Abstract
In April 2020, in light of COVID-19-related blood shortages, the US Food and Drug Administration (FDA) reduced the deferral period for men who have sex with men (MSM) from its previous duration of 1 year to 3 months.
Although originally born out of necessity, the decades-old restrictions on MSM donors have been mitigated by significant advancements in HIV screening, treatment, and public education. The severity of the ongoing COVID-19 pandemic—and the urgent need for safe blood products to respond to such crises—demands an immediate reconsideration of the 3-month deferral policy for MSM.
We review historical HIV testing and transmission evidence, discuss the ethical ramifications of the current deferral period, and examine the issue of noncompliance with donor deferral rules. We also propose an eligibility screening format that involves an individual risk-based screening protocol and, unlike current FDA guidelines, does not effectively exclude donors on the basis of gender identity or sexual orientation. Our policy proposal would allow historically marginalized community members to participate with dignity in the blood donation process without compromising blood donation and transfusion safety outcomes.
In March 2020, as COVID-19 rapidly proliferated in its new epicenter, New York City’s blood supply dwindled. With social distancing measures and stay-at-home orders in effect, blood drives were cancelled citywide, cutting off more than 75% of the city’s blood supply sources.1 During a call for blood donations in the initial weeks of the shortage, one group was consistently denied the chance to donate solely on the basis of sexual practices: men who have sex with men (MSM).2 According to the recommendations of the US Food and Drug Administration (FDA), men who had sex with other men within the past year were ineligible to donate and were required to stay celibate for at least a year to regain eligibility. However, the deteriorating blood supply, as well as pressure from the media and various advocacy organizations, catalyzed changes in the federal recommendation. On April 3, 2020, the FDA shortened the blood donation deferral period for MSM from 1 year to 3 months.3
Shortly afterward, the need for donations surged again as researchers investigated convalescent plasma as a promising therapeutic option for COVID-19. MSM who had recovered from the novel coronavirus and had not had sex with another man in more than 3 months eagerly pursued donation at blood centers. Despite this change, many blood centers continued to turn away MSM donors.2 Mainstream media outlets such as NBC News and The Daily Show captured the public’s attention by opening the doors to a nationwide conversation about this policy.2,4 Organizations such as the National Alliance of State and Territorial AIDS Directors and the HIV Medicine Association also addressed the issue, asking for a complete rescission of the deferral period in their comments on the most recent regulation (https://www.regulations.gov/comment?D=FDA-2015-D-1211-0109).
The FDA officially placed the first lifelong ban on blood-product donations from MSM in 1985 during the early phase of the US AIDS epidemic. At the time, the ban was necessary because HIV had not been fully characterized, no effective treatment existed, and diagnostics were severely constrained by high false-negative rates and a lengthy period between HIV infection and test positivity. There was also a perception that policymakers were slow to implement a ban on then high-risk groups, leading to thousands of new HIV cases that arose from the blood supply.4,5 The 3-decade span between the 1985 MSM ban and the 2015 MSM 1-year deferral policy was partly the result of the morbidity and mortality related to transfusion-associated HIV; importantly, however, it also arose from homophobic public perceptions of lesbian, gay, bisexual, transgender, and queer people that led to an incoherent approach to blood donor qualification policies.
Today, testing is highly accurate and sexual preference is not synonymous with risk status. In developing equitable screening practices, we must remember to continually assess standing policies and be willing to change them in light of new information. Here we propose an individual risk assessment–based screening tool as an alternative to the FDA’s current MSM deferral policy. We review the current best evidence surrounding HIV testing and transmission rates, examine the limitations of the FDA’s current recommendations, and discuss the social implications of such blood donation policies. As we evaluate current regulations and petition for new ones, we emphasize that the ethics surrounding blood donation policies exist at the intersection of public health and human rights and should be considered within that context.
