Abstract
The self-renewal transcription factor Nanog and the phosphoinositide 3-kinase (PI3K)–Akt pathway are known to be essential for maintenance of mesenchymal stem cells. We evaluated their contribution to the maintenance of CD133(+) cancer stem-like cells (CSCs) and spheroid-forming cells in patient-derived cell lines from three human sarcoma subtypes: HT1080 fibrosarcoma, SK-LMS-1 leiomyosarcoma, and DDLS8817 dedifferentiated liposarcoma. Levels of Nanog and activated Akt were significantly higher in sarcoma cells grown as spheroids or sorted for CD133 expression to enrich for CSCs. shRNA knockdown of Nanog decreased spheroid formation 10- to 14-fold, and reversed resistance to both doxorubicin and radiation in vitro and in H1080 flank xenografts. In the HT1080 xenograft model, doxorubicin and Nanog knockdown reduced tumor growth by 34% and 45%, respectively, and the combination reduced tumor growth by 74%. Using a human phospho-kinase antibody array, Akt1/2 signaling, known to regulate Nanog, was found to be highly activated in sarcoma spheroid cells compared with monolayer cells. Pharmacologic inhibition of Akt using LY294002 and Akt1/2 knockdown using shRNA in sarcoma CSCs decreased Nanog expression and spheroid formation and reversed chemotherapy resistance. Akt1/2 inhibition combined with doxorubicin treatment of HT1080 flank xenografts reduced tumor growth by 73%. Finally, in a human sarcoma tumor microarray, expression of CD133, Nanog, and phospho-Akt were 1.8- to 6.8-fold higher in tumor tissue compared with normal tissue. Together, these results indicate that the Akt1/2–Nanog pathway is critical for maintenance of sarcoma CSCs and spheroid-forming cells, supporting further exploration of this pathway as a therapeutic target in sarcoma.
Subject terms: Cancer stem cells, Cancer stem cells
Introduction
Mesenchymal tissues are derived from the mesoderm (i.e. middle layer of the embryo) and include the musculoskeletal system, circulatory and lymphatic systems, and connective tissues. Sarcomas are malignant tumors of mesenchymal tissues, with an annual incidence of approximately 15,000 new diagnoses in the United States, of which about 40% lead to fatality1. Treatment of primary tumors often includes aggressive surgical resection and radiation therapy, but local recurrence remains a significant problem, especially for tumors in difficult locations such as the head and neck, paraspinal region, retroperitoneum, and pelvis. Up to half of patients with large, high-grade sarcomas develop distant metastases, most frequently to the lung2. The efficacy of chemotherapy and radiation in treating local and distant recurrence is modest at best, and for the minority of patients who do respond, nearly all eventually develop resistance3.
Cancer stem-like cells (CSCs) in sarcomas and other cancers may be a source of therapy resistance as well as distant metastasis4,5. CSCs share characteristics with normal adult stem cells, specifically the capacity for self-renewal and pluripotent differentiation6. Methods to identify CSCs include tumor initiation in immunodeficient mice, spheroid colony formation in vitro, and expression of certain cell surface markers. The most commonly identified cell surface marker for CSCs in sarcomas is CD133 (refs. 7,8). Given there are over 80 distinct histologic subtypes of sarcoma, many have also suggested that sarcomas originate from multipotent cells such as mesenchymal stem cells9.
Thus, targeting CSCs is a worthy goal in the development of more effective treatments for advanced sarcomas, as such strategies would circumvent treatment resistance and prevent metastasis. CSCs may be regulated by stemness-related proteins, including Nanog, Sox2, Oct4, and c-Myc10–13. Nanog plays a multifaceted role in pluripotency and embryonic stem cells by regulating the expression of Sox2, Oct4, KLF4, and itself14. Several reports have also found that Nanog expression drives CSC function and oncogenesis in multiple human cancers, including prostate cancer15, hepatocellular carcinoma16, leukemia, glioblastoma multiforme17, colorectal cancers18, lung cancer19, and breast cancer20. Similarly, we have shown that Nanog expression correlates with response to therapy in sarcoma stem cells21.
Nanog is regulated by several pathways, including indirect association with the mitogen-activated protein kinase pathway22. The PI3K–Akt pathway has similarly been shown to play a key role in cancer stem cell biology23, and some studies suggest that it may regulate the stemness and reprogramming in various solid tumors24,25. In keeping with that conclusion, we previously showed that inhibition of PI3K significantly reduced expression of the CSC marker CD44 and the mesenchymal transdifferentiation controller Slug in gastric CSCs26.
Here, we sought to examine the role of Nanog in the maintenance of stem-like properties in sarcoma CSCs and to determine if Nanog inhibition could reverse resistance to chemotherapy and radiation. Using a protein kinase array, we found that the PI3K/Akt pathway was upregulated in sarcoma CSCs. As we also found that PI3K/Akt regulates Nanog, we further investigated the role of the PI3K–Akt pathway in sarcoma CSCs.
Results
Nanog promotes stemness in human sarcoma cells
To explore the function of Nanog in sarcoma CSCs, we first examined its expression in sarcoma cells grown as spheroids, which enriches for CSCs27–29. In all three sarcoma cell lines (HT1080 fibrosarcoma cells, SK-LMS-1 leiomyosarcoma cells, and DDLS8817 dedifferentiated liposarcoma cells), growth as spheroids increased expression of Nanog compared with that in monolayer-grown cells, while expression of other self-renewal proteins Sox2, Oct4, and c-Myc was not uniformly increased (Fig. 1A). We then assessed Nanog expression in flow-sorted CSCs, distinguished by the marker CD133 (ref. 30), and Nanog expression was dramatically higher in CD133(+) cells (Fig. 1B).
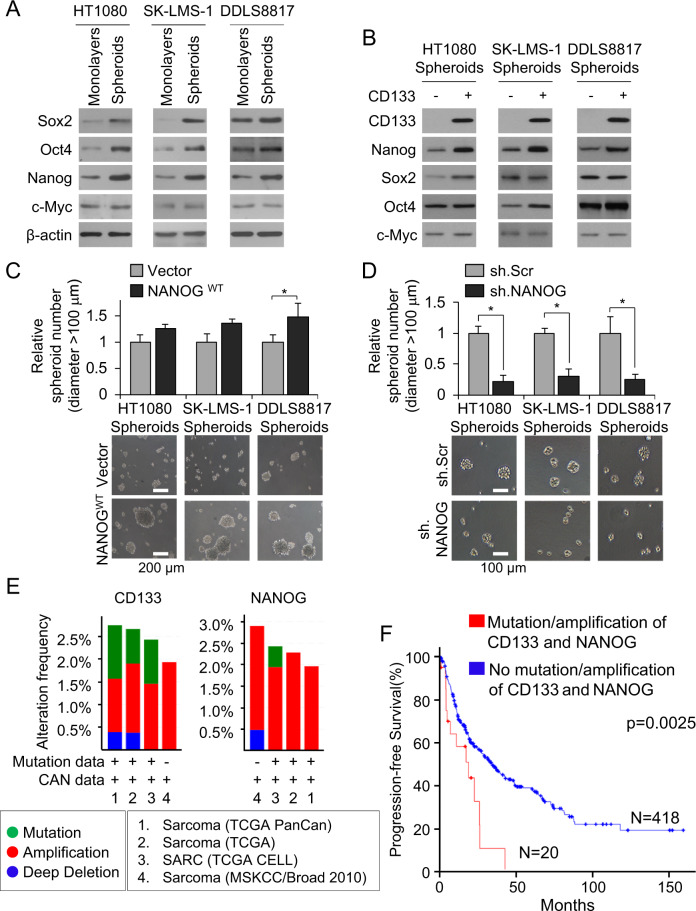
Fig. 1. Nanog promotes stemness in human sarcoma cells.
