Abstract
The generation of β-amyloid protein (Aβ) is considered a key step in the pathogenesis of Alzheimer's disease (AD) and the regulation of its production is an important therapeutic strategy. It was hypothesized in the present study that Nogo-A may be involved in AD and may regulate the generation of Aβ. Nogo-A is known to act as a major inhibitor of neuron regeneration in the adult central nervous system. A recent study indicated that Nogo-A is associated with AD; however, the underlying effect and molecular mechanisms remain largely elusive. In the present study, the potential effects of Nogo-A on AD were investigated. ELISA was used to detect the levels of Aβ, enzymatic activity detection kits were used to determine the activity of secretase enzymes in amyloid precursor protein (APP) metabolism, and western blot analysis was used to detect the expression levels of proteins associated with the APP processing and Nogo-A/Nogo-66 receptor (NgR) signaling pathways. The results revealed that Nogo-66, the major inhibitory region of Nogo-A, promoted neuronal Aβ secretion by increasing the activity of β-secretase 1 via the NgR/Rho-associated coiled-coil containing kinases pathway in a dose-dependent manner. The present data suggested that Nogo-A may facilitate the onset and development of AD by promoting Aβ secretion, providing information on a potential novel target for AD therapy.
Keywords: Nogo-A, Nogo-66 receptor, β-amyloid, β-secretase 1, Alzheimer's disease
Introduction
Alzheimer's disease (AD) is the most common type of dementia; the two disease-defining pathological features of AD are senile plaques (SPs) predominantly consisting of β-amyloid protein (Aβ), and neurofibrillary tangles (NFTs) comprising p-Tau (1,2). The excessive production and accumulation of Aβ are essential steps in the formation of SPs. The amyloid hypothesis, which states that the accumulation of Aβ induces neuronal loss and cognitive impairment (3), holds a dominant position in AD pathogenesis. Aβs are a group of polypeptides containing 39–43 amino acids produced by the proteolysis of amyloid precursor protein (APP) by β-secretase and γ-secretase (4). The tau theory is another hypothesis of AD, which states that the hyperphosphorylation of tau leads to the formation of NFTs and degeneration of neurons. The apolipoprotein (APO)E4 variant is considered a genetic risk factor for sporadic AD; in a previous study, converting APOE4 to APOE3 in brain cell types, including neurons, astrocytes and microglia-like cells, was sufficient to attenuate numerous AD-related pathologies (5). In addition, there are several possible novel etiologies of AD, including defective mitophagy (6), lack of nicotinamide adenine dinucleotide (7) and Aβ-induced oligodendrocyte progenitor cell senescence (8).
Nogo-A is one of three subtypes of the Nogo protein, which is a member of the reticulon (RTN) family; Nogo-A consists of a long N-terminal segment and a C-terminal reticulon homology domain (RHD) (9). There is a special domain between the two hydrophobic segments of the RHD domain of Nogo-A, known as Nogo-66, which is the major inhibitory region of Nogo-A (9). Nogo-A has been reported to be expressed by oligodendrocytes and is considered a major component of myelin in the central nervous system (CNS) (10). In addition, Nogo-A has been identified as a key molecule that limits axon regeneration and is considered to be a major obstacle to nerve regeneration following injury in the adult mammalian CNS (11). Nogo-A interacts with the specific receptor complex on neurons to prevent neurite outgrowth; this receptor complex is composed of Nogo-66 receptor 1 (NgR1), and two complementary co-receptors p75 and Ig-like domain-containing NgR1-interacting protein 1 (LINGO-1) (12).
It has been suggested that Nogo-A may be involved in the pathology of AD. Nogo-A is normally expressed in the hippocampus of healthy elderly individuals, but it is overexpressed in the brain tissues of patients with AD and is associated with Aβ deposits in SPs (13). It has previously been demonstrated that inhibition of the Nogo-A/NgR pathway by Nogo-66 antagonist peptide (NEP1-40) attenuated amyloidogenic processing of APP and reduced Aβ plaque deposition in APP/PS1 mice (14). It has also been hypothesized that Nogo-A may alter neuronal APP metabolism and Aβ production. Our previous study reported that Nogo-P4, the 31–55 amino acid of the Nogo-66 peptide, promoted Aβ secretion in cortical neurons (15). However, whether the full-length Nogo-66 protein has the same effect, as well as the underlying mechanisms of Nogo-66 in APP regulation, are not clear. Therefore, the aim of the present study was to investigate whether Nogo-66 promotes Aβ secretion in cortical neurons, Neuro2a (N2a) cells and Sprague-Dawley rats. Furthermore, the present study explored the possible underlying molecular mechanism of Nogo-66 on Aβ secretion, which in turn may provide insight into possible effects and mechanisms of the Nogo-A/NgR1 pathway in AD pathogenesis.
Materials and methods
Reagents
Soluble Nogo-66 protein was obtained via SUMO fusion in Escherichia coli from our laboratory (16). Y-27632 and the NEP1-40 were obtained from Merck KGaA and Tocris Bioscience, respectively. The Cy3-conjugated anti-rabbit secondary antibody was obtained from Abcam (cat. no. ab6939). Rabbit monoclonal IgG anti-APP was purchased from LifeSpan BioSciences, Inc. In addition, the following primary antibodies were obtained: Anti-β-secretase 1 (BACE1; cat. no. 5606; Cell Signaling Technology, Inc.), anti-ROCK2 (cat. no. ab125025; Abcam), anti-phosphorylated (p)-collapsin response mediator protein-2 (CRMP2; Thr514; cat. no. 9397; Cell Signaling Technology, Inc.), anti-CRMP2 (cat. no. 35672; Cell Signaling Technology, Inc.), anti-GAPDH (cat. no. BS72410; Bioworld Technology, Inc.) and anti-microtubule-associated protein 2 (MAP2; cat. no. 05-346; Merck KGaA). HRP-conjugated goat anti-rabbit IgG antibody (cat. no. E030120-01; Earthox Life Sciences) was used. All reagents and drugs used were of analytical grade.
Animals
Sprague-Dawley rats (n=24; age, 3 months; weight, 330±20 g) were provided by the Experimental Animal Center of Guangdong Province. Animals were randomly divided into the following groups (n=6 per group): i) Control; ii) low dose; iii) middle dose; and iv) high dose. Rats were housed under standard temperature (24±1°C) with 60±10% humidity, diurnal conditions (lights on, 08:00-20:00), and ad libitum access to food and water. The present study was approved by the Animal Research Committee of the School of Medicine, Jinan University (Guangzhou, China; approval no. IACUC-20180120-05).
