Abstract
Introduction:
Colorectal cancer remains the second leading cause of cancer death in the United States, underscoring the need for novel therapies. Despite the successes of new targeted agents for other cancers, colorectal cancer suffers from a relative scarcity of actionable biomarkers. In this context, the intestinal receptor, guanylyl cyclase C (GUCY2C), has emerged as a promising target.
Areas Covered:
GUCY2C regulates a tumor suppressive signaling axis that is silenced through loss of its endogenous ligands at the earliest stages of tumorigenesis. A body of literature supports a cancer chemoprevention strategy involving reactivation of GUCY2C through FDA-approved cGMP-elevating agents such as linaclotide, plecanatide, and sildenafil. Its limited expression in extra-intestinal tissues, and retention on the surface of cancer cells, also positions GUCY2C as a target for immunotherapies to treat metastatic disease, including vaccines, chimeric antigen receptor T-cells, and antibody-drug conjugates. Likewise, GUCY2C mRNA identifies metastatic cells, enhancing colorectal cancer detection and staging. Pre-clinical and clinical programs exploring these GUCY2C-targeting strategies will be reviewed.
Expert Opinion:
Recent mechanistic insights characterizing GUCY2C ligand loss early in tumorigenesis, coupled with results from the first clinical trials testing GUCY2C-targeting strategies, continue to elevate GUCY2C as an ideal target for prevention, detection, and therapy.
Keywords: Colorectal cancer, guanylin, GUCY2C, hormone replacement, immunotherapy, linaclotide, plecanatide
1.0. Introduction
Since our previous review in 2017 [1], the relative incidence and mortality of colorectal cancer in the United States has remained unchanged - colorectal cancer represents the fourth most incident cancer and second leading cause of cancer death in men and women [2]. Widespread adoption of colonoscopy as a screening and preventative measure represents a success story, producing remarkable declines in colorectal cancer incidence through the past two decades. However, this decline has tapered as of 2016, and hides an alarming 2% annual increase in incidence among individuals <55 years of age since the mid-1990s [2]. Furthermore, a sizable fraction of patients (21%) still present with late-stage disease at the time of diagnosis, and the 5-year survival rate drops precipitously when comparing patients presenting with local disease (90% survival) to those presenting with metastases (14% survival)[3]. These latest statistics underscore the importance of early detection and the unmet need for late-stage therapies.
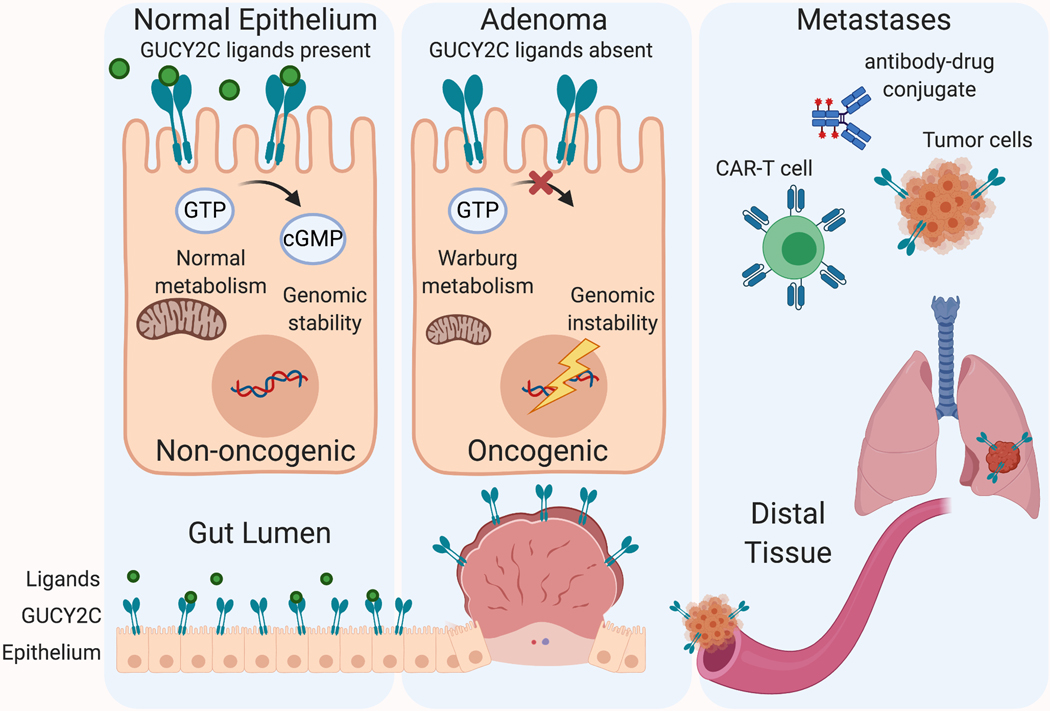
Here we highlight the most recent updates on preventative, diagnostic, and therapeutic approaches targeting the colorectal cancer biomarker, guanylyl cyclase C (GUCY2C). GUCY2C (also commonly known as GC-C, or the STa receptor, STaR) is a transmembrane receptor expressed on the surface of intestinal epithelial cells [4]. In normal physiology, it plays a tumor-suppressive role, restricting epithelial transformation through a variety of pathways regulated by its second messenger, cyclic guanosine monophosphate (cGMP) [5, 6, 7, 8, 9, 10, 11, 12]. In colorectal adenomas, loss of expression of the endogenous ligand for GUCY2C, guanylin, orphans the receptor, which is thought to contribute to tumor progression [13, 14, 15, 16, 17, 18]. Importantly, GUCY2C protein expression is retained in the primary tumor and metastases [16, 18, 19, 20, 21]. These features create an opportunity for (1) primary chemoprevention, through reactivation of the receptor with FDA-approved GUCY2C agonists, (2) immunotherapies targeting extra-intestinal GUCY2C as a tumor-specific antigen, and (3) detection of metastatic cells expressing the otherwise intestinally restricted protein (Figure 1). We will first briefly review the molecular features of colon cancer and the GUCY2C/cGMP signaling axis, and then describe efforts to target this axis for colorectal cancer therapy.
Figure 1.
GUCY2C-targeting strategies. GUCY2C is an intestinal epithelial receptor that regulates homeostatic circuits commonly dysregulated during tumorigenesis. The loss of GUCY2C ligands, silencing receptor signaling, represents an early and near-universal step in tumorigenesis, leading to genomic instability, metabolic reprogramming, and uncontrolled proliferation. Oral replacement of GUCY2C ligands represents a strategy for colorectal cancer chemoprevention. GUCY2C expression is retained on metastatic cancer cells, providing a biomarker that can be targeted for immunotherapies, including vaccines, CAR-T cells, immunotoxins.
2.0. Colorectal cancer genetics and targeted therapies
Since Vogelstein and Fearon first proposed their description of the adenoma carcinoma sequence, colorectal cancer has provided the prototypic example of an oncogenic mutational event [22]. This model proposes that colorectal cancer arises from series of sequential mutations in key growth-regulatory genes, corrupting normal intestinal epithelial renewal, and enabling the formation of an adenomatous polyp. The most commonly mutated gene in sporadic (non-hereditary) colorectal cancer is the tumor suppressor, adenomatous polyposis coli (APC), mutated in 80% of tumors [23]. APC serves several roles in the nucleus, including regulation of DNA damage repair, replication fork dynamics, and spindle assembly during cell division [24, 25, 26, 27]. However, its canonical role is in the cytoplasm, where APC serves as the scaffold for the sequestration, polyubiquitination, and proteasomal degradation of β-catenin, the transcriptional mediator of the pro-proliferative Wnt signaling pathway that regulates intestinal crypt-villus differentiation [28, 29]. Spontaneous mutation of one allele of APC produces allelic heterozygosity, and loss of the second allele (loss of heterozygosity) eliminates APC expression, permitting intracellular β-catenin accumulation, oncogenic transcription, and growth of a polyp. Accumulation of subsequent mutations in oncogenes, like KRAS (mutated in 43% of tumors), and in tumor suppressors, like TP53 (mutated in 64% of tumors), enable the progression from adenoma to carcinoma [23, 30]. These genetics also underlie a hereditary form of colorectal cancer, familial adenomatous polyposis (FAP), in which patients harbor a germline mutation in one allele of APC, develop hundreds of colorectal adenomas, and typically require full colectomy before the age of 40.
The availability of large datasets of sequenced tumors, such as The Cancer Genome Atlas, has refined our understanding of colon cancer to encompass a genetically heterogeneous disease that arises through three distinct mutational sequences (although considerable overlap between these pathways occurs, and acquired mutations frequently converge on key pathways, like Wnt signaling, regardless of the initiating mutations) [31]. APC loss is thought to be the driving mutation in the most common pathway, the chromosomal instability pathway (CIN; 65–70% of tumors), which is characterized by aneuploidy, insertions, deletions, loss of heterozygosity at tumor suppressor gene loci, and other alterations in large sections of DNA [32, 33]. CIN tumors harbor relatively few single base pair mutations, but do generally acquire KRAS and TP53 mutations.
Less frequently (<15%), colon tumors arise through the microsatellite instability pathway (MSI), defined by loss of DNA mismatch repair proteins, most commonly MLH1 or MSH2 [23, 31]. Their loss disrupts normal DNA repair, permitting the accumulation of mutations in short, repetitive DNA sequences called microsatellite sequences. These frequent DNA base pair mutations phenotypically distinguish MSI from CIN tumors, and contribute to a more rapid tumor progression (1–3 years, vs. 10 or more years for CIN tumors) [34]. MSI tumors also exhibit aberrant methylation of key DNA regulatory regions, described as the CpG island methylator phenotype (CIMP), producing epigenetic silencing of key tumor suppressor genes. MLH1 promoter methylation represents the most common driver of the MSI phenotype. Although most MSI tumors arise sporadically, the most common hereditary colon cancer syndrome, hereditary non-polyposis colorectal cancer (HNPCC, or Lynch syndrome), arises from germline mutations in MLH1 or MSH2.
