Abstract
This study aimed to evaluate the effect of methionine dipeptide supplementation on the meat quality of broilers subjected to heat stress. A completely randomized 3 × 2 factorial design with four repetitions of each treatment was used. Three diets, unsupplemented (U), supplemented with methionine (M), and supplemented with methionine dipeptide (MM), were fed to 96 broilers subjected to thermal comfort (TC) or heat stress (HS, 32 °C for 24 h) conditions antemortem. Meat quality parameters, total antioxidant capacity (TAC), protein and lipid oxidation, and ryanodine receptor type 3 (RYR3) gene expression in breast muscle of 35-day-old broilers were evaluated. Methionine supplementation (M and MM) enhanced the nutritional quality of breast meat. Diet had a significant effect on breast meat pH, color (a*), and nitrogen and lipid contents. Interaction effects of diet and HS on TAC and protein oxidation were not observed. Diet and HS influenced lipid oxidation of breast meat after 7 days of refrigerated storage. High RYR3 expression was observed in breast meat of broilers subjected to heat stress and fed the U diet. No differences were observed between M and MM diets in any of the parameters evaluated. The results showed that both sources of methionine (M and MM) can be supplemented in broiler diets with beneficial effects on breast yield and meat nutritional quality. In addition, HS has made chickens more susceptible to biomolecule oxidation, and MM can potentiate chicken TAC. Further study is needed to better understand the effects of MM on broilers.
Keywords: Heat stress, Methionine, Dipeptide, Antioxidant, Oxidation
Introduction
Ambient temperature is one of the most important factors in broiler production. When temperature exceed the thermal comfort zone, broilers can suffer heat stress, leading to considerable economic losses (Lara and Rostagno 2013). Heat stress affects digestion and nutrient absorption in broilers by altering the expression of genes encoding digestive enzymes (Wang et al. 1997). In an attempt to dissipate heat, birds increase blood flow to the periphery of the body in detriment to the intestine. The low supply of blood and oxygen to gut tissues impairs the integrity of the intestinal barrier (Leon and Helwig 2010. Furthermore, when under stress, broilers direct their energy and nutrients toward the maintenance of vital functions (such as thermoregulation) instead of anabolic processes (Costa et al. 2014).
Heat stress can impair muscle growth and structure (Van Laack et al. 2000). The biochemical changes occurring in response to stressful conditions reduce the efficiency with which birds convert food into muscles (Classen 2000). In particular, antemortem heat stress may affect the nutritional and functional quality of meat (Zhang et al. 2015). The physiological imbalance caused by stress influences the expression of the ryanodine receptor gene, which controls calcium release (Goes et al. 2015), and the levels of muscle glycogen, responsible for postmortem biochemical reactions that determine meat quality (Rodrigues and Silva 2016).
Other bioprocesses affected by heat stress are antioxidant activity and free radical generation. Free radicals, such as reactive oxygen and nitrogen species, are highly toxic substances capable of reacting with important macromolecules, such as proteins and lipids (Stadtman and Levine 2003). Even a transient state of oxidative stress is believed to affect animal performance (Del Vesco and Gasparino 2013). Studies have investigated the use of antioxidant compounds, such as vitamins A and E (Souza et al. 2011) and amino acids (Del Vesco et al. 2015), in broiler diets as a means of mitigating the adverse effects of heat stress.
Methionine is a proteogenic amino acid with an important role in initiating the translation of proteins associated with body functions (e.g., muscle deposition). Its direct and indirect antioxidant properties help minimize the harmful effects of free radicals (Levine et al. 1996). Methionine is involved in glutathione synthesis (Wang et al. 1997) and is one of the most readily oxidized residues in proteins (Levine et al. 1996). The beneficial effects of methionine supplementation on antioxidant activity in heat-stressed birds have been demonstrated (Del Vesco et al. 2015; Gasparino et al. 2018).
Methionine is commonly supplemented as a free amino acid. Recent studies, however, have pointed to the use of methionine dipeptide. Khatlab et al. (2019) demonstrated that methionine supplementation (whether as a free amino acid or dipeptide) helped protect broiler intestinal cells from oxidative damage caused by Eimeria infection.
The purpose of the present study was to test the hypotheses that heat stress before slaughter influences broiler meat quality and oxidative status and that methionine supplementation minimizes its harmful effects. To test the hypotheses, we evaluated meat quality, total antioxidant capacity, protein and lipid oxidation, and ryanodine receptor type 3 (RYR3) gene expression in breast meat of 35-day-old broilers subjected to antemortem heat stress for 24 h. Differences in the effects of free methionine and methionine dipeptide were also investigated.
Material and methods
This experiment was approved by the Animals Ethics Committee (CEUA) of the State University of Maringá, Brazil (protocol no. 4000170615).
Animals and experimental design
A total of 96 Cobb 500 male broiler chicks aged 1 day were used in this study. Broilers were reared in suspended metal cages (1 m2, 4 chicks per cage) in a temperature-controlled environment under a 24 h photoperiod. The temperature was gradually reduced according to bird age, as recommended by the Cobb 500 management guide.
Birds were raised conventionally up to 10 days of age, after which they were reared following a 3 × 2 factorial completely randomized design with four repetitions per treatment. The first factor was diet: U, unsupplemented diet; M, diet supplemented with free dl-methionine (99%); and MM, diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine, 95%). The second factor was antemortem ambient temperature condition (AT): TC, thermal comfort condition (7 days: 31 ºC; 21 days: 27 ºC; 28 days: 24 ºC; 35 days: 21 ºC - in accordance with the Cobb 500 management guide), and HS, heat stress condition (32 °C for 24 h).
