Abstract
Cellular senescence, a state of irreversible growth arrest triggered by various stressors, engages in a category of pathological processes, whereby senescent cells accumulate in mitotic tissues. Senolytics as novel medicine against aging and various diseases through the elimination of senescent cells has emerged rapidly in recent years. Exercise is a potent anti‐aging and anti‐chronic disease medicine, which has shown the capacity to lower the markers of cellular senescence over the past decade. However, whether exercise is a senolytic medicine for aging and various diseases remains unclear. Here, we have conducted a systematic review of the published literature studying the senolytic effects of exercise or physical activity on senescent cells under various states in both human and animal models. Exercise can reduce the markers of senescent cells in healthy humans, while it lowered the markers of senescent cells in obese but not healthy animals. The discrepancy between human and animal studies may be due to the relatively small volume of research and the variations in markers of senescent cells, types of cells/tissues, and health conditions. These findings suggest that exercise has senolytic properties under certain conditions, which warrant further investigations.
Keywords: cellular senescence, exercise, senescent cells, senolytic medicine, senolytics
Cellular senescent upregulates certain markers such as p16INK4a, p21Cip1, SA‐beta‐Gal, and/or SASP, which can also be shown in accelerated aging animals. This review discusses by using human and experimental laboratory animal studies to show how exercise attenuates all these senescent markers and serves as senolytic medicine.
1. INTRODUCTION
Aging, especially when coupled with unhealthy lifestyles, is the leading contributor to most physical dysfunctions and chronic diseases in humans, whereby senescent cells defined as division‐arrested normal cells progressively accumulate in tissues (Childs et al., 2015; Collado et al., 2007). While gradual cellular senescence throughout the whole aging process was identified by Hayflick and Moorhead in 1961, to date, rare anti‐aging and age‐associated disease medicine has been developed targeting these biological aging processes at the cellular level. Moreover, unhealthy lifestyles, such as physical inactivity, unhealthy diets, and cigarette smoking, have been found to be associated with the accumulation of senescent cells in humans independent of chronological age (Liu et al., 2009; Song et al., 2010; Tchkonia et al., 2010). Importantly, cellular senescence also participates in other vital biological processes, not only aging but tumor suppression, tumor promotion, and tissue repair (Rodier & Campisi, 2011). Therefore, while cellular senescence is a promising therapeutic target of multiple diseases, especially aging‐related diseases and cancer, the consequences and safety of up‐ or downregulating these complex biological processes should be taken into consideration.
Senescent cells are the hallmark and therapeutic target of cellular senescence involved in a wide range of biological processes, including tumor suppression, embryonic development, wound healing and tissue repair, and aging (Van Deursen, 2014). Although senescent cells are detrimental to the body during aging and can lead to chronic diseases, such as obesity, diabetes, and sarcopenia, they also suppress cancer and fibrosis (Muñoz‐Espín & Serrano, 2014). Under the complex functions, there are two major senescent pathways: p53/p21Cip1 and p16INK4a/RB; and both p21Cip1 and p16INK4a are the key markers of senescent cells (Ben‐Porath & Weinberg, 2005). Moreover, other markers of senescent cells have been reported, including senescence‐associated heterochromatic foci (SAHFs), ARF, DNA segments with chromatin alterations reinforcing senescence (DNA‐SCARS), senescence‐associated beta‐galactosidase (SA‐β‐Gal), shorter telomere length, lower proliferation, and senescence‐associated secretory phenotype (SASP) (He & Sharpless, 2017). Exploration of these senescent markers as targets might contribute to the development and testing of anti‐senescent cell therapeutic strategies.
Senolytics is a new class of medicines that target senescent cells, which has emerged rapidly in the past few years (Kirkland et al., 2017). Preclinical studies on rodents have been applied to explore the potential targets of senescent cells and the preliminary effects of senolytic medicine in vivo. In 2011, an INK‐ATTAC transgenic mouse with a BubR1 progeroid background was first established and showed restored aging‐associated dysfunctions following ablation of senescent cells by AP20187 (Baker et al., 2011). Moreover, another transgenic line, p16‐3MR, was generated, where senescent cells can also be eliminated via ganciclovir and further mitigated the post‐traumatic osteoarthritis and age‐related intervertebral disk degeneration (Jeon et al., 2017; Patil et al., 2019). In addition to transgenic mice, senolytic drugs, including dasatinib and quercetin, ABT263, and SSK1, also showed the therapeutic effects on senescent cells and alleviated the radiation and age‐related symptoms and pathology (Cai et al., 2020; Chang et al., 2016; Zhu et al., 2015). A combined treatment of senolytic medicines in transgenic mice also presented the therapeutic effects on obesity‐induced metabolic dysfunction and fibrotic pulmonary disease (Palmer et al., 2019; Schafer et al., 2017). Strikingly, two small clinical trials on senolytic treatments with dasatinib and quercetin were completed last year and reported therapeutic effects for patients with diabetic kidney disease (N = 9) and idiopathic pulmonary fibrosis (N = 14) (Hickson et al., 2019; Justice et al., 2019). More recently, other novel senolytic agents such as ABT‐737, navitoclax, flavone, fisetin, A1331852, and A1155463 have been developed; ongoing research will determine their effects against various diseases in the future (Yosef et al., 2016; Zhu et al., 2015, 2017).
Physical exercise is widely recognized as a safe, effective, and cost‐effective “medicine” for a broad range of age‐related diseases. Moreover, a lack of exercise is a major contributing factor to accelerated aging and age‐associated chronic conditions, including cancer, obesity, and cardiovascular diseases (Booth et al., 2011). World Health Organization (WHO) thus encourages adults aged 18–64 years to engage in over 150 min of moderate‐intensity physical activity each week to reduce the risk of these chronic conditions. Unfortunately, 23% of men and 32% of women worldwide failed to meet this recommendation according to data from WHO, (Guthold et al., 2018). Therefore, a clearer delineation of anti‐aging and anti‐disease effects and underlying mechanisms of exercise is needed. While the accumulation of senescent cells has been identified as the mechanism of aging and multiple diseases for decades, senolytics targeting senescent cells has just been developed in recent years. In addition, exercise has shown its capacity to lower the marker of senescent cells over the past decade. In the current systematic review of all available literature, we explored the potential senolytic effects of exercise in both human and animal models under healthy or disease states. We aimed to improve the understanding of the cellular senescence‐based mechanisms underlying exercise as anti‐aging medicine. This may impel people to engage in more exercise and lead to the development of more precise “exercise prescriptions” and “exercise mimetics” for the aging population and patients with various age‐related diseases (Li & Laher, 2015).
2. RESULTS
2.1. Study characteristics
The systematic search in the database and manually searching the reference list yielded 2182 articles. After the exclusion of duplicates, 1704 articles were screened for the abstract and title. Of these, 60 articles were further screened for full text and finally included 21 articles in this review, which contained eight human studies, twelve animal studies, and one study included both human and animal experiments (Figure 1). The total number of included participants in human studies was 535 (Table 1). Participants, including sedentary or active volunteers, athletes, and patients, aged 18–81 years were recruited. Four out of nine were cohort studies, while three of them were intervention studies.
FIGURE 1.
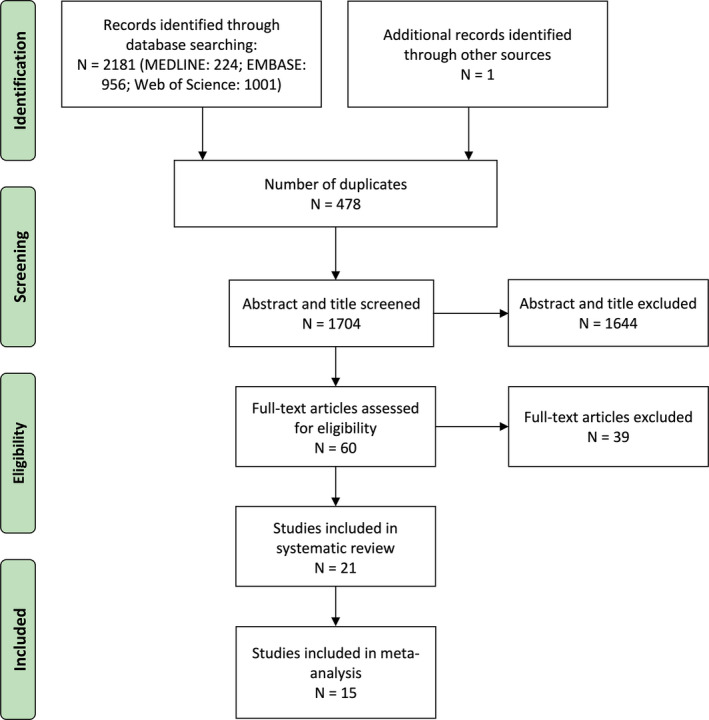
PRISMA flowchart of search strategy
TABLE 1.
Senolytic effects of exercise on senescent cells in human studies
| Author (year) | Participant | Exercise | PA Levels/Training protocol | Organ or cell | Marker of SCs | Senolytics |
|---|---|---|---|---|---|---|
| Habitual PA (Untrained) | ||||||
| Liu et al. (2009) | Healthy participants (18–80 years); n = 170 | Habitual PA | 0–1900 min/month (n = 170). Comparison between <= 240 and >240 min/month | T lymphocyte | p16INK4a↓ | Yes |
| Tsygankov et al. (2009) a | Healthy participants (18–80 years ); n = 170 | Habitual PA | 0–1900 min/month (n = 170). Comparison between <= 240 and >240 min/month | T lymphocyte | p16INK4a↓ | Yes |
| Song et al. (2010) | Healthy participants (18–80 years); n = 104 | Habitual PA | >= 0 and <2000 min/month (n = 104) | T lymphocyte | p16INK4a↓ | Yes |
| Pustavoitau et al. (2016) | Older (56–81 years) patients undergoing coronary artery bypass surgery; n = 47 | Habitual PA | >= 0 and <4000 min/month (n = 47). Comparison between <= 240 and >240 min/month | T lymphocyte | p16INK4a→ | No |
| Chronic exercise training | ||||||
| Werner et al. (2009) | Healthy middle‐aged marathon runners or triathletes (51.1 ± 1.6 years) and sedentary control (50.9 ± 1.6 years); n = 46 | Exercise training (running) | Trained: 80 ± 7.5 km/week for 35 ± 2.7 years; control: < 1 hr per week for 1 years | Mononuclear cells | p16INK4a↓ | Yes |
| Rossman et al. (2017) | Healthy trained older participants (59 ± 1 years, LTPA: 83 ± 9 MET hr/week) and sedentary control (62 ± 1 years, LTPA: 32 ± 6 MET hr/week) control; n = 25 | Exercise training | Trained: >= 45 min/day, 5 days/week for 5 years; control: <30 min/day, 2 days/week for 2 years | Vascular endothelial cell |
p16INK4a↓ & p21Cip1↓ |
Yes |
| Justice et al. (2018) | Older overweight/obese women (67–77 years); n = 8 | Resistance training (machine) | 3 sets of 10 repetitions at 70% 1RM per day, 3 day/week for 5 month | Adipose tissue | p16INK4a↓ | Yes |
| Acute exercise bout | ||||||
| Yang et al. (2018) | Healthy recreationally active (>= twice a week for >3 years) college student (22.5 ± 1.7 years); n = 12 | Resistance exercise (squat) | 6 sets of 12 repetitions at 70% of 1 RM | Endothelial progenitor cells | p16INK4a↓ | Yes |
| Wu et al. (2019) | Healthy recreationally active men (21 ± 0.2 years); n = 12 | Bicycle ergometer | 1 h on bike ergometer at 70% power output | Muscle | SA‐β‐gal→ | No |
Abbreviations: ↓ deceased and →unchanged compared with control (non‐exercise);LTPA, leisure time physical activity; MET, metabolic equivalent; PA, physical activity; RM, repetition maximum; SA‐β‐gal, senescence‐associated beta‐galactosidase; SCs, senescent cells.
The same cohort as (Liu et al., 2009) used for development of mathematical model that links p16INK4a with aging.
Overall, most human studies used p16INK4a and T lymphocyte as markers of senescent cells and targeted tissue or cell, respectively. For physical exercise, habitual physical activity (via questionnaire) was determined in most human studies, while resistance training and an acute bout of moderate‐high intensity cycling were applied as the exercise intervention. On the other hand, mice (nine studies) and rat (five studies) were utilized as animal models to investigate the senolytic effects of exercise on senescent cells (Table 2). Wild‐type and spontaneous or accelerated aging animals were used in most studies. Overall, p16INK4a, p21Cip1, and/or SA‐β‐Gal were applied as markers of senescent cells in various tissues or cells. For chronic exercise training, long‐term running, including both wheel/voluntary and treadmill/forced running, was reported in most animal studies, while swimming was also applied as the exercise intervention in three studies.
TABLE 2.
