Abstract
Background:
Severe Acute Respiratory Syndrome - Coronavirus – 2 (SARS-CoV-2) is a virus, primarily transmitted through droplets, able to persist on different surfaces and in the air for several hours. During the COVID-19 pandemic, Health Care Workers should be considered a high risk profession. Beside social distancing rules and the proper use of Personal Protective Equipment, sanitization measures and ventilation system disinfection are essential to reduce viral transmission.
Objectives:
This is the first Italian study aiming to assess the magnitude of environmental contamination in a COVID-19 non-Intensive Care Unit.
Methods:
In addition to ordinary cleaning procedures, surface and air samplings have been performed before and after the application of two different sanitization devices. Samples have been analyzed with Real Time-Polymerase Chain Reaction in order to find viral RNA.
Results:
All samples obtained from surfaces and air before and after extra-ordinary sanitization procedures turned out negative for viral detection.
Discussion:
These findings highlight the efficiency of ordinary cleaning procedures in guaranteeing a safer workplace. The adoption of additional sanitization protocols should be considered in order to further reduce environmental viral contamination.
Key words: Contamination, environmental sampling, industrial hygiene, SARS-CoV-2, sanitization, ventilation system
Abstract
«Valutazione della contaminazione di aria e superfici in un reparto COVID-19 a bassa intensità di cure».
Introduzione:
Il Severe Acute Respiratory Syndrome - Coronavirus – 2 (SARS-CoV-2) è un virus prevalentemente trasmesso tramite droplets, in grado di persistere su differenti tipologie di superfici e nell’aria per diverse ore. Gli operatori sanitari, coinvolti in prima linea durante la recente pandemia, devono essere considerati una professione ad alto rischio di infezione. In aggiunta alle norme di distanziamento sociale e del rigoroso utilizzo dei Dispositivi di Protezione Individuale, l’adozione di procedure di sanificazione e la disinfezione dei sistemi di ventilazione sono misure essenziali per ridurre la probabilità di trasmissione del virus.
Obiettivi:
Questo studio si propone di indagare l’entità della contaminazione ambientale di SARS-CoV-2 in un reparto COVID-19 a bassa intensità di cure.
Metodi:
In aggiunta alle procedure di pulizia ordinaria, sono stati effettuati campionamenti su aria e superfici prima e dopo l’adozione di due diversi meccanismi di sanificazione. I campioni ottenuti sono stati analizzati con la metodica di Real Time- Polymerase Chain Reaction.
Risultati:
Tutti i campioni effettuati, sia prima che dopo le misure di sanificazione non ordinarie, sono risultati negativi per la ricerca del virus.
Discussione:
I risultati evidenziano l’efficacia delle misure di pulizia ordinaria per garantire una maggior sicurezza sul posto di lavoro. L’adozione di procedure di sanificazione non ordinarie dovrebbe essere presa in considerazione per ridurre ulteriormente l’entità della contaminazione virale.
Introduction
Severe Acute Respiratory Syndrome - Coronavirus – 2 (SARS-CoV-2) is a RNA virus of the family of Coronaviridae (11) primarily transmitted between people through respiratory droplets and contact routes (17). Until May 2020, SARS-CoV-2, responsible for a pathology known as COronaVIrus Disease-19 (COVID-19), caused over 5.5 million infections and almost 350,000 deaths worldwide (6).
Coronaviruses have been recently involved in nosocomial outbreaks and, likewise, a nosocomial transmission of SARS-CoV-2 has been reported. Thus, Health Care Workers (HCWs) should be considered an occupational group at high risk for SARS-CoV-2 infection (5). The occurrence of HCWs-associated infections might be associated with viral contamination of the air and surfaces surrounding COVID-19 positive patients and carriers. Recent publications (8, 13) demonstrated that the environmental stability of SARS-CoV-2 on surfaces is up to four hours on copper, up to 24 hours on cardboard and up to two to three days on plastic and stainless steel. Its persistence in the air post-aerosolization is up to three hours. Because of evidence on the role of airborne transmission in the spread of other two zoonotic Coronaviruses (13), recent studies have attempted to test this hypothesis also for SARS-CoV-2. In this regard, recent studies (4) suggest that Heating, Ventilation and Air Conditioning (HVAC) systems may have a role in viral transmission, especially in buildings such as hospitals or other healthcare facilities. Ventilation systems have already been reported as a way of transmission of other infectious diseases such as measles, tuberculosis, chickenpox, influenza, smallpox and legionellosis (1).
