The spleen has the primary function of filtering our blood, clearing it from pathogens through immune cells of the white pulps, and removing senescent red blood cells (RBCs) through reticuloendothelial macrophages of the red pulp.1 The erythrocytic lifecycle is a highly regulated process whereby erythrocytes are produced in the bone marrow and live in the circulation for an average of 120 days before being removed by splenic red pulp macrophages and, to some extent, hepatic Kupffer macrophages. While recent discoveries have shown how loss of deformability as well as surface molecules contribute to senescent erythrocyte retention in the spleen, questions remain about how cell recognition by red pulp macrophages and subsequent erythrophagocytosis occur.
Specifically, the inability to reproduce the in vivo erythrophagocytic process by cultured macrophages indicates that the underlying mechanism is complex, likely requires features that cannot be fully recapitulated in vitro (eg, splenic architecture) and goes beyond the simple interaction of macrophages with senescent RBCs. The recent study by Klei et al2 attempts to solve the issue by investigating the role of red pulp macrophages on erythrocytic turnover. The authors uncovered the unique role of splenic architecture and fluid dynamics in the retention of senescent RBCs and their removal by macrophages through the intermediated hemolytic formation of erythrocytic ghosts.
The isolation of human red pulp macrophages revealed that while only the 3% of macrophages were actively digesting senescent erythrocytes, a proportion of engulfed erythrocytes appeared as ghosts devoid of hemoglobin. Ghost formation in vivo indicates that erythrocytes are prone to hemolysis within the spleen and, due to the faster rate of ghost degradation compared to intact RBCs (3 versus 24 h), likely explains the low frequency of erythrophagocytosis observed in ex vivo macrophages.3,4 In vivo experiments with transfused aged erythrocytes showed that in the spleen an abundant amount of ghosts are generated, which maintain at flow cytometry a specific membrane staining (PKH26 dye) but lose the intracellular staining (calcein) consequent to hemolysis.2 The decrease in splenic and hepatic plasma levels of haptoglobin and hemopexin compared with circulating plasma levels suggests an active local consumption of the hemoglobin (Hb) and heme scavengers occurring upon senescent RBC hemolysis and ghost formation.2 The preference of red pulp macrophages for ghosts was confirmed in vitro, where erythrocyte ghosts were readily picked up and quickly digested by red pulp macrophages compared with intact senescent RBCs.2,5
Finally, Klei et al2 demonstrated the critical role of the spleen architecture in the retention of senescent RBCs, ghost generation, and macrophage-mediated clearance. The authors hypothesized that the interplay of splenic architecture, adhesion molecule-mediated trapping of aged erythrocytes, and shear stress resulted in hemolysis, thereby inducing ghost formation. Taking advantage of an in vitro system where fresh, aged, and spleen-derived erythrocytes were flown over a surface coated with extracellular matrix components as laminin-α5 and hyaluronic acid, they showed a specific retention of both aged and spleen-derived but not fresh erythrocytes. The interaction between the adhesion molecule Lutheran blood group and basal cell adhesion molecule on the surface of senescent RBCs and its ligand laminin-α5 abundant in the matrix of splenic red pulp sinuses is responsible for senescent erythrocyte trapping in the splenic microenvironment, and the combination of prolonged matrix adhesion and low shear stress exposure ultimately triggers aged RBC shrinkage and hemolysis with ghost formation.2
In vivo, the complexity of the architecture becomes clear. While discontinuous vascular endothelial (VE)-Cadherin expression in the red pulp selectively allows deformable healthy RBCs to pass through splenic endothelial fenestrae, elevated laminin levels wrapping red pulp sinuses in a web-like structure allows for specific capturing of senescent erythrocytes.2
Overall, the findings from this provocative study demonstrate a novel mechanism of macrophage-mediated removal of senescent erythrocytes through the formation of intermediate ghosts via hemolysis and highlight how the complex evolution of the splenic architecture is as important as the biomolecular interactions in erythrocyte turnover (Figure 1). Without the unique filtration design created by reduced VE-Cadherin and increased laminin-α5 in red pulp sinuses, which allows to selectively retain rigid senescent RBCs, while releasing deformable younger erythrocytes, the spleen would be unable to efficiently recognize and remove aged erythrocytes (Figure 1).
Figure 1.
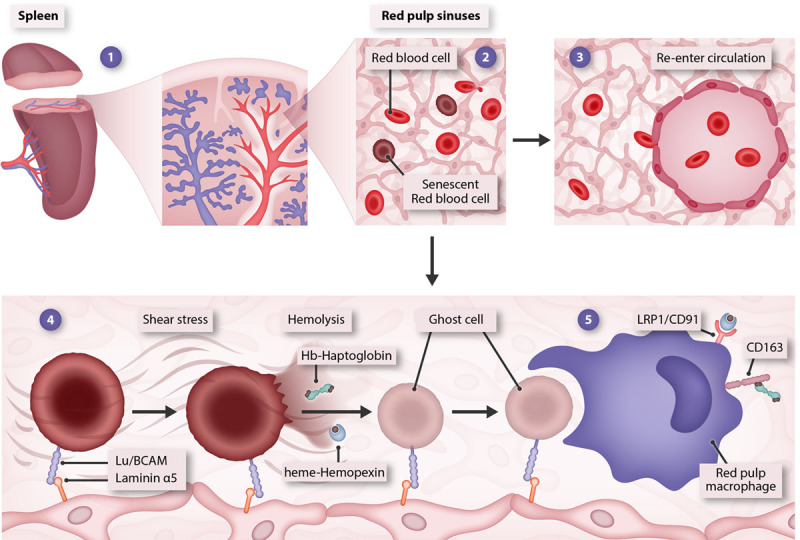
Novel model of erythrophagocytosis. Upon entry in the spleen, erythrocytes flow through red pulp sinuses (1). The splenic architecture allows deformable healthy RBCs to pass through the web-like structure of red pulp sinuses and selectively retains senescent RBCs (2). While young RBCs leave the spleen and re-enter circulation (3), aged RBCs are captured through the interaction of laminin-α5 on the extracellular matrix with Lu/BCAM on aged RBC membrane and undergo shear stress-induced shrinkage and hemolysis (4). Red pulp macrophages phagocytize ghost remnants of aged RBCs, while Hb and heme content released in the extracellular space is scavenged by haptoglobin and hemopexin that facilitate macrophