Abstract
Purpose
To determine whether supplementation with turmeric or curcumin extract effects pain and physical function in individuals with knee osteoarthritis (OA). Second, we investigated the therapeutic response (pain and function) of turmeric compared with non-steroidal anti-inflammatory drugs (NSAIDs).
Methods
A search was conducted in MEDLINE, Embase, CINAHL and Cochrane Review. Inclusion criteria included randomised controlled trials reporting pain and physical function in humans with knee OA comparing turmeric therapy with NSAIDs or no therapy. Two reviewers screened 5273 abstracts. Risk of bias and quality were assessed via Cochrane Collaboration tool and CONSORT (Consolidated Standards of Reporting Trials) 2010, respectively.
Results
Ten studies were included in the final analysis. Eight had high methodological quality and two were categorised as good with a mean CONSORT quality score of 21.1. Nine studies had adequate sequence generation and six had adequate allocation concealment. Participants and outcome assessors were blinded in eight studies. Three of the studies compared turmeric therapy to NSAIDs. All 10 studies showed improvement in pain and function from baseline with turmeric therapy (p≤0.05). In three studies comparing turmeric to NSAIDs, there were no differences in outcome scores (p>0.05). In all studies there were no significant adverse events in the turmeric therapy group.
Conclusion
Compared with placebo, there appears to be a benefit of turmeric on knee OA pain and function. Based on a small number of studies the effects are similar to that of NSAIDs. Variables such as optimal dosing, frequency and formulation remain unclear at this time.
Keywords: osteoarthritis, nutrition, knee, meta-analysis
Summary box.
What is already known
Turmeric is a widely used nutraceutical for various ailments due to its anti-inflammatory properties.
In-vitro studies have shown turmeric modulates the NF kappa Beta immune response in a similar way to non-steroidal anti-inflammatory drugs (NSAIDs).
American College of Rheumatology and Osteoarthritis Research Society International do not specifically address turmeric in the management of osteoarthritis.
What are the new findings
Based on evidence from randomised controlled trials, there is data supporting the use of turmeric therapy on patients with knee osteoarthritis to improve pain and physical function. Though findings do not suggest improvement in performance-based outcomes.
Turmeric appears to be safe and without severe side effects.
There are no conclusive findings as to best formulation or dosage.
Based on a limited number of studies in this systematic review evidence suggests that turmeric therapy may have similar efficacy to NSAID therapy for pain and function.
Current studies do not examine the use of turmeric therapy both against and in conjunction with traditional therapies such as physical therapy.
Introduction
Osteoarthritis (OA) is the most prevalent joint disease worldwide and is the 11th cause of years lived with disability according to the 2010 WHO global burden of disease report.1 Roughly 80% of the OA disease burden is secondary to knee OA (KOA) which impacts over 300 million people worldwide and 19% of Americans above the age of 45.2 3 Current best practice management is often characterised as palliative and reactive, rather than a combination of shared decision-making coupled with proactive and preventive measures that are the goal of chronic disease management in today’s environment.
Rather than simply a ‘wear-and-tear’ disease, it is now recognised that OA involves mechanical, inflammatory and metabolic factors. Advances in the methodology of DNA sequencing have also advanced our understanding of the genetics and epigenetics of the disease.4 The underlying process of KOA includes a variety of factors, including biomechanical forces, pro-inflammatory mediators, metabolic dysregulation and alterations in local tissue metabolism.5 Following the initial loss of cartilage integrity, compositional changes in the joint allow increased susceptibility to damage from physical impact.6 As joint damage continues, chondrocyte activity increases leading to secondary degradation and pro-inflammatory mediators throughout the synovium.7 Protein complexes such as NF-kB which contribute to joint homoeostasis then become upregulated leading to increased cytokine activity.6 Taken together, these factors suggest that inflammation plays a greater role in the pathogenesis of OA than previously appreciated.
OA is a complex chronic disease that is frequently compounded by the presence of multiple comorbidities. In recognition of the various underlying aetiologies of KOA and coexistence of comorbidities, recent efforts have focussed on identifying specific phenotypes with distinct features that could potentially aid in molecular targeting of disease management. For example, Dell’Isola et al8 proposed six phenotypes including chronic pain, inflammation, metabolic syndrome, bone and cartilage metabolism, mechanical overload and minimal joint disease. On the other hand, van der Esch et al9 suggested phenotypes based on lower extremity muscle strength, body habitus and psychological components of KOA pain. To date, there have not been any validated guidelines grouping patients with KOA into specific phenotypes. Despite this lack of consensus, inflammation is a common feature of many of these emerging phenotypic classification systems.
Given the recognition that inflammation may play a critical role in the pathogenesis and progression of OA, particularly that of post-traumatic OA, it is not surprising that researchers and clinicians have turned to strategies to address this feature of the disease as a way to manage symptoms and ideally slow its progression. A recent review summarises the current evidence for the role of diet and nutrition in patients with OA.10 Both the Osteoarthritis Research Society International (OARSI) and American College of Rheumatology (ACR) have published recent recommendations on conservative treatment of KOA.11 12 There is near-uniform consensus on the use of exercise, weight loss, topical non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections and hyaluronic acid injections from both societies. In the OARSI statement, expert consensus was derived from a review of 60 popular treatments and a subsequent meta-analysis. Interestingly, the ACR report did evaluate some nutraceuticals and recommended that there was insufficient evidence for the vast majority including glucosamine, vitamin D, chondroitin sulfate and fish oil.11 A previous meta-analysis by Liu et al published in the British Journal of Sports Medicine examined the use of various nutraceutical supplements in which two randomised controlled trials (RCTs) evaluating turmeric were included. Though the author noted positive outcomes with turmeric therapy, no recommendations could be provided due to limitations in quantity and quality of the data reviewed.13
Turmeric is a spice from the ginger family that is widely used in Indo-Asian cuisine and traditional eastern medicine. Curcumin is one of the active components in turmeric making up roughly 3% to 10% of the turmeric powder which can be extracted.14 The isolated curcumin extract (CE) has anti-inflammatory properties similar to that of non-steroidal anti-inflammatories.15 It has been shown that CE affects the signalling of pro-inflammatory cytokines such as interleukins, phospholipase A2, 5-lipoxygenase enzyme and COX-2 by influencing NF kappa Beta activity.16 Its anti-inflammatory properties may be particularly important at the chondrocyte level where curcumin has been shown to have an inhibitory effect on macrophage inhibitory factor-induced upregulation of matrix metalloproteinase MMP-1 (interstitial collagenase) and MMP-3 (stromelysin) enzymes.17 These enzymes when activated in synovial fibroblasts accelerate catabolic changes of the articular cartilage and subsequent development of OA.
To date, there has been one systematic review assessing the therapeutic effects of turmeric in patients with either OA or rheumatoid arthritis.18 This review highlighted the potential benefits of this supplement and evaluated a Pain Visual Analogue Score and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as primary outcomes. Eight RCTs were included and judged to exhibit low-to-moderate risk of bias. The authors concluded that there was evidence supporting the use of turmeric extract in treatment of arthritis but, a lack of sufficient power to draw definitive conclusions. Given the promise of their conclusions and the increasing recognition of inflammation and its role in the pathogenesis of OA, our goal was to evaluate the efficacy of turmeric in the treatment of patients with KOA exclusively. Our primary purpose was to determine whether turmeric affects pain and physical function in individuals with KOA exclusively. Second, we investigated the therapeutic responses of turmeric in studies that compared its efficacy to NSAID treatment.
Materials and methods
Protocol and registration
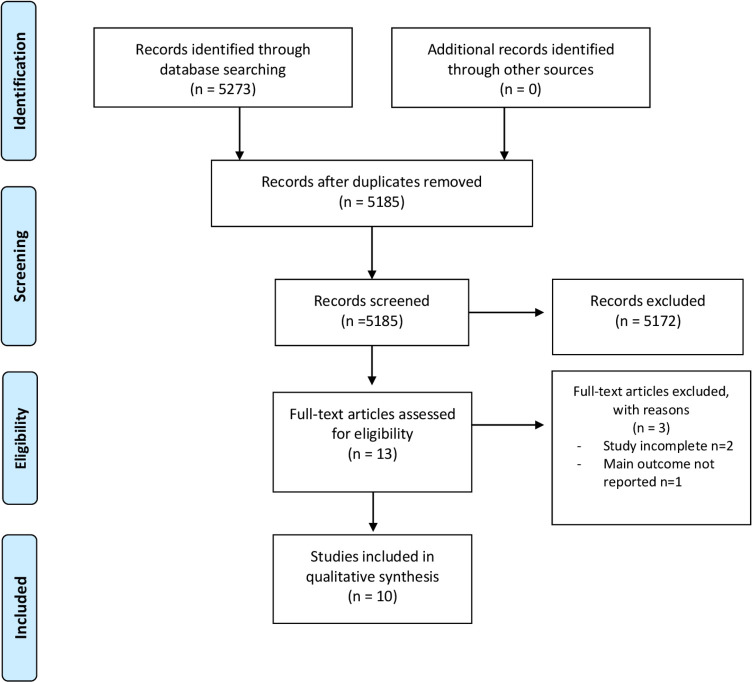
Study selection, eligibility criteria and data extraction calculations were conducted based on a prespecified protocol and registered on PROSPERO (ID CRD42020152828).19 For the selection of articles to review, PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and PRISMA flowchart were used (figure 1).
Figure 1.
PRISMA flow diagram. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097.
Eligibility criteria
Inclusion criteria comprised of human subjects with symptomatic KOA in at least one knee. Included studies were RCTs that evaluated and reported the outcome measures of pain and physical function in humans with knee OA that were treated with turmeric therapy alone or in combination with NSAID therapy. Turmeric therapy was defined as any formulation of turmeric or curcumin extract developed for therapeutic use. No restrictions were made with regards to age, body mass index (BMI) or gender. Excluded were studies which evaluated arthritis secondary to inflammatory or rheumatological conditions.
Information sources
A comprehensive literature search was performed between August 2019 through November 2019. In conjunction with a professional medical librarian (JMR), search strategies using a combination of subject headings and keywords for KOA and turmeric or curcumin extract was developed by one of the authors (KP). A comprehensive search for RCTs was then conducted in MEDLINE (PubMed), Embase (Elsevier, Embase.com), Cochrane Library (CENTRAL) and CINAHL (EBSCO), from each database’s inception date. Authors identified RCT’s on humans using the sensitivity and precision maximising version of Cochrane Highly Sensitive Search Strategies (2011 revision).20 For the purposes of this search, date restrictions were not enforced. Complete search strategies are available in online supplemental appendix 1.
bmjsem-2020-000935supp001.pdf (21.4KB, pdf)
Study selection
Two reviewers (KP and DH) independently performed eligibility assessments on each trial in the database search. Studies that did not investigate KOA (such as those that evaluated rheumatoid arthritis) were removed. Titles and abstracts were screened and if trial was deemed eligible by at least one reviewer, further evaluation with a full-text review was performed. If there was any discordance with regard to study eligibility, a third reviewer (TB) screened, and a final consensus was reached.
Data collection
Using Microsoft Excel, data extraction included the following: authors, year of publication, number of participants, formulation of turmeric therapy, control intervention and baseline and follow-up outcome measures for knee pain. The following details of the interventions were also recorded: adverse events, BMI, mean age and participant dropout rate (table 1). Trials that compared more than one outcome measure for knee pain were treated as separate comparisons and analysed independently.
Table 1.
