Key Points
Question
Does the efficacy of varenicline, bupropion, and nicotine patch differ for US Black and White smokers, and what variables explain the difference?
Findings
In this secondary analysis of US participants in the EAGLES randomized clinical trial, Black smokers were less likely than White smokers to quit. All treatments had greater efficacy than placebo for White smokers, but only varenicline had greater efficacy than placebo for Black smokers; baseline, postbaseline, and safety characteristics did not explain the race difference in abstinence.
Meaning
Understanding variables (eg, socioeconomic, biological) beyond those explored that underlie Black vs White differences in pharmacotherapy efficacy may improve treatment outcomes.
This secondary analysis of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) randomized clinical trial compares the pharmacotherapy efficacy of varenicline, bupropion, and nicotine replacement therapy in quitting smoking for Black vs White US smokers.
Abstract
Importance
Understanding Black vs White differences in pharmacotherapy efficacy and the underlying reasons is critically important to reducing tobacco-related health disparities.
Objective
To compare pharmacotherapy efficacy and examine variables to explain Black vs White differences in smoking abstinence.
Design, Setting, and Participants
This study is a secondary analysis of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) double-blind, placebo-controlled, randomized clinical trial, which took place at clinical trial centers, academic centers, and outpatient clinics in 29 states in the US. US Black and White smokers who smoked 10 or more cigarettes per day with and without psychiatric comorbidity were enrolled between November 2011 and January 2015. Data analysis was performed from July 2019 to January 2020.
Interventions
Participants were randomized (1:1:1:1) in a double-blind, triple-dummy, placebo- and active-controlled (nicotine patch) trial of varenicline and bupropion for 12 weeks with follow-up through week 24.
Main Outcomes and Measures
Biochemically verified continuous cigarette abstinence rate (CAR) from weeks 9 to 24. Baseline, postbaseline treatment, and safety characteristics were examined as variables to explain race differences in abstinence.
Results
Of the 1065 Black smokers enrolled, 255 were randomized to receive varenicline, 259 received bupropion, 286 received nicotine replacement therapy (NRT [ie, nicotine patch]), and 265 received placebo. Among the 3044 White smokers enrolled, 778 were randomized to receive varenicline, 769 received bupropion, 738 received NRT, and 759 received placebo. Participants were predominantly female (614 Black [57.7%] and 1786 White [58.7%] women) and heavy smokers (mean [SD] cigarettes per day, 18.2 [7.9] for Black and 20.0 [7.5] for White smokers), with a mean (SD) age of 47.2 (11.2) years for Black and 46.5 (12.7) years for White participants. Treatment and race were associated with CAR for weeks 9 to 24. The CAR was 4.9% lower for Black vs White participants (odds ratio [OR], 0.53; 95% CI, 0.41-0.69; P < .001); differences were found across all treatments. Pooling psychiatric and nonpsychiatric cohorts, varenicline (OR, 2.63; 95% CI, 1.90-3.63; P < .001), bupropion (OR, 1.75; 95% CI, 1.25-2.46; P = .001), and NRT (OR, 1.52; 95% CI, 1.07-2.16; P = .02) had greater efficacy than placebo for White participants. Only varenicline (OR, 2.63; 95% CI, 1.26-5.48; P = .01) had greater efficacy than placebo for Black participants. Baseline, postbaseline, and safety characteristics differed by race, but these variables did not eliminate the association of race with CAR. Black participants had 49% reduced odds of CAR for weeks 9 to 24 compared with White participants in the adjusted model (OR, 0.51; 95% CI, 0.39-0.66; P < .001).
Conclusions and Relevance
Black and White smokers achieved the highest rate of abstinence while taking varenicline, suggesting that it is the best first-line therapy for these groups. However, Black smokers were less responsive to all therapies, including placebo. Understanding variables (eg, socioeconomic or biological) beyond those may lead to improved treatment outcomes for Black smokers.
Trial Registration
ClinicalTrials.gov Identifier: NCT01456936
Introduction
Tobacco remains the leading cause of preventable morbidity and mortality in the US, accounting for more than 480 000 total deaths and more than 34% of all cancer deaths annually.1 Although non-Hispanic Black individuals (referred to hereafter as Black) have a prevalence of smoking that is comparable to that of non-Hispanic White individuals (referred to hereafter as White), smoke fewer cigarettes per day (CPD), and smoke on fewer days of the month,2,3,4,5 they have higher rates of smoking-related morbidity and mortality.6,7,8
Racial/ethnic differences in quitting are not well understood.9 Cross-sectional and cohort studies3,10,11,12,13,14,15,16,17 consistently find that Black smokers are less likely than White smokers to quit despite Black smokers making more quit attempts. The results of randomized clinical trials (RCTs) have been more mixed, with approximately half finding no difference in cessation rates between Black and White smokers,18,19,20,21,22,23,24 half finding higher cessation rates among White smokers,25,26,27,28,29,30,31,32,33 and only 1 finding higher cessation rates among Black smokers.34 None of these RCTs examined variables to explain the race difference, leaving unanswered questions about why Black smokers experience lower cessation rates compared with White smokers. Secondary analyses of Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES), the largest and most comprehensive smoking cessation study to date, also found that Black smokers were significantly less likely than White smokers to quit across all treatments, including varenicline, bupropion, nicotine replacement therapy (NRT [ie, nicotine patch]), and placebo, but did not explore reasons to explain the race difference in abstinence.33
Elucidating variables that explain racial/ethnic differences in abstinence is critically important to reducing tobacco-related health disparities.5 Most studies examine barriers and facilitators to abstinence in Black and White smokers separately.35,36,37,38,39,40 This contributes to knowledge about the variables associated with quitting for Black smokers and for White smokers, including variables such as CPD, nicotine dependence, and nicotine metabolism,11,18,41,42,43,44,45,46,47,48,49,50,51,52,53 but does little to explain why Black vs White differences in abstinence exist. A recent study by Nollen et al,31 prospectively designed to examine this question, found that Black smokers achieved lower quit rates than White smokers when provided the same treatment of varenicline in combination with smoking cessation counseling.31 Preplanned secondary analyses demonstrated that the difference in abstinence was not associated with race or biological differences in nicotine metabolism, but rather was explained by socioeconomic factors, treatment process, and smoking characteristics. A better understanding of variables associated with race that make it harder for Black smokers in the US to quit smoking compared with White smokers is needed to reduce tobacco-related health disparities.
EAGLES was a large, multicenter, placebo-controlled RCT examining the safety and efficacy of varenicline, bupropion, and NRT for smoking cessation.25 The size, design, and number of Black and White smokers enrolled provide an opportunity to assess variables to explain race-related differences in quitting. The present article reports post hoc secondary analyses that (1) compare the efficacy of varenicline, bupropion, NRT, and placebo between and within US Black and White smokers; and (2) examine baseline, postbaseline, and safety characteristics associated with race to explain the Black vs White difference in abstinence.
Methods
Design
EAGLES was a randomized (1:1:1:1), double-blind, triple-dummy, placebo-controlled and active-controlled (NRT; 21 mg per day with taper) trial of varenicline (1 mg twice daily) and bupropion (150 mg twice daily) for 12 weeks with 12-week nontreatment follow-up. The published trial report provides full details of study design and outcomes.25 The trial protocol is available in Supplement 1. Reporting follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized studies. Written consent forms and study procedures were approved by the institutional review boards or ethics committees at participating institutions.
Participants
Participants in EAGLES were 8144 male and female smokers, aged 18 to 75 years, with and without prespecified current or lifetime psychiatric diagnoses, who smoked 10 or more CPD. They were recruited from 16 countries, including the US, between November 2011 and January 2015. A list of participating study sites was previously published.25 The sample included smokers meeting diagnostic criteria for psychotic, anxiety, mood, and borderline personality disorders per structured clinical interview, with stable treatment and no imminent risk of self-harm.54
Most EAGLES participants (95.9%) were either White or Black. Race was self-identified by the participant. Yet with 52.3% of participants enrolled in the US, there was a race imbalance for US vs non-US participants (US, 71.5% White vs 25.0% Black participants; non-US, 92.8% White vs 2.5% Black participants). To avoid potential confounding issues, the current analyses restricted inclusion to US White or Black participants. The sample was not powered, a priori, to detect differences in efficacy associated with race and possible interactions with treatment group or cohort.
Measures
Outcomes
As per the Russell Standard, the continuous cigarette abstinence rate (CAR) for weeks 9 to 24 was used as the primary end point here because longer periods of abstinence are more strongly associated with lifetime abstinence.55 The CAR for weeks 9 to 12 and 7-day point prevalence abstinence (PPA) each week from weeks 0 to 24 were also explored. Participants were considered abstinent if they self-reported cigarette abstinence throughout the specified period in conjunction with exhaled carbon monoxide concentration less than 10 ppm. Missing self-reported data were classified as smokers.55
Variables Explored
Demographic characteristics included sex, age, and body mass index (calculated as weight in kilograms divided by height in meters squared). Smoking characteristics included CPD in the month prior to enrollment, cigarette dependence (Fagerström Test for Cigarette Dependence),56 age of starting smoking, length of time as smoker, and lifetime serious quit attempts (yes or no and number). Treatment process characteristics included prior use of varenicline, bupropion, or NRT (none vs any) and history of suicidal ideation and/or behavior (none vs any), captured through the Columbia-Suicide Severity Rating Scale.57 Psychosocial characteristics included history of a psychiatric diagnosis (defined as the primary diagnosis: none, mood disorder, anxiety disorder, psychotic disorder, or borderline personality disorder), alcohol or substance abuse disorder via structured clinical interview,54 anxiety score (Hospital Anxiety and Depression Scale),58 depression score (Hospital Anxiety and Depression Scale),58 aggression score (Buss-Perry Aggression Questionnaire),59 and use of psychotropic medication, including sleep aids (none vs any).
Postbaseline characteristics included discontinuation from the study, discontinuation from the study drug, lost to follow-up, days’ exposure to study drug, treatment compliance (80% or 68-day threshold), change in anxiety and depression during the treatment period, treatment-emergent composite neuropsychiatric end point, and mild, moderate, or severe adverse events (AEs). Measurement and computation of postbaseline characteristics have been defined in detail in Anthenelli et al25 and are included in eTable 1 in Supplement 2.
Statistical Analysis
Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute). Tests and 95% CIs are 2-sided and use a 5% level of significance (eg, stepwise regression entry and stay thresholds were P < .05) except for candidate covariate assessment, which invoked a nominal P < .10 rule.
For weeks 9 to 24 (and separately for weeks 9 to 12), the CAR was analyzed via logistic regression with a fully crossed model of treatment group, psychiatric cohort, and race to produce odds ratio (OR) and 95% CI summaries. An analogous general linear model (with identity link) yielded risk difference (RD) and 95% CI summaries.
Covariate inclusion was a multistep process, where first a simple χ2 test (categorical case) or t test (numerical case) compared a potential variable (1) across race and (2) across cigarette abstinence. Variables that differed by both race and abstinence (P < .10 for each) were candidate variables for inclusion in the subsequent stepwise logistic regression analysis, as was race and each race-by-candidate interaction. Treatment and cohort were forced inclusion terms as study design factors. The identified pool of candidate variables was assessed in a stepwise fashion. Stepwise logistic regression was performed separately for baseline variables and for postbaseline variables (the latter being anecdotal in nature). Finally, for each cigarette abstinence end point, the included covariates from these 2 models became candidates in another stepwise logistic regression.
Seven-day PPA and CPD among continuing smokers were examined for Black and White participants in a descriptive fashion by treatment and psychiatric cohort. The presented analyses were performed July 2019 to January 2020, were not preplanned, and should be considered exploratory.
Results
Participant Characteristics
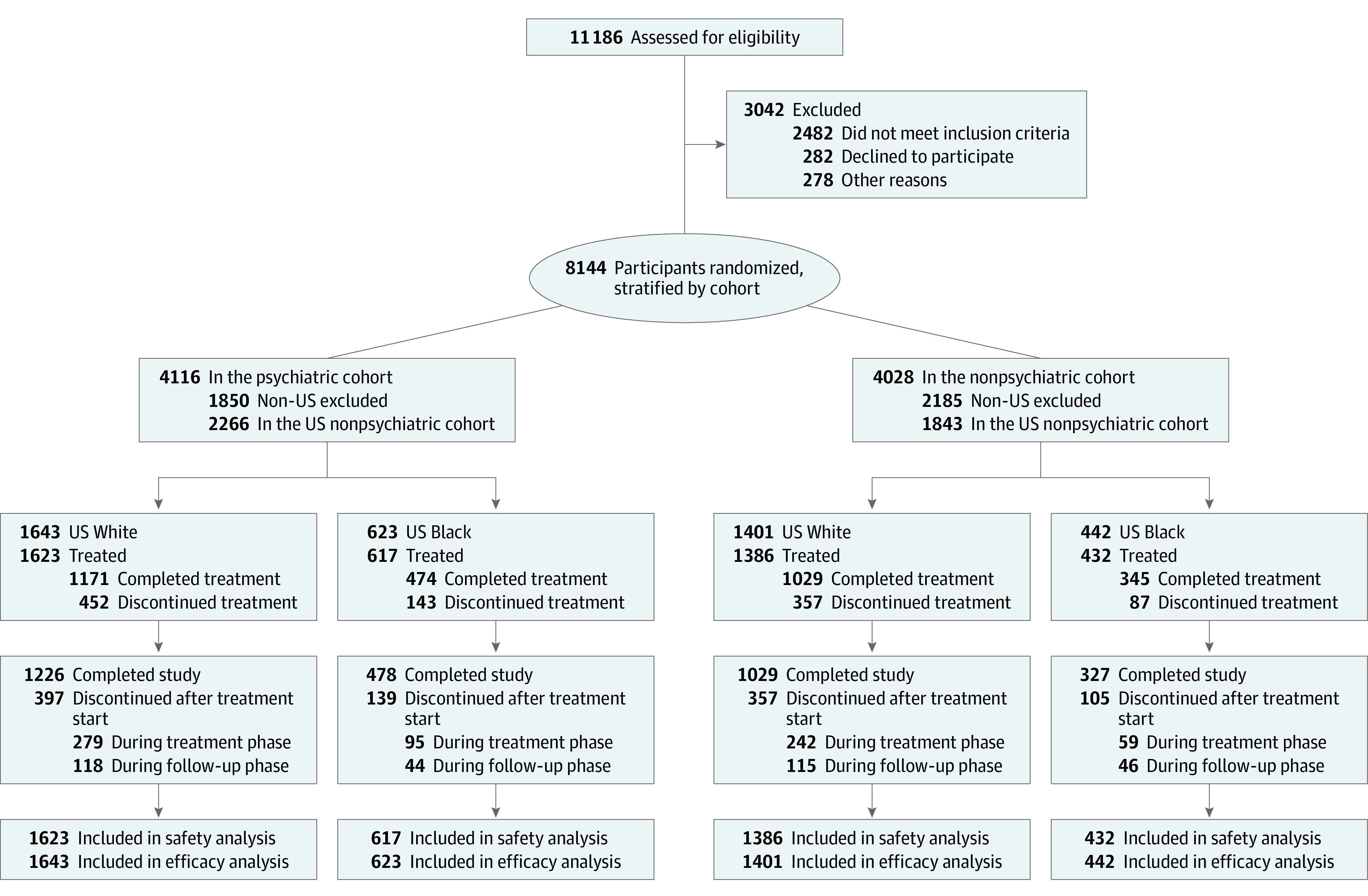
This study included 1065 US Black participants (255 receiving varenicline, 259 receiving bupropion, 286 receiving NRT, and 265 receiving placebo) and 3044 US White participants (778 receiving varenicline, 769 receiving bupropion, 738 receiving NRT, and 759 receiving placebo) with and without psychiatric disorders (Figure 1). Characteristics of the sample are shown in eTable 1 in Supplement 2. Participants were predominantly female (614 Black [57.7%] and 1786 White [58.7%] women) and heavy smokers (mean [SD] CPD, 18.2 [7.9] for Black participants and 20.0 [7.5] for White participants), with a mean (SD) age of 47.2 (11.2) years for Black participants and 46.5 (12.7) years for White participants.
Figure 1. CONSORT Flow Diagram.
Cigarette Abstinence
Only treatment and race were associated with CAR for weeks 9 to 24 (eTable 2 in Supplement 2). The CAR for weeks 9 to 24 was 12.5% (382 of 3044) for White participants and 7.0% (75 of 1065) for Black participants. The absolute difference in CAR for weeks 9 to 24 was 4.9% less for Black participants compared with White participants (OR, 0.53; 95% CI, 0.41-0.69; P < .001) (eTable 3 in Supplement 2). Figure 2 illustrates CAR for weeks 9 to 24 for race by treatment and cohort. Pooling psychiatric cohorts, the CAR for weeks 9 to 24 for Black participants compared with White participants was 10.3% vs 18.1% for varenicline (OR, 0.52; 95% CI, 0.33-0.82, P = .004), 5.8% vs 12.9% for bupropion (OR, 0.42; 95% CI, 0.24-0.74, P = .003), 7.9% vs 11.4% for NRT (OR, 0.67; 95% CI, 0.41-1.09, P = .11), and 4.2% vs 7.8% for placebo (OR, 0.52; 95% CI, 0.27-1.01; P = .05). For the psychiatric cohort only, the ORs for Black participants compared with White participants were 0.40 (95% CI, 0.20-0.79; P = .009) for varenicline, 0.40 (95% CI, 0.19-0.83; P = .01) for bupropion, 0.53 (95% CI, 0.26-1.05; P = .07) for NRT, and 0.48 (95% CI, 0.20-1.17; P = .11) for placebo. For the nonpsychiatric cohort only, the ORs for Black participants compared with White participants were 0.68 (95% CI, 0.38-1.21; P = .19) for varenicline, 0.44 (95% CI, 0.18-1.07; P = .07) for bupropion, 0.84 (95% CI, 0.42-1.71; P = .64) for NRT, and 0.56 (95% CI, 0.21-1.50; P = .25) for placebo.
Figure 2. Continuous Cigarette Abstinence Rates for Weeks 9 to 24 for Black and White Smokers by Treatment, Cohort, and Overall .
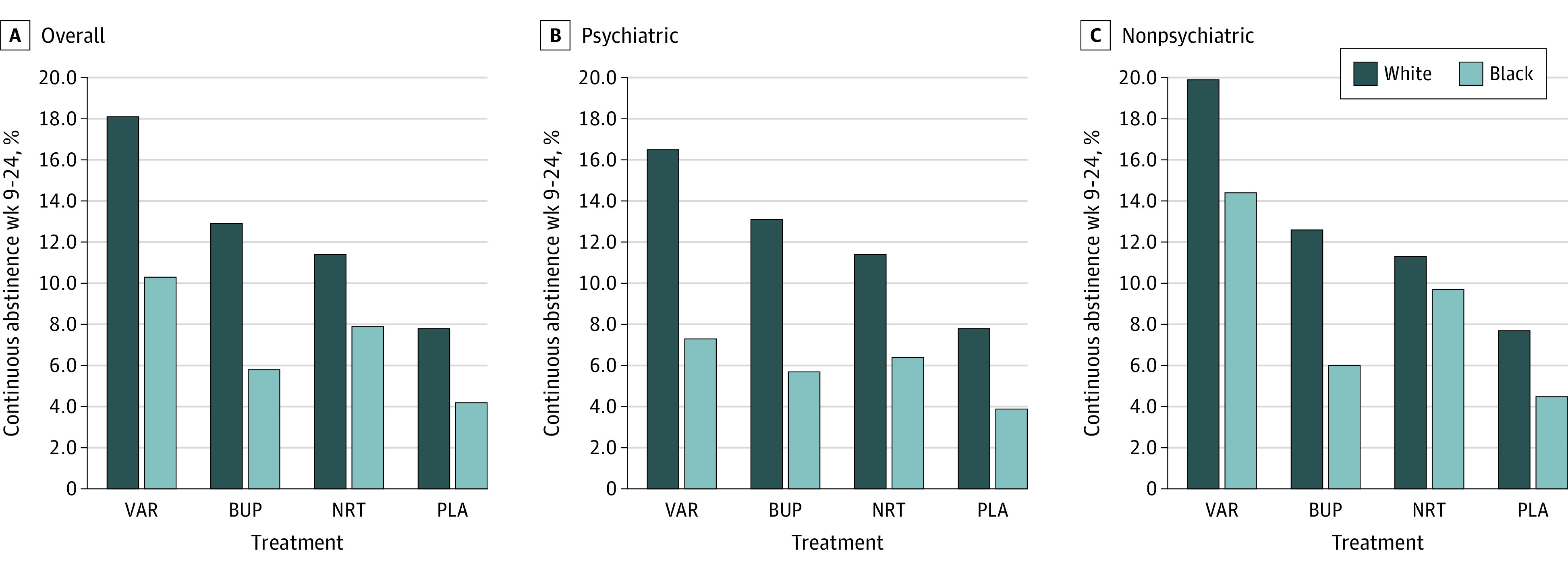
BUP indicates bupropion; NRT, nicotine replacement therapy; PLA, placebo; and VAR, varenicline.
Similarly, the CAR for weeks 9 to 12 was 19.2% (585 of 3044) for White participants and 11.6% (124 of 1065) for Black participants. The absolute difference in CAR for weeks 9 to 12 was 6.6% less for Black participants compared with White participants (OR, 0.56; 95% CI, 0.45-0.69; P < .001) (eTable 3 in Supplement 2). At the end of treatment, cohort was also associated with CAR for weeks 9 to 12. Figure 3 presents abstinence by cohort. For the pooled cohorts, the ORs for Black participants compared with White participants were 0.54 (95% CI, 0.38-0.76; P < .001) for varenicline, 0.43 (95% CI, 0.27-0.69; P < .001) for bupropion, 0.73 (95% CI, 0.50-1.08; P = .12) for NRT, and 0.50 (95% CI, 0.28-0.90; P = .02) for placebo. For the psychiatric cohort only, the ORs for Black participants compared with White participants were 0.58 (95% CI, 0.36-0.96; P = .03) for varenicline, 0.30 (95% CI, 0.15-0.59; P < .001) for bupropion, 0.52 (95% CI, 0.30-0.91; P = .02) for NRT, and 0.64 (95% CI, 0.32-1.27; P = .20) for placebo. For the nonpsychiatric cohort only, the ORs for Black participants compared with White participants were 0.49 (95% CI, 0.30-0.82; P = .006) for varenicline, 0.64 (95% CI, 0.33-1.20; P = .16) for bupropion, 1.03 (95% CI, 0.60-1.78; P = .91) for NRT, and 0.39 (95% CI, 0.15-1.01; P = .05) for placebo.
Figure 3. Continuous Cigarette Abstinence Rates for Weeks 9 to 12 for Black and White Smokers by Treatment, Cohort, and Overall.
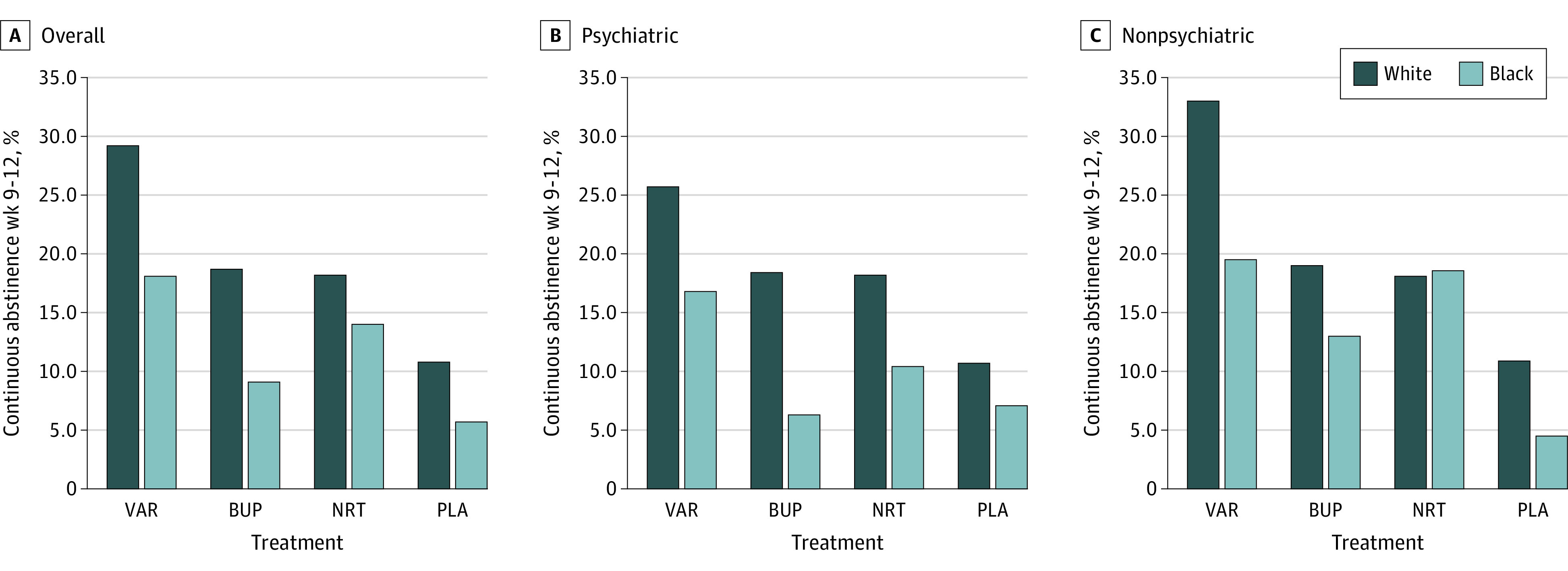
BUP indicates bupropion; NRT, nicotine replacement therapy; PLA, placebo; and VAR, varenicline.
Comparison of the relative efficacy of treatments within race is shown in Table 1. Among White participants and pooling psychiatric cohorts, varenicline resulted in significantly higher odds of CAR for weeks 9 to 24 than bupropion (OR, 1.50; 95% CI, 1.14-1.99; P = .004), NRT (OR, 1.73; 95% CI, 1.29-2.31; P < .001), and placebo (OR, 2.63; 95% CI, 1.90-3.63; P < .001); compared with placebo, bupropion (OR, 1.75; 95% CI, 1.25-2.46; P = .001) and NRT (OR, 1.52; 95% CI, 1.07-2.16; P = .02) resulted in significantly higher odds of quitting. The relative efficacy of bupropion vs NRT did not differ for White participants (OR, 1.15; 95% CI, 0.84-1.57; P = .38). Among Black participants and pooling across psychiatric cohorts, varenicline resulted in significantly higher odds of CAR for weeks 9 to 24 than placebo (OR, 2.63; 95% CI, 1.26-5.48; P = .01), but no significant differences were seen in the relative efficacy of varenicline vs bupropion or NRT, bupropion and NRT vs placebo, and bupropion vs NRT.
Table 1. Comparison of the Relative Efficacy of Treatments via RDs and ORs for White and Black Smokers Overall and by Psychiatric Cohort.
| Variable | Overall | Psychiatric cohort | Nonpsychiatric cohort | |||
|---|---|---|---|---|---|---|
| White smokers | Black smokers | White smokers | Black smokers | White smokers | Black smokers | |
| CAR wk 9-24 | ||||||
| VAR vs BUP | ||||||
| RD, % (95% CI) | 5.3 (1.7 to 9.0) | 5.0 (0.2 to 9.9) | 3.4 (–1.4 to 8.2) | 1.6 (–4.0 to 7.3) | 7.3 (1.8 to 12.7) | 8.4 (0.5 to 16.3) |
| Pa | .004 | .04 | .16 | .57 | .009 | .04 |
| OR (95% CI) | 1.50 (1.14 to 1.99) | 1.86 (0.95 to 3.65) | 1.31 (0.90 to 1.92) | 1.31 (0.52 to 3.33) | 1.72 (1.14 to 2.59) | 2.64 (1.00 to 6.97) |
| Pb | .004 | .07 | .16 | .57 | .009 | .05 |
| VAR vs NRT | ||||||
| RD, % (95% CI) | 6.8 (3.2 to 10.4) | 2.8 (–2.2 to 7.9) | 5.1 (0.4 to 9.8) | 0.9 (–4.7 to 6.6) | 8.6 (3.2 to 13.9) | 4.7 (–3.7 to 13.0) |
| Pa | <.001 | .28 | .04 | .75 | .002 | .27 |
| OR (95% CI) | 1.73 (1.29 to 2.31) | 1.35 (0.74 to 2.45) | 1.53 (1.02 to 2.29) | 1.16 (0.48 to 2.82) | 1.94 (1.27 to 2.96) | 1.56 (0.70 to 3.50) |
| Pb | <.001 | .33 | .04 | .74 | .002 | .28 |
| VAR vs PLA | ||||||
| RD, % (95% CI) | 10.4 (7.1 to 13.8) | 6.7 (2.1 to 11.2) | 8.7 (4.4 to 13.1) | 3.4 (–1.9 to 8.7) | 12.1 (7.1 to 17.2) | 9.9 (2.5 to 17.3) |
| Pa | <.001 | .004 | <.001 | .21 | <.001 | .009 |
| OR (95% CI) | 2.63 (1.90 to 3.63) | 2.63 (1.26 to 5.48) | 2.34 (1.50 to 3.63) | 1.94 (0.69 to 5.49) | 2.96 (1.84 to 4.75) | 3.57 (1.27 to 10.0) |
| Pb | <.001 | .01 | <.001 | .21 | <.001 | .02 |
| BUP vs NRT | ||||||
| RD, % (95% CI) | 1.5 (–1.8 to 4.8) | –2.2 (–6.6 to 2.2) | 1.7 (–2.9 to 6.2) | –0.7 (–5.8 to 4.4) | 1.3 (–3.5 to 6.0) | –3.7 (–10.9 to 3.4) |
| Pa | .38 | .32 | .47 | .79 | .60 | .31 |
| OR (95% CI) | 1.15 (0.84 to 1.57) | 0.72 (0.36 to 1.44) | 1.17 (0.76 to 1.79) | 0.88 (0.36 to 2.19) | 1.13 (0.72 to 1.78) | 0.59 (0.21 to 1.66) |
| Pb | .38 | .36 | .47 | .79 | .60 | .32 |
| BUP vs PLA | ||||||
| RD, % (95% CI) | 5.1 (2.0 to 8.1) | 1.6 (–2.2 to 5.5) | 5.3 (1.1 to 9.5) | 1.8 (–3.0 to 6.5) | 4.9 (0.4 to 9.3) | 1.5 (–4.6 to 7.5) |
| Pa | .001 | .41 | .01 | .46 | .03 | .63 |
| OR (95% CI) | 1.75 (1.25 to 2.46) | 1.42 (0.63 to 3.17) | 1.78 (1.12 to 2.82) | 1.48 (0.51 to 4.26) | 1.72 (1.04 to 2.84) | 1.35 (0.40 to 4.58) |
| Pb | .001 | .40 | .01 | .47 | .03 | .63 |
| NRT vs PLA | ||||||
| RD, % (95% CI) | 3.6 (0.6 to 6.6) | 3.8 (–0.3 to 7.9) | 3.6 (–0.5 to 7.7) | 2.5 (–2.3 to 7.2) | 3.6 (–0.7 to 7.9) | 5.2 (–1.5 to 11.9) |
| Pa | .02 | .07 | .08 | .31 | .10 | .13 |
| OR (95% CI) | 1.52 (1.07 to 2.16) | 1.96 (0.93 to 4.13) | 1.52 (0.94 to 2.46) | 1.67 (0.60 to 4.64) | 1.52 (0.91 to 2.54) | 2.29 (0.77 to 6.81) |
| Pb | .02 | .08 | .08 | .32 | .11 | .14 |
| CAR wk 9-12 | ||||||
| VAR vs BUP | ||||||
| RD, % (95% CI) | 10.6 (6.4 to 14.9) | 8.5 (2.4 to 14.6) | 7.2 (1.7 to 12.8) | 10.5 (3.2 to 17.8) | 14.0 (7.6 to 20.4) | 6.5 (–3.2 to 16.2) |
| Pa | <.001 | .006 | .01 | .005 | <.001 | .19 |
| OR (95% CI) | 1.79 (1.41 to 2.27) | 2.21 (1.29 to 3.78) | 1.53 (1.10 to 2.12) | 3.01 (1.38 to 6.57) | 2.10 (1.48 to 2.97) | 1.62 (0.77 to 3.39) |
| Pb | <.001 | .004 | .01 | .006 | <.001 | .20 |
| VAR vs NRT | ||||||
| RD, % (95% CI) | 11.2 (6.9 to 15.5) | 3.6 (–2.7 to 10.0) | 7.5 (1.9 to 13.1) | 6.4 (–1.4 to 14.1) | 14.9 (8.5 to 21.3) | 0.9 (–9.2 to 11.0) |
| Pa | <.001 | .26 | .009 | .11 | <.001 | .86 |
| OR (95% CI) | 1.86 (1.46 to 2.38) | 1.36 (0.85 to 2.16) | 1.56 (1.11 to 2.18) | 1.74 (0.90 to 3.37) | 2.23 (1.57 to 3.17) | 1.06 (0.55 to 2.05) |
| Pb | <.001 | .20 | .01 | .10 | <.001 | .86 |
| VAR vs PLA | ||||||
| RD, % (95% CI) | 18.6 (14.6 to 22.5) | 12.3 (6.8 to 17.8) | 15.0 (9.9 to 20.0) | 9.6 (2.2 to 17.1) | 22.2 (16.2 to 28.1) | 15.0 (6.9 to 23.1) |
| Pa | <.001 | <.001 | <.001 | .01 | <.001 | <.001 |
| OR (95% CI) | 3.41 (2.58 to 4.50) | 3.67 (1.95 to 6.89) | 2.88 (1.97 to 4.20) | 2.62 (1.23 to 5.61) | 4.04 (2.69 to 6.06) | 5.13 (1.88 to 14.0) |
| Pb | <.001 | <.001 | <.001 | .01 | <.001 | .001 |
| BUP vs NRT | ||||||
| RD, % (95% CI) | 0.6 (–3.3 to 4.5) | –4.8 (–10.5 to 0.8) | 0.3 (–5.1 to 5.6) | –4.1 (–10.0 to 1.8) | 0.9 (–4.8 to 6.6) | –5.6 (–15.3 to 4.2) |
| Pa | .77 | .10 | .92 | .17 | .75 | .26 |
| OR (95% CI) | 1.04 (0.80 to 1.35) | 0.62 (0.35 to 1.07) | 1.02 (0.71 to 1.46) | 0.58 (0.26 to 1.29) | 1.06 (0.73 to 1.55) | 0.65 (0.31 to 1.39) |
| Pb | .77 | .08 | .92 | .18 | .75 | .27 |
| BUP vs PLA | ||||||
| RD, % (95% CI) | 7.9 (4.4 to 11.5) | 3.8 (–0.9 to 8.5) | 7.7 (2.9 to 12.5) | –0.9 (–6.4 to 4.7) | 8.2 (2.9 to 13.4) | 8.5 (0.9 to 16.1) |
| Pa | <.001 | .11 | .002 | .76 | .002 | .03 |
| OR (95% CI) | 1.90 (1.42 to 2.55) | 1.66 (0.83 to 3.33) | 1.88 (1.26 to 2.81) | 0.87 (0.36 to 2.12) | 1.93 (1.26 to 2.95) | 3.17 (1.09 to 9.23) |
| Pb | <.001 | .15 | .002 | .76 | .003 | .04 |
| NRT vs PLA | ||||||
| RD, % (95% CI) | 7.3 (3.8 to 10.9) | 8.7 (3.6 to 13.8) | 7.5 (2.6 to 12.3) | 3.3 (–2.8 to 9.4) | 7.2 (2.1 to 12.4) | 14.1 (5.9 to 22.2) |
| Pa | <.001 | <.001 | .003 | .30 | .006 | <.001 |
| OR (95% CI) | 1.83 (1.36 to 2.46) | 2.70 (1.42 to 5.13) | 1.85 (1.23 to 2.77) | 1.51 (0.69 to 3.31) | 1.81 (1.18 to 2.79) | 4.84 (1.75 to 13.3) |
| Pb | <.001 | .002 | .003 | .30 | .007 | .002 |
Abbreviations: BUP, bupropion; CAR, continuous cigarette abstinence rate; NRT, nicotine replacement therapy (transdermal nicotine patch); OR, odds ratio; PLA, placebo; RD, risk difference; VAR, varenicline.
P value for RD.
P value for OR.
Differences in Baseline and Postbaseline Variables by Race and CAR
Baseline age, heaviness of smoking, cigarette dependence, age of smoking initiation, lifetime (serious) quit attempts, psychiatric diagnosis, aggression, anxiety, and depression scores differed between Black participants and White participants and were also associated with CAR for weeks 9 to 24 (eTable 1 in Supplement 2). Postbaseline differences demonstrated that Black participants were less likely to be discontinued from the study drug, had greater study drug compliance overall and days of exposure to the study drug, greater reduction in depression, and fewer psychiatric and nonpsychiatric AEs over the study period (eTable 1 in Supplement 2). Of these postbaseline variables, all but neuropsychiatric AEs were also associated with CAR for weeks 9 to 24.
Variables Associated With CAR
Results of the baseline and postbaseline variables, modeled separately, are shown in eTable 4 in Supplement 2. Results of the final stepwise logistic regression of baseline and postbaseline variables that together modeled CAR for weeks 9 to 24 are shown in Table 2. The effect of race was not statistically eliminated from the model, with Black participants having a 49% reduced odds of abstinence compared with White participants (OR, 0.51; 95% CI, 0.39-0.66; P < .001). Other variables that decreased the likelihood of abstinence included discontinuation from the study drug (OR, 0.27; 95% CI, 0.19-0.37; P < .001), greater cigarette dependence (OR, 0.91; 95% CI, 0.86-0.97; P < .001), heavier smoking (OR, 0.97; 95% CI, 0.95-0.99; P < 0.001), higher baseline anxiety (OR, 0.94; 95% CI, 0.90-0.97; P < .001), and an increase in depression over time (OR, 0.89; 95% CI, 0.85-0.93; P < .001). Older age (OR, 1.02; 95% CI, 1.01-1.03; P < .001), lifetime quit attempts (OR, 1.77; 95% CI, 1.20-2.62; P = .002), and experiencing more treatment-related AEs (OR, 1.03; 95% CI, 1.00-1.06; P = .02) increased the likelihood of abstinence. A nearly identical pattern was found in the modeling of CAR for weeks 9 to 12 (eTable 5, eTable 6, and eTable 7 in Supplement 2); race remained in the model, with Black participants having a 47% reduced odds of abstinence compared with White participants (OR, 0.53; 95% CI, 0.43-0.67; P < .001).
Table 2. Final Stepwise Logistic Regression Modeling of Continuous Cigarette Abstinence Rates for Weeks 9 to 24 With Baseline and Postbaseline Factorsa.
| Factors | OR (95% CI) | P value |
|---|---|---|
| Discontinued from study drug | ||
| No | [Reference] | |
| Yes | 0.27 (0.19-0.37) | <.001 |
| Cigarette dependence (Fagerström Test for Cigarette Dependence) | 0.91 (0.86-0.97) | <.001 |
| Race | ||
| White | [Reference] | |
| Black | 0.51 (0.39-0.66) | <.001 |
| Change in depression (HADS) | 0.89 (0.85-0.93) | <.001 |
| Age | 1.02 (1.01-1.03) | <.001 |
| Cigarettes per day in the past month | 0.97 (0.95-0.99) | <.001 |
| Anxiety (HADS) | 0.94 (0.90-0.97) | <.001 |
| Lifetime quit attempts | ||
| No | [Reference] | |
| Yes | 1.77 (1.20-2.62) | .002 |
| No. of mild, moderate, or severe treatment-emergent adverse events | 1.03 (1.00-1.06) | .02 |
Abbreviations: HADS, Hospital Anxiety and Depression Scale; OR, odds ratio.
Baseline and postbaseline factors associated with race and abstinence at P < .10 were modeled jointly. The main effects of race, each factor, and the race by factor interaction subsequently entered in a stepwise fashion. Only factors associated with abstinence at P < .05 were retained in the final model. The model is adjusted for treatment group and cohort (psychiatric, nonpsychiatric), which were entered and retained in the model because they were inherent in the study design.
PPA and CPD
Because prior studies have found that PPA increases over time with varenicline,60,61 we explored the time course in 7-day self-reported PPA and cigarettes per day in a given week by race within treatment and cohort (eFigure 1 and eFigure 2 in Supplement 2). Seven-day PPA mirrored continuous abstinence, with generally higher rates of abstinence among White participants and the nonpsychiatric cohorts. Among the varenicline-treated group, abstinence peaked between weeks 9 and 13 and stayed the same or declined from week 13 to week 23, whereas a posttreatment benefit of NRT emerged in the Black nonpsychiatric cohort.
Discussion
This study confirmed prior Black-White differences in abstinence reported in RCTs.25,26,27,28,29,30,31,32,33 Black participants were significantly less likely to quit overall, irrespective of treatment or placebo, although differences in quit rates were somewhat greater for varenicline and bupropion at long-term follow-up than for NRT or placebo. Differences in abstinence between Black and White smokers do not appear to be a pharmacological interaction in that the same effect is seen with placebo. Differences are similar to those reported in previous studies,25,26,27,28,29,30,31,32,33 and should not be interpreted to mean that pharmacotherapies are not effective for Black smokers but rather that, on average, Black smokers have lower rates of quitting on these therapies compared with White smokers. Rates of abstinence were lower across all treatments than those reported in prior studies.26,29,31,43,48,62,63,64 Lower rates of abstinence are likely due to inclusion of a large psychiatric cohort, lower-intensity behavioral counseling, and the use of the more stringent continuous abstinence vs PPA measure.65
All active treatments had greater efficacy than placebo for White smokers at long-term follow-up (week 24) but only varenicline had greater relative efficacy than placebo for Black smokers. To our knowledge, this is the first comparison of the relative efficacy of 3 active treatments and placebo by race in a single study, with the data indicating that varenicline appears to be the best first-line treatment for both Black and White smokers. This is consistent with previous research that suggests that varenicline is the most effective of available smoking cessation pharmacotherapies.66
Lack of difference in the long-term relative efficacy of bupropion and NRT to placebo in Black smokers should be interpreted in light of the time frame (short or long term) and cohort (psychiatric or nonpsychiatric). Among Black smokers in the nonpsychiatric cohort, both bupropion and NRT had significantly higher relative efficacy than placebo during active treatment (weeks 9-12). Specifically, Black smokers in the nonpsychiatric cohort had 3 times the odds of quitting while taking bupropion vs placebo and nearly 5 times the odds of quitting with NRT vs placebo from weeks 9 to 12. These findings suggest that long-term pharmacotherapy (>12 weeks), particularly bupropion and NRT, may be important for Black smokers given that the effects of these therapies diminished at the end of treatment and were not significantly different from placebo at long-term follow-up. Similar relapse effects have been seen with Black smokers taking bupropion in previous trials.43 Extended treatment has been found to be effective in White smokers but is understudied in Black smokers.60,63,67,68 Future research is needed in this area. Little is known about pharmacotherapy efficacy in Black smokers with psychiatric comorbidities. Findings from this study suggest that this group may be particularly recalcitrant to treatment and that it is an important group for future studies.
Race remained significantly associated with short- and long-term abstinence after accounting for variables that differed by race. Race differences in abstinence may be explained by variables not measured in EAGLES. The study by Nollen et al31 evaluated many of the same smoking, treatment, and psychosocial variables but also included comprehensive measures of socioeconomic and biological characteristics; analyses found that socioeconomic variables, namely home ownership, income, and neighborhood disadvantage, in addition to visit attendance and cotinine levels, explained the Black-White difference in abstinence among varenicline-treated smokers. Socioeconomically disadvantaged adults are less likely to achieve abstinence,5,69 and Black smokers in the US are more socioeconomically disadvantaged than White smokers.41,47,50,51,52,70,71,72,73,74,75,76,77,78,79,80 Socioeconomic variables were not measured in EAGLES and, therefore, we cannot speculate about their impact, but results of Nollen et al31 and other studies26,33,73,81 suggest that socioeconomic variables should be considered within the context of clinical trials. Addressing the pathways linking socioeconomic status to cessation (ie, stress, coping, psychological distress) has been found to attenuate the impact of socioeconomic status on abstinence and could be particularly important for the most socioeconomically disadvantaged smokers.41
Limitations
Several limitations warrant consideration. EAGLES was not designed or powered to examine race differences in abstinence. The large sample size allowed us to detect significant effects that may lack clinical relevance. It is unlikely, however, that a smoking cessation pharmacotherapy study of the size and scope of EAGLES will be conducted any time in the future and, therefore, limitations must be considered within the context of this strength. The results are also hypothesis-generating and point to important areas of future study regarding the relative efficacy of smoking cessation pharmacotherapy for Black participants and White participants and the need to prospectively design studies to examine variables underlying Black-White differences in abstinence. In addition to socioeconomic status, other key covariates known to be associated with abstinence, including menthol,42,45,46,49,82 nicotine metabolism,48,83,84,85,86 and social and environmental contexts associated with racism, discrimination, and increased stress50,70,71,72,73,74,75,76,77,78,79,80 were not assessed and may have contributed to our inability to explain the race difference in abstinence. Information on history, treatment, or level of care for psychiatric comorbidities was not assessed in EAGLES. Black participants and continued smokers had greater likelihood of a psychiatric diagnosis, but this candidate variable did not enter models estimating abstinence. Furthermore, follow-up was limited to 24 weeks, the sample was composed of moderate to heavy smokers and is not representative of most US Black smokers, and findings may have limited generalization to non-US Black participants and White participants.
Conclusions
US Black smokers in the EAGLES study were significantly less likely to quit than White smokers. Lower rates of abstinence were not explained by age, heaviness of smoking, treatment-related AEs, reduction in depression, and study drug compliance, which all favored Black smokers. To advance the important public health goal of reducing the prevalence of cigarette smoking, researchers and clinicians need to be aware that race is a proxy for powerful social and contextual factors that shape all aspects of life, including the likelihood of quitting smoking. Studies that identify race as a variable associated with abstinence do little to advance the field. Rather, prospectively designed studies are needed to identify the variables associated with race that make it harder for Black smokers to quit.
Trial Protocol
eTable 1. Relation of Demographic, Smoking, Treatment Process, and Psychosocial Variables With Race and Continuous Abstinence Rates Weeks 9-24 for the Sample Overall
eTable 2. General Linear Regression Models Examining the Effects of Psychiatric Cohort, Treatment Group, Race, and Interactions on Continuous Abstinence Rates Weeks 9-24 and 9-12
eTable 3. Risk Differences and Odds Ratios From a Main Effects Model (Cohort, Race, and Treatment) for Continuous Abstinence Rates Weeks 9-24 and 9-12
eTable 4. Final Stepwise Logistic Regression Modeling of Continuous Abstinence Rates Weeks 9-24 With Baseline and Postbaseline Factors Modeled Separately
eTable 5. Relation of Demographic, Smoking, Treatment Process, and Psychosocial Variables With Race and Continuous Abstinence Rates Weeks 9-12 for the Sample Overall
eTable 6. Final Stepwise Logistic Regression Modeling of Continuous Abstinence Rates Weeks 9-12 With Baseline and Postbaseline Factors Modeled Separately
eTable 7. Final Stepwise Logistic Regression Modeling of Continuous Abstinence Rates Weeks 9-12 With Baseline and Postbaseline Factors Modeled Together
eFigure 1. 7-Day Point Prevalence Abstinence for Black and Whites by Treatment and Psychiatric Cohort
eFigure 2. Cigarettes per Day for Black and White Continuing Smokers by Treatment and Psychiatric Cohort
eReference
Data Sharing Statement
References
- 1.US Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults: United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205-1211. doi: 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- 3.Trinidad DR, Pérez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11(2):203-210. doi: 10.1093/ntr/ntn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699-706. doi: 10.2105/AJPH.2010.191668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US National Cancer Institute . A Socioecological Approach to Addressing Tobacco Related Health Disparities. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017. [Google Scholar]
- 6.American Cancer Society . Cancer Facts & Figures for African Americans, 2016-2018. American Cancer Society; 2017. [Google Scholar]
- 7.Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital signs: racial disparities in age-specific mortality among blacks or African Americans—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2017;66(17):444-456. doi: 10.15585/mmwr.mm6617e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi: 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- 9.Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the International Tobacco Control US Survey. Nicotine Tob Res. 2016;18(1)(suppl):S79-S87. doi: 10.1093/ntr/ntv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 11.Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34(5):404-412. doi: 10.1016/j.amepre.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Davila EP, Zhao W, Byrne M, et al. Correlates of smoking quit attempts: Florida Tobacco Callback Survey, 2007. Tob Induc Dis. 2009;5(1):10. doi: 10.1186/1617-9625-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahende JW, Malarcher AM, Teplinskaya A, Asman KJ. Quit attempt correlates among smokers by race/ethnicity. Int J Environ Res Public Health. 2011;8(10):3871-3888. doi: 10.3390/ijerph8103871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy DT, Blackman K, Tauras J, et al. Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health. 2011;101(7):1241-1247. doi: 10.2105/AJPH.2011.300178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messer K, Trinidad DR, Al-Delaimy WK, Pierce JP. Smoking cessation rates in the United States: a comparison of young adult and older smokers. Am J Public Health. 2008;98(2):317-322. doi: 10.2105/AJPH.2007.112060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahre M, Okuyemi KS, Joseph AM, Fu SS. Racial/ethnic differences in menthol cigarette smoking, population quit ratios and utilization of evidence-based tobacco cessation treatments. Addiction. 2010;105(1)(1):75-83. doi: 10.1111/j.1360-0443.2010.03200.x [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services . Tobacco Use Among U.S. Racial/Ethnic Minority Groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. US Department of Health and Human Services; 1998. [Google Scholar]
- 18.Cropsey KL, Clark CB, Zhang X, Hendricks PS, Jardin BF, Lahti AC. Race and medication adherence moderate cessation outcomes in criminal justice smokers. Am J Prev Med. 2015;49(3):335-344. doi: 10.1016/j.amepre.2015.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daza P, Cofta-Woerpel L, Mazas C, et al. Racial and ethnic differences in predictors of smoking cessation. Subst Use Misuse. 2006;41(3):317-339. doi: 10.1080/10826080500410884 [DOI] [PubMed] [Google Scholar]
- 20.Fu SS, Burgess DJ, Hatsukami DK, et al. Race and nicotine replacement treatment outcomes among low-income smokers. Am J Prev Med. 2008;35(6)(suppl):S442-S448. doi: 10.1016/j.amepre.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 21.Hymowitz N, Sexton M, Ockene J, Grandits G; MRFIT Research Group . Baseline factors associated with smoking cessation and relapse. Prev Med. 1991;20(5):590-601. doi: 10.1016/0091-7435(91)90057-B [DOI] [PubMed] [Google Scholar]
- 22.Kendrick JS, Zahniser SC, Miller N, et al. Integrating smoking cessation into routine public prenatal care: the Smoking Cessation in Pregnancy project. Am J Public Health. 1995;85(2):217-222. doi: 10.2105/AJPH.85.2.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray RP, Connett JE, Buist AS, Gerald LB, Eichenhorn MS. Experience of Black participants in the Lung Health Study smoking cessation intervention program. Nicotine Tob Res. 2001;3(4):375-382. doi: 10.1080/14622200110081435 [DOI] [PubMed] [Google Scholar]
- 24.Windsor RA, Lowe JB, Perkins LL, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Public Health. 1993;83(2):201-206. doi: 10.2105/AJPH.83.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi: 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 26.Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. 2016;315(4):371-379. doi: 10.1001/jama.2015.19284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croghan IT, Hurt RD, Ebbert JO, et al. Racial differences in smoking abstinence rates in a multicenter, randomized, open-label trial in the United States. Z Gesundh Wiss. 2010;18(1):59-68. doi: 10.1007/s10389-009-0277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cropsey KL, Weaver MF, Eldridge GD, Villalobos GC, Best AM, Stitzer ML. Differential success rates in racial groups: results of a clinical trial of smoking cessation among female prisoners. Nicotine Tob Res. 2009;11(6):690-697. doi: 10.1093/ntr/ntp051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. J Subst Abuse Treat. 2009;37(3):247-255. doi: 10.1016/j.jsat.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine Tob Res. 2003;5(2):215-222. doi: 10.1080/0955300031000083436 [DOI] [PubMed] [Google Scholar]
- 31.Nollen NL, Mayo MS, Sanderson Cox L, et al. Factors that explain differences in abstinence between black and white smokers: a prospective intervention study. J Natl Cancer Inst. 2019;111(10):1078-1087. doi: 10.1093/jnci/djz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens VJ, Solberg LI, Bailey SR, et al. Assessing trends in tobacco cessation in diverse patient populations. Nicotine Tob Res. 2016;18(3):275-280. doi: 10.1093/ntr/ntv092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West R, Evins AE, Benowitz NL, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction. 2018;113(8):1507-1516. doi: 10.1111/add.14208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess DJ, van Ryn M, Noorbaloochi S, et al. Smoking cessation among African American and white smokers in the Veterans Affairs health care system. Am J Public Health. 2014;104(4)(suppl):S580-S587. doi: 10.2105/AJPH.2014.302023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chenoweth MJ, Ware JJ, Zhu AZX, et al. ; PGRN-PNAT Research Group . Genome-wide association study of a nicotine metabolism biomarker in African American smokers: impact of chromosome 19 genetic influences. Addiction. 2018;113(3):509-523. doi: 10.1111/add.14032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faseru B, Nollen NL, Mayo MS, et al. Predictors of cessation in African American light smokers enrolled in a bupropion clinical trial. Addict Behav. 2013;38(3):1796-1803. doi: 10.1016/j.addbeh.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635-643. doi: 10.1038/clpt.2009.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nollen NL, Mayo MS, Ahluwalia JS, et al. Factors associated with discontinuation of bupropion and counseling among African American light smokers in a randomized clinical trial. Ann Behav Med. 2013;46(3):336-348. doi: 10.1007/s12160-013-9510-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979-1986. doi: 10.1111/j.1360-0443.2007.02010.x [DOI] [PubMed] [Google Scholar]
- 40.Zhu AZ, Cox LS, Nollen N, et al. CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther. 2012;92(6):771-777. doi: 10.1038/clpt.2012.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Businelle MS, Kendzor DE, Reitzel LR, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29(3):262-273. doi: 10.1037/a0019285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caraballo RS, Giovino GA, Pechacek TF, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988-1991. JAMA. 1998;280(2):135-139. doi: 10.1001/jama.280.2.135 [DOI] [PubMed] [Google Scholar]
- 43.Cox LS, Nollen NL, Mayo MS, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst. 2012;104(4):290-298. doi: 10.1093/jnci/djr513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu SS, Sherman SE, Yano EM, van Ryn M, Lanto AB, Joseph AM. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20(2):108-116. doi: 10.4278/0890-1171-20.2.108 [DOI] [PubMed] [Google Scholar]
- 45.Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63(3):360-367. doi: 10.1111/j.1742-1241.2008.01969.x [DOI] [PubMed] [Google Scholar]
- 46.Gundersen DA, Delnevo CD, Wackowski O. Exploring the relationship between race/ethnicity, menthol smoking, and cessation, in a nationally representative sample of adults. Prev Med. 2009;49(6):553-557. doi: 10.1016/j.ypmed.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 47.Kotz D, West R. Explaining the social gradient in smoking cessation: it’s not in the trying, but in the succeeding. Tob Control. 2009;18(1):43-46. doi: 10.1136/tc.2008.025981 [DOI] [PubMed] [Google Scholar]
- 48.Lerman C, Schnoll RA, Hawk LW Jr, et al. ; PGRN-PNAT Research Group . Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131-138. doi: 10.1016/S2213-2600(14)70294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Stable EJ, Herrera B, Jacob P III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152-156. doi: 10.1001/jama.280.2.152 [DOI] [PubMed] [Google Scholar]
- 50.Robinson CD, Pickworth WB, Heishman SJ, et al. Black cigarette smokers report more attention to smoking cues than white smokers: implications for smoking cessation. Nicotine Tob Res. 2015;17(8):1022-1028. doi: 10.1093/ntr/ntu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: results from a prospective study of an Australian national sample. Addiction. 2006;101(1):121-127. doi: 10.1111/j.1360-0443.2005.01292.x [DOI] [PubMed] [Google Scholar]
- 52.Siahpush M, Yong HH, Borland R, Reid JL, Hammond D. Smokers with financial stress are more likely to want to quit but less likely to try or succeed: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104(8):1382-1390. doi: 10.1111/j.1360-0443.2009.02599.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webb Hooper M, Dietz NA, Wilson JC. Smoking urges during treatment and long-term cessation among low-income African Americans. Ethn Dis. 2017;27(4):395-402. doi: 10.18865/ed.27.4.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. American Psychiatric Press; 2000. [Google Scholar]
- 55.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299-303. doi: 10.1111/j.1360-0443.2004.00995.x [DOI] [PubMed] [Google Scholar]
- 56.Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75-78. doi: 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- 57.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 59.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63(3):452-459. doi: 10.1037/0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- 60.Laude JR, Bailey SR, Crew E, et al. Extended treatment for cigarette smoking cessation: a randomized control trial. Addiction. 2017;112(8):1451-1459. doi: 10.1111/add.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am J Psychiatry. 2013;170(8):860-867. doi: 10.1176/appi.ajp.2013.12070919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468-474. doi: 10.1001/jama.288.4.468 [DOI] [PubMed] [Google Scholar]
- 63.Schnoll RA, Patterson F, Wileyto EP, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144-151. doi: 10.7326/0003-4819-152-3-201002020-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb Hooper M, Antoni MH, Okuyemi K, Dietz NA, Resnicow K. Randomized controlled trial of group-based culturally specific cognitive behavioral therapy among African American smokers. Nicotine Tob Res. 2017;19(3):333-341. doi: 10.1093/ntr/ntw181 [DOI] [PubMed] [Google Scholar]
- 65.Piper ME, Bullen C, Krishnan-Sarin S, et al. Defining and measuring abstinence in clinical trials of smoking cessation interventions: an updated review. Nicotine Tob Res. 2019;22(7):1098-1106. doi: 10.1093/ntr/ntz110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiore M, Jaen C, Baker T, et al. Treating Tobacco Use and Dependence Clinical Practice Guideline: 2008 Update. US Department of Health and Human Services; 2008. [Google Scholar]
- 67.Hays JT, Hurt RD, Rigotti NA, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation: a randomized, controlled trial. Ann Intern Med. 2001;135(6):423-433. doi: 10.7326/0003-4819-135-6-200109180-00011 [DOI] [PubMed] [Google Scholar]
- 68.Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175(4):504-511. doi: 10.1001/jamainternmed.2014.8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leventhal AM, Bello MS, Galstyan E, Higgins ST, Barrington-Trimis JL. Association of cumulative socioeconomic and health-related disadvantage with disparities in smoking prevalence in the United States, 2008 to 2017. JAMA Intern Med. 2019;179(6):777-785. doi: 10.1001/jamainternmed.2019.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Catley D, Ahluwalia JS, Resnicow K, Nazir N. Depressive symptoms and smoking cessation among inner-city African Americans using the nicotine patch. Nicotine Tob Res. 2003;5(1):61-68. doi: 10.1080/1462220021000060464 [DOI] [PubMed] [Google Scholar]
- 71.Catley D, Harris KJ, Okuyemi KS, Mayo MS, Pankey E, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine Tob Res. 2005;7(6):859-870. doi: 10.1080/14622200500330118 [DOI] [PubMed] [Google Scholar]
- 72.Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. Am J Prev Med. 1996;12(5):378-387. doi: 10.1016/S0749-3797(18)30295-2 [DOI] [PubMed] [Google Scholar]
- 73.Kendzor DE, Businelle MS, Costello TJ, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health. 2010;100(4):702-706. doi: 10.2105/AJPH.2009.172676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kendzor DE, Businelle MS, Reitzel LR, et al. Everyday discrimination is associated with nicotine dependence among African American, Latino, and White smokers. Nicotine Tob Res. 2014;16(6):633-640. doi: 10.1093/ntr/ntt198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kendzor DE, Reitzel LR, Mazas CA, et al. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Soc Sci Med. 2012;74(9):1394-1401. doi: 10.1016/j.socscimed.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH. Associations between smoking cessation and anxiety and depression among U.S. adults. Addict Behav. 2009;34(6-7):491-497. doi: 10.1016/j.addbeh.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 77.Reitzel LR, Businelle MS, Kendzor DE, et al. Subjective social status predicts long-term smoking abstinence. BMC Public Health. 2011;11:135. doi: 10.1186/1471-2458-11-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reitzel LR, Mazas CA, Cofta-Woerpel L, et al. Subjective social status affects smoking abstinence during acute withdrawal through affective mediators. Addiction. 2010;105(5):928-936. doi: 10.1111/j.1360-0443.2009.02875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reitzel LR, Vidrine JI, Businelle MS, et al. Neighborhood perceptions are associated with tobacco dependence among African American smokers. Nicotine Tob Res. 2012;14(7):786-793. doi: 10.1093/ntr/ntr285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams DR. Race, socioeconomic status, and health: the added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896(1):173-188. doi: 10.1111/j.1749-6632.1999.tb08114.x [DOI] [PubMed] [Google Scholar]
- 81.Piper ME, Cook JW, Schlam TR, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647-657. doi: 10.1093/ntr/ntq067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith SS, Fiore MC, Baker TB. Smoking cessation in smokers who smoke menthol and non-menthol cigarettes. Addiction. 2014;109(12):2107-2117. doi: 10.1111/add.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al Koudsi N, Ahluwalia JS, Lin SK, Sellers EM, Tyndale RF. A novel CYP2A6 allele (CYP2A6*35) resulting in an amino-acid substitution (Asn438Tyr) is associated with lower CYP2A6 activity in vivo. Pharmacogenomics J. 2009;9(4):274-282. doi: 10.1038/tpj.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho MK, Mwenifumbo JC, Zhao B, Gillam EM, Tyndale RF. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black-African descent. Pharmacogenet Genomics. 2008;18(1):67-75. doi: 10.1097/FPC.0b013e3282f3606e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mwenifumbo JC, Al Koudsi N, Ho MK, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum Mutat. 2008;29(5):679-688. doi: 10.1002/humu.20698 [DOI] [PubMed] [Google Scholar]
- 86.Mwenifumbo JC, Zhou Q, Benowitz NL, Sellers EM, Tyndale RF. New CYP2A6 gene deletion and conversion variants in a population of Black African descent. Pharmacogenomics. 2010;11(2):189-198. doi: 10.2217/pgs.09.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Relation of Demographic, Smoking, Treatment Process, and Psychosocial Variables With Race and Continuous Abstinence Rates Weeks 9-24 for the Sample Overall
eTable 2. General Linear Regression Models Examining the Effects of Psychiatric Cohort, Treatment Group, Race, and Interactions on Continuous Abstinence Rates Weeks 9-24 and 9-12
eTable 3. Risk Differences and Odds Ratios From a Main Effects Model (Cohort, Race, and Treatment) for Continuous Abstinence Rates Weeks 9-24 and 9-12
eTable 4. Final Stepwise Logistic Regression Modeling of Continuous Abstinence Rates Weeks 9-24 With Baseline and Postbaseline Factors Modeled Separately
eTable 5. Relation of Demographic, Smoking, Treatment Process, and Psychosocial Variables With Race and Continuous Abstinence Rates Weeks 9-12 for the Sample Overall
eTable 6. Final Stepwise Logistic Regression Modeling of Continuous Abstinence Rates Weeks 9-12 With Baseline and Postbaseline Factors Modeled Separately
eTable 7. Final Stepwise Logistic Regression Modeling of Continuous Abstinence Rates Weeks 9-12 With Baseline and Postbaseline Factors Modeled Together
eFigure 1. 7-Day Point Prevalence Abstinence for Black and Whites by Treatment and Psychiatric Cohort
eFigure 2. Cigarettes per Day for Black and White Continuing Smokers by Treatment and Psychiatric Cohort
eReference
Data Sharing Statement


