Abstract
Purpose:
To report the long-term outcomes of amniotic membrane (AM) use in the form of transplantation (AMT) and self-retained amniotic membrane (ProKera® device, PD) in acute Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).
Methods:
Electronic records of all patients with a diagnosis of SJS/TEN at Massachusetts Eye and Ear between January 2008 and January 2018 were reviewed. Patients who received AM in acute SJS/TEN were selected. Only patients with follow-up ≥ 3 months after discharge were included.
Results:
Data of 55 eyes of 29 patients were analyzed. All 55 eyes received the first AM at a median interval of 5 days (inter-quartile range (IQR): 3–7 days) after onset of skin rash. Fifty-six percent of eyes (31/55) received AMT while 44% (24/55) received PD. Forty percent of eyes (22/55) required a repeat AMT or PD. Median follow-up after initial AM was 2.5 years (IQR: 1.2–3.6 years). At last follow-up, the best-corrected visual acuity was ≥ 20/40 in 87% of eyes (48/55). The most common complications in the chronic phase were meibomian gland disease and dry eye, seen in 78% of eyes (43/55) and 58% of eyes (32/55) respectively.
Conclusions:
Long-term results show that early use of AM in the acute phase of SJS/TEN may be effective in mitigating severe vision loss after SJS/TEN. However, eyelid-related complications and dry eye remain a common problem even with the use of AM.
Keywords: amniotic membrane, dry eye disease, ocular surface, ProKera device, Stevens-Johnson syndrome, Toxic epidermal necrolysis
1. Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) belong to a spectrum of disease characterized by acute sloughing of the skin and the mucous membranes. Acute SJS/TEN manifests at the ocular surface by eyelid margin inflammation, conjunctival pseudomembrane formation and epithelial defects of the cornea and the conjunctiva.1 Acute ocular involvement is reported to occur in 40–84% of cases,2–5 out of which 43–89% progress to suffer from chronic ocular complications.6–9 The severity of the acute ocular disease has been identified as a significant risk factor for chronic ocular complications.5 These ocular complications range from moderate to severe dry eye and total limbal stem cell deficiency (LSCD) with subsequent bilateral corneal blindness. Such chronic ocular sequelae affect 20–75% of survivors of SJS/TEN.10
Transplantation of cryopreserved amniotic membrane (AM) in the acute phase of SJS/TEN as a biological bandage over the entire ocular surface was first described in 2002 by John et al.11 Since then, there have been multiple case reports, case series,12,13 a case-control study,9 a randomized controlled trial,14 and a comparative case series,15 all of which demonstrated improved outcomes in cases where amniotic membrane transplantation (AMT) was performed early in acute SJS/TEN when compared to traditional supportive therapy in the form of topical medications and symblepharon lysis. In AMT, AM is applied in sheets to cover the entire ocular surface and eyelid margins, from upper eyelid to lower eyelid. A detailed description of the original technique can be found by Gregory et al.16 Newer approaches have been developed which can more readily be performed at the bedside.16,17 In contrast, the ProKera® device [(PD) (Bio-Tissue, Inc., Miami, FL)] only covers the cornea and perilimbal conjunctiva and consists of a polycarbonate ring-set with a sheet of AM clipped in between and stretched across the lumen of the ring. The use of a PD has also been previously described in acute SJS/TEN.12,19,20 Because PDs only cover and treat the cornea and conjunctiva and not the eyelid margin, it may not prevent eyelid margin and forniceal complications to the same degree as AMT. (SHAY REF 21) Collectively, the use of AMT or PD is referred to as the use of AM. We have previously shown that a standardized acute treatment protocol which includes AM placement significantly improves outcomes.15 Our standard acute treatment protocol, which determines which patients receive AMT versus PD can be seen in Figure 1. Although short term studies have shown the benefit of AM, long-term outcomes of the AM cohort specifically have not yet been reported, and there is no information on the long-term complications of SJS/TEN which may still arise.9,12 We have previously reported that complications from SJS/TEN continue well past the acute stage.15 This present study describes the outcomes in 55 eyes of 29 patients with SJS/TEN which were treated with AM (AMT or PD) in the acute phase, after a median follow-up period of 2.5 years.
Figure 1. Flow diagram outlining the protocol for management of ocular manifestations in acute SJS/TEN.