Patient demographics
| Study first author, year, reference | Patients (M:F) |
Groups (M:F) | Age, years, mean±SD | BMI (kg/m2)±SD | KL grade | Dropout rate (n) | Adverse events N (%) |
| Panda (2018)26 | 50; N/A | Curene 25 (N/A) Placebo 25 (N/A) |
Total: 54.16±8.40. Curene: 55.20±8.58. Placebo: 53.12±8.25 | 25.18±2.36 | Inclusion criteria of KL grade of 3 or 4 set; no breakdown provided | Curene: n=1 Placebo: n=3 |
Curene: n=2 (8%) Placebo: n=3 (12%) |
| Nakagawa (2014)27 | 41; (9:32) | Theracurcumin 18 (4:14) Placebo 23 (5:18) |
Total:68.7±7 Theracurcumin: 71.9±5.3 Placebo: 66.1±7.2 |
24.9±2.4 | Grade 1:0 Grade 2:33 Grade 3:8 Grade 4:0 | Theracurcumin: n=5 Placebo: n=2 |
None |
| Madhu (2013)28 | 120; (37:83) | Turmacin 30 (13:17) Placebo 30 (13:17) Glucosamine 30 (5:25) Glucosamine+Turmacin 30 (6:24) | Total: 57.09±9.41 Turmacin: 56.63±10.58 Glucosamine: 56.80±7.99 Turmacin+glucosamine: 58.17±9.30 |
27.67±4.30 | Inclusion criteria of KL grade of 2 or 3; no breakdown provided | Turmacin: n=1 Placebo: n=1 Glucosamine: n=2 Glucosamine+Turmacin: n=6 |
Turmacin: n=2 (6.7%) Placebo: n=2 (6.7%) Glucosamine: n=5 (16.7%) Turmacin+glucosamine: n=4 (14.3%) |
| Belcaro (2010)29 | 100; (51:49) | Meriva 50 (23:27) Control group 50 (28:22) | Total: 43.9±5.73 Meriva: 43.6±5.5 Control group: 44.2±6 | N/A | N/A | Meriva: n=5 Control group: n=6 |
None documented |
| Shep (2019)30 | 139; (93:46) | Curcumin (BCM-95) 70 (45:25) Control group 69 (48:21) |
Total: 52.62±3.99 Curcumin (BCM-95): 53.09±4.17 Control group: 52.14±3.76 |
N/A | N/A | Curcumin (BCM-95): n=4 Control group: n=6 |
Curcumin (BCM-95): n=9 (13%) Control group: n=26 (28%) |
| Panahi (2014)31 | 40; (9:31) | Curcuminoids 21 (5:16) Placebo 19 (4:15) |
Total: 57.44±8.83 Curcuminoids: 57.32±8.78 Control group: 57.57±9.05 |
29.22±3.88 | N/A | Curcuminoids: n=8 Placebo: n=5 | Curcuminoids: n=3 (14%) Placebo group: n=4 (21%) |
| Henrotin (2013)32 | 150; (28:113) | Flexoyfytol high dose 49 (10:39) Flexofytol low dose 47 (7:40) Placebo 45 (11:34) |
Total: 61.83±8.42 High dose Flexoyfytol: 60.9±9.78 Low dose Flexoyfytol: 61.4±7.49 Placebo: 63.3±7.69 |
29.73±5.08 | Grade 1: 0 Grade 2: 87 Grade 3: 53 Grade 4: 1 |
High dose Flexoyfytol: n=21 Low dose Flexoyfytol: n=17 Placebo: n=11 |
High dose Flexoyfytol: n=18 (37%) Low dose Flexoyfytol n=10 (21%) Placebo: n=6 (13%) |
| Kuptniratsaikul (2009)33 | 107; (21:86) | Curcumin extract 52 (11:41) Control group 55 (10:45) |
Total: 60.68±8.54 Curcumin extract: 61.4±8.7 Control group: 60.0±8.4 |
26.61±4.29 | N/A | Curcumin extract: n=7 Control group: n=9 | Curcumin extract: n=16 (33.3%) Control group: n=23 (44.2%) |
| Kuptniratsaikul (2014)34 | 367; (71:296) | Curcumin extract 171 (14:157) Control group 160 (21:139) |
Total: 60.60±6.85 Curcumin extract: 60.3±6.8 Control group: 60.9±6.9 |
26.54±3.84 | Grade 1: 42 Grade 2: 131 Grade 3: 102 Grade 4: 55 |
Curcumin extract: n=11 Control group: n=14 |
Curcumin extract: n=55 (32%) Control group: n=65 (40.6) |
| Srivastava (2016)35 | 60; (57:103) | Curcumin extract 78 (25:53) Control group 82 (32:50) |
Total: 50.25±8.34 Turmeric group: 50.23±8.08 Control group: 50.27±8.63 |
27.84±5.43 | Grade 1: 11 Grade 2: 30 Grade 3: 66 Grade 4: 53 |
Curcumin extract: n=12 Control group: n=15 | Curcumin extract: n=2 (2.5%) Placebo: n=4 (4.8%) |
BMI, body mass index; KL grade, Kellgren-Lawrence grade.
Assessment of methodological quality and risk of bias
CONSORT (Consolidated Standards of Reporting Trials) 201021 is an evidence-based set of recommendations for reporting randomised trials. It provides greater accuracy, transparency and a reduction of bias in randomised studies by standardising the reporting of trial findings.22 The CONSORT scale is a 25-item checklist based on the CONSORT 2010 recommendations and grades the reporting of how trials are designed, analysed and interpreted.23 Studies were independently reviewed by two reviewers (KP and DH) for methodological quality and risk of bias CONSORT recommendations. Scoring discrepancies were resolved by a third independent reviewer (TB). For the purposes of this systematic review, a score greater than 20 was considered high quality, a score between 16 and 20 was considered good quality, a score between 11 and 15 was deemed fair quality and a score of 10 or less was considered poor quality.
The Cochrane Collaboration tool for assessing the risk of bias in randomised trials was used to further assess the risk of potential bias.24 The two reviewers (KP and DH) independently assessed the risk of bias using this tool and supplementary material from the Cochrane handbook criteria. Any disagreement was resolved by a third independent reviewer (TB).
Data synthesis—effect sizes
For study designs using treatment(s), along with a control group, patient-reported pain and physical function scales (eg, Visual Analogue Scale (VAS), WOMAC and Knee injury Osteoarthritis Outcome Score (KOOS)) were determined, independently, to express the size of the treatment (ie, Intervention) effect in each study relative to the variability observed in the particular study’s patient-reported outcome measure(s) of follow-up (Effect size: magnitude of the difference in the means of the control and experimental groups in a study with respect to the pooled SD). Thereby, the standardised mean difference for each group, was determined by calculating the mean difference between reported baseline and follow-up assessments and dividing the result by their pooled SD. For studies without requisite measures to quantify variation (eg, SD, SEM or CIs), effect sizes were indeterminate (ie, N/A). Notably, the longest reported time frame of follow-up was established to identify the mean difference, among studies with multiple post-intervention time periods. Measures of effect size, typically reported as Cohen’s d, are characterised by the standard interpretation (0.8=large, 0.5=moderate, 0.2=small) offered by Cohen (1988).25
Results
Study selection
Our search identified a total of 5273 potentially relevant studies from MEDLINE, Embase, CENTRAL and CINAHL from conception to November 2019. After the removal of 88 duplicate studies, the remaining 5185 were screened by title and 5032 were removed secondary to study design. Abstracts were reviewed for the remaining 153 studies and 140 studies were further excluded due to being animal studies and non-relevance to KOA. Of the 13 full articles reviewed and assessed for eligibility, 3 were excluded due to lack of data/incomplete study. Therefore, 10 studies met the inclusion criteria for subsequent analysis. Figure 1 provides the systematic review flow chart that demonstrates the inclusion and exclusion process and results.
