Abstract
Objectives:
This pilot trial tested the effect of adding a multi-level, technology-based physical activity intervention module to a standard survivorship care plan for breast and colorectal cancer survivors. The objective of this analysis was to determine whether the physical activity module improved health-related quality of life, sleep, and factors key to lasting behavior change (e.g., social support, self-efficacy).
Methods:
Breast and colorectal cancer survivors (n=50) were enrolled alongside a support partner. Survivors were assigned to receive a standard survivorship care plan either alone or augmented by a 12-week multi-component physical activity module. The module included a Fitbit tracker (with the physical activity data integrated into the electronic health record for clinician review) and customized email feedback. Physical activity was measured using the ActiGraph GT3X+. Psychosocial outcomes included the SF-36, FACT, ISEL, PROMIS sleep measures, and physical activity beliefs. Data were analyzed using linear mixed modeling.
Results:
Cancer survivors were aged 54.4±11.2 years and were 2.0±1.5 years from diagnosis. Relative to comparison, the intervention was associated with moderate-to-large improvements in physical health (effect size: d=0.39, 95% CI=0.0,0.78), mental health (d=0.59, 95% CI=0.19,0.99), sleep impairment (d=0.62, 95% CI=−1.02,−0.22), and exercise self-efficacy (d=0.60, 95% CI=0.20,1.0).
Conclusion:
The intervention delivered meaningful improvements in survivors’ quality of life, social support, and sleep impairment. If replicated in a larger sample, adding a technology-supported physical activity module to survivorship care plans may be a practical strategy for supporting healthy survivorship.
Trial registration:
Keywords: Breast, cancer, colorectal, exercise, Fitbit, Psycho-Oncology, psychosocial, quality of life, survivorship, survivorship care plan
Introduction
Over ninety percent of breast cancer patients and 90% of colorectal cancer patients will survive past five years (1, 2) and there are 3.8 million breast and 1.5 million colorectal cancer survivors in the United States (3). While recent evidence suggests that quality of life has been improving among these cancer survivors (4), a significant proportion still have clinically relevant levels of post-treatment stress, depression, anxiety and lower quality of life (5, 6). Proposed mechanisms stem from cancer-related psychosocial distresses such as a heightened fear of recurrence (7), concerns about family and finances (8), and difficulty resuming social life (9). Physical issues such as fatigue, pain, and functional impairments can reduce quality of life (10), while changes in body image and sexuality affect emotional health (11, 12). Post-treatment cancer survivors also have a high prevalence of sleep-wake disturbances that adversely affect their quality of life (13).
Regular physical activity (PA) is a safe and effective way to improve physical and psychological health in cancer survivors (14). Several randomized trials among breast cancer survivors have shown that PA improves physical functioning and quality of life and reduced fatigue (15–18). Similarly, for colorectal cancer survivors, systematic reviews report benefits of PA on a range of health-related quality of life (HRQOL) outcomes (19). Due to these benefits, guidelines for PA are typically included in the survivorship care planning (SCP) document that many cancer survivors receive at the end of primary treatment (20). However, general lifestyle recommendations are unlikely to result in sustained behavior change (21) unless augmented by additional behavior change support.
This study reports psychosocial outcomes from a pilot randomized trial that used a novel approach – implementing a technology-based PA intervention as an add-on module to standard-of-care survivorship care planning – in a sample of insufficiently active post-treatment cancer survivors diagnosed within the past five years. The module, which included Fitbit trackers with data imported into the electronic health record (EHR), email feedback, and a support partner, was designed to be scalable and accessible. The main outcomes – feasibility and changes in PA – have been reported previously. Specifically, survivors in the intervention group increased moderate-to-vigorous-intensity physical activity (MVPA) by 69 ± 84 min/week vs. a 20 ± 71 min/week (23). For this analysis, we hypothesized that the PA module would deliver improvements in health-related quality of life and sleep relative to standard care planning alone. Furthermore, we hypothesized that because the module would improve factors that are key to lasting PA behavior change, namely social support, self-efficacy, processes of change, and decisional balance.
Methods
Data collection occurred from August 2016 to January 2018. All procedures were approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board. Informed consent was obtained from all participants.
Participants and randomization
The trial had a two-arm parallel randomized controlled design. Fifty cancer survivor and support partner dyads were enrolled (n=100 total). This pilot focused on breast and colorectal cancers due to the feasibility constraints in recruiting from more than two groups of oncology providers. Inclusion criteria for cancer survivors were: (a) patient of the UW Health system, (b) 28-75 years of age, (c) diagnosed with Stage I-III colorectal cancer or female breast cancer within the past 5 years, (d) finished with primary treatment (completed all definitive cancer surgery, (neo)adjuvant chemotherapy and/or radiation), (e) had a computer, tablet, or smartphone and internet access, (f) could exercise safely, (g) fluent in English, and (h) willing and able to attend study visits. Exclusion criteria were (a) recurrent or metastatic disease, (b) previously received a survivorship care plan, (c) performing >100 min/week of MVPA, (d) unable to identify a support partner. Survivors were recruited through the UW’s Carbone Comprehensive Cancer Center. A randomization scheme in REDCap (24) assigned dyads with equal probability to standard survivorship planning either alone (comparison group) or augmented with the PA module (intervention group). The randomization sequence was generated by L.C.B. and uploaded to REDCap. Participants were enrolled by B.V.R. and P.D. Randomization was stratified by cancer type (breast vs. colorectal) and chemotherapy (yes/no). Chemotherapy was chosen as a stratification criterion due to its role in risk of late effects that affect quality of life (25). The primary aim of the trial was feasibility; sample size was determined based on the secondary aim (change in MVPA), to enable us to estimate the SD to a precision (CI half-width) of ±22%.The trial was completed as planned after reaching the target sample size.
