Summary
We resolve debate over the evolution of vertebrate hypermineralized tissues through analyses of matrix protein-encoding secretory calcium-binding phosphoprotein (SCPP) genes and phylogenetic inference of hypermineralized tissues. Among these genes, AMBN and ENAM are found in both sarcopterygians and actinopterygians, whereas AMEL and SCPP5 are found only in sarcopterygians and actinopterygians, respectively. Actinopterygian AMBN, ENAM, and SCPP5 are expressed during the formation of hypermineralized tissues on scales and teeth: ganoin, acrodin, and collar enamel in gar, and acrodin and collar enameloid in zebrafish. Our phylogenetic analyses indicate the emergence of an ancestral enamel in stem-osteichthyans, whereas ganoin emerged in stem-actinopterygians and true enamel in stem-sarcopterygians. Thus, AMBN and ENAM originated in concert with ancestral enamel, SCPP5 evolved in association with ganoin, and AMEL evolved with true enamel. Shifts in gene expression domain and timing explain the evolution of different hypermineralized tissues. We propose that hypermineralized tissues in osteichthyans coevolved with matrix SCPP genes.
Subject areas: Evolutionary Biology, Evolutionary Processes, Phylogenetics, Paleobiology
Graphical Abstract
Highlights
-
•
Ganoin emerged in actinopterygians; true enamel arose in sarcopterygians
-
•
Dental enamel, acrodin, and enameloid in actinopterygians are related to ganoin
-
•
SCPP5 evolved in association with ganoin, whereas AMEL evolved with true enamel
-
•
Shifts in SCPP gene expression explain the evolution of hypermineralized tissues
Evolutionary Biology; Evolutionary Processes; Phylogenetics; Paleobiology
Introduction
The vertebrate skeleton is composed principally of cartilage, bone, dentine, enamel, and enameloid (Donoghue et al., 2006; Hall, 2015), all of which are critical to vertebrate adaptations, including protective body armor, an endoskeleton for locomotion, and teeth for feeding (Donoghue and Keating, 2014). As such, mineralized tissues comprise a key innovation underlying much of vertebrate evolutionary success. Among these skeletal tissues, the origin of hypermineralized tissues, enamel, enameloid, and their histological derivatives, remains controversial, partly because their classification in fossils varies among researchers (Friedman and Brazeau, 2010; Schultze, 2016) and partly because genes encoding their matrix proteins are well understood only for true enamel in sarcopterygians. Here we investigate the evolution of various hypermineralized tissues through genomic and developmental analyses, combined with estimation of ancestral states based on data available from living and fossil vertebrates. We draw these disparate approaches together to obtain a holistic understanding of the origin and diversification of vertebrate hypermineralized tissues. Our results reveal evidence for the coevolution of hypermineralized tissues in osteichthyans and their matrix secretory calcium-binding phosphoprotein (SCPP) genes.
Results
Enamel and enameloid have been classified into different types depending on their location and histological characteristics (Figure 1A). Mineralization of enamel and enameloid progresses in organic matrices (Berkovitz and Shellis, 2016) that are subsequently removed as they mature into hypermineralized inorganic tissues (Sasagawa, 1997; Fincham et al., 1999). Enamel grows in a non-collagenous matrix secreted by ameloblasts of epithelial origin (Fincham et al., 1999) and occurs in three main types: (1) true enamel, considered equivalent to mammalian tooth enamel (Smith, 1989); (2) multilayered ganoin (Schultze, 2016) on scales and their derivatives, found only in bichirs and gars among extant clades, as well as in diverse fossil actinopterygians (Sire et al., 2009); and (3) tooth collar enamel, which occurs in actinopterygians, including bichirs, gars, and extinct clades (Smith, 1995; Ishiyama et al., 1999; Sasagawa et al., 2013). Enameloid forms in a collagenous matrix secreted by both inner dental epithelial (IDE) cells and mesenchyme-derived odontoblasts (Poole, 1967), often characterized histologically by protruding dentine tubules (Smith, 1995). Enameloid constitutes an acrodin tooth cap in various extant and extinct actinopterygians (Shellis and Miles, 1974; Sasagawa et al., 2013; Schultze, 2016). Tooth collar enameloid occurs in teleosts (Shellis and Miles, 1974; Sasagawa, 1988; Smith, 1995). Enameloid is also found on the dental and dermal skeleton in chondrichthyans (including acanthodians), as well as in extinct jawed and jawless stem-gnathostomes (Donoghue et al., 2006; Rücklin et al., 2011; Keating et al., 2015).
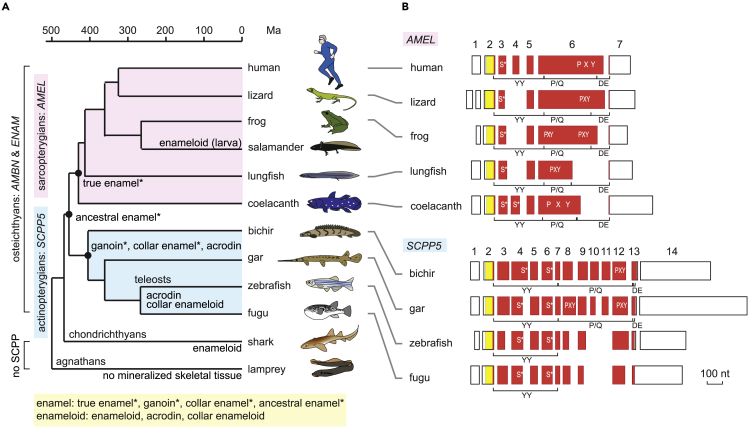
Figure 1.
Phylogenetic distribution of enamel and enameloid matrix genes, and exon-intron structure of AMEL and SCPP5
(A) Phylogenetic distribution of enamel, enameloid, and their matrix genes. Hypermineralized tissues classified as enamel are denoted by an asterisk. Divergence times are based on previous studies: most sarcopterygian taxa (Hedges and Kumar, 2009), actinopterygian taxa (Near et al., 2012), lungfish-tetrapods and chondrichthyans-osteichthyans (Giles et al., 2017), and agnathans-gnathostomes (Kuraku and Kuratani, 2006).
(B) Exon-intron structure of AMEL and SCPP5. Separate boxes represent exons. Open areas in exons show 5′ and 3′ untranslated regions. The areas encoding the signal peptide and mature protein are shown in yellow and vermilion, respectively. Exon numbers are indicated for human AMEL (AMELX) and bichir scpp5. Locations encoding phospho-Ser residues (S∗) (Kawasaki and Amemiya, 2014) and Pro-Xaa-Yaa (Xaa and Yaa represent any amino acids; PXY) repeats are illustrated within exon boxes. Regions encoding the three modules, the N-terminal aromatic residue-rich region (YY), the Pro/Gln-rich core region containing uninterrupted PXY repeats (P/Q), and the C-terminal hydrophilic region (DE), are indicated below exon boxes. Scale bar, 100 nucleotides (nt). See Figure S1A for details.