Broilers received diets based on corn and soybean meal, formulated according to the recommendations of Rostagno et al. (2011). Broilers had free access to feed and water throughout the experimental period. Diet ingredients and analyzed and calculated nutrient composition are shown in Table 1. All animals were raised under thermal comfort conditions up to 34 days of age; then, 48 birds were kept in a heated environment (32 °C) for 24 h, while the other 48 birds remained under the thermal comfort condition for 24 h. At 35 days of age, all broilers were killed by cervical dislocation.
Table 1.
Analyzed and calculated nutrient composition of experimental diets
| Ingredients (kg) | 1–20 days | 21–35 days | ||||
|---|---|---|---|---|---|---|
| Ua | M | MM | U | M | MM | |
| Corn (7.8% crude protein) | 54.89 | 54.89 | 54.89 | 59.81 | 59.81 | 59.81 |
| Soybean oil | 3.8 | 3.8 | 3.8 | 4.5 | 4.5 | 4.4 |
| Soybean meal (46% crude protein) | 37.30 | 37.30 | 37.30 | 32.40 | 32.40 | 32.40 |
| Common salt | 0.45 | 0.45 | 0.45 | 0.43 | 0.43 | 0.43 |
| Calcitic lime (38% calcium) | 1.16 | 1.16 | 1.16 | 0.93 | 0.93 | 0.93 |
| Dicalcium phosphate (20% phosphorous) | 1.53 | 1.53 | 1.53 | 1.07 | 1.07 | 1.07 |
| dl-Methionyl-dl-methionine (95%) | – | – | 0.29 | – | – | 0.28 |
| dl-Methionine (99%) | – | 0.28 | – | – | 0.27 | – |
| l-Threonine (98.5%) | 0.04 | 0.04 | 0.04 | 0.02 | 0.02 | 0.02 |
| l-Lysine HCl (78%) | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Vitamin–mineral premixb | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Inert (sand) | 0.30 | 0.02 | 0.01 | 0.29 | 0.02 | 0.01 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrients | ||||||
| Analyzed compositionc (g/kg) | ||||||
| Crude protein | 217 | 220 | 216 | 193 | 195 | 194 |
| Lysine | 12.87 (100)d | 12.78 (100) | 12.59 (100) | 11.42 (100) | 11.32 (100) | 11.26 (100) |
| Methionine | 3.05 (24) | 5.78 (45) | 5.84 (46) | 2.77 (24) | 5.67 (50) | 5.56 (49) |
| Methionine + cysteine | 6.53 (51) | 9.10 (71) | 9.17 (73) | 5.92 (52) | 8.86 (78) | 8.76 (736) |
| Threonine | 8.54 (66) | 8.73 (68) | 8.41 (67) | 7.43 (65) | 7.51 (66) | 7.40 (67) |
| Tryptophan | 10.39 (81) | 10.56 (83) | 10.12 (80) | 9.24 (81) | 9.33 (82) | 9.25 (82) |
| Valine | 9.56 (74) | 9.75 (76) | 9.27 (74) | 8.42 (74) | 8.48 (75) | 8.36 (74) |
| Isoleucine | 14.52 (113) | 14.89 (117) | 14.34 (114) | 12.72 (111) | 12.64 (112) | 12.58 (112) |
| Calculated composition | ||||||
| AMEe (kcal/kg) | 3.053 | 3.052 | 3.052 | 3.169 | 3.169 | 3.169 |
| Calcium (g/kg) | 8.76 | 8.76 | 8.76 | 6.80 | 6.80 | 6.80 |
| Available phosphorus (g/kg) | 4.50 | 4.50 | 4.50 | 3.50 | 3.50 | 3.50 |
| Sodium (g/kg) | 2.00 | 2.00 | 2.00 | 1.90 | 1.90 | 1.90 |
aU, unsupplemented diet, M diet supplemented with dl-methionine, MM diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine)
bDiets supplied the following compounds (per kg): retinyl acetate, 3.44 mg; cholecalciferol, 50 µg; dl-α-tocopherol, 15 mg; thiamine, 1.63 mg; riboflavin, 4.9 mg; pyridoxine, 3.26 mg; cyanocobalamin, 12 mg; d-pantothenic acid, 9.8 mg; d-biotin, 0.1 mg; menadione, 2.4 mg; folic acid, 0.82 mg; niacinamide, 35 mg; selenium, 0.2 mg; iron, 35 mg; copper, 8 mg; manganese, 60 mg; zinc, 50 mg; iodine, 1 mg; butylated hydroxytoluene, 80 mg
cDiets were formulated using the total amino acid contents of corn and soybean meal, determined by near-infrared reflectance spectroscopy. Total amino acid and other nutrient values are shown as g/kg and not as a percentage of the diet. Total amino acid composition was analytically determined by Evonik Industries (Hanau, Germany) using high-performance liquid chromatography
dValues in parentheses indicate the amino acid to lysine ratio (ideal protein concept)
eAME apparent metabolizable energy
Breast meat yield
Broilers were weighed individually before slaughter (live weight). Immediately after slaughter, the breast muscle (pectoralis superficialis) was removed from the carcass and weighed without skin for determination of the relative muscle weight, as shown in Eq. (1).
| 1 |
Breast meat quality
The pH, color, water-holding capacity (WHC), shear force, and moisture, ash, nitrogen, and lipid contents were determined. Broilers were fasted for 12 h prior to slaughter. Then, the breast meat of 8 birds from each treatment was collected, placed on ice, and stored at − 20 °C until analysis.
pH
The pH of breast meat at 24 h postmortem was measured using a portable probe pH meter (Testo 205, Testo, Campinas, Brazil). Measurements were made at three different points in breast muscle.
Color analysis
Breast meat color was analyzed 24 h postmortem at three different points on the surface of the muscle using a CR-400 Chroma Meter (Konica Minolta Ltd., Tokyo, Japan). The colorimeter was previously calibrated using a white plate. Results are expressed as CIELab coordinates L* (lightness), a* (redness), and b* (yellowness).