Senolytic effects of exercise on senescent cells in animal studies
| Author (year) | Animal model | Exercise | Exercise protocol | Organ or tissue | Marker of SCs | Senolytics |
|---|---|---|---|---|---|---|
| Chronic exercise training | ||||||
| Werner et al. (2008) | Mice (8‐week‐old); n = 8–12 a ; WT | Wheel running (voluntary) | 5100 ± 800 m/24 h for 21 days | Heart | p16INK4a↓ | Yes |
| Werner et al. (2009) | Mice (8‐week‐old); n = 6–8; WT | Wheel running (voluntary) | 4280 ± 670 m/24 h for 21 days | MNC & Vessel | p16INK4a↓ | Yes |
| Kröller‐Schön et al. (2012) | Mice (8‐week‐old); n = 6; WT | Wheel running (voluntary) | 4336 ± 842 m/24 h for 8 weeks | Endothelium | p16INK4a↓ | Yes |
| Huang et al. (2013) | Rat (7‐week‐old); n = 5–6; Accelerated aging | Swimming | 60 min/time, 5 times/week for 12 weeks | Liver | p21Cip1↓& SA‐β‐gal↑ | Opposite & Yes |
| Schafer et al. (2016) | Mice (8‐month‐old); n = 6–7; Obesity | Wheel running (voluntary) | 3291 ± 1166 m/24 h for 16 weeks | Adipose tissue, liver, aorta, skeletal muscle, pancreas, kidney, heart, | p16INK4a↓→, p21Cip1↓→& SA‐β‐gal↓→ | Yes & No |
| Zhang et al. (2016) | Rat (20‐month‐old); n = n.a.; Spontaneous Aging | Treadmill running | 30 min/day at 13 m/min, 5 days/week for 4–8 weeks | Tendons | SA‐β‐gal↓ | Yes |
| Fan et al. (2017) | Rat (4‐month‐old); n = 7; Accelerated aging | Swimming | 45 min/day, 5 day/week for 6 weeks | Muscle | SA‐β‐gal↓ | Yes |
| Yoon et al. (2019) | Mice (9‐week‐old/19‐month‐old); n = 5; Spontaneous Aging | Treadmill running | 30 min, twice per day, 5 days/week for 4 weeks | Muscle | p16INK4a↓→& p21Cip1↓→ | Yes & No |
| Wong et al. (2019) | Mice (3–5‐month‐old/23‐24‐month‐old); n = 5–7; Spontaneous Aging | Treadmill running | 33d at 10 m/min and 5 day at 8 m/min | Epidermal and dermal cell | p16INK4a→& p21Cip1→ | No |
| Liu et al. (2019) | Rat (8–10‐week‐old); n = 3; WT | Swimming | 60 min/day for 5 week with sinker weight | Hippocampus cell | p21Cip1↑& SA‐β‐gal↑ | Opposite |
| Jang et al. (2019) | Mice (10‐week‐old); n = 3; Obesity | Treadmill running | 12‐week running, 1 h/day, 5 day/week with increased speed (12–14–15 m/min) | Hippocampus cell | p16INK4a↓, p21Cip1↓, SA‐β‐gal↓ and lipofuscin↓ | Yes |
| Bao et al. (2020) | Mice (19‐month‐old); n = 3; Spontaneous Aging | Rotatable treadmill running | 15–60 min/day, 5 day/week for 6 weeks with increased time and speed (3.2–4.8 m/min) | Kidney | SA‐β‐gal↓ | Yes |
| Acute exercise bout | ||||||
| Saito et al. (2020) | Mice (13‐week‐old); n = 3–5; WT | Treadmill running | −20° downhill treadmill for 30 min at 17 m/min | Fibro‐adipogenic progenitors | p16INK4a→& p21Cip1↑ | Opposite & No |
Per group; WT, wild‐type; SCs: senescent cells; SA‐β‐gal: senescence‐associated beta‐galactosidase; ↑ increased, ↓deceased and → unchanged compared with control (non‐exercise).
2.2. Qualitative description of study findings
Of all 21 articles, 16 articles reported senolytic effects of exercise on the markers of senescent cells characterized by declined levels of specific molecular machinery (p16INK4a, p21Cip1, and SA‐β‐Gal), while five and three articles reported no and opposite effects of exercise on senescent cells, respectively. Interestingly, exercise showed distinct effects on different senescent markers (e.g., p16INK4a and p21Cip1) in one study.
For human studies (Table 1), the level of habitual physical activity is negatively associated with the level of p16INK4a in immune cells of healthy untrained participants aged 18–80 years (Liu et al., 2009; Song et al., 2010; Tsygankov et al., 2009), but not in older coronary bypass patients aged 56–81 years (Pustavoitau et al., 2016). In addition, chronic exercise training reduced the level of p16INK4a or p21Cip1 in mononuclear cells or vascular endothelial cells of healthy middle‐age athletes (marathon runners or triathletes) and healthy older trained participants compared to sedentary controls (Rossman et al., 2017; Werner et al., 2009). On the other hand, chronic resistance training reduced the level of p16INK4a in aged and overweight/obese women (4 out of 8 participants underwent caloric restriction during training were excluded) (Justice et al., 2018). A single bout of resistance training acutely lowered the level of p16INK4a in healthy recreationally active college students (Yang et al., 2018), but an acute bout of moderate‐high intensity cycling failed to reduce the level of SA‐β‐Gal in the muscle of healthy recreationally active young men (Wu et al., 2019). Collectively, physically active people have lower levels of p16INK4a‐positive senescent cells than sedentary people, while resistance training contributed to a lower level of p16INK4a.
For animal studies (Table 2), prolonged voluntary wheel running decreased the level of p16INK4a in various organs/tissues or cells in young, old, or obese mice/rat, including heart, vessel, endothelium, and adipose tissue (Kröller‐Schön et al., 2012; Schafer et al., 2016; Werner et al., 2008, 2009), while it remained unchanged in some other organs and tissues, such as kidney and pancreas. Moreover, long‐term forced treadmill running showed contradictory effects on the markers of senescent cells, including p16INK4a, p21Cip1, and SA‐β‐Gal (Bao et al., 2020; Jang et al., 2019; Wong et al., 2019; Yoon et al., 2019; Zhang et al., 2016), although four out of five studies showed senolytic effects of exercise in specific tissues under obesity or aging conditions. Conversely, studies on an acute bout of downhill running and prolonged swimming reported an increased level of p21Cip1 and SA‐β‐Gal in fibro/adipogenic progenitors (Saito et al., 2020), and liver and brain (Huang et al., 2013; Liu et al., 2019), respectively, while another swimming study indicated a reduced level of SA‐β‐Gal in the muscle (Fan et al., 2017). In summary, 10 out of 13 animal studies showed a senolytic effect of exercise, but this effect was influenced by the form and dosage of exercise, type of senescent tissue or cells, and healthy or aging/disease conditions.
2.3. Meta‐analysis
Overall, 16 out of 21 articles were included in the meta‐analysis. However, two studies were excluded because of a lack of essential data (Wu et al., 2019; Zhang et al., 2016), one studies were excluded because of less than two data with similar outcomes and design (Saito et al., 2020), one study was excluded because the data were taken from cohort reported in another study (Tsygankov et al., 2009), and one study was excluded because of the fact that exercise was not an independent factor (Yang et al., 2018).
For cross‐sectional studies in humans, a negative correlation between habitual physical activity and the level of p16INK4a in T lymphocytes (r = −0.30, 95% CI = −0.54, 0.01, I 2 = 84%, p = 0.04), subgroup by healthy participants (r = −0.30, 95% CI = −0.53, −0.34, I 2 = 0%, p < 0.001) and patients (one article) were identified (Figure 2a). Moreover, exercise training significantly reduced the level of p16INK4a in humans (−64%, 95% CI = −72%, −55%, I 2 = 0%, p < 0.001) (Figure 2b). While both habitual physical activity and exercise training studies supported the senolytic effects of exercise on p16INK4a‐positive senescent cells, more studies on various exercise and markers of senescent cells in different populations are still required.
FIGURE 2.