Enamel and enameloid matrix genes in sarcopterygians and actinopterygians
The amelogenin (AMEL), ameloblastin (AMBN), and enamelin (ENAM) genes encode the principal enamel matrix proteins (Fincham et al., 1999) in tetrapods (presumably also coelacanth [Kawasaki and Amemiya, 2014]), whereas SCPP5 is thought to encode an enameloid matrix protein in Fugu rubripes (fugu) and Danio rerio (zebrafish) (Kawasaki et al., 2005, 2009). AMEL has been found only in sarcopterygians and SCPP5 only in actinopterygians (Figure S1A) (Qu et al., 2015; Braasch et al., 2016; Kawasaki et al., 2017). AMBN and ENAM are also found in actinopterygians, including Lepisosteus oculatus (referred to below as “gar”) and zebrafish (Figures S1B and S1C) (Braasch et al., 2016; Kawasaki et al., 2017). All four genes belong to the SCPP gene family that arose by gene duplication (Kawasaki and Weiss, 2003; Kawasaki et al., 2017). It is notable that no SCPP genes have been identified in chondrichthyans (Figure 1A) (Venkatesh et al., 2014; Enault et al., 2018).
We identified amel and ambn in Lepidosiren paradoxa (lungfish); ambn, enam, and scpp5 in Polypterus senegalus (referred to below as “bichir”) and various teleosts; and ambn in Acipenser sinensis (sturgeon; Figure S1); AMEL was not identified in actinopterygians. All three modules characteristic of tetrapod and coelacanth amelogenins (YY-, P/Q-, and DE-rich regions; Figures 1B and S1A) (Toyosawa et al., 1998; Fincham et al., 1999) were detected in proteins encoded by amel in L. paradoxa and scpp5 in bichir and gar. Nevertheless, in most sarcopterygians these modules are encoded by five exons in AMEL, but twelve exons in bichir and gar scpp5 genes (Figure 1B). Furthermore, AMEL differs from SCPP5 in genomic location, exon-intron organization, and exons encoding phospho-Ser residues (Figure S1A). These data support interpretation independent origin of AMEL and SCPP5. Teleosts do not possess enamel, and their scpp5 genes lack two of these three modules (P/Q and DE; Figures 1B and S1A).
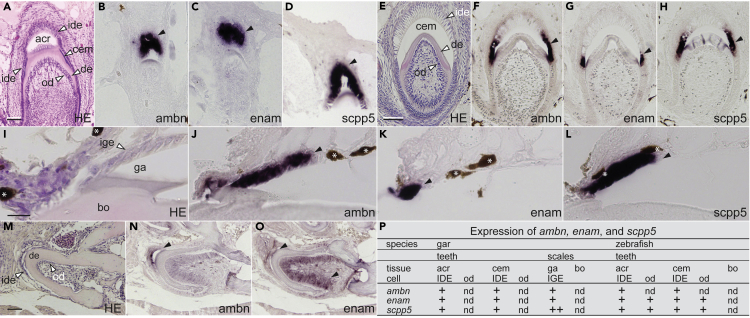
Expression of ambn, enam, and scpp5 in teeth and scales of gar and teeth of zebrafish
In gar, expression of ambn, enam, and scpp5 was detected during tooth formation in IDE cells, initially during acrodin matrix formation (matrix formation stage of enameloid; Figures 2A–2D) and then during collar enamel matrix formation (secretory stage of enamel; Figures 2E–2H) (Sasagawa and Ishiyama, 2005; Sasagawa et al., 2008). During scale formation, expression of ambn, enam, and scpp5 was detected in inner ganoin epithelial (IGE) cells that secrete the ganoin matrix on scales (secretory stage; Figures 2I–2L). We detected no other expression domains of these genes (Figure S2A). Given the limited expression domain of these genes in gar skin, their relative expression levels in IGE cells can be determined by RNA sequencing (RNA-seq) analysis of the skin (Qu et al., 2015; Braasch et al., 2016). Expression of scpp5 was the highest among these genes and among all SCPP genes (Table S1).
Figure 2.
Expression of ambn, enam, and scpp5 in teeth and scales of gar and teeth of zebrafish
(A) Hematoxylin-eosin (H&E) staining of a developing gar tooth immediately after acrodin formation. Scale bar, 50 μm.
(B–D) Our in situ hybridization (ISH) analysis reveals expression of gar ambn (B), enam (C), and scpp5 (D) in IDE cells during the matrix formation stage of acrodin formation (closed arrowheads).
(E) H&E staining of a developing gar tooth forming collar enamel. Scale bar, 50 μm.
(F–H) ISH analysis reveals expression of gar ambn (F), enam (G), and scpp5 (H) in IDE cells during the secretory stage of collar enamel formation (closed arrowheads).
(I) H&E staining of a gar scale forming ganoin. Ganoin was lost during decalcification. Scale bar, 50 μm.
(J–L) ISH analysis reveals expression of gar ambn (J), enam (K), and scpp5 (L) in IGE cells during the secretory stage of ganoin formation (closed arrowheads). Brown pigments are shown by asterisks.
(M) H&E staining of a developing zebrafish tooth. Scale bar, 50 μm.
(N and O) ISH analysis reveals expression of zebrafish ambn (N) in IDE cells (a closed arrowhead) and enam (O) in matrix-formation stage IDE cells and odontoblasts (closed arrowheads).
(P) Summary of matrix SCPP gene expression in mineralized tissues. In gar scales, relative expression levels were determined by RNA-seq analysis (Table S1) and the highest expression level of scpp5 among all SCPP genes is shown as “++.” Expression of zebrafish scpp5 (Kawasaki et al., 2017) and stages of hypermineralized tissue formation in gar and teleosts were described previously (Sasagawa, 1995, 1997; Sasagawa and Ishiyama, 2005; Sasagawa et al., 2008, 2013). See Figure S2A for negative controls and more results. Abbreviations: acr, acrodin; bo, bone; cem, collar enamel; de, dentine; ga, ganoin; ide, inner dental epithelial cells; ige, inner ganoin epithelial cells; od, odontoblasts.
In zebrafish, expression of ambn and enam was detected in IDE cells in the matrix formation stages of acrodin and collar enameloid (Figures 2M–2O and S2A); enam was also expressed in odontoblasts, but we detected no significant expression of enam or ambn in bone cells (Figures 2N and 2O). Expression of enam in odontoblasts appears to initiate during acrodin matrix formation and continue throughout collar enameloid and dentine matrix formation (Figures 2O and S2A) (Shellis and Miles, 1974). Results of our expression analysis are summarized in Figure 2P.
