Abstract
The goal of the study was testing the effects of chlorogenic acid (CA) supplementation on small intestine healthiness, growth performance, oxidative stress, inflammatory response, and blood biochemical indices in specific-pathogen-free (SPF) chickens after infection with Clostridium perfringens (CP) type A. In this study, 324 1-day-old male SPF chickens were randomly distributed into 6 groups: control group; CA group; CP infection group; CA + CP group; antibiotic group; antibiotic + CP group. All 1-day-old chickens were fed with CA or antibiotic in corresponding treatment group for 13 d. On the 14 d, the chickens in corresponding infection group were challenged with CP type A for 3 d. Samples in each group were collected when the chickens were 17 and 21 d old. This study proves for the first time that CA, a Chinese herbal medicine, can effectively improve growth performance, inhibit small intestine structural damage, improve antioxidant capacity, inhibit damage to ileal mucosal layer construction and tight junctions, inhibit inflammatory cytokines, and ameliorate blood biochemical indices. Therefore, this study provides data for CA being able to effectively alleviate small intestine damage caused by CP type A infection in chickens.
Key words: C. perfringens type A, chlorogenic acid, intestinal injury, oxidative stress, inflammation
Introduction
Clostridium perfringens (CP) is one of the main pathogens causing necrotizing enteritis, enterotoxemia, food poisoning, and traumatic gas gangrene in various animal species (Nowell et al., 2010). At present, 12 kinds of toxins have been found in CP, among which a, β, ε, and t are the most lethal (Rood, 1998). In addition, CP can be divided into 5 types: A, B, C, D, and E (Meer and Songer, 1997).
Chickens are very susceptible to CP (Chalmers et al., 2008). In chickens, necrotizing enteritis (necrotic enteritis) is an intestinal infectious disease caused by CP type A (occasionally CP type C) that is characterized by fibrinous necrosis and bleeding in the small intestinal mucosa (Annett et al., 2002; Olkowski et al., 2006). Clostridium perfringens is a common inhabitant in the intestinal tract of normal chickens (McCourt et al., 2005). In normal circumstances, CP lives in the ileum and usually does not cause disease, but when the bowel membrane is damaged or the composition of the intestinal microflora changes, it will multiply and proliferate in the front of the digestive tract, leading to necrotizing enteritis and death (Stanley et al., 2014). Clostridium perfringens is widely found all over the world (Craven et al., 2003; Siragusa et al., 2006; Miller et al., 2010; Zhang et al., 2018a). Clostridium perfringens infection can induce oxidative stress and cause inflammation and serious pathological changes in the intestinal mucosa (Rood et al., 2016; Liu et al., 2018). In chickens, CP type A infections are associated with necrotic enteritis and severe mortality, which causes serious economic losses to the poultry industry; therefore, CP type A has had a serious, negative impact on poultry production over the years (Van Immerseel et al., 2004). With the increasingly stringent use of antibiotics, the incidence of CP type A infections and necrotic enteritis in chickens has been increasing every year, and necrotic enteritis was responsible for annual losses of US $6 billion to the poultry industry in the United States (Li et al., 2017). Therefore, it is necessary to explore the efficacy of various nonantibiotic drugs for the treatment of necrotic enteritis caused by CP type A infections.
Chlorogenic acid (CA), also known as coffee tannic acid, is a polyphenol synthesized through the condensation of caffeic acid and quinic acid (Tajik et al., 2017). Chlorogenic acid is widely found in many plants and Chinese herbal medicines, such as green tea, coffee tea, chrysanthemum, and honeysuckle (Farah et al., 2008; Leiss et al., 2009; Yan et al., 2016; da Silveira et al., 2017). In animals, CA can improve growth performance, immunity, and intestinal barrier functioning by enhancing biological functions such as anti-inflammatory activities, bacteriostasis, antioxidant activities and by regulating lipid metabolism (Chen et al., 2018a, b; Exon et al., 1998). Studies have also showed inhibiting effects of CA on bacteria in vitro, so it might be possible that CA could partly replace antibiotics and hormone drugs (Santana-Galvez et al., 2017). Until now, little information has been available concerning the protective effects of dietary CA supplementation in chickens infected with CP type A in terms of intestinal injury, such as the small intestine structure and inflammation. It is well known that chickens infected with CP often have intestinal inflammation and oxidative stress (Liu et al., 2012). Hence, it is plausible to hypothesize that CA has the potential to improve the intestinal health of infected chickens by alleviating inflammation, enhancing antioxidant abilities, etc.
This study aimed to verify the above hypothesis by evaluating the effects of CA on small intestine injury, antioxidant capacity, changes in tight-junction-related proteins, anti-inflammatory activities, and blood biochemical indices in chickens infected with CP type A.
Materials and methods
Ethics Statement
The use of animals in this study was approved by the Animal Care Committee of South China Agricultural University (approval ID: SYXK-2014-0136).
Strains, Chlorogenic Acid, and Experimental Animals
Clostridium perfringens type A (No. ATCC13124) was purchased from the Guangdong microbial strain Preservation Center. The strain was aseptically inoculated into fluid thioglycollate medium overnight at 37°C in an anaerobic environment before being used for the challenge. Chlorogenic acid was purchased from Beijing Shengtaier Technology Co., Ltd. and supplied in the feed. Enramycin antibiotic (each 1,000 g, containing 0.125 g of 8% enramycin) in feed diets was purchased from Schering-Plough, China. About 324 healthy, 1-day-old male specific-pathogen-free (SPF) White Leghorn chickens with basically the same body weight were bred and purchased from the SPF Experimental Animal Center of Guangdong Dahuanong Animal Health Co., Ltd.
Experimental Design
About 324 1-day-old male SPF chickens were randomly distributed into 6 groups with 9 replicates per group and 6 chickens per replicate. The chickens were reared in an over pressure isolator. The temperature of the isolator was maintained 32°C during the first week, and then, it was reduced 2°C each week until the third week. Chickens were exposed to 23 h of white light a day and 1 h a dark. The specific experimental groups are shown in Table 1. Plastic drinkers and plastic feeders were put in the negative pressure isolators that used for supplying water and feed. All chickens in different groups were allowed liberal feed intake and water every day. The chickens received either a basal diet or a basal diet supplemented with 500 mg/kg CA or a basal diet supplemented with 0.125 g/kg antibiotic before being infected with CP type A. The composition and nutritional level of basal feeding were shown in Table 2. In this study, after 10-fold continuous dilution of the culture medium of CP, the diluent of each dilution gradient was evenly coated on TSC agar medium and anaerobically cultured at 37°C for 24 h. The number of characteristic black presumptive CP colonies was then counted and the concentration of CP in the culture medium was determined to be 108 cfu. A round-tipped animal feeding needle attached to a repeating syringe was used to orally gavage (Fasina et al., 2016) in CP type A–infected chickens, at a dose of 1 × 108 cfu/mL, and at 14 d old, each chicken was fed 3 mL of CP type A for 3 d, whereas chickens were mock-challenged with freshly prepared fluid thioglycollate medium.
Table 1.
The experimental groups in this study.
| Group | Treatment | Number of chickens | The examined number of chickens |
|---|---|---|---|
| Control group | Basal diet | 54 | 9 |
| CA group | Basal diet and CA (500 mg/kg) | 54 | 9 |
| CP infection group | Basal diet, challenge at 14 d old (1 × 108 cfu/mL, total of 3 mL per chicken) | 54 | 9 |
| CA + CP group | Basal diet and chlorogenic acid, challenge at 14 d old (1 × 108 cfu/mL, total of 3 mL per chicken) | 54 | 9 |
| Antibiotic group | Basal diet and antibiotic (0.125 g/kg) | 54 | 9 |
| Antibiotic + CP group | Basal diet and antibiotic (0.125 g/kg), challenge at 14 d old (1 × 108 cfu/mL, total of 3 mL per chicken) | 54 | 9 |
Abbreviations: CA, chlorogenic acid; CP, Clostridium perfringens.
Table 2.