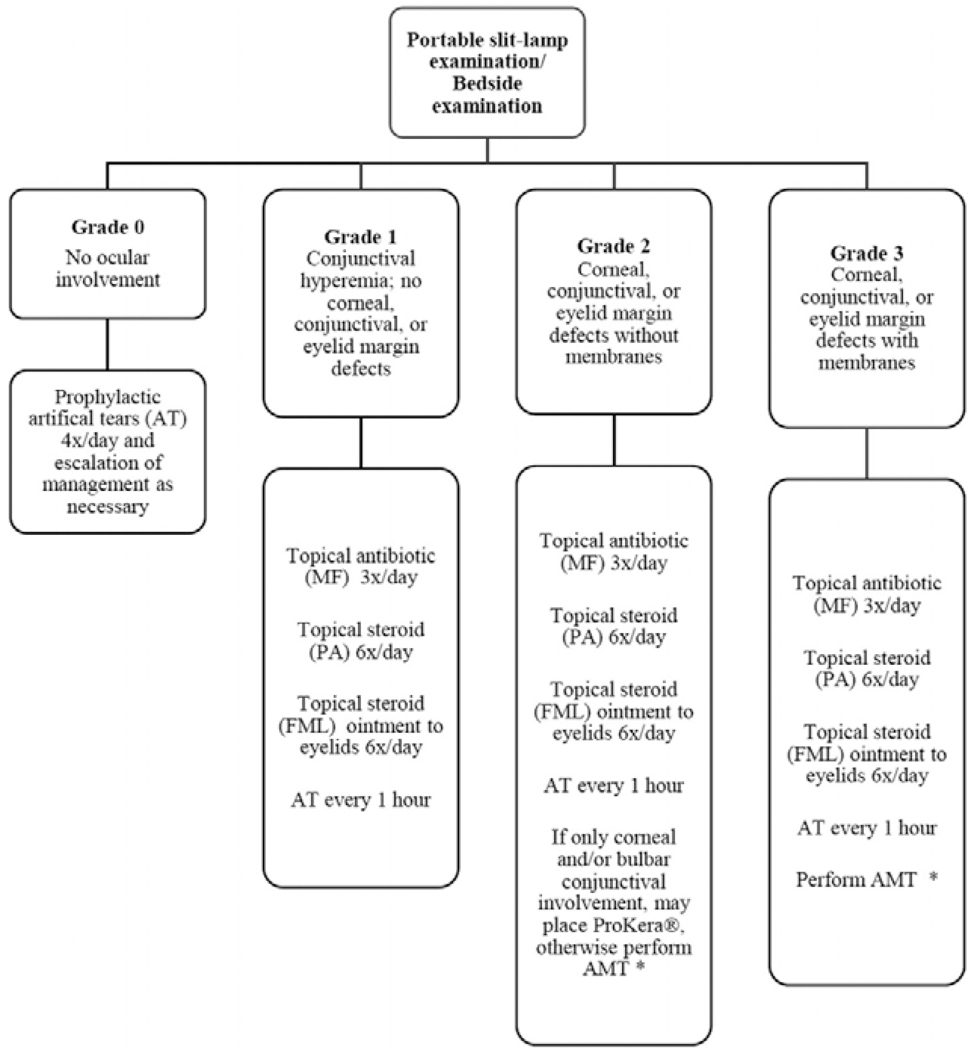
MF [ moxifloxacin 0.5%; PA [ prednisolone acetate 1%; FML [0.1%; AT [ artificial tears; AMT [ amniotic membrane transplantation. *Decision to perform AMT was based on feasibility (intubation status, cooperation, etc.). ProKera is acceptable only if bulbar conjunctival or corneal involvement is present or when AMT is not feasible. Modified from Shanbhag et al. with permission from Elsevier.15
2. Methods
This study was approved by the institutional review board of the Massachusetts Eye and Ear (MEE) and the Massachusetts General Hospital (MGH). The study was conducted under Health Insurance Portability and Accountability Act (HIPAA) compliance and adhered to the tenets of the Declaration of Helsinki.
Electronic records of all patients with a diagnosis of SJS/TEN at MEE between January 2008 and January 2018 who received AM were reviewed. Only patients with follow-up ≥ 3 months after AM were included. In eyes which received AMT (AmnioGraft®; Bio-Tissue, Inc., Miami, FL), the amniotic membrane (AM) was anchored to the external eyelid skin with bolsters or cyanoacrylate glue and coverage of the fornices was ensured with a customized symblepharon ring made of sterile intravenous tubing as previously described.17,18 Eyes which received a PD were not secured with any additional procedures. All eyes received topical medications, including corticosteroid eyedrops, corticosteroid eye ointment to the eyelid margins, topical antibiotics, and topical lubricants (See Figure 1).