Cell culture
Cortical neurons were prepared from postnatal rats (n=20; age, 1–3 days; weight, 6–8 g) (17), which were obtained from the Guangdong Medical Laboratory Animal Center. Animals were housed at a standard temperature (24±1°C) with 60±10% humidity, diurnal conditions (lights on, 08:00-20:00), and ad libitum access to food and water. Briefly, the pups were sacrificed by cervical dislocation without anesthesia, in accordance with the procedures approved by the local ethics review board, and the cerebral tissues were dissociated by combined trituration and 0.25% trypsin (Thermo Fisher Scientific, Inc.) at 37°C for 30 min. The debris was removed and the supernatant was centrifuged at 1,000 × g for 5 min at 4°C to collect the cell pellet. Cortical neurons were plated at a density of 2.1×105/well into 24-well plates on poly-L-lysine (PLL; Merck KGaA)-coated borosilicate glass coverslips (16-mm diameter; VWR International, LLC) or at a density of 1×106/well in 6-well plates coated with 100 µg/ml PLL in neurobasal medium (Thermo Fisher Scientific, Inc.) containing 25 mM KCl, 2% B27 (Thermo Fisher Scientific, Inc.), 1.5 mM glutamine (Thermo Fisher Scientific, Inc.) and 0.01% penicillin/streptomycin (Merck KGaA).
N2a mouse neuroblastoma cells stably expressing the human Swedish mutant APP695 (APP695 N2a cells) (18) were used in the present study, and were kindly provided by Dr Cuizan Cai (Macau University of Science and Technology, Macao, China). The cells were maintained in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Shanghai Shuangwei Biological Technology Co., Ltd.), 200 µg/ml G418 (Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 before the experiments.
Following treatment with different concentrations of Nogo-66 (0.25, 0.5 and 1 µM Nogo-66) or a buffer control (PBS) for 48 h at 37°C, cortical neurons and N2a mouse neuroblastoma cells were cultured for 48 h. Cells were prepared in RIPA lysis buffer containing a cocktail of complete protease inhibitors (Merck KGaA). The lysates were clarified by centrifugation at 12,000 × g for 5 min at 4°C to remove the insoluble components.
Following culture for 48 h, cortical neurons and N2a mouse neuroblastoma cells were treated with Y-27632 (100 µM) and NEP1-40 (2 µM) for 1 h of pre-protection at 37°C. Subsequently, cells were treated with different concentrations of Nogo-66 (1 µM) for 48 h at 37°C. Cells were then prepared in RIPA lysis buffer containing a cocktail of complete protease inhibitors (Merck KGaA). The lysates were clarified by centrifugation at 12,000 × g for 5 min at 4°C to remove the insoluble components.
Neurite outgrowth assay
Cortical neuron cells were plated (2.1×105/well) onto 0.01% PLL-coated coverslips in 24-well culture dishes for 48 h following treatment with different concentrations of Nogo-66 (0.25, 0.5 and 1 µM) and a buffer control (PBS) for 48 h at 37°C. The cells were fixed for 20 min in 4% paraformaldehyde at room temperature and washed with PBS. After rinsing several times with PBS, the cells were blocked and permeabilized for 30 min at room temperature with PBS containing 5% goat serum (Gibco; Thermo Fisher Scientific, Inc.), 1% (w/v) BSA (GenView Scientific, Inc.) and 0.05% Triton X-100. The cells were then rinsed and incubated with anti-MAP2 antibody (1:500; Merck KGaA) at 4°C overnight. The cells were then rinsed again and incubated for 1 h with goat anti-rabbit Cy3 secondary antibody (1:1,000; cat. no. ab6939; Abcam) at room temperature. All slides were visualized under a laser scanning confocal microscope (LSM 700; Carl Zeiss AG). Each group was assessed in triplicate and the experiments were repeated four times. The average length of each neuron was measured with Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.). Neurites were measured only if they were >20 µm in length. A total of 100 neurons were measured from each group in each experiment. These counts were averaged and the SD values were calculated using Graph Pad Prism v5.02 (GraphPad Software, Inc.). Mean values were obtained by averaging values from the measurement of ~100 neurons per well in three separate wells per group.
Intra-hippocampal injection of Nogo-66
Male Sprague-Dawley rats (age, 12 weeks; weight, 330±20 g; Guangdong Medical Laboratory Animal Center) were anesthetized via an intravenous injection of 30 mg/kg pentobarbital sodium (Merck KGaA) and were placed in a stereotaxic frame. Unilateral injections of 2.5 µl physiological saline, or 0.5, 1 and 2 mg/kg Nogo-66, were administered at a rate of 0.5 µl/min into the left and right hippocampus (3.0 mm posterior to the bregma, 1.8 mm lateral to the midline and 2.6 mm below the dura mater). Following the intra-hippocampal injection, the scalp was sutured and sterilized. The rats were kept warm for 2 h before being returned to their cages. After 4 days, the rats were euthanized by CO2 asphyxiation; the filling rate of CO2 was 20% chamber volume per minute. Subsequently, hippocampal tissues from the rats were rapidly harvested and stored at −80°C until further processing for biochemical analysis.
After 4 days, hippocampal tissue lysates were extracted for biochemical assays. Hippocampal tissues were dissected and homogenized in RIPA buffer (Beyotime Institute of Biotechnology) containing a cocktail of complete protease inhibitors (Merck KGaA). The lysate was centrifuged at 12,000 × g for 15 min at 4°C. Protein concentrations were determined using a BCA assay kit (Thermo Fisher Scientific, Inc.).
Western blotting
The cortical neurons and APP-overexpression (induced by the human Swedish mutant APP695) N2a cells were prepared in RIPA lysis buffer containing a cocktail of complete protease inhibitors (Merck KGaA). The lysates were clarified by centrifugation at 12,000 × g for 5 min at 4°C to remove the insoluble components. The protein concentration in the supernatant was detected using a BCA assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of proteins (0.125–2 mg/ml) were separated by SDS-PAGE on 10 or 12% gels and were transferred to PVDF membranes. The membranes were blocked with TBS-Tween-20 (0.1%) containing 5% non-fat dry milk at room temperature for 1 h, and incubated overnight at 4°C with the following primary antibodies: Anti-APP (1:1,000; Lifespan Biosciences, Inc.), anti-APP-C99 (1:2,000; Merck KGaA), anti-secreted APP-α (sAPPα; 1:1,000; BioLegend, Inc.), anti-BACE1 (1:1,000; Cell Signaling Technology, Inc.), anti-ROCK2 (1:1,000; Abcam), anti-CRMP2 (1:1,000; Cell Signaling Technology, Inc.), anti-p-CRMP2 Thr 509/514 (1:1,000; Cell Signaling Technology, Inc.) and anti-GAPDH (1:10,000; Bioworld Technology, Inc.). The membranes were then incubated with HRP-conjugated goat anti-rabbit IgG antibody (1:2,000; cat. no. E030120-01; Earthox Life Sciences) for 1 h at room temperature. The blots were detected using an ECL detection kit (Tanon Science and Technology Co., Ltd.), according to the manufacturer's instructions. The results were analyzed using Quantity-One software (version 4.6.6; Bio-Rad Laboratories, Inc.) to determine the relative ratio. The hippocampal tissue was prepared and analyzed in the same way.