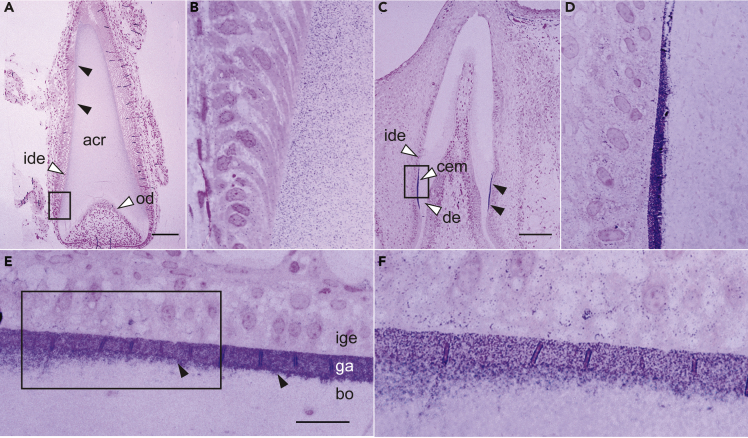
Distribution of gar Scpp5 in acrodin and collar enamel on teeth and in ganoin on scales
The results of our in situ hybridization analysis for gar scpp5 are consistent with the results of our immunohistochemical (IHC) analysis using an antibody raised against gar Scpp5. In the mineralization stage of acrodin formation, which begins after the matrix formation stage (Sasagawa and Ishiyama, 2005), high levels of Scpp5 were detected near the outer surface, decreasing toward the core, close to odontoblasts (Figures 3A and 3B). In the collar enamel matrix, Scpp5 was detected uniformly and strongly in the secretory stage, except in proximity to acrodin (Figures 3C and 3D), where Scpp5 was presumably absorbed by IDE cells that advanced to the maturation stage (Sasagawa et al., 2008). Scpp5 was also detected weakly in dentine along the border with collar enamel. In scales, Scpp5 was detected uniformly and strongly in the ganoin matrix in the secretory stage (Figure 3E) and underlying bone, decreasing immediately in a deeper region of the bone (Figure 3F).
Figure 3.
Distribution of gar Scpp5 in the matrix of teeth and scales detected using optical IHC analysis
(A–F) Optical IHC analysis of gar Scpp5 in acrodin (A and B) and collar enamel (C and D) in teeth, and ganoin in a scale (E and F). The rectangular region in (A), (C), and (E) is enlarged in (B), (D), and (F), respectively. IHC signals in (A), (C), and (E) are shown by closed arrowheads. Vertical lines in the ganoin layer (E and F) are artifacts of sectioning. See the legend of Figure 2 for abbreviations. Scale bar, 100 μm (A and C) or 20 μm (E). The contrast of these images was enhanced uniformly over the entire field (see Figure S2B for original images).
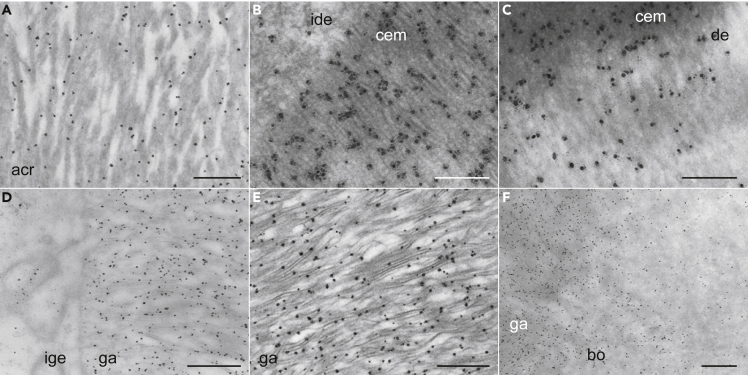
Since our optical IHC analysis detected specific distributions of Scpp5 in the matrix of developing teeth and scales, we furthered IHC analysis using transmission electron microscopy (TEM). The results revealed an association of Scpp5 with the edge of collagen fibrils in the mineralization stage of acrodin formation (Figure 4A). In the developing collar enamel, Scpp5 was associated with electron-dense fibrils (Figure 4B) postulated to form as organic sheaths surrounding slender crystals (Warshawsky, 1989). Scpp5 was also detected in the underlying dentine near the border with collar enamel (Figure 4C), corroborating optical microscopic observations (Figure 3D). As in collar enamel, most Scpp5 signals in developing ganoin were associated with electron-dense fibrils (Figures 4D and 4E), especially along fibril edges, suggesting interactions of Scpp5 with minerals or mineral-associated organic molecules. Weak but significant signals were also detected in bone underlying scale ganoin, but not in deeper regions (Figure 4F), as in dentine underlying collar enamel (Figure 4C).
Figure 4.
Distribution of gar Scpp5 in the matrix of teeth and scales detected using TEM IHC analysis
(A–F) TEM IHC analysis of gar Scpp5 in acrodin (A), collar enamel near IDE cells (B), collar enamel near dentine (C), ganoin near IGE cells (D), a middle portion of ganoin (E), and ganoin near bone (F). Dark dots show the distribution of Scpp5. Scale bar, 500 nm (A, D, E, and F) or 200 nm (B and C). See the legend of Figure 2 for abbreviations and Figure S2C for negative controls.
Reconstructing ancestral states of hypermineralized tissues
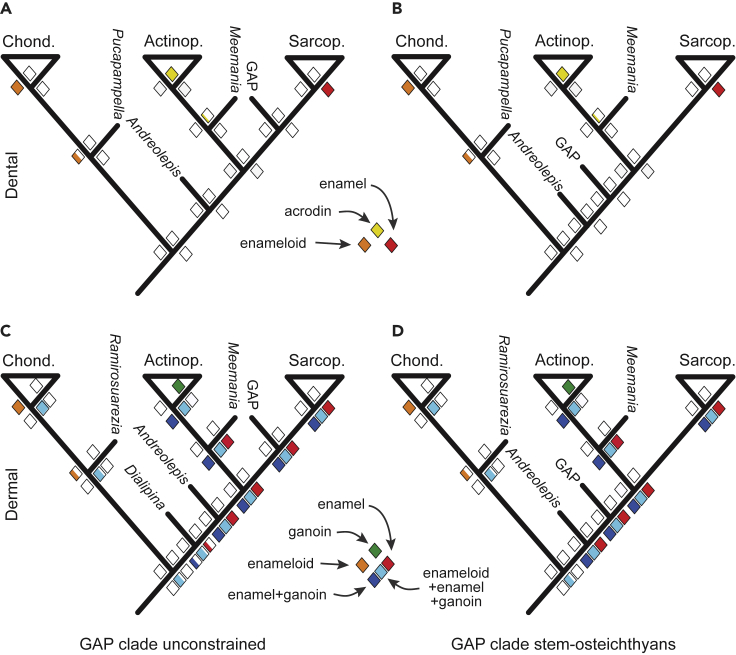
We explored the evolution of acrodin, enameloid, enamel, and ganoin by reconstructing the ancestral states using data from living and fossil taxa (Table S2), stochastic character state mapping (Huelsenbeck et al., 2003), and phylogenetic hypotheses in which (1) Guiyu, Achoania, and Psarolepis (GAP clade) are constrained to stem-osteichthyans (King et al., 2017; Lu et al., 2017) and (2) these taxa are resolved as stem-sarcopterygians (Lu et al., 2016; Qiao et al., 2016; Choo et al., 2017) (Figures S3A and S3B).