Study characteristics
The total number of participants across the 10 studies was 1287. Of the 10 RCT’s one did not provide gender data.26 The remaining nine studies included 1237 participants, 340 men and 897 women. The mean age across the 10 studies was 56.85±9.19 years. Eight studies reported BMI (table 1) and the mean BMI reported was 27.52±4.14 kg/m2. Inclusion criteria across the 10 RCT’s varied and subject selection was based on diagnosis of primary KOA. In 8 of the 10 studies, ACR criteria were used as the basis for diagnosing participants with KOA. The remaining two studies used the Kellgren-Lawrence (KL) grading system.27 28 Duration of intervention for the 10 studies varied with the shortest being 4 weeks and the longest at 8 months.
Assessment of methodological quality and risk of bias
Based on the CONSORT quality score, two studies met classification for good quality and eight were classified as high quality. Mean score among the 10 studies was 21.1, with the lowest graded at 15.5 and the highest at 24.5.
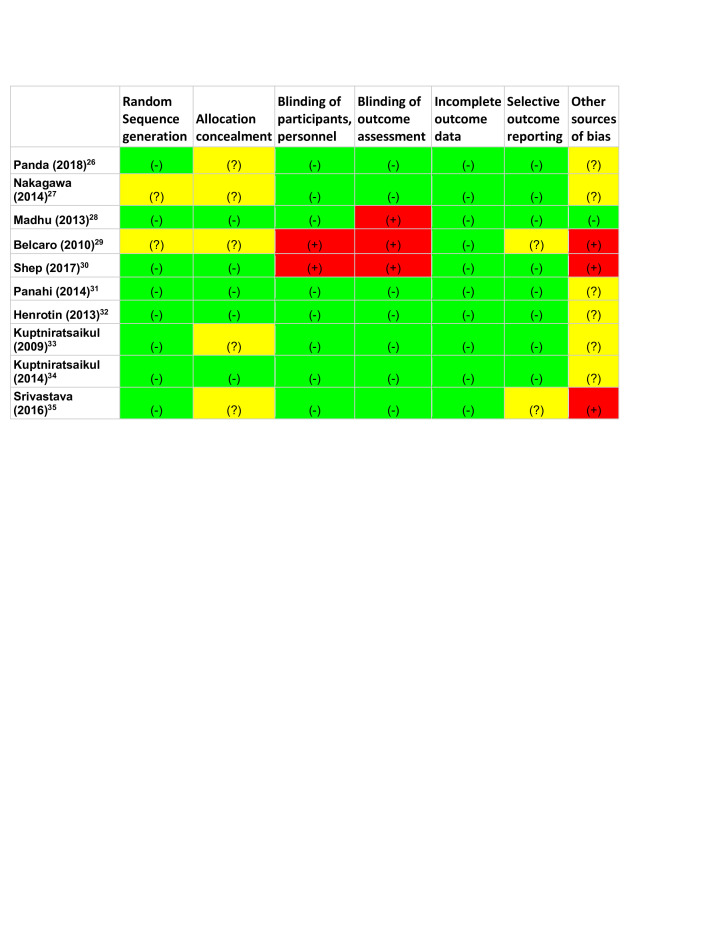
Figure 2 classified the risk of bias among the 10 studies, 6 were double blinded, 2 were single blinded and 2 were not blinded. Eight of the studies reported adequate sequence generation and five had adequate allocation concealment. Two studies were found to have an increased risk of bias in more than one section of the Cochrane collaboration tool.29 30 Both Belcaro et al and Shep et al were open label studies that did not have blinding among participants or those who assessed outcomes leading to an increased risk of performance and detection bias. Additionally, Belcaro et al was unclear in their method of sequence generation and allocation concealment leading to a possible increase in selection bias.
Figure 2.
Cochrane Collaboration’s tool for assessing risk of bias. (-), low risk; (?), unclear risk; (+), high risk.
Rescue therapy
Of six studies comparing turmeric to placebo, five documented rescue therapy with NSAIDs or acetaminophen.26–28 31 32 These five studies tracked the use of rescue therapy throughout their respected studies and reported an overall reduction in the use of rescue therapy in the turmeric group over placebo. Two studies compared turmeric to NSAID therapy for which rescue medication was not used.30 33 Of the studies, comparing turmeric therapy to NSAID therapy or as an adjunct therapy to NSAIDs, Shep et al30 reported use of paracetamol (acetaminophen) as rescue therapy and Kuptniratsaikul et al34 reported use of tramadol as rescue therapy. There was no significant difference reported in the reduction of rescue therapy use between turmeric and NSAID therapy. (table 2)
Table 2.
Summary of interventions
| Study first author, year, reference | Study design | Inclusion criteria | Preparation of tumeric/curcumin | Control intervention (type) | Rescue intervention (dose) |
| Panda (2018)26 | RCT with two parallel groups |
|
Curene: Bio-optimised turmuric/Curcuma longa extract comprising of curcuminoids developed via Aqueosome technology | Placebo capsules with similar size and shape to intervention | Acetaminophen: 2000 mg per day |
| Nakagawa (2014)27 | RCT with two parallel groups |
|
Theracurcumin: a surface-controlled water dispersible curcumin extract with high bioavailability | Placebo capsules with similar size and shape to intervention | Celecoxib 100 mg per day |
| Madhu (2013)28 | RCT with four parallel groups |
4. KL grades: grades 2 and 3 |
NR-INF-02 (Turmacin): extract from rhizome of Curcuma longa with increased bioavailability | 1. Placebo capsules. 2. Glucosamine 1500 mg per day. 3. NR-INF-02+GS |
Acetaminophen: Dose not provided |
| Belcaro (2010)29 | RCT with two parallel groups |
|
Meriva: A mixture of 75 per cent curcumin, 15 per cent demethoxycurcumin and 10 per cent bisdemethoxycurcumin. | ‘Best available treatment’ | N/A |
| Shep (2019)30 | RCT with two parallel groups |
|
Curcumin (BCM-95): containing curcuminoids and essential oil of turmeric complex (curcumin, demethoxycurcumin, bisdemethoxycurcumin and volatile oils from turmeric rhizome) | Diclofenac 50 mg two times per day | Paracetamol 500 mg tablet |
| Panahi (2014)31 | RCT with two parallel groups |
|
Curcuminoids (C3 complex) with 5 mg of BioPerine pepper extract for increased bioavailability | Placebo capsules with similar size and shape to intervention | Naproxen: Dose not provided |
| Henrotin (2013)32 | RCT with three parallel groups |
|
Flexofytol: Bio-optimised turmeric rhizome extract | Placebo tablets with similar size and shape to intervention | 1. Acetaminophen: up to 500 mg three times per day 2. NSAIDs as secondary rescue |
| Kuptniratsaikul (2009)33 | RCT with two parallel groups |
|
Curcumon domestica extract: Bio-optimised turmeric extract each capsule contained 250 mg of curcurminoids | Ibuprofen at a dose of 800 mg per day | N/A |
| Kuptniratsaikul (2014)34 | RCT with two parallel groups |
|
Curcumin domestica extract: Bio-optimised turmeric extract each capsule contained 250 mg of curcurminoids | Ibuprofen at dose of 1200 mg per day | Tramadol: Dose not provided |
| Srivastava (2016)35 | RCT with two parallel groups |
|
Curcumin longa extract | Control group: Placebo capsules+diclofenac 25 mg two times per day | N/A |
ACR, American College of Rheumatology; BMI, body mass index; KL grade, Kellgren-Lawrence grade; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis; OTC, over-the-counter; RCT, randomised controlled trial; TVAS, targeted visual analog scale; VAS, Visual Analogue Score.
Adverse events
Excluding the study by Belcaro et al,29 a total of nine studies recorded adverse events (AEs).26–28 30–35 There were no severe AEs recorded and those reported were non-specific with gastrointestinal discomfort secondary to nausea, diarrhoea and dyspepsia being most prevalent. Among the studies comparing turmeric to placebo, five studies displayed no significant difference in AEs. One study identified a dose dependent rise in AEs in the turmeric groups.32 When compared with placebo, high dose turmeric therapy had a significant increase in AEs while low dose therapy displayed no significant changes. Three studies compared turmeric therapy to NSAID’s, two studies showed no significant difference in AEs33 34 and one study showed a significant increase in the NSAID group.30 In the Shep et al study, 38% of the NSAID group participants reported AEs versus 13% in the turmeric group (p<0.01). Additionally, 19 of the participants required secondary medication to tolerate gastrointestinal (GI) discomfort in the NSAID group. One study which used turmeric as an adjuvant therapy to NSAIDs did not show significant changes in AEs when compared with placebo.35 (table 1)
Dropout rate
Rates of dropout were reported across all 10 studies. With the exception of Henrotin et al32 there were no statistical differences among dropout rates between intervention and control groups. Henrotin et al found a small but statistically significant increase in dropout rate in the high dose turmeric therapy group versus the placebo group. Despite that observation there was no difference in dropout rate between the two groups. Loss to follow-up in the high dose group was secondary to adverse events (n=10), non-compliance (n=3), withdrawn consents (n=3) and loss to follow-up (n=2). (table 1)
Formulation and dosing
Formulation of turmeric among the studies varied with two studies using the same compound at different doses.33 34 All studies used a different formulation (ie, Turmacin, Meriva, Theracurcumin, Curene) that modified the pharmacokinetics of turmeric in order to increase bio-availability. Dosing of the turmeric therapies varied from 93.34 mg per day to 2 g per day. Although, it is possible that the actual strength of turmeric therapy across studies significantly differed as there is no standardised scale to compare the different preparations. (table 3)
Table 3.
Combined outcome scores
| Study | Treatment(s) | Control | Follow-up | Parameters | Treatment. mean (std) | Control mean (std) | Effect size | Magnitude of effect |
| Panda (2014)26 | Curene (n=25) | Placebo (n=25) | Day 7 | VAS | 46.59 (8.19) | 51.94 (4.16) | 0.8 | Large |
| WOMAC total score | 34.88 (5.27) | 36.40 (3.88) | 0.3 | Small | ||||
| Pain | 7.36 (1.73) | 8 (1.12) | 0.4 | Small | ||||
| Stiffness | 4.16 (1.07) | 4.64 (0.91) | 0.5 | Moderate | ||||
| PF | 23.36 (3.05) | 23.76 (2.73) | 0.1 | Small | ||||
| Day 15 | VAS | 41.71 (9.35) | 49.28 (4.40) | 1.0 | Large | |||
| WOMAC total score | 31.44 (4.85) | 34.36 (3.79) | 0.7 | Moderate | ||||
| Pain | 6.6 (1.66) | 7.64 (0.91) | 0.8 | Large | ||||
| Stiffness | 3.52 (0.96) | 4.4 (0.87) | 1.0 | Large | ||||
| PF | 21.32 (2.85) | 22.32 (2.94) | 0.4 | Small | ||||