Beside social distancing and proper Personal Protective Equipment (PPE) use, it is essential, in order to reduce the infection risk, to establish and follow procedures for a correct sanitization of the environments that could have been contaminated with SARS-CoV-2. The World Health Organization (WHO) recommends ensuring that environmental cleaning and disinfection procedures are followed consistently and correctly (16). Various types of biocidal agents such as hydrogen peroxide, alcohols, sodium hypochlorite or benzalkonium chloride are used effectively worldwide for surfaces disinfection, mainly in healthcare settings. In particular, alcohol-base disinfectants have been shown to significantly reduce the infectivity of enveloped virus like SARS-CoV-2 (8). Furthermore, correct air recirculation strategies and ventilation system disinfection measures need to be adopted in order to reduce this possible route of transmission.
However, to date, the extent and the role of SARS-CoV-2 environmental contamination as well as the persistence of the virus on surfaces and in the air are not fully elucidated and the present knowledge is mostly based on studies of other Coronaviruses. A better understanding of the spreading mechanisms of SARS-CoV-2 could have an important impact on prevention and control strategies.
Therefore, the primary aim of this study was to measure the presence of viral RNA on surfaces and in the air of potentially contaminated environments in a COVID-19 non-Intensive Care Unit (ICU). A secondary aim of the study was to investigate the effect of additional sanitization procedures.
Methods
From May 5th to May 6th, 2020 environmental samplings on air and surfaces have been carried out in a COVID-19 non-ICU of a Trauma Center in Northern Italy.
Batch of samplings were performed at two times, the first day at 12.00 and the second day after 24 hours. The second day samples were collected after the adoption of extra-ordinary sanitization procedures.
The first day, surface samplings were collected 18 hours after the last daily ordinary cleaning procedure with disinfectants made with chlorhexidine, quaternary ammonium salts with high alcohol concentration and sodium hypochlorite. Before the beginning of the study, ordinary cleaning procedures were carried out twice a day by qualified personnel.
The second day, surface samplings were performed after an extra-ordinary sanitization procedure with a hot disinfection system (based on a solution with high alcohol concentration and quaternary ammonium salts) (Figure 1).
Figure 1.

Surface sanitization procedure
Air samplings were also collected at two times, on day one they were performed at basal condition and on day two after a procedure of disinfection of air conditioning ducts via an atomizer that supplies high quantities of hydrogen peroxide microparticles (0.3-0.5 µm).
A total of 34 environmental samples were collected (Table 1). Main sites of sampling are represented in Figure 2. Before every batch of samplings, room temperature, humidity and atmospheric pressure were measured. Twenty-four surface samples were obtained inside the COVID-19 non intensive care unit: 12 samplings were collected before extra-ordinary sanitization procedures and 12 after extra-ordinary sanitization procedures. One surface sample from an administration office was obtained outside the unit as a control sample. Eight samples were obtained from PPE: four from the inner layer of two surgical masks worn by two patients (two before and two after the extra-ordinary sanitization procedure) and four from the outer layer of two disposable gowns worn by two nurses for one working shift (two before and two after the sanitization extra-procedure).
Table 1.
Surface and air sampling sites inside the COVID-19 Unit.
| Patient 1 room surfaces | PPE | HVAC | Air pumps sites |
| Bed raila | Surgical mask Patient 1a | Air Handling Units (AHU) filter of supply air duct.b | Patient 1 roomb |
| Sheets and pillowa | Surgical mask Patient 2a | AHU filter nearby return air duct. b | Patient 2 roomb |
| Floor within 1 m of beda | Disposable Gown Patient 1a | Return air vents in Patient 1 roomb | Empty room near patients roomsb |
| Wall within 1 m of beda | Disposable Gown Patient 2a | Return air vents in Patient 2 roomb | Corridor outside the roomsb |
(a): sites treated with a hot disinfection system based on a solution with high alcohol concentration and quaternary ammonium salts;
(b): sites treated with hydrogen peroxide atomizer.
