Abstract
Mycoheterotrophic plants (MHPs) are leafless, achlorophyllous, and completely dependent on mycorrhizal fungi for their carbon supply. Mycorrhizal symbiosis is a mutualistic association with fungi that is undertaken by the majority of land plants, but mycoheterotrophy represents a breakdown of this mutualism in that plants parasitize fungi. Most MHPs are associated with fungi that are mycorrhizal with autotrophic plants, such as arbuscular mycorrhizal (AM) or ectomycorrhizal (ECM) fungi. Although these MHPs gain carbon via the common mycorrhizal network that links the surrounding autotrophic plants, some mycoheterotrophic lineages are associated with saprotrophic (SAP) fungi, which are free-living and decompose leaf litter and wood materials. Such MHPs are dependent on the forest carbon cycle, which involves the decomposition of wood debris and leaf litter, and have a unique biology and evolutionary history. MHPs associated with SAP fungi (SAP-MHPs) have to date been found only in the Orchidaceae and likely evolved independently at least nine times within that family. Phylogenetically divergent SAP Basidiomycota, mostly Agaricales but also Hymenochaetales, Polyporales, and others, are involved in mycoheterotrophy. The fungal specificity of SAP-MHPs varies from a highly specific association with a single fungal species to a broad range of interactions with multiple fungal orders. Establishment of symbiotic culture systems is indispensable for understanding the mechanisms underlying plant–fungus interactions and the conservation of MHPs. Symbiotic culture systems have been established for many SAP-MHP species as a pure culture of free-living SAP fungi is easier than that of biotrophic AM or ECM fungi. Culturable SAP-MHPs are useful research materials and will contribute to the advancement of plant science.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10265-020-01244-6.
Keywords: In vitro culture, Litter decay fungi, Orchid, Stable isotopes, Wood decay fungi
Introduction
Mycoheterotrophic plants (MHPs) are non-photosynthetic and thus completely reliant on mycorrhizal fungi for carbon uptake throughout their lifecycle (Leake 1994). Most MHPs have small vegetative organs and have an underground root/rhizome system as the main body, emerging aboveground only for reproduction. Such extreme evolution occurred independently over 40 times in all divisions of land plants, and there are ca. 580 species of MHPs (Jacquemyn and Merckx 2019). The evolution of mycoheterotrophy was accompanied by dramatic changes in a variety of characteristics, such as morphology (Leake 1994), mycorrhizal symbiosis (Ogura-Tsujita et al. 2012), pollination systems (Suetsugu 2015), seed dispersal systems (Suetsugu et al. 2015; Suetsugu 2018), and genome size and content (Barrett and Davis 2012). MHPs are therefore expected to be useful models in plant science.
Mycorrhizal symbiosis between plants and fungi is a ubiquitous type of mutualism, in which autotrophic plants exchange photosynthesized carbon for mineral nutrients obtained by mycorrhizal fungi (Smith and Read 2008). However, mycoheterotrophy represents a breakdown of this mutualism, as plants obtain carbon from fungi without photosynthesis (Merckx and Bidartondo 2008). Molecular studies for mycobiont identification have revealed two main mycorrhizal systems supporting carbon gain by MHPs; namely, via the mycorrhizal fungi of autotrophic plants —arbuscular mycorrhizal (AM) and ectomycorrhizal (ECM) fungi—and via free-living saprotrophic (SAP) fungi (Waterman et al. 2013). These two mycorrhizal systems use different carbon sources —MHPs associated with AM (AM-MHPs) and ECM fungi (ECM-MHPs) obtain carbon from surrounding autotrophic plants through shared mycorrhizal fungi, whereas MHPs associated with SAP fungi (SAP-MHPs; Fig. 1) obtain carbon from plant debris through the decomposition of wood and leaf litter. Most of the litter- or wood-decay fungi associated with SAP-MHPs are rarely found as mycorrhizal fungi in autotrophic land plants. The biology of AM- and ECM-MHPs has been reviewed by others (Bidartondo 2005; Leake 1994; Merckx 2013). However, the diversity of SAP-MHPs was elucidated only recently despite its discovery by Kusano in 1911 A critical advantage of SAP-MHPs is the feasibility of symbiotic culture. Because a pure culture of free-living SAP fungi is easier than that of biotrophic AM or ECM fungi, culture systems for several SAP-MHPs have been established (Burgeff 1936; Xu and Guo 2000; Yagame et al. 2007). These enable key questions of mycoheterotrophy to be addressed and facilitate the conservation of endangered species. Here, we review SAP-MHPs with emphasis on their evolutionary history and mycorrhizal associations. We also introduce case studies of symbiotic culture of SAP-MHPs and discuss future perspectives.
Fig. 1.
Mycoheterotrophic species associated with saprotrophic (SAP) fungi. a Cremastra aphylla, b Gastrodia confusa, c Cyrtosia septentrionalis, d Erythrorchis altissima, e Yoania flava, f Gastrodia nipponica, and g a tuber of Gastrodia elata. Black rhizomorphs are attached to the surface of the tuber. h In vitro symbiotic culture of E. altissima (photo by H Umata), and i in vitro symbiotic germination of G. nipponica seeds
Mycoheterotrophy
The evolution from autotrophy to mycoheterotrophy is a stepwise process involving the reduction of foliage leaves and chlorophyll content. Leafless and achlorophyllous MHPs are categorized as fully mycoheterotrophic; partial and initial mycoheterotrophy are also recognized in land plants (Merckx 2013). Partial MHPs retain normal chlorophyllous leaves and have the ability to obtain carbon from both photosynthesis and mycorrhizal fungi (Gebauer and Meyer 2003). Initial MHPs are dependent on their mycorrhizal fungi for carbon supply during the early stages of their life history and subsequently develop into autotrophic mature plants (Merckx 2013). Initial mycoheterotrophy has been observed in seed plants producing small dust-like seeds, such as in Orchidaceae and Pyroleae in Ericaceae, and also in the gametophytes of lycophytes and pteridophytes (Merckx 2013). Partial and initial mycoheterotrophy are thought to be an intermediate stage in the transition from autotrophy to mycoheterotrophy and provide insight into the evolution of the latter (Ogura-Tsujita et al. 2012).
The definitions of partial and full mycoheterotrophy are unclear. Foliage leaves are substantially reduced but still develop in some species, such as Cephalanthera subaphylla Miyabe and Kudô (Orchidaceae). Furthermore, some leafless MHPs have chlorophyllous reproductive shoots, indicating that photosynthesis is active during flowering and fruiting (Suetsugu et al. 2018; Zimmer et al. 2008 but also see Cameron et al. 2009). Partial and full mycoheterotrophy can be distinguished by their stable isotope signatures (Suetsugu et al. 2018; Zimmer et al. 2008; see also the section “Isotopic signature of SAP-MHPs”), but the level of mycoheterotrophy has not been evaluated for most SAP-MHPs. Therefore, in this review, species that lack foliage leaves are defined as full MHPs. Leafless species that develop chlorophyllous reproductive shoots are included as full MHPs because photosynthesis is limited to the reproductive phase. Species that have small, chlorophyllous foliage leaves are excluded from full MHP status because the leaves, the principal photosynthetic apparatus, function during the growth period. Further, leafless lianas with chlorophyllous stems such as Pseudovanilla foliata (F.Muell.) Garay and Vanilla aphylla Blume (Orchidaceae), and leafless epiphytes with chlorophyllous roots such as Dendrophylax and Taeniophyllum (Orchidaceae) are not considered MHPs because the stems or roots are photosynthetic and function throughout the life history of such species.
Mycorrhizal symbiosis in MHPs
Three phylogenetically and physiologically distinct fungal groups are involved in mycorrhizal symbiosis in MHPs—AM, ECM, and SAP fungi (Waterman et al. 2013). AM or ECM fungi obtain carbon from their autotrophic host plants through mutualistic relationships. The AM association is the most dominant mycorrhizal symbiosis type in land plants, with more than 71% of mycorrhizal plant species associated with AM fungi (Brundrett and Tedersoo 2018). ECM fungi are mostly associated with particular tree families, such as Pinaceae and Fagaceae, and are the dominant mycorrhizal type in boreal and temperate forests (Smith and Read 2008). AM or ECM fungi are simultaneously associated with MHPs, and thus, AM- or ECM-MHPs obtain photosynthesized carbon from the surrounding autotrophic plants via shared mycorrhizal mycelia. This tripartite symbiosis allows MHPs access to the common mycorrhizal networks of AM or ECM fungi that link the surrounding autotrophic plants.
By contrast, SAP-MHPs depend on nonliving biomass. Mycoheterotrophic associations with free-living litter- or wood-decay fungi are dependent on the forest carbon cycle (Ogura-Tsujita et al. 2018; Suetsugu et al. 2020b). The decomposition of woody debris and leaf litter by SAP fungi plays a key role in regulating carbon and nutrient cycles in forest ecosystems (Berg and McClaugherty 2008). Woody debris is a major component of forest biomass, and this large carbon store represents up to 20% of the total aboveground biomass (Bradford et al. 2009; Laiho and Prescott 1999). MHPs dependent on SAP fungi can access the carbon pool via associations with litter- or wood-decay fungi, a pathway of carbon gain unique among land plants. Although carbon flow in tripartite symbiosis has been studied using stable isotopic signatures (Gebauer and Meyer 2003; Hynson et al. 2016) and labeled isotopes (Bougoure et al. 2010; McKendrick et al. 2000), carbon acquisition from plant debris in SAP-MHPs is less well understood than that in AM- and ECM-MHPs.
Phylogeny and evolution of SAP-MHPs
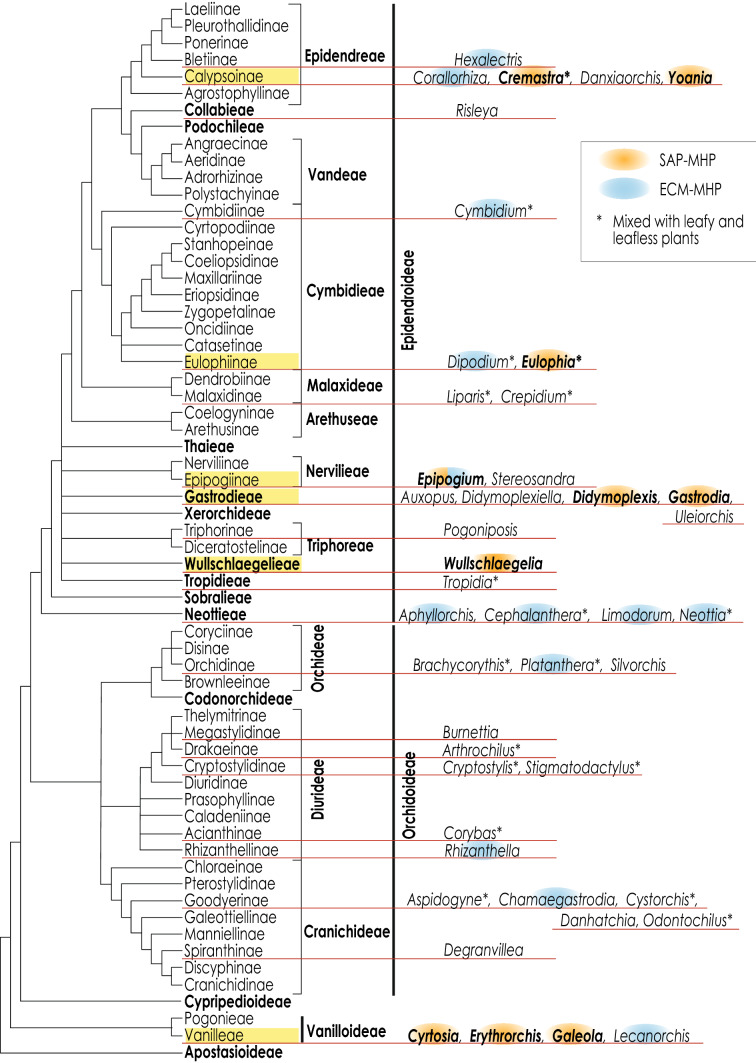
Among the MHPs for which the mycorrhizal fungi have been surveyed, SAP-MHPs include 28 species from 10 genera, all belonging to Orchidaceae (Fig. 2; Table 1). The evolutionary tracks of mycoheterotrophy within Orchidaceae were traced in a phylogenetic tree covering all of the major clades of the family (Chase et al. 2015; Fig. 2). It is likely that full mycoheterotrophy evolved independently at least 41 times, and that SAP-associated mycoheterotrophy (SAP-MH) evolved at least nine times within Orchidaceae (Fig. 2). When a group includes only SAP-MHPs, SAP-MH evolved in their common ancestor, whereas when a group comprises a mixture of SAP-MHPs and species associated with other mycorrhizal types, SAP-MH evolved in that clade. SAP-MHPs are found in the second-basal-most subfamily Vanilloideae and five tribes of the latest diverged subfamily Epidendroideae, showing that SAP-MH evolved in various lineages in the family.
Fig. 2.
Occurrence of mycoheterotrophy within Orchidaceae. Evolutionary tracks of mycoheterotrophy are traced on a phylogenetic tree covering all major clades of the family (Chase et al. 2015). Tribes or subtribes that include saprophytic fungi-associated mycoheterotrophic plants (SAP-MHPs) are colored yellow. Genera that include SAP or ectomycorrhizal (ECM) fungi-associated MHPs (ECM-MHPs) are colored red and blue, respectively. The genus Epipogium comprises both SAP- and ECM-associated species. Genera of unknown mycorrhizal status are not colored. Asterisks indicate genera that include both leafy and leafless species
Table 1.
Mycoheterotrophic species associated with litter- or wood-decaying fungi, and the taxonomic affiliations of their mycobionts
| Plant taxa | Taxonomic affiliation of mycobionts | Analysesa | References | Notes | |
|---|---|---|---|---|---|
| Order | Taxa | ||||
| Cremastra aphylla | Agaricales | Coprinellus domesticus, Coprinellus sp. | Molecular identification, sporocarp formation | Yagame et al. (2018) | One of the isolates was identified as C. domesticus. |
| Cyrtosia javanica | Polyporales | Meripilaceae | Molecular identification, stable isotopes | Lee et al. (2015) | The Meripilaceae fungi were identified as Physisporinus by Yamashita et al. (2020). |
|
C. septentrionalis (Galeola septentrionalis)b |
Agaricales | Armillaria mellea | Rhizomorph morphology, isolate characteristics | Hamada (1939, 1940) | |
| Armillaria mellea | Symbiotic culture | Terashita (1985) | Aseptic seedlings formed mycorrhizae with A. mellea. | ||
| Armillaria tabescens | Sporocarp formation | Terashita and Chuman (1987) | |||
| Armillaria borealis, A. cepistipes, A. gallica (A. bulbosa), A. mellea, A. tabescens | SI test | Terashita and Chuman (1989), Terashita (1996) | Possibly A. borealis, but further identification is required. | ||
| Armillaria | Isozyme | Matsushita et al. (1996) | The fungal isolates were assigned to four biological species. | ||
| Armirallia jezoensis | SI test | Cha and Igarashi (1996) | |||
| Armirallia jezoensis | PCR-RFLP | Terashima et al. (1998) | |||
| Armillaria mellea | SI test, RAPD | Ota et al. (2000) | |||
| Armillaria gallica, A. mellea, A. tabescens | Symbiotic culture | Umata et al. (2013) | Seed germination was stimulated, but no further growth was observed. | ||
| Polyporales | Meripilaceae | Symbiotic culture, molecular identification | Umata et al. (2013) | Seed germination and following seedling growth were promoted. The Meripilaceae fungus was identified as Physisporinus by Yamashita et al. (2020). | |
| Russulales | Xylobolus annosus | Symbiotic culture | Umata et al. (2013) | Seed germination was stimulated, but no further growth was observed. | |
| Cantharellales | Rhizoctonia repens | Symbiotic culture | Masuhara and Katsuya (1991) | Aseptic seedlings formed mycorrhizae with R. repens. | |
| – | – | – | Nakamura et al. (1975), Nakamura (1982) | The aseptic seed germination was observed. | |
| – | – | – | Umata et al. (2006) | No fungal peloton was observed in the protocorms obtained from in situ seed germination. | |
| – | – | Stable isotopes | Motomura et al. (2010) | ||
| – | – | Radiocarbon | Suetsugu et al. (2020b) | ||
| Didymoplexis micradenia (D. minor) b | Agaricales | Marasmius coniatus var. didymoplexis | Symbiotic culture, sporocarp formation | Burgeff (1932, 1936, 1959) | |
| D. pallens | Agaricales | Marasmius coniatus var. didymoplexis | Symbiotic culture, sporocarp formation | Burgeff (1932, 1936, 1959) | |
| – | – | – | Irawati (2002) | Aseptic seedlings produced inflorescences. | |
| Epipogium roseum | Agaricales | Coprinellus (Coprinus)b, Psathyrella | Molecular identification | Yamato et al. (2005) | |
| Coprinellus | Molecular identification, symbiotic culture | Yagame et al. (2007) | |||
| Coprinellus disseminatus | Sporocarp formation | Yagame et al. (2008) | |||
|
Erythrorchis altissima (Galeola altissima, E. ochobiensis) b |
Hymenochaetales | Erythromyces crocicreas (Hymenochaete crociceras)b | Isolate characteristics | Hamada and Nakamura (1963) | |
| Agaricales | Lentinula edodes | Symbiotic culture | Umata (1998a) | See also supplemental information of Ogura-Tsujita et al. (2018) for a series of studies by Umata. | |
| Lyophyllum shimeji | Symbiotic culture | Umata (1997a) | Seed germination was stimulated, but no promotive effect for further development. | ||
| Pleurotus ostreatus | Symbiotic culture | Umata et al. (2000a) | |||
| Gymnopus, Hypholoma, Mycena, Neonothopanus | Molecular identification | Ogura-Tsujita et al. (2018) | The Gymnopus sequence was nested within the Marasmiellus clade (Fig. S2). | ||
| Atheliales | Athelia | Molecular identification | Ogura-Tsujita et al. (2018) | ||
| Auriculariales | Auricularia polytricha | Symbiotic culture | Umata (1997b) | ||
| Cantharellales | Ceratobasidium, Tulasnella | Molecular identification | Ogura-Tsujita et al. (2018) | ||
| Corticiales | Vuilleminia | Molecular identification | Ogura-Tsujita et al. (2018) | ||
| Hymenochaetales | Erythromyces crocicreas | Symbiotic culture | Umata (1995, 1998b) | ||
| Phellinus sp. | Symbiotic culture | Umata (1995, 1998b) | |||
| Phellinus gilvus, Phellinus wahlbergii | Symbiotic culture | Umata et al. (2000a) | |||
| Fuscoporia, Hymenocaetaceae | Molecular identification | Ogura-Tsujita et al. (2018) | |||
| Polyporales | Fomitopsis vinosa, Lentinus sajor-caju, Panus tigrinus | Symbiotic culture | Umata et al. (2000a) | ||
| Ganoderma australe, Loweporus tephroporus, Microporus affinis | Symbiotic culture | Umata (1995, 1998b) | |||
| Lenzites betulinus, Trametes hirsuta | Symbiotic culture | Umata (1999) | |||
| Ceriporia, Hyphoderma, Ischnoderma, Microporus, Phanerochaete, Phanerocaetaceae, Phlebia, Phlebiopsis, Stereum | Molecular identification | Ogura-Tsujita et al. (2018) | |||
| Russulales | Hericium erinaceus, Xylobolus annosus | Symbiotic culture | Umata et al. (2000a) | ||
| Asterostroma, Coniophorafomes matsuzawae, Russulac, Scytinostroma | Molecular identification | Ogura-Tsujita et al. (2018) | |||
| Sebacinales Russula | Serendipitaceae | Molecular identification | Ogura-Tsujita et al. (2018) | ||
| Trechisporales | Hyphodontia, Sitstostremastrum, Trechispora, Trechisporales, Trichaptum cf. durum | Molecular identification, stable isotopes | Ogura-Tsujita et al. (2018) | ||
| Erythrorchis cassythoides | Agaricales, Russulales | Russulac, Gymnopus | Molecular identification | Dearnaley (2006) | The Gymnopus sequence was nested within the Marasmiellus clade (Fig. S2). |
| Eulophia zollingeri | Agaricales | Psathyrella cf. candolleana | Molecular identification | Ogura-Tsujita and Yukawa (2008) | |
| – | – | Radiocarbon | Suetsugu et al. (2020b) | ||
| Galeola falconeri | Polyporales | Meripilaceae | Molecular identification, stable isotopes | Lee et al. (2015) | The Meripilaceae fungus was identified as Physisporinus by Yamashita et al. (2020). |
| G. nudifolia (G. hydra)b | Polyporales | Fomes | Burgeff (1959) | ||
| Gastrodia appendiculata | Agaricales | Mycena | Molecular identification, stable isotopes | Lee et al. (2015) | |
| G. callosa | – | Mycelia without clamp connection | Microscopic observation | Burgeff (1932, 1959) | |
| G. confusa | Agaricales | Clitocybula, Gymnopus, Mycena | Molecular identification, stable isotopes | Ogura-Tsujita et al. (2009) | Mycena was the most dominant. The sequences of Clitocybula and Gymnopus were nested within the hydropoid and Marasmiellus clades, respectively (Fig. S2). |
| Mycena | Molecular identification, symbiotic culture | Shimaoka et al. (2017) | |||
| Cantharellales | Ceratobasidium | Molecular identification | Ogura-Tsujita et al. (2009) | ||
| G. cunninghamii | Agaricales | Armillaria mellea | Morphology of rhizomorph | Campbell (1962) | Rhizomorphs were attached to the tuber surfaces. |
| G. elata | Agaricales | Armillaria mellea | Rhizomorph morphology | Kusano (1911) | See also the review by Xu and Guo (2000) and Liu et al. (2010) for G. elata study. |
| A. gallica | SI test | Mohammed et al. (1994) | |||
| A. gallica, A. jezoensis, A. ostoyae, A. sinapina, A. singula | SI test, isozyme | Cha and Igarashi (1995) | |||
| A. gallica | SI test, sporocarp formation | Kikuchi et al. (2008b) | |||
| A. cepistipes, A. gallica, A. nabsnona | SI test | Kikuchi et al. (2008a) | |||
| A. nabsnona | SI test, molecular identification | Sekizaki et al. (2008) | |||
| Armirallia (seven lineages) | Molecular identification | Guo et al. (2016) | |||
| Armirallia | Molecular identification, symbiotic culture | Yeh et al. (2017) | |||
| Mycena anoectochila | Symbiotic culture | Guo et al. (1997) | |||
| Mycena dendrobii | Symbiotic culture | Guo et al. (1999), Pan et al. (2015) | |||
| Mycena orchidicola | Symbiotic culture | Fan et al. (1996) | |||
| Mycena osmundicola, Mycena | Symbiotic culture | Hong et al. (2002) | |||
| Mycena osmundicola | Sporocarp formation, symbiotic culture | Xu and Guo (1989) | |||
| Mycena osmundicola | Symbiotic culture | Kim et al. (2006) | |||
| Armillaria mellea, Mycena osmundicola | Symbiotic culture | Park et al. (2012) | |||
| Mycena | Molecular identification, symbiotic culture | Park and Lee (2013a) | |||
| Armillaria mellea, Mycena | Symbiotic culture | Park and Lee (2013b) | |||
| Mycena | Symbiotic culture, TEM | Li et al. (2020) | |||
| Agaricales and others | Unidentified Agaricales and others | Illumina sequencing | Chen et al. (2019) | Identified from seedlings. | |
| Hymenochaetales | Resinicium | Illumina sequencing | Chen et al. (2019) | Identified from seedlings. | |
| – | – | Radiocarbon | Suetsugu et al. (2020b) | ||
| G. flabilabella | Agaricales | Hydropus | Molecular identification, stable isotopes | Lee et al. (2015) | |
| Agaricales and others | Mycena and others | Illumina sequencing | Liu et al. (2015) | ||
| G. fontinalis | Agaricales | Gymnopus, Mycena | Molecular identification, stable isotopes | Lee et al. (2015) | |
| G. javanica | Agaricales | Xerotus javanicus | Sporocarp formation | Burgeff (1936, 1959) | |
| G. lacista | – | – | Stable isotopes | Sommer et al. (2012) | |
| G. minor | – | Clamp bearing fungus | Isolate characteristics | Campbell (1963) | |
| G. nantoensis | Agaricales | Mycena | Molecular identification, stable isotopes | Lee et al. (2015) | |
| G. nipponica | Agaricales | Crinipellis, Clitocybula, Gymnopus, Marasmiellus, Marasmius, Mycena | Molecular identification | Kinoshita et al. (2016) | The sequences of Crinipellis, Critocybula, Gymnopus, Marasmiellus and Marasmius were spread into the Omphalotaceae, Marasmiaceae and hydropoid clades (Fig. S2). |
| Auriculariales | Auricularia | Molecular identification | Kinoshita et al. (2016) | ||
| Corticiales | Corticium | Molecular identification, symbiotic culture | Shimaoka et al. (2017) | ||
| Hymenochaetales | Resinicium | Molecular identification | Kinoshita et al. (2016) | ||
| Polyporales | Meruliaceae, Phlebiopsis, Polyporales | Molecular identification | Kinoshita et al. (2016) | ||
| Theleporus | Molecular identification, symbiotic culture | Shimaoka et al. (2017) | |||
| Russulales | Lactariusc, Peniophoraceae, Russulac | Molecular identification | Kinoshita et al. (2016) | ||
| Sebacinales | Sebacinac | Molecular identification | Kinoshita et al. (2016) | ||
| Trechisporales | Trechispora | Molecular identification, symbiotic culture | Shimaoka et al. (2017) | ||
| G. pubilabiata | Agaricales | Crinipellis, Clitocybula, Gymnopus, Marasmiellus, Marasmius, Mycena, Pterulaceae | Molecular identification | Kinoshita et al. (2016) | The sequences of Crinipellis, Clitocybula, Gymnopus, Marasmiellus and Marasmius were spread into the Omphalotaceae, Marasmiaceae and hydropoid clades (Fig. S2). |
| Mycena | Molecular identification, symbiotic culture | Higaki et al. (2017) | |||
| Cantharellales | Tulasnella | Molecular identification | Kinoshita et al. (2016) | ||
| Polyporales | Diplomitoporus rimosus | Molecular identification | Kinoshita et al. (2016) | Fungal ITS sequences had 100% similarity with D. rimosus. | |
| Diplomitoporus rimosus | Molecular identification, symbiotic culture | Shimaoka et al. (2017) | Fungal ITS sequence from protocorm shared 558/559 bp identity with that from D. rimosus. | ||
| – | Isolates from G. confusa (G. verrucosa) | Symbiotic culture | Umata et al. (2000b) | ||
| G. sesamoides | Polyporales | Probably Fomes mastoporus | Field observation | Campbell (1964) | Mycelium, that was similar to the mycobiont of G. sesamoides, was traced to the sporocarp of F. mastoporus. |
| Agaricales | Campanella, Marasmius | Molecular identification, stable isotopes | Dearnaley and Bougoure (2010) | The sequences of Campanella and Marasmius were nested within the campanelloids and Omphalotaceae clades, respectively (Fig. S2). | |
| – | Clamp bearing fungus | Microscopic observation | McLennan (1959) | ||
| – | – | Stable isotopes | Gomes et al. (2020) | ||
| G. similis | Hymenochaetales | Resinicium, Mycena, Gymnopus | Molecular identification, stable isotopes | Martos et al. (2009) | Resinicium is the most dominant. The Gymnopus sequence was nested within the Marasmiellus clade (Fig. S2). |
| G. verrucosa | – | Clamp bearing isolates from G. nipponica and G. verrucosa | Symbiotic culture | Tashima et al. (1978) | Plant identification is erroneous and may represent either Gastrodia confusa or G. pubilabiata (H. Umata, personal communication). |
| Wullschlaegelia calcarata | Agaricales | Gymnopus, Mycena | Molecular identification | Martos et al. (2009) | A species was wrongly identified as W. aphylla in Martos et al. (2009) (see Hatté et al. 2020). The Gymnopus sequences were nested within the two clades of Omphalotaceae (Fig. S2). |
| Radiocarbon | Hatté et al. (2020) | ||||
| Yoania amagiensis | Polyporales | Physisporinus (four OTUs) | Molecular identification | Yamashita et al. (2020) | |
| Y. flava | – | Unidentified isolate from Y. flava rhizome | Symbiotic culture | Tsuda et al. (2004) | Asymbiotic culture was also achieved. |
| Polyporales | Physisporinus (a single OTU), Thelephoraceaec | Molecular identification | Yamashita et al. (2020) | A single Physisporinus OTU is dominantly detected. | |
| Y. japonica | Polyporales | Physisporinus (two OTUs) | Molecular identification | Yamashita et al. (2020) | A single OTU is dominantly detected. |
| – | – | Radiocarbon | Suetsugu et al. (2020b) | ||
aMethods for fungal identification are shown. Molecular identification: Identification by using extract DNA from the fungal isolates or mycorrhizal roots. Sporocarp formation: Identification by morphology of sporocarp formed from fungal isolates. Isolate characteristics: Fungal isolates were identified by morphological and cultural characteristics. SI test: Somatic incompatibility test of fungal isolates. Symbiotic culture: Plants were co-cultured with fungi. Illumina sequencing: Fungal community in mycorrhizal tissue was assessed by llumina sequencing. TEM: Transmission electron microscopy. Stable isotope analysis and radiocarbon approach are also indicated as “Stable isotopes” and “Radiocarbon”, respectively
bBinomials in parentheses are those used in the original publications. Current taxonomic literature suggests that these names are appropriate to treat as synonyms, c Ectomycorrhizal fungi
Mycoheterotrophy dependent on SAP fungi probably evolved twice or more in Vanilloideae, which encompasses three SAP-MH genera—Galeola, Cyrtosia, and Erythrorchis. Cameron et al. (2009) and Cameron (2011) showed that the paired genera Galeola-Cyrtosia and Erythrorchis-Pseudovanilla form a clade (Fig. S1). Pseudovanilla is the only chlorophyllous genus in this clade. There are two alternative hypotheses on the evolution of mycoheterotrophy in this clade. One is that mycoheterotrophy evolved twice from the common ancestor of Galeola-Cyrtosia and the ancestor of Erythrorchis. The other is that mycoheterotrophy evolved once from the common ancestor of the four genera and was subsequently reversed in Pseudovanilla, a photosynthetic plant. The latter is implausible because evolutionary processes to achieve full mycoheterotrophy cause the loss of multiple genes regulating photosynthesis (Delannoy et al. 2011; Li et al. 2020), and reversal from full mycoheterotrophy to autotrophy requires the reorganization of these functional genes. Thus, SAP-MHPs likely evolved at least twice in Vanilloideae.
In Epidendroideae, SAP-MH likely evolved at least seven times, viz., Wullschlaegelia, Gastrodia, Didymoplexis, Epipogium roseum (D.Don) Lindl., Eulophia zollingeri (Rchb.f.) J.J.Sm., Cremastra aphylla T.Yukawa, and Yoania (Fig. 2). Wullschlaegelia comprises two mycoheterotrophic species and W. calcarata Benth. is recognized as an SAP-MHP (Hatté et al. 2020; Martos et al. 2009). Gastrodia is the largest genus of SAP-MHPs and includes ca. 100 species (WCSP 2020), among which 13 have been reported to be SAP-MHPs (Table 1). Didymoplexis comprises 20 species, two of which—D. micradenia Hemsl. (synonym, D. minor J.J.Sm.) and D. pallens Griff.—exhibit SAP-MH (Burgeff 1932, 1936). The mycoheterotrophic genus Epipogium comprises both SAP- and ECM-associated species. Epipogium roseum was reported to be an SAP-MHP (Yamato et al. 2005). Eulophia and Cremastra contain both leafy and leafless species, and SAP-MH was reported for both Eulophia zollingeri (Ogura-Tsujita and Yukawa 2008; Suetsugu et al. 2020b) and C. aphyllla (Yagame et al. 2018). The mycoheterotrophic genus Yoania includes four species, three of which are SAP-MHPs (Suetsugu et al. 2020b; Yamashita et al. 2020). In Cremastra and Epipogium, speciation stopped occurring subsequent to SAP-MH evolution. By contrast, SAP-MH did not lead to an evolutionary dead end in Gastordia, and this genus was likely diversified by the establishment of novel symbioses with various SAP fungi (Kinoshita et al. 2016).
Diversity of mycobionts in SAP-MHPs
Mycobionts of SAP-MHPs include leaf-litter or wood basidiomycete fungi (Table 1). Leaf-litter-decaying fungi colonize the topsoil and decompose plant leaf litter and other soil organic matter (Osono 2007), whereas wood-decaying fungi inhabit living trees, the trunks of standing dead trees, stumps, or fallen logs and degrade wood lignocellulose (Stokland et al. 2012). The activities of enzymes that catalyze the degradation of natural polymers, including lignin and plant cell-wall polysaccharides (mainly cellulose and hemicellulose), in both fungal groups are higher than those in AM and ECM fungi (Kohler et al. 2015). Because of their saprotrophic nature, mycobionts of MHPs isolated from plant roots are amenable to pure culture, facilitating the identification of fungal species. Fungal isolates often develop basidiocarps on culture medium and can be identified morphologically (Burgeff 1932; Kikuchi et al. 2008a; Terashita and Chuman 1987; Xu and Guo 2000; Yagame et al. 2008, 2018).
Most leaf-litter-decaying fungi that associate with MHPs belong to families in Agaricales, such as Mycenaceae, Marasmiaceae, and Omphalotaceae, which are the main fungal partners of Gastrodia and Didymoplexis (Table 1). These fungi are found worldwide as common saprobes in decaying plant materials (Kirk et al. 2008), but their taxonomic status is controversial (Wilson and Desjardin 2005). We conducted a phylogenetic analysis using published internal transcribed spacer (ITS) sequences of mycobionts of SAP-MHPs, including fungi molecularly identified as Marasmius, Marasmiellus, Gymnopus, Clitocybula, Crinipellis, Campanella, and Hydropus (Fig. S2). Recent studies that updated the phylogenetic placement of these fungal lineages (Antonín et al. 2019; Oliveira et al. 2019; Sandoval-Leiva et al. 2016) were employed as references. The sequences mostly clustered in Marasmiaceae and Omphalotaceae, with some in the hydropoid clade, which mainly includes wood-decaying fungi (Antonín et al. 2019). Most of the sequences clustered in the Marasmiellus clade, and the mycobionts of Gastrodia similis Bosser, Gastrodia pubilabiata Sawa, and Gastrodia confusa Honda and Tuyama were closely related with 100% bootstrap support (BS). Furthermore, mycobionts of G. pubilabiata and Gastrodia nipponica (Honda) Tuyama were closely related within the Marasmiellus, campanelloid, and Porotheleum clades (99–100% BS). These results indicate that particular fungal lineages are associated with SAP-MHPs, although a variety of fungal species participate in mycoheterotrophy. Members of Mycena are the most common mycobionts of Gastrodia species (Table 1). Although Mycena species are pure saprophytes, recent in vitro investigations revealed that several Mycena species have saprotrophic and biotrophic abilities (Thoen et al. 2020). These species can penetrate tree roots, and one Mycena species facilitated nutrient transfer to the plant. Interestingly, mycobionts of G. pubilabiata and G. nipponica detected by Kinoshita et al. (2016) exhibit high sequence similarity (> 98%) with three of these Mycena species— Mycena galopus (Pers.) P.Kumm., Mycena albidolilacea Kühner and Maire, and Mycena olivaceomarginata (Massee) Massee. Therefore, Mycena species that associate with SAP-MHPs could have biotrophic potential, and further evaluation of their trophic mode is warranted.
The wood-decaying fungi that associate with SAP-MHPs are predominantly members of Agaricales (Basidiomycota), such as Armillaria (Physalacriaceae), Psathyrella (Psathyrellaceae), and Coprinellus (Psathyrellaceae) (Table 1). Armillaria species are the main symbionts of Gastrodia elata Blume (Kusano 1911) and Cyrtosia septentrionalis (Rchb.f.) Garay (Fig. 1c; Hamada 1939). Mycobionts from G. elata and C. septentrionalis formed sporocarps and were identified as Armillaria gallica Marxm. & Romagn. (Kikuchi et al. 2008a) and Armillaria tabescens (Scop.) Emel (Terashita and Chuman 1987), respectively. Psathyrella and Coprinellus, both belonging to the Psathyrellaceae, were found in Epipogium roseum and Eulophia zollingeri (Ogura-Tsujita and Yukawa 2008; Yamato et al. 2005). Mycobionts of E. roseum and Cremastra aphylla (Fig. 1a) were identified as Coprinellus disseminatus (Pers.) J.E. Lange (Yagame et al. 2008) and Coprinellus domesticus (Bolton) Vilgalys, Hopple & Jacq. Johnson (Yagame et al. 2018), respectively, by sporocarp morphology and molecular identification. Besides Agaricales fungi, SAP symbionts include wood-decay fungi from other Basidiomycota orders. Gastrodia similis, a tropical MHP, associates with Resinicium of the Hymenocaetales (Martos et al. 2009). Wood-decay basidiomycetes of the Trechisporales, Polyporales, Corticiales, Russulales, and Atheriales were found in roots of a climbing MH orchid, Erythrorchis altissima (Blume) Blume (Fig. 1d; Ogura-Tsujita et al. 2018). Symbioses between multiple species of wood-decay fungi and E. altissima were confirmed by in vitro symbiotic culture by Umata (1995, 1997a,b, 1998a,b, 1999; Table 1). Further, Yamashita et al. (2020) found Physisporinus (Meripilaceae, Polyporales) as a fungal partner of SAP-MHPs. This genus is predominantly associated with Yoania species (Fig. 1e) as well as two other SAP-MH genera, Cyrtosia and Galeola. Although the litter-decay fungi found in SAP-MHPs comprise three Agaricales families; i.e., Mycenaceae, Marasmiaceae, and Omphalotaceae, highly divergent wood-decay fungal families are involved in SAP-MH associations.
A wood-decaying fungus, Armillaria mellea (Vahl) P. Kumm. sl, was reported to be a symbiont of SAP-MHPs by Kusano (1911) and Hamada (1939). This fungus is one of the largest and longest-lived terrestrial organisms and has been reported to cover an area of up to 965 ha with an age of up to ca. 8650 years (Ferguson et al. 2003). Therefore, associating with A. mellea sl allows MHPs access to the huge carbon pool in forests. This fungus has been recognized as a species complex (Korhonen 1978), and seven Armillaria species associated with Japanese Gastrodia elata have been recognized for A. mellea sl (Cha and Igarashi 1995; Kikuchi et al. 2008b). At least seven Armillaria lineages are associated with Chinese G. elata (Guo et al. 2016). Although A. mellea sl includes pathogens that cause root-rot disease in woody plants, Armillaria gallica, a major symbiont of G. elata, is a weak pathogen that inhabits decayed wood and litter (Mohammed et al. 1994). Rhizomorphs, i.e., linear mycelial organs, are well-developed in A. mellea sl (Roll-Hansen 1985) and are often attached to the tuber surface of G. elata (Fig. 1g; Kusano 1911). Such rhizomorphs can be traced from plant tubers or roots to decayed wood (Cha and Igarashi 1996; Kikuchi et al. 2008b). A. mellea rhizomorphs transport water and phosphate efficiently (Cairney et al. 1988), indicating that fungal rhizomorphs play an important role in nutrient transport between MHPs and rhizomorph-forming mycobionts.
ECM fungi occasionally associate with SAP-MHPs although dominant fungal partners are SAP fungi (more than half of total abundance). In such cases, SAP-MHPs are simultaneously associated with both ECM and SAP fungi. ECM Russula fungi were found to associate with Erythrorchis cassythoides (A.Cunn. ex Lindl.) Garay (Dearnaley 2006) and Erythrorchis altissima (Ogura-Tsujita et al. 2018). Mycobionts of Gastrodia nipponica (Fig. 1f) included ECM fungi in Russulaceae (8.6% in frequency) and Sebacinaceae (6.2%) as well as SAP fungi (Kinoshita et al. 2016). Because those ECM fungi are the main symbionts in ECM-MHPs (Ogura-Tsujita et al. 2012; Selosse et al. 2002; Taylor and Bruns 1999), they could also be symbiotic with SAP-MHPs although they are not main symbionts.
Most orchids are associated with so-called rhizoctonia fungi, including those in the basidiomycete families Tulasnellaceae, Ceratobasidiaceae, and Serendipitaceae (Rasmussen 2002; Yukawa et al. 2009). Associations with rhizoctonia fungi are occasionally observed in SAP-MHPs, such as Erythrorchis altissima (Ogura-Tsujita et al. 2018) and Gastrodia species (Kinoshita et al. 2016). Rhizoctonia fungi exhibit divergent trophic strategies; they can be plant pathogens, endophytes, saprophytes, orchid mycorrhizal or ectomycorrhizal fungi (Roberts 1999; Veldre et al. 2013). However, rhizoctonia fungi isolated from leafy orchid roots are saprophytes and are thus able to obtain nutrients from plant debris (Rasmussen 1995). These fungi are involved in initial or partial mycoheterotrophy in leafy Orchidaceae species (Schiebold et al. 2018; Stöckel et al. 2014). Although rhizoctonia fungi are involved in mycoheterotrophy with the albino forms of usually chlorophyllous orchid species (Suetsugu et al. 2019), saprophytic rhizoctonia fungi are only occasionally associated with fully mycoheterotrophic species. This suggests that rhizoctonia fungi possess insufficient physiological functionality to support the growth of full MHPs (Martos et al. 2009).
Specificity of mycobionts
Most land plants have generalized associations with AM or ECM fungi (Smith and Read 2008), but AM-MHPs (Gomes et al. 2017; Merckx and Bidartondo 2008; Yamato et al. 2011) and ECM-MHPs (Ogura-Tsujita et al. 2012; Selosse et al. 2002; Taylor and Bruns 1999) typically have highly specific associations with a narrow phylogenetic range of fungi. In SAP-MHPs, the specificity varies among plant species, from a highly specific association with a single fungal species to broad interactions with multiple fungal orders. Individuals of the SAP-MHP Eulophia zollingeri from seven populations in Japan, Taiwan, and Myanmar associate exclusively with Psathyrella candolleana (Fr.) Maire sl (Ogura-Tsujita and Yukawa 2008). Most mycobionts of Gastrodia confusa from 10 populations separated by 5–1000 km belong to three fungal groups in the genus Mycena (Fig. 1b; Ogura-Tsujita et al. 2009). By contrast, Gastrodia pubilabiata, a species closely related to G. confusa, associates with multiple groups of litter-decaying fungi in the families Mycenaceae, Marasmiaceae, and Omphalotaceae (Fig. S2; Kinoshita et al. 2016). Further, a close relative of these Gastrodia species, Gastrodia nipponica, associates with wood-decaying and ECM fungi in addition to litter-decaying fungi, and its mycobionts exhibited significantly higher sequence divergence than those of G. confusa and G. pubilabiata (Kinoshita et al. 2016). The giant mycoheterotroph, Erythrorchis altissima, is an extreme example of low fungal specificity in full MHPs. In total, 37 fungal species belonging to nine orders of Basidiomycota, which mainly include wood-decaying fungi but also ECM and rhizoctonia fungi, have been identified in the roots of this MHP (Ogura-Tsujita et al. 2018). Mycobiont specificity in SAP-MHPs often varies within a single host plant genus, as in Gastrodia (Kinoshita et al. 2016) and Yoania (Yamashita et al. 2020). Therefore, specificity can fluctuate greatly during host-plant speciation.
Fungal partner shift during the plant life cycle
The fungal partner often changes during the life cycle of an SAP-MHP. A mycoheterotrophic orchid, Gastrodia elata, switches its fungal partner upon transitioning from the juvenile to adult stage. The litter-decaying fungus, Mycena, induces seed germination, whereas the wood-decaying Armillaria supports further development of the mature plant (Xu and Guo 2000). A recent high-throughput sequencing study suggests that more diverse fungal groups than previously assumed are involved at the juvenile stage of G. elata (Chen et al. 2019). Partner switching also seems to occur in Cyrtosia septentrionalis, the adult stage of which is associated with Armillaria (e.g., Hamada 1939). However, C. septentrionalis seeds failed to germinate with Armillaria isolates in vitro (Terashita 1985), and Physisporinus, a wood-decaying fungus, promoted germination in situ (Umata et al. 2013). Changing the fungal partner at some stage of the life cycle seems riskier than living with the same partner. Switching to a fungus with a large biomass, such as Armillaria, allows access to a large carbon pool, thus possibly outweighing the risk of partner shifting. Armillaria produces abundant rhizomorphs in soil (Smith et al. 1992), which increases the likelihood of successful colonization.
Evolution of mycorrhizal interactions
During the evolution from autotrophy to mycoheterotrophy, the associated mycorrhizal fungi have switched to different fungal communities in some instances (Jacquemyn and Merckx 2019; Ogura-Tsujita et al. 2012; Yagame et al. 2016). Most leafy relatives of SAP-MHPs are associated with rhizoctonia fungi, suggesting that the mycorrhizal community shifted from rhizoctonia to litter- or wood-decaying fungi during the evolution of SAP-MHP lineages. The chlorophyllous genus Vanilla is most closely related to three genera containing SAP-MHPs in the tribe Vanilleae (Cameron 2011; Cameron et al. 2009; Fig. S1) and mainly associates with rhizoctonia fungi, including Tulasnellaceae and Ceratobasidiaceae (Porras-Alfaro and Bayman 2007). Mycobionts may have shifted from rhizoctonia to diverse wood-decaying fungi in accordance with the evolution of Galeola-Cyrtosia and Erythorchis. The leafy genus Calypso is likely sister to SAP-MHP-containing Yoania (Freudenstein et al. 2017) and forms associations with Tulasnellaceae and Ceratobasidiaceae (Currah et al. 1988; Taylor and McCormick 2008). This suggests that fungal partners have been switched from rhizoctonia fungi to the wood-decaying Physisporinus with the shift to SAP-MH in Yoania. Rhizoctonia fungi are often found as free-living saprotrophs (Roberts 1999), and mycorrhizal associations with these fungi are unique to Orchidaceae (Yukawa et al. 2009). This ability to associate with free-living fungi in orchids might have triggered the evolution of full mycoheterotrophy dependent on saprotrophic non-rhizoctonia fungi.
Mycorrhizal communities are typically switched via a phase of dual association, which involves mycobionts of both leafy and leafless plants, during the evolution of mycoheterotrophy. In the orchid genus Cymbidium, mycobionts were compared within a clade in which mycoheterotrophy evolved (Ogura-Tsujita et al. 2012). A leafy outgroup species, Cymbidium dayanum Rchb.f., associates mainly with rhizoctonia fungi from Tulasnellaceae, whereas two MHPs, Cymbidium macrorhizon Lindl. and Cymbidium aberrans (Finet) Schltr., mostly associate with ECM fungi in Sebacinaceae. By contrast, the leafy sister taxa of the two MHPs, Cymbidium lancifolium Hook. and Cymbidium goeringii (Rchb.f.) Rchb.f., associate with both rhizoctonia fungi and several ECM fungal families, suggesting that fungal partners have switched via a dual association with both rhizoctonia and ECM fungi. Such dual associations could trigger mycorrhizal switching and play an important role in the evolution of MHPs. Another case of evolution via a dual association with wood-decaying fungi was found in the genus Cremastra (Yagame et al. 2018). A SAP-MHP, Cremastra aphylla, which mainly associates with Coprinellus, a wood-decaying fungus, and its leafy sister species, Cremastra appendiculata (D.Don) Makino, can associate with both rhizoctonia fungi and Coprinellus (Freudenstein et al. 2017; Nishikawa and Ui 1976; Yagame et al. 2013). This suggests that the type of mycorrhizal fungi in the symbiosis had been switched to wood-decaying fungi via a dual association. A novel association with Coprinellus probably triggered the evolution of the SAP-MHP.
Symbiotic associations with SAP fungi are occasionally found in leafy orchid species. The wood-decaying fungus Psathyrella is often found associating with leafy orchids, such as Oeceoclades maculata (Lindl.) Lindl. (Bayman et al. 2016) and Satyrium nepalense D.Don (Jyothsna and Purushothama 2014). Seeds of the former orchid species germinated in vitro only in association with Psathyrella, whereas adult plants associate with rhizoctonia fungi in addition to Psathyrella (Bayman et al. 2016). Such high specificity for SAP fungi during seed germination could represent an initial stage of the evolution of an SAP-MHP. Mycena fungi are one of the main symbionts of Gastrodia SAP-MHPs and also promote seed germination and seedling growth in Dendrobium epiphytic green orchids (Guo et al. 1997; Zhang et al. 2012). Interestingly, Mycena anoectochila L. Fan & S.X. Guo, which was isolated from the leafy orchid Anoectochilus roxburghii (Wall.) Lindl., induced seed germination in the mycoheterotrophic Gastrodia elata (Guo et al. 1997). These results suggest that the same fungus can associate with both leafy and leafless orchids. Mycena fungi were also found to associate with the terrestrial orchids Cymbidium sinense (Andrews) Willd. (Fan et al. 1996), Goodyera repens (L.) R.Br. (Voronina et al. 2018), and Bletilla striata (Thunb.) Rchb.f. (Guo and Xu 1992). Members of Trechisporales, Polyporales, Corticiales, and Hymenochaetales, which largely comprise wood-decaying fungi, have been occasionally found in tropical epiphytic orchids (Kartzinel et al. 2013; Martos et al. 2012). Although these cases could reflect opportunistic associations in leafy orchids, a symbiotic relationship with SAP fungi may be more adaptive than that with rhizoctonia fungi in some environments and trigger the evolution of SAP-MHPs (Selosse et al. 2010).
Convergent evolution of mycorrhizal specificity toward particular fungal lineages has occurred in several SAP-MH lineages. Associations with Armillaria have been observed for Cyrtosia septentrionalis (Subfamily Vanilloideae) and Gastrodia elata (Subfamily Epidendroideae) (Fig. 2). For instance, Armillaria gallica associates with both these phylogenetically distant orchids exhibiting SAP-MH (Kikuchi et al. 2008a; Terashita 1996). Two SAP-MHPs among several tribes of Epidendroideae, Epipogium roseum and Eulophia zollingeri, associate with Psathyrellaceae fungi (Ogura-Tsujita and Yukawa 2008; Yamato et al. 2005). Sequences of the nuclear ribosomal ITS region of Psathyrella fungi isolated from these two orchids had > 97% similarity (Ogura-Tsujita and Yukawa 2008). Such convergent evolution has also been found in AM- and ECM-MHPs. AM fungi from AM-MHPs belonging to different plant families, such as Burmanniaceae and Corsiaceae, were grouped into the same taxa (> 97% small subunit rRNA sequence similarity; Gomes et al. 2019; Merckx et al. 2012). The mycobionts in Sebacinaceae from the ECM-MHP Hexalectris (Epidendreae) are closely related to those from Neottia nidus-avis (L.) Rich. (Neottieae) (> 98% ITS sequence similarity; Kennedy et al. 2011). Mycobionts of MHPs may converge on particular fungal taxa that support high nutrient acquisition.
Mycorrhizal interactions have changed during the speciation of MHPs. The species of mycobionts in the associations have changed or fungal specificity has changed from broad to narrow, such that the same mycobiont species is shared among several plant species (Kinoshita et al. 2016). In SAP-MHPs, fungal specificity differed among three closely related Gastrodia species, G. confusa, G. pubilabiata, and G. nipponica, all of which are associated with litter-decaying fungi within Mycenaceae, Marasmiaceae, and Omphalotaceae (Kinoshita et al. 2016). The fungal specificity of G. confusa was significantly greater than that of G. pubilabiata and G. nipponica, indicating that specificity fluctuates during speciation. Interestingly, G. confusa exclusively inhabits bamboo thickets, the mycorrhizal communities of which differ significantly from those of other vegetation. This suggests that adaptation to particular fungi inhabiting bamboo thickets triggered the speciation of G. confusa. The mycobionts of 15 Gastrodia species have been surveyed; Mycenaceae, Marasmiaceae or Omphalotaceae fungi were reported to associate with ten species (Table 1). This suggests that these litter-decaying fungi are main fungal partners for Gastrodia species. Associations with the wood-decaying fungi Armillaria and Resinicium could have appeared during the evolution of Gastrodia elata and Gastrodia simillis. Furthermore, a symbiotic relationship with ECM fungi has been observed in G. nipponica, which associates with both litter-decaying and ECM fungi. Both SAP- and ECM-MHPs have appeared in the genus Epipogium (Roy et al. 2009; Yamato et al. 2005). Although because of the poor phylogenetic resolution of this genus it is unclear which MHP type evolved earlier, the fungal partner could be switched to SAP or ECM fungi even in closely related taxa. Fungal partner switching during speciation has been reported in AM- and ECM-MHPs. Two sister ECM-MHPs, Corallorhiza maculata (Raf.) Raf. and Corallorhiza mertensiana Bong., specifically associate with different Russulaceae fungal taxa (Taylor and Bruns 1999). Fungal specificity differs among the six ECM-MHPs within Neottia (Yagame et al. 2016). Five AM-MHPs in the genus Afrothismia exhibit high fungal specificity for different Glomeraceae fungal taxa (Merckx and Bidartondo 2008; Merckx et al. 2012). These results suggest that switching between mycorrhizal partners accelerated the speciation of MHPs.
Isotopic signature of SAP-MHPs
Nutrient fluxes between MHPs and mycorrhizal fungi have been studied using stable isotope natural abundance analysis (Gebauer and Meyer 2003; Hynson et al. 2013). Because fungal-derived carbon and nitrogen are highly enriched in 13C and 15N, the tissues of MHPs are expected to also be enriched in carbon and nitrogen isotopes compared to the surrounding autotrophic plants (Gebauer and Meyer 2003). This approach has been applied to examine a variety of ECM-MHPs (Liebel and Gebauer 2011; Motomura et al. 2010; Roy et al. 2009), AM-MHPs (County et al. 2011; Gomes et al. 2020; Merckx et al. 2010), and several SAP-MHPs (Lee et al. 2015; Martos et al. 2009; Ogura-Tsujita et al. 2009). The relative enrichment levels of isotopes confirmed the mycoheterotrophy of those species. All three types of MHPs are highly enriched in 13C, but the 15N enrichment level is lower in SAP- and AM-MHPs than in ECM-MHPs. The difference is attributable to the greater enrichment of nitrogen isotopes in ECM fungi than in AM and SAP fungi, as a result of their different nitrogen acquisition strategies. Interestingly, litter-decaying fungi are generally depleted in carbon isotopes relative to wood-decaying fungi (Kohzu et al. 1999), likely because wood is more enriched in 13C than leaf tissue (Gebauer and Schulze 1991). Lee et al. (2015) showed that 13C was significantly less enriched in SAP-MHPs associated with the litter-decaying fungi—i.e., Gastrodia appendiculata C.S.Leou & N.J.Chung, Gastrodia fontinalis T.P.Lin, and Gastrodia nantoensis T.C.Hsu & C.M.Kuo ex T.P.Lin—than in those associated with wood-decaying fungi. However, there are insufficient studies of the isotopic signature of SAP-MHPs, and further work is required to clarify the isotopic signatures of litter- and wood-decaying fungi and elucidate the physiological ecology of SAP-MHPs.
Stable isotope analysis can be used to distinguish between partial and full mycoheterotrophy (Gebauer and Meyer 2003). The linear two-source mixing model estimates the proportion of carbon and nitrogen gain from photosynthesis and mycorrhizal fungi. The endpoint of this model is a value that falls between those of co-occurring autotrophic plants (0% nutrient gain from fungi) and full MHPs (100% nutrient gain from fungi) (Preiss and Gebauer 2008). The level of mycoheterotrophy has been quantitatively assessed for various leafy plant species associated with ECM (Abadie et al. 2006; Bidartondo et al. 2004; Matsuda et al. 2012) and AM (Suetsugu et al. 2020a) fungi using this model, but little is known of those associated with SAP fungi. A preliminary isotope analysis by Yagame et al. (2015) showed that the leafy orchid Cremastra appendiculata, which associates with wood-decaying fungi, is partially mycoheterotrophic. Stable isotope analysis may reveal more diverse partial SAP-MHPs if applied to leafy orchids associated with non-rhizoctonia SAP fungi.
Suetsugu et al. (2020b) estimated the age of carbon in SAP-MHP tissue by measuring the natural abundance of radiocarbon (nuclear weapon-derived 14C). This approach traces the time elapsed since carbon isotopes derived from the nuclear-weapon tests of the 1950s and 1960s were fixed from atmospheric CO2 by photosynthesis. The carbon utilized by wood decaying fungus-dependent MHPs was fixed 10–40 years before that fixed by ECM-MHPs (Suetsugu et al. 2020b). The carbon in SAP-MHPs associated with litter-decaying fungi was estimated to be 6.7–9.9 years old (Hatté et al. 2020), suggesting that SAP-MHPs associated with wood-decaying fungi use older carbon than those associated with litter-decaying fungi. This technique will enable investigations of nutrient flows via mycoheterotrophy from decomposing plant debris.
Culturing SAP-MHPs
AM and ECM fungi are almost obligately biotrophic, i.e., dependent on autotrophic plants for their carbon supply. Thus, AM- and ECM-MHPs require a chlorophyllous host plant for co-culture with appropriate symbiotic fungi in vitro (Mckendrick et al. 2000). By contrast, mycobionts of SAP-MHPs can grow in pure culture and stimulate seed germination and further seedling growth in vitro (Burgeff 1932; Yagame et al. 2007). Field or container culture has been established for several SAP-MHPs (Shimaoka et al. 2017; Xu and Guo 2000). These culture techniques will enhance our understanding of the physiology of mycoheterotrophy.
In vitro symbiotic culture with MHP seeds and their mycobiont was achieved for SAP-MHPs by Burgeff (1932). Leaf litter or wood debris is the main carbon source for fungal isolates from SAP-MHPs, and so culture medium containing dead organic material, such as fallen leaves, twigs, or ground wood chips, can sustain isolates. Pieces of Quercus leaves inoculated with Mycena osmundicola J.E. Lange induced seed germination in Gastrodia elata on water agar without additives (Kim et al. 2006). In a preliminary study, we induced germination of Gastrodia nipponica using this system (Fig. 1i). A medium containing Fagus crenata Blume sawdust, water, glucose, and yeast powder enabled co-culture of seeds of Erythrorchis altissima and fungi in a series of studies by Umata (1995, 1997a, b, 1998a, b, 1999) (Fig. 1h; Table 1). The seedlings continued to grow after they were transplanted into large culture bottles with fresh sawdust medium (Fig. 1h), attained a stem length of more than 30 cm, and were successfully transplanted to their natural habitat (Umata et al. 2007). Symbiotic culture in a medium containing bamboo leaves and nutrient solution induced flower bud formation in Gastrodia verrucosa Blume (Tashima et al. 1978). Mycobionts of the mycoheterotrophic orchids Galeola nudifolia Lour. (previously known as Galeola hydra Rchb.f.) and Gastrodia javanica Endl. grew on medium containing pure cellulose or lignin from Populus wood (Burgeff 1936; Hollander 1932); thus, these components may be crucial for the growth of SAP-MHPs.
Artificial cultivation other than in vitro culture, such as in the field or in a container, has been established for several SAP-MHPs. Gastrodia elata, a component of a traditional Chinese medicine, has been cultivated in the field using wood logs inoculated with Armillaria mellea (Park and Lee 2013a; Xu and Guo 2000). The development of this cultivation technique has been useful to the pharmaceutical industry. Flower induction under symbiotic cultivation in a container was achieved for Epipogium roseum using sawdust and volcanic soil (Yagame et al. 2007). Container cultivation of Gastrodia species has been achieved using organic materials from their natural habitats. Seed germination in Gastrodia nipponica (Umata and Nishi 2010) and Gastrodia pubilabiata (Higaki et al. 2017) was induced in a plastic box containing leaf litter from their habitats. Further, the life cycles of G. pubilabiata and Gastrodia confusa were completed in culture with wood logs, cedar cones, and humus from a forest (Shimaoka et al. 2017).
Asymbiotic culture is difficult for full MHPs, but several studies have demonstrated in vitro asymbiotic germination and subsequent growth of SAP-MHPs. Controlling the O2 and CO2 concentrations within the culture vessel stimulates seed germination in Cyrtosia septentrionalis (Nakamura et al. 1975). Interestingly, the concentrations were similar to those in soil, implying that in its natural habitat, the seed does not require direct mycobiont contact for germination (Umata et al. 2013). Aseptically germinated seedlings developed inflorescences in Didymoplexis pallens (Irawati 2002), and rhizome formation was observed in an asymbiotic culture of Yoania flava K.Inoue & T.Yukawa (Tsuda et al. 2004). Aseptic propagation via an embryogenic callus was demonstrated in Gastrodia elata, and the regenerated tubers continued to grow after inoculation of Armillaria isolates (Yeh et al. 2017). Seeds of Gastrodia pubilabiata successfully germinated without symbionts, and their subsequent development was controlled by illumination (Godo et al. 2020).
Future perspectives
Fully mycoheterotrophic plants associated with SAP fungi have to date been found only in Orchidaceae and have evolved independently at least nine times within two subfamilies, Vanillioideae and Epidendroideae. A variety of litter- and wood-decaying fungi are involved in mycoheterotrophy in association with SAP-MHPs, and several SAP-MHPs can be cultured with or without mycorrhizal fungi. Culturable SAP-MHPs may be key to addressing many unsolved questions regarding mycoheterotrophy and will contribute to a range of scientific fields. A critical event in the evolution from autotrophy to mycoheterotrophy is fungal partner switching, the replacement of the associated fungal community by another. The mycobionts of most SAP-MHPs have been switched from rhizoctonia fungi to leaf-litter- or wood-decaying basidiomycetes. The benefits gained by plants from mycobionts differ between rhizoctonia and SAP fungi. Plants may select the best fungal partners for nutrient acquisition (Jacquemyn and Merckx 2019; Ogura-Tsujita et al. 2012), thus triggering the evolution of mycoheterotrophy. Symbiotic culture will allow direct comparisons of the relative fitness between plants with rhizoctonia and those with SAP mycobionts. Leafy sister species of fully mycoheterotrophic species, such as Cremastra appendiculata, often associate with rhizoctionia and wood-decaying fungi and could be suitable model plants for such assays. Comparing gene expression between SAP-MHPs and their leafy relatives will clarify the mechanism underlying the evolution from autotrophy to mycoheterotrophy. Field samples are subject to environmental effects, but culture systems facilitate comparisons between autotrophs and mycoheterotrophs.
The physiological mechanisms underlying plant–fungus interactions in MHPs are unclear, but recent studies of SAP-MHPs have provided information on the interactions between MHPs and their mycorrhizal fungi. Transcriptomic and proteomic analyses of Gastrodia elata co-cultured with Mycena fungi revealed differentially accumulated mRNAs and proteins involved in energy metabolism, plant defense, molecular signaling, and secondary metabolism (Zeng et al. 2017, 2018). Fungal digestion was demonstrated during seed germination in G. elata co-cultured with Mycena (Li et al. 2020). The factors transported from the fungus to the plant are unknown in MHPs, but two sucrose transporter-like genes, GeSUT4 and GeSUT3, were highly expressed in Armillaria‐colonized G. elata tubers, suggesting that sucrose is the major sugar transported between the fungus and G. elata (Ho et al. 2020). Symbiotic culture of SAP-MHPs enables broader approaches for physiological studies of mycoheterotrophy, such as those investigating the mechanism of recognition between plant and fungus, and of nutrient transfer from fungus to plant. Furthermore, asymbiotic culture allows the comparison of gene expression profiles of plants with and without mycorrhizal fungi, thus providing insights into the physiology of mycoheterotrophy.
The ecology of MHPs is poorly understood because they spend most of their life cycle underground and shoot systems appear only during reproductive phases. For example, the processes of plant development and seasonal growth in many MH species are considered “black boxes”. A culture system would expand the understanding of the life cycle and phenological properties of MHPs. Container culture of Epipogium roseum revealed the developmental process of subterranean seedlings, with stolons and rhizomes produced (Yagame et al. 2007). Rapid clonal propagation of this orchid was also achieved, with a single protocorm producing 80 tubers. Development from seed to flower in several SAP-MHPs was successfully monitored in symbiotic or asymbiotic culture and required 6 months in E. roseum (Yagame et al. 2007), 4 months in Gastrodia pubilabiata (Shimaoka et al. 2017), and 4–6 months in Didymoplexis pallens (Irawati 2002). Many MHPs are endangered worldwide because of habitat loss and climate change (Merckx 2013). A culture system would contribute to the recovery of the SAP-MHP populations. The rhizomes of Yoania flava that developed under symbiotic culture were transplanted to the natural habitat and survived for 490 days thereafter (Tsuda et al. 2004). Because MHPs require mycobionts for survival, ex situ conservation of plants with their mycobionts is a good strategy for preventing extinction. The cryopreservation of seeds and culture of SAP-MHP symbiont isolates will contribute greatly toward the long-term conservation of SAP-MHPs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank H. Umata and Y. Yamashita for the helpful comments on this manuscript.
Footnotes
Yuki Ogura-Tsujita is the recipient of the BSJ Award for Young Scientist, 2013.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abadie JC, Püttsepp Ü, Gebauer G, et al. Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Can J Bot. 2006;84:1462–1477. doi: 10.1139/B06-101. [DOI] [Google Scholar]
- Antonín V, Borovička J, Holec J, et al. Taxonomic update of Clitocybula sensu lato with a new generic classification. Fungal Biol. 2019;123:431–447. doi: 10.1016/j.funbio.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Davis JI. The plastid genome of the mycoheterotrophic Corallorhiza striata (Orchidaceae) is in the relatively early stages of degradation. Am J Bot. 2012;99:1513–1523. doi: 10.3732/ajb.1200256. [DOI] [PubMed] [Google Scholar]
- Bayman P, Mosquera-Espinosa AT, Saladini-Aponte CM, et al. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am J Bot. 2016;103:1880–1889. doi: 10.3732/ajb.1600127. [DOI] [PubMed] [Google Scholar]
- Berg B, McClaugherty C. Plant litter: decomposition, humus formation, carbon sequestration. Berlin: Springer; 2008. [Google Scholar]
- Bidartondo MI. The evolutionary ecology of myco-heterotrophy. New Phytol. 2005;167:335–352. doi: 10.1111/j.1469-8137.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Burghardt B, Gebauer G, et al. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc B Biol Sci. 2004;271:1799–1806. doi: 10.1098/rspb.2004.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougoure JJ, Brundrett MC, Grierson PF. Carbon and nitrogen supply to the underground orchid, Rhizanthella gardneri. New Phytol. 2010;186:947–956. doi: 10.1111/j.1469-8137.2010.03246.x. [DOI] [PubMed] [Google Scholar]
- Bradford J, Weishampel P, Smith ML, et al. Detrital carbon pools in temperate forests: magnitude and potential for landscape-scale assessment. Can J For Res. 2009;39:802–813. doi: 10.1139/X09-010. [DOI] [Google Scholar]
- Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018;220:1108–1115. doi: 10.1111/nph.14976. [DOI] [PubMed] [Google Scholar]
- Burgeff H. Saprophytismus und symbiose. Studien an tropischen orchideen. Jena: Gustav Fischer; 1932. [Google Scholar]
- Burgeff H. Samenkeimung der orchideen. Jena: Gustav Fischer; 1936. [Google Scholar]
- Burgeff . Mycorrhiza of orchids. In: Withner CL, editor. The orchids, a scientific survey. New York: Wiley; 1959. pp. 361–395. [Google Scholar]
- Cairney JWG, Jennings DH, Ratcliffe RG, Southon TE. The physiology of basidiomycete linear organs II. Phosphate uptake by rhizomorphs of Armillaria mellea. New Phytol. 1988;109:327–333. doi: 10.1111/j.1469-8137.1988.tb04202.x. [DOI] [Google Scholar]
- Cameron KM. On the value of nuclear and mitochondrial gene sequences for reconstructing the phylogeny of vanilloid orchids (Vanilloideae, Orchidaceae) Ann Bot. 2009;104:377–385. doi: 10.1093/aob/mcp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KM (2011) Vanilla phylogeny and classification. In: Havkin-Frenkel D, Belanger FC (eds) Handbook of vanilla science and technology. Wiley-Blackwell, Chichester, pp 241–255
- Cameron DD, Preiss K, Gebauer G, Read DJ. The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytol. 2009;183:358–364. doi: 10.1111/j.1469-8137.2009.02853.x. [DOI] [PubMed] [Google Scholar]
- Campbell EO. The mycorrhiza of Gastrodia cunninghamii Hook. f. Trans R Soc NZ Bot. 1962;1:289–296. [Google Scholar]
- Campbell EO. Gastrodia minor Petrie, an epiparasite of manuka. Trans R Soc NZ Bot. 1963;2:73–81. [Google Scholar]
- Campbell EO. The fungal association in a colony of Gastrodia sesamoides R.Br. Trans R Soc NZ Bot. 1964;2:237–246. [Google Scholar]
- Cha JY, Igarashi T. Armillaria species associated with Gastrodia elata in Japan. Eur J For Pathol. 1995;25:319–326. doi: 10.1111/j.1439-0329.1995.tb01347.x. [DOI] [Google Scholar]
- Cha JY, Igarashi T. Armillaria jezoensis, a new symbiont of Galeola septentrionalis (Orchidaceae) in Hokkaido. Mycoscience. 1996;37:21–24. doi: 10.1007/BF02461451. [DOI] [Google Scholar]
- Chase MW, Cameron KM, Freudenstein JV, et al. An updated classification of Orchidaceae. Bot J Linn Soc. 2015;177:151–174. doi: 10.1111/boj.12234. [DOI] [Google Scholar]
- Chen L, Wang YC, Qin LY, et al. Dynamics of fungal communities during Gastrodia elata growth. BMC Microbiol. 2019;19:158. doi: 10.1186/s12866-019-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courty PE, Walder F, Boller T, et al. Carbon and nitrogen metabolism in mycorrhizal networks and mycoheterotrophic plants of tropical forests: a stable isotope analysis. Plant Physiol. 2011;156:952–961. doi: 10.1104/pp.111.177618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currah RS, Hambleton S, Smreciu A. Mycorrhizae and mycorrhizal fungi of Calypso bulbosa. Am J Bot. 1988;75:739–752. doi: 10.2307/2444206. [DOI] [PubMed] [Google Scholar]
- Dearnaley J. The fungal endophytes of Erythrorchis cassythoides---is this orchid saprophytic or parasitic? Austral Mycol. 2006;25:51–57. [Google Scholar]
- Dearnaley JDW. Further advances in orchid mycorrhizal research. Mycorrhiza. 2007;17:475–486. doi: 10.1007/s00572-007-0138-1. [DOI] [PubMed] [Google Scholar]
- Dearnaley JDW, Bougoure JJ. Isotopic and molecular evidence for saprotrophic Marasmiaceae mycobionts in rhizomes of Gastrodia sesamoides. Fungal Ecol. 2010;3:288–294. doi: 10.1016/j.funeco.2009.11.003. [DOI] [Google Scholar]
- Delannoy E, Fujii S, Colas Des Francs-Small C, et al. Rampant gene loss in the underground orchid Rhizanthella gardneri highlights evolutionary constraints on plastid genomes. Mol Biol Evol. 2011;28:2077–2086. doi: 10.1093/molbev/msr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Guo S, Cao W, Xiao P, Xu J. Isolation, culture, identification and biological activity of Mycena orchidicola sp. nov. in Cymbidium sinense (Orchidaceae) Acta Mycol Sin. 1996;15:251–255. [Google Scholar]
- Ferguson BA, Dreisbach TA, Parks CG, Filip GM, Schmitt CL. Coarse-scale population structure of pathogenic Armillaria species in a mixed-conifer forest in the Blue Mountains of northeast Oregon. Can J For Res. 2003;33:612–623. doi: 10.1139/x03-065. [DOI] [Google Scholar]
- Freudenstein JV, Yukawa T, Luo YB. A reanalysis of relationships among Calypsoinae (Orchidaceae: Epidendroideae): floral and vegetative evolution and the placement of Yoania. Syst Bot. 2017;42:17–25. doi: 10.1600/036364417X694944. [DOI] [Google Scholar]
- Gebauer G, Meyer M. 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol. 2003;160:209–223. doi: 10.1046/j.1469-8137.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- Gebauer G, Schulze ED. Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia. 1991;87:198–207. doi: 10.1007/BF00325257. [DOI] [PubMed] [Google Scholar]
- Godo T, Hashimoto T, Nakata M, Miyoshi K. The effects of illumination, temperature and 6-benzylaminoprine on asymbiotic seed germination and protocorm development in vitro in the achlorophyllous orchid Gastrodia pubilabiata Sawa. Vitro Cell Dev Biol Plant. 2020;56:230–235. doi: 10.1007/s11627-020-10061-4. [DOI] [Google Scholar]
- Gomes SIF, Aguirre-Gutiérrez J, Bidartondo MI, Merckx VSFT. Arbuscular mycorrhizal interactions of mycoheterotrophic Thismia are more specialized than in autotrophic plants. New Phytol. 2017;213:1418–1427. doi: 10.1111/nph.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S, Fortuna M, Bascompte J, Merckx V (2019) Plant cheaters preferentially target arbuscular mycorrhizal fungi that are highly connected to mutualistic plants. bioRxiv. 10.1101/867259 [DOI] [PMC free article] [PubMed]
- Gomes SIF, Merckx VSFT, Kehl J, Gebauer G. Mycoheterotrophic plants living on arbuscular mycorrhizal fungi are generally enriched in 13C, 15N and 2H isotopes. J Ecol. 2020;108:1250–1261. doi: 10.1111/1365-2745.13381. [DOI] [Google Scholar]
- Guo SX, Xu JT. The relation between the seed germination and seedling development of Bletilla striata and Mycena osmundicola etc. fungi. Acta Acad Med Sin. 1992;14:51–54. [PubMed] [Google Scholar]
- Guo SX, Fan L, Cao WQ, et al. Mycena anoectochila sp. nov. isolated from mycorrhizal roots of Anoectochilus roxburghii from Xishuangbanna, China. Mycologia. 1997;89:952–954. doi: 10.2307/3761116. [DOI] [Google Scholar]
- Guo S, Fan L, Cao W, Chen X. Mycena dendrobii, a new mycorrhizal fungus. Mycosystema. 1999;18:141–144. [Google Scholar]
- Guo T, Wang HC, Xue WQ, et al. Phylogenetic analyses of Armillaria reveal at least 15 phylogenetic lineages in China, seven of which are associated with cultivated Gastrodia elata. PLoS One. 2016;11:e0154794. doi: 10.1371/journal.pone.0154794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M. Studien über die mykorrhiza von Galeola septentrionalis Reichb. f.—Ein neuer fall der mykorrhiza-bildung durch intraradicale rhizomorpha. Jpn J Bot. 1939;10:151–211. [Google Scholar]
- Hamada M. Physiologisch--morphologische studien über Armillaria mellea (Vahl) Quél., mit besonderer rücksicht auf die oxalsäurebildung. Ein nachtrag zur mykorrhiza von Galeola septentrionalis Reichb. f. Jpn J Bot. 1940;10:387–463. [Google Scholar]
- Hamada M, Nakamura S. Wurzelsymbiose von Galeola altissima Reichb. F., einer chlorophyllfreien Orchidee, mit dem holzzerstorenden Pilz Hymenochaete crocicreas Berk et Br. Sci Rep Tohuku Univ Ser IV. 1963;29:227–238. [Google Scholar]
- Hatté C, Zazzo A, Selosse MA. The radiocarbon age of mycoheterotrophic plants. New Phytol. 2020;227:1284–1288. doi: 10.1111/nph.16637. [DOI] [PubMed] [Google Scholar]
- Higaki K, Rammitsu K, Yamashita Y, et al. A method for facilitating the seed germination of a mycoheterotrophic orchid, Gastrodia pubilabiata, using decomposed leaf litter harboring a basidiomycete fungus, Mycena sp. Bot Stud. 2017;58:59. doi: 10.1186/s40529-017-0214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LH, Lee YI, Hsieh SY et al (2020) GeSUT4 mediates sucrose import at the symbiotic interface for carbon allocation of heterotrophic Gastrodia elata (Orchidaceae). Plant Cell Environ (in press). 10.1111/pce.13833 [DOI] [PubMed]
- Hollander S (1932) Ernahrungs-physiologische untersuchung an wurzelpilzen saprophytische lebender Orchideen. Dissertation, Wurzburg (reported in Burgeff, 1936)
- Hong IP, Kim HK, Park JS, et al. Physiological characteristics of symbiotic fungi associated with the seed germination of Gastrodia elata. Mycobiology. 2002;30:22–26. doi: 10.4489/myco.2002.30.1.022. [DOI] [Google Scholar]
- Hynson NA, Madsen TP, Selosse MA, et al. et al. The physiological ecology of mycoheterotrophy. In: Merckx VSFT, et al.et al., editors. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer; 2013. pp. 297–342. [Google Scholar]
- Hynson NA, Schiebold JMI, Gebauer G. Plant family identity distinguishes patterns of carbon and nitrogen stable isotope abundance and nitrogen concentration in mycoheterotrophic plants associated with ectomycorrhizal fungi. Ann Bot. 2016;118:467–479. doi: 10.1093/aob/mcw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irawati HJ (2002) Seed germination of the mycotrophic orchid, Didymoplexis pallens. In: Proceedings of 17th World Orchid conference. Natural History Publications, Borneo, pp 299–301
- Jacquemyn H, Merckx VSFT. Mycorrhizal symbioses and the evolution of trophic modes in plants. J Ecol. 2019;107:1567–1581. doi: 10.1111/1365-2745.13165. [DOI] [Google Scholar]
- Jyothsna BS, Purushothama KB. Psathyrella candolleana (Fr.) Marie, a saprophytic fungus forming orchid mycorrhiza in Satyrium nepalense D. Don from India. Can J Pure Appl Sci. 2014;8:2691–2697. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TK et al (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Meth 14:587–589 [DOI] [PMC free article] [PubMed]
- Kartzinel TR, Trapnell DW, Shefferson RP. Highly diverse and spatially heterogeneous mycorrhizal symbiosis in a rare epiphyte is unrelated to broad biogeographic or environmental features. Mol Ecol. 2013;22:5949–5961. doi: 10.1111/mec.12536. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780 [DOI] [PMC free article] [PubMed]
- Kennedy AH, Taylor DL, Watson LE. Mycorrhizal specificity in the fully mycoheterotrophic Hexalectris Raf. (Orchidaceae: Epidendroideae) Mol Ecol. 2011;20:1303–1316. doi: 10.1111/j.1365-294X.2011.05000.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi G, Higuchi M, Morota T, et al. Fungal symbiont and cultivation test of Gastrodia elata Blume (Orchidaceae) J Jpn Bot. 2008;83:88–95. [Google Scholar]
- Kikuchi G, Higuchi M, Yoshimura H, et al. In vitro symbiosis between Gastrodia elata Blume (Orchidaceae) and Armillaria Kummer (Tricholomataceae) species isolated from the orchid tuber. J Jpn Bot. 2008;83:77–87. [Google Scholar]
- Kim YI, Chang KJ, Ka KH, et al. Seed germination of Gastrodia elata using symbiotic fungi, Mycena osmundicola. Mycobiology. 2006;34:79–82. doi: 10.4489/myco.2006.34.2.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Ogura-Tsujita Y, Umata H, et al. How do fungal partners affect the evolution and habitat preferences of mycoheterotrophic plants? A case study in Gastrodia. Am J Bot. 2016;103:207–220. doi: 10.3732/ajb.1500082. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. Dictionary of the fungi. 10. Wallingford: CAB International; 2008. [Google Scholar]
- Kohler A, Kuo A, Nagy LG, et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet. 2015;47:410–415. doi: 10.1038/ng.3223. [DOI] [PubMed] [Google Scholar]
- Kohzu A, Yoshioka T, Ando T, et al. Natural 13C and 15N abundance of field-collected fungi and their ecological implications. New Phytol. 1999;144:323–330. doi: 10.1046/j.1469-8137.1999.00508.x. [DOI] [Google Scholar]
- Korhonen K. Interfertility and clonal size in the Armillariella mellea complex. Karstenia. 1978;18:31–42. doi: 10.29203/ka.1978.135. [DOI] [Google Scholar]
- Kusano S. Gastrodia elata and its symbiotic association with Armilllaria mellea. J Coll Agric Univ Tokyo. 1911;4:1–65. [Google Scholar]
- Laiho R, Prescott CE. The contribution of coarse woody debris to carbon, nitrogen, and phosphorus cycles in three Rocky mountain coniferous forests. Can J For Res. 1999;29:1592–1603. doi: 10.1139/x99-132. [DOI] [Google Scholar]
- Leake J. Tansley Review No. 69. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 1994;127:171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- Lee YI, Yang CK, Gebauer G. The importance of associations with saprotrophic non-Rhizoctonia fungi among fully mycoheterotrophic orchids is currently under-estimated: novel evidence from sub-tropical Asia. Ann Bot. 2015;116:423–435. doi: 10.1093/aob/mcv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Han M, Choi MN, et al. Enhancement of the germination efficiency of Gastrodia elata seeds using a new Mycena species. J Plant Biotechnol. 2017;44:56–60. doi: 10.5010/JPB.2017.44.1.056. [DOI] [Google Scholar]
- Li YY, Guo SX, Lee YI. Ultrastructural changes during the symbiotic seed germination of Gastrodia elata with fungi, with emphasis on the fungal colonization region. Bot Stud. 2020;61:4. doi: 10.1186/s40529-019-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Jiang Y, Ma X, et al. Plastid genome evolution in the subtribe Calypsoinae (Epidendroideae, Orchidaceae) Genome Biol Evol. 2020;12:867–870. doi: 10.1093/gbe/evaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebel HT, Gebauer G. Stable isotope signatures confirm carbon and nitrogen gain through ectomycorrhizas in the ghost orchid Epipogium aphyllum Swartz. Plant Biol. 2011;13:270–275. doi: 10.1111/j.1438-8677.2010.00369.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Luo Y, Liu H. Studies of mycorrhizal fungi of Chinese orchids and their role in orchid conservation in China-a review. Bot Rev. 2010;76:241–262. doi: 10.1007/s12229-010-9045-9. [DOI] [Google Scholar]
- Liu T, Li C, Han Y, et al. Highly diversified fungi are associated with the achlorophyllous orchid Gastrodia flavilabella. BMC Genom. 2015;16:185. doi: 10.1186/s12864-015-1422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martos F, Dulormne M, Pailler T, et al. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol. 2009;184:668–681. doi: 10.1111/j.1469-8137.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- Martos F, Munoz F, Pailler T, et al. The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Mol Ecol. 2012;21:5098–5109. doi: 10.1111/j.1365-294X.2012.05692.x. [DOI] [PubMed] [Google Scholar]
- Masuhara G, Katsuya K. Fungal coil formation of Rhizoctonia repens in seedlings of Galeola septentrionalis (Orchidaceae) Bot Mag Tokyo. 1991;104:275–281. doi: 10.1007/BF02488381. [DOI] [Google Scholar]
- Matsuda Y, Shimizu S, Mori M, et al. Seasonal and environmental changes of mycorrhizal associations and heterotrophy levels in mixotrophic Pyrola japonica (Ericaceae) growing under different light environments. Am J Bot. 2012;99:1177–1188. doi: 10.3732/ajb.1100546. [DOI] [PubMed] [Google Scholar]
- Matsushita N, Fukuda K, Nagasawa E, et al. Armillaria species in Japan identified by isozyme patterns with special reference to the biological species of the Northern Hemisphere. J For Res. 1996;1:155–160. doi: 10.1007/BF02348194. [DOI] [Google Scholar]
- McKendrick SL, Leake JR, Read DJ. Symbiotic germination and development of myco-heterotrophic plants in nature: transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytol. 2000;145:539–548. doi: 10.1046/j.1469-8137.2000.00592.x. [DOI] [PubMed] [Google Scholar]
- McLennan EI. Gastrodia sesamoides R.Br. and its endophyte. Aust J Bot. 1959;7:225–229. doi: 10.1071/BT9590225. [DOI] [Google Scholar]
- Merckx VSFT. Mycoheterotrophy: an introduction. In: Merckx VSFT, editor. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer; 2013. pp. 1–17. [Google Scholar]
- Merckx VSFT, Bidartondo MI. Breakdown and delayed cospeciation in the arbuscular mycorrhizal mutualism. Proc R Soc B Biol Sci. 2008;275:1029–1035. doi: 10.1098/rspb.2007.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx V, Stöckel M, Fleischmann A, et al. ) 15N and 13C natural abundance of two mycoheterotrophic and a putative partially mycoheterotrophic species associated with arbuscular mycorrhizal fungi. New Phytol. 2010;188:590–596. doi: 10.1111/j.1469-8137.2010.03365.x. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT, Janssens SB, Hynson NA, et al. Mycoheterotrophic interactions are not limited to a narrow phylogenetic range of arbuscular mycorrhizal fungi. Mol Ecol. 2012;21:1524–1532. doi: 10.1111/j.1365-294X.2012.05472.x. [DOI] [PubMed] [Google Scholar]
- Mohammed C, Guillaumin JJ, Berthelay S. Armillaria species identified in China and Japan. Mycol Res. 1994;98:607–613. doi: 10.1016/S0953-7562(09)80406-1. [DOI] [Google Scholar]
- Motomura H, Selosse MA, Martos F, et al. Mycoheterotrophy evolved from mixotrophic ancestors: evidence in Cymbidium (Orchidaceae) Ann Bot. 2010;106:573–581. doi: 10.1093/aob/mcq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura SJ. Nutritional conditions required for the non-symbiotic culture of an achlorophyllous orchid Galeola septentrionalis. New Phytol. 1982;90:701–715. doi: 10.1111/j.1469-8137.1982.tb03279.x. [DOI] [Google Scholar]
- Nakamura SJ, Uchida T, Hamada M. Atmospheric condition controlling the seed germination of an achlorophyllous orchid, Galeola septentrionalis. Bot Mag Tokyo. 1975;88:103–109. doi: 10.1007/BF02491245. [DOI] [Google Scholar]
- Nishikawa T, Ui T. Rhizoctonias isolated from wild orchids in Hokkaido. Trans Mycol Soc Jpn. 1976;17:77–84. [Google Scholar]
- Ogura-Tsujita Y, Yukawa T. High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae) Am J Bot. 2008;95:93–97. doi: 10.3732/ajb.95.1.93. [DOI] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Gebauer G, Hashimoto T, et al. Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc R Soc B Biol Sci. 2009;276:761–767. doi: 10.1098/rspb.2008.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Yokoyama J, Miyoshi K, Yukawa T. Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae) Am J Bot. 2012;99:1158–1176. doi: 10.3732/ajb.1100464. [DOI] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Gebauer G, Xu H, et al. The giant mycoheterotrophic orchid Erythrorchis altissima is associated mainly with a divergent set of wood-decaying fungi. Mol Ecol. 2018;27:1324–1337. doi: 10.1111/mec.14524. [DOI] [PubMed] [Google Scholar]
- Oliveira JJS, Vargas-Isla R, Cabral TS, Rodrigues SP, Ishikawa NK. Progress on the phylogeny of the Omphalotaceae: Gymnopus s. str., Marasmiellus s. str., Paragymnopus gen. nov. and Pusillomyces gen. nov. Mycol Prog. 2019;18:713–739. doi: 10.1007/s11557-019-01483-5. [DOI] [Google Scholar]
- Osono T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res. 2007;22:955–974. doi: 10.1007/s11284-007-0390-z. [DOI] [Google Scholar]
- Ota Y, Intini M, Hattori T. Genetic characterization of heterothallic and non-heterothallic Armillaria mellea sensu stricto. Mycol Res. 2000;104:1046–1054. doi: 10.1017/S0953756200002550. [DOI] [Google Scholar]
- Pan Y, Liang X, Fan Q. Study on the primary metabolites of Mycena dendrobii, a fungus stimulating the germination of Gastrodia elata. Sydowia. 2015;67:127–132. doi: 10.12905/0380.sydowia67-2015-0127. [DOI] [Google Scholar]
- Park EJ, Lee WY. In vitro symbiotic germination of myco-heterotrophic Gastrodia elata by Mycena species. Plant Biotechnol Rep. 2013;7:185–191. doi: 10.1007/s11816-012-0248-x. [DOI] [Google Scholar]
- Park EJ, Lee WY. Quantitative effects of various tree species on tuber growth and pharmacological compositions of Gastrodia elata. Hortic Environ Biotechnol. 2013;54:357–363. doi: 10.1007/s13580-013-0030-1. [DOI] [Google Scholar]
- Park EJ, Lee WY, Ahn JK. In vitro propagation of myco-heterotrophic Gastrodia elata. Hort Environ Biotechnol. 2012;53:415–420. doi: 10.1007/s13580-012-0046-y. [DOI] [Google Scholar]
- Porras-Alfaro A, Bayman P. Mycorrhizal fungi of Vanilla: diversity, specificity and effects on seed germination and plant growth. Mycologia. 2007;99:510–525. doi: 10.3852/mycologia.99.4.510. [DOI] [PubMed] [Google Scholar]
- Preiss K, Gebauer G. A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isotopes Environ Health Stud. 2008;44:393–401. doi: 10.1080/10256010802507458. [DOI] [PubMed] [Google Scholar]
- Preiss K, Adam IKU, Gebauer G. Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proc Biol Sci. 2010;277:1333–1336. doi: 10.1098/rspb.2009.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HN. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Rasmussen HN. Recent developments in the study of orchid mycorrhiza. Plant Soil. 2002;244:149–163. doi: 10.1023/A:1020246715436. [DOI] [Google Scholar]
- Roberts P. Rhizoctonia-forming fungi. Kew: Royal Botanic Gardens; 1999. [Google Scholar]
- Roll-Hansen F. The Armillaria species in Europe. Eur J For Pathol. 1985;15:22–31. doi: 10.1111/j.1439-0329.1985.tb01039.x. [DOI] [Google Scholar]
- Roy M, Watthana S, Stier A, et al. Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol. 2009;7:51. doi: 10.1186/1741-7007-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Yagame T, Yamato M, et al. Ectomycorrhizal Inocybe species associate with the mycoheterotrophic orchid Epipogium aphyllum but not its asexual propagules. Ann Bot. 2009;104:595–610. doi: 10.1093/aob/mcn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Leiva PA, McDonald JV, Thorn RG. Gymnopanella nothofagi, a new genus and species of gymnopoid fungi (Omphalotaceae) from Chilean Nothofagus forest. Mycologia. 2016;108:820–827. doi: 10.3852/15-303. [DOI] [PubMed] [Google Scholar]
- Schiebold JM, Bidartondo MI, Lenhard F, et al. Exploiting mycorrhizas in broad daylight: partial mycoheterotrophy is a common nutritional strategy in meadow orchids. J Ecol. 2018;106:168–178. doi: 10.1111/1365-2745.12831. [DOI] [Google Scholar]
- Sekizaki H, Kuninaga S, Yamamoto M, et al. Identification of Armillaria nabsnona in Gastrodia tubers. Biol Pharm Bull. 2008;31:1410–1414. doi: 10.1248/bpb.31.1410. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Weiß M, Jany JL, Tillier A. Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich. and neighbouring tree ectomycorrhizae. Mol Ecol. 2002;11:1831–1844. doi: 10.1046/j.1365-294X.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- Selosse M, Martos F, Perry BA, et al. Saprotrophic fungal mycorrhizal symbionts in achlorophyllous orchids: finding treasures among the ‘molecular scraps’? Plant Signal Behav. 2010;5:349–353. doi: 10.4161/psb.5.4.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka C, Fukunaga H, Inagaki S, Sawa S. Artificial cultivation system for Gastrodia spp. and identification of associated mycorrhizal fungi. Int J Biol. 2017;9:27–34. doi: 10.5539/ijb.v9n4p27. [DOI] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3. New York: Acacemic Press; 2008. [Google Scholar]
- Smith ML, Bruhn JN, Anderson JB. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature. 1992;356:428–431. doi: 10.1038/356428a0. [DOI] [Google Scholar]
- Sommer J, Pausch J, Brundrett MC, et al. Limited carbon and mineral nutrient gain from mycorrhizal fungi by adult Australian orchids. Am J Bot. 2012;99:1133–1145. doi: 10.3732/ajb.1100575. [DOI] [PubMed] [Google Scholar]
- Stöckel M, Těšitelová T, Jersáková J, et al. Carbon and nitrogen gain during the growth of orchid seedlings in nature. New Phytol. 2014;202:606–615. doi: 10.1111/nph.12688. [DOI] [PubMed] [Google Scholar]
- Stokland JN, Siitonen J, Jonsson BG. Biodiversity in dead wood. Cambridge: Cambridge University Press; 2012. [Google Scholar]
- Suetsugu K. Autonomous self-pollination and insect visitors in partially and fully mycoheterotrophic species of Cymbidium (Orchidaceae) J Plant Res. 2015;128:115–125. doi: 10.1007/s10265-014-0669-4. [DOI] [PubMed] [Google Scholar]
- Suetsugu K. Independent recruitment of a novel seed dispersal system by camel crickets in achlorophyllous plants. New Phytol. 2018;217:828–835. doi: 10.1111/nph.14859. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Kawakita A, Kato M. Avian seed dispersal in a mycoheterotrophic orchid Cyrtosia septentrionalis. Nature Plants. 2015;1:15052. doi: 10.1038/nplants.2015.52. [DOI] [Google Scholar]
- Suetsugu K, Ohta T, Tayasu I. Partial mycoheterotrophy in the leafless orchid Cymbidium macrorhizon. Am J Bot. 2018;105:1595–1600. doi: 10.1002/ajb2.1142. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Yamato M, Matsubayashi J, Tayasu I. Comparative study of nutritional mode and mycorrhizal fungi in green and albino variants of Goodyera velutina, an orchid mainly utilizing saprotrophic rhizoctonia. Mol Ecol. 2019;28:4290–4299. doi: 10.1111/mec.15213. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Matsubayashi J, Ogawa NO, et al. Isotopic evidence of arbuscular mycorrhizal cheating in a grassland gentian species. Oecologia. 2020;192:929–937. doi: 10.1007/s00442-020-04631-x. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Matsubayashi J, Tayasu I. Some mycoheterotrophic orchids depend on carbon from dead wood: novel evidence from a radiocarbon approach. New Phytol. 2020;227:1519–1529. doi: 10.1111/nph.16409. [DOI] [PubMed] [Google Scholar]
- Tashima Y, Terashita T, Umata H, Matsumoto M. In vitro development from seed to flower in Gastrodia verrucosa under fungal symbiosis. Trans Mycol Soc Jpn. 1978;19:449–453. [Google Scholar]
- Taylor DL, Bruns TD. Population, habitat and genetic correlates of mycorrhizal specialization in the “cheating” orchids Corallorhiza maculata and C. mertensiana. Mol Ecol. 1999;8:1719–1732. doi: 10.1046/j.1365-294X.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- Taylor DL, McCormick MK. Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytol. 2008;177:1020–1033. doi: 10.1111/j.1469-8137.2007.02320.x. [DOI] [PubMed] [Google Scholar]
- Terashima K, Cha JY, Yajima T, et al. Phylogenetic analysis of Japanese Armillaria based on the intergenic spacer (IGS) sequences of their ribosomal DNA. Eur J For Pathol. 1998;28:11–19. doi: 10.1111/j.1439-0329.1998.tb01161.x. [DOI] [Google Scholar]
- Terashita T. Fungi inhabiting wild orchids in Japan (III) A symbiotic experiment with Armillariella mellea and Galeola septentrionalis. Trans Mycol Soc Jpn. 1985;26:47–53. [Google Scholar]
- Terashita T. Biological species of Armillaria symbiotic with Galeola septentrionalis. Trans Mycol Soc Jpn. 1996;37:45–49. [Google Scholar]
- Terashita T, Chuman S. Fungi inhabiting wild orchids in Japan (IV). Armillariella tabescens, a new symbiont of Galeola septentrionalis. Trans Mycol Soc Jpn. 1987;28:145–154. [Google Scholar]
- Terashita T, Chuman S (1989) Armillaria species isolated from the wild orchid, Galeola septentrionalis. In: Proceedingsof the 7th International Conference on Root and Butt Rots. International Union of Forestry Research Organisations, Working Party S2.06.01, British Columbia, pp 364–374
- Thoen E, Harder CB, Kauserud H, et al. In vitro evidence of root colonization suggests ecological versatility in the genus Mycena. New Phytol. 2020;227:601–612. doi: 10.1111/nph.16545. [DOI] [PubMed] [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235 [DOI] [PMC free article] [PubMed]
- Tsuda S, Moriya H, Tomita M, Harada Y. Nagoya International Orchid Congress 2004. Nagoya: Organizing Committee of Nagoya International Orchid Show; 2004. Artificial propagation and the new symbiotic mycorrhiza of Yoania flava, a terrestrial saprophytic orchid, central Japan; pp. 36–40. [Google Scholar]
- Umata H. Seed germination of Galeola altissima, an achlorophyllous orchid, with aphyllophorales fungi. Mycoscience. 1995;36:369–372. doi: 10.1007/BF02268616. [DOI] [Google Scholar]
- Umata H. In vitro germination of Erythrorchis ochobiensis (Orchidaceae) in the presence of Lyophyllum shimeji, an ectomycorrhizal fungus. Mycoscience. 1997;38:355–357. doi: 10.1007/BF02464097. [DOI] [Google Scholar]
- Umata H. Formation of endomycorrhizas by an achlorophyllous orchid, Erythrorchis ochobiensis, and Auricularia polytricha. Mycoscience. 1997;38:335–339. doi: 10.1007/BF02464092. [DOI] [Google Scholar]
- Umata H. A new biological function of Shiitake mushroom, Lentinula edodes, in a myco-heterotrophic orchid, Erythrorchis ochobiensis. Mycoscience. 1998;39:85–88. doi: 10.1007/BF02461583. [DOI] [Google Scholar]
- Umata H. In vitro symbiotic association of an achlorophyllous orchid, Erythrorchis ochobiensis, with orchid and non-orchid fungi. Mem Fac Agric Kagoshima Univ. 1998;34:97–107. [Google Scholar]
- Umata H. Germination and growth of Erythrorchis ochobiensis (Orchidaceae) accelerated by monokaryons and dikaryons of Lenzites betulinus and Trametes hirsuta. Mycoscience. 1999;40:367–371. doi: 10.1007/BF02463883. [DOI] [Google Scholar]
- Umata H, Fujimoto T, Arai K (2000a) Species richness of the symbiont in Erythrorchis ochobiensis, an achlorophyllous orchid. In: Proceedings of 7th international symposium of the Mycological Society of Japan. Tsukuba Center for Institute, Science and Technology Agency, Tsukuba, Japan, pp 52–56
- Umata H, Yamauchi H, Hashimoto T. In vitro culture of a myco-heterotrophic orchid Gastrodia pubilabiata with a mycorrhizal symbiont of G. verrucosa. Res Bull Kagoshima Univ For. 2000;28:27–30. [Google Scholar]
- Umata H, Kubota K, Inoue I, Shiiba Y, Nagasawa H (2006) Naturally germinating seeds of the achlorophyllous orchid Galeola septentrionalis contains no fungal pelotons. Res Bull Kagoshima Univ For 34:69–74
- Umata H, Kaneko M, Miyagi T, Nakahira Y. The application and utilization of fungi for the propagation of the endangered achlorophyllous plant, Erythrorchis ochobiensis (Hayata) Garay (Orchidaceae) in natural situations. Res Bull Kagoshima Univ For. 2007;35:31–48. [Google Scholar]
- Umata H, Nishi M (2010) Ecological characteristics and water supply effect on in situ conservation in Gastrodia nipponica and G. pubilabiata, the endangered myco-heterotrophic orchids. Res Bull Kagoshima Univ For 37:137–149 (in Japanese with English abstract)
- Umata H, Ota Y, Yamada M, et al. Germination of the fully myco-heterotrophic orchid Cyrtosia septentrionalis is characterized by low fungal specificity and does not require direct seed-mycobiont contact. Mycoscience. 2013;54:343–352. doi: 10.1016/j.myc.2012.12.003. [DOI] [Google Scholar]
- Veldre V, Abarenkov K, Bahram M, et al. Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol. 2013;6:256–268. doi: 10.1016/j.funeco.2013.03.004. [DOI] [Google Scholar]
- Voronina EY, Malysheva EF, Malysheva VF, et al. A mixotrophy is in question: new data on fungal community associated with photosynthetic terrestrial orchid Goodyera repens. Bot Pac. 2018;7:51–61. doi: 10.17581/bp.2018.07106. [DOI] [Google Scholar]
- Waterman RJ, Klooster MR, Hentrich H, Bidartondo MI. Species interactions of mycoheterotrophic plants: specialization and its potential consequences. In: Merckx VSFT, editor. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer; 2013. pp. 267–296. [Google Scholar]
- WCSP (2016) World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/. Accessed 21 July 2020
- Wilson AW, Desjardin DE. Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade) Mycologia. 2005;97:667–679. doi: 10.3852/mycologia.97.3.667. [DOI] [PubMed] [Google Scholar]
- Xu J, Guo S. Fungus associated with nutrition of seed germination of Gastrodia elata--Mycena osmundicola Lange. Acta Mycol Sin. 1989;8:221–226. [Google Scholar]
- Xu J, Guo S. Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chin Med J. 2000;113:686–692. [PubMed] [Google Scholar]
- Xu J, Mu C. The relation between growth of Gastrodia elata protocorms and fungi. Acta Bot Sin. 1990;32:26–31. [Google Scholar]
- Yagame T, Yamato M, Mii M, et al. Developmental processes of achlorophyllous orchid, Epipogium roseum: from seed germination to flowering under symbiotic cultivation with mycorrhizal fungus. J Plant Res. 2007;120:229–236. doi: 10.1007/s10265-006-0044-1. [DOI] [PubMed] [Google Scholar]
- Yagame T, Fukiharu T, Yamato M, et al. Identification of a mycorrhizal fungus in Epipogium roseum (Orchidaceae) from morphological characteristics of basidiomata. Mycoscience. 2008;59:18–23. doi: 10.1007/s10267-007-0396-y. [DOI] [Google Scholar]
- Yagame T, Funabiki E, Nagasawa E, et al. Identification and symbiotic ability of Psathyrellaceae fungi isolated from a photosynthetic orchid, Cremastra appendiculata (Orchidaceae) Am J Bot. 2013;100:1823–1830. doi: 10.3732/ajb.1300099. [DOI] [PubMed] [Google Scholar]
- Yagame T, Funabiki E, Selosse MA et al (2015) Mycoheterotrophic level of Cremastra appendiculata is affected by mycobiont species. In: Proceedings of the 79th annual meeting of the Botanical Society of Japan, Niigata, p 152 (in Japanese)
- Yagame T, Ogura-Tsujita Y, Kinoshita A, et al. Fungal partner shifts during the evolution of mycoheterotrophy in Neottia. Am J Bot. 2016;103:1630–1641. doi: 10.3732/ajb.1600063. [DOI] [PubMed] [Google Scholar]
- Yagame T, Funabiki E, Yukawa T, Nagasawa E. Identification of mycobionts in an achlorophyllous orchid, Cremastra aphylla (Orchidaceae), based on molecular analysis and basidioma morphology. Mycoscience. 2018;59:18–23. doi: 10.1016/j.myc.2017.08.001. [DOI] [Google Scholar]
- Yamashita Y, Kinoshita A, Yagame T et al (2020) Physisporinus is an important mycorrhizal partner for mycoheterotrophic plants: identification of mycorrhizal fungi of three Yoania species. Mycoscience (in press). 10.1016/j.myc.2020.05.003
- Yamato M, Yagame T, Suzuki A, Iwase K. Isolation and identification of mycorrhizal fungi associating with an achlorophyllous plant, Epipogium roseum (Orchidaceae) Mycoscience. 2005;46:73–77. doi: 10.1007/s10267-004-0218-4. [DOI] [Google Scholar]
- Yamato M, Yagame T, Shimomura N, et al. Specific arbuscular mycorrhizal fungi associated with non-photosynthetic Petrosavia sakuraii (Petrosaviaceae) Mycorrhiza. 2011;21:631–639. doi: 10.1007/s00572-011-0373-3. [DOI] [PubMed] [Google Scholar]
- Yamato M, Ogura-Tsujita Y, Takahashi H, Yukawa T. Significant difference in mycorrhizal specificity between an autotrophic and its sister mycoheterotrophic plant species of Petrosaviaceae. J Plant Res. 2014;127:685–693. doi: 10.1007/s10265-014-0661-z. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Liao FS, Huang KL, et al. An efficient protocol of protocorm-like bodies regeneration from callus cultures of Gastrodia elata Blume and the further associations with mycorrhizal fungi. J Fac Agric Kyushu Univ. 2017;62:39–46. [Google Scholar]
- Yukawa T, Ogura-Tsujita Y, Shefferson RP, Yokoyama J. Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. Am J Bot. 2009;96:1997–2009. doi: 10.3732/ajb.0900101. [DOI] [PubMed] [Google Scholar]
- Zeng X, Li Y, Ling H, et al. Transcriptomic analyses reveal clathrin-mediated endocytosis involved in symbiotic seed germination of Gastrodia elata. Bot Stud. 2017;58:31. doi: 10.1186/s40529-017-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Li Y, Ling H, et al. Revealing proteins associated with symbiotic germination of Gastrodia elata by proteomic analysis. Bot Stud. 2018;59:8. doi: 10.1186/s40529-018-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen J, Lv Y, et al. Mycena sp., a mycorrhizal fungus of the orchid Dendrobium officinale. Mycol Prog. 2012;11:395–401. doi: 10.1007/s11557-011-0754-1. [DOI] [Google Scholar]
- Zimmer K, Meyer C, Gebauer G. The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytol. 2008;178:395–400. doi: 10.1111/j.1469-8137.2007.02362.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.