| Day 30 | VAS | 36.63 (8.85) | 46.77 (3.84) | 1.5 | Large | |||
| WOMAC total score | 25.84 (5.54) | 32.76 (3.88) | 1.5 | Large | ||||
| Pain | 5.8 (1.80) | 7.32 (1.07) | 1.0 | Large | ||||
| Stiffness | 2.84 (1.07) | 4 (0.91) | 1.2 | Large | ||||
| PF | 17.2 (3.65) | 21.44 (3.03) | 1.3 | Large | ||||
| Day 60 | VAS | 27.26 (11.95) | 44.83 (4.27) | 2.0 | Large | |||
| WOMAC total score | 18.44 (4.75) | 30.76 (4.84) | 2.6 | Large | ||||
| Pain | 4.28 (1.54) | 6.96 (1.43) | 1.8 | Large | ||||
| Stiffness | 2.12 (0.97) | 3.76 (1.09) | 1.6 | Large | ||||
| PF | 12.04 (3.12) | 20.04 (3.77) | 2.3 | Large | ||||
| Nakagawa (2014)27 | Theramicin (n=18) | Placebo (n=23) | 8 weeks | VAS | N/A | N/A | N/A | |
| Madhu (2013)28 | NR-INF-02 (n=29) | Placebo (n=29) | 21st day | VAS | 38.83 (21.36) | 53.83 (15.84) | 0.8 | Large |
| WOMAC | 36.67 (16.08) | 52.23 (9.63) | 1.17 | Large | ||||
| CGIC | 3.73 (1.92) | 5.53 (0.97) | 1.18 | Large | ||||
| 42nd day | VAS | 19.48 (17.84) | 46.03 (20.84) | 1.37 | Large | |||
| WOMAC | 27.14 (16.13) | 47.90 (12.59) | 1.43 | Large | ||||
| CGIC | 2.21 (1.80) | 4.72 (1.27) | 1.61 | Large | ||||
| Belcaro (2010)29 | *Meriva (n=50) | Placebo (n=50) | 8 months | WOMAC subscale scores | N/A | N/A | N/A | N/A |
| Shep (2019)30 |
Curcumin (n=70) | Diclofenac (n=69) | Day 14 | VAS | 4.69 (0.79) | 4.58 (0.60) | 0.2 | Small |
| KOOS | ||||||||
| Pain | 72.70 (4.89) | 73.69 (3.97) | 0.2 | Small | ||||
| Symptoms | 61.58 (5.65) | 61.93 (5.47) | 0.1 | Small | ||||
| Function in daily living | 77.67 (5.90) | 80.54 (6.10) | 0.5 | Moderate | ||||
| Function in sport and recreation | 68.43 (5.81) | 71.88 (5.01) | 0.6 | Moderate | ||||
| Quality of Life | 59.64 (6.08) | 59.15 (7.73) | 0.1 | Small | ||||
| Day 28 | VAS | 2.20 (0.81) | 2.20 (0.61) | 0.0 | Null | |||
| KOOS | ||||||||
| Pain | 88.77 (5.62) | 90.38 (3.61) | 0.3 | Small | ||||
| Symptoms | 67.95 (5.86) | 69.13 (5.50) | 0.2 | Small | ||||
| Function in daily living | 94.58 (6.58) | 92.63 (2.71) | 0.4 | Small | ||||
| Function in sport and recreation | 89.14 (6.43) | 91.88 (4.38) | 0.5 | Moderate | ||||
| Quality of Life | 73.57 (7.46) | 72.26 (8.61) | 0.2 | Small | ||||
| Panahi (2010)31 | Curcuminoids (n=19) | Placebo (n=21) | 6 weeks | WOMAC global score | 25.0 (13.0) | 40.6 (12.6) | 1.2 | Large |
| WOMAC pain | 6.1 (2.9) | 9.4 (3.4) | 1.0 | Large | ||||
| WOMAC stiffness | 0.15 (0.5) | 0.76 (0.9) | 0.8 | Large | ||||
| *Henrotin (2019)32 | BCL high dose (n=49) |
Placebo (n=45) |
Month 1 | VAS | N/A | N/A | *0.4 | Small |
| KOOS global score and subscales | N/A | N/A | ||||||
| Month 3 | VAS | N/A | N/A | *0.4 | Small | |||
| BCL low dose | Month 1 Month 3 |
KOOS global score and subscales VAS KOOS global score and subscales VAS KOOS global score and subscales |
N/A N/A N/A N/A N/A |
N/A N/A N/A N/A N/A |
*0.35
*0.4 |
Small Small |
||
| Kuptniratsaikul (2009)33 |
C.domestica (n=45) | Ibuprofen (n=46) | Week 6 | Pain | ||||
| Level of walking | 2.7 (2.5) | 3.1 (2.3) | 0.2 | Small | ||||
| Stairs | 3.1 (1.5) | 3.8 (2.4) | 0.4 | Moderate | ||||
| Function | ||||||||
| Time spent on 100 m walk | 96.7 (17.0) | 97.0 (25.7) | 0.0 | Null | ||||
| Time spent on going up and down of flight of stairs | 24.8 (10.2) | 25.9 (12.3) | 0.1 | Small | ||||
| Kuptniratsaikul (2014)34 |
C.domestica (n=171) |
Ibuprofen (n=160) |
4 weeks | WOMAC total score | 3.36 (2.04) | 3.23 (1.97) | 0.1 | Small |
| Pain | 3.25 (2.11) | 3.17 (1.98) | 0.0 | Null | ||||
| Stiffness | 3.28 (2.38) | 3.16 (2.36) | 0.1 | Small | ||||
| PF | 3.41 (2.09) | 3.26 (2.05) | 0.1 | Small | ||||
| 6 min walk (metres) | 345.43 (91.66) | 347.99 (86.60) | 0.0 | Null | ||||
| Srivastava (2016)35 | CL extract (n=78) | Placebo (n=82) | Day 60 | VAS | 4.96 (0.07) | 6.00 (0.11) | 11 | Large |
| WOMAC | ||||||||
| Pain | 11.19 (0.26) | 12.05 (0.21) | 3.6 | Large | ||||
| Stiffness | 4.51 (0.21) | 4.70 (0.23) | 0.9 | Large | ||||
| PF | 41.28 (0.51) | 45.11 (0.37) | 8.6 | Large | ||||
| Day 120 | VAS | 4.03 (0.08) | 5.11 (0.14) | 9.5 | Large | |||
| WOMAC | ||||||||
| Pain | 9.48 (0.17) | 10.16 (0.16) | 4.1 | Large | ||||
| Stiffness | 4.08 (0.17) | 4.16 (0.18) | 0.5 | Moderate | ||||
| PF | 32.14 (0.40) | 33.88 (0.50) | 3.8 | Large | ||||
BCL, bio-optimised Curcuma longa; CGIC, Clinician Global Impression Change; CL, Curcuma longa; KOOS, Knee injury Osteoarthritis Outcome Score; PF, Pain and Function; VAS, Visual Analogue Score; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Patient-reported outcomes
Of the 10 studies 6 used WOMAC scores to evaluate pain and function.26 28 29 31 34 For one study, Belcaro et al,29 effect sizes were indeterminate due to lack of requisite information. The remaining four studies compared effect sizes for WOMAC scores in turmeric therapy versus placebo groups and showed a large effect size.26 28 31 35 The remaining study comparing WOMAC scores between turmeric therapy and NSAIDs had a small effect size.34 (table 3)
Five studies analysed VAS scores from turmeric therapy versus placebo.26 28 35 Three of these studies showed a large effect size. In contrast, one study had a small effect size,32 and another was indeterminate.27 One study evaluated VAS scores from turmeric therapy versus NSAIDs, resulting in a small effect size at 14 days of treatment though the effect size was indeterminate at 28 days.30
KOOS scoring was used in two studies, one comparing turmeric to placebo and the other to NSAID therapy. When compared against NSAID therapy the effect size was small in all categories save for ‘function in recreational sport’ which had a moderate effect size.30 When comparing turmeric therapy to placebo the effect size was indeterminate.32
Kuptniratsaikul (2009) et al used a separate pain and function scale to compare turmeric to NSAID therapy.33 On measures of pain there was a small magnitude of effect with walking but a moderate effect size while going up a flight of stairs. In the functional assessment portion of the study, the 100-metre walk was indeterminate while going up and down flights of stairs was found to have a small effect size.