ELISA for Aβ40 and Aβ42
The total levels of Aβ40 and Aβ42 in the culture medium of cultured neurons and the hippocampal homogenate of rats were detected using ELISA kits (cat. nos. 294-62501 and 292-64501; Wako Chemicals GmbH), according to the manufacturer's instructions. For human or rat Aβ40 and Aβ42 ELISA, the monoclonal BAN50 antibody, which specifically detected the N-terminal portion of human Aβ1-16, was used as the capturing antibody, and the monoclonal BA27 and BC05 antibodies, which detect the C-terminal portion of Aβ1-40 and Aβ1-42, respectively, were used as detector antibodies. The amount of Aβ was calculated by comparing these absorbance values with the control solutions.
Enzymatic activity assay
The β-Secretase Activity Assay Kit (cat. no. 565785; Merck KGaA) was used to measure the BACE1 activity of cells and hippocampal tissue according to manufacturer's protocol. Protein lysates (20–30 µl; 5–7 mg/ml) were used in vitro assays. All fluorescent values were detected on a plate reader with excitation (Ex) at 320 nm and emission (Em) at 405 nm (BioTek China). The ADAM10 Activity Detection kit (AS-72226; AnaSpec) was used to measure ADAM10 activity in cells and hippocampal tissue according to the manufacturer's protocol. Samples (50 µl) were used for in vitro assays. Fluorescence intensity was measured at Ex/Em=490/520 nm after incubating the reaction at 37°C for 30 min in the dark.
Statistical analysis
All experiments were repeated 3–4 times. All values are expressed as the mean ± SD. Analyses were performed using SPSS 16.0 statistical software (SPSS, Inc.). Comparison of parameters among more than two groups was made by one-way ANOVA followed by the post hoc Tukey test. P<0.05 was considered to indicate a statistically significant difference.
Results
Nogo-66 inhibits neurite outgrowth and promotes Aβ secretion in a dose-dependent manner
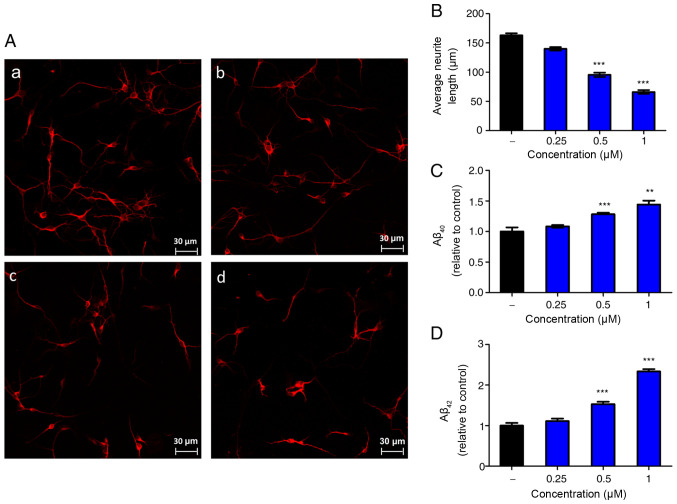
To observe the effect of Nogo-66 on neurite growth, neurons from the cerebral cortex of newborn rats were analyzed in vitro. A laser scanning confocal microscope was used to analyze the average neurite length. Notably, Nogo-66 had a dose-dependent inhibitory effect on neurite outgrowth (Fig. 1A and B). Quantification of the neurite length demonstrated that 0.25, 0.5 and 1 µM Nogo-66 markedly decreased neurite outgrowth compared with that in the control group; the neurite length was ~87, 62 and 37% of the control values respectively (Fig. 1B).
Figure 1.
Nogo-66 inhibits neurite outgrowth and promotes Aβ secretion in primary cortical neurons. (A) Immune-fluorescence micrographs of microtubule-associated protein 2 in cultured neurons treated with different concentrations of Nogo-66. (a) PBS (vehicle control); (b) 0.25 µM Nogo-66; (c) 0.5 µM Nogo-66; (d) 1 µM Nogo-66. Scale bar, 30 µm. (B) Semi-quantification of neurite outgrowth in (A). Data are presented as the mean ± SD (n=8). ***P<0.001 vs. the PBS group. Nogo-66 induced (C) Aβ40 and (D) Aβ42 production in a dose-dependent manner in cortical neurons. Data are presented as the mean ± SD (n=4). **P<0.01, ***P<0.001 vs. control. Aβ, β-amyloid protein.
To determine whether Nogo-66 had an effect on the production of Aβ, the cortical neurons were exposed to various concentrations of Nogo-66 (0.25, 0.5 and 1 µM) and a buffer control (PBS), and secreted Aβ40 and Aβ42 levels were measured by ELISA. Treatment with 0.5 or 1 µM Nogo-66 significantly increased the levels of Aβ40 and Aβ42 compared with those in the control group (Fig. 1C and D). ELISA analysis indicated that the levels of Aβ40 and Aβ42 in the Nogo-66 group (0.5 and 1 µM) were higher than those detected in the PBS groups.
Inhibition of NgR1/ROCK pathway reduces Aβ secretion
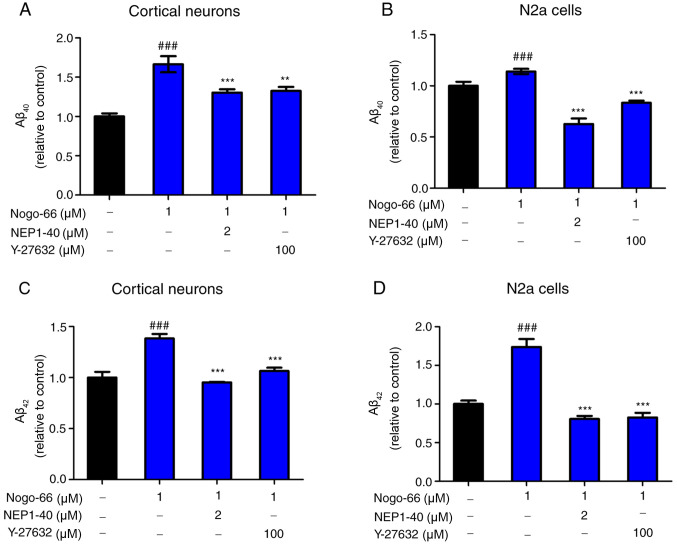
Primary cortical neurons and N2a cells were used, which were transduced with lentivirus expressing human APP695, to determine whether the pharmacological inhibition of ROCK and NgR1 reduced Aβ production. To determine how Nogo-66 influenced APP processing to Aβ, secreted Aβ40 and Aβ42 levels were measured in primary cortical neurons and APP-overexpressing N2a cells by ELISA, following treatment with NEP1-40 or Y-27632 prior to treatment with 1 µM Nogo-66. The optimum concentrations of NEP1-40 and Y-27632 had already been explored by adding a series of concentrations of NEP1-40 (0, 0.5, 1 and 2 µM) or Y-27632 (0, 12.5, 25, 50 and 100 µM) with Nogo-66 to the cortical neurons; the levels of Aβ40 and Aβ42 were decreased in a dose-dependent manner (data not shown). To explore whether NEP1-40 and Y-27632 could reduce Aβ secretion, the cortical neurons were exposed to NEP1-40 and Y-27632. Following treatment with 2 µM NEP1-40, secreted Aβ40 and Aβ42 levels were lower compared with those detected in the Nogo-66-only group (Fig. 2A and C). In addition, the effects of treatment with 100 µM Y-27632 were similar to those detected following treatment with NEP1-40 (Fig. 2A and C). In addition, compared with in the PBS group, Nogo-66 (1 µM) significantly increased the levels of secreted Aβ40 and Aβ42. The results of APP-overexpressing N2a cells were consistent with those for cortical neurons (Fig. 2B and D).