Ingredient composition and nutrient content of the basal diet (%, as-fed basis).
| Ingredient | Proportion (kg) | Nutrient levels2 | Content |
|---|---|---|---|
| Corn | 59.00 | Metabolic energy/(MJ/kg) | 12.64 |
| 46% Soybean meal | 29.50 | Crude protein | 21.30 |
| Soybean oil IV | 2.80 | Calcium | 0.83 |
| Corn gluten meal | 4.50 | Available phosphorus | 0.34 |
| Calcium hydrogen phosphate | 1.30 | Lysine | 1.15 |
| Limestone | 1.20 | Methionine | 0.47 |
| Sodium chloride | 0.30 | ||
| L-lysine | 0.25 | ||
| Methionine | 0.15 | ||
| 1Premix | 1.00 | ||
| Total | 100 |
Premix is provided per kilogram of diet: Mn (MnSO4·H2O) 60 mg; Fe (FeSO4·H2O) 66.5 mg; Zn (ZnSO4·7H2O) 88 mg; Cu (CuSO4·5H2O) 8.8 mg; I (CaI2) 0.7 mg; Se (Na2SeO3) 0.288 mg; VA11 500 IU; VD33 500 IU; VE 30 mg; VK 33 mg; VB1 3.38 mg; VB2 9.00 mg; VB6 8.96 mg; VB12 0.025 mg; choline chloride 800 mg; calcium pantothenate 13 mg; niacin 45 mg; biotin 0.08 mg; folic acid 1.20 mg.
The metabolic energy and available phosphorus were calculated values and others were analyzed values.
Growth Performance
All chickens were weighed daily from day 1 to day 21 of the feeding-challenge trial per treatment group. Daily feed intake was also recorded. The average daily gain (ADG), average daily feed intake (ADFI), feed to gain ratio (F/G), and survival rate were evaluated.
The Histological Observation of the Jejunum and Histomorphological Measurements of the Small Intestine
At 17 and 21 days old, a total of 9 chickens per group were selected with one chicken randomly selected from each replicate. After carotid artery bloodletting, a 1-cm jejunum of 9 chickens in each group was collected to observe the histopathologic changes. Segment from each chicken was cut off, rinsed repeatedly with phosphate buffered saline, fixed in formalin, and used as paraffin embedding to make hematoxylin and eosin staining sections. The intestinal sections were observed using a Nikon Eclipse ci optical microscope and were analyzed and photographed using NIS-Elements F 4.00.00 software. Nine jejunum sections from 9 chickens (one jejunum section/one chicken) in different treatment groups were selected and 5-μm stained sections were observed and analyzed histopathologically.
Ten villi from each histological section were chosen to assess the histological changes. Individual segments of the duodenum, jejunum, and ileum were rinsed with 0.9% of physiological saline and then fixed in 4% paraformaldehyde and embedded in paraffin. The samples were sliced into 5-μm section that was stained with hematoxylin and eosin. Morphometric measurements were performed at a 40× magnification using a light microscope. The criterion for villus section was the presence of intact lamina propria. Villus length was measured from the tip of the villus to the villus–crypt junction, whereas crypt depth was defined as the depth of the invagination between adjacent villi (Awad et al., 2009). The ratio of villus length to crypt depth was calculated, and the average value was taken as the final measured value.
Detection of Blood Biochemical Indices and Serum Antioxidant Indexes
Venous blood from 9 chickens per treatment group was collected at 17 and 21 d old and was placed in a water bath at 37°C for 30 min without anticoagulant treatment. The serum was isolated after 2,000 r/min centrifugation for 10 min (after agglutination). Serum biochemical indices, including blood glucose, triglycerides, total cholesterol, high-density cholesterol, low-density cholesterol, total proteins, albumin, and globulin, were measured with a Mindray BS-5800M automatic biochemical analyzer.
Venous blood samples from 9 chickens per treatment group were collected at 17 and 21 d old. The collected venous blood was placed in a water bath at 37°C for 30 min without anticoagulant treatment, and the serum was isolated after 2,000 r/min centrifugation for 10 min (after agglutination). The kits used for the determination of total antioxidant capacity, Glutathione peroxidase, superoxide dismutase, and malondialdehyde were purchased from the Nanjing Jiancheng company (Nanjing, China). The procedures were performed in accordance with the instructions in the different kits.
Detection of Ileum Immune-Related Cytokines, the Ileum Mucosal Layer Structure, and Tight-Junction–Related Factors Using Quantitative Real-Time PCR (RT-qPCR)
Nine chickens from each group were randomly selected and euthanized by cranial stunning immediately followed by cervical dislocation before necropsy. In an aseptic environment, a segment of ileum was cut off and put into a 2-mL grinding tube containing 1 mL TRIzol (Invitrogen, Carlsbad, CA). Total RNA was extracted from the ileum epithelium samples with TRIzol (Invitrogen, Carlsbad, CA). The purity and concentration of the total RNA were measured in a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific Co., Waltham, MA) using the 260/280 nm absorbance ratio and then a 1.0 μg RNA of each sample was reverse-transcribed into cDNA using the RecerTra Ace qPCR RT Master Mix with gDNA remover (TOYOBO Co., Ltd. Life Science Department, OSAKA, Japan), and the cDNA was stored at −20°C. Quantitative real-time PCR (RT-qPCR) was performed in duplicate reactions including 1.5 μL nuclease-free water, 1 μL of each forward and reverse primers of each gene (5 μmol/L), 1.5 μL cDNA (1:20 dilution) and 5 μL SYBR Premix Ex Taq II kit (Thermo Fisher Scientific Co., Waltham, MA), as a detector on an CFX96 Touch real-time PCR detection system (Bio-Rad Laboratories, Mississauga, ON, Canada). In accordance with the sequences published in GenBank, the primers for the ileum mucosal layer structure and the tight-junction–related factors, and ileum inflammation–related cytokines were designed using Primer Express 3.0 (Applied Biosystems) for RT-qPCR detection and are shown in Table 3. Each sample was analyzed in triplicate under the following PCR conditions: initial denaturation at 95°C for 3 min, followed by 40 cycles at 95°C for 5 s and at 60°C for 30 s; specificity of the PCR products was evaluated by the analysis of the melting curve; GAPDH as an internal reference gene; the relative expression of each gene was calculated using 2−△△Ct (Livak and Schmittgen, 2001).
Table 3.
Primers for RT-qPCR detection of ileum mucosal barrier related indexes and inflammation-related cytokines.
| Gene names | Primers | Accession no. |
|---|---|---|
| ZO-1 | F: TATGAAGATCGTGCGCCTCC R: TGCCGGATAATAGCTGCGTT |
Accession: XM_015278981 |
| Claudin-3 | F: CTATGGGGCTGGAGATCGGT R: ATGTTGTTGCCGATGAAGGC |
Accession: NM_204202 |
| TFF2 | F: CTGCTTCAATGCGTCGGTG R: AGGGTAGCCACAGTTCACTC |
Accession: XM_416743 |
| MUC2 | F: ATGCCCTTGCGTCCATAACA R: AGGAGCAGTGTCCGTCAAAG |
Accession: NM_002457 |
| IFN-γ | F: GATGCCACCTTCTCTCACGA R: GGATGTCGTGGGTGGTTTTG |
Accession: NM_205427 |
| IFN-β | F: CAGCCCACACACTCCAAAAC R: ACGAAGCATTGCTCAAGGTG |
Accession: NM_001024836 |
| IL-1 | F: CCCGCTTCATCTTCTACCGC R: GCTTGTAGGTGGCGATGTTG |
Accession: NM_204524 |
| IL-17A | F: CTCTGACCCTGCCTCTAGGA R: CTGTGTGATGGGGACGGAAT |
Accession: XM_426223 |
| IL-22 | F: CATCAGGGAGAACAACCGCT R: TGCCACATCCTCAGCATACG |
Accession: XM_025147965 |
| TNF-α | F: TGTATGTGCAGCAACCCGTA R: CCACACGACAGCCAAGTCAA |
Accession: NM_204267 |
| GAPDH | F: AGGCTGAGAACGGGAAACTTG R: CACCTGCATCTGCCCATTTG |
Accession: NM_204305 |
Statistical Analysis
The individual chicken was considered as the experimental unit for all measures, with 9 replicate individuals per treatment group. Statistical analysis was conducted using SPSS. The normality of residuals was verified using liner regression procedure. Growth performance was analyzed by one-way ANOVA. Histomorphological measurements, blood biochemical indices, serum antioxidant indexes, inflammation-related cytokines, mucosal layer structure, and tight-junction–related factors were analyzed by 2-way ANOVA to determine the main effects (stage and treatment) and their interaction using the generalized linear model procedure as a 2 × 6 factorial arranged treatment. Survival rate was analyzed by the nonparametric Kruskal-Wallis test, as the data were not normally distributed. When interactions were significant (P < 0.05), differences between means were determined using least significant difference. The results were presented as the means and SEMs.
