Abstract
A recently developed network perspective on tobacco withdrawal posits that withdrawal symptoms causally influence one another across time, rather than simply being indicators of a latent syndrome. Evidence supporting a network perspective would shift the focus of tobacco withdrawal research and intervention toward studying and treating individual withdrawal symptoms and inter-symptom associations. Here we construct and examine temporal tobacco withdrawal networks that describe the interplay among withdrawal symptoms across time using experience-sampling data from 1210 participants (58.35% female, 86.24% white) undergoing smoking cessation treatment. We also construct person-specific withdrawal networks and capture individual differences in the extent to which withdrawal symptom networks promote the spread of symptom activity through the network across time using impulse response analysis. Results indicate substantial moment-to-moment associations among withdrawal symptoms, substantial between-person differences in withdrawal network structure, and reductions in the interplay among withdrawal symptoms during combination smoking cessation treatment. Overall, findings suggest the utility of a network perspective and also highlight challenges associated with the network approach stemming from vast between-person differences in symptom networks.
Keywords: networks, withdrawal, nicotine, tobacco, symptoms
General Scientific Summary
This study uses a network approach to examine how symptoms of tobacco withdrawal may impact one another from one moment to the next during a smoking cessation attempt. We find substantial moment-to-moment associations among withdrawal symptoms. Participants undergoing combination treatment have networks in which symptom activity is less likely to linger across time due to moment-to-moment inter-symptom associations relative to participants in a placebo condition.
Introduction
During efforts to quit smoking, aversive withdrawal symptoms appear that are prominent as primary motives for the resumption of smoking in many theories of addiction (Baker et al., 2004; Piper, 2015; Solomon & Corbit, 1974). Withdrawal symptoms are often treated as interchangeable indicators of an underlying syndrome (Toll et al., 2007). During diagnosis (American Psychiatric Association, 2013), for example, criteria for tobacco withdrawal is met if a patient presents with four or more withdrawal symptoms (anger, anxiety, depressed mood, difficulty concentrating, increased appetite, insomnia, and restlessness). Yet, there is increasing evidence that individual withdrawal symptoms are not interchangeable; rather, they exhibit distinct time profiles during the course of cessation attempts (Hendricks et al., 2006; Leventhal et al., 2010; Piasecki et al., 2000; West, Hajek, & Belcher, 1987) and unique responses to treatment (Foulds et al., 2013).
A network approach to tobacco withdrawal has recently been proposed to complement a syndrome perspective on withdrawal (Lydon-Staley et al., 2018). A network theory of psychopathology highlights symptoms of disorders as units that causally impact one another across time, forming networks of causally connected symptoms (Borsboom, 2017; Bringmann et al., 2013; Schmittman et al., 2013). From this perspective, the experience of tobacco withdrawal can be described as a network in which the nodes of the network represent symptoms and the edges (or lines between nodes) represent temporal associations among symptoms across time. Studies provide preliminary support for the network perspective’s proposal that individual tobacco withdrawal symptoms influence one another in a potentially causal fashion over time. For example, positive associations between anhedonia, negative affect, and craving have been observed over the course of a smoking cessation attempt (Cook et al., 2017) and strong positive associations have been observed between sleep problems and restlessness, as well as among affective symptoms (anger, anxiety, and depressed mood), in smokers undergoing smoking cessation treatment (Lydon-Staley et al., 2018). Recent work making use of experience sampling protocols has considered the dynamic associations among cessation fatigue, negative affect, nicotine craving, and self-efficacy in smokers undergoing cessation treatment (Bekiroglu et al., 2017). This work, which makes use of the temporal precedence available in time series data to model how the reporting of symptoms at a previous time point (t-1) predicts symptom intensity at the current time point (t), found that these four constructs changed over time in response to each other during the course of smoking cessation.
Although there is empirical support for the notion that individual symptoms of withdrawal influence one another over time, key questions about a network approach to withdrawal remain to be addressed. We use experience-sampling data from participants providing momentary reports of withdrawal symptoms over 14 days to estimate a temporal network that describes the moment-to-moment interplay among withdrawal symptoms. The use of experience-sampling data to construct temporal networks (Holme & Saramäki, 2012) is an important complement to existing studies modeling cross-sectional withdrawal networks (Lydon-Staley et al., 2018). The edges in cross-sectional networks indicate between-person associations among symptoms, with positive edges indicating that if, for example, an individual experiences high levels of anxiety, then they are also likely to experience high levels of anger. This symptom co-occurrence, from a network perspective, is theorized to result from causal associations among symptoms. Yet, between-person associations (i.e., people high in anxiety experience high levels of anger) do not always translate to the within-person associations (i.e., at times when a person is more anxious than usual, they are also angrier than usual; Bos et al., 2017; Hamaker, Dolan, & Molenaar, 2005). One of the strengths of experience-sampling data is that it can probe time-lagged, within-person associations between symptoms.
Further, the use of temporal networks allows a test for the presence of a potential source of distress experienced during smoking cessation, unique to a network approach to withdrawal, that may undermine efforts to remain abstinent. In particular, individuals undergoing cessation may experience continued symptom activation long after the cause of symptom activity (i.e., deprivation) has disappeared due to symptom interplay across time (Borsboom, 2017; Cramer et al., 2016). Network theory posits that this failure for symptoms to de-activate arises in strongly connected symptom networks that facilitate the reverberation of symptom activity through the network across time as activity in one symptom is transferred to another symptom via causal associations. This spreading of symptom activity through networks is analogous to the behavior of two sets of domino tiles: one in which the tiles are far apart and a second in which the tiles are close together. In a densely connected symptom network, the set of domino tiles are close together, and when one tile falls it causes other tiles to topple and activity ripples through the system (Borsboom & Cramer, 2013). In a less densely connected symptom network, the set of domino tiles are far apart, and when one tile falls the other tiles are unaffected and remain standing. Such an explanation of spreading activity following symptom network perturbation would fit with empirical evidence of extended withdrawal symptom experiences among some smokers (Piasecki et al., 1998; 2003) when other research suggests that withdrawal should peak after the first 14 days (Hughes, 2007).
As yet, the phenomenon of self-perpetuating symptom networks in the context of tobacco withdrawal awaits empirical examination. Complicating this examination is the need to identify an appropriate statistical framework that can capture the complex theoretical notion of self-perpetuating symptom networks. Existing efforts to capture the reverberation of symptom activity through symptom networks have focused on node centrality and network density. Centrality indices have been hypothesized to identify the most important nodes in a network. Degree centrality, for example, is a common measure of node centrality and is defined as the number of edges emanating from a node (Newman, 2010). Network density is defined as a fraction of possible edges that exist in a network or, in networks with weighted edges, network strength. Both node centrality and network density have been identified as potential indices for identifying networks that would facilitate the spread of activity through the network, activating self-perpetuating sequences of symptom activity across time (Bello et al., 2017; Fried et al., 2016; Hasmi et al., 2017; Lydon-Staley et al., 2019a; Pe et al., 2015). Yet, the use of both centrality and density are limited. Centrality and density focus on direct connections and contain little information about how symptoms might affect each other indirectly as activity flows through the entire network (Bringmann et al., 2019).
Impulse response analysis is a tool with the potential to capture the complex notion of the spread of symptom activity through a network (Lütkepohl, 2005). Once a temporal network indicating the predictive associations between symptoms across time has been constructed, impulse response analysis simulates an instantaneous, exogenous impulse (sometimes termed a shock) to certain network symptoms. Through simulation, the propagation of this impulse through the network, along the time-lagged symptom network edges, is charted. The impulse response function shows the hypothetical change in a symptom in response to a simulated increase in one of the other symptoms over a horizon of several time points. Importantly, activity in a symptom observed after the symptom network receives a shock contains system-level information. The impulse experienced by non-shocked symptoms is due to the flow of activity along the network’s edges emanating from the shocked node.