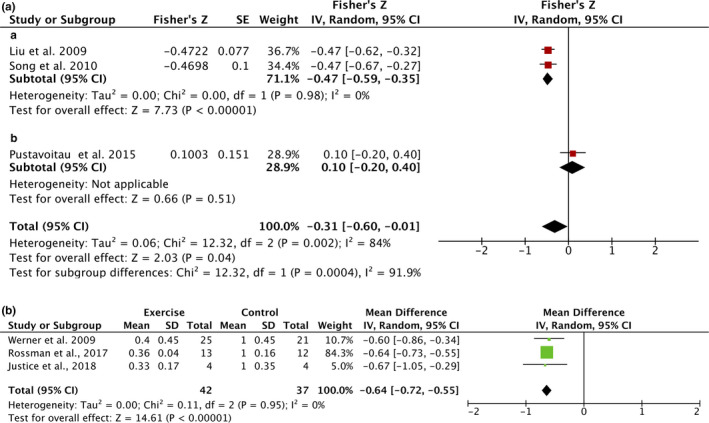
Forest plots of the meta‐analysis for the effect of exercise on the level of p16INK4a in human studies. A: association of habitual exercise and the level of p16INK4a in T lymphocyte with subgroup analysis. (a) healthy participants; (b) patients. B: the effect of exercise training on p16INK4a
On the other hand, diverse effects of exercise on the level of senescent cells were observed in animal studies. p16INK4a and p21Cip1 were conventional markers of senescent cells used in previous animal studies. Specifically, no significant effects of exercise on p16INK4a were found in animal studies with high heterogeneity (−11%, 95% CI = −31%, 10%, I 2 = 99%, p = 0.32) (Figure S1). To identify the source of the high heterogeneity, studies were subgrouped by tissues, including heart, vessel, muscle, fat, skin, brain, liver, pancreas, and kidney. Of these, a senolytic effect of exercise was observed in vessel with a lower heterogeneity (−56%, 95% CI = −72%, −39%, I 2 = 73%, p < 0.001), while no significant effect of exercise was found in muscle (42%, 95% CI = −51%, 136%, I 2 = 59%, p = 0.37). For p21Cip1, a significant senolytic effect of exercise was observed in animal studies, though a high heterogeneity was observed (21%, 95% CI = −32%, −9%, I 2 = 92%, p < 0.001) (Figure 3). To identify the source of the high heterogeneity, studies were subgrouped by healthy states, including healthy and young, aged, and HFD‐induced obesity. Strikingly, no effect of exercise on p21Cip1 was found in a healthy state (−5%, 95% CI = −14%, 4%, I 2 = 45%, p = 0.32). In contrast, a significant senolytic effect of exercise on senescent cells was found in obese (−57%, 95% CI = −69%, −46%, I 2 = 62%, p < 0.001) but not aged animals (p = 0.44). SA‐β‐gal, another marker of senescent cells, was also examined in the animal studies; however, high heterogeneity was observed together with the senolytic effects of exercise, which cannot be reduced by subgroup (−40%, 95% CI = −64%, −16%, I 2 = 99%, p < 0.001) (Figure S2). In summary, senolytic effects of exercise were observed in the vessel (p16INK4a) and obese animals (p21Cip1), but not in the muscle (p16INK4a) or healthy animals (p21Cip1). In this context, the senolytic effects of exercise remain unclear, especially in various organs or tissues and states. More studies with similar outcomes and designs are needed due to the high level of heterogeneity observed in these studies.
FIGURE 3.
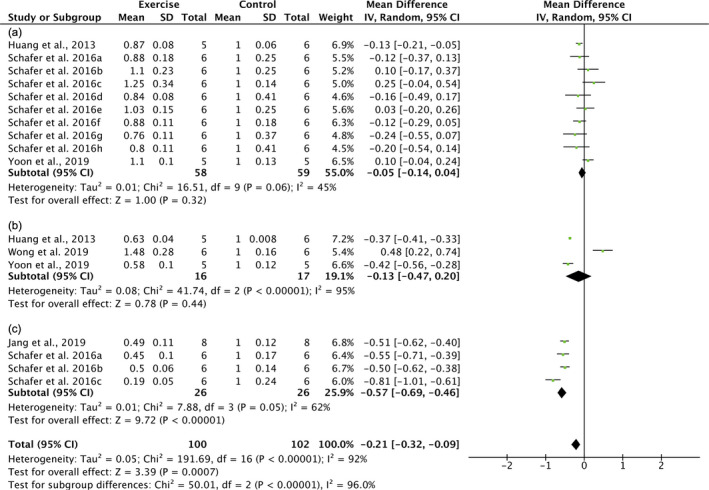
Forest plots of the subgroup meta‐analysis for effect of exercise on the level of p21Cip1 in healthy and young animals. Subgroup by healthy states: (a) health; (b) aging; (c) high‐fat‐diet (HFD)‐induced obesity. Schafer et al. (2016) a‐h (various organs and tissues): a, visceral fat; b, subcutaneous fat; c, liver; d, gastrocnemius (muscle); e, pancreas; f, kidney; g, heart; h, aorta
2.4. Study quality
The quality scores of each study included in this systematic review are shown in the supplemental materials. For human cross‐sectional studies, the score of Newcastle‐Ottawa quality assessment ranged from 6 (satisfactory studies) to 8 (good studies) (Table S2). Moreover, AUB KQ1 risk of bias assessment of human intervention studies reported a low risk of random sequence generation (80%), allocation concealment (60%), incomplete outcome data (100%), selective outcome reporting (100%), and other bias (100%), but not blinding of patients and personnel (20%) and blinding of outcome assessment (20%) (Table S3). On the other hand, the median number of CAMARADES checklist score for animal studies was 4 ranged from 3 to 6 (Table S4). Although the quality assessment of animal studies was provided, the risk of bias remained unclear in these studies due to the lack of sufficient details for assessment that has long been identified as the limitation of animal research.
2.5. Publication bias
Publication bias was examined for outcomes with over nine data/studies by the asymmetry of funnel plots. No major bias was detected in animal studies on p16INK4a (Figure S3) or p21Cip1 (Figure S4), while some asymmetry was observed in the funnel plots, but this was unlikely to be due to publication bias.
3. DISCUSSION
Our systematic review and meta‐analysis were the first to investigate the senolytic effects of exercise on senescent cells in both human and animal studies. Despite the limited number of studies, the evidence on the senolytic effect of exercise on senescent cells in humans and animals appears convincing. A higher level of habitual physical activity and exercise training decreased the level of p16INK4a in humans. In addition, high heterogeneity was observed in animal studies, probably due to study design and sample heterogeneity rather than a real heterogeneity in results. A further subgroup analysis revealed that exercise reduced the level of p21Cip1 in various organs/tissues under high‐fat‐diet (HFD)‐induced obesity. In contrast, no senolytic effect of exercise on the level of p21Cip1 was found in the healthy state. The evidence for other outcomes and conditions is less clear. Thus, more human and animal studies investigating the senolytic effects of exercise on senescent cells are required.
Cellular senescence serves pleiotropic roles in aging, chronic diseases, and cancer (Van Deursen, 2014). In addition to advancing age, unhealthy lifestyles, such as physical inactivity, unhealthy diets, and cigarette smoking, also contribute to the accumulation of senescent cells in the body, especially during aging, and subsequent accelerated aging and chronic diseases (Liu et al., 2009; Song et al., 2010). In this context, the elimination of senescent cells (senolytics) is a potential therapy for aging and chronic diseases, such as obesity, type 2 diabetes, and atherosclerosis (He & Sharpless, 2017). Senolytic medicine has been developed in recent years and some of them are currently undergoing clinical trials, which may require years to be clinically available (Hickson et al., 2019; Justice et al., 2019). Our systematic review and meta‐analysis found evidence that exercise has senolytic properties in healthy humans, where a higher level of habitual physical activity or physically active (Liu et al., 2009; Song et al., 2010) and chronic exercise training (Justice et al., 2018; Rossman et al., 2017; Werner et al., 2009) reduced the level of p16INK4a in various tissues/cells, especially the senescent lymphocytes. These findings supported the hypothesis that exercise can be a senolytic medicine, although there was only very limited evidence demonstrating a negative association between habitual exercise/exercise training and the level of senescent markers. Besides, the optimal or the lowest effective dose of exercise remains unclear (Eijsvogels & Thompson, 2015). Therefore, studies on the different forms (e.g., exercise alone or together with other senolytics such as dasatinib and quercetin) and dosages of exercise on senescent cells in various cells/tissues, populations, and health conditions are urgently needed. Moreover, exercise may have potential anti‐tumor properties (Pedersen et al., 2016), which raises the question of whether exercise is beneficial for cancer patients in the context of senescent cells as an emerging target of cancer (Lee & Schmitt, 2019). Collectively, physical exercise shows promise as a senolytic medicine against aging and multiple diseases, which calls for more preclinical and clinical studies.