Results
CA Improved the Growth Performance in SPF Chickens After CP Type A Infection
Effect of adding CA on the growth performance in SPF chickens after CP type A infection is shown in Table 4. At day 1 to 14, 14 to 17, 17 to 21, and the overall period of 1 to 21 d, compared with the control group, ADG in the CA group were significantly increased (P < 0.05). Besides, the ADFI and F/G in the CA group were significantly lower than that in the control group at day 17 to 21 and day 1 to 21 (P < 0.05). Compared with the control group, ADG in the CP group was significantly decreased at day 1 to 14, 14 to 17, 17 to 21, and 1 to 21. F/G in the CP group was significantly higher than that in the control group at day 1 to 14, 14 to 17, 17 to 21, and 1 to 21 (P < 0.05). Furthermore, the ADG was significantly higher in the CA + CP group than in the CP group (P < 0.05), whereas F/G was lower in the CA + CP group than in the CP group at day 1 to 14, 14 to 17, 17 to 21, and 1 to 21 (P < 0.05). The survival rate of chickens in CA + CP group was reached to 100% from 1 to 21 d old.
Table 4.
Effects of different treatments on growth performance of SPF chickens.
| Stage | Indicator | Control | CP | CA | CA + CP | AB | AB + CP | SEM | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Day 1–14 | ADG(g) | 4.98c | 4.36d | 5.35a | 5.10b | 5.27a | 5.13b | 0.057 | <0.001 |
| ADFI(g) | 8.38b | 8.43b | 8.40b | 8.22c | 8.77a | 8.41b | 0.051 | <0.001 | |
| F/G | 1.68b | 1.93a | 1.57c | 1.61c | 1.66b,c | 1.64b,c | 0.030 | <0.001 | |
| Survival rate (%) | 100 | 94 | 100 | 100 | 100 | 100 | 1.000 | 0.416 | |
| Day 14–17 | ADG(g) | 9.38d | 9.04e | 9.77a | 9.53c | 9.72b | 9.49c | 0.022 | <0.001 |
| ADFI(g) | 20.09a,b | 20.15a,b | 19.81b | 19.75b | 20.36a | 20.09a,b | 0.222 | 0.114 | |
| F/G | 2.14b | 2.23a | 2.03c | 2.07c | 2.09b,c | 2.12b,c | 0.024 | <0.001 | |
| Survival rate (%) | 100 | 98 | 100 | 100 | 100 | 100 | 0.333 | 0.416 | |
| Day 17–21 | ADG(g) | 12.04c | 9.97f | 12.36a | 11.81d | 12.11b | 10.77e | 0.016 | <0.001 |
| ADFI(g) | 27.04a | 24.74b,c | 24.26c | 23.90c | 24.25c | 24.89b | 0.259 | <0.001 | |
| F/G | 2.26c | 2.48a | 1.97e | 2.03d | 1.99d,e | 2.32b | 0.027 | <0.001 | |
| Survival rate (%) | 100 | 98 | 100 | 100 | 100 | 100 | 0.043 | 0.416 | |
| Day 1–21 | ADG(g) | 6.95c | 6.10e | 7.32a | 7.01c | 7.21b | 6.83d | 0.039 | <0.001 |
| ADFI(g) | 13.61a | 13.21c | 13.05e | 12.85d | 13.37b | 13.22c | 0.070 | <0.001 | |
| F/G | 1.96b | 2.17a | 1.78d | 1.83c | 1.86c | 1.94b | 0.020 | <0.001 | |
| Survival rate (%) | 100 | 92 | 100 | 100 | 100 | 100 | 1.333 | 0.416 |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: AB, antibiotic group; AB + CP, antibiotic + C. perfringens group; ADFI, average daily feed intake (g/head/d); ADG, average daily gain (g/head/d); CA, chlorogenic acid group; CA + CP, chlorogenic acid + C. perfringens group; CP, Clostridium perfringens group; F/G, feed to gain ratio; specific-pathogen-free; SR, survival rate.
CA Minimized the Pathological Injury to the Jejunum Structure in SPF Chickens After CP Type A Infection
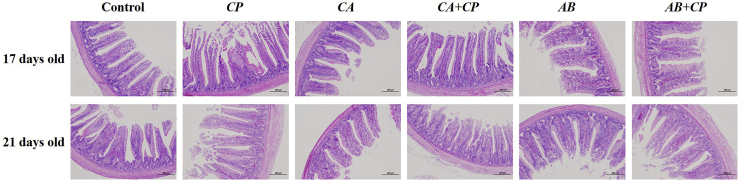
Histopathological sections were performed to analyze the jejunum structure injury caused by CP type A infection and to verify whether CA could inhibit injury. A jejunum histopathology analysis showed that at 17 d of age, the epithelial cells in the jejunum mucosa of the SPF chickens infected with CP type A were exfoliated, and the villi were both damaged and exfoliated. At 21 d of age, the jejunum of CP type A infection group showed mucosal epithelial cells were degenerated and shed, and the villi were severely damaged and shed. However, there was no injury in the jejunum of the examined 20 SPF chickens (17 and 21 d old) that were fed CA and infected with CP type A, whereas there was slight exfoliation of mucosal epithelial cells in the jejunum of the group fed with antibiotics and infected with CP type A, indicating that CA could minimize the pathological injury to the jejunum caused by CP type A infection (Figure 1).
Figure 1.
The addition of chlorogenic acid alleviated jejunum histopathological changes caused by CP type A infection in the jejunum of 17- and 21-day-old SPF chickens. The histological features of the jejunum mucosa in the control group, CP infection group, CA group, CA plus CP infection group (CA + CP), antibiotic group, and antibiotic plus CP group (antibiotic + CP) are shown together with the hematoxylin and eosin staining of 17- and 21-day-old SPF chickens. For histological observation, images at a lower magnification (100×) are provided. n = 9 chickens/group. Abbreviations: CA, chlorogenic acid; CP, Clostridium perfringens; SPF, specific-pathogen-free.
CA Protected the Small Intestine Morphology of SPF Chickens After CP Type A Infection
As shown in Table 5, the villus length of the duodenum and ileum at day 17 was significantly higher than that at day 21 (P < 0.05); furthermore, the villus length and villus length/crypt depth of the jejunum at day 21 was significantly higher than that at day 17 (P < 0.05). The villus length, villus length/crypt depth of the duodenum and jejunum in the CA treatment group were significantly higher than that in other treatment groups (P < 0.05). The villus length and length/crypt depth of the duodenum, jejunum, and ileum in the CP group were significantly lower than that in the control group (P < 0.05), whereas the crypt depth of the duodenum, jejunum, and ileum was significantly higher than that in the control group (P < 0.05). Furthermore, the villus length and villus length/crypt depth of the CA + CP group were significantly higher than that in the CP group and AB + CP group, respectively (P < 0.05). The effect of interaction (P < 0.05) between stage and treatments was found in the crypt depth and villus length/crypt depth of the duodenum and jejunum, also was found in villus length of the duodenum and villus length/crypt depth of the ileum. These results indicated that the addition of CA minimized small intestine structure injury in chickens after CP type A infection.
Table 5.
