Abstract
Introduction
Vitamin D insufficiency is much more common among patients with cancer than the general population. Previous meta-analyses of controlled trials showed an approximately 15% reduction of cancer mortality by vitamin D supplementation compared with placebo or no treatment in the general population.
On top of updating the latest systematic review on vitamin D supplementation and cancer mortality in the general population, we aim to conduct the first meta-analyses of trials on vitamin D3 supplementation and cancer-specific and overall survival of patients with cancer. Besides, we will conduct for the first time subgroup analyses based on individual patient data collected from randomised controlled trials.
Methods and analysis
A systematic review and individual patient data meta-analysis will be performed on randomised placebo-controlled trials with a vitamin D3 intervention. All databases are searched from inception without time restriction. The addressed outcomes are cancer mortality in the general population as well as cancer-specific and overall survival of patients with cancer. The quality appraisal of the studies will be evaluated by the Cochrane risk-of-bias tool for randomised trials. Trial results will be reanalysed using adjusted and unadjusted Cox proportional hazard regression models and meta-analyses are planned. Cochran’s Q-Test and the I2 index will be used to statistically assess the level of heterogeneity, while sensitivity and subgroup analyses serve to identify potential causes of heterogeneity. Subgroup analyses will be conducted for vitamin D3 dosing, follow-up time, age, sex, obesity, vitamin D deficiency/insufficiency, history of cancer and compliance. Publication bias will be assessed by funnel plots and Egger’s test.
Ethics and dissemination
Ethical approval is not required since no human beings are involved in this systematic review. Results will be published in a peer-reviewed journal with open access. They will be presented at conferences and sent to patient advocacy groups and German oncological rehabilitation centres.
PROSPERO registration number
CRD42020185566
Keywords: nutritional support, oncology, nutrition & dietetics, public health, protocols & guidelines, epidemiology
Strengths and limitations of this study.
First meta-analysis on vitamin D3 supplementation and cancer survival as well as first individual patient data meta-analysis on this research topic.
Results of subgroup analyses based on individual patient data allow fundamental insights for personalised medicine and may be used as guidance for future clinical trials targeting patients with cancer that presumably profit most from vitamin D supplementation.
Conduction of the systematic review according to this protocol and a thorough assessment of study quality, sources of heterogeneity and bias in meta-analyses minimise the risk of bias and will gather reproducible results.
Number of studies with eligible data for subgroup analyses may be limited.
Introduction
Background
The global cancer burden is estimated to have risen to 18.1 million new cases and 9.6 million deaths in 2018.1 There is accumulating evidence from epidemiological studies that a low vitamin D status goes along with increased risks of several types of cancer. Meta-analyses of observational studies reported increased risks of lung cancer, colorectal cancer, breast cancer, bladder carcinoma and lymphoma in subjects with low 25-hydroxyvitamin D (25(OH)D) serum concentrations.2–5 Furthermore, epidemiological studies have shown that low serum levels of 25(OH)D, the acknowledged best biomarker to measure vitamin D status, were strongly associated with substantially increased cancer mortality.6 For example, in a German population-based cohort study of older adults, the risk to die of cancer was increased by 42% in study participants with vitamin D deficiency (defined as 25(OH)D <30 nmol/L) compared with individuals with sufficient 25(OH)D levels >50 nmol/L (HR and 95% CIs: 1.42 (1.08 to 1.87)).7
The molecular links between vitamin D and carcinogenesis and progression have been previously described.8 In brief, genomic mechanisms of the active hormone 1,25(OH)2D impact signalling pathways that regulate cell proliferation, differentiation and cell survival. 1,25(OH)2D may primarily act as an antiproliferative agent in many tissues and may slow down malignant cellular growth. Thus, there is biological plausibility that a sufficient vitamin D supply is especially essential for a good cancer prognosis. A causal relationship of low 25(OH)D levels and cancer mortality was furthermore supported by a Mendelian randomisation study conducted within three large cohorts from Denmark.9
Several randomised trials with vitamin D supplementation have been conducted with mostly the aim to improve skeletal outcomes at older ages. Cancer mortality was a secondary outcome in all trials and therefore the trials were not specifically designed for this outcome.10 Despite strong heterogeneity in study populations, intervention schemes and other important design aspects, three out of four meta-analyses demonstrated a statistically significant reduction in cancer mortality.11–15
However, most trials have not been restricted to patients who were vitamin D deficient.10 The latter is important because the association of 25(OH)D levels and adverse health outcomes is not linear.6 Neglecting this dose–response relationship by treating subjects without hypovitaminosis D is expected to have led to a substantial underestimation of the potential efficacy of vitamin D supplementation in previous clinical trials.16 Therefore, there is a need for a systematic review that reanalyses individual patient data (IPD) from previous trials restricted to subjects with vitamin D insufficiency (25(OH)D <50 nmol/L) or deficiency (25(OH)D <30 nmol/L).
