 2010; Kim et al., 2019).
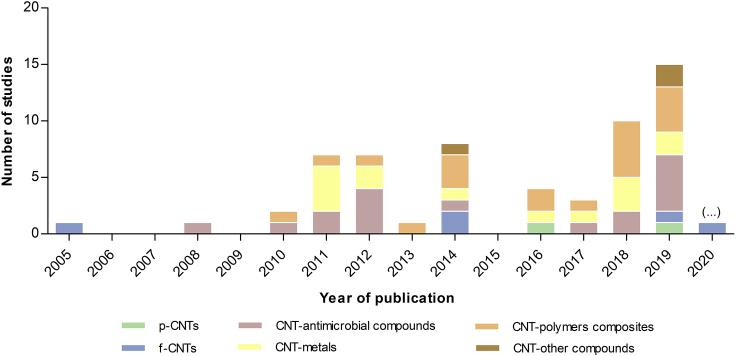
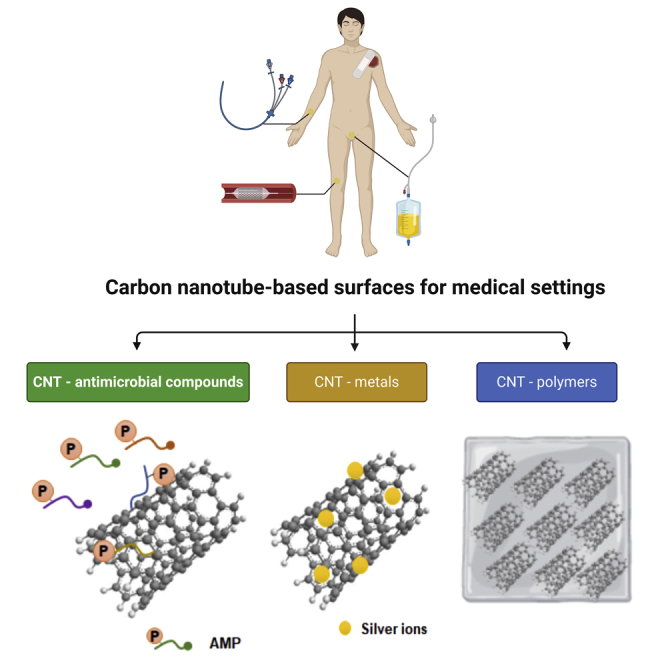
In this systematic review, 42 studies addressed the antimicrobial and anti-adhesive properties of CNT-based surfaces, whereas 17 studies referred to promising CNT composite solutions that may be immobilized. Among the selected articles, 81% studies used MWCNTs, possibly because MWCNTs have higher toxicity for microorganisms than SWCNTs (Young et al., 2012). Additionally, two studies focused on pristine carbon nanotubes (p-CNTs) and five studies explored the potential of functionalized CNTs (f-CNTs). Between the studies addressing the association of CNTs with other compounds, 17 studies explored the conjugation of CNTs with compounds displaying antimicrobial activity, 14 studies evaluated the efficacy of CNTs in association with different formulations of silver and other metals, and 19 studies addressed the efficacy of CNTs conjugated with different polymers.
Figure 3 summarizes the research progress on antimicrobial and anti-adhesive properties of CNTs and their composites with potential applications in the medical field. The increasing number of studies in recent years is notorious, especially for CNT-polymers and -antimicrobial compounds composites.
Figure 3.
Number of published studies addressing the antimicrobial and anti-adhesive properties of carbon nanotubes with application in the medical field, by year
In this review, special attention was given to the CNT-based surfaces with application in the medical field and their antimicrobial and anti-adhesive potential.
Pristine carbon nanotubes
In 2007, Kang et al. (2007) demonstrated for the first time the antimicrobial potential of p-SWCNTs, revealing that bacterial cells in contact with p-SWCNT-deposited layers suffered membrane damage and lost their viability by 80%. Since then, the antimicrobial activity of p-SWCNTs and p-MWCNTs has been demonstrated against a broad range of Gram-positive and -negative bacteria (Kang et al., 2007, 2008; Chen et al., 2013; Zhang et al., 2015; Hartono et al., 2018). However, despite the promising potential of p-CNTs, their medical application may be limited by their hydrophobic nature and cytotoxicity on human cells (Upadhyayula and Gadhamshetty, 2010), which possibly explains the lower number of studies in this field.
According to collected data, only the p-MWCNT-based surfaces have been applied in the medical field. Malek et al. (2016) showed that silicone surfaces immobilized with vertically aligned MWCNTs were able to reduce bacterial biofilm formation by up to 60%, recommending the application of these contact mechanics-based surfaces for the construction of medical devices. In 2019, Vagos et al. (2019) demonstrated that the incorporation of a small concentration of p-MWCNTs (0.1%) in a polydimethylsiloxane (PDMS) matrix was able to reduce the E. coli adhesion by 20% under hydrodynamic conditions that simulated the flow in the urinary tract, representing a step forward in the design of novel surfaces for urinary tract devices. Recently, the same authors incorporated 1% p-MWCNTs pre-treated by ball-milling in PDMS and achieved a 60% reduction of E. coli adhesion (Vagos et al., 2020). These results suggested that even at low CNT loading values, p-CNTs are promising compounds for the design of antibacterial surfaces for biomedical applications.
Functionalized carbon nanotubes
CNTs can be functionalized to increase their interaction with microbial cells and thus improve their antimicrobial potential (Arias and Yang, 2009). Moreover, CNT functionalization is an excellent approach to enhance their dispersion in different matrices, increase biocompatibility, and decrease toxicity for human cells (Upadhyayula and Gadhamshetty, 2010). Table 1 describes different f-MWCNT-based surfaces and their antimicrobial and anti-adhesive efficacy against a broad range of bacterial species.
Table 1.
Studies demonstrating the antimicrobial efficacy of functionalized carbon nanotubes with application in the medical field
| Functionalized CNTs | Type | Medical application | Species | Major conclusions | Reference |
|---|---|---|---|---|---|
| Pulsed laser deposition of graphite and bombardment of nitrogen ions | MWCNT | Medical devices |
S. aureus Staphylococcus warneri |
Staphylococcus sp. did not grow on f-MWCNT films | (Narayan et al., 2005) |
| Surface groups -COOH, -NH2 |
MWCNT | Orthopedic applications | E. coli | Bacteria exposed to f-MWCNT composites showed lower optical density compared with control (p < 0.01) | (Kumar et al., 2014) |
| Ethanolamine | MWCNT | Antimicrobial agents | A broad range of Gram-positive and -negative bacteria | Based on MIC determination, the antimicrobial activity of f-MWCNTs was higher than p-MWCNTs (2.87 ± 0.11–14.22 ± 0.17 μg/mL versus 6.12 ± 0.16–36.41 ± 0.06 μg/mL) | (Zardini et al., 2014) |
| Surface groups -COOH |
MWCNT | Medical devices |
E. coli S. aureus |
The percentage of killed bacteria in f-MWCNT membranes was over 98% | (Alizadeh et al., 2019) |
MWCNT, multi-walled carbon nanotubes; MIC, minimum inhibitory concentration.
Narayan et al. (2005) synthesized MWCNT composite films by pulsed laser ablation of graphite and bombardment of nitrogen ions. In vitro testing revealed that Staphylococcus sp. did not grow on f-MWCNT films, suggesting that these films may be useful for inhibiting microorganism attachment and biofilm formation in hemodialysis catheters and other medical devices.
The functionalization of CNTs with amine and carboxyl moieties has also been explored. Kumar et al. (2014) demonstrated that amine-functionalized MWCNT (a-MWCNT) films significantly reduced E. coli optical density compared with poly(ε-caprolactone) (PCL) control (p < 0.01). The high bactericidal activity of a-MWCNT/PCL films seems to be associated with an extensive cell membrane lysis on the composite surface induced by quaternary ammonium groups (Kumar et al., 2014). Similar evidence was reported in another study, where CNTs were functionalized with ethanolamine groups and evaluated against a broad spectrum of Gram-positive and -negative bacteria displaying higher antimicrobial activity than p-MWCNTs (Zardini et al., 2014). Amino groups present in the chains of ethanolamine have a strong antimicrobial activity due to their cationic nature that increases the interaction between CNTs and negatively charged cell walls of microorganisms (Zardini et al., 2014).
Alizadeh et al. (2019) improved the antimicrobial and anti-adhesive properties of nanoporous solid-state membranes by modification with carboxylated MWCNTs (c-MWCNTs). Results showed that the percentage of killed bacteria in f-MWCNT membranes was over 98%. Additionally, the inherent properties of c-MWCNTs increased the roughness and hydrophilicity of membranes, conferring them a high resistance to bacterial adhesion.
Altogether these results indicated that functionalized CNT surfaces can be successfully applied for the construction of medical devices and bioimplants.
Carbon nanotubes associated with compounds with antimicrobial activity
Several authors have reported the synergic association between CNTs and different compounds with antimicrobial activity. Table 2 lists studies reporting the efficacy of CNT and antimicrobial compound conjugates.
Table 2.