PPE: Personal Protective Equipment;
HVAC: Heat,Ventilation and Air Conditioning.
Figure 2.
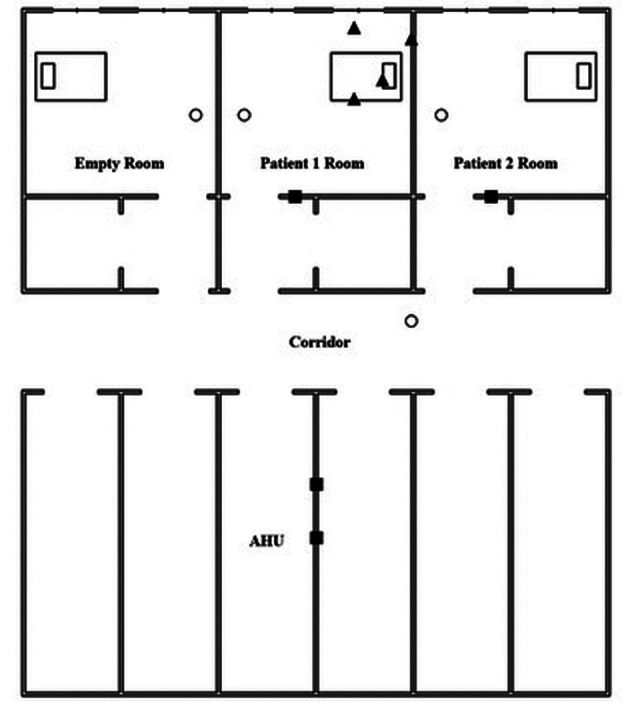
Sampling sites in COVID-19 non-Intensive Care Unit
Sampling sites: Pump sites HVAC Patient 1 Room surfaces
Following WHO recommendations (15), samples were taken by brushing a 25-cm2 area with a swab with a synthetic tip and a plastic shaft (Swab 490CE.A produced by Copan Italia S.p.A) and, subsequently, stored in a vial containing 3 ml of Universal Transport Medium (UTM-RT).
Air sampling (3) was performed using SKC Flite pumps (47 mm filter cassettes and 0.45 µm filters in polytetrafluoroethylene-PTFE) positioned 1 meter above the floor for 340 minutes at 15 l/min. The calibration and the verification of the pump flow rates were carried out using a certified primary flow meter.
A total of four pumps were placed in four different sites: patient 1 room, patient 2 room, an empty room nearby patients’ rooms, corridor outside the rooms. One air control sample was obtained outside the hospital. Each patient room had a volume of about 90 cubic meters.
All samples were stored at 4°C and sent to a microbiology laboratory within 3 hours from the collection and afterwards processed with Real Time - Polymerase Chain Reaction (RT-PCR) to detect SARS-CoV-2 RNA.
The two patients (patient 1 and patient 2), admitted to the hospital rooms investigated (room 1 and room 2) were positive to COVID-19 for more than a week and underwent a nasopharyngeal swab before starting the environmental sampling.
Results
Surface samples collected before and after sanitization (n=24) were all negative for SARS-CoV-2 RNA, as was the single control sample.
Air samples before and after sanitization (n=8), obtained filtering 25000 litres of air, failed to detected the presence of SARS-CoV-2 at the RT-PCR test. Also the PTFE filter of the pump placed outside the hospital was negative for RNA detection.
Patient 1 rhinopharingeal swab was positive while patient 2 rhinopharingeal swab was negative (Table 2).
Table 2.
