Abstract
Background
Non-invasive biomarkers for the assessment of disease severity in idiopathic pulmonary fibrosis (IPF) are urgently needed. Calprotectin belongs to the S-100 proteins produced by neutrophils, which likely contribute to IPF pathogenesis. Calprotectin is a well-established biomarker in inflammatory bowel diseases. In this cross-sectional study, we aimed to establish the potential role of calprotectin as a biomarker in IPF. Specifically, we hypothesised that patients with IPF have higher serum calprotectin levels compared with healthy controls, and that calprotectin levels are associated with disease severity.
Methods
Blood samples were obtained from healthy volunteers (n=26) and from two independent IPF cohorts (derivation cohort n=26, validation cohort n=66). Serum calprotectin levels were measured with a commercial kit adapted for that purpose and compared between healthy controls and patients with IPF. Clinical parameters, including forced vital capacity, diffusing capacity for carbon monoxide (DLCO) and the Composite Physiologic Index (CPI), were correlated with calprotectin serum levels.
Results
The IPF derivation cohort showed increased serum calprotectin levels compared with healthy controls (2.47±1.67 vs 0.97±0.53 µg/mL, p<0.001). In addition, serum calprotectin levels correlated with DLCO% predicted (r=−0.53, p=0.007) and with CPI (r=0.66, p=0.007). These findings were confirmed in an independent IPF validation cohort.
Conclusion
Serum calprotectin levels are significantly increased in patients with IPF compared with healthy controls and correlate with DLCO and CPI. Calprotectin might be a potential new biomarker for disease severity in IPF.
Keywords: interstitial fibrosis
Key message.
We investigated the relevance/association of serum calprotectin levels with the disease severity of patients with idiopathic pulmonary fibrosis (IPF).
Serum calprotectin levels are significantly higher in patients with IPF compared with healthy controls and correlate with disease severity in two independent IPF cohorts.
This is the first study demonstrating a potential role of serum calprotectin as a biomarker of disease severity in patients with IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF) is the prototype of severe fibrosing interstitial lung diseases (ILD).1 The progressive loss of alveolar epithelial cells (AEC) in IPF induces abnormal lung fibroblast accumulation and extracellular matrix (ECM) deposition,2 leading to progressive loss of pulmonary function in affected patients.3 Two antifibrotic drugs are available to slow down disease progression.4 5 The assessment of disease severity and progression are important to evaluate the natural course of disease, predict prognosis and estimate treatment response in the individual patient. Pulmonary function tests (PFT) are the standard to assess disease progression. However, PFTs have important weaknesses, including intrapatient variability, poor sensitivity to change, as well as possible difficulties in acquisition (eg, in patients with advanced disease). Alternative non-invasive biomarkers that capture changes in disease severity and progression of IPF are urgently needed for clinical practice and to optimise future clinical trial design.6
Calprotectin is an heterodimer complex that belongs to the S-100 family and has been investigated as biomarker and treatment target in various diseases.7 This complex is formed by S100A8 and S100A9 subunits, also mentioned in the literature as calgranulin A and B, MRP8 and MRP14 or 27E10 antigen.8 Calprotectin is expressed in several immune cells, including macrophages and monocytes, as well as neutrophils,9 and belongs to the damage-associated molecular pattern proteins (DAMP), an important group of proteins of the innate immune system that promote inflammatory responses.10 Involvement in the inflammatory and the innate immune response have been described.11 In clinical routine, calprotectin is a well-established biomarker for disease activity in inflammatory bowel diseases (IBD).12 Furthermore, calprotectin has already been investigated in fibrotic diseases such as systemic sclerosis13 and skin fibrosis.14
Although the role of inflammation is controversial in IPF,15 some previous observations support the idea of a moderate role of inflammation in IPF. For example, neutrophil count is elevated in bronchoalveolar lavage fluid (BALF) from patients with IPF which correlates as well with mortality.16 BALF levels of calprotectin subunits (ie, S100A9) are higher in IPF compared with other ILDs and healthy controls.17–19 Collectively, these findings underline the potential role of calprotectin as a biomarker for disease activity and severity in fibrotic diseases, specifically IPF.