Survivorship Care Planning
Survivors in both groups received a survivorship care plan, which was reviewed with a provider either in the clinic or over the phone. Care plans contained a written script on the benefits of PA for cancer survivors and PA recommendations of 150 min/week of MVPA (26).
Multi-level Technology-based Physical Activity Module (Intervention Group)
Dyads in this group received a 12-week, multi-component intervention. Participants were asked to gradually increase their MVPA to 150 min/week and daily steps to 10,000. Due to the exclusion of individuals currently performing >100 min/week of MVPA, all survivors were in the contemplation or preparation stages of the Transtheoretical Model (27). Both dyad members received the following four components: (a) Fitbit tracker (Charge HR or Charge 2, which are comparable with respect to key features); (b) educational handbook, adapted from our previous trial (23), addressing benefits of PA for cancer survivorship, self-efficacy and goal-setting, and instructions on how to use the Fitbit to reach the study goals; (c) Social support: survivors and support partners were asked to assist each other in achieving and maintaining their activity goals (e.g., exercising together, providing encouragement, helping a spouse find time to exercise); and (d) coaching emails sent by study staff to each dyad member at 1, 2, 4, and 8 weeks. Although other consumer-based activity trackers (e.g., Garmin, Apple Watch) offer monitoring of MVPA minutes, we chose the Fitbit because (a) it has the most validation data; (b) integrates with the Fitabase research platform; (c) had an Epic flowsheet available to pull data into the EHR; and (d) can be used on multiple platforms (e.g., computer, Android, iOS). Fitbit data was tracked via Fitabase (Small Steps, LLC) and used to tailor e-mail coaching to each dyad member based on personalized goals and recent progress, to help set updated goals, and provide suggestions for individuals to support their dyad partner. Additionally, each survivor’s Fitbit data (steps/day) was linked to their health record via the patient portal and was viewable from the clinician side of EHR.
Attention Control Components (Comparison Group)
Survivors and support partners assigned to the comparison group received the following components, intended to support retention and reduce differences in the level of attention provided to each group: (a) the 2015 US Dietary Guidelines for Americans, which was reviewed with each dyad at the randomization visit (28); (b) standardized e-mails at 1, 2, 4, and 8 weeks with information on healthy eating and stress management (PA coaching was not provided).
Measures
Participant characteristics.
Demographic characteristics were self-reported. Height and weight were measured using standard protocols described previously (23). Diagnosis and treatment details were abstracted from the EHR to REDCap. Physical activity was measured at baseline and 12-weeks using the ActiGraph GT3X+, worn on the hip during waking hours for 7 consecutive days. Details of PA measurement were described previously (23).
Quality of Life and Sleep Outcomes.
Two quality of life instruments were used: the SF-36 Health Survey and the Functional Assessment of Cancer Therapy (FACT). The SF-36 has 8 sub-scales (physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, mental health, role limitations due to emotional problems, social functioning and vitality) (29). Scores range from 0-100 with higher scores reflecting better health. The FACT has four sub-scales: physical well-being (range=0-28), social/family well-being (range=0-28), emotional well-being (range=0-24) and functional well-being (range=0-28). They also completed the FACT breast cancer subscale (BCS) or colorectal cancer subscale (CCS) with a score ranges of 0-40 and 0-28 respectively, with higher scores indicating better quality of life (30). Sleep was measured using the Patient Reported Outcomes Measurement Information System’s (PROMIS) 8-item short forms for sleep disturbance and sleep-related impairment. Raw scores were rescaled to standardized t-scores ranging from 28.9-76.5 for sleep disturbance and 30-80.1 for sleep-related impairment, with higher scores reflecting worse sleep (31).
Process Outcomes.
Four instruments were used to assess relevant factors that support lasting PA behavior change. Perceived social support was assessed using the Interpersonal Support Evaluation List (ISEL), consisting of four sub-scales: appraisal support (the perceived availability of another person to offer advice/information), tangible support (perceived availability of aid, material, or instrumental support), self-esteem (self-perceptions through social comparisons), and belonging (perceived availability of others for companionship). Sub-scale scores were summed (range=0-30), with a higher score reflecting stronger social support (32). The self-efficacy for PA scale measured confidence in performing PA in a variety of situations; scores range from 1-5 with higher scores indicating greater self-efficacy (33). Processes of behavior change used ten sub-scales to measure increasing knowledge of PA, awareness of risks of inactivity, caring about consequences of PA, comprehending benefits of PA, increasing activity opportunities, substituting alternatives with PA, enlisting social support for PA, rewarding oneself for being active, committing oneself to be active, and reminding oneself to be active. Scores range from 1-5 with greater scores reflecting higher likelihood that the individual is trying to change their PA-related thinking and behavior (33). Decisional balance used 16 items to assess perceived benefits and barriers to PA (33).