Clinical and microbiological features of patients
| Time elapsed between diagnosis and sampling | Rhinopharingeal swab at the time of sampling | Symptoms at the time of sampling | |
| Patient 1 | 9 days | positive | none |
| Patient 2 | 12 days | negative | none |
Discussion
To the best of our knowledge, this is the first study aiming to assess the magnitude of environmental contamination in a COVID-19 non-ICU in Italy. All samples obtained from surfaces and air have turned out negative, while other scientific studies report different results. These differences may have several explanations, as discussed below.
Ye G et al. (18), analysing 626 surface samples from 13 different hospital areas of Zhorgnan Medical Center of Wuhan Universitiy, showed 85 (13,6%) positive results: ICU was the most contaminated area (p<0.01). Another study, performed by Ong SWX et al. (12), reported RNA presence in 17/28 surface samples, in an ICU with symptomatic COVID-19 patients, while our study was carried out in a non-ICU, without patients requiring assisted ventilation.
Beside patients’ settings, also clinical features should be considered. Patients recruited in our study were asymptomatic and only one of two rinopharingeal swabs was positive at the RT-PCR and, thus, it is plausible to expect that our cases show a lower viral load, according to the results of Liu Y et al. (9) that found that the mean viral load of severe COVID-19 positive cases was around sixty times higher than that of mild ones. Moreover, mild cases had an earlier viral clearance, compared to severe ones.
Also, the time elapsed between diagnosis and environmental sampling may play a role. As shown by Po Ying Chia et al. (2), the probability to find viral RNA on surfaces decreased with time elapsed from diagnosis. These Authors analyzed 245 surface samples on 30 single rooms of COVID-19 positive patients: floor, bed rail and beside locker surfaces were the most likely to be contaminated. They found at least one positive sample in 10/15 rooms of patients with recent (< 7 days) diagnosis while only in 3/15 rooms of patients with an older diagnosis (> 7 days) there were positive samples. Our results well agree with Po Ying Chia et al. (2) since our patients had a microbiological diagnosis older than a week.
Furthermore, our study was conducted in an advanced phase of the COVID-19 pandemic. On one side, the number of patients at the time of samplings was lower compared to a few weeks before and, on the other, there were an implementation over the ordinary sanitization measures. The contribution of a lower number of patients and of sanitization procedures is highlighted in a study carried out by Liu et al. (10). These authors investigated the airborne diffusion of SARS-CoV-2 in different areas of two Wuhan hospitals. Measurements were carried out in two different points in time: the second batch of air samples was collected after a reduction of the number of patients from over 200 to less than 100 per zone with the implementation of more rigorous and frequent sanitization measures such as spraying of chlorinated disinfectant on the floor, additional disinfection by 3% hydrogen peroxide at least once a week and prolonged operation time of indoor air purifiers. The samples from this second batch, unlike the first one, showed all non-detectable results. In addition, the survey performed by Jie Wang et al. (7) did not find any traces of viral RNA on 36 surfaces analyzed in COVID-19 isolation wards. Surface samplings were done after 4 hours of environmental disinfection with 1000 mg/L chlorine containing disinfectant. These data, according to our study, seem to confirm the importance of sanitization in reducing SARS-CoV-2 environmental contamination (19).
Our study has some limitations. Firstly, the sample size was small, reducing the probability to detect viral RNA contamination. Secondly, it must be considered that the air volume sampled was only a fraction of total volume, and periodic air exchanges in the room could have diluted the presence of SARS-CoV-2 in the air. Another limitation is related to the reliability of the sampling method and of the laboratory technique. On one side, the macroscopic and microscopic characteristics of the examined surfaces could have influenced the accuracy of the sampling method increasing the risk of false negative results. On the other, RT-PCR for SARS-CoV-2 detection has a 70% sensitivity and a 95% specificity, with a probability of 30% of false negative results (14).
Finally, our study took place in a limited period of time, without the possibility of repeated samplings over the time.
