Abstract
Current therapies for Parkinson’s disease (PD) are palliative, of which the levodopa/carbidopa therapy remains the primary choice but is unable to modulate the progression of neurodegeneration. Due to the complication of such a multifactorial disorder and significant limitations of the therapy, numerous genetic approaches have been proved effective in finding out genes and mechanisms implicated in this disease. Following the observation of a higher frequency of PD in Gaucher’s disease (GD), a lysosomal storage condition, mutations of glycosylceramidase beta (GBA) encoding glucocerebrosidase (GCase) have been shown to be involved and have been explored in the context of PD. GBA mutations are the most common genetic risk factor of PD. Various studies have revealed the relationships between PD and GBA gene mutations, facilitating a better understanding of this disorder. Various hypotheses delineate that the pathological mutations of GBA minimize the enzymatic activity of GCase, which affects the proliferation and clearance of α-synuclein; this affects the lysosomal homeostasis, exacerbating the endoplasmic reticulum stress or encouraging the mitochondrial dysfunction. Identification of the pathological mechanisms underlying the GBA-associated parkinsonism (GBA + PD) advances our understanding of PD. This review based on current literature aims to elucidate various genetic and clinical characteristics correlated with GBA mutations and to identify the numerous pathological processes underlying GBA + PD. We also delineate the therapeutic strategies to interfere with the mutant GCase function for further improvement of the related α-synuclein–GCase crosstalks. Moreover, the various therapeutic approaches such as gene therapy, chaperone proteins, and histone deacetylase inhibitors for the treatment of GBA + PD are discussed.
Keywords: Parkinson’s disease, Glycosylceramidase, Glucocerebrosidase, Gaucher’s disease, Mutations, α-Synuclein
Background
Parkinson’s disease (PD) is the second most common neurodegenerative disease that affects 2%–3% of the elderly (above 65 years) around the world, which is characterized by progressive dopaminergic neuronal degeneration in the substantia nigra and α-synuclein aggregation. PD is clinically defined as the combination of restful tremor, rigidity, bradykinesia, and postural instability [1–4]. Research advances in human genetics have significantly improved the understanding of PD. As PD is a multifactorial disorder and current therapies have significant limitations, numerous genetic approaches have been proven effective in sequencing the whole genome, which has led to the recognition of unpredictable genes and mechanisms implicated in this disease. Nevertheless, the most important genetic risk element in the case of PD in trials of patients with an uncommon lysosomal storage condition is the “Gaucher’s disease (GD)” [5]. The glycosylceramidase beta (GBA) gene located on chromosome 1 (1q21) encodes glucocerebrosidase (GCase), a lysosomal enzyme engaged in the glucosylceramide metabolism. Mutations in the gene have been widely correlated with GD, which is an inherited, systemic disorder with a significant level of central nervous system involvement [6–9]. Amazingly, research in the past 14 years has suggested that mutations within this gene are linked with elevated incidence of PD as well as the PD incidence in GD patients and asymptomatic carriers [2]. GCase is involved in the endolysosomal path, which appears to be essential in PD pathogenesis, in which a link among PD, GBA mutations, and GCase function has been discovered through clinical observations and the genes engaged in this process are responsible for several specific monogenic familial variants of PD [10, 11]. GBA gene mutations are much more persistent in most PD populations than other genes involved such as α-synuclein SNCA, PARK2, and LRKK2 [12, 13]. Mutations of GBA are the most significant risk factor for PD and the variants of GBA can raise the PD risk by up to 10 folds [7, 14]. Nowadays, over 300 mutations have been identified in GBA [14–16]. It has been proposed that not only a persistent lack of action of GCase enzyme, but also the probable toxic retain-of-function of mutant GCase, may lead to lysosome dysfunction and endoplasmic reticulum (ER) stress that facilitate the disease pathogenesis [17]. In addition, mutant GCase may not fold appropriately and will thus concentrate in various dopaminergic neuronal cells, triggering cellular stress, which can cause further damage to the cells; however, impeded activity of GCase tends to cause the aggregation of α-synuclein [18]. In addition to the contribution of GBA in PD development, researchers have highlighted different clinical characteristics in PD patients with GBA mutations as compared to the idiopathic PD [19].
In this review, we outline how GCase was recognized as the risk element for PD, the involvement of numerous molecular pathways in GBA + PD, and the identification of clinically relevant and new fields of PD research and therapies arising from this finding. Also, we summarize the genetic alterations and factors linked with the gene mutations in GD and PD and the associated clinical features. GCase deficiency leads to α-syn accumulation, and α-syn in turn can inhibit GCase activity, thereby enhancing α-syn aggregation. It will help to navigate a key role of GBA mutations in the pathogenesis. Hereby, we review the current knowledge on the GCase-α-synuclein pathway as a therapeutic target for the sporadic PD type. The most important literature was searched in order to explore the most recent and relevant published papers in the field.
Main text
An overview: GBA gene and GCase
The GBA gene encoding the lysosomal enzyme GCase comprises 10 introns and 11 exons and is located on chromosome 1q21, which is a gene-rich site composed of 2 pseudogenes (GBAP and MTX1) and 9 genes within a long sequence of about 100 kb. The highly untranslated pseudogene GBAP is homologous to GBA, which share a 96% exonic sequence identity in the coding region, complicating the detection of GBA mutations. The second convergently transcribed pseudogene MTX1 (Metaxin-1) encodes a protein found in the mitochondrial external (outer) membrane and is located downstream to the GBAP sequence [5, 12, 13, 20–22]. GCase (D-glucosyl-N-acylspingosine glucohydrolase) is a 497-amino-acid (AA) active protein with 5 glycosylation regions [17, 23–27]. The protein is synthesized in the ER, transported by the lysosomal integral membrane protein-2 (LIMP2), which is a GCase transporter encoded by SCARB2 gene, and becomes active upon reaching the acidic lumen of the lysosome by interacting with its activator protein saposin C. In the lysosomal compartment, the enzyme hydrolyses glucose moieties from glucosylceramide and glucosylsphingosine [21]. GCase consists of 3 domains: domain-1, an antiparallel β-sheet; domain-2, an immunoglobulin-like domain comprised of two closely associated β sheets; and domain-3, a (β/α)8 triosephosphate isomerase (TIM) barrel [17, 23].
GCase goes through several structural alterations during the transportation to the lysosome. It has 2 functional ATG-initiation sites, transcribed as a 516- or 536-AA protein, which is further processed into the 497-AA functional enzyme [23, 28]. It has been proposed that the transport of GCase into the ER is differentially affected by the different leaders and the 19-AA leader peptide cleavage takes place when it enters the ER. Oligosaccharide modulations also occur but they do not affect the intracellular stabilization or the catalytic activity of GCase; hence, their significance remains unclear [29, 30]. The transportation of GCase to the lysosome is independent of mannose-6-phosphate as suggested by the following lines of evidence. In the lysosomal disease state, the transportation of lysosomal enzymes into the lysosome could not be targeted via the mannose-6-phosphate-dependent pathway [31, 32]. Studies in different animal and cellular models have suggested that LIMP2, which is responsible for GCase transportation to the lysosome, is independent of mannose-6-phosphate [26].
An important function of GCase is to degrade glucocerebroside (also known as glucosylceramide) into glucose and ceramide. It also potentially cleaves β-glucosides such as glucosylsphingosine. GCase deficiency renders glucocerebroside accumulation within cells of the reticuloendothelial system [21].
The occurrence of GBA gene mutations: from GD to PD
The correlation between GBA mutations and a higher risk for developing PD was initially observed in GD clinics about 14 years ago [33, 34], when GD patients and their relatives, who were supposed to be GBA mutation carriers, were found to have a higher incidence of PD than the general population [35]. This finding promoted investigation of patients diagnosed at the GD clinic, which revealed a PD incidence of about 25% [36]. A survey conducted at the GD clinic (Jerusalem) elucidated a similar finding to the earlier report [6, 37, 38]. In 2004, it was reported that the first-degree relatives of GD patients had a much higher incidence of PD relative to the normal population [6, 38]. This was when various Parkinsonism-associated GD populations were screened for this gene mutation to assess the vital role of GBA in the PD pathogenesis worldwide [38]. Animal models for the analysis of GBA mutations in GD had been established before the recognition of its role as a risk factor for PD and now new models have been designed especially for the evaluation of the correlation between GBA mutations and PD. In addition, there is a wide range of cell-based designs using e.g., the pluripotential induced stem cells (iPSCs) [39, 40]. Broad Gaucher Registry statistics suggest that the risks for developing PD in GD patients are 9%–12% and 5%–7% before the age of 80 years and 70 years, respectively, although the GBA mutations occur in 5%–10% of PD patients [41]. This made GBA mutations the most significant genetic risk factor for PD [42]. Macrophages of GD patients are associated with glycolipid stress and load and are thus regarded as “Gaucher cells”, which are characteristic of the disease pathology. The single heterozygous mutations were originally believed to be harmless, but after analysis of early cases of Parkinsonism in GD patients, the heterozygous mutations were considered to present a substantial risk of developing PD [6, 43, 44].