TESTING AND TRANSMISSION: CURRENT EVIDENCE
The first-generation HIV diagnostic test, which came to market in 1985, had a sensitivity of 99% and a specificity of 95% to 98%. However, the accompanying serological test had a window period of up to 10 weeks and therefore could not effectively detect a new HIV infection until several months after exposure.1,6,7 At the time, blood transfusions conferred a risk of HIV transmission in 1 of 153 123 units.8
Decades of HIV research and technological advancements have since revolutionized HIV testing. At present, there are several HIV screening and diagnostic options available, including a chemiluminescent immunoassay to detect HIV-1 and HIV-2 antibodies and a duplex nucleic acid test with confirmatory western blots and enzyme-linked immunosorbent assays.9 The nucleic acid test, in particular, has a sensitivity and specificity of virtually 100% and boasts a window period of just under 3 days, although more conservative organizations report a window of up to 7 to 10 days.10,11 Currently, the Centers for Disease Control and Prevention requires a 2-pronged approach to testing blood donations for HIV-1 and HIV-2, and every donated unit undergoes both nucleic acid and antibody testing.11 Given these newer testing parameters, recent studies have estimated the risk of HIV transmission through blood products to be 1 in 1.5 million.12 For perspective, the risk of other transfusion-related complications, such as transfusion-related acute lung injury, is far greater.5,13
Furthermore, prophylactic measures for HIV prevention have simultaneously become more pervasive. From 2014 to 2017, knowledge of preexposure prophylaxis increased from 60% to 90% among MSM, and the prevalence of its use increased from 6% to 35%.14 Daily preexposure prophylaxis is highly effective in reducing the risk of seroconversion after exposure by up to 99%.15,16 Concerns regarding false-negative screening results may also be assuaged by an open-label randomized trial conducted by McCormack et al., who found no cases of breakthrough HIV infections in a study of 544 participants taking preexposure prophylaxis.17
FOREIGN BLOOD TRANSFUSION POLICIES
Nations around the world employ one of a pair of broad blood donation strategies: time-based deferrals or risk-based deferrals. Time-based strategies, such as those used in Australia, Canada, France, New Zealand, and the United Kingdom, delineate groups of potential donors according to risk and defer the members of each group identically.18 Currently, the shortest deferral period for MSM is 3 months, which is nearly 10 times longer than even the most conservative window period for the HIV nucleic acid test. Empirical and modeling studies in various countries have repeatedly shown that shortening deferral periods does not meaningfully increase rates of HIV transmission.18–21
By contrast, risk-based strategies, as implemented in countries such as Italy and Spain, stratify donors individually on the basis of self-reported questionnaires.18 Donors typically undergo an interview with a provider to determine their risk, which is based on factors such as having sex with a partner whose HIV status is unknown and having unprotected sex. These behaviors, among others, result in a deferral period that can span any duration from weeks to lifelong.18 Importantly, after Italy shifted from a time-based to a risk-based strategy in 2001, a study by Suligoi et al. showed no significant increase in MSM donor seropositivity relative to heterosexual donors. In addition, the study researchers reviewed patients’ awareness of sexually risky behavior and found no difference between the 2 groups, suggesting that education initiatives rather than deferral periods could improve outcomes.22
In September 2015, Argentina implemented a risk-based approach that was “gender neutral” and did not enforce policies on the basis of sexual orientation or gender identity.23 In 2020, a large cohort study by Blanco et al. demonstrated no significant difference in the prevalence of HIV in the blood donor population, despite an increase in the total number of donors.24 This is clear evidence that inclusive donor qualification policies do not confer increased risk to the blood supply.
Whereas time-based deferral mitigates donation-associated transmission of HIV, risk-based deferrals provide equal public safety and are a reflection of just policy-making.
ETHICAL AND LOGICAL PERSPECTIVES
We must also consider the social implications of an MSM deferral policy. The desire to donate blood alone should not outweigh the recipient’s right to receive safe blood. However, consideration of the evidence outlined here indicates that including greater numbers of MSM in the donor pool would not threaten blood safety. Instead, turning away MSM donors during times of great need and public solidarity, such as after the 2016 Pulse nightclub shooting or during the COVID-19 crisis, stigmatizes these individuals by deeming them unworthy and dangerously perpetuates the myth of HIV as a purely “gay disease.”25,26 Furthermore, given that MSM are estimated to compose 2% of the overall United States population27 and that approximately 10% of eligible donors donate blood on an annual basis,28 revising eligibility guidelines to include more MSM could add up to 600 000 annual donors to the blood supply.
