Abstract
Background and Purpose:
Stroke is a major cause of chronic neurological disability. There is considerable interest in understanding how acute transcriptome changes evolve into subacute and chronic patterns that facilitate or limit spontaneous recovery. Here we mapped longitudinal changes in gene expression at multiple time points after stroke in mice out to 6 months.
Methods:
Adult C57BL/6 mice were subjected to transient middle cerebral artery occlusion. Longitudinal transcriptome levels were measured at 10 time points after stroke from acute to recovery phases of ischemic stroke. Localization and the number of mononuclear phagocytes were determined in the post-ischemic brain. Whole mount brain imaging was performed in asplenic mice receiving GFP+-tagged splenocytes.
Results:
Sustained stroke-induced mRNA abundance changes were observed in both hemispheres with 2989 ipsilateral and 822 contralateral genes significantly perturbed. In the hemisphere ipsilateral to the infarct, genes associated with immune functions were strongly affected, including temporally overlapping innate and adaptive immunity and macrophage M1 and M2 phenotype related-genes. The strong immune gene activation was accompanied by the sustained infiltration of peripheral immune cells at acute, subacute and recovery stages of stroke. The infiltrated immune cells were found in the infarcted area, but also in remote regions at 2 months after stroke.
Conclusions:
The study identifies that immune components are the predominant molecular signatures and they may propagate or continuously respond to brain injury in the subacute to chronic phase after CNS injury. The study suggests a potential immune-based strategy to modify injury progression and tissue remodeling in ischemic stroke, even months after the initiating event.
Keywords: gene profiling, stroke, immune signature, acute and recovery phase
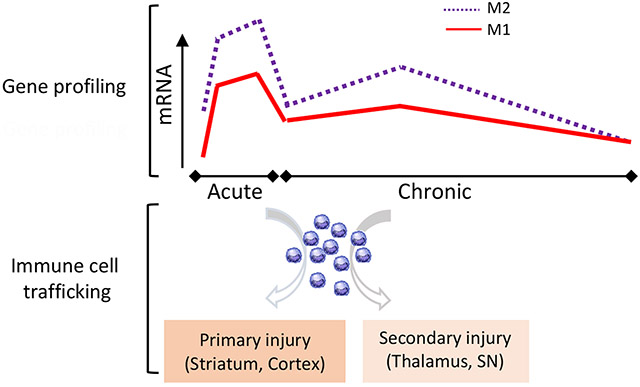
Graphical Abstract
The spatio-temporal relationship between immune gene expression and the presence of immune cells in the post-ischemic brain. M1 and M2 macrophage gene profiles showed sustained elevation of immune rassociated gene expression across different phases of stroke. Immune cell trafficking occurs mainly in the primary injury sites in the acute phase of stroke. The presence of immune cells during the chronic phase of stroke in remote areas, including the thalamus and substantia nigra (SN), shows an involvement of immunity in secondary injury.
Introduction
Stroke is the leading cause of serious, long-term disability in the United States and worldwide. This chronic disability reflects suboptimal recovery1, 2 and few medical treatments are available to treat long-term functional impairments after stroke. Typically, most preclinical studies exploring genetic and cellular changes after stroke have focused on early stages. The longer-term recovery phases of stroke represent a promising window for intervention, but the development of effective clinical therapies that target recovery phases has been challenging due to limited understanding of the genetic and cellular changes that occur in the later stages after stroke.
Gene expression changes in the peripheral blood and immune cells of stroke patients have been proposed as potential diagnostic and prognostic biomarkers in acute stroke3, 4. In blood5, 6 and brain7, 8, results of both preclinical and clinical studies have shown that acute changes in gene expression profiles differ based on the type of stroke and extent of CNS injury. A substantial caveat, however, is that gene expression profiling of human brain tissue after stroke has been conducted largely on patients who do not survive, and thus are poorly representative of the full range of damage and time points after stroke. Generating gene expression profiles across time using an animal model of brain ischemia might allow us to formulate hypothesis about potential blood, CSF, or inflammation imaging biomarkers in non-fatal stroke, leading to a better monitoring of disease progression and stroke recovery.
A fundamental challenge of gene profiling studies is that the results are generally limited to a snapshot of gene expression levels that do not reflect the complex cascades of cellular changes that occur over time in stroke recovery. It is thought to be a transition in the milieu from an acute toxic state to a long-term microenvironment that allows regenerative events to occur9. Since there may be gene mRNA abundance changes related to the control of neurogenesis, angiogenesis, and axonal sprouting beyond the acute phases of stroke10, 11, establishing longitudinal transcriptomic changes across stages would provide a framework for a deeper understanding of stroke pathology and recovery processes.
Results of recent clinical trials demonstrated the benefit of reperfusion in stroke patients receiving mechanical thrombectomy12, 13, and re-perfusion has become a key goal of human stroke treatment. Transient middle cerebral artery occlusion (MCAO) in rodents reflects the clinical features of thrombectomy that result from endovascular recanalization or other methods of reperfusion. The current study uses this model to characterize how stroke-induced gene expression changes shift across acute, sub-acute and chronic (recovery) phases of brain ischemia. Here, we report a persistent elevation of peripheral immune markers and cells after stroke, including during recovery processes in the chronic phases of stroke. The involvement of the immune response following stroke appears to be more expansive and longer lasting than previously recognized.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Details are described in the Supplemental materials.
Animals:
Procedures for the use of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medicine in accordance with NIH and ARRIVE guidelines.
Transient middle cerebral artery occlusion (MCAO):
C57BL/6 mice (12-week-old, males and females) were subjected to 30 min transient MCAO and post-stroke care was performed as previously described 14, 15.