A, B Western blot for self-renewal proteins Sox2, Oct4, Nanog, and c-Myc in human sarcoma cell lines grown as A monolayers and as spheroids and B after separation of spheroid cells into CD133+ and CD133− fractions. β-Actin, loading control. C, D Spheroid formation following lentiviral transduction with C Nanog or vector and D Nanog shRNA (sh.NANOG) or scramble control shRNA (sh.Scr). Scale bar, 100, 200 µm. *p < 0.05 compared to control. E Frequency and types of CD133 and Nanog alterations in sarcoma samples according to previous genomic studies from TCGA31, Liu and colleagues32, and University of Tokyo33. F Kaplan–Meier curves of progression-free survival of sarcoma patients following potentially curative resection, stratified by the presence or absence of CD133 and Nanog mutation/amplification. Graphs in E, F generated using cBioPortal (http://www.https://cbioportal.mskcc.org).
To investigate whether Nanog influences spheroid formation, we stably overexpressed it by lentiviral transduction; this did not alter cell proliferation in vitro (Suppl. Fig. S1A). Nanog overexpression increased expression of CD133 (Suppl. Fig. S1B), increased spheroid formation by 23–49% (Fig. 1C), and increased spheroid size by 28–43% compared with control cells (Suppl. Fig. S1C). Conversely, stable knockdown of Nanog using shRNA, which did not alter cell proliferation (Suppl. Fig. S1D), in sarcoma spheroid cells resulted in decreased expression of CD133 as determined by western blot and immunofluorescence (Suppl. Fig. S1E, S1F). Nanog shRNA also decreased spheroid size by 71–74% (Suppl. Fig. S1G) and spheroid number by 70–81% (Fig. 1D) as compared with control cells. Together, these data show that Nanog is upregulated in sarcoma CSCs and promotes spheroid formation.
We finally confirmed the association of CD133 and Nanog gene mutations and copy number alterations in sarcomas with patient survival data in four publicly available databases (http://cancergenome.nih.gov)31–33. The frequency of CD133 mutation or amplification in these cohorts was 0.8–1.9% and the frequency of Nanog mutation or amplification was 0.5–2.4% (Fig. 1E). Patients whose sarcomas had gene mutation or gene amplification of both CD133 and Nanog had significantly worse disease-free survival compared to patients whose sarcomas did not have gene mutation or gene amplification of CD133 and Nanog (Fig. 1F).
Nanog inhibition reverses chemotherapy and radiation resistance
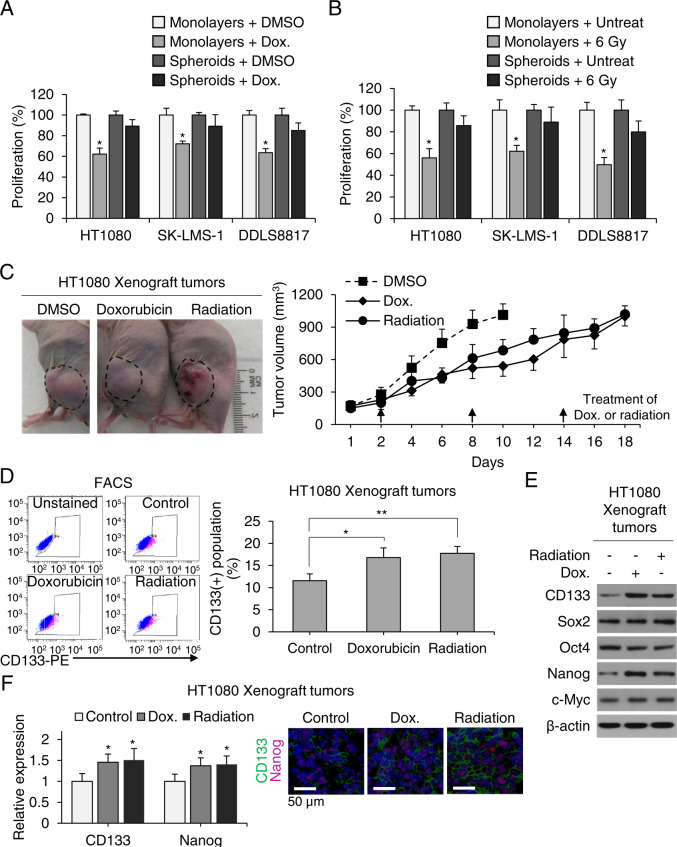
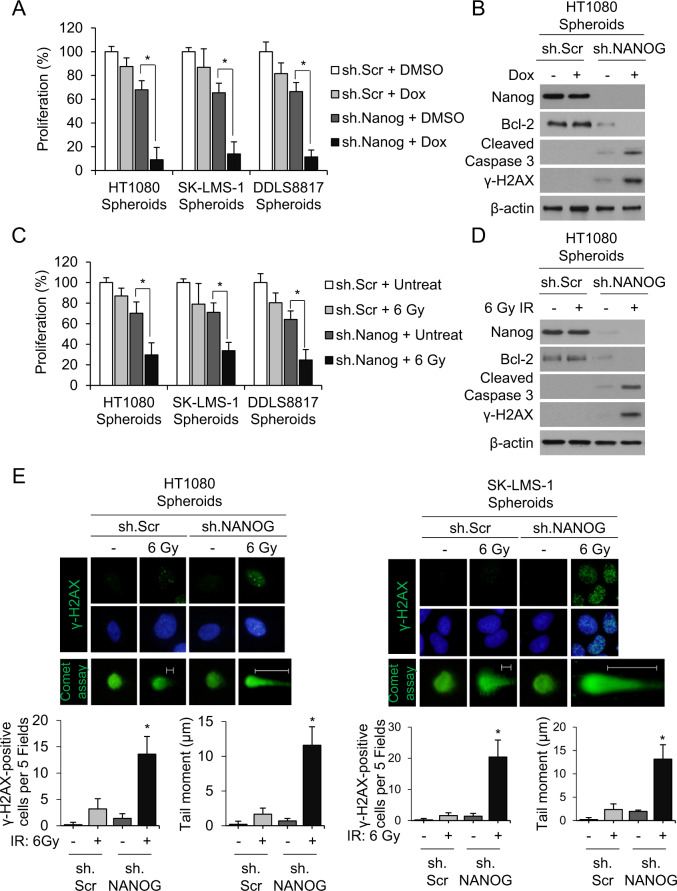
As numerous studies have demonstrated that CSCs are generally resistant to chemotherapy13,29,34 and radiation35,36, we tested sarcoma cells’ sensitivity to these modalities in vitro and in vivo. Figure 2A shows proliferation assays for HT1080, SK-LMS-1, and DDLS8817 monolayer cells and spheroids cells treated with DMSO control or doxorubicin (1.5 μg/mL). For the three cell lines grown as monolayer cells, doxorubicin decreased proliferation by 28–38%. For the three cell lines grown as spheroids, doxorubicin decreased proliferation by only 11–15%. Figure 2B shows proliferation assays for monolayer cells and spheroids cells treated with DMSO control or 6 Gy of radiation. For the three cell lines grown as monolayer cells, radiation decreased proliferation by 38–50%. For the three cell lines grown as spheroids, radiation decreased proliferation by only 11–20%. HT1080 spheroids cells were next used to generate flank xenografts in mice. Upon reaching about 100 mm3, mice were treated every 6 days with doxorubicin 4 mg/kg or radiation 6 Gy for three total treatments. HT1080 xenografts were similarly resistant to doxorubicin and radiation. Control tumors grew to over 1000 mm3 in about 10 days while tumors treated with doxorubicin or radiation grew to over 1000 mm3 in about 18 days. These treatments also