Discussion
Osteoarthritis continues to be a tremendous patient and societal burden despite intense pre-clinical and clinical efforts to more completely address its pathophysiology and ideally patient-specific treatment. The primary goal of this systematic review was to determine the efficacy of turmeric therapy on pain and physical function in patients with KOA. Ten studies were reviewed and overall judged to have a ‘good’ quality based on the CONSORT quality assessment scale. The major finding was that large effect sizes were correlated with improvement in both pain and physical function when compared with placebo. A second important finding was that when comparing turmeric to NSAID therapy, small effect sizes indicate similar effectiveness of these two strategies for pain and function. The only study found to have a small effect size when comparing turmeric to placebo was Henrotin et al.32 One possible explanation is that turmeric was being studied as an adjunct to NSAID and paracetamol therapy. Additionally, it was reported that the high and low dose turmeric groups had a reduction in the use of paracetamol and NSAID therapy, respectively. It is worthwhile noting that the studies using rescue therapy when comparing turmeric to placebo showed not only an improvement in outcome scores but also a significant reduction in rescue therapy usage. Of the studies documenting rescue therapy when comparing turmeric to NSAIDs, (Shep et al30 and Kuptniratsaikul et al34) the effect sizes were found to have no significant difference in regards to outcome scores or in the reduction of rescue therapy usage. In summary, we conclude that turmeric is a safe adjunct to mainstay pharmaceutical therapies in patients with KOA and may even allow for the reduction in dosing of medications such as NSAIDs.
Current approved drugs commonly used to treat KOA such as NSAIDs and corticosteroids have well documented and potentially significant side effect profiles. Given our major finding that turmeric appears efficacious for pain and function in KOA, the safety of this approach across the 10 RCTs was evaluated. For all 10 studies, adverse events were not significantly different from placebo and reported as mild with GI discomfort the most prevalent. There was a possible dose-related relationship between turmeric and adverse events in one study with increased GI side effects at higher doses.32 Based on the overall incidence and the low severity of AEs when compared with placebo, turmeric therapy can be considered to have a low risk profile making it an attractive alternative in patients with contraindications to NSAIDs.
It is well established that the bioavailability of turmeric is low and requires sizeable doses for therapeutic effect.36 While there is some evidence that preparations with additive such as black pepper extract aid in the absorptions turmeric, in this review all studies used trademarked formulations that extracted the active curcuminoids and encapsulated them in bio-optimised preparations.37 It is for this reason that conclusions on optimal dosing for pain and function were not able to be drawn, as there is an inability to quantify the strength of the turmeric formulation used in each study. Conclusions on optimal dosing for pain and function were not able to be drawn due to variations in both dosing and the inability to quantify the strength of the turmeric formulation for each study. Interestingly, Henrotin et al32 studied both a high dose and low dose turmeric therapy group and showed similar effect sizes for both pain and function although the high dose group had a significant increase in adverse events when compared with placebo (37% and 13% respectively p=0.012). There was however no significant difference between the low dose therapy and placebo groups. Therefore, although no standardisation of dosing exists, there is likely a therapeutic index based on factors of bioavailability to consider when dosing this supplement.
Across the 10 studies the average participant age ranged between mid 40s to upper 60s. This corresponds to the average age groups affected by KOA in the general population.38 One noticeable trend among the study populations is the overall ratio of male to female study participants. The nine studies reporting gender showed a 3:1 female:male enrolment, respectively. With the exclusion of Panda et al26 which did not report gender, only Shep et al30 studied a higher number of men than women. Therefore, the results mirror those of the general population where women are known to have radiographically diagnosed KOA more frequently than men (1.7:1 ratio).38
Our systematic review is not without limitations. Both the AORSI and the ACR have recommended in their guidelines for OA management that non-pharmacological interventions such as physical therapy and weight loss be part of first-line treatment. For the RCTs included in this review there was a lack of reporting if these interventions, or similar ones, were concomitantly prescribed and followed by the patients making it difficult to conclude that our findings are due to turmeric or its equivalent exclusively. Second, it is increasingly recognised that OA is a multiphenotype disease and not simply a mechanical ‘wear and tear’ problem. None of the studies included herein discuss any phenotype classification, as this concept is advanced both scientifically and clinically it may be important to consider when prescribing agents such as turmeric. Third, we attempted to determine if KL grade or degree of OA is an important consideration when prescribing turmeric. Although studies reported degree of OA or KL grade as inclusion criteria, there was no analysis of the effectiveness of turmeric at each level of disease severity. Additionally, inference on the efficacy of turmeric therapy over longer periods of time cannot be made as this study is limited by length of follow-up. None of the studies evaluated the effects of turmeric therapy beyond an 8-month period. Finally, race and ethnicity are important factors which are known to affect the severity and presentation of OA.38 Unfortunately, these variables were unable to be further investigated, which may limit the generalisability of our findings to specific populations not included in the studies we reviewed. Though promising, there is still a limited breadth of high-quality data available to draw a final consensus on the overall utility in treating KOA with turmeric therapy. As KOA continues increase in prevalence across an ageing population with numerous comorbidities, the need for safe alternate therapies has grown. The most significant finding of this review is that due to the overall improved safety profile when compared with NSAIDs and acetaminophen, turmeric therapy can be used as a monotherapy or adjunct therapy without a significant increase of risk to the patient. Factors to keep in mind by healthcare professionals relate to the uncertainty of proper dosing and formulation of this therapy.