Gene expression analysis:
Detailed methods for RNA-seq library generation, differential gene expression and heat maps, and pathway and cluster analyses are described in supplementary methods.
Flow cytometry analysis:
Isolation, single cell preparation of brain immune cells, and flow cytometer analyses of CD45low and CD45high mononuclear phagocyte subsets were performed according to the methods described16.
Immunostaining of brain tissue:
Paraformaldehyde-perfused mouse brains were sectioned at 30 μm thickness and incubated with anti-rabbit Iba-1 (1:1000, Wako, Richmond, VA, 019-19741) followed by Alexa Fluor 594 goat anti-rabbit IgG (1:2000, Life Technologies, A11012). The sections were mounted and examined under a laser scanning confocal microscope (Carl Zeiss, Thornwood, NY, USA).
Statistics:
Immune cell assessments at different time points were analyzed using Two-way ANOVAs (mixed model) followed by post-hoc Fisher’s LSD comparison between the two hemispheres at each time point. Statistical analyses were performed using Prism software (GraphPad Software Inc., La Jolla, CA). Differences were considered significant if p<0.05.
Results
Perturbations in gene expression persist in the brain following ischemic stroke
There is an incomplete understanding of how time points after stroke in experimental rodent models translate to recovery phases after human stroke. Considering differences in metabolic rate between the species17, we estimated that 1-2 weeks in mice would be approximately equivalent to 2-4 months in humans. Thus, we chose time points that would represent an acute phase (hours to 3d), sub-acute phase (1w to 2w), and recovery phase (1m, 2m, and 6m) of stroke.
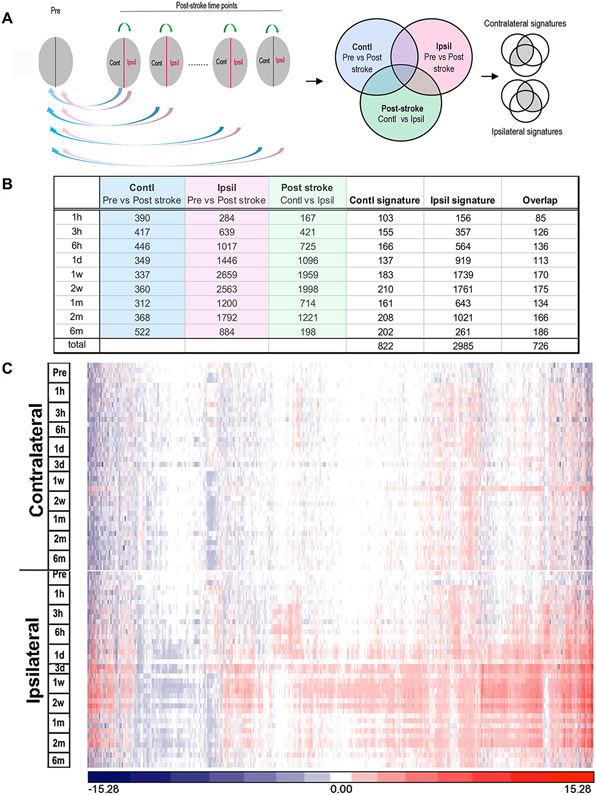
Identification of perturbed gene expression at each time point was performed using the analysis algorithm by 3 pairs of comparisons (Figure 1A): 1) each post-stroke time point vs. the pre-stroke values, both in contralateral hemisphere (blue circle); 2) each post-stroke time point vs. the pre-stroke values, both in ipsilateral hemisphere (pink circle); 3) post-stroke contralateral vs. post-stroke ipsilateral hemisphere at each time point (green circle). Contralateral and ipsilateral gene lists were constructed using the following criteria: a) overlapping genes with expression levels significantly perturbed in both hemispheres in the post-stroke vs pre-stroke (naïve) comparisons (the overlap of the top 2 circles); b) overlapping genes in the contralateral vs. ipsilateral hemisphere comparisons (the overlap of each top circle with the bottom circle). Since the analysis algorithm relied on pairwise comparisons between hemispheres, the initial analyses to identify genes with perturbed expression levels excluded 3-day samples because they did not meet the minimum requirement of 3 pairs at each time point due to unexpected RNA degradation. The 3-day samples were included in later clustering and pathway analyses that did not involve pairwise analyses.
Figure 1.
Identification of perturbed gene expression in the post-ischemic brain across post-stroke stages. A, Algorithm based on 3 separate categories of comparisons. Blue and red arrows indicate comparisons of post-ischemic vs. pre-ischemic brain in the respective contralateral and ipsilateral hemispheres. Green arrows indicate comparisons between hemispheres in post-ischemic brain. Color coded areas in the Venn diagrams indicate the genes perturbed in each of the 3 categories of pairwise comparisons. The gray area contains overlapping genes that are selectively perturbed in the contralateral or ipsilateral hemipsheres. B, Number of perturbed gene expression signatures after all 3 pairwise comparisons and signatures at the post-stroke time points. C, Heat map of the 3081 genes (union of all signature genes) that were significantly perturbed across the post-ischemic time points.
From 24,475 genes analyzed (NCBI Short Read Archive (SRS), accession number: PRJNA525413), mRNA abundance levels for 822 genes in contralateral and 2985 genes in ipsilateral were significantly changed. The ipsilateral hemisphere at 1-2 weeks after stroke contained the greatest number of gene changes (Figure 1B). There was a significant overlap between the mRNA abundance changes identified in the two hemispheres, with the vast majority of genes in the contralateral hemisphere also being identified in the ipsilateral hemisphere (726/822); however, most perturbed genes were specific to the ipsilateral hemisphere. The heat map for the union of the combined 3081 perturbed genes demonstrates that stroke induced a substantially greater change in gene expression in the ipsilateral hemisphere, which peaked from 3 days to 2 weeks after stroke (Figure 1C).
Immunity-related signaling pathways are the most perturbed pathways following stroke
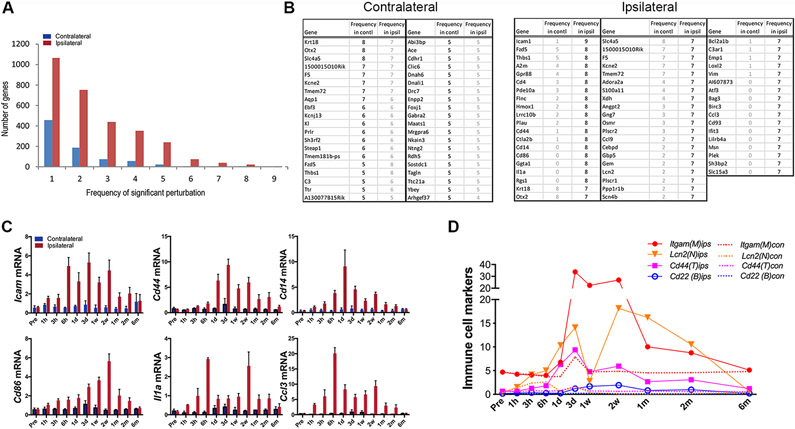
mRNA abundance levels for many genes were significantly altered at multiple time points in both hemispheres, but there were more genes with high frequency perturbations (i.e., perturbed across multiple time points) in the ipsilateral hemisphere (Figure 2A). The most frequently perturbed genes in the contralateral hemisphere were also among the most frequently perturbed in the ipsilateral hemisphere, suggesting that the most frequently perturbed genes on the contralateral side represented genes that were part of a global response to stroke (Figure 2B).
Figure 2.
Perturbation frequency and frequently perturbed gene profiles. A, Frequency of perturbed genes across post-stroke time points. B, List of the most frequently perturbed genes in the both hemispheres. The frequency of perturbation of the same gene on the other hemipshere was indicated in gray. C, Temporal profiles of genes that were perturbed ≥7 times in the post-ischemic brain. D, Temporal gene expression profiles of representative immune cell markers, Itgam, microglia/macrophages; Lcn2, neutrophils; Cd44, T cells; Cd22, B cells.
The ipsilateral hemisphere also contained a set of genes with high frequency perturbations that were not identified in the contralateral hemisphere (Figure 2B right panel). The gene with the most consistent perturbation within this unique ipsilateral subset was Icam1 (perturbed in 9 out of 10 time points). The Icam1 gene encodes an adhesion protein involved in leukocyte rolling, which is an early step in extravasation of immune cells through the brain endothelium (Figure 2B, C). Other genes with high perturbation frequency (> 7 time points) included Cd44, Cd86, Cd14, Il1a, and Ccl3, which also facilitate activation, adhesion and endothelial transmigration of immune cells. The upregulation of these genes was associated with significant expression of immune cell markers for mononuclear phagocytes, neutrophils, and lymphocytes between 3 days and 2 months after stroke, suggesting that infiltration of peripheral immune cells occurs beyond the acute phases following stroke (Figure 2D).
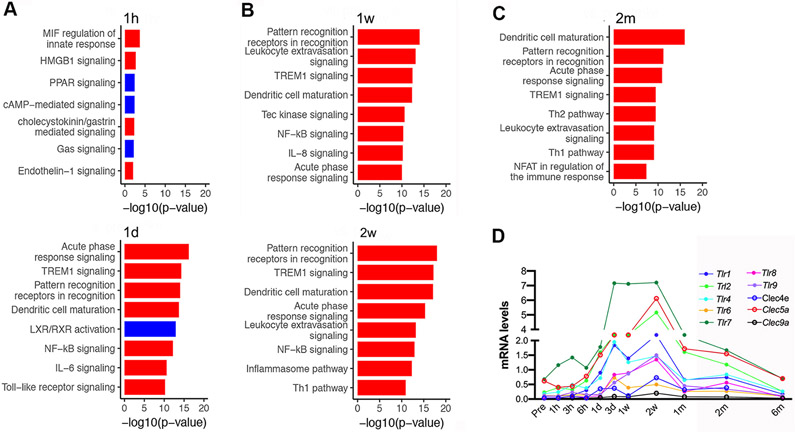
Pathway analyses of the ipsilaterally regulated genes revealed the acute activation of pathways related to MIF regulation, HMGB1 signaling, cholecystokinin signaling, and Endothelin-1 signaling (red bar, Figure 3A), as well as a down-regulation of PPAR, cAMP and LXR/RXR signaling (blue bars, Figure 3A). Gene expression associated with immune system signaling was activated in acute (1h and 1d; Figure 3A), sub-acute (1-2 weeks; Figure 3B), and recovery phases (2m; Figure 3C). Among them, the most prominent immune pathways involving pattern recognition receptors were repeatedly up-regulated across all time points. Gene profiling showed continuous up-regulation of Toll-like receptor (Tlrs) and c-type lectin receptor (Clec9a, Clec4e) expression across all stroke stages, with a peak between 3d to 1m post-stroke (Figure 3D).
Figure 3.
Ingenuity Canonical Pathway analysis of signature genes identified from ipsilateral hemipshere after stroke. A-C, Significantly perturbed genes at acute (A, 1h and 1d)), sub-acute (B, 1w and 2w) and recovery (C, 2m) time points. Significant pathways are presented with blue and red bars indicating down and up-regulation, respectively. D, Expression profiles of pattern recognition receptors over time. Tlr8, Tlr9, Toll-like receptor; Clec9a, Clec4e, Clec5a, lectin-receptor families.