Another important reason to reanalyse the previous trial data is that most studies were not restricted to patients with cancer. Vitamin D deficiency or insufficiency are much more common among patients with cancer than among the general population. In a study with 2912 patients with colorectal cancer, vitamin D deficiency (25(OH)D levels <30 nmol/L) was found among 59% of patients with colorectal cancer during or shortly after first-line treatment, and, in agreement with previous evidence, low 25(OH)D levels were strongly associated with poorer survival.17 18 Systematic reviews of observational studies on 25(OH)D levels and cancer prognosis concluded that sufficient 25(OH)D levels are associated with a better prognosis of breast and colorectal cancer, whereas there are too few studies for other cancer sites up to date to draw conclusions.17 19
Further important potential effect modifiers that deserve close investigation are obesity and compliance. People with low compliance and/or obesity, who may need higher vitamin D doses because vitamin D is stored in adipocytes, might have attenuated the overall treatment effect in the trials.20
Objective
The objective of our systematic review is to assess the efficacy of vitamin D3 supplementation on cancer mortality in the general population and the prognosis of patients with cancer with special attention to potential effect modifiers, including baseline 25(OH)D levels, cancer at baseline, body mass index (BMI) and compliance.
The main outcomes include ‘cancer mortality in the general population’, ‘cancer-specific survival of patients with cancer’ and ‘overall survival of patients with cancer’. These outcomes are universally used in cancer studies and do not need further refinement during the review.
In a first step, we intend to update the previous systematic reviews on vitamin D supplementation and cancer mortality in the general population by including newly published trials and unpublished data from trials with outcome data on cancer incidence or all-cause mortality by asking the authors for data on cancer mortality. Second, we will obtain data for an IPD meta-analysis. Third, we will conduct IPD meta-analyses on vitamin D3 supplementation and overall and cancer-specific survival among patients with cancer. Fourth, we will conduct subgroup analyses to explore sources of heterogeneity and to identify effect modifiers. The timetable for the review is shown in table 1.
Table 1.
Proposed timetable for conducting the review
| Step | Time frame for completion |
| Literature search, abstract and full-text selection | 2.5 months |
| Data extraction and individual patient data acquisition | 2.5 months |
| Quality appraisal | 2 months |
| Data analysis and meta-analysis | 3 months |
| Writing of manuscript | 2 months |
| Total | 12 months |
Methods and analysis
Study selection criteria/eligibility criteria
We will follow a two-step approach for the study selection: first, all trials will be selected that could potentially have published or unpublished data on the research topic. All authors of trials with potentially unpublished data on cancer mortality/survival will be contacted to provide data. In the second step, only trials with eligible data for a meta-analysis will be included.
Step 1: inclusion criteria for trials
Participants
We will include studies investigating the adult population (18 years or older). We will also include studies conducted solely with cancer populations or patients with other conditions (eg, studies that recruited only patients with type 2 diabetes).
Interventions
We will focus on trials that used vitamin D3 in any dose and any regimen (eg, daily/weekly/monthly intake) as the intervention. However, the minimum time of the intervention shall be 6 months to exclude studies with one-time bolus interventions or very short intervention periods. The first reason is that cancer mortality is highly unlikely to be influenced by very brief intervention periods. The second reason is that after initiating daily, weekly or monthly supplementation schedules, it takes 3 to 6 months for 25(OH)D levels to reach homeostasis.
Besides, we will also include studies using vitamin D3 bioequivalent substances such as calcitriol, being the active vitamin D hormone 1,25(OH)2D, as well as alfacalcidol and calcifediol, which are both equally metabolised to 1,25(OH)2D.
We will exclude studies with vitamin D2 supplementation since the Cochrane review of Bjelakovic et al and other recent data showed clearly no efficacy on mortality.10 11 15 21 Cosupplementation with calcium or other dietary supplements in the intervention arm will not be an exclusion criterion. A sensitivity analysis will elucidate whether the inclusion of these studies had an impact on the overall effect estimate of the meta-analysis.
Comparators
We will include only studies, which used placebo as the comparator.
Outcomes
To be eligible for inclusion in a meta-analysis trials need to have assessed the outcome of cancer mortality, cancer survival or cancer-specific survival. In an intermediate step of the systematic review, we will also record studies with the outcomes of cancer incidence or all-cause mortality and contact the authors if they have data for the outcomes needed for the planned meta-analyses. The definitions of all outcomes are shown in table 2.
Table 2.