Impulse response analysis has recently been applied to networks of symptoms and behaviors to capture individual differences in the spread of activity through networks of time-lagged edges. In a sample of subclinically depressed individuals, impulse response analysis was used to quantify the extent to which stress impacted affect in participants with anhedonic symptoms relative to participants without anhedonic symptoms (Bos et al., 2018). A shock to stress was simulated and the spread of activity associated with this simulated increase in stress along time-lagged edges in a network of six variables (high-arousal positive affect, low-arousal positive affect, high-arousal positive affect, low-arousal negative affect, stress, and physical activity) was charted over a horizon of ten time points. When an increase in stress was simulated, affect in non-anhedonic individuals showed a stronger increase relative to affect in anhedonic individuals. By using impulse response analysis, the observed association between stress and affect consisted of the effect of a simulated increase in stress filtering through direct pathways to impact affect, but also via indirect pathways emerging from the complex interplay between stress and affect with other variables in the network, (e.g., physical activity) across time.
In addition to capturing the spread of symptom activity through networks, impulse response analysis has been used to capture individual differences in the extent to which networks facilitate the persistence of activity through complex systems of temporally associated components, long after the initiating shock has ended. In 13-variable, person-specific, temporal networks indicating the moment-to-moment associations between a range of socioemotional processes (e.g., anger, sadness, ratings of interpersonal behaviors and perceptions), impulse response analysis was used to simulate a shock to sadness (Yang et al., 2018). Changes in sadness intensity was charted over 150 time steps and the time step at which sadness intensity returned a level consistent with its pre-shock level was identified. This time step was taken as a measure of the recovery time of sadness following a perturbation and, importantly, consisted of activity related to the exogeneous shock, the autoregressive effect of sadness on itself from one timepoint to the next, but also indirect effects on sadness as activity spread to other nodes and returned via time-lagged edges to impact sadness. Participants with networks associated with longer recovery times of sadness exhibited higher levels of depressive symptoms, especially during periods of higher than usual stressful life events.
Impulse response analysis, then, is a framework capable of capturing the potential for symptom activity to spread through a network via time-lagged edges and for quantifying the time it takes for symptoms to return to baseline levels of intensity following hypothetical perturbations. Here, we extend this work to tobacco withdrawal. We estimate temporal networks of tobacco withdrawal, articulating the moment-to-moment associations between withdrawal symptoms across time. After constructing temporal networks, we quantify individual differences in networks by using impulse response analysis to simulate a shock to network symptoms and chart the spread of symptom activity along time-lagged edges through each network. To quantify the extent to which symptom networks facilitate persistent symptom activity, we identify the number of timesteps after the initial shock at which symptom activity returns to a baseline level of intensity comparable to the intensity of activity preceding the shock to the symptom network. We test whether participants undergoing smoking cessation treatment show a lower potential for symptom activity spread and reverberation through symptom networks across time relative to participants in a placebo condition. We hypothesize that treatment is associated with a quicker return to symptom intensity baseline (i.e., shorter recovery time) following a simulated shock.
Method
Participants
Participants were 1210 smokers (58.35% female, 86.24% white), previously reported on in an existing study (Piper et al., 2009; Bolt et al., 2012). Participants were recruited via TV, radio, and newspaper advertisements, community flyers, and earned media (e.g., radio and TV interviews, press releases) in the greater Madison and Milwaukee (Wisconsin) areas. The primary inclusion criteria were smoking at least 10 cigarettes per day for the past 6 months and being motivated to quit smoking. Exclusion criteria included: use of certain medications (including MAO inhibitors, bupropion); any history of psychosis, bipolar disorder, or an eating disorder; consuming six or more alcohol beverages daily 6 or 7 days a week; pregnancy or breast-feeding; and a serious health condition that might prevent study completion. The study was approved by the University of Wisconsin Health Sciences Institutional Review Board, study title “A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies”.
Procedure
Participants passing a phone screen were invited to an information session at which a study description was provided and written informed consent was obtained. Participants then completed multiple baseline screenings, including a medical history screening, vital signs measurements, and a CO breath test. Participants also completed demographic, smoking history, and tobacco dependence questionnaires.
Eligible participants were randomized to one of six treatment conditions: bupropion sustained-release (SR) (n=215), nicotine lozenge (n=202), nicotine patch (n=210), nicotine patch + nicotine lozenge (n=228), buproprion SR + nicotine lozenge (n=214), or placebo (n=141). Five placebo conditions matched the five active conditions, with three monotherapy placebo conditions (n=80) and two combination therapy placebo conditions (n=61). All medications were provided for 8 weeks post-quit except the nicotine lozenge, which was provided for 12 weeks post-quit, consistent with prescribing instructions at the time (Fiore et al., 2008).
Measures
Participants completed ecological momentary assessments (EMA) four times a day (once just after waking, once prior to going to bed, and twice at randomly chosen times between waking and sleeping) for 1 week pre-quit and 2 weeks post-quit. We analyzed data from the 2-week post-quit period. The EMA prompted participants to rate their withdrawal symptoms within the last 15 minutes using 11 items from the Wisconsin Smoking Withdrawal Scale (Welsch et al., 1999) and 1 item adapted from the Questionnaire of Smoking Urges (Sweeney et al., 1996) to asses 6 symptoms: anxiety (tense or anxious, impatient), craving (bothered by desire to smoke, urge to smoke), sadness (sad or depressed, hopeless or discouraged), irritability (irritable or easily angered, bothered by negative moods such as anger, frustration, and irritability), hunger (hungry, thinking about food a lot), and difficulty concentrating (hard to pay attention, difficult to think clearly). The mean of the 2-items making up each symptom scale was computed. Participants also reported the number of cigarettes smoked since the last report at each prompt. Prior to the EMA protocol, participants completed the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991). Carbon monoxide (CO) confirmed 7-day point-prevalence abstinence data was collected at 8 weeks and 6 months post-quit. Alveolar CO was assessed using a Bedfont Smokerlyzer and smokers with a CO < 10ppm were considered abstinent.
Data Preparation
Temporal network models assume stationarity of symptoms such that symptoms do not exhibit a trend in the mean or variance of their intensity across time (Lütkepohl, 2005). We removed linear trends from each symptom by regressing each participants’ ratings of symptom intensity on time in the study to remove the linear trend, and then by taking the residuals forward, which is a common practice to handle violations of stationarity in the means. A second assumption of the models used is that there are equal time lags between consecutive measurements (de Haan-Rietdijk et al., 2017). To accommodate this assumption, we did not regress the first measurement of each day on the last measurement of the previous day.
Data Analysis
We used the R package mlVAR (Epskamp et al., 2018) to estimate the dynamic associations among withdrawal symptoms at the group level (Epskamp et al., 2016). Each symptom was regressed on the values of all other symptoms at the previous time point. To accommodate the nested nature of the data, with participants providing multiple reports, a multilevel extension of VAR was used (Bringmann et al., 2013; Lydon-Staley et al., 2019a). The resulting coefficients represent the degree to which changes in one symptom at time t predict changes in another symptom at time t+1 for the average individual in the sample. Importantly, data are person-mean centered by default, allowing a consideration of within-person associations between variables that are disentangled from between-person differences in levels of symptom intensity (Curran & Bauer, 2011). From this within-person perspective, edges may be interpreted as how deviations in symptom intensity from one’s average level of symptom intensity is associated with change in another symptom’s intensity. In addition to producing a temporal network, the mlVAR approach also estimates a contemporaneous network. Edges in the temporal network encode information regarding how symptoms at one measurement occasion predict symptoms at the next measurement occasion. This network has the potential to provide information about the directionality of effects (Epskamp et al., 2018), and thus estimates of influence are depicted as directed edges with arrows indicating the direction of effects across time. Edges in the contemporaneous network show associations between symptoms occurring within consecutive measurements and indicate partial correlations between nodes at the same time, controlling for both temporal effects and all other variables within the same measurement occasion. Edges in the contemporaneous network are computed by correlating the residuals of the temporal effects (Dahlhaus & Eichler, 2003). The contemporaneous network provides insight into the co-occurrence of symptoms, and may also indicate the presence of causal associations occurring on a shorter timescale than the timescale of measurement (Epskamp et al., 2018). One of the advantages of multilevel models is their tolerance to heterogeneity of time points across participants. As such, data from all participants were included in the model.