Our results indicate that acrodin evolved in the actinopterygian stem-lineage (Cheirolepis and more crown-ward taxa); its presence in Ligulalepis is a consequence of convergence or the isolated tooth from which these data derive does not belong to Ligulalepis (Figures 5A, 5B, S3C, and S3D). Dental enameloid evolved late in the chondrichthyan stem-lineage (Pucapampella or more crown-ward taxa; Figures 5A, 5B, S3E, and S3F); the presence of dental enameloid in acanthodians is convergent. Dermal enameloid was resolved as primitive to the chondrichthyan total-group and, although it is also present in ostracoderms (Donoghue and Sansom, 2002; Keating et al., 2015), it is inferred absent from the dermal skeleton of stem-osteichthyans (Figures 5C, 5D, S3G, and S3H). Thus, dermal enameloid was lost in placoderms (Giles et al., 2013) and evolved convergently in stem-chondrichthyans after the divergence of Ramirosuarezia (Figures S3G and S3H). Taken together, these results suggest that, in both the dental and dermal skeletons, osteichthyan enameloid originated independently from chondrichthyan enameloid (but see below).
Figure 5.
Reconstructing ancestral states of hypermineralized tissues
(A–D) Ancestral state reconstruction of the presence of hypermineralized tissues in the dental (A and B) and dermal (C and D) skeletons for trees, in which the GAP clade is resolved through unconstrained analysis to a stem-sarcopterygian affinity (A and C), or constrained to a stem-osteichthyan affinity (B and D). Diamonds at nodes express probability of the presence of a specific hypermineralized tissue, with the proportion of the color fill reflecting probability. The crown-chondrichthyans (Chond.), crown-actinopterygians (Actinop.), and crown-sarcopterygians (Sarcop.) are shown as triangles, and important stem taxa by bars (e.g., Meemannia). Tables S2 and S3 show data used to construct these trees.
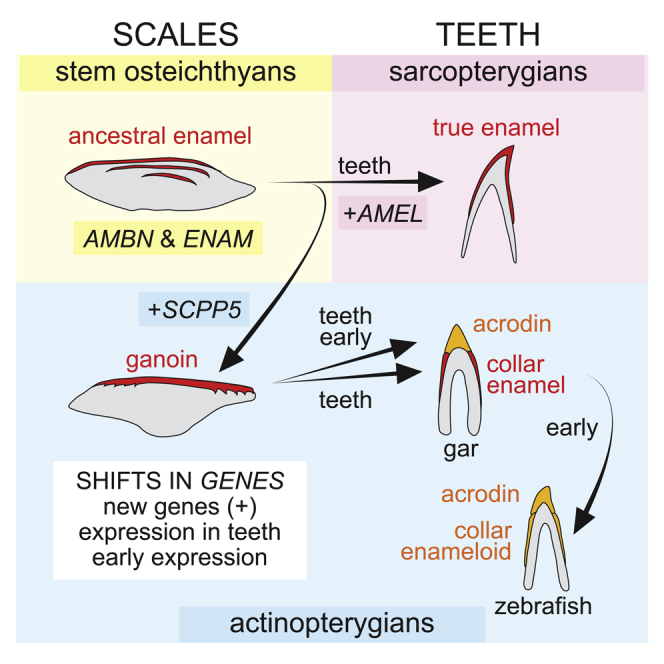
Our estimates of ancestral states were invariant to whether the GAP clade is resolved as stem-osteichthyan or stem-sarcopterygian (Figure 5). Dental enamel evolved early in the sarcopterygian stem-lineage (Onychodus and more crown-ward taxa), arising convergently as collar enamel in non-teleost actinopterygians (bichir and gar; Figures 5A, 5B, S3I, and S3J). Dermal enamel evolved deep in the osteichthyan stem-lineage (Andreolepis and more crown-ward taxa), was lost among early stem-actinopterygians (Cheirolepis and more crown-ward taxa), but retained in crown-sarcopterygians (Figures 5C, 5D, S3K, and S3L). Ganoin evolved among early stem-actinopterygians (Cheirolepis and more crown-ward taxa; Figures 5C, 5D, S3M, and S3N); the ganoin-like (Ørvig, 1978; Schultze, 2016) overlapping enamel in Ligulalepis (Schultze and Märss, 2004; Schultze, 2016) is resolved as convergent. If dermal enamel and ganoin are considered homologous, consistent with the expression patterns of AMBN and ENAM in both sarcopterygian enamel and gar ganoin, the ancestral tissue evolved deep in the osteichthyan-stem (Andreolepis and more crown-ward taxa; Figures 5C, 5D, S3O, and S3P). Given the absence of SCPP genes in chondrichthyans, early enamel matrix SCPP genes likely evolved in concert with a prototypic enamel in stem-osteichthyans, from which true enamel and ganoin arose, respectively, in sarcopterygians and actinopterygians, as discussed below (Figure 1A).
Dermal enameloid is present in stem-gnathostomes, early members of placoderm plesia, and chondrichthyans (Giles et al., 2013). It has been hypothesized that enamel replaced enameloid through shifts in the timing of ameloblast and odontoblast activity (Smith, 1992, 1995). Ancestral state estimation provides support for this switch in a combined coding of dermal enamel, ganoin, and enameloid (Figures 5C, 5D, S3Q, and S3R). The initial enamel matrix SCPP gene may have originated in stem-gnathostomes and may have subsequently been lost in stem-chondrichthyans. As we discuss below, however, it is more likely that the enamel matrix SCPP genes are primitively absent from chondrichthyans and were never involved in dermal enameloid development. The origin of AMBN and ENAM in stem-osteichthyans may be invoked in the developmental evolution of enamel from an ancestral dermal enameloid (Donoghue and Sansom, 2002; Donoghue et al., 2006; Keating et al., 2015).
Discussion
Similar formation of ganoin and collar enamel, and of acrodin and collar enameloid
We detected a similar expression pattern of gar scpp5, ambn, and enam in secretory-stage IGE cells and the uniform distribution of gar Scpp5 in the ganoin matrix (Figures 2J–2L, 3E, and 3F), which suggests that all these three genes encode ganoin matrix proteins. During collar enamel formation, expression of scpp5, ambn, and enam in IDE cells (Figures 2F–2H) and the uniform distribution of Scpp5 in the collar enamel matrix (Figures 3C and 3D) were also detected. These results imply that ganoin and collar enamel form in a similar manner. Thus, collar enamel of gar is better described as collar ganoin, which corroborates the hypothesis obtained by histological studies of gars and many fossil actinopterygians (Ørvig, 1978; Schultze, 2016).