Definition of outcomes
| Outcome | Definition |
| All-cause mortality | Rate of deaths during a specific time period in a population at risk |
| Cancer mortality | Rate of cancer deaths during a specific time period in a population at risk |
| Cancer incidence | Rate of newly diagnosed cancer cases during a specific time period in a population at risk |
| Overall cancer survival | Proportion of patients from a cancer population at risk alive at some point subsequent to the diagnosis of their cancer |
| Cancer-specific survival | Proportion of patients from a cancer population at risk who did not die of cancer at some point subsequent to the diagnosis of their cancer |
Study design
We will include randomised controlled trials (RCTs) in which, analogous to the intervention period, the follow-up period is at least 6 months. The follow-up time should not be longer than the time under treatment. We will focus on parallel-group designs and exclude single-arm studies. We will further exclude all types of cohort studies and case–control studies as well as the following types of records: reviews, dissertations, theses, editorials, study protocol, clinical guidelines, commentaries and letters.
Setting
There will be no restrictions by type of setting.
Minimum sample size
The studies need to have at least one cancer death in the verum and placebo group.
Geographical location
No restrictions are defined regarding the geographical location.
Step 2: inclusion criteria for pooling in meta-analysis
Studies will be included for pooling in the meta-analysis if the risk ratio and 95% CI for at least one outcome of interest (cancer mortality in the general population, cancer-specific survival of patients with cancer or overall survival of patients with cancer) were either reported in the publication or could be obtained from authors or individual participant data. In the case of double publication from the same trial, only the publication with the largest amount of information, for example, the longest follow-up, will be included in the meta-analysis.
Information sources and search strategy
The search strategy will be elaborated by SK, BS and A Heppert. Mrs Heppert is a specialist for systematic bibliographic searches at the Central Library of the German Cancer Research Centre and is not otherwise associated with the project. Finally, it will be peer-reviewed by HB and carried out by SK.
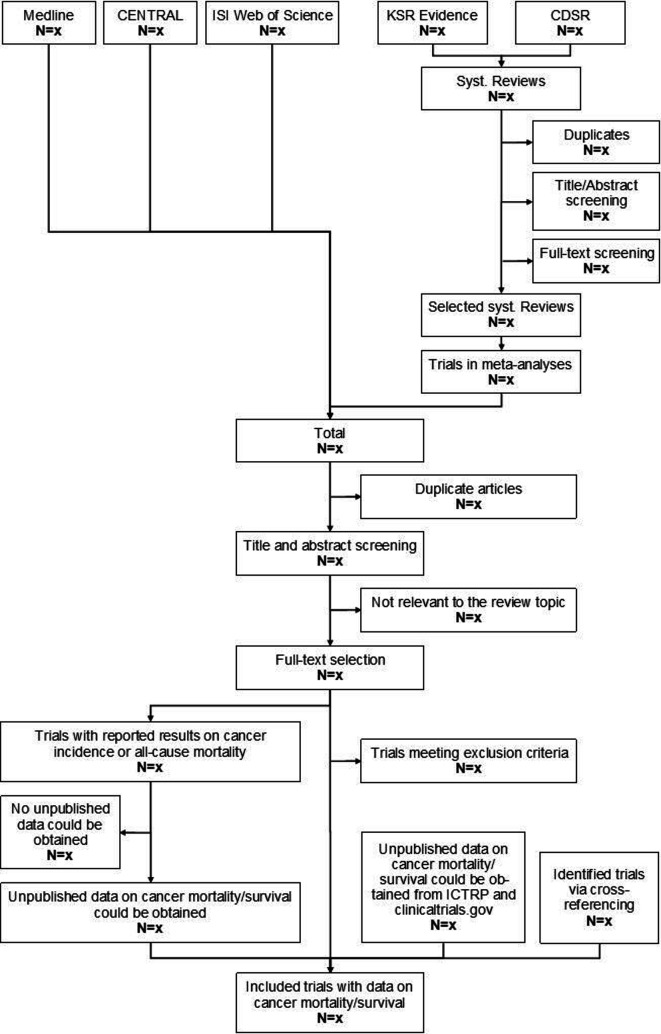
The bibliographic databases MEDLINE (Pubmed interface), ISI Web of Science (WoS; Clarivate Analytics interface) and the Cochrane Central Register of Controlled Trials (CENTRAL; OVID interface) will be searched systematically. We will also carry out a systematic search for previous systematic reviews in the Cochrane Database of Systematic Reviews (CDSR, OVID interface) and KSR Evidence (https://ksevidence.com), which are both specialised search engines for systematic reviews. RCTs included in meta-analyses on the topics vitamin D supplementation and cancer mortality, cancer incidence, all-cause mortality or cancer survival will be extracted and merged with the hits found in the bibliographic database search. The electronic database search will be complemented by searching the WHO’s International Clinical Trials Research Portal (ICTRP) and clinicaltrials.gov to capture results from ongoing or recently completed RCTs that have not been published in scientific journals, yet. We will also scan the reference lists of eligible studies to yield additional trial articles via cross-referencing. A draft of the search strategy is presented in figure 1.
Figure 1.
Draft of the study selection process. CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; ICTRP, International Clinical Trials Research Portal.
We will search in MEDLINE, ISI WoS and CENTRAL for medical subject headings (MeSH), free-text words, synonyms and related search terms for the concepts ‘vitamin D’, ‘mortality’, ‘cancer’, ‘randomized controlled trial’ and ‘placebo’. Besides, standard search terms for RCTs will be used additionally wherever available. No restrictions are planned in the search strategy to prevent overlooking important studies that have not been correctly classified in the respective bibliographic databases. All databases will be searched from the inception of the databases without time restriction. Moreover, we will not limit the search to studies in English as relevant studies might also be published in other languages. The search string for MEDLINE is shown in table 3.
Table 3.
Search string for MEDLINE
| Step | Search string |
| 1 | ‘vitamin d’(tw) OR ‘vitamin D’(MeSH) OR cholecalciferol(MeSH) OR cholecalciferol*(tw) OR calciol(tw) OR hydroxycholecalciferols(MeSH) OR hydroxycholecalciferol*(tw) OR dihydroxycholecalciferol*(tw) OR ‘vitamin d3’(tw) OR ‘vitamin d 3’(tw) OR calcitriol(MeSH) OR calcitriol(tw) OR ‘1-hydroxycholecalciferol’(tw) OR calcifediol(MeSH) OR calcifediol(tw) OR calcidiol(tw) OR alfacalcidol(Supplementary Concept))OR alphacalcidol(tw) OR alfacalcidol(tw) |
| 2 | mortality(tw) OR mortality(MeSH) OR death(MeSH) OR death(tw) OR died(tw) OR dead(tw) OR survival(tw) OR surviv*(tw) OR survival(MeSH) |
| 3 | neoplasms(MeSH) OR neoplas*(tw) OR malignanc*(tw) OR cancer*(tw) OR tumour*(tw) OR tumour*(tw) OR carcinoma*(tw) |
| 4 | (((((((((‘randomized controlled trial’(pt)) OR ‘controlled clinical trial’(pt)) OR randomized(tiab)) OR placebo(tiab)) OR ‘drug therapy’(sh)) OR randomly(tiab)) OR trial(tiab)) OR groups(tiab))) NOT ((animals(mh) NOT humans(mh))) |
| 5 | placebos(MeSH) OR placebo(tw) |
| 6 | 2 OR 3 |
| 7 | 1 AND 4 AND 5 AND 6 |
MeSH, medical subject headings.
A shortened version of the MEDLINE search string will be used to search for systematic reviews in CDSR and KSR Evidence. Only the first three search steps are needed because the study design is ‘systematic review’ and not ‘placebo-controlled RCT’. The search string for CDSR is shown in table 4. The literature search will be updated during the peer-review process of the publication in order to include the most up to date literature.
Table 4.
Search string for the Cochrane database of systematic reviews
| Step | Search string |
| 1 | #1 MeSH descriptor: (Vitamin D) explode all trees |
| #2 MeSH descriptor: (Cholecalciferol) explode all trees | |
| #3 MeSH descriptor: (Calcifediol) explode all trees | |
| #4 MeSH descriptor: (Calcitriol) explode all trees | |
| #5 MeSH descriptor: (Hydroxycholecalciferols) explode all trees | |
| #6 ((‘alfacalcidol’) OR (‘alphacalcidol’) OR (‘hydroxycholecalciferol*’) OR (‘1-hydroxycholecalciferol’) OR (‘hydroxyvitamin* D’) OR (‘calcifediol’) OR (‘calcidiol’) OR (‘calcitriol’) OR (‘dihydroxycholecalciferol*’) OR (‘dihydroxyvitamin d*’) OR (‘vitamin D’) OR (cholecalciferol*) OR (‘vitamin D3’) OR (‘vitamin D 3’) OR (‘calciol’)) (Word variations have been searched) | |
| #7 (‘vitamin d*’):ti, ab, kw (Word variations have been searched) | |
| #8 {OR #1-#7} | |
| 2 | #9 MeSH descriptor: (Mortality) explode all trees |
| #10 MeSH descriptor: (Death) explode all trees #11 MeSH descriptor: (Survival) explode all trees | |
| #12 (‘mortality’ OR ‘dea*’ OR ‘died’ OR ‘survival’ OR ‘surviv*’) (Word variations have been searched) | |
| #13 {OR #9-#12} | |
| 3 | #14 MeSH descriptor: (Neoplasms) explode all trees |
| #15 (carcinoma* OR tumour* OR tumor* OR cancer* OR malignanc* OR neoplas*) (Word variations have been searched) | |
| #16 #14 OR #15 | |
| 4 | #17 #13 OR #16 |
| 5 | #18 #8 AND #17 in Cochrane Reviews (Word variations have been searched) |
MeSH, medical subject headings.