After estimating a group level dynamic withdrawal network, we used the same participants and measures to estimate person-specific dynamic networks of withdrawal. The raw time series data for the 6 withdrawal symptoms were regressed on time in order to remove trends and meet the assumption of stationarity. These data were then within-person standardized so that each symptom for each person had a mean of 0 and a standard deviation of 1. This standardization was performed to render the coefficients representing different edges in the network comparable to one another (Bulteel et al., 2016). Within-person standardization also allows us to focus on within-person associations among the various symptoms when comparing networks between participants. The edges in the network represent how fluctuations in one symptom beyond a participant’s typical level (captured by 0 due to the within-person standardization) are associated with fluctuations in a different symptom (Curran & Bauer, 2011). We then modeled each participant’s 6-symptom time series as a 6-node network using a unified Structural Equation Model (uSEM) (Gates et al., 2010).
In this approach, the observed multivariate time series γ(t)is modeled as the output of a latent variable time series η(t) ,
| (1) |
where Λ is a factor loading matrix and ε(t) is a time series of residuals given by a matrix Θ, that is assumed diagonal. We then model the temporal associations among the set of latent withdrawal symptoms represented in η(t) as,
| (2) |
Where η(t-1) is a vector of the lag-1 version of the multivariate latent withdrawal symptom time series, A is a matrix of regression parameters describing the contemporaneous associations among the latent withdrawal symptom variables, Φ1 is a matrix of regression parameters describing the lag-1 associations (auto- and cross-regressions) among the latent withdrawal symptom variables, and ζ(t) contains the errors in prediction. Both the contemporaneous associations in A and the auto- and cross-regressive associations in Φ1 indicate directed temporal associations among the withdrawal symptoms through which exogenous input can diffuse. Note that although the contemporaneous networks estimated in mlVAR are undirected, in the uSEM approach, directed edges are estimated in the contemporaneous networks (e.g., Wright et al., 2019; Yang et al., 2018; Yang et al., 2019). Directed contemporaneous edges in structural equation models provide insight into the co-occurrence of symptoms and may also indicate the presence of causal associations occurring on a faster timescale than the timescale of measurement (Granger, 1969), especially when autoregressive influences are simultaneously estimated (Gates et al., 2010), as in the current case.
The uSEM model is estimated using an iterative search process. A series of models is constructed and improvements to model fit are determined. With each iteration, Lagrange Multiplier tests (Sörbom, 1989) are used to select the edge which, if free to be estimated, would have the maximum improvement on model fit. We constrained the iteration process such that only A and Φ1 blocks of the parameter matrix may be freed. This choice serves to maintain the time-series structure of the model. We avoided bidirectional edges in the contemporaneous associations by including all potential autoregressive associations in the initial model and deeming the reverse association between two nodes unavailable when any edge in A was freed (see also Yang et al., 2018). By specifying unidirectional edges in the contemporaneous network, only one causal edge between two variables is allowed. This restriction still allows reciprocal edges in withdrawal networks given that the reverse association can be observed in the lagged network. We configured the factor loading matrix Λ as an identity matrix I and we fixed all elements of Θ, the variance-covariance matrix of εt1 and εt , to 0. Model fit is determined according to a predetermined α level on a χ-square distribution with one degree of freedom. Here, as elsewhere (Beltz et al., 2013), we set α to 0.05. This edge is freed, the model is re-estimated, and a new set of modification indices are calculated. Edges are freed or added until further addition does not significantly improve model fit.
To quantify individual differences in symptom activity spread across symptom network edges, we used impulse response analysis (Lütkepohl, 2005) to provide insight into how the experience of one symptom propagates through the withdrawal symptom network by predicting other, connected symptoms. In this approach, an impulse is given to an individual node and the behavior of the symptom network is observed, through simulation, over many time steps. This dynamical process simulates how a symptom’s activity moves through the symptom network along edges when it becomes transiently increased due to an external perturbation. Formally, the impulse response simulation is derived from Equation 2 as a one step ahead forecasting process through conversion into a vector auto-regression model (Amisano & Giannini, 2012). The system is set in motion with an initial impulse vector, ζ(0), and latent states are calculated for t=0 as:
| (3) |
to accommodate the contemporaneous associations among latent states and for each subsequent t as:
| (4) |
and the system evolution over 100 time steps is simulated. The time profile, showing how symptom intensity changes over the time steps following the initial impulse, is examined. Specifically, the recovery time of symptom intensity, defined as the time taken to return to within +/−0.01 of equilibrium, is derived via a backward search. We searched backward from the end of the time profile to identify the time step k where the intensity of a symptom is first outside the +/−0.01 boundary. Recovery time is then quantified as the number of time steps from perturbation to equilibrium. Implementation of person-specific uSEM and impulse response analysis was done using the R package pompom (Yang, Ram, & Molenaar, 2018).
We next quantified individual differences in the extent to which withdrawal symptom networks facilitate self-perpetuating symptom activity across time by estimating system recovery time. Impulses were given to all 6 symptoms, each time tracking changes in all 6 symptoms. The result is a 6×6 matrix of symptom activity trajectories. We took the mean value of the column means in the 6×6 matrix as an indication of system recovery time, indicating the average time taken for a symptom to return to equilibrium after a hypothetical perturbation.
To explore the extent to which system recovery time may be impacted by treatment, we compared the system recovery time of participants receiving combination therapy during the quit attempt (nicotine patch + nicotine lozenge and buproprion SR + nicotine lozenge conditions) to participants in combination therapy placebo conditions using independent samples t-tests. A second independent samples t-test was used to determine whether system recovery time differed between participants in the monotherapy conditions (buproprion SR, nicotine lozenge, and nicotine patch) and the associated placebo conditions.
Results
We provide descriptive statistics of the study sample before presenting the results for the group and person-specific withdrawal networks.
Descriptive statistics
The average intensity of withdrawal symptoms across the EMA period are shown in Table 1. Smoking lapses were common with only 400 participants (33.06%) reporting not smoking since the previous EMA report. On average, smoking since the previous prompt was reported at 18.00% of prompts (SD=27.00). The mean number of EMA reports available per participant was 27.91 (SD=21.51). Participants completing a greater number of EMA reports exhibited lower intensity of withdrawal symptoms (all rs<0.20 indicating weak associations) during the EMA protocol (see Table 1).
Table 1.
Descriptive statistics
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Anxietya | - | |||||||
| 2. Irritabilitya | 0.85 | - | ||||||
| 3. Difficulty concentratinga | 0.75 | 0.72 | - | |||||
| 4. Cravinga | 0.52 | 0.47 | 0.41 | - | ||||
| 5. Sadnessa | 0.69 | 0.73 | 0.65 | 0.35 | - | |||
| 6. Hungera | 0.44 | 0.41 | 0.40 | 0.34 | 0.32 | - | ||
| 7. Smokingb | 0.14 | 0.17 | 0.14 | 0.30 | 0.24 | 0.05 | - | |
| 8. EMA completion rate | −0.17 | −0.16 | −0.14 | −0.15 | −0.14 | −0.08 | −0.25 | - |
| Variables | ||||||||
| Mean | 1.90 | 1.57 | 1.37 | 4.38 | 1.06 | 2.57 | 0.18 | 27.91 |
| Standard Deviation | 1.69 | 1.60 | 1.62 | 2.55 | 1.40 | 1.60 | 0.27 | 12.51 |
Notes:
Variables represent the intraindividual mean of participant’s repeated measures; n=1210. All correlations greater than 0.05 are significant at p<0.05.
Indicates the proportion of prompts at which smoking since the previous prompt was reported.
Group-level withdrawal networks
Results from the multilevel vector autoregressive model are in Table S1. The edges of the temporal network (Fig. 1A) represent the prediction of symptoms at one measurement occasion by symptoms at the previous measurement occasion for the prototypical individual in the sample. We find that all estimated edges are positive, such that withdrawal symptoms at one moment predict increases (rather than decreases) in the experience of other withdrawal symptoms (including themselves) at the next moment. Craving has many outgoing edges, indicating that increased craving at one moment has a relatively strong prediction of other withdrawal symptoms from moment to moment. Both sadness and irritability have the largest number of ingoing edges, indicating that increases in sadness and irritability are predicted by increases in the experience of other withdrawal symptoms. The majority of symptoms show positive self-loops (edges pointing towards themselves), which indicate that current symptom experiences are predictive of subsequent experiences. Notable is the presence of reciprocal edges between sadness and irritability, and between anxiety and irritability. These reciprocal edges represent positive feedback loops whereby the experience of one symptom (e.g., irritability) predicts increases in the experience of another symptom (e.g., anxiety), which in turn predicts the experience of the original symptom (i.e., irritability). Also notable is hunger, which is disconnected from the other withdrawal symptoms; the lack of incoming or outgoing edges to and from hunger indicates that fluctuations in the experience of hunger over time do not play a large role in predicting other withdrawal symptoms.