Finally, the third pathway of tumorigenesis, the serrated neoplasia pathway, is named for the histologically distinct, sawtooth appearance of its polyps. Accounting for 15% of colorectal cancers, serrated polyps are highly associated with early mutations in BRAF, a relatively rare mutation in conventional colorectal tumors, suggesting a distinct route of tumor initiation [31, 35]. Subsequent mutations converge with the MSI or CIN pathways; serrated lesions acquire mutations in mismatch repair genes, producing an MSI phenotype, or mutations in TP53 and activation of oncogenic Wnt signaling with a microsatellite-stable phenotype.
Despite the relatively well-defined genetic basis for the disease, a persistent challenge in colorectal cancer management is the relative scarcity of effective drug targets [36]. Our current pharmacologic repertoire reflects this issue: nonsteroidal anti-inflammatory drugs (NSAIDs) represent the only widely accepted chemoprevention agents. Aspirin is the most studied NSAID in chemoprevention clinical trials, and the only agent accepted for non-hereditary colorectal cancer, but its attributable risk reduction is modest, and it is only recommended for patients with concomitant cardiovascular risk or Lynch syndrome [37]. Aspirin’s mechanism of cancer prevention remains debated, but it inhibits several pathways linked to tumorigenesis, including prostaglandin synthesis, inflammation, platelet activation, and β-catenin activity [37]. Non-aspirin NSAIDs, including sulindac and celecoxib have also been shown to reduce colorectal polyp number, although gastrointestinal and cardiovascular safety concerns have relegated their use to highest-risk populations, such as patients with FAP [37]. The mainstay of tumor prevention remains modification of risk factors (smoking, alcohol consumption, body mass index) and removal of precancerous adenomatous polyps during colonoscopy.
With regards to advanced disease, the antimetabolite, 5-fluorouracil (5-FU), and vitamin, leucovorin, have remained the backbone of first-line chemotherapy since the early 1990s [38]. Their combination with oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) are the current standard. These chemotherapeutic agents broadly target replicating cells, including both cancer and normal cells. In contrast, targeted therapies recognize features unique to cancer tissue, ideally limiting off-target cell-death. FDA-approved agents for targeted therapy include agents opposing angiogenesis (bevacizumab), immune checkpoints (pembrolizumab and nivolumab), and oncogenic pathways such as those regulated by the epidermal growth factor receptor (EGFR; cetuximab and panitumumab) and BRAF (vemurafenib) [36]. The molecular profile of a tumor dictates the choice of targeted agent. For example, EGFR inhibitors have proven effective in the treatment of metastatic colorectal cancer because cancer cells often depend on the proliferation, angiogenesis, and survival signals generated by EGFR. However, these agents are ineffective for the large percentage of tumors harboring mutant KRAS or BRAF mutations because these mutations constitutively activate the oncogenic signaling circuits downstream of EGFR. Likewise, the use of BRAF inhibitors is restricted to the 8–12% of colon tumors harboring activating mutations in BRAF, and immune checkpoint inhibitors, although lauded in other cancers, only appear to benefit the 3–7% of patients with the microsatellite instability-high phenotype [36]. Despite these promising new agents, as noted in the introductory statistics, patients with metastatic disease are rarely cured. The identification of actionable molecular targets remains of critical importance.
3.0. The intestinal GUCY2C/cGMP signaling axis
Recent studies have revealed a role for the intestinal epithelial receptor, GUCY2C, as a novel target for colorectal cancer therapy. Canonically involved in intestinal fluid secretion [39], our understanding of the axis has evolved to include roles in epithelial proliferation and apoptosis [5, 6, 15, 40], DNA damage response [8, 10, 41, 42], GI barrier integrity [7, 8, 43], epithelial-mesenchymal cross-talk [11], inflammation [44], satiety [45, 46], and other aspects of epithelial homeostasis [9]. Importantly, GUCY2C is ubiquitous throughout the intestine and retained on primary tumor and metastatic tissue derived from the intestine, significant features for diagnostic and therapeutic goals [19, 20, 21].
3.1. Canonical biology of the GUCY2C/cGMP axis
Guanylyl cyclases are an enzyme class responsible for the catalysis of guanosine triphosphate (GTP) to the second messenger, cGMP [4, 47]. GUCY2C is a transmembrane receptor expressed on the brush border of the intestinal epithelium. Its endogenous ligands, collectively known as guanylins, are small peptide hormones secreted by the epithelium into the intestinal lumen. Uroguanylin, secreted in the small intestine, and guanylin, secreted in the large intestine, are synthesized as propeptides and processed into 16-mer and 15-mer mature peptides, respectively [48, 49]. Both mature peptides harbor two disulfide bonds, conferring stability in the intestinal lumen. This paracrine hormone axis, regulated by the guanylins and GUCY2C, canonically controls intestinal secretion. Agonist stimulation of the GUCY2C extracellular ligand binding domain activates its intracellular catalytic domain, leading to the production of cGMP. cGMP in turn targets several effector proteins, including cGMP-dependent protein kinases (PKGs), phosphodiesterases (PDEs), and cGMP-gated ion channels [4]. Direct activation of PKG II, as well as indirect activation of protein kinase A, drives phosphorylation and translocation of the cystic fibrosis transmembrane regulator (CFTR) to the cell surface [50, 51, 52]. Simultaneously, PKG inhibits the apical Na+/H+ exchanger (NHE3) also present on the cell surface [50, 51, 52]. Together, modulation of these channels results in HCO3− and Cl− secretion, with Na+ retention in the intestinal lumen; this osmotic gradient results in fluid secretion into the intestine.
Disorders of the GUCY2C/cGMP signaling axis result in intestinal secretion syndromes. GUCY2C was originally discovered as the receptor for the bacterial heat stable enterotoxins, STs, the agents responsible for traveler’s diarrhea [39]. STs are a family of peptides sharing a conserved C-terminal region, which is processed to a mature peptide with three disulfide bonds conferring heat and pH stability [4, 53]. Enterotoxigenic bacteria, such as E. coli, K. pneumonia, V. cholera, and Y. enterocolitica, colonize the gut and secrete these peptides, which have high affinity for the GUCY2C extracellular domain and produce uncontrolled CFTR-mediated secretion, manifesting as secretory diarrhea [4, 53]. In addition to aberrant agonist stimulation, mutations in GUCY2C itself have been reported in small populations, resulting in hereditary hypo- and hyper-secretion syndromes. The recently described familial GUCY2C diarrhea syndrome arises from a single missense mutation in the GUCY2C catalytic domain, producing receptor hyperactivation [54, 55, 56]. Conversely, hereditary inactivating mutations in the ligand-binding and catalytic domains produce meconium ileus (neonatal intestinal obstruction), due to diminished intestinal secretion and stool motility [57, 58].
3.2. GUCY2C/cGMP axis and colorectal cancer
Beyond regulating fluid secretion, the GUCY2C/cGMP axis has emerged as a regulator of homeostatic circuits dysregulated during tumorigenesis. For example, evidence from genetic mouse models suggests that cGMP signaling regulates the proliferation, differentiation, and turnover circuits that underlie normal epithelial renewal in the intestine. Genetic ablation of guanylin, GUCY2C, or the downstream effector, PKGII, produces mice with crypt abnormalities, including hyperproliferation, expansion of the pool of transit amplifying cells, and loss of differentiation of the secretory cell lineage [6, 15, 59]. GUCY2C−/− mice also exhibit changes in the relative populations of crypt stem cells, with an increase in the reserve BMI1+ cells responsible for crypt injury response [60]. Transcriptomic profiling of the intestinal epithelium from GUCY2C−/− mice suggests that this phenotype reflects activation of the pro-proliferative AKT signaling pathway, resulting in reprogramming to a glycolytic metabolism, commonly associated with oncogenic processes [12]. In turn, activation of GUCY2C signaling opposes these processes through PTEN, the canonical inhibitor of AKT [12]. Furthermore, cGMP regulates proliferation through cell cycle arrest. Intestinal epithelium from GUCY2C−/− mice exhibits accelerated progression through the G1/S transition, and altered expression of regulators of the cell cycle (e.g. pRb, CDK4, cyclin D1, p27) [6]. Administration of agents that increase intracellular cGMP, including ST, uroguanylin, cell-permeable cGMP, or the PDE inhibitor, zaprinast, slow cell cycle progression in human colorectal cancer cell lines and mice [6, 8, 61].
In addition to opposing pro-proliferative circuits in the gut, the GUCY2C/cGMP axis also regulates DNA damage sensing and repair. Mice harboring one mutant allele of APC (the APCmin/+ mouse model) develop spontaneous intestinal tumors, mimicking the driving mutation of human colorectal cancer. Concomitant deletion of GUCY2C in these mice increases the frequency of DNA double strand breaks, genomic instability, APC loss of heterozygosity, and tumorigenesis [8, 42]. Likewise, in a chemical model of tumorigenesis, where mice are exposed to the carcinogen, azoxymethane, GUCY2C deletion exacerbates tumorigenesis [42]. This phenotype in part reflects increased oxidative DNA damage arising from metabolic reprogramming and ROS generation in proliferative cells. But it was recently reported that GUCY2C−/− mice also exhibit an impaired response to genotoxic injury, such as ionizing radiation, through a mechanism mediated by p53 [10]. Oral administration of the GUCY2C ligand, ST, reduced gastrointestinal radiation toxicity, including a reduction of double strand breaks, aneuploidy, and mitotic disruption, suggesting that cGMP circuits directly oppose hallmarks of chromosomal instability.
