Abstract
Objective
To assess the effect of transabdominal amnioinfusion or no intervention on long‐term outcomes in children born after second‐trimester prelabour rupture of the membranes (PROM between 16+0/7–24+0/7 weeks) and oligohydramnios.
Population
Follow up of infants of women who participated in the randomised controlled trial: PPROMEXIL‐III (NTR3492).
Methods
Surviving infants were invited for neurodevelopmental assessment up to 5 years of corrected age using a Bayley Scales of Infant and Toddler Development or a Wechsler Preschool and Primary Scale of Intelligence. Parents were asked to complete several questionnaires.
Main outcome measures
Neurodevelopmental outcomes were measured. Mild delay was defined as −1 standard deviation (SD), severe delay as −2 SD. Healthy long‐term survival was defined as survival without neurodevelopmental delay or respiratory problems.
Results
In the amnioinfusion group, 18/28 children (64%) died versus 21/28 (75%) in the no intervention group (relative risk 0.86; 95% confidence interval [CI] 0.60–1.22). Follow‐up data were obtained from 14/17 (82%) children (10 amnioinfusion, 4 no intervention). In both groups, 2/28 (7.1%) had a mild neurodevelopmental delay. No severe delay was seen. Healthy long‐term survival occurred in 5/28 children (17.9%) after amnioinfusion versus 2/28 (7.1%) after no intervention (odds ratio 2.50; 95% CI 0.53–11.83). When analysing data for all assessed survivors, 10/14 (71.4%) survived without mild neurodevelopmental delay and 7/14 (50%) were classified healthy long‐term survivor.
Conclusions
In this small sample of women suffering second‐trimester PROM and oligohydramnios, amnioinfusion did not improve long‐term outcomes. Overall, 71% of survivors had no neurodevelopmental delay.
Tweetable abstract
Healthy long‐term survival was comparable for children born after second‐trimester PROM and treatment with amnioinfusion or no intervention.
Keywords: Follow up, infant development, neurodevelopment, oligohydramnios, second‐trimester prelabour rupture of the membranes
Tweetable abstract
Healthy long‐term survival was comparable for children born after second‐trimester PROM and treatment with amnioinfusion or no intervention.
Introduction
Second‐trimester prelabour rupture of membranes (PPROM between 16+0/7 and 24+0/7 weeks of gestation) complicates 0.4–0.7% of all pregnancies and results in high rates of perinatal morbidity and mortality.1, 2, 3 After second‐trimester PROM, an immature delivery may arise. Ongoing pregnancies are challenged by lack of amniotic fluid (oligohydramnios), which is crucial for pulmonary development.4, 5 Consequently, outcomes of pregnancies complicated by second‐trimester PROM are poor due to high rates of pulmonary hypoplasia, neonatal and maternal infection, and extremely premature delivery.6
Serial transabdominal amnioinfusion could restore residual amniotic fluids and thus reduce the rate of pulmonary hypoplasia, severe respiratory failure, and cardiovascular problems such as pneumothorax and persistent pulmonary hypertension of the neonate (PPHN). Furthermore, it may prevent compression of the umbilical cord and skeletal deformities, and increase time to delivery.7 Recently, two multicentre randomised controlled trials (RCTs; AMIPROM trial and PPROMEXIL‐III trial) have been published investigating whether amnioinfusion improves outcomes for PPROM in the previable period.8, 9 No statistically significant differences were observed in perinatal, pregnancy or maternal outcomes. The AMIPROM trial was the first trial to evaluate not only short‐term outcomes but also long‐term respiratory and neurodevelopmental outcomes. Performing a pilot study, the trial found no significant difference in the overall chance of survival without long‐term respiratory or neurodevelopmental disability (4/28 children [14.3%] in the amnioinfusion group versus 0/28 children in the no intervention group, relative risk [RR] 9.0; 95% confidence interval [CI] 0.51–159.70). Even though a significant beneficial effect from serial transabdominal amnioinfusion could not be demonstrated in women with second‐trimester PROM, the AMIPROM study did suggest that amnioinfusion might lead to an improvement in long‐term healthy survival of children. Observation of the children in early and later childhood seems appropriate, as children born after second‐trimester PROM are more likely to show long‐term respiratory symptoms and neurodevelopmental impairments due to perinatal morbidity caused by intrauterine infection or oligohydramnios. Therefore, we aimed to compare the neurodevelopmental outcome and healthy survival of children up to 5 years of age, born to mothers with second‐trimester PROM and oligohydramnios, randomised to amnioinfusion or no intervention.
Material and methods
Participants
We performed a follow‐up study of the PPROMEXIL‐III trial (NTR 3492). Details of the PPROMEXIL‐III trial have been published elsewhere.9, 10 In summary, 56 women with second‐trimester PROM between 16+0/7 and 24+0/7 weeks’ gestation were included, 28 women randomised to amnioinfusion and 28 to no intervention.
The follow‐up study took place between November 2017 and March 2018, and was approved by the Medical Ethics Committee of the Academic Medical Centre (AMC), Amsterdam, The Netherlands (ref. no. MEC 2016_218, NL58495.018.16). All children born to women who had participated in the PPROMEXIL‐III trial and were alive at discharge (n = 17) were invited for an extensive follow‐up assessment up to 5 years of corrected age, calculated from estimated date of delivery until the date of assessment. Children were assessed for neurodevelopmental and respiratory outcomes. Secondary outcomes such as behavioural, sensory processing, and health outcomes were assessed. A power calculation before start of the follow‐up study showed that a sample size of six children in each group would be sufficient to detect a difference of 30 points (−2 standard deviations [SD]) in the Bayley Scales of Infant Development – 3rd edn Dutch version (Bayley‐III‐NL)11 or Wechsler Preschool and Primary Scale of Intelligence – 3rd edn Dutch version (WPPSI‐III‐NL)12 test with a power of 80%, a two‐sided α of 0.05, and ß of 0.20.
