Abstract
The toxicity and environmental persistence of anthropogenic per- and poly-fluoroalkyl substances (PFAS) are of concern globally. To address legacy PFAS concerns in the US, industry developed numerous replacement PFAS that commonly are treated as confidential information. To investigate the distribution of PFAS in New Jersey (NJ), soils collected from across the state were subjected to nontargeted mass-spectral analyses. Ten chloro-perfluoro-polyether-carboxylates were tentatively identified, with ≥3 congeners in all samples. Nine congeners are ≥(CF2)7. Distinct chemical formulas and structures, as well as geographic distribution, suggest airborne transport from an industrial source. Lighter congeners dispersed more widely than heavier, with the most widely dispersed detected in an in-stock New Hampshire sample. Additional data were used to develop a legacy-PFAS fingerprint for historical PFAS sources in NJ.
Per- and poly-fluoroalkyl substances (PFAS) are anthropogenic compounds used to impart surfactant, anti-staining, anti-sticking and related properties to a wide array of consumer and industrial products. Spurred by concerns regarding potential toxicity and environmental persistence of long-chain PFAS (1–5), in 2006 the United States Environmental Protection Agency (USEPA) and eight leading PFAS manufacturers and users negotiated a voluntary “PFOA Stewardship Program” in which the companies agreed to work toward the elimination of perfluorooctanoic acid (PFOA or C8), as well as C8 precursors and related longer-chain homologues from emissions and product content by 2015. With establishment of the Stewardship Program, numerous PFAS manufacturers and users initiated efforts to develop substitute compounds for legacy long-chain PFAS, commonly settling on structures that are treated as confidential business information. With proliferation of these substitute PFAS, environmental chemists have set about attempting to identify them using nontargeted, high-resolution mass spectrometry (HRMS) to assemble formulas and likely structures from molecular-precursor and -fragment data (6). High mass-resolution enables chemists to identify those molecular formulas having exact masses within a user-specified mass-error threshold, and molecular-fragment masses and spectra of the molecules help narrow possible formulas further, ideally informing molecular structure as well (e.g., 7).
Among participants in the Stewardship Program several have operated industrial facilities, ongoing or in the past, in or near densely populated New Jersey. As part of efforts to elucidate industrial chemical sources, chemical species and distribution of legacy and possible substitute PFAS in New Jersey, in late 2017 the New Jersey Department of Environmental Protection (NJDEP) collected soil samples. For this survey, samples primarily were collected in southern New Jersey where two Stewardship Program signatories are located, Solvay in West Deptford Township and DuPont (now Chemours) in Pennsville Township. Historically, Solvay produced polyvinylidene fluoride (PVDF) which entailed use of Surflon, a surfactant containing C9, C11 and C13 (perfluorononanoate, perfluoroundecanoate and perfluorotridecanoate) perfluorocarboxylates (PFCAs) (8). In contrast, the DuPont/Chemours facility manufactured and used fluorotelomers (i.e., compounds synthesized from perfluoroalkyl iodide, comprised of perfluorinated-carbon straight chains, e.g. F(CF2)6-, and usually two hydrogen-bearing carbons, e.g. -CH2CH2-) from 1962 until no later than 2014 (9). Sampling transects were collected in the dominant downwind directions as recorded at nearby Philadelphia International Airport, and remote locations around the state were sampled as well (For sampling campaign details, see Supplementary Materials - SM). These samples were sent to the USEPA, Office of Research and Development laboratory (USEPA/ORD) in Athens, Georgia.
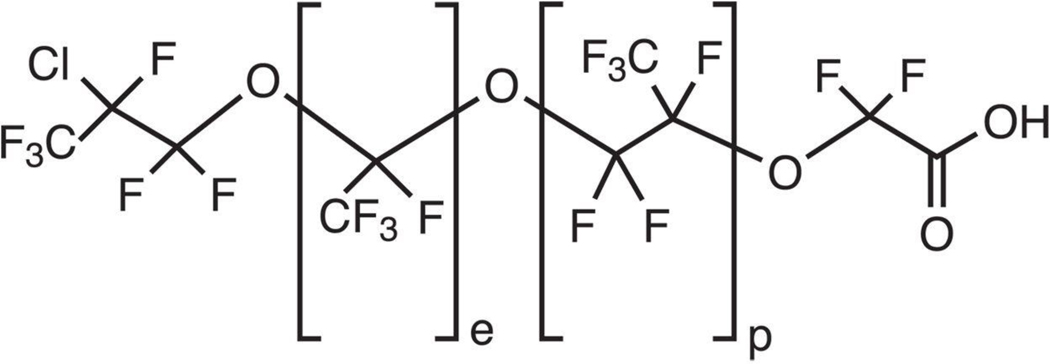
At the ORD laboratory, soil samples were extracted (see SM) in triplicate and selected samples analyzed (see SM) for PFAS unknown to our research team using ultra-performance liquid chromatograph (UPLC) coupled to a quadrupole time-of-flight (QToF) mass spectrometer (MS) operating in negative electrospray ionization (ESI), MSe (no mass filtering) mode. Output data were sorted by signal intensity, high-intensity molecular features were plotted on mass-defect plots (7) ranging in defect from −0.10 to +0.05 Da, and molecular features appearing in the plots of multiple samples were culled for further scrutiny. Using low collision-energy precursor masses, high collision-energy fragment masses, a distinctive mono-chloro M+2 spectral feature, and carbon-isotopic ratios (10), we tentatively identified a molecular feature as a chloro perfluoro polyether carboxylate (ClPFPECA) described in the literature as “Solvay’s product (CAS No. 329238–24-6)” (11) as reported in a product assessment by the European Food Safety Authority (EFSA) at the request of “Solvay Solexis, Italy” (12). With these reports, together with compound-synthesis papers by Solvay chemists (13, 14), the structure of these ClPFPECAs appears to be as shown in Fig. 1 for 70% of production, with 30% having an alternative terminus of ClCF2CF(CF3)O-.