Figure 1.
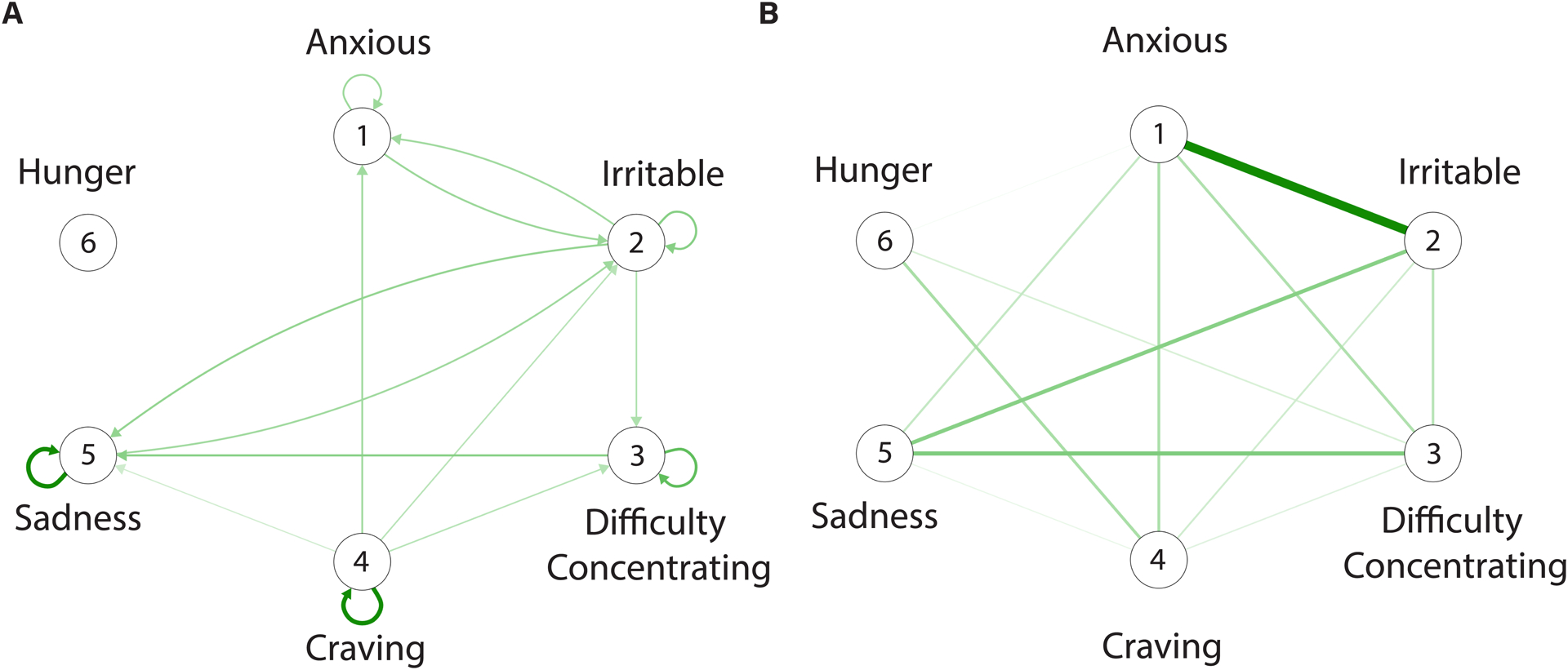
Dynamic networks of tobacco withdrawal. A. A temporal network in which the nodes (circles) represent symptoms and the edges represent the association between symptoms from one measurement occasion to the next. B. A contemporaneous network in which the edges represent the co-occurrence of symptoms at the same measurement occasion while controlling for temporal effects and the effects of other symptoms. Only green edges are observed in the networks indicating that the experience of increases in withdrawal symptoms tend to be associated with increases in the experience of other symptoms (e.g., when sadness is higher than usual, anxiety is higher than usual). Both temporal and contemporaneous networks are estimated by pooling data across all participants in the sample and, thus, edges represent the prototypical associations among symptoms.
The edges of the contemporaneous network (Fig. 1B) represent the co-occurrence of symptoms, controlling for both temporal effects and the effects of all other variables at the same measurement occasion. Edges are unidirectional (without arrows) but they may indicate causal associations playing out at timescales shorter than the timescale of symptom measurement (Epskamp et al., 2018). Similar to the temporal network, all edges are positive, indicating that when an increase in one symptom is experienced (e.g., sadness) an increase in a second symptom is experienced (e.g., irritability).
Person-specific withdrawal networks
We next moved from a group model indicating the temporal associations between symptoms across time for the prototypical individual in the sample to the estimation of person-specific temporal networks. The estimation of person-specific networks requires substantially more data than the estimation of a group model that pools data across individuals. A person-specific model of the associations among the 6 withdrawal symptoms fit the data of 290 individual participants well; the goodness of fit is indicated by at least three of four following criteria: RMSEA ≤ 0.08, SRMRs ≤ 0.08, CFIs ≥ 0.95, NNFI ≥ 0.95 (Beltz et al., 2013; Yang et al., 2018). Comparing the number of days available for participants for whom a person-specific model fit well relative to those for whom a person-specific model could not be fit well indicate that, in line with previous work (Yang et al., 2018), the length of the time series impacted model fit, with a significantly greater amount of time series data available for participants with good (M=37.21, n=290) versus poor (M=23.16, n=998) model fit (t(745.72)=21.692, p<0.001, d=1.14).
Substantial heterogeneity in symptom network structure is evident (Figure 2A; see Figures S1–S7 for distributions of edge estimates). We quantify individual differences in participants’ withdrawal symptom networks and their implications for the extent to which symptom activity persists across time due to the predictive associations among network symptoms by using impulse response analysis (Lütkepohl, 2005; Yang et al., 2018). The average system recovery time (Fig. 2B) for the sample is 3.18 (SD=2.55) time steps and exhibits substantial positive skew (min=0.89, max=26.86, skew=4.23). We used the common logarithm to reduce the positive skew and remove three outliers (all greater than 3 SDs above the mean). In the sample of 287 participants, there were no differences across the treatment conditions in the average number of EMA data points available, in self-reported cigarettes smoked per day reported at baseline, in FTND total score reported at baseline, or in the average intensity of the different withdrawal symptoms across the EMA period (all p-values > 0.05). There was a difference in the proportion of prompts during the EMA period for which participants across the different treatment groups reported smoking since the previous report such that participants in the monotherapy placebo group reported the most smoking occasions (Mean=0.33, SD=0.34), followed by the combination placebo group (Mean=0.14, SD=0.19), then the monotherapy group (Mean=0.13, SD=0.22), and finally the combination therapy group (M=0.10, SD=0.17). Only the difference between the combination therapy and the monotherapy placebo group, however, was statistically significant, t(12.45)=−2.43, p=0.03, d=1.25.
Figure 2.