Acrodin forms below the basal lamina (BL) that originally separates odontoblasts from IDE cells (Smith, 1995). During acrodin formation in gar, odontoblasts retreat from the BL and secrete the bulk of the collagenous matrix (Sasagawa and Ishiyama, 2005), similar to dentine formation, while IDE cells secrete Scpp5, and presumably also Ambn and Enam, as suggested by the expression of ambn and enam in matrix formation-stage IDE cells. Thus, the region distal to the BL is formed principally by odontoblasts, whereas the contribution of IDE cells is large in the BL-proximal region (we observed the distribution of Scpp5 only near the outer surface; Figures 3A and 3B). This result suggests that gar acrodin forms as a dentine-ganoin composite, supporting the hypothesis for acrodin formation in teleosts (Shellis and Miles, 1974). Association of Scpp5 with collagen in the acrodin matrix during mineralization (Figure 4A) probably affects the mineralization and/or maturation processes, as previously inferred for teleosts (Shellis and Miles, 1974).
During acrodin formation in zebrafish, ambn and enam are expressed in IDE cells and enam are additionally expressed in odontoblasts. Similar to zebrafish enam, zebrafish and fugu scpp5 genes are also expressed in both IDE cells and odontoblasts (Kawasaki et al., 2005; Kawasaki, 2009), unlike their gar orthologs (Figure 2P). Although the expression of these genes suggests conservation of acrodin in gar and zebrafish, expression of zebrafish scpp5 and enam and fugu scpp5 in odontoblasts implies a modification of acrodin. Because ENAM is expressed primarily in cells of epithelial origin during the formation of hypermineralized tissues in gar and sarcopterygians, the modification of acrodin is inferred to have occurred in teleosts. In zebrafish, a similar expression pattern of ambn, enam, and scpp5 in IDE cells and odontoblasts was also detected during collar enameloid formation (Figure 2P) (Kawasaki, 2009). These results suggest that acrodin and collar enameloid form in a similar matrix in zebrafish and support the inference, obtained by studying various teleosts, that acrodin and collar enameloid are homologous, formed by both IDE cells and odontoblasts in a similar manner (Shellis and Miles, 1974; Sasagawa and Ishiyama, 1988).
Coevolution of hypermineralized tissues and SCPP genes
Our results suggest that enameloid evolved independently in chondrichthyans and actinopterygians. Although it is difficult to distinguish enameloid of chondrichthyans from enameloid of actinopterygians by histological characteristics (Reif, 1979), independent evolution of these tissues is supported by their different mineralization processes. In various actinopterygians, mineralization of enameloid initiates in matrix vesicles, and fine crystals accumulate along collagen fibrils, similar to mineralization of bone and dentine (Shellis and Miles, 1976; Sasagawa, 1988, 1997; Sasagawa et al., 2019). By contrast, mineralization of enameloid in various chondrichthyans begins in tubular vesicles, which are not found in osteichthyans, and no crystals concentrate along fibrillar structures (Sasagawa, 1998, 2002). The unique mineralization process of chondrichthyan enameloid reinforces the idea that this tissue evolved independently of SCPP genes (Kawasaki et al., 2017).
In gar, scpp5 and presumably also ambn and enam encode ganoin matrix proteins, whereas AMEL, AMBN, and ENAM encode true enamel matrix proteins in sarcopterygians. The difference in matrix proteins of ganoin in gar and true enamel in sarcopterygians supports histological classification of true dental enamel in most stem- and crown-sarcopterygians and ganoin on scales in total-group actinopterygians (Qu et al., 2015). AMEL is found only in sarcopterygians; SCPP5 only in actinopterygians (Figure S1). Gar scpp5 shows the highest expression level among all SCPP genes in the skin during ganoin formation (Table S1), reminiscent of AMEL that encodes the most abundant enamel matrix protein (Fincham et al., 1999). Sarcopterygian AMEL genes and gar and bichir scpp5 genes encode a similar modular structure, which is not encoded by scpp5 in teleosts that do not possess enamel (Figure 1). Furthermore, expression of bichir scpp5 was confirmed in the skin during ganoin formation (Figure S1A). These results suggest that sarcopterygian AMEL genes and gar and bichir scpp5 genes have overlapping functions and that either AMEL or scpp5 is sufficient for these overlapping functions during the formation of true enamel and ganoin. We thus assume that AMEL evolved in concert with true enamel and SCPP5 evolved with ganoin.
Given the narrow expression domains and timings of AMBN and ENAM, similar spatiotemporal expression patterns of these genes during the formation of true enamel in sarcopterygians and ganoin in gar imply an evolutionary relationship of these two tissues, rather than independent and coincidental employment of AMBN and ENAM in sarcopterygian true enamel and gar ganoin. Since sarcopterygians and gar phylogenetically bracket sarcopterygians and actinopterygians (Figure 1A) (Witmer, 1995), we hypothesize that both AMBN and ENAM were expressed during the formation of ancestral enamel (see below) in the most recent common ancestor of sarcopterygians and actinopterygians. The evolutionary relationship of true enamel and ganoin in gar and many fossil actinopterygians is supported by the common rod-like arrangement of crystallites, known as the protoprismatic microstructure (Ørvig, 1978; Smith, 1989; Sasagawa et al., 2016). A unique mineralization process of true enamel and ganoin in gar and bichir also supports their evolutionary relationship. During the formation of true enamel and ganoin, enamel ribbons form the mineralization front along the distal membrane of ameloblasts or IGE cells (Sire, 1995; Simmer et al., 2010). The close relationship of true enamel and ganoin suggests their homology: either one tissue derived from the other or both derived from an ancestral enamel.
Enamel and ganoin are considered homologous (Sire et al., 1987; Sasagawa et al., 2013; Qu et al., 2015), and the ancestral character estimation analysis assuming this (Figures 5C and 5D) is phylogenetically congruent, requiring an ancestral dermal hypermineralized tissue in stem-osteichthyans. Within the context of SCPP gene evolution, either AMEL or SCPP5 was initially employed during the formation of an ancestral enamel in stem-osteichthyans; SCPP5 was subsequently replaced by AMEL in sarcopterygians, or AMEL was replaced by SCPP5 in actinopterygians. It is also conceivable, however, that AMEL arose in sarcopterygians and SCPP5 in actinopterygians. Studies of gene-disrupted mice showed that both AMBN and ENAM are necessary for enamel ribbon formation, the unique mineralization process of enamel, whereas AMEL is not (Smith et al., 2016; Liang et al., 2019). Furthermore, a thin enamel can form in AMEL-deficient mice and in toothed whales that have deleterious mutations in AMEL, if both AMBN and ENAM are functional (Gibson et al., 2001; Kawasaki et al., 2020). These studies support the presence of ancestral enamel that formed enamel ribbons in a matrix containing AMBN and ENAM, but no AMEL or SCPP5. We hypothesize that both true enamel and ganoin originated from this ancestral enamel (Figures 1A and 5).
Dental acrodin arose with dermal ganoin among stem-actinopterygians. It was previously hypothesized that acrodin of teleosts is a composite of dentine and enamel (Shellis and Miles, 1974). Our observation of acrodin formation in gar confirms this hypothesis. Thus, acrodin evolution can be explained partly by early expression of ganoin matrix genes on the tooth cap during dentine formation, as previously suggested (Smith, 1992, 1995). Acrodin is similar in gar and zebrafish, but odontoblasts contribute more to acrodin formation in zebrafish by expressing scpp5 and enam.