Fig. 1:
A chloro perfluoro polyether carboxylate (ClPFPECA) identified by nontargeted MS analyses in soil samples from New Jersey. In the NJ samples, perfluoroethyl (e) plus perfluoropropyl (p) groups were observed to range in sum from one to four. The example congener depicted here would be designated (e,p)=1,1. Isomers likely include an alternative terminal structure of ClCF2CF(CF3)O- (13, 14) as well as relative positions for the perflluoroethyl and perfluoropropyl groups.
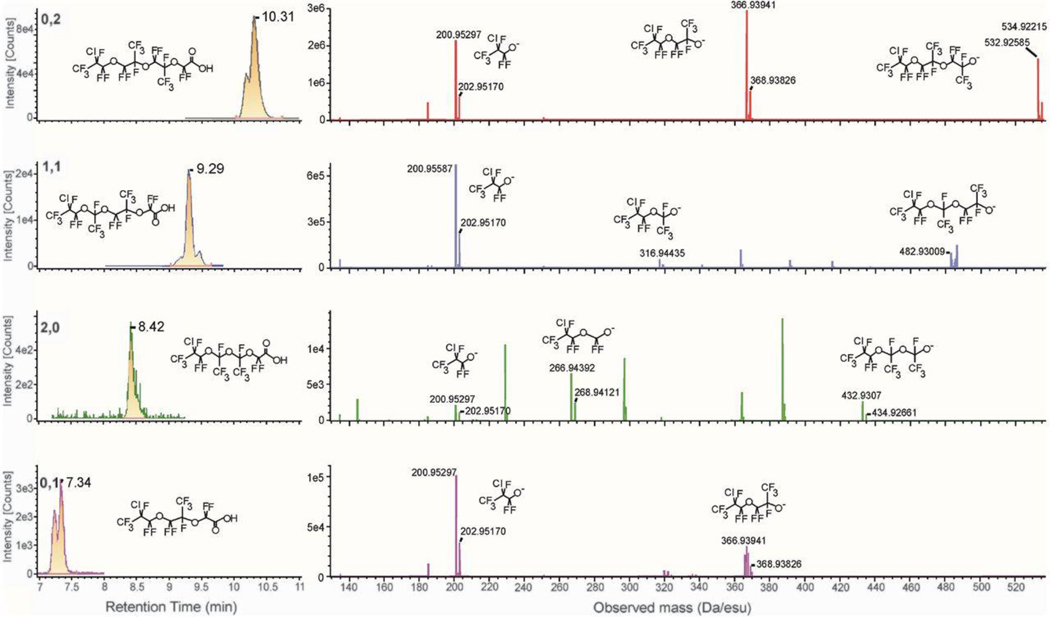
We have not had access to a standard of the Solvay product. However, based on tentative identification of one Solvay product congener in our data, and the literature report that ClPFPECA congeners can include 0 to 2 perfluoroethyl groups (e) and 1 to 4 perfluoropropyl groups (p) (11, 12) separated by ether linkages (Fig. 1), we carried out suspect screening of our MSe data by extracting hypothetical masses to determine what other congeners might be present. Following this effort, all tentatively identified congeners were further elucidated on the QToF operating in MS/MS mode wherein the quadrupole magnets were focused on suspected precursor m/z values, fragmented with ramped collision energy, then precursors and fragments isolated/detected in the ToF (see SM). Results for the nine ClPFPECA congeners tentatively identified on QToF are depicted in Fig. 2 and Fig. S2. Within conventional HRMS-identification confidence context (15, 16), these compounds fall at Level 2b (diagnostic probable structure) and Level 3 (tentative candidate), but considering the nine congeners together, confidence of their general identity is high.