The associations of GBA mutations with dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD) have also been well established. GBA1 mutations are a risk factor for the development of DLB, suggesting some correlations among GBA1, dementia and parkinsonism. Patients with DLB are 8 times more likely to be the GBA mutation-carriers than controls. Such a risk is greater than that reported for PD, and seems to correlate with disease severity, earlier age onset and progression of disease. GBA is involved in the DLB pathology, but the precise cause of this predisposition is not known. Recently, the association of DLB with the PD-linked SCARB2 has highlighted the significance of the lysosomal pathway in DLB. Genetically, DLB seems to be heterogeneous, with common risk factors and relatively rare contribution from pathogenic causative mutations [45, 46]. There is no significant difference between DLB patients with and without a GBA mutation in the neuropathological data, though the GBA - mutation-carriers show a reduced activity of GCase and more prominent lipid profile alteration in the brain. GBA expression is reduced in PDD and DLB cases in the caudate nucleus and the temporal cortex [36, 47]. PD mild cognitive impairment (MCI) and PDD are two types of cognitive impairment and the most prevalent and disabling non-motor symptoms of PD. MCI is the early warning signal of late dementia in PD. Research evidence has shown that about 30% of newly diagnosed PD patients have MCI, > 40% of PD patients with normal cognitive function will develop MCI within 6 years, and about 80% of patients may develop PDD at the late phase of PD. Various studies have suggested that the GBA mutations are more likely to be associated with PD-MCI [47, 48].
GBA mutations associated with PD: a clear link
About 495 mutations and rearrangements across the exons of GBA are known to be associated with GD, including splicing mutations, frameshift mutations, point mutations, deletions, insertions and null alleles, and most of them are “missense mutations”. The most common mutations are point mutations including N370S (seen exclusively in type 1 GD patients in the Europe, USA and Israel) and L444P (observed worldwide) [21, 35, 38]. There is a 10%–30% probability of developing PD among the heterozygous mutation carriers at the age of 80, a 20-fold rise as compared to the non-carriers [49–53]. The idiopathic PD is undifferentiated from GBA + PD, with the only distinctive characteristic being the earlier age of onset and a greater incidence of cognitive features [49, 54, 55]. Moreover, the variant E326K is a notable example that is not considered a GD-causing mutation but a risk factor for PD [54, 56]. This implies a precise, and perhaps a different process by which mutations make their carriers prone to PD. The enzymatic activity of GCase is differentially influenced by different GBA mutations, as many of the mutations lead to no residual activity whereas others result in decreased activity [39, 45].
In comparison to GD, a smaller number of GBA mutations has been observed in PD patients (~ 130 GBA mutations) [33, 34]. Only certain mutations that are most generally linked with PD have been screened in several studies. Hence, the PD-related mutants that are less persistent could go unidentified. Generally, c.1448 T > C (L444P) and C.1226A > G (N370S) are the two most persistent mutations amongst others. They even contribute to 70%–80% of GBA mutants linked with PD in certain populations [57]. In a population of Colombian PD patients, an increased frequency of the variant K198E was observed in comparison with controls, consistent with the recent finding in GD patients [33]. Severe GBA mutations such as c.115 + 1 G > A (IVS2 + 1), c.84dupG (84GG), c.1297 G > T (V394L), c.1263del + RecTL, c.1342 G > C (D409H), and c.1448 T > C (L444P), appear to be correlated with a higher risk of PD development compared to the mild mutations such as c.84dupG (84GGG) and N370S [58]. p.T369M is one of the GBA variants that do not cause GD in homozygous carriers and may modify the GCase activity. In some studies, the substitution of p.T369M was associated with PD, while in other studies it had similar or increased frequency in controls. It is of interest that the GBA p.T369M substitution was demonstrated to be correlated with declined GCase activity in PD patients and controls compared to that in non-carriers. A recent meta-analysis showed that the p.T369M allele was associated with an increased risk for PD [59, 60]. Besides, the severe mutations mentioned above are also linked with an earlier onset age, and also have greater incidence and elevated cognitive ability involvement [58–62]. A previous study showed that PD patients with extreme GBA mutations had significantly worse motor symptoms together with some non-motor symptoms such as insomnia and rapid-eye-movement sleep disturbances, compared to subjects with moderate mutations or idiopathic PD [63]. Interestingly, GBA is nothing more than a risk element for PD. The latter implies that the disease will not develop in every carrier. The implications of all these mutations remain to be clarified. Information from a wide array of human studies shows that around 9.1% of GBA carriers will develop PD. Several reports have indicated that the prevalence of PD in GD patients at age of 80 is about 30%, but more research is needed to validate this data [50]. Homozygous GBA variant-linked patients that are affected by GD have a greater risk of developing PD and generally have an earlier age of symptom onset [63]. It is important to note that even in the case of severe mutations, most GD subjects do not develop PD. The heterozygous and homozygous GBA variant-carriers exhibit a 5- and 10-times greater risk of having PD, respectively [50, 64, 65]. GBA mutations are present in ~ 2%–30% of PD patients [66] (Table 1).
Table 1.
Frequency of GBA mutations in various populations of PD patients
| GBA variant | Population | No. of participants | Carrier frequency (%) |
P value |
Most common variants | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Cases | Control | Cases | Control | |||||
|
N370S, L444P, T369M, IVS6, IVS10, K303K, R262H, E326K, RecNci1 |
Mixed | 1325 | 359 | 12.6% | 5.3% | – | E326K, L444P, N370S, T369M | [55] |
|
N370S, c.84dupG, R496H, L444P, V394L, IVS2 + 1, RecTL, D409H |
Ashkenazi | 420 | 333 | 17.9% | 4.2% | ≤0.0001 | N370S | [57] |
|
N370S, IVS2 + A > G, K198T, L444P, R329C, RecNci1, c.84dupG |
Canadian | 88 | 122 | 5.7% | 0.8% | 0.48% | RecNci1 | [67] |
| L444P, N370S | Chinese population in Singapore | 331 | 347 | 2.4% | 0.0% | 0.06 | L444P | [68] |
| L444P, N370S | Italian | 395 | 483 | 2.8% | 0.2% | 0.0018 | L444P | [69] |
|
L444P, N370S, c.84dupG, V394L, IVS2 + A > G, R496H |
Ashkenazi | 99 | 1543 | 31.3% | 6.2% | ≤0.0001 | N370S | [70] |
| L444P, N370S | Russian | 330 | 240 | 2.7% | 0.4% | 0.038 | N370S | [71] |
| GBA exons | Japanese | 534 | 544 | 9.4% | 0.1% | ≤0.0001 | R120W, RecNci1 | [72] |
| GBA exons | Korean | 277 | 291 | 3.2% | 0.0% | 0.01 | N188S, R257Q, P201H, L444P, S271G | [73] |
| GBA exons | Portugese | 230 | 430 | 6.1% | 0.7% | – | N370S, N396T | [74] |
| L444P, R353W, F213I, N370S | PD patients in China's mainland | 402 | 413 | 2.7% | 0.0% | 0.0007 | L444P | [75] |
| N370S, G377S, L444P | Brazilian | 65 | 267 | 3.0% | 0.0% | 0.037 | L444P | [76] |
| L444P, R120W, RecNci1 | PD patients in Taiwan region | 518 | 339 | 3.1% | 1.2% | 0.07 | L444P, RecNci1 | [77] |
| GBA exons | Greek | 172 | 132 | 4.7% | 0.8% | 0.048 | H255Q, L444P | [78] |
|
N370S, 84GG, L444P, IVS2 + 1G > A, V394L, D409H, del55bp, R496H |
Ashkenazi | 250 | – | 12.8% | – | – | N370S | [79] |
| LRRK2 (G2019S), GBA exons | North African (Morocco, Algeria, Libya, Tunisia) | 194 | 177 | 4.6% | 0.5% | 0.01 | N370S, L444P, RecNci1 | [57] |
Evidence for the potential mechanisms of GBA mutation involvement in PD
Cross-talks among GBA mutations, GCase, and α-synuclein, and their roles in PD
There has been a significant attempt over the past couple of years to reveal the pathogenic function of GBA mutations in PD. Accumulating evidence has suggested the autophagic and endolysosomal pathway failure in PD [57]. The autophagic and endolysosomal pathways are essential for α-synuclein depletion, while the aggregation of α-synuclein is one of the defining characteristics of PD that renders dopaminergic neuronal death [80, 81]. GBA mutations can structurally alter the GCase protein, leading to the loss of function or reduced enzymatic activity. Theoretically, these effects may arise in many forms through the following pathways:
-
i.