Silencing of cGMP signaling represents an early and near universal feature of colorectal cancer. Suppression of cGMP effectors have been widely reported, including downregulation of PKGI expression and upregulation of PDEs that hydrolyze cGMP [62, 63, 64, 65]. Interestingly however, GUCY2C is retained throughout tumor progression, and its normal cellular localization remains intact [16, 18, 20, 21, 66, 67]. Furthermore, GUCY2C mutations are rare, suggesting that the receptor remains functional; only 4% (22/537) of colorectal tumors in The Cancer Genome Atlas harbor GUCY2C mutations (TCGA-COAD and -READ datasets)[68]. Bashir et. al. recently observed a nuance to this observation however; while GUCY2C expression is retained in the most frequent histological and molecular subtypes of colon cancer (conventional and microsatellite unstable tubular adenomas), expression is lost in human and mouse serrated adenomas [18]. Loss of GUCY2C expression in these tumors arises from loss of CDX2 expression, a necessary transcription factor for GUCY2C [69].
Although GUCY2C itself is largely spared, the process of transformation orphans the receptor through near-universal loss of its ligands. Guanylin and uroguanylin are among the most commonly lost gene products in colorectal cancer; the loss of their paracrine signaling is thought to be the dominant mechanism of silencing cGMP signaling during tumorigenesis [13, 14, 66, 70, 71]. Hormone loss is conserved across mice and humans, and occurs early in tumorigenesis, at the precancerous adenoma stage [16, 18]. The loss of guanylin was recently attributed to the altered transcriptional program downstream of mutant APC [16]. Conditional biallelic deletion of Apc in mice eliminates guanylin expression and downstream cGMP signaling within days. Conversely, re-expression of wild type APC in human colorectal cell lines reconstitutes guanylin expression, suggesting that hormone loss may be a reversible phenomenon, controlled by mutant Wnt signaling. These observations lead to a tempting hypothesis that GUCY2C ligand insufficiency silences the tumor suppressive functions of cGMP signaling, producing a microenvironment conducive to transformation.
4.0. Targeting GUCY2C/cGMP for colorectal cancer chemoprevention
The loss of GUCY2C activating ligands in tumorigenesis, coupled with retention of the receptor in the most common tumor subtypes, lends itself to a paradigm of pharmacologic reconstitution of cGMP signaling. Two primary approaches have been explored in pre-clinical and clinical settings – (1) administration of GUCY2C ligands, aiming to replace those lost in the course of disease, and (2) administration of small molecule PDE inhibitors, opposing the hydrolysis of intracellular cGMP.
4.1. GUCY2C ligand replacement
The disappearance of the endogenous GUCY2C ligands, guanylin and uroguanylin, coupled with retention of the receptor on the luminal aspect of the epithelium, creates an opportunity for oral hormone replacement therapy. The earliest evidence of efficacy comes from the observation that APCmin/+ mice fed uroguanylin in the diet develop fewer intestinal adenomas [66]. Subsequent studies have demonstrated that restoration of cGMP signaling with natural GUCY2C ligands has a tumor suppressive effect. For example, transgenic guanylin opposes tumorigenesis associated with diet-induced obesity in mice [72]. Likewise, mice colonized with E. coli engineered to express ST develop fewer tumors than those colonized with wild type E. coli [73]. Indeed, administration of GUCY2C agonists has been widely shown in cell and mouse models to oppose the oncogenic circuits regulated by cGMP, such as proliferation [5, 6, 61], matrix remodeling [74, 75], DNA damage sensing [10], and metabolic reprogramming [12].
FDA-approved synthetic GUCY2C agonists represent a promising new avenue to translate cGMP-dependent chemoprevention into man. Two agents have been approved, both targeting the canonical secretory function of GUCY2C for the treatment of chronic idiopathic constipation and constipation predominant irritable bowel syndrome [53]. Linaclotide (Linzess; Ironwood Pharmaceuticals), approved in 2012, is a 14 amino acid analog of ST [76]. Like ST, it is stabilized by three disulfide bonds and differs only by a single tyrosine/leucine substitution. Plecanatide (Trulance; Synergy Pharmaceuticals), approved in 2017, is a 16 amino acid analog of uroguanylin, again stabilized by two disulfide bonds and differing only by a single aspartate/glutamate substitution [77]. Both agents have been explored in pre-clinical chemoprevention studies. A recent report in APCmin/+ mice showed that linaclotide administered in drinking water reduced polyp counts by 67%, and reproduced features of cGMP signaling, including a reduction of crypt proliferation and restoration of differentiation of the secretory cell lineage [78]. Likewise, in a model of inflammation-induced tumorigenesis (mediated by dextran sodium sulfate, DSS) orally-administered plecanatide opposed the formation of dysplastic lesions [79].
These observations formed the basis for the first clinical trial in a small human cohort examining colorectal bioactivity of linaclotide [80]. Linaclotide is formulated for gastric release, and perhaps unsurprisingly, failed to stimulate colorectal cGMP production at baseline; however, in patients administered a polyethylene glycol bowel preparation, linaclotide stimulated cGMP synthesis and markers of epithelial cGMP activity, including phosphorylation of VASP and reduced expression of the epithelial proliferation marker, Ki67. The authors speculated that the bowel prep flushed active agent further down the intestinal tract. Promisingly, the findings suggested that signaling mechanisms reported in mice are likely conserved in humans. A subsequent trial evaluating oral linaclotide bioactivity in patients with a history of colorectal adenoma or carcinoma is ongoing, with a predicted completion date in 2021 (NCT03796884). Study participants receive oral linaclotide or placebo daily for seven days, followed by standard of care colonoscopy or surgical resection of tumors. Cyclic GMP, GUCY2C, guanylin, and Ki67 levels will be assessed in biopsy specimens of tumor and matched normal adjacent tissue.
Several potential obstacles to the translation of GUCY2C agonists for chemoprevention are worth noting. While oral GUCY2C agonists have been shown to reduce intestinal polyp burden in mice, it remains an open-ended question if the utility of these agents lies in tumor prevention or tumor therapy. In part, this question remains unanswered due to limitations of colon cancer mouse models, which do not fully recapitulate human disease progression; the most-widely used model, the APCmin/+ mouse, succumbs to disease before adenomas progress to invasive carcinoma. Pathologic reorganization of the epithelium and invasion beyond the basement membrane may make the GUCY2C ligand binding domain inaccessible to peptides in the intestinal lumen. Hence, while oral GUCY2C agonists have the potential to restore cGMP signaling in the colonic epithelium in the early stages of disease, their limited systemic absorption may restrict utility as the disease progresses. An additional obstacle is the relatively common side-effect of diarrhea in patients receiving linaclotide or plecanatide. A recent meta-analysis reported rates of diarrhea ranging from 3.2–22%, depending on the study’s definition of diarrhea [81]. This side-effect is consistent with their mechanism of action as anti-constipation agents, and dose-reduction may be a reasonable means of management [81]. Given these obstacles, at-risk patient populations, such as those with a genetic predisposition (i.e. FAP or Lynch syndrome), or a history of previous polyps, may be the best candidates for chemoprevention with GUCY2C agonists.
4.2. Phosphodiesterase inhibitors
An alternative approach to chemoprevention recognizes the role of PDEs in antagonizing GUCY2C signaling through the hydrolysis of cGMP [82]. PDE5 and PDE10 overexpression has been observed in colorectal cancer cell lines, biopsy samples, and tumors from mice, representing a potential mechanism of silencing cGMP signaling [62, 63, 64, 65]. Similar to observations in cell and mouse models treated with GUCY2C agonists, administration of PDE inhibitors increases homeostatic signaling by cGMP, including opposing epithelial proliferation and inducing apoptosis [40, 62, 63, 64, 65]. Importantly, PDE inhibitors exhibit systemic bioavailability, enabling treatment of cancer cells that may be inaccessible to GUCY2C agonists. Co-administration of PDE inhibitors and GUCY2C ligands may represent a means to amplify cGMP signaling beyond that of a single agent alone.
Initial efforts to test PDE inhibition for chemoprevention in humans focused on the pan-PDE inhibitor, exisulind, in patients with FAP and sporadic adenomas [83, 84, 85]. Although exisulind treatment resulted in polyp regression, these trials were largely abandoned as a result of significant hepatotoxicity. Another agent, the non-aspirin NSAID, sulindac, has been shown to reduce polyp formation in patients with FAP, although its use is restricted by gastrointestinal toxicity arising from cyclooxygenase(COX)-inhibition [86, 87]. The chemopreventative effects of sulindac may be mediated by a COX-independent mechanism involving PDE inhibition and activation of cGMP signaling [65, 88]. Sulindac derivatives retaining PDE-inhibitory activity and lacking COX-inhibitory activity have been proposed for chemoprevention without gastrointestinal toxicity [89]. Recent efforts have focused on inhibition of PDE5 with sildenafil (Viagra), which benefits from decades of use and a well-documented safety profile. In APCmin/+ mice, sildenafil administered in drinking water reduced the number of polyps by 50% [78]. Similar results were observed in an inflammatory model of tumorigenesis, where sildenafil administration opposed intestinal inflammation and polyp multiplicity [90]. Interestingly, the anti-neoplastic effect of sildenafil was only observed when administered prior to the neoplastic insult (azoxymethane), consistent with reported geno-protective roles of cGMP.
Despite the body of pre-clinical evidence supporting the potential of cGMP elevating agents for chemoprevention, the first (and only) study to address the relationship between tumor incidence and cGMP-elevating agents was just published in 2019 [91]. This nationwide retrospective cohort study in Sweden examined the incidence of colorectal cancer in 36,020 men, of which 4849 were prescribed a PDE inhibitor during the ten-year study period (2005–2015). Encouragingly, patients prescribed a PDE inhibitor had a significantly lower incidence of colorectal cancer (hazard ratio = 0.65; 95% CI, 0.49–0.85). While these results do not imply causation, they certainly support further investigation of chemoprevention targeting the GUCY2C/cGMP axis.