Ancestral state estimates suggest that dental enamel arose independently in stem-sarcopterygians and non-teleost actinopterygians. As we discussed above, collar enamel is better described as collar ganoin in gar and many fossil actinopterygians, which suggests that ganoin matrix gene expression on the tooth collar was critical to the evolution of collar enamel. Expression of scpp5, ambn, and enam during the formation of collar enamel in gar and collar enameloid in zebrafish suggests an evolutionary relationship of these two tissues and reinforces the previous hypothesis that the evolution of collar enameloid involved early expression of ganoin matrix genes during dentine formation (Smith, 1992, 1995). Consequently, collar enamel found in various non-teleost actinopterygians (Ørvig, 1978; Schultze, 2016) was replaced by collar enameloid in teleosts (Figure 1A). Enameloid is also found on teeth in larval urodeles (Assaraf-Weill et al., 2014; Berkovitz and Shellis, 2016), but in no other sarcopterygians (Figures S3C–S3F), indicating its independent origin in urodeles.
The results of our present study and previous studies using other methods and other species suggest that various hypermineralized tissues in modern osteichthyans can be classified genetically into true enamel, ganoin, and a diversity of enameloids; collar enamel, acrodin, and collar enameloid in actinopterygians are evolutionarily related to scale ganoin. A previous study supported the homology between ganoin and enamel largely based on the expression of gar ambn and enam in the skin (Qu et al., 2015); however, if acrodin and collar enameloid arose as a composite of dentine and ganoin, neither acrodin nor collar enameloid could be a ganoin homolog, even though AMBN and ENAM are expressed during formation. Our results show that orthologous SCPP gene expression may be insufficient to explain the differences between these tissues. The evolution of true enamel, ganoin, and enameloids can be better explained by various changes of matrix SCPP genes, including spatiotemporal shifts in their expression. Hypermineralized tissues in osteichthyans appear to have coevolved with their matrix SCPP genes.
Limitations of the study
In extant actinopterygians, ganoin is found only in bichirs and gars. Although scales of bichirs consist of ganoin, dentine, and bone, scales of gars lack dentine. However, ganoin formation is similar in both clades (Sire, 1995), and bichir ambn, enam, and scpp5 are presumably also expressed during scale and tooth formation in a manner similar to their gar orthologs. In teleosts, comprising ~30,000 species (Nelson et al., 2016), both acrodin and collar enameloid are found in various species. It was reported, however, that acrodin and collar enameloid of the common eel are covered with a hypermineralized layer formed by IDE cells, hence a type of enamel (Shellis and Miles, 1976). Although this layer remains to be confirmed, hypermineralized tissues may vary in some teleosts. Moreover, ambn, enam, and scpp5 are all found in zebrafish and fugu (Figure S1), whereas ambn and/or enam were secondarily lost in some teleosts (Lv et al., 2017). The lack of these genes suggests modifications of acrodin and collar enameloid, or a loss of hypermineralized tissues. Examination of hypermineralized tissues in various teleosts would elucidate the evolution and adaptation of hypermineralized tissues in diverse teleosts.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kazuhiko Kawasaki (kuk2@psu.edu).
Materials availability
The anti-gar Scpp5 antibody generated in this study is available from the Lead Contact with a completed Materials Transfer Agreement.
Data and code availability
The nucleotide sequences generated during this study are available at GenBank (accession numbers: MG010658-MG010662).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
K.K. is grateful to Prof. Joan T. Richtsmeier at Penn State University for critical reading of the manuscript. We acknowledge funding from the National Science Foundation (BCS0725227) and the Penn State Evan Pugh Professors Research Fund to Prof. Kenneth M. Weiss, from the European Commission Marie Curie scheme to M.W. (626424), from the Royal Commission for the Exhibition of 1851 (1851 Research Fellowship) to M.N.P., from a Royal Society Wolfson Merit Award to P.C.J.D., from the Natural Environment Research Council (NE/N002067/1; NE/P013678/1) to P.C.J.D., from the Biotechnology and Biological Sciences Research Council (BB/N000919/1) to P.C.J.D., from the Ministry of Education, Science, and Culture of Japan (Grant-in-Aid 18592013) to M.I., and from the Nippon Dental University to I.S. (NDUF-13-10, NDUF-14-12, and NDU Grants N-15015) and to M.I. (NDUF-10-04, NDUF-11-03, NDUF-14-07, NDUF-15-04, and NDU Grants N-17006).
Author contributions
Conceptualization, K.K., P.C.J.D., and M.I.; Investigation, all authors; Writing and Supervision, K.K. and P.C.J.D.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.102023.
Supplemental Information
References
- Assaraf-Weill N., Gasse B., Silvent J., Bardet C., Sire J.Y., Davit-Beal T. Ameloblasts express type I collagen during amelogenesis. J. Dent. Res. 2014;93:502–507. doi: 10.1177/0022034514526236. [DOI] [PubMed] [Google Scholar]
- Berkovitz B., Shellis R.P. Academic Press; 2016. The Teeth of Non-mammalian Vertebrates. [Google Scholar]
- Braasch I., Gehrke A.R., Smith J.J., Kawasaki K., Manousaki T., Pasquier J., Amores A., Desvignes T., Batzel P., Catchen J. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 2016;48:427–437. doi: 10.1038/ng.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo B., Zhu M., Qu Q., Yu X., Jia L., Zhao W. A new osteichthyan from the late Silurian of Yunnan, China. PLoS One. 2017;12:e0170929. doi: 10.1371/journal.pone.0170929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue P.C.J., Keating J.N. Early vertebrate evolution. Palaeontology. 2014;57:879–893. [Google Scholar]
- Donoghue P.C.J., Sansom I.J. Origin and early evolution of vertebrate skeletonization. Microsc. Res. Tech. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Donoghue P.C.J., Sansom I.J., Downs J.P. Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J. Exp. Zool. B Mol. Dev. Evol. 2006;306:278–294. doi: 10.1002/jez.b.21090. [DOI] [PubMed] [Google Scholar]
- Enault S., Munoz D., Simion P., Venteo S., Sire J.-Y., Marcellini S., Debiais-Thibaud M. Evolution of dental tissue mineralization: an analysis of the jawed vertebrate SPARC and SPARC-L families. BMC Evol. Biol. 2018;18:127. doi: 10.1186/s12862-018-1241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham A.G., Moradian-Oldak J., Simmer J.P. The structural biology of the developing dental enamel matrix. J. Struct. Biol. 1999;126:270–299. doi: 10.1006/jsbi.1999.4130. [DOI] [PubMed] [Google Scholar]