Studies reporting the efficacy of single- and multi-walled CNTs conjugated with compounds displaying antimicrobial activity
| CNT-antimicrobial compounds | Type | Medical application | Species | Major conclusions | Reference |
|---|---|---|---|---|---|
| Antimicrobial agents | |||||
| Cefalexin | MWCNT | Nonspecified |
B. subtilis E. coli P. aeruginosa S. aureus |
MWCNT/cefalexin films reduced bacteria viability by 50%–80% | (Qi et al., 2012) |
| AZ | SWCNT | Drug delivery | M. luteus | SWCNT/AZ composites had a significant in vitro activity against M. luteus (p < 0.05) | (Darabi et al., 2014) |
| Rifampicin | MWCNT | Implant materials | S. epidermidis | MWCNT/rifampicin-coated surfaces caused a significant inhibition of biofilm formation for up to 5 days (50%) | (Hirschfeld et al., 2017) |
| Photosensitizers | |||||
| APT | SWCNT | Nonspecified | S. aureus | A high reduction of bacterial growth (≈90%) was observed when bacteria were exposed to porphyrin-SWCNT composite | (Sah et al., 2018) |
| DTTC fluorophores | MWCNT | Nonspecified |
P. aeruginosa S. aureus |
P. aeruginosa exposed to MWNT/DTTC hybrid films and irradiated with NIR laser light were inactivated by 77% | (Oruc and Unal, 2019) |
| Antimicrobial peptides | |||||
| EP | MWCNT | Medical devices |
E. coli P. aeruginosa S. aureus |
MWCNT/EP composites killed 97.6%, 91.5%, and 88.5% of E. coli, P. aeruginosa, and S. aureus, respectively | (Zhou and Qi, 2011) |
| PLL and PGA | SWCNT | Medical devices |
E. coli S. epidermidis |
SWCNT/AMP films inactivated E. coli and S. epidermidis up to 90% | (Aslan et al., 2012) |
| Nisin | MWCNT | Antimicrobial surfaces |
B. subtilis E. coli P. aeruginosa S. aureus |
MWCNT/nisin composites showed up to 7-fold higher antimicrobial activity than p-MWCNTs. Additionally, deposited films exhibited a 100-fold higher anti-biofilm activity than the p-MWCNT deposited film | (Qi et al., 2011) |
| Enzymes | |||||
| LSZ | SWCNT | Medical devices |
M. lysodeikticus S. aureus |
Coatings terminating in SWNT/LSZ layer exhibited an antimicrobial activity of 84% | (Nepal et al., 2008) |
| Lysostaphin | MWCNT | Antimicrobial surfaces |
B. cereus E. coli MRSA S. epidermidis |
Enzyme-based composites were highly efficient in killing MRSA (> 99%) | (Pangule et al., 2010) |
| Laccase | MWCNT | Antimicrobial surfaces |
B. anthracis B. cereus E. coli S. aureus |
The laccase-CNT films showed > 99% bactericidal activity against E. coli and S. aureus, and > 98% sporicidal activity against B. anthracis and B. cereus | (Grover et al., 2012) |
APT, antimicrobial photodynamic therapy; AMP, antimicrobial peptide; APT, amine-functionalized porphyrin; AZ, azithromycin; DTTC, 3,3′-diethylthiatricarbocyanine; EP, epsilon-polylysine; LSZ, lysozyme; MWCNT, multi-walled carbon nanotubes; PGA, poly(L-glutamic acid); PLL, polyelectrolytes poly(L-lysine); MRSA, MRSA, methicillin-resistant Staphylococcus aureus; NIR, near-infrared; SWCNT, single-walled carbon nanotubes.
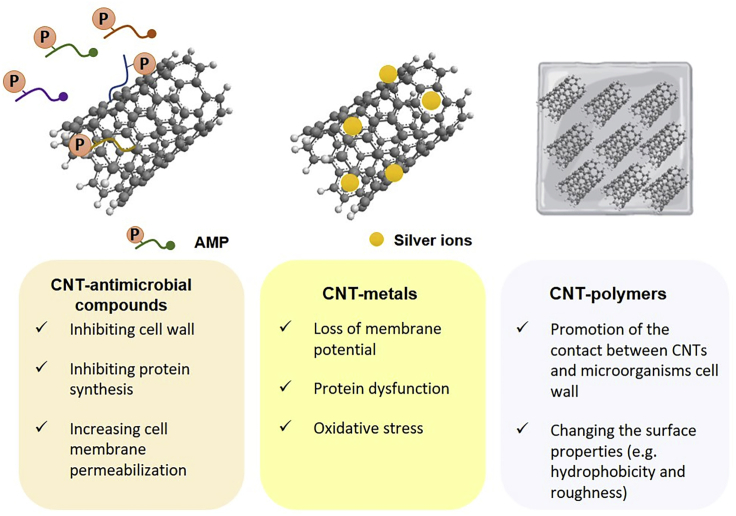
In the last decade, the combination of CNTs with antimicrobial agents received great attention as an alternative to fight antimicrobial resistance and create new therapeutic options. Qi et al. (2012) demonstrated that the covalent immobilization of cefalexin in MWCNT/poly(ethylene glycol) (PEG) films improved the antimicrobial and anti-adhesive properties of CNTs against Gram-positive and -negative bacteria, inhibiting bacterial adhesion and reducing cell viability between 50% and 80%. The conjugation of azithromycin (AZ) with SWCNTs also showed high antimicrobial activity against Micrococcus luteus. Besides, the AZ release from these SWCNT composites was confirmed, thus supporting their potential for the development of drug delivery systems (Darabi et al., 2014). Lastly, to decrease the incidence of prosthetic joint infections, Hirschfeld et al. (2017) developed titanium discs coated with MWCNTs and impregnated with rifampicin. Rifampicin-MWCNT surfaces significantly inhibited Staphylococcus epidermidis biofilm formation (approximately 50%) for up to 5 days (Figure 4).
Figure 4.
Synergic effect of antimicrobial compounds, metals, and polymers associated with carbon nanotubes
Alternative approaches to inactivate microorganisms through physical damage may solve important problems associated with colonization of material surfaces, particularly by drug-resistant microorganisms (Oruc and Unal, 2019). In this context, antimicrobial photodynamic therapy (APT) is gaining significant interest as a non-antibiotic way of inactivating microorganisms. Nanocomposites based on SWCNTs and amine-functionalized porphyrin were able to reduce S. aureus growth by 90% (Sah et al., 2018). The interaction between the porphyrin conjugated nanotubes and the bacterial cells in the presence of visible light led to cell membrane damage, improving the CNT antimicrobial activity (Sah et al., 2018). Likewise, the association of MWCNTs with near-infrared (NIR)-absorbing 3,3′-diethylthiatricarbocyanine fluorophores (DTTC) inactivated 77% bacteria through laser-activated heat generation (Oruc and Unal, 2019). The efficacy of MWCNT/DTTC nanohybrids was improved when they were embedded in a waterborne polyurethane matrix. The resulting surface presented temperatures reaching 120°C upon 2 min of laser irradiation, killing all Pseudomonas aeruginosa cells attached to the surface (Oruc and Unal, 2019). These results support the application of APT in the development of antimicrobial and anti-adhesive surfaces.
In the last years, antimicrobial peptides (AMPs) have emerged as contact-killing coatings for diverse medical surfaces and are currently one of the most promising alternatives to conventional antimicrobial agents. Zhou and Qi (2011) synthesized a novel antimicrobial nanocomposite by the covalent attachment of epsilon-polylysine (MEPs) on MWCNT surfaces. The deposited film of MEPs showed high antibacterial activity against E. coli (97.6%), P. aeruginosa (91.5%), and S. aureus (88.5%), as well as excellent anti-adhesive activity (Zhou and Qi, 2011). It is known that epsilon-polylysines display high stability, low toxicity, and broad biocidal activity and are one of the best AMPs for covalent incorporation (Shih et al., 2006). In another study, Aslan et al. (2012) investigated the antimicrobial activity of SWCNTs layer-by-layer assembled with poly(L-lysine) (PLL) and poly(L-glutamic acid) (PGA). The growth of E. coli and S. aureus was significantly inhibited (up to 90%) upon 24 h incubation with SWCNT-based films compared with control (20%), suggesting the potential usefulness of SWCNT/PLL and/PGA films as antimicrobial biomaterials (Aslan et al., 2012). Also, the immobilization of nisin (a natural AMP) with PEG as a linker enhanced the antimicrobial and anti-adhesive properties of MWCNTs (Qi et al., 2011). MWCNT/nisin composite showed up to 7-fold higher antimicrobial activity against E. coli, P. aeruginosa, S. aureus, and B. subtilis, and a 100-fold higher antibiofilm activity than p-MWCNT control film (Qi et al., 2011). These excellent results may be due to the strong antimicrobial activity of nisin, which interferes with cell wall synthesis, increasing cell membrane permeabilization (Peschel and Sahl, 2006). Overall, CNT/AMP composites display strong antimicrobial and anti-adhesive activities (Figure 4), with the advantage of being less prone to the development of antimicrobial resistance.
Cell lytic enzymes have also emerged as potential alternatives to antimicrobial agents in combating resistant pathogens. Nepal et al. (2008) demonstrated that SWCNT/lysozyme films produced by layer-by-layer assembly exhibited high antimicrobial activity against Micrococcus lysodeikticus and S. aureus (Nepal et al., 2008). Likewise, Pangule et al. (2010) produced antimicrobial surfaces by incorporating CNT/lysostaphin conjugates and demonstrated that these enzyme-based composites were highly efficient in killing methicillin-resistant Staphylococcus aureus (> 99% within 2 h). These results indicated that the conjugation of CNTs with cell wall-degrading enzymes can be an advantageous approach to prevent biofilm formation on medical surfaces. Moreover, enzymes able to catalyze halide oxidation may also act as potent biocides. Grover et al. (2012) immobilized laccase and chloroperoxidase separately onto MWCNT surfaces and mixed with a commercial paint to generate biocatalytic coatings. These enzyme-nanotube formulations showed more than 99% bactericidal activity against E. coli and S. aureus and 98% sporicidal activity against Bacillus anthracis and Bacillus cereus (Grover et al., 2012). In general, these results suggest that enzyme-CNT composites have a promising self-decontamination activity with application in a wide range of medical surfaces.
Carbon nanotubes associated with silver and other metals
Up to date, several studies have reported the effectiveness of silver and other metal-coated films in the prevention of biofilm-related infections. Table 3 describes the studies reporting the efficacy of CNTs conjugated with metals.
Table 3.