Failure of GCase to escape the ER,
-
ii.
Failure of GCase to bind to its trafficking transporter LIMP2,
-
iii.
Degradation of unstable and misfolded GCase by the proteasome,
-
iv.
Failure of GCase to leave the Golgi,
-
v.
GCase inactivity due to the mutations at the active site, and
-
vi.
Degradation of GCase by the proteasome [82].
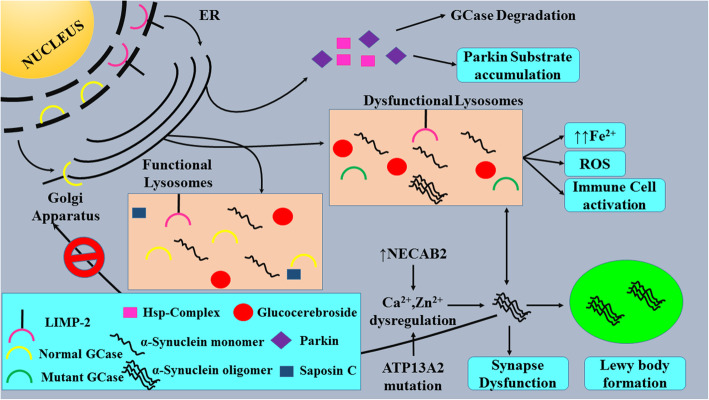
All the pathways that lead to PD development are presented in Fig. 1.
Fig. 1.
Feed-forward cascade of all the pathways that lead to PD development
Several recent studies have postulated a relationship between elevated levels of α-synuclein and diminished GCase activity in both in vitro and in vivo models. α-Synuclein deposition occurs in many forms in both the peripheral and central nervous systems. The progression of PD was proposed to be related to the expansion of α-synuclein aggregates between neurons. Neuronal cell lines overexpressing α-synuclein tend to release exosomes containing α-synuclein. Such exosomes will transfect regular neurons. The external α-synuclein may co-aggregate with native α-synuclein, ejected from the neuron, and transfect other neurons. Lysosomal impairment and deterioration of GCase expression facilitate the proliferation of α-synuclein aggregates [83, 84]. A correlated mutation study on all 72 vertebrate species with known complete sequences of α-synuclein and GCase revealed that α-synuclein and GCase probably co-evolve, and mutations could disrupt the beneficial interactions between them [85].
The association between GCase and α-synuclein
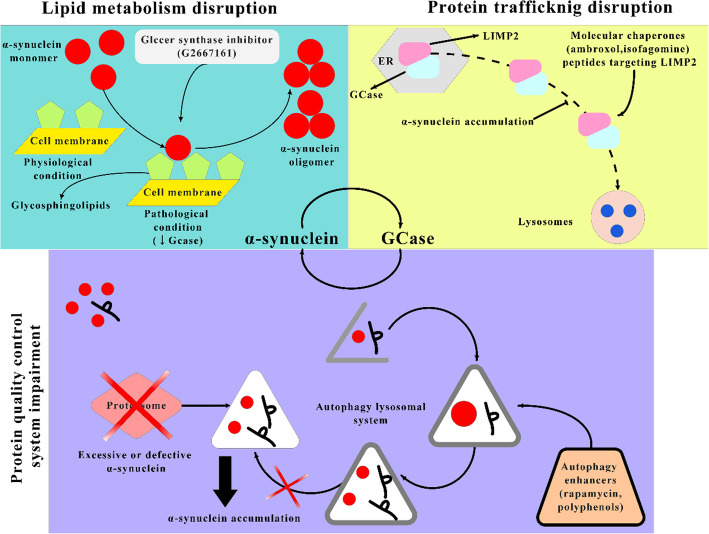
The relationship between α-synuclein and GCase particularly depends on the pH and the cellular site. The GCase substrate glucosylceramide could contribute to the aggregation of α-synuclein, which in turn results in decreased activity of GCase. In vitro experiments indicate that α-synuclein (membrane-bound) communicates with GCase in a different way from that between the unbound type of α-synuclein and GCase, creating a matrix that decreases the GCase activity at the lysosomal pH. The GCase–α-synuclein communication on the membrane surface involves a larger α-synuclein region than that in solution, and the bound α-synuclein (α-helix) acts as a mixed inhibitor for GCase. Generally, GCase binds to the lipid bilayer of the membrane where it is inserted partly. The active site of GCase possibly resides above the interface between the membrane and water. GCase is moved away from the membrane following contact with α-synuclein, which is membrane-bound. This can block access to substrates and disrupt the active GCase site. As a response, GCase shifts the attached α-synuclein helical residues farther from the bilayer and may have a detrimental effect on α-synuclein degradation by lysosomes. Saposin C is a protein co-factor that is usually required by GCase. It competes with α-synuclein for binding to the active site of GCase, thereby preventing some inhibition of GCase [86–89]. Transgenic mice expressing GBA L444P together with wild-type human SNCA have a 40% drop in GCase activity, which elicits the aggregation of α-synuclein in cortical neurons. The co-expression of GBA L444P and SNCA A53T leads to the exacerbation of the intensity of gastrointestinal and motor effects as compared to those expressing the A53T mutation alone [90]. Studies showing brain areas with aggregation of α-synuclein have implied a persuasive reduction in GCase function. This connection has been shown at the periphery, with correlations of lower GCase leukocyte activity with greater plasma levels of oligomeric α-synuclein. The mechanisms underlying the implication of the GCase–α-synuclein crosstalk in pathological conditions are presented in Fig. 2.
Fig. 2.
Mechanisms underlying the GCase–α-synuclein cross-talk that results in pathological conditions. GCase alterations induce disruptions of lipid composition and protein trafficking, inducing aggregation of α-synuclein
Under normal conditions, α-synuclein is unfolded and attains a tertiary arrangement on certain biochemical interactions. The abnormal aggregation of α-synuclein is toxic to the survival of dopaminergic neurons, contributing to the PD-associated neurodegeneration. GBA gene mutations and overexpression can affect the aggregation and conformational changes of α-synuclein. Moreover, some species can stimulate neuroinflammatory responses, which can spread the pathology of α-synuclein from one cell to another. Most importantly, there are growing data suggesting failure of the endolysosomal and autophagic pathways in PD [81]. These pathways are essential for α-synuclein degradation, while α-synuclein accumulation in the dopaminergic neurons is a key hallmark of PD. GCase plays an important role in the lysosomal degradation of α-synuclein and has close cross-talks with α-synuclein [80]. In patients with mutations that decrease GCase, the degradation of α-synuclein within the cell is impaired and lysosomal functioning is compromised, leading to increased levels of oligomeric α-synuclein, which results in dopaminergic neuronal death in PD. Such condition may be enhanced by ageing, which is accompanied by impaired functions of lysosome and increased concentrations of cellular α-synuclein. This is how mutant α-synuclein influences the survival of dopaminergic neurons, causing cell demise [5].
PD is associated with mutations in both SNCA (which encodes α-synuclein) and GBA [90]. Murphy et al. reported a decrease in the functions of brain GCase in the early phase of intermittent PD, which then remains unchanged during PD development. The production and function of GCase are decreased in brain areas with α-synuclein deposition. While the lysosome malfunction is important in these areas, it is not sufficient to induce neuronal death. It has been shown that the decreased activity of GCase in cultured neurons leads to the declined clearance and increased levels of α-synuclein. The declined GCase function in the lysosome is associated with glucosyl sphingosine and glucosylceramide substrate aggregation, the latter of which confers more cytotoxicity [80, 91].
Other potential pathways involved in GBA-associated PD
Many mechanisms have been proposed to be engaged in GBA-associated neurodegeneration. A recent study showed that treatment with a glucosylceramide synthase inhibitor miglustat to reduce glycosphingolipid accumulation and overexpression of GBA1 to augment GBA1 activity can reverse the GBA1 deficiency-induced destabilization of α-synuclein tetramers and related multimers and protect against the α-synuclein preformed fibril-induced toxicity in hDA neurons derived from GBA + PD iPSCs, which suggest therapeutic opportunities for GD and PD patients [91]. This study suggested a neurotoxic connection between GCase and α-synuclein that can partly describe the process underlying GBA + PD. Therefore, no research till date has demonstrated increased glucosylceramide production in the presence of heterozygote GBA mutation.