Beyond these considerations, there are several inconsistencies in the FDA’s MSM deferral policy. One is the FDA’s recommendation that gender be “self-identified” or “self-reported” in donor questionnaires.3 For instance, gender nonbinary individuals or heterosexual trans women, despite being recorded as male at birth, are eligible to donate blood even if they have cis male sexual partners. In addition, cis females who have had sex with cis MSM partners are deferred, relying entirely on the expectation that an individual could know every sexual partner’s partners, making enforcement impractical if not impossible. Moreover, non-MSM donors may engage in risky behaviors. As noted by Galarneau, sexual orientation “is not a valid proxy for high-risk behavior,”26(p36) and sexual intercourse between men is not synonymous with high-risk sexual behavior.
Also, the FDA’s current recommendations police at-risk populations inconsistently. For instance, the FDA tests all donated units of blood for Trypanosoma cruzi,29 a pathogen endemic to Latin America that afflicts up to 300 000 people in the United States. This parasite causes Chagas disease, which is often asymptomatic.30 Although blood banks test all blood samples for this pathogen, they do not screen specifically for Chagas disease when considering donors who have spent time in Latin America.31,32 This allows donors who may be unaware of a latent infection to donate blood. Screening practices should be consistent among all high-risk groups.8,33
ISSUES RELATED TO NONCOMPLIANCE
In a survey of male blood donors in the United States before the FDA instituted a 1-year deferral policy in 2015, 2.6% of respondents reported that they had, in fact, not complied with the lifelong ban and donated blood despite a history of having sexual encounters with other men. In a more recent study, Wentz et al. reported that 70% of 305 young MSM who had donated blood had done so within 12 months of having unprotected anal intercourse.34 Many voiced concerns about stigma stemming from this discriminatory policy as a reason for noncompliance, whereas others noted a widespread desire for equity and confidence surrounding one’s negative HIV status.23,34,35 In this way, perceptions of a policy’s injustice can engender distrust of the policy itself, and many sexually active MSM have expressed frustration with the policy’s outdated rationale.34,35 Moreover, shortening deferral periods has paradoxically improved compliance with blood donation regulations.18
Another frequently cited reason for noncompliance in other countries is ambiguity in or miscommunication of the regulations themselves. Inaccessible medical jargon and ill-defined “high-risk” behaviors often confuse the self-reporting donor and impair a proper assessment of that individual’s eligibility.20,36 This complication is not specific to MSM; in a study of 32 HIV-positive participants, most of whom were not MSM, several donors did not read the screening form carefully enough to individually assess and answer each item.25 As such, incorrect completion of screening questionnaires affects all blood donors, and new screening policies must address this contributor to noncompliance to ensure the safety of the blood supply.
PROPOSED ELIGIBILITY SCREENING FORMAT
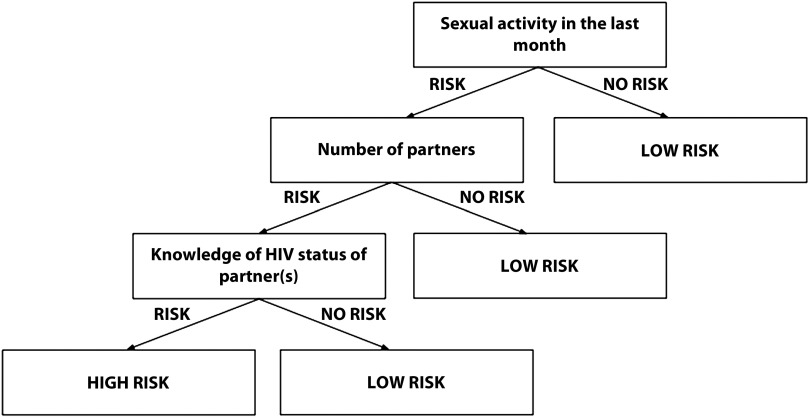
In light of this evidence, we propose an individual risk-based screening protocol that is not informed by a donor’s sexual orientation or gender identity and integrates a branched question format. Figure 1 provides a blueprint for branched risk stratification that will require stakeholder input and interdisciplinary collaboration for protocol design. Many have advocated for a risk-based protocol in the past decade, including Cohen et al., who stated that “a thoughtfully reformulated risk level–focused assessment of donor eligibility should be coupled with rigorous testing (and retesting).”37,38(p338) With this approach, all potential blood donors would be asked to answer the same set of risk stratification questions. Donors classified as low risk would be eligible for blood donation, whereas donors classified as high risk would undergo a deferral period.