Data collection and management
Study selection and data extraction will be performed in duplicate by two reviewers. Both are blinded to each other’s decision but not to journal titles, study authors or institutions. The screening will be conducted by using the Rayyan QCRI web application (Qatar Computing Research Institute (Data Analytics), Doha, Qatar).22 The software EndNote will be used to store, organise and manage all the references and allow a transparent and reproducible systematic search. To assure validity and high quality of the data, the data extraction will be performed by using standard and predefined data extraction forms (see online supplemental appendix 1). Both reviewers will scan independently the titles and abstracts of studies obtained by the aforementioned search strategy against the eligibility criteria. For those studies that meet the inclusion criteria or that cannot yet be fully excluded, full-text reports will be acquired and screened again towards the inclusion criteria. In the next step, the results of both reviewers will be compared and in cases of disagreement, critical points will be discussed until a consensus is reached. If necessary, we will contact study authors to resolve questions about eligibility. We will document the reasons for excluding trials.
bmjopen-2020-041607supp001.xlsx (15.8KB, xlsx)
After completing the abstract and full-text selection with eligible studies, the two reviewers will extract independently the predefined data (see online supplemental appendix 1). Extracted items will include first author, publication year, country, number of participants, general population or medical condition (including cancer site and stage(s)), sex, mean/median age, race/ethnicity, mean/median BMI, mean/median 25(OH)D levels at baseline, vitamin D3 dosing regimen, duration of vitamin D3 supplementation, compliance, mean/median and maximum follow-up time, number of cancer deaths and effect estimates (including confidence intervals) reported for cancer mortality/cancer survival. Individual patient data for the aforementioned variables will be obtained from all trials with at least 20 cancer deaths (see online supplemental appendix 1). If summary data are not published, they shall be calculated from the obtained data. All authors will be contacted by e-mail with a maximum of three attempts sent at 2-week intervals.
For the meta-analyses on cancer survival and cancer-specific survival, we will ask all authors who conducted trials in the general population to provide IPD for cancer diagnoses in the 5 years prior to baseline and during the trial (including cancer site with ICD code, stage and diagnosis date). The following IPD will be additionally collected: age, sex, BMI, race/ethnicity, baseline 25(OH)D levels, compliance, randomisation group allocation, baseline date, deaths during follow-up with date, cancer deaths with date, censoring dates for survival outcomes and censoring date for patients not dying of cancer (see online supplemental appendix 1). If IPD cannot be shared, the authors of the studies will be asked to conduct the analyses in-house and to provide the summary estimates for the meta-analysis. If trial authors do not collaborate, their study cannot be included in subgroup analyses for which no effect estimates were published but the result from the total trial population will remain included in the main meta-analysis.
Quality assessment
The protocol of the systematic review with all planned statistical analyses has been registered in PROSPERO before data collection to preclude data-driven analyses and selective reporting of only statistically significant findings. The study protocol has been developed in line with the ‘Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols’ (PRISMA-P, see online supplemental appendix 2), the Cochrane Handbook for Systematic Reviews of Interventions as well as the Institute of Medicine guideline.23–26 We will ensure to fulfil all requirements recommended by the current PRISMA guideline when writing the publication of the systematic review.27 28
bmjopen-2020-041607supp002.pdf (188.6KB, pdf)
The Cochrane risk-of-bias (RoB 2) tool for randomised trials will be used to assess various domains of bias including aspects of trial design, conduct and reporting (see online supplemental appendix 1).29 30 The following domains will be covered during the evaluation: sequence generation, allocation concealment, blinding, incomplete outcome data (eg, withdrawals and dropouts) and selective outcome reporting. A summary assessment will be made based on the extracted items, judging whether the risk of bias in the respective study is low, high or has some concerns. If only insufficient data are reported, the risk of bias is ‘unclear’ and the original study authors will be contacted for further information. The assessment will be conducted by two independent reviewers using the RoB 2 tool.29 30 In cases of disagreement, critical points will be discussed until a consensus is reached. The risk of bias evaluation will be incorporated into the data synthesis by performing a sensitivity analysis that excludes studies with high or unknown risk of bias.
Descriptive analysis and meta-analysis
Measures of treatment effect
The mortality/survival outcomes shall be addressed by estimating HRs and 95% CIs. Results of the intention-to-treat (ITT) approach will be used, including all patients randomised when both ITT and per-protocol results are given.
Data synthesis
As far as study quality and differences between studies allow, effect estimates of all eligible studies with data for the following three main meta-analyses will be pooled deriving random effects results with the DerSimonian and Laird method (primary analysis) and fixed-effects summary estimates using the Mantel-Haenzel method (secondary analysis).
Association of vitamin D3 supplementation and cancer mortality in the general population
Association of vitamin D3 supplementation and cancer-specific survival of patients with cancer
Association of vitamin D3 supplementation and overall survival of patients with cancer
For all studies that provide IPD, unadjusted Cox proportional hazard regression models will be used to estimate HRs and 95% CIs for the main meta-analyses in which we will pool effect size data from studies who do and who do not provide IPD in a two-step approach. For studies that cannot send IPD to the coordinating centre (German Cancer Research Centre, Heidelberg), authors are being asked to estimate the HRs and 95% CIs themselves and send the summary data for the meta-analyses. To assess cancer survival as time-to-event data from general population cohorts, the study will be restricted to patients with a history of cancer in the 5 years preceding baseline or a cancer diagnosis during the trial. For the former, the survival time will be calculated from baseline to death/end of the trial, and for the latter, survival time will be counted from the date of cancer diagnosis till death/end of the trial.