On the other hand, animal studies on the senolytic effects of exercise provided more evidence about its effects on various tissues and organs in detail, which cannot be directly investigated in humans. A recent systematic review and meta‐analysis reported that the expression levels of selected markers of senescent cells varied from different tissues during aging (Tuttle et al., 2020). Similarly, we found no significant change of p16INK4a in animal studies with high heterogeneity. However, a significant decline of p16INK4a was detected in the vessels (Kröller‐Schön et al., 2012; Schafer et al., 2016; Werner et al., 2009) but not the muscle (Saito et al., 2020; Schafer et al., 2016; Yoon et al., 2019) after subgroup analysis by tissues. In accordance with a previous study that p16INK4a expressed in various organs and tissues, such as the brain, liver, and spleen, but not in the muscle, during aging (Idda et al., 2020), while senescent cells may be the therapeutic target of cardiovascular diseases (Childs et al., 2018). It suggested that senolytic medicine, including exercise, may only be suitable to target the aging and disease states for certain tissues and organs. The comprehensive cellular senescence‐based effects of exercise on various organs and tissues remain to be investigated; the results of these studies may contribute to the development of more precise exercise therapeutic strategies in the future.
In addition to p16INK4a, p21Cip1 is another conventional marker of senescent cells. Unlike p16INK4a which is involved in the maintenance of senescent phenotype, p21Cip1 is essential for establishing senescence (Stein et al., 1999). Strikingly, we found exercise showed no effect on the level of p21Cip1 in healthy and young animals (Huang et al., 2013; Schafer et al., 2016; Yoon et al., 2019). Since cellular senescence serve vital roles in health maintenance and cancer suppression (He & Sharpless, 2017), a lack of significant senolytic effects of exercise on p21Cip1 in healthy animals may imply potential safety of using exercise as senolytic medicine, since exercise may only “remove” excess senescent cells induced by pathological stressors. On the contrary, in the HFD‐induced obesity, exercise reduced the level of p21Cip1 in obese animals after subgroup analysis by the disease states, including aging and obesity, while more studies are required to understand its effects on senescent cells in aged animals (Jang et al., 2019; Schafer et al., 2016). Cellular senescence is considered to be involved in and serves as the therapeutic target of obesity and metabolic dysfunction (Palmer et al., 2019). However, few disease states have been examined in previous animal studies. Exercise may be a potent and safe senolytic medicine against a specific disease, especially for senescent cells characterized by p21Cip1.
While the senolytic effects of exercise have been identified in the current review, the underlying mechanisms are less well understood. Previous studies have reported that exercise regulated DNA damage (Radák et al., 2002), telomere erosion (Puterman et al., 2010), oxidative stress (Gomez‐Cabrera et al., 2008), in various tissues and cells, are the major drivers of cellular senescence. On the other hand, exercise‐induced autophagy (He, Bassik, et al., 2012; He et al., 2012) and apoptosis (Phaneuf & Leeuwenburgh, 2001), which have been shown to elicit regulating effects on cellular senescence (Kang et al., 2015; Vicencio et al., 2008), might also be involved in the senolytic effects of exercise. A clearer picture of mechanisms underlying senolytic effects of exercise may contribute to the discovery and development of “exercise mimetics” or “exercise pills” against senescent cells (Li & Laher, 2015) and encourage people to engage in more exercise to tackle physical inactivity as one of the leading health problems in the world (Blair, 2009; Kohl et al., 2012) and to develop exercise as an effective treatment for specific diseases.
The present systematic review and meta‐analysis provide an up‐to‐date summary of the senolytic effects of exercise on senescent cells based on both human and animal studies. However, there are some limitations that should be mentioned. The major limitation of this study is the limited number of studies included in this review, though the effects of exercise on senescent cells have been studied for over a decade. In addition, the relatively high heterogeneity was observed among studies due to the complexity of research on cellular senescence, such as the types of cells/tissues and markers of senescent cells. Besides, the search strategy was limited to the articles written in English, which may lead to a language bias and a potential loss of studies met other items of inclusion criteria.
The findings of this systematic review and meta‐analysis provided some evidence that exercise may be a senolytic medicine for p16INK4a‐positive senescent cells in humans and for p21Cip1‐positive senescent cells in obese but not healthy animals. Future studies should examine the optimal form and dosage of exercise, targeted cells/tissues, different disease states, and the underlying cellular mechanisms in humans and animals. A greater understanding of the senolytic effects of exercise can lead to significant clinical and public health impact.
4. METHODS
4.1. Search strategy and data sources
A systematic search of the literature was conducted on May 20, 2020, in three databases: MEDLINE (OVID), EMBASE (OVID), and Web of Science using the Medical Subject Headings (MeSH) terms “exercise” and “cellular senescence” without date limit (a full list of search items used in systemic search is listed in Table S1). Articles were imported into Mendeley reference management software (Elsevier, USA), and the duplicates were identified and removed. Titles and abstracts of all identified records were independently screened by two reviewers to exclude irrelevant articles. The specific inclusion criteria were as follows: (a) original study; (b) peer‐reviewed article; (c) articles written in English; (d) full text available; (e) animal or human studies; (f) “senescence” or “senescent” means “cellular senescence”; (g) “exercise” means physical exercise. Subsequently, full text of the remaining articles was retrieved and reviewed by the same independent reviewers with the following additional inclusion criteria: (a) markers of senescent cells (p16INK4a, p21Cip1, SA‐β‐Gal, and lipofuscin) were reported; (b) exercise as a sole intervention/contributing factor; (c) for immunosenescence, articles were included if cellular senescence in immune cells was reported.
4.2. Study quality assessment
The quality of included studies was assessed independently by two reviewers using (a) Newcastle‐Ottawa quality assessment scale for cohort studies, (b) AUB KQ1 risk of bias assessment, and (c) collaborative approach to meta‐analysis and review of animal data from experimental studies (CAMARADES) checklist (Anon n.d.) for (a) habitual physical activity studies in humans, (b) exercise training studies in humans, and (c) animal studies, respectively. The information of each article was then summarized after independent assessment and the disagreement was resolved by discussion.