Proximate composition analysis
Moisture was determined by volatilization and ash content by combustion at 550 °C for 5 h, according to AOAC (2005). Total nitrogen was quantified by the Kjeldahl method using a correction factor of 6.25 (AOAC 2005). Total lipid content was determined according to Bligh and Dyer (1959).
WHC
WHC was determined following the method of Nakamura and Katoh (1981). Briefly, 1 g of pectoralis superficialis was added to a 1.5 mL microtube containing filter paper. The material was centrifuged at 1500 × g for 4 min at 4 °C. Then, the sample was weighed (W1) and oven dried at 70 °C for 12 h. Samples were cooled to room temperature (25 °C) and weighed again (W2). WHC was calculated according to Eq. (2) and is expressed as a percentage.
| 2 |
Shear force
The shear force of raw breast meat was determined using a TA.XTplus texture analyzer (Stable Micro Systems, Surrey, England) equipped with a Warner–Bratzler shear blade. A cut speed of 2 mm/s and return speed of 5 mm/s were used. Texture analysis was performed according to the procedure of the U.S. Meat Animal Research Center (Wheeler et al. 1997). Samples were thawed, wrapped in aluminum foil, labeled, and roasted using a George Foreman electric oven at 180 °C until reaching a core temperature of 40 °C. Then, samples were turned and roasted until the internal temperature reached 71 °C. Cooked meat was packed, cooled at room temperature (25 °C), and cut parallel to muscle fibers into 1 cm cubes. Shear force peaks were recorded and are expressed as kgf/cm2.
Oxidative parameter
In order to evaluate possible oxidative damage, lipid oxidation analyzes of broiler breast were performed after seven days of refrigeration and protein oxidation of broiler breast muscle, as well as the total antioxidant capacity determined in plasma.
Plasma total antioxidant capacity (TAC)
Blood samples of 8 broilers from each treatment were collected from the jugular vein shortly after slaughter using heparin tubes. Samples were centrifuged at 3000 rpm for 10 min, and plasma was separated and stored at − 80 °C until analysis. TAC was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging method, as described in Janaszewska and Bartosz (2002), with modifications. Briefly, 96 µL of 0.1 M potassium phosphate buffer (pH 7.4), 4 µL of plasma, and 100 µL of 0.06 mM DPPH in methanol (Sigma–Aldrich, St. Louis, USA) were added to the wells of a 96-well microplate. Plates were incubated for 30 min at room temperature (25 °C) in the dark. Then, the absorbances of samples and 0.06 mM DPPH in methanol were read at 515 nm using a microplate reader (VersaMax™, Molecular Devices, Sunnyvale, USA). TAC (%) was determined using the equation TAC = [1 − (sample absorbance/DPPH absorbance)] × 100.
Protein oxidation: carbonylated protein content
The concentration of carbonyl groups in breast meat was measured using 2,4-dinitrophenylhydrazine (DNPH, Sigma–Aldrich, St. Louis, USA), as described by Levine et al. (1994). Absorbance was read at 370 nm using an Evolution™ 300 UV-Vis spectrophotometer (Thermo Fisher Scientific, Massachusett, USA). Carbonyl group concentration was quantified using the Beer–Lambert law, A = c × b × ε, where A is the difference between sample absorbance and control absorbance, c is the carbonylated protein concentration, b is the optical path length, and ε is the molar extinction coefficient (22,000 mol/cm). Results are expressed as nmol of carbonyl groups/mg of protein.
Lipid oxidation
Lipid oxidation was determined by the 2-thiobarbituric acid reactive substances (TBARS) method, according to Raharjo et al. (1992). Breast samples (5 g) were stored for seven days under refrigeration at 7° C until the day of analysis. A standard curve of malonaldehyde bis (diethyl acetal) (1 × 10−8 to 10 × 10−8 mol/mL) was constructed. Results are expressed as mg of malonaldehyde/kg of sample.
Gene expression in breast muscle
After slaughter, pectoralis superficialis samples from 6 animals per treatment were collected, mixed with TRIzol™ (Invitrogen, Carlsbad, USA), and stored at − 80 °C until total RNA extraction. Total RNA extraction was performed using TRIzol ™ (Invitrogen, Carlsbad, USA), according to the manufacturer’s recommendations. All materials used in sample collection and gene expression analysis were previously treated with RNase inhibitor (RNase AWAY®, Invitrogen, Carlsbad, USA). Total RNA concentration was determined at 260 nm using a NanoDrop™ 2000c spectrophotometer (Thermo Fisher Scientific). RNA purity and integrity were assessed by determining the absorbance ratios 260/280 and 260/230 and electrophoresis in 1% agarose gel. Bands were stained with SYBR™ Safe DNA gel stain (Invitrogen TM, Carlsbad, CA, USA) and visualized under ultraviolet light (L L-PIX TOUCH, Loccus Biotechnology, Brazil).
To avoid genomic DNA contamination, 1 µg of total RNA was treated with amplification grade DNase I (Invitrogen, Carlsbad CA, USA), according to the manufacturer’s recommendations. Complementary DNA synthesis (cDNA) was performed using the SuperScript™ III First-Strand Synthesis SuperMix kit (Invitrogen Corporation, Brazil), following the manufacturer’s instructions. cDNA samples were stored at − 20 °C until use.
Real-time polymerase chain reaction (qPCR) was carried out on a StepOne™ Real-Time PCR system version 2.3 thermal cycler (Applied Biosystems™, USA) using 5 μL of cDNA diluted to 40–80 ng/mL, 0.5 or 1 μL of each primer (forward and reverse) at 10 µM (final concentration of 400 μm), 12.5 mL of SYBR™ Green PCR Master Mix (Applied Biosystems™, USA), and ultrapure water to complete the volume to 25 μL.