Studies reporting the efficacy of single- and multi-walled CNTs conjugated with silver and other metals
| CNT-metals | Type | Medical application | Species | Major conclusions | Ref. |
|---|---|---|---|---|---|
| AgNPs stabilized with DNA | SWCNT | Drug delivery | A broad range of Gram-positive and -negative bacteria | The bactericidal ratio of the AgNP-nanofilm against the major pathogenic organisms was between 57.78% and 78.89% | (Subbiah et al., 2011) |
| AgNPs | MWCNT | Medical devices |
E. coli S. epidermidis |
Bacteria viability on the Ag-CNT films decreased by 32% and 13% for S. epidermidis and E. coli, respectively | (Jung et al., 2011) |
| SWCNT | Nonspecified | E. coli | The number of bacteria colonies decreased by 4 log in the presence of Ag-CNT hybrid films | (Misra et al., 2012) | |
| MWCNT | Medical implants |
E. coli S. aureus |
Polymer shielded with AgNP-CNT inactivated E. coli and S. aureus by 89.3% and 95%, respectively, and reduced the number of adhered bacteria by 80% for both species | (Nie et al., 2017) | |
| MWCNT | Medical devices |
K. pneumoniae S. aureus |
The Ag- plasma polymer fluorocarbon nanocomposite films suppressed the growth and proliferation of bacteria by up 92.2% compared with uncoated films | (Cho et al., 2019) | |
| Zn-HA | MWCNT | Bioimplants |
B. subtilis E. coli S. aureus Shigella flexneri |
Bacterial growth was clearly inhibited by Zn-HA-MWCNT (inhibition zone ≥12 mm) | (Sivaraj and Vijayalakshmi, 2018) |
| PdNPs | MWCNT | Nonspecified |
B. subtilis E. coli K. pneumoniae P. aeruginosa |
PdNP-MWCNT composites deposited in a polypyrrole matrix showed 65–81.71% biofilm inhibition | (Murugesan et al., 2018) |
AgNPs, silver nanoparticles; MWCNT, multi-walled carbon nanotubes; PdNPs, palladium nanoparticles; SWCNT, single-walled carbon nanotubes; Zn-HA, Zinc-hydroxyapatite.
Subbiah et al. (2011) produced a “bio-nanofilm” composed of silver nanoparticles (AgNPs) coated on SWCNT surfaces, which were later hybridized with DNA and stabilized in poly(vinyl alcohol). This bio-nanofilm displayed high antimicrobial activity (58%–79%) against a broad range of Gram-positive and -negative bacteria (Subbiah et al., 2011). Ever since numerous studies have described the potential of AgNP-CNT nanocomposites as a coating for medical devices and implants (Jung et al., 2011; Misra et al., 2012; Nie et al., 2017; Cho et al., 2019). Data showed that these composites reduced bacterial adhesion by 80% (Nie et al., 2017) and the viability of adhered bacteria up to 32% for S. epidermidis (Jung et al., 2011), 92% for E. coli and Klebsiella pneumoniae (Cho et al., 2019), and 95% for S. aureus (Nie et al., 2017). These results demonstrated that the inherent antimicrobial activity of CNTs was enhanced by their association with AgNPs (Figure 4). Indeed, the mechanisms of action of silver are already well characterized and include loss of membrane potential, protein dysfunction, and oxidative stress (Zhu et al., 2019).
The association of CNTs with other metals also seems to generate promising results. Sivaraj and Vijayalakshmi (2018) developed a hybrid zinc-hydroxyapatite/MWCNT film to improve the antibacterial activity and corrosion resistance of stainless-steel implants. This hybrid composite clearly inhibited the bacterial growth, possibly because Zn ions have a bacteriostatic activity, changing cellular permeability and causing cell dysfunction (Sivaraj and Vijayalakshmi, 2018). Similarly, the modification of MWCNT surfaces with palladium nanoparticles resulted in a composite film with high antibiofilm activity (65%–82%) (Murugesan et al., 2018). The high bacterial biofilm reduction seems to be caused by reactive oxygen species (ROS) production resulting from the catalytic behavior of palladium, which destroys the biofilm structure (Murugesan et al., 2018) (Figure 4). Overall, these data indicate that the association of CNTs and metals successfully prevents the microbial adhesion on medical surfaces.
Carbon nanotubes conjugated with different polymers
Several studies involving the use of CNTs as fillers in polymer matrices have been developed with the intent to produce nanocomposites with improved antimicrobial and antifouling activity. Moreover, the use of polymers during the fabrication of CNT composites allow (1) the improvement of structural, mechanical, and degradation properties of the final composite; (2) increment of composite hydrophilicity and stability in biological environments; and (3) the lower final cost (the use of pure CNT materials/surfaces is more expensive) (Aslan et al., 2010; Liu et al., 2007). With the considerable number of available polymers, a set of versatile and multifunctional biomaterials can be produced. Table 4 reports the efficacy of SWCNT and MWCNT/polymer composites against different microorganisms.
Table 4.
Studies reporting the efficacy of single- and multi-walled CNTs conjugated with polymers
| CNT-Polymer composites | Type | Medical application | Species | Major conclusions | Reference |
|---|---|---|---|---|---|
| PLGA | SWCNT | Medical devices |
E. coli S. epidermidis |
Up to 98% bacteria were inactivated on SWCNT/PLGA versus 15%–20% on pure PLGA | (Aslan et al., 2010) |
| PEI | MWCNT | Medical devices | E. coli | Almost no adhered E. coli were found on the superhydrophobic poly(dopamine)-modified CNT/PEI films (p < 0.05) | (Wang et al., 2014) |
| Poly(ester amide) | MWCNT | Wound dressing |
B. subtilis E. coli K. pneumonia Mycobacterium smegmatis S. aureus |
Nanocomposites exhibited high antibacterial efficacy against B. subtilis (2–3 log reduction) and S. aureus (2 log reduction), when compared with E. coli and K. pneumonia (1 log reduction) | (Pramanik et al., 2014) |
| PEG | MWCNT | Wound dressing | E. coli | CNT/PEG-grafted polyurethane/electrospun nanofibers reduced adherent bacteria by 85.3% | (Shi et al., 2016) |
| MWCNT | Tissue engineering of bone or cartilage | Gram-positive and -negative bacteria | The inhibition zone for drug-loaded c-MWCNT-PEG/gelatin-CS nanocomposite was higher against all the bacterial species compared with drug-loaded c-MWCNT/gelatin-CS composite | (Sharmeen et al., 2018) | |
| PE | MWCNT | Nonspecified |
M. smegmatis Pseudomonas fluorescens |
After 56 days of biofilm growth, the number of cells for the 4% MWCNT/PE composites decreased by 89.3% for P. fluorescens and 29.0% for M. smegmatis compared with the pristine PE | (Jing et al., 2018) |
| Lignin in PVA | MWCNT | Wound healing | S. aureus | After 18 h of incubation with the lignin/PVA and lignin/t-MWCNT/PVA NFs, bacterial growth decreased by 60% and 69%, respectively, compared with the control | (Lee et al., 2018) |
| PMMA | MWCNT | Dental biomaterials |
Candida albicans S. aureus Streptococcus mutans |
Compared with the PMMA control group, the CNT/PMMA composites showed a significant decrease in microbial adhesion (35%–95% less adhesion) by all three microbial species | (Kim et al., 2019) |
| PNIPAM hybrid brush | SWCNT | Nonspecified | E. sibiricum | CNT/PNIPAM films showed > 85% bacterial inactivation | (Pangilinan et al., 2013) |
| PVK | MWCNT | Nonspecified | E. coli | The MWCNT/PVK- and MWNT-modified substrates inhibited bacterial growth by up to 83% and 87%, respectively | (Santos et al., 2012) |
| Polypyrrole | MWCNT | Wound dressing |
E. coli S. aureus |
The anti-biofilm activity is field-dependent, reaching a reduction of 40% for E. coli and 90% for S. aureus | (Da Silva et al., 2019) |
| CS | MWCNT | Nonspecified |
Candida tropicalis E. coli S. aureus |
CNT/CS hydrogels showed a strong antimicrobial activity (∼1 log reduction, except for Gram-negative bacteria) | (Venkatesan et al., 2014) |
| MWCNT | Drug delivery |
E. coli S. aureus |
Bacterial killing efficacy of Ag-MWCNT/biopolymer composites was above 80% | (Nie et al., 2016) | |
| MWCNT | Nonspecified |
E. coli S. aureus |
MWCNT/CMCS biocomposites exhibited high inhibition zone diameters: 22.3 ± 0.21 mm against S. aureus and 21.3 ± 0.72 mm against E. coli | (El-Ghany, 2017) | |
| MWCNT | Nonspecified |
Aspergillus niger Cryptococcus neoformans C. tropicalis E. coli Enterococcus faecalis S. epidermidis |
MWCNT/aminohydrazide cross-linked CS composites displayed high antimicrobial activity as demonstrated by their inhibition zones for bacteria (≥18.4 ± 0.20 mm) and fungi (≥17.2 ± 0.45 mm) | (Mohamed and Abd El-Ghany, 2018) | |
| MWCNT | Nonspecified |
A. niger C. neoformans C. tropicalis E. coli E. faecalis S. epidermidis |
MWCNT/aminosalicylhydrazide cross-linked CS composites displayed high antimicrobial activity as judged by their inhibition zones for bacteria (> 19.8 ± 0.20 mm) and fungi (> 20.1 ± 0.20 mm) | (Mohamed and Abd El-Ghany, 2019) | |
| MWCNT | Nonspecified | Gram-positive and -negative bacteria; Fungi | MWCNT/trimellitic anhydride isothiocyanate cross-linked CS hydrogel composites showed high antimicrobial activity as demonstrated by their inhibition zones for bacteria (≥16.7 ± 0.50 mm) and fungi (≥18.4 ± 0.63 mm) | (Bellingeri et al., 2018) | |
| MWCNT | Drug delivery | S. aureus | The number of adhered cells in the hydrogel decreased sharply (4 log reduction) by the incorporation of CS-MWCNT | (Mohamed et al., 2019) |
CS, chitosan; CMCS, carboxymethyl chitosan; MWCNT, multi-walled carbon nanotubes; PE, polyethylene; PEG, Polyethylene glycol; PEI, poly(ethyleneimine); PLGA, poly(lactic-co-glycolic acid); PVA, poly(vinyl alcohol); PMMA, poly(methyl methacrylate); PNIPAM, poly(N-isopropylacrylamide); PVK, poly(N-vinylcarbazole); SWCNT, single-walled carbon nanotubes.
Among all the studies included in this systematic review, only two refer to the antimicrobial properties of SWCNT/polymer composites. However, in both cases, significant bacterial inactivation was achieved. Aslan et al. (2010) reported an inactivation rate of E. coli and S. epidermidis of up to 98% after incubation with SWCNT/poly(lactic-co-glycolic acid) composites, whereas Pangilinan et al. (2013) demonstrated a decrease in the viability of Exiguobacterium sibiricum of about 85% after cell incubation with temperature-responsive c-CNT/poly(N-isopropylacrylamide) hybrid brush films.