4.3. Extraction of data
Data were extracted independently by the same reviewers. Any disagreement was resolved by discussion or a third reviewer. The following study characteristics were extracted onto a pre‐designed data collection form: author, publication year, participant or animal characteristics (e.g., age, health and exercise status, type of animal), sample size, types of exercise, physical activity level/training protocol, types of organ, tissue or cell, markers of senescent cells and their responses to exercise, senolytic effect (yes, no, or opposite). For analysis purposes, means, standard deviation, and sample size were collected for each study. If the data were reported in graphical form, means and standard deviation were extracted using WebPlotDigitizer (Rohatgi, 2011). Studies were excluded if the mean, standard deviation or sample size was not available or equal to 0. If the sample size was provided as a range, we utilized the smallest value. To examine the effects of exercise in different markers of senescent cells, these data were extracted separately. Moreover, to determine whether the senolytic effects of exercise were specific to certain disease state, data from healthy/young samples and disease /old samples were extracted separately as well. Therefore, the total number of data points included in the meta‐analysis was greater than the number of articles.
4.4. Data analysis
For all meta‐analyses, review manager (RevMan) version 5.3 (Copenhagen: The Nordic Cochrane Center) was used where at least two studies of similar study design had reported the same outcomes (i.e., markers of senescent cells). For each study, the mean differences based on the changes from control to exercise group, or from baseline to post‐exercise, were calculated and pooled using the random‐effects model. Correlation coefficient (r) was used to calculate the effect size after transforming to Fisher's z in meta‐analysis Fisher's z was then transformed back to r for presentation. Heterogeneity was determined using Cochran's chi‐square test and Higgins's I 2 test while publication bias was explored using funnel plots. To assess the specific senolytic effects of exercise on senescent cells in animal studies, we pre‐defined the following subgroups for analyses: markers of senescent cells, healthy and young/disease or old, and organ/tissue. Sensitivity analysis was performed by removing the lowest quality study among included studies from the analysis.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
XKC, RCC, ACM, and JSK contributed to the conception and design of this study. XKC, ZNY, and KMH contributed to literature searching and eligible study screening. XKC and ZNY contributed to the data extraction and analysis. XKC, ZNY, KMH, GTW, RCC, ACM, and JSK contribute to the manuscript preparation and reviewed the final version.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
XKC is supported by a postgraduate studentship by the Graduate School, The University of Hong Kong. The study is partially supported by a research grant provided by the Department of Medicine.
Contributor Information
Joseph Shiu‐Kwong Kwan, Email: joseph.kwan@nhs.net.
Alvin Chun‐Hang Ma, Email: alvin.ma@polyu.edu.hk.
Raymond Chuen‐Chung Chang, Email: rccchang@hku.hk.
DATA AVAILABILITY STATEMENT
We believe this systematic review is exempt under the rule of data availability.
REFERENCES
- Anon Collaborative Approach to Meta‐Analysis and Review of Animal Data from Experimental Studies . Retrieved from http://www.dcn.ed.ac.uk/camarades/default.htm [Accessed July 21, 2020].
- Baker, D. J. , Wijshake, T. , Tchkonia, T. , LeBrasseur, N. K. , Childs, B. G. , Van De Sluis, B. , Kirkland, J. L. , & van Deursen, J. M. (2011). Clearance of p16 Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature, 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, C. , Yang, Z. , Li, Q. , Cai, Q. , Li, H. , & Shu, B. (2020). Aerobic endurance exercise ameliorates renal vascular sclerosis in aged mice by regulating PI3K/AKT/mTOR signaling pathway. DNA and Cell Biology, 39, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Porath, I. , & Weinberg, R. A. (2005). The signals and pathways activating cellular senescence. International Journal of Biochemistry & Cell Biology, 37, 961–976. [DOI] [PubMed] [Google Scholar]
- Blair, S. N. (2009). Physical inactivity: the biggest public health problem of the 21st century. British Journal of Sports Medicine, 43, 1–2. [PubMed] [Google Scholar]
- Booth, F. W. , Roberts, C. K. , & Laye, M. J. (2011). Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology, 2, 1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. , Zhou, H. , Zhu, Y. , Sun, Q. , Ji, Y. , Xue, A. , Wang, Y. , Chen, W. , Yu, X. , & Wang, L. (2020). Elimination of senescent cells by β‐galactosidase‐targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Research, 30, 574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. , Wang, Y. , Shao, L. , Laberge, R.‐M. , Demaria, M. , Campisi, J. , Janakiraman, K. , Sharpless, N. E. , Ding, S. , & Feng, W. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs, B. G. , Durik, M. , Baker, D. J. , & van Deursen, J. M. (2015). Cellular senescence in aging and age‐related disease: From mechanisms to therapy. Nature Medicine, 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs, B. G. , Li, H. , & Van Deursen, J. M. (2018). Senescent cells: A therapeutic target for cardiovascular disease. Journal of Clinical Investigation, 128, 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado, M. , Blasco, M. A. , & Serrano, M. (2007). Cellular senescence in cancer and aging. Cell, 130, 223–233. [DOI] [PubMed] [Google Scholar]
- Eijsvogels, T. M. H. , & Thompson, P. D. (2015). Exercise is medicine: at any dose? JAMA, 314, 1915–1916. [DOI] [PubMed] [Google Scholar]
- Fan, J. , Yang, X. , Li, J. , Shu, Z. , Dai, J. , Liu, X. , Li, B. , Jia, S. , Kou, X. , Yang, Y. , & Chen, N. (2017). Spermidine coupled with exercise rescues skeletal muscle atrophy from D‐gal‐induced aging rats through enhanced autophagy and reduced apoptosis via AMPK‐FOXO3a signal pathway. Oncotarget, 8, 17475–17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Cabrera, M.‐C. , Domenech, E. , & Viña, J. (2008). Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radical Biology and Medicine, 44, 126–131. [DOI] [PubMed] [Google Scholar]
- Guthold, R. , Stevens, G. A. , Riley, L. M. , & Bull, F. C. (2018). Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population‐based surveys with 1·9 million participants. The Lancet Global Health, 6, e1077–e1086. [DOI] [PubMed] [Google Scholar]