The addition of chlorogenic acid protected small intestine morphology damaged by Clostridium perfringens type A infection in 17- and 21-day-old SPF chickens.
| Interaction | Treatments | Duodenum villus length (μmol/L) | Duodenum crypt depth (μmol/L) | Duodenum villus length/crypt depth | Jejunum villus length (μmol/L) | Jejunum crypt depth (μmol/L) | Jejunum villus length/crypt depth | Ileum villus length (μmol/L) | Ileum crypt depth (μmol/L) | Ileum villus length/crypt depth | Lesion score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 17 | Control | 1,216.97b | 86.06c | 13.76c | 476.68d | 59.48b,c | 8.77c | 532.60b | 56.78b | 9.05c | 0.00i |
| CP | 1,099.33c | 171.24a | 6.77e | 426.44e | 67.72a | 6.41e | 456.07c | 62.32a | 7.44d | 2.50a | |
| CA | 1,334.01a | 78.88c | 19.32a,b | 560.83b,c | 42.57e | 12.63a | 598.97a | 45.83d | 11.64a | 0.23g | |
| CA + CP | 1,245.07b | 82.91c | 14.14c | 529.17c | 56.73c | 9.56b | 539.42b | 47.96c,d | 10.83a,b | 0.33f | |
| AB | 1,191.25b,c | 85.33c | 14.15c | 471.05d,e | 60.02b,c | 7.90d | 527.75b | 59.47a,b | 8.90c,d | 0.77d | |
| AB + CP | 1,079.08c | 89.20c | 12.35c,d | 455.73d,e | 60.52b,c | 7.59d,e | 518.30b | 58.64a,b | 8.89c,d | 0.83c | |
| Day 21 | Control | 1,098.12c | 84.82c | 13.59c | 552.81b,c | 59.53b,c | 9.79b | 412.83d | 49.71c,d | 8.38c,d | 0.00i |
| CP | 968.81d | 103.08b | 10.22d | 464.41d,e | 70.27a | 6.87e | 327.98e | 57.31b | 5.21e | 1.50b | |
| CA | 1,412.31a | 65.08d | 21.09a | 676.60a | 51.08d | 12.57a | 513.72b | 41.81d,e | 10.64b | 0.12h | |
| CA + CP | 1,109.88c | 60.38d | 17.41b | 579.27b | 49.53d | 12.88a | 444.70c,d | 40.33e | 10.34b | 0.13h | |
| AB | 1,124.52c | 85.42c | 13.19c | 545.77b,c | 57.25c | 9.64b | 421.15c,d | 49.77c,d | 8.47c,d | 0.33f | |
| AB + CP | 1,071.85c | 86.24c | 12.81c | 545.26b,c | 63.15b | 8.67c,d | 413.59d | 50.53c | 8.21d | 0.37e | |
| SEM | 19.598 | 4.418 | 0.797 | 15.860 | 1.502 | 0.272 | 12.242 | 1.511 | 0.285 | 0.012 | |
| Stage | |||||||||||
| Day 17 | 1,200.02a | 98.94a | 13.42b | 486.65b | 57.84 | 8.81b | 528.85a | 55.17a | 9.46a | 0.78a | |
| Day 21 | 1,130.92b | 80.84b | 14.72a | 560.69a | 58.47 | 10.07a | 422.33b | 48.24b | 8.54b | 0.40b | |
| SEM | 8.001 | 1.804 | 0.326 | 6.475 | 0.613 | 0.111 | 4.998 | 0.617 | 0.116 | 0.005 | |
| Treatments | |||||||||||
| Control | 1,157.33b | 85.44b | 13.68c | 514.74c | 59.50b,c | 9.28c | 472.72b,c | 53.24b | 8.72b | 0.00f | |
| CP | 1,034.15d | 137.16a | 8.50d | 445.42d | 69.00a | 6.64e | 392.02d | 59.82a | 6.32c | 2.00a | |
| CA | 1,390.08a | 71.98c | 20.20a | 618.72a | 46.82e | 12.60a | 556.34a | 43.82c | 11.14a | 0.16e | |
| CA + CP | 1,177.53b | 71.64c | 15.78b | 554.22b | 53.13d | 11.22b | 492.06b | 44.14c | 10.58a | 0.23d | |
| AB | 1,157.84b | 85.38b | 13.67c | 508.41c | 58.64c | 8.77c | 474.45b,c | 54.62b | 8.68b | 0.55c | |
| AB + CP | 1,075.87c | 87.72b | 12.58c | 500.50c | 61.84b | 8.13d | 465.94c | 54.58b | 8.55b | 0.60b | |
| SEM | 13.858 | 3.124 | 0.564 | 11.215 | 1.062 | 0.192 | 8.657 | 1.069 | 0.201 | 0.009 | |
| P-values | |||||||||||
| Stage | <0.001 | <0.001 | 0.008 | <0.001 | 0.473 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Treatments | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage × Treatments | <0.001 | <0.001 | 0.040 | 0.204 | <0.001 | <0.001 | 0.541 | 0.460 | 0.031 | <0.001 |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: AB, antibiotic group; AB + CP, antibiotic + C. perfringens group; CA, chlorogenic acid group; CA + CP, chlorogenic acid + C. perfringens group; CP, Clostridium perfringens group; SPF, specific-pathogen-free.
CA Improved the Antioxidant Capacity of SPF Chickens After CP Type A Infection
As shown in Table 6, the total serum antioxidant capacity, serum glutathione peroxidase, and the content of serum malondialdehyde at day 21 were significantly higher than that at day 17 (P < 0.05). The total serum antioxidant capacity, serum superoxide dismutase, and serum glutathione peroxidase in the CA treatment group were significantly higher than that in other treatment groups (P < 0.05); however, serum malondialdehyde in the CA treatment group was significantly lower than that in other treatment groups (P < 0.05). The total serum antioxidant capacity, serum superoxide dismutase, and serum glutathione peroxidase in the CP group were significantly lower than that in the control group (P < 0.05), whereas the serum malondialdehyde in the CP group was significantly higher than that in the control group (P < 0.05). Furthermore, the total serum antioxidant capacity, serum superoxide dismutase, and serum glutathione peroxidase in the CA + CP group were significantly higher than that in CP group and AB + CP group (P < 0.05), whereas serum malondialdehyde in the CA + CP group was significantly lower than that in the CP group and AB + CP group (P < 0.05). The effect of interaction (P < 0.05) between stage and treatments was found in total serum antioxidant capacity, serum glutathione peroxidase, and serum malondialdehyde. These results indicated that the addition of CA improved the antioxidant capacity of SPF chickens after CP type A infection.
Table 6.
The addition of chlorogenic acid improved the antioxidant capacity affected by Clostridium perfringens type A infection in 17-and 21-day-old SPF chickens.
| Interaction | Treatments | Total antioxidant capacity (mmol/L) | Superoxide dismutase (U/mL) | Glutathione peroxidase (μmol/L) | Malondialdehyde (nmol/mL) |
|---|---|---|---|---|---|
| Day 17 | Control | 6.56d | 67.44c | 1,024.90d | 18.15d |
| CP | 3.32e | 59.31d | 800.29e | 28.22a | |
| CA | 10.77b | 78.56a | 1,319.53b | 12.69e | |
| CA + CP | 7.44d | 69.88b,c | 1,191.34c | 16.81d | |
| AB | 6.60d | 66.42c,d | 1,017.16d | 18.70c,d | |
| AB + CP | 6.36d | 66.77c | 1,049.87d | 18.37d | |
| Day 21 | Control | 9.83b,c | 66.91c | 1,140.70c,d | 23.63b |
| CP | 1.66f | 59.15d | 570.73f | 30.06a | |
| CA | 19.20a | 79.46a | 1,533.56a | 13.80e | |
| CA + CP | 9.68b,c | 73.60b | 1,351.62b | 21.01c | |
| AB | 9.74b,c | 64.16c,d | 1,083.82c,d | 23.50b | |
| AB + CP | 8.96c | 61.90d | 1,040.70d | 25.77b | |
| SEM | 0.472 | 1.642 | 39.716 | 0.825 | |
| Stage | |||||
| Day 17 | 6.84b | 68.06 | 1,067.18b | 18.82b | |
| Day 21 | 9.84a | 67.53 | 1,120.19a | 22.96a | |
| SEM | 0.193 | 0.670 | 16.214 | 0.337 | |
| Treatments | |||||
| Control | 8.20b | 67.18c | 1,082.80c | 20.89b | |
| CP | 2.49c | 59.23d | 685.51d | 29.14a | |
| CA | 14.98a | 79.01a | 1,426.54a | 13.24d | |
| CA + CP | 8.56b | 71.74b | 1,271.48b | 18.91c | |
| AB | 8.17b | 65.29c | 1,050.49c | 21.10b | |
| AB + CP | 7.66b | 64.34c | 1,045.28c | 22.07b | |
| SEM | 0.334 | 1.161 | 28.083 | 0.584 | |
| P-values | |||||
| Stage | <0.001 | 0.577 | 0.027 | <0.001 | |
| Treatments | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage × Treatments | <0.001 | 0.196 | <0.001 | 0.005 | |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: AB, antibiotic group; AB + CP, antibiotic + C. perfringens group; CA, chlorogenic acid group; CA + CP, chlorogenic acid + C. perfringens group; CP, Clostridium perfringens group; SPF, specific-pathogen-free.