Fig. 2:
Mass chromatograms (MS/MS mode), spectra and precursor/fragment structures of four smaller ClPFPECA congeners detected in NJ samples, identified in the upper left of the chromatograms by ethyl#,propyl#. Results for larger congeners are shown in Fig. S2. Chromatogram peaks consist of signal from precursors and selected major fragments. Note congeners elute in order according to molecular mass, small to large. Also note on major spectra the diagnostic mono-chlorine signal of 3:1 for 35Cl:37Cl.
Having tentatively identified nine congeners in these NJ soil samples as “Solvay’s product,” we re-examined in-house nontargeted results for a water sample from the Bormida di Spigno River, downstream of Solvay Specialty Polymers Italy S.p.A. (Spinetta Marengo AL, Italy). In this Italian water sample, we identified five ClPFPECA congeners (Fig. S3) consistent with our NJ soil samples, bolstering confidence still further in our identification of these compounds as “Solvay’s product.”
Informed by the fragmentation patterns of the QToF suspect screening, we developed a method for routine analysis of the detected congeners on a conventional-resolution tandem mass spectrometer (LC/MS/MS), adding monitoring for a possible ethyl,propyl (e,p)=1,0 congener (Table S2; Fig. S4). Whereas this method was not developed with the benefit of authentic standards, it was informed by masses for ~30 precursors and fragments uniformly having mass error <4 mDa when MS signal is ≥105 (Fig. S5). With an objective of assessing relative concentrations among samples, we performed analyses on the triplicate soil extracts with a matrix internal standard labeled with five heavy carbons, 13C5-perfluorononanoic acid (13C5-PFNA; 13C5-C9), then reported ClPFPECAs “as C9,” by simple peak-area ratios (see SM). We also performed LC/MS/MS analyses on the triplicate soil-extract replicates for legacy PFCAs, quantitating on mass-labeled internal matrix standards (see SM). Results of ClPFPECA analyses are summarized in Table S4, and PFCA analyses are summarized in Table S5.
Of the ten congeners we report by QToF or tandem MS: i) six were expected based on EFSA (11, 12) information (e,p=0,1; 1,1; 0,2; 2,1; 1,2; and 0,3 congeners); ii) four were not included as congeners in the EFSA information (1,0; 2,0; 3,0; and 4,0 congeners); and iii) six congeners anticipated based on EFSA information were not detected (2,2; 1,3; 2,3; 0,4; 1,4; 2,4 congeners) (Fig. S6). In Fig. S7, we summarize the fractional composition of the ten ClPFPECA congeners detected in our study in terms of mean, maximum and minimum fraction observed amongst our soil samples. Addressing the mean fractions, at roughly 40% each, the e,p=0,1 and 1,1 congeners are dominant, followed by ~15% for the 0,2 and lesser to trace amounts of all other congeners (Fig. S7).
Several ClPFPECAs eluted as split peaks (Fig. 2 & S2). We investigated whether this splitting reflected the presence of isomers by extracting spectral patterns of visually distinct chromatographic peak ranges, looking for unique fragmentation patterns across aggregate peaks (see SM; Fig. S8–S10). Based on these efforts, we suspect the presence of group-regioisomerism for congeners having both ethyl and propyl groups, as well as regioisomers based on chlorine position (Fig 1).
These NJ soil samples generally were elevated in legacy PFCAs relative to global background soil estimates (17), and particularly elevated in C9 and longer homologues. For example, the mean C9 in our NJ soils is 785 pg/g dry soil (Table S5) (compared to global background of 18 pg/g (17)); mean C10=437 pg/g (perfluorodecanoate; background=11 pg/g); mean C11=1618 pg/g (background=9.6 pg/g); mean C12=167 pg/g (Perfluorodecanoate; background=9.0 pg/g); and mean C13=222 pg/g (background not reported). Also, the lowest NJ soil concentrations in our study for C9 through C12 PFCAs (Table S5) were 5- to 30-fold that of mean global background values (17). These elevated long-chains resulted in an anomalous PFCA-homologue profile for the NJ samples relative to global background. Whereas the PFCA profile for global background soils tended to be highest in C6, C7 and C8 PFCAs (perfluorohexanoate, perfluoroheptanoate and perfluorooctanoate), in this order, these NJ samples were most highly represented by C11 and C9, in this order (Fig. S11).
Taken altogether, these data for ClPFPECAs and the elevated levels of legacy PFAS strongly suggest the presence of regional PFAS sources.
Probing for possible relationships suggested by variation in the data, principal component analysis (PCA) was performed (Fig. S12) to guide directed testing as described below. In sum, principal component 1 (PC1) and PC2 account for 96.8% of variation in the data, with PC1 alone accounting for 90.6%. The 95% confidence-interval ellipsoids in the PCA score plot (Fig. S12) encompass the two chemical families almost exclusively, the ClPFPECAs and the legacy PFCAs. The major ellipsoidal axis of the ClPFPECA cluster is oriented more closely parallel to PC1 reflecting considerable variance among these data that can be characterized dominantly by a single component, as might be expected for a single physical source. Also noteworthy is that C11 and C13 fall within the ClPFPECA ellipsoid (Fig. S12), suggesting similarities in the pattern of variation for C11 and C13 with at least some of the ClPFPECAs.
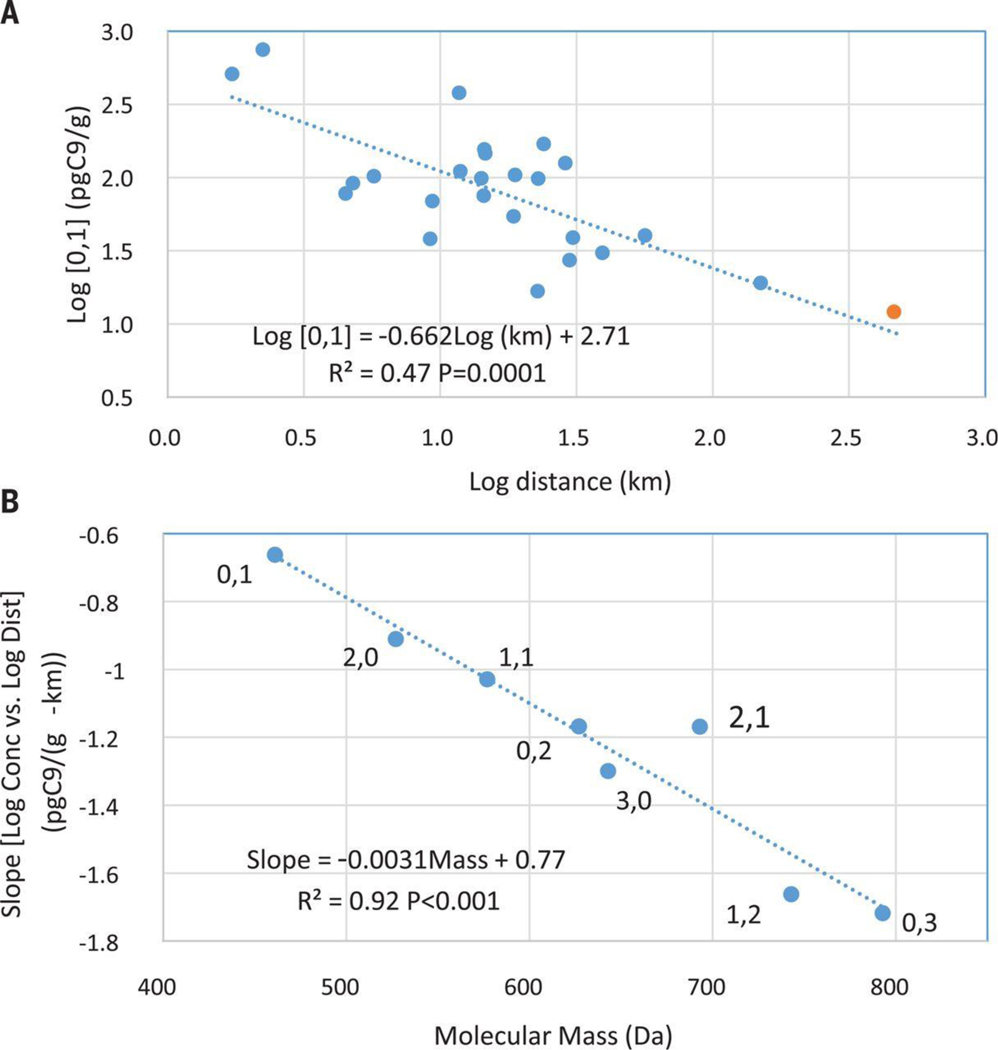
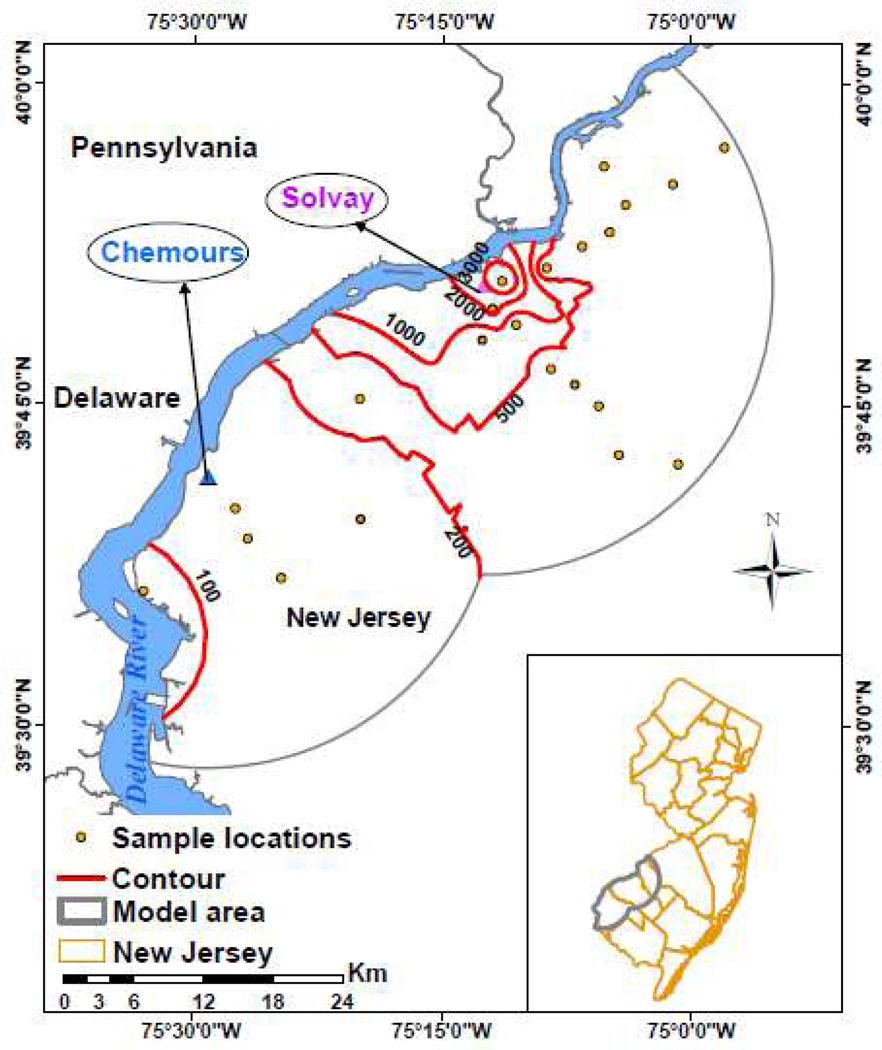
Exploring variation in the ClPFPECA data (Fig. S12), we regressed the eight ClPFPECA congeners detected in most samples (excluding rarely detected 1,0 and 4,0 congeners) against distance from Solvay in log-transformed space (e.g., Fig. 3A). All eight congeners decreased with distance from Solvay with high degrees of significance (i.e., P<0.0002; Table 1). Examining the data in three dimensions, the ClPFPECA concentration contours form a concentric focus on Solvay, consistent with Solvay being the source of these compounds (Fig. 4). The slope of diminishing concentration with distance from Solvay (Table 1) also increases with molecular mass (P<0.001; Fig. 3B) suggesting smaller congeners were dispersed more widely than larger congeners. This sorting by mass