Abstract
Background
Prenatal alcohol exposure is a leading cause of neurobehavioral and neurocognitive deficits collectively known as fetal alcohol spectrum disorders, including eating disorders and increased risk for substance abuse as very common issues. In this context, the present study aimed to assess the interaction between prenatal and lactation alcohol exposure (PLAE) and a high-fat diet (HFD) during childhood and adolescence.
Methods
Pregnant C57BL/6 mice underwent a procedure for alcohol binge drinking during gestation and lactation periods. Subsequently, PLAE female offspring were fed with an HFD for 8 weeks, and thereafter, nutrition-related parameters as well as their response to cocaine were assessed.
Results
In our model, feeding young females with an HFD increased their triglyceride blood levels but did not induce overweight compared with those fed with a standard diet. Moreover, PLAE affected how females responded to the fatty diet as they consumed less food than water-exposed offspring, consistent with a lower gain of body weight. HFD increased the psychostimulant effects of cocaine. Surprisingly, PLAE reduced the locomotor responses to cocaine without modifying cocaine-induced reward. Moreover, PLAE prevented the striatal overexpression of cannabinoid 1 receptors induced by an HFD and induced an alteration of myelin damage biomarker in the prefrontal cortex, an effect that was mitigated by an HFD-based feeding.
Conclusion
Therefore, in female offspring, some effects triggered by one of these factors, PLAE or an HFD, were blunted by the other, suggesting a close interaction between the involved mechanisms.
Keywords: drinking-in-the-dark, alcohol, high-fat diet, cocaine, female
Significance Statement.
Prenatal alcohol exposure is a leading cause of neurobehavioral and neurocognitive deficits, including eating disorders and increased risk for substance abuse as very common issues. Furthermore, eating conditions can also induce long-lasting modifications in the responses to drugs.
In this context, we considered of particular relevance to assess the interaction between alcohol exposure during gestation and lactation (PLAE) and a high-fat diet (HFD) during childhood and adolescence of female offspring and their consequences on nutrition-related parameters as well as on the response to the most consumed psychostimulant worldwide, cocaine.
Our results shed light on and suggest the existence of a crosstalk between the effects induced by a HFD and PLAE, so there is a mutual attempt to compensate the effects induced by each condition (PLAE and HFD). However, further investigation would be needed to unveil what would occur with a longer exposure to a fatty diet.
Introduction
Prenatal alcohol exposure is a leading cause of significant neurobehavioral and neurocognitive deficits. This can contribute to increased risk for substance abuse among people with fetal alcohol spectrum disorders (FASD) (Grant et al., 2013). In this sense, Barbier et al. (2008) reported that alcohol-exposed male offspring are more sensitive to the anxiolytic effect of ethanol, a feature that could partially explain the altered pattern of consumption of alcohol observed in these animals. Furthermore, it has been described that maternal binge-like alcohol consumption during gestation and lactation alters sensitivity to the reinforcing effects of cocaine and thus enhances vulnerability to cocaine addiction in adult mice (Cantacorps et al., 2020).
Additionally, prenatal alcohol exposure increases the hypothalamic-pituitary-adrenal axis tone, resulting in hypothalamic-pituitary-adrenal dysregulation throughout life. This dysregulation is thought to affect energy homeostasis and eating behavior (Yau and Potenza, 2013). Nevertheless, the potential consequences of maternal alcohol exposure for eating behaviors and on nutritional issues of young offspring are relatively under-researched. In this regard, eating disorders seem to be common; thereby, children with FASD show an altered food intake self-regulation. In this way, these children seem to prefer calorie-dense foods, which can increase obesity risk (Fuglestad et al., 2014; Amos-Kroohs et al., 2018). Indeed, rates of overweight and obesity are increased among children with FASD diagnosis, particularly in females (Werts et al., 2014). Altogether, evidence is suggestive of possible metabolic and/or endocrine disruptions in children with FASD. Furthermore, eating conditions, including body weight and type of food, can also induce long-lasting modifications on the responses to drugs (therapeutic and recreational) (Baladi et al., 2010).
In this context, the present study aims to assess the interaction between alcohol exposure during gestation and lactation, and a high-fat diet (HFD) during the female offspring’s childhood and adolescence, and their consequences on the psychostimulant-induced effects of cocaine (both behavioral and molecular) and on nutrition-related parameters.
It is important to highlight that the experimental model used in the present study intends to mimic as much as possible real-life exposition to these issues. Hence, C57BL/6 female mice were exposed to alcohol following the drinking-in-the-dark (DID) paradigm during the entire gestational and lactational periods. Subsequently, prenatal and lactation alcohol exposed (PLAE) female offspring were fed ad libitum with an HFD for 8 weeks. Thereafter, they were assessed for the psychostimulant and rewarding effects of cocaine using the horizontal locomotor test and the conditioned place preference (CPP) paradigm. In parallel, we investigated possible changes in some parameters related with neurotoxicity, cocaine response, and its main target, the dopaminergic system, to explore how alcohol and HFD could influence the vulnerability to this psychostimulant in female mice. These changes were studied in specific brain areas: the ventral striatum and the prefrontal cortex (PFC), respectively. Additionally, nutrition-related parameters such as food intake, glucose and triglyceride blood levels, and the expression of genes induced by ghrelin were determined in the hypothalamus.
Cannabinoid CB1 receptor levels were also determined due to their implication in feeding regulation and drug abuse (Kirkham and Williams, 2001; Maldonado et al., 2006). The stimulation of CB1 receptors is a key component in the development of obesity, so that CB1 knockout mice are resistant to diet-induced obesity and antagonists of these receptors have anti-obesity potential (Di Marzo et al., 2001; Ravinet Trillou et al., 2004). On the other hand, endocannabinoids also play an important role in the modulation of synaptic plasticity in the dorsal striatum and nucleus accumbens (NAcc) (for reviews, see Freund et al., 2003; Gerdeman and Lovinger, 2003; Piomelli, 2003). Moreover, dopamine agonists and psychostimulants increase the striatal release of endocannabinoids, suggesting that they could participate in the effects of psychostimulant drugs (Giuffrida et al., 1999; Patel et al., 2003; Centonze et al., 2004).
Our results point to a mutual interaction between some of the effects induced by PLAE and HFD, which deserves further investigation.
Materials and Methods
Animals
Twelve-week-old male and female C57BL/6 inbred mice were purchased from Charles River (Barcelona, Spain) and shipped to our animal facility (UBIOMEX, PRBB) to be used as breeders. On arrival, they were housed in standard cages at constant temperature (21°C ± 1°C ) and humidity (55% ± 10%) under a reversed light-dark cycle (white lights on 8:00 pm-8:00 am). After 1 week of acclimatization, breeding pairs were mated, and pregnant females were observed daily for parturition. For each litter, the date of birth was designated as postnatal day (PND) 0. Pups remained with their mothers for 21 days and were then weaned (PND 21). After weaning, male offspring were used for other experiments, and female mice from each litter were randomly assigned to the different diets to avoid potential litter effect. All animal care and experimental procedures were approved by the local ethics committee (CEEA-PRBB) and conducted in accordance with the European Union Directive 2010/63/EU guidelines on the protection of animals used for scientific purposes. ARRIVE guidelines for reporting animal research have been followed.
Materials
Ethyl alcohol was purchased from Merck (Darmstadt, Germany) and diluted in tap water to obtain a 20% (v/v) alcohol solution for the DID procedure. Cocaine hydrochloride was purchased in Alcaliber S.A. (Madrid, Spain) and prepared in 0.9% NaCl, pH 7.4 (saline), immediately before administration.
[3H]Dopamine ([3H]DA) was from Perkin Elmer (Boston, MA). All the other reagents were of analytical grade and purchased from several commercial sources.
Experimental Design
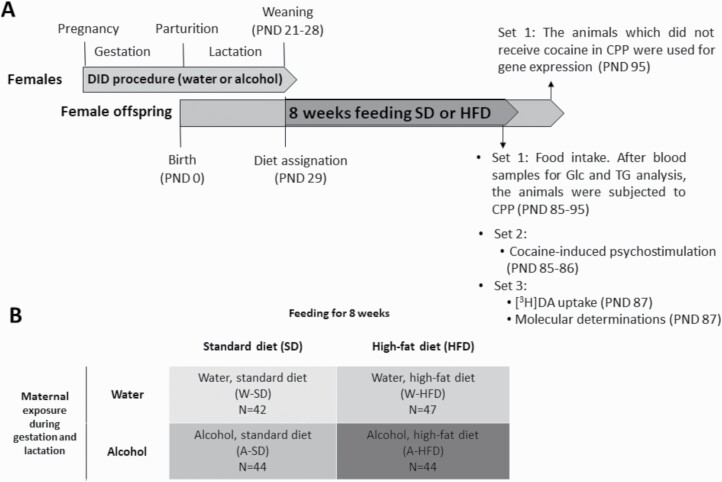
The experimental design and the 4 experimental groups involved in the study are depicted in Figure 1. Three different sets of animals were used in this study. The first (N = 106) had food intake, glucose, and triglyceride blood levels measured. Then the animals were subjected to the cocaine-induced CPP that lasted for 10 days. Throughout the whole CPP process, the animals continued being fed with the corresponding diet. Afterwards, the animals that did not receive cocaine (N = 20) were killed to assess the expression of ghrelin-induced genes in the hypothalamus. The second set was used to assess the psychostimulant properties of cocaine (N = 37), and the third to evaluate [3H]DA uptake (hemi-striatum) (N = 33) and the expression of factors regarding dopaminergic neurotransmission (ventral hemi-striatum) and neuronal damage (frontal cortex) (N = 20).
Figure 1.
(A) Experimental design. Pregnant C57BL/6 females were exposed to alcohol or water (control) during the gestation and lactation periods, following the drinking-in-the-dark (DID) procedure. On postnatal day (PND) 29, female offspring were assigned a standard diet or a high-fat diet (HFD). After feeding for 8 weeks, their glucose (Glc) and triglyceride (TG) blood levels were determined, and mice were tested for cocaine-induced psychostimulation, cocaine-induced conditioned place preference (CPP), or molecular determinations. (B) Experimental groups: there are 4 experimental groups according to the exposure of their mothers to water (W) or alcohol (A) during the gestation and lactation periods, and the diet they were fed (standard diet or HFD).
DID procedure
Pregnant and nursing C57BL/6 mice were exposed to alcohol following the DID paradigm (Rhodes et al., 2005). The procedure was conducted as previously reported (Cantacorps et al., 2017, 2020) starting 2 days after mating. Pregnant female mice were randomly assigned to 2 groups: alcohol and water exposed (control). Briefly, water bottles were replaced with 10-mL graduated cylinders fitted with sipper tubes containing either 20% (v/v) alcohol in tap water or only tap water 3 hours after the lights were turned off. Following a 2-hour access period, individual intake was recorded, and the original water drinking bottles were returned to the home cage. This procedure was repeated on days 2 and 3 and fresh fluids were provided each day. On day 4, alcohol or water cylinders were left for 4 hours and fluid intakes were recorded. Fluid intakes (g/kg body weight) were calculated based on average 2-day body weight values as dams were weighed at 2-day intervals. The procedure was maintained throughout the 3-week gestation and the 3-week lactation periods.
Feeding conditions
Two different types of diet were assigned to female offspring: a standard diet (831193 RM1; Special Diets Services, Essex, UK) and an HFD (D05122301; Research Diets, Inc.). The standard diet had an energy density of 3.52 kcal/g (energy contribution from 75.1% carbohydrates, 17.49% protein, and 7.42% fat). The HFD had an energy density of 4.73 kcal/g (35% carbohydrate energy, 20% protein energy, and 45% fat energy). The fat source was composed of 91% hydrogenated coconut oil and 9% soybean oil.
On PND 29, female offspring were randomly divided into 4 experimental groups and assigned either a standard diet or an HFD (Figure 1B). Animals were fed with 1 of both diets from weaning up to time of killing. All behavioral experiments were carried out during the youth of the animals (PND 85–105) (Flurkey et al., 2007). The weight of the animals and food intake were monitored weekly during the whole feeding period. The weight gain throughout the feeding period was calculated for each animal as the difference of weight between the last and the first week of diet exposure, expressed in grams.
Glucose and triglyceride determination
Fasting triglycerides and glucose levels were measured following 8 weeks of standard diet or HFD feeding immediately before the CPP (Set 1). In brief, mice were fasted for 6 hours, and blood samples were extracted from the tail between 3 pm and 4 pm. The tests were performed in a quiet room using an appropriate measuring device (Accutrend Plus System Cobas, Roche, Spain).
Behavioral Tests
CPP
The CPP procedure consisted of 3 different phases: preconditioning, conditioning, and testing day (Luján et al., 2019). During preconditioning, mice could freely explore both compartments for 20 minutes. The conditioning phase consisted of 4 pairings: mice received an i.p. injection of 15 mg/kg cocaine immediately prior to confinement to the drug-paired compartment for 30 minutes on days 2, 4, 6, and 8, while on alternate days (3, 5, 7, and 9) mice received physiological saline before being confined to the vehicle-paired compartment for 30 minutes. The time spent in each compartment during the preconditioning and testing sessions, as well as the distance travelled, were recorded by computerized monitoring software (CIBERTEC APL software). A CPP score was calculated for each subject as the difference between the time spent in the drug‐paired compartment during the testing and the pre‐conditioning sessions.
Cocaine-induced locomotor activity
After a 2-day habituation period (day −2), all mice (set 2) received a single dose of cocaine (8 mg/kg i.p.) and were immediately placed into the open field arena where their horizontal locomotor activity was recorded by a computerized monitoring software (Smart 3.0 Panlab, Barcelona, Spain) for 30 minutes.
Molecular Determinations
Tissue samples preparation
Mice were killed by cervical dislocation. The whole striatum, ventral striatum, PFC, or hypothalamus, when appropriate, were quickly dissected out and, except the whole striatum, stored at −80°C until use.
For the [3H]DA uptake experiments, mouse synaptosome from fresh hemi-striatums were prepared as described by Pubill et al. (2005).
Total protein extracts were isolated from ventral hemi-striatum and PFC and processed as described by Pubill et al. (2013) with minor modifications. Briefly, tissue samples were thawed and homogenized through sonication at 4°C in 20 volumes of lysis buffer (20 mM Tris-HCl, 1% NP40, 137 mM NaCl, and 2 mM EDTA, pH 8) containing a protease and phosphatase inhibitor cocktail. Thereafter, the homogenates were shaken and rolled for 2 hours at 4°C and subsequently centrifuged at 15 000 × g for 30 minutes at 4°C. Protein content of the supernatants was determined using the Bio-Rad Protein Reagent (Bio Rad, Inc. Spain).
[3H]DA uptake in striatal synaptosomes
Reaction tubes were composed of 25 µL of the radioligand [3H]DA (final concentration 5 nM), 100 µL of the synaptosome suspension, and 125 µL of Hank’s HEPES-buffered solution containing pargyline (20 mM) and ascorbic acid (1 mM). The incubation was performed for 5 minutes at 37°C. Uptake reactions were terminated by rapid vacuum filtration through Whatman GF/B glass fiber filters (Whatman Intl Ltd, Maidstone, UK) presoaked with 0.5% polyethyleneimine. Tubes and filters were washed 3 times with ice-cold 50 mM Tris-HCl. The radioactivity trapped on the filters was measured by liquid scintillation spectrometry. Nonspecific uptake value was determined in parallel samples containing cocaine (final concentration 300 µM) at 4°C and was subtracted from total uptake to yield specific uptake.
Western-blot analysis
A general western blotting protocol was used as described by Duart-Castells et al. (2019) with minor modifications. Membranes were incubated overnight at 4°C with anti CB1 receptor (1:1000, Frontiers Institute, South Africa), myelin basic protein (MBP) (1:1000, Abcam, Cambridge, UK), NeuN (1:10 000 Abcam, Cambridge, UK), NFκB (1:1000, Cell Signaling Technology, Inc), or TH (1:5000, Transduction Laboratories, Lexington, KY) primary antibodies. After washing, membranes were incubated for 1 hour at room temperature with their respective secondary peroxidase-conjugated anti-IgG antibody: donkey anti-rabbit, sheep anti-mouse, or goat anti-rat (1:5000, GE Healthcare). GAPDH (1:5000, Merck Millipore) antibody was used as a control for loading.
Total RNA extraction and gene expression determination
RNA extraction and quantitative RT-PCR were performed from hypothalamus as described by Mir et al. (2018). The sequences of the primers used for each gene were 5’- TGCAGACCGAGCAGAAGAAG (forward) and 5’- GACTCGTGCAGCCTTACACA (reverse) for AgRP; 5’- TATCTCTGCTCGTGTGTTTG (forward) and 5’-GTTCT GGGGGCGTTTTCTG (reverse) for Npy; and 5’- TATGCAGTCGCCC TTCCT (forward) and 5’- ACATCAATCAGGTGTGTCTGCT (reverse) for Cpt1c.
Data Acquisition and Statistical Analysis
Data are expressed as mean ± SEM. Data from biochemical analysis (western blot and [3H]DA uptake experiments) were normalized with 100% defined as the mean of the technical replicates in the control group (water-standard diet). In qPCR analysis, data were expressed as fold-change variations relative to the water-standard diet group.
Differences between groups were compared using 1-way or 2-way ANOVA with diet (standard diet or HFD) and maternal exposure (water or alcohol) as factors of variation. To analyze the food intake- and cocaine-induced CPP, a 3-way ANOVA was performed, with diet (standard diet or HFD), mother’s exposure (water or alcohol), and time (day or week) as factors of variations. The α error probability was set at 0.05. Significant differences (P < .05) were analyzed using the Bonferroni post-hoc test for multiple comparison measures only if the F value resulting from the ANOVA achieved the necessary level of statistical significance (P < .05) and no significant variance in homogeneity was observed. Statistical calculations were performed using GraphPad Prism 8.0 software.
Results
Maternal Alcohol Consumption
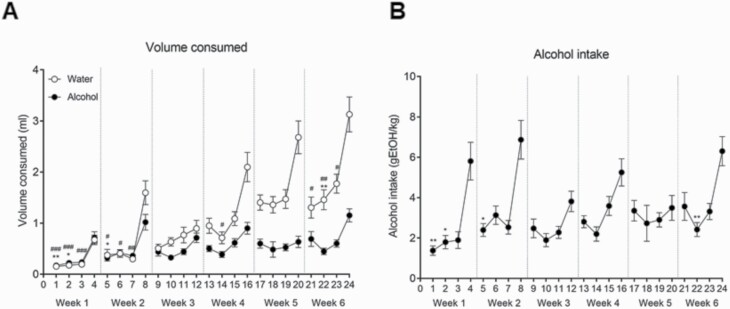
Two-way ANOVA with repeated-measures analysis of the volumes of water and alcohol consumed during DID testing showed a significant effect of day [F(23,1173) = 27.693; P < .001] and alcohol [F(1,51) = 66.795; P < .001], with interaction between factors [F(23,1173) = 10.482; P < .001] (Figure 2A). Bonferroni post-hoc comparisons revealed a significant increase in water consumption on day 4 compared with day 1 (P < .001), day 2 (P < .001), and day 3 (P < .001); on day 8 compared with day 5 (P < .05), day 6 (P < .05), and day 7 (P < .01); on day 16 compared with day 14 (P < .05); and on day 24 compared with day 21 (P < .05), day 22 (P < .01), and day 23 (P < .05). Furthermore, a significant increase in alcohol consumption on day 4 compared with day 1 (P < .01) and day 2 (P < .05); on day 8 compared with day 5 (P < .05); and on day 24 compared with day 22 (P < .01) was found. Additionally, 1-way ANOVA with repeated-measures analysis of alcohol intake showed a significant effect of day [F(23,575) = 7.389; P < 0.001] (Figure 2B). Bonferroni post-hoc comparisons revealed a significant increase in alcohol intake on day 4 compared with day 1 (P < .01) and day 2 (P < .05), on day 8 compared with day 5 (P < .05), and on day 24 compared with day 22 (P < .01).
Figure 2.
Maternal alcohol drinking. (A) Volume of water or alcohol consumed and (B) alcohol intake (g ethanol [EtOH]/kg) throughout the gestation (week 1–3) and lactation (week 4–6) periods during the drinking-in-the-dark (DID) procedure test. Results are expressed as mean ± SEM. *P < .05, **P < .01 day 4 of each week compared with the 3 previous days in the alcohol-exposed group. #P < .05, ##P < .01 and ###P < .001 day 4 of each week compared with the 3 previous days in the water-exposed group. (N=26–27/group).
Body Weight and Food Intake
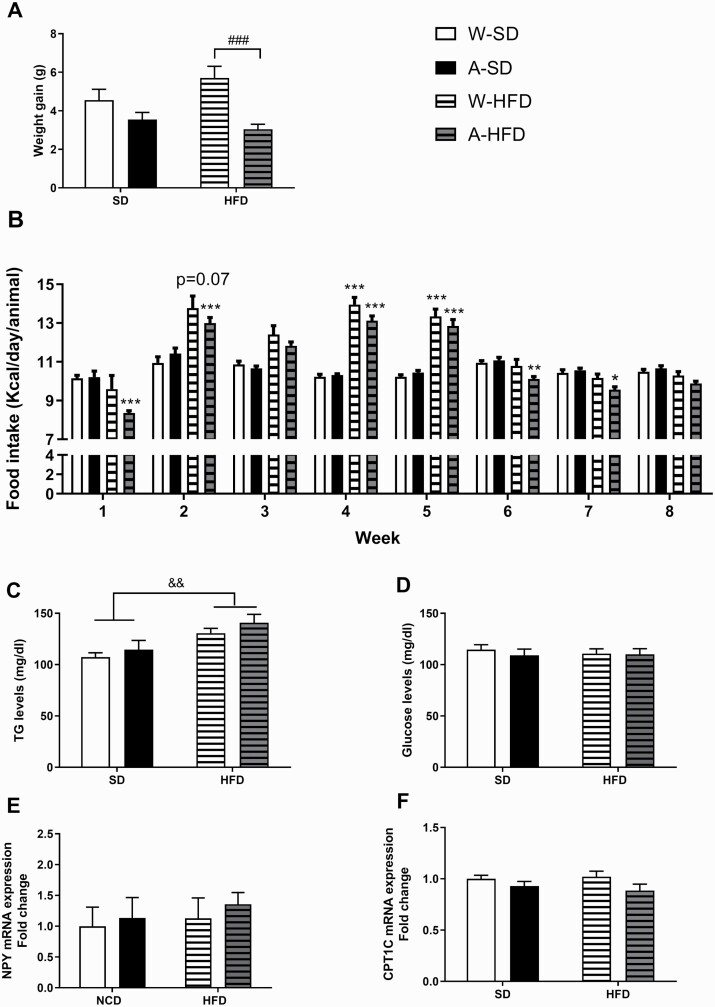
Initially, body weight after weaning was not affected by alcohol exposure [F(3,85) = 1.241, n.s.]. From that moment on, all groups of animals gained weight over time across the 8-week exposure period. Two-way ANOVA of the weight gain revealed a significant effect of the alcohol factor [F(1,100) = 14.96; P < .001] but no effect of the diet [F(1,100) = 0.451; P > .05]. Accordingly, no significant differences in weight gain between the groups fed with HFD vs those fed with standard diet were observed. Bonferroni post-hoc multiple comparisons revealed that the weight gain of the alcohol (A)-HFD group was significantly lower than that of the water (W)-HFD group. Conversely, no differences were observed in weight gain between animals fed with standard diet whether exposed to water or alcohol (Figure 3A).
Figure 3.
(A) Body weight gain after 8 weeks of feeding, expressed in grams (N = 25–27/group). (B) Mean daily kcal ingested by mice exposed to water (W) or alcohol (A) during the gestation and lactation periods and exposed to a standard diet or a high-fat diet (HFD) (N = 25–27/group). (D) Fasting triglycerides (TG) and glucose blood levels (N = 6/group) and (E) NPY and (F) CPT1C gene expression in the hypothalamus (N = 5/group) measured following 8 weeks of standard diet or HFD feeding. Results are expressed as mean ± SEM. ###P < .001 vs W-HFD; *P < .05, **P < .01, ***P < .001 vs the corresponding group (water or alcohol) fed with standard diet; &&P < .01 vs standard diet.
Weekly food intake, calculated either as an average of grams/day or kcal/day, was also measured. Three-way ANOVA (alcohol × diet × week) with repeated measures of grams/day ingested yielded a significant effect of diet [F(1,100) = 305.5; P < .001] and time [F(7,651) = 68.85; P < .001], with interaction between alcohol × diet [F(1,100) = 6.034; P < .05] and time × diet [F(7,651) = 62.20; P < .01]. Subsequent Bonferroni post-hoc tests revealed significant differences in food intake between standard diet and HFD groups in mice exposed to alcohol (P < .001). Furthermore, significant differences between standard diet and HFD levels of food intake were found on weeks 1, 2, 3, 6, 7, and 8 (P < .001 in all cases). Overall, mice receiving HFD ingested less grams of diet than those fed with standard diet.
Regarding the weekly food intake in kcal/day, 3-way ANOVA analysis (alcohol × diet × week) with repeated measures showed a significant effect of time [F(7,651) = 74.44; P < .001] and diet [F(1,100) = 27.86; P < .001], with significant interactions between time × diet [F(7,651) = 68.85; P < .001] and alcohol × diet [F(1,100) = 6.36; P < .05] (Figure 3B). Bonferroni post-hoc comparisons indicated that, from the second week of diet consumption until the sixth, the HFD groups (water and alcohol) significantly ingested more kcal/day than standard diet-fed mice (P < .001; in all cases). Nevertheless, from week 6 until the end, these groups reduced their kcal intake until being equated to that of the standard diet-fed groups.
In accordance with the significant interaction found (alcohol × diet), maternal exposure to alcohol did not modify the food and kcal intake when female offspring were fed with standard diet, but it did when fed with HFD.
Glucose and Triglyceride Blood Levels
Two-way ANOVA analysis of triglyceride blood levels showed a significant effect of the diet [F(1,20) = 13.07; P < .01]. Thus, HFD exposure significantly increased triglyceride blood levels compared with standard diet (Figure 3C). By contrast, neither the diet nor the maternal alcohol exposure altered glucose blood levels by the end of exposure (Figure 3D).
Basal Locomotor Activity and Cocaine-Induced CPP
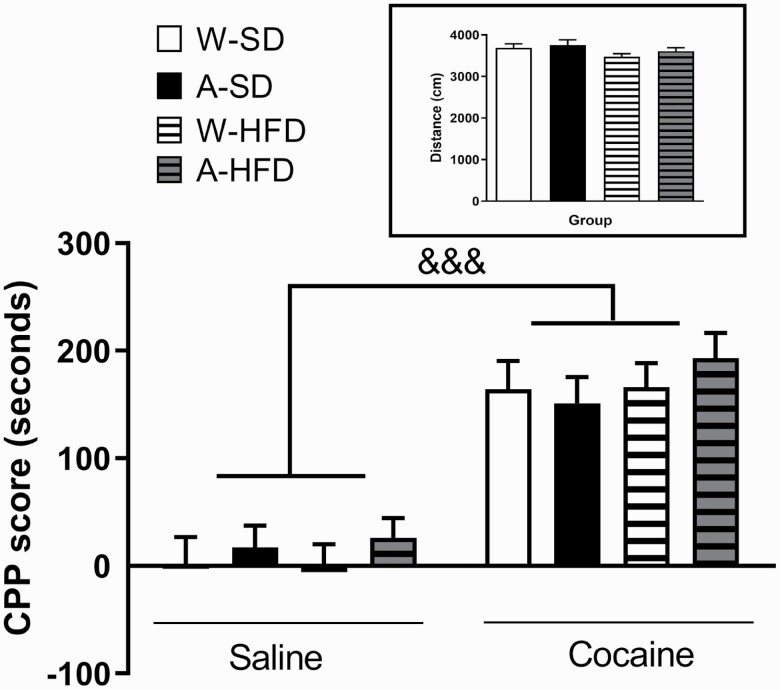
The first set of mice were finally subjected to the cocaine-induced CPP. Firstly, 2-way ANOVA of the results did not show any significant effect of the diet or alcohol exposure on the distance travelled by the animals in the apparatus, so no changes in basal locomotor activity were found between the different experimental groups (Figure 4, inset).
Figure 4.
Cocaine-induced conditioned place preference (CPP) in mice exposed to water (W) or alcohol (A) during the gestation and lactation periods after 8 weeks of feeding with a standard diet SD or a high-fat diet (HFD). Bars represent the CPP score, which is the difference in seconds between the time spent in the drug‐paired compartment during the testing and the pre‐conditioning sessions. (Inset) Distance travelled by each experimental group during the preconditioning phase. Results are expressed as mean ± SEM (N = 10–12/group). &&&P < .001 vs saline-treated animals. *P < .05 and **P < .01 vs the corresponding CPP group (water or alcohol).
Regarding the CPP experiments, 3-way ANOVA analysis (alcohol × diet × day) revealed a significant effect of cocaine treatment [F(1,78) = 93.314; P < .001], indicating that the repeated administration of cocaine (15 mg/kg, i.p.) produced a preference for the cocaine-paired compartment in all groups (Figure 4). However, no effect of alcohol [F(1,78) = 0.913; P > .05] or diet [F(1,78) = 0.496; P > .05] was found.
Cocaine Effect on Locomotor Activity
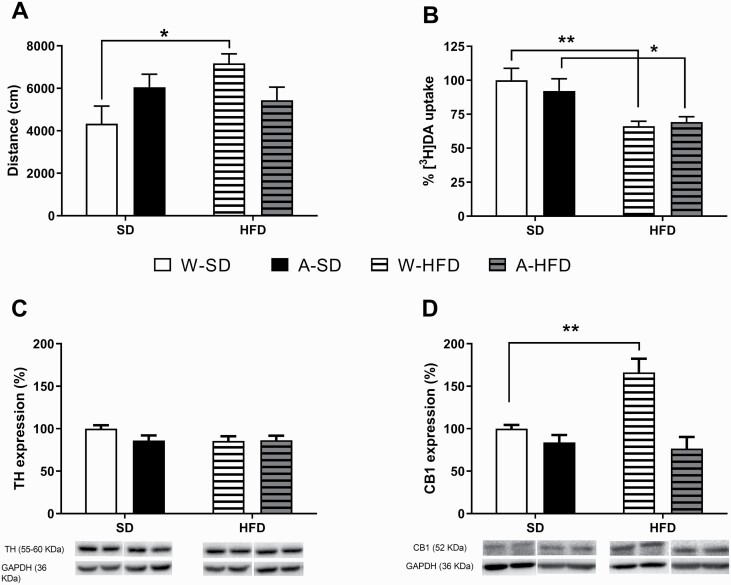
When the acute effect of cocaine was assessed in the second set of mice, an interesting result was obtained. Two-way ANOVA yielded a significant influence of the interaction alcohol × diet [F(1,34) = 7.12; P = .01]. As shown in Figure 5A, an acute administration of cocaine (8 mg/kg i.p.) elicited a similar effect in all groups of animals but an increased response in the W-HFD group compared with the water-standard diet group (P < .05).
Figure 5.
(A) Cocaine-induced locomotor activity immediately after the drug injection (8 mg/kg i.p.) for 30 minutes. Results are expressed as mean ± SEM of the distance travelled (cm) (N = 9–10/group). (B) Dopamine ([3H]DA) uptake in striatal synaptosomes. Data are expressed as a percentage of control uptake relative to W-standard diet (mean ± SEM) (N = 7–9/group). (C) Effects on TH and (D) CB1 protein expression in ventral striatum induced by PLAE and the subsequent exposure to a standard diet or a high-fat diet (HFD) for 8 weeks. Representative pictures of the immunoblots are shown below the bar charts. Images of the whole immunoblot membranes can be found in supplementary Figure 1. Results are expressed as mean ± SEM (N = 5/group). *P < .05, **P < .01 vs water-standard diet.
Molecular Determinations
To find a possible explanation to the differences observed in food intake, the expression of genes induced by ghrelin was determined in the hypothalamus (set 1 of animals). In parallel, as an attempt to explain the results observed from the cocaine-induced psychostimulation, [3H]DA uptake in striatal synaptosomes (set 2) and the expression of tyrosine hydroxylase (TH) and cannabinoid 1 receptor (CB1) in ventral striatum was evaluated (set 3). Finally, the expression levels of proteins involved in neuronal and myelin damage were measured in the PFC (set 3) in view of previous studies using the male offspring from mice subjected to the DID procedure (Cantacorps et al., 2017): NeuN, nuclear factor κB (NF-κB), and myelin basic protein (MBP).
Effects on Ghrelin-Induced Genes
In the hypothalamus, we determined by qPCR the mRNA levels of genes that are induced by ghrelin and promote food intake: neuropeptide Y (NPY) (Figure 3E) and Agouti-related protein (AgRP) (water-standard diet:1.000 ± 0.31, alcohol-standard diet: 1.193 ± 0.425, W-HFD: 0.873 ± 0.191, A-HFD: 1.226 ± 0.409), and no significant changes were observed (N = 5/group). We also measured the mRNA levels of CPT1C as a mediator of ghrelin signaling, showing reduced levels in the alcohol-exposed groups that almost reached statistical significance [F(1,16) = 4.263; P = .055] (Figure 3F).
Effects on [3H]DA Uptake
Striatal synaptosome suspensions were prepared to perform [3H]DA uptake experiments. Two-way ANOVA yielded a significant effect of diet [F(1,29) = 16.28; P < .001]. Accordingly, mice exposed to HFD presented lower DA uptake activity than those fed with standard diet (P < .01 and P < .05 vs the water and alcohol-matching groups, respectively) (Figure 5B).
Effects on TH and CB1 Receptor Expression in Ventral Striatum
As shown in Figure 5C, the expression of TH was not affected by either alcohol or diet. By contrast, 2-way ANOVA of the results of CB1 expression yielded a significant effect of diet [F(1,16) = 6.576; P < .05] and alcohol [F(1,16 ) = 21.14; P < .001] with interaction between both factors [F(1,16) = 10.27; P < .01] (Figure 5D). Bonferroni post-hoc multiple comparisons showed a significant increase in CB1 receptor expression in W-HFD mice compared with water-standard diet (P < .01). However, such overexpression was not present in animals fed with HFD and exposed to alcohol. (Pictures of the whole immunoblot membranes can be seen as supplementary Materials).
Neuronal and Myelin Damage Within the Prefrontal Cortex
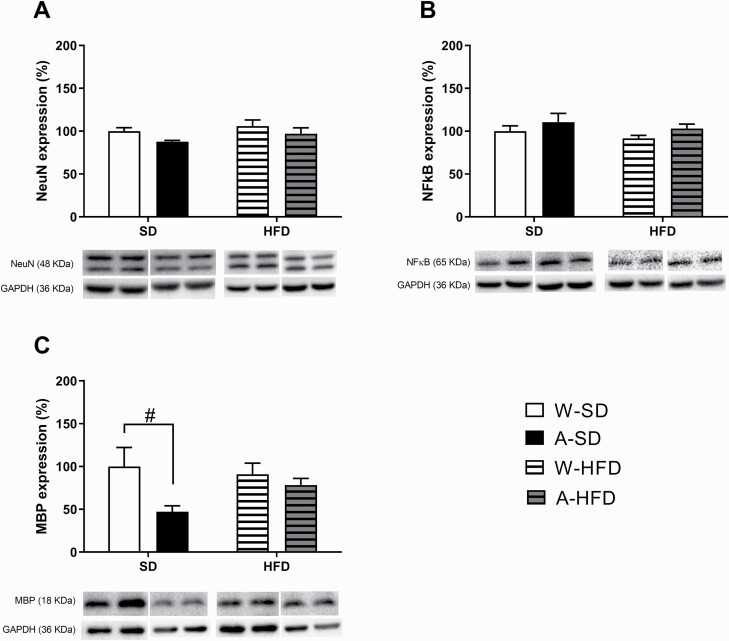
In our study using female offspring, no differences were observed in NeuN or NFκB/p65 expression (Figure 6A–B). However, 2-way ANOVA of the results of MBP expression revealed a significant effect of alcohol [F(1,16) = 5.000; P < .05] (Figure 6C). Therefore, alcohol exposure significantly decreased MBP protein levels, mainly in animals fed with SD (P = .05).
Figure 6.
Effects on (A) NeuN, (B) NFκB, and (C) MBP protein expression in prefrontal cortex induced by PLAE and the subsequent exposure to a standard diet or a high-fat diet (HFD) for 8 weeks. Results are expressed as mean ± SEM. #P < .05 vs water-standard diet. (N = 5/group).
Discussion
The present study aimed to assess the interaction between alcohol exposure during gestation and lactation and an HFD during the female offspring’s childhood and adolescence on some nutrition-related parameters as well as on the response to cocaine.
In our study, there was no contribution of PLAE to offspring’s body weight either, in agreement with previous studies from our group (Cantacorps et al., 2017). Moreover, we did not observe any significant weight gain in mice fed an HFD with respect to the standard diet group with previous studies (Hwang et al., 2010; Sims et al., 2013; Hicks et al., 2016). When food intake was measured, HFD-fed mice reduced the grams of ingested food from week 6 until the end of the exposure, thus equaling their kcal/day intake to those of the standard diet group. Importantly, the equal intake of kcal is in line with the nonsignificant differences observed in body weight between W-HFD and W-standard diet mice at the end of the experiment. Lin et al. (2000), using a similar HFD-feeding protocol, showed that HFD-induced obesity in mice could be divided into early (1 week), middle (until 8 weeks), and late stages. Mice fed for 8 weeks with HFD showed a very slight increased body weight, but energy intake fell below control levels during this period, which began to increase gradually after 8 weeks. In addition, previous studies have described that male C57BL/6J mice on HFD are more susceptible to weight gain than females (Gelineau et al., 2017), and weight gain appears earlier in males (46 days old) than in females (129 days old) (Hwang et al., 2010). Overall, we can reasonably deduce that, in our model, female offspring were probably in a pre-obesity state and thus they needed a longer exposure period on the HFD (more than 16–20 weeks) (Wang and Liao, 2013) to develop an obesity status.
TG and glucose blood levels were also assessed. The HFD significantly increased TG blood levels but not those of glucose. It is already known that C57BL/6 mice, even when fed with high-sucrose diets (65%), do not develop insulin resistance (Gajda et al., 2007). Nonetheless, female C57BL/6J mice are particularly resistant to the physiological changes caused by HFD, and they exhibit a better glucose tolerance than males (Gelineau et al., 2017). Although we used a strain of C57BL/6 mice with a slightly different genetic background (from Charles River Labs) and strain-dependent differences could not be completely ruled out, our results for glucose levels and metabolic effects are in line with those previously reported by other groups using the J strain. Importantly, no additional effects of PLAE were evidenced on TG and glucose blood levels.
Additionally, PLAE affected how C57BL/6 mice responded to the HFD in such a way that, during almost the whole feeding period, A-HFD mice ate less grams of food, and thus, less kcal, than the W-HFD, consistent with their lower weight gain. Indeed, similar results were observed by Amos-Kroohs et al. (2018). Importantly, such lower weight gain cannot be attributed to an increased basal locomotor activity of these animals since no differences were observed in their basal locomotion. However, regarding the effects of PLAE and HFD on ghrelin-induced genes, although no changes were observed in NPY or AgRP mRNA expression, an apparent decrease of the CPTC1 expression in A-HFD mice should be noted. Hypothalamic CPT1C mediates the central effects of leptin and ghrelin on feeding behavior (Gao et al., 2011; Ramírez et al., 2013). More specifically, besides other mechanisms, ghrelin induces food intake through regulation of hypothalamic CPT1C, so the orexigenic action of ghrelin is totally blunted in CPT1C knockout mice (Ramírez et al., 2013). Accordingly, aside from the metabolic change induced by HFD, which caused an overall decrease in food intake, PLAE seemed to additionally induce an apparent decrease in CPTC1 expression, a feature that might be involved in the lower weight gain observed in the A-HFD animals.
FASD is associated with a higher risk of later developing drug abuse. Our results showed that PLAE and/or a HFD did not modify CPP acquisition; thus, the rewarding effects of cocaine were not altered. Consistent with our findings, Blanco-Gandía et al. (2017) suggested that consumption of an HFD during adolescence in male mice induces neurobiochemical changes that increased sensitivity to cocaine, but only when fat is withdrawn, acting as an alternative reward. Moreover, locomotion induced by an acute dose of cocaine was assayed as indicative of its psychostimulant effect. Data in rats indicate that the consumption of fat and, perhaps, the resulting hormonal changes markedly alter dopamine systems (Baladi et al., 2010). In agreement with Collins et al. (2015), we observed an increased sensitivity to the psychostimulant effect of acute cocaine in animals fed with HFD. This is of chief importance since it provides evidence that consuming an HFD during early development enhances the psychostimulant effect of cocaine and thus might favor and increase the probability of repeating and perpetuating the use of the substance, ultimately leading to its abuse. Surprisingly, the A-HFD mice did not show the increased response to the first dose of cocaine compared with the standard diet group as the W-HFD group did. It is known that reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after an HFD (Barnes et al., 2020). In our study, when assessing [3H]DA uptake in striatal synaptosomes, we found that the HFD reduced the overall [3H]DA uptake in both the A-HFD and W-HFD groups, implicating diet in the regulation of DA function, which can lead to functional modifications in DA signaling (South and Huang, 2008; Cone et al., 2013). By decreasing DA uptake, HFD consumption could promote adaptations such as downregulation of DA receptors, a feature of both human and rodent models of obesity (Wang et al., 2009; Johnson and Kenny, 2010). Cone et al. (2013) described that prolonged HFD appears to reduce dopamine transporter (DAT) trafficking or perhaps maturation but not DAT gene expression. Therefore, diet-related decreases in membrane DAT could precede and contribute to the onset of DA receptor downregulation as well as obesity and compulsive eating behavior that develop over the course of HFD consumption (Johnson and Kenny, 2010).
Cocaine increases the motor activity by facilitating DA signaling via the mesostriatal pathway through both DAT-dependent and independent mechanisms (Gardner and Ashby, 2000). Given the effects on DA uptake, it seems reasonable that the W-HFD group elicited an enhanced psychostimulant response to an acute dose of cocaine, as more DA would be available in the synapses and the proportion of DAT blocked by the same dose of cocaine would be higher. However, the A-HFD mice did not show enhanced hyperlocomotion by cocaine. Several mechanisms can be hypothesized to explain such difference: probably alcohol reduced the cocaine-induced hyperlocomotion in the HFD group by other mechanisms than DA synthesis or DA uptake, which could not be explained in this study but correlates with other parameters induced by HFD but dampened by PLAE. Another possible cause may be the increased CB1 receptors, which appeared only in the W-HFD group and play an important role in dopamine transmission, as discussed below. Finally, an increased sensitivity of postsynaptic dopamine receptors as a result of CB1 increase or any other undetermined mechanism cannot completely be ruled out.
We also assessed 2 proteins related to dopaminergic neurotransmission in the ventral striatum: TH and CB1 receptor. In our model, the expression of the rate-limiting enzyme in DA synthesis, TH, was not affected by alcohol or diet. By contrast, and as observed for the acute response to cocaine, HFD induced an increase in CB1 receptors but only in the animals not exposed to alcohol. Stimulation of CB1 receptors is a key component in the development of diet-induced obesity in such a way that CB1 receptor knockout mice display a hypofagic behavior and reduced body weight (Ravinet Trillou et al., 2004). The fact that the A-HFD group in our study did not present an increase in CB1 receptors probably contributed to a reduced predisposition to obesity due to early alcohol exposure.
There are functional interactions between endocannabinoid and dopaminergic systems that may contribute to striatal signaling. More concretely, the endogenous cannabinoid system is a key regulatory element of the plastic changes associated with cocaine-induced behavioral responses in the rat. Corbillé et al. (2007) investigated the role of CB1 receptor in the effects of a single injection of psychostimulants and found that locomotor responses to cocaine and D-amphetamine were decreased in CB1 receptor-deficient mice. Their results provided strong evidence for the role of the endocannabinoid system in regulating neuronal circuits critical for cocaine effects, as ERK phosphorylation, presumably by acting on CB1 receptors located on terminals of striatal medium spiny neurons. Additionally, Gessa et al. (1998) used CB1−/− mice with a C57BL/6J genetic background to further investigate the role of CB1 receptors in cocaine action. CB1−/− mice displayed a significant reduction in cocaine-enhanced locomotion related to a reduction in DA release. Accordingly, pharmacological blockade of CB1 receptors inhibited cocaine-induced hyperlocomotion and DA release in CB1+/+ mice. All these findings suggest an important role for CB1 receptors in mediating the psychostimulant effects of cocaine. Therefore, an increase of CB1, as seen in the W-HFD group, combined with the reduced DA uptake could contribute to the enhanced hyperlocomotion induced by this psychostimulant.
Recent studies have demonstrated that alcohol intake activates the innate immune system in the central nervous system, leading to neuroinflammation and contributing to brain damage and behavioral dysfunctions (Montesinos et al., 2016). In this context, we have already reported that PLAE increases pro-inflammatory markers, alters the expression of myelin proteins, and induces neuron cell damage in the PFC of male C57BL/6 mice (Cantacorps et al., 2017). Reduction of MBP levels in certain brain areas after alcohol exposure during development has also been described by other research groups (Bichenkov and Ellingson, 2009). In the present study, effects of PLAE were evident in MBP protein expression in such a way that alcohol exposure induced a decrease in MBP. Regarding NeuN expression, although a significant decrease was found in alcohol-standard diet mice compared with water-standard diet mice (t7 = 2.557, P < .05), such difference was blunted until being nonsignificant when also considering the variable diet in the analysis. Therefore, we could confirm that the effects of PLAE on NeuN and on MBP expression were the same in both sexes, albeit female offspring seemed more resistant to such effects than males. Furthermore, all the alterations induced by PLAE were blunted in HFD groups; thereby, a short exposure to a fatty diet seems to counteract the deleterious effects of prenatal and lactational alcohol exposure. It could be hypothesized that the fatty diet would supply extra lipids that could overcome the deleterious effects of alcohol on myelinization.
In summary, in our model, feeding young female mice with an HFD for 8 weeks increased their TG blood levels but did not induce overweight compared with those fed a standard diet. Moreover, PLAE affected how females responded to the fatty diet as they consumed less amount of food, consistent with their lower weight gain. Regarding their response to cocaine, the HFD reduced DA uptake and increased the acute hyperlocomotion induced by the drug without modifying its rewarding effects. Surprisingly, PLAE attenuated such increment in the cocaine-induced locomotion and overexpression of CB1 receptors induced by the HFD. At the same time, the effect of PLAE on MBP was reduced by the HFD.
Altogether, the results suggest the existence of a crosstalk among the mechanisms involved in such changes so that in the first period of exposition to an HFD, there might be a mutual attempt to compensate the effects induced by both conditions (PLAE and HFD). However, further investigation is needed to unveil what would occur with a longer exposure to a fatty diet.
Supplementary Materials
Supplementary data are available at International Journal of Neuropsychopharmacology (IJNPPY) online
Acknowledgments
This study was supported by Ministerio de Economia y Competitividad (grant nos. SAF2016-75347-R, SAF2016-75966-R-FEDER, SAF2017-83813-C3-1-R), Ministerio de Sanidad, Asuntos Sociales e Igualdad (Retic-ISCIII, RD16/017/010), and Plan Nacional sobre Drogas 2018I007, 2016I004). Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y la Nutrición (CIBEROBN) (grant CB06/03/0001 to D.S.). Fundació LaMarató de TV3 (grant 201627–30 to D.S.). L.D.C. received FPU grants from the Ministerio de Economía y Competitividad (15/02492). J.C., D.P., R.L.A., and E.E. belong to institutionally-recognized research consolidated groups 2017SGR979 and O.V. to 2017SGR109. The funding sources had no further involvement in the study.
Statement of Interest
None.
References
- Amos-Kroohs RM, Nelson DW, Hacker TA, Yen CE, Smith SM (2018) Does prenatal alcohol exposure cause a metabolic syndrome? (Non-)evidence from a mouse model of fetal alcohol spectrum disorder. PLoS One 13:e0199213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP (2010) Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behav Pharmacol 21:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Pierrefiche O, Vaudry D, Vaudry H, Daoust M, Naassila M (2008) Long-term alterations in vulnerability to addiction to drugs of abuse and in brain gene expression after early life ethanol exposure. Neuropharmacology 55:1199–1211. [DOI] [PubMed] [Google Scholar]
- Barnes CN, Wallace CW, Jacobowitz BS, Fordahl SC (2020) Reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after a diet high in saturated but not unsaturated fat. Nutr Neurosci 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichenkov E, Ellingson JS (2009) Ethanol alters the expressions of c-Fos and myelin basic protein in differentiating oligodendrocytes. Alcohol 43:627–634. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandía MC, Cantacorps L, Aracil-Fernández A, Montagud-Romero S, Aguilar MA, Manzanares J, Valverde O, Miñarro J, Rodríguez-Arias M (2017) Effects of bingeing on fat during adolescence on the reinforcing effects of cocaine in adult male mice. Neuropharmacology 113:31–44. [DOI] [PubMed] [Google Scholar]
- Cantacorps L, Alfonso-Loeches S, Moscoso-Castro M, Cuitavi J, Gracia-Rubio I, López-Arnau R, Escubedo E, Guerri C, Valverde O (2017) Maternal alcohol binge drinking induces persistent neuroinflammation associated with myelin damage and behavioural dysfunctions in offspring mice. Neuropharmacology 123:368–384. [DOI] [PubMed] [Google Scholar]
- Cantacorps L, Montagud-Romero S, Luján MÁ, Valverde O (2020) Prenatal and postnatal alcohol exposure increases vulnerability to cocaine addiction in adult mice. Br J Pharmacol 177:1090–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agrò A, Bernardi G, Calabresi P, Maccarrone M (2004) A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology 29:1488–1497. [DOI] [PubMed] [Google Scholar]
- Collins GT, Chen Y, Tschumi C, Rush EL, Mensah A, Koek W, France CP (2015) Effects of consuming a diet high in fat and/or sugar on the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice. Exp Clin Psychopharmacol 23:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF (2013) Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. Plos One 8:e58251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, Hervé D, Girault JA (2007) Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci 27:6937–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G (2001) Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410:822–825. [DOI] [PubMed] [Google Scholar]
- Duart-Castells L, López-Arnau R, Buenrostro-Jáuregui M, Muñoz-Villegas P, Valverde O, Camarasa J, Pubill D, Escubedo E (2019) Neuroadaptive changes and behavioral effects after a sensitization regime of MDPV. Neuropharmacology 144:271–281. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE (2007) Mouse models in aging research. The mouse in biomedical research. 2nd ed. Vol. 3. Normative Biology, Husbandry, and Models. Cambridge, MA: Academic Press. Faculty Research 2000–2009. 1685. [Google Scholar]
- Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066. [DOI] [PubMed] [Google Scholar]
- Fuglestad AJ, Boys CJ, Chang PN, Miller BS, Eckerle JK, Deling L, Fink BA, Hoecker HL, Hickey MK, Jimenez-Vega JM, Wozniak JR (2014) Overweight and obesity among children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 38:2502–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda AM, Pellizzon MA, Ricci MR, Ulman EA (2007) Diet-induced metabolic syndrome in rodent models. Animal Lab News 74:775–793. [Google Scholar]
- Gao S, Zhu G, Gao X, Wu D, Carrasco P, Casals N, Hegardt FG, Moran TH, Lopaschuk GD (2011) Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc Natl Acad Sci U S A 108:9691–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Ashby CR Jr (2000) Heterogeneity of the mesotelencephalic dopamine fibers: physiology and pharmacology. Neurosci Biobehav Rev 24:115–118. [DOI] [PubMed] [Google Scholar]
- Gelineau RR, Arruda NL, Hicks JA, Monteiro De Pina I, Hatzidis A, Seggio JA (2017) The behavioral and physiological effects of high-fat diet and alcohol consumption: sex differences in C57BL6/J mice. Brain Behav 7:e00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM (2003) Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol 140:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M (1998) Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341:39–44. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2:358–363. [DOI] [PubMed] [Google Scholar]
- Grant TM, Brown NN, Dubovsky D, Sparrow J, Ries R (2013) The impact of prenatal alcohol exposure on addiction treatment. J Addict Med 7:87–95. [DOI] [PubMed] [Google Scholar]
- Hicks JA, Hatzidis A, Arruda NL, Gelineau RR, De Pina IM, Adams KW, Seggio JA (2016) Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL6/J mice exposed to a high-fat diet. Behav Brain Res 310:1–10. [DOI] [PubMed] [Google Scholar]
- Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC (2010) Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 18:463–469. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM (2001) Endogenous cannabinoids and appetite. Nutr Res Rev 14:65–86. [DOI] [PubMed] [Google Scholar]
- Lin S, Thomas TC, Storlien LH, Huang XF (2000) Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 24:639–646. [DOI] [PubMed] [Google Scholar]
- Luján MA, Cantacorps L, Valverde O (2019) The pharmacological reduction of hippocampan neurogenesis attenuates the protective effects of cannabidiol on cocaine voluntary intake. Addict Biol 4:e12778. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232. [DOI] [PubMed] [Google Scholar]
- Mir JF, Zagmutt S, Lichtenstein MP, García-Villoria J, Weber M, Gracia A, Fabriàs G, Casas J, López M, Casals N, Ribes A, Suñol C, Herrero L, Serra D (2018) Ghrelin causes a decline in GABA release by reducing fatty acid oxidation in cortex. Mol Neurobiol 55:7216–7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S, Guerri C (2016) Impact of the innate immune response in the actions of ethanol on the central nervous system. Alcohol Clin Exp Res 40:2260–2270. [DOI] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ (2003) Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther 306:880–888. [DOI] [PubMed] [Google Scholar]
- Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884. [DOI] [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallàs M, Camarasa J, Escubedo E (2005) Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol 204:57–68. [DOI] [PubMed] [Google Scholar]
- Pubill D, Garcia-Ratés S, Camarasa J, Escubedo E (2013) 3,4-Methylenedioxy-methamphetamine induces in vivo regional up-regulation of central nicotinic receptors in rats and potentiates the regulatory effects of nicotine on these receptors. Neurotoxicology 35:41–49. [DOI] [PubMed] [Google Scholar]
- Ramírez S, Martins L, Jacas J, Carrasco P, Pozo M, Clotet J, Serra D, Hegardt FG, Diéguez C, López M, Casals N (2013) Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 62:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Sims EK, Hatanaka M, Morris DL, Tersey SA, Kono T, Chaudry ZZ, Day KH, Moss DR, Stull ND, Mirmira RG, Evans-Molina C (2013) Divergent compensatory responses to high-fat diet between C57Bl6J/J and C57BLKS/J inbred mouse strains. Am J Psysiol Endocrinol Metab 305:E1495–E1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T, Huang XF (2008) High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res 33:598–605. [DOI] [PubMed] [Google Scholar]
- Wang CY, Liao JK (2013) A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 821:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS (2009) Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med 3:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts RL, Van Calcar SC, Wargowski DS, Smith SM (2014) Inappropriate feeding behaviors and dietary intakes in children with fetal alcohol spectrum disorder or probable prenatal alcohol exposure. Alcohol Clin Exp Res 38:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau YH, Potenza MN (2013) Stress and eating behaviors. Minerva Endocrinol 38:255–267. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.