Data Analyses
Baseline characteristics were compared using t-tests and chi-squared tests. Intervention effects were examined using linear mixed effects regression models. Effect size (Cohen’s d) was also computed. Additionally, 95% confidence intervals are presented for all longitudinal analyses. All analyses were performed in SAS 9.4 (SAS, Cary, NC). In sensitivity analyses, adjusting for age and chemotherapy as covariates, results did not meaningfully change for any outcome.
Results
Participant characteristics
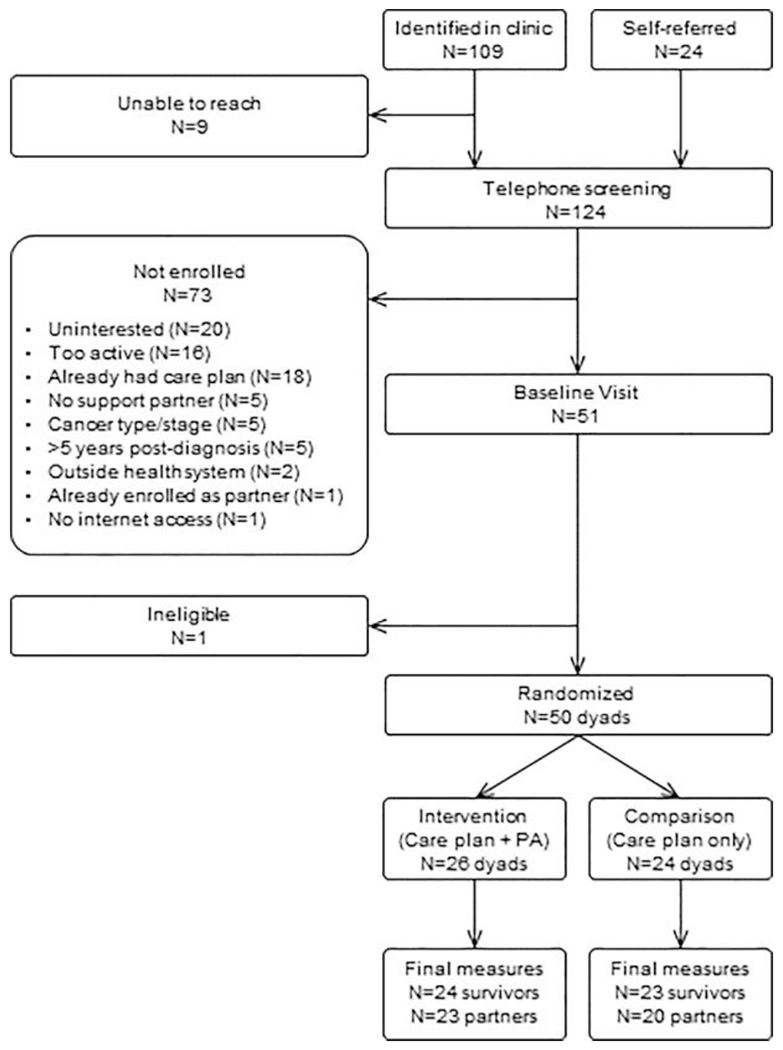
Fifty cancer survivors were enrolled between August 2016 and November 2017 (Figure 1). As shown in Table 1, survivors were 96% women, 29-73 years of age, and 94% were non-Hispanic whites. Average time since diagnosis was 2.0±1.5 years and most (90%) were breast cancer survivors. Thirty-two percent had Stage 1 cancer; 50.0% had Stage 2, and 18.0% had Stage 3. All had undergone surgery; 64% received chemotherapy and 72.0% received radiation. Mean BMI was 32.2±7.4 kg/m2, which is generally comparable to national averages for this age group (34).
Figure 1:
Effect of a technology-supported physical activity intervention on health-related quality of life, sleep, and processes of behavior change in cancer survivors: A randomized controlled trial
Table 1:
Baseline characteristics of cancer survivors.
| Total | Intervention | Comparison | ||
|---|---|---|---|---|
| Mean±SD or N(%) | Mean±SD or N(%) | Mean±SD or N(%) | p | |
| N | 50 | 26 | 24 | |
| General characteristics | ||||
| Sex (% female) | 48(96.0%) | 26(100.0%) | 22(91.7%) | .23 |
| Age | 54.4±11.2 | 52.5±12.2 | 56.5±9.8 | .21 |
| % with a college degree | 43(86%) | 23(88.5%) | 20(83.3%) | .70 |
| BMI (kg/m2) | 32.2±7.4 | 32.4±6.2 | 33.4±6.5 | .56 |
| Race/ethnicity | .37 | |||
| Non-Hispanic White | 47(94.0%) | 25(96.2%) | 22(91.7%) | |
| Hispanic | 1(2.0%) | 1(3.9%) | 0(0.0%) | |
| Black | 1(2.0%) | 0(0.0%) | 1(4.2%) | |
| More than one race | 1(2.0%) | 0(0.0%) | 1(4.2%) | |
| Physical activity | ||||
| MVPA min/week | 170±131 | 174±123 | 165±142 | .80 |
| MVPA in 10+ min bouts | 15±26 | 19±29 | 11±22 | .27 |
| Steps/day | 5,252±2,237 | 5,318±2115 | 5,181±2405 | .83 |
| Sedentary time (hrs/day) | 9.2±3.6 | 9.3±3.5 | 9.14 (3.74) | .89 |
| Cancer characteristics | ||||
| Tumor type (breast) | 45(90.0%) | 25(96.2%) | 20(83.3%) | .18 |
| Years since diagnosis | 2.0±1.5 | 1.9±1.4 | 2.0±1.7 | .72 |
| Stage | .08 | |||
| I | 16(32.0%) | 11(42.3%) | 5(20.8%) | |
| II | 25(50.0%) | 9(34.6%) | 16(66.7%) | |
| III | 9(18.0%) | 6(23.1%) | 3(12.5%) | |
| Treatment | ||||
| Chemotherapy | 32(64.0%) | 14(53.9%) | 18(75.0%) | .12 |
| Radiation | 36(72.0%) | 20(76.9%) | 16(66.7%) | .42 |
Health-related quality of life and sleep
Across all eight domains of SF-36, from baseline to 12-weeks, survivors in the intervention group showed medium to large improvements in physical functioning (d=0.40), role limitations due to physical health problems (d=0.43) general health (d=0.88), mental health (d=0.45), role limitations due to emotional problems (d=0.60), social functioning (d=0.51) and vitality (d=0.61) and small improvement in bodily pain (d=0.15) relative to the comparison group (see Table 2 for 95% CIs). Among the aggregate scores, mental health improved significantly in the intervention group relative to the comparison group (d=0.59). The results from FACT-B (breast cancer) corroborated the findings from the SF-36. Physical well-being measured by the FACT-B improved more in the intervention group relative to comparison (d=0.56). Emotional well-being (d=0.54) and the breast cancer subscale (d=0.48) also showed a medium standardized mean difference between groups. Results from FACT-C are not presented due to the small number of colorectal cancer survivors enrolled. There was a greater reduction in both sleep disturbance and impairment among the cancer survivors in the intervention group from baseline to 12-weeks relative to the comparison group. Both scales were associated with medium effect sizes, with impairment (d=−0.62) improving more than disturbance (d=−0.46).
Table 2:
Mean changes in health-related quality of life and sleep related outcomes in cancer survivors.
| Intervention Group | Comparison Group | d | 95% CI for d | p* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-weeks | Change | 95% CI | Baseline | 12-weeks | Change | 95% CI | ||||||
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||||||
| SF-36 | |||||||||||||
| Aggregate physical health | 46.6±8.7 | 52.1±7.9 | 4.3±9.5 | 0.2 | 8.4 | 46.5±7.1 | 47.6±8.9 | 1.2±5.9 | −1.4 | 3.8 | .39 | 0,.78 | .12 |
| Physical functioning | 48.2±7.3 | 52.4±5.0 | 3.1±6.3 | 0.4 | 5.8 | 47.1±7.4 | 47.7±8.6 | 0.3±7.5 | −3.0 | 3.6 | .40 | .01,.80 | .13 |
| Role-physical | 43.5±10.9 | 50.7±9.5 | 6.8±15.3 | 0.2 | 13.4 | 45.9±10.6 | 46.6±10.4 | 1.6±6.5 | −1.3 | 4.5 | .43 | .04,.83 | .085 |
| Bodily pain | 48.8±10.4 | 51.1±11.6 | 1.6±11.0 | −3.1 | 6.4 | 49.8±9.7 | 50.1±11.3 | 0.2±7.3 | −3.0 | 3.4 | .15 | −.24,.54 | .55 |