CA Inhibited Damage to the Ileal Mucosal Layer Construction and Tight Junctions of SPF Chickens After CP Type A Infection
As shown in Table 7, the mRNA expression of Claudin-3 and ZO-1 at day 17 was significantly higher than that at day 21 (P < 0.05). The mRNA expression of Claudin-3, MUC-2, TFF-2, and ZO-1 in the CP group was significantly lower than that in the control group (P < 0.05). The mRNA expression of Claudin-3 and ZO-1 in the CA + CP group was significantly higher than that in the CP group (P < 0.05). Furthermore, the mRNA expression of ZO-1 in the CA + CP group was significantly higher than that in the AB + CP group (P < 0.05). Besides, the mRNA expression of TFF-2 in the CA + CP group was significantly higher than that in the CP group (P < 0.05). Moreover, the mRNA expression of MUC-2 in the CA group was significantly higher than that in the AB group (P < 0.05). The effect of interaction (P < 0.05) between stage and treatments was found in Claudin-3 and ZO-1. These results indicated that the addition of CA inhibited damage to the ileal mucosal layer construction and tight junctions of SPF chickens after CP type A infection.
Table 7.
The addition of CA affected the mRNA expression levels of TFF2, MUC2, ZO-1, and Claudin-3 in the ileal epithelial cells of 17- and 21-day-old SPF chickens, which had been affected by CP type A infection (as found by RT-qPCR).
| Interaction | Treatments | Claudin-3 | MUC-2 | TFF-2 | ZO-1 |
|---|---|---|---|---|---|
| Day 17 | Control | 1.00a,b | 1.02a,b | 0.99a,b | 1.00a,b |
| CP | 0.95a,b | 0.51e | 0.51c | 0.59c | |
| CA | 1.00a,b | 1.05a,b | 0.95a,b | 1.05a | |
| CA + CP | 0.99a,b | 0.92b,c | 0.89b | 1.04a | |
| AB | 0.99a,b | 0.98b | 0.99a,b | 0.99a,b | |
| AB + CP | 0.93b | 0.83c | 0.81b | 0.97a,b | |
| Day 21 | Control | 0.99a,b | 0.99b | 1.00a | 1.03a |
| CP | 0.63c | 0.70d | 0.49c | 0.14d | |
| CA | 1.03a | 1.11a | 0.95a,b | 0.99a,b | |
| CA + CP | 0.93b | 0.98b | 0.88b | 1.06a | |
| AB | 1.02a | 0.98b | 1.01a | 0.99a,b | |
| AB + CP | 1.00a,b | 0.86c | 0.87b | 0.91b | |
| SEM | 0.03 | 0.037 | 0.037 | 0.04 | |
| Stage | |||||
| Day 17 | 0.98a | 0.88b | 0.86 | 0.94a | |
| Day 21 | 0.93b | 0.94a | 0.87 | 0.85b | |
| SEM | 0.012 | 0.015 | 0.015 | 0.016 | |
| Treatments | |||||
| Control | 1.00a | 1.00a,b | 1.00a | 1.02a,b | |
| CP | 0.79b | 0.60d | 0.50c | 0.36c | |
| CA | 1.02a | 1.08a | 0.95a,b | 1.02a,b | |
| CA + CP | 0.96a | 0.95b | 0.88b | 1.05a | |
| AB | 1.00a | 0.98b | 1.00a | 0.99a,b | |
| AB + CP | 0.96a | 0.84c | 0.84b | 0.94b | |
| SEM | 0.021 | 0.026 | 0.026 | 0.028 | |
| P-values | |||||
| Stage | 0.017 | 0.021 | 0.639 | 0.001 | |
| Treatments | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage × Treatments | <0.001 | 0.088 | 0.910 | <0.001 | |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: AB, antibiotic group; AB + CP, antibiotic + C. perfringens group; CA, chlorogenic acid group; CA + CP, chlorogenic acid + C. perfringens group; CP, Clostridium perfringens group; SPF, specific-pathogen-free.
CA Alleviated the Inflammatory Response in the Ileum of SPF Chickens After CP Type A Infection by Inhibiting the mRNA Expression of the Proinflammatory Cytokines IFN-β, IFN-γ, IL-1, IL-17A, IL-22, and TNF-α
As shown in Table 8, the mRNA expression of IFN-β and IFN-γ at day 17 was significantly higher than that at day 21 (P < 0.05), the mRNA expression of IL-22 at day 21 was significantly higher than that at day 17 (P < 0.05). Furthermore, the mRNA expression of the proinflammatory cytokines IFN-β, IFN-γ, IL-1, IL-17A, IL-22, and TNF-α in the CP group was significantly higher than that in the control group; however, the adding of CA in the CP group could significantly decrease the mRNA expression of the IFN-β, IFN-γ, IL-1, IL-17A, IL-22, and TNF-α compared with the CP group (P < 0.05). Besides, the mRNA expression of IFN-β, IFN-γ, IL-22, and TNF-α in the CA + CP group was significantly lower than that in the AB + CP group (P < 0.05). The effect of interaction (P < 0.05) between stage and treatments was found in the proinflammatory cytokines IFN-β, IFN-γ, IL-1, IL-17A, IL-22, and TNF-α. These results indicated that the addition of CA alleviated the inflammatory response in the ileum of SPF chickens after CP type A infection.
Table 8.
The addition of CA alleviated the inflammatory response induced by CP type A infection in 17- and 21-day-old SPF chickens by inhibiting the mRNA expression of the proinflammatory cytokines IFN-β, IFN-γ, IL-1, IL-17A, IL-22, and TFN-a in the ileal epithelial cells (as found by RT-qPCR).
| Interaction | Treatments | IFN-β | IFN-γ | IL-1 | IL-22 | IL-17A | TNF-α |
|---|---|---|---|---|---|---|---|
| Day 17 | Control | 1.00e | 1.00e | 0.98f | 0.98d | 1.01c | 0.99e |
| CP | 2.90a | 2.94a | 1.58d | 2.12b | 2.35a | 1.47c | |
| CA | 1.01e | 0.99e | 0.97f | 1.00d | 1.02c | 0.98e | |
| CA + CP | 1.81d | 1.25d | 1.67c | 0.96d | 0.99c,d | 1.07d | |
| AB | 1.01e | 1.02e | 1.02f | 1.00d | 0.99c,d | 1.01d,e | |
| AB + CP | 2.10c | 2.10c | 1.85b | 1.36c | 0.94d | 2.03b | |
| Day 21 | Control | 1.00e | 0.99e | 0.99f | 1.00d | 1.01c | 1.00d,e |
| CP | 2.51b | 2.22b | 2.77a | 2.84a | 2.23b | 2.70a | |
| CA | 0.99e | 1.02e | 0.98f | 0.96d | 0.98c,d | 0.98e | |
| CA + CP | 1.06e | 1.01e | 1.12e | 0.99d | 1.00c,d | 1.02d,e | |
| AB | 0.99e | 0.98e | 0.99f | 0.98d | 1.01c | 1.02d,e | |
| AB + CP | 1.04e | 1.02e | 1.02f | 1.06d | 1.01c | 0.99e | |
| SEM | 0.036 | 0.033 | 0.031 | 0.048 | 0.024 | 0.025 | |
| Stage | |||||||
| Day 17 | 1.64a | 1.55a | 1.34 | 1.24b | 1.22 | 1.26 | |
| Day 21 | 1.26b | 1.21b | 1.31 | 1.30a | 1.21 | 1.28 | |
| SEM | 0.015 | 0.014 | 0.013 | 0.019 | 0.010 | 0.01 | |
| Treatments | |||||||
| Control | 1.00d | 1.00d | 0.98c | 0.99c | 1.01b | 1.00c,d | |
| CP | 2.70a | 2.58a | 2.18a | 2.48a | 2.29a | 2.08a | |
| CA | 1.00d | 1.01d | 0.98c | 0.98c | 1.00b | 0.98d | |
| CA + CP | 1.44c | 1.13c | 1.40b | 0.98c | 1.00b | 1.04c | |
| AB | 1.00d | 1.00d | 1.00c | 0.99c | 1.00b | 1.02c,d | |
| AB + CP | 1.57b | 1.56b | 1.44b | 1.21b | 0.98b | 1.51b | |
| SEM | 0.026 | 0.024 | 0.022 | 0.034 | 0.017 | 0.018 | |
| P-values | |||||||
| Stage | <0.001 | <0.001 | 0.071 | 0.02 | 0.474 | 0.073 | |
| Treatments | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage × Treatments | <0.001 | <0.001 | <0.001 | <0.001 | 0.009 | <0.001 | |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: AB, antibiotic group; AB + CP, antibiotic + C. perfringens group; CA, chlorogenic acid group; CA + CP, chlorogenic acid + C. perfringens group; CP, Clostridium perfringens group; SPF, specific-pathogen-free.