FIGURE 1—
Simplified Schematic of a Branched Risk Stratification Format
Note. This figure serves as a conceptual blueprint. Creation of the screening instrument should involve a transdisciplinary approach with a team comprising experts in all relevant fields.
We suggest that these screening questions be simple and free of medical jargon and acknowledge that specific behaviors are associated with an increased risk for blood-borne diseases. Potential risk stratification questions include “Have you had unprotected sex in the last month?” and “How many sexual partners have you had in the last month?” If the algorithm suggests higher risk, the individual would be prompted to answer additional questions. For instance, individuals indicating that they recently had unprotected sex would subsequently be asked “Have you been tested for sexually transmitted infections since this encounter?”
After stratifying donors, we recommend deferring high-risk individuals on the basis of empirically determined window periods for infectious blood-borne diseases. These periods can be conservatively extended to 7 to 10 days to uphold maximum blood supply safety. We acknowledge that the FDA is currently assessing the feasibility of behavioral risk assessments for MSM donors and that time, resources, and personnel are all nontrivial limitations to implementing our recommendations. However, our aim is to draw renewed attention and focus to this critical issue. As Jay Epstein, director of the FDA Office of Blood Research and Review, stated more than 20 years ago: “The FDA is not supposed to look at cost. We’re supposed to look at . . . safety, effectiveness. We can go as far as to look at . . . public health, risk/benefit, but not the C word.”26(p33)
ADDRESSING COUNTERARGUMENTS
There is valid concern that shifting to a risk-based deferral policy could allow an influx of eligible MSM into the donor pool but disqualify otherwise eligible non-MSM donors. In the interest of public safety, however, all individuals, regardless of sexual orientation or gender identity, should be screened for high-risk sexual behaviors.
Others note that the prevalence of HIV in the MSM population in the United States is roughly 11% to 12%,3 representing a disproportionate percentage of HIV cases. However, according to the FDA’s revised April 2020 recommendations, the prevalence of HIV among MSM blood donors is just 0.25%. Put simply, the prevalence of HIV among MSM who seek out blood donations is demonstrably lower than the prevalence in the general population. Self-selection among MSM donors likely contributes to this discrepancy, but regardless of the reason, it is critical to note that increased HIV prevalence among MSM is not proportionally associated with increased MSM donor prevalence.35
Proponents of a 3-month deferral period contend that these policies protect not only against HIV transmission but also against other blood-borne illnesses. Although the window period for HIV is short, the period for hepatitis B virus is notably longer.9 Still, a recent survey of HIV-positive blood donors showed that 5.8% of those who reported a hepatitis B diagnosis and 4.8% of those who reported a hepatitis C diagnosis were MSM donors. MSM donors made up 60% of the HIV-positive blood donor cohort in that study, suggesting that rates of these other blood-borne illnesses among MSM donors are appreciably lower than is the case with HIV.3,39 Thus, MSM self-identification cannot justify a lengthy deferral period for these other diseases when transmission has demonstrated a stronger relationship with other high-risk behaviors such as intravenous drug use.
CONCLUSION
Although born out of necessity, the current national blood product donation policy as it relates to MSM is anachronistic. Currently, there is substantial evidence that individual risk-based policies are equally effective in protecting the safety of the blood supply. The existing policy defers a group in a manner that is inextricably linked with donors’ sexual orientation and gender identity. By discounting current evidence and relying on factors bound up with past and present bias, this policy has shown itself to be particularly susceptible to noncompliance, public dissatisfaction, and missed opportunities to strengthen the blood supply.
In lieu of these shortcomings, we hope that the FDA will adopt a policy that reflects scientific evidence and rejects the illogical and unsubstantiated premise that fundamental aspects of personal identity dictate the suitability of one’s blood to save another’s life. As physicians and scientists, we must advocate for policies rooted in science and against ones that unnecessarily marginalize groups of people. The ongoing crisis calls for reconsideration of blood donation screening practices and provides the opportunity to champion equity without compromising public safety.