Another process through which GBA mutations lead to PD is the impairment of ER-associated degradation (ERAD) and cell death correlated with ER stress. The accumulation of α-synuclein will trigger ER tension, impede the ER-related substrate degradation, and restrict the ER-to-Golgi traffic [92]. Some PD-associated genes like PARK-2 are involved in ERAD. Collectively, the ERAD and ER tension may significantly participate in the PD pathogenesis [93]. The consistent findings of ER in experiments with a few of the mutant types of GCase indicate that ER stress is often engaged in PD pathogenesis in some GBA mutation-carriers [94–96].
It has also been demonstrated that the aggregation of GCase in aggresomal-like structures is promoted via the interactions of mutated GCase with PARK-2 [93]. The ER stress found in GBA mutation models may be attributed to the aggregation of α-synuclein, instead of aggregation of GCase. The disturbance of ceramide metabolism may also be implicated in the PD pathogenesis. Although Lewy bodies are the pathological signature of PD [97], they are also present in several other diseases. The genes governing such diseases have been noted (such as SMPD1, GALC, PLA2G6, ASAH1, and PANK2) to play a vital role in ceramide metabolism [98, 99].
Ceramide is linked to PD through its involvement in inflammation and stress-induced cell death [100, 101]. Hence the pathway of ceramide metabolism may be associated with the formation of LB in GBA + PD [98] (Table 2).
Table 2.
Cell and animal models used in GBA mutation studies
| Model | Model characteristics | Reference |
|---|---|---|
| Fibroblasts of patients | Carriers of heterozygous GBA mutations without or with PD | [102] |
| Fibroblasts of PD patients | Heterozygous GBA L444P and N370S mutations | [103] |
| Dopaminergic neurons derived from iPSC (patient midbrain) | Carriers of heterozygous GBA variant N370S in twins discordant for PD. | [104] |
| Mouse model | Heterozygous GBA variant L444P | [105] |
| SH-SY5Y cells (human α-synuclein) | siRNA knockdown of GBA | [106] |
| Mouse | GBA knockout | [107] |
| Mouse | GBA point mutations (D409H, N370S, D409V, and V394L) | [108] |
| Zebrafish | Deletion of 23 bp GBA | [109] |
| Mouse | GBA L444P mutation + α-synuclein A53T mutation | [90] |
| NSC and dopaminergic cells derived from the iPSC and fibroblasts of patients | Heterozygous GBA N370S mutations | [110] |
| Embryonic fibroblasts from mice | GBA heterozygous mutation | [111] |
ER endoplasmic reticulum; GCase glucocerebrosidase; LIMP2 lysosomal integral membrane protein-2
Mitochondrial dysfunction, reactive oxygen species (ROS), and neuroinflammation in GBA-associated PD
Mitochondrial dysfunction, neuroinflammation, and excessive ROS formation are key processes that lead to dopaminergic neuronal death, wherein the mitochondrial dysfunction is involved in the pathophysiology of both familial and idiopathic PD [112, 113]. Evidence has suggested that α-synuclein interacts with the mitochondrial import proteins through a cryptic mitochondrial import signal [114]. Mutations of PTEN-induced putative kinase (PINK1) and PARK2 contribute to the monogenic PD, and are assumed to affect mitochondrial function by elevating the susceptibility to toxins [115]. In a neuronopathic GD mouse model (K14-lnl/lnl), Ossellame et al. found that the proteasomal and autophagic pathways in astrocytes and neurons were impaired and that insoluble α-synuclein deposits occurred in neurons [107, 116]. Depletion in GCase function in cell studies led to a gradual deterioration of the potential of mitochondrial membrane needed for ATP output, fragmented mitochondria, respiratory complex function loss, and oxidative stress.
Ultimately, dysregulation of calcium occurred in the mitochondria, resulting in a distorted potential of the membrane [117]. In addition, ROS are generated after mitochondrial dysfunction, inducing persistent oxidative damage that may trigger α-synuclein misfolding and activate other deteriorating channels in the neuron [118, 119]. Accordingly, secondary mitochondrial dysfunction may arise from the primary lysosomal insult (i.e., GCase activity loss). Cellular disturbances including ROS, impairment of mitophagy, and ER stress can further deteriorate the cellular homeostasis and promote α-synuclein accumulation [120]. The transcript level of the antioxidant NQ01 has been found to be augmented in GD patients and GBA heterozygotes with or without PD, which may serve as a potential compensatory process. Thus, the GCase insufficiency can increase the oxidative stress of neurons. Elevating the levels of glutathione as an antioxidant may be a therapeutic strategy, which can be achieved by administration of N-acetylcysteine [121, 122].
Chemotactic factors are a central part of the process of neuroinflammation in that they can allocate immunologic facilitators to the inflammatory site [123, 124]. Iron is increased in PD patients in the substantia nigra. Excessive iron can normally be chelated by neuromelanin or ferritin. However, with natural ageing, the residual iron is stored in the brain. Excessive levels of iron induced by reactive nitrogen species or ROS can contribute to neuronal death [125]. Disruption of zinc homeostasis can aggravate or cause neurodegeneration via induction of protein misfolding and oxidative stress. Zinc augmentation also occurs in the substantia nigra of PD patients [126]. Mutations of PARK9/ATP13A2, which encode a zinc pump that brings zinc into the membraned components, are correlated with juvenile-onset PD. ATP13A2 overexpression causes extracellular zinc tolerance and facilitates the exosomal transfer of α-synuclein [127, 128]. Silenced ATP13A2 expression in primary neurons causes a decline in lysosomal chelation of Zn2+ and increases the expression of Zn2+ transporters. The increased Zn2+ leads to lysosome dysfunction that leads to the GCase–α-synuclein pathological cascade. Either chelation of zinc or improvement of ATP13A2 expression can improve the phenotype [129, 130].
Prospects for the treatment of GBA-associated PD
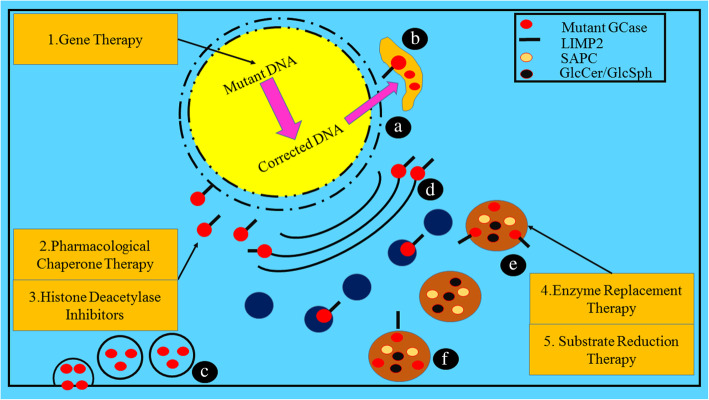
Current promising therapies for GD include enzyme replacement therapy (ERT) and substrate replacement therapy (SRT), both have been approved by the FDA and work through the production and maintenance of a more standard ratio of GCase substrate in patients. These therapies have markedly enhanced the visceral symptoms of GD but are unable to gain access to the blood-brain barrier, so the neuronopathic symptoms of GD cannot be alleviated or reversed [2]. Considering the significant involvement of GCase in the PD pathogenesis, innovative therapies that can restore the levels of neural GCase, may not only enhance the life quality of neuronopathic patients, but also delay the development of PD in populations vulnerable to the GD-associated PD or idiopathic PD. Currently, brain-penetrating variants of SRT are under clinical trials with PD patients who are carriers of heterozygous GBA mutation [36, 129]. Numerous companies have been trying to tackle this problem, achieving very encouraging outcomes in animal and cell models. The interventions available in clinical trials so far mainly resolve pathways that are considered to be counterproductive in connecting GBA mutations to PD. The hypothetical pathways that lead to impairment of GCase and the associated therapies targeting these pathways are shown in Fig. 3.
Fig. 3.