increased the percentage of CD133(+) cells in tumors (Fig. 2D), as well as expression of CD133 and Nanog (Fig. 2E, F); levels of other self-renewal proteins Sox2, Oct4, and c-Myc were unchanged as measured by western blot and immunofluorescence. To determine whether Nanog influences sarcoma chemotherapy and radiation resistance, Nanog was knocked down in all three sarcoma cells lines using shRNA; stable knockdown was confirmed by western blot (Suppl. Fig. S2). In Nanog-deficient cells (all three lines), doxorubicin and radiation reduced proliferation in spheroid cells by 86–91% and 66–75%, respectively (Fig. 3A, B). To investigate how elimination of Nanog affects susceptibility to these agents, we next measured DNA damage using ɣ-H2AX expression and apoptosis using Bcl-2 expression. In Nanog-deficient HT1080 spheroid cells, doxorubicin and radiation led to a significant increase in DNA damage and a significant reduction in apoptosis (Fig. 3C, D). The DNA damage response was confirmed by immunofluorescence for γ‐H2AX as an increased number of foci and by comet assay as an increased tail moment (Fig. 3E). Thus, these data suggest that sarcoma spheroid-forming cells are relatively resistant to chemotherapy and radiotherapy, and this resistance can be overcome by Nanog inhibition.
Fig. 2. CSCs contribute to sarcomas’ resistance to chemo- and radiotherapy.
A, B Proliferation of monolayers and spheroids following treatment with doxorubicin (Dox) or radiation therapy. C Growth curve of HT1080 fibrosarcoma xenografts in athymic nude mice treated with Dox or 6 Gy radiation. D Fluorescence-activated cell sorting (FACS) analysis for CD133+ cells in untreated control and Dox- or 6-Gy-treated tumors. E Western blot of tumor lysates for CD133, Sox2, Oct4, Nanog, and c-Myc. β-Actin is the loading control. F Immunofluorescence of tumors for CD133 (green) and Nanog (red) with DAPI (blue) staining in athymic nude mice treated with doxorubicin or 6 Gy radiation. Scale bar, 50 µm. *p < 0.05, **p < 0.01 compared to control.
Fig. 3. Nanog inhibition reverses chemo- and radiotherapy resistance.
A–D Proliferation of sarcoma cells grown as spheroids (A, C) and western blot for Nanog, Bcl-2, cleaved caspase-3, and γ-H2AX (B, D) following treatment with sh.NANOG or sh.Scr and doxorubicin (Dox) or 6 Gy radiotherapy. β-Actin, loading control. *p < 0.01 compared to control. E Immunofluorescence of sarcoma spheroid cells (HT1080, SK-LM-1). Top panel shows γ-H2AX in green. Middle panel shows γ-H2AX in green and nuclei in blue (DAPI). Bottom panel shows Comet assay in green. All photos are of different cells under specified conditions. Graphs show quantification of γ-H2AX positive staining and mean tail moment. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; blue).
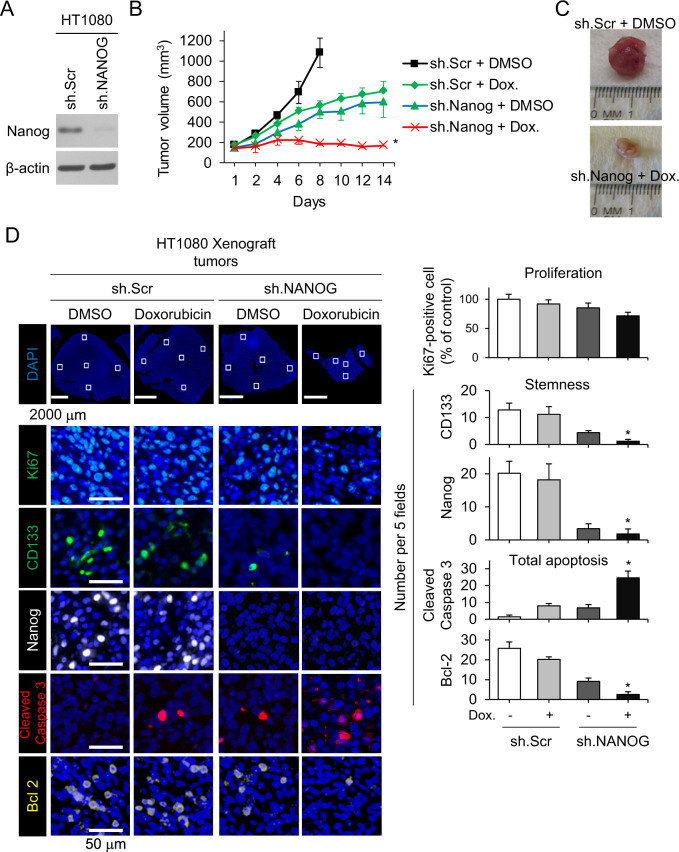
We next evaluated the combination of Nanog knockdown and doxorubicin chemotherapy in vivo using flank xenografts of HT1080 cells that were stably transduced with Nanog shRNA or control shRNA (Fig. 4A). After tumors reached 100–150 mm3 in size, mice were randomized to treatment with either doxorubicin or PBS. Nanog shRNA and doxorubicin reduced tumor growth by 45% and 35%, respectively, while the combination of Nanog shRNA and doxorubicin inhibited tumor growth by 84% (Fig. 4B, C). The combination of Nanog shRNA and doxorubicin reduced proliferation as measured by Ki76 immunofluorescence in tumors after 14 days of treatment by only 28.4% compared to control tumors (Fig. 4D). In contrast, this combination reduced expression of CD133 and Nanog by >90% compared with controls and increased apoptosis by 17.5-fold as measured by cleaved caspase-3 expression, corresponding to 90% reduced expression of the anti-apoptotic protein Bcl-2(+) cells. Thus, Nanog inhibition overcomes chemotherapy resistance in HT1080 xenografts.