Stroke induces expression of distinct gene clusters with robust immune signatures in the ipsilateral hemisphere
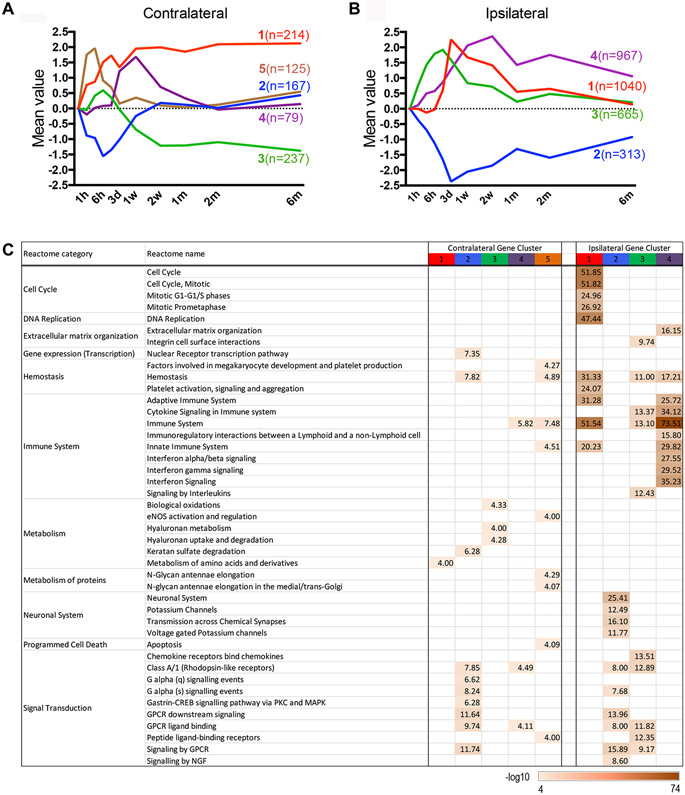
Several gene clusters remained either up- or down-regulated for months after stroke in both hemispheres. In the contralateral hemisphere, clustering algorithms of perturbed genes revealed 5 distinct temporal expression patterns. Clusters 1, 4, and 5 were acutely up-regulated and clusters 2 and 3 were down-regulated (Figure 4A). Genes in clusters 2, 4 and 5 returned to baseline by 6 months after stroke, but clusters 1 and 3 remained up- and down-regulated, respectively, through all recovery stages examined. Pathway analyses (Figure 4C) revealed that the down-regulation of cluster 2 genes was associated with hemostasis and signal transduction. Up-regulated genes in cluster 1 were associated with amino acid metabolism, while genes in cluster 3 were associated with biological oxidation and hyaluronan metabolism. Cluster 4 genes included both immune system and signal transduction reactomes. The identification of these distinct clusters in the contralateral hemisphere suggests changes that could reflect remodeling to compensate for the loss of function on the stroked side.
Figure 4.
Clustering and pathway analysis of genes perturbed in the post-stroke brain. A,B, Five gene clusters in the contralateral (A) and 4 in the ipsilateral hemisphere (B) were identified. Average expression of each cluster is color coded with content in parenthesis indicating the number of genes in each cluster. C, MSigDB reactome enrichment analysis. The values of each gene across all time points were log2 transformed and normalized as described in Method section.
In the ipsilateral hemisphere, 4 distinct temporal clusters were identified (Figure 4B). Genes in ipsilateral cluster 2 were down-regulated, whereas cluster 1, 3 and 4 genes were up-regulated. Several highly enriched reactome categories were found in the ipsilateral clusters (Figure 4C, right). Down-regulated genes in cluster 2 were mostly associated with neuronal systems. Consistent with stroke-induced cellular death in the striatum, cluster 2 contained neuronal genes associated with the striato-nigral pathway. These genes included an acute down-regulation of Ppp1r1a/Darpp32, Slc6a3, and Drd2, which was followed by reduced expression of Th (tyrosine hydroxylase, an enzymatic marker for dopaminergic neurons) that started at 1 week after stroke (Supplementary Figure IA). Th expression remained reduced through 6 months after injury, but there was no change in the expression of marker genes for cholinergic, serotonergic and glutamatergic neurons (Supplementary Figure IB). Genes up-regulated in cluster 1 included cell cycle, DNA replication, and immune system reactomes. These genes were induced at day 1, peaked at day 3, and eventually recovered to pre-stroke levels by 6 months. Extracellular organization, hemostasis, and immune system genes were enriched in both clusters 3 and 4, with cluster 3 also containing an enrichment of signal transduction genes. Notably, clusters 1, 3, and 4 were all enriched in immune system genes, but the peak induction of these gene clusters was temporally different. Peak expression of clusters 1, 3 and 4 were at 3-day, 1-day, and 2-weeks after stroke, respectively. Unlike clusters 1 and 3, however, the genes in cluster 4 did not recover to their pre-stroke levels after 6 months. Collectively, the pathway analyses of gene clusters in the ipsilateral hemisphere identify strong immune signatures across multiple stages after stroke. These persistent changes implicate immune system involvement not only in the initial events after stroke, but also in long-term tissue remodeling and whole brain recovery. Complete lists of genes in the clusters for both hemispheres are presented (Supplementary Table I).
Stroke induces temporally overlapping innate and adaptive as well as M1 and M2 immune profiles
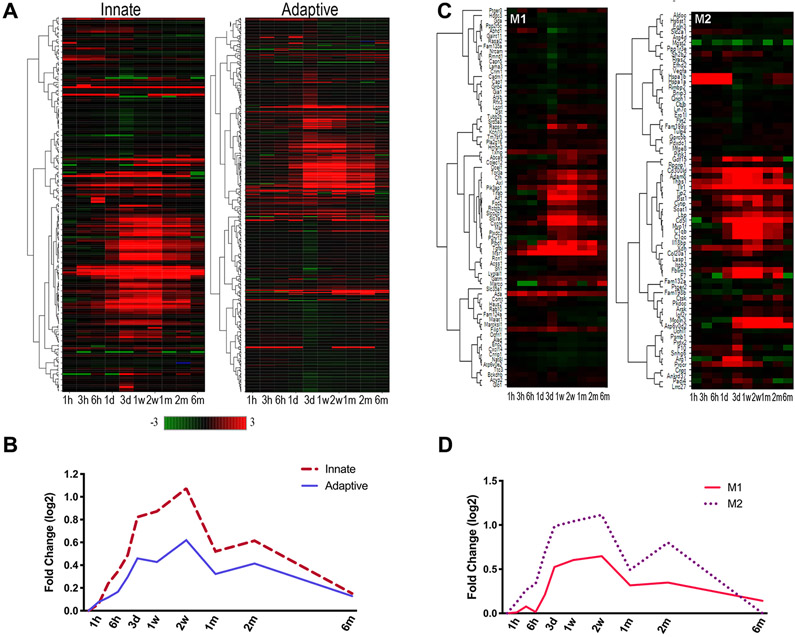
With the robust immune signatures, we analyzed innate and adaptive immune genes in the ipsilateral hemisphere (Figure 5A). The most significantly and frequently up-regulated innate immune genes included pattern recognition receptors (Tlrs), cell interaction and immune activation (Lgals3), and complement cascades (C3, C1qa, C1qa, C1qc). Up-regulated adaptive immune genes included adhesion and cytokine signaling genes (Icam1, Socs3). Both innate and adaptive immunity-related genes remained elevated during the chronic recovery period, but mostly returned to baseline by 6 months after stroke (Figure 5B). The similarity pattern between the innate and adaptive immune systems suggests that their activation may be temporally coupled in the post-stroke brain.
Figure 5.
Expression of key immune genes in the ipsilateral hemisphere. Heat map and average expression of of innate or adaptive genes (A, B) and M1 and M2 genes (C,D) in the post-stroke brain. Green (decreased) and red (increased) intensities indicate log2 transformed-fold change compared to pre-stroke.
Among peripheral immune cells, monocyte-derived macrophages (MMs) are the major innate immune cells observed in the post-ischemic brain. These MMs polarize to either classically activated M1 macrophages that elicit inflammation or alternatively activated reparative M2 macrophages that resolve inflammation in the injured brain18. Based on the longitudinal immune transcriptome signatures observed across multiple phases of stroke, we further analyzed temporal changes of inflammatory and phagocytic M1 and M2 markers. We found an immediate induction of M2 genes, followed by elevated M1 and M2 gene expression (Figure 5C). Notably, M2 gene profiles showed a biphasic response with a second rise in expression that peaked 2 months after stroke including increases in Cd5l, Atp6v0d2, and Thsp1. In addition, Cd36 and Lipa, which are required for lipid uptake and lysosomal lipolysis19 during alternative activation of macrophages showed biphasic responses (Supplementary Figure II).
Stroke induces the infiltration of peripheral MMs in the brain at acute and recovery phases
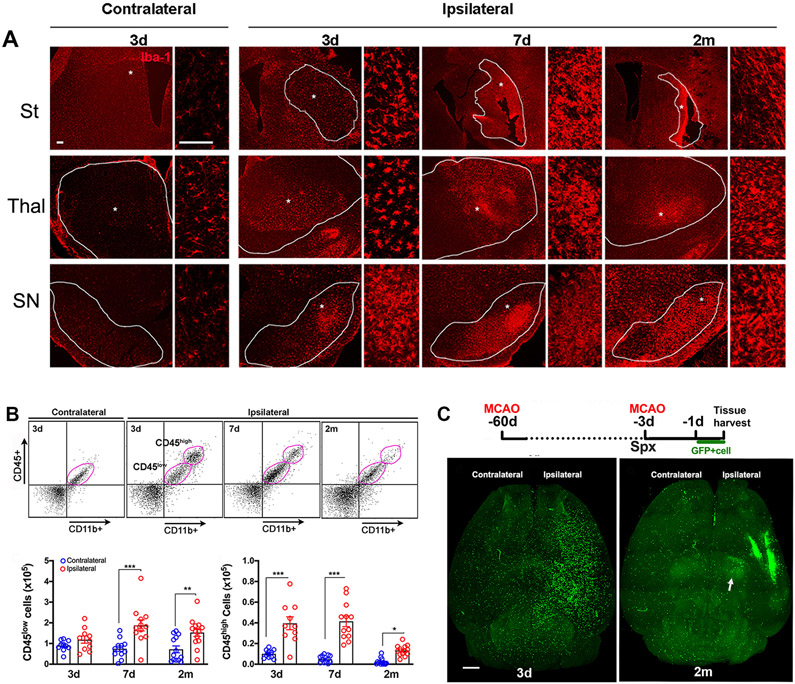
Iba-1 is a common marker that is expressed on both resident microglia and infiltrated monocyte-derived macrophages, both of which share common innate immune gene sets. Given the persistent immune activation and MM signatures, we determined the presence Iba+ cells in several brain sub-regions at 3 days, 1 week, and 2 months after stroke. At 3 days after stroke, Iba1+ cells in the contralateral striatum had a ramified shape with fine processes typical of microglia (Figure 6A). In the infarcted striatum of the ipsilateral hemisphere (outlined areas in Figure 6A), Iba-1+ cells had activated ameboid shapes with thicker processes. These cells were abundant at 3 and 7 days, and persisted to 2 months after stroke in the scar region. Activated Iba1+ cells also presented in remote areas (thalamus and substantia nigra [SN]) from infarct at 3 and 7 days and their presence persisted at 2 months (Figure 6A). The study shows that in addition to the infarcted region, there is a continuous presence of mononuclear phagocytes in regions away from primary injury site 20.
Figure 6.
Mononuclear phagocyte localization and trafficking. A, Iba-1 immunofluorescence. Dotted area in striatum (St) indicates infarct zone. Dotted lines in the thalamus (thal) and substantia nigra (SN) demarcate the sub-regions. * high magnification. Scale bar, 50μm. B, Representative flow cytometry plots and qunatification for CD45low and CD45high subsets in the pose-stroke brain. n=10-13/timepoint. Values are expressed as mean ± s.e.m. Two way ANOVA, Fisher’s LSD test, *, **, *** p<0.05, 0.01, 0.001 versus contralateral. C, Experimental timeline. GFP+ splenocytes was adoptively transferred to asplenic (Spx) mice one day prior to sacrifice. Representative horizontal view images from cleared whole brain at 3d and 2 m post-stroke. Scale bar,1mm.
To address the significance of transcriptome changes in immune cells, especially MMs, we used the differential expression of CD45 in CD11b+ population to distinguished infiltrating CD45high cells from CD45low resident microglia in the ischemic brain 21, 22. The number of CD45low cells was similar between hemispheres at 3 days, but their number was increased at 7 days and 2 months (Figure 6B). Compared to the contralateral hemisphere, CD45high cells were increased in the ipsilateral hemisphere at all time points, suggesting continuous infiltration of peripheral MMs during sub-acute and recovery phases.
The presence of Iba1+ cells and CD45+/CD11b+ subsets in the brain suggest immune cell infiltration in the brain. To accurately characterize the source and distribution of long-lasting changes in the immune transcriptome, immune cell trafficking from the periphery was investigated in an acute (3 days) and recovery (2 months) phase after stroke. Since the spleen is the major reservoir of monocytes deployed in response to injury23, mice received splenectomy 2 days prior to GFP+ splenocyte transfer to reduce the pool of endogenous immune cells, thereby increasing the visibility of transferred GFP+ cells (Figure 6C). Whole mount brain imaging showed prominent GFP+ cells in the striatum and cortex of ipsilateral hemispheres at 3 days (Figure 6C). At 2 months after stroke, GFP+ cells were densely packed around the glial scar of the infarcted zone. Large numbers of GFP+ cells were also present in the ipsilateral thalamus and SN (arrow in 6C). Supplementary videos for both time points are included. The trafficking and physical presence of peripheral immune cells in the primary injury site and remote areas over different phases after stroke was consistent with the observed sustained upregulation of immune transcriptome profiles.
Discussion
A likely key factor in the inability to successfully translate results from preclinical stroke studies to human stroke recovery is a poor understanding of the molecular and cellular changes that occur long after the acute phase of stroke. Unlike most previous studies, which have mainly examined the brain in the acute phase, the current study comprehensively profiled ~25,000 mouse genes across multiple phases after stroke. These gene analyses suggest a key role for immune-related genes and a likely contribution of peripheral immune cells trafficked into regions of the injured brain, even months after stroke. To better validate that immune infiltration persists past the acute phase after stroke, we transplanted exogenously-derived GFP-labeled peripheral immune cells into non-GFP mice to unambiguously demonstrate peripheral immune cell infiltration after stroke. The findings from this transplant study show for the first time that peripheral immune cell trafficking into the brain occurs not only in the acute phase, but for at least 2 months after stroke. Thus, the novelty of these studies is the demonstration of a previously unrecognized expansive and longer-lasting involvement of peripheral immunity on stroke pathology and recovery processes during the chronic phases of stroke.
Stroke has been known to trigger a rapid and massive infiltration of peripheral immune cells that differentiate into inflammatory or reparative macrophage phenotypes, depending on the ischemic millieu24, 25. The functional significance of the persistent perturbation of immune-related genes in the post-ischemic brains is the suggestion of a temporally and spatially distinct pattern of immune cell trafficking. The transplant study with exogenous immune cells confirmed this pattern, but also demonstrated that peripheral immune cell infiltration occurs in regions far from primary injury sites during the progression of brain injury and repair.
Icam1 was among the most frequently and significantly perturbed genes identified in the ipsilateral hemisphere at all time points. The up-regulation of Icam1 suggests a persistent enhancement in leukocyte-endothelial interaction and increased transmigration of leukocytes into brain tissue, which has been associated with injury development. Despite this potential negative association, worsened stroke outcomes have been observed in acute ischemic stroke patients treated with an anti-ICAM1 antibody26, suggesting a possible protective role for Icam1 up-regulation, perhaps via remodeling and recovery processes. In addition to immunity-related genes, persistent upregulation of extracellular matrix genes (Figure 4C) also suggests overlapping inflammatory and remodeling processes during the recovery phases of stroke.
There was a striking resemblance of the ipsilateral gene cluster 4 profile to M2-related genes that displayed biphasic response with an early rise 1 week after stroke and a second rise 2 months after stroke. An example of this profile is the gene Cd36, which encodes a fatty acid translocase and a marker for M2 polarization19 as well as cluster 4 genes that are involved in cholesterol transporter activity and in synaptic plasticity (e.g., Apoe, Abca1, Abcg1, and Tbsp2). While stage-specific roles of M2 genes in brain remodeling remain to be elucidated, the biphasic responses in these genes likely reflect both an early role in the acute inflammatory response to brain injury and a later role as brain circuits work to compensate and remodel.
Another notable observation is the overlapping profiles between M1 and M2-related genes (Figure 5B). M1 macrophages recruited into inflamed tissue have been reported to convert to M2 macrophages when inflammation recedes27, 28. A report that blocking the recruitment of CCR2+ monocyte-derived macrophages hinders subsequent functional recovery in stroke29, 30 suggests the importance of either the early recruitment of inflammatory monocytes, the initial differentiation to M1 macrophages, and/or the transition to M2 macrophages, for subsequent inflammation resolution processes. This role may be related to a phagocytic function of MMs in the early resolution phase of inflammation31. The sustained elevation of both the M1 and M2 signatures could also indicate long-lasting and overlapping inflammation and resolution in this animal model.
It is unclear whether these overlapping inflammation and resolution processes play a role in the heterogeneity of stroke recovery in human patients. Some patients experience a substantial recovery while others remain profoundly disabled or experience progressive decline, despite a similar magnitude of initial stroke32. Thus, the sustained and widespread immune involvement during the recovery phase suggests the possibility of variability in spatial and temporal immune responses as a candidate contributor to this heterogeneity. In particular, the sustained immune infiltration could potentially contribute to secondary degeneration and/or repair in remote areas, helping to define the final full extent of brain atrophy after stroke. This possibility is supported by neuroimaging studies in stroke patients showing atrophy in both the ipsilateral hemisphere and also in the less-affected contralateral thalamus33-36.
Translating preclinical findings to human patients is only marginally successful and not without controversy. There are differing views of whether or not genomic responses in animal models reflect human disease37,38. However, several common mechanistic aspects of CNS injury are shared in animals and humans, including immune cell infiltration, temporal and spatial activation patterns (based on imaging in humans and histological findings in animal models), and the similarity in blood biomarkers and peripheral immune system depression39. Together, these similarities further support the utility of experimental brain ischemia models to model key, salient features of human stroke pathophysiology40.
The persistent presence of peripheral immune cells in the injured hemisphere across post-stroke phase likely identifies cellular trafficking as a contributing factor driving the upregulation of immune-associated gene expression levels. With overlapping marker expression and function, MMs and resident microglia possess bivalent features with both toxic pro-inflammatory and reparative phenotypes depending on the ischemic environment25. It has been a challenge to distinguish function between MMs and resident microglia. However, emerging studies show convergence between their phenotypes in CNS tissue during neuroinflammation, likely due to the influence of the CNS milieu on the behavior of infiltrating immune cells, as well as a regulatory role of peripherally derived macrophages on microglia phagocytic function 25, 41, 42. All of these studies indicate complex bidirectional changes in marker expression between microglia and infiltrating MMs in the inflamed tissue by the influence of stage-specific ischemic milieu, suggesting that these two types of cells interact to become a functional unit in the post-ischemic brain.
Other caveats include that our global gene database is unable to address differences in gene changes between core and periinfarct, and have not been correlated with functional significance using recovery behavior. Since infarct development is dynamic, expanding and shrinking over time, it is challenging to define specific regions across multiple phases of stroke for gene profiling. Moreover, stroke induces transneuronal degeneration in remote areas away from primary injury sites20 and regional plastic changes including angiogenesis and white matter remodeling. A separate full investigation of detailed gene expression and cellular function in different brain sub-regions at multiple phases after stroke using anatomical dissection is warranted.
In summary, longitudinal gene expression profiling in the brain after experimental stroke revealed a persistent enrichment of immune signatures. The long-lasting gene expression perturbations after stroke, including in the non-stroked hemisphere, suggest that pathologic and repair processes in cerebral ischemia are prolonged and dynamic. The persistent upregulation of immune pathway gene expression was associated with the presence of MMs in the primary injury site and remote areas. The overlapping innate and adaptive immunity and M1 and M2 gene signatures for weeks and months after stroke suggest a persistent immune influence on the progression of injury development and the repair process. Considering the fact that comorbid conditions including diabetes, hypertension, and obesity influence peripheral immunity as well as the progression of brain injury after stroke,14, 43, 44, it is tempting to propose a potential immune explanation for the role of these comorbidities in stroke outcome. Regardless, targeting of the peripheral immune system should be further studied as a potential strategy to intervene in brain injury progression or repair in both typical and comorbid stroke.
Supplementary Material
Acknowledgments
We thank John Cave and Matthew Sleeman on insightful comments on manuscript.
Source of Funding
This work was supported by NIH Grants HL82511S1, NS095359 and NS111568 (SC) and Regeneron resources to generate gene profiling data.
Footnotes
Disclosures
WF, YB, LEM, and SDC are employees and shareholders of Regeneron, a for-profit corporation.
References
- 1.Collaborators GBDS. Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:439–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll WM. The global burden of neurological disorders. Lancet Neurol. 2019;18:418–419 [DOI] [PubMed] [Google Scholar]
- 3.Adamski MG, Li Y, Wagner E, Yu H, Seales-Bailey C, Soper SA, et al. Expression profile based gene clusters for ischemic stroke detection. Genomics. 2014;104:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr TL, Conley Y, Ding J, Dillman A, Warach S, Singleton A, et al. Genomic biomarkers and cellular pathways of ischemic stroke by rna gene expression profiling. Neurology. 2010;75:1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp FR, Jickling GC, Stamova B, Tian Y, Zhan X, Liu D, et al. Molecular markers and mechanisms of stroke: Rna studies of blood in animals and humans. J Cereb Blood Flow Metab. 2011;31:1513–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jickling GC, Stamova B, Ander BP, Zhan X, Tian Y, Liu D, et al. Profiles of lacunar and nonlacunar stroke. Ann Neurol. 2011;70:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford G, Xu Z, Gates A, Jiang J, Ford BD. Expression analysis systematic explorer (ease) analysis reveals differential gene expression in permanent and transient focal stroke rat models. Brain Res. 2006;1071:226–236 [DOI] [PubMed] [Google Scholar]
- 8.Rickhag M, Wieloch T, Gido G, Elmer E, Krogh M, Murray J, et al. Comprehensive regional and temporal gene expression profiling of the rat brain during the first 24 h after experimental stroke identifies dynamic ischemia-induced gene expression patterns, and reveals a biphasic activation of genes in surviving tissue. J Neurochem. 2006;96:14–29 [DOI] [PubMed] [Google Scholar]
- 9.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311 [DOI] [PubMed] [Google Scholar]
- 10.Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, et al. Gdf10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015;18:1737–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautheron V, Xie Y, Tisserand M, Raoult H, Soize S, Naggara O, et al. Outcome after reperfusion therapies in patients with large baseline diffusion-weighted imaging stroke lesions: A thrace trial (mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke) subgroup analysis. Stroke. 2018 [DOI] [PubMed] [Google Scholar]
- 13.Chamorro A, Blasco J, Lopez A, Amaro S, Roman LS, Llull L, et al. Complete reperfusion is required for maximal benefits of mechanical thrombectomy in stroke patients. Sci Rep. 2017;7:11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E, Tolhurst AT, Cho S. Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. J Neuroinflammation. 2014;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin L, Jing D, Parauda S, Carmel J, Ratan RR, Lee FS, et al. An adaptive role for bdnf val66met polymorphism in motor recovery in chronic stroke. J Neurosci. 2014;34:2493–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Balkaya M, Beltran C, Heo JH, Cho S. Remote postischemic conditioning promotes stroke recovery by shifting circulating monocytes to ccr2(+) proinflammatory subset. J Neurosci. 2019;39:7778–7789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terpstra AH. Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: Role of metabolic rate. J Nutr. 2001;131:2067–2068 [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070 [DOI] [PubMed] [Google Scholar]
- 19.Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nature immunology. 2014;15:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronenberg G, Balkaya M, Prinz V, Gertz K, Ji S, Kirste I, et al. Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry. 2012;72:273–281 [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014;34:1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Balkaya M, Beltran C, Hoe Heo J, Cho S. Remote post-ischemic conditioning promotes stroke recovery by shifting circulating monocytes to ccr2+ pro-inflammatory subset. J Neurosci. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miro-Mur F, Perez-de-Puig I, Ferrer-Ferrer M, Urra X, Justicia C, Chamorro A, et al. Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain Behav Immun. 2016;53:18–33 [DOI] [PubMed] [Google Scholar]
- 25.Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enlimomab Acute Stroke Trial I. Use of anti-icam-1 therapy in ischemic stroke: Results of the enlimomab acute stroke trial. Neurology. 2001;57:1428–1434 [DOI] [PubMed] [Google Scholar]
- 27.Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL Jr., Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS ONE. 2014;9:e86660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J, et al. Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain. J Neuroinflammation. 2016;13:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J Neurosci. 2016;36:4182–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu HX, Broughton BR, Kim HA, Lee S, Drummond GR, Sobey CG. Evidence that ly6c(hi) monocytes are protective in acute ischemic stroke by promoting m2 macrophage polarization. Stroke. 2015;46:1929–1937 [DOI] [PubMed] [Google Scholar]
- 31.Woo MS, Yang J, Beltran C, Cho S. Cell surface cd36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J Biol Chem. 2016;291:23654–23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitz C, Luchsinger JA, Tang M-X, Manly J, Mayeux R. Stroke and memory performance in elderly without dementia. Archives of neurology. 2006;63:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassi N, Malpas CB, Campbell BC, Moffat B, Steward C, Parsons MW, et al. Contralesional thalamic surface atrophy and functional disconnection 3 months after ischemic stroke. Cerebrovasc Dis. 2015;39:232–241 [DOI] [PubMed] [Google Scholar]
- 34.Ohe Y, Hayashi T, Uchino A, Tanahashi N. [secondary degeneration of the substantia nigra after cerebral infarctions including the striatum]. Brain Nerve. 2013;65:289–295 [PubMed] [Google Scholar]
- 35.Ogawa T, Okudera T, Inugami A, Noguchi K, Kado H, Yoshida Y, et al. Degeneration of the ipsilateral substantia nigra after striatal infarction: Evaluation with mr imaging. Radiology. 1997;204:847–851 [DOI] [PubMed] [Google Scholar]
- 36.Nakane M, Teraoka A, Asato R, Tamura A. Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke. 1992;23:328–332 [DOI] [PubMed] [Google Scholar]
- 37.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2015;112:1167–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirnagl U, Endres M. Found in translation: Preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke. 2014;45:1510–1518 [DOI] [PubMed] [Google Scholar]
- 40.Dirnagl U Modeling immunity and inflammation in stroke: Can mice be trusted? Stroke. 2014;45:e177–178 [DOI] [PubMed] [Google Scholar]
- 41.Grassivaro F, Menon R, Acquaviva M, Ottoboni L, Ruffini F, Bergamaschi A, et al. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J Neurosci. 2020;40:784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarruk JG, Greenhalgh AD, David S. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp Neurol. 2018;301:120–132 [DOI] [PubMed] [Google Scholar]
- 43.Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. Cd36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28:4661–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim E, Febbraio M, Bao Y, Tolhurst AT, Epstein JM, Cho S. Cd36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol. 2012;71:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.