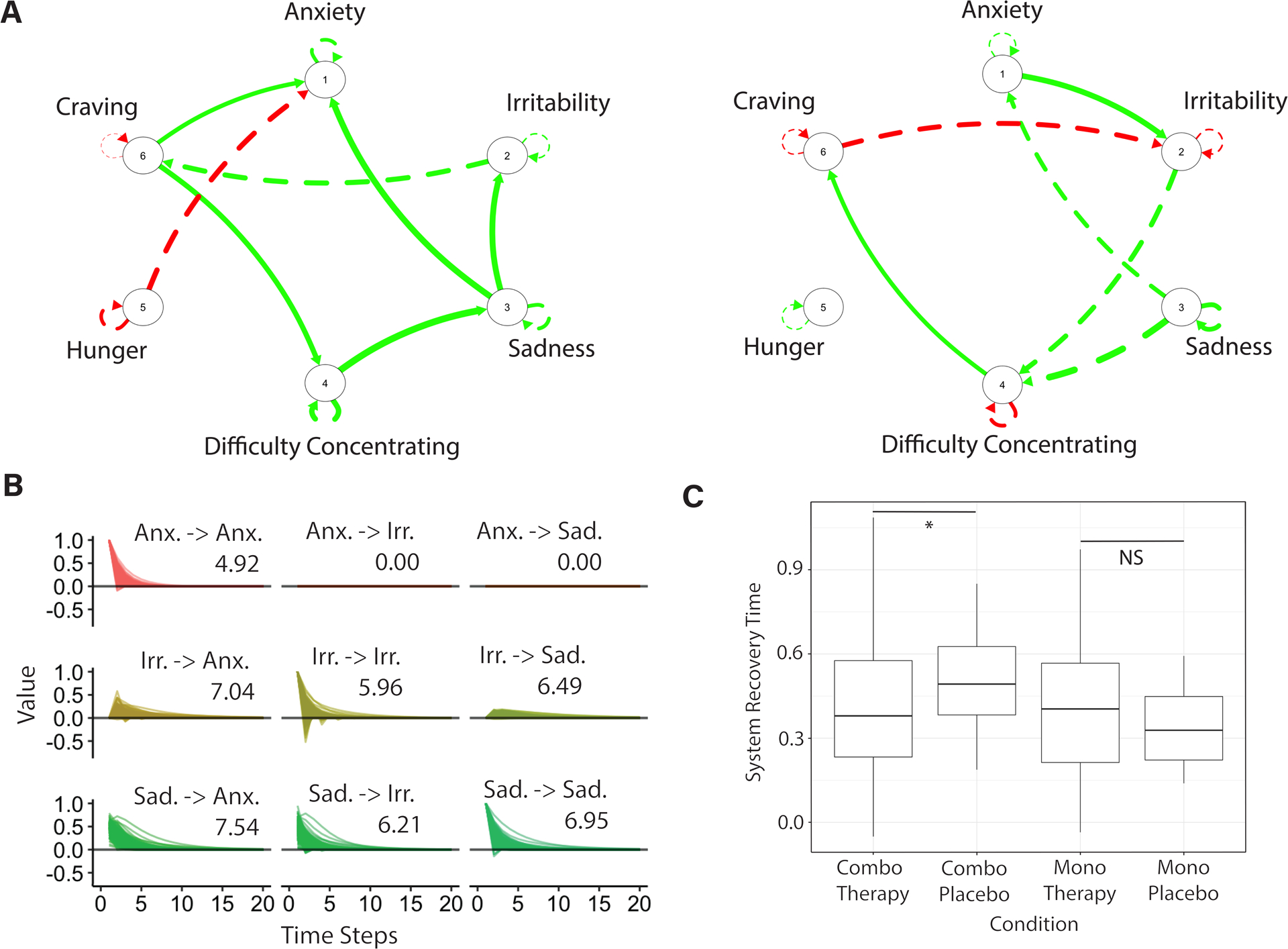
Person-specific networks of tobacco withdrawal. A. Two graphs estimated using a unified Structural Equation Model on experience-sampling data show the temporal associations (edges) among individual withdrawal symptoms (nodes) across time for two different participants. Red edges show negative associations; green edges show positive associations; dashed edges are lagged associations; and continuous edges are contemporaneous associations. Notable is the heterogeneity in network structure across the two participants. B. A sample matrix of recovery times associated with the network on the left in panel A, and estimated using impulse response analysis, indicates the time it takes for a symptom to return to equilibrium after being perturbed. For example, when an increase in anxiety is simulated, it takes 4.92 time steps for anxiety to return to baseline (as indicated by the Anx. -> Anx label). A subsample of three symptoms (anxiety, irritability, and sadness) of the full six symptom matrix is shown to enhance readability. C. Independent samples t-tests show higher log system recovery times in the symptom networks of participants in the combination therapy placebo condition (non-log mean value = 3.55) relative to participants in the active combination therapy condition (non-log mean value = 3.05). No differences in system recovery time emerges for participants in the active (non-log mean value = 2.88) or placebo monotherapy (non-log mean value = 2.39) groups. Notes: Anx.=anxiety; Irr. = irritablility; Sad. = sadness; *p=0.03; NS = non-significant.
Using independent samples t-tests we found that participants undergoing combination treatment have faster recovery times relative to participants in a matched placebo condition, t(26.76)=−2.23, p=0.03, d=0.43, a small effect size (Cohen, 1988; Fig. 2C). There was no significant difference between participants in monotherapy treatment and a matched placebo condition, t(21.04)=1.00, p=0.33, d=0.24. Beyond these planned contrasts, we also observed that participants in the monotherapy condition had shorter recovery time relative to participants in the combination placebo group, t(34.60)=−2.29, p=0.03, d=0.52, and that participants in the monotherapy placebo group had shorter recovery time relative to participants in the combination placebo group, t(28.67)=−2.69, p=0.01, d=0.92. There was no significant difference in recovery time between the combination therapy and monotherapy groups, t(155.39)=0.34, p=0.74, d=0.04 or between the combination therapy and monotherapy placebo group, t(16.40)=1.29, p=0.21, d=0.26.
Average system recovery time was not associated with CO-verified smoking abstinence at end of treatment (8 weeks post quit; t(277.31)=1.05, p=0.30, d=0.12) or at 6-months post quit, t(228.19)=0.87, p=0.38, d=0.11. Correlations revealed that participants with longer system recovery time experience more intense anxiety, r(285)=0.12, p=0.04, sadness, r(285)=0.12, p=0.04, irritability, r(285)=0.14, p=0.03, and difficulty concentrating, r(285)=0.15, p=0.01, but did not report more intense craving, r(285)=0.05, p=0.37, or hunger, r(285)=−0.01, p=0.83, during the EMA protocol.
Discussion
Smoking cessation is notoriously difficult, with the vast majority of quit attempts ending in relapse (Alterman, Gariti, & Mulvaney, 2001; Fiore, Bailey, & Cohen, 2000). Withdrawal symptoms emerge following reductions in smoking that are associated with decreased quit intentions as well as increased smoking relapse (Orleans et al., 1991; Allen et al., 2008; West et al., 1987). The present study examined the potential utility of a network perspective on tobacco withdrawal, particularly in providing insight into smokers’ withdrawal experiences.
A key contribution of the present paper was to extend cross-sectional network findings of associations among symptoms of tobacco withdrawal (Lydon-Staley et al., 2018) to model the interplay among withdrawal symptoms using experience-sampling data containing temporal information on the moment-to-moment activity of withdrawal symptoms. The edges in cross-sectional networks indicate between-person associations, with strong edges indicating that if an individual experiences high levels of anxiety, for example, they are likely to also experience high levels of anger. This symptom co-occurrence, from a network perspective, is theorized to result from causal associations among symptoms. Yet, between-person associations (i.e., people high in anxiety experience high anger) do not always translate to the within-person associations (i.e., at times when a person is more anxious than usual, they are also angrier than usual; Bos et al., 2017; Hamaker et al., 2005).
To address this open challenge in the field, we estimated networks in which the edges indicated the within-person association between symptom activity at one time point and symptom activity at the next time point. The group-level networks of withdrawal exhibited substantial interplay among symptoms from moment to moment. Comparison of temporal networks to cross-sectional withdrawal networks estimated in previous work (Lydon-Staley et al., 2018) is difficult due to the minimal overlap between symptoms examined across studies. However, the positive associations among anxiety, sadness, and difficulty concentrating observed in the group-level contemporaneous network in the current study were also observed in cross-sectional withdrawal networks that also included these three constructs (Lydon-Staley et al., 2018), suggesting that some findings from cross-sectional networks may translate to within-person associations. However, no associations were observed between sadness and anxiety and difficulty concentrating and anxiety in the present study in the group-level temporal network wherein edges represented the extent to which symptom intensity at the current timepoint predicted symptom intensity at the next timepoint. Thus, although some edges present in cross-sectional networks may be observed in temporal networks, the limited overlap suggests that, like previous work in the context of depression (Bos et al., 2017), cross-sectional networks do not reflect how symptoms are associated with one another across time. This limited overlap between cross-sectional and temporal networks limits the extent to which cross-sectional networks, which require data that are more easily obtained than repeated measures data, provide information relevant for clinical applications aimed at identifying potentially causal associations between symptoms that exacerbate illness (Kroeze, 2017; Fisher & Boswell, 2016).