We hypothesised that serum calprotectin levels are higher in patients with IPF than in healthy controls, and correlate with disease severity in patients with IPF. We tested our hypotheses in a derivation cohort of Swiss patients with IPF and validated our findings in an independent cohort of Spanish patients with IPF.
Methods
Study participants and measurements
Healthy controls and Swiss IPF derivation cohort
Blood serum samples from 26 healthy controls without known respiratory disease and normal pulmonary function, and from 26 patients with IPF were collected within the prospective Swiss IIP cohort study in Bern. Patients with IPF were diagnosed according to the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) guidelines.3 Clinical features such as age, sex, body mass index, smoking (pack-years) and PFTs were collected.
The Composite Physiologic Index (CPI), which reflects the morphological extent of pulmonary fibrosis, was calculated by using forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and diffusing capacity for carbon monoxide (DLCO), as previously described.20
Spanish IPF validation cohort
The validation cohort consisted of 66 patients with IPF diagnosed at the ILD Unit of Bellvitge University Hospital, in Barcelona. Patients’ diagnosis was made following the ATS/ERS/JRS/ALAT guidelines.3 In addition, patient’s characteristics and PFTs were recorded. All patients signed written informed consent before inclusion in both cohorts.
Calprotectin measurement
Peripheral blood samples were obtained from all participants and serum fraction was separated by centrifugation at 2000 x g for 15 min within an hour of blood extraction before storage at −20°C until use. All available samples from both cohorts were analysed in the Centre of Laboratory Medicine at the Bern University Hospital, Switzerland.
A commercially available enzyme-linked chemiluminescent immunoassay (CLIA) kit for faecal calprotectin (701350, QuantaFlash, INOVA Diagnostics, San Diego, California, USA) was adapted to measure circulating calprotectin in serum samples. Serum concentrations of calprotectin were converted from µg/g stool into µg/mL by multiplying the values by a factor of 0.023. Using a receiver operating characteristic (ROC) analysis in our Swiss cohort, a cut-off of 1.133 µg/mL calprotectin resulted in a sensitivity of 76.92% (95% CI 57.95% to 88.97%) and a specificity of 80.77% (95% CI 62.12% to 91.49%).
Patient and public involvement
Patients and the public were not involved in the design and conduct of this study, choice of outcome measures, nor recruitment. The results of this study will not be disseminated to the participants and linked communities.
Statistical analysis
Clinical features and serum calprotectin levels were reported as mean (SD) or median (IQR) depending on the distribution of the variables assessed by histograms.
Wilcoxon signed-rank tests were used to compare calprotectin levels between two groups, Spearman’s correlation was used for unadjusted correlation between serum calprotectin levels and continuous clinical variables. Multivariable linear regression models were used to determine an independent relationship between serum calprotectin levels and IPF severity. Calprotectin values were log transformed to account for the non-normal distribution, and potential confounders of conceptual importance (eg, age, sex or smoking) or a relevant unadjusted correlation with serum calprotectin levels (predictor) and IPF severity (outcome) were included in the models. The coefficient of determination (R2) was used to assess the percent of variance in calprotectin that was explained by the linear models. The Akaike information criterion was used to compare the model fit. Kaplan-Meier curves were used to illustrate the association of serum calprotectin levels with survival, HRs from Cox proportional hazard models and ORs from logistic regression models were used to quantify the relationship between calprotectin levels, overall and 1-year survival. A two-sided p<0.05 indicated statistical significance for all comparisons. Data were analysed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria)
Results
Serum calprotectin levels in healthy controls and in patients with IPF
Clinical features of all three groups of participants included in this study are shown in table 1. More than half of healthy controls (54%) were male with an average age of 47.6 years (±12) and 4.5 (0–15) smoked pack-years. Patients with IPF from the derivation and the validation cohort were predominantly male, and older than healthy controls (derivation cohort 69.6±11.5, validation cohort 71.3±7.1) with a more important smoking history. As expected, healthy controls showed normal values in PFTs; while patients with IPF had impaired pulmonary function parameters. The patients in the Swiss derivation cohort showed lower FVC % predicted than patients in the Spanish validation cohort (62.4±16.1 vs 81.7%±17.9% predicted, respectively).