5.0. GUCY2C-targeted immunotherapies for metastatic colorectal cancer
While cancer prevention remains the clinical ideal, patients frequently present with late-stage disease, for which existing therapies are inadequate. The five-year survival for patients presenting with late stage colorectal cancer, for example, remains less than 10%. This past decade has witnessed a transformation in the field of oncology with the introduction of cancer immunotherapies, which harness the body’s immune system to target neoplastic tissue. Despite successes in other cancers, results for colorectal cancer have been mixed, with the most common subtypes proving stubbornly unresponsive. For example, immune checkpoint inhibitors, which prevent neoplastic tissue from evading immune surveillance, have proven very successful for MSI tumors (69–77% disease control rates)[92]. MSI tumors are characterized by a high mutational burden, expression of abnormal/immunogenic proteins, and comparatively high cytotoxic T cell and natural killer cell infiltration of the tumor. However, the MSI phenotype is only observed in 15% of sporadic colorectal cancers, requiring alternative approaches for patients presenting with the most common forms of the disease. Thus, beyond checkpoint blockade for patients with metastatic MSI colorectal cancer, no immunotherapies exist for primary or secondary prevention of colorectal cancer, or for the treatment of metastatic MSS colorectal cancer.
The unique anatomic characteristics of GUCY2C make it an ideal antigenic target for cancer immunotherapies. As described above, GUCY2C is expressed primarily by the intestinal mucosa. The extracellular domain is distinct from other guanylyl cyclases and faces the lumen of the intestine, an “immune privileged” site inaccessible by the systemic immune system. These key features limit the potential for autoimmune toxicity from GUCY2C-targeted agents in systemic circulation [93, 94, 95]. Furthermore, GUCY2C expression is retained throughout tumorigenesis, in the majority of molecular subtypes, and carried by metastatic cells to distal sites, making it a tumor-selective target antigen for recognition by the immune system [18, 19, 20, 21]. These features serve to maximize the potential targetability of metastatic cells, while minimizing autoimmunity against normal tissue. Approaches to targeting GUCY2C fall into three major strategies: vaccines, adoptive T cell therapies, and immunotoxins.
5.1. GUCY2C vaccines
Cancer vaccines consist of a tumor antigen and an adjuvant designed to stimulate an immune response against tumor cells [96]. These vaccines can serve a therapeutic role for patients presenting with active metastatic disease, stimulating the expansion of the adaptive immune response (i.e. CD4+ T-helper cells, cytotoxic CD8+ T cells, and antibody responses) to destroy existing tumor cells [97]. Vaccines can also serve a preventative role, priming the immune system to protect against future tumor development or recurrence.
A cancer vaccine targeting GUCY2C was first described in 2008 and incorporated the extracellular domain of the receptor in a replication-deficient type 5 recombinant adenovirus (Ad5-GUCY2C)[93]. In a proof-of-concept study in mice, vaccination with Ad5-GUCY2C stimulated a cytotoxic CD8+ T-cell response against GUCY2C-expressing cancer cells, eliminated metastases in the liver and lung (the most common sites of human colorectal metastases), and extended survival without autoimmunity [93, 94]. Unexpectedly, immunization of wild type and Gucy2c−/− mice produced striking differences in the immune response: both mice generated a CD8+ T cell response; however, robust CD4+ and B-cell responses, and superior anti-tumor efficacy, could only be generated in Gucy2c −/− mice [95]. Helper CD4+ T cells play a necessary role in priming CD8+ T cell cytolytic activity, generating CD8+ T and B-cell memory responses, and inducing antibody production. These studies revealed a selective CD4+ T-cell tolerance, the specific elimination of CD4+ T cells recognizing the GUCY2C antigen, which limited vaccine efficacy in GUCY2C wild type animals. To overcome this barrier, the vaccine was modified to include an influenza epitope (S1) known to stimulate CD4+ T-cell responses (Ad5-GUCY2C-S1) [95]. The new vaccine generated CD4+ T-cell responses, long-lived memory CD8+ T cells, antibodies, and enhanced anti-tumor immunity in wild type animals. Subsequent studies further refined the vaccine efficacy by incorporating a prime-boost regimen, where animals were first vaccinated with a GUCY2C-DNA vaccine to “prime” the immune response, followed by Ad5-GUCY2C-S1 vaccination to “boost” GUCY2C-specific immunity [98]. Such an approach enhanced CD8+ T cell counts and antitumor efficacy due to the induction of a more potent T cell pool with higher T-cell receptor avidity.
A modified GUCY2C vaccine was developed for testing in human clinical trials, substituting S1 with a different helper epitope, the pan HLA DR-binding epitope (PADRE)[99]. Efficacy and immunogenicity of the new vaccine, Ad5-GUCY2C-PADRE, were evaluated in a phase I study in a small cohort of ten colorectal cancer patients.[100] Ad5-GUC2C-PADRE was well tolerated, with no toxicity in GUCY2C-expressing tissues, or significant adverse events. Four of the ten patients developed GUCY2C-specific CD8+ T-cell responses, but none developed GUCY2C-specific CD4+ T-cell responses, recapitulating the GUCY2C-specific CD4+ T-cell tolerance observed in mice. Interestingly, pre-existing immunity to Ad5 opposed the development of a GUCY2C-immune response in some patients. Ad5 is a relatively common respiratory pathogen, and the acquisition of neutralizing antibodies against the vaccine vector may represent a barrier to efficacy. A subsequent iteration of the vaccine incorporates a chimeric adenoviral vector derived from Ad5 in which the fiber protein has been replaced with that of the rare Ad35 serotype, limiting neutralization by Ad5-fiber-specific antibodies (Ad5.F35-GUCY2C-PADRE) [101]. A Phase II trial (NCT04111172) testing this new vaccine format for secondary prevention of recurrence in colorectal, esophageal, gastric, and pancreatic cancer patients is scheduled to start in 2020.
5.2. GUCY2C chimeric antigen receptor (CAR) T cell therapy
While vaccines may be effective in primary or secondary prevention strategies, their utility in treating metastatic disease in patients is likely to be low, necessitating the development of more robust therapies for active disease. CAR-T cell therapies are a form of adoptive cell therapy in which a patient’s T cells are isolated, modified ex vivo to express an engineered receptor recognizing a tumor antigen, and re-introduced into the patient [102]. The engineered receptor is typically a chimeric antigen receptor (CAR), consisting of an antigen-specific antibody, coupled with intracellular T-cell receptor signaling motifs that activate cytolytic killing upon binding the tumor antigen [103]. Importantly, the CAR recognizes antigens in an MHC/HLA-independent fashion and theoretically can be designed with antibodies targeting any surface antigen. Two CAR-T therapies have been FDA approved, both targeting the B-cell antigen CD19: Tisagenlecleucel (Kymriah) for refractory acute lymphoblastic leukemia, and Axicabtagene ciloleucel (Yescarta) for refractory diffuse large B-cell lymphoma. Although a breakthrough for hematological malignancies, no CAR-T therapies have yet been approved for colorectal cancer, or for solid tumors in general [102].
The first description of GUCY2C-targeted CAR-T cell therapy was reported in 2016 [104]. The proof-of-concept study compared the safety and efficacy in mice, of CAR constructs employing two different antibodies against the extracellular domain of mouse GUCY2C. The GUCY2C-directed CAR-T cells specifically lysed colorectal cancer cell lines expressing GUCY2C, and in a colorectal lung metastasis model, the CAR-T cells harboring the higher-affinity receptor effectively cleared metastases and prolonged survival. Animals receiving the control CAR-T cells died within 30 days, but 25% of animals receiving the GUCY2C-directed CAR-T cells survived beyond 200 days. Importantly, no CAR-T-cell-mediated autoimmunity or intestinal infiltration was observed. A subsequent study employing CAR-T cells targeting human GUCY2C reported similarly efficacious results in mice with human colorectal cancer xenografts [105].
5.3. GUCY2C immunotoxins
Immunotoxins consist of an antibody conjugated to a cytotoxic agent, enabling targeting, endocytic uptake, and intracellular delivery of the agent to tissues expressing the target antigen [106]. Compared to traditional chemotherapeutics, antibody-drug conjugates can overcome widespread systemic toxicity through targeted delivery to tumor cells and overcome intracellular efflux pumps (a common drug resistance mechanism) through endocytic uptake [107]. Spurred by the success of monoclonal antibodies as therapeutic agents, the growing class of antibody-drug conjugates now includes six agents FDA approved for breast and hematologic malignancies [108].
Of all GUCY2C-targeted therapeutics, immunotoxins have progressed the furthest in clinical trials. Despite initially promising results however, the most recent Phase II trials were halted due to lack of efficacy, and the status of these agents remains to be seen. The first GUCY2C-immunotoxin, described in 2014, consisted of a GUCY2C antibody linked to a ricin toxin payload [109]. The immunotoxin was shown to target GUCY2C, undergo endocytosis, and deliver its payload to the lysosome in colorectal cancer cell lines in vitro. In mouse models of colorectal lung metastases, the immunotoxin prolonged survival by 25% without systemic toxicity. A subsequent GUCY2C-immunotoxin design, TAK-264, consisted of a human IgG1 monoclonal antibody to GUCY2C conjugated via a protease-cleavable linker to monomethyl auristatin E, an anti-mitotic agent [110]. A Phase I safety and tolerability trial of TAK-264 enrolled 41 patients with advanced GUCY2C-positive gastrointestinal malignancy (85% were metastatic colorectal cancer patients) [110]. The dose-escalation study identified a maximum tolerated dose of 1.8 mg/kg, with a manageable safety profile; 41% of patients reported drug-related Grade ≥ 3 adverse events, most commonly neutropenia. Two subsequent phase II trials were initiated, enrolling patients with GUCY2C+ gastric and gastroesophageal junction adenocarcinoma [111], or GUCY2+ pancreatic adenocarcinoma [112]. Unfortunately, both trials were discontinued due to lack of efficacy (6% and 3% clinical response rates, respectively). The authors speculated that drug potency, linker chemistry, tumor penetration, and/or cell internalization kinetics may have contributed to poor efficacy. It remains to be seen if TAK-264, or subsequent GUCY2C-immunotoxin design, will demonstrate efficacy in colorectal cancer patients.