Follow‐up assessment
Medical records were checked for possible occurrence of death of women’s offspring before contacting parents for participation in the follow‐up study by telephone. Written informed consent was obtained prior to the examination. Parents were asked to complete four different questionnaires: a respiratory questionnaire, Child Behaviour Checklist (CBCL 1.5‐5),13 the Infant/Toddler Sensory Profile (ITSP)14 or Sensory Profile (SP‐NL),15 and a health questionnaire. To assess neurodevelopmental outcome, a neuropsychologist and/or a neonatologist, blinded for the study group, administered a Bayley‐III‐NL or WPPSI‐III‐NL, as appropriate for the child’s age. In case neurodevelopmental tests (i.e. Bayley‐III‐NL) or behavioural questionnaires (CBCL) had already been administered during standardised neonatal follow‐up visits (a national follow‐up programme for extremely premature infants16), these results would be collected after written informed consent of the parents had been obtained.
Neurodevelopmental assessment and respiratory outcomes
The Bayley‐III‐NL and WPPSI‐III‐NL are both designed to chart a child’s cognitive developmental level. The Bayley‐III‐NL also assesses motor development.11, 17 The test appropriate for the child’s age was administered. A validation study showed significant correlation between the Bayley‐III‐NL and WPPSI‐III‐NL, and underlines the relation between both tests.18 Dutch versions of both tests were used, with scores based on Dutch validated norms scores.12 The Bayley‐III‐NL assessments were used for children with a corrected age of <42 months. This test reports outcomes in two subscales: the Cognitive Composite Score (CCS) and the Motor Composite Score (MCS). The WPPSI‐III‐NL was used to measure cognitive development of children ≥42 months old. Outcomes were reported in three index scores: Performance IQ (PIQ), Verbal IQ (VIQ), and Full‐Scale IQ (FSIQ). Both tests have a mean score of 100 points with a SD of 15 points. An index score ≤70 (i.e. more than −2 SD below the mean score) was considered severe neurodevelopmental delay, a score >70 and ≤85 (i.e. −1 SD) mild delay. Normal neurodevelopment outcome was defined as no severe or mild neurodevelopmental delay (i.e. score >85) on both Bayley‐III‐NL index scores or on any of the three WPPSI‐III‐NL index scores. Below 42 months, the MCS of the Bayley was included in the outcome measure neurodevelopmental delay because cognitive and motor development are strongly interconnected at early ages.19
Assessment of respiratory problems: respiratory questionnaire
Respiratory problems were assessed using a parental respiratory questionnaire and defined as symptoms interfering with daily activities (i.e. not able to attend school or not able to play) at least once a week over the past 4 weeks, or use of anti‐asthmatic medication at least one or more times/week (from birth until current age), or visits to a pulmonologist (from birth until current age).
Secondary outcomes
Behavioural assessment
The CBCL for age 1.5–5 years (CBCL 1.5–5) was used to assess behavioural and emotional problems and informs on eight subscales: emotionally reactive, anxious or depressed, somatic complaints, withdrawn, sleep problems, attention problems, and aggressive behaviour.13, 20 Data from these scales can be summed to provide a combined total problem score and two broad dimensions scores (internalising problems and externalising problems). A score >90th percentile in one of the two broad dimensions or the total problem score of the CBCL was defined as abnormal and clinically relevant for indicating serious behavioural problems.
Assessment of sensory processing
The ITSP and SP‐NL were used to assess sensory processing in children <36 months (ITSP) and ≥36 months old (SP‐NL).14, 15 The test appropriate for the child’s age was used. The ITSP and SP‐NL report four sensory quadrants: Low Registration, Sensory Sensitivity, and Sensation Avoiding. The higher the quadrant score, the lower the responsiveness of the child.21 Quadrant scores are interpreted relative to age norms and described as typical performance (≤1 SD), probable difference (between −1.0 and −2.0 SD), and definite difference (± 2 SD). A score of ± 2 SD in any four sensory quadrants of the ITSP and SP‐NL was considered an abnormal outcome and indicated a definite difference in sensory processing.
Assessment of health outcomes
A health questionnaire was used to address demographic variables and healthcare use of the children until 5 years of age. Healthcare use was clustered into clinically relevant groups (i.e. visits to healthcare provider or developmental care specialists, use of medication, hospital admission, and need for surgery) and divided into different age groups (i.e. before 2 years of age and after 2 years of age). Visits to a healthcare provider were divided into visits to the general practitioner or a specialist. Medication use was categorised as use of one or more medicines or no medication use at all. To prevent multiple comparisons, one analysis per clinically relevant group was performed.
Statistical analysis
Differences in infant and demographic characteristics, maternal, pregnancy, and delivery characteristics, as well as short‐term neonatal characteristics were compared between the amnioinfusion versus the no intervention group in children who participated in follow up, between follow‐up participants versus children lost to follow up, and between deceased children versus all surviving children using an independent sample t‐test, Mann–Whitney U‐test, Chi‐square test or Fisher’s exact test as appropriate. Considering age differences, only validated cut‐off scores were used, correlating to means, SDs or percentiles, adjusted per questionnaire for specific age groups. The clinically important composite outcome ‘healthy long‐term survival’, defined as survival with normal neurodevelopment and without respiratory problems, was calculated. All outcomes were reported in relation to trial assignment and for all surviving children participating in follow up (irrespective of trial assignment) All outcomes were analysed using the intention‐to‐treat principle. If possible, RR or odds ratio (OR), mean or median difference (MD), and their corresponding 95% CIs were calculated. Due to small groups, adjusting for potential confounders was not possible, and therefore no adjusted RRs were calculated. A P‐value of <0.05 was considered to indicate statistical significance. All analyses were conducted in IBM SPSS statistics version 24.0 (IBM Corp., Armonk, NY, USA).