The primer used for RYR3 amplification were designed specifically for Gallus gallus on the basis of gene sequences deposited in the National Center for Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov) using the Integrated DNA Technologies system (www.idtdna.com). The β-actin gene was used as an endogenous control. All reactions were performed in duplicate, and the results are expressed as arbitrary units (AU). The 2−∆CT method was used for the relative quantification of RYR3 expression (Livak and Schmittgen 2001). Primer sequences used in qPCR were as follows: RYR3 (F: 5′-CTGGCAGGAGTCATTTGTGT-3′) (R: 5′-TGGAAGAAATCCCAGCATCT-3′, 59 base pairs), registered under accession no. NM_206874.2, and β-actin (F: 5′-GCCAACAGAGAGAGAGAAGATGAC-3′) (R: 5′-CACCAGTCATCACAATAC-3′, 130 base pairs), registered under accession no. l08165.1. The annealing temperature was 60 °C.
Statistical analysis
Data normality was tested using the Shapiro–Wilk test. Normality assumptions were met, and data were subjected to two-way analysis of variance. The model used to evaluate main and interaction effects was Yijk = µ + αi + τj + ατij + eijk, where Yijk is the dependent variable, µ is the overall mean, αi is the main effect of diet (i = U, M, or MM), τj is the main effect of stress (j = TC or HS), ατij is the interaction effect between diet and stress, and eijk is the residual error. Means were compared by Tukey’s test (P < 0.05). Analyses were performed using SAS version 9.00 (SAS Institute Inc. Cary, USA).
Results
Breast meat yield
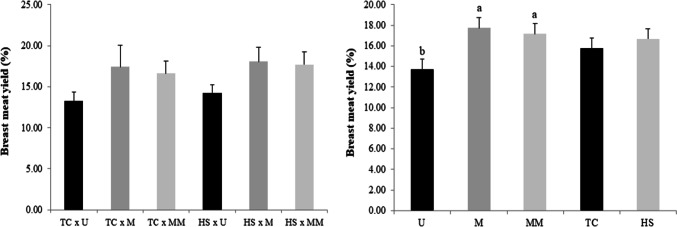
Figure 1 shows the breast meat yield of 35-day-old broilers exposed to thermal comfort and heat stress conditions antemortem. Diet was found to influence breast meat yield (P < 0.01). Broilers fed the U diet had a 21% lower breast yield than methionine-supplemented birds. This result was independent of the form of methionine used. Neither the interaction effect of diet and heat stress (P = 0.91) nor the main effect of heat stress on breast yield (P = 0.06) was significant.
Fig. 1.
Effect of diet and heat stress on the breast meat yield (%) of 35-day-old broilers. a,bDifferent letters represent significant differences by Tukey’s test (P < 0.05). Results are presented as mean and standard deviation. U unsupplemented diet, M diet supplemented with dl-methionine, MM diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine), TC thermal comfort condition, HS heat stress condition
pH and instrumental color of breast meat
The pH and color (L*, a*, and b*) of the muscle pectoralis superficialis of 35-day-old broilers were determined at 24 h postmortem (Table 2). There was an interaction effect of diet and heat stress on the parameter b* (P = 0.04). Heat-stressed broilers fed the U diet had the lowest b* values in breast meat. Diet influenced breast meat pH and a*; these variables were higher in birds fed the U diet. Antemortem ambient temperature was also found to influence the pH of breast meat (P < 0.01). Exposure to heat stress was associated with lower pH values. No treatment effects were observed for L* (P > 0.05).
Proximate composition, WHC, and shear force of breast meat
Table 3 presents the proximate composition, WHC, and textural characteristics of the breast meat of 35-day-old broilers. No interaction effect was found. The main effect of diet on nitrogen and lipid contents in breast meat was significant (P = 0.03 and P < 0.01, respectively). Birds fed M and MM diets had higher nitrogen content and lower lipid content than those fed the U diet. Diet had no effect on moisture, ash content, WHC, or shear force of breast meat (P > 0.05). Broilers exposed to antemortem heat stress had lower breast meat moisture. There was no effect of ambient temperature on ash, nitrogen, lipid content, WHC, or shear force (P > 0.05).
Table 2.
pH and color (L*, a*, and b*) of breast meat (pectoralis superficialis) of 35-day-old broilers at 24 h postmortem
| Treatment | pH | L* | a* | b* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| TC | |||||||||
| U | 5.99 | 0.11 | 54.50 | 3.01 | 1.68 | 1.08 | 8.38b | 1.98 | |
| M | 5.96 | 0.09 | 54.55 | 2.18 | 0.35 | 0.82 | 7.88b | 1.29 | |
| MM | 5.94 | 0.10 | 55.34 | 2.92 | 0.18 | 1.44 | 11.13a | 3.91 | |
| HS | |||||||||
| U | 5.95 | 0.06 | 55.30 | 2.17 | 3.46 | 1.50 | 8.09b | 0.75 | |
| M | 5.79 | 0.09 | 55.85 | 2.89 | 0.73 | 1.10 | 9.65a | 1.73 | |
| MM | 5.84 | 0.09 | 55.7 | 1.85 | 1.24 | 2.55 | 8.96ab | 1.43 | |
| Main effects | |||||||||
| Diet | |||||||||
| U | 5.97a | 0.09 | 54.90 | 2.57 | 2.57a | 1.57 | 8.24 | 1.45 | |
| M | 5.87b | 0.12 | 55.20 | 2.56 | 0.54b | 0.96 | 8.77 | 1.74 | |
| MM | 5.89b | 0.11 | 55.55 | 2.37 | 0.71b | 2.07 | 10.05 | 3.05 | |
| AT | |||||||||
| TC | 5.96a | 0.10 | 54.80 | 2.64 | 0.74b | 1.29 | 9.13 | 2.91 | |
| HS | 5.86b | 0.11 | 55.64 | 2.25 | 1.81a | 2.12 | 8.90 | 1.46 | |
| P-value | |||||||||
| Diet | 0.008 | 0.773 | 0.001 | 0,054 | |||||
| AT | 0.003 | 0.259 | 0.018 | 0,706 | |||||
| Diet × AT | 0.126 | 0.883 | 0.432 | 0.039 | |||||
Results are presented as mean and standard deviation (SD).