CA Affected Blood Biochemical Indices that Had Been Affected by the CP Type A Infection Including Blood Glucose and Triglyceride, Whereas Did Not Affect Total Cholesterol, Density Cholesterol, Low-Density Cholesterol, Total Protein, Albumin, and Globulin
As shown in Table 9, the triglyceride, total cholesterol, high-density cholesterol, total proteins, albumin, globulin, and albumin/globulin at day 21 was significantly higher than that at day 17 (P < 0.05). The blood glucose and triglyceride in the CP group were significantly lower than that in the control group (P < 0.05); however, the adding of CA in the CP group could significantly increase the blood glucose and triglyceride compared with the CP group (P < 0.05). Clostridium perfringens type A infection had no significant effect on total cholesterol, high-density cholesterol, low-density cholesterol, total protein, albumin, and globulin (P > 0.05). Similarly, the adding of CA in the CP group could not significantly affect the total cholesterol, high-density cholesterol, low-density cholesterol, total protein, albumin, and globulin (P > 0.05). The effect of interaction (P < 0.05) between stage and treatments was found in blood glucose and triglyceride. These results indicated that the addition of CA affected blood biochemical indices that had been affected by the CP type A infection including blood glucose and triglyceride.
Table 9.
The addition of chlorogenic acid inhibited injury in 17- and 21-day-old SPF chickens infected with Clostridium perfringens type A by affecting blood biochemical indices (as found by RT-qPCR).
| Interaction | Treatments | Glucose | Triglyceride | Total cholesterol | High-density cholesterol | Low-density cholesterol | Total proteins | Albumin | Globulin | Albumin/Globulin |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 17 | Control | 11.23a,b | 1.21d | 19.64b | 2.09b,c | 1.26a | 19.64b | 11.20c | 11.62b | 0.97b,c |
| CP | 9.01c | 1.23d | 19.92b | 2.00c | 1.22a,b | 19.92b | 11.96b,c | 12.04b | 0.99b,c | |
| CA | 11.20a,b | 1.28d | 19.70b | 2.14b,c | 1.23a,b | 19.70b | 11.64b,c | 11.76b | 0.99b,c | |
| CA + CP | 10.35b | 1.25d | 19.91b | 2.11b,c | 1.22a,b | 19.91b | 12.24b | 12.00b | 1.02a,b | |
| AB | 11.01a,b | 1.23d | 19.54b | 2.20b | 1.28a | 19.54b | 11.52c | 12.04b | 0.96b,c | |
| AB + CP | 9.32c | 1.09e | 17.36c | 1.87c | 1.09b | 17.36c | 10.46d | 10.46c | 0.88c | |
| Day 21 | Control | 11.24a,b | 2.13b | 20.44a,b | 2.43a | 1.23a,b | 20.44a,b | 12.66a,b | 12.62a,b | 1.00b |
| CP | 8.03d | 1.78c | 19.98b | 2.49a | 1.26a | 19.98a,b | 13.10a | 12.08b | 1.09a | |
| CA | 10.54b | 2.25a | 20.40a,b | 2.52a | 1.23a,b | 20.40a,b | 12.72a,b | 12.40a,b | 1.03a,b | |
| CA + CP | 11.41a | 2.19a,b | 20.58a | 2.44a | 1.24a,b | 20.58a | 12.96a | 12.78a | 1.02a,b | |
| AB | 10.77b | 2.17a,b | 20.02a,b | 2.48a | 1.27a | 20.02a,b | 12.66a,b | 12.10b | 1.05a,b | |
| AB + CP | 9.29c | 1.85c | 17.83c | 2.17b | 1.10b | 17.83c | 11.29c | 10.98c | 0.91c | |
| SEM | 0.205 | 0.030 | 0.208 | 0.049 | 0.027 | 0.227 | 0.245 | 0.189 | 0.029 | |
| Stage | ||||||||||
| Day 17 | 10.35 | 1.22b | 19.34b | 2.07b | 1.22 | 19.34b | 11.50b | 11.65b | 0.97b | |
| Day 21 | 10.21 | 2.06a | 19.88a | 2.42a | 1.22 | 19.88a | 12.56a | 12.16a | 1.02a | |
| SEM | 0.084 | 0.012 | 0.085 | 0.020 | 0.011 | 0.093 | 0.100 | 0.077 | 0.012 | |
| Treatments | ||||||||||
| Control | 11.24a | 1.67b | 20.04a,b | 2.26a | 1.24a | 20.04a,b | 11.93b | 12.12a | 0.98a | |
| CP | 8.52c | 1.50c | 19.95a,b | 2.24a | 1.24a | 19.95a,b | 12.53a,b | 12.06a | 1.04a | |
| CA | 10.87a | 1.76a | 20.05a,b | 2.33a | 1.23a | 20.05a,b | 12.18a,b | 12.08a | 1.01a | |
| CA + CP | 10.88a | 1.72a,b | 20.24a | 2.28a | 1.23a | 20.24a | 12.60a | 12.39a | 1.02a | |
| AB | 10.89a | 1.70b | 19.78b | 2.34a | 1.28a | 19.78b | 12.09b | 12.07a | 1.00a | |
| AB + CP | 9.30b | 1.47c | 17.60c | 2.02b | 1.10b | 17.60c | 10.88c | 10.72b | 0.90b | |
| SEM | 0.145 | 0.021 | 0.147 | 0.035 | 0.019 | 0.160 | 0.173 | 0.133 | 0.020 | |
| P-values | ||||||||||
| Stage | 0.245 | <0.001 | <0.001 | <0.001 | 0.747 | <0.001 | <0.001 | <0.001 | 0.006 | |
| Treatments | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage × Treatments | <0.001 | <0.001 | 0.555 | 0.345 | 0.835 | 0.643 | 0.724 | 0.086 | 0.494 | |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: AB, antibiotic group; AB + CP, antibiotic + C. perfringens group; CA, chlorogenic acid group; CA + CP, chlorogenic acid + C. perfringens group; CP, C. perfringens group; SPF, specific-pathogen-free.
Discussion
In this study, CA was chosen to test its effect on CP type A infection in chickens. Production performance measured as ADG was improved, whereas ADFI and F/G were decreased by adding CA in the CP infection group compared with the CP infection group at the overall period of 1 to 21 d. Small intestine structural was damaged by CP infection, while dietary CA could inhibit this damage. Besides, CA could improve antioxidant capacity, inhibit damage to ileal mucosal layer structure and tight -junction–related factors, inhibit inflammatory cytokines, and ameliorate blood biochemical indices that had been affected by CP type A infection in chickens. These results indicate a beneficial effect on reducing negative effects of CP infection which causing necrotic enteritis in chickens.
Chlorogenic acid is present in various plants and Chinese herbal medicines and has been proved to improve the production performance of animals under different treatment conditions. Study showed that CA had positive effect on growth performance of Litopenaeus vannamei under normal and stress conditions (Wang et al., 2015). Dietary CA could improve growth performance of weaned pigs through maintain intestinal digestion and absorption function (Chen et al., 2018b). In 2019, researchers showed that dietary CA-enriched extract supplementation could alleviate the adverse effects of heat stress on growth performance and improve oxidative stability and fatty acid profile of breast meat in heat-stressed broilers (Zhao et al., 2019a). So far, it has not been reported that dietary CA can improve the production performance of chickens infected with CP. Hence, this study reveals for the first time that dietary CA could reduce F/G, while increasing the survival rate that had been affected by CP type A infection. This may be because CA not only could improve the function of enhancing the intestinal barrier and immune function, but also could improve the ability to digest and absorb nutrients in chickens infected with CP.