Figure 2.
NEP1-40 or Y-27632 overcome Nogo-66-induced production of Aβ40 and Aβ42 in cortical neurons and N2a cells. NEP1-40 and Y-27632 decreased the levels of (A) Aβ40 and (C) Aβ42 in cortical neurons. NEP1-40 and Y-27632 decreased the levels of (B) Aβ40 and (D) Aβ42 in N2a cells. Data are presented as the mean ± SD (n=6). ###P<0.001 vs. control; **P<0.01 vs. control; ***P<0.001 vs. Nogo-66 group. NEP1-40, Nogo-66 antagonist peptide; N2a, Neuro2a; Aβ, β-amyloid protein.
Effect of Nogo-66 on processing of APP
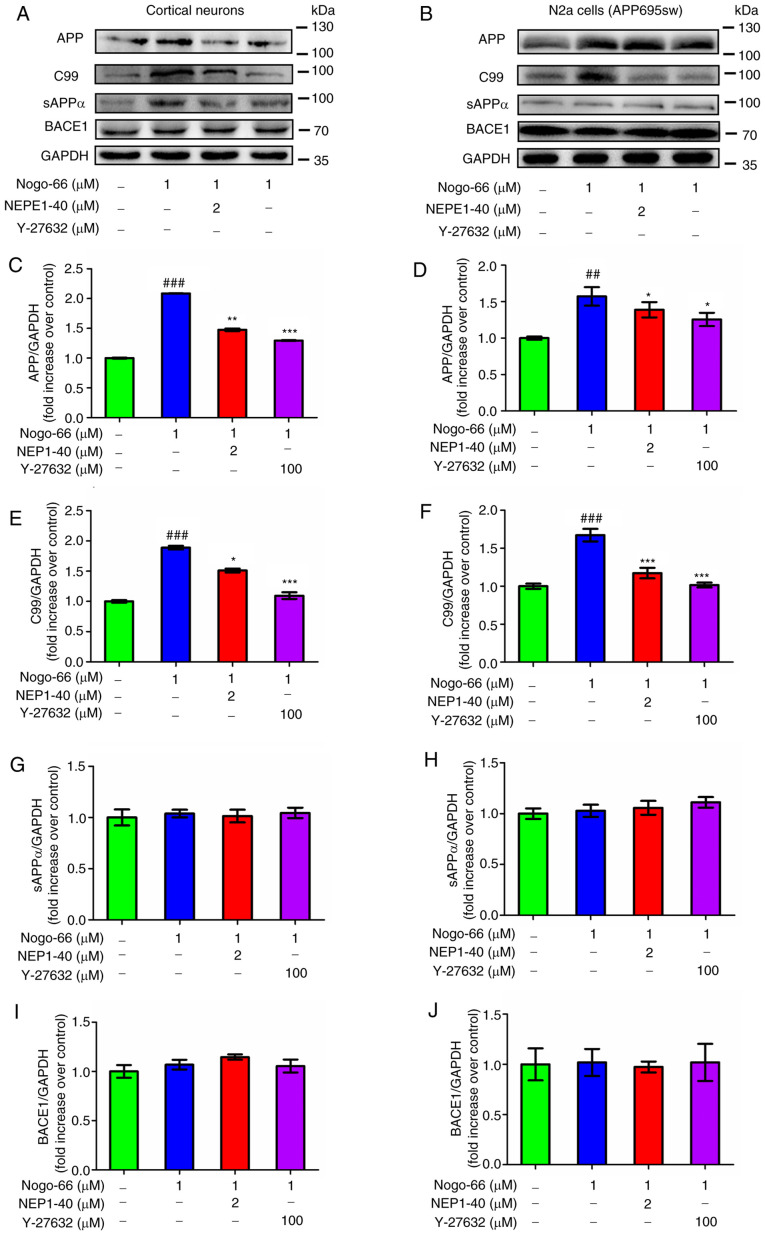
To further characterize the effects of Nogo-66 on APP processing to Aβ, the primary neurons were exposed to Nogo-66, NEP1-40 and Y-27632. Subsequently, the protein expression levels of cell-associated full-length APP, C99 and sAPPα were measured by western blot analysis. Densitometric analysis indicated that treatment with 1 µM Nogo-66 significantly increased the protein expression levels of APP and C99 levels, whereas treatment with NEP1-40 and Y-27632 decreased the expression levels of these proteins (Fig. 3A, C and E). Conversely, treatment with Nogo-66, NEP1-40 or Y-27632 had little effect on the protein expression levels of sAPPα and BACE1 (Fig. 3A, G and I). To determine whether Nogo-66 had a similar effect on APP-overexpressing N2a cells, these cells were treated in the same manner. Consistent with the aforementioned findings, treatment with 1 µM Nogo-66 significantly increased the protein expression levels of APP and C99, whereas the effects of Nogo-66 were reversed following treatment with NEP1-40 and Y-27632 (Fig. 3B, D and F). Furthermore, the protein expression levels of sAPPα and BACE1 were not affected by the same treatment (Fig. 3B, H and J). In combination, these findings suggested that Nogo-66 may increase the production of Aβ by augmenting the amyloidogenic processing of APP. Notably, the opposing outcomes of NEP1-40 or Y-27632 on Aβ levels suggested that inhibition of NgR1 and the receptor downstream signaling molecule ROCK may reduce the amyloidogenic processing of APP.
Figure 3.
Effects of Nogo-66, NEP1-40 and Y-27632 on the protein expression levels of APP, C99, sAPPα and BACE1 in (A, C, E, G and I) primary cortical neurons and (B, D, F, H and J) N2a cells. Data are presented as the mean ± SD from three biological repeats. ##P<0.01, ###P<0.001 vs. control; *P<0.05, **P<0.01, ***P<0.001 vs. Nogo-66 group. NEP1-40, Nogo-66 antagonist peptide; APP, amyloid precursor protein; sAPPα, secreted APP-α; BACE1, β-secretase 1; N2a, Neuro2a.