Conclusion
The results of this systematic review suggest that turmeric therapy may be a beneficial addition to our current treatment regimen for patients with KOA. Although limitations exist within the 10 RCTs reviewed, this small set of studies show a reduction in pain and improvement in function similar to that of NSAIDs but with a reduced incidence of adverse events. Turmeric appears to be a safe adjunct to NSAID therapy allowing for additional analgesic benefit as well as a reduced dosage requirement for NSAIDs. Future research should focus on standardising the dosing and formulation of turmeric therapy. Emphasis should also be placed on assessing the effect of turmeric therapy on different clinical variables including degree OA and phenotype for which optimal benefits can be derived.
Footnotes
Correction notice: This article has been corrected since it first published. The provenance and peer review statement has been included.
Contributors: Kristopher Paultre is the primary author of paper and involved in all portions. Daniel Hernandez is a co-author who contributed to methods, review of the literature and bias assessment. William Cade provided statistical analysis and contributed to Table 3. Dylan Greif provided formatting of manuscript, tables and charts. John Reynolds provided guidance on strategies for database search. Thomas Best was the supervising PI for the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Havelaar AH, Kirk MD, Torgerson PR, et al. World Health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 2015;12:e1001923. 10.1371/journal.pmed.1001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35. 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffries MA. Osteoarthritis year in review 2018: genetics and epigenetics. Osteoarthritis Cartilage 2019;27:371–7. 10.1016/j.joca.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen J, Abu-Amer Y, O'Keefe RJ, et al. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res 2017;58:49–63. 10.1080/03008207.2016.1208655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:412–20. 10.1038/nrrheum.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter D, Pietro-Alhambra D, Arden N. Osteoarthritis: the facts. Oxford: Oxford University Press, 2014. [Google Scholar]
- 8.Dell'Isola A, Allan R, Smith SL, et al. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2016;17:425. 10.1186/s12891-016-1286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Esch M, Knoop J, van der Leeden M, et al. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthritis Cartilage 2015;23:544–9. 10.1016/j.joca.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 10.Thomas S, Browne H, Mobasheri A, et al. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology 2018;57:iv61–74. 10.1093/rheumatology/key011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 12.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2020;72:149–62. 10.1002/acr.24131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Machado GC, Eyles JP, et al. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med 2018;52:167–75. 10.1136/bjsports-2016-097333 [DOI] [PubMed] [Google Scholar]
- 14.Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods 2017 10.3390/foods6100092. [Epub ahead of print: 22 10 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 2017;57:2889–95. 10.1080/10408398.2015.1077195 [DOI] [PubMed] [Google Scholar]
- 16.Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene 1999;18:6013–20. 10.1038/sj.onc.1202980 [DOI] [PubMed] [Google Scholar]
- 17.Mobasheri A, Henrotin Y, Biesalski H-K, et al. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int J Mol Sci 2012;13:4202–32. 10.3390/ijms13044202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daily JW, Yang M, Park S. Efficacy of Turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food 2016;19:717–29. 10.1089/jmf.2016.3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth A, Clarke M, Dooley G, et al. The nuts and bolts of Prospero: an international prospective register of systematic reviews. Syst Rev 2012;1:2. 10.1186/2046-4053-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:e1–37. 10.1016/j.jclinepi.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandis N, Chung B, Scherer RW, et al. Consort 2010 statement: extension checklist for reporting within person randomised trials. BMJ 2017;357:j2835. 10.1136/bmj.j2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. Academic press, 2013. [Google Scholar]
- 26.Panda SK, Nirvanashetty S, Parachur VA, et al. A randomized, double blind, placebo controlled, parallel-group study to evaluate the safety and efficacy of Curene® versus placebo in reducing symptoms of knee oa. Biomed Res Int 2018;2018:1–8. 10.1155/2018/5291945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa Y, Mukai S, Yamada S, et al. Short-Term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci 2014;19:933–9. 10.1007/s00776-014-0633-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology 2013;21:129–36. 10.1007/s10787-012-0163-3 [DOI] [PubMed] [Google Scholar]
- 29.Belcaro G, Cesarone MR, Dugall M, et al. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev 2010;15:337–44. [PubMed] [Google Scholar]
- 30.Shep D, Khanwelkar C, Gade P, et al. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: a randomized open-label parallel-arm study. Trials 2019;20:214. 10.1186/s13063-019-3327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panahi Y, Rahimnia A-R, Sharafi M, et al. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res 2014;28:1625–31. 10.1002/ptr.5174 [DOI] [PubMed] [Google Scholar]
- 32.Henrotin Y, Malaise M, Wittoek R, et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: a double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res Ther 2019;21:179. 10.1186/s13075-019-1960-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, et al. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med 2009;15:891–7. 10.1089/acm.2008.0186 [DOI] [PubMed] [Google Scholar]
- 34.Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging 2014;9:451–8. 10.2147/CIA.S58535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava S, Saksena AK, Khattri S, et al. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016;24:377–88. 10.1007/s10787-016-0289-9 [DOI] [PubMed] [Google Scholar]
- 36.Shen L, Liu C-C, An C-Y, et al. How does curcumin work with poor bioavailability? clues from experimental and theoretical studies. Sci Rep 2016;6:6. 10.1038/srep20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Sun D, Bi X, et al. Pharmacokinetic based study on "lagged stimulation" of Curcumae Longae Rhizoma - Piper nigrum couplet in their main active components' metabolism using UPLC-MS-MS. Phytomedicine 2017;27:15–22. 10.1016/j.phymed.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 38.Roberts J, Burch TA. Osteoarthritis prevalence in adults by age, sex, race, and geographic area. Vital Health Stat 11 1966;15:1–27. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2020-000935supp001.pdf (21.4KB, pdf)