Concerning the MWCNTs, several studies have described their application in the production of composites that substantially hinder biofilm growth. The fact is, whether through the use of thin organic films of MWCNT/poly(N-vinylcarbazole), MWCNT/polyethylene composite disks, or the application of drug-loaded MWCNT-PEG/gelatin-chitosan nanocomposites, significant cell viability losses associated with Gram-negative and -positive bacteria could already be achieved (Santos et al., 2012; Jing et al., 2018; Sharmeen et al., 2018). Another study also demonstrated good antimicrobial properties of electrospun nanofibers consisting of lignin-decorated thin MWCNTs (t-MWNTs) incorporated in poly(vinyl alcohol) (Lee et al., 2018). In a different approach, Da Silva et al. (2019) evaluated the antibacterial activity of electrically charged MWCNT/polypyrrole (PPy) composites, and the results have shown a decrease in the order of 40% for E. coli and 90% for S. aureus. In this case, the use of the polymer PPy and the application of the electric field brought an added value to the fabrication of antimicrobial composites. If on the one hand, PPy has an intrinsic antibacterial activity (Da Silva et al., 2019; Varesano et al., 2009), on the other hand, the electric field has already proved its worth in the intensification of the antibacterial activity (Del Pozo et al., 2008; Ronen et al., 2015; Zhang et al., 2014).
Early attempts to repress the initial adhesion of pathogens to the material surface have also been reported. Wang et al. (2014) designed thin organic films through the application of layer-by-layer assembly of poly(dopamine)-modified CNTs and poly(ethyleneimine), and the resulting composites showed effective inhibition of platelet adhesion and activation, along with excellent resistance against E. coli adhesion. Later studies confirmed the previous findings regarding the antifouling activity of MWCNT/polymer composites, either by incorporating carboxylated MWCNTs into poly(methyl methacrylate) (Kim et al., 2019) or through the dispersion of chitosan-decorated MWCNTs into a copolymer matrix of acrylamide and acrylic acid (Bellingeri et al., 2018).
The incorporation of MWCNTs into chitosan has been increasingly explored for the production of potentially useful antimicrobial surfaces. An essential contribution to this is the unique physicochemical properties of chitosan that offer the final composite a huge potential in different biomedical applications, including wound dressing, tissue engineering, biosensing, and drug delivery (El-Ghany, 2017). In spite of the inherent antibacterial activity of chitosans against bacteria and fungi (Kong et al., 2010), the incorporation of MWCNTs constitutes an added value to the production of composites with improved antimicrobial activity (Venkatesan et al., 2014). In a study combining Ag-loaded oxidized MWCNTs with chitosan, a significant antimicrobial action against both Gram-positive and -negative bacteria was observed (with a bacterial killing efficacy of 80% and 100% for nanocomposite concentrations of 5 and 10 μg/mL, respectively) (Nie et al., 2016).
Other authors have demonstrated the importance of the use of chemically modified chitosans and functionalized MWCNTs on the antimicrobial activity of the resulting nanocomposites. In a study carried out by El-Ghany (2017), the incorporation of MWCNTs in a matrix of carboxymethyl chitosan (CMCS, a water-soluble derivative of chitosans) showed significant antibacterial activity, as demonstrated by the inhibition zone diameters and the minimum inhibitory concentrations of the composite. Compared with CMCS, MWCNT/CMCS composites with different MWCNT loading presented higher inhibition zone diameters ranging from 17.3 ± 0.12 to 22.3 ± 0.21 mm and 14.3 ± 0.58 to 21.3 ± 0.72 mm for S. aureus and E. coli, respectively (El-Ghany, 2017). Furthermore, it was reported that aminohydrazide and aminosalicylhydrazide-cross-linked chitosan hydrogels filled with MWCNTs present stronger antimicrobial activity against different microorganisms (Mohamed et al., 2019; Mohamed and Abd El-Ghany, 2019). In fact, they exhibited equal or even better antimicrobial activity than the standard antimicrobial agents, a consequence of the synergistic action between MWCNTs and the hydrazide/hydrazone derivatives (Mohamed and Abd El-Ghany, 2018; Mohamed and Abd El-Ghany, 2019). Mohamed et al. (2019) have also recently shown that by combining MWCNTs with chitosan and functionalized groups of trimellitic anhydride isothiocyanate (the incorporated cross-linker) in the same system, the original antimicrobial properties of the polymer can be enriched. In general, MWCNT/chemically modified chitosan composites were more effective against Gram-positive bacteria than Gram-negative ones (Venkatesan et al., 2014; El-Ghany, 2017; Mohamed and Abd El-Ghany, 2018; Mohamed and Abd El-Ghany, 2019; Mohamed et al., 2019).
Other research studies combined the antimicrobial and anti-adhesive properties of CNT/polymer composites (Shi et al., 2016). Pramanik et al. (2014) fabricated f-MWCNT/hyperbranched poly(ester amide) (HBPEA) thin films capable of efficiently reduce the cell viability of different species of bacteria. The bio-based f-MWCNT/HBPEA nanocomposites exhibited pronounced antibacterial efficacy against Gram-positive bacteria when compared with the Gram-negative ones, possibly due to the release of a significant amount of cytoplasmic constituents from the Gram-positive cells. A decrease in cell adhesion to the films with the enhancement of the f-MWCNT content was also observed. Additionally, the importance of the use of PEG into CNT biomaterials was addressed by Shi et al. (2016), who employed a chemical grafting modification technique for the preparation of thermoplastic polyurethane (TPU)-PEG electrospun nanofibers (TPU-g-PEG nanofibers). MWCNT/TPU-g-PEG nanofibers showed to combine both bactericidal and bacteria-repelling strategies (with a total reduction of 85.3% on E. coli adhesion) (Shi et al., 2016). Overall, these two properties can be enhanced by increasing the CNT loading (Aslan et al., 2010; Schiffman and Elimelech, 2011; Goodwin et al., 2015). The role of polymer matrices on the overall antimicrobial activity of CNT/polymer composites can be attributed not only to the preservation of the inherent cytotoxicity of SWCNTs and MWCNTs (Goodwin et al., 2015) but also to the promotion of the contact between CNTs and microorganism cell walls by reducing CNT autoaggregation (Mohamed et al., 2019) (Figure 4). Regarding the anti-adhesive effect of CNT/polymer composites, it seems to be strictly related with the uniform layer of polymer that surrounds the CNT surface and protect them from nonspecific protein adsorption, as well as with the CH-π interactions established between the polymer and the CNT surface (Upadhyayula and Gadhamshetty, 2010; Beigbeder et al., 2008), and the topographic structure of the CNT/polymer composite surface (rough surfaces revealed to be more vulnerable to microbial adhesion than smooth surfaces) (Kim et al., 2019; Schubert et al., 2019; Dantas et al., 2016).
Promising carbon nanotube composites
In this review, solutions of CNT composites with proved antimicrobial activity were addressed as promising materials for surface immobilization (Table 5). Recently, Murugesan et al. (2020) reported for the first time the application of heteroatom (NFB/P) doped-MWCNT nanocomposites to combat biofilms associated with wound healing. When compared with p-MWCNT, NFB-MWCNT and NFP-MWCNT revealed greater effectiveness against K. pneumoniae, P. aeruginosa, E. coli, and B. subtilis, as well as exceptional healing ability in Wistar rats. The capacity to inhibit biofilm formation is possibly related to the surface charge created by the different electronegativity between the heteroatoms. This, together with the mineralization provided by the presence of different atoms, helps in the formation of new tissue in the wound-healing process (Murugesan et al., 2020).
Table 5.
Promising CNT composite solutions for medical applications
| CNT composites | Type | Medical application | Species | Major conclusions | Reference |
|---|---|---|---|---|---|
| Functionalized | |||||
| Heteroatoms (N, F, P/B) | MWCNT | Wound healing |
K. pneumoniae P. aeruginosa E. coli B. subtilis |
f-MWCNT showed 82.53%, 80.98%, 76.83%, and 77.41% biofilm inhibition against B. subtilis, E. coli, K. pneumoniae, and P. aeruginosa, respectively | (Murugesan et al., 2020) |
| Compounds displaying antimicrobial activity: | |||||
| Surfactants | |||||
| Sodium cholate | SWCNT | Antimicrobial agents |
E. coli S. enterica |
Antibacterial effect increased with increasing SWCNT concentration as demonstrated by the optical density reduction from 0.8 to 0.4 | (Dong et al., 2012) |
| Photosensitizers | |||||
| Rose Bengal (RB) (APT) |
MWCNT | Antimicrobial agents | E. coli | RB-CNT-mediated photodestruction resulted in 5.46 log reduction for E. coli | (Anju et al., 2018) |
| Malachite green (MG) (APT) |
MWCNT | Medical devices |
P. aeruginosa S. aureus |
Upon MG-c-MWCNT treatment, S. aureus and P. aeruginosa viability was reduced by 5.16 and 5.55 log, respectively | (Anju et al., 2019a) |
| Methylene blue (MB) (APT) |
MWCNT | Medical devices |
E. coli S. aureus |
Photodynamic activation of MB-c-MWCNT resulted in 4.86 and 5.55 log reductions in E. coli and S. aureus viability, respectively | (Parasuraman et al., 2019) |
| Toluidine blue (TB) (APT) |
MWCNT | Medical devices |
P. aeruginosa S. aureus |
P. aeruginosa and S. aureus planktonic cells treated with TB-c-MWCNT and exposed to light irradiation reduced their viability by 4.91 and 5.47 log, respectively | (Anju et al., 2019b) |
| Antimicrobial peptides | |||||
| AMPs: TP359, TP226, and TP557 | SWCNT | Antimicrobial agents | S. aureus | Non-treated 3D skin showed 4 log CFU/g increase in 2 h after incubation with bacteria, whereas the f-SWCNT-s-Ag-treated skin showed only 1 log CFU/g increase in bacterial counts | (Chaudhari et al., 2019) |
| Metals: | |||||
| Silver nanoparticles-deposited f-CNT with an amphiphilic poly(propyleneimine) dendrimer (MWCNTs-APPI-AgNPs) |
MWCNT | Drug delivery Bioimaging Medical devices |
B. subtilis E. coli S. aureus |
f-MWCNTs-AgNPs showed an inactivation percentage of 99.8% ± 0.2%, 99.7% ± 0.1%, and 93.1% ± 0.5%, for B. subtilis, S. aureus, and E. coli, respectively | (Murugan and Vimala, 2011) |
| Silver sulfide quantum dots (Ag2S) | MWCNT | Antimicrobial surfaces |
E. coli P. aeruginosa S. aureus |
The bacteria killing ability of f-MWCNT-Ag2S was 97.8% ± 2.1%, 78.5% ± 2.9%, and 55.7% ± 1.5% for E. coli, P. aeruginosa, and S. aureus, respectively | (Neelgund et al., 2012) |
| AgNP-decorated c-CNT | MWCNT | Nonspecified |
Methylobacterium spp. Sphingomonas spp. |
Ag-MWCNT (40 or 50 μg/mL) completely inhibited bacterial growth | (Seo et al., 2014) |
| Silver and copper nanoparticles | MWCNT | Medical devices | E. coli | Ag-MWCNT and Cu-MWCNT exhibited 97% and 89% growth inhibition against E. coli, respectively | (Mohan et al., 2011) |
| Copper nanoparticles | MWCNT | Nonspecified | Gram-positive and -negative bacteria; Fungi | Cu-MWCNT showed an enhanced inhibitory effect when compared with MWCNT | (Yallappa et al., 2016) |
| Titanium oxide-gold (TiO2-Au) | MWCNT | Drug delivery | A broad range of Gram-positive and -negative bacteria | More than 90% biofilm inhibition was observed in the presence of TiO2-Au-MWCNT | (Karthika et al., 2018) |
| Titanium dioxide (TiO2) | MWCNT | Antimicrobial agents |
E. coli S. aureus |
TiO2-MWCNT displayed high antimicrobial activity against both bacteria, as demonstrated by the diameter of inhibition zones (≥18 mm) | (Sukkar et al., 2019) |
| Cadmium quantum dots (CdS) | MWCNT | Antimicrobial agents |
E. coli P. aeruginosa S. aureus |
The bacteria-killing ability of f-MWCNT-CdS was 87.2% ± 4.1%, 68.9% ± 2.5%, and 46.7% ± 1.4% against E. coli, P. aeruginosa, and S. aureus, respectively | (Neelgund et al., 2012) |
| Polymers: | |||||
| Amphiphilic poly(propyleneimine) Dendrimer (APPI) |
MWCNT | Drug delivery Bioimaging Medical devices |
B. subtilis E. coli S. aureus |
MWCNTs-APPI hybrid inhibited bacterial growth by 96.6% ± 0.3%, 96.5% ± 0.2%, and 87% ± 0.5% for B. subtilis, S. aureus, and E. coli, respectively | (Murugan and Vimala, 2011) |
| Other compounds: | |||||
| Antibodies to group A Streptococcus (GAS) | MWCNT | Antimicrobial agents | Streptococcus pyogenes | GAS-MWCNT induced 97%–100% killing of planktonic cells, depending on the time of laser exposure, and 99.99% killing of bacteria in biofilm | (Levi-Polyachenko et al., 2014) |
| Pyrazole and pyrazolone derivates | MWCNT | Antimicrobial agents |
S. aureus B. subtilis E. coli C. albicans A. niger |
The antibacterial activity of MWCNTs conjugated with pyrazole derivates ranged between 17.5% and 95.2% | (Metwally et al., 2019) |
| Mannose derivates | SWCNT | Anti-adhesive agents | Uropathogenic E. coli |
Mannose derivate-SWCNT induced a considerable reduction in the CFU (around 50%) when compared with the control | (Romero-Ben et al., 2019) |
APT, antimicrobial photodynamic therapy; AMP, antimicrobial peptide; MWCNT, multi-walled carbon nanotubes; SWCNT, single-walled carbon nanotubes; CFU, colony forming unit.
The dispersion of SWCNTs in a surfactant solution of sodium cholate, for example, has shown to exhibit an antibacterial effect against both Salmonella enterica and E. coli (Dong et al., 2012). The importance of the use of surfactants to disperse CNT relies on the capacity of these compounds to efficiently disperse bundled CNTs into suspensions of individual CNTs (Moore et al., 2003).
Another interesting approach is the employment of APT, an alternative method of killing pathogens and their biofilms through the generation of ROS. Anju et al. (2018) reported for the first time an efficient photodestruction of E. coli biofilms through the combination of rose Bengal (RB) with MWCNTs. After irradiation, the reduction in the biofilm formation by RB and RB-MWCNT conjugates was measured to be 42.1% ± 1.7% and 64.9% ± 2.9%, respectively; a reduction in the EPS production of RB-MWCNT-treated cells was also observed (about 50.2% ± 2.0%). RB and RB-MWCNT photodynamic inactivation of planktonic cells of E. coli has shown a reduction of 3.6 and 5.5 log, respectively. APT mediated by malachite green (cationic dye of the triarylmethane family) and methylene blue (cationic phenothiazine dye)/MWCNT composites also proved to be efficient against planktonic and biofilm cells of Gram-positive and -negative bacteria (Anju et al., 2019a; Parasuraman et al., 2019). For the same radiant exposure (58.5 J/cm2), toluidine blue/MWCNT conjugates showed to be more efficient in the inactivation of P. aeruginosa (69.9%) and S. aureus (75.5%) biofilms compared with the remaining photosensitizers (Anju et al., 2019b). It should be noted that enhanced photoinactivation was observed in Gram-positive bacteria compared with Gram-negative ones.
AMP-functionalized silver-coated SWCNTs (f-SWCNT-s-Ag) represent another promising alternative to conventional antimicrobial agents. Using a 3D skin model, Chaudhari et al. (2019) have recently shown that bacterial proliferation in f-SWCNT-s-Ag-treated skin was significantly inhibited (105 CFU/g) compared with non-treated skin (108 CFU/g). SWCNTs' activity was complemented by the synergic action of Ag nanoparticles and AMPs, the latter being well known for their antimicrobial activity against both Gram-negative and -positive bacteria, fungi, and viruses (Zhu et al., 2019). The use of titanium, silver, and copper-coated MWCNTs constitutes another potential alternative for the available antimicrobial composite surfaces (Mohan et al., 2011; Yallappa et al., 2016; Sukkar et al., 2019). Along with the complete inhibition of Methylobacterium spp. and Sphingomonas spp. after treatment with silver nanoparticle (AgNP)-decorated carboxylated MWCNTs (Seo et al., 2014), the literature reports high inactivation rates of E. coli, B. subtilis, and S. aureus (> 90%) after cell incubation with AgNP-deposited MWCNTs functionalized with an amphiphilic poly(propyleneimine) dendrimer (Murugan and Vimala, 2011). Likewise, more than 90% biofilm inhibition was observed after incubation of Shigella dysenteriae, Proteus vulgaris, K. pneumoniae, Streptococcus pneumoniae, B. subtilis, S. aureus, and C. albicans with titanium oxide-gold (Karthika et al., 2018). Silver sulfide and cadmium sulfide quantum dots immobilized on poly(amidoamine)-grafted MWCNTs have also shown to efficiently reduce the viability of E. coli, P. aeruginosa, and S. aureus (Neelgund et al., 2012).
Other compounds that are known to exhibit excellent antibacterial and antibiofilm activities when combined with MWCNTs include the antibodies to group A Streptococcus (GAS), pyrazole and pyrazolone derivates, and mannose derivatives (Levi-Polyachenko et al., 2014; Romero-Ben et al., 2019; Metwally et al., 2019).
Altogether, these data provide important findings that should be considered in the development of new antimicrobial and anti-adhesive CNT-based surfaces.
Cytotoxicity of carbon nanotubes on human cells
Cytotoxicity is one of the major drawbacks of CNTs and is the main obstacle for their translation into medical applications. As such, the clinical usage of CNTs and their composites have not yet been approved by the US Food and Drug Administration or equivalent organizations (Kim et al., 2019). In the literature, there is still little consensus about this topic, with some studies revealing toxicity, whereas others showed different levels of biocompatibility. Indeed, because SWCNT and MWCNT have their own structures and features, they can exert different toxicity on human cells (Zeinabad et al., 2016). CNT cytotoxicity is influenced by various factors including diameter, length, purity, functionalization moieties, and concentration (Karthika et al., 2018; Kavosi et al., 2018).
Several in vivo and in vitro studies have demonstrated that CNTs cause cytotoxic effects via oxidative stress induction (Kayat et al., 2011). A panoply of CNT side effects, from persistent or transient pulmonary inflammation, cardiovascular diseases, to liver and spleen inflammation, have been demonstrated using animal models and have been associated with their structure and concentration (Kavosi et al., 2018).
Although functionalization could indeed help to reduce CNTs' toxicity to human cells (Chaudhari et al., 2019), not all treatments decrease the toxicity risks and only reactions that can render CNTs short and stable without aggregation in the human fluids can provide safe results (Shi et al., 2016).
Based on the reviewed studies, the conjugation of CNTs with silver or titanium plays an important role in decreasing CNTs' toxicity (Subbiah et al., 2011; Seo et al., 2014; Karthika et al., 2018; Chaudhari et al., 2019). Likewise, the incorporation of CNTs into polymeric matrices was also effective in suppressing its toxicity (Subbiah et al., 2011; Nie et al., 2017; Sharmeen et al., 2018). However, although the results are promising, further research is needed to clarify the effects of CNTs on biological systems.
Conclusions and future perspectives
The development of biomaterials capable of preventing biofilm-related infections is necessary for several medical applications, including the construction of medical devices and implants. This review demonstrated that there has been an increasing interest in the CNT composites for surface engineering due to their outstanding properties. Most of the reviewed studies investigated the efficacy of CNTs conjugated with different polymers, antimicrobial compounds, or metals. The modification of CNT surfaces was crucial to increase their hydrophilicity and, consequently, biocompatibility. Data also indicated that surfaces containing modified CNTs and in particular CNT/polymer composites exerted strong antimicrobial and anti-adhesive activities against a broad spectrum of microorganisms. However, although CNTs showed an evident efficacy to reduce biofilm formation, there is still little consensus about the CNTs mode of action. Further scientific and empirical research is therefore required to clearly elucidate the exact antimicrobial and anti-adhesive mechanisms behind the use of CNTs. Despite their antimicrobial activity, CNTs cannot yet compete with the conventional antimicrobials as their toxicity profile on the human body has not been thoroughly addressed, along with their environmental risks. Additionally, it should be noted that the large-scale manufacturing of CNTs remains a challenge as the production process is slow, costly, and highly energy consuming. Future research addressing the safety of CNTs, as well as their production in a cost-effective way, can thus help to overcome these limitations, creating new opportunities in the biomedical field. The implementation of in vivo assays is also needed for a better understanding of the clinical performance of CNTs, allowing their translation into medical applications.