- He, C. , Bassik, M. C. , Moresi, V. , Sun, K. , Wei, Y. , Zou, Z. , An, Z. , Loh, J. , Fisher, J. , Sun, Q. , Korsmeyer, S. , Packer, M. , May, H. I. , Hill, J. A. , Virgin, H. W. , Gilpin, C. , Xiao, G. , Bassel‐Duby, R. , Scherer, P. E. , & Levine, B. (2012). Exercise‐induced BCL2‐regulated autophagy is required for muscle glucose homeostasis. Nature, 481, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C. , Sumpter Rhea, J. , & Levine, B. (2012). Exercise induces autophagy in peripheral tissues and in the brain. Autophagy, 8, 1548–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S. , & Sharpless, N. E. (2017). Senescence in health and disease. Cell, 169, 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson, L. T. J. , Langhi Prata, L. G. P. , Bobart, S. A. , Evans, T. K. , Giorgadze, N. , Hashmi, S. K. , Herrmann, S. M. , Jensen, M. D. , Jia, Q. , Jordan, K. L. , Kellogg, T. A. , Khosla, S. , Koerber, D. M. , Lagnado, A. B. , Lawson, D. K. , LeBrasseur, N. K. , Lerman, L. O. , McDonald, K. M. , McKenzie, T. J. , … Kirkland, J. L. (2019). Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 47, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. C. , Chiang, W. D. , Huang, W. C. , Huang, C. Y. , Hsu, M. C. , & Lin, W. T. (2013) Hepatoprotective effects of swimming exercise against D‐galactose‐induced senescence rat model. Evidence‐Based Complementary and Alternative Medicine, 2013 (no p.) Retrieved from http://easyaccess.lib.cuhk.edu.hk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed14&AN=369321922275431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idda, M. L. , McClusky, W. G. , Lodde, V. , Munk, R. , Abdelmohsen, K. , Rossi, M. , & Gorospe, M. (2020). Survey of senescent cell markers with age in human tissues. Aging (Albany NY), 12, 4052–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, Y. , Kwon, I. , Cosio‐Lima, L. , Wirth, C. , Vinci, D. M. , & Lee, Y. (2019). Endurance exercise prevents metabolic distress‐induced senescence in the hippocampus. Medicine and Science in Sports and Exercise, 51, 2012–2024. [DOI] [PubMed] [Google Scholar]
- Jeon, O. H. , Kim, C. , Laberge, R.‐M. , Demaria, M. , Rathod, S. , Vasserot, A. P. , Chung, J. W. , Kim, D. H. , Poon, Y. , & David, N. (2017). Local clearance of senescent cells attenuates the development of post‐traumatic osteoarthritis and creates a pro‐regenerative environment. Nature Medicine, 23, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, J. N. , Gregory, H. , Tchkonia, T. , Lebrasseur, N. K. , Kirkland, J. L. , Kritchevsky, S. B. , & Nicklas, B. J. (2018). Cellular senescence biomarker p16 INK4a + cell burden in thigh adipose is associated with poor physical function in older women. The Journals of Gerontology: Series A, 73, 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, J. N. , Nambiar, A. M. , Tchkonia, T. , LeBrasseur, N. K. , Pascual, R. , Hashmi, S. K. , Prata, L. , Masternak, M. M. , Kritchevsky, S. B. , Musi, N. , & Kirkland, J. L. (2019). Senolytics in idiopathic pulmonary fibrosis: results from a first‐in‐human, open‐label, pilot study. EBioMedicine, 40, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, C. , Xu, Q. , Martin, T. D. , Li, M. Z. , Demaria, M. , Aron, L. , Lu, T. , Yankner, B. A. , Campisi, J. , & Elledge, S. J. (2015). The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science, 349, 6255 10.1126/science.aaa5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland, J. L. , Tchkonia, T. , Zhu, Y. , Niedernhofer, L. J. , & Robbins, P. D. (2017). The clinical potential of senolytic drugs. Journal of the American Geriatrics Society, 65, 2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl, 3rd., H. W. , Craig, C,. L. , Lambert, E. V. , Inoue, S. , Alkandari, J. R. , Leetongin, G. , Kahlmeier, S. , & Group LPASW (2012). The pandemic of physical inactivity: global action for public health. Lancet, 380, 294–305. [DOI] [PubMed] [Google Scholar]
- Kröller‐Schön, S. , Jansen, T. , Hauptmann, F. , Schüler, A. , Heeren, T. , Hausding, M. , Oelze, M. , Viollet, B. , Keaney, J. F. , Wenzel, P. , Daiber, A. , Münzel, T. , & Schulz, E. (2012). α1AMP‐activated protein kinase mediates vascular protective effects of exercise. Arteriosclerosis, Thrombosis, and Vascular Biology, 32, 1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , & Schmitt, C. A. (2019). The dynamic nature of senescence in cancer. Nature Cell Biology, 21, 94–101. [DOI] [PubMed] [Google Scholar]
- Li, S. , & Laher, I. (2015). Exercise pills: At the starting line. Trends in Pharmacological Sciences, 36, 906–917. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Liu, W. , Liu, P. , Liu, X. , Song, X. , Hayashi, T. , Onodera, S. , & Ikejima, T. (2019). Silibinin alleviates the learning and memory defects in overtrained rats accompanying reduced neuronal apoptosis and senescence. Neurochemical Research, 44, 1818–1829. Retrieved from http://easyaccess.lib.cuhk.edu.hk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emexa&AN=627809668 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Sanoff, H. K. , Cho, H. , Burd, C. E. , Torrice, C. , Ibrahim, J. G. , Thomas, N. E. , & Sharpless, N. E. (2009). Expression of p16INK4a in peripheral blood T‐cells is a biomarker of human aging. Aging Cell, 8, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz‐Espín, D. , & Serrano, M. (2014). Cellular senescence: From physiology to pathology. Nature Reviews Molecular Cell Biology, 15, 482–496. [DOI] [PubMed] [Google Scholar]
- Palmer, A. K. , Xu, M. , Zhu, Y. I. , Pirtskhalava, T. , Weivoda, M. M. , Hachfeld, C. M. , Prata, L. G. , Dijk, T. H. , Verkade, E. , Casaclang‐Verzosa, G. , Johnson, K. O. , Cubro, H. , Doornebal, E. J. , Ogrodnik, M. , Jurk, D. , Jensen, M. D. , Chini, E. N. , Miller, J. D. , Matveyenko, A. , … Kirkland, J. L. (2019). Targeting senescent cells alleviates obesity‐induced metabolic dysfunction. Aging Cell, 18, e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, P. , Dong, Q. , Wang, D. , Chang, J. , Wiley, C. , Demaria, M. , Lee, J. , Kang, J. , Niedernhofer, L. J. , & Robbins, P. D. (2019). Systemic clearance of p16INK4a‐positive senescent cells mitigates age‐associated intervertebral disc degeneration. Aging Cell, 18, e12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, L. , Idorn, M. , Olofsson, G. H. , Lauenborg, B. , Nookaew, I. , Hansen, R. H. , Johannesen, H. H. , Becker, J. C. , Pedersen, K. S. , Dethlefsen, C. , Nielsen, J. , Gehl, J. , Pedersen, B. K. , thor Straten, P. , & Hojman, P. (2016). Voluntary running suppresses tumor growth through epinephrine‐ and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metabolism, 23, 554–562. Retrieved from http://www.sciencedirect.com/science/article/pii/S1550413116300031 [DOI] [PubMed] [Google Scholar]
- Phaneuf, S. , & Leeuwenburgh, C. (2001). Apoptosis and exercise. Medicine and Science in Sports and Exercise, 33, 393–396. [DOI] [PubMed] [Google Scholar]
- Pustavoitau, A. , Barodka, V. , Sharpless, N. E. , Torrice, C. , Nyhan, D. , Berkowitz, D. E. , Shah, A. S. , Roche, K. J. B. , & Walston, J. D. (2016). Role of senescence marker p16INK4a measured in peripheral blood T‐lymphocytes in predicting length of hospital stay after coronary artery bypass surgery in older adults. Experimental Gerontology, 74, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman, E. , Lin, J. , Blackburn, E. , O'Donovan, A. , Adler, N. , & Epel, E. (2010). The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One, 5, e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radák, Z. , Naito, H. , Kaneko, T. , Tahara, S. , Nakamoto, H. , Takahashi, R. , Cardozo‐Pelaez, F. , & Goto, S. (2002). Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflügers Archiv European Journal of Physiology, 445, 273–278. [DOI] [PubMed] [Google Scholar]