6.0. GUCY2C as a colorectal cancer biomarker
Decisions regarding the course of colorectal cancer treatment rely heavily on the initial survey of disease progression at the time of diagnosis. Staging refers to the evaluation of the extent of primary tumor invasion through the bowel wall, and the spread to nearby lymph nodes and extraintestinal tissues. While patients presenting with cancer restricted to the bowel wall (stage I and II) can often be cured with surgery alone, the detection of metastatic cells in lymph nodes (stage III) often requires aggressive treatment including adjuvant or neoadjuvant chemotherapy [113]. Traditional staging involves histopathological examination of resected lymph nodes, which is subject to several limitations. A recent report suggested that less than 0.01% of available tissue is typically reviewed, and undetected metastatic cells lead to disease recurrence in death in as many as 25% of histologically-node negative patients [113].
The features that make GUCY2C an appealing immunotherapeutic target have also been leveraged to improve disease detection. GUCY2C mRNA and protein are expressed predominantly by the intestine and are retained by cancer cells. As metastatic cells seed extraintestinal tissues, the selective expression of GUCY2C mRNA in the cancer cells can be used to identify occult disease. This principle was first proposed in 1996, when the authors detected GUCY2C mRNA in colorectal tumors and patient blood samples by polymerase chain reaction (PCR) [20]. A subsequent retrospective study examined preserved lymph node samples from patients with histologically node-negative disease [21]. GUCY2C mRNA could be detected by reverse transcriptase PCR (RTPCR) in samples from all patients that ultimately developed disease recurrence (21/21), but not from those without recurrence. Finally, a prospective trial across nine centers enrolled 257 patients with node-negative colorectal cancer, and examined time-to-recurrence relative to GUCY2C mRNA expression [114]. 225/257 patients had lymph nodes positive for GUCY2C, and 20.9% developed recurrent disease in a five year period between 2002–2007. Conversely, 93% of patients with lymph nodes negative for GUCY2C remained disease-free, confirming GUCY2C mRNA as a sensitive staging tool and prognostic marker of disease recurrence. Molecular staging by GUCY2C RTPCR has since been independently validated by multiple laboratories [115, 116, 117].
7.0. Conclusion
Colorectal cancer remains the second leading cause of cancer death in the United States, underscoring an unmet need for new preventative and therapeutic tools. Recent studies have elevated the intestinal receptor, GUCY2C, as a desirable target for preventative, diagnostic, and therapeutic agents. The GUCY2C/cGMP signaling axis has emerged as a putative tumor suppressor, opposing proliferative, metabolic, genomic instability, and other oncogenic circuits that contribute to tumorigenesis. The loss of GUCY2C endogenous ligands represents an early and near-universal feature of the disease, silencing the GUCY2C/cGMP axis and potentially lifting a block on tumor progression. Retention of wild type GUCY2C expression by healthy and cancer tissue creates a new chemoprevention paradigm consisting of exogenous oral hormone replacement to enhance cGMP generation, or downstream inhibition of cGMP hydrolysis by phosphodiesterases. Synthetic GUCY2C ligands and phosphodiesterase inhibitors are already FDA approved, and a clinical program testing one of these, linaclotide, is underway. Furthermore, the dichotomous GUCY2C expression profile between metastatic cells and extraintestinal tissue has made the receptor amenable for an array of immunotherapies, including vaccines, CAR-T cells, and antibody drug conjugates, all in varying stages of clinical translation. Preclinical and clinical evidence suggests that targeting GUCY2C for immunotherapy can be done safely without autoimmunity. Finally, GUCY2C mRNA is a demonstrated clinical biomarker for occult disease, offering improved sensitivity and staging over traditional histopathology. Taken together, the unique features of GUCY2C and its downstream signaling axis provide a promising platform for a new class of targeted therapies for the most common forms of colorectal cancer.
8.0. Expert Opinion
A recent special issue of Gastroenterology (Volume 158, Issue 2) summarized our current understanding of the pathophysiological mechanisms contributing to tumorigenesis and the latest advances in therapeutic approaches. The authors frequently noted two key observations: (1) the success of colonoscopy adoption in reducing the incidence of colorectal cancer over the last two decades, and (2) the relatively small fraction of colorectal cancer patients appearing to benefit from the new targeted agents that have generated excitement for other cancers.
Preventative strategies represent the clinical ideal. Like the Pap smear for cervical cancer, colonoscopy is one of the rare screening measures able to reduce the incidence of disease by removing lesions before they progress to cancer. Screening prevalence in individuals aged 50 years and older has increased from 38% to 66% since 2000, paralleling declines in mortality of 3% per year for much of that time [3]. Less invasive screening approaches have also entered clinical use, such as the fecal Cologuard test, which detects mutant KRAS, promoter methylation of BMP3 and NDRG4, and hemoglobin [118]. Additional assays, including a recently approved blood test for SEPT9 DNA and investigational circulating tumor cell assays, add more promising options to the screening toolbox [119, 120].
Despite screening, patients frequently present with advanced disease requiring a combination of surgical, radio-, and chemotherapeutic approaches. First-line chemotherapeutic approaches, such as the fluoropyrimidines used for colorectal cancer, broadly target dividing cells with considerable off-target effects. In contrast, much enthusiasm surrounds precision medicine, the effort to tailor treatment to cancer- and patient-specific features. Progress has been mixed for colorectal cancer. The molecular phenotyping of colorectal tumors for specific markers, including MSI-status and somatic mutations in BRAF and KRAS, now plays a central role in guiding therapeutic decisions. For example, KRAS mutations predict a lack of benefit from anti-EGFR therapies, but a subset of BRAF/KRAS wild type tumors harbor HER2/neu amplifications and benefit from anti-HER2 therapies more commonly used with HER2/neu-amplified breast cancer [92]. Significantly, patients with treatment-refractory MSI-high tumors have seen remarkable benefit from immune checkpoint inhibitors (69–77% disease control rates). These agents are even being explored for chemoprevention in patients with Lynch Syndrome, the hereditary predisposition to MSI-tumors (NCT03631641). But these represent a small fraction of colorectal cancers; while remarkable strides have been made in identifying and targeting new biomarkers in other cancers, actionable targets for the most common forms of colorectal cancer remain stubbornly elusive.
In part, this challenge reflects the genetic heterogeneity of colorectal cancer; precision therapy may necessarily consist of an array of agents tailored to individual tumor genotypes. But the challenge also reflects an incomplete understanding of pathophysiological mechanisms driving tumorigenesis. Despite acquiring many mutations over the course of disease progression, colorectal tumors are thought to primarily arise from a specific few – BRAF, MLH1, MSH2, and most commonly APC. The steps between these initial “hits” and progression to cancer continue to be refined. In this context, GUCY2C, and its downstream cGMP-dependent signaling axis, has emerged as one of the earliest tumor suppressive circuits dysregulated in cancer. The loss of the endogenous GUCY2C-activating ligand in the colon, guanylin, has been observed in tumors arising from the three major genetic pathways (CIN, MSI, serrated) [18], and was recently directly attributed APC loss [16], therefore proceeding, and possibly enabling, the subsequent proliferation and genomic instability underlying tumorigenesis. Reactivation of GUCY2C/cGMP signaling with FDA-approved GUCY2C ligands or downstream inhibitors of cGMP-degrading phosphodiesterases represents an exciting new chemoprevention paradigm that may be applicable to most colorectal cancers.
There has been some debate in the field regarding the precise timeline of events linking mutant APC to GUCY2C-inactivation. One hypothesis suggests that GUCY2C ligand loss precedes and contributes to APC loss of heterozygosity. This hypothesis is supported by the observations that GUCY2C/cGMP signaling opposes genomic instability [8, 10, 41, 42], GUCY2C−/− mice acquire more APC loss-of-heterozygosity [42], and several APC-independent conditions that predispose to tumorigenesis also lead to GUCY2C-hormone (e.g. inflammation, obesity) [44, 121, 122], potentially creating an environment conducive to APC loss. Indeed, a recent study in mice indicated that stimulation of cGMP signaling with sildenafil most-effectively opposed tumorigenesis when administration preceded the genotoxic insult (azoxymethane), suggesting that cGMP protected the mice from the insult [90]. However, an alternative hypothesis suggests that APC loss of heterozygosity precedes guanylin loss, necessarily lifting a barrier to tumorigenesis imposed by GUCY2C. A recent pair of studies demonstrated that guanylin is comparably expressed in wild type and APCmin/+ mice, and is only lost following biallelic APC deletion, suggesting that guanylin expression is unable to prevent sporadic APC loss of heterozygosity [16, 17]. Reconstitution of wild type APC in colorectal cancer cells was sufficient to restore guanylin expression in vitro, supporting the hypothesis that APC loss drives guanylin loss [16]. From a therapeutic perspective, these observations suggest that colorectal cancer chemoprevention by targeting GUCY2C may not oppose the initiating APC mutation leading to transformation, but may help retain normal epithelial homeostasis despite these mutations. Given that the majority of polyps take years to progress to carcinoma, and most naturally regress, GUCY2C-reactivation could tip the scale further towards polyp regression. The necessity of guanylin loss for tumorigenesis to proceed would fundamentally reframe the nature of colon cancer from a disease of genetic mutation, to a disease of hormone insufficiency.
The same feature that makes GUCY2C amenable to pharmaceutical reactivation - its retention on cancer cells - has also made it desirable as a biomarker and immunotherapeutic target (vaccines, CAR-T cells, immunotoxins). These strategies have reached varying levels of clinical translation, and clinical trials have revealed challenges to overcome. For example, initial studies in mice with a GUCY2C-targeted vaccine revealed a surprising GUCY2C immunological tolerance mechanism, preventing activation of all arms of the adaptive immune system. This was overcome by redesigning the vaccine to include a CD4-helper epitope (S1), enhancing anti-tumor immunity in mice [95]; however, the helper epitope chosen for the first clinical trial (PADRE) failed to stimulate a CD4 response [100]. Furthermore, patient immunity to the adenoviral vector used to deliver the vaccine limited vaccine efficacy. A reformulated vaccine, incorporating insights from the initial trial, is scheduled to enter a Phase II clinical trial in 2020 (NCT04111172). Additionally, recent clinical trials testing the GUCY2C-targeted immunotoxin TAK-264 were terminated due to lack of efficacy [111, 112]. The authors speculate that optimization of linker chemistry, drug internalization kinetics, or tumor penetrance may be necessary. These trials recruited treatment-refractory patients with GUCY2C-expressing pancreatic and gastric cancer, and it remains to be seen if patients with colorectal cancer will be incorporated into future trials.