Effect of Nogo-66 on the NgR/ROCK pathway in cortical neurons and N2a cells
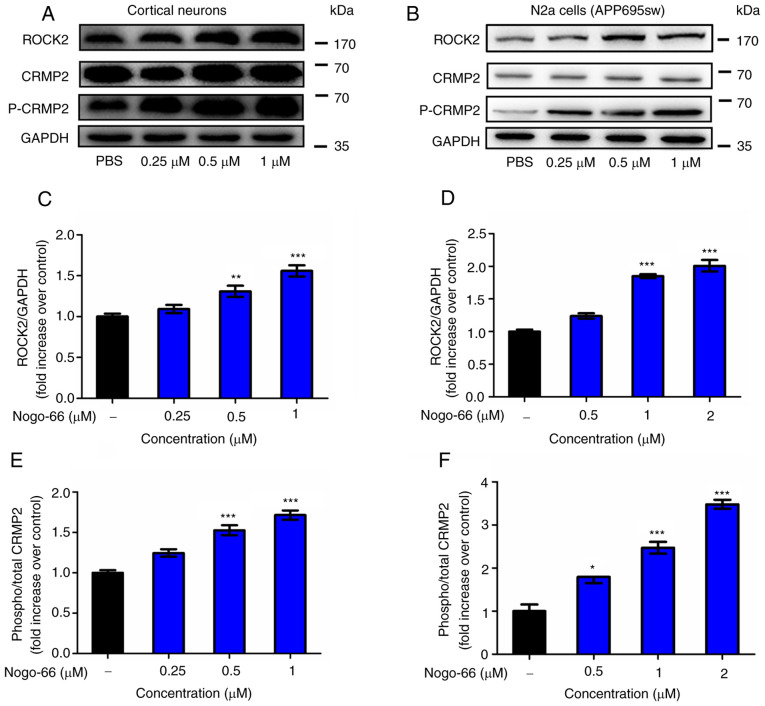
Western blot analysis was performed to explore whether Nogo-66 increased the secretion of Aβ and inhibited neurite regeneration by activating RhoA/ROCK2 signaling. As shown in Fig. 4, the expression levels of ROCK2 and p-CRMP2 were significantly enhanced by Nogo-66 in cortical neurons (Fig. 4A, C and E). Therefore, the results indicated that treatment with Nogo-66 led to a significant dose-dependent increase in the activation of the relevant signaling molecules, including ROCK2 and p-CRMP2. The results in APP-overexpressing N2a cells were consistent with the findings in cortical neurons (Fig. 4B, D and F). These results suggested that the Rho/ROCK2 pathway may have an important role in the effect of Nogo-66, including the increase in Aβ generation and the inhibition of neurite outgrowth in cortical neurons. These findings suggested that Nogo-66 regulated the expression of ROCK2 and activated the phosphorylation of CRMP2, which was associated with increasing APP levels.
Figure 4.
Nogo-66 inhibits neurite outgrowth and induces Aβ production by regulating ROCK2 protein expression and CRMP2 phosphorylation. (A, C and E) Cortical neurons were treated with various concentrations of Nogo-66. Western blot analysis results showing the protein expression levels of ROCK2, p-CRMP2 and CRMP2. Equal protein loading was confirmed using GAPDH. Data are presented as the mean OD values ± SD of triplicate samples. **P<0.01, ***P<0.001 vs. control. (B, D and F) N2a cells were treated with various concentrations of Nogo-66. Western blot analysis results showing the protein expression levels of ROCK2, p-CRMP2 and CRMP2. Equal protein loading was confirmed using GAPDH. Data are presented as the mean OD values ± SD of triplicate samples. *P<0.05, ***P<0.001 vs. control. Aβ, β-amyloid protein; N2a, Neuro2a; ROCK2, Rho-associated coiled-coil containing kinase 2; CRMP2, collapsin response mediator protein-2; p-, phosphorylated; OD, optical density.
Nogo-66 promotes APP processing by activating BACE1
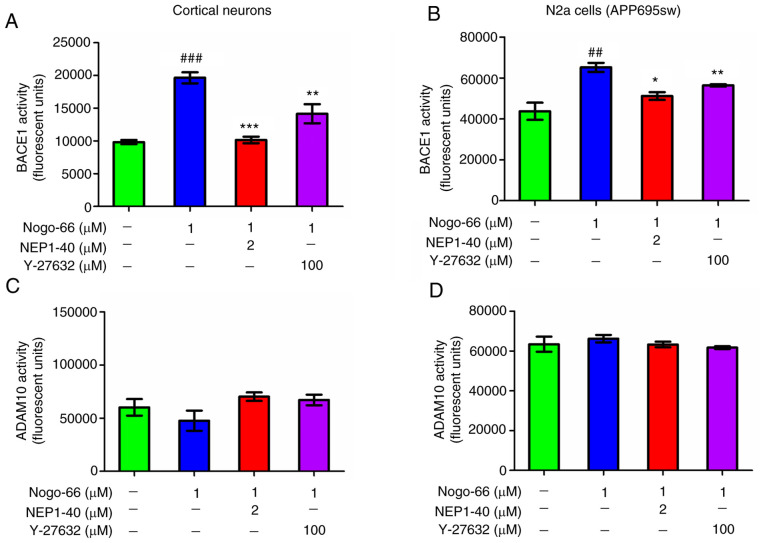
Based on the considerable increase in APP and C99 expression levels following exposure to Nogo-66, it was speculated that Nogo-66 may stimulate APP processing by activating BACE1. To test this speculation, BACE1 activity in cortical neurons was assessed using an Activity Detection kit. The results revealed that BACE1 activity was almost twice as high in the Nogo-66 group compared with that in the control group (Fig. 5A); however, the results of western blotting indicated that the protein expression levels of BACE1 were not altered in the presence of Nogo-66 (Fig. 3I and J). Furthermore, NEP1-40 and Y-27632 prevented BACE1 activity from being increased by Nogo-66 in the primary cultured cortical cells (Fig. 5A). ADAM10 activity was also detected following exposure to Nogo-66, and inhibitors NEP1-40 and Y-27632. The results revealed that ADAM10 activity was almost constant in Nogo-66-treated cells, and ADAM10 activity remained almost unchanged following treatment with the inhibitors (Fig. 5C). To determine whether Nogo-66 had a comparable effect on APP-overexpressing cells, N2a cells were treated in the same manner. Consistent with the aforementioned findings, 1 µM Nogo-66 significantly increased BACE1 activity compared with that in the control group, whereas the effects of Nogo-66 were reversed after the addition of NEP1-40 and Y-27632 to the cells (Fig. 5B). Furthermore, ADAM10 activity remained the same after treatment with Nogo-66 or the two inhibitors (Fig. 5D).
Figure 5.
Effects of Nogo-66, NEP1-40 and Y-27632 on the enzymatic activity of BACE1 and ADAM10 in primary cortical neurons and N2a cells. Enzymatic activity was measured in (A and C) primary cortical neurons and (B and D) N2a cells. Data are presented as the mean ± SD from data in triplicate. ##P<0.01, ###P<0.001 vs. control; *P<0.05, **P<0.01, ***P<0.001 vs. Nogo-66 group. NEP1-40, Nogo-66 antagonist peptide; BACE1, β-secretase 1; N2a, Neuro2a.
These results indicated that Nogo-66 increased Aβ secretion by activating the enzymatic activity of BACE1. Based on these findings, it was proposed that the hydrolysis of APP may be increased through upregulation of BACE1 activity via the downstream signaling pathway of Nogo-66 interacting with NgR, which could result in increased Aβ.