Sensitivity analysis
A sensitivity analysis assessing children lost to follow up was performed using multiple imputation (10 datasets). The following variables were used as predictors when imputing missing values using multiple imputation techniques: gestational age, latency period (time from PPROM to delivery), ethnicity, maternal age, smoking at beginning of pregnancy, neonatal outcomes: birthweight, neonatal sepsis, IRDS, PVL, IVH, NEC, CLD, gender of the neonate, congenital deformities. Also, we performed a best‐case scenario (all children lost to follow up were healthy) and worst‐case scenario (all children lost to follow up had an abnormal outcome) for the composite outcome ‘healthy long‐term survival’.
Patient involvement
No patients or patient/parent organisations have been involved in the development of this trial.
Results
In the PPROMEXIL‐III trial, 56 women were randomised to amnioinfusion (n = 28) or no intervention (n = 28). In the amnioinfusion group, 18 children died: 13 (46%) antepartum and five (18%) postpartum. In the no intervention group, 21 children died: 15 (54%) antepartum and six (21%) postpartum (RR for death at any time after amnioinfusion versus no intervention 0.86; 95% CI 0.60–1.22). There were no deaths after discharge. Seventeen surviving children (30%) were eligible for participation in this follow‐up study: 10 (35.7%) in the amnioinfusion group versus 7 (25%) in the no intervention group (RR for being alive at follow‐up assessments 1.43; 95% CI 0.63–3.22) (Figures 1 and S1). Children were seen for a follow‐up assessment between 2 and 5 years of corrected age. Follow‐up data were obtained from 14/17 (82%) surviving children, 10/10 (100%) in the amnioinfusion and 4/7 (57%) in the no intervention group. One child could not be approached because of social services’ involvement and a secret living address, and for two children no informed consent was provided by the parents due to lack of time and no interest in participating in research.
Figure 1.
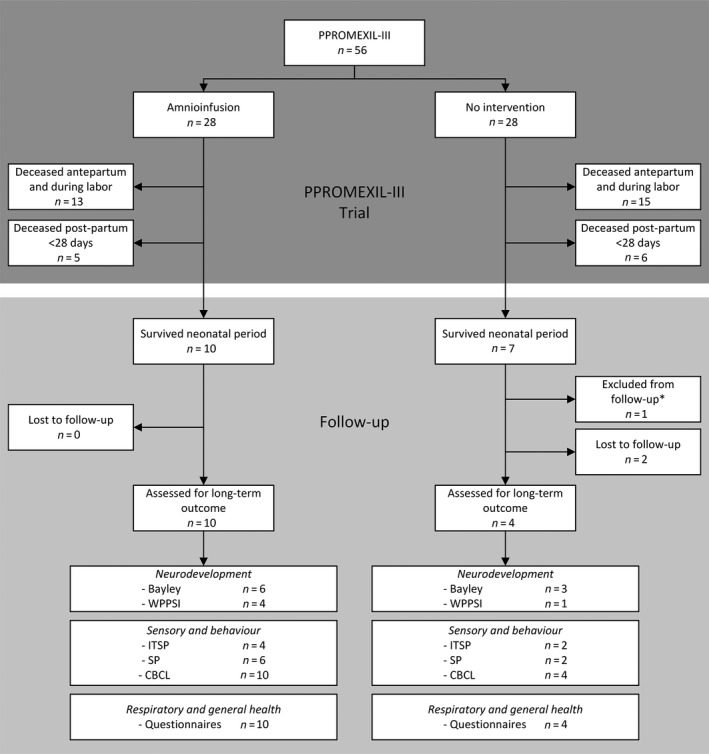
Flowchart of all participating mothers in the PPROMEXIL‐III trial and their children in the follow‐up study.
Baseline characteristics
No differences in infant, maternal, pregnancy or delivery characteristics were found for surviving children participating in follow up according to randomisation group, apart from white cell count in maternal serum at randomisation, which was significantly lower in the amnioinfusion group than in the no intervention group: 10.1 × 109/l (7.8–11.5) versus 13.6 × 109/l (10.5–16.2); P = 0.045 (Tables 1 and S1). When comparing children who participated in follow up (n = 14) with children lost to follow up (n = 3) a difference was seen in gestational age at second‐trimester PROM: 19.5 weeks (1.9) in follow up versus 16.6 weeks (0.8) in lost to follow‐up group;, P = 0.018. Furthermore, attrition was not related to baseline characteristics (Table S2).
Table 1.
Baseline characteristics of infants at follow up, and maternal, pregnancy, and delivery characteristics during PPROMEXIL‐III trial
| PPROMEXIL‐Trial | Follow‐up study | |||
|---|---|---|---|---|
|
Amnioinfusion n = 28 |
No intervention n = 28 |
Amnioinfusion n = 10 |
No intervention n = 4 |
|
| Maternal characteristics at randomisation | ||||
| Age at randomisation, mean ± SD in years | 33.9 ± 4.9 | 33.0 ± 6.9 | 34.4 ± 5.1 | 29.8 ± 5.6 |
| Parental education*, n (%) | ||||
| High | Not reported | Not reported | 7 (70%) | 2 (50%) |
| Middle | 3 (30%) | 2 (50%) | ||
| Low | 0 | 0 | ||
| Pregnancy and at delivery characteristics | ||||
| Gestational age at PPROM, mean ± SD in days | 18.7 ± 1.9 | 18.6 ± 2.3 | 19.6 ± 1.3 | 18.9 ± 2.9 |
| Gestational age at delivery, median (IQR) in days | 24.2 (21.5–28.0) | 23.6 (20.7–27.4) | 29.6 (26.9–35.9) | 30.0 (27.9–35.9) |
| Pregnancy and short‐term neonatal outcomes | Neonates born n = 15 | Neonates born n = 13 | ||
| Gender male, n (%) | ||||
| All pregnancies | 18 (64.3%) | 17 (60.7%) | 10 (100%) | 3 (75%) |
| Live‐born neonates | 13 (86.7%) | 7 (53.8%) | ||
| Apgar score <7 after 5 min, n (%) | 10 (66.7%) | 9 (69.2%) | 5 (50%) | 2 (50%) |
| Birthweight, median (IQR) in kg | 1050 (735–1950) | 1015 (790–1478) | 1517.5 (1020.0–2397.5) | 1562.5 (900.0–2723.8) |
| Infant characteristics at follow up | ||||
| Living in two‐parent family,** n (%) | n/a | n/a | 9 (90%) | 4 (100%) |
N/A, not applicable.