Patients were characterized as having SJS, SJS-TEN overlap, or TEN based on the percentage of total body surface area (TBSA) of epidermal detachment (SJS: <10% TBSA; SJS-TEN overlap: 10–30% TBSA; TEN: >30% TBSA).22 The acute phase was characterized as the period between symptom onset of SJS/TEN and 2 months post-onset; the sub-acute phase was defined as between 2–6 months after symptom onset, while the chronic phase was defined as > 6 months after symptom onset. Ocular involvement in the acute phase was retrospectively graded based on clinical exam notes for each eye using the grading system proposed by Sotozono et al.23 Ocular examination was carried out daily in the first week of hospitalization and continued every 2–3 days until the patient was discharged. The highest grade of severity that was observed during admission was used for this study. The follow-up period was calculated based on the date of first AM placement (day 1). The primary outcome measures were best-corrected visual acuity (BCVA) at final follow-up examination and ocular complications in the chronic phase. BCVA was measured with full corrective aids in place including glasses, contact lenses, and/or prosthetic replacement of the ocular surface ecosystem (PROSE) devices in place. PROSE is discussed in Section 3.4 of the manuscript.
Statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC, Version 9.4), with a p value < 0.05 considered statistically significant. Continuous parametric data were reported as means (± standard deviation) and non-parametric data were reported as median with inter-quartile range (IQR).
3. Results
From January 2008 to January 2018, 128 patients were screened for ocular involvement secondary to acute SJS/TEN. Out of 128 patients, 45 patients (35%) and 87 eyes received AM. Sixty-seven percent of patients (30/45) were female. Four patients who received AMT and one patient who received PD died during the course of acute SJS/TEN and were excluded from analysis. Eleven patients had follow-up less than 3 months after AM and were excluded from analysis. Altogether, 55 eyes of 29 patients were included, of which 31 eyes had undergone AMT and 24 eyes PD.
3.1. Demographics and baseline characteristics of patients who underwent AM
Overall, seventy-two percent of patients (21/29) included in our study were female. Sixty-six percent of patients (19/29) who underwent AM had TEN. Demographics and baseline characteristics are presented in Table 1. Median duration of follow up after first AM was 2.5 years (inter-quartile range: 1.2–3.6 years). The most common presumed etiology for SJS/TEN was sulfonamide antibiotics (primarily cotrimoxazole) in 35% of patients followed by antiepileptic medications (primarily lamotrigine) in 17% of patients. Seventy-nine percent of patients (23/29) were hospitalized in the burn unit during acute SJS/TEN. Eighty-six percent of patients (25/29) received systemic immunosuppression including systemic corticosteroid, intravenous immunoglobulin, cyclosporine, and etanercept (see Table 1).
Table 1.
Characteristics of patients who underwent amniotic membrane transplantation or ProKera device implantation in the acute phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Demographics and features in acute SJS/TEN | Patients with AM in the acute phase of SJS/TEN, N=29 patients |
|---|---|
| Median age in years at the time of acute SJS/TEN (IQR) | 23 (6 – 69) |
| Number of adults:children at the time of acute SJS/TEN* | 19:10 |
| Sex (female:male) | 21:8 |
| Median duration of follow-up after AM (IQR) | 2.5 years (1.2 – 3.6) |
| Number of patients, n (%) | |
| with SJS | 5 (17) |
| with SJS-TEN overlap | 5 (17) |
| with TEN | 19 (66) |
| Presumed etiology, n (%) | |
| Antibiotics | |
| Sulfonamides | 10 (35) |
| Other antibiotics | 1 (3) |
| Anti-epileptics | 5 (17) |
| NSAIDs | 4 (14) |
| Allopurinol | 1 (3) |
| Other drugs | 4 (14) |
| Infectious etiology (Mycoplasma pneumoniae) | 2 (7) |
| Unknown etiology | 2 (7) |
| Number of patients treated in the | |
| Burn service ICU | 23 (79) |
| Medical ICU | 3 (10.5) |
| Ward floor | 3 (10.5) |
| Mucous membranes affected (other than ocular) | |
| Oral | 29(100) |
| Vaginal/ urethral | 20 (69) |
| Penile/ urethral | 6 (21) |
| Respiratory | 3 (10) |
| Gastro-intestinal | 2 (7) |
| Median duration of hospital stay (IQR) | 24 days (16–37 days) |
| Systemic immunosuppression in the acute phase | |
| None | 4 (14) |
| Systemic steroids | 16 (55) |
| IVIG | 12 (41) |
| Cyclosporine | 5 (17) |
| Etanercept | 2 (7) |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; IQR = inter-quartile range; AMT = amniotic membrane transplantation; PD = ProKera device; NSAIDs = nonsteroidal anti-inflammatory drugs; ICU = intensive care unit; IVIG = intravenous immunoglobulin
Children defined as younger than 18 years
3.2. Acute phase for eyes which underwent AM placement
The BCVA at onset could not be measured in 10 eyes due to sedation/intubation. The number of eyes with involvement of the eyelids, conjunctiva and cornea in the acute phase are shown in Table 2. For all 55 eyes, the median interval at which AM was received from the onset of skin rash was 5 days [IQR: 3–7 days]. Of note, eyes which received AMT had earlier treatment than eyes which received PD, likely because eyes that received PD had less severe initial disease resulting in later placement as disease progressed (AMT = median 4 days, PD = median 7 days, p = 0.002).