Nogo-66 promotes Aβ secretion via the NgR/ROCK-dependent activation of BACE1 in rats
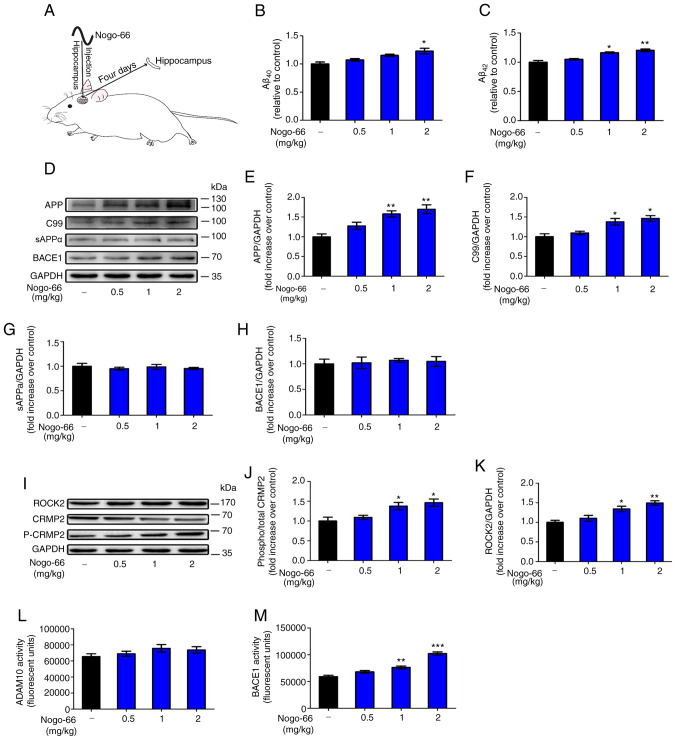
The present study aimed to examine whether exposure to Nogo-66 could influence Aβ secretion in Sprague-Dawley rats (Fig. 6A).
Figure 6.
Effects of Nogo-66 on the expression levels and enzymatic activity of proteins involved in the Aβ pathway in the hippocampus of Sprague-Dawley rats. (A) Schematic diagram of treatment of rats with Nogo-66. Nogo-66 induced (B) Aβ40 and (C) Aβ42 production in a dose-dependent manner in the brains of rats. Data are presented as the mean ± SD (n=6). *P<0.05, **P<0.01 vs. control. (D) Western blotting results showing the protein expression levels of APP, C99, sAPPα and BACE1 in the hippocampus. (E-H) Semi-quantification of western blotting. (I) Western blot analysis of the expression levels of ROCK2, p-CRMP2 and CRMP2 in the hippocampus. Semi-quantification of (J) p-CRMP2 and (K) ROCK2 protein. Equal protein loading was confirmed using GAPDH. (L) ADAM10 and (M) BACE1 activity in the hippocampus of Sprague-Dawley rats was measured. Data are presented as the mean OD values ± SD of triplicate samples (n=3). *P<0.05, **P<0.01, ***P<0.001 vs. control. APP, amyloid precursor protein; sAPPα, secreted APP-α; BACE1, β-secretase 1; Aβ, β-amyloid protein; CRMP2, collapsin response mediator protein-2; p-, phosphorylated; ROCK2, Rho-associated coiled-coil containing kinase 2; OD, optical density.
Hippocampal homogenates were prepared for western blotting, in order to examine the expression levels of proteins associated with APP processing. Densitometric analysis revealed that the protein expression levels of APP and C99 were significantly increased following treatment with 1 and 2 mg/kg Nogo-66 (Fig. 6D-F). Conversely, treatment with various concentrations of Nogo-66 had little effect on the protein expression levels of sAPPα and BACE1 (Fig. 6D, G and H). In addition, soluble Aβ levels were measured by ELISA; Nogo-66-treated rats exhibited a substantial increase in the levels of Aβ40 and Aβ42 compared with those in the control groups (Fig. 6B and C). The results were consistent with those obtained in vitro.
The protein expression levels of ROCK2 and p-CRMP2 were increased in the hippocampus of rats exposed to Nogo-66 in a dose-dependent manner. High (2 mg/kg) and medium (1 mg/kg) doses of Nogo-66 significantly increased the expression of phosphorylated CRMP2 and ROCK2 in the hippocampus of rats (Fig. 6I-K). A low dose (0.5 mg/kg) of Nogo-66 also increased the protein expression levels of ROCK2 and p-CRMP2 in the hippocampus, but without a significant effect (Fig. 6I-K). These trends in the hippocampus were consistent with those observed in vitro, indicating that Nogo-66 may promote the secretion of Aβ by increasing the expression of APP and activating NgR1 downstream signal proteins, such as ROCK2 and p-CRMP2.
The activity of BACE1 in the hippocampus of rats was increased in a dose-dependent manner in response to Nogo-66. High (2 mg/kg) and medium (1 mg/kg) doses of Nogo-66 significantly increased BACE1 activity in the hippocampus of rats (Fig. 6M). A low dose (0.5 mg/kg) of Nogo-66 also increased the enzymatic activity of in the hippocampus; however, this was not significant. In addition, Nogo-66 treatment had a slight effect on ADAM10 enzymatic activity, but there was no significant difference (Fig. 6L). The effects of Nogo-66 on APP metabolic enzymatic activity in the hippocampus were consistent with those observed in vitro, and indicated that Nogo-66 mainly promoted the cleavage of the APP protein through the BACE1 pathway by increasing the activity of BACE1, thereby increasing the secretion of Aβ.
Discussion
Nogo-A is a member of the Nogo family found in the myelin sheath of the CNS, which inhibits axonal regeneration. Nogo-A is considered a major obstacle to neurological regeneration following traumatic injury in mammals (19). Nogo-66 is a 66-amino acid residue in the extracellular domain of Nogo-A, which inhibits neurite outgrowth by binding to the NgR1 in the CNS (20). In vitro, Nogo-66 was previously shown to inhibit neurite outgrowth in a variety of nerve cells, such as dorsal root ganglion cells (21,22), PC12 cells (21) and cerebellar granule cells (23). In the present study, Nogo-66 was revealed to inhibit neurite outgrowth of cortical neurons in a dose-dependent manner.
Previous studies have reported that Nogo-A and its receptors are involved in the regulation of synaptic plasticity, as well as the metabolism of Aβ, suggesting that it may serve an important role in AD. In a previous study, the levels of Aβ deposition were decreased in NgR2-knockout mice (24). However, whereas NgR1 has been shown to bind to APP (24), contrasting effects of this interaction have also been reported; Park et al (25) observed decreased Aβ production following NgR1-overexpression in neuroblastoma in vitro. These previous studies have suggested a strong linkage between Nogo-A and Aβ; however, the exact roles need to be elucidated. The excess production of Aβ is considered the initiating factor of AD, which is generated from the hydrolysis of APP via BACE-1 and γ-secretase. NgR can cause parallel changes in sAPPα and Aβ, indicating that NgR blocks APP hydrolysis (26). In contrast to these findings, Zhou et al (24) reported that the increased interaction between NgR and APP reduced the surface expression of APP and favored the processing of APP via the BACE1 pathway.