| General health | 46.1±8.8 | 51.9±7.4 | 5.6±6.7 | 2.7 | 8.5 | 46.5±6.6 | 46.7±6.2 | 0.3±5.1 | −2.0 | 2.6 | .88 | .47,1.29 | .004 |
| Aggregate mental health | 46.8±9.9 | 45.6±8.3 | −0.1±6.2 | −2.7 | 2.6 | 50.2±7.1 | 45.0±11.6 | −4.8±9.3 | −8.9 | −0.6 | .59 | .19,.99 | .06 |
| Mental health | 48.4±9.0 | 51.7±9.2 | 4.0±5.8 | 1.5 | 6.5 | 49.9±9.5 | 49.7±12.5 | 0.4±9.3 | −3.7 | 4.5 | .45 | .06,.85 | .13 |
| Role-emotional | 46.8±9.9 | 49.8±8.9 | 3.7±8.2 | 0.1 | 7.2 | 50.5±6.9 | 48.6±10.0 | −1.4±8.8 | −5.3 | 2.5 | .60 | .20,1.0 | .05 |
| Social functioning | 41.3±9.6 | 28.9±3.3 | −11.9± 8.9 | −15.8 | −8.1 | 43.3±7.8 | 27.4±4.2 | −16.2±7.4 | −19.4 | −12.9 | .51 | .12,.92 | .14 |
| Vitality | 49.4±7.6 | 55.5±8.5 | 6.1±6.5 | 3.3 | 8.9 | 52.4±7.0 | 53.3±10.1 | 1.3±8.8 | −2.6 | 5.2 | .61 | .22,1.02 | .03 |
| FACT | |||||||||||||
| FACT-G total† | 85.0±15.7 | 89.5±15.2 | 4.1±10.4 | −0.6 | 8.9 | 84.6±13.3 | 85.1±16.3 | 0.9±7.8 | −3.0 | 4.8 | .35 | −.05,.75 | .26 |
| Physical well-being | 22.6±4.1 | 24.5±3.8 | 1.4±3.6 | −0.2 | 3.1 | 22.3±6.6 | 23.1±4.4 | −0.3±2.3 | −1.4 | 0.9 | .56 | .17,.97 | .12 |
| Social/family well-being | 22.8±5.0 | 22.5±5.6 | 0.2±2.9 | −1.1 | 1.6 | 21.2±5.2 | 21.4±6.2 | 0.7±3.2 | −0.8 | 2.3 | −.17 | −.56,.22 | .60 |
| Emotional well-being | 18.1±4.9 | 19.6±3.7 | 1.1±3.8 | −0.6 | 2.9 | 20.9±2.5 | 19.9±2.7 | −0.6±2.5 | −1.9 | 0.6 | .54 | .14,.94 | .06 |
| Functional well-being | 21.4±5.6 | 23.0±5.1 | 1.3±3.2 | −0.1 | 2.8 | 20.2±4.8 | 20.7±5.0 | 1.1±3.7 | −0.8 | 2.9 | .08 | −.31,.47 | .68 |
| FACT-B total‡ | 111±20 | 118.1±20.2 | 6.2±13.8 | −0.1 | 12.5 | 109.5± 19.5 | 110.2±22.9 | 0.9±9.7 | −3.9 | 5.7 | .44 | .04,.84 | .16 |
| Breast cancer subscale | 26±6.5 | 28.7±6.3 | 2.1±5.0 | −0.2 | 4.3 | 24.9±7.1 | 25.2±8.0 | 0±3.2 | −1.6 | 1.6 | .48 | .09,.88 | .13 |
| Trial outcome index§ | 70±13.9 | 76.1± 13.6 | 4.8±9.8 | 0.3 | 9.3 | 67.4±14.9 | 68.9±16.0 | 0.8±6.5 | −2.4 | 4.0 | .48 | .09,.88 | .13 |
| Sleep | |||||||||||||
| Sleep disturbance | 46.8±10.3 | 47.0±8.6 | −2.9±6.8 | −6.1 | 0.3 | 49.5±7.6 | 49.5±9.3 | 0.4±7.4 | −3.1 | 3.9 | −.46 | −.86,−.07 | .33 |
| Sleep impairment | 47.6±8.9 | 44.7±9.3 | −3.6±9.8 | −7.8 | 0.6 | 49.5±9.3 | 52.0±10.5 | 2.1±8.5 | −1.7 | 5.8 | −.62 | −1.02,−.22 | .04 |
FACTG total=Physical+Social/family+Emotional+Functional
FACTB total=Physical+Social/family+Emotional+Functional+Breast Cancer sub-scale
FACTB Trial Outcome Index=Physical+Functional+Breast Cancer sub-scale
P-value from the mixed effects regression model and for comparing changes from baseline to 12-weeks between intervention and comparison groups
Process Variables
Two of the four items on the social support scale showed a greater improvement among the survivors in the intervention group relative to comparison: tangible (d=0.53) and belonging (d=0.55) scores were associated with medium effect sizes. However, appraisal (d=0.13) and self-esteem (d=0.12) scores did not improve and were associated with lower effect sizes and confidence intervals that included zero (see Table 3 for 95% CIs). The participants improved on the self-efficacy scale more in the intervention group relative to the comparison (d=0.60). Survivors in the intervention group also showed a significantly greater improvement in three constructs of processes of behavior change as compared to the comparison group (p=0.00). These three were substituting alternatives with PA (d=1.12), enlisting social support for PA (d=1.12) and reminding oneself to be active (d=0.95).
Table 3:
Mean changes in social support, self-efficacy, processes of behavior change and decisional balance outcomes in cancer survivors.