Table 1.
Baseline characteristics of healthy controls and patients with IPF
| Healthy controls (n=26) | Patients with IPF | ||
| Derivation cohort (n=26) | Validation cohort (n=66) | ||
| Sex, men | 14 (54%) | 24 (92%) | 59 (89%) |
| Age, years | 47.6 (12) | 69.6 (11.5) | 71.3 (7.1) |
| BMI, kg/m2 | 24.4 (3.4) | 26.2 (3.1) | 28.6 (3.6) |
| Ever-smokers | 14 (54%) | 20 (77%) | 47 (71%) |
| Pack-years | 4.5 (0–15) | 30 (20–40) | 20 (0–35) |
| Diabetes | 17 (26%) | ||
| Dyslipidaemia | 29 (44%) | ||
| DLCO, % predicted | 89.4 (11.1) | 44.3 (15.2) | 53.7 (16.9) |
| FVC, % predicted | 101.8 (10.4) | 62.4 (16.1) | 81.7 (17.9) |
| FEV1, % predicted | 95.9 (10.7) | 73.5 (11) | 83.6 (17.2) |
| CPI | 11.6 (8.9) | 50.5 (11.2) | 41.0 (14.2) |
| Calprotectin, µg/mL | 0.89 (0.57–1.12) | 2.16 (1.12–2.85) | 2.90 (1.60–3.78) |
Data expressed in number (percentage), mean (SD) or median (IQR).
BMI, body mass index; CPI, Composite Physiological Index; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis.
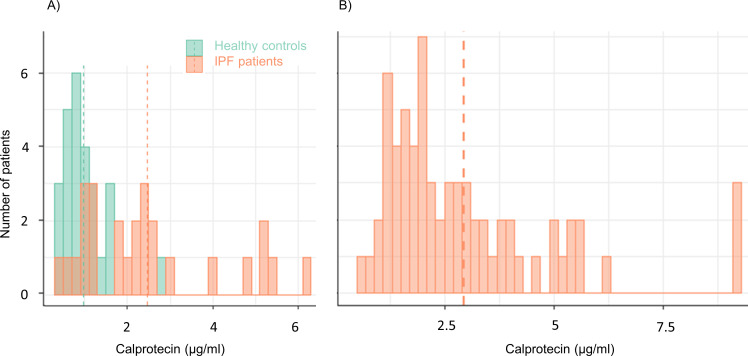
The CLIA analyses of calprotectin revealed significantly higher serum calprotectin levels in patients with IPF compared with healthy controls (2.47±1.67 vs 0.97±0.53 µg/mL, p<0.001, figure 1A). After the adjustment for confounding factors such as age and sex, serum calprotectin levels remained 1.3 µg/mL higher in patients with IPF (95% CI 0.29 to 2.31 µg/mL, p=0.01) compared with healthy controls (online supplemental figure 1).
Figure 1.
Distribution of serum calprotectin levels in patients with IPF and in healthy controls. (A) healthy controls (green) and patients with IPF (orange) in the derivation cohort. (B) Patients with IPF from the validation cohort. Dashed lines indicate mean. IPF, idiopathic pulmonary fibrosis.