Distinctive hypothetical pathways through which impairment of GCase occurs and therapies targeting these pathways. a: GCase failure to escape the ER; b GCase failure to link with LIMP2 transporter; c misfolded and degraded GCase; d GCase failure to escape Golgi; e inactive GCase due to the mutation at the active site; f Altered GCase function due to the defective saposin C. The targeted therapies are (1) Gene therapy: direct replacement of mutant DNA with the correct DNA via viral infections; (2) Chaperone therapy to refold and stabilize misfolded proteins; (3) Histone deacetylase inhibitors that inhibit the response of unfolded protein; (4) Enzyme replacement therapy: substituting the dysfunctional enzyme with the recombinant enzyme in the targeted lysosome; and (5) Substrate replacement therapy: diminishing the accumulation of substrate independent of the enzyme level
One hypothesis demonstrates that the mutated GBA proteins are not adequately foldable in the ER, triggering protein accumulation in the cellular compartment, which induces dopaminergic neurons to respond to the stress, contributing to their damage and death. In addition, the β-GCase entanglement in the ER causes a declined enzyme level within the cell, inducing the aggregation of α-synuclein [102]. To target this pathogenic mechanism, distinctive chaperones, which are capable of facilitating the substrate replenishment, have been tested [103, 104, 131, 132]. A clinical trial on ambroxol, a chaperone that demonstrated extremely interesting preliminary findings, was established in 2016 (NCT02914366). This clinical trial at Phase 2 was aimed to evaluate the efficacy and safety of ambroxol to enhance the cognitive and motor characteristics of GBA + PD patients [125, 133]. Research has shown that administering chaperones to raise the native protein levels of chaperone could be the gateway to GCase refolding and restoration of normal enzymatic function in the brain. Arimoclomol is one of those chemical substances, which activates the heat shock reaction, thus magnifying Hsp70 and other proteins from heat shock. The administration of arimoclomol to fibroblasts from L444P genotype patients increased GCase activity at a frequency comparable to about 1 unit of the regular ERT drug, alglucerase [132]. Another pharmacological chaperone isofagomine, has been studied in vivo and in vitro to determine the capacity to modify the GBA mutation-induced phenotype and to stabilize the GCase [134].
Another pathway to be explored for the treatment of GBA + PD is the accumulation of glucosylceramide (substrate of GCase) in the dopaminergic neurons due to GBA mutation [131, 135, 136, 137]. Recently, a phase 2 multicenter, placebo-controlled, randomized, double-blind study was initiated to analyze the pharmacokinetics, pharmacodynamics, and safety of an oral molecule, ibiglustat (SAR402671), which is capable of reducing beta-GCase levels in early-stage GBA + PD. Compared with the wild-type form, mutated GCases are much more unstable. Modification of GCase degradation may be another effective technique to increase the enzymatic function and thereby combate aggregation of α-synuclein and neurodegeneration. Hsp90 is responsible for the breakdown of misfolded GCase, together with another heat shock protein PARK-2. In general, specific HSP inhibitors and histone deacetylase inhibitors (HDACis) can enhance the levels of GCase, limiting its degradation. HDACis, in effect, prevent contact between GCase and Hdp90 by hyperactivating one of its domains [36, 138, 139, 140]. It has been proposed that the inactivation of protein phosphatase 2A may reflect the possible process via which the deficiency of GCase blocks autophagy and facilitates the aggregation of α-synuclein. Autophagy upregulation by the mTOR inhibitors polyphenols and rapamycin showed favorable effects on synucleinopathy in animal and cell models by decreasing the intracytoplasmic aggregates of protein and ultimately the death of cells. Surprisingly, these pathways tend to underlie the cross-talk between α-synuclein and GCase and can influence the propagation of disease [136, 141, 142].
Conclusion and future interventions
The most important genetic risk factor for PD was an unintended finding from GD patient trials. The identification of the link between GBA mutations and PD has given rise to vital implications and findings that advance the understanding of PD pathogenesis. The mechanism underlying the GBA mutation-induced elevated risk of PD has gained much attention in genetic research. A substantial body of evidence has supported the relationship between GCase and α-synuclein. GCase activity in PD patients is decreased in brain regions where α-synuclein builds up. The decreased activity of GCase causes neuronal aggregation of α-synuclein, which accelerates PD. Nevertheless, the lysosomal dysfunctioning has been widely investigated in PD following the initial findings. The endolysosomal pathway is implicated in α-synuclein accumulation and degeneration of dopaminergic neurons, in which a variety of genes observed in monogenic variants of PD or hereditary risk factors for the disease are involved, such as ATP13A2, SNCA, PINK-1, PARK, and LRRK2. GCase forms a complex together with membrane-bound α-synuclein, which decreases its activity. The α-synuclein–GCase complex also has a deleterious impact on α-synuclein degradation in the lysosome. There is an inverse association between α-synuclein levels and GCase activity and designed therapies that could increase the GCase activity may be useful for the treatment of PD. In addition, carriers of extreme mutations have faster disease advancement than mild mutation-carriers and homozygous mutations confer faster advancement than heterozygous mutations. The GBA + PD patients have an earlier onset age and are more likely to demonstrate cognitive dysfunction than PD patients without GBA mutation. The current promising therapies for GD are ERT and SRT, which have been approved by the FDA and are developed for the production and maintenance of GCase. These therapies have markedly enhanced the visceral symptoms of GD but have poor accessibility to the blood-brain barrier. Therapies directly targeting GBA + PD are now under clinical studies, such as chaperons (ambroxol, arimoclomol, and isofagomine), autophagy enhancers (rapamycin and polyphenols), ibiglustat (which reduces GCase levels), and GZ667161 (a glucosylceramide synthase).
Acknowledgments
The authors would like to thank Chitkara University, Punjab, India for providing the basic facilities for the completion of the current article.
Abbreviations
- ER
Endoplasmic reticulum
- ERAD
Endoplasmic reticulum associated degradation
- ERT
Enzyme replacement therapy
- GBA
Glycosylceramidase beta
- GBA+PD
GBA-associated Parkinson’s disease
- GBAP
GBA pseudogene
- GCase
Glucocerebrocidase
- GD
Gaucher’s disease
- HDACis
HSP direct inhibitors and histone deacetylase inhibitors
- IPSC
Induced pluripotential stem cells
- LB
Lewy body
- LIMP2
Lysosomal integral membrane protein-2
- MCI
Mild cognitive impairment
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- SRT
Substrate replacement therapy
Authors’ contributions
T.B. and G.K.: Conceived the idea and wrote the first draft; O.F. and C.B.: Figure work and review of literature; C.T.J-P. and N.N.: Improvement of the draft; C.B. and S.B.: Proofreading of the manuscript. The author(s) read and approved the final manuscript.
Funding
The present review received no external funding.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Tapan Behl, Gagandeep Kaur, Ovidiu Fratila, Camelia Buhas, Claudia Teodora Judea-Pusta, Nicoleta Negrut, Cristiana Bustea and Simona Bungau contributed equally to this work.
References
- 1.. Kaur G, Behl T, Bungau S, Kumar A, Uddin MS, Mehta V, Zengin G, Mathew B, Shah MA, Arora S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of numerous factors on Gut Microbiota associated Parkinson's Disease. Curr Neuropharmacol. 2020. 10.2174/1570159X18666200606233050. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 2.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. 10.1038/nrdp.2017.13. [DOI] [PubMed]
- 3.Goyal V, Radhakrishnan D. Parkinson's disease: a review. Neurol India. 2018;66(7):26. doi: 10.4103/0028-3886.226451. [DOI] [PubMed] [Google Scholar]
- 4.Zeng XS, Geng WS, Jia JJ, Chen L, Zhang PP. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front Aging Neurosci. 2018;10:109. doi: 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11(11):986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goker-Alpan O. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004;41(12):937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lwin A. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81(1):70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Sidransky E. Heterozygosity for a Mendelian disorder as a risk factor for complex disease. Clin Genet. 2006;70(4):275–282. doi: 10.1111/j.1399-0004.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 9.Eblan M, Walker J, Sidransky E. The Glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2005;352(7):728–731. doi: 10.1056/NEJM200502173520719. [DOI] [PubMed] [Google Scholar]
- 10.Klein AD, Mazzulli JR. Is Parkinson’s disease a lysosomal disorder? Brain. 2018;141(8):2255–2262. doi: 10.1093/brain/awy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, et al. Occurrence of Parkinson's syndrome in type 1 Gaucher disease. QJM. 1996;89(9):691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz M, Wilder S, Horowitz Z, Reiner O, Gelbart T, Beutler E. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989;4(1):87–96. doi: 10.1016/0888-7543(89)90319-4. [DOI] [PubMed] [Google Scholar]
- 13.Winfield SL, Tayebi N, Martin BM, Ginns EI, Sidransky E. Identification of three additional genes contiguous to the glucocerebrosidase locus on chromosome 1q21: implications for Gaucher disease. Genome Res. 1997;7(10):1020–1026. doi: 10.1101/gr.7.10.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Regan G, de Souza RM, Balestrino R, Schapira AH. Glucocerebrosidase mutations in Parkinson disease. J Park Dis. 2017;7(3):411–422. doi: 10.3233/JPD-171092. [DOI] [PubMed] [Google Scholar]
- 15.Fan K, Tang BS, Wang YQ, Kang JF, Li K, Liu ZH, et al. The GBA, DYRK1A and MS4A6A polymorphisms influence the age at onset of Chinese Parkinson patients. Neurosci Lett. 2016;621:133–6. [DOI] [PubMed]
- 16.Guo JF, Li K, Yu RL, Sun QY, Wang L, Yao LY, et al. Polygenic determinants of Parkinson's disease in a Chinese population. Neurobiol Aging. 2015;36(4):1765.e1761–1765.e1766. doi: 10.1016/j.neurobiolaging.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Sardi SP, Cheng SH, Shihabuddin LS. Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog Neurobiol. 2015;125:47–62. doi: 10.1016/j.pneurobio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Blandini F, Cilia R, Cerri S, Pezzoli G, Schapira AHV, Mullin S, et al. Glucocerebrosidase mutations and synucleinopathies: toward a model of precision medicine. Mov Disord. 2018;34(1):9–21. doi: 10.1002/mds.27583. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Sun QY, Zhao YW, Shu L, Guo JF, Xu Q, Yan XX, Tang BS. Effect of GBA Mutations on Phenotype of Parkinson's Disease: A Study on Chinese Population and a Meta-Analysis. Parkinsons Dis. 2015;2015:916971. 10.1155/2015/916971. [DOI] [PMC free article] [PubMed]
- 20.Gan-Or Z, Liong C, Alcalay RN. GBA-Associated Parkinson's Disease and Other Synucleinopathies. Curr Neurol Neurosci Rep. 2018;18(8):44. 10.1007/s11910-018-0860-4. [DOI] [PubMed]
- 21.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29(5):567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Arias R. Sequence variability of a human Pseudogene. Genome Res. 2001;11(6):1071–1085. doi: 10.1101/gr.GR-1677RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, et al. X-ray structure of human acid-β-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003;4(7):704–709. doi: 10.1038/sj.embor.embor873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginns EI, Choudary PV, Tsuji S, Martin B, Stubblefield B, Sawyer J, et al. Gene mapping and leader polypeptide sequence of human glucocerebrosidase: implications for Gaucher disease. Proc Natl Acad Sci U S A. 1985;82(20):7101–7105. doi: 10.1073/pnas.82.20.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mistry PK, Lopez G, Schiffmann R, Barton NW, Weinreb NJ, Sidransky E. Gaucher disease: Progress and ongoing challenges. Mol Genet Metab. 2017;120(1–2):8–21. doi: 10.1016/j.ymgme.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell. 2007;131(4):770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Tamargo RJ, Velayati A, Goldin E, Sidransky E. The role of saposin C in Gaucher disease. Mol Genet Metab. 2012;106(3):257–263. doi: 10.1016/j.ymgme.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorge J, West C, Kuhl W, Treger L, Beutler E. The human glucocerebrosidase gene has two functional ATG initiator codons. Am J Hum Genet. 1987;41(6):1016. [PMC free article] [PubMed] [Google Scholar]
- 29.Pasmanik-Chor M, Elroy-Stein O, Aerts H, Agmon V, Gatt S, Horowitz M. Overexpression of human glucocerebrosidase containing different-sized leaders. Biochem J. 1996;317(1):81–88. doi: 10.1042/bj3170081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Weely S, Aerts JM, Van Leeuwen MB, Heikoop JC, Donker-Koopman WE, Barranger JA, et al. Function of oligosaccharide modification in glucocerebrosidase, a membrane-associated lysosomal hydrolase. Eur J Biochem. 1990;191(3):669–677. doi: 10.1111/j.1432-1033.1990.tb19173.x. [DOI] [PubMed] [Google Scholar]
- 31.Aerts JMFG, Schram A, Strijland A, van Weely S, Jonsson LMV, Tager JM, et al. Glucocerebrosidase, a lysosomal enzyme that does not undergo oligosaccharide phosphorylation. Biochim Biophys Acta. 1988;964(3):303–308. doi: 10.1016/0304-4165(88)90030-X. [DOI] [PubMed] [Google Scholar]
- 32.Dierks T, Schlotawa L, Frese MA, Radhakrishnan K, von Figura K, Schmidt B. Molecular basis of multiple sulfatase deficiency, mucolipidosis II/III and Niemann–pick C1 disease — Lysosomal storage disorders caused by defects of non-lysosomal proteins. Biochim Biophys Acta Mol Cell Res. 2009;1793(4):710–25. [DOI] [PubMed]
- 33.Velez-Pardo C, Lorenzo-Betancor O, Jimenez-Del-Rio M, Moreno S, Lopera F, Cornejo-Olivas M, et al. The distribution and risk effect of GBA variants in a large cohort of PD patients from Colombia and Peru. Parkinsonism Relat Disord. 2019;63:204–8. [DOI] [PMC free article] [PubMed]
- 34.Zhang Y, Shu L, Zhou X, et al. A Meta-Analysis of GBA-Related Clinical Symptoms in Parkinson's Disease. Parkinsons Dis. 2018;2018:3136415. 10.1155/2018/3136415. [DOI] [PMC free article] [PubMed]
- 35.Tayebi N, Stubblefield BK, Park JK, Orvisky E, Walker JM, LaMarca ME, et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: implications for complexity in Gaucher disease. Am J Hum Genet. 2003;72(3):519–34. [DOI] [PMC free article] [PubMed]
- 36.Riboldi GM, Di Fonzo AB. GBA, Gaucher disease, and Parkinson’s disease: from genetic to clinic to new therapeutic approaches. Cells. 2019;8(4):364. doi: 10.3390/cells8040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36(3):426–8. [DOI] [PubMed]
- 38.Tayebi N, Walker J, Stubblefield B, Orvisky E, LaMarca M, Wong K, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79(2):104–109. doi: 10.1016/S1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 39.Irfan Maqsood M, Matin MM, Bahrami AR, Ghasroldasht MM. Immortality of cell lines: challenges and advantages of establishment. Cell Biol Intl. 2013;37(10):1038–1045. doi: 10.1002/cbin.10137. [DOI] [PubMed] [Google Scholar]
- 40.Tran J, Anastacio H, Bardy C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Parkinson's Dis. 2020;6(1):1–8. doi: 10.1038/s41531-019-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbloom B, Balwani M, Bronstein JM, Kolodny E, Sathe S, Gwosdow AR, et al. The incidence of parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher registry. Blood Cells Mol Dis. 2011;46(1):95–102. doi: 10.1016/j.bcmd.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schapira AHV, Gegg ME. Glucocerebrosidase in the pathogenesis and treatment of Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(9):3214–3215. doi: 10.1073/pnas.1300822110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Carvalho GB, Valente Pereira AC, da Costa RF, Vaz dos Santos A, Júnior MC, Mendonça dos Santos J, et al. Glucocerebrosidase N370S and L444P mutations as risk factors for Parkinson’s disease in Brazilian patients. Parkinsonism Relat Disord. 2012;18(5):688–689. doi: 10.1016/j.parkreldis.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Liu L, Xiong J, Zhang X, Chen Z, Yu L, et al. Glucocerebrosidase L444P mutation confers genetic risk for Parkinson’s disease in Central China. Behav Brain Funct. 2012;8(1):57. doi: 10.1186/1744-9081-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener. 2019;14:5. doi: 10.1186/s13024-019-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orme T, Hernandez D, Ross OA, Kun-Rodrigues C, Darwent L, Shepherd CE, et al. Analysis of neurodegenerative disease-causing genes in dementia with Lewy bodies. Acta Neuropathol Commun. 2020;8:5. doi: 10.1186/s40478-020-0879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burbulla LF, Krainc D. The role of dopamine in the pathogenesis of GBA1-linked Parkinson's disease. Neurobiol Dis. 2019;132:104545. doi: 10.1016/j.nbd.2019.104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Z, Huang Y, Zhang P, Han C, Lu Y, Mo Z, et al. Characterization of a pathogenic variant in GBA for Parkinson's disease with mild cognitive impairment patients. Mol Brain. 2020;13(1):102. [DOI] [PMC free article] [PubMed]