L* lightness, a* redness, b* yellowness, U unsupplemented diet, M diet supplemented with dl-methionine, MM diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine), AT ambient temperature, TC thermal comfort condition, HS heat stress condition
a,b,cMeans in a column followed by different letters differ significantly by Tukey’s test (P < 0.05).
The main and interaction effects of diet and heat stress on lipid oxidation, TAC, and protein oxidation in breast meat are presented in Table 4. The interaction of diet and heat stress on lipid oxidation was not significant. However, the main effects of diet (P = 0.03) and heat stress (P < 0.01) were. At 7 days postmortem, the breast meat of U broilers exposed to heat stress had a high malonaldehyde concentration (0.20 mg/kg). Birds fed the MM diet had the lowest malonaldehyde content in breast meat (about 23% lower than that of U broilers).
Table 4.
Lipid oxidation (after 7 days of storage at 7 °C), protein oxidation (carbonylated proteins, CP), and plasma total antioxidant capacity (TAC) of 35-day-old broilers
| Treatment | Lipid oxidation1 | CP2 | TAC (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| TC | |||||||
| U | 0.14 | 0.04 | 0.58 | 0.18 | 42.16 | 6.26 | |
| M | 0.12 | 0.03 | 0.68 | 0.09 | 46.64 | 2.55 | |
| MM | 0.12 | 0.02 | 0.57 | 0.11 | 48.34 | 3.90 | |
| HS | |||||||
| U | 0.20 | 0.08 | 0.70 | 0.15 | 38.47 | 7.76 | |
| M | 0.16 | 0.04 | 0.71 | 0.09 | 45.31 | 8.65 | |
| MM | 0.14 | 0.03 | 0.67 | 0.11 | 37.05 | 6.26 | |
| Main effects | |||||||
| Diet | |||||||
| U | 0.17a | 0.09 | 0.64 | 0.16 | 40.32b | 7.00 | |
| M | 0.14ab | 0.04 | 0.70 | 0.09 | 41.85ab | 7.87 | |
| MM | 0.13b | 0.03 | 0.63 | 0.12 | 46.83a | 5.22 | |
| AT | |||||||
| TC | 0.13b | 0.03 | 0.61b | 0.13 | 45.71a | 5.01 | |
| HS | 0.17a | 0.06 | 0.70a | 0.11 | 40.28b | 8.06 | |
| P-value | |||||||
| Diet | 0.003 | 0.370 | 0.040 | ||||
| AT | < 0.001 | 0.030 | 0.010 | ||||
| Diet × AT | 0.230 | 0.510 | 0.380 | ||||
Results are presented as mean and standard deviation (SD).
U unsupplemented diet, M diet supplemented with dl-methionine, MM diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine), AT ambient temperature, TC thermal comfort condition, HS heat stress condition
a,b,cMeans in a column followed by different letters differ significantly by Tukey’s test (P < 0.05).
1Determined in breast muscle and expressed as mg of malonaldehyde/kg of sample
2Determined in breast muscle and expressed as nmol of carbonyl groups/mg of protein
Table 3.
Proximate composition, water-holding capacity (WHC), and shear force (SF) of broiler breast meat
| Treatment | Moisture (%) | Ash (%) | Nitrogen (%) | Lipids (%) | WHC (%) | SF (kgf/cm2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| TC | |||||||||||||
| U | 74.78 | 0.76 | 1.22 | 0.08 | 22.44 | 0.23 | 1.77 | 0.42 | 61.99 | 0.55 | 1.83 | 0.49 | |
| M | 75.38 | 0.47 | 1.33 | 0.18 | 23.79 | 1.03 | 1.48 | 0.43 | 59.50 | 1.84 | 2.52 | 0.72 | |
| MM | 75.11 | 0.76 | 1.21 | 0.19 | 24.71 | 1.67 | 1.19 | 0.37 | 60.99 | 1.83 | 2.28 | 0.70 | |
| HS | |||||||||||||
| U | 74.40 | 0.54 | 1.14 | 0.07 | 23.33 | 1.86 | 2.00 | 0.50 | 61.83 | 2.35 | 2.32 | 0.19 | |
| M | 74.53 | 1.36 | 1.27 | 0.20 | 23.90 | 1.15 | 1.30 | 0.45 | 60.11 | 3.82 | 2.34 | 0.38 | |
| MM | 74.37 | 0.69 | 1.38 | 0.11 | 23.73 | 1.48 | 1.21 | 0.26 | 62.12 | 0.7 | 2.61 | 0.70 | |
| Main effects | |||||||||||||
| Diet | |||||||||||||
| U | 74.59 | 0.66 | 1.18 | 0.09 | 22.89b | 1.36 | 1.89a | 0.46 | 60.82 | 1.79 | 2.08 | 0.44 | |
| M | 74.93 | 1.10 | 1.32 | 0.19 | 23.85ab | 1.05 | 1.39b | 0.43 | 61.35 | 2.63 | 2.42 | 0.55 | |
| MM | 74.74 | 0.80 | 1.29 | 0.17 | 24.25a | 1.61 | 1.20b | 0.31 | 59.80 | 2.88 | 2.44 | 0.70 | |
| AT | |||||||||||||
| TC | 75.11a | 0.68 | 1.26 | 0.16 | 23.70 | 1.47 | 1.48 | 0.46 | 61.55 | 1.45 | 2.23 | 0.68 | |
| HS | 74.44b | 0.92 | 1.26 | 0.16 | 23.65 | 1.47 | 1.51 | 0.54 | 61.91 | 1.63 | 2.42 | 0.47 | |
| P-value | |||||||||||||
| Diet | 0.525 | 0.083 | 0.035 | 0.001 | 0.050 | 0.200 | |||||||
| AT | 0.015 | 0.998 | 0.988 | 0.839 | 0.467 | 0.248 | |||||||
| Diet × AT | 0.760 | 0.072 | 0.191 | 0.387 | 0.762 | 0.276 | |||||||
Results are presented as mean and standard deviation (SD).
a,b,cMeans in a column followed by different letters differ significantly by Tukey’s test (P < 0.05).