bmjresp-2020-000827supp001.pdf (240.2KB, pdf)
Correlation of serum calprotectin levels with IPF disease severity in the derivation cohort
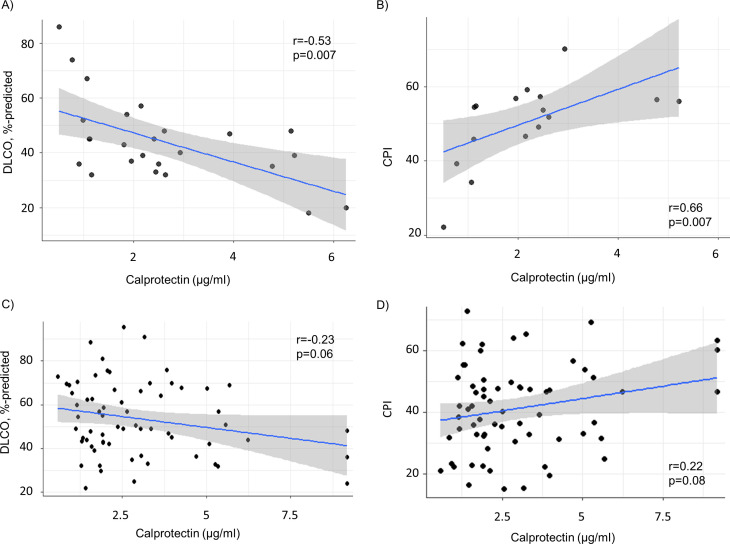
In the Swiss IPF derivation cohort, serum calprotectin levels showed a moderate to strong negative and positive correlation with DLCO% predicted and CPI, respectively (r=−0.53, and r=0.66, respectively, both p<0.01) (table 2 and figure 2A, B).
Table 2.
Unadjusted correlation of baseline characteristics and disease severity with calprotectin serum levels in the three study groups
| Calprotectin levels | Healthy controls | Derivation IPF cohort | Validation IPF cohort | |||
| Variables | r | P value | r | P value | r | P value |
| Sex, men | 0.03 | 0.81 | 0.15 | |||
| Age, years | 0.17 | 0.39 | −0.02 | 0.91 | −0.16 | 0.20 |
| BMI, kg/m2 | 0.50 | 0.009 | 0.12 | 0.63 | 0.01 | 0.94 |
| Ever-smokers | 0.60 | 0.35 | 0.08 | |||
| Pack-years | 0.03 | 0.88 | 0.62 | 0.07 | 0.14 | 0.28 |
| DLCO, % predicted | 0.31 | 0.12 | −0.53 | 0.007 | −0.23 | 0.06 |
| FVC, % predicted | 0.12 | 0.56 | 0.02 | 0.90 | −0.13 | 0.29 |
| FEV1, % predicted | 0.13 | 0.54 | 0.33 | 0.20 | −0.03 | 0.78 |
| CPI | −0.31 | 0.13 | 0.66 | 0.007 | 0.22 | 0.08 |
BMI, body mass index; CPI, Composite Physiological Index; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; r, correlation coefficient.
Figure 2.
Unadjusted correlation between calprotectin levels and predicted DLCO and CPI in the derivation (A, B) and the validation cohort (C, D). CPI, Composite Physiological Index; DLCO, diffusing capacity for carbon monoxide.
In the adjusted models accounting for potential confounding by age, sex and smoked pack-years, DLCO% predicted and CPI both remained significantly correlated with serum calprotectin levels in patients with IPF (table 3).
Table 3.
Multivariable linear regression models for the adjusted correlation of calprotectin with disease severity in the derivation cohort
| Log (calprotectin) | Adjusted for age, sex, smoked pack-years | ||
| Coefficient (95% CI) | P value | Adjusted R2 | |
| DLCO, % predicted | −0.05 (−0.09 to −0.01) | 0.02 | 0.74 |
| CPI | 0.08 (0.03 to 0.12) | 0.01 | 0.85 |
CPI, Composite Physiological Index; DLCO, diffusing capacity for carbon monoxide; R2, coefficient of determination.
Levels of serum calprotectin levels and correlation with IPF disease severity in the validation cohort
To validate our findings from the Swiss IPF cohort, we repeated calprotectin measurements in serum samples from an independent Spanish IPF cohort (baseline characteristics shown in table 1). Increased serum calprotectin levels were confirmed in the IPF validation cohort (figure 1B and online supplemental figure 1).
Correlation of serum calprotectin levels with clinical features of the validation cohort are shown in table 2. Without adjustment for potential confounders, the correlation between serum calprotectin levels and measurements of disease severity was weak (DLCO r=−0.23, p=0.06; and CPI r=0.22, p=0.08) (table 2 and figure 2C, D). After adjustment for age, sex, smoking status and treatment in individual multivariable linear regression models (table 4), DLCO% predicted and CPI were independently associated with serum calprotectin levels in the Spanish IPF cohort.