- 49.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18):1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, Viallet F, et al. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology. 2012;78(6):417–420. doi: 10.1212/WNL.0b013e318245f476. [DOI] [PubMed] [Google Scholar]
- 51.Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132(7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 2012;15(2):146–149. doi: 10.1038/gim.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimran A, Altarescu G, Elstein D. Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells Mol Dis. 2013;50(2):134–137. doi: 10.1016/j.bcmd.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 54.McNeill A, Wu RM, Tzen KY, Aguiar PC, Arbelo JM, Barone P, et al. Dopaminergic neuronal imaging in genetic Parkinson's disease: insights into pathogenesis. PLoS One. 2013;8(7):e69190. doi: 10.1371/journal.pone.0069190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, Halter CA, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2008;72(4):310–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horowitz M, Pasmanik-Chor M, Ron I, Kolodny EH. The enigma of the E326K mutation in acid β-glucocerebrosidase. Mol Genet Metab. 2011;104(1–2):35–38. doi: 10.1016/j.ymgme.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Lesage S, Condroyer C, Hecham N, Anheim M, Belarbi S, Lohman E, et al. Mutations in the glucocerebrosidase gene confer a risk for Parkinson disease in North Africa. Neurology. 2011;76(3):301–303. doi: 10.1212/WNL.0b013e318207b01e. [DOI] [PubMed] [Google Scholar]
- 58.Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 59.Mallett V, Ross JP, Alcalay RN, Ambalavanan A, Sidransky E, Dion PA, et al. GBA p.T369M substitution in Parkinson disease: Polymorphism or association? A meta-analysis. Neurol Genet. 2016;2(5):e104. [DOI] [PMC free article] [PubMed]
- 60.Gallagher DA, Schapira AH. Etiopathogenesis and treatment of Parkinson's disease. Curr Top Med Chem. 2009;9(10):860–8. [PubMed]
- 61.Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson's disease: the mutation matters. Ann Neurol. 2016;80(5):662–73. [DOI] [PubMed]
- 62.Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, et al. Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's. Ann Neurol. 2016;80(5):674–685. doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thaler A, Gurevich T, Shira AB, Weisz MG, Ash E, Shiner T, et al. A “dose” effect of mutations in the GBA gene on Parkinson's disease phenotype. Parkinsonism Relat Disord. 2017;36:47–51. doi: 10.1016/j.parkreldis.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Bultron G, Kacena K, Pearson D, Boxer M, Yang R, Sathe S, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis. 2010;33(2):167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNeill A, Duran R, Hughes DA, Mehta A, Schapira AHV. A clinical and family history study of Parkinson's disease in heterozygous glucocerebrosidase mutation carriers. J Neurol Neurosurg Psychiatry. 2012;83(8):853–4. [DOI] [PMC free article] [PubMed]
- 66.McNeill A, Duran R, Proukakis C, Bras J, Hughes D, Mehta A, et al. Hyposmia and cognitive impairment in Gaucher disease patients and carriers. Mov Disord. 2012;27(4):526–532. doi: 10.1002/mds.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato C, Morgan A, Lang AE, Salehi-Rad S, Kawarai T, Meng Y, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov Disord. 2005;20:367–370. doi: 10.1002/mds.20319. [DOI] [PubMed] [Google Scholar]
- 68.Tan EK, Tong J, Fook-Chong S, Yih Y, Wong MC, Pavanni R, et al. Glucocerebrosidase mutations and risk of Parkinson disease in Chinese patients. Arch Neurol. 2007;64:1056–1058. doi: 10.1001/archneur.64.7.1056. [DOI] [PubMed] [Google Scholar]
- 69.De Marco EV, Annesi G, Tarantino P, Rocca FE, Provenzano G, Civitelli D, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in southern Italy. Mov Disord. 2008;23:460–463. doi: 10.1002/mds.21892. [DOI] [PubMed] [Google Scholar]
- 70.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. New Eng J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 71.Emelyanov A, Boukina T, Yakimovskii A, Usenko T, Drosdova A, Zakharchuk A, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in Russia. Mov Disord. 2011;27:158–159. doi: 10.1002/mds.23950. [DOI] [PubMed] [Google Scholar]
- 72.Mitsui J, Mizuta I, Toyoda A, Ashida R, Takahashi Y, Goto J, Fukuda Y, Date H, Iwata A, Yamamoto M, Hattori N, Murata M, Toda T, Tsuji S. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66(5):571–6. 10.1001/archneurol.2009.72. [DOI] [PubMed]
- 73.Choi JM, Kim WC, Lyoo CH, Kang SY, Lee PH, Baik JS, et al. Association of mutations in the glucocerebrosidase gene with Parkinson disease in a Korean population. Neurosci Lett. 2012;514:12–15. doi: 10.1016/j.neulet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 74.Bras JM, Singleton A. Genetic susceptibility in Parkinson's disease. Biochim Biophys Acta. 2009;1792(7):597–603. doi: 10.1016/j.bbadis.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun QY, Guo JF, Wang L, Yu RH, Zuo X, Yao LY, et al. Glucocerebrosidase gene L444P mutation is a risk factor for Parkinson's disease in Chinese population. Mov Disord. 2010;25:1005–11. [DOI] [PubMed]
- 76.Spitz M, Rozenberg R, da Veiga PL, Barbosa ER. Association between Parkinson's disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord. 2008;14:58–62. doi: 10.1016/j.parkreldis.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Wu YR, Chen CM, Chao CY, Ro LS, Lyu RK, Chang KH, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78:977–979. doi: 10.1136/jnnp.2006.105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452:87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 79.Saunders-Pullman R, Hagenah J, Dhawan V, Stanley K, Pastores G, Sathe S, et al. Gaucher disease ascertained through a Parkinson's center: imaging and clinical characterization. Mov Disord. 2010;25:1364–1372. doi: 10.1002/mds.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzulli Joseph R, Xu YH, Sun Y, Knight Adam L, McLean Pamela J, Caldwell Guy A, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moors T, Paciotti S, Chiasserini D, Calabresi P, Parnetti L, Beccari T, et al. Lysosomal dysfunction and α-synuclein aggregation in Parkinson's disease: diagnostic links. Mov Disord. 2016;31(6):791–801. doi: 10.1002/mds.26562. [DOI] [PubMed] [Google Scholar]
- 82.Gündner AL, Duran-Pacheco G, Zimmermann S, Ruf I, Moors T, Baumann K, et al. Path mediation analysis reveals GBA impacts Lewy body disease status by increasing α-synuclein levels. Neurobiol Dis. 2019;121:205–213. doi: 10.1016/j.nbd.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJA, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42(3):360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bae EJ, Yang NY, Lee C, Lee HJ, Kim S, Sardi SP, et al. Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and α-synuclein aggregation. Exp Mol Med. 2015;47(3):e153. doi: 10.1038/emm.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gruschus JM. Did α-synuclein and glucocerebrosidase coevolve? Implications for Parkinson’s disease. PLoS ONE. 2015;10(7):e0133863. doi: 10.1371/journal.pone.0133863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gruschus JM, Jiang Z, Yap TL, Hill SA, Grishaev A, Piszczek G, et al. Dissociation of glucocerebrosidase dimer in solution by its co-factor, saposin C. Biochem Biophys Res Comm. 2015;457(4):561–566. doi: 10.1016/j.bbrc.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yap TL, Gruschus JM, Velayati A, Sidransky E, Lee JC. Saposin C protects glucocerebrosidase against α-synuclein inhibition. Biochemistry. 2013;52(41):7161–7163. doi: 10.1021/bi401191v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yap TL, Jiang Z, Heinrich F, Gruschus JM, Pfefferkorn CM, Barros M, et al. Structural features of membrane-bound glucocerebrosidase and α-synuclein probed by neutron reflectometry and fluorescence spectroscopy. J Biol Chem. 2015;290(2):744–754. doi: 10.1074/jbc.M114.610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound α-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol Genet Metab. 2013;108(1):56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fishbein I, Kuo YM, Giasson BI, Nussbaum RL. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain. 2014;137(12):3235–47. [DOI] [PMC free article] [PubMed]
- 91.Kim S, Yun SP, Lee S, Umanah GE, Bandaru VVR, Yin X, et al. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc Natl Acad Sci U S A. 2018;115(4):798–803. doi: 10.1073/pnas.1700465115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cooper AA. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313(5785):324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson's disease. Antioxid Redox Signal. 2007;9(5):553–561. doi: 10.1089/ars.2006.1524. [DOI] [PubMed] [Google Scholar]
- 94.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14(16):2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 95.Schmitz M, Alfalah M, Aerts JMFG, Naim HY, Zimmer KP. Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher's disease. Int J Biochem Cell Biol. 2005;37(11):2310–20. [DOI] [PubMed]