Additional considerations raised by examining the group-level networks are the insights gained from the group-level temporal network. The temporal network contained many positive edges, such that if symptom activity was high at one time point, it was more likely to increase, rather than decrease, in activity at the next time point. Indeed, the group temporal network contained numerous potential positive feedback loops that might render symptom activity vulnerable to exhibiting self-perpetuation of symptom activity due to the reverberation of symptom activity through the network. For example, anxiety at one time point was associated with increased irritability and anger at the next time point and, in turn, irritability and anger at one time point was associated with increased anxiety at the next time point. The capacity for one’s current emotions to give rise to other emotions has long been emphasized in emotion theory (e.g., Gross & Muñoz, 1995) and there is empirical support for the moment-to-moment transfer of emotions across time and states (Anand et al., 2016; Lydon-Staley et al., 2019a; Pe & Kuppens, 2012).
Additional findings of interest include the many out-going edges from craving to other withdrawal symptoms. Although the association between negative affect and craving is prominent in models of withdrawal, the greatest emphasis is placed on the effect of affect to craving rather than the reverse direction (Baker et al., 2004; Conklin & Perkins, 2005; Delfino et al., 2001; Otto et al., 2007). The current findings suggest that the experience of craving at one moment predict the emergence of aversive states (e.g., anxiety, irritability) at the next moment. Thus, craving may be a useful treatment target, not simply to avoid drug use in response to craving, but also to ameliorate aversive states resulting from its experience (Tiffany & Wray, 2012). We also note that the current findings do not preclude the possibility that negative affect predicts craving given the edges between affect and craving in the contemporaneous group network.
Estimation of person-specific networks revealed that the group model masks substantial individual differences. The most striking difference includes observing negative edges in the networks of individual participants (red edges in Figure 2A) when the networks estimated at the group level contained only positive edges. The extensive heterogeneity in withdrawal symptom structure echoes heterogeneity in temporal symptom networks observed in disorders beyond tobacco withdrawal (e.g., Fisher et al., 2017; Reeves & Fisher, 2020) as well as long-standing calls for the need for an idiographic science to inform psychological processes (Molenaar, 2004). The heterogeneous experience of tobacco withdrawal is increasingly appreciated (Piper, 2015). There are individual differences in the extent to which smokers endorse the experience of various withdrawal symptoms during periods of smoking abstinence (Pang et al., 2019; Weinberger et al., 2017) and tracking withdrawal symptoms across pre-quit and post-quit periods reveals substantial individual differences in the trajectories of craving and negative affect intensity (McCarthy et al., 2006; see also Robinson et al., 2007). The heterogeneity in withdrawal experiences, observed in this study and in others, may impact the extent to which treatments can successfully target withdrawal in the service of facilitating smoking cessation. Developing the type of data and methodologies that capture person-specific withdrawal experiences provides a pathway to precision medicine, in which treatments are tailored to individuals (Allenby et al., 2016).
In addition to allowing an appreciation for the vast heterogeneity of tobacco withdrawal, constructing person-specific, temporal networks of withdrawal symptoms enabled us to probe the notion of self-perpetuating symptom networks and its implications for the experience and treatment of withdrawal. At the core of network theory in psychology are notions of individual differences in the extent to which symptom networks are vulnerable to the reverberation of symptom activity across time as symptoms influence one another. We quantified the extent to which withdrawal symptom networks were vulnerable to self-perpetuation by capturing the time it took for symptom activity to recover after a simulated increase in activity that spread along the estimated directed paths of the symptom network. Findings showed that combination nicotine replacement smoking cessation treatment was associated with reduced symptom network recovery time relative to a placebo condition. While combination nicotine replacement has been shown to work by reducing craving (Bolt et al., 2012), this research suggests that this treatment may also reduce the time it takes for a smoker to recover and return to baseline levels of withdrawal (i.e., negative affect, cognitive disturbance, craving) after an acute increase in withdrawal symptom activity. Further evidence for the validity of symptom recovery time are observations that longer system recovery time are associated with more intense experiences of anxiety, sadness, irritability, and difficulty concentrating during the EMA protocol.
Perhaps surprisingly, the monotherapy placebo group showed faster recovery time relative to the combination placebo group. One potential explanation for this observation is that the monotherapy placebo group exhibited the most smoking lapses during the quit period. As such, the short network recovery time observed in the monotherapy group may stem less from treatment effects (or lack thereof in this case) and more from continued smoking which alleviated the monotherapy placebo group’s withdrawal experiences to a greater extent than the other groups showing fewer smoking lapses.
In considering differences between the group-level and person-specific networks, it is important to highlight a number of methodological differences. The group temporal networks incorporate data from all individuals in estimating edges and, thus, estimates represent the prototypical individual in the sample. In contrast, the person-specific networks are estimated in an approach that models only data from the individual. Additionally, estimates from six different models are combined to create the group temporal networks. The person-specific approach, in contrast, leverages structural equation modeling to estimate all potential edges in the network simultaneously. Advantages of the person-specific approach are not, however, without limitations. Estimating a well-fitting person-specific model requires substantially more data than approaches that aggregate data across groups of people. Indeed, in the present case, person-specific networks could not be constructed for each individual due to the length of the time series required for estimation.
Going forward, it will be important to identify the mechanisms underlying the network edges. In the context of the cross-talk observed between emotions across time, for example, emerging work suggests the relevance of individual differences in the neural correlates of cognitive control as well individual differences in the use of emotion regulation strategies involving the adoption of an accepting and open attitude to one’s current experience for understanding the spread of emotions to other emotions across time (Drake et al., 2019; Lydon-Staley et al., 2019a; Lydon-Staley et al., 2019b).
Limitations and future outlook
Despite observing associations between symptom recovery time and treatment, we observe no association between symptom recovery time and biochemically-confirmed smoking abstinence at end of treatment (8 weeks post-quit) or at 6 months post-quit. The lack of association between symptom network recovery time and smoking cessation abstinence may be interpreted in a number of ways. We emphasize that recovery time provides information on how individual differences in tobacco withdrawal symptom network structure might promote symptom spread across time following a hypothetical, simulated perturbation. As such, recovery time represents a potential risk factor through which events external to the withdrawal network may act to promote persistent symptom activity. Indeed, in a study using impulse response analysis to examine the recovery time of sadness to a simulated perturbation, longer recovery time was associated with overall higher levels of depression symptoms in participants experiencing high exposure to stressful life events but not in participants experiencing low exposure to stressful life events. In examining the association between withdrawal network recovery time and smoking cessation outcomes going forward, it will be important to concurrently measure the occurrence of events outside of the withdrawal network that could plausible lead to increases in symptom activity in ways that mirror the simulated shock of the current study.
The person-specific approach allowed an appreciation for the heterogeneous experience of withdrawal as well as the computation of system recovery time, allowing us to capture theoretical notions of self-perpetuating symptom networks. However, estimating good-fitting person-specific models required substantially more data than approaches that aggregate data across groups of people. In the present case, person-specific networks could not be constructed for each individual in the sample due to the length of the time series required for estimation, a difficulty observed in applications beyond tobacco withdrawal (Yang et al., 2018). We hope that highlighting the difficulties of estimating person-specific networks using the type of time series data that has provided substantial insights into withdrawal dynamics to date, as well as the important insights associated with the heterogeneous experience of withdrawal, encourages future collection efforts of denser time series data of tobacco withdrawal. An additional potential limitation is the use of random assessments twice per day between waking and sleeping. The use of random assessments minimizes potential problems with participant anticipation of measurement occasions but also complicates the assumption of equal intervals between pairs of EMA observations.
Smoking lapses are very common during smoking quit attempts (Ashare et al., 2014; Shiffman et al., 2006) and the prototypical participant in the present study reported smoking since the last EMA report at approximately 18% of their EMA prompts. As such, the symptom networks should be interpreted as withdrawal networks estimated during a quit attempt marked by occasional lapses. With denser withdrawal symptom time series data, extensions of the unified structural equation modeling approach used here to examine person-specific networks could incorporate information on lapses into the modeling approach, treating smoking lapses as events that may moderate network edges (Gates et al., 2011).