Table 4.
Multivariable linear regression models for the adjusted correlation of calprotectin with disease severity in the validation cohort
| Log (calprotectin) | Adjusted for age, sex, smoking status and pirfenidone treatment | ||
| Coefficient (95% CI) | P value | Adjusted R2 | |
| DLCO, % predicted | −0.01 (−0.002 to −0.020) | 0.01 | 0.16 |
| CPI | 0.014 (0.003 to 0.024) | 0.01 | 0.17 |
CPI, Composite Physiological Index; DLCO, diffusing capacity for carbon monoxide; R2, coefficient of determination.
Levels of serum calprotectin and mortality
In the derivation cohort, patients with calprotectin levels above the highest quartile (≥2.85 µg/mL) had significantly worse survival compared with patients with calprotectin levels (HR 6.10, 95%CI 1.45 to 25.6, p=0.01) (online supplemental figure 2A). The relationship between high calprotectin and mortality remained significant after adjustment for age and sex (HR 4.59, 95% CI 1.10 to 19.1, p=0.04).
In the validation cohort, the highest calprotectin quartile (≥3.78 µg/mL) did not significantly discriminate long-term mortality (HR 1.54, 95%CI 0.53 to 4.44, p=0.43). However, calprotectin levels were significantly associated with 1-year survival, with an OR for mortality per 1 µg/mL increase of 1.46 (95% CI 1.01 to 2.16, p=0.04) (online supplemental figure 2B). This relationship remained significant after adjustment for age and sex (OR 1.53, 95%CI 1.03 to 2.41, p=0.04).
Discussion
Our cross-sectional study including two independent European IPF cohorts shows that patients with IPF have higher serum calprotectin levels compared with healthy controls, and that calprotectin levels correlate with disease severity in IPF. These findings suggest a potential role of calprotectin as a blood biomarker for disease severity in IPF.
The need of new simple, reliable, valid and preferably non-invasive biomarkers in fibrotic lung diseases is undisputable. Over the last decade, our understanding of IPF pathogenesis has grown substantially, and anti-fibrotic drugs have been approved for treatment. However, establishing valid biomarkers for the assessment of disease severity and progression remains challenging.
PFTs are widely available and well-validated to monitor the course of fibrotic ILDs, particularly in the setting of clinical trials.21 However, pulmonary function decline only indirectly reflects disease progression, and the high noise-to-signal ratio in the individual patient makes it challenging to evaluate disease progression from FVC and DLCO trajectories.21–23 Very frequent (even daily) FVC measurements might improve this poor noise-to-signal ratio but have not been established in clinical practice yet. Some patients with advanced disease have difficulties to perform spirometry and DLCO assessment, and alternative methods to assess pulmonary function are emerging.24
Multidimensional indices including demographics and multiple pulmonary function parameters are valid tools to predict radiological disease severity and mortality in IPF.25 A recent study confirmed the prognostic validity of isolated predicted DLCO% measurement and the multidimensional CPI,26 which we used to estimate disease severity in our study population.
Current antifibrotic drugs have improved IPF treatment substantially over the last years. Therefore, the effectiveness of potential new drugs needs to be investigated on the background of currently available antifibrotic medications. This highlights the need for easy-to-measure clinical trial outcomes that are highly sensitive to change. Blood-based biomarkers such as calprotectin might improve outcome assessment in future clinical trials and in individual patients in addition to currently used pulmonary function measurements.
The potential of calprotectin as a biomarker in IPF is underlined by previous studies. Higher levels of the S100A9 subunit of calprotectin have been detected in BALF from patients with IPF compared with patients with sarcoidosis and healthy controls, with BALF S100A9 levels correlating with FVC and DLCO.18 19 The S100A9 subunit of calprotectin might also be involved in other fibrotic ILDs. Greater levels of the S100A9 subunit have also been reported in BALF from patients with idiopathic non-specific interstitial pneumonia with fibrotic pattern,27 suggesting a link between the this subunit and fibrosis in general.