A study showed that CA could attenuate cadmium-induced intestinal injury in Sprague-Dawley rats (Xue et al., 2019). Another study showed that dietary CA supplementation can affect gut morphology in weaned piglets (Zhang et al., 2018b); however, whether dietary CA supplementation has the similar effect, such on improving the intestine morphology in chickens, was unknown. Previous study has been reported that in broiler chickens infected with CP, the jejunal villi lamina propria was increased and severely congested, and the inflammatory reaction was dramatic in the lamina propria and included the infiltration of heterophilic granulocytes and lymphocytes (Zhao et al., 2019b). Subclinical CP infection in chickens also manifests as macroscopic lesions in the small intestine (Van Immerseel et al., 2004). Similarly, in this study, CP type A infection could cause serious pathological changes in the jejunum of infected chickens, causing degeneration and exfoliation in the mucosal epithelial cells at 17 and 21 d old. The villus length and crypt depth are considered to be important parameters in evaluating intestinal health and recovery (Zhao et al., 2019b). Shorter villi and a deeper crypt may lead to decreased resistance to disease, lower growth performance, poor nutrient absorption, and increased secretion in the gastrointestinal tract (Mohammadagheri et al., 2016). The present study showed that CA can alleviate the intestine injury caused by CP type A infection in chickens by decreasing crypt depth while increasing villus length and villus length/crypt depth of the jejunum, ileal, and duodenum. These results indicate that CA can protect the small intestine structure from the damage caused by CP type A infection.
Studies have shown that CA has good antioxidative stress effects: CA protects against liver fibrosis in vivo and in vitro through the inhibition of oxidative stress (Shi et al., 2016); it can protect against injury to the liver and kidneys induced by D-galactose via antioxidation and anti-inflammatory effects (in mice) (Feng et al., 2016); and it can relieve oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2 (Gong et al., 2019). However, it is unknown whether CA can alleviate the oxidative stress induced by CP type A infection. This study showed that CA can effectively alleviate the oxidative stress caused by CP type A infection by increasing serum antioxidant capacity, superoxide dismutase activity, glutathione peroxidase activity, and inhibiting malondialdehyde content. In addition, this study found that dietary CA has good antioxidant effects, indicating that it is a good feed additive.
In intestinal epithelial cells, tight junction proteins play an important role in the intestinal mucosal barrier, and damage to these proteins will lead to an increase in cell-to-cell permeability (Chasiotis et al., 2012). Chlorogenic acid has been reported to have the effect on decreasing intestinal permeability and increasing expression of intestinal tight junction proteins in weaned rats challenged with LPS (Ruan et al., 2014), improving intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs (Chen et al., 2018a). However, it is unknown whether CA can inhibit damage to the ileal mucosal layer construction and tight junctions of SPF chickens after CP type A infection. Previous study showed that CP infection affects the ileal gene expression of Claudin-1, Occluding, and Mucin-2 in broilers (Du et al., 2016); the results of our study first shed light on the effects of CA on repairing the ileal tight junction through increasing the mRNA level of Claudin-3, MUC-2, TFF-2, and ZO-1 of chickens infected with CP type A.
Cytokines play an important role in the immune response. During the occurrence of a disease, the expression of proinflammatory cytokines in the body is abnormal, resulting in low immune functioning and/or pathological damage (Yockey and Iwasaki, 2018). Studies have shown that oxidative stress can cause inflammation (Hald and Lotharius, 2005); besides, bacteria can enter the portal vein and lymphatic system through the damaged intestinal mucosa, and even cause systemic inflammation in severe cases (Gosain and Gamelli, 2005). Because we have proven that CP type A infection can induce oxidative stress and damage the intestinal mucosa, we speculated that CP type A infection has the effect on inducing proinflammatory cytokines. Our results supported our hypothesis that CP type A infection could induce an increase in proinflammatory factors in intestinal epithelial cells, including IL-1, IL-17A, IL-22, INF-β, IFN-γ, and TNF-α. Furthermore, we found that dietary CA could significantly inhibit the expression of these proinflammatory cytokines in ileal epithelia; this is consistent with the conclusions of other studies that CA has good anti-inflammatory effects (dos Santos et al., 2006; Hwang et al., 2014). Our results may lead to new ideas for the treatment of inflammation induced by CP infection in chickens.
Serum biochemical indices generally include blood glucose, liver function indicators, kidney function indicators, and other indices: changes in various components in sera can help determine the health status of a body, which is important in clinical diagnosis and treatment (Liao et al., 2007). Researchers showed that lower blood sugar content and triglyceride are common with impaired liver disease (Nishikawa et al., 2016; Soohoo et al., 2019). High-density lipoprotein cholesterol can transport cholesterol to the liver and then be excreted by bile, whereas low-density lipoprotein cholesterol can transport cholesterol to extrahepatic tissue (Ayonrinde et al., 2019). The total serum protein level is a reflection of a body's protein metabolism and immune functioning: it is mainly composed of albumin and globulin, and its contents can indicate the strength of protein synthesis metabolism (Stohl et al., 2019). The ratio of albumin to globulin reflects the state of the immune system in the body, and if the ratio decreases, this indicates that globulin synthesis is increased and that immune performance in the body is improved (Yoshino et al., 2019). In this study, we confirmed that CP type A infection could decrease blood glucose and triglyceride. Abnormalities in blood glucose and triglycerides are closely related to liver disease, suggesting that CP type A infection also has a damaging effect on the livers of SPF chickens. Interestingly, the addition of CA could upregulate the content of blood glucose and triglycerides after it was affected by CP type A, illustrating that CA has a certain protective effect in livers damaged by CP type A infection.
Conclusions
This study showed for the first time that CA minimizes the pathological damage to the small intestine structure, improves antioxidant capacity, inhibits damage to ileal mucous layer construction and tight junctions, and inhibits the transcriptional activity of inflammatory cytokines to alleviate the inflammatory response and ameliorate harmful blood biochemical indices in chickens infected with CP type A. This study provides new data for the clinical prevention and control of necrotic enteritis caused by CP type A infections.
Acknowledgments
This work was supported by the Chief Expert Project of Agricultural Industry Technology System in Guangdong Province (grant no. 2019KJ128), Creation of Triple Chimeric Vaccine (rIBV-ND-H9) based on attenuated Avian Infectious bronchitis virus D90 (grant no. 2017KZDM008), Special Project of National Modern Agricultural Industrial Technology System (grant no. CARS-41) and Guangdong Provincial Promotion Project on Preservation and Utilization of Local Breed of Livestock and Poultry.