- Friedman M., Brazeau M.D. A reappraisal of the origin and basal radiation of the Osteichthyes. J. Vertebr. Paleontol. 2010;30:36–56. [Google Scholar]
- Gibson C.W., Yuan Z.A., Hall B., Longenecker G., Chen E., Thyagarajan T., Sreenath T., Wright J.T., Decker S., Piddington R. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 2001;276:31871–31875. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- Giles S., Rücklin M., Donoghue P.C.J. Histology of "placoderm" dermal skeletons: implications for the nature of the ancestral gnathostome. J. Morphol. 2013;274:627–644. doi: 10.1002/jmor.20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles S., Xu G.H., Near T.J., Friedman M. Early members of 'living fossil' lineage imply later origin of modern ray-finned fishes. Nature. 2017;549:265–268. doi: 10.1038/nature23654. [DOI] [PubMed] [Google Scholar]
- Hall B.K. Elsevier; 2015. Bones and Cartilage. [Google Scholar]
- Hedges S.B., Kumar S. Oxford University Press; 2009. The Time Tree of Life. [Google Scholar]
- Huelsenbeck J.P., Nielsen R., Bollback J.P. Stochastic mapping of morphological characters. Syst. Biol. 2003;52:131–158. doi: 10.1080/10635150390192780. [DOI] [PubMed] [Google Scholar]
- Ishiyama M., Inage T., Shimokawa H. An immunocytochemical study of amelogenin proteins in the developing tooth enamel of the gar-pike, Lepisosteus oculatus (Holostei, Actinopterygii) Arch. Histol. Cytol. 1999;62:191–197. doi: 10.1679/aohc.62.191. [DOI] [PubMed] [Google Scholar]
- Kawasaki K. The SCPP gene repertoire in bony vertebrates and graded differences in mineralized tissues. Dev. Genes Evol. 2009;219:147–157. doi: 10.1007/s00427-009-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K., Amemiya C.T. SCPP genes in the coelacanth: tissue mineralization genes shared by sarcopterygians. J. Exp. Zool. B Mol. Dev. Evol. 2014;322:390–402. doi: 10.1002/jez.b.22546. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Buchanan A.V., Weiss K.M. Biomineralization in humans: making the hard choices in life. Annu. Rev. Genet. 2009;43:119–142. doi: 10.1146/annurev-genet-102108-134242. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Mikami M., Goto M., Shindo J., Amano M., Ishiyama M. The evolution of unusually small amelogenin genes in cetaceans; pseudogenization, X-Y gene conversion, and feeding strategy. J. Mol. Evol. 2020;88:122–135. doi: 10.1007/s00239-019-09917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K., Mikami M., Nakatomi M., Braasch I., Batzel P., J H.P., Sato A., Sasagawa I., Ishiyama M. SCPP genes and their relatives in gar: rapid expansion of mineralization genes in osteichthyans. J. Exp. Zool. B Mol. Dev. Evol. 2017;328:645–665. doi: 10.1002/jez.b.22755. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Suzuki T., Weiss K.M. Phenogenetic drift in evolution: the changing genetic basis of vertebrate teeth. Proc. Natl. Acad. Sci. U S A. 2005;102:18063–18068. doi: 10.1073/pnas.0509263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K., Weiss K.M. Mineralized tissue and vertebrate evolution: the secretory calcium-binding phosphoprotein gene cluster. Proc. Natl. Acad. Sci. U S A. 2003;100:4060–4065. doi: 10.1073/pnas.0638023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating J.N., Marquart C.L., Donoghue P.C. Histology of the heterostracan dermal skeleton: insight into the origin of the vertebrate mineralised skeleton. J. Morphol. 2015;276:657–680. doi: 10.1002/jmor.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Qiao T., Lee M.S.Y., Zhu M., Long J.A. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Syst. Biol. 2017;66:499–516. doi: 10.1093/sysbio/syw107. [DOI] [PubMed] [Google Scholar]
- Kuraku S., Kuratani S. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool. Sci. 2006;23:1053–1064. doi: 10.2108/zsj.23.1053. [DOI] [PubMed] [Google Scholar]
- Liang T., Hu Y., Smith C.E., Richardson A.S., Zhang H., Yang J., Lin B., Wang S.K., Kim J.W., Chun Y.H. AMBN mutations causing hypoplastic amelogenesis imperfecta and Ambn knockout-NLS-lacZ knockin mice exhibiting failed amelogenesis and Ambn tissue-specificity. Mol. Genet. Genom. Med. 2019;7:e929. doi: 10.1002/mgg3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Giles S., Friedman M., den Blaauwen J.L., Zhu M. The oldest actinopterygian highlights the cryptic early history of the hyperdiverse ray-finned fishes. Curr. Biol. 2016;26:1602–1608. doi: 10.1016/j.cub.2016.04.045. [DOI] [PubMed] [Google Scholar]
- Lu J., Giles S., Friedman M., Zhu M. A new stem sarcopterygian illuminates patterns of character evolution in early bony fishes. Nat. Commun. 2017;8:1932. doi: 10.1038/s41467-017-01801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Kawasaki K., Li J., Li Y., Bian C., Huang Y., You X., Shi Q. A genomic survey of SCPP family genes in fishes provides novel insights into the evolution of fish scales. Int. J. Mol. Sci. 2017;18:2432. doi: 10.3390/ijms18112432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near T.J., Eytan R.I., Dornburg A., Kuhn K.L., Moore J.A., Davis M.P., Wainwright P.C., Friedman M., Smith W.L. Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl. Acad. Sci. U S A. 2012;109:13698–13703. doi: 10.1073/pnas.1206625109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.S., Grande T.C., Wilson M.V.H. Wiley; 2016. Fishes of the World. [Google Scholar]
- Ørvig T. Microstructure and growth of dermal skeleton in fossil actinopterygian fishes - Birgeria and Scanilepis. Zool. Scr. 1978;7:33–56. [Google Scholar]
- Poole D.F.G. Phylogeny of tooth tissues: enameloid and enamel in recent vertebrates, with a note on the history of cementum. In: Miles A.E.W., editor. Structural and Chemical Organization of Teeth. Academic Press; 1967. pp. 111–149. [Google Scholar]
- Qiao T., King B., Long J.A., Ahlberg P.E., Zhu M. Early gnathostome phylogeny revisited: multiple method consensus. PLoS One. 2016;11:e0163157. doi: 10.1371/journal.pone.0163157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q., Haitina T., Zhu M., Ahlberg P.E. New genomic and fossil data illuminate the origin of enamel. Nature. 2015;526:108–111. doi: 10.1038/nature15259. [DOI] [PubMed] [Google Scholar]
- Reif W.-E. Structural convergences between enameloid of actinopterygian teeth and of shark teeth. Scan. Electron. Micros. 1979;II:547–554. [Google Scholar]