might be a factor in the absence of our detection of several of the largest ClPFPECA congeners expected for the Solvay product (12) (Fig. S6); the heaviest congener we detected is the e,p=0,3 at 792.9 Da and the lightest of the six congeners expected, but not detected (Fig. S6), was the 2,2 with a mass of 858.9 Da.
Fig. 3A:
Log 0,1-congener soil concentration (pg/g) vs log distance from Solvay (km). The regression statistics are for the NJ soil samples (blue) located as far as 150 km removed from Solvay (Table S1). Other ClPFPECA congeners are still more highly correlated with distance from Solvay (Table 1). Also shown is the 0,1 congener detected in a soil from Merrimack, NH at 12.1 pg/g (orange), some 460 km distant from Solvay (Table S1), falling closely proximate to the regression line for NJ 0,1 congeners. The 0,1 congener is the most-widely dispersed of the ClPFPECAs (Fig. 3B) and the only ClPFPECA detected in the NH soil. Inclusion of the NH data point in the regression (R2=0.55; P=10−5) increases the significance of the relationship roughly an order of magnitude beyond that of NJ data alone. 3B: Regression slope (log [ClPFPECA] vs log distance from Solvay) for each of eight ClPFPECA congeners vs congener molecular mass. Given the statistically significant relationship (P=0.001), this observation suggests sorting by molecular mass in an atmospheric plume, with lighter molecules generally being dispersed more remotely than heavier molecules. Mechanisms of atmospheric mass sorting remain uncertain, but regression slope also is correlated with congener-acid vapor pressure (R2=0.91; P<0.001) and congener-anion octanol-water partition coefficient (R2=0.92; P<0.001) as estimated by the USEPA Chemical Transformation Simulator (25).