ACKNOWLEDGMENTS
We acknowledge Amy Ahn, Aislyn DiRisio, Michael Fernando, and Akila Pai for their contributions toward needs assessments, development of the related advocacy campaign, and preparation of this article, all of which helped inspire the article and permitted its timely and efficient composition.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
HUMAN PARTICIPANT PROTECTION
No protocol approval was needed for this research because no human participants were involved.
Footnotes
See also Bruhn, p. 188.
REFERENCES
- 1.American Red Cross. American Red Cross faces severe blood shortage as coronavirus outbreak threatens availability of nation’s supply. Available at: https://www.redcross.org/about-us/news-and-events/press-release/2020/american-red-cross-faces-severe-blood-shortage-as-coronavirus-outbreak-threatens-availability-of-nations-supply.html. Accessed May 7, 2020.
- 2.NBC News. Gay men still unable to donate blood, plasma despite new FDA rules. Available at: https://www.nbcnews.com/health/health-care/gay-men-still-unable-donate-blood-plasma-despite-new-fda-n1182926. Accessed May 7, 2020.
- 3.US Department of Health and Human Services. Revised recommendations for reducing the risk of human immunodeficiency virus transmission by blood and blood products. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/revised-recommendations-reducing-risk-human-immunodeficiency-virus-transmission-blood-and-blood. Accessed October 20, 2020.
- 4. Shilts R. And the Band Played On. Rev. ed. New York, NY: St. Martin’s Griffin; 2007.
- 5.Sacks CA, Goldstein RH, Walensky RP. Rethinking the ban—the US blood supply and men who have sex with men. N Engl J Med. 2017;376(2):174–177. doi: 10.1056/NEJMms1613425. [DOI] [PubMed] [Google Scholar]
- 6.Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249–253. doi: 10.1128/CVI.00053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pear R. AIDS blood test to be available in 2 to 6 weeks. Available at: https://www.nytimes.com/1985/03/03/us/aids-blood-test-to-be-available-in-2-to-6-weeks.html. Accessed May 7, 2020.
- 8.Cumming PD, Wallace EL, Schorr JB, Dodd RY. Exposure of patients to human immunodeficiency virus through the transfusion of blood components that test antibody-negative. N Engl J Med. 1989;321(14):941–946. doi: 10.1056/NEJM198910053211405. [DOI] [PubMed] [Google Scholar]
- 9.American Red Cross. Infectious disease testing. Available at: https://www.redcrossblood.org/biomedical-services/blood-diagnostic-testing/blood-testing.html. Accessed May 7, 2020.
- 10.America’s Blood Centers. Regional blood supply. Available at: https://americasblood.org/for-donors/americas-blood-supply/regional-blood-supply. Accessed May 7, 2020.
- 11.Centers for Disease Control and Prevention. Blood safety basics. Available at: https://www.cdc.gov/bloodsafety/basics.html. Accessed May 7, 2020.
- 12.Centers for Disease Control and Prevention. HIV transmission through transfusion—Missouri and Colorado. 2008. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5941a3.htm. Accessed May 12, 2020.
- 13.Carson JL, Grossman BJ, Kleinman S et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson T, Cha S, Xia M et al. Changes in HIV preexposure prophylaxis awareness and use among men who have sex with men—20 urban areas, 2014 and 2017. MMWR Morb Mortal Wkly Rep. 2019;68(27):597–603. doi: 10.15585/mmwr.mm6827a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddell J, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319(12):1261–1268. doi: 10.1001/jama.2018.1917. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PL, Glidden DV, Liu A et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormack S, Dunn DT, Desai M et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman M, Shih AWY, O’Brien SF, Devine D. Donor deferral policies for men who have sex with men: past, present and future. Vox Sang. 2018;113(2):95–103. doi: 10.1111/vox.12623. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien SF, Grégoire Y, Pillonel J et al. HIV residual risk in Canada under a three-month deferral for men who have sex with men. Vox Sang. 2020;115(2):133–139. doi: 10.1111/vox.12867. [DOI] [PubMed] [Google Scholar]
- 20.Pillonel J, Pelat C, Tiberghien P et al. The evolving blood donor deferral policy for men who have sex with men: impact on the risk of HIV transmission by transfusion in France. Transfusion. 2020;60(3):525–534. doi: 10.1111/trf.15677. [DOI] [PubMed] [Google Scholar]
- 21.Oakes K. FDA examines changing donation policies for men who have sex with men. Available at: https://www.mdedge.com/hematology-oncology/article/197105/transfusion-medicine/fda-examines-changing-donation-policies-men. Accessed May 8, 2020.