With all studies that agree to send IPD data to the coordinating centre or to do additional analyses in-house, we will also conduct an additional multivariate Cox proportional hazards regression model. The model for the outcome cancer mortality among general population studies will contain the variables vitamin D3 intervention (vs placebo), age (continuous; <70 vs ≥70 years), sex (male, female, unknown), BMI (<25 vs 25 to 29.9 vs ≥30 kg/m² vs unknown), ethnicity (white vs black/brown vs other), 25(OH)D baseline level (<30 vs 30 to 49.9 nmol/L vs ≥50 nmol/L vs unknown), diagnosis of cancer (except non-melanoma skin cancer and benign tumours) in 5 years before baseline (yes vs no vs unknown), health status (general healthy population vs diseased population) and compliance (<80% vs ≥80% vs unknown). The models for the outcomes overall and cancer-specific survival of patients with cancer will be adjusted for the same variables but the variable ‘diagnosis of cancer in 5 years before baseline’ will be replaced by more specific variables for cancer stage (only advanced stages III and/or IV vs unknown), cancer site (prostate vs colorectal vs breast vs lung vs other vs unknown) and time since cancer diagnosis (<1 year vs 1–5 years). We will test for interactions of the treatment variable (vitamin D3 vs placebo) with these covariates to identify potential effect modifiers. Again, a two-step approach will be used for the meta-analyses, whereby the analyses are carried out on a study-specific basis, and then the effect estimates are pooled. To further explore the variation of the treatment effect by methodological or patient characteristics differences of the studies, the following subgroup analyses will be performed with IPD data and studies that published eligible data.
Subgroup analyses according to trial design
Daily dose versus weekly/monthly bolus dose versus bolus dose at the beginning of the trial followed by a daily dose
Low versus moderate versus high vitamin D3 dosing (<1000 IU vs 1000–2000 IU vs >2000 IU per day or equivalent weekly or monthly taken dose).
Vitamin D3 supplementation duration (<5 vs ≥5 years).
Health status (general population vs diseased population).
Region (North America vs Europe vs Other)
Subgroup analyses according to patient characteristics
Age (<70 vs ≥70 years)
Sex (male vs female)
Ethnicity (white vs black/brown vs other)
BMI (<25 vs 25 to 29.9 vs ≥30 kg/m²)
Baseline 25(OH)D levels (<30 vs 30 to 49.9 nmol/L vs ≥50 nmol/L)
Compliance rate (<80% vs ≥80%)
For meta-analyses conducted in patients with cancer in addition
Cancer stage (only advanced stages III and/or IV vs unknown).
Cancer site (prostate vs colorectal vs breast vs lung vs other).
Time since cancer diagnosis (<1 year vs 1–5 years).
Analyses in the coordinating centre will be done with the statistical software SAS V.9.4. The meta-analyses will be performed with Comprehensive Meta-Analysis V.2.0 (Biostat, Englewood, New Jersey, USA).
Assessment of heterogeneity
Heterogeneity will be presented visually by forest plots and assessed statistically by Cochran’s Q test (significance level=0.05) as well as the I² index (<25% low, 25%–50% moderate, >50% high heterogeneity). Meta-Analyses will be conducted even if high heterogeneity is being detected and the results will be discussed taking the heterogeneity into consideration. Sources of heterogeneity will be explored by the subgroup analyses outlined in the previous section and the following sensitivity analyses:
Excluding studies with a high or unknown risk of bias according to assessment with the Cochrane RoB 2 tool for randomised trials.
Excluding studies not reporting ITT results.
Excluding trials with cosupplementation of calcium.
Excluding events in the first year of follow-up.
Assessment of publication bias
Publication bias will be accessed visually in funnel plots and tested for with Egger’s test.
Dealing with missing data
In case of missing data, we will seek contact with the original investigators. If possible, we will calculate missing numerical data from the given reported data.
Strength of the body of evidence
The quality of the evidence for each outcome will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The four levels of evidence comprise very low, low, moderate and high. Evidence from RCTs starts as high quality but can be decreased for reasons such as the risk of bias, imprecision, inconsistency, indirectness and publication bias.
Amendments
In the case of protocol amendments, we will document the date, the description of the change and the rationale in a predefined log sheet in Microsoft Word (see online supplemental appendix 3).
bmjopen-2020-041607supp003.pdf (12.1KB, pdf)
Patient and public involvement
Patients and the public were not involved in the development of the study design. Since this is a protocol for a systematic review and no participant recruitment will take place, the involvement of patients in the recruitment, the conduct of the study and the dissemination of findings to study participants are not applicable.