- 96.Zimmer KP, le Coutre P, Aerts HM, Harzer K, Fukuda M, O'Brien JS, et al. Intracellular transport of acid β-glucosidase and lysosome-associated membrane proteins is affected in Gaucher's disease (G202R mutation) J Pathol. 1999;188(4):407–414. doi: 10.1002/(SICI)1096-9896(199908)188:4<407::AID-PATH377>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 97.Grosu F, Ungureanu A, Bianchi E, Moscu B, Coldea L, Stupariu AL, et al. Multifocal and multicentric low-grade oligoastrocytoma in a young patient. Romanian J Morphol Embryol. 2017;58(1):207–210. [PubMed] [Google Scholar]
- 98.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275(23):5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gan-Or Z, Ozelius LJ, Bar-Shira A, Saunders-Pullman R, Mirelman A, Kornreich R, et al. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology. 2013;80(17):1606–1610. doi: 10.1212/WNL.0b013e31828f180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grassmé H, Riethmüller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46(3–4):161–170. doi: 10.1016/j.plipres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 101.Kitatani K, Sheldon K, Anelli V, Jenkins RW, Sun Y, Grabowski GA, et al. Acid β-glucosidase 1 counteracts p38δ-dependent induction of interleukin-6. J Biol Chem. 2009;284(19):12979–88. [DOI] [PMC free article] [PubMed]
- 102.Ambrosi G, Ghezzi C, Zangaglia R, Levandis G, Pacchetti C, Blandini F. Ambroxol-induced rescue of defective glucocerebrosidase is associated with increased LIMP-2 and saposin C levels in GBA1 mutant Parkinson's disease cells. Neurobiol Dis. 2015;82:235–242. doi: 10.1016/j.nbd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Sanchez-Martinez A, Beavan M, Gegg ME, Chau KY, Whitworth AJ, Schapira AH. Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci Rep. 2016;6:31380. 10.1038/srep31380. [DOI] [PMC free article] [PubMed]
- 104.Woodard CM, Campos BA, Kuo SH, Nirenberg MJ, Nestor MW, Zimmer M, et al. iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell Rep. 2014;9:1173–1182. doi: 10.1016/j.celrep.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Migdalska-Richards A, Daly L, Bezard E, Schapira AHV. Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice. Ann Neurol. 2016;80:766–75. [DOI] [PMC free article] [PubMed]
- 106.Holleran WM, Ginns EI, Menon GK, Grundmann JU, Fartasch M, McKinney CE, et al. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest. 1994;93(4):1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, et al. Mitochondria and quality control defects in a mouse model of Gaucher disease—links to Parkinson’s disease. Cell Metab. 2013;17:941–53. [DOI] [PMC free article] [PubMed]
- 108.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid β-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003;163:2093–2101. doi: 10.1016/S0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keatinge M, Bui H, Menke A, Chen YC, Sokol AM, Bai Q, et al. Glucocerebrosidase 1 deficientDanio reriomirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death. Hum Mol Genetics. 2015;24:6640–6652. doi: 10.1093/hmg/ddv369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Momcilovic O, Sivapatham R, Oron TR, Meyer M, Mooney S, Rao MS, et al. Derivation, characterization, and neural differentiation of integration-free induced pluripotent stem cell lines from Parkinson’s disease patients carrying SNCA, LRRK2, PARK2, and GBA mutations. PLoS One. 2016;11:e0154890. doi: 10.1371/journal.pone.0154890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, Schapira AHV. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genetics. 2016;25:3432–3445. doi: 10.1093/hmg/ddw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makkar R, Behl T, Bungau S, Zengin G, Mehta V, Kumar A, et al. Nutraceuticals in neurological disorders. Int J Mol Sci. 2020;21(12):4424. [DOI] [PMC free article] [PubMed]
- 113.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–9. [DOI] [PMC free article] [PubMed]
- 114.Devi L, Anandatheerthavarada HK. Mitochondrial trafficking of APP and alpha synuclein: relevance to mitochondrial dysfunction in Alzheimer's and Parkinson's diseases. Biochim Biophys Acta. 2010;1802(1):11–19. doi: 10.1016/j.bbadis.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu X, Mao C, Fan L, Luo H, Hu Z, Zhang S, et al. Modeling Parkinson's disease using induced pluripotent stem cells. Stem Cells Int. 2020;2020:1061470. doi: 10.1155/2020/1061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Enquist IB, Bianco CL, Ooka A, Nilsson E, Mansson JE, Ehinger M, et al. Murine models of acute neuronopathic Gaucher disease. Proc Natl Acad Sci U S A. 2007;104:17483–17488. doi: 10.1073/pnas.0708086104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rostovtseva TK, Gurnev PA, Protchenko O, Hoogerheide DP, Yap TL, Philpott CC, et al. α-Synuclein shows high affinity interaction with voltage-dependent anion channel, suggesting mechanisms of mitochondrial regulation and toxicity in Parkinson disease. J Biol Chem. 2015;290:18467–18477. doi: 10.1074/jbc.M115.641746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61:187–196. doi: 10.1016/j.survophthal.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 119.Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P. Triggers, facilitators, and aggravators: redefining Parkinson’s disease pathogenesis. Trends Neurosci. 2019;42:4–13. doi: 10.1016/j.tins.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davidson BA, Hassan S, Garcia EJ, Tayebi N, Sidransky E. Exploring genetic modifiers of Gaucher disease: the next horizon. Hum Mutat. 2018;39:1739–1751. doi: 10.1002/humu.23611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G, et al. N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103–106. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abdel-Daim MM, El-Tawil OS, Bungau SG, Atanasov AG. Applications of antioxidants in metabolic disorders and degenerative diseases: mechanistic approach. Oxidative Med Cell Longev. 2019;2019:4179676. doi: 10.1155/2019/4179676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chahine LM, Qiang J, Ashbridge E, Minger J, Yearout D, Horn S, et al. Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 2013;70:852. doi: 10.1001/jamaneurol.2013.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pandey MK, Jabre NA, Xu YH, Zhang W, Setchell KDR, Grabowski GA. Gaucher disease: chemotactic factors and immunological cell invasion in a mouse model. Mol Genet Metab. 2014;111(2):163–71. [DOI] [PubMed]
- 125.Sian-Hülsmann J, Mandel S, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem. 2011;118(6):939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- 126.Kozlowski H, Luczkowski M, Remelli M, Valensin D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer's, Parkinson's and prion diseases) Coord Chem Rev. 2012;256(19–20):2129–2141. doi: 10.1016/j.ccr.2012.03.013. [DOI] [Google Scholar]
- 127.Kong SMY, Chan BKK, Park JS, Hill KJ, Aitken JB, Cottle L, et al. Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-synuclein externalization via exosomes. Hum Mol Genet. 2014;23(11):2816–33. [DOI] [PubMed]
- 128.Tsunemi T, Hamada K, Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J Neurosci. 2014;34(46):15281–15287. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsunemi T, Krainc D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet. 2014;23(11):2791–2801. doi: 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Selvaraj S, Piramanayagam S. Impact of gene mutation in the development of Parkinson's disease. Genes Dis. 2019;6(2):120–128. doi: 10.1016/j.gendis.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, Lee HJ, Kim S, Masliah E, Sardi SP, Lee SJ. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat Commun. 2014;5:4755. 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed]
- 132.Fog CK, Zago P, Malini E, Solanko LM, Peruzzo P, Bornaes C, et al. The heat shock protein amplifier arimoclomol improves refolding, maturation and lysosomal activity of glucocerebrosidase. EBioMedicine. 2018;38:142–153. doi: 10.1016/j.ebiom.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schneider SA, Alcalay RN. Precision medicine in Parkinson's disease: emerging treatments for genetic Parkinson's disease. J Neurol. 2020;267(3):860–869. doi: 10.1007/s00415-020-09705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Migdalska-Richards A, Ko WKD, Li Q, Bezard E, Schapira AHV. Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse. 2017;71:e21967. doi: 10.1002/syn.21967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, et al. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci U S A. 2011;108(29):12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164(2):541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 137.Nuzhnyi E, Emelyanov A, Boukina T, Usenko T, Yakimovskii A, Zakharova E, et al. Plasma oligomeric alpha-synuclein is associated with glucocerebrosidase activity in Gaucher disease. Mov Disord. 2015;30(7):989–991. doi: 10.1002/mds.26200. [DOI] [PubMed] [Google Scholar]
- 138.Yang C, Swallows CL, Zhang C, Lu J, Xiao H, Brady RO, et al. Celastrol increases glucocerebrosidase activity in Gaucher disease by modulating molecular chaperones. Proc Natl Acad Sci U S A. 2014;111(1):249–254. doi: 10.1073/pnas.1321341111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, et al. Reversible conformational conversion of α-synuclein into toxic assemblies by glucosylceramide. Neuron. 2018;97(1):92–107. e110. doi: 10.1016/j.neuron.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, et al. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137(5):1481–95. [DOI] [PMC free article] [PubMed]
- 141.Bové J, Martínez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12(8):437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 142.Hajieva P. The effect of polyphenols on protein degradation pathways: implications for neuroprotection. Molecules. 2017;22(1):159. doi: 10.3390/molecules22010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.