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Annett C.B., Viste J.R., Chirino-Trejo M., Classen H.L., Middleton D.M., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Ayonrinde O., Chin J., Mori T., Olynyk J., Adams L., Beilin L. Associations between remnant lipoprotein cholesterol and non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019;34:60–61. [Google Scholar]
- Chalmers G., Bruce H.L., Hunter D.B., Parreira V.R., Kulkarni R.R., Jiang Y.F., Prescott J.F., Boerlin P. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. J. Clin. Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis H., Kolosov D., Bui P., Kelly S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: a review. Respir. Physiol. Neurobiol. 2012;184:269–281. doi: 10.1016/j.resp.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Chen J.L., Li Y., Yu B., Chen D.W., Mao X.B., Zheng P., Luo J.Q., He J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018;96:1108–1118. doi: 10.1093/jas/skx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu B., Chen D., Huang Z., Mao X., Zheng P., Yu J., Luo J., He J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018;59:84–92. doi: 10.1016/j.jnutbio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Craven S.E., Cox N.A., Bailey J.S., Cosby D.E. Incidence and tracking of Clostridium perfringens through an integrated broiler chicken operation. Avian Dis. 2003;47:707–711. doi: 10.1637/6010. [DOI] [PubMed] [Google Scholar]
- da Silveira T.F.F., Meinhart A.D., de Souza T.C.L., Cunha E.C.E., de Moraes M.R., Teixeira J., Godoy H.T. Optimization of the preparation conditions of yerba mate tea beverage to maximize chlorogenic acids extraction. Plant Food Hum. Nutr. 2017;72:219–223. doi: 10.1007/s11130-017-0613-6. [DOI] [PubMed] [Google Scholar]
- dos Santos M.D., Almeida M.C., Lopes N.P., de Souza G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006;29:2236–2240. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- Du E.C., Wang W.W., Gan L.P., Li Z., Guo S.S., Guo Y.M. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7 doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exon J.H., Magnuson B.A., South E.H., Hendrix K. Effect of dietary chlorogenic acid on multiple immune functions and formation of aberrant crypt foci in rats. J. Toxicol. Environ. Health A. 1998;53:375–384. doi: 10.1080/009841098159231. [DOI] [PubMed] [Google Scholar]
- Farah A., Monteiro M., Donangelo C.M., Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008;138:2309–2315. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- Fasina Y.O., Newman M.M., Stough J.M., Liles M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016;95:247–260. doi: 10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- Feng Y., Yu Y.H., Wang S.T., Ren J., Camer D., Hua Y.Z., Zhang Q., Huang J., Xue D.L., Zhang X.F., Huang X.F., Liu Y. Chlorogenic acid protects d-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016;54:1027–1034. doi: 10.3109/13880209.2015.1093510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W.F., Li J., Zhu G.Y., Wang Y.C., Zheng G.Y., Kan Q.C. Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2. Cell Cycle. 2019;18:1549–1559. doi: 10.1080/15384101.2019.1612697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosain A., Gamelli R.L. Role of the gastrointestinal tract in burn sepsis. J. Burn Care Rehabil. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- Hald A., Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp. Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hwang S.J., Kim Y.W., Park Y., Lee H.J., Kim K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- Leiss K.A., Maltese F., Choi Y.H., Verpoorte R., Klinkhamer P.G.L. Identification of chlorogenic acid as a resistance factor for thrips in Chrysanthemum. Plant Physiol. 2009;150:1567–1575. doi: 10.1104/pp.109.138131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lillehoj H.S., Gadde U.D., Ritter D., Oh S. Characterization of Clostridium perfringens strains isolated from healthy and necrotic enteritis-afflicted broiler chickens. Avian Dis. 2017;61:178–185. doi: 10.1637/11507-093016-Reg.1. [DOI] [PubMed] [Google Scholar]
- Liao F., Zhu X.Y., Wang Y.M., Zhao Y.S., Zhu L.P., Zuo Y.P. Correlation of serum arylesterase activity on phenylacetate estimated by the integrated method to common classical biochemical indexes of liver damage. J. Zhejiang Univ. Sci. B. 2007;8:237–241. doi: 10.1631/jzus.2007.B0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Guo S., Guo Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012;41:291–298. doi: 10.1080/03079457.2012.684089. [DOI] [PubMed] [Google Scholar]
- Liu N., Lin L., Wang J., Zhang F., Wang J.P. Dietary cysteamine hydrochloride protects against oxidation, inflammation, and mucosal barrier disruption of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. 2018;96:4339–4347. doi: 10.1093/jas/sky292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCourt M.T., Finlay D.A., Laird C., Smyth J.A., Bell C., Ball H.J. Sandwich ELISA detection of Clostridium perfringens cells and alpha-toxin from field cases of necrotic enteritis of poultry. Vet. Microbiol. 2005;106:259–264. doi: 10.1016/j.vetmic.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Meer R.R., Songer J.G. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 1997;58:702–705. [PubMed] [Google Scholar]
- Miller R.W., Skinner E.J., Sulakvelidze A., Mathis G.F., Hofacre C.L. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 2010;54:33–40. doi: 10.1637/8953-060509-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mohammadagheri N., Najafi R., Najafi G. Effects of dietary supplementation of organic acids and phytase on performance and intestinal histomorphology of broilers. Vet. Res. Forum. 2016;7:189–195. [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H., Yoh K., Enomoto H., Iwata Y., Kishino K., Shimono Y., Hasegawa K., Nakano C., Takata R., Nishimura T., Aizawa N., Sakai Y., Ikeda N., Takashima T., Ishii A., Iijima H., Nishiguchi S. Factors associated with protein-energy malnutrition in chronic liver disease analysis using indirect calorimetry. Medicine. 2016;95 doi: 10.1097/MD.0000000000002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell V.J., Poppe C., Parreira V.R., Jiang Y.F., Reid-Smith R., Prescott J.F. Clostridium perfringens in retail chicken. Anaerobe. 2010;16:314–315. doi: 10.1016/j.anaerobe.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Olkowski A.A., Wojnarowicz C., Chirino-Trejo M., Drew M.D. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res. Vet. Sci. 2006;81:99–108. doi: 10.1016/j.rvsc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Rood J.I. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 1998;52:333–360. doi: 10.1146/annurev.micro.52.1.333. [DOI] [PubMed] [Google Scholar]
- Rood J.I., Keyburn A.L., Moore R.J. NetB and necrotic enteritis: the hole movable story. Avian Pathol. 2016;45:295–301. doi: 10.1080/03079457.2016.1158781. [DOI] [PubMed] [Google Scholar]
- Ruan Z., Liu S., Zhou Y., Mi S., Liu G., Wu X., Yao K., Assaad H., Deng Z., Hou Y., Wu G., Yin Y. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS One. 2014;9:e97815. doi: 10.1371/journal.pone.0097815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Galvez J., Cisneros-Zevallos L., Jacobo-Velazquez D.A. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22 doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.T., Shi A., Dong L., Lu X.L., Wang Y., Zhao J.H., Dai F., Guo X.Y. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016;35:1366–1373. doi: 10.1016/j.clnu.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Siragusa G.R., Danyluk M.D., Hiett K.L., Wise M.G., Craven S.E. Molecular subtyping of poultry-associated type A Clostridium perfringens isolates by repetitive-element PCR. J. Clin. Microbiol. 2006;44:1065–1073. doi: 10.1128/JCM.44.3.1065-1073.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soohoo M., Moradi H., Obi Y., Kovesdy C.P., Kalantar-Zadeh K., Streja E. Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million US veterans. J. Clin. Lipidol. 2019;13:744–753. doi: 10.1016/j.jacl.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S.B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:e104739. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl W., Kenol B., Kelly A.J., Correa A.A., Panush R.S. Elevated serum globulin gap as a highly reliable marker of elevated erythrocyte sedimentation rate in patients with systemic rheumatic diseases. Semin. Arthritis Rheum. 2019;49:485–492. doi: 10.1016/j.semarthrit.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Tajik N., Tajik M., Mack I., Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur. J. Nutr. 2017;56:2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li Z., Li J., Duan Y.F., Niu J., Wang J., Huang Z., Lin H.Z. Effects of dietary chlorogenic acid on growth performance, antioxidant capacity of white shrimp Litopenaeus vannamei under normal condition and combined stress of low-salinity and nitrite. Fish Shellfish Immunol. 2015;43:337–345. doi: 10.1016/j.fsi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Xue Y.W., Huang F., Tang R.X., Fan Q.S., Zhang B., Xu Z.J., Sun X.M., Ruan Z. Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague-Dawley rats. Food Chem. Toxicol. 2019;133 doi: 10.1016/j.fct.2019.110751. [DOI] [PubMed] [Google Scholar]
- Yan K., Cui M.X., Zhao S.J., Chen X.B., Tang X.L. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica thunb.) Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey L.J., Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49:397–412. doi: 10.1016/j.immuni.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino Y., Taguchi A., Shimizuguchi T., Nakajima Y., Takao M., Kashiyama T., Furusawa A., Kino N., Yasugi T. A low albumin to globulin ratio with a high serum globulin level is a prognostic marker for poor survival in cervical cancer patients treated with radiation based therapy. Int. J. Gynecol. Cancer. 2019;29:17–22. doi: 10.1136/ijgc-2018-000025. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Chen D., Yu B., Zheng P., Mao X., Luo Y., Li Y., He J. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. 2018;9:4968–4978. doi: 10.1039/c8fo01126e. [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhang W., Ai D., Zhang R., Lu Q., Luo Q., Shao H. Prevalence and characterization of Clostridium perfringens in broiler chickens and retail chicken meat in central China. Anaerobe. 2018;54:100–103. doi: 10.1016/j.anaerobe.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Zhao J.S., Deng W., Liu H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019;98:3040–3049. doi: 10.3382/ps/pez081. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zeng D., Wang H., Qing X., Sun N., Xin J., Luo M., Khalique A., Pan K., Shu G., Jing B., Ni X. Dietary probiotic Bacillus licheniformis H2 enhanced growth performance, morphology of small intestine and liver, and antioxidant capacity of broiler chickens against Clostridium perfringens-induced subclinical necrotic enteritis. Probiotics Antimicrob. Proteins. 2019;12 doi: 10.1007/s12602-019-09597-8. [DOI] [PubMed] [Google Scholar]