Table 1:
Regression statistics for chemical data (pg/g) against distance (km) from selected facilities in log-transformed space
| Analyte Atmospheric Precursor (1) | Distance from Solvay (km) (maximum n=24) |
Distance from Chemours (km) (anomalous background SS22 excluded; n=23) |
||||||
|---|---|---|---|---|---|---|---|---|
| Compound(s) | Pearson R | P (2) | Slope | Compound(s) | Pearson R | P (2) | Slope | |
| 0,1 (ND=0; n=24) | 0.688 | 2.0E-04 | −0.662 | |||||
| 2,0 (ND=2; n=22) | 0.766 | 3.2E-05 | −0.911 | |||||
| 1,1 (ND=0; n=24) | 0.791 | 4.1E-06 | −1.029 | |||||
| 0,2 (ND=0; n=24) | 0.845 | 2.0E-07 | −1.167 | |||||
| 3,0 (ND=3; n=21) | 0.822 | 4.9E-06 | −1.300 | |||||
| 2,1 (ND=2; n=22) | 0.831 | 1.7E-06 | −1.169 | |||||
| 1,2 (ND=7; n=17) | 0.846 | 1.9E-05 | −1.662 | |||||
| 0,3 (ND=4; n=20) | 0.849 | 2.2E-06 | −1.718 | |||||
| Ĉongeners (ND=0; n=24) | 0.796 | 3.3E-06 | −0.937 | |||||
| 8:2FTOH | PFNA (C9) | 0.130 | Nonsig. | PFOA (C8) | 0.202 | Nonsig. | ||
| 10:2FTOH | PFUA(Cll) | 0.620 | 1.2E-03 | −0.464 | PFDA (CIO) | 0.514 | 1.2E-02 | −0.404 |
| 112FTOH | PFTrA(C13) | 0.482 | 1.7E-02 | −0.356 | PFDoA (C12) | 0.478 | 2.1E-02 | −0.394 |
| 14:2FTOH | (C15 not analyzed) | PFTeA (C14) | 0.426 | 4.3E-02 | −0.337 | |||
| (C9fCll+C13) | 0.519 | 4.7E-03 | −0.324 | (C8+C10+C12) | 0.204 | Nonsig. | ||
| (C11+C13) | 0.604 | 1.8E-03 | −0.449 | (C10+C12) | 0.519 | 1.1E-02 | −0.402 | |
| (C9+C11+C13)-(C8+C10+C12) | 0.383 | Nonsig. | ||||||
| (C11+C13)-(C10+C12) | 0.660 | 4.5E-04 | −0.608 | |||||
Ellis et al. (20); 2)
=significance level; ND = not-detected sample count {Additional Supplementary Materials References added here for inclusion in reference list: (26, 27); do not include these parenthetical notes in the paper}
Fig. 4:
∑ClPFPECAs in surface soils (pg/g). Contours lines were generated using an algorthim in ArcMAP 10.6.1 that weighted the five nearest data points according to inverse-square distance. Despite some geographic sporadicity in the data and numerical artifacts where data are sparsely spaced, taken as a group the contours depict a clear pattern of increasing ∑ClPFPECAs with proximity to Solvay.
Considering that these soil samples chiefly are from positions that are not hydraulically downgradient in the watershed of any Solvay waste-water discharge (Fig. 4; Fig. S1), aqueous discharge cannot explain these observations, so these correlations strongly suggest atmospheric release from Solvay as the principal mode of occurrence for these soils.
The observation that three of the lightest congeners (i.e., 0,1; 1,1; 0,2) were detected in all study samples including the most remote NJ sample near the northern state border (Sample SS22; Fig. S1), suggests that light congeners might be dispersed beyond NJ state boundaries. To explore this possibility, we analyzed an in-stock sample from Merrimack, NH that falls roughly parallel with the downwind transect extending northeasterly from Solvay (Fig. S13). To determine whether unrelated samples might have ClPFPECAs, we also analyzed an in-stock sample from Conyers, GA, roughly 1000 km SW from Solvay (Fig. S13). We detected the 0,1 congener in the downwind Merrimack sample and no other congeners, and we detected no ClPFPECAs in the remote Conyers sample. The 0,1 congener is the most widely dispersed (Fig. 4, Table 1), and the NH sample, some 450 km removed, plots closely proximate to the regression line for the 0,1 congener in NJ samples as a function of distance to Solvay. However, whether this NH 0,1-congener detection is from Solvay, or some unknown source, requires more study.