- 22.Suligoi B, Pupella S, Regine V, Raimondo M, Velati C, Grazzini G. Changing blood donor screening criteria from permanent deferral for men who have sex with men to individual sexual risk assessment: no evidence of a significant impact on the human immunodeficiency virus epidemic in Italy. Blood Transfus. 2013;11(3):441–448. doi: 10.2450/2013.0162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Economy and Public Finance. Argentina. InfoLEG. Available at: http://servicios.infoleg.gob.ar/infolegInternet/anexos/250000-254999/252134/norma.htm. Accessed May 12, 2020.
- 24.Blanco S, Carrizo LH, Moyano RW, Mangeaud A, Gallego SV. Gender-neutral donor deferral policies: experience in Argentina implementing individual risk-assessment policies. Vox Sang. 2020;115(7):548–554. doi: 10.1111/vox.12933. [DOI] [PubMed] [Google Scholar]
- 25.Blankschaen KM. The ethics of ordinary and exact justification in blood donation deferral categories for men who have sex with men. Bioethics. 2018;32(7):445–453. doi: 10.1111/bioe.12461. [DOI] [PubMed] [Google Scholar]
- 26.Galarneau C. Blood donation, deferral, and discrimination: FDA donor deferral policy for men who have sex with men. Am J Bioeth. 2010;10(2):29–39. doi: 10.1080/15265160903487619. [DOI] [PubMed] [Google Scholar]
- 27.Purcell DW, Johnson CH, Lansky A et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6(1):98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AABB. Blood FAQ. Available at: http://www.aabb.org/tm/Pages/bloodfaq.aspx. Accessed August 14, 2020.
- 29.US Food and Drug Administration. Keeping blood transfusions safe: FDA’s multi-layered protections for donated blood. Available at: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/keeping-blood-transfusions-safe-fdas-multi-layered-protections-donated-blood. Accessed May 7, 2020.
- 30.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49(5):e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 31.Dean CL, Wade J, Roback JD. Transfusion-transmitted infections: an update on product screening, diagnostic techniques, and the path ahead. J Clin Microbiol. 2018;56(7):e00352–18. doi: 10.1128/JCM.00352-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AABB Center for Cellular Therapies. Blood donor history questionnaires. Available at: http://www.aabb.org/tm/questionnaires/Pages/dhqaabb.aspx. Accessed May 12, 2020.
- 33.Wendel S. Transfusion transmitted Chagas disease: is it really under control? Acta Trop. 2010;115(1):28–34. doi: 10.1016/j.actatropica.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Wentz AE, Merchant RC, Clark MA et al. Blood donation, sexual practices, and self-perceived risk for HIV in the United States among young adult men who have sex with men. Public Health Rep. 2018;134(1):36–46. doi: 10.1177/0033354918815182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grace D, Gaspar M, Lessard D et al. Gay and bisexual men’s views on reforming blood donation policy in Canada: a qualitative study. BMC Public Health. 2019;19(1):772. doi: 10.1186/s12889-019-7123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duquesnoy A, Danic B, Santos A et al. Context and social perceptions of blood donation in donors found positive for human immunodeficiency virus in France. Transfusion. 2017;57(9):2240–2247. doi: 10.1111/trf.14187. [DOI] [PubMed] [Google Scholar]
- 37.AABB Center for Cellular Therapies. Infectious disease rates higher among MSM blood donors compared with overall blood donor population. Available at: http://www.aabb.org/press/Pages/pr181015.aspx. Accessed May 13, 2020.
- 38.Cohen IG, Feigenbaum J, Adashi EY. Reconsideration of the lifetime ban on blood donation by men who have sex with men. JAMA. 2014;312(4):337–338. doi: 10.1001/jama.2014.8037. [DOI] [PubMed] [Google Scholar]
- 39.Custer B, Kessler D, Vahidnia F et al. Risk factors for retrovirus and hepatitis virus infections in accepted blood donors. Transfusion. 2015;55(5):1098–1107. doi: 10.1111/trf.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]