Ethics and dissemination
An ethics approval is not required for this systematic review because it is only a summary of already published trial data. All studies to be included in the systematic review have their own ethics approvals, which are named in the original publications. For the IPD meta-analysis, we will take care that the additional analyses are in adherence with the ethics approvals of the trials.
The systematic review will be published in an international peer-reviewed journal for clinical oncology or general medicine with open access option and presented in national and international meetings. If the meta-analyses of the systematic review obtain statistically significant findings, we expect the result to be reflected in national and international guidelines and to change the current practice of tertiary prevention among patients with cancer. Vitamin D3 is already on the market in various doses and at low costs because it is not patented.
Patients will be informed via a press release from the German Cancer Research Centre. Moreover, we will send a summary of the results in a language suitable for laypersons to all patient advocacy groups recommended by the Cancer Information Service of the German Cancer Research Centre (up to data n=30) for further dissemination among their members.31 With respect to oncologists, we will disseminate the results to all German rehabilitation centres having a ward for oncological rehabilitation, as listed in the register of the Bundesarbeitsgemeinschaft für Rehabilitation e.V.32 As the topic of the review is in the field of tertiary prevention, oncologists in the rehabilitation setting are the target audience for information dissemination.
Discussion
One of the strengths of this systematic review comprises the first meta-analysis on vitamin D supplementation and cancer survival and additionally the first IPD meta-analysis on this research topic. The IPD meta-analysis will allow the investigation of potential effect modifiers. Especially 25(OH)D levels at baseline, BMI and compliance are candidates that could have had a great impact on the overall trial results.
The creation of this research protocol prompted us to plan carefully all the details of the systematic review and to anticipate and address potential problems before their actual occurrence. Arbitrary decision-making concerning any procedure of this systematic review is prevented, resulting again in a decreased risk of publication bias and selective reporting bias. The protocol allows reproducible and transparent research for future reviewers.
Possible limitations of our review include a potentially insufficient number of cancer deaths in the studies and high heterogeneity, which could both negatively influence the statistical power of the meta-analyses. However, it is still too early to judge whether these limitations occur.
The quality of selected studies will be assessed and the quality of the evidence will be judged. The ultimate goal is to ensure the reporting of highly meaningful findings for clinicians and patients. Oncologists are well aware that vitamin D deficiency and insufficiency are very common in patients with cancer but there is uncertainty about whether and how they should routinely perform preventive screening and treatments. In some clinics, patients with cancer receive a uniform dose of vitamin D with a ‘one-dose-fits-all’ approach, which does not take individual 25(OH)D levels or other patient characteristics into account. The optimal dose for one person may be utterly insufficient for another one to achieve beneficial vitamin D levels. Since vitamin D products are readily available in pharmacies or drug stores, many patients use low-dose vitamin D supplementation as self-medication. Yet, it can be doubted whether this untargeted intervention has any effect on cancer prognosis. Consequently, evidence-based recommendations for high-dose vitamin D supplementation are highly relevant for both, clinicians and patients.
If the planned systematic review determines the efficacy of vitamin D supplementation on cancer prognosis in the expected magnitude of 10%–15%, the review will be used to provide clear suggestions on how vitamin D can be appropriately dosed to overcome vitamin D deficiency or insufficiency in patients with cancer.12 Furthermore, our systematic review would provide the evidence for statutory health insurances to cover the costs for screening for vitamin D deficiency or insufficiency in patients with cancer and a subsequent vitamin D supplementation. With expected relatively large effects and very low screening and treatment costs (a vitamin D blood test costs approximately €20, and 1 year of vitamin D therapy costs less than €100), vitamin D supplementation will be highly cost-effective. The costs would be close to negligible compared with other current cancer treatment costs.
Supplementary Material
Acknowledgments
The authors thank the Helmholtz International Graduate School for Cancer Research (HIGS) at the German Cancer Research Centre, Heidelberg, for supporting this research with a doctoral scholarship to Sabine Kuznia.
Footnotes
BS and SK contributed equally.
Contributors: All authors meet the ICMJE criteria for authorship as follows. BS and SK are the guarantors of the systematic review, therefore, are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors made substantial contributions to the conception of the work. BS and SK designed the search strategy and the risk of bias assessment strategy. BS developed the selection criteria, the data extraction criteria and the statistical methods. SK drafted the protocol publication, which BS and HB revised critically for important intellectual content. All authors approved the final version to be published.
Funding: The authors did not receive any funding for the work on this systematic review protocol. The open access publication fee was paid from a research grant of the Wereld Kanker Onderzoek Fonds (WKOF), member of the World Cancer Research Fund network based in Amsterdam, the Netherlands (grant reference no.: 2018/1696).