However, the exact source as well as the role of elevated serum calprotectin in patients with IPF remain unclear. Calprotectin is produced by neutrophils and a correlation between the amount of neutrophils in BALF from patients with IPF with the levels of S100A9 subunit of calprotectin has been described.17 18 In addition, BALF neutrophilia is associated with poor prognosis in IPF,16 and other neutrophil-associated proteins have been described as markers of disease severity in patients with IPF.28 In analogy, other chronic lung diseases showing increased neutrophils, such as COPD, plasma calprotectin levels were associated with neutrophil granulocyte count and even mortality.29
Calprotectin has been previously associated with exacerbation and lung functional decline in inflammatory lung diseases such as cystic fibrosis.30 However, the role of inflammation in IPF remains controversial.15 Activation and development of lung fibrosis may involve several inflammatory mechanisms, but the strong effect of transforming growth factor-β (TGF-β) during fibrosis is thought to limit the role of inflammation in IPF.31 Furthermore, previous use of anti-inflammatory and immunosuppressive treatments have been unsuccessful and even harmful in patients with IPF.32
Nevertheless, these therapeutic failures do not exclude a relevant role of inflammation in the IPF disease process. Although inflammatory cells are not a predominant finding in the histopathology of IPF, they might regulate lung fibroblasts behaviour and ECM deposition.33 Involvement of the S100A8 and S100A9 subunits of calprotectin in the fibrotic process and activation was previously suggested.14 In lung fibrosis, S100A9 activates proliferation of lung fibroblasts and induces production of proinflammatory cytokines in vitro through the receptor for advanced glycation endproducts (RAGE).34 RAGE is a multiligand receptor of S-100 family proteins highly expressed in the AECs and is responsible for acceleration of the immune and inflammatory responses.35 Furthermore, the soluble isoform of RAGE might exert a protective response against proinflammatory signals of calprotectin by interfering with receptor binding,36 which is downregulated in patients with IPF.37 Thus, the presence of calprotectin in BALF and serum and the downregulation of inhibitory proteins suggest the involvement of calprotectin in the pathogenesis of IPF. However, with our current study we cannot answer the pathogenetic role of calprotectin.
The present study has some limitations. Our healthy controls were younger, and less frequently men and smokers, which likely confounds the higher calprotectin levels in IPF compared with those controls. We adjusted our analysis for these differences, to account for potential confounding. Unlike to previous reports, we did not assess neutrophils in blood or BALF to confirm a relationship of serum calprotectin with neutrophilia. Furthermore, the cross-sectional design of this study does not allow definite conclusions on a causal relationship between calprotectin and IPF progression; further studies are needed to assess calprotectin as a marker of disease progression, and its prognostic role either in isolation or in combination with clinical and functional parameters. By establishing an age, sex and exposure independent association of serum calprotectin with disease severity in a cohort of patients with IPF with advanced disease and a second cohort with less severe disease, we ensure generalisability of our findings to most IPF cohorts. This pilot project did not aim for integration of calprotectin serum measurements in clinical practice, but demonstrates feasibility and provides prognostic validity of this biomarker in patients with IPF.
In conclusion, we demonstrate that serum calprotectin levels are significantly higher in patients with IPF compared with healthy controls and show a relevant correlation of calprotectin with disease severity measured by DLCO and CPI with confirmation of these findings in an independent validation cohort. Taken together with findings from previous studies we propose serum calprotectin as a new biomarker for disease severity assessment in IPF.
Acknowledgments
Liselotte McEvoy, Tanja Hermann, Anja Renner and all participating patients with IPF and healthy volunteers.
Footnotes
Correction notice: This article has been corrected since it first published. The provenance and peer review statement has been included.