Conclusions
These preliminary results show the absence of SARS-CoV-2 RNA in surface and air samples in a COVID-19 non-Intensive Care Unit. Possible explanations to these preliminary findings are related to the limited number of hospitalized patients at the time of the samplings, to patients’ clinical features and their likely low viral load, to the significant amount of time elapsed between diagnosis and environmental samplings and to the daily adoption of ordinary sanitization procedures.
Since the pre-sanitization samplings were negative, we were not able to assess the secondary aim of our investigation in order to evaluate the efficacy of extra-ordinary disinfection procedures. Further investigations are needed to better analyze this topic.
No potential conflict of interest relevant to this article was reported by the authors
References
- 1.Ather B, Edemekong PF. Airborne Precautions. Treasure Island (FL): StatPearls Publishing; 2020 Feb 17. [PubMed] [Google Scholar]
- 2.Chia PY, Coleman KK, Tan YK, et al. Detection of Air and Surface Contamination by Severe Acute Respiratory Syndrome 2 Coronavirus 2 (SARS-CoV-2) in Hospital Rooms of Infected Patients. MedRxiv, 2020 April 9. Preprint. doi: https://doi.org/10.1101/2020.03.29.20046557 . [Google Scholar]
- 3.Donato F, Garzaro G, Pira E, Boffetta P. Mortality and cancer morbidity among cement production workers: a meta-analysis. Int Arch Occup Environ Health. 2016 Nov;89(8):1155–1168. doi: 10.1007/s00420-016-1167-x. doi: 10.1007/s00420-016-1167-x. Epub 2016 Sep 7. PMID: 27604876. [DOI] [PubMed] [Google Scholar]
- 4.Faridi S, Niazi S, Sadeghi K, et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020 Apr 6;725:138401. doi: 10.1016/j.scitotenv.2020.138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garzaro G, Clari M, Ciocan C, et al. COVID-19 infection and diffusion among the healthcare workforce in a large university-hospital in northwest Italy. Med Lav. 2020;111:184–194. doi: 10.23749/mdl.v111i3.9767. doi: 10.23749/mdl.v111i3.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. https://www.worldometers.info/coronavirus/ [accessed 2020 May 25] [Google Scholar]
- 7.Jie Wang J, Feng H, Zhang S, et al. SARS-CoV-2 RNA Detection of Hospital Isolation Wards Hygiene Monitoring During the Coronavirus Disease 2019 Outbreak in a Chinese Hospital. Int J Infect Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hospit Infect. 2020 Feb 06 doi: 10.1016/j.jhin.2020.01.022. doi: https://doi.org/10.1016/j.jhin.2020.01.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 April 27 doi: 10.1038/s41586-020-2271-3. doi.org/10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 11.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Doremalen N, Morris D, Bushmaker T, et al. Aerosol and Surface Stability of SARS-CoV-2 as compared with SARS-CoV-1. New Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson J, Whiting PF, Brush E. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. doi: 10.1136/bmj.m1808. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Surface sampling of coronavirus disease (COVID-19): a practical “how to” protocol for health care and public health professionals, 2020 February 18, version 1.1. Available at https://apps.who.int/iris/handle/10665/331058 . [Google Scholar]
- 16.World Health Organisation. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected. Interim Guidance. 2020 Mar 19. Available at https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 . [Google Scholar]
- 17.World Health Organisation. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020 Mar 29. Available at https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations . [Google Scholar]
- 18.Ye G, Lin H, Chen L, et al. Environmental contamination of the SARS-CoV-2 in healthcare premises: An urgent call for protection for healthcare workers. 2020 Mar 16, medRxiv preprint. doi: https://doi.org/10.1101/2020.03.11.20034546 . [Google Scholar]
- 19.Yuan XN, Meng QY, Shen N, et al. Detection and evaluation of SARS-CoV-2 nucleic acid contamination in corona virus disease 19 ward surroundings and the surface of medical staff’s protective equipment. Beijing Da Xue Xue Bao Yi Xue Ban. 2020 Oct 18;52(5):803–808. doi: 10.19723/j.issn.1671-167X.2020.05.002. PMID: 33047711. [DOI] [PMC free article] [PubMed] [Google Scholar]