| Intervention Group | Comparison Group | d | 95% CI for d | p* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-weeks | Change | 95% CI | Baseline | 12-weeks | Change | 95% CI | ||||||
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||||||
| Social Support | |||||||||||||
| Appraisal | 25.7±5.8 | 25.6±5.6 | 0.1±3.4 | −1.4 | 1.6 | 23.5±6.1 | 23.2±6.5 | −0.4±4.5 | −2.4 | 1.6 | .13 | −.27,.52 | .72 |
| Tangible | 26.1±5.0 | 26.1±4.3 | 0.2±3.1 | −1.2 | 1.6 | 24.4±5.7 | 22.7±7.1 | −1.5±3.2 | −2.9 | −0.1 | .53 | .13,.93 | .08 |
| Self-esteem | 22.6±3.4 | 23.0±4.2 | 0.5±3.3 | −1.0 | 2.0 | 20.2±3.7 | 20.2±4.7 | 0.1±4.4 | −1.9 | 2.0 | .12 | −.28,.51 | .75 |
| Belonging | 24.3±5.0 | 25.1±4.1 | 1.4±3.8 | −0.3 | 3.0 | 22.9±5.3 | 21.9±6.6 | −0.9±4.3 | −2.8 | 1.0 | .55 | .15,.95 | .08 |
| Self-efficacy | 2.8±0.7 | 3.1±0.8 | 0.3±1.0 | −0.1 | 0.7 | 2.7±0.8 | 2.4±1.0 | −0.3±0.9 | −0.7 | 0.1 | .60 | .20,1.00 | .03 |
| Processes of change | |||||||||||||
| Increasing knowledge | 3.3±0.8 | 3.3±0.9 | 0.0±0.9 | −0.4 | 0.4 | 2.9±0.8 | 3.1±0.9 | 0.1±0.9 | −0.3 | 0.5 | −.07 | −.46,.32 | .78 |
| Being aware of risks | 2.6±1.1 | 2.6±1.1 | −0.1± 1.0 | −0.5 | 0.4 | 2.7±1.1 | 2.7±0.9 | −0.1±1.0 | −0.5 | 0.3 | .01 | −.38,.40 | .98 |
| Caring about consequences | 3.2±1.2 | 3.1±1.0 | −0.0±1.0 | −0.4 | 0.4 | 3.1±0.8 | 3.0±0.8 | −0.2±0.7 | −0.4 | 0.2 | .17 | −.22,.56 | .62 |
| Comprehending benefits | 3.9±1.0 | 3.9±0.9 | −0.0±0.6 | −0.3 | 0.3 | 4.0±1.0 | 3.7±1.0 | −0.2±0.6 | −0.4 | 0.1 | .22 | −.17,.62 | .42 |
| Increasing health opportunities | 2.9±1.0 | 3.3±1.0 | 0.3±1.0 | −0.1 | 0.8 | 2.7±1.1 | 2.7±0.9 | 0.0±0.6 | −0.2 | 0.3 | .39 | 0,.79 | .17 |
| Substituting alternatives | 2.7±0.9 | 3.4±0.9 | 0.8±0.9 | 0.4 | 1.2 | 2.9±0.8 | 2.6±0.9 | −0.2±0.8 | −0.5 | 0.2 | 1.12 | .71,1.55 | .00 |
| Enlisting social support | 2.7±0.9 | 3.3±1.1 | 0.7±1.0 | 0.3 | 1.1 | 2.7±1.0 | 2.4±1.1 | −0.3±0.8 | −0.6 | 0.1 | 1.12 | .71,1.55 | .00 |
| Rewarding oneself | 3.0±0.8 | 3.6±0.7 | 0.5±1.0 | 0.0 | 0.9 | 2.8±0.8 | 3.0±1.0 | 0.1±0.6 | −0.2 | 0.4 | .40 | 0,.79 | .18 |
| Committing oneself | 3.8±0.7 | 4.2±0.7 | 0.4±0.8 | 0.1 | 0.8 | 3.5±0.6 | 3.6±0.8 | 0.1±0.7 | −0.2 | 0.4 | .43 | .04,.83 | .14 |
| Reminding oneself | 1.9±0.6 | 3.0±1.0 | 1.0±1.1 | 0.5 | 1.5 | 2.1±0.6 | 2.3±0.9 | 0.2±0.5 | −0.0 | 0.4 | .95 | .54,1.37 | .00 |
| Decisional balance | 2.0±1.0 | 1.9±1.1 | −0.1 (0.9 | −0.5 | 0.3 | 1.8±1.1 | 1.5±1.0 | −0.3 (1.1) | −0.8 | 0.2 | .26 | −.14,.65 | .44 |
P-value from the mixed effects regression model and for comparing changes from baseline to 12-weeks between intervention and comparison groups
Discussion
The survivors in the intervention group improved their general health and vitality, both of which are important constructs of physical functioning in SF-36. Of note, we also found evidence that the two composite scores in SF-36 (physical and mental health) were associated with fairly large positive changes in the intervention group relative to the comparison. These findings were corroborated by scores from the FACT-B scale, wherein emotional well-being improved significantly in the intervention group relative to the comparison group. These associations are consistent with previous research among cancer survivors (35, 36). Despite the small sample size in our study, these favorable findings hold promise for future research on the use of physical activity programs as an add-on module to survivorship care planning.
Another major finding is that the intervention delivered significant improvement in sleep impairment. The measure of sleep disturbance was also associated with a medium effect size, though the difference between the groups was not significant. As previously reported, MVPA increased by 69±84 min/week in the intervention group and decreased by 20±71 min/week in the comparison group (p=0.001) (23). Physical activity is an effective non-pharmacologic treatment for insomnia and other sleep disturbances. Research has shown that by influencing the circadian system, PA results in an increased total sleep time as well as time in deep sleep, which improves sleep quality (37). The BEAT Cancer intervention trial, which examined the effect of a PA intervention on sleep quality of breast cancer survivors, found that the intervention was associated with improvements in several sleep related variables (sleep quality, sleep disturbances, and daytime dysfunction) (38).