Given the role of Solvay as potentially the dominant or sole source of ClPFPECAs in our study, plots of legacy PFCAs against ClPFPECAs potentially guide which, if any, legacy PFCAs remain diagnostic of pre-Stewardship Solvay releases. Fig. S14, plotting concentrations of each legacy PFCA, chain lengths C4 (perfluorobutanoic acid) thru C13 (perfluorotridecanoic acid), against the sum of ClPFPECAs, shows three samples from closely proximate to Solvay that are high in ClPFPECAs also are high in C9, C11 and C13 PFCAs. Based on this observation, C9, C11 and C13 were regressed against distance from Solvay. Results of these regressions indicated that C9 is not correlated with distance from Solvay, but consistent with the PCA (Fig. S12), C11 (P=1.2×10−3) and C13 (P=1.7×10−2) were statistically related with distance from Solvay (Fig. S15; Table 1). The seeming inconsistency of C9 plotting anomalously in Fig. S14 but not being statistically related to distance from Solvay likely is due in large part to the relatively much higher mobility of C9 than C11 and C13 in soils. For example, in a study of PFCAs in Decatur, AL soils, Washington et al. (18) reported deep/surface soil ratios for C9 as high as 50-fold that of C11 or C13, suggesting much higher rates of loss for C9 than C11 and C13 from surface soils by leaching and percolation.
Although Figs. S14–S15 and Table 1 suggest a relationship of C11 and C13 with Solvay, considerable spread remains in the data (Fig. S15), perhaps reflecting noise imparted from other sources. The majority of all environmental releases of PFCAs longer than C8 from 1951 to 2015 arose from fluorotelomer- and C9-based products (19). Based on smog-chamber experiments (20), and global-scale modeling using a complex suite of kinetic constants estimated from literature (21), atmospheric oxidation of n:2FTOHs (where n is an even integer) yields roughly equimolar nPFCAs and (n+1)PFCAs or preferentially nPFCAs in urban areas where nitrogen oxides can be elevated. In soils, microbially mediated degradation of n:2FTOHs has been shown to proceed through beta oxidation to yield dominantly nPFCAs (22, 23). Consistent with these studies, in their global soil survey, Rankin et al. (24) reported PFOA/PFNA (i.e., nPFCA/(n+1)PFCA) ratios commonly fall in roughly equimolar to dominantly PFOA (nPFCA) range, and argued atmospheric or soil degradation of fluorotelomers as a dominant mode of PFCAs occurrence globally. Given, (i) historical production/use of fluorotelomers at the large-scale NJ Chemours facility, (ii) the generally prevalent contribution of fluorotelomers to C10 and C12, and (iii) atmospheric and soil fluorotelomer-degradation stoichiometry favoring roughly equimolar or dominantly even-chain PFCAs, the difference of nPFCAs minus (n+1)PFCAs, (C11+C13)-(C10+C12), has the potential to deconvolute potential signals from Solvay and Chemours for these legacy PFAS. Large positive excesses in this difference suggest direct release of C11 and C13 PFCAs, whilst near-zero or negative values of this difference would be consistent with atmospheric or soil degradation of fluorotelomer precursors as a source.
Indeed, applying the difference (C11+C13)-(C10+C12) to our NJ soil data accentuates signal-to-noise in that the strength of correlation with distance from Solvay (Fig. S16) increases nearly an order of magnitude beyond that of C11 or C13 alone, with P=4.5×10−4 (Table 1). Fig. S17 plots (C11+C13)-(C10+C12) as a function of the sum of ClPFPECAs, illustrating a relationship significant at P=4.0×10−5 and bolstering that these parameters reflect a common mode of occurrence -- airborne transport.
Contours of the difference (C11+C13)-(C10+C12) are mapped in Fig. S18. The resulting pattern depicts a strongly expressed positive anomaly focusing on Solvay as well as a negative anomaly proximate to Chemours consistent with the reasoning above (Fig. S18). These results are consistent with values reported in Rankin et al. (24) in that three of four samples collected ~20 km southeast of Chemours calculate to negative values for the difference (C11+C13)-(C10+C12). Taken altogether then, the difference (C11+C13)-(C10+C12) evidently fingerprints two potential PFAS sources in concert by accentuating differences in mode of occurrence, direct odd-chain PFCA release of Solvay vs fluorotelomer degradation in the atmosphere or soil from the Chemours facility.
Here we have reported tentative identification of ten ClPFPECA congeners distributed across an expansive breadth of soils in densely populated New Jersey and likely beyond. In light of these findings, numerous near-term pressing uncertainties merit investigation including the presence and mobility of the congeners in soil profiles, in surface and ground waters, in vegetation (e.g., agricultural crops) and in animals including humans, as well as whether there is evidence that these ClPFPECAs degrade in the environment. In the longer term, investigation of whether these ClPFPECAs might be toxic is prudent.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Nidal Azzam, Tim Buckley, Tim Collette, Andy Gillespie, Myriam Medina-Vera, Gloria Post, Brian Schumacher, Caroline Stevens, Kate Sullivan and Eric Weber for support and technical review.
Funding: This research was supported by the United States Environmental Protection Agency, Office of Research and Development and the New Jersey Department of Environmental Protection, Site Remediation and Waste Management Program. It has been subjected to Agency’s administrative review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the USEPA. JWW is an adjunct faculty member at the University of Georgia, Department of Geology.