Overall, this systematic review provides important findings that should be considered to produce efficient CNT-based surfaces with improved antimicrobial and anti-adhesive activities.
Limitations of the study
This study has demonstrated the antimicrobial and anti-adhesive activities of CNT-based surfaces in the biomedical field. However, the CNT mode of action against microbial biofilms was not fully explored due to the lack of sound knowledge in the literature regarding this topic. Additionally, although the techniques used to synthesize CNT-based surfaces may influence their effectiveness, they were not addressed in this work due to the very recent introduction of these materials in medical scenarios. These two limitations highlight the need for further research to optimize the performance of CNT-based surfaces.
Resource availability
Lead contact
Further requests for resources and materials should be directed to and will be fulfilled by the Lead Contact, Filipe Mergulhão (filipem@fe.up.pt).
Materials availability
This study did not yield new unique reagents.
Data and code availability
This study did not produce datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was financially supported by: Base Funding— UIDB/00511/2020 of the Laboratory for Process Engineering, Environment, Biotechnology and Energy LEPABE—funded by national funds through the FCT/MCTES (PIDDAC), and by Project PTDC/BIIBIO/29589/2017 - POCI-01-0145-FEDER-029589, funded by FEDER funds through COMPETE2020, Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES. R.T.-S. acknowledges the receipt of a junior researcher fellowship from this project. L.C.G. acknowledges the Portuguese Foundation for Science and Technology (FCT) for the financial support of her work contract through the Scientific Employment Stimulus—Individual Call—(CEECIND/01700/2017).
Author contributions
R.T.-S. and M.G. were responsible for the systematic search. R.T.-S., M.G., and L.C.G. wrote the manuscript. F.J.M. critically revised the manuscript. All authors provided substantial contributions to the conception of the work and approved the final version.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2020.102001.
Supplemental information
References
- Ahmed F., Santos C.M., Vergara R.a.M.V., Tria M.C.R., Advincula R., Rodrigues D.F. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ. Sci. Technol. 2012;46:1804–1810. doi: 10.1021/es202374e. [DOI] [PubMed] [Google Scholar]
- Al-Jumaili A., Alancherry S., Bazaka K., Jacob M.V. Review on the antimicrobial properties of carbon nanostructures. Materials. 2017;10:1066. doi: 10.3390/ma10091066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh A., Razmjou A., Ghaedi M., Jannesar R. Nanoporous solid-state membranes modified with multi-wall carbon nanotubes with anti-biofouling property. Int. J. Nanomedicine. 2019;14:1669–1685. doi: 10.2147/IJN.S189728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anju V.T., Paramanantham P., Sruthil S.B., Sharan A., Alsaedi M.H., Dawoud T.M.S., Asad S., Busi S. Antimicrobial photodynamic activity of rose bengal conjugated multi walled carbon nanotubes against planktonic cells and biofilm of Escherichia coli. Photodiagnosis Photodyn. Ther. 2018;24:300–310. doi: 10.1016/j.pdpdt.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Anju V.T., Paramanantham P., Siddhardha B., Sruthil Lal S.B., Sharan A., Alyousef A.A., Arshad M., Syed A. Malachite green-conjugated multi-walled carbon nanotubes potentiate antimicrobial photodynamic inactivation of planktonic cells and biofilms of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Nanomedicine. 2019;14:3861–3874. doi: 10.2147/IJN.S202734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anju V.T., Paramanantham P., Sruthil S.B., Sharan A., Syed A., Bahkali N.A., Alsaedi M.H., Kaviyarasu K., Busi S. Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus - understanding the mechanism of action. Photodiagnosis Photodyn. Ther. 2019;27:305–316. doi: 10.1016/j.pdpdt.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Arias L.R., Yang L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir. 2009;25:3003–3012. doi: 10.1021/la802769m. [DOI] [PubMed] [Google Scholar]
- Aslan S., Deneufchatel M., Hashmi S., Li N., Pfefferle L.D., Elimelech M., Pauthe E., Van Tassel P.R. Carbon nanotube-based antimicrobial biomaterials formed via layer-by-layer assembly with polypeptides. J. Colloid Interface Sci. 2012;388:268–273. doi: 10.1016/j.jcis.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Aslan S., Loebick C.Z., Kang S., Elimelech M., Pfefferle L.D., Van Tassel P.R. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid) Nanoscale. 2010;2:1789–1794. doi: 10.1039/c0nr00329h. [DOI] [PubMed] [Google Scholar]
- Beigbeder A., Linares M., Devalckenaere M., Degée P., Claes M., Beljonne D., Lazzaroni R., Dubois P. CH-π interactions as the driving force for silicone-based nanocomposites with exceptional properties. Adv. Mater. 2008;20:1003–1007. [Google Scholar]
- Bekyarova E., Ni Y., Malarkey E.B., Montana V., Mcwilliams J.L., Haddon R.C., Parpura V. Applications of carbon nanotubes in Biotechnology and biomedicine. J. Biomed. Nanotechnol. 2005;1:3–17. doi: 10.1166/jbn.2005.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingeri R., Mulko L., Molina M., Picco N., Alustiza F., Grosso C., Vivas A., Acevedo D.F., Barbero C.A. Nanocomposites based on pH-sensitive hydrogels and chitosan decorated carbon nanotubes with antibacterial properties. Mater. Sci. Eng. C. 2018;90:461–467. doi: 10.1016/j.msec.2018.04.090. [DOI] [PubMed] [Google Scholar]
- Chaudhari A.A., Joshi S., Vig K., Sahu R., Dixit S., Baganizi R., Dennis V.A., Singh S.R., Pillai S. A three-dimensional human skin model to evaluate the inhibition of Staphylococcus aureus by antimicrobial peptide-functionalized silver carbon nanotubes. J. Biomater. Appl. 2019;33:924–934. doi: 10.1177/0885328218814984. [DOI] [PubMed] [Google Scholar]
- Chen H., Wang B., Gao D., Guan M., Zheng L., Ouyang H., Chai Z., Zhao Y., Feng W. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small. 2013;9:2735–2746. doi: 10.1002/smll.201202792. [DOI] [PubMed] [Google Scholar]
- Cho E., Kim S.H., Kim M., Park J.-S., Lee S.-J. Super-hydrophobic and antimicrobial properties of Ag-PPFC nanocomposite thin films fabricated using a ternary carbon nanotube-Ag-PTFE composite sputtering target. Surf. Coat. Technol. 2019;370:18–23. [Google Scholar]
- Da Silva F.a.G., Jr., Alcaraz-Espinoza J.J., Da Costa M.M., De Oliveira H.P. Low intensity electric field inactivation of Gram-positive and Gram-negative bacteria via metal-free polymeric composite. Mater. Sci. Eng. C. 2019;99:827–837. doi: 10.1016/j.msec.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Dantas L.C.D.M., Silva-Neto J.P.D., Dantas T.S., Naves L.Z., Das Neves F.D., Da Mota A.S. Bacterial adhesion and surface roughness for different clinical techniques for acrylic polymethyl methacrylate. Int. J. Dent. 2016;2016:8685796. doi: 10.1155/2016/8685796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi H.R., Roozkhosh A., Jafar Tehrani M., Aghapoor K., Sayahi H., Balavar Y., Mohsenzadeh F. Characterization of ester- or thioamide-functionalized single-walled carbon nanotube-azithromycin conjugates. Appl. Surf. Sci. 2014;288:122–129. [Google Scholar]
- Del Pozo J.L., Rouse M.S., Patel R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs. 2008;31:786–795. doi: 10.1177/039139880803100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Henderson A., Field C. Antimicrobial activity of single-walled carbon nanotubes suspended in different surfactants. J. Nanotechnol. 2012;2012:928924. [Google Scholar]
- Dong X., Yang L. Inhibitory effects of single-walled carbon nanotubes on biofilm formation from Bacillus anthracis spores. Biofouling. 2014;30:1165–1174. doi: 10.1080/08927014.2014.975797. [DOI] [PubMed] [Google Scholar]
- El-Ghany N.a.A. Antimicrobial activity of new carboxymethyl chitosan–carbon nanotube biocomposites and their swell ability in different pH media. J. Carbohydr. Chem. 2017;36:31–44. [Google Scholar]
- Elmekawy A., Hegab H.M., Losic D., Saint C.P., Pant D. Applications of graphene in microbial fuel cells: the gap between promise and reality. Renew. Sust. Energ. Rev. 2017;72:1389–1403. [Google Scholar]
- Galano A. Carbon nanotubes: promising agents against free radicals. Nanoscale. 2010;2:373–380. doi: 10.1039/b9nr00364a. [DOI] [PubMed] [Google Scholar]
- Goodwin D.G., Marsh K.M., Sosa I.B., Payne J.B., Gorham J.M., Bouwer E.J., Fairbrother D.H. Interactions of microorganisms with polymer nanocomposite surfaces containing oxidized carbon nanotubes. Environ. Sci. Technol. 2015;49:5484–5492. doi: 10.1021/acs.est.5b00084. [DOI] [PubMed] [Google Scholar]
- Goodwin D.G., Xia Z., Gordon T.B., Gao C., Bouwer E.J., Fairbrother D.H. Biofilm development on carbon nanotube/polymer nanocomposites. Environ. Sci. Nano. 2016;3:545–558. [Google Scholar]
- Grover N., Borkar I.V., Dinu C.Z., Kane R.S., Dordick J.S. Laccase- and chloroperoxidase-nanotube paint composites with bactericidal and sporicidal activity. Enzyme Microb. Technol. 2012;50:271–279. doi: 10.1016/j.enzmictec.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Güler Ö., Bağcı N. A short review on mechanical properties of graphene reinforced metal matrix composites. J. Mater. Res. Technol. 2020;9:6808–6833. [Google Scholar]