U unsupplemented diet, M diet supplemented with dl-methionine, MM diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine), AT ambient temperature, TC thermal comfort condition, HS heat stress condition
There was no interaction effect between diet and heat stress on TAC or protein oxidation. On the other hand, the main effect of diet on TAC was significant (P = 0.04). Birds fed the MM diet had higher TAC, whereas those fed U and MM diets did not differ from each other. No effect of diet on protein oxidation (P = 0.37) was observed. Heat stress influenced both TAC (P = 0.01) and protein oxidation (P = 0.03). Broilers exposed to heat stress had a 12% reduction in TAC and a 13% increase in protein oxidation compared to broilers kept under moderate temperature conditions.
RYR3 gene expression in breast muscle
The results of RYR3 expression in the pectoralis superficialis muscle of broilers are shown in Fig. 2. Note that both diet (P = 0.02) and heat stress (P < 0.01) had a significant effect on RYR3 expression. The highest expression levels were seen in broilers fed the U diet and exposed to heat stress before slaughter.
Fig. 2.
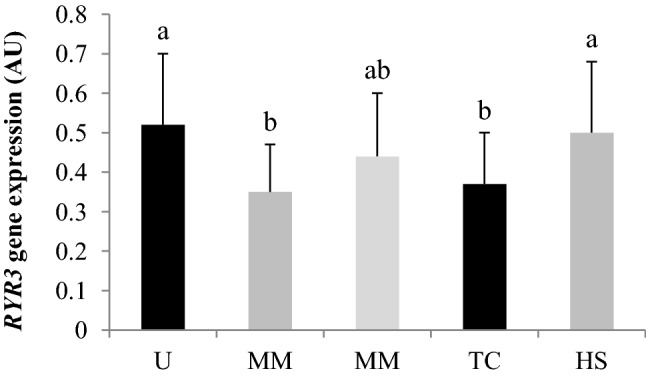
Expression of the ryanodine receptor type 3 (RYR3) gene in the breast muscle (pectoralis superficialis) of 35-day-old broilers. a,bDifferent letters represent significant differences by Tukey’s test (P < 0.05). Results are expressed as arbitrary units (AU) and presented as mean and standard deviation. U unsupplemented diet, M diet supplemented with dl-methionine, MM diet supplemented with methionine dipeptide (dl-methionyl-dl-methionine), TC thermal comfort condition, HS heat stress condition
Discussion
Broilers supplemented with free methionine or methionine dipeptide showed improved breast meat yield, higher total nitrogen content, and lower lipid content, indicating that methionine supplementation increased feed efficiency. These results may be attributed to the stimulatory effect of methionine on the expression of genes coding for growth-related hormones (e.g., growth hormone receptor and type I insulin-like growth factor) and myogenic regulatory factors involved in muscle development (e.g., myogenic factor 5 and myocyte enhancer factor 2) as well as to its inhibitory effects on the expression of genes related to protein degradation (e.g., cathepsin L2 and atrogin-1) and negative regulators of muscle growth (e.g., myostatin) (Del Vesco et al. 2015).
Because one of the goals of animal production is to maximize performance, poultry diets are carefully formulated to meet the nutritional needs for maintenance, growth, and reproduction (Costa et al. 2014). Non-specific nitrogen sources are required for the synthesis of non-essential amino acids. Some amino acids cannot be synthesized and must be obtained from the diet (Zhang et al. 2015). Not only the quantity but also the quality of proteins should be adequate for each developmental stage (Costa et al. 2014). Broilers have high nutritional requirements for methionine. Supplementing broiler diets with a commercial form of this essential amino acid improves animal performance. Studies have shown the benefits of free methionine supplements on performance parameters (Moghadam et al. 2017; Wang et al. 2019), but there is little information available on the effects of methionine dipeptide. To address this gap, whether free methionine and methionine dipeptide differed in their effects on broiler breast meat yield, nitrogen content, and lipid content were examined. We found no differences in any of the analyzed parameters between broilers fed diets supplemented with methionine and methionine dipeptide, indicating that the two forms of the amino acid are equally effective in healthy broilers. These results are in agreement with the findings of Mencalha et al. (2016), who reported that broilers supplemented with the two forms of methionine had similar carcass characteristics.
The U diet led to reduced breast yield, lower nitrogen content, and higher lipid content in the pectoralis superficialis muscle. This was probably a result of amino acid imbalance, which limits protein synthesis and stimulates protein catabolism and nitrogen excretion, culminating in an excess of carbon skeletons that are directed to lipid synthesis (Cauwenberghe and Burnham 2001). Overall, the results show that methionine deficiency is detrimental to animal performance.
We also examined the effects of diet and heat stress on meat quality parameters. Postmortem glycolytic reactions influence breast meat pH, color parameters, WHC, and texture (Venturini et al. 2007). Heat stress can alter the rate of glycolytic reactions (Carvalho 2017). In this study, broilers exposed to heat stress had lower breast meat pH (5.86) than birds kept at moderate temperatures in the 24 h prior to slaughter (5.96). However, although the pH values of both groups differed significantly, the results are within the normal range for broiler meat (5.70–5.96) (Van Laack et al. 2000). The treatments had no effect on WHC, but the breast meat of heat-stressed broilers had lower moisture content, which may be related to the lower pH. Stress conditions lead to increased production of lactic acid in muscles, thereby decreasing pH and moisture content in meat (Rodrigues and Silva 2016).