Conclusions
The present study examined the temporal network structure of tobacco withdrawal. In doing so, we find that that there are substantial associations between individual withdrawal symptoms across time, and that smoking cessation treatments may impact the extent to which withdrawal symptom activity lingers across time due to moment-to-moment inter-symptom associations. These findings suggest that a network perspective of tobacco withdrawal is a promising approach to understanding withdrawal and its treatment to help all smokers succeed in quitting smoking. The study highlights the challenges associated with using cross-sectional networks to inform within-person associations between symptoms across time (Bos et al., 2017), reinforcing the need to collect intensive repeated measures data to articulate theories of within-person variation in withdrawal symptoms. The vast heterogeneity observed in withdrawal symptom experiences also provide challenges for generalizability, necessitating consideration of methodological approaches capable of capturing both idiographic experiences as well as experiences that may be common to most smokers (Beltz et al., 2016).
Supplementary Material
Acknowledgments
D.M.L. acknowledges support from the National Institute on Drug Abuse (K01-A047417-01A1). D.S.B. and D.M.L. acknowledge support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the ISI Foundation, the Paul Allen Foundation, the Army Research Laboratory (W911NF-10-2-0022), the Army Research Office (W911NF-14-1-0679, W911NF-16-1-0474, W911NF-17-2-0181), the Office of Naval Research, the National Institute of Mental Health (2-R01-DC-009209-11, R01-MH112847, R01-MH107235, R21-M MH-106799), the National Institute of Child Health and Human Development (1R01HD086888-01), National Institute of Neurological Disorders and Stroke (R01 NS099348), and the National Science Foundation (BCS-1441502, BCS-1430087, NSF PHY-1554488, and BCS-1631550).
M.E.P. acknowledges support from the National Institute on Drug Abuse (P50 DA019706), the General Clinical Research Centers Program of the National Center for Research Resources NIH (M01 RR03186), and an Institutional Clinical and Translational Science Award (UW-Madison; KL2 RR025012). A.M.L. acknowledges support from grant K24DA048160 and RSG-13-163-01 and the American Cancer Society. R.A.S. acknowledges support from grant K24 DA045244. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. A pre-peer reviewed version of this manuscript is available on PsyArXiv 10.31234/osf.io/t2a9q. Collection of the data used in this study was approved by the University of Wisconsin Health Sciences Institutional Review Board, study title “A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies”.
References
- Allen SS, Bade T, Hatsukami D, & Center B (2008). Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine & Tobacco Research, 10, 35–45. [DOI] [PubMed] [Google Scholar]
- Allenby CE, Boylan KA, Lerman C, & Falcone M (2016). Precision medicine for tobacco dependence: development and validation of the nicotine metabolite ratio. Journal of Neuroimmune Pharmacology, 11, 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI, Gariti P, & Mulvaney F (2001). Short- and long-term smoking cessation for three levels of intensity of behavioral treatment. Psychology of Addictive Behaviors, 15, 261–264. [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed). Washington, D.C: American Psychiatric Association. [Google Scholar]
- Amisano G, & Giannini C (2012). Topics in structural VAR econometrics (2nd ed.). New York, NY: Springer-Verlag. [Google Scholar]
- Anand D, Wilt J, & Revelle W (2017). Within-subject covariation between depression-and anxiety-related affect. Cognition and Emotion, 31, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Perkins KA, & Schnoll RA (2013). The first seven days of a quit attempt predicts relapse: Validation of a measure for screening medications for nicotine dependence. Journal of Addiction Medicine, 7, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review, 111, 33–51. [DOI] [PubMed] [Google Scholar]
- Bekiroglu K, Russell MA, Lagoa CM, Lanza ST, & Piper ME (2017). Evaluating the effect of smoking cessation treatment on a complex dynamical system. Drug and Alcohol Dependence, 180, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello MS, Pang RD, Chasson GS, Ray LA, & Leventhal AM (2017). Obsessive-compulsive symptoms and negative affect during tobacco withdrawal in a non-clinical sample of African American smokers. Journal of Anxiety Disorders, 48, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Wright AG, Sprague BN, & Molenaar PC (2016). Bridging the nomothetic and idiographic approaches to the analysis of clinical data. Assessment, 23, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Gates KM, Engels AS, Molenaar PC, Pulido C, Turrisi R, … & Wilson SJ (2013). Changes in alcohol-related brain networks across the first year of college: a prospective pilot study using fMRI effective connectivity mapping. Addictive Behaviors, 38, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, & Baker TB (2012). Why two smoking cessation agents work better than one: Role of craving suppression. Journal of Consulting and Clinical Psychology, 80, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D (2017). A network theory of mental disorders. World Psychiatry, 16, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, & Cramer AO (2013). Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. [DOI] [PubMed] [Google Scholar]
- Bos FM, Blaauw FJ, Snippe E, Van der Krieke L, De Jonge P, & Wichers M (2018). Exploring the emotional dynamics of subclinically depressed individuals with and without anhedonia: an experience sampling study. Journal of AAffective Disorders, 228, 186–193. [DOI] [PubMed] [Google Scholar]
- Bos FM, Snippe E, de Vos S, Hartmann JA, Simons CJ, van der Krieke L, … & Wichers M (2017). Can we jump from cross-sectional to dynamic interpretations of networks implications for the network perspective in psychiatry. Psychotherapy and Psychosomatics, 86, 175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann LF, Elmer T, Epskamp S, Krause RW, Schoch D, Wichers M, Wigman JTW, & Snippe E (2019). What do centrality measures measure in psychological networks? Journal of Abnormal Psychology. Advanced online publication. doi: 10.1037/abn0000446. [DOI] [PubMed] [Google Scholar]
- Bringmann LF, Vissers N, Wichers M, Geschwind N, Kuppens P, Peeters F, … & Tuerlinckx F (2013). A network approach to psychopathology: new insights into clinical longitudinal data. PloS one, 8, e60188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulteel K, Tuerlinckx F, Brose A, & Ceulemans E (2016). Using raw VAR regression coefficients to build networks can be misleading. Multivariate Behavioral Research, 51, 330–344. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic [Google Scholar]
- Cook JW, Lanza ST, Chu W, Baker TB, & Piper ME (2017). Anhedonia: its dynamic relations with craving, negative affect, and treatment during a quit smoking attempt. Nicotine & Tobacco Research, 19, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, & Perkins KA (2005). Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology, 114, 153–64. [DOI] [PubMed] [Google Scholar]
- Cramer AO, van Borkulo CD, Giltay EJ, van der Maas HL, Kendler KS, Scheffer M, & Borsboom D (2016). Major depression as a complex dynamic system. PloS one, 11, e0167490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, & Bauer DJ (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology, 62, 583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan-Rietdijk S, Voelkle MC, Keijsers L, & Hamaker EL (2017). Discrete-vs. continuous-time modeling of unequally spaced experience sampling method data. Frontiers in Psychology, 8, 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhaus R, & Eichler M (2003). Causality and graphical models in time series analysis. Oxford Statistical Science Series, 115–137. [Google Scholar]
- Delfino RJ, Jamner LD, & Whalen CK (2001). Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine & Tobacco Research, 3, 235–248. [DOI] [PubMed] [Google Scholar]
- Drake A, Dore BP, Falk EB, Zurn P, Bassett DS, & Lydon-Staley DM (2019, August 24). Flourishing and its Associations with Affective Reactivity and Recovery to Daily Stress. 10.31234/osf.io/ahxq5 [DOI] [Google Scholar]
- Epskamp S, van Borkulo CD, van der Veen DC, Servaas MN, Isvoranu AM, Riese H, & Cramer AO (2018). Personalized network modeling in psychopathology: The importance of contemporaneous and temporal connections. Clinical Psychological Science, 6, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Deserno MK, & Bringmann LF (2016). mlVAR: multi-level vector autoregression. R package version 0.3, 3. [Google Scholar]
- Fiore MC, Bailey WC, & Cohen SJ (2000). Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: Department of Health and Human Services. [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, … & Henderson PN (2008). Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. [Google Scholar]
- Fisher AJ, Reeves JW, Lawyer G, Medaglia JD, & Rubel JA (2017). Exploring the idiographic dynamics of mood and anxiety via network analysis. Journal of AAbnormal Psychology, 126, 1044–1056. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, & Boswell JF (2016). Enhancing the personalization of psychotherapy with dynamic assessment and modeling. Assessment, 23(4), 496–506. [DOI] [PubMed] [Google Scholar]