Several obstacles may arise in any upcoming GUCY2C vaccine and CAR-T trials that are worth considering. GUCY2C expression has been reported in specific nuclei of the brain [123], creating a potential for autoimmunity that should be carefully monitored. To date, adverse neurologic effects have not been noted in mice or humans treated with any GUCY2C immunotherapy. We can only speculate, but the presence of the blood brain barrier, or differences in GUCY2C subcellular localization between brain and intestine, may make it inaccessible to systemic immunotherapies. Another obstacle to efficacy may be the immuno-suppressive niche of solid tumors. A variety of tools have been explored to overcome this barrier, such as local injection of CAR-T cells into the tumor, co-administration of immune checkpoint inhibitors, and designing the CAR-T cells to co-express stimulatory cytokines (e.g. IL-12, IL-18) [102]. Steps to further refine these GUCY2C immunotherapies will undoubtedly be informed by the upcoming trials.
The most clinically successful efforts so far have been those exploring GUCY2C mRNA as a biomarker to detect occult metastases and inform staging. The strategy accurately identifies metastatic cells in patients with histologically-negative lymph nodes, predicts time-to-recurrence, and has been validated across multiple laboratories and patient cohorts [114, 115, 116, 117]. Aberrant GUCY2C expression has been reported in other cancers of the GI tract as well, owing to oncogenic transcriptional reprogramming of these tissues, broadening the potential applicability of these platforms [111, 112, 124]. A large body of preclinical data provides a strong foundation for clinical translation into man, and the coming years will undoubtedly reveal new insights.
9.0. Five Year View
The translation of GUCY2C-targeted therapies to man remains in its infancy and continues to hold promise. This promise is underscored by the recent Swedish study, published in 2019, and the first to examine a relationship between usage of cGMP-stimulating agents and incidence of colorectal cancer [91]. In a cohort of 36,020 men, those taking a PDE inhibitor over the ten-year study period had a significantly lower incidence of colorectal cancer (hazard ratio = 0.65; 95% CI, 0.49–0.85). Given that most sporadic colorectal cancers are slow to develop (as many as ten years), and the first synthetic GUCY2C ligand, linaclotide, was only approved in 2012, we expect similar data for patients taking linaclotide, and possibly plecanatide, to become available over the next five years. Furthermore, we look forward to results from upcoming trials testing linaclotide for chemoprevention in colorectal cancer patients (NCT03796884; predicted completion in 2021), the GUCY2C-targeted vaccine (NCT04111172; predicted initiation in 2020), and GUCY2C CAR-T cells (not-yet scheduled). We also anticipate continued insight into the molecular steps linking GUCY2C/cGMP signaling to circuits underlying tumorigenesis, which may be further leveraged for therapy.
Article highlights.
The intestinal receptor, GUCY2C, and its downstream signaling axis regulated by cGMP, has emerged as tumor-suppressive regulator of several oncogenic circuits underlying colorectal cancer
Inactivation of the receptor occurs early and near-universally in colorectal cancer, through mutant APC-mediated inactivation of GUCY2C ligand expression. A clinical trial is underway examining reactivation of the receptor with the FDA-approved GUCY2C agonist, linaclotide, for colorectal cancer chemoprevention.
GUCY2C expression is retained in cancer cells, enabling its use as a biomarker for cancer staging and for accurate prediction of disease recurrence
GUCY2C represents a putative target for immunotherapies, including vaccines, CAR-T cells, and immunotoxins. A clinical trial testing efficacy of a GUCY2C-targeted vaccine is scheduled to begin this year.
Acknowledgments
Funding
This paper was funded by the National Institutes of Health (R01 CA204481, R01 CA206026, and P30 CA056036). It was also supported by a PhRMA Predoctoral Fellowship Award in Pharmacology/Toxicology and an NIH Ruth Kirschstein Individual Predoctoral MD/PhD Fellowship (F30 CA232469).
S.A. Waldman is a member of Scientific Advisory Board, and the Board of Directors of Targeted Diagnostics and Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Aka AA, Rappaport JA, Pattison AM, Sato T, Snook AE, Waldman SA. Guanylate cyclase C as a target for prevention, detection, and therapy in colorectal cancer. Expert Rev Clin Pharmacol. 2017; 10:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020; 70:145–64. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev. 2016; 96:751–804. [DOI] [PubMed] [Google Scholar]
- 5.Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem. 2014; 289:581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007; 171:1847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011; 6:e16139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S, Waldman SA. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012; 7:e31686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappaport JA, Waldman SA. The Guanylate Cyclase C-cGMP Signaling Axis Opposes Intestinal Epithelial Injury and Neoplasia. Front Oncol. 2018; 8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Wuthrick E, Rappaport JA, Kraft C, Lin JE, Marszalowicz G, Snook AE, Zhan T, Hyslop TM, Waldman SA. GUCY2C signaling opposes the acute radiation-induced GI syndrome. Cancer Res. 2017; 77:5095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons AV, Lin JE, Kim GW, Marszalowicz GP, Li P, Stoecker BA, Blomain ES, Rattan S, Snook AE, Schulz S, Waldman SA. Intestinal GUCY2C prevents TGF-beta secretion coordinating desmoplasia and hyperproliferation in colorectal cancer. Cancer Res. 2013; 73:6654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, Gibbons AV, Marszlowicz G, Pitari GM, Waldman SA. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010; 138:241–54.* Describes the mechanism by which GUCY2C acts as a colorectal cancer tumor suppressor
- 13.Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, Liu C, Girondo MA, Rui H, Hyslop T, Waldman SA. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014; 23:2328–37.** Describes the near universal loss of guanylin in primary colorectal tumors compared to matched normal adjacent tissue.
- 14.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun. 2000; 273:225–30. [DOI] [PubMed] [Google Scholar]
- 15.Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol. 2002; 161:2169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blomain ES, Rappaport JA, Pattison AM, Bashir B, Caparosa E, Stem J, Snook AE, Waldman SA. APC-beta-catenin-TCF signaling silences the intestinal guanylin-GUCY2C tumor suppressor axis. Cancer Biol Ther. 2020; 21:441–51.** First demonstration that oncogenic Wnt signaling mediates the loss of guanylin expression, linking the driving mutations in colorectal cancer to the silencing of GUCY2C signaling
- 17.Pattison AM, Barton JR, Entezari AA, Zalewski A, Rappaport JA, Snook AE, Waldman SA. Silencing the intestinal GUCY2C tumor suppressor axis requires APC loss of heterozygosity. Cancer Biol Ther. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashir B, Merlino DJ, Rappaport JA, Gnass E, Palazzo JP, Feng Y, Fearon ER, Snook AE, Waldman SA. Silencing the GUCA2A-GUCY2C tumor suppressor axis in CIN, serrated, and MSI colorectal neoplasia. Hum Pathol. 2019; 87:103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005; 36:170–9. [DOI] [PubMed] [Google Scholar]
- 20.Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD, Waldman SA. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci U S A. 1996; 93:14827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cagir B, Gelmann A, Park J, Fava T, Tankelevitch A, Bittner EW, Weaver EJ, Palazzo JP, Weinberg D, Fry RD, Waldman SA. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann Intern Med. 1999; 131:805–12. [DOI] [PubMed] [Google Scholar]
- 22.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990; 61:759–67. [DOI] [PubMed] [Google Scholar]
- 23.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011; 6:479–507. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst. 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouzmenko AP, Takeyama K, Kawasaki Y, Akiyama T, Kato S. Truncation mutations abolish chromatin-associated activities of adenomatous polyposis coli. Oncogene. 2008; 27:4888–99. [DOI] [PubMed] [Google Scholar]
- 26.Brocardo MG, Borowiec JA, Henderson BR. Adenomatous polyposis coli protein regulates the cellular response to DNA replication stress. Int J Biochem Cell Biol. 2011; 43:1354–64. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal AS, Narayan S. Assembly of the base excision repair complex on abasic DNA and role of adenomatous polyposis coli on its functional activity. Biochemistry. 2011; 50:1901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlach JP, Emmink BL, Nojima H, Kranenburg O, Maurice MM. Wnt signaling induces accumulation of phosphorylated beta-catenin in two distinct cytosolic complexes. Open Biol. 2014; 4:140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014; 27:9–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012; 487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020; 158:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008; 135:1079–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992; 359:235–7. [DOI] [PubMed] [Google Scholar]
- 34.Umar A, Risinger JI, Hawk ET, Barrett JC. Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer. 2004; 4:153–8. [DOI] [PubMed] [Google Scholar]
- 35.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004; 53:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019; 125:4139–47. [DOI] [PubMed] [Google Scholar]
