Abstract
OBJECTIVE
To compare the long-term efficacy of initiating therapy with metformin/pioglitazone/exenatide in patients with new-onset type 2 diabetes mellitus (T2DM) versus sequential addition of metformin followed by glipizide and insulin.
RESEARCH DESIGN AND METHODS
Drug-naive patients (N = 318) with new-onset T2DM were randomly assigned to receive for 3 years either 1) combination therapy with metformin, pioglitazone, and exenatide (triple therapy) or 2) sequential addition of metformin followed by glipizide and insulin (conventional therapy) to maintain HbA1c at <6.5% (48 mmol/mol). Insulin sensitivity and β-cell function were measured at baseline and 3 years. The primary outcome was the difference in HbA1c between the groups at 3 years.
RESULTS
Baseline HbA1c ± SEM values were 9.0% ± 0.2% and 8.9% ± 0.2% in the triple therapy and conventional therapy groups, respectively. The decrease in HbA1c resulting from triple therapy was greater at 6 months than that produced by conventional therapy (0.30% [95% CI 0.21–0.39]; P = 0.001), and the HbA1c reduction was maintained at 3 years in patients receiving triple therapy compared with conventional therapy (6.4% ± 0.1% and 6.9% ± 0.1%, respectively), despite intensification of antihyperglycemic therapy in the latter. Thus, the difference in HbA1c between the two treatment groups at 3 years was 0.50% (95% CI 0.39–0.61; P < 0.0001). Triple therapy produced a threefold increase in insulin sensitivity and 30-fold increase in β-cell function. In conventional therapy, insulin sensitivity did not change and β-cell function increased by only 34% (both P < 0.0001 vs. triple therapy).
CONCLUSIONS
Triple therapy with agents that improve insulin sensitivity and β-cell function in patients with new-onset T2DM produces greater, more durable HbA1c reduction than agents that lower glucose levels without correcting the underlying metabolic defects.
Introduction
Hyperglycemia is a sine qua non in type 2 diabetes mellitus (T2DM). Multiple pathophysiologic defects contribute to hyperglycemia and collectively these are known as the Ominous Octet (1). Although insulin resistance (IR) can be demonstrated early in the natural history of T2DM (2–4), diabetic levels of hyperglycemia do not occur in the absence of β-cell failure, which is the primary factor responsible for the development and progression of hyperglycemia (1–8). It follows that to achieve an effective, durable reduction in HbA1c and reduce the risk of microvascular complications, 1) no single agent can correct the multiple metabolic abnormalities present in T2DM, and 2) the therapeutic regimen should include antidiabetic agents that improve β-cell function, as well as IR.
The current strategy in the U.S. and worldwide for glycemic control in T2DM (9–11) is starting metformin at the time of diagnosis and then following with stepwise addition of agents after failure of the previous agent without considering the disease pathophysiology (9). Because of the central role of IR and β-cell dysfunction in the pathogenesis of T2DM (1–8), we hypothesized that early administration of antidiabetic agents that improve insulin sensitivity and β-cell dysfunction would be more effective than agents that focus on glucose lowering without affecting insulin sensitivity and β-cell function (e.g., metformin, sulfonylurea, insulin). Glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) potentiate glucose-stimulated insulin secretion (12,13), suppress food intake, and promote weight loss, which secondarily can enhance insulin sensitivity (1,12). Pioglitazone improves insulin sensitivity in skeletal muscle, liver, and adipocytes (1,14) and also has a potent effect on improving β-cell function (15,16). Thus, the combination of GLP-1 RA plus pioglitazone addresses or improves seven of the eight defects that make up the Ominous Octet (1).
The combination of pioglitazone plus GLP-1 RA is very effective in lowering HbA1c in patients with new-onset diabetes (17), as well as in patients with poorly controlled T2DM who have longstanding disease (18). Furthermore, the combination of pioglitazone plus GLP-1 RA effectively reduces HbA1c independent of the starting HbA1c level (17,19). In this report, we present the long-term (3-year) effects of initiating triple therapy with pioglitazone, exenatide, and metformin at the time of T2DM diagnosis on glucose control, insulin sensitivity, and β-cell function versus initiating therapy with metformin followed by sequential addition of glipizide and then basal insulin.
Research Design and Methods
The Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT) study is an open-label, 3-year, single-center, randomized controlled trial (ClinicalTrials.gov reg. no. NCT01107717) designed to compare the efficacy and durability of the following two therapeutic approaches for the treatment of patients with new-onset T2DM: 1) initial combination therapy with medications (metformin, pioglitazone, and exenatide [triple therapy]) that correct core metabolic defects present in T2DM, versus 2) stepwise addition of medications that lower plasma glucose (PG) levels without correcting the underlying pathophysiologic abnormalities (conventional therapy). The study protocol was approved by Institutional Review Board of University of Texas Health Science Center at San Antonio and informed written consent was obtained from all participants before enrolment. Herein, we described the 3-year glycemia results, the physiologic measurements of β-cell function and insulin sensitivity, and the effect of therapy on cardiovascular risk factors.
The study design has been published (17). Briefly, drug-naive patients (aged 18–75 years) with new-onset (<2 years) T2DM, who otherwise were healthy, were recruited to the study. Key exclusion criteria were use of medications known to affect glucose metabolism; previous treatment with any antidiabetic agent; evidence of proliferative retinopathy; albumin excretion >300 mg/day, or major organ-system disease as determined by physical examination, medical history, and screening blood tests.
Study Design
After screening, eligible participants underwent 1) a 75-g oral glucose tolerance test (OGTT) and 2) ultrasound measurement of carotid intima-media thickness (CIMT) (15). After completing baseline studies, patients were consecutively randomly assigned to receive either initial triple therapy or conventional therapy to maintain HbA1c at <6.5% (48 mmol/mol) (17). The treatment algorithm for each arm has been published (17) and is described in detail in the Supplementary Material. Briefly, patients in the conventional therapy group began treatment with metformin 1,000 mg/day, and the dose was escalated and followed by sequential addition of glipizide followed by glargine insulin (up to 60 units per day) within 6 months upon failure to achieve the treatment goal (HbA1c <6.5%). Patients in the triple therapy group began treatment with metformin 100 mg/day, pioglitazone 15 mg, and exenatide 5 µg twice daily, and the dose was escalated to the maximal tolerated dose within 6 months.
OGTT and CIMT
Each patient underwent a 75-g OGTT for the measurement of insulin secretion and insulin sensitivity, and CIMT measurement. Both tests were repeated at the end of the study. Detailed description of the two procedures is provided in the Supplementary Material.
Data Analysis and Statistical Methods
The primary end point was HbA1c difference between participants receiving triple therapy versus conventional therapy at the study end. The intention-to-treat (ITT) analysis was used to compare the two treatment arms. Per-protocol analysis and last observation carried forward also were used to compare the two treatment arms (Supplementary Material).
Overall frequency of hypoglycemia was calculated as the total number of hypoglycemic events divided by the number of patient-years of follow-up in each arm. The percentage of participants experiencing hypoglycemia was calculated as the number of participants in a study arm experiencing at least a single event divided by number of patients in that arm.
OGTT-derived indices of insulin sensitivity and insulin secretion were calculated from the PG, insulin, and C-peptide (C-pep) concentrations during the OGTT, as previously described (5,20,21). The Matsuda Index was used to quantitate insulin sensitivity (21). This index strongly correlates with total body insulin sensitivity measured with the euglycemic insulin clamp. Plasma C-pep concentration during the OGTT was used to quantitate insulin secretion. The ratio between incremental area under the plasma C-pep concentration curve to incremental area under PG concentration curve during the OGTT (∆C-pep/∆G)0–120 was used to provide a measure of insulin secretion. β-Cell function was measured by expressing insulin secretion per prevailing level of IR, as follows: (∆C-pep/∆G)0–120/IR). The incremental area under PG and C-pep concentration curves during the OGTT was calculated using the trapezoid rule.
Values are presented as mean ± SEM. Two-sided t test was used to compare mean differences between treatment arms, and the χ2 test was used to test the significance of discrete variables. The Cox proportional hazards model was used to estimate the influence of therapy on failure to maintain the treatment goal. The model was adjusted for other confounders (namely, age, sex, BMI, disease duration, and baseline HbA1c level).
The study was powered to detect a 0.5% (±0.95 SD) HbA1c difference between the two treatment arms based on the HbA1c decrease in the PROactive study (22). We calculated that 76 patients who completed the study would be required in each arm to detect significant difference between the two groups at α < 0.05. A detailed description of the sample size calculation is given in the Supplementary Material.
Results
A total of 487 patients with newly diagnosed T2DM were screened between 2009 and 2018, and 318 eligible participants were randomly assigned to study arms as follows: 157 participants were randomly assigned to receive triple therapy and 161 to receive conventional therapy (Supplementary Fig. 1). Participants in both treatment arms were well matched for age, BMI, diabetes duration, and HbA1c (Supplementary Table 1). Participants were generally obese, had mean HbA1c value of 8.9% (74 mmol/mol; range, 6.5–14.0% [48–130 mmol/mol]) and mean diabetes duration of 5.1 months. All patients were drug naïve. The mean follow-up was 28.1 ± 0.9 and 27.0 ± 0.9 months in the conventional and triple therapy arms, respectively (P = NS).
At study end, all patients in the triple therapy group were receiving metformin (1,929 ± 23 mg), pioglitazone (37 ± 1 mg), and exenatide twice daily (45 patients received 5 µg twice daily and 112 received 10 µg twice daily). In the conventional therapy group, 29% of patients were receiving metformin only, and 71% were receiving metformin plus glipizide (mean dose, 9.3 ± 0.1, 11.2 ± 0.1, and 14.2 ± 0.6 mg/day at year 1, 2, and 3, respectively) and glargine (37 ± 1, 42 ± 2, and 53 ± 2 units/day at year 1, 2, and 3, respectively).
Forty-seven patients in the conventional therapy arm (12.5% per year) and 54 in the triple therapy arm (15.3% per year) dropped out of the study (P = NS). The baseline characteristics in patients who dropped out of the study were comparable to those who completed the study (Supplementary Table 2). The long-term effect of treatment was recorded in 114 and 103 patients in conventional therapy and triple therapy arms, respectively. Sixty-seven and 34 patients in conventional therapy and triple therapy arms, respectively, failed to maintain the treatment HbA1c goal of <6.5% (48 mmol/mol) and were considered treatment failures, whereas 47 (41%) and 69 (67%), respectively, maintained HbA1c at <6.5% (48 mmol/mol) over 3 years (P = 0.0002).
The difference in HbA1c at study end between the two treatment groups (ITT analysis) was 0.50% (95% CI 0.39–0.61, P < 0.0001; 6.4% ± 0.1% vs. 6.9% ± 0.1% in the triple therapy [n = 157] and conventional therapy [n = 161] groups, respectively). The conclusion from the ITT analysis (Fig. 1A) was virtually identical to that from the per-protocol analysis (HbA1c, 6.3% ± 0.1%, and 6.9% ± 0.1% for the triple therapy [n = 103] and conventional therapy [n = 114] groups, respectively, P < 0.0001) (Supplementary Fig. 2) or last observation carried forward analysis (change in HbA1c, 0.50% ± 0.1%) (Supplementary Fig. 3). Baseline HbA1c was similar in both groups (8.8% [73 mmol/mol] and 9.0% [75 mmol/mol] in the conventional and triple therapy arms, respectively; P = NS) and progressively decreased after initiating therapy in both treatment arms. At 6 months, there was a small but significant HbA1c difference (0.30% [95% CI 0.21–0.39]; P = 0.001) between groups (triple therapy, 6.0% [42 mmol/mol] vs. conventional therapy, 6.3% [45 mmol/mol]). After 6 months, the difference in HbA1c between the two groups gradually increased, reaching 0.50% (95% CI 0.39–0.61; P < 0.0001) at study end (Supplementary Fig. 4).
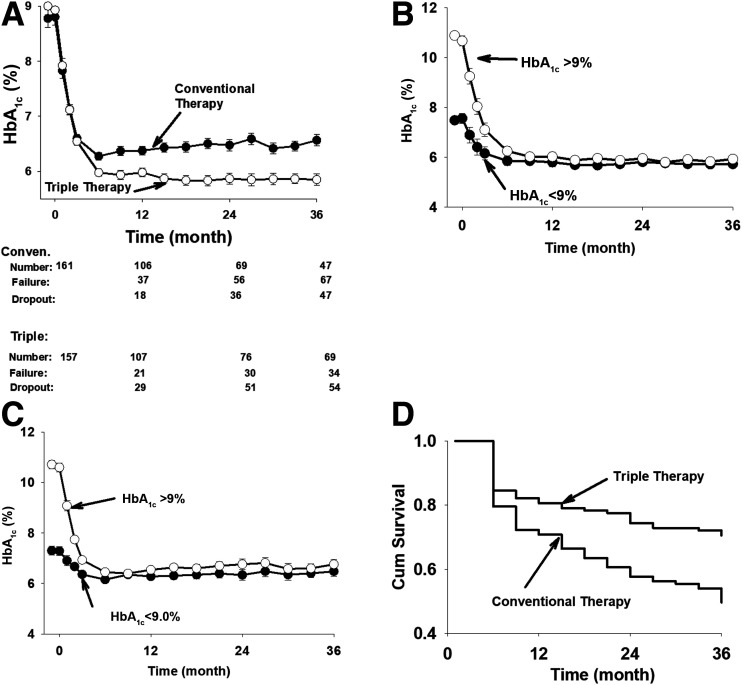
Figure 1.
Time-related change in HbA1c in the ITT analysis. Conven., conventional (A). Decrease in HbA1c in patients receiving triple therapy who had baseline HbA1c values ≥9.0% and <9.0% (B). Decrease in HbA1c in patients in the conventional therapy group who had baseline HbA1c values ≥9.0% and <9.0% (C). Kaplan-Meier plot of time to treatment failure in the triple therapy and conventional therapy groups. Cum, cumulative (D). Patients who dropped out of the study were not included.
The efficacy of triple therapy was independent of the starting HbA1c value and was equally effective in patients with starting HbA1c above or below 9% (75 mmol/mol). In patients in the triple therapy group with HbA1c ≥9.0% (75 mmol/mol) (n = 70) and HbA1c <9.0% (75 mmol/mol) (n = 87), the baseline HbA1c was 10.9% ± 0.2% (96 mmol/mol) and 7.5% ± 0.2% (58 mmol/mol), respectively, and decreased to 5.9% (41 mmol/mol) and 5.7% (39 mmol/mol), respectively, at 36 months (Fig. 1B). The decrease in HbA1c value in patients receiving triple therapy was significantly greater than in patients in the conventional therapy group, with starting HbA1c ≥9 (75 mmol/mol) and <9% (75 mmol/mol) (Supplementary Figs. 5 and 6). Similarly, conventional therapy was equally effective in reducing the HbA1c in patients with baseline HbA1c >9.0% versus below 9.0% (Fig. 1C).
More participants receiving triple therapy achieved and maintained the treatment goal compared with those receiving conventional therapy (67% vs. 41%; P = 0.0002) (Supplementary Fig. 7). The Kaplan-Meier plot of time to treatment failure (i.e., HbA1c >6.5%, [48 mmol/mol]) showed an early and progressive separation of the HbA1c curves for the two groups (Fig. 1D). Also, more participants receiving triple therapy (51%) reduced their HbA1c value to the normal range (<6.0%; 42 mmol/mol) compared with those receiving conventional therapy (26%; P < 0.0001).
Effect of Therapy on Fasting PG and 2-Hour PG Levels
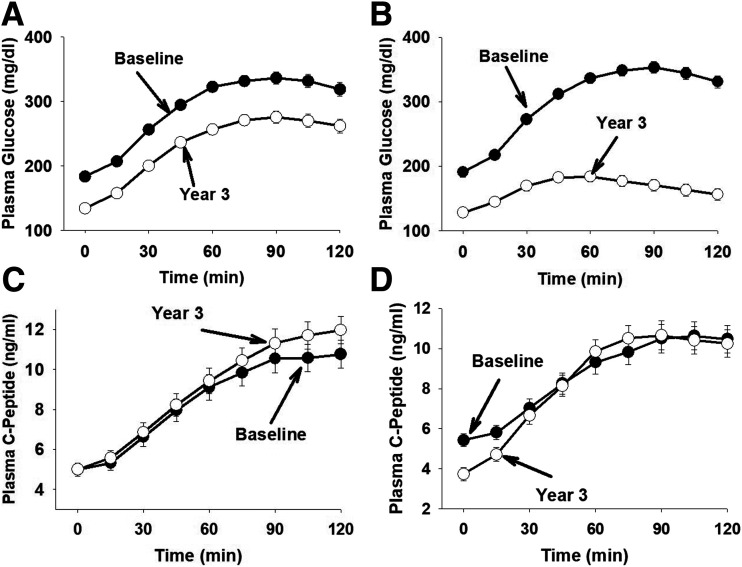
The fasting PG (FPG) concentration was similar in the conventional and triple therapy groups (11.1 ± 0.3 mmol/L [200 ± 6 mg/dL] and 11.3 ± 0.3 mmol/L [203 ± 6 mg/dL], respectively; P = NS) and decreased rapidly after starting therapy. At 6 months (Supplementary Fig. 8), the decrease in FPG was greater in the triple therapy group than in the conventional therapy group (6.7 ± 0.2 mmol/L [121 ± 3 mg/dL] vs. 7.3 ± 0.2 mmol/L [132 ± 3 mg/dL]; P = 0.001). The FPG concentration decreased further at 36 months in both groups but remained lower in the triple therapy group (6.2 ± 0.2 mmol/L [111 ± 4 mg/dL] vs. 7.0 ± 0.2 mmol/L [126 ± 4 mg/dL]; P = 0.003). Both therapies reduced the 2-h PG concentration (Fig. 2) and the incremental area (∆G0–120) under the PG concentration during the OGTT at 36 months (Table 1), but the decreases were markedly greater (both P < 0.0001) in the triple therapy group versus the conventional therapy group.
Figure 2.
PG and C-pep concentrations during the OGTT in the conventional therapy (A and C) and triple therapy (B and D) groups.
Table 1.
Metabolic effects of triple therapy and conventional therapy
| Triple therapy | P | Conventional therapy | P | ANOVA | |||
|---|---|---|---|---|---|---|---|
| Baseline | 3-years | Baseline | 3-years | ||||
| FPG (mg/dL) | 191 ± 7 | 128 ± 6 | <0.0001 | 183 ± 7 | 134 ± 7 | <0.0001 | NS |
| 2-h PG (mg/dL) | 320 ± 11 | 156 ± 10 | <0.0001 | 330 ± 9 | 265 ± 8 | <0.0001 | <0.0001 |
| HbA1c (%) | 9.0% ± 0.2 | 6.4% ± 0.1 | <0.0001 | 8.8% ± 0.2 | 6.9% ± 0.1 | <0.0001 | <0.0001 |
| ∆G0–120 (mg/dL/h) | 227 ± 5 | 80 ± 9 | <0.0001 | 216 ± 6 | 195 ± 8 | 0.006 | <0.0001 |
| ∆I0–120 (µU/mL/h) | 55 ± 8 | 64 ± 11 | NS | 58 ± 6 | 54 ± 5 | NS | NS |
| ∆C-pep0–120 (ng/mL/h) | 6.5 ± 0.6 | 9.5 ± 0.9 | 0.004 | 7.0 ± 0.7 | 7.9 ± 0.7 | NS | NS |
| (∆C-pep/∆G)0–120 | 0.029 ± 0.003 | 0.64 ± 0.15 | 0.0002 | 0.035 ± 0.004 | 0.047 ± 0.050 | 0.02 | 0.0001 |
| Matsuda Index | 2.9 ± 0.3 | 9.3 ± 1.5 | 0.0006 | 4.1 ± 0.9 | 3.4 ± 0.6 | NS | 0.0002 |
| (∆C-pep/∆G)0–120 × Matsuda Index | 0.079 ± 0.015 | 2.7 ± 0.5 | <0.0001 | 0.090 ± 0.012 | 0.137 ± 0.018 | 0.01 | <0.0001 |
∆I0–120, change in insulin concentration. Data are mean ± SEM.
Effect of Therapy on Insulin Sensitivity and β-Cell Function
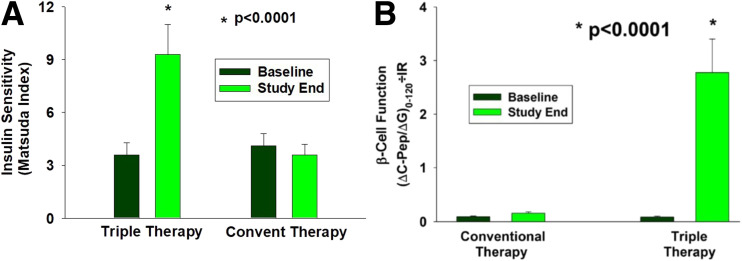
At baseline, insulin sensitivity, measured with the Matsuda Index, was comparable in both treatment groups. Triple therapy caused a threefold increase in Matsuda Index values compared with a small, nonsignificant decrease with conventional therapy (P < 0.0001) (Fig. 3A). At study end, insulin secretion, (∆C-pep/∆G)0–120, improved with both therapies, but the increase in triple therapy (greater than threefold) was significantly greater than the increase (34%) observed with conventional therapy (P < 0.0001) (Table 1). The improvement in β-cell function—(∆C-pep/∆G)0–120/IR—caused by triple therapy was markedly greater (30-fold) than the increase (52%) resulting from conventional therapy (P < 0.0001) (Fig. 3B and Table 1).
Figure 3.
Matsuda Index of insulin sensitivity (A) and β-cell function measured with (∆C-pep/∆G)0–120 × Matsuda Index (B) in the conventional therapy and triple therapy groups at baseline and study end.
Body Weight
Both therapies caused small but significant weight gain: 1.3 ± 0.9 kg with triple therapy and 0.6 ± 0.8 kg with conventional therapy (P < 0.001), and the increase in body weight was comparable between the two treatment groups. The increment in body weight correlated with the HbA1c at study end (r = 0.43; P < 0.001) and was independent of treatment (i.e., the greater the weight gain, the higher the HbA1c value) (Supplementary Fig. 9). The means indicated there was weight gain in both groups. However, 39% and 48% of patients in the triple therapy and conventional therapy groups, respectively, lost weight during the study. The decrease in HbA1c at study end resulting from triple therapy was significantly greater than that with conventional therapy in patients who lost weight (5.7%% ± 0.1 [39 mmol/mol] vs. 6.2% ± 0.1% [44 mmol/mol], respectively; P < 0.0001) or gained weight (6.5% ± 0.1% [48 mmol/mol] vs. 7.0% ± 0.1% [53 mmol/mol], respectively; P = 0.0003).
Effect of Therapy on Lipid Profile
Triple therapy produced a greater decrease in plasma triglyceride concentration than did conventional therapy (−0.50 ± 0.12 vs. −0.07 ± 0.16 mmol/L, respectively; P < 0.05). Plasma HDL concentration increased in patients receiving triple therapy (+0.13 ± 0.05) and decreased in patients receiving conventional therapy (−0.08 ± 0.03 mmol/L) (P = 0.004 between groups). Total cholesterol concentration decreased slightly by −0.41 ± 0.07 and −0.49 ± 0.13 mmol/L in the conventional and triple therapy groups, respectively (P = NS).
Carotid Intima Media Thickness
CIMT at baseline was comparable in the two treatment arms and increased in both groups at the study end. The increment above baseline in patients receiving triple therapy (0.005 ± 0.002 mm) did not reach statistical significance, whereas the increment above baseline in patients receiving conventional therapy (+0.015 ± 0.002 mm) was highly statistically significant (P < 0.0001) and significantly greater than in patients receiving triple therapy (P = 0.01) (Supplementary Fig. 10).
Adverse Events
Two-thirds of participants receiving conventional therapy and one-half of participants receiving triple therapy experienced at least one adverse event. The majority of adverse events were unrelated to the study treatment. The most common adverse event related to the study intervention was hypoglycemia, reported by 46% and 14% of participants receiving conventional therapy and triple therapy, respectively (P < 0.0001). The overall frequency of hypoglycemic events was greater in participants receiving conventional therapy (2.2 vs. 0.31 events per participant per year; P < 0.0001). It should be noted that patients in the conventional therapy group whose HbA1c was well controlled with metformin only did not experience hypoglycemia. Furthermore, the rate of hypoglycemia in patients receiving glipizide and/or insulin increased over time in parallel to the escalation of glipizide and insulin doses (Supplementary Fig. 11). No patient experienced a severe hypoglycemic event.
Of participants receiving triple therapy, 25% experienced nausea related to initiation of exenatide; the nausea was mild and subsided within 2 to 3 months. In 45 patients, the exenatide dose was reduced to 5 μg twice daily to avoid gastrointestinal side effects. The incidence of peripheral edema was low and mild: 1.3% versus 5.3% in conventional and triple therapy groups, respectively. The pioglitazone dose was reduced to 15 and 30 mg/day in 20 and 44 patients, respectively, to avoid fluid retention and minimize wait gain. Two deaths occurred in the conventional therapy group; both were unrelated to treatment (sleep apnea; complication of surgical procedure for hernia). The number of participants who withdrew because of adverse events was small: seven in the triple therapy group and three in the conventional therapy group.
Conclusions
The results of this study demonstrate that initial triple therapy with metformin, pioglitazone, and exenatide produces greater and more durable HbA1c reduction in patients with new-onset diabetes than sequential add-on therapy with metformin followed by sulfonylurea and then basal insulin. In patients receiving triple therapy, the HbA1c value at the study end was significantly lower than that of patients receiving conventional therapy. Furthermore, in patients receiving conventional therapy, the HbA1c gradually increased over time, whereas it remained stable in patients receiving triple therapy. Thus, more patients receiving triple therapy achieved and maintained the treatment goal of HbA1c <6.5% (48 mmol/mol) at study end compared with patients receiving conventional therapy. When analyzed on the basis of starting HbA1c greater than or less than 9.0% (75 mmol/mol), both therapies were equally effective in reducing HbA1c.
In addition, the number of patients who did not reduce their HbA1c value to <6.5% (48 mmol/mol) had largely plateaued after 24 months in patients receiving triple therapy, whereas it was still increasing in the conventional therapy group (Fig. 1C). In the triple therapy group, both the FPG concentration and the incremental area under the PG concentration during OGTT were significantly reduced and contributed to the decline in HbA1c. In contrast, conventional therapy reduced the FPG concentration, but the reduction in the incremental area under the PG curve after glucose ingestion was markedly smaller than that with triple therapy (Table 1). To the extent that the postmeal increase in PG concentration contributes to the development of diabetic microvascular complications, this would favor triple therapy.
This study also helps elucidate the mechanisms that contribute to the greater efficacy and more durable HbA1c reduction with triple therapy. Although HbA1c was markedly reduced in patients in the conventional therapy group (∼2.5%) and was reasonably well controlled at study end, rigorous escalation of therapy upon failure was required to achieve the treatment goal of HbA1c <6.5%. Furthermore, the intensification of conventional therapy to maintain the treatment goal was associated with increased risk of hypoglycemia. Failure to rigorously intensify therapy upon failure to achieve the treatment goal and fear of hypoglycemia are major causes of therapy inertia in the real-world setting. Importantly, despite the rigorous intensification of therapy, the HbA1c value continued to slowly increase over time because the agents used in the conventional therapy group (i.e., metformin, glipizide, or insulin) did not improve the underlying defects responsible for the hyperglycemia (Fig. 3). Conversely, patients in the triple therapy group experienced a threefold increase in insulin sensitivity measured with the Matsuda Index (Fig. 3A) and 30-fold increase in β-cell function (Fig. 3B). In marked contrast, there was no improvement in tissue sensitivity to insulin or β-cell function in patients receiving conventional therapy. It is noteworthy that, despite the modest weight gain in the triple therapy group, insulin sensitivity still improved, most likely due to the insulin-sensitizing effect of pioglitazone (14).
Pioglitazone (15,16) and GLP-1 RAs (12,13) have potent effects to enhance β-cell function, which, in the present study, resulted in a marked and durable increase in β-cell function measured with the gold standard disposition index [(∆C-pep/∆G)0–120 × Matsuda Index]. The failure to observe any improvement in either insulin sensitivity or β-cell function with metformin, glipizide, and insulin is consistent with the known lack of long-term effect of these agents on these pathophysiologic disturbances (1,23). Because of the central role of β-cell function in the development and progression of hyperglycemia, and the important role of IR in the pathogenesis of T2DM (1), these actions of triple therapy explain the greater efficacy and durability of triple therapy over conventional therapy. With respect to this, there is little to no emphasis in the national guidelines about selecting antidiabetic agents on the basis of their ability to reverse the underlying pathophysiologic disturbances responsible for the development of T2DM (9).
Although at study end, the majority of patients (∼70%) in the conventional (stepwise) therapy group required combination therapy to maintain glucose control, the primary aim of this study was to compare two approaches: 1) the standard approach in the U.S. and worldwide that uses medications (metformin → sulfonylurea → insulin) that have little effect on the underlying pathophysiologic disturbances present in T2DM versus medications that have well established actions to improve the two major defects in T2DM: IR and impaired insulin secretion. We believe the metabolic actions of the agents used in each arm of this study, rather than the time of their addition to therapy, are the principal factors responsible for the different outcome between the tfgtwo treatment arms. It should be noted that this study was not designed to compare simultaneous versus sequential therapy. That would require a different study design in which the three agents were administered simultaneously to initiate therapy in one group while the comparative group received the same three agents in sequential order.
It is important to note that in 29% of participants in the conventional therapy group, the HbA1c decreased to ∼6.0% (from a mean baseline at 8.1%) at 3 months after starting metformin and remained stable at ∼6% during the entire study period (3 years). Thus, unlike the other 71% of participants in the conventional therapy group, this subgroup of patients with T2DM did not require escalation of therapy. This finding emphasizes the heterogeneity of T2DM (24).
Despite the significantly higher HbA1c values in patients receiving conventional therapy, they experienced a sevenfold greater risk of hypoglycemia. This demonstrates that use of therapeutic agents that correct the underlying defects present in T2DM not only produces a greater and more durable HbA1c reduction but does so without increasing the risk of hypoglycemia. Other than transient gastrointestinal side effects with the initiation of exenatide and an increase in incidence of mild edema in patients receiving pioglitazone (and these side effects were well managed by reduction of the medication dose), triple therapy was well tolerated.
Both groups gained, on mean, a small amount of weight, which was associated with a higher HbA1c value at study end in both groups. The weight gain, 1.3 kg over 3 years, in the triple therapy group was modest despite the ingestion of pioglitazone. This most likely was related to the simultaneous use of the GLP-1 RA exenatide.
The results of this study support the concept that initiating therapy at the time of T2DM diagnosis with a combination of agents (i.e., pioglitazone plus GLP-1 RA) that correct the underlying pathophysiologic defects (IR and impaired insulin secretion) results in superior and more durable reduction of HbA1c than sequential addition of agents that do not correct the underlying defects of T2DM. Short-acting exenatide was used in this study because it was the only GLP-1 RA approved at the time the study wtttt,klas initiated; however, we believe that other GLP-1 RA would have yielded similar results.
This study has some limitations. Although the number of participants was relatively large for a single-center study, approximately three-fourths of participants were of Mexican American ethnicity. The type of intervention between the two arms precluded blinding of the study interventions. Furthermore, capping the glargine dose at 60 units/day could have resulted in more therapy failure in the conventional therapy group. The dropout rate in the study was relatively high (29% and 34% in the conventional and triple therapy groups, respectively). Although this is the major limitation of the study, the baseline characteristics of patients who dropped out were similar to those who had their outcome known in the two groups (Supplementary Table 2).
In conclusion, this study provides a proof of concept that early intensive therapy in patients with new-onset diabetes with agents that improve underlying disease pathophysiology produces a large, long-lasting reduction in HbA1c compared with agents that lower PG levels without influencing the underlying disease pathophysiology (i.e., IR and β-cell dysfunction). A larger and longer multiethnic study is warranted to examine the generalizability of the results and whether the difference in HbA1c at this range is translated to a difference in diabetic microvascular complications.
Article Information
Acknowledgments. The authors thank Lorrie Albarado (University of Texas Health Science Center, San Antonio, TX) for her expert assistance in preparation of the manuscript.
Funding. This study was supported by National Institutes of Health grant R01DK24092-34 to R.A.D. Exenatide was provided by AstraZeneca.
Duality of Interest. R.A.D. receives grant support from Janssen, Merck, and AstraZeneca; is a member of the advisory boards of AstraZeneca, Janssen Pharmaceuticals, Boehringer Ingelheim, Intarcia, and Novo Nordisk; and is a member of the speakers bureaus of Novo Nordisk and AstraZeneca. E.C. receives grant support from AstraZeneca and Janssen Pharmaceuticals; is a member of the advisory boards of VeroScience, the Boehringer Ingelheim and Lilly Diabetes Alliance, and Sanofi; and is a member of the speakers bureaus of AstraZeneca, Janssen Pharmaceuticals, and the Boehringer Ingelheim and Lilly Diabetes Alliance. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. The study was designed and the study protocol was drafted by M.A.-G. and R.A.D. C.P., J.A., A.K., and G.B. contributed to data generation and collection. E.C. and C.T. reviewed the manuscript. M.A.-G. analyzed the data and wrote the manuscript. R.A.D. reviewed and revised the manuscript. M.A.-G. and R.A.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability. Data will be available upon proper request to the corresponding author after the study has been completed and all blood samples are analyzed.
Footnotes
Clinical trial reg. no. NCT01107717, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13148381.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Defronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulli G, Ferrannini E, Stern M, Haffner S, DeFronzo RA. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 1992;41:1575–1586 [DOI] [PubMed] [Google Scholar]
- 3.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990;113:909–915 [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 [DOI] [PubMed] [Google Scholar]
- 5.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2004;47:31–39 [DOI] [PubMed] [Google Scholar]
- 6.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 7.United Kingdom Prospective Diabetes Study 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995;310:83–88 [PMC free article] [PubMed] [Google Scholar]
- 8.Weir GC, Gaglia J, Bonner-Weir S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol 2020;8:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [published correction appears in Diabetes Care 2020;43:1670]. Diabetes Care 2020;43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 2018;41:69–78 [DOI] [PubMed] [Google Scholar]
- 11.Le P, Chaitoff A, Misra-Hebert AD, Ye W, Herman WH, Rothberg MB. Use of antihyperglycemic medications in U.S. adults: an analysis of the National Health and Nutrition Examination Survey. Diabetes Care 2020;43:1227–1233 [DOI] [PubMed] [Google Scholar]
- 12.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul-Ghani M, DeFronzo RA. Is it time to change the type 2 diabetes treatment paradigm? Yes! GLP-1 RAs should replace metformin in the type 2 diabetes algorithm. Diabetes Care 2017;40:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldor R, DeFronzo RA, Abdul-Ghani M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 2013;36(Suppl. 2):S162–S174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Defronzo RA, Tripathy D, Schwenke DC, et al.; ACT NOW Study . Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes 2013;62:3920–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–E883 [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015;17:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdul-Ghani M, Migahid O, Megahed A, et al. Combination therapy with exenatide plus pioglitazone versus basal/bolus insulin in patients with poorly controlled type 2 diabetes on sulfonylurea plus metformin: the Qatar Study. Diabetes Care 2017;40:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdul-Ghani M, Migahid O, Megahed A, DeFronzo RA, Zirie M, Jayyousi A. Efficacy of exenatide plus pioglitazone vs basal/bolus insulin in T2DM patients with very high HbA1c. J Clin Endocrinol Metab 2017;102:2162–2170 [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 22.Dormandy JA, Charbonnel B, Eckland DJ, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 23.RISE Consortium; RISE Consortium Investigators Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]