- Foulds J, Russ C, Yu CR, Zou KH, Galaznik A, Franzon M, … & Hughes JR (2013). Effect of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials. Nicotine & Tobacco Research, 15, 1849–1857. [DOI] [PubMed] [Google Scholar]
- Fried EI, Epskamp S, Nesse RM, Tuerlinckx F, & Borsboom D (2016). What are ‘good’ depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. Journal of Affective Disorders, 189, 314–320. [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PC, Hillary FG, & Slobounov S (2011). Extended unified SEM approach for modeling event-related fMRI data. NeuroImage, 54, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PC, Hillary FG, Ram N, & Rovine MJ (2010). Automatic search for fMRI connectivity mapping: an alternative to Granger causality testing using formal equivalences among SEM path modeling, VAR, and unified SEM. NeuroImage, 50, 1118–1125. [DOI] [PubMed] [Google Scholar]
- Granger CW (1969). Investigating causal relations by econometric models and cross-spectral methods. Econometrica: Journal of the Econometric Society, 424–438. [Google Scholar]
- Gross JJ, & Muñoz RF (1995). Emotion regulation and mental health. Clinical psychology: Science and Practice, 2, 151–164 [Google Scholar]
- Hamaker EL, Dolan CV, & Molenaar PC (2005). Statistical modeling of the individual: Rationale and application of multivariate stationary time series analysis. Multivariate Behavioral Research, 40, 207–233. [DOI] [PubMed] [Google Scholar]
- Hasmi L, Drukker M, Guloksuz S, Menne-Lothmann C, Decoster J, van Winkel R, … & Thiery E. (2017). Network approach to understanding emotion dynamics in relation to childhood trauma and genetic liability to psychopathology: replication of a prospective experience sampling analysis. Frontiers in Psychology, 8, 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–27. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, & Brandon TH (2006). The early time course of smoking withdrawal effects. Psychopharmacology, 187, 385–396. [DOI] [PubMed] [Google Scholar]
- Holme P, & Saramäki J (2012). Temporal networks. Physics Reports, 519, 97–125. [Google Scholar]
- Hughes JR (2007). Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research, 9, 3, 315–327. [DOI] [PubMed] [Google Scholar]
- Kroeze R, Van Veen D, Servaas MN, Bastiaansen JA, Oude Voshaar R, Borsboom D, & Riese H (2017). Personalized feedback on symptom dynamics of psychopathology: A proof-of-principle study. Journal for Person-Oriented Research, 3, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, & Pickworth WB (2010). A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors, 35, 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütkephol H (2005). New introduction to multiple time-series analysis. Berlin, Germany: Springer. [Google Scholar]
- Lydon-Staley DM, Xia M, Mak HW, & Fosco GM (2019a). Adolescent emotion network dynamics in daily life and implications for depression. Journal of Abnormal Child Psychology, 47, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon-Staley DM, Kuehner C, Zamoscik V, Huffziger S, Kirsch P, & Bassett DS (2019b). Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Translational Psychiatry, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon-Staley DM, Schnoll R, Hitsman B, & Bassett DS (2018). The Network Structure of Tobacco Withdrawal in a Community Sample of Smokers Treated with Nicotine Patch and Behavioral Counseling. Nicotine & Tobacco Research. Advanced online publication. doi: 10.1093/ntr/nty250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, & Baker TB (2006). Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology, 115, 454–466. [DOI] [PubMed] [Google Scholar]
- Molenaar PC (2004). A manifesto on psychology as idiographic science: Bringing the person back into scientific psychology, this time forever. Measurement, 2(4), 201–218. [Google Scholar]
- Newman M (2010). Networks: An introduction. Oxford, UK: Oxford University Presss. [Google Scholar]
- Orleans CT, Rimer BK, Cristinzio S, Keintz MK, & Fleisher L (1991). A national survey of older smokers: Treatment needs of a growing population. Health Psychology, 10, 343–351. [DOI] [PubMed] [Google Scholar]
- Otto MW, O’cleirigh CM, & Pollack MH (2007). Attending to emotional cues for drug abuse: bridging the gap between clinic and home behaviors. Science & Practice Perspectives, 3, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, Bello MS, Liautaud MM, Weinberger AH, & Leventhal AM (2019). Gender differences in negative affect during acute tobacco abstinence differ between African American and White adult cigarette smokers. Nicotine and Tobacco Research, 21, 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe ML, Kircanski K, Thompson RJ, Bringmann LF, Tuerlinckx F, Mestdagh M, … & Kuppens P. (2015). Emotion-network density in major depressive disorder. Clinical Psychological Science, 3, 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe ML, & Kuppens P (2012). The dynamic interplay between emotions in daily life: Augmentation, blunting, and the role of appraisal overlap. Emotion, 12, 1320–1328. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB (2003). Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology, 112, 1, 3–13. [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, & Baker TB (2000). Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology, 109, 74–86. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB (1998). Profiles in discouragement: Two studies of variability in the time course of smoking withdrawal symptoms. Journal of Abnormal Psychology, 107, 2, 238–251. [DOI] [PubMed] [Google Scholar]
- Piper ME (2015). Withdrawal: expanding a key addiction construct. Nicotine & Tobacco Research, 17, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D & Baker TB (2009). A randomized placebo-controlled clinical trial of five smoking cessation pharmacotherapies. Archives of General Psychiatry, 66, 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JW, & Fisher AJ (2020). An examination of idiographic networks of posttraumatic stress disorder symptoms. Journal of Traumatic Stress, 33, 84–95. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Minnix JA, Wetter DW, Tomlinson GE, Minna JD, … & Cinciripini PM (2007). The DRD2 Taq IB polymorphism and its relationship to smoking abstinence and withdrawal symptoms. The Pharmacogenomics Journal, 7, 266–274. [DOI] [PubMed] [Google Scholar]
- Schmittmann VD, Cramer AO, Waldorp LJ, Epskamp S, Kievit RA, & Borsboom D (2013). Deconstructing the construct: A network perspective on psychological phenomena. New Ideas in Psychology, 31, 43–53. [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, & Clark DB (2006). Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology, 74, 276–285. [DOI] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD (1974). An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review, 81, 119–145. [DOI] [PubMed] [Google Scholar]
- Sörbom D (1989). Model modification. Psychometrika, 54, 371–84. [Google Scholar]
- Sweeney CT, Pillitteri JL, & Kozlowski LT (1996). Measuring drug urges by questionnaire: do not balance scales. Addictive Behaviors, 21, 199–204. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, & Wray JM (2012). The clinical significance of drug craving. Annals of the New York Academy of Sciences, 1248, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, O’Malley SS, McKee SA, Salovey P, & Krishnan-Sarin S (2007). Confirmatory factor analysis of the Minnesota nicotine withdrawal scale. Psychology of Addictive Behaviors, 21, 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Platt JM, Smith PH, & Goodwin RD (2017). Racial/ethnic differences in self-reported withdrawal symptoms and quitting smoking three years later: a prospective, longitudinal examination of US adults. Nicotine & Tobacco Research, 19, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7, 354–361. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, & Belcher M (1987). Time course of cigarette withdrawal symptoms during four weeks of treatment with nicotine chewing gum. Addictive Behaviors, 12, 199–203. [DOI] [PubMed] [Google Scholar]
- Wright AG, Gates KM, Arizmendi C, Lane ST, Woods WC, & Edershile EA (2019). Focusing personality assessment on the person: Modeling general, shared, and person specific processes in personality and psychopathology. Psychological Assessment, 31(4), 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ram N, Lougheed JP, Molenaar P, & Hollenstein T (2019). Adolescents’ emotion system dynamics: Network-based analysis of physiological and emotional experience. Developmental Psychology, 55(9), 1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ram N, Gest SD, Lydon-Staley DM, Conroy DE, Pincus AL, & Molenaar P (2018). Socioemotional Dynamics of Emotion Regulation and Depressive Symptoms: A Person-Specific Network Approach. Complexity, 2018, Article ID 5094179. doi: 10.1155/2018/5094179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ram N, & Molenaar P (2018). pompom: Person-Oriented Method and Perturbation on the Model. R Package version 0.2.0. Available from: https://cran.r-project.org/package=pompom [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