Contributors: Study design: MF-C, MPH, CM and SG. Clinical data and sample collection from derivation cohort: MF-C and SG. Clinical data and sample collection from validation cohort: LP-C, AM-W, CM and MM-M. Calprotectin measurement: MPH. Data analysis and interpretation: MPH, SG, CM, MF-C. Manuscript draft: CM, SG and MF-C. Supervision and coordination of the study: MF-C, MM-M and TKG. Intellectual contribution and manuscript revision: all authors.
Funding: Swiss IIP cohort received unrestricted grants form Roche and Boehringer Ingelheim. Spanish IPF cohort was funded by the Institute of Health Carlos III (ISCIII, PI18/00367), cofunded by FEDER funds/European Regional Development Fund (ERDF)—'a Way to Build Europe' and the Biomedical National Research Network CIBER.
Competing interests: MM-M reports grants and personal fees from Roche, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Esteve-Teijin, personal fees from Pfizer, outside the submitted work. MF-C reports unrestricted grants from Boehringer Ingelheim, and Roche, during the conduct of the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Swiss Ethics Committee (Bern, Switzerland) approved collection and analysis within the prospective Swiss IIP cohort study (Swiss Ethics Committee, Bern, approval number KEK 246/15 PB_2016-01524). The Ethical Committee on Clinical Research from Bellvitge University Hospital (Barcelona, Spain) approved collection and analysis of samples (PR082/15).
References
- 1.Leonard-Duke J, Evans S, Hannan RT, et al. Multi-Scale models of lung fibrosis. Matrix Biol 2020;91-92:35–50. 10.1016/j.matbio.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc 2015;12 Suppl 1:S16–20. 10.1513/AnnalsATS.201410-448MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 6.Drakopanagiotakis F, Wujak L, Wygrecka M, et al. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol 2018;68-69:404–21. 10.1016/j.matbio.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 7.Pruenster M, Vogl T, Roth J, et al. S100A8/A9: from basic science to clinical application. Pharmacol Ther 2016;167:120–31. 10.1016/j.pharmthera.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 8.Stríz I, Trebichavský I. Calprotectin - a pleiotropic molecule in acute and chronic inflammation. Physiol Res 2004;53:245–53. [PubMed] [Google Scholar]
- 9.Edgeworth J, Gorman M, Bennett R, et al. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem 1991;266:7706–13. [PubMed] [Google Scholar]
- 10.Foell D, Wittkowski H, Vogl T, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007;81:28–37. 10.1189/jlb.0306170 [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Song R, Wang Z, et al. S100A8/A9 in inflammation. Front Immunol 2018;9:1298. 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mari A, Baker FA, Mahamid M, et al. Clinical utility of fecal calprotectin: potential applications beyond inflammatory bowel disease for the primary care physician. Ann Gastroenterol 2019;32:425–30. 10.20524/aog.2019.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Bon L, Cossu M, Loof A, et al. Proteomic analysis of plasma identifies the Toll-like receptor agonists S100A8/A9 as a novel possible marker for systemic sclerosis phenotype. Ann Rheum Dis 2014;73:1585–9. 10.1136/annrheumdis-2013-205013 [DOI] [PubMed] [Google Scholar]
- 14.Zhong A, Xu W, Zhao J, et al. S100A8 and S100A9 are induced by decreased hydration in the epidermis and promote fibroblast activation and fibrosis in the dermis. Am J Pathol 2016;186:109–22. 10.1016/j.ajpath.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 15.Heukels P, Moor CC, von der Thüsen JH, et al. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 2019;147:79–91. 10.1016/j.rmed.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 16.Kinder BW, Brown KK, Schwarz MI, et al. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008;133:226–32. 10.1378/chest.07-1948 [DOI] [PubMed] [Google Scholar]