- 37.Katona BW, Weiss JM. Chemoprevention of Colorectal Cancer. Gastroenterology. 2020; 158:368–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O’Connell M, Sargent P, Piedbois P, Meta-Analysis Group in C. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004; 22:3766–75. [DOI] [PubMed] [Google Scholar]
- 39.Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990; 63:941–8. [DOI] [PubMed] [Google Scholar]
- 40.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000; 60:3338–42. [PubMed] [Google Scholar]
- 41.Garin-Laflam MP, Steinbrecher KA, Rudolph JA, Mao J, Cohen MB. Activation of guanylate cyclase C signaling pathway protects intestinal epithelial cells from acute radiation-induced apoptosis. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Baran AA, Siracusa LD, Pitari GM, Waldman SA. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007; 133:599–607.* Describes the mechanism by which GUCY2C acts as a colorectal cancer tumor suppressor
- 43.Mann EA, Harmel-Laws E, Cohen MB, Steinbrecher KA. Guanylate cyclase C limits systemic dissemination of a murine enteric pathogen. BMC Gastroenterol. 2013; 13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenna O, Bruland T, Furnes MW, Granlund A, Drozdov I, Emgard J, Bronstad G, Kidd M, Sandvik AK, Gustafsson BI. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol. 2015; 50:1241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011; 121:3578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim GW, Lin JE, Snook AE, Aing AS, Merlino DJ, Li P, Waldman SA. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes. 2016; 6:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000; 52:375–414. [PubMed] [Google Scholar]
- 48.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992; 89:947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, Duffin KL, Siegel NR, Currie MG. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993; 90:10464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahsan MK, Tchernychev B, Kessler MM, Solinga RM, Arthur D, Linde CI, Silos-Santiago I, Hannig G, Ameen NA. Linaclotide activates guanylate cyclase-C/cGMP/protein kinase-II-dependent trafficking of CFTR in the intestine. Physiological reports. 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pattison AM, Blomain ES, Merlino DJ, Wang F, Crissey MA, Kraft CL, Rappaport JA, Snook AE, Lynch JP, Waldman SA. Intestinal enteroids model guanylate cyclase C-dependent secretion induced by heat-stable enterotoxins. Infect Immun. 2016; 84:3083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bijvelds MJ, Loos M, Bronsveld I, Hellemans A, Bongartz JP, Ver Donck L, Cox E, de Jonge HR, Schuurkes JA, De Maeyer JH. Inhibition of Heat-Stable Toxin-Induced Intestinal Salt and Water Secretion by a Novel Class of Guanylyl Cyclase C Inhibitors. J Infect Dis. 2015; 212:1806–15. [DOI] [PubMed] [Google Scholar]
- 53.Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Volkmann HL, Bronstad I, Gilja OH, R RT, Sangnes DA, Nortvedt R, Hausken T, Dimcevski G, Fiskerstrand T, Nylund K. Prolonged intestinal transit and diarrhea in patients with an activating GUCY2C mutation. PLoS One. 2017; 12:e0185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Volkmann HL, Nylund K, Tronstad RR, Hovdenak N, Hausken T, Fiskerstrand T, Gilja OH. An activating gucy2c mutation causes impaired contractility and fluid stagnation in the small bowel. Scand J Gastroenterol. 2016; 51:1308–15. [DOI] [PubMed] [Google Scholar]
- 56.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Havik B, Tonder SL, Levy SE, Brackman D, Boman H, Biswas KH, Apold J, Hovdenak N, Visweswariah SS, Knappskog PM. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012; 366:1586–95. [DOI] [PubMed] [Google Scholar]
- 57.Romi H, Cohen I, Landau D, Alkrinawi S, Yerushalmi B, Hershkovitz R, Newman-Heiman N, Cutting GR, Ofir R, Sivan S, Birk OS. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012; 90:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith A, Bulman DE, Goldsmith C, Bareke E, Majewski J, Boycott KM, Nikkel SM. Meconium ileus in a Lebanese family secondary to mutations in the GUCY2C gene. Eur J Hum Genet. 2015; 23:990–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R, Kwon IK, Thangaraju M, Singh N, Liu K, Jay P, Hofmann F, Ganapathy V, Browning DD. Type 2 cGMP-dependent protein kinase regulates proliferation and differentiation in the colonic mucosa. Am J Physiol Gastrointest Liver Physiol. 2012; 303:G209–19. [DOI] [PubMed] [Google Scholar]
- 60.Kraft CL, Rappaport JA, Snook AE, Pattison AM, Lynch JP, Waldman SA. GUCY2C maintains intestinal LGR5(+) stem cells by opposing ER stress. Oncotarget. 2017; 8:102923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001; 98:7846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, Zhou J, Xu G, Zhi F. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res. 2017; 7:41–52. [PMC free article] [PubMed] [Google Scholar]
- 63.Lee K, AP. G The interaction between the Wnt/beta-catenin signaling cascade and PKG activation in cancer. Journal of biomedical research. 2017; 31:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li N, Lee K, Xi Y, Zhu B, Gary BD, Ramirez-Alcantara V, Gurpinar E, Canzoneri JC, Fajardo A, Sigler S, Piazza JT, Chen X, Andrews J, Thomas M, Lu W, Li Y, Laan DJ, Moyer MP, Russo S, Eberhardt BT, Yet L, Keeton AB, Grizzle WE, Piazza GA. Phosphodiesterase 10A: a novel target for selective inhibition of colon tumor cell growth and beta-catenin-dependent TCF transcriptional activity. Oncogene. 2015; 34:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, Keeton AB, Xi Y, Abadi AH, Grizzle WE, Piazza GA. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res (Phila). 2012; 5:822–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, Boddupalli SS, Currie MG, Forte LR. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000; 60:5151–7.* First pre-clinical demonstration of oral delivery of a GUCY2C agonist for tumor prevention
- 67.Danaee H, Kalebic T, Wyant T, Fassan M, Mescoli C, Gao F, Trepicchio WL, Rugge M. Consistent expression of guanylyl cyclase-C in primary and metastatic gastrointestinal cancers. PLoS One. 2017; 12:e0189953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The Cancer Genome Atlas [Acessed July 20, 2020]. Available from: https://portal.gdc.cancer.gov/.
- 69.Park J, Schulz S, Waldman SA. Intestine-specific activity of the human guanylyl cyclase C promoter is regulated by Cdx2. Gastroenterology. 2000; 119:89–96. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997; 276:1268–72. [DOI] [PubMed] [Google Scholar]
- 71.Cohen MB, Hawkins JA, Witte DP. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab Invest. 1998; 78:101–8. [PubMed] [Google Scholar]
- 72.Lin JE, Colon-Gonzalez F, Blomain E, Kim GW, Aing A, Stoecker B, Rock J, Snook AE, Zhan T, Hyslop TM, Tomczak M, Blumberg RS, Waldman SA. Obesity-induced colorectal cancer is driven by caloric silencing of the guanylin-GUCY2C paracrine signaling axis. Cancer Res. 2016; 76:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li P, Lin JE, Snook AE, Waldman SA. ST-producing E. coli oppose carcinogen-induced colorectal tumorigenesis in mice. Toxins. 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lubbe WJ, Zuzga DS, Zhou Z, Fu W, Pelta-Heller J, Muschel RJ, Waldman SA, Pitari GM. Guanylyl cyclase C prevents colon cancer metastasis by regulating tumor epithelial cell matrix metalloproteinase-9. Cancer Res. 2009; 69:3529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuzga DS, Pelta-Heller J, Li P, Bombonati A, Waldman SA, Pitari GM. Phosphorylation of vasodilator-stimulated phosphoprotein Ser239 suppresses filopodia and invadopodia in colon cancer. Int J Cancer. 2012; 130:2539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoenfeld P, Lacy BE, Chey WD, Lembo AJ, Kurtz CB, Reasner DS, Bochenek W, Tripp K, Currie MG, Fox SM, Blakesley RE, O’Dea CR, Omniewski ND, Hall ML. Low-Dose Linaclotide (72 mug) for Chronic Idiopathic Constipation: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Am J Gastroenterol. 2018; 113:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brenner DM, Fogel R, Dorn SD, Krause R, Eng P, Kirshoff R, Nguyen A, Crozier RA, Magnus L, Griffin PH. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol. 2018. [DOI] [PubMed] [Google Scholar]
- 78.Sharman SK, Islam BN, Hou Y, Singh N, Berger FG, Sridhar S, Yoo W, Browning DD. Cyclic-GMP-elevating agents suppress polyposis in Apc(Min) mice by targeting the preneoplastic epithelium. Cancer Prev Res (Phila). 2018; 11:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang WL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, Palejwala VA, Clapper ML, Shailubhai K. Plecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc+/Min-FCCC mice. World J Gastrointest Pharmacol Ther. 2017; 8:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinberg DS, Lin JE, Foster NR, Della’Zanna G, Umar A, Seisler D, Kraft WK, Kastenberg DM, Katz LC, Limburg PJ, Waldman SA. Bioactivity of oral linaclotide in human colorectum for cancer chemoprevention. Cancer Prev Res (Phila). 2017; 10:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018; 113:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Browning DD. The enduring promise of phosphodiesterase 5 inhibitors for colon cancer prevention. Translational Gastroenterology and Hepatology. 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoner GD, Budd GT, Ganapathi R, DeYoung B, Kresty LA, Nitert M, Fryer B, Church JM, Provencher K, Pamukcu R, Piazza G, Hawk E, Kelloff G, Elson P, van Stolk RU. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv Exp Med Biol. 1999; 470:45–53. [DOI] [PubMed] [Google Scholar]
- 84.van Stolk R, Stoner G, Hayton WL, Chan K, DeYoung B, Kresty L, Kemmenoe BH, Elson P, Rybicki L, Church J, Provencher K, McLain D, Hawk E, Fryer B, Kelloff G, Ganapathi R, Budd GT. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin Cancer Res. 2000; 6:78–89. [PubMed] [Google Scholar]
