Abstract
Since 2007, electronic cigarette (e-cigarette) sales in the U.S. have surpassed those of tobacco cigarettes. This is due, in part, to manufacturer’s claims that they are a safer alternative to tobacco cigarettes. However, formaldehyde, acrolein, and diacetyl have been detected in e-cigarettes and public knowledge of e-cigarette composition and ingredient bioactivity is conspicuously lacking. We evaluated the toxicity of nine e-cigarette flavor mixtures and their constituents in the developmental zebrafish, an excellent whole animal biosensor of chemical hazard. Seven of the nine flavors (78%) elicited adverse developmental responses at 1% by volume. The number of toxic endpoints varied greatly between flavors. Two flavors, Grape and Bubble Gum, had similar chemical compositions, but different toxicity profiles. We hypothesized that the toxicity was driven by a constituent present only in the Bubble Gum flavor, cinnamaldehyde. To replicate this toxicity, we built our own defined mixture. The addition of varying concentrations of cinnamaldehyde suggested that it drove the toxicity of these mixtures and that e-cigarette hazard can be flavor dependent.
Keywords: Development, Toxicity, Behavior, morphological, bioactivity, vaping
1. Introduction
1.1. Electronic Cigarette Devices
Electronic cigarettes, e-cigarettes, or “vape” have become a popular alternative to tobacco cigarettes. These products are touted as “safer” alternatives based upon the belief that they neither produce emissions nor contain toxins such as carbon monoxide, phenol, or arsenic (Al-Delaimy, 2015; Grana et al., 2014; Kennedy et al., 2017a; Muthumalage et al., 2017; Palpant et al., 2015; Spindel and McEvoy, 2016), a gross misconception because their sole operating principle is low-temperature combustion of organic compounds, a process fraught with undesirable by-products (Goniewicz et al., 2014; Jensen et al., 2017; Kosmider et al., 2018). The use of e-cigarettes among children, young adults, and pregnant women is high (Arrazola et al., 2015; Suter et al., 2015b). In 2012, the CDC determined that at least 1.78 million American students between grades 6 – 12 had used e-cigarettes once (Arrazola et al., 2015). As of July 2015, the FDA banned sales of e-cigarettes to anyone under the age of 18 (FDA, 2016; HHS, 2013; Smith, 2016; Tierney et al., 2016). Roughly ten percent of female smokers in the US continue to smoke while pregnant and may turn to e-cigarettes as an ostensibly safer tobacco alternative (Cnattingius, 2004; Colman, 2003; Kennedy et al., 2017a; Kennedy et al., 2017b; Spindel and McEvoy, 2016; Suter et al., 2015b; Wickstrom, 2007). While the effects of tobacco cigarette exposure on the fetus are well documented, few studies have evaluated the developmental hazards of e-cigarette components (Al-Delaimy, 2015; Bahl et al., 2012; Bruin et al., 2010; Grana et al., 2014; Longo et al., 2013; Muthumalage et al., 2017; Slotkin, 2004; Smith, 2016; Spindel and McEvoy, 2016; Suter et al., 2015a; Wickstrom, 2007).
E-cigarette devices contain a battery and a heating element to vaporize the contents of a cartridge mixture. Cartridges contain propylene glycol (PG), vegetable glycerine (VG), nicotine (from 0 – 36 mg/mL), and various chemical flavorings (Bahl et al., 2012; Grana et al., 2014; Massarsky et al., 2017; Muthumalage et al., 2017; Spindel and McEvoy, 2016; Suter et al., 2015b; Tierney et al., 2016). Zebrafish exposure to PG concentrations as low as 1.25% by volume during development was associated with reduced body size, hyperactivity, and oedemas (Massarsky et al., 2017). The average concentration of PG in an e-cigarette mixture was estimated at 500 – 600 mg/mL, while the concentration of VG was estimated at 400 – 500 mg/mL (Schober et al., 2014). Together, PG and VG make up more than 90% of a given e-cigarette cartridge, suggesting that both inhalation hazard and long-term hazard potential of these compounds be investigated (Jensen et al., 2017; Massarsky et al., 2017; Suter et al., 2015a).
1.2. Electronic Cigarette Flavorings
Under the FDA, e-cigarette chemicals are required to be generally recognized as safe (GRAS). This certification only applies to oral ingestion of the chemical, not inhalation (Borgerding et al., 2012; Carmines and Gaworski, 2005; Kennedy et al., 2017b; Sears et al., 2017; Tierney et al., 2016). There is little information regarding the safety of e-cigarette flavoring agents (Grana et al., 2014; Kennedy et al., 2017a; Kennedy et al., 2017b; Muthumalage et al., 2017; Smith, 2016; Suter et al., 2015b; Tierney et al., 2016). In and in vitro mixture study by Bahl et. al. (2012), cytotoxicity was related to the concentrations of individual e-cigarette flavors rather than nicotine content (Bahl et al., 2012; Behar et al., 2014). Xenopus laevis craniofacial defects were more severe in embryos exposed to e-cigarette flavors with a fruit, candy, or vanilla flavor profile than exposures to PG, VG or nicotine alone. This suggests that flavor-specific, and thus chemical-specific, effects can exist within a given mixture (Kennedy et al., 2017a). Tierney et. al. (2016) determined that flavoring chemicals make up roughly 1 – 4% of cartridges by weight, and consist of known or probable respiratory irritants such as benzaldehyde, vanillin, and cinnamaldehyde (Bahl et al., 2012; Behar et al., 2014; Tierney et al., 2016). Many adult users have reported throat, mouth and lung irritation after using various cinnamon-flavored cartridges, of which cinnamaldehyde and cinnamic acid are major constituents (Bahl et al., 2012; Behar et al., 2014; Czégény et al., 2016; Tierney et al., 2016). Thermal decomposition of cinnamic acid at 300°C in the presence of oxygen leads to the formation of toluene, phenol, and phenanthrene (Czégény et al., 2016). Combustion aside, the base toxicity of the flavoring agents remains an important knowledge gap in the risk these compounds may present to human health.
1.3. Animal Model
To overcome the limited ability to translate in vitro data into human hazard potential, we leveraged the advantages of the developmental zebrafish (Bugel et al., 2014; Garcia et al., 2016; Massarsky et al., 2017; Palpant et al., 2015; Truong et al., 2014). With a short generation time and significant physiological and genetic homology to humans, the zebrafish has proven a useful model to study phenotypic and genotypic outcomes in response to chemical insults (Garcia et al., 2016; Howe et al., 2013; Truong et al., 2016; Truong et al., 2014). By assessing biological activity, chemicals that are a “hit” in morphological and behavioral endpoints can be prioritized for further studies (Geier et al., 2018a; Truong et al., 2014).
Few studies have utilized the zebrafish model to evaluate the effects of e-cigarette exposure. Palpant et. al. (2015) showed that developmental zebrafish heart defects associated with exposure to tobacco and e-cigarette smoke extracts were more severe than in animals exposed to nicotine alone (Palpant et al., 2015). The effects of native e-cigarette flavorings, alone or in mixtures, on zebrafish development have not been examined (Palpant et al., 2015).
1.4. Rationale
In the present study, we observed significant developmental toxicity associated with exposure to some e-cigarette flavors, while others were apparently inactive in our assays. For instance, Grape and Bubble Gum had purportedly similar chemical compositions but produced different morphological outcomes. We showed that the presence of a single natural ingredient, cinnamaldehyde, unique to the Bubble Gum flavor and not previously known to be a developmental hazard, was the likely driver of toxicity. We have begun a whole animal-response dataset that may ultimately inform better consumer choices and better regulatory decisions regarding e-cigarette health impacts.
2. Materials and Methods
2.1. Chemicals and Reagents
Eight e-cigarette flavors previously identified and measured in e-cigarette fluid by Tierney et. al. (2016) study: Bubble Gum, Coffee, Cotton Candy, French Vanilla, Grape, Nicotine (24 mg/mL), Unflavored, and 555 were ordered online from Mt. Baker Vapor (Lynden, WA; https://www.mtbakervapor.com/) (Tierney et al., 2016). Each cartridge had a total volume of 15 mL, and each flavor contained 12 mg/mL of nicotine, except for the Nicotine (24 mg/mL) and Unflavored (0 mg/mL) cartridges. We received an additional flavor as a gift with our order: Banana Crème Pie; which was not included in the Tierney study (Tierney et al., 2016). Analytical grade glycerine (≥99%), propylene glycol (≥99%), nicotine (≥99%), DMSO (≥99.5%), cinnamaldehyde (≥95%), ethyl butyrate (≥99%), ethyl vanillin (≥98%), maltol (≥98.5%), and vanillin (99%) were ordered from Sigma-Aldrich (St. Louis, MO) [See Table 1 for CAS]. Benzyl alcohol (99%) and ethyl acetate (≥99%) were ordered from Alfa Aesar (Haverhill, MA). All chemical constituents listed in Table 1, were solubilized in 100% dimethyl sulfoxide, and the maximum DMSO concentration in the exposure solutions was 0.64%.
Table 1: E-cigarette Chemical Components Screened in Zebrafish.
The seven most common chemical components detected in six different e-cigarette mixtures from Mount Baker Vapor in Lynden, WA (Tierney et al., 2016) and concentration range tested in zebrafish. Nicotine and the Unflavored cartridges do not appear as neither contained chemicals in addition to PG, VG, and nicotine. Banana Crème Pie flavor is not included in the table as its chemical constituents could not be confirmed.
| Chemical Constituent Name | CAS Number | Present in E-Cigarette Flavor(s) | Concentration Range (μg/mL) |
|---|---|---|---|
| Benzyl alcohol | 100–51–6 | Coffee | 1,000; 100; 10; 1; 0.1 |
| Cinnamaldehyde | 104–55–2 | Bubble Gum | 1,000; 100; 10; 1; 0.1 |
| Ethyl acetate | 141–78–6 | Bubble Gum, Grape | 1,000; 100; 10; 1; 0.1 |
| Ethyl butyrate | 105–54–4 | Bubble Gum, Grape | 1,000; 100; 10; 1; 0.1 |
| Ethyl vanillin | 121–32–4 | 555, Cotton Candy, French Vanilla | 1,000; 100; 10; 1; 0.1 |
| Maltol | 118–71–8 | 555, French Vanilla | 1,000; 100; 10; 1; 0.1 |
| Vanillin | 148–53–8 | 555, Coffee, Cotton Candy, French Vanilla | 10,000; 1,000; 100; 10; 1 |
2.2. Zebrafish Maintenance and Embryo Collection
Adult zebrafish were housed following the approved Institutional Animal Care and Use Committee (IACUC) protocols at the Oregon State University Sinnhuber Aquatic Research Laboratory (SARL) (Corvallis, OR) and maintained on a 28°C recirculating water system with a 14:10 h light/dark cycle. The fish were fed twice daily with the appropriate Gemma Micro (Skretting Inc. Tooele, France) without supplementation of any live feed. All experiments were conducted using the wild type (WT) 5D Tropical line. Spawning funnels were placed in tanks the night prior to spawning. The following morning embryos were collected, staged, and maintained in an incubator at 28°C (Kimmel, 1995; Westerfield, 2007). To increase bioavailability, the chorion was enzymatically removed using 22.1 Units of pronase (Roche, Indianapolis, IN, USA)) per dechorionation reaction, at 4 hours post-fertilization (hpf) using a custom automated dechorionator (Mandrell et al., 2012).
2.3. Morphological and Behavioral Endpoints Measured in Larval Zebrafish
All exposures were conducted on zebrafish embryos singulated in 96 well plates in a 100 uL final test volume per well. Exposures commenced at 6 hpf and ended at 120 hpf. Animals were evaluated for morphological endpoints and photomotor behavior endpoints at 24 and 120 hpf. Behavioral endpoints were examined prior to morphology. At 24 hpf, mortality, developmental progression, spontaneous movement, and notochord distortion were evaluated under a dissecting microscope in 96 well plates (Hagstrom et al., 2018; Truong et al., 2016). Embryonic photomotor response (EPR) was assessed in all animals at 24 hpf (Reif et al., 2016). The EPR background period consisted of 30 seconds of darkness (IR light), the excitatory period consisted of a 1-second pulse of intense visible light followed 9 seconds later by a second pulse. The refractory period consisted of the 10 seconds of darkness that immediately followed the second pulse of visible light (Hagstrom et al., 2018; Truong et al., 2014). At 120 hpf, larval photomotor response (LPR) behavior was assessed using the ZebraBox instrument platform (Viewpoint Behavior Technology, Montreal, CAN) (Truong et al., 2014; Zhang et al., 2017) over 3 cycles of 3-minute light/3-minute dark phases (Truong et al., 2014; Zhang et al., 2017). Wells with mortality or malformed animals were excluded from the subsequent analysis (Truong et al., 2016). Larvae were then evaluated for abnormality in 18 morphological endpoints: mortality, yolk sac edema, curved or bent body axis, missing or smaller/larger eye(s), shortened or malformed snout, malformed jaw, malformed or missing otic vesicle, pericardial edema, malformation of the brain, malformed, missing, or disorganized somites, malformed or missing pectoral and/or caudal fins, hypo or hyperpigmentation, lack of circulation, truncated body, failure of swim bladder to inflate, bent notochord and/or tail, and response to touch (Hagstrom et al., 2018; Truong et al., 2016).
2.4. Developmental Exposure to E-Cigarette Vehicles and Flavors
Zebrafish embryos were first exposed to the major e-cigarette vehicle components propylene glycol (PG) and vegetable glycerine (VG). Test chemical introduction to the embryos was always as a 10 uL added to 90 μL of embryo medium (EM) (Westerfield, 2007) that already contained a 6 hpf embryo. Exposures were thus 1:100, 1:1000, 1:10,000, 1:100,000, or 1:1,000,000 dilution of PG, VG or a 50:50 mix (by volume) of PG and VG in EM. Exposures to e-cigarette favors were similarly conducted using dilution flavour dilutions made in a 1:1,000 PG:EM vehicle. After chemical introduction, plates were sealed with silicone plate mats (to prevent volatilization) and gently shaken overnight at 235 RPM at 28°C (Truong et al., 2016). Embryos were not exposed to visible light until the EPR test at 24 hpf (Reif et al., 2016). Two replicate plates were used for a total of 32 animals for each dilution. Embryos were statically exposed until 120 hpf.
2.5. Developmental Exposure to Nicotine and Flavor Constituent Chemicals
Zebrafish embryos were exposed to a nicotine standard for comparison with e-cigarette mixture effects. A Hewlett Packard D300e digital chemical dispenser was used to achieve nicotine at 0, 0.2, 0.8, 2.4, 3.2, and 4.9 μg/mL from a 1.62 mg/mL stock in 100% DMSO. Wells contained 100 uL of EM prior to chemical dispensation and the dispenser software automatically compensated for the << 1 uL volume additions to the standard 100 uL of EM. All wells were normalized to 0.64% DMSO. Replicate plates totaled 64 animals for each concentration. The seven most common chemical constituents in the flavors examined in our study appear in Table 1 (Tierney et al., 2016). Stock solutions of 100 mg/mL were made in 100% DMSO for benzyl alcohol, cinnamaldehyde, ethyl acetate, ethyl vanillin, and maltol. A stock solution of 200 mg/mL in 100% DMSO was made for ethyl butyrate, and a stock solution of 1g/mL was made for vanillin. Digital chemical dispensation was used to achieve the target concentration (Table 1) of each constituent. Replicate plates totaled 32 animals for each concentration. All plates were normalized to 0.64% DMSO.
2.6. Recreating a Flavor Mixture
To determine which compound(s) was driving the toxicity of an e-cigarette flavor, we compared two flavors with similar compositions but different morphological outcomes, Bubble Gum and Grape. The major tested constituents of each were ethyl acetate and ethyl butyrate, while Bubble Gum also contained cinnamaldehyde. We first conducted a concentration-response test with ethyl acetate or ethyl butyrate Replicate plates were used for a total of 32 animals per concentration. To decrease the likelihood of carryover effects (due to ethyl acetate’s high volatility) controls were on a separate plate (n=96) in the 1:1,000 PG:EM vehicle. The concentration-response exposures led us to select 10 μg/mL of ethyl acetate and 10 μg/mL ethyl butyrate for further testing in combination with 1000, 750, 500, 250, 100, 10, 1, and 0.1 μg/mL cinnamaldehyde. Replicate plates were used for a total of 32 animals per concentration. Exposures took place as described above, and control animals were on a separate plate.
To determine whether cinnamaldehyde was driving toxicity in a real mixture, we added the same concentrations of cinnamaldehyde to the Grape flavor (which contained ethyl acetate and ethyl butyrate). Replicate plates were used for a total of 32 animals per concentration. Exposures took place as described above, and control animals were on a separate plate.
2.7. Statistics
EPR –
The recorded periods at the beginning and end of the experiment (immediately surrounding the initiation/termination of camera recording) were truncated to assure equivalence in recorded experimental period for all chemicals. The statistical analysis of activity considered only the Background (B), Excitatory (E), and Refractory (R) intervals. The overall pattern of activity within each B, E, or R interval was compared to that interval’s negative control activity using a combination of percent change (50% peak difference from control in either direction) and a Kolmogorov-Smirnov test (Bonferroni-corrected p-value threshold = 0.05 /5 concentrations = 0.01) (Hagstrom et al., 2018; Reif et al., 2016).
LPR –
Movement data from the 15 frames s-1 capture was integrated into 6-sec bins. A statistical modeling method previously published by our group (Zhang et al., 2017) was applied. Briefly, the data was adapted to a uniform distribution with 95% and 5% quartiles to reduce bias caused by outliers. The differential entropy model allows us to measure the average surprisal of continuous probability distribution. The area under the curve using the differential entropy values was computed for each exposure plate and averaged per chemical. The differential entropy for each treatment was statistically compared to the control using a 2 sample K-S test (p<0.01). The LPR at a given exposure concentration was considered valid only when statistical significance (K-S test, p < 0.01) was reached and the relative AUC treatment: AUC control ratio was ≥ 1 or ≤ −0.3. Animals dead or malformed at the 120 hpf time point were excluded from the LPR data analysis.
Mortality and morphology response –
Statistical analysis of the morphology endpoints was performed using code developed in R (R-Core-Team, 2016). The data were binary incidences recorded for each endpoint (Truong et al., 2016; Truong et al., 2014) and retrieved from our laboratory information management system. We computed the lowest effect level (LEL) as the concentration at which the incidence exceeded a significance threshold over the background (control) incidence rate. Because the endpoints were binary and replicates were measured in separate wells, the 0/1 responses for each chemical-endpoint-concentration-replicate combination translated to a series (sample size n) of Bernoulli trials, or “coin-flips.” The LEL significance threshold was estimated using a binomial test which maximized power versus a typical logistic/curve-fit approach by in case of false “nonmonotonic” responses where mortality could cause missing morphology endpoint measurements at higher concentrations. Because background incidence rate varied slightly across chemicals and endpoints, the significance threshold (x) was determined independently from the binomial distribution function for each chemical-endpoint pair as: F (x;nc,e, pc,e) = P(X > x) ≤ 0.05, where nc,e = number of controls (trials) for this chemical, for this endpoint; pc,e = observed incidence (positive responses) in controls for this chemical, for this endpoint. EC50 values were calculated using the drm function within the drc package in R, as described (Truong et al., 2016; Truong et al., 2014).
3. Results and Discussion
3.1. E-Cigarette Flavor Developmental Toxicity
The hazard potential of e-cigarette use is via inhalation, an exposure route not readily compatible with a fish model. Our goal was simply to query the bioactivity of the most common e-cigarette flavors and their components, in a sensitive whole animal model, under the reasonable assumption that adverse developmental impacts of the native products would likely be even more severe as partially combusted, inhaled byproducts. We used the developmental zebrafish to profile the biological responses to nine different e-cigarette flavour mixtures and 10 of their most common constituents. Embryos were exposed from 6 – 120 hpf to a dilution range of e-cigarette flavors in propylene glycol (PG) and assessed for morphological and behavioral outcomes at 24 and 120 hpf (Figures 1A, 1B, S1 and Tables S1 and S2).
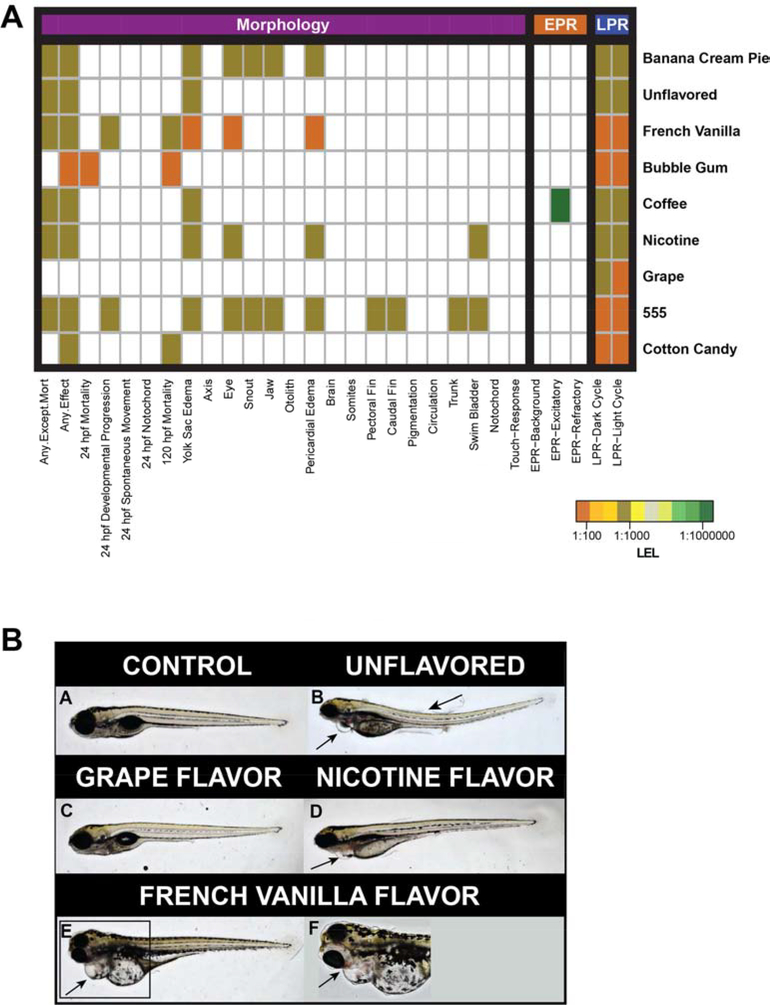
Figure 1.
A. Morphology and behavior outcomes in embryos exposed to nine commercial e-cigarette flavors. Color scale indicates relative potency, based upon the Lowest Effect Level (LEL) determined for a given mixture and endpoint. E-Cigarette flavors appear in the top right column. All morphological and behavioral endpoints are listed across the bottom of the figure. Orange color denotes greater potency (1:100 dilution factor), while green-blue colors indicate less potency (1:1000000 dilution factor). White indicates that there was no observable LEL for a particular mixture and endpoint. “Any Effect” and “Any Except Mortality” (left two columns) are aggregates of all morphological end points. The Unflavored mixture contained only PG and VG while the Nicotine flavor contained PG, VG and 24 mg/mL of Nicotine. Figure 1B. Representative images of 120 hpf zebrafish exposed to four different e-cigarette flavors. Images A-E were taken at 2X magnification using the bright field setting. Image F was taken at 10X magnification under the bright field setting. A: Control animal, exposed to 1:1000 PG:EM. B: 1:100 dilution of Unflavored e-cigarette flavor, presence of some edemas and a slightly curved body axis. C: 1:100 dilution of Grape flavor, note the lack of malformations. D: 1:100 dilution of Nicotine e-cigarette flavor, presence of some minor edemas. E and F: Zebrafish exposed to a 1:1000 dilution of French Vanilla flavor. Note the blood pooling in the eye and midbrain regions.
The embryo photomotor response (EPR) at 24 hpf in animals exposed to the e-cigarette flavors did not differ significantly from controls (Table S1). The larval photomotor response (LPR) at 120 hpf associated with the majority of e-cigarette flavors, except for Nicotine, exhibited hyperactive behavior (Table S2). Previous studies demonstrated that exposure to 1.25% PG resulted in hyperactive larval zebrafish (Maes et al., 2012; Massarsky et al., 2017). Here, propylene glycol exposures alone were not associated with hyperactivity in the LPR (Table S2). While the concentration of PG in the e-cigarette flavors we tested could not be established from the manufacturer’s information, it was likely high enough to result in an exposure concentration above 1.25%. E-cigarette flavors are typically ≥ 90% propylene glycol by weight (Jensen et al., 2017; Tierney et al., 2016), and elsewhere reported at 500 – 600 mg/mL (Schober et al., 2014). It is possible that PG exposure significantly above 1.25% imparted additional bioactivity that no longer manifested as a hyperactive LPR.
Zebrafish exposed to the Nicotine flavor or a nicotine standard (12.2 μg/mL) exhibited hypoactivity, an effect previously associated with nicotine in zebrafish (Table S2) (Klee et al., 2011; Svoboda, 2002; Thomas et al., 2009). In the LPR assay, animals exposed to nicotine were unable to move. This effect was previously characterized as exogenous overstimulation of nicotinic acetylcholine receptors (nAChR) (Thomas et al., 2009) homologous to those in humans (Papke et al., 2012; Svoboda, 2002; Thomas et al., 2009). We note that the Nicotine flavor contained 24 mg/mL of nicotine versus 12 mg/mL in all of the other flavors (except unflavored), thus the hypoactive LPR associated with Nicotine flavor likely outweighed any hyperactive effects of PG.
At the 1:1000 dilution, malformations were associated with exposure to the majority of e-cigarette flavors (Figure 1A). These included yolk sac and pericardial edemas (YSE, PE, respectively), and eye malformations. YSE was associated with exposure to six of the nine flavors – 555, Banana Crème Pie, Coffee, French Vanilla, Nicotine and Unflavored. Four of these six flavors were also associated with eye malformations and PE (exceptions were Coffee and Unflavored). There was no significant bioactivity associated with exposure to the Grape mixture in our study (Figure 1A, 1B). From Figure 1A, we suggest that flavor had additive toxicity with the nicotine content, and drove the toxic response of a given e-cigarette mixture. This was consistent with previous work in both in vivo and in vitro models (Bahl et al., 2012; Behar et al., 2014; Kennedy et al., 2017a) which showed that toxicity was either not due to nicotine, or could be exacerbated by nicotine, but was most correlated with the number and concentration of chemicals used to flavor fluids. All zebrafish morphology concentration-response plots are available in the Appendix (Figure S1).
The highest incidence of malformations in the cranial and cardiac regions was associated with exposure to 555, Banana Crème Pie, and French Vanilla (Figure 1A, 1B). The main chemical constituents in 555 and French Vanilla were ethyl vanillin, maltol, and vanillin (Table 1; (Tierney et al., 2016)). The chemical constituents of Banana Crème Pie were not reported by the manufacturer (Table 1), but the bioactivity profiles would suggest that Banana Crème Pie flavor contained some of the same or related flavoring agents as 555 and French Vanilla. The embryonic zebrafish results appear consistent with the craniofacial deformity promoting activity of these flavor profiles in X. laevis (Kennedy et al., 2017a).
Exposure to the Bubble Gum or Cotton Candy flavors was associated exclusively with mortality, though sufficient animals survived to estimate incidences of some malformations at the 1:1000 and 1:10000 dilutions (Figure S1). Bubble Gum was the only flavor associated with a high incidence of 24 hpf mortality (MO24), at the 1:100 dilution. Cotton Candy and French Vanilla were associated with a high incidence of mortality only at 120 hpf at the 1:100 dilution (Figure S1).
3.2. Chemical Constituent Developmental Toxicity
To profile the developmental toxicity of the flavor component, it was essential to expose animals to a concentration range that included a no observable adverse effect level (NOAEL). The concentration of each component in the commercial flavor was unavailable except that each was no more than 4% of the mixture by weight, and often less than 1% (Tierney et al., 2016). Thus, a one percent measure by weight would roughly correspond to 10 mg/mL (Tierney et al., 2016). We note that our maximum exposure concentrations to individual flavor components were one-tenth the minimum estimated concentration in a commercial e-cigarette flavor (Table 1). Figure 2 shows the morphology and behavioral readouts associated with exposure to seven common e-cigarette flavor components. Embryos at 6 hpf were exposed to 0.1, 1, 10, 100 and 1000 μg/mL of each of 6 components and to 1, 10, 100, 1,000 and 10,000 μg/mL of vanillin. All stocks were made in 100% DMSO to enhance solubility.
Figure 2. Morphology and behavior outcomes associated with exposure to common e-cigarette flavor components.
Colour scale indicates relative potency, based upon the Lowest Effect Level (LEL) determined for a given chemical and morphological or behavioral endpoint. Chemical names appear in the top right column. All morphological and behavioral endpoints are listed across the bottom of the figure. Orange colour denotes smaller LEL (μg/mL) and thus greater potency, while green-blue colours indicate a higher LEL and less potency. White indicates that there was no significant observable LEL for a particular endpoint and mixture. “Any Effect” and “Any Except Mortality” (left two columns) are aggregates of all morphological end points.
In Figure 2, benzyl alcohol, cinnamaldehyde, ethyl vanillin, and vanillin were associated with YSE, PE, and trunk defects in the 100 to 10,000 μg/mL concentration range. Cinnamaldehyde, ethyl vanillin, and vanillin were also associated with bent body axis, eye abnormality, and jaw malformations by 120 hpf, in the same concentration range. The craniofacial endpoints affected were similar to those affected by French Vanilla, 555, and Banana Crème Pie flavors (Figure 1A, Table 3). Table 3 compares the flavor and component associated outcomes which revealed that YSE and PE were associated with ethyl vanillin, vanillin and their respective flavors (Table 3). Our study appeared uncover flavor mixture and flavor component-specific developmental toxicity profiles. Future work could anchor these phenotypic profiles to the underlying transcriptomic and metabolomic changes to explore what pathway targets and are perturbed by what chemical structures (Bugel et al., 2014; Garcia et al., 2016; Haggard et al., 2017; Truong et al., 2014; Zoupa and Machera, 2017). Since more than 80% of human disease-related genes have at least one zebrafish ortholog, the probability is high that the same cellular processes and responses impacted in zebrafish are impacted by e-cigarette use in humans.
Table 3: Summary Table.
Morphological endpoints affected by, and LELs associated with, developmental exposures to e-cigarette flavors (Figure 1A) and chemical constituents (Figure 2). The left hand column lists each e-cigarette flavor or created mixture and its chemical constituents. The three right-hand columns list the most common endpoints affected, LEL values, and, where applicable, calculated EC50 values. The top row is each chemical constituent and flavor it was part of, represented by an ✖. The bottom three rows in the left-hand column list the most common endpoints affected, LELs, and, where applicable, calculated EC50 values. All EC50 values were calculated using the mortality endpoint (MORT). ND stands for Not Determined. As in Figures 1 and 2, endpoints common to 555 and French Vanilla were also common to their chemical constituents (Ethyl Vanillin, Vanillin, and Maltol), suggesting a similar mechanism of toxicity.
| Mixture/Chemical | Benzyl Alcohol | Ethyl Vanillin | Vanillin | Cinnamaldehyde | Maltol | Ethyl Butyrate | Ethyl Acetate | Endpoints | LEL (Dilution Factor) | EC50 |
|---|---|---|---|---|---|---|---|---|---|---|
| 555 | ✖ | ✖ | ✖ | YSE, EYE, SNOUT, JAW, PE | 1:1000 | ND | ||||
| Banana Crème Pie | YSE, Eye, SNOUT, JAW, PE | 1:1000 | ND | |||||||
| Coffee | ✖ | YSE | 1:1000 | ND | ||||||
| French Vanilla | ✖ | ✖ | ✖ | MORT, YSE, EYE, PE | 1:100 | ND | ||||
| Cotton Candy | ✖ | ✖ | MORT | 1:1000 | ND | |||||
| Bubble Gum | ✖ | ✖ | ✖ | MO24, MORT | 1:1000 | 1 to 100 | ||||
| Grape | ✖ | ✖ | NA | NA | NA | |||||
| Nicotine | YSE, EYE, PE | 1:1000 | ND | |||||||
| Unflavored | YSE | 1:1000 | ND | |||||||
| EA+EB | ✖ | ✖ | MO24, MORT | 100 ug/mL EB | 405 ug/mL | |||||
| EA+EB+C | ✖ | ✖ | ✖ | MO24, MORT | 10 ug/mL C | 45.1 ug/mL | ||||
| G+C | ✖ | ✖ | ✖ | MO24, MORT | 10 ug/mL C | 28.6 ug/mL | ||||
| Endpoints | MORT, YSE, EYE, JAW | YSE, EYE, JAW, PE | YSE, EYE, SNOUT, JAW, PE | MORT | MO24, MORT | MO24, MORT | DP24 | |||
| LEL (μg/mL) | 100 | 10 | 100 | 0.1 | 1000 | 100 | 100 | |||
| EC50 | ND | ND | ND | 11.4 ug/mL | ND | 117 ug/mL | NA |
For instance, in our screen, cinnamaldehyde was the most bioactive chemical, with the lowest effect level (LEL) of 0.1 μg/mL (Figure 2). Cinnamon-flavored cartridges have been ostensibly associated with throat, mouth, and lung irritations in e-cigarette user forums (Behar et al., 2014). Cinnamaldehyde is recognized as an irritant by the North American Skin Contact Dermatitis Group (Nguyen, 2007). Future studies using the developmental zebrafish model could employ forward genetics techniques to uncover the underlying mechanism(s) (Garcia et al., 2016; Zoupa and Machera, 2017).
Bahl et. al. (2012) found that the most cytotoxic refill fluids to human stem cells, human pulmonary fibroblasts, and mouse neural stem cells had cinnamon, caramel, butterscotch, and vanilla flavor profiles. These flavors had lower IC50 values than propylene glycol, vegetable glycerine, and menthol flavorings (Bahl et al., 2012). Cells in this study were exposed to a percent by volume solution of refill cartridges, similar to our study, although the exposure concentrations did not exceed 1% (Bahl et al., 2012). A follow-up study using GC-MS and HPLC identified cinnamaldehyde (from 0.005 – 0.75M) and vanillin (from 0.0025 – 0.075M) in the cinnamon-flavored cartridges (Behar et al., 2014).
3.3. Mixture Recreation
Bubble Gum and Grape mixtures had similar components but were associated with different toxicity outcomes (Figure 1A, Table 3). Only the cinnamaldehyde component was associated with high incidences of mortality malformation (Figure 2). We used a component-based mixture approach and varied the final concentration of a given component (Geier et al., 2018b; Monosson, 2004; Simmons et al., 2004). Since we have established that the flavoring agents, not nicotine, drove the adverse outcomes in Figure 2, nicotine was omitted from the mixture (Figure 2). Additionally, vegetable glycerine was omitted as it was previously associated with relatively little bioactivity (Bahl et al., 2012; Carmines and Gaworski, 2005; Kennedy et al., 2017a). Finally, since e-cigarettes typically contain 90% (or more) propylene glycol by weight, PG and embryo medium served as the base for each mixture (Kimmel, 1995; Massarsky et al., 2017; Tierney et al., 2016).
Three mixtures: ethyl acetate + ethyl butyrate (EA+EB), ethyl acetate + ethyl butyrate + cinnamaldehyde (EA+EB+C), and the Grape flavor + cinnamaldehyde (G+C) were tested. At 120 hpf, mortality incidence was used to calculate EC50 values (Table 2, 3). Figure 3 shows the concentration-response curves of the mortality incidence associated with each mixture. The estimated EC50 values for each chemical or mixture are listed in Table 2. A range of cinnamaldehyde concentrations was added to the 10 μg/mL ethyl acetate + 10 μg/mL ethyl butyrate vials. We were not able to account for all of the possible sub-components in the mixtures, thus we added different concentrations of cinnamaldehyde to a 1% solution of the Grape flavor, as this concentration of Grape was not associated with significant incidences of adverse effects (Figure 1A and 1B).
Table 2: E-cigarette Flavor and Component EC50 Values for Mortality at 120 hpf.
Bubble Gum and Grape as well as their mixture components: ethyl acetate, ethyl buytrate and cinnamaldehyde, in addition to the component-based mixtures created in the study, were tested. For the Grape mixture and ethyl acetate, we were unable to estimate EC50 due to the lack of mortality. Ethyl acetate + ethyl butyrate = EA+EB; ethyl acetate + ethyl butyrate + cinnamaldehyde = EA+EB+C; Grape mixture + cinnamaldehyde = G+C.
| Chemical or Mixture | Estimated EC50 Value |
|---|---|
| Bubble Gum Flavor | 1:100 dilution factor |
| Grape Flavor | NA (dilution factor) |
| Ethyl Acetate | NA μg/mL |
| Ethyl Butyrate | 117 μg/mL |
| Cinnamaldehyde | 11.4 μg/mL |
| EA+EB | 406 μg/mL |
| EA+EB+C | 101 μg/mL |
| G+C | 28.6 μg/mL |
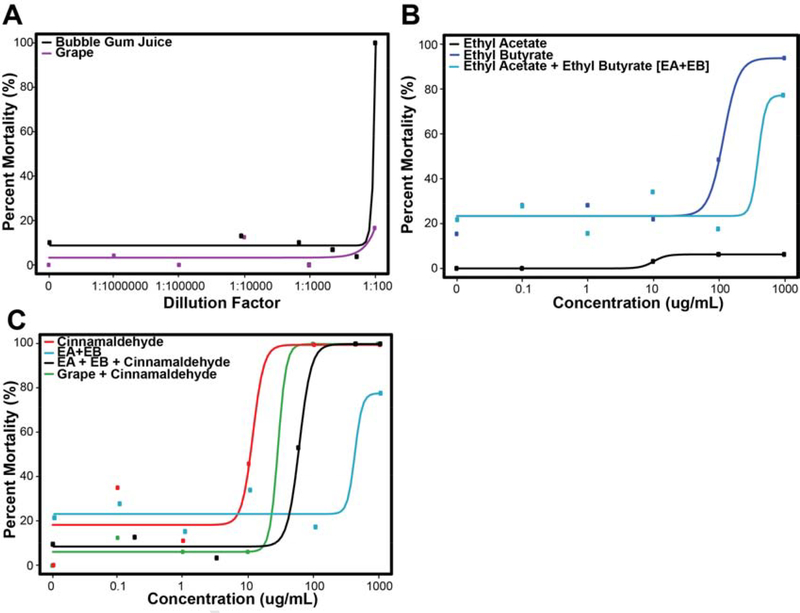
Figure 3. Concentration-mortality curves at 120 hpf associated with Bubble Gum and Grape flavors.
3A: Concentration-mortality curve for Bubble Gum is much steeper than Grape, suggesting an additional component(s) driving toxicity. 3B: Concentration-mortality curve of ethyl acetate, ethyl butyrate, and ethyl acetate + ethyl butyrate. Concentration is in units of ug/mL. 3C: Cinnamaldehyde, the ethyl acetate + ethyl butyrate + cinnamaldehyde mixture and the Grape mixture + cinnamaldehyde. The high mortality rate is recapitulated at the 100 and 1000 ug/mL cinnamaldehyde concentrations for both mixtures, suggesting the large role that cinnamaldehyde is likely playing in driving toxicity.
The percent mortality associated with Bubble Gum flavor (80%) was at least four-fold higher than Grape (<20%; Figure 3A). Ethyl acetate (EA) alone was associated with < 10% mortality at any concentration, thus an EC50 value could not be estimated due to lack of significant bioactivity (Table 2, Figure 3B). Ethyl butyrate (EB) alone was associated with a near five-fold mortality increase between 10 and 100 μg/mL (Figure 3B) and the estimated EC50 was 117 ug/mL (Table 2). The estimated EC50 for the EA+EB mixture was approximately four-fold higher than the EC50 associated with EB alone. This was anticipated given the lack of bioactivity from the EA component (Figure 3B).
The concentration-response curves for the cinnamaldehyde mixtures appear in Figure 3C. The EA+EB+C and G+C mixtures had much steeper concentration-response curves than their counterparts without cinnamaldehyde (Figure 3A, 3B). The G+C mixture was associated with 100% mortality at both the 100 and 1000 μg/mL cinnamaldehyde concentrations and thus was more toxic than the EA+EB+C mixture (Figure 3C). Cinnamaldehyde alone was associated with an EC50 of 11.4 μg/mL while the EA+EB+C mixture was associated with an EC50 of 45.1 μg/mL. The G+C mixture-associated toxicity was intermediate with an EC50 of 28.6 μg/mL. Thus, cinnamaldehyde primarily drove the mortality response of the flavor mixtures that contained it (Figure 3C).
4. Conclusion
The present study demonstrated that exposure of zebrafish embryos to non-combusted commercial e-cigarette flavors was developmentally hazardous (Figure 1). Further, the hazards that we detected were not entirely due to nicotine, but rather to flavoring agents meant to impart fruity odors and flavors to e-cigarette use. Our study was somewhat limited by not knowing the concentration of each component in each flavor (Tierney et al., 2016). Due to the lack of regulation of e-cigarette flavors, batch-to-batch effects are likely, and concentration values may only offer rough estimates of actual exposures (Bahl et al., 2012; Behar et al., 2014; FDA, 2016; Grana et al., 2014; Sears et al., 2017; Smith, 2016; Suter et al., 2015a; Tierney et al., 2016). There are currently thousands of different e-cigarette brand-flavor combinations on the market. Here, flavor-specific teratogenicity targeted different aspects of vertebrate development. Our results suggest that bioactivity assessments in the developmental zebrafish can rapidly uncover those flavors and their components which may be the greatest hazard potential to e-cigarette users.
Supplementary Material
Highlights.
7 of 9 flavors tested in the developmental zebrafish model induced adverse responses at 1% by volume
Chemical composition and bioactivity varies by flavoring mixture
Developmental zebrafish is an excellent whole animal biosensor of chemical hazard
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences (NIEHS) [P30 ES000210 and T32 ES007060]. The authors would like to thank members of the Tanguay Laboratory and the Sinnhuber Aquatic Research Laboratory, especially Carrie Barton and Greg Gonnerman, for their assistance with fish husbandry and chemical screening. Additionally, we would also like to thank Chenglian Bai for her assistance imaging the larval zebrafish that appear in Figure 1B.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Delaimy W,K, Myers M,G, Leas E,C, Strong D,R, and Hofstetter R, 2015. E-Cigarette Use in the Past and Quitting Behavior in the Future: A Population-Based Study. American Journal of Public Health 105, 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS, 2015. Tobacco use among middle and high school students - United States, 2011–2014. MMWR. Morbidity and mortality weekly report 64, 381–385. [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P, 2012. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 34, 529–537. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P, 2014. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicology in Vitro 28, 198–208. [DOI] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE, 2012. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol 64, 367–387. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC, 2010. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci 116, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, Tanguay RL, Planchart A, 2014. Zebrafish: A marvel of high-throughput biology for 21(st) century toxicology. Curr Environ Health Rep 1, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmines EL, Gaworski CL, 2005. Toxicological evaluation of glycerin as a cigarette ingredient. Food Chem Toxicol 43, 1521–1539. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, 2004. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res 6 Suppl 2, S125–140. [DOI] [PubMed] [Google Scholar]
- Colman G,J, and Joyce T, 2003. Trends in Smoking, Before, During, and After Pregnancy in Ten States. American Journal of Preventive Medicine 24, 29–35. [DOI] [PubMed] [Google Scholar]
- Czégény Z, Bozi J, Sebestyén Z, Blazsó M, Jakab E, Barta-Rajnai E, Forster M, Nicol J, McAdam KG, Liu C, 2016. Thermal behaviour of selected flavour ingredients and additives under simulated cigarette combustion and tobacco heating conditions. Journal of Analytical and Applied Pyrolysis 121, 190–204. [Google Scholar]
- FDA, 2016. The “Deeming Rule” - New Requirements for Tobacco Retailers, in: O.o.C.a. Enforcement (Ed.). [Google Scholar]
- Garcia GR, Noyes PD, Tanguay RL, 2016. Advancements in zebrafish applications for 21st century toxicology. Pharmacol Ther 161, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier MC, Chlebowski AC, Truong L, Massey Simonich SL, Anderson KA, Tanguay RL, 2018a. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Arch Toxicol 92, 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier MC, James Minick D, Truong L, Tilton S, Pande P, Anderson KA, Teeguardan J, Tanguay RL, 2018b. Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicol Appl Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA, 2014. E-cigarettes: a scientific review. Circulation 129, 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard DE, Das SR, Tanguay RL, 2017. Comparative Toxicogenomic Responses to the Flame Retardant mITP in Developing Zebrafish. Chem Res Toxicol 30, 508–515. [DOI] [PubMed] [Google Scholar]
- Hagstrom D, Truong L, Zhang S, Tanguay R, Collins ES, 2018. Comparative analysis of zebrafish and planarian model systems for developmental neurotoxicity screens using an 87-compound library. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HHS, 2013. NIOSH Alert: Preventing Lung Disease in Workers Who Use or Making Flavoring, in: CDC (Ed.). National Institute for Occupational Safety and Health, Cincinnati. [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL, 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RP, Strongin RM, Peyton DH, 2017. Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci Rep 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AE, Kandalam S, Olivares-Navarrete R, Dickinson AJG, 2017a. E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLoS One 12, e0185729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RD, Awopegba A, De Leon E, Cohen JE, 2017b. Global approaches to regulating electronic cigarettes. Tob Control 26, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C,B, Ballard W,W, Kimmel S,R, Ullmann B, and Schilling T,F, 1995. Stages of Embryonic Development of the Zebrafish. Developmental Dynamics 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Klee EW, Ebbert JO, Schneider H, Hurt RD, Ekker SC, 2011. Zebrafish for the study of the biological effects of nicotine. Nicotine Tob Res 13, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Spindle TR, Gawron M, Sobczak A, Goniewicz ML, 2018. Nicotine emissions from electronic cigarettes: Individual and interactive effects of propylene glycol to vegetable glycerin composition and device power output. Food Chem Toxicol 115, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo CA, Fried PA, Cameron I, Smith AM, 2013. The long-term effects of prenatal nicotine exposure on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol 39, 9–18. [DOI] [PubMed] [Google Scholar]
- Maes J, Verlooy L, Buenafe OE, de Witte PA, Esguerra CV, Crawford AD, 2012. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS One 7, e43850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, Simonich MT, Tanguay RL, 2012. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom 17, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarsky A, Abdel A, Glazer L, Levin ED, Di Giulio RT, 2017. Exposure to 1,2-Propanediol Impacts Early Development of Zebrafish (Danio rerio) and Induces Hyperactivity. Zebrafish 14, 216–222. [DOI] [PubMed] [Google Scholar]
- Monosson E, 2004. Chemical Mixtures: Considering the Evolution of Toxicology and Chemical Assessment. Environmental Health Perspectives 113, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I, 2017. Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used e-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front Physiol 8, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SH, Dang TP, MacPherson C, Maibach H, Maibach HI, 2007. Prevalance of patch test results from 1970 to 2002 in a multi-centre population in North America (NACDG). Contact Dermatitis 58, 101–106. [DOI] [PubMed] [Google Scholar]
- Palpant NJ, Hofsteen P, Pabon L, Reinecke H, Murry CE, 2015. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PLoS One 10, e0126259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Ono F, Stokes C, Urban JM, Boyd RT, 2012. The nicotinic acetylcholine receptors of zebrafish and an evaluation of pharmacological tools used for their study. Biochem Pharmacol 84, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R-Core-Team, 2016. R: A language environment for statistical computing [Google Scholar]
- Reif DM, Truong L, Mandrell D, Marvel S, Zhang G, Tanguay RL, 2016. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol 90, 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jorres RA, Fromme H, 2014. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 217, 628–637. [DOI] [PubMed] [Google Scholar]
- Sears CG, Hart JL, Walker KL, Robertson RM, 2017. Generally Recognized as Safe: Uncertainty Surrounding E-Cigarette Flavoring Safety. Int J Environ Res Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JE, Teuschler LK, Gennings C, Speth TF, Richardson SD, Miltner RJ, Narotsky MG, Schenck KD, Hunter ES 3rd, Hertzberg RC, Rice G, 2004. Component-based and whole-mixture techniques for addressing the toxicity of drinking-water disinfection by-product mixtures. J Toxicol Environ Health A 67, 741–754. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, 2004. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 198, 132–151. [DOI] [PubMed] [Google Scholar]
- Smith L, Brar K, Srinivasan K, Enja M, and Lippman S, 2016. E-cigarettes: How “safe” are they? The Journal of Family Practice 65, 380–385. [PubMed] [Google Scholar]
- Spindel ER, McEvoy CT, 2016. The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease. Implications for Dangers of E-Cigarettes. Am J Respir Crit Care Med 193, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Abramovici AR, Griffin E, Branch DW, Lane RH, Mastrobattista J, Rehan VK, Aagaard K, 2015a. In utero nicotine exposure epigenetically alters fetal chromatin structure and differentially regulates transcription of the glucocorticoid receptor in a rat model. Birth Defects Res A Clin Mol Teratol 103, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Mastrobattista J, Sachs M, Aagaard K, 2015b. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res A Clin Mol Teratol 103, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Vijayaraghavan S, Tanguay RL, 2002. Nicotinic Receptors Mediate Changes in Spinal Motoneuron Development and Axonal Pathfinding in Embryonic Zebrafish Exposed to Nicotine. The Journal of Neuroscience 22, 10731–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LT, Welsh L, Galvez F, Svoboda KR, 2009. Acute nicotine exposure and modulation of a spinal motor circuit in embryonic zebrafish. Toxicol Appl Pharmacol 239, 1–12. [DOI] [PubMed] [Google Scholar]
- Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF, 2016. Flavour chemicals in electronic cigarette fluids. Tob Control 25, e10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Bugel SM, Chlebowski A, Usenko CY, Simonich MT, Simonich SL, Tanguay RL, 2016. Optimizing multi-dimensional high throughput screening using zebrafish. Reprod Toxicol 65, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL, 2014. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M, 2007. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed. University of Oregon Press, Eugene, OR. [Google Scholar]
- Wickstrom R, 2007. Effects of Nicotine During Pregnancy: Human and Experimental Evidence. Curr Neuropharmacology 5, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Roell KR, Truong L, Tanguay RL, Reif DM, 2017. A data-driven weighting scheme for multivariate phenotypic endpoints recapitulates zebrafish developmental cascades. Toxicol Appl Pharmacol 314, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoupa M, Machera K, 2017. Zebrafish as an Alternative Vertebrate Model for Investigating Developmental Toxicity-The Triadimefon Example. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.