- Rücklin M., Giles S., Janvier P., Donoghue P.C. Teeth before jaws? Comparative analysis of the structure and development of the external and internal scales in the extinct jawless vertebrate Loganellia scotica. Evol. Dev. 2011;13:523–532. doi: 10.1111/j.1525-142X.2011.00508.x. [DOI] [PubMed] [Google Scholar]
- Sasagawa I. The appearance of matrix vesicles and mineralization during tooth development in three teleost fishes with well-developed enameloid and orthodentine. Arch. Oral Biol. 1988;33:75–86. doi: 10.1016/0003-9969(88)90049-0. [DOI] [PubMed] [Google Scholar]
- Sasagawa I. Fine structure of the cap enameloid and of the dental epithelial cells during enameloid mineralisation and early maturation stages in the tilapia, a teleost. J. Anat. 1997;190(Pt 4):589–600. doi: 10.1046/j.1469-7580.1997.19040589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa I. Fine structure of tooth germs during the formation of enameloid matrix in Tilapia nilotica, a teleost fish. Arch. Oral Biol. 1995;40:801–814. doi: 10.1016/0003-9969(95)00050-y. [DOI] [PubMed] [Google Scholar]
- Sasagawa I. Mechanisms of mineralization in the enameloid of elasmobranchs and teleosts. Connect. Tissue Res. 1998;39:207–214. doi: 10.3109/03008209809023928. discussion 221-225. [DOI] [PubMed] [Google Scholar]
- Sasagawa I. Mineralization patterns in elasmobranch fish. Microsc. Res. Tech. 2002;59:396–407. doi: 10.1002/jemt.10219. [DOI] [PubMed] [Google Scholar]
- Sasagawa I., Ishiyama M. Fine structural and cytochemical mapping of enamel organ during the enameloid formation stages in gars, Lepisosteus oculatus, Actinopterygii. Arch. Oral Biol. 2005;50:373–391. doi: 10.1016/j.archoralbio.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Sasagawa I., Ishiyama M. The structure and development of the collar enameloid in two teleost fishes, Halichoeres poecilopterus and Pagrus major. Anat. Embryol. (Berl.) 1988;178:499–511. doi: 10.1007/BF00305037. [DOI] [PubMed] [Google Scholar]
- Sasagawa I., Ishiyama M., Yokosuka H., Mikami M. Fine structure and development of the collar enamel in gars, Lepisosteus oculatus, Actinopterygii. Front. Mater. Sci. China. 2008;2:134–142. [Google Scholar]
- Sasagawa I., Ishiyama M., Yokosuka H., Mikami M. Teeth and ganoid scales in Polypterus and Lepisosteus, the basic actinopterygian fish: an approach to understand the origin of the tooth enamel. J. Oral Biosci. 2013;55:76–84. [Google Scholar]
- Sasagawa I., Ishiyama M., Yokosuka H., Mikami M., Oka S., Shimokawa H., Uchida T. Immunolocalization of enamel matrix protein-like proteins in the tooth enameloid of spotted gar, Lepisosteus oculatus, an actinopterygian bony fish. Connect. Tissue Res. 2019;60:291–303. doi: 10.1080/03008207.2018.1506446. [DOI] [PubMed] [Google Scholar]
- Sasagawa I., Oka S., Mikami M., Yokosuka H., Ishiyama M., Imai A., Shimokawa H., Uchida T. Immunohistochemical and Western blotting analyses of ganoine in the ganoid scales of Lepisosteus oculatus: an actinopterygian fish. J. Exp. Zool. B Mol. Dev. Evol. 2016;326:193–209. doi: 10.1002/jez.b.22676. [DOI] [PubMed] [Google Scholar]
- Schultze H.-P. Scales, enamel, cosmine, ganoine, and early osteichthyans. C. R. Palevol. 2016;15:83–102. [Google Scholar]
- Schultze H.-P., Märss T. Revisiting Lophosteus, a primitive osteichthyan. Acta Univ. Latv. 2004;679:57–78. [Google Scholar]
- Shellis R.P., Miles A.E.W. Autoradiographic study of formation of enameloid and dentin matrices in teleost fishes using tritiated amino-acids. Proc. R. Soc. Lond. B. 1974;185:51–72. [Google Scholar]
- Shellis R.P., Miles A.E.W. Observations with electron microscope on enameloid formation in common eel (Anguilla anguilla; Teleostei) Proc. R. Soc. Lond. B. 1976;194:253–269. [Google Scholar]
- Simmer J.P., Papagerakis P., Smith C.E., Fisher D.C., Rountrey A.N., Zheng L., Hu J.C. Regulation of dental enamel shape and hardness. J. Dent. Res. 2010;89:1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sire J.Y. Ganoine formation in the scales of primitive actinopterygian fishes, lepisosteids and polypterids. Connect. Tissue Res. 1995;33:213–222. doi: 10.3109/03008209509017006. [DOI] [PubMed] [Google Scholar]
- Sire J.Y., Donoghue P.C., Vickaryous M.K. Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates. J. Anat. 2009;214:409–440. doi: 10.1111/j.1469-7580.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sire J.Y., Geraudie J., Meunier F.J., Zylberberg L. On the origin of ganoine: histological and ultrastructural data on the experimental regeneration of the scales of Calamoichthys calabaricus (Osteichthyes, Brachyopterygii, Polypteridae) Am. J. Anat. 1987;180:391–402. doi: 10.1002/aja.1001800409. [DOI] [PubMed] [Google Scholar]
- Smith C.E., Hu Y., Hu J.C., Simmer J.P. Ultrastructure of early amelogenesis in wild-type, Amelx(-/-), and Enam(-/-) mice: enamel ribbon initiation on dentin mineral and ribbon orientation by ameloblasts. Mol. Genet. Genom. Med. 2016;4:662–683. doi: 10.1002/mgg3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.M. Distribution and variaion in enamel structure in the oral teeth of sarcopterygians: its significance for the evolution of a protoprismatic enamel. Hist. Biol. 1989;3:97–126. [Google Scholar]
- Smith M.M. Heterochrony in the evolution of enamel in vertebrates. In: McNamara K.J., editor. Evolutionary Change and Heterochrony. John Wiley & Sons; 1995. pp. 125–150. [Google Scholar]
- Smith M.M. Microstructure and evolution of enamel amongst osteichthyan fishes and early tetrapods. In: Smith P., Tchernov E., editors. Structure, Function, and Evolution of Teeth. Freund Publishing House; 1992. pp. 73–101. [Google Scholar]
- Toyosawa S., O'HUigin C., Figueroa F., Tichy H., Klein J. Identification and characterization of amelogenin genes in monotremes, reptiles, and amphibians. Proc. Natl. Acad. Sci. U S A. 1998;95:13056–13061. doi: 10.1073/pnas.95.22.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh B., Lee A.P., Ravi V., Maurya A.K., Lian M.M., Swann J.B., Ohta Y., Flajnik M.F., Sutoh Y., Kasahara M. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshawsky H. Organization of crystals in enamel. Anat. Rec. 1989;224:242–262. doi: 10.1002/ar.1092240214. [DOI] [PubMed] [Google Scholar]
- Witmer L.M. The extant phylogeny bracket and the importance of reconstructing soft tissues in fossils. In: Thompson J.J., editor. Functional Morphology in Vertebrate Paleontology. Cambridge University Press; 1995. pp. 19–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nucleotide sequences generated during this study are available at GenBank (accession numbers: MG010658-MG010662).