Fig. 4. Nanog knockdown restores sensitivity to chemotherapy in mouse xenograft models.
A Western blot of HT1080 cells transduced with sh.NANOG lentivirus for Nanog and β-actin. B Growth of HT1080 fibrosarcoma xenografts in athymic nude mice treated with sh.NANOG or sh.Scr and PBS or doxorubicin (Dox). C Photos of representative tumors. D Immunohistochemical analysis of tumors for Ki67 (green), CD133 (green), Nanog (white), cleaved caspase-3 (red), and Bcl-2 (yellow). Scale bar, 50 µm. *p < 0.01 compared to control.
PI3K/Akt signaling regulates Nanog expression
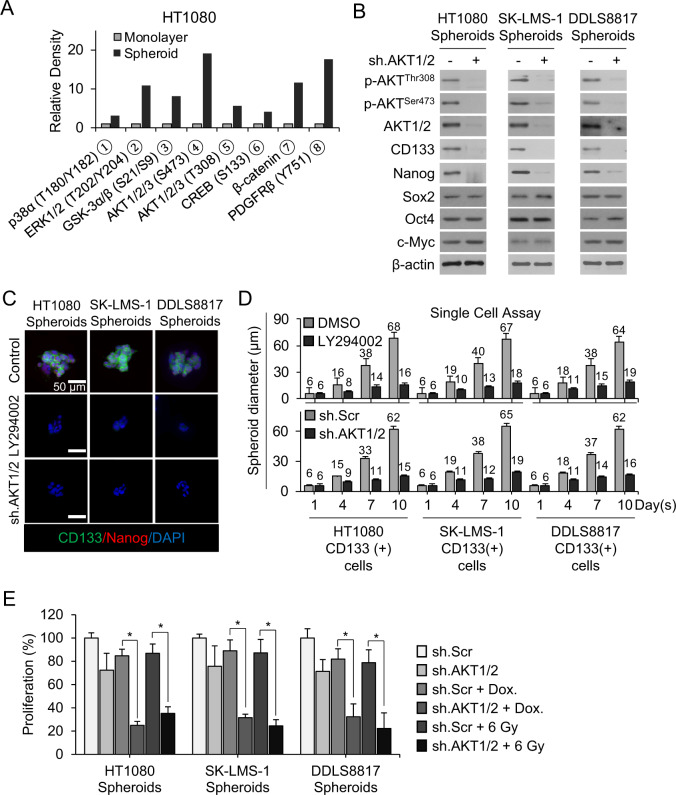
We next searched for signaling pathways that regulate Nanog in sarcoma CSCs and spheroid-forming cells by using a human phospho-kinase array to identify kinases and kinase substrates that were differentially phosphorylated between sarcoma cell lines grown as monolayers vs. spheroids. Following quantification by densitometric analysis, we found that phosphorylation of Akt1/2 and platelet-derived growth factor receptor β (PDGFR-β) were significantly increased in spheroids (Fig. 5A and Suppl. Fig. S3A). We confirmed that the PI3K/Akt pathway is highly activated in sarcoma spheroid-forming cells by western blot for phosphorylated Akt (T308 or S473) and total Akt1/2; phosphorylation was higher in sarcoma cells grown as spheroids compared with monolayers (Suppl. Fig. S3B) and in CD133+ compared with CD133− cells (Suppl. Fig. S3C). Akt shRNA knockdown or pharmacologic inhibition using LY294002 dramatically reduced levels of CD133 and Nanog but had no effect on levels of other stemness factors such as Sox2, Oct4, and c-Myc (Fig. 5B and Suppl. Fig. S3D). Furthermore, following separation of spheroids into CD133+ and CD133− cells, PI3K inhibition with LY294002 reduced the expression of CD133 and Nanog in CD133+ cells from all sarcoma lines (Suppl. Fig. S3E). Inhibition of the PI3K/Akt signaling pathway with LY294002 or Akt1/2 shRNA in CD133+ cells reduced spheroid formation under standard conditions (Fig. 5C) and from single cells (Fig. 5D and Suppl. Fig. S3F) by 71–77%.
Fig. 5. PI3K/Akt signaling regulates Nanog expression.
A Activated proteins in HT1080 monolayers and spheroids identified by human phospho-kinase array kit. B Western blot for indicated proteins following transduction with sh.Akt1/2 or sh.Scr in sarcoma spheroid cells. C CD133 (green) and Nanog (red) immunofluorescence with and DAPI (blue) counterstaining following treatment with PI3K inhibitor LY294002 or sh.Akt1/2 in spheroid cells. D Diameter of spheroids grown from single CD133+ cell cells showing following treatment with LY294002 or sh.Akt1/2 vs, DMSO or sh.Scr, respectively. E Proliferation of spheroid cells following treatment with sh.Akt1/2 and doxorubicin or 6 Gy radiation. β-Actin, loading control. Scale bar, 50 µm. *p < 0.01 compared to control.
We next examined whether PI3K inhibition increased the sensitivity of sarcoma cell lines grown as spheroids to doxorubicin chemotherapy and radiation. The PI3K inhibitor LY294002 inhibited growth by 31–35%, doxorubicin by 11–16%, and the combination by 77–84% (Suppl. Fig. S4A). LY294002 combined with doxorubicin led to significant induction of apoptosis, as measured by western blotting for cleaved caspase-3 and Bcl-2, in HT1080 spheroid cells (Suppl. Fig. S4B). Similar results were obtained when Akt1/2 shRNA was used in place of the PI3K inhibitor (Fig. 5E and Suppl. Fig. S4C). Akt1/2 shRNA with radiation enhanced the reduction in proliferation of HT1080, SK-LMS-1, and DDLS8817 sarcoma spheroid cells caused by 6-Gy radiation from 13–21% to 65–78% (Fig. 5E). Similar to results with doxorubicin, sh.Akt1/2 combined with radiation led to significant induction of apoptosis in HT1080 spheroid cells (Suppl. Fig. S4D). These studies demonstrate that the PI3K/Akt pathway regulates the expression of Nanog in sarcoma spheroid-forming cells and promotes CSC phenotypes such as spheroid formation and therapy resistance.
Combined Akt1/2 inhibition and chemotherapy inhibits stemness and tumor growth in vivo
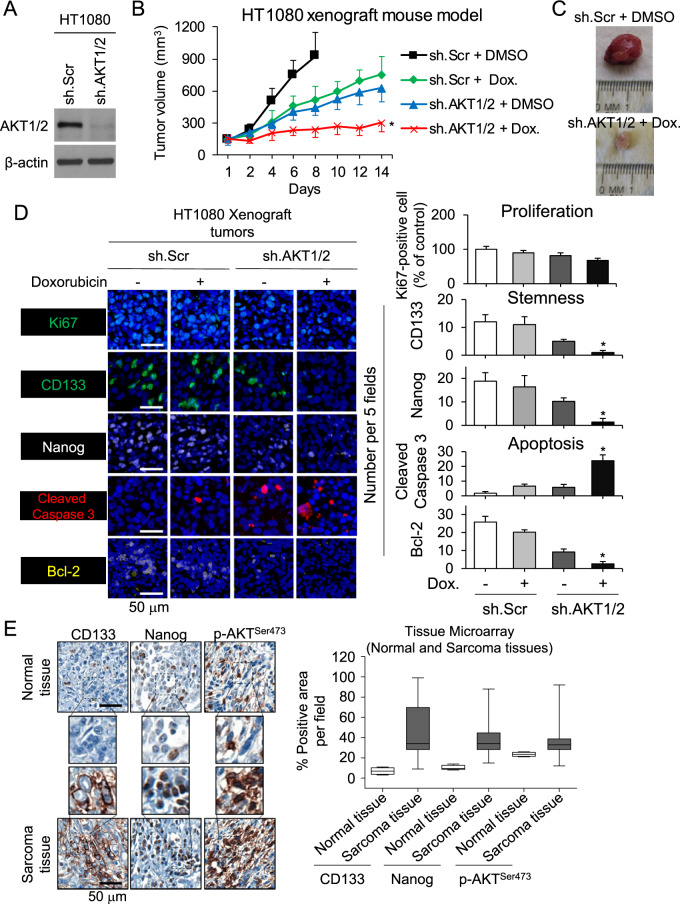
To evaluate the efficacy of combining Akt1/2 inhibition with doxorubicin, we xenografted HT1080 cells stably transduced with Akt1/2 shRNA or control shRNA into the flanks of immunodeficient mice following confirmation of stable knockdown (Fig. 6A). Once tumors reached 100–150 mm3, mice were randomized and treated with doxorubicin or PBS. Akt1/2 shRNA or doxorubicin slowed tumor growth by approximately 40–50% (Fig. 6B), while the combination of Akt1/2 shRNA and doxorubicin inhibited growth by 74% without affecting body weight (Suppl. Fig. S5). After 14 days, xenografts were harvested and analyzed (Fig. 6C). The combination of Akt1/2 shRNA and doxorubicin resulted in a 13-fold increase in the apoptosis marker cleaved caspase-3 compared with control, and ≥90% decreases in expression of the CSC markers CD133 and Nanog and in the anti-apoptosis marker Bcl-2 as measured by immunofluorescence (Fig. 6D). To examine the role of Akt1/2 activation in human sarcoma CSCs and spheroid-forming cells, we assessed expression of CD133, Nanog, and phosphorylated Akt1/2 (S473) by immunostaining a commercially available tissue microarray containing 79 human sarcomas and 4 human normal tissues. Levels of CD133, Nanog, and phospho-Akt were 1.8–6.8-fold higher in tumor tissue compared with normal tissue (Fig. 6E). Thus, targeting the Akt1/2–Nanog signaling axis may be relevant to multiple types of sarcoma.
Fig. 6. Combined Akt1/2 inhibition and chemotherapy inhibits stemness and tumor growth in vivo.
A Western blot confirmation of Akt1/2 knockdown by shRNA in HT1080 cells. B Growth of HT1080 fibrosarcoma xenografts stably transduced with sh.Scr or sh.Akt1/2 in athymic nude mice treated and PBS or doxorubicin. C Photos of representative tumors. D Immunohistochemical analysis of tumors for proliferation using Ki67 (green), stemness using CD133 (green) and Nanog (white), and apoptosis using cleaved caspase-3 (red) and Bcl-2 (yellow). E Immunohistochemical staining of commercially available tissue array slide containing 79 human sarcomas and 4 human normal tissues for CD133, Nanog, and p-Akt1/2 (S473). β-Actin, loading control. Scale bar, 50 µm. *p < 0.01 compared to control.
Discussion
In this study, we demonstrate the central role of Nanog and PI3K/Akt signaling in maintaining sarcoma CSC phenotypes including spheroid formation and therapy resistance in sarcomas. Knockdown of Nanog in spheroid cells or CD133(+) cells using shRNA inhibited spheroid formation and reversed their resistance to chemotherapy and radiation. In the HT1080 flank xenograft model, inhibition of Nanog synergized with doxorubicin to reduce tumor growth, and histologic examination of treated tumors found dramatic increases in tumor cell apoptosis and depletion of CD133+ cells. Moreover, pharmacologic inhibition or knockdown of Akt in sarcoma CSCs reduced expression of Nanog, reduced spheroid formation, and reversed therapy resistance.
The existence of CSCs has been shown in multiple human cancers including several sarcoma subtypes, their potential as therapeutic targets remains less examined. Whether this population of cells is responsible for therapeutic resistance and recurrence has not been established. Sarcomas are a heterogeneous group of tumors with over 80 subtypes, each with its own tumor biology and response to therapy. However, sarcomas may originate from multipotent stem cells such as mesenchymal stem cells9, and so targeting such cells may have broad application to all sarcoma subtypes. Here we examined three common sarcoma subtypes (fibrosarcoma, leiomyosarcoma, and liposarcoma) and found similar effects when targeting Nanog and PI3K/Akt signaling in spheroid-forming cells and CD133(+) cells from these cell lines. Our reliance on CD133 as a marker of CSCs is based on several studies from our lab and others showing that this molecule plays a role in the growth and development of tumors including sarcomas21,37. CD133(+) cells are enriched in CSCs and show greater tumorigenic potential than CD133()- cells in multiple cancers38–41.
Multidrug chemotherapy with agents such as doxorubicin, ifosfamide, gemcitabine, and docetaxel is standard treatment for patients with advanced or metastatic sarcomas. These drugs can lead to tumor shrinkage but resistance ultimately develops. Our results implicate Nanog in the resistance of sarcoma CSCs to chemotherapy and radiation; we found that Nanog in spheroid-forming cells reduced chemo- and radiotherapy resistance, increased apoptosis, and attenuated the tumor growth of flank xenografts. These data are in line with extensive evidence supporting Nanog’s vital role in malignant disease; its expression correlates with cell proliferation, tumorigenicity, and invasiveness42–44.
Our phospho-kinase array analysis revealed that Akt1/2 is more highly activated in CD133(+) vs. CD133(−) sarcoma cells and spheroid vs. monolayer sarcoma cells. This finding is in agreement with prior reports that the Akt pathway contributes to the self-renewal and maintenance of CSCs25,45, and with the central role of Akt activation in sarcomagenesis46–49. The key advance in our study is that we show that Akt signaling supports the maintenance of sarcoma CSC phenotypes such as self-renewal50, and chemo- or radiotherapy resistance and its activation in human sarcomas, as well as the correlation of that activation with expression of CD133 and Nanog. These findings demonstrate that Akt signaling contributes to the control of expression of CSC transcription factors in human sarcoma. Our results support targeting the Akt pathway to prevent resistance to chemotherapy and radiotherapy and increase the likelihood of durable remission, as has been suggested for many cancers40, including several sarcomas49,51–55.
In conclusion, our findings indicate that Akt1/2–Nanog signaling plays a critical role in sarcoma CSC maintenance. Thus, targeting the Akt1/2–Nanog signaling pathway may prevent or reverse CSC phenotypes, tumor progression, and radiotherapy resistance in sarcomas.
Materials and methods
Cell lines and reagents
HT1080 human fibrosarcoma cells and SK-LMS-1 human leiomyosarcoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The DDLS8817 dedifferentiated liposarcoma cell line was established from a patient sample following informed consent and were confirmed by cytogenetic analysis and by DNA copy number arrays (Agilent 244K) to harbor chromosome 12q amplification. HT1080 and SK-LMS-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), and DDLS8817 cells were maintained in DMEM-F12. All media were supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM) (hereafter termed “regular media”). Cancer cell lines were actively passaged for less than 6 months from the time that they were received, following United Kingdom Coordinating Committee on Cancer Research guidelines56. Doxorubicin (RYG02) was purchased from Biotang Inc. (Lexington, MA). LY294002 (cat. 440202) and Puromycin (cat. 508838) were purchased from Calbiochem.
Spheroid generation
Cells were resuspended in spheroid media, comprised of DMEM/F12 containing epidermal growth factor (EGF, E9644; Sigma-Aldrich, St. Louis, MO), basic fibroblast growth factor (bFGF, 341583; Sigma-Aldrich), N-2 supplement (A13707-01; Thermo Fisher Scientific, Waltham, MA), and B27 (0080085SA; Sigma-Aldrich), and then plated on Ultra-Low Attachment culture dishes (Corning Life Sciences, Tewksbury, MA). Spheroids were collected after 5–7 days except when noted otherwise. Proteins were extracted for analysis, or cells were dissociated with Accutase (561527, BD Biosciences) and used for other experiments. Spheroid growth was quantified as the average number of spheroids >50–100 μm in diameter among five fields after image processing using Imaris 7.6 (Bitplane, Concord, MA).
Single-cell spheroid growth assay
Spheroids were treated with LY294002 (10 μM) or DMSO, dissociated into single cells, and plated onto Ultra-Low Attachment 96-well plates at a density of 1 cell per well. Clone formation was monitored at 1, 4, 7, and 10 days on an inverted microscope (Leica Microsystems, Buffalo Grove, IL). Clone size was measured using MetaMorph software, version 7.8.2 (Molecular Devices, Sunnyvale, CA). Data reflect the mean of three samples.
Western blot analysis
Proteins were extracted by collecting cells in RIPA buffer (Sigma-Aldrich) containing Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN), and protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Proteins were detected using the following antibodies: CD133 (MBS462020) from MyBioSource (San Diego, CA); Sox2 (#2748), Oct4 (#2788), Nanog (#4893), p-Akt (T308) (#9275), p-AKT(Ser 473) (#9271), p-Erk1/2 (#9101), cleaved caspase-3 (#96610, all from Cell Signaling Technology; c-Myc (sc-40), Akt1/2 (sc-8312), all from Santa Cruz Biotechnology, Bcl-2 (ab692), both from Abcam), γ-H2AX (05-636; Millipore), and β-actin (A5441; Sigma).
FACS and magnetic-activated cell sorting
For FACS, cells were dissociated using Accutase and resuspended in PBS containing 0.5% BSA. The cells were stained with PE-conjugated CD133 (BD566593) or isotype control antibody (BD555742) from BD Biosciences on ice for 30 min. Cells were then washed with PBS and analyzed on a BD FACSCalibur (BD Biosciences) using Cell Quest software.
CD133+ cells were sorted by a magnetic-activated cell sorting system (Miltenyi Biotech). After collecting spheroids, cells were washed with PBS, dissociated to single cells using Accutase, and stained with CD133-Micro Beads on ice for 30 min. Cells were then passed through an LS magnetic column, where CD133+ cells were retained. The CD133+ cells were then eluted from the column after removal from the magnet. Quantitative analysis of CD133+ cells was performed by immunofluorescence using PE-conjugated CD133 antibody.
Lentiviral transduction
Nanog and Akt1/2 expression was silenced via lentiviral transduction of human Nanog shRNA (sc-43958-V; Santa Cruz) and human Akt1/2 shRNA (sc-43609-V; Santa Cruz) per the manufacturer’s protocol. Nanog was overexpressed via Nanog lentiviral activation particles (sc-418033-LAC; Santa Cruz) per the manufacturer’s protocol. Controls were scramble shRNA control (sc-108080; Santa Cruz) or control Lentiviral Activation Particles (sc-437282, Santa Cruz). Maximal knockdown of genes occurred 72–96 h after transduction.
Cell proliferation
Spheroids were dissociated with Accutase, and monolayer cells were collected following detachment using trypsin. For proliferation assays, 2 ×103 cells were plated onto 96-well flat-bottom plates and maintained in regular media overnight. After 1 day, cells were treated with doxorubicin (1.5 μg/mL) or radiation (6 Gy). A colorimetric MTT assay was used to assess cell number by optical density after 5 days or the specified number of days, as previously described57. Data reflect the mean of 6 samples.
NANOG and CD133 mutation and copy number alteration analysis from various databases
To determine the frequency of CD133 and Nanog mutations and copy number alterations (amplifications and deletions) in sarcoma samples from previous genomic studies, we selected sarcoma samples from TCGA31, Liu et al.32, other groups33 and Memorial Sloan Kettering Cancer Center (https://cbioportal.mskcc.org/), along with types of alterations in cBioPortal (http://www.cbioportal.org) and http://cancergenome.nih.gov. Progression-free survival data were available for these patients. Kaplan–Meier estimates were used to generate progression-free survival plots. The log-rank test was used to compare survival outcomes.
Comet assay
HT1080 and SK-LMS-1 cells were transduced with Nanog shRNA or control scrambled shRNA and grown as spheroids. Some spheroids with treated with 6 Gy of radiation. After 24 h, spheroids were dissociated into single cells. The single-cell gel electrophoresis assay, also known as the comet assay, was carried out using Comet Assay Kit (Trevigen Inc., Gaithersburg, MD, USA) per the manufacturer’s instructions. After electrophoresis, the slides were stained with 20 μL of 1 μg/mL DAPI and analyzed with a fluorescence microscope. Bleomycin was not added. Olive tail moment values (equal to the product of the tail length and the fraction of total DNA in the tail) were calculated from at least 50 cells for each group using Komet 5.5 software (Andor Technology, Belfast, UK).
Immunocytochemistry
Spheroid cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. Following cell fixation, cells were incubated with antibodies recognizing CD133 (MBS462020; MyBioSource), Nanog (#4893; Cell Signaling), and γ-H2AX (05-636; Millipore) in a solution of PBS with 1% FBS and 0.1% Triton X-100 at 4 °C overnight. Cells were then stained with secondary antibodies tagged with Alexa Fluor 488 (A-11029) or Alexa Fluor 568 (A-11061), both from Thermo Fisher. Nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI, 28718-90-3; Sigma-Aldrich). Stained cells were visualized on an inverted confocal microscope (Leica Microsystems) and images were processed using Imaris version 7.6 (Bitplane). Three samples were examined for each group, and each sample was visualized for five fields.
Human phospho-kinase array
Phospho-antibody array analysis was performed using the Proteome Profiler Kit ARY003B (R&D Systems). The array membranes were incubated with 500 µg lysate overnight at 4 °C. The following day, chemiluminescent detection was done according to the manufacturer’s protocols24. Densitometry analysis was performed using ImageJ.
Mouse xenograft model
All mouse protocols were approved by the Institutional Animal Care and Use Committee. To generate subcutaneous flank tumors, 2 × 106 HT1080 cells (following shRNA knockdown of Nanog or Akt1/2 where indicated) were resuspended in 100 μL of Hank’s balanced salt solution (HBSS) and injected subcutaneously into the right flank of athymic 6–8-week-old male BALB/c nu/nu mice (Taconic, Hudson, NY) following isoflurane anesthesia. Mice were randomly assigned by coin flip into treatment groups (6 mice per group) when tumors reached 100–150 mm3 in volume. The investigator was not blinded. Sample size was determined using the sample a size program (http://hedwig.mgh.harvard.edu/sample_size/size.html). Doxorubicin (4 mg/kg) or PBS was administered two times per week by intraperitoneal injection or as indicated. Tumor volume was calculated using the formula length × (width)2 × 0.52. To radiate tumors, mice were anesthetized using ketamine (125 mg/kg) and xylazine (10 mg/kg), placed in shielded device to expose only the flank tumor, and irradiated using a Gammacell 40 Exactor Irradiator (Best Theratronics, Ottawa, ON, Canada).
Immunohistochemistry of xenograft tumors
At least four sections were analyzed from each tumor. Paraffin-embedded sections were deparaffinized51, and then incubated with one or more of the following primary antibodies recognizing Sox2 (ab93689), Oct4 (ab18976), Nanog (ab80892), c-Myc (ab125275; Abcam), Ki67 (ab15580) (all from Abcam), cleaved caspase-3 (#9661; Cell Signaling), Bcl-2 (Santa Cruz, sc-65392), or CD133 (MBS462020; MyBioSource) in a solution of PBS with 1% FBS and 0.1% Triton X-100 at 4 °C overnight. Staining was visualized using secondary antibodies tagged with Alexa Fluor 488 (A-21206) or Alexa Fluor 568 (A-11011), both from Thermo Fisher. Nuclei were counterstained using DAPI. Slides were digitally scanned with Panoramic Flash 250 (3DHistech, Budapest, Hungary) using a ×20/0.8 NA objective, and stained cells were counted in five microscopic fields. Images were processed using MetaMorph version 7.8.2 (Molecular Devices).
Tumor microarray
Commercially available paraffin-embedded tissue array slides containing 79 human sarcomas and 4 human normal tissues (NBP2-30332; Novus Biologicals, and T242, US Biomax, Inc., USA) were purchased. Sections were deparaffinized, then incubated with antibodies recognizing human CD133 (MBS462020; MyBioSource), Nanog (ab80892; Abcam), and p-Akt1/2 (#9271; Cell Signaling) in a solution of PBS with 1% BSA and 0.1% Triton X-100 at 4 °C overnight. Staining was visualized using secondary antibodies tagged with Alexa Fluor 488 (A32766, A10042) and Alexa Fluor 594 (A32744, A32754), from Thermo Fisher, with nuclear counterstaining using DAPI. Images were collected on an inverted confocal microscope (Leica Microsystems) and processed using Imaris 7.6. Five fields were examined for each sample.
Statistical analysis
Statistical analyses were performed using Microsoft Office Excel 2010. P values were calculated using Student’s t-test. For comparisons between more than two groups, treatment groups were compared to the control using one-way ANOVA with the Bonferroni adjustment for multiple comparisons. Variance was assumed to similar between groups. All experiments were repeated independently at least twice. P values < 0.05 were considered significant. Sample sizes were using a sample size program (http://hedwig.mgh.harvard.edu/sample_size/size.html).
Supplementary information
Acknowledgements
This research was supported by the National Cancer Institute of the US National Institutes of Health through R01 CA158301 (to M.C.S.) and Cancer Center Support Grant P30 CA008748 (to M.S.K.), and by the DeGregorio Family Foundation (to S.S.Y.). We thank MSKCC senior editor Jessica Moore for reviewing this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41389-024-00519-0
Change history
5/29/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41389-024-00519-0
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41389-020-00300-z).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Billingsley KG, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann. Surg. 1999;229:602–610. doi: 10.1097/00000658-199905000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Glabbeke M, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens—a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 4.Allegra A, et al. The cancer stem cell hypothesis: a guide to potential molecular targets. Cancer Invest. 2014;32:470–495. doi: 10.3109/07357907.2014.958231. [DOI] [PubMed] [Google Scholar]
- 5.Izumiya M, et al. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32:3847–3853. [PubMed] [Google Scholar]
- 6.Tan BT, Park CY, Ailles LE, Weissman IL. The cancer stem cell hypothesis: a work in progress. Lab. Invest. 2006;86:1203–1207. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- 7.Terry J, Nielsen T. Expression of CD133 in synovial sarcoma. Appl. Immunohistochem. Mol. Morphol. 2010;18:159–165. doi: 10.1097/PAI.0b013e3181b77451. [DOI] [PubMed] [Google Scholar]
- 8.Feng BH, et al. CD133+ subpopulation of the HT1080 human fibrosarcoma cell line exhibits cancer stem-like characteristics. Oncol. Rep. 2013;30:815–823. doi: 10.3892/or.2013.2486. [DOI] [PubMed] [Google Scholar]
- 9.Xiao W, Mohseny AB, Hogendoorn PC, Cleton-Jansen AM. Mesenchymal stem cell transformation and sarcoma genesis. Clin. Sarcoma Res. 2013;3:10. doi: 10.1186/2045-3329-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dela Cruz FS. Cancer stem cells in pediatric sarcomas. Front. Oncol. 2013;3:168. doi: 10.3389/fonc.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon CH, et al. c-Jun N-terminal kinase has a pivotal role in the maintenance of self-renewal and tumorigenicity in glioma stem-like cells. Oncogene. 2012;31:4655–4666. doi: 10.1038/onc.2011.634. [DOI] [PubMed] [Google Scholar]
- 12.Ueda K, et al. Aldehyde dehydrogenase 1 identifies cells with cancer stem cell-like properties in a human renal cell carcinoma cell line. PLoS ONE. 2013;8:e75463. doi: 10.1371/journal.pone.0075463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon C, et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014;20:3974–3988. doi: 10.1158/1078-0432.CCR-14-0011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Torres-Padilla ME, Chambers I. Transcription factor heterogeneity in pluripotent stem cells: a stochastic advantage. Development. 2014;141:2173–2181. doi: 10.1242/dev.102624. [DOI] [PubMed] [Google Scholar]
- 15.Jeter CR, et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai W, et al. Prognostic evaluation of NANOG and OCT4 expression for posttransplantation hepatocellular carcinoma recurrence. J. Cell Biochem. 2019;120:8419–8429. doi: 10.1002/jcb.28128. [DOI] [PubMed] [Google Scholar]
- 17.Bien-Moller S, et al. Association of glioblastoma multiforme stem cell characteristics, differentiation, and microglia marker genes with patient survival. Stem Cells Int. 2018;2018:9628289. doi: 10.1155/2018/9628289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim EE, et al. Embryonic NANOG activity defines colorectal cancer stem cells and modulates through AP1- and TCF-dependent mechanisms. Stem Cells. 2012;30:2076–2087. doi: 10.1002/stem.1182. [DOI] [PubMed] [Google Scholar]
- 19.Ye T, et al. Nr5a2 promotes cancer stem cell properties and tumorigenesis in nonsmall cell lung cancer by regulating Nanog. Cancer Med. 2019;8:1232–1245. doi: 10.1002/cam4.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, Mazur SJ, Lin T, Appella E, Xu Y. The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene. 2014;33:2655–2664. doi: 10.1038/onc.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon C, et al. Multimodal targeting of tumor vasculature and cancer stem-like cells in sarcomas with VEGF-A inhibition, HIF-1alpha inhibition, and hypoxia-activated chemotherapy. Oncotarget. 2016;7:42844–42858. doi: 10.18632/oncotarget.10212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Chen H, et al. Erk signaling is indispensable for genomic stability and self-renewal of mouse embryonic stem cells. Proc. Natl Acad. Sci. USA. 2015;112:E5936–E5943. doi: 10.1073/pnas.1516319112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martelli AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in cancer stem cells. Curr. Med. Chem. 2011;18:2715–2726. doi: 10.2174/092986711796011201. [DOI] [PubMed] [Google Scholar]
- 24.Chang L, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivas, S., Gomez-Oro, C., Anton, I. M. & Wandosell F. Role of Akt isoforms controlling cancer stem cell survival, phenotype and self-renewal. Biomedicines6, 29 (2018). [DOI] [PMC free article] [PubMed]
- 26.Yoon C, et al. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol. Cancer Res. 2017;15:1106–1116. doi: 10.1158/1541-7786.MCR-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saygin C, Matei D, Majeti R, Reizes O, Lathia JD. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. 2019;24:25–40. doi: 10.1016/j.stem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Yoon C, et al. Chemotherapy resistance in diffuse-type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin. Cancer Res. 2016;22:971–983. doi: 10.1158/1078-0432.CCR-15-1356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Chang KK, et al. Platelet-derived growth factor receptor-alpha and -beta promote cancer stem cell phenotypes in sarcomas. Oncogenesis. 2018;7:47. doi: 10.1038/s41389-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Cancer Genome Atlas Research Network. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171:950–965. e928. doi: 10.1016/j.cell.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu JAD, et al. An integrated TCGA Pan-Cancer Clinical Data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–416. e411. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shern JF, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat. Rev. 2012;38:589–598. doi: 10.1016/j.ctrv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Yang CF, et al. Cancer stem-like cell characteristics induced by EB virus-encoded LMP1 contribute to radioresistance in nasopharyngeal carcinoma by suppressing the p53-mediated apoptosis pathway. Cancer Lett. 2014;344:260–271. doi: 10.1016/j.canlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 37.Yoon C, et al. Hypoxia-activated chemotherapeutic TH-302 enhances the effects of VEGF-A inhibition and radiation on sarcomas. Br. J. Cancer. 2015;113:46–56. doi: 10.1038/bjc.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 39.Ma S, et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7:694–707. doi: 10.1016/j.stem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Fang DD, et al. Expansion of CD133(+) colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br. J. Cancer. 2010;102:1265–1275. doi: 10.1038/sj.bjc.6605610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto O, et al. Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1alpha-dependent manner in pancreatic cancer cells. Pathobiology. 2011;78:181–192. doi: 10.1159/000325538. [DOI] [PubMed] [Google Scholar]
- 42.Noh KH, et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J. Clin. Invest. 2012;122:4077–4093. doi: 10.1172/JCI64057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TK, et al. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Jeter CR, Yang T, Wang J, Chao HP, Tang DG. Concise review: NANOG in cancer stem cells and tumor development: an update and outstanding questions. Stem Cells. 2015;33:2381–2390. doi: 10.1002/stem.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am. J. Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 46.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat. Rev. Cancer. 2003;3:685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 47.Tomita Y, et al. Prognostic significance of activated AKT expression in soft-tissue sarcoma. Clin. Cancer Res. 2006;12:3070–3077. doi: 10.1158/1078-0432.CCR-05-1732. [DOI] [PubMed] [Google Scholar]
- 48.Hernando E, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat. Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 49.Zhu QS, et al. Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45 alpha. Cancer Res. 2008;68:2895–2903. doi: 10.1158/0008-5472.CAN-07-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 51.Patel S. Exploring novel therapeutic targets in GIST: focus on the PI3K/Akt/mTOR pathway. Curr. Oncol. Rep. 2013;15:386–395. doi: 10.1007/s11912-013-0316-6. [DOI] [PubMed] [Google Scholar]
- 52.Mery B, et al. Biological aspects of chondrosarcoma: leaps and hurdles. Crit. Rev. Oncol./Hematol. 2018;126:32–36. doi: 10.1016/j.critrevonc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Setsu N, et al. Prognostic impact of the activation status of the Akt/mTOR pathway in synovial sarcoma. Cancer. 2013;119:3504–3513. doi: 10.1002/cncr.28255. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed AA, Sherman AK, Pawel BR. Expression of therapeutic targets in Ewing sarcoma family tumors. Hum. Pathol. 2012;43:1077–1083. doi: 10.1016/j.humpath.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Cen L, et al. PDK-1/AKT pathway as a novel therapeutic target in rhabdomyosarcoma cells using OSU-03012 compound. Br. J. Cancer. 2007;97:785–791. doi: 10.1038/sj.bjc.6603952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.UKCCR. UKCCCR guidelines for the use of cell lines in cancer research. Br. J. Cancer. 2000;82:1495–1509. doi: 10.1054/bjoc.1999.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon SS, et al. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.