- Rodier, F. , & Campisi, J. (2011). Four faces of cellular senescence Four faces of senescence. Journal of Cell Biology, 192, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi, A. (2011). WebPlotDigitizer. Retrieved from http://arohatgi.info.WebPlotDigitizer/app [Google Scholar]
- Rossman, M. J. , Kaplon, R. E. , Hill, S. D. , McNamara, M. N. , Santos‐Parker, J. R. , Pierce, G. L. , Seals, D. R. , & Donato, A. J. (2017). Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function. American Journal of Physiology‐Heart and Circulatory Physiology, 313, H890–H895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, Y. , Chikenji, T. S. , Matsumura, T. , Nakano, M. , & Fujimiya, M. (2020). Exercise enhances skeletal muscle regeneration by promoting senescence in fibro‐adipogenic progenitors. Nature Communications, 11 Retrieved from http://easyaccess.lib.cuhk.edu.hk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emexb&AN=2004251579 889–. 10.1038/s41467-020-14734-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, M. J. , White, T. A. , Evans, G. , Tonne, J. M. , Verzosa, G. C. , Stout, M. B. , Mazula, D. L. , Palmer, A. K. , Baker, D. J. , Jensen, M. D. , Torbenson, M. S. , Miller, J. D. , Ikeda, Y. , Tchkonia, T. , van Deursen, J. M. , Kirkland, J. L. , & LeBrasseur, N. K. (2016). Exercise prevents diet‐induced cellular senescence in adipose tissue. Diabetes, 65, 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, M. J. , White, T. A. , Iijima, K. , Haak, A. J. , Ligresti, G. , Atkinson, E. J. , Oberg, A. L. , Birch, J. , Salmonowicz, H. , & Zhu, Y. (2017). Cellular senescence mediates fibrotic pulmonary disease. Nature Communications, 8, 14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Z. F. , von Figura, G. , Liu, Y. , Kraus, J. M. , Torrice, C. , Dillon, P. , Rudolph‐Watabe, M. , Ju, Z. Y. , Kestler, H. A. , Sanoff, H. , & Rudolph, K. L. (2010). Lifestyle impacts on the aging‐associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell, 9, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, G. H. , Drullinger, L. F. , Soulard, A. , & Dulić, V. (1999). Differential roles for cyclin‐dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Molecular and Cellular Biology, 19, 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia, T. , Morbeck, D. E. , Von Zglinicki, T. , Van Deursen, J. , Lustgarten, J. , Scrable, H. , Khosla, S. , Jensen, M. D. , & Kirkland, J. L. (2010). Fat tissue, aging, and cellular senescence. Aging Cell, 9, 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankov, D. , Liu, Y. , Sanoff, H. K. , Sharpless, N. E. , & Elston, T. C. (2009). A quantitative model for age‐dependent expression of the p16INK4a tumor suppressor. Proceedings of the National Academy of Sciences, 106, 16562–16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle, C. S. L. , Waaijer, M. E. C. , Slee‐Valentijn, M. S. , Stijnen, T. , Westendorp, R. , & Maier, A. B. (2020). Cellular senescence and chronological age in various human tissues: A systematic review and meta‐analysis. Aging Cell, 19, e13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deursen, J. M. (2014). The role of senescent cells in ageing. Nature, 509, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicencio, J. M. , Galluzzi, L. , Tajeddine, N. , Ortiz, C. , Criollo, A. , Tasdemir, E. , Morselli, E. , Ben Younes, A. , Maiuri, M. C. , Lavandero, S. , & Kroemer, G. (2008). Senescence, apoptosis or autophagy? Gerontology, 54, 92–99. [DOI] [PubMed] [Google Scholar]
- Werner, C. , Furster, T. , Widmann, T. , Poss, J. , Roggia, C. , Hanhoun, M. , Scharhag, J. , Buchner, N. , Meyer, T. , Kindermann, W. , Haendeler, J. , Bohm, M. , & Laufs, U. (2009). Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation, 120, 2438–2447. Retrieved from http://easyaccess.lib.cuhk.edu.hk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed11&AN=358044064 [DOI] [PubMed] [Google Scholar]
- Werner, C. , Hanhoun, M. , Widmann, T. , Kazakov, A. , Semenov, A. , Pöss, J. , Bauersachs, J. , Thum, T. , Pfreundschuh, M. , Müller, P. , Haendeler, J. , Böhm, M. , & Laufs, U. (2008) Effects of physical exercise on myocardial telomere‐regulating proteins, survival pathways, and apoptosis. Journal of the American College of Cardiology, 52(6), 470–482. 10.1016/j.jacc.2008.04.034 [DOI] [PubMed] [Google Scholar]
- Wong, W. , Crane, E. D. , Kuo, Y. , Kim, A. , & Crane, J. D. (2019). The exercise cytokine interleukin‐15 rescues slow wound healing in aged mice. Journal of Biological Chemistry, 294, 20024–20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Saovieng, S. , Cheng, I. S. , Liu, T. , Hong, S. , Lin, C. Y. , Su, I. C. , Huang, C. Y. , & Kuo, C. H. (2019). Ginsenoside Rg1 supplementation clears senescence‐associated beta‐galactosidase in exercising human skeletal muscle. Journal of Ginseng Research, 43, 580–588. Retrieved from http://easyaccess.lib.cuhk.edu.hk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emexb&AN=2000981473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. , Jiao, Y. , Wei, B. , Yang, Z. , Wu, J.‐F. , Jensen, J. , Jean, W.‐H. , Huang, C.‐Y. , & Kuo, C.‐H. (2018). Aged cells in human skeletal muscle after resistance exercise. Aging (Albany NY), 10, 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, K. J. , Zhang, D. , Kim, S. J. , Lee, M. C. , & Moon, H. Y. (2019) Exercise‐induced AMPK activation is involved in delay of skeletal muscle senescence. Biochemical and Biophysical Research Communications, 512(3), 604–610. 10.1016/j.bbrc.2019.03.086 [DOI] [PubMed] [Google Scholar]
- Yosef, R. , Pilpel, N. , Tokarsky‐Amiel, R. , Biran, A. , Ovadya, Y. , Cohen, S. , Vadai, E. , Dassa, L. , Shahar, E. , & Condiotti, R. (2016). Directed elimination of senescent cells by inhibition of BCL‐W and BCL‐XL. Nature Communications, 7, 11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Yuan, T. , & Wang, J. H. C. (2016). Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget, 7, 8498–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Doornebal, E. J. , Pirtskhalava, T. , Giorgadze, N. , Wentworth, M. , Fuhrmann‐Stroissnigg, H. , Niedernhofer, L. J. , Robbins, P. D. , Tchkonia, T. , & Kirkland, J. L. (2017). New agents that target senescent cells: the flavone, fisetin, and the BCL‐XL inhibitors, A1331852 and A1155463. Aging (Albany NY), 9, 955–963. 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. I. , Tchkonia, T. , Pirtskhalava, T. , Gower, A. C. , Ding, H. , Giorgadze, N. , Palmer, A. K. , Ikeno, Y. , Hubbard, G. B. , Lenburg, M. , O'Hara, S. P. , LaRusso, N. F. , Miller, J. D. , Roos, C. M. , Verzosa, G. C. , LeBrasseur, N. K. , Wren, J. D. , Farr, J. N. , Khosla, S. , … Kirkland, J. L. (2015). The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
We believe this systematic review is exempt under the rule of data availability.