Disclaimer: The views of the authors do not necessarily reflect those of the German Cancer Research Centre.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.International Agency for Research on Cancer Press release no 263: latest global cancer data: cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Available: https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf [Accessed 9 May 2020].
- 2.Zhang L, Wang S, Che X, et al. . Vitamin D and lung cancer risk: a comprehensive review and meta-analysis. Cell Physiol Biochem 2015;36:299–305. 10.1159/000374072 [DOI] [PubMed] [Google Scholar]
- 3.Garland CF, Gorham ED. Dose-Response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: a meta-analysis. J Steroid Biochem Mol Biol 2017;168:1–8. 10.1016/j.jsbmb.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Li M, Chen P, Li J, et al. . Review: the impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2014;99:2327–36. 10.1210/jc.2013-4320 [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Zhang H, Wen X, et al. . Vitamin D deficiency and increased risk of bladder carcinoma: a meta-analysis. Cell Physiol Biochem 2015;37:1686–92. 10.1159/000438534 [DOI] [PubMed] [Google Scholar]
- 6.Heath A, Kim I, Hodge A, et al. . Vitamin D status and mortality: a systematic review of observational studies. Int J Environ Res Public Health 2019;16:383 10.3390/ijerph16030383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöttker B, Haug U, Schomburg L, et al. . Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr 2013;97:782–93. 10.3945/ajcn.112.047712 [DOI] [PubMed] [Google Scholar]
- 8.Fleet JC, DeSmet M, Johnson R, et al. . Vitamin D and cancer: a review of molecular mechanisms. Biochem J 2012;441:61–76. 10.1042/BJ20110744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afzal S, Brøndum-Jacobsen P, Bojesen SE, et al. . Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014;349:g6330. 10.1136/bmj.g6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rejnmark L, Bislev LS, Cashman KD, et al. . Non-Skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One 2017;12:e0180512. 10.1371/journal.pone.0180512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjelakovic G, Gluud LL, Nikolova D. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2011;7. [DOI] [PubMed] [Google Scholar]
- 12.Keum N, Lee DH, Greenwood DC, et al. . Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol 2019;30:733–43. 10.1093/annonc/mdz059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulão B, Stewart F, Ford JA, et al. . Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr 2018;107:652–63. 10.1093/ajcn/nqx047 [DOI] [PubMed] [Google Scholar]
- 14.Corrigendum for Goulao B Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr 2018;111:729–30. 10.1093/ajcn/nqx047 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Fang F, Tang J, et al. . Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 2019;366:l4673 10.1136/bmj.l4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner H, Jansen L, Saum K-U, et al. . Vitamin D supplementation trials aimed at reducing mortality have much higher power when focusing on people with low serum 25-hydroxyvitamin D concentrations. J Nutr 2017;147:1325–33. 10.3945/jn.117.250191 [DOI] [PubMed] [Google Scholar]
- 17.Maalmi H, Walter V, Jansen L, et al. . Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients 2018;10:896. 10.3390/nu10070896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maalmi H, Walter V, Jansen L, et al. . Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol 2017;32:961–71. 10.1007/s10654-017-0298-z [DOI] [PubMed] [Google Scholar]
- 19.Toriola AT, Nguyen N, Scheitler-Ring K, et al. . Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev 2014;23:917–33. 10.1158/1055-9965.EPI-14-0053 [DOI] [PubMed] [Google Scholar]
- 20.Heaney RP, Armas LAG. Quantifying the vitamin D economy. Nutr Rev 2015;73:51–67. 10.1093/nutrit/nuu004 [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury R, Kunutsor S, Vitezova A, et al. . Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014;348:g1903. 10.1136/bmj.g1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, et al. . Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Shamseer L, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamseer L, Moher D, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine Finding what works in health care: standards for systematic reviews. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- 26.Higgins JPT, Thomas J, Chandler J. Cochrane Handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. Available: www.training.cochrane.org/handbook
- 27.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JAC, Savović J, Page MJ, et al. . Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Savović J, Page MJ. Chapter 8: Assessing risk of bias in a randomized trial : Higgins JPT, Thomas J, Chandler J, Cochrane Handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook [Google Scholar]
- 31.Krebsinformationsdienst Deutsches Krebsforschungszentrum Krebs-Selbsthilfegruppen und Patientenverbände. updated 06 August 2018. Available: https://www.krebsinformationsdienst.de/service/adressen/selbsthilfe.php#inhalt17 [Accessed 9 May 2020].
- 32.BAR e.V BAR-Verzeichnis von stationären Einrichtungen Der medizinischen rehabilitation. Available: https://www.bar-frankfurt.de/service/datenbanken-verzeichnisse/rehaklinikenverzeichnis/rehastaetten-suche.html [Accessed 9 May 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041607supp001.xlsx (15.8KB, xlsx)
bmjopen-2020-041607supp002.pdf (188.6KB, pdf)
bmjopen-2020-041607supp003.pdf (12.1KB, pdf)