The Nogo-A protein, also termed RTN4A, is a member of the RTN family. RTNs are characterized by the highly conserved C-terminal RHD, which is composed of two putative transmembrane domains separated by one hydrophilic loop, plus a hydrophilic tail. Notably, all four human RTN proteins appear to be involved in the pathogenesis of AD. He et al (27) discovered that BACE1 co-immunoprecipitated with RTN1, RTN2, RTN3 and RTN4, and revealed that RTN family members are binding partners of BACE1. Furthermore, increased RTN3 expression caused a dose-dependent reduction in Aβ, whereas suppressing the expression of RTN3 by RNA interference increased the secretion of Aβ, thus suggesting that RTN3 may block access of BACE1 to APP, reduce the cleavage of this protein and inhibit the production of Aβ. RTN4-B/C has also been reported to interact with BACE1 and inhibit its ability to produce Aβ (28). Certain studies have reported that Nogo-A promotes the pathogenesis of AD. Masliah et al (29) demonstrated that deleting Nogo-A ameliorated learning and memory deficits of APP-transgenic mice in the Morris water maze at an early/intermediate stage of the disease, thus suggesting that Nogo-A may influence the metabolism of APP; however, the detailed effect and mechanism are not clear. In the present study, ELISA results revealed that Nogo-66 promoted the levels of Aβ40 and Aβ42 in primary cortical neurons in a dose-dependent manner. The present results showed that increased Nogo-66 increased production of Aβ, which is the opposite effect to other RTNs. Notably, Nogo-A may be considered a very different protein compared with others from the RTN family, as it not only has the unique function of inhibiting neurite outgrowth but it may also increase Aβ.
When cortical neurons were treated with NEP1-40 in the present study, the levels of Aβ40 and Aβ42 were decreased. After treatment with Nogo-66, the protein expression levels of APP and activation of BACE1 were increased in cortical neurons and N2a cells, indicating that Nogo-66 simultaneously increased the levels of APP and activated BACE1, which may eventually lead to an increase in the secretion of Aβ.
The Rho/ROCK signaling pathway was activated when Nogo-A interacts with NgR1 (30). The downstream event included activation of RhoA-GTP, ROCK, MAP and CRMP2, which eventually exerted effects on the cytoskeleton associated with neurite growth. The results of western blotting revealed that higher ROCK2 expression and increased p-CRMP2 expression was induced by Nogo-66. It was therefore suggested that Nogo-66-stimulated ROCK2-induced regulation of CRMP2 phosphorylation may contribute to neurite outgrowth inhibition.
The present results also revealed that the ROCK inhibitor Y-27632 suppressed Nogo-66-stimulated Aβ42 and Aβ40 secretion. These findings indicated that Y-27632 may reduce Aβ42 by inhibiting the activation of ROCK and Nogo-66 may increase Aβ42 secretion by activating the ROCK pathway. Subsequently, western blot analysis was performed to explore whether Nogo-66 stimulated Aβ secretion by regulating ROCK2 expression. It was revealed that Nogo-66 significantly increased the expression levels of ROCK2 in cortical neurons and N2a cells. A previous study used a variety of non-steroidal anti-inflammatory drugs to demonstrate that the activation of Rho/ROCK2 led to a large production of Aβ42, whereas the inhibition of ROCK2 activity reduced the APP amyloid enzymatic degradation pathway and the levels of Aβ42 in the brain (31). Another study confirmed that inhibition of RhoA or ROCK2 knockdown could interfere with the β-cleavage of APP, and significantly reduced the levels of brain Aβ in AD model mice (32). It was therefore hypothesized that Nogo-66 bound to NgR, and potentially exerted its regulatory effects on Aβ levels by increasing the expression of ROCK2 and promoting the catalytic action of BACE1, which may then facilitate amyloidogenic metabolism of APP, thus resulting in increased Aβ42 secretion.
In the in vivo experiment conducted in the present study, Nogo-66 promoted the levels of Aβ40 and Aβ42 in rat tissues. Nogo-66 also enhanced the expression levels of proteins associated with the APP processing pathway and activated BACE1 in rats, which may eventually lead to increased secretion of Aβ. Subsequently, western blot analysis was used to explore the expression levels of ROCK2 and p-CRMP2 in the hippocampus; it was revealed that Nogo-66 increased the expression levels of ROCK2 and p-CRMP2 in vivo. The results regarding the effects of Nogo-66 on the rat hippocampus and related mechanism were consistent with the results obtained for neurons in vitro.
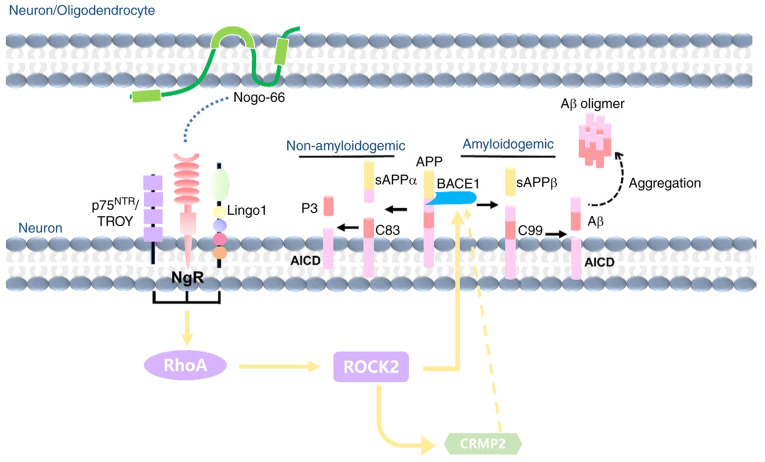
In conclusion, although Nogo-A has an important role in AD pathology, its effects and mechanisms are not clear. The present study demonstrated that Nogo-66 inhibited neurite outgrowth, and increased the levels of Aβ42 and Aβ40 in a dose-dependent manner. The underlying mechanism may be as follows (Fig. 7): Nogo-66 increases Aβ generation by activating BACE1 and increasing APP expression, which is associated with activation of NgR1 and its downstream signaling molecule ROCK2, as well as the phosphorylation of CRMP2. Therefore, Nogo-A may be a precipitating factor that causes the onset and development of AD; however, the exact mechanism still requires further exploration. From the viewpoint of drug therapeutic targets, NgR and ROCK2 appear to be potentially useful and effective therapeutic targets for AD treatment, and targeting them may promote neurite outgrowth and inhibit the neuronal production of Aβ.
Figure 7.
Proposed schematic working model: Nogo-66 promotes Aβ secretion through the NgR/ROCK/BACE1 pathway. Aβ, β-amyloid protein; ROCK, Rho-associated coiled-coil containing kinase; BACE1, β-secretase 1; APP, amyloid precursor protein; sAPP, secreted APP; NgR, Nogo-66 receptor.