Table 2.
Characteristics of eyes of patients who underwent amniotic membrane transplantation or ProKera device implantation in the acute phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Category | Patients with AM in the acute phase of SJS/TEN; n=55 eyes (%) |
|---|---|
| BCVA at onset of SJS/TEN | |
| ≥ 20/40 | 45 (82) |
| Could not be measured | 10 (l8) |
| Number of eyes in acute phase with, n (%) | |
| Conjunctival hyperemia | 55 (100) |
| Lid margin involvement (denudation of epithelium) | 47 (85) |
| Conjunctival pseudomembranes | 22 (40) |
| Conjunctival epithelial defects | 44 (80) |
| Corneal epithelial defects | 31 (56) |
| Number of eyes in acute phase with, n (%)* | |
| Grade 2 involvement | 34 (62) |
| Grade 3 involvement | 21 (38) |
| Number of eyes which received AM at | |
| Bedside | 45 (82) |
| Operating room | 10 (18) |
| Median period at which AM was received from the onset of skin rash (IQR) | 5 days (3–7) |
| Median time to dissolution of AM (IQR) | 9 days (7–9) |
| Number of eyes with primary AMT which required | |
| Repeat AM in the form of AMT | 3/31 (10) |
| Repeat AM in the form of PD | 15/31 (48) |
| Number of eyes with primary PD placement which required | |
| Repeat AM in the form of AMT | 4/24 (17) |
| Repeat AM in the form of PD | 6/24 (25) |
| Number of eyes which required more than two applications of AM | 6 (11) |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; IQR = inter-quartile range; AMT = amniotic membrane transplantation; PD = ProKera device; BCVA = best-corrected visual acuity
Grade of involvement was adapted from Sotozono et al.23
PD implantation is a bedside procedure while AMT was initially performed either at bedside or in the operating room. Techniques to facilitate AMT at the bedside were developed later in the study period. Hence, 10 eyes underwent AMT in the operating room while 21 eyes underwent AMT at bedside. The median time to dissolution of the amniotic membrane in all 55 eyes was 9 days (IQR: 7–9 days). However, there was a significant difference in the 2 groups with slower dissolution of the AM in patients that underwent a full AMT compared to the patients who underwent PD (AMT = median 9 days, PD = median 7.5 days, p = 0.02). Forty percent of eyes (22/55) required a repeat AMT or PD. Eleven percent of eyes (6/55) required more than two AM procedures due to persistent corneal and conjunctival epithelial defects. In eight eyes where AMT was advised, PD was performed instead secondary to patient refusal, inability to co-operate for bedside AMT, and/or contraindications for general anesthesia.
3.3. Complications in the chronic phase in eyes which underwent AMT/PD
The complications in the chronic phase are detailed in Table 3. Serious vision-threatening complications like persistent epithelial defects (PED), sterile corneal perforations, and infectious keratitis were most common in the first year after onset of SJS. Of the 55 eyes, the most common complications in the chronic phase were meibomian gland disease, which affected 78% of eyes and dry eye which affected 58% of eyes. The most common vision-threatening complication in the chronic phase was moderate to severe eyelid margin keratinization in 31% of eyes. Other vision-threatening complications such as central corneal vascularization and central corneal opacity occurred in 7% of eyes. None of the eyes had total LSCD or keratinization of the ocular surface in the chronic phase. Eighty-seven percent of eyes had BCVA ≥ 20/40 at last follow-up.