Parental education: ‘low’, defined as total years post elementary schooling <6 years. Classified as ‘low’ if at least one of the parents has a low level of education (but not if one parent is highly educated). ‘Middle’ defined as total years post elementary schooling 6–8 years. Classified as ‘middle’ if both parents had this level of education. ‘High’ defined as total years post elementary schooling >8 years. Classified as ‘high’ if one of the parents was highly educated. Parental education was measured at the time of the follow‐up study.
Living in two parent family: children living with one or two biological parents, new marriage and de facto relationship.
Neurodevelopmental outcome and respiratory outcomes
No severe abnormal neurodevelopmental delay was seen in surviving children (Table 2). Also, there were no differences in mean index scores of the CCS and MCS for Bayley‐III‐NL and of the PIQ for WPPSI‐III‐NL (Table S3). Due to missing scores in the no intervention group, the VIQ and FSIQ could not be compared. A mild delay (−1 SD) was seen in two children in each treatment group (Table 2. For pregnancy details of children with a mild neurodevelopmental delay, please see Table S4. By accident, for one child, the MCS was not administered by the neuropsychologist; however, the neurodevelopmental parental report indicated a normal motor development and the outcome was therefore classified as normal. For two children, a number of subscales of the WPPSI‐III‐NL were not performed, due to delayed performance or child’s limited understanding of tasks. A team consisting of a neuropsychologist and a neonatologist classified the missing subscales as −1 SD index scores based on the neurodevelopmental reports (Table S5).
Table 2.
Neurodevelopmental outcomes, respiratory outcomes and long‐term healthy survival of children participating in follow‐up
| Complete case analysis (N = 56) | |||
|---|---|---|---|
| Assessed for neurodevelopment and health outcomes | Amnioinfusion (n = 10) | No intervention (n = 4) | RR (95% CI) |
| No neurodevelopmental delay a | 8/28 | 2/28 | 4.00 (0.93–17.19) |
| Percentage of all assessed surviving infants b | 80% | 50% | |
| Percentage of all included participants c | 28.6% | 7.1% | |
| Percentage of all participants with known outcome d | 28.6% | 8% | |
| Bayley/WPSSI mild delay (−1SD) | 8 (28.6%) | 2 (7.1%) | 4.00 (0.93–17.19) |
| Bayley/WPSSI severe delay (−2SD) | 0 (0%) | 0 (0%) | — |
| No respiratory problems e | 6/28 | 3/28 | 2.00 (0.55–7.22) |
| Percentage of all assessed surviving infants b | 60% | 75% | |
| Percentage of all included participants c | 21.4% | 10.7% | |
| Percentage of all participants with known outcome d | 21.4% | 12% | |
| Respiratory symptoms disturbing daily activities f | 1 (3.6%) | 0 (0%) | — |
| Anti‐asthmatic medication g | 2 (7.1%) | 1 (3.6%) | 2.00 (0.19–20.82) |
| Visits to a paediatric pulmonologist h | 1 (3.6%) | 1 (3.6%) | 1.00 (0.7–15.21) |
| Composite healthy survivor i | 5/28 | 2/28 | 2.50 (0.53–11.83) |
| Percentage of all assessed surviving infants b | 50% | 50% | |
| Percentage of all included participants c | 17.9% | 7.1% | |
| Percentage of all participants with known outcome d | 17.9% | 8% | |
Data presented as n (%).
Neurodevelopmental delay defined as: scores for a mild neurodevelopmental delay with a cut off of −1 SD in any of the 2 indexes of the Bayley‐III‐NL or in any of the 3 scales of the WPPSI‐III‐NL.
Percentage of all infants that were assessed for follow‐up, n = 10 in the amnioinfusion group, n = 4 in the no intervention group.
Percentage of all included participants, n = 28 in the amnioinfusion group, n = 28 in the no intervention group.
Percentage of participants with a known outcome, n = 28 in the amnioinfusion group, n = 25 in the no intervention group (three infants lost to follow‐up).
Respiratory problems defined as: at least once a week respiratory symptoms interfering with daily activities (i.e. not able to attend school or not able to play) in the past four weeks, or visits to a paediatric pulmonologist from birth until current age, or use of anti‐asthmatic medication for respiratory symptoms at least ≥1 time/week.
Respiratory symptoms interfering with daily activities (i.e. not able to attend school or not able to play) at least once a week over the past four weeks.
Use of anti‐asthmatic medication at least ≥1 time/week from birth until current age.
Visits to a pulmonologist from birth until current age.
Healthy survivor defined as: no neurodevelopmental delay or no respiratory problems.
Table 2 shows the descriptive data regarding respiratory problems. Parents of five children reported respiratory problems, four in the amnioinfusion and one child in the no intervention group, with three children reporting respiratory problems interfering with daily activities (n = 1), use of anti‐asthmatic medication (n = 1) or visits to a pulmonologist (n = 1). Two children reported anti‐asthmatic medication use as well as pulmonologist visits.