- Hartono M.R., Kushmaro A., Chen X., Marks R.S. Probing the toxicity mechanism of multiwalled carbon nanotubes on bacteria. Environ. Sci. Pollut. Res. 2018;25:5003–5012. doi: 10.1007/s11356-017-0782-8. [DOI] [PubMed] [Google Scholar]
- He H., Pham-Huy L.A., Dramou P., Xiao D., Zuo P., Pham-Huy C. Carbon nanotubes: applications in pharmacy and medicine. Biomed. Res. Int. 2013;2013:578290. doi: 10.1155/2013/578290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld J., Akinoglu E.M., Wirtz D.C., Hoerauf A., Bekeredjian-Ding I., Jepsen S., Haddouti E.-M., Limmer A., Giersig M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine. 2017;13:1587–1593. doi: 10.1016/j.nano.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- Jing H., Sahle-Demessie E., Sorial G.A. Inhibition of biofilm growth on polymer-MWCNTs composites and metal surfaces. Sci. Total Environ. 2018;633:167–178. doi: 10.1016/j.scitotenv.2018.03.065. [DOI] [PubMed] [Google Scholar]
- Jung J.H., Hwang G.B., Lee J.E., Bae G.N. Preparation of airborne Ag/CNT hybrid nanoparticles using an aerosol process and their application to antimicrobial air filtration. Langmuir. 2011;27:10256–10264. doi: 10.1021/la201851r. [DOI] [PubMed] [Google Scholar]
- Kang S., Herzberg M., Rodrigues D.F., Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24:6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- Kang S., Pinault M., Pfefferle L.D., Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23:8670–8673. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- Karthika V., Kaleeswarran P., Gopinath K., Arumugam A., Govindarajan M., Alharbi N.S., Khaled J.M., Al-Anbr M.N., Benelli G. Biocompatible properties of nano-drug carriers using TiO2-Au embedded on multiwall carbon nanotubes for targeted drug delivery. Mater. Sci. Eng. C. 2018;90:589–601. doi: 10.1016/j.msec.2018.04.094. [DOI] [PubMed] [Google Scholar]
- Kavosi A., Hosseini Ghale Noei S., Madani S., Khalighfard S., Khodayari S., Khodayari H., Mirzaei M., Kalhori M.R., Yavarian M., Alizadeh A.M. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci. Rep. 2018;8:8375. doi: 10.1038/s41598-018-26790-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kayat J., Gajbhiye V., Tekade R.K., Jain N.K. Pulmonary toxicity of carbon nanotubes: a systematic report. Nanomedicine. 2011;7:40–49. doi: 10.1016/j.nano.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Kim K.-I., Kim D.-A., Patel K.D., Shin U.S., Kim H.-W., Lee J.-H., Lee H.-H. Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci. Rep. 2019;9:4921. doi: 10.1038/s41598-019-41381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kumar S., Bose S., Chatterjee K. Amine-functionalized multiwall carbon nanotubes impart osteoinductive and bactericidal properties in poly(ε-caprolactone) composites. RSC Adv. 2014;4:19086–19098. [Google Scholar]
- Lee E.-S., Kim Y.-O., Ha Y.-M., Lim D., Hwang J.Y., Kim J., Park M., Cho J.W., Jung Y.C. Antimicrobial properties of lignin-decorated thin multi-walled carbon nanotubes in poly(vinyl alcohol) nanocomposites. Eur. Polym. J. 2018;105:79–84. [Google Scholar]
- Levi-Polyachenko N., Young C., Macneill C., Braden A., Argenta L., Reid S. Eradicating group A streptococcus bacteria and biofilms using functionalised multi-wall carbon nanotubes. Int. J. Hyperthermia. 2014;30:490–501. doi: 10.3109/02656736.2014.966790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wei L., Hao L., Fang N., Chang M.W., Xu R., Yang Y., Chen Y. Sharper and faster “nano darts” kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano. 2009;3:3891–3902. doi: 10.1021/nn901252r. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sun X., Nakayama-Ratchford N., Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1:50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- Maas M. Carbon nanomaterials as antibacterial colloids. Materials. 2016;9:617. doi: 10.3390/ma9080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek I., Schaber C.F., Heinlein T., Schneider J.J., Gorb S.N., Schmitz R.A. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B. 2016;4:5228–5235. doi: 10.1039/c6tb00942e. [DOI] [PubMed] [Google Scholar]
- Metwally N.H., Saad G.R., Abd El-Wahab E.A. Grafting of multiwalled carbon nanotubes with pyrazole derivatives: characterization, antimicrobial activity and molecular docking study. Int. J. Nanomedicine. 2019;14:6645–6659. doi: 10.2147/IJN.S182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R.D.K., Girase B., Depan D., Shah J.S. Hybrid nanoscale Architecture for enhancement of antimicrobial activity: immobilization of silver nanoparticles on thiol-functionalized polymer crystallized on carbon nanotubes. Adv. Eng. Mater. 2012;14:B93–B100. [Google Scholar]
- Mocan T., Matea C.T., Pop T., Mosteanu O., Buzoianu A.D., Suciu S., Puia C., Zdrehus C., Iancu C., Mocan L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017;74:3467–3479. doi: 10.1007/s00018-017-2532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N.A., Abd El-Ghany N.A. Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int. J. Biol. Macromol. 2018;115:651–662. doi: 10.1016/j.ijbiomac.2018.04.101. [DOI] [PubMed] [Google Scholar]
- Mohamed N.A., Abd El-Ghany N.A. Synthesis, characterization and antimicrobial activity of novel aminosalicylhydrazide cross linked chitosan modified with multi-walled carbon nanotubes. Cellulose. 2019;26:1141–1156. [Google Scholar]
- Mohamed N.A., Al-Harby N.F., Almarshed M.S. Synthesis and characterization of novel trimellitic anhydride isothiocyanate-cross linked chitosan hydrogels modified with multi-walled carbon nanotubes for enhancement of antimicrobial activity. Int. J. Biol. Macromol. 2019;132:416–428. doi: 10.1016/j.ijbiomac.2019.03.195. [DOI] [PubMed] [Google Scholar]
- Mohan R., Shanmugharaj A.M., Sung Hun R. An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. J. Biomed. Mater. Res. B Appl. Biomater. 2011;96:119–126. doi: 10.1002/jbm.b.31747. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore V.C., Strano M.S., Haroz E.H., Hauge R.H., Smalley R.E., Schmidt J., Talmon Y. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003;3:1379–1382. [Google Scholar]
- Murugan E., Vimala G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J. Colloid Interface Sci. 2011;357:354–365. doi: 10.1016/j.jcis.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Murugesan B., Pandiyan N., Kasinathan K., Rajaiah A., Arumuga M., Subramanian P., Sonamuthu J., Samayanan S., Arumugam V.R., Marimuthu K. Fabrication of heteroatom doped NFP-MWCNT and NFB-MWCNT nanocomposite from imidazolium ionic liquid functionalized MWCNT for antibiofilm and wound healing in Wistar rats: synthesis, characterization, in-vitro and in-vivo studies. Mater. Sci. Eng. C. 2020;111:110791. doi: 10.1016/j.msec.2020.110791. [DOI] [PubMed] [Google Scholar]
- Murugesan B., Sonamuthu J., Samayanan S., Arumugam S., Mahalingam S. Highly biological active antibiofilm, anticancer and osteoblast adhesion efficacy from MWCNT/PPy/Pd nanocomposite. Appl. Surf. Sci. 2018;434:400–411. [Google Scholar]
- Narayan R.J., Berry C.J., Brigmon R.L. Structural and biological properties of carbon nanotube composite films. Mater. Sci. Eng. B. 2005;123:123–129. [Google Scholar]
- Neelgund G.M., Oki A., Luo Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf. B Biointerfaces. 2012;100:215–221. doi: 10.1016/j.colsurfb.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal D., Balasubramanian S., Simonian A.L., Davis V.A. Strong antimicrobial coatings: single-walled carbon nanotubes armored with biopolymers. Nano Lett. 2008;8:1896–1901. doi: 10.1021/nl080522t. [DOI] [PubMed] [Google Scholar]
- Nie C., Cheng C., Peng Z., Ma L., He C., Xia Y., Zhao C. Mussel-inspired coatings on Ag nanoparticle-conjugated carbon nanotubes: bactericidal activity and mammal cell toxicity. J. Mater. Chem. B. 2016;4:2749–2756. doi: 10.1039/c6tb00470a. [DOI] [PubMed] [Google Scholar]
- Nie C., Yang Y., Cheng C., Ma L., Deng J., Wang L., Zhao C. Bioinspired and biocompatible carbon nanotube-Ag nanohybrid coatings for robust antibacterial applications. Acta Biomater. 2017;51:479–494. doi: 10.1016/j.actbio.2017.01.027. [DOI] [PubMed] [Google Scholar]
- Oruc B., Unal H. Fluorophore-decorated carbon nanotubes with enhanced photothermal activity as antimicrobial nanomaterials. ACS Omega. 2019;4:5556–5564. [Google Scholar]
- Pangilinan K.D., Santos C.M., Estillore N.C., Rodrigues D.F., Advincula R.C. Temperature-responsiveness and antimicrobial properties of CNT–PNIPAM hybrid brush films. Macromol. Chem. Phys. 2013;214:464–469. [Google Scholar]
- Pangule R.C., Brooks S.J., Dinu C.Z., Bale S.S., Salmon S.L., Zhu G., Metzger D.W., Kane R.S., Dordick J.S. Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates. ACS nano. 2010;4:3993–4000. doi: 10.1021/nn100932t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman P., Anju V.T., Sruthil Lal S.B., Sharan A., Busi S., Kaviyarasu K., Arshad M., Dawoud T.M.S., Syed A. Synthesis and antimicrobial photodynamic effect of methylene blue conjugated carbon nanotubes on E. coli and S. aureus. Photochem. Photobiol. Sci. 2019;18:563–576. doi: 10.1039/c8pp00369f. [DOI] [PubMed] [Google Scholar]