Two of four sub-scales of the social support scale showed significant between-group differences with the intervention group showing improvements in the ‘tangible’ and ‘belonging’ scores. The improvements in these subscales could be explained by the fact that the intervention module was aimed at the survivors performing PA with support partners who could be spouses, other family members, or friends. The survivors in the comparison group were enrolled with support partners but were not provided with any PA support. It is possible that recreational activity and exercises with support partners fostered a sense of belonging, friendship, and mutual dependability among the survivors. The reason why ‘appraisal’ and ‘self-esteem’ scores did not change in response to the intervention is not clear and requires further inquiry.
The intervention was also hypothesized to affect the processes of behavior change, which are rooted in the stages of motivational readiness for change model. These processes of change are the strategies and techniques that people use to modify their behavior (39). We found that, within processes of behavior change, all the behavioral strategies, including substituting alternatives, enlisting social support, rewarding oneself, committing oneself and reminding oneself, were associated with at least a medium effect size. The cognitive strategies (increasing knowledge, being aware of risks, caring about consequences, comprehending benefits), for most part, did not show change in response to the intervention. This is consistent with previous similar research (40) among cancer survivors. Additionally, our intervention had a significant positive effect on self-efficacy. These results provide insights into how at an individual level, the intervention had an effect on the stages of motivational readiness, on more proximal PA behavior, and eventually the distal HRQOL and psychosocial outcomes of the survivors.
Strengths and limitations:
Strengths include a randomized controlled design and high retention (94% of survivors at 12 weeks). However, several limitations should be noted. First, the trial had a short intervention period with no post-intervention follow-up, thus long-term effects are unknown. Also, our study used only self-reported measures of sleep variables. In future studies, these could be supplemented by device-measured sleep. Moreover, our sample was highly educated, predominantly non-Hispanic white, and, due to differences in referral rates, included few colorectal cancer survivors, limiting generalizability. There is also a possibility that the effects on the psychosocial outcomes could have occurred through other avenues (e.g., frequency of social contact with support partners or study staff) and not entirely through increased PA. In future studies with larger samples, mediation analyses could be performed to understand the underlying mechanisms responsible for changes in outcomes.
Clinical Implications:
Given the growing numbers of cancer survivors, the positive results from the present study have important implications for healthy and a long survivorship of millions. Augmenting the SCP with a more proactive technology-supported PA intervention may be an effective and practical strategy for improving psychosocial health of cancer survivors.
Conclusions:
urvivorship care planning serves as an important tool in outlining lifestyle recommendations after the completion of primary treatment. However, research shows that in order for survivors to successfully meet PA recommendations, lifestyle recommendations alone are not sufficient. We previously published a study using the data from the parent pilot randomized controlled trial and showed that augmenting the SCP with a technology supported PA module results in increased moderate-to-vigorous PA among the survivors (23). In addition, in the current study, the intervention has been found to be positively associated with several psychosocial and quality of life outcomes of survivors. In summary, this pilot study showed a positive effect of the intervention on quality of life of breast cancer survivors.
Acknowledgements
This project was supported by NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. Dr. Cadmus-Bertram’s time was supported by NIH grant 1K07CA178870.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical approval statement
All procedures performed in the trial were in accordance with the ethical standards of the University of Wisconsin Health Sciences IRB (protocol 2015-1295) and with the 1964 Helsinki declaration.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures 2017-2018. Atlanta; 2017. [Google Scholar]
- 2.American Cancer Society. Colorectal Cancer Facts and Figures 2017-2019. Atlanta; 2017. [Google Scholar]
- 3.Miller KDNL, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019; 69(5): 363–385. [DOI] [PubMed] [Google Scholar]
- 4.Hsu T, Ennis M, Hood N, Graham M, Goodwin PJ. Quality of Life in Long-Term Breast Cancer Survivors. J Clin Oncol. 2013; 31(28): 3540–8. [DOI] [PubMed] [Google Scholar]
- 5.Paraskevi T Quality of life outcomes in patients with breast cancer. Oncol Rev. 2012; 6(1): e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen LKL, Brenner H, Arndt V. Quality of life among long-term (≥5years) colorectal cancer survivors--systematic review. Eur J Cancer. 2010; 46(16): 2879–88. [DOI] [PubMed] [Google Scholar]
- 7.Thewes B BP, Zachariae R, Christensen S, Simard S, Gotay C. Fear of Cancer Recurrence: a systematic literature review of self-report measures. Psychooncology. 2012; 21(6): 571–87. [DOI] [PubMed] [Google Scholar]
- 8.Kale HP CN. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer. Cancer. 2016; 122(8): 283–9. [DOI] [PubMed] [Google Scholar]
- 9.Keesing S, Rosenwax L, & McNamara B The implications of women’s activity limitations and role disruptions during breast cancer survivorship. Women’s Health. 2018; 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanHoose L BL, Doty K, Sabata D, Twumasi-Ankrah P, Taylor S, Johnson R. An analysis of the distress thermometer problem list and distress in patients with Cancer. Support Care Cancer. 2015; 23(5): 1225–32. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. Body Image and Sexuality After Breast Cancer. Atlanta: American Cancer Society, Inc. [Google Scholar]
- 12.Traa MJ DVJ, Roukema JA, Rutten HJ, Den Oudsten BL. The sexual health care needs after colorectal cancer: the view of patients, partners, and health care professionals. Support Care Cancer. 2014; 22(3): 763–72. [DOI] [PubMed] [Google Scholar]
- 13.Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage. 2010; 39(3): 535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019; 51(11): 2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017; 123(7): 1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courneya KS, Tamburrini AL, Woolcott CG, McNeely ML, Karvinen KH, Campbell KL, et al. The Alberta Physical Activity and Breast Cancer Prevention Trial: quality of life outcomes. Prev Med. 2011; 52(1): 26–32. [DOI] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Morey MC, Clipp EC, Snyder DC, Sloane R, Pieper CF, et al. Results of Project LEAD (Leading the Way in Exercise and Diet) - A trial testing an intervention of telephone-counseling and mailed materials in improving physical functioning among older breast and prostate cancer survivors. J Clin Oncol. 2005; 23(16_suppl): 8138–8138. [Google Scholar]
- 18.Demark-Wahnefried W, Colditz GA, Rock CL, Sedjo RL, Liu J, Wolin KY, et al. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Cancer Res Treat. 2015; 154(2): 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch BM, van Roekel EH, Vallance JK. Physical activity and quality of life after colorectal cancer: overview of evidence and future directions. Expert Rev Qual Life Cancer Care. 2016; 1(1): 9–23. [Google Scholar]
- 20.Institute of Medicine. Implementing Cancer Survivorship Care Planning: Workshop Summary. Maria H, Patricia AG, editors. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 21.Jacobsen PB, DeRosa AP, Henderson TO, Mayer DK, Moskowitz CS, Paskett ED, et al. Systematic Review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol. 2018; 36(20): 2088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michie S AC, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009; 28(6): 690–701. [DOI] [PubMed] [Google Scholar]
- 23.Cadmus-Bertram L, Tevaarwerk AJ, Sesto ME, Gangnon R, Van Remortel B, Date P. Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: a randomized controlled pilot trial. J Cancer Surviv. 2019; 13(4): 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA TR, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42(2): 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016; 34(6): 611–635. [DOI] [PubMed] [Google Scholar]
- 26.Rock CL DC, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012; 62(4): 243–74. [DOI] [PubMed] [Google Scholar]
- 27.Prochaska JO, & DiClemente CC The transtheoretical approach In Norcross JC & Goldfried MR (Eds.), Oxford series in clinical psychology. Handbook of psychotherapy integration. Oxford University Press; 2005: 147–171. [Google Scholar]
- 28.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington DC; 2015. [Google Scholar]
- 29.John E Ware J, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 1993, 2000. [Google Scholar]
- 30.Cella D Facit Questionnaires. https://www.facit.org/FACITOrg/Questionnaires. Accessed November 19, 2019.
- 31.HealthMeasures. Sleep Disturbance- A brief guide to the PROMIS sleep disturbance instruments. http://www.healthmeasures.net/images/promis/manuals/PROMIS_Sleep_Disturbance_Scoring_Manual.pdf. Accessed November 15, 2019.
- 32.Cohen SMR, Kamarck T, Hoberman HM. Measuring the functional components of social support . In: (Eds.) IIGSBRS, editor. Social support: Theory, research and application. The Hague, Holland: 1985: 73–94. [Google Scholar]
- 33.Marcus BH, Forsyth LH. Motivating people to be physically active: Human Kinetics; 2003. [Google Scholar]
- 34.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999-2000 Through 2015-2016. Natl Health Stat Report. 2018; (122): 1–16. [PubMed] [Google Scholar]
- 35.Frensham LJ, Parfitt G, Dollman J. Effect of a 12-Week Online Walking Intervention on Health and Quality of Life in Cancer Survivors: A Quasi-Randomized Controlled Trial. Int J Environ Res Public Health. 2018; 15(10): 2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson MC, Lyons EJ, Song J, Cox-Martin M, Li Y, Green CE, et al. Change in physical activity and quality of life in endometrial cancer survivors receiving a physical activity intervention. Health Qual Life Outcomes. 2019; 17(1): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferioli M, Zauli G, Martelli AM, Vitale M, McCubrey JA, Ultimo S, et al. Impact of physical exercise in cancer survivors during and after antineoplastic treatments. Oncotarget. 2018; 9(17): 14005–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers LQ, Courneya KS, Oster RA, Anton PM, Robbs RS, Forero A, et al. Physical activity and sleep quality in breast cancer survivors: A randomized trial. Med Sci Sports Exerc. 2017; 49(10): 2009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prochaska JO, Velicer WF, DiClemente CC, Fava J. Measuring processes of change: Applications to the cessation of smoking. J Consult Clin Psychol. 1988; 56(4): 520–8. [DOI] [PubMed] [Google Scholar]
- 40.Scruggs S, Mama SK, Carmack CL, Douglas T, Diamond P, Basen-Engquist K. Randomized trial of a lifestyle physical activity intervention for breast cancer survivors: Effects on transtheoretical model variables. Health Promot Pract. 2017; 19(1): 134–44. [DOI] [PubMed] [Google Scholar]