- 17.Hara A, Sakamoto N, Ishimatsu Y, et al. S100A9 in BALF is a candidate biomarker of idiopathic pulmonary fibrosis. Respir Med 2012;106:571–80. 10.1016/j.rmed.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 18.Korthagen NM, Nagtegaal MM, van Moorsel CHM, et al. Mrp14 is elevated in the bronchoalveolar lavage fluid of fibrosing interstitial lung diseases. Clin Exp Immunol 2010;161:no–347. 10.1111/j.1365-2249.2010.04181.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bargagli E, Olivieri C, Cintorino M, et al. Calgranulin B (S100A9/MRP14): a key molecule in idiopathic pulmonary fibrosis? Inflammation 2011;34:85–91. 10.1007/s10753-010-9210-7 [DOI] [PubMed] [Google Scholar]
- 20.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962–9. 10.1164/rccm.2111053 [DOI] [PubMed] [Google Scholar]
- 21.Paterniti MO, Bi Y, Rekić D, et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2017;14:1395–402. 10.1513/AnnalsATS.201606-458OC [DOI] [PubMed] [Google Scholar]
- 22.Nathan SD, Yang M, Morgenthien EA, et al. FVC variability in patients with idiopathic pulmonary fibrosis and role of 6-min walk test to predict further change. Eur Respir J 2020;55:1902151. 10.1183/13993003.02151-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt SL, Tayob N, Han MK, et al. Predicting pulmonary fibrosis disease course from past trends in pulmonary function. Chest 2014;145:579–85. 10.1378/chest.13-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyilas S, Schreder T, Singer F, et al. Multiple breath washout: a new and promising lung function test for patients with idiopathic pulmonary fibrosis. Respirology 2018;23:764–70. 10.1111/resp.13294 [DOI] [PubMed] [Google Scholar]
- 25.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684–95. 10.7326/0003-4819-156-10-201205150-00004 [DOI] [PubMed] [Google Scholar]
- 26.Sharp C, Adamali HI, Millar AB. A comparison of published multidimensional indices to predict outcome in idiopathic pulmonary fibrosis. ERJ Open Res 2017;3:00096-2016–2016. 10.1183/23120541.00096-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett D, Salvini M, Fui A, et al. Calgranulin B and KL-6 in bronchoalveolar lavage of patients with IPF and NSIP. Inflammation 2019;42:463–70. 10.1007/s10753-018-00955-2 [DOI] [PubMed] [Google Scholar]
- 28.Ikezoe K, Handa T, Mori K, et al. Neutrophil gelatinase-associated lipocalin in idiopathic pulmonary fibrosis. Eur Respir J 2014;43:1807–9. 10.1183/09031936.00192613 [DOI] [PubMed] [Google Scholar]
- 29.Sørensen AK, Holmgaard DB, Mygind LH, et al. Neutrophil-to-lymphocyte ratio, calprotectin and YKL-40 in patients with chronic obstructive pulmonary disease: correlations and 5-year mortality - a cohort study. J Inflamm 2015;12:20. 10.1186/s12950-015-0064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid PA, McAllister DA, Boyd AC, et al. Measurement of serum calprotectin in stable patients predicts exacerbation and lung function decline in cystic fibrosis. Am J Respir Crit Care Med 2015;191:233–6. 10.1164/rccm.201407-1365LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 2009;175:3–16. 10.2353/ajpath.2009.081170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghu G, Anstrom KJ, Talmadge E. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968–77. 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai O, Winkler J, Minasyan M, et al. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med 2018;5:43. 10.3389/fmed.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Chen H, Zhu X, et al. S100A9 promotes human lung fibroblast cells activation through receptor for advanced glycation end-product-mediated extracellular-regulated kinase 1/2, mitogen-activated protein-kinase and nuclear factor-κB-dependent pathways. Clin Exp Immunol 2013;173:523–35. 10.1111/cei.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt AM, Yan SD, Yan SF, et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001;108:949–55. 10.1172/JCI200114002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bramhall M, Rich K, Chakraborty A, et al. Differential expression of soluble receptor for advanced glycation end-products in mice susceptible or resistant to chronic colitis. Inflamm Bowel Dis 2020;26:360–8. 10.1093/ibd/izz311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machahua C, Montes-Worboys A, Planas-Cerezales L, et al. Serum AGE/RAGEs as potential biomarker in idiopathic pulmonary fibrosis. Respir Res 2018;19:215. 10.1186/s12931-018-0924-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000827supp001.pdf (240.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request from the corresponding author.