Table 3.
Complications in the chronic phase in eyes which underwent amniotic membrane transplantation or ProKera device implantation in the acute phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Complications | Number of eyes = 55 (%) |
|---|---|
| Persistent epithelial defect | 8 (15) |
| Sterile corneal perforation | 1 (2) |
| Infectious keratitis | 2 (4) |
| Meibomian gland disease | 43 (78) |
| Punctal closure by scarring | 18 (33) |
| Epiphora due to punctal closure by scarring | 3 (5) |
| Lid margin keratinization | |
| Mild | 17 (31) |
| Moderate - Severe | 17 (31) |
| Trichiasis / Distichiasis | 25 (45) |
| Entropion | 8 (15) |
| Tarsal conjunctival scarring | 31 (56) |
| Dry eye* | |
| Grade 1 | 6 (11) |
| Grade 2 | 5 (9) |
| Grade 3 | 9 (16) |
| Grade 4 | 12 (22) |
| Symblepharon | |
| Mild, peripheral, not threatening vision | 19 (34) |
| Moderate, threatening vision | 0 |
| Conjunctivalization of cornea | |
| Peripheral corneal surface | 3 (5) |
| Entire corneal surface | 0 |
| Corneal neovascularization | |
| Peripheral | 10 (18) |
| Central | 4 (7) |
| Corneal opacity | |
| Peripheral | 3 (5) |
| Central | 4 (7) |
| Total LSCD | 0 |
| Total keratinization of the ocular surface | 0 |
| BCVA at last follow-up | |
| ≥ 20/40 | 48 (87) |
| 20/50 to 20/200 | 5 (9) |
| < 20/20 | 2 (4) |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; NLDO = nasolacrimal duct obstruction; LSCD = limbal stem cell deficiency; BCVA = best-corrected visual acuity; CF = counting fingers; LP = light perception; NLP = no perception of light
Grading of dry eye as per DEWS criteria
3.4. Subsequent procedures required in the chronic phase for management of complications
Thirty-eight percent of eyes (21/55) were referred for prosthetic replacement of the ocular surface ecosystem devices (PROSE, BostonSight, Needham, MA). PROSE devices are customized scleral lens devices that vault over the corneal and rest on the sclera, protecting the corneal epithelium from eyelid trauma and allowing for maintenance of a fluid reservoir over the cornea. The median time interval between first AM and the use of PROSE devices in the 21 eyes that required PROSE was 5.6 months (IQR: 4.7–8.9 months). Fifteen percent of eyes (8/55) underwent mucous membrane grafting (MMG), which involves harvesting labial mucosa and using it to replace the affected eyelid margin mucosa in moderate to severe eyelid margin keratinization. The median time interval between first AM and MMG in these 8 eyes was 2.73 months (IQR: 1.7–22 months). It should be noted that the oral mucosa was completely healed after the acute SJS/TEN before undertaking MMG. Six eyes received both MMG and PROSE.
3.5. Challenges in AM placement and retention
In one of the first AMT procedures performed in 2009, the AM was dislodged from the eyelids in both eyes of a patient after cleaning of the face by the nursing staff. In a patient with relatively shallow orbits, sizing of the intravenous tube required more trial and error than usual. In a patient with delirium, AMT was inadvertently removed from one eye by the patient within a day and had to be repeated. Another patient had an episode of severe vomiting in the acute phase of SJS/TEN which resulted in the PD being dislodged from one eye. While education of the nursing staff on proper care of AM can reduce these challenges, there will always be some risk of loss of the AM. Close follow up ensures that the AM is replaced promptly.
4. Discussion
SJS/TEN is a devastating disease, and a substantial number of survivors suffer profound disability in the chronic phase.24–26 Importantly, disabling ocular complications have been shown to be the most common of all complications in survivors of TEN.10,27 In countries where acute care for SJS/TEN is not available, SJS/TEN is the cause of bilateral LSCD in 30% of such cases.28 In a study of pediatric SJS/TEN in which 99% of the children did not have access to AM in the acute phase of their disease, 66% had BCVA<20/60 a year or more later.29 Despite this, a recent survey noted that only 66% of burn intensive care units in the United States routinely seek an ophthalmologic consultation for patients with acute SJS/TEN.30 Our study shows that an ophthalmology consult in the immediate acute phase of SJS/TEN followed by aggressive use of AM when indicated by specific clinical signs, can substantially reduce the visual morbidity of SJS/TEN in the chronic phase, with 87% of the 55 eyes in this study maintaining BCVA ≥ 20/40 at a median follow-up period of 2.5 years after disease onset.
AM when used in the acute phase in these highly inflamed eyes exhibits an anti-inflammatory and anti-scarring action.31 However, the timing of AM placement is crucial. Preliminary findings by Cherof and colleagues suggest that AMT is most effective at preventing ocular sequelae of SJS when applied within the first 6 days after symptom onset.32 They noted increased risk of moderate/severe dry eye, moderate tarsal scarring and visual acuity < 20/30 in eyes receiving AMT after 6 days of disease onset. Additionally, Ciralsky and coworkers noted increased complications and ocular surface disease in an untreated eye as compared to the fellow eye treated with AMT.33 Given a lack of serious complications from AM placement, it is reasonable to have a low threshold for placement and to perform the procedure as early in the course of disease as possible, when indicated. In our study, although the median time interval between disease onset and first AM was 5 days, delayed intervention occurred in a few cases mainly because of lack of continuity in care, or uncertainty about the diagnosis. Many of our patients were transferred to the burn intensive care unit at MGH from outside hospitals and therefore, the time from skin rash onset to AM placement was suboptimal.
PD implantation is an easy technique which provides coverage of the cornea and perilimbal conjunctiva with amniotic membrane in eyes that may not require AMT. Our protocol recommends PD or AMT depending on disease severity.15 In some cases where AMT is recommended but cannot be performed because the patient cannot tolerate a bedside procedure and/or general anesthesia carries significant medical risk, PD is performed instead. These patients were included in our analysis to depict a real-world implementation analysis of AM placement in SJS/TEN. Our results may have shown an even greater impact of AM if those patients who could not adhere to the treatment protocol were excluded. Recently, we have developed a bedside technique to perform AMT at the bedside more easily, precluding the need for general anesthesia.18 We advise performing AM at the bedside in every patient with acute SJS/TEN and grade 2 or 3 acute phase ocular disease as early as possible.
In our series, 87% of eyes retained BCVA ≥ 20/40. However, AM placement does not eliminate ocular complications of SJS/TEN. Out of seven eyes which had BCVA < 20/40 in the chronic phase, five had severe vision threatening complications including PED, sterile corneal necrolysis, and infectious keratitis in the sub-acute and early chronic phase of the disease. Therefore, eyes which receive AM placement should still be monitored closely.
All patients in our study were seen in the outpatient setting within a week of discharge from the hospital and were examined with the slit-lamp every 2–3 weeks for the first 3 months after acute SJS/TEN. Some patients later required MMG or PROSE lenses because of eyelid margin keratinization or severe dry eye. These patients were referred quickly upon recognition of severe eyelid changes, as demonstrated by the short median time interval between AM and MMG or PROSE intervention. AM is not a panacea in SJS/TEN and will not prevent all eyelid-related complications in the chronic phase. In such patients, it is critical to offer these interventions as early as possible to prevent blinding eyelid-related damage to the ocular surface.32–35
In our study, 58% of the 55 eyes suffered from varying degrees of dry eye symptoms in the chronic phase. In a randomized clinical trial which compared patients who had undergone AMT in the acute phase with patients who underwent medical therapy alone, better tear film break-up times and higher Schirmer values were seen in the eyes which underwent AMT at a follow-up period of 6 months.14 The study did not report on patient symptoms of dry eye and/or degree of ocular surface disease. Development of dry eye symptoms after SJS/TEN is multifactorial, but likely derives in part from damage to the lacrimal and meibomian glands, which may not be effectively addressed with AM placement.
The principal strength of our study is the long overall follow-up of our cases. Maintaining ophthalmology follow-up at the same center where the patient is seen in the acute phase may be difficult as patients are often referred to burn intensive care units from a large surrounding geographical area and they may prefer to follow-up with a local ophthalmologist after their acute care. The limitations of our study include its retrospective nature, necessitating the use of hospital records of ocular examinations in the acute phase. Additionally, various systemic treatments were given by the primary inpatient team which may have influenced ocular outcomes. Lastly, the means by which AMT was secured to the eyelid skin evolved over the study period of 10 years from use of sutures and bolsters to cyanoacrylate glue. We neither expected nor did we find differences in clinical outcomes between AMT techniques (data not shown).
5. Conclusions
SJS/TEN is a source of severe ocular surface disease and can result in bilateral corneal blindness. We believe that an aggressive approach toward AM placement in acute SJS/TEN with a low threshold for performing bedside AM early after symptom onset, combined with close monitoring in the sub-acute and chronic phases, may decrease visual morbidity and serious chronic complications. However, sight-threatening complications do occur even with the use of AM and long-term follow-up in this patient population is of utmost importance.
Table 4.
Details of subsequent procedures in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Procedures in the chronic phase of SJS/TEN | Number of eyes (%) (out of total 55 eyes) |
|---|---|
| PROSE device | |
| Fitted | 21 (38) |
| Continuous usage | 21 (38) |
| Procedures | |
| Punctal plugs | 4 (7) |
| Punctal cautery | 4 (7) |
| Cyanoacrylate glue | 1 (2) |
| Lateral tarsorrhaphy | 2 (4) |
| AMT in chronic phase | 1 (2) |
| PD in chronic phase | 3 (5) |
| Mucous membrane graft | 8 (15) |
| Surgery for entropion | 4 (7) |
SJS = Stevens-Johnson syndrome, TEN = Toxic epidermal necrolysis, PROSE = Prosthetic replacement of the ocular surface ecosystem, AMT = Amniotic membrane transplantation
Highlights:
Every patient with Stevens Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) should have an ophthalmology consult immediately after diagnosis.
There should be a low threshold for treatment with amniotic membrane for acute ocular involvement.
Amniotic membrane placement acutely, when indicated, aids in maintaining visual acuity in the chronic phase.
Sight-threatening complications may still develop after treatment with amniotic membrane.
Long-term follow-up is necessary in all patients who have SJS/TEN.
Footnotes
Financial Disclosure: None
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kohanim S, Palioura S, Saeed HN, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - A comprehensive review and guide to therapy. II. Ophthalmic Disease. Ocul Surf 2016;14:168–188. [DOI] [PubMed] [Google Scholar]
- 2.Yip LW, Thong BY, Lim J, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy 2007;62:527–531. [DOI] [PubMed] [Google Scholar]
- 3.Morales ME, Purdue GF, Verity SM, Arnoldo BD, Blomquist PH. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol 2010;150:505–510.e501. [DOI] [PubMed] [Google Scholar]
- 4.Chang YS, Huang FC, Tseng SH, Hsu CK, Ho CL, Sheu HM. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea 2007;26:123–129. [DOI] [PubMed] [Google Scholar]
- 5.Gueudry J, Roujeau JC, Binaghi M, Soubrane G, Muraine M. Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Arch Dermatol 2009;145:157–162. [DOI] [PubMed] [Google Scholar]
- 6.Yang MS, Lee JY, Kim J, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: A nationwide population-based study using National Health Insurance Database in Korea. PloS One 2016;11:e0165933. doi: 10.1371/journal.pone.0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang CW, Cho YT, Chen KL, Chen YC, Song HL, Chu CY. Long-term sequelae of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Acta Derm Venereol 2016;96:525–529. [DOI] [PubMed] [Google Scholar]
- 8.Jongkhajornpong P, Lekhanont K, Siriyotha S, Kanokrungsee S, Chuckpaiwong V. Factors contributing to long-term severe visual impairment in Stevens-Johnson syndrome and toxic epidermal necrolysis. J Ophthalmol 2017;2017:2087578. doi: 10.1155/2017/2087578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu M, Jayaram A, Verner R, Lin A, Bouchard C. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea 2012;31:1394–1402. [DOI] [PubMed] [Google Scholar]
- 10.Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol 2017;177:924–935. [DOI] [PubMed] [Google Scholar]
- 11.John T, Foulks GN, John ME, Cheng K, Hu D. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology 2002;109:351–360. [DOI] [PubMed] [Google Scholar]
- 12.Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology 2011;118:908–914. [DOI] [PubMed] [Google Scholar]
- 13.Shammas MC, Lai EC, Sarkar JS, Yang J, Starr CE, Sippel KC. Management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis utilizing amniotic membrane and topical corticosteroids. Am J Ophthalmol 2010;149:203–213. [DOI] [PubMed] [Google Scholar]
- 14.Sharma N, Thenarasun SA, Kaur M, et al. Adjuvant role of amniotic membrane transplantation in acute ocular Stevens-Johnson syndrome: A randomized control trial. Ophthalmology 2016;123:484–491. [DOI] [PubMed] [Google Scholar]
- 15.Shanbhag SS, Rashad R, Chodosh J, Saeed HN. Long-term impact of a treatment protocol for acute ocular involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis. Am J Ophthalmol. 2019. July 18. doi: 10.1016/j.ajo.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory DG. The ophthalmologic management of acute Stevens-Johnson syndrome. Ocul Surf. 2008;6(2):87–95. [DOI] [PubMed] [Google Scholar]
- 17.Ma KN, Thanos A, Chodosh J, Shah AS, Mantagos IS. A novel technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf 2016;14:31–36. [DOI] [PubMed] [Google Scholar]
- 18.Shanbhag SS, Chodosh J, Saeed HN. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis.Ocul Surf 2019;17:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol 2009;54:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlins PJ, Parulekar MV, Rauz S. “Triple-TEN” in the treatment of acute ocular complications from toxic epidermal necrolysis. Cornea 2013;32:365–369. [DOI] [PubMed] [Google Scholar]
- 21.Shay E, Khadem JJ, Tseng SCCornea. Efficacy and limitation of sutureless amniotic membrane transplantation for acute toxic epidermal necrolysis. 2010. March;29(3):359–61. [DOI] [PubMed] [Google Scholar]
- 22.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129:92–96. [PubMed] [Google Scholar]
- 23.Sotozono C, Ueta M, Nakatani E, et al. Predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol 2015;160:228–237. [DOI] [PubMed] [Google Scholar]
- 24.Butt TF, Cox AR, Lewis H, Ferner RE. Patient experiences of serious adverse drug reactions and their attitudes to medicines: a qualitative study of survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. Drug Saf 2011;34:319–328. [DOI] [PubMed] [Google Scholar]
- 25.Butt TF, Cox AR, Oyebode JR, Ferner RE. Internet accounts of serious adverse drug reactions: a study of experiences of Stevens-Johnson syndrome and toxic epidermal necrolysis. Drug Saf 2012;35:1159–1170. [DOI] [PubMed] [Google Scholar]
- 26.Dodiuk-Gad RP, Olteanu C, Feinstein A, et al. Major psychological complications and decreased health-related quality of life among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2016;175:422–424. [DOI] [PubMed] [Google Scholar]
- 27.Haber J, Hopman W, Gomez M, Cartotto R. Late outcomes in adult survivors of toxic epidermal necrolysis after treatment in a burn center. J Burn Care Rehabil 2005;26:33–41. [DOI] [PubMed] [Google Scholar]
- 28.Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol 2018;188:99–103. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children: Long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol 2018;189:17–28. [DOI] [PubMed] [Google Scholar]
- 30.Le HG, Saeed H, Mantagos IS, Mitchell CM, Goverman J, Chodosh J. Burn unit care of Stevens Johnson syndrome/toxic epidermal necrolysis: A survey. Burns 2016;42:830–835. [DOI] [PubMed] [Google Scholar]
- 31.Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf 2004;2:177–187. [DOI] [PubMed] [Google Scholar]
- 32.Cherof AM CA, Lynch A, Wagner B, Gregory DG. Acute Stevens-Johnson syndrome: the effect the timing of amniotic membrane transplantation has on the occurrence of significant ocular sequelae. Paper presented at AAO Annual Meeting; November 16, 2015; Las Vegas. [Google Scholar]
- 33.Ciralsky JB, Sippel KC. Prompt versus delayed amniotic membrane application in a patient with acute Stevens-Johnson syndrome. Clin Ophthalmol 2013;7:1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papakostas TD, Le HG, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology 2015;122:248–253. [DOI] [PubMed] [Google Scholar]
- 35.Iyer G, Pillai VS, Srinivasan B, Guruswami S, Padmanabhan P. Mucous membrane grafting for lid margin keratinization in Stevens-Johnson syndrome: results. Cornea 2010;29:146–151. [DOI] [PubMed] [Google Scholar]
- 36.Iyer G, Srinivasan B, Agarwal S, Kamala Muralidharan S, Arumugam S. Comprehensive approach to ocular consequences of Stevens Johnson Syndrome - the aftermath of a systemic condition. Graefes Arch Clin Exp Ophthalmol 2014;252:457–467. [DOI] [PubMed] [Google Scholar]
- 37.Iyer G, Srinivasan B, Agarwal S, Pillai VS, Ahuja A. Treatment modalities and clinical outcomes in ocular sequelae of Stevens-Johnson syndrome over 25 years--a paradigm shift. Cornea 2016;35:46–50. [DOI] [PubMed] [Google Scholar]