Acknowledgements
The authors would like to thank Dr Fei Fang (Department of Clinical Molecular Biology, Akershus University Hospital, University of Oslo, Norway) for critical discussion and assistance in the preparation of the manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (grant nos. 81202519, 81873739, 81572497 and 81703011), the Science and Technology Program of Guangzhou Province (grant no. 201607010216) and the Natural Science Foundation of Guangdong Province (grant nos. 2019A1515010936, 2014A030313362 and 2017A030313487).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FX, QZ, HY and FCL were involved in revising the manuscript critically for important intellectual content, making substantial contributions to conception and design, and giving final approval of the version to be published. QQX participated in the entire process, drafting of the article and data analysis. XF, YYH, NF, QYC, GFL, JPP, YH and ZJW fed the animals, performed the experiments and collected samples from the rats. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal experiments were conducted according to the Jinan University ethical guidelines and were approved by the Animal Research Committee of the School of Medicine of Jinan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer's disease: Cellular and molecular mechanisms. Trends Neurosci. 2017;40:151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson-Jones M, Yu K, Ahrens S, Tucciarone JM, van Huijstee AN, Mejia LA, Penzo MA, Tai LH, Wilbrecht L, Li B. A basal ganglia circuit for evaluating action outcomes. Nature. 2016;539:289–293. doi: 10.1038/nature19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira JB, Janelidze S, Ossenkoppele R, Kvartsberg H, Brinkmalm A, Mattsson-Carlgren N, Stomrud E, Smith R, Zetterberg H, Blennow K, et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer's disease. Brain awaa395. 2020 doi: 10.1093/brain/awaa395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y-T, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer's disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154.e7. doi: 10.1016/j.neuron.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci. 2019;22:719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brösamle C, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandpré T, Strittmatter SM. Nogo: A molecular determinant of axonal growth and regeneration. Neuroscientist. 2001;7:377–386. doi: 10.1177/107385840100700507. [DOI] [PubMed] [Google Scholar]
- 12.Mosyak L, Wood A, Dwyer B, Buddha M, Johnson M, Aulabaugh A, Zhong X, Presman E, Benard S, Kelleher K, et al. The structure of the Lingo-1 ectodomain, a module implicated in central nervous system repair inhibition. J Biol Chem. 2006;281:36378–36390. doi: 10.1074/jbc.M607314200. [DOI] [PubMed] [Google Scholar]
- 13.Gil V, Nicolas O, Mingorance A, Ureña JM, Tang BL, Hirata T, Sáez-Valero J, Ferrer I, Soriano E, del Río JA. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:433–444. doi: 10.1097/01.jnen.0000222894.59293.98. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Yao L, Li C, Wang J, Wang J, Chen S, Zhou XF, Liao H. The blockage of the Nogo/NgR signal pathway in microglia alleviates the formation of Aβ plaques and tau phosphorylation in APP/PS1 transgenic mice. J Neuroinflammation. 2016;13:56. doi: 10.1186/s12974-016-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao F, Lin LF, Cheng X, Gao Q, Luo HM. Nogo-66 receptor activation inhibits neurite outgrowth and increases β-amyloid protein secretion of cortical neurons. Mol Med Rep. 2012;5:619–624. doi: 10.3892/mmr.2011.692. [DOI] [PubMed] [Google Scholar]
- 16.Dai X, Sun Z, Liang R, Li Y, Luo H, Huang Y, Chen M, Su Z, Xiao F. Recombinant Nogo-66 via soluble expression with SUMO fusion in Escherichia coli inhibits neurite outgrowth in vitro. Appl Microbiol Biotechnol. 2015;99:5997–6007. doi: 10.1007/s00253-015-6477-5. [DOI] [PubMed] [Google Scholar]
- 17.zur Nedden S, Doney AS, Frenguelli BG. Modulation of intracellular ATP determines adenosine release and functional outcome in response to metabolic stress in rat hippocampal slices and cerebellar granule cells. J Neurochem. 2014;128:111–124. doi: 10.1111/jnc.12397. [DOI] [PubMed] [Google Scholar]
- 18.Gao R, Wang Y, Pan Q, Huang G, Li N, Mou J, Wang D. Fuzhisan, a chinese herbal medicine, suppresses beta-secretase gene transcription via upregulation of SIRT1 expression in N2a-APP695 cells. Int J Clin Exp Med. 2015;8:7231–7240. [PMC free article] [PubMed] [Google Scholar]
- 19.Jin SG, Ryu HH, Li SY, Li CH, Lim SH, Jang WY, Jung S. Nogo-A inhibits the migration and invasion of human malignant glioma U87MG cells. Oncol Rep. 2016;35:3395–3402. doi: 10.3892/or.2016.4737. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Zhou X, Guo JJ, Mao L, Wang YJ, Sun J, Sun LX, Zhang LY, Zhou XF, Liao H. Nogo-66 inhibits adhesion and migration of microglia via GTPase Rho pathway in vitro. J Neurochem. 2012;120:721–731. doi: 10.1111/j.1471-4159.2011.07619.x. [DOI] [PubMed] [Google Scholar]
- 21.GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 22.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 23.Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Hu X, He W, Tang X, Shi Q, Zhang Z, Yan R. Interaction between amyloid precursor protein and Nogo receptors regulates amyloid deposition. FASEB J. 2011;25:3146–3156. doi: 10.1096/fj.11-184325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang R, Wu XF, Wang B, Guan RX, Lv LM, Li AP, Lei L, Ma Y, Li N, Li QF, et al. Reduction of NgR in perforant path decreases amyloid-β peptide production and ameliorates synaptic and cognitive deficits in APP/PS1 mice. Alzheimers Res Ther. 2020;12:47. doi: 10.1186/s13195-020-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He W, Lu Y, Qahwash I, Hu X-Y, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-β peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- 28.Murayama KS, Kametani F, Saito S, Kume H, Akiyama H, Araki W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid β-protein. Eur J Neurosci. 2006;24:1237–1244. doi: 10.1111/j.1460-9568.2006.05005.x. [DOI] [PubMed] [Google Scholar]
- 29.Masliah E, Xie F, Dayan S, Rockenstein E, Mante M, Adame A, Patrick CM, Chan AF, Zheng B. Genetic deletion of Nogo/Rtn4 ameliorates behavioral and neuropathological outcomes in amyloid precursor protein transgenic mice. Neuroscience. 2010;169:488–494. doi: 10.1016/j.neuroscience.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, Xiao P, Fan S, Chen Y, Huang W, Chen X, Liu G, Dang C, Zeng J, Xing S. Blockade of Nogo-A/Nogo-66 receptor 1 (NgR1) inhibits autophagic activation and prevents secondary neuronal damage in the thalamus after focal cerebral infarction in hypertensive rats. Neuroscience. 2020;431:103–114. doi: 10.1016/j.neuroscience.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- 32.Herskowitz JH, Feng Y, Mattheyses AL, Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC, Thambisetty M, Seyfried NT, et al. Pharmacologic inhibition of ROCK2 suppresses amyloid-β production in an Alzheimer's disease mouse model. J Neurosci. 2013;33:19086–19098. doi: 10.1523/JNEUROSCI.2508-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.