Healthy long‐term survival
The composite outcome ‘healthy long‐term survival’ occurred in 5/28 (17.9%) children in the amnioinfusion and 2/28 (7.1%) children in the no intervention group (RR 2.50; 95% CI 0.53–11.83) (Table 2). When assessing children lost to follow up for a sensitivity analysis using multiple imputations, being a healthy survivor occurred more often in children in the amnioinfusion group than children in no intervention group (Table S6). However, when assessing healthy long‐term survival for the best case (all children lost to follow up were healthy) or worst‐case scenario (all children lost to follow up had an abnormal outcome) for a sensitivity analysis, no differences between the amnioinfusion group and control group were shown (Table S7).
Secondary outcomes: behaviour, sensory processing and health
Abnormal cut‐off scores for behaviour and sensory processing outcomes are shown in Table S8. No differences were observed between the treatment groups. Table S9 shows that there were no differences in health outcomes of children in the amnioinfusion group compared with the no intervention group.
Analysing data irrespectively of trial assignment
Of 56 pregnancies complicated by second‐trimester PROM, there were 17 live births (30.4%) (Figure 2). When comparing baseline characteristics of survivors versus non‐survivors, we show a difference in gestational age (median 29.1 weeks, interquartile range [IQR] 27.4–33.9 versus 22.3 weeks, IQR 20.4–24.4, difference in medians 7.7 weeks; 95% CI 5.7–9.9), latency period from PPROM to delivery (11 weeks, IQR 8.2–15.2 versus 3.3 weeks, IQR 1.9–5.6, difference in medians 7.4 weeks; 95% CI 5.4–10.1), and single deepest pocket measured at randomisation: 14.0 mm (7.3–16.5) versus 6.0 mm (0–12.0), difference in medians 6.0 mm; 95% CI 2.0–11.0) (Table S10). Mild neurodevelopmental delay was observed in 4/14 children (29%), abnormal sensory processing in 3/14 children (21%), and abnormal behaviour in two children (14.3%). Five of 14 children (36%) reported respiratory problems. The composite outcome ‘healthy long‐term survival’ occurred in 7/14 children (50%).
Figure 2.
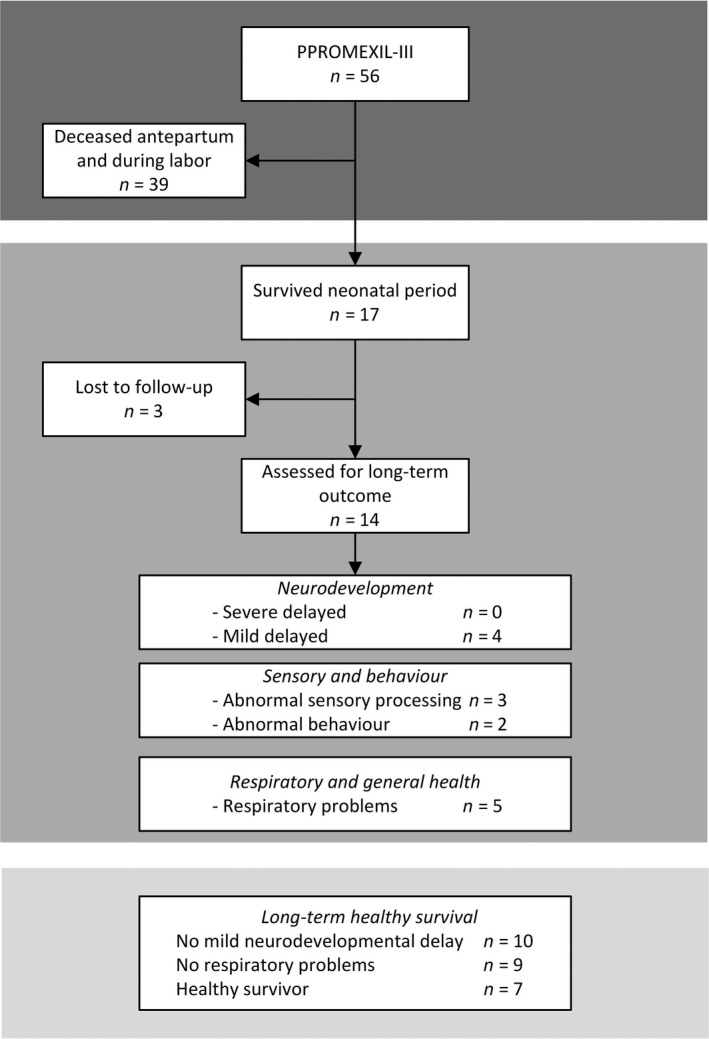
Neurodevelopmental outcomes of children participating in follow up, when analysing data irrespectively of trial assignment.
Discussion
Main findings
In women suffering from second‐trimester PROM and oligohydramnios, 30% of children survived until childhood. Survival with normal development and without respiratory problems was comparable between children born to mothers treated with amnioinfusion and mothers managed with no intervention. Thus, in this small sample, amnioinfusion does not significantly improve long‐term developmental or respiratory childhood outcomes. These results do not suggest changing standard care after second‐trimester PROM in clinical practice; however, a larger sample size is warranted (>56 subjects, >17 survivors) to draw definitive conclusions.
Strengths and limitations
Our study has several strengths. We have provided an extensive assessment of long‐term outcomes after second‐trimester PROM and amnioinfusion or no intervention, and investigated a child’s neurodevelopment, behaviour, sensory processing, respiratory problems, and health outcomes in detail using validated instruments and parental questionnaires. The Bayley‐III‐NL and WPPSI‐III‐NL were used, both of which are standardised, well‐validated developmental tests that are highly recommended to evaluate neurodevelopment in young childhood.11, 12 Data were collected by trained healthcare professionals blinded for study group and in a standardised care setting.