Methionine-supplemented diets resulted in lower meat pH (M = 5.87 and MM = 5.89) than the U diet (5.97). The breast meat pH of broilers fed the U diet was outside the normal range. A high pH in breast meat is associated with meat quality problems, such as dark, firm, and dry meat. These results suggest that, regardless of the form of methionine used, supplementation contributes to meat quality development during the transformation of muscle to meat.
There were no treatment effects on breast meat lightness (L*), but all treatments influenced meat redness (a*) and yellowness (b*). Heat-stressed birds fed the U diet yielded breast meat with higher a*, whereas heat-stressed broilers fed the M and MM diets, as well as unstressed broilers fed the MM diet, produced breast meat with higher b*. A stressful situation leads to increased glycogen consumption, which implies lower glycogen reserves in postmortem muscle for lactic acid formation. This can result in a pH above the isoelectric point of myofibrillar proteins, increasing the amount of positively charged proteins available for interaction with water molecules and, consequently, the absorption of light in meat, resulting in a darker color (Rodrigues and Silva 2016).
Carvalho (2017) investigated the effects of supplementation with different levels of methionine and cystine on hematological parameters of broilers and found that supplemented birds had lower hemoglobin concentration. Hemoglobin and myoglobin are the main molecules responsible for meat color (Wilkinson et al. 2006). Together, these findings suggest that methionine supplementation can result in lighter broiler breast meat by reducing hemoglobin levels.
Little is known about the molecular mechanisms that determine meat quality. In this study the expression of RYR3, a gene linked to muscular contraction was studied (Sporer et al. 2012). Heat-stressed broilers fed the U diet had high RYR3 expression. Ryanodine receptors are incorporated into the sarcoplasmic reticulum of muscle cells, where their main function is to control the release of calcium from the reticulum to the sarcoplasm (Sporer et al. 2012). When activated, RYR3 releases calcium for long periods, resulting in accumulation of calcium ions in the sarcoplasm and altering the structural configuration of thin filaments of the sarcomere (Petracci and Cavani 2012). Calcium ions participate in the generation of tension within muscle fibers (Petracci and Cavani 2012). Goes et al. (2015) associated the low expression of the ryanodine receptor gene in Nile tilapia to antemortem stress. Sporer et al. (2012) observed that broilers and turkeys with pale, soft, and exudative meat showed lower RYR3 expression. Our results indicate that broilers were able to adapt to heat-stress and methionine deficiency conditions. Their meat had a normal appearance, and pH levels were within normal limits.
Heat-stressed birds showed lower TAC and higher protein oxidation. Studies show that heat stress induces oxidative stress in animals, that is, an imbalance between reactive oxygen species (ROS) generation and elimination. Increased ROS levels in animals under stress occur as a result of both increased production and impaired antioxidant activity (Del Vesco and Gasparino 2013). Protein oxidation affects meat quality by compromising nutritional quality and digestibility. In agreement with this result, broilers subjected to heat stress and fed the U diet also showed higher lipid oxidation. Inadequate environment and nutritional factors are capable of causing stress and affecting the quality and shelf-life of meat products.
Broilers fed methionine-supplemented diets had higher TAC than those fed the U diet, showing that methionine promotes the elimination of toxic oxidative substances. Methionine has direct and indirect antioxidant properties (Levine et al. 1996), which allow it to mitigate the harmful effects of free radicals and provide antioxidant protection at the molecular and cellular levels (Stadtman and Levine, 2003).
Heat stress and methionine deficiency were detrimental to animal performance. Enrichment of broiler diets with free methionine or methionine dipeptide had a positive effect on breast meat yield and nutritional quality. Methionine supplementation was able to counteract heat stress-induced lipid oxidation and TAC reduction, maintaining the quality of breast meat for an extended period. No differences were found between supplementation with free methionine and methionine dipeptide for any of the parameters. Thus, both forms of methionine are equally applicable to improving meat quality in broiler.
Acknowledgements
The authors are grateful for the support of the Araucaria Research of the State of Paraná - (Maringá, PR, Brazil) by provide funding for research publication.
Compliance with ethical standard
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC Association Official Analytical Chemist . Official Methods of Analysis. 18. Gaitherburg: AOAC; 2005. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Carvalho GB. Níveis e fontes de metionina na nutrição de frangos de corte. Goiânia: Tese (Doutorado em Zootecnia) - Universidade Federal de Goiás; 2017. p. 126. [Google Scholar]
- Cauwenberghe SV, Burnham D (2001) New developments in amino acid protein nutrition of poultry, as related to optimal performance and reduced nitrogen excretion. In: European symposium of poultry nutrition, Blankenberge. Anais, Blankenberge, p. 234.
- Classen HL (2000) Managing metabolic disease in rapidly growing strains of poultry. In: Hill WG, Bishop SC, McGuirk B, McKay JC, Simm G, Webb A (eds) The challenge of genetic change in animal production. Edinburgh: Journal of the British Society of Animal Science, pp 63–64. (Occasional publication, 27).