- 85.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006; 55:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993; 328:1313–6. [DOI] [PubMed] [Google Scholar]
- 87.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002; 122:641–5. [DOI] [PubMed] [Google Scholar]
- 88.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, Moyer MP, Grizzle WE, Chang WC, Clapper ML, Piazza GA. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/beta-catenin signaling. Mol Cancer Ther. 2013; 12:1848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tinsley HN, Grizzle WE, Abadi A, Keeton A, Zhu B, Xi Y, Piazza GA. New NSAID targets and derivatives for colorectal cancer chemoprevention. Recent Results Cancer Res. 2013; 191:105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Islam BN, Sharman SK, Hou Y, Bridges AE, Singh N, Kim S, Kolhe R, Trillo-Tinoco J, Rodriguez PC, Berger FG, Sridhar S, Browning DD. Sildenafil suppresses inflammation-driven colorectal cancer in mice. Cancer Prev Res (Phila). 2017; 10:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang W, Sundquist J, Sundquist K, Ji J. Use of Phosphodiesterase 5 Inhibitors Is Associated With Lower Risk of Colorectal Cancer in Men With Benign Colorectal Neoplasms. Gastroenterology. 2019; 157:672–81 e4.* This retrospective trial was the first to show a correlation between PDE5 inhibition and reduced colorectal cancer incidence
- 92.Muller C, Yurgelun M, Kupfer SS. Precision Treatment and Prevention of Colorectal Cancer-Hope or Hype? Gastroenterology. 2020; 158:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snook AE, Stafford BJ, Li P, Tan G, Huang L, Birbe R, Schulz S, Schnell MJ, Thakur M, Rothstein JL, Eisenlohr LC, Waldman SA. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J Natl Cancer Inst. 2008; 100:950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snook AE, Li P, Stafford BJ, Faul EJ, Huang L, Birbe RC, Bombonati A, Schulz S, Schnell MJ, Eisenlohr LC, Waldman SA. Lineage-specific T-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009; 69:3537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Snook AE, Magee MS, Schulz S, Waldman SA. Selective antigen-specific CD4(+) T-cell, but not CD8(+) T- or B-cell, tolerance corrupts cancer immunotherapy. Eur J Immunol. 2014; 44:1956–66.**Describes the Ad5-GUCY2C vaccine and provides the first demonstration of CD4+ T-cell tolerance as a mechanism of cancer vaccine resistance
- 96.Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014; 20:3738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Snook AE, Waldman SA. Advances in cancer immunotherapy. Discov Med. 2013; 15:120–5. [PMC free article] [PubMed] [Google Scholar]
- 98.Xiang B, Baybutt TR, Berman-Booty L, Magee MS, Waldman SA, Alexeev VY, Snook AE. Prime-Boost Immunization Eliminates Metastatic Colorectal Cancer by Producing High-Avidity Effector CD8(+) T Cells. J Immunol. 2017; 198:3507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Snook AE, Baybutt TR, Hyslop T, Waldman SA. Preclinical evaluation of a replication-deficient recombinant adenovirus serotype 5 vaccine expressing guanylate cyclase C and the PADRE T-helper epitope. Human gene therapy methods. 2016; 27:238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Snook AE, Baybutt TR, Xiang B, Abraham TS, Flickinger JC Jr., Hyslop T, Zhan T, Kraft WK, Sato T, Waldman SA. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J Immunother Cancer. 2019; 7:104.* First clinical trial of the Ad5-GUCY2C vaccine
- 101.Flickinger JC Jr., Singh J, Carlson R, Leong E, Baybutt TR, Barton JR, Caparosa E, Pattison A, Rappaport JA, Roh J, Zhan T, Bashir B, Waldman SA, Snook AE. Chimeric Ad5.F35 Vector Evades Anti-Adenovirus Serotype 5 Neutralization Opposing GUCY2C-Targeted Antitumor Immunity. Journal for Immunotherapy of Cancer. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baybutt TR, Flickinger JC Jr., Caparosa EM, Snook AE. Advances in Chimeric Antigen Receptor T-Cell Therapies for Solid Tumors. Clin Pharmacol Ther. 2019; 105:71–8. [DOI] [PubMed] [Google Scholar]
- 103.Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989; 21:127–30. [PubMed] [Google Scholar]
- 104.Magee MS, Kraft CL, Abraham TS, Baybutt TR, Marszalowicz GP, Li P, Waldman SA, Snook AE. GUCY2C-directed CAR-T cells oppose colorectal cancer metastases without autoimmunity. OncoImmunology. 2016; 5:e1227897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Magee MS, Abraham TS, Baybutt TR, Flickinger JC Jr., Ridge NA, Marszalowicz GP, Prajapati, Hersperger AR, Waldman SA, Snook AE Human GUCY2C-Targeted Chimeric Antigen Receptor (CAR)-Expressing T Cells Eliminate Colorectal Cancer Metastases. Cancer Immunol Res. 2018; 6:509–16.** Pre-clinical study demonstrating the efficacy of a GUCY2C-targeted CAR-T cell against human colorectal cancer xenografts
- 106.Papachristos A, Pippa N, Demetzos C, Sivolapenko G. Antibody-drug conjugates: a mini-review. The synopsis of two approved medicines. Drug Deliv. 2016; 23:1662–6. [DOI] [PubMed] [Google Scholar]
- 107.Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015; 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leung D, Wurst JM, Liu T, Martinez RM, Datta-Mannan A, Feng Y. Antibody Conjugates-Recent Advances and Future Innovations. Antibodies (Basel). 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marszalowicz GP, Snook AE, Magee MS, Merlino D, Berman-Booty LD, Waldman SA. GUCY2C lysosomotropic endocytosis delivers immunotoxin therapy to metastatic colorectal cancer. Oncotarget. 2014; 5:9460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Almhanna K, Kalebic T, Cruz C, Faris JE, Ryan DP, Jung J, Wyant T, Fasanmade AA, Messersmith W, Rodon J. Phase I study of the investigational anti-guanylyl cyclase antibody-drug conjugate TAK-264 (MLN0264) in adult patients with advanced gastrointestinal malignancies. Clin Cancer Res. 2016; 22:5049–57. [DOI] [PubMed] [Google Scholar]
- 111.Almhanna K, Miron ML, Wright D, Gracian AC, Hubner RA, Van Laethem JL, Lopez CM, Alsina M, Munoz FL, Bendell J, Firdaus I, Messersmith W, Ye Z, Fasanmade AA, Danaee H, Kalebic T. Phase II study of the antibody-drug conjugate TAK-264 (MLN0264) in patients with metastatic or recurrent adenocarcinoma of the stomach or gastroesophageal junction expressing guanylyl cyclase C. Invest New Drugs. 2017; 35:235–41. [DOI] [PubMed] [Google Scholar]
- 112.Almhanna K, Wright D, Mercade TM, Van Laethem JL, Gracian AC, Guillen-Ponce C, Faris J, Lopez CM, Hubner RA, Bendell J, Bols A, Feliu J, Starling N, Enzinger P, Mahalingham D, Messersmith W, Yang H, Fasanmade A, Danaee H, Kalebic T. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Invest New Drugs. 2017; 35:634–41. [DOI] [PubMed] [Google Scholar]
- 113.Rahbari NN, Bork U, Motschall E, Thorlund K, Buchler MW, Koch M, Weitz J. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012; 30:60–70. [DOI] [PubMed] [Google Scholar]
- 114.Waldman SA, Hyslop T, Schulz S, Barkun A, Nielsen K, Haaf J, Bonaccorso C, Li Y, Weinberg DS. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA. 2009; 301:745–52.** Multi-center trial demonstrating the utility of GUCY2C RT-PCR for molecular staging of colorectal cancer
- 115.Beaulieu M, Desaulniers M, Bertrand N, Deschesnes RG, Beaudry G, Garon G, Haince JF, Houde M, Holzer TJ. Analytical performance of a qRT-PCR assay to detect guanylyl cyclase C in FFPE lymph nodes of patients with colon cancer. Diagn Mol Pathol. 2010; 19:20–7. [DOI] [PubMed] [Google Scholar]
- 116.Sargent DJ, Resnick MB, Meyers MO, Goldar-Najafi A, Clancy T, Gill S, Siemons GO, Shi Q, Bot BM, Wu TT, Beaudry G, Haince JF, Fradet Y. Evaluation of guanylyl cyclase C lymph node status for colon cancer staging and prognosis. Ann Surg Oncol. 2011; 18:3261–70. [DOI] [PubMed] [Google Scholar]
- 117.Haince JF, Houde M, Beaudry G, L’Esperance S, Garon G, Desaulniers M, Hafer LJ, Heald JI, Lyle S, Grossman SR, Tetu B, Sargent DJ, Fradet Y. Comparison of histopathology and RT-qPCR amplification of guanylyl cyclase C for detection of colon cancer metastases in lymph nodes. J Clin Pathol. 2010; 63:530–7. [DOI] [PubMed] [Google Scholar]
- 118.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014; 370:1287–97. [DOI] [PubMed] [Google Scholar]
- 119.Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, Castanos-Velez E, Blumenstein BA, Rosch T, Osborn N, Snover D, Day RW, Ransohoff DF, Presept Clinical Study Steering Committee I, Study T. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014; 63:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamada T, Matsuda A, Koizumi M, Shinji S, Takahashi G, Iwai T, Takeda K, Ueda K, Yokoyama Y, Hara K, Hotta M, Matsumoto S, Yoshida H. Liquid Biopsy for the Management of Patients with Colorectal Cancer. Digestion. 2019; 99:39–45. [DOI] [PubMed] [Google Scholar]
- 121.Lan D, Niu J, Miao J, Dong X, Wang H, Yang G, Wang K, Miao Y. Expression of guanylate cyclase-C, guanylin, and uroguanylin is downregulated proportionally to the ulcerative colitis disease activity index. Sci Rep. 2016; 6:25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Guglielmo MD, Perdue L, Adeyemi A, van Golen KL, Corao DU. Immunohistochemical staining for uroguanylin, a satiety hormone, is decreased in intestinal tissue specimens from female adolescents with obesity. Pediatr Dev Pathol. 2017:1093526617722912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Merlino DJ, Barton JR, Charsar BA, Byrne MD, Rappaport JA, Smeyne RJ, Lepore AC, Snook AE, Waldman SA. Two distinct GUCY2C circuits with PMV (hypothalamic) and SN/VTA (midbrain) origin. Brain Struct Funct. 2019; 224:2983–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park J, Schulz S, Haaf J, Kairys JC, Waldman SA. Ectopic expression of guanylyl cyclase C in adenocarcinomas of the esophagus and stomach. Cancer Epidemiol Biomarkers Prev. 2002; 11:739–44. [PubMed] [Google Scholar]