Footnotes
Competing interests: The authors declare no competing interests nor outside consulting.
Data and materials availability: All data included in this study are available in the main text and in the supplementary materials, underlying data are available on-line at the EPA Environmental Dataset Gateway (https://edg.epa.gov/metadata/catalog/main/home.page then search by article title).
References:
- 1.Giesy JP, Kannan K, Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35, 1339–1342 (2001). [DOI] [PubMed] [Google Scholar]
- 2.De Silva AO, Mabury SA, Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations. Environ. Sci. Technol. 38, 6538–6545 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Lau C, Butenhoff JL, Rogers JM, The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 198, 231–241 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Kannan K et al. , Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Archives of Environmental Contamination and Toxicology 48, 559–566 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG, Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 40, 3463–3473 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Lim X, The fluorine detectives. Nature 566, 26–29 (2019).30728524 [Google Scholar]
- 7.Newton S et al. , Novel polyfluorinated compounds identified using high resolution mass spectrometry downstream of manusfacturing facilities near Decatur, Alabama. Environ. Sci. Technol. 51, 1544–1552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH, Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 40, 32–44 (2006). [DOI] [PubMed] [Google Scholar]
- 9.AECOM, “Conceptual site model (CSM) for poly- and perfluoroalkyl substances (PFAS). prepared by AECOM on behalf of The Chemours Company,” (Newark, DE, 2017). [Google Scholar]
- 10.Washington JW, Rosal CG, Ulrich EM, Jenkins TM, Use of carbon isotopic ratios in nontargeted analysis to screen for anthropogenic compounds in complex environmental matrices. J. Chromatogr. A 1583, 73–79 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Cousins IT, Scheringer M, Hungerbuehler K, Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environment International 60, 242–248 (2013). [DOI] [PubMed] [Google Scholar]
- 12.EFSA, Scientific Opinion on the safety evaluation of the substance perfluoro acetic acid, α-substituted with the copolymer of perfluoro-1,2-propylene glycol and perfluoro-1,1-ethylene glycol, terminated with chlorohexafluoropropyloxy groups. EFSA Journal 8, 1519 (2010). [Google Scholar]
- 13.Tonelli C, Di Meo A, Picozzi R, New hydrofluoropolyethers I Synthesis and reaction pathway evaluation. J. Fluorine Chem. 128, 46–51 (2007). [Google Scholar]
- 14.Tonelli C, Di Meo A, Picozzi R, Bassi M, New hydrofluoropolyethers II: Physico-chemical characterization. J. Fluorine Chem. 132, 356–362 (2011). [Google Scholar]
- 15.et al SLW., Proposed minimum reporting standards for chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 3, 211–221 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schymanski EL et al. , Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ. Sci. Technol. 48, 2097–2098 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Washington JW, Rankin K, Libelo EL, Lynch DG, Cyterski M, Determining global background soil PFAS loads and the fluorotelomer-based polymer degradation rates that can account for these loads. Sci. Total Environ. 651, 2444–2449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washington JW, Yoo H, Ellington JJ, Jenkins TM, Libelo EL, Concentrations, Distribution, and Persistence of Perfluoroalkylates in Sludge-Applied Soils near Decatur, Alabama, USA. Environ. Sci. Technol. 44, 8390–8396 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbuehler K, Global emission inventories for C-4-C-14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environment International 70, 62–75 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Ellis DA et al. , Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 38, 3316–3321 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Wallington TJ et al. , Formation of C7F15COOH (PFOA) and other perfluorocarboxylic acids during the atmospheric oxidation of 8 : 2 fluorotelomer alcohol. Environ. Sci. Technol. 40, 924–930 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Wang N et al. , 8–2 Fluorotelomer alcohol aerobic soil biodegradation: Pathways, metabolites, and metabolite yields. Chemosphere 75, 1089–1096 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Washington JW, Jenkins TM, Weber EJ, Identification of Unsaturated and 2H Polyfluorocarboxylate Homologous Series and Their Detection in Environmental Samples and as Polymer Degradation Products. Environ. Sci. Technol. 49, 13256–13263 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Rankin K, Mabury SA, Jenkins TM, Washington JW, A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 161, 333–341 (2015). [DOI] [PubMed] [Google Scholar]
- 25.USEPA. (2019. CTS: On-line Chemical Transformation Simulator). [Google Scholar]
- 26.Washington JW, Naile JE, Jenkins TM, Lynch DG, Characterizing Fluorotelomer and Polyfluoroalkyl Substances in New and Aged Fluorotelomer-Based Polymers for Degradation Studies with GC/MS and LC/MS/MS. Environ. Sci. Technol. 48, 5762–5769 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Washington JW, Jenkins TM, Rankin K, Naile JE, Decades-scale degradation of commercial, side-chain, fluorotelomer-based polymers in soils & water. Environ. Sci. Technol. 49, 915–923 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.