- Costa FGP, Silva JHV, Goulart CC, Nogueira ET, Sá LM (2014) Exigências de aminoácidos para aves. In: Sakomura NK, Silva JHV, Costa FGP, Fernandes JBK, e Hauschild L, (eds) Nutrição de não ruminantes. Funep, Jaboticabal, pp. 241–261
- Del Vesco AP, Gasparino E, Grieser DO, Zancanela V, Soares MAM, Oliveira Neto AR. Effects of methionine supplementation on the expression of oxidative stress-related genes in acute heat stress-exposed broilers. Br J Nutr. 2015;113(4):549–559. doi: 10.1017/S0007114514003535. [DOI] [PubMed] [Google Scholar]
- Del Vesco AP, Gasparino E. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute heat stress. J Anim Sci. 2013;91(2):582–587. doi: 10.2527/jas.2012-5498. [DOI] [PubMed] [Google Scholar]
- Gasparino E, Del Vesco AP, Khatlab AS, Zancanela V, Grieser DO, Silva SCC. Effects of methionine hydroxy analogue supplementation on the expression of antioxidant-related genes of acute heat stress-exposed broilers. Animal. 2018;12(5):931–939. doi: 10.1017/S1751731117002439. [DOI] [PubMed] [Google Scholar]
- Janaszewska A, Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Investiga. 2002;62(3):231–236. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]
- Khatlab AS, Del Vesco AP, Oliveira Neto AR, Fernandes RPM, Gasparino E. Dietary supplementation with free methionine or methionine dipeptide mitigates intestinal oxidative stress induced by Eimeria spp. challenge in broiler chickens. J Anim Sci Biotechnol. 2019;10(58):1–17. doi: 10.1186/s40104-019-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L, Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3(2):356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79(4):583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93(26):15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mencalha R, Helmbrecht A, Arruda N, Batista L, Bertechini A. Comparing bioefficacy of different sources of methionine relative to DL-methionine in the grower phase (22 to 42 days) of broilers chickens. Poult Sci. 2016;95(Suppl 2):135. [Google Scholar]
- Moghadam MHB, Shehab A, Cherian G. Methionine supplementation augments tissue n-3 fatty acid and tocopherol content in broiler birds fed flaxseed. Anim Feed Sci Technol. 2017;228(2017):149–158. doi: 10.1016/j.anifeedsci.2017.04.014. [DOI] [Google Scholar]
- Nakamura M, Katoh K. Influence of thawing method on several properties of rabbit meat. Bull Ishikawa Prefect Coll Agric Ishikawa. 1981;11(1):45–49. [Google Scholar]
- Petracci M, Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4(1):1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raharjo S, Sofos JN, Schmidt GR. Improved speed, specificity, and limit of determination of an aqueous acid extraction thiobarbituric acid-C18 method for measuring lipid peroxidation in beef. J Agric Food Chem. 1992;40(11):2182–2185. doi: 10.1021/jf00023a027. [DOI] [Google Scholar]
- Rodrigues TP, Silva TJP. Caracterização do processo de rigor mortis e qualidade da carne de animais abatidos no Brasil. Arquivos de Pesquisa Animal. 2016;1(1):1–20. [Google Scholar]
- Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes DC, Ferreira AS, Barreto SLT. Brazilian tables for birds and pigs: composition of foods and nutritional requirements. 3. Viçosa: Universidade Federal de Viçosa; 2011. [Google Scholar]
- Souza MG, Oliveira RFM, Donzele JL, Assis Maia AP, Balbino EM, Oliveira WP. Utilização das vitaminas C e E em rações para frangos de corte mantidos em ambiente de alta temperatura. R Bras Zootec. 2011;40(10):2192–2198. doi: 10.1590/S1516-35982011001000019. [DOI] [Google Scholar]
- Sporer KRB, Zhou HR, Linz JE, Booren AM, Strasburg GM. Differential expression of calcium-regulating genes in heat-stressed turkey breast muscle is associated with meat quality. Poult Sci. 2012;91(6):1418–1424. doi: 10.3382/ps.2011-02039. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Goes ESR, Lara JA, Gasparino E, Del Vesco AP, Goes MD, Alexandre Filho L, Ribeiro RP. Pre-slaughter stress affects ryanodine receptor protein gene expression and the water-holding capacity in fillets of the Nile tilapia. PLoS ONE. 2015;10(6):e0129145. doi: 10.1371/journal.pone.0129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laack RLJM, Liu CH, Smith MO, Loveday HD. Characteristics of pale, soft, exudative broiler breast meat. Poult Sci. 2000;79:1057–1061. doi: 10.1093/ps/79.7.1057. [DOI] [PubMed] [Google Scholar]
- Venturini KS, Sarcinelli MF, Silva LC (2007) Características da carne de frango. Espírito Santo: UFES, p. 7. (Boletim Técnico: 01307 PIE)
- Wang S-T, Chen H-W, Sheen L-Y, Lii C-K. Methionine and cysteine affect glutathione level, glutathione-related enzyme activities and the expression of glutathione s-transferase isozymes in rat hepatocytes. J Nutr. 1997;127(11):2135–2141. doi: 10.1093/jn/127.11.2135. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin X, Yin D, Lei Z, Mahmood T, Yuan J. Antioxidant response and bioavailability of methionine hydroxy analog relative to DL-methionine in broiler chickens. Anim Nutr. 2019;5(3):241–247. doi: 10.1016/j.aninu.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TL, Shackelford SD, Koohmaraie M (1997) Standardizing collection and interpretation of Warner-Bratzler shear force and sensory tenderness data. In: Proceedings of the Reciprocal Meat Conference, vol 50, pp.68–77. Acess: %3chttps://www.ars.usda.gov/ARSUserFiles/30400510/1997500068.pdf%3e.
- Wilkinson BHP, Janz JAM, Morel PCH, Purchas RW, Hendriks WH. The effect of modified atmosphere packaging with carbon monoxide on the storage quality of master-packaged fresh pork. Meat Sci. 2006;73(4):605–610. doi: 10.1016/j.meatsci.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Hu ZP, Lu CH, Yang MX, Zhang LL, Wang T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J Anim Sci. 2015;93(4):1656–1665. doi: 10.2527/jas.2014-8244. [DOI] [PubMed] [Google Scholar]