- Peschel A., Sahl H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- Pramanik S., Konwarh R., Barua N., Buragohain A.K., Karak N. Bio-based hyperbranched poly(ester amide)–MWCNT nanocomposites: multimodalities at the biointerface. Biomater. Sci. 2014;2:192–202. doi: 10.1039/c3bm60170f. [DOI] [PubMed] [Google Scholar]
- Qi X., Gunawan P., Xu R., Chang M.W. Cefalexin-immobilized multi-walled carbon nanotubes show strong antimicrobial and anti-adhesion properties. Chem. Eng. Sci. 2012;84:552–556. [Google Scholar]
- Qi X., Poernomo G., Wang K., Chen Y., Chan-Park M.B., Xu R., Chang M.W. Covalent immobilization of nisin on multi-walled carbon nanotubes: superior antimicrobial and anti-biofilm properties. Nanoscale. 2011;3:1874–1880. doi: 10.1039/c1nr10024f. [DOI] [PubMed] [Google Scholar]
- Rodrigues D.F., Elimelech M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Environ. Sci. Technol. 2010;44:4583–4589. doi: 10.1021/es1005785. [DOI] [PubMed] [Google Scholar]
- Romero-Ben E., Cid J.J., Assali M., Fernández-García E., Wellinger R.E., Khiar N. Surface modulation of single-walled carbon nanotubes for selective bacterial cell agglutination. Int. J. Nanomedicine. 2019;14:3245–3263. doi: 10.2147/IJN.S179202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen A., Duan W., Wheeldon I., Walker S., Jassby D. Microbial attachment inhibition through low-voltage electrochemical reactions on electrically conducting membranes. Environ. Sci. Technol. 2015;49:12741–12750. doi: 10.1021/acs.est.5b01281. [DOI] [PubMed] [Google Scholar]
- Rosen Y., Elman N.M. Carbon nanotubes in drug delivery: focus on infectious diseases. Expert Opin. Drug Deliv. 2009;6:517–530. doi: 10.1517/17425240902865579. [DOI] [PubMed] [Google Scholar]
- Sah U., Sharma K., Chaudhri N., Sankar M., Gopinath P. Antimicrobial photodynamic therapy: single-walled carbon nanotube (SWCNT)-Porphyrin conjugate for visible light mediated inactivation of Staphylococcus aureus. Colloids Surf. B Biointerfaces. 2018;162:108–117. doi: 10.1016/j.colsurfb.2017.11.046. [DOI] [PubMed] [Google Scholar]
- Saliev T. The advances in biomedical applications of carbon nanotubes. C. 2019;5:29. [Google Scholar]
- Santos C.M., Milagros Cui K., Ahmed F., Tria M.C.R., Vergara R.a.M.V., De Leon A.C., Advincula R.C., Rodrigues D.F. Bactericidal and anticorrosion properties in PVK/MWNT nanocomposite coatings on stainless steel. Macromol. Mater. Eng. 2012;297:807–813. [Google Scholar]
- Schiffman J.D., Elimelech M. Antibacterial activity of electrospun polymer mats with incorporated narrow diameter single-walled carbon nanotubes. ACS Appl. Mater. Interfaces. 2011;3:462–468. doi: 10.1021/am101043y. [DOI] [PubMed] [Google Scholar]
- Schubert A., Wassmann T., Holtappels M., Kurbad O., Krohn S., Bürgers R. Predictability of microbial adhesion to dental materials by roughness parameters. Coatings. 2019;9:456. [Google Scholar]
- Seo Y., Hwang J., Kim J., Jeong Y., Hwang M.P., Choi J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int. J. Nanomedicine. 2014;9:4621–4629. doi: 10.2147/IJN.S69561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen S., Rahman A.F.M.M., Lubna M.M., Salem K.S., Islam R., Khan M.A. Polyethylene glycol functionalized carbon nanotubes/gelatin-chitosan nanocomposite: an approach for significant drug release. Bioact. Mater. 2018;3:236–244. doi: 10.1016/j.bioactmat.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Liu H., Luan S., Shi D., Yan S., Liu C., Li R.K.Y., Yin J. Effect of polyethylene glycol on the antibacterial properties of polyurethane/carbon nanotube electrospun nanofibers. RSC Adv. 2016;6:19238–19244. [Google Scholar]
- Shih I.-L., Shen M.-H., Van Y.-T. Microbial synthesis of poly(ε-lysine) and its various applications. Bioresour. Technol. 2006;97:1148–1159. doi: 10.1016/j.biortech.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Sivaraj D., Vijayalakshmi K. Enhanced corrosion resistance and antibacterial activity of Zn-HA decorated MWCNTs film coated on medical grade 316L SS implant by novel spray pyrolysis technique. J. Anal. Appl. Pyrolysis. 2018;134:176–182. [Google Scholar]
- Subbiah R.P., Lee H., Veerapandian M., Sadhasivam S., Seo S.-W., Yun K. Structural and biological evaluation of a multifunctional SWCNT-AgNPs-DNA/PVA bio-nanofilm. Anal. Bioanal. Chem. 2011;400:547–560. doi: 10.1007/s00216-011-4757-1. [DOI] [PubMed] [Google Scholar]
- Sukkar K., Ahmed D., Hussein A., Mohammad M. Synthesis and characterization hybrid materials (TiO2/MWCNTS) by chemical method and evaluating antibacterial activity against common microbial pathogens. Acta Phys. Pol. A. 2019;135:588–592. [Google Scholar]
- Teixeira-Santos R., Gomes M., Mergulhão F.J. Carbon nanotube-based antimicrobial and antifouling surfaces. In: Snigdha S., Thomas S., Radhakrishnan E.K., Kalarikkal N., editors. Engineered Antimicrobial Surfaces. Springer; 2020. pp. 65–93. [Google Scholar]
- Upadhyayula V.K.K., Gadhamshetty V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: a review. Biotechnol. Adv. 2010;28:802–816. doi: 10.1016/j.biotechadv.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Vagos M.R., Gomes M., Moreira J.M.R., Soares O.S.G.P., Pereira M.F.R., Mergulhão F.J. Carbon nanotube/poly(dimethylsiloxane) composite materials to reduce bacterial adhesion. Antibiotics. 2020;9:434. doi: 10.3390/antibiotics9080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagos M.R., Moreira J.M.R., Soares O.S.G.P., Pereira M.F.R., Mergulhão F.J. Incorporation of carbon nanotubes in polydimethylsiloxane to control Escherichia coli adhesion. Polym. Composites. 2019;40:E1697–E1704. [Google Scholar]
- Varesano A., Aluigi A., Florio L., Fabris R. Multifunctional cotton fabrics. Synth. Met. 2009;159:1082–1089. [Google Scholar]
- Vecitis C.D., Zodrow K.R., Kang S., Elimelech M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 2010;4:5471–5479. doi: 10.1021/nn101558x. [DOI] [PubMed] [Google Scholar]
- Venkatesan J., Jayakumar R., Mohandas A., Bhatnagar I., Kim S.K. Antimicrobial activity of chitosan-carbon nanotube hydrogels. Mater. Mater. 2014;7:3946–3955. doi: 10.3390/ma7053946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-L., Ren K., Chang H., Zhang S.-M., Jin L.-J., Xu J.-P. Facile fabrication of robust superhydrophobic multilayered film based on bioinspired poly(dopamine)-modified carbon nanotubes. Phys. Chem. Chem. Phys. 2014;16:2936–2943. doi: 10.1039/c3cp54354d. [DOI] [PubMed] [Google Scholar]
- Yallappa S., Manjanna J., Dhananjaya B.L., Vishwanatha U., Ravishankar B., Gururaj H., Niranjana P., Hungund B.S. Phytochemically functionalized Cu and Ag nanoparticles embedded in MWCNTs for enhanced antimicrobial and anticancer properties. Nano-micro Lett. 2016;8:120–130. doi: 10.1007/s40820-015-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Mamouni J., Tang Y., Yang L. Antimicrobial activity of single-walled carbon nanotubes: length effect. Langmuir. 2010;26:16013–16019. doi: 10.1021/la103110g. [DOI] [PubMed] [Google Scholar]
- Yick S., Mai-Prochnow A., Levchenko I., Fang J., Bull M.K., Bradbury M., Murphy A.B., Ostrikov K. The effects of plasma treatment on bacterial biofilm formation on vertically-aligned carbon nanotube arrays. RSC Adv. 2015;5:5142–5148. [Google Scholar]
- Young Y.-F., Lee H.-J., Shen Y.-S., Tseng S.-H., Lee C.-Y., Tai N.-H., Chang H.-Y. Toxicity mechanism of carbon nanotubes on Escherichia coli. Mater. Chem. Phys. 2012;134:279–286. [Google Scholar]
- Zardini H.Z., Davarpanah M., Shanbedi M., Amiri A., Maghrebi M., Ebrahimi L. Microbial toxicity of ethanolamines--multiwalled carbon nanotubes. J. Biomed. Mater. Res. A. 2014;102:1774–1781. doi: 10.1002/jbm.a.34846. [DOI] [PubMed] [Google Scholar]
- Zeinabad H.A., Zarrabian A., Saboury A.A., Alizadeh A.M., Falahati M. Interaction of single and multi wall carbon nanotubes with the biological systems: tau protein and PC12 cells as targets. Sci. Rep. 2016;6:26508. doi: 10.1038/srep26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Neoh K.G., Hu X., Kang E.-T. Mechanistic insights into response of Staphylococcus aureus to bioelectric effect on polypyrrole/chitosan film. Biomaterials. 2014;35:7690–7698. doi: 10.1016/j.biomaterials.2014.05.069. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Nghiem J., Silverberg G.J., Vecitis C.D. Semiquantitative performance and mechanism evaluation of carbon nanomaterials as cathode coatings for microbial fouling reduction. Appl. Environ. Microbiol. 2015;81:4744. doi: 10.1128/AEM.00582-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang Z., Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011;6:555. doi: 10.1186/1556-276X-6-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Qi X. Multi-walled carbon nanotubes/epilson-polylysine nanocomposite with enhanced antibacterial activity. Lett. Appl. Microbiol. 2011;52:76–83. doi: 10.1111/j.1472-765X.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Wang Z., Li S., Yuan X. Antimicrobial strategies for urinary catheters. J. Biomed. Mater. Res. A. 2019;107:445–467. doi: 10.1002/jbm.a.36561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not produce datasets/code.