Our study also has limitations. The absolute number of participating children in this follow‐up study was low due to high perinatal mortality rates, even though the follow‐up rate was above the recommended follow‐up thresholds of 60–80%.22 Therefore, results should be interpreted cautiously. Three children lost to follow up were all assigned to the no intervention group and had PPROM at a lower gestational age compared with the group of children assessed for developmental outcomes. As we were unsure whether these children are growing up without health problems, we performed a sensitivity analysis, analysing all children lost to follow up as either having normal outcome or neurodevelopmental delay. Also, multiple imputations were used to analyse all children, even though unavoidably, the results of multiple imputation techniques in small sample clinical trials will still be complicated by the problem of attrition.23
It is known that parental socio‐economic disadvantage, independent of pregnancy and delivery complications, is associated with abnormal child neurodevelopment.24, 25 Adjusting for this confounder was not possible due to the small sample size.26 Additionally, as socio‐economic status was not assessed for all women in the original PPROMEXIL‐III trial, but only in the follow‐up study, it could not be used as a predictor for imputation for missing values.
This follow‐up study included enough subjects to detect a difference of −2 SD in neurodevelopmental test scores with a power of 80% and a two‐sided α of 0.05. However, no −2 SD differences in test scores were seen in our study. To detect a difference of 15 points in neurodevelopmental tests (−1 SD), a larger number of subjects would have been needed (i.e. 17 children per group) to give adequate power.
One of the mayor limitations concerning all follow‐up studies of RCTs investigating perinatal interventions is that the power of a follow‐up study is determined by the sample and perinatal mortality of the original RCT. Even though a sample size calculation is often performed in follow‐up studies, a sample large enough to detect statistical significance is regularly not reached. Additionally, when investigating a rare complication in pregnancy, such as second‐trimester PPROM with an incidence of 0.4–07.%, large study samples might be hard to obtain unless collaborating internationally and intercontinentally.
Furthermore, both RCTs (PPROMEXIL‐III and AMIPROM) based their power analysis upon the mortality rates of observational studies.27, 28 However, when calculating a sample size using perinatal mortality rates of these two RCTs combined in a traditional meta‐analysis, we would need a sample of 1352 women per group to detect a decrease of 5% (71–66%), with an alpha of 0.05 and a power of 80%.9 Thus, both RCTs were underpowered, and consequently their follow‐up studies as well; larger future RCTs are therefore needed.
Interpretations
In the AMIPROM study,22 the only other RCT investigating the efficacy of amnioinfusion for second‐trimester PROM, long‐term survival without respiratory or neurodevelopmental disabilities occurred in 4/28 (14.3%) in the amnioinfusion and 0/28 in the control group (RR 9.0; 95% CI 0.51–159.70)29 compared with 5/28 (17.9%) and 2/28 (7.1%) (RR 2.50; 95% CI 0.53–11.83) in this study. For neurodevelopmental outcome, Roberts et al. assessed eight children in the amnioinfusion group of which five had severe or mild delay compared with four of the five assessed children in the expectant management. In our study, we found two children in both treatment groups with neurodevelopmental delay. Of note, Roberts et al. show a significant delay (−2 SD) in three surviving infants, whereas in our follow‐up study no severe delay was reported. The overall chance of survival without neurodevelopmental disability was 4/56 (7.1%) in the AMIPROM trial and 10/56 (17.9%) in our follow‐up study.
When assessing our study population, irrespectively of trial assignment, 71% of the children survived without neurodevelopmental delay and 50% had a ‘normal child outcome’, and were classified as healthy long‐term survivors. Few other studies have investigated long‐term outcomes of children born after second‐trimester PROM.30, 31, 32 Comparable to our study, all these studies suggest some neuromotor abnormalities. A retrospective cohort study published in 2007 reviewed obstetric and neonatal records for 87 pregnancies with PPROM between 14 and 24 weeks.31 They demonstrated a normal neurological and developmental outcome at 2 years of age in half of all (6/12) surviving infants. A comparable cohort study by Rib et al., published in 1993, showed that 72% of surviving infants after PPROM <26 weeks would survive without developmental delay as assessed at 2 years of age.32 Finally, a small study investigating pregnancies with second‐trimester (spontaneous and iatrogenic) PPROM between 14 and 24 weeks’ gestation showed that 71% of infants survived without neurological and developmental sequelae at age 4.30
Conclusion
The findings of this follow‐up study suggest that survival without neurodevelopmental delay or respiratory problems does not differ between management with amnioinfusion versus no intervention in the assessed sample of this follow‐up study. The power of our study was insufficient to identify the ‘true’ potentially beneficial effect of amnioinfusion. Our findings do not suggest a change in management of second‐trimester PROM and oligohydramnios. Assessment of all survivors in this follow‐up study suggests no severe or mild delay at school age in 71% of surviving children and could be helpful in counselling parents regarding long‐term developmental sequelae after second‐trimester PROM and oligohydramnios.
Disclosure of interests
Dr Ben Willem Mol is supported by an NHMRC Practitioner Fellowship (GNT1082548). Dr Ben Willem Mol reports consultancy for ObsEva, Merck KGaA, and Guerbet. The other authors have not reported any potential conflicts of interest. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to authorship
AdR and NE participated in protocol development, trial management, data collection, data analysis, interpretation, and writing. JvtH, AvT, RD, AvWL, CAM, CvdB, DO, MH, MW, MP, JD, LvK, TR, BWM, and EP participated in protocol development, data analysis, data interpretation, and writing. All collaborators saw and approved the final version. All collaborators were sent the paper as prepared for submission and given the opportunity to comment on the draft manuscript.
Details of ethics approval
This follow‐up study was approved by the Medical Ethics Committee of the Academic Medical Centre (AMC), Amsterdam, The Netherlands (ref. no. MEC 2016_218, NL58495.018.16). Clinical Trial Registration: NTR Dutch Trial Register (www.trialregister.nl): NTR3492.
Funding
No funding.
Acknowledgements
Not applicable.
Supporting information
Figure S1 . CONSORT flow diagram.
Figure S2 . Forest plot for the risk of the outcome: healthy survival (survival without neurodevelopmental delay and without respiratory problems) in children who have been randomised prenatally to amnioinfusion or no intervention.
Table S1 . All baseline characteristics of infants at follow up, and maternal, pregnancy, and delivery characteristics during PPROMEXIL‐III trial.
Table S2. Baseline characteristics of mothers participating in follow up versus lost to follow up; maternal characteristics at randomisation, during pregnancy, and delivery. Total surviving children n = 17.
Table S3. Neurodevelopmental scores (abnormal scores and mean scores) for 14 survivors who have been randomised prenatally to amnioinfusion or no intervention.
Table S4. Pregnancy characteristics and neonatal outcome in children with a mild neurodevelopmental delay (–1 SD).
Table S5. Missing subscales Wechsler Preschool and Primary Scale of Intelligence – 3rd edn Dutch version (WPPSI‐III‐NL).
Table S6. Neurodevelopmental outcomes, respiratory outcomes, and long‐term healthy survival of children participating in follow up, born to 56 women with second‐trimester PROM according to treatment assignment.
Table S7. Sensitivity analysis: best case scenario (i.e. all children lost to follow up are healthy) and worst case scenario (i.e. all children lost to follow up are unhealthy).
Table S8. Behaviour and sensory processing (abnormal cut‐off scores) for 14 survivors who have been randomised prenatally to amnioinfusion or no intervention.
Table S9. Health outcomes for 14 survivors who have been randomised prenatally to amnioinfusion or no intervention.
Table S10. Maternal, pregnancy, and delivery characteristics of women with a deceased child versus women with a surviving child.
de Ruigh AA, Simons NE, van ‘t Hooft J, van Teeffelen AS, Duijnhoven RG, van Wassenaer‐Leemhuis AG, Aarnoudse‐Moens C, van de Beek C, Oepkes D, Haak MC, Woiski M, Porath MM, Derks JB, van Kempen LEM, Roseboom TJ, Mol BW, Pajkrt E. Child outcomes after amnioinfusion compared with no intervention in women with second‐trimester rupture of membranes: a long‐term follow‐up study of the PROMEXIL‐III trial. BJOG: Int J Obstet Gy. 2021;128:292–301.
Each author has confirmed compliance with the journal’s requirements for authorship.
Presentation: Presented as a poster at the 38th Annual Meeting of the Society of Maternal Fetal Medicine, 11–16 February 2019, Las Vegas, Nevada, USA
Linked article This article is commented on by DW Skupski, p. 302 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16187.
References
- 1. Williams O, Michel B, Hutchings G, Debauche C, Hubinont C. Two‐year neonatal outcome following PPROM prior to 25 weeks with a prolonged period of oligohydramnios. Early Hum Dev 2012;88:657–61. [DOI] [PubMed] [Google Scholar]
- 2. Nourse CB, Steer PA. Perinatal outcome following conservative management of mid‐trimester pre‐labour rupture of the membranes. J Paediatr Child Health 1997;33:125–30. [DOI] [PubMed] [Google Scholar]
- 3. Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am 2005;32:411–28. [DOI] [PubMed] [Google Scholar]
- 4. Hibbard JU, Hibbard MC, Ismail M, Arendt E. Pregnancy outcome after expectant management of premature rupture of the membranes in the second trimester. J Reprod Med 1993;38:945–51. [PubMed] [Google Scholar]
- 5. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Degree of oligohydramnios and pregnancy outcome in patients with premature rupture of the membranes. Obstet Gynecol 1985;66:162–7. [PubMed] [Google Scholar]
- 6. Kilbride HW, Thibeault DW. Neonatal complications of preterm premature rupture of membranes: Pathophysiology and management. Clin Perinatol 2001;28:761–85. [DOI] [PubMed] [Google Scholar]
- 7. Porat S, Amsalem H, Shah PS, Murphy KE. Transabdominal amnioinfusion for preterm premature rupture of membranes: a systematic review and metaanalysis of randomized and observational studies. Am J Obstet Gynecol 2012;207:393.e1–e11. [DOI] [PubMed] [Google Scholar]
- 8. Roberts D, Vause S, Martin W, Green P, Walkinshaw S, Bricker L, et al. Amnioinfusion in preterm premature rupture of membranes (AMIPROM): a randomised controlled trial of amnioinfusion versus expectant management in very early preterm premature rupture of membranes—a pilot study. Health Technol Assess 2014;18:1–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Kempen LEM, van Teeffelen AS, de Ruigh AA, Oepkes D, Haak MC, van Leeuwen E, et al. Amnioinfusion compared with no intervention in women with second‐trimester rupture of membranes: a randomized controlled trial. Obstet Gynecol 2018;133:129–36. [DOI] [PubMed] [Google Scholar]
- 10. van Teeffelen AS, van der Ham DP, Willekes C, Al Nasiry S, Nijhuis JG, van Kuijk S, et al. Midtrimester preterm prelabour rupture of membranes (PPROM): expectant management or amnioinfusion for improving perinatal outcomes (PPROMEXIL‐III trial). BMC Pregnancy Childbirth 2014;14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayley N. The Bayley Scales of Infant and Toddler Development (3rd edn). San Antonio: Psychological Corporation; 2006. [Google Scholar]
- 12. Wechsler D. WPPSI‐III‐NL Nederlandstalige bewerking: Technische handleiding [Dutch version of the WPPSI‐III‐NL: Technical and Interpretive Manual] ne, Pearson Assessment and Information BV, Amsterdam, The Netherlands, 2010 Dutch adaptation by: Hendriksen J., Hurks P.
- 13. Achenbach TM, Rescorla LA. Manual for ASEBA Preschool Forms & Profiles. Burlington: University of Vermont, Department of Psychiatry; 2000. [Google Scholar]
- 14. Keizer H. Infant/Toddler Syptom Checklist‐NL. Een Screeningslijst Voor Ouders. Pearson: Amsterdam.
- 15. Dunn W, Rietman A. Sensory Profile‐NL. Amsterdam: Harcourt Test Publishers; 2006. [Google Scholar]
- 16. https://www.nvk.nl/Kwaliteit/Richtlijnen‐overzicht/Details/articleType/ArticleView/articleId/1241/Aanbeveling‐Landelijke‐Neonatale‐Follow‐up‐NICU‐follow‐up. Aanbeveling Landelijke Neonatale Follow‐up‐ NICU follow‐up.
- 17. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence – Third Ed. – NL (WPPSI‐III‐NL) A, The Netherlands, Pearson Assessment and Information B.V, 2009.
- 18. Kerkmeer MZJ, Dek J. Bayley–III‐NL ‐ Psychometrische eigenschappen. White paper 2. Pearson Clin 2015.
- 19. Van Baar AL, SL, Verhoeven M, Hessen D. Bayley‐III‐NL; Technische handleiding. Amsterdam: Pearson Assessment and Information B.V; 2014. [Google Scholar]
- 20. Koot HM, Van Den Oord EJ, Verhulst FC, Boomsma DI. Behavioral and emotional problems in young preschoolers: cross‐cultural testing of the validity of the Child Behavior Checklist/2‐3. J Abnorm Child Psychol 1997;25:183–96. [DOI] [PubMed] [Google Scholar]
- 21. Ermer J, Dunn W. The sensory profile: a discriminant analysis of children with and without disabilities. Am J Occup Ther 1998;52:283–90. [DOI] [PubMed] [Google Scholar]
- 22. Kristman V, Manno M, Cote P. Loss to follow‐up in cohort studies: how much is too much? Eur J Epidemiol 2004;19:751–60. [DOI] [PubMed] [Google Scholar]
- 23. Barnes SA, Lindborg SR, Seaman JW Jr. Multiple imputation techniques in small sample clinical trials. Stat Med 2006;25:233–45. [DOI] [PubMed] [Google Scholar]
- 24. Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci 2010;11:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chin‐Lun Hung G, Hahn J, Alamiri B, Buka SL, Goldstein JM, Laird N, et al. Socioeconomic disadvantage and neural development from infancy through early childhood. Int J Epidemiol 2015;44:1889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenland S, Schwartzbaum JA, Finkle WD. Problems due to small samples and sparse data in conditional logistic regression analysis. Am J Epidemiol 2000;151:531–9. [DOI] [PubMed] [Google Scholar]
- 27. Ogunyemi D, Thompson W. A case controlled study of serial transabdominal amnioinfusions in the management of second trimester oligohydramnios due to premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol 2002;102:167–72. [DOI] [PubMed] [Google Scholar]
- 28. Vergani P, Locatelli A, Strobelt N, Mariani S, Cavallone M, Arosio P, et al. Amnioinfusion for prevention of pulmonary hypoplasia in second‐trimester rupture of membranes. Am J Perinatol 1997;14:325–9. [DOI] [PubMed] [Google Scholar]
- 29. Roberts D, Vause S, Martin W, Green P, Walkinshaw S, Bricker L, et al. Amnioinfusion in very early preterm prelabor rupture of membranes (AMIPROM): pregnancy, neonatal and maternal outcomes in a randomized controlled pilot study. Ultrasound Obstet Gynecol 2014;43:490–9. [DOI] [PubMed] [Google Scholar]
- 30. Chauleur C, Rochigneux S, Seffert P, Chene G, Billiemaz K, Collet F. Neonatal outcomes and four‐year follow‐up after spontaneous or iatrogenic preterm prelabor rupture of membranes before 24 weeks. Acta Obstet Gynecol Scand 2009;88:801–6. [DOI] [PubMed] [Google Scholar]
- 31. Pristauz G, Bauer M, Maurer‐Fellbaum U, Rotky‐Fast C, Bader AA, Haas J, et al. Neonatal outcome and two‐year follow‐up after expectant management of second trimester rupture of membranes. Int J Gynaecol Obstet 2008;101:264–8. [DOI] [PubMed] [Google Scholar]
- 32. Rib DM, Sherer DM, Woods JR Jr. Maternal and neonatal outcome associated with prolonged premature rupture of membranes below 26 weeks' gestation. Am J Perinatol 1993;10:369–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 . CONSORT flow diagram.
Figure S2 . Forest plot for the risk of the outcome: healthy survival (survival without neurodevelopmental delay and without respiratory problems) in children who have been randomised prenatally to amnioinfusion or no intervention.
Table S1 . All baseline characteristics of infants at follow up, and maternal, pregnancy, and delivery characteristics during PPROMEXIL‐III trial.
Table S2. Baseline characteristics of mothers participating in follow up versus lost to follow up; maternal characteristics at randomisation, during pregnancy, and delivery. Total surviving children n = 17.
Table S3. Neurodevelopmental scores (abnormal scores and mean scores) for 14 survivors who have been randomised prenatally to amnioinfusion or no intervention.
Table S4. Pregnancy characteristics and neonatal outcome in children with a mild neurodevelopmental delay (–1 SD).
Table S5. Missing subscales Wechsler Preschool and Primary Scale of Intelligence – 3rd edn Dutch version (WPPSI‐III‐NL).
Table S6. Neurodevelopmental outcomes, respiratory outcomes, and long‐term healthy survival of children participating in follow up, born to 56 women with second‐trimester PROM according to treatment assignment.
Table S7. Sensitivity analysis: best case scenario (i.e. all children lost to follow up are healthy) and worst case scenario (i.e. all children lost to follow up are unhealthy).
Table S8. Behaviour and sensory processing (abnormal cut‐off scores) for 14 survivors who have been randomised prenatally to amnioinfusion or no intervention.
Table S9. Health outcomes for 14 survivors who have been randomised prenatally to amnioinfusion or no intervention.
Table S10. Maternal, pregnancy, and delivery characteristics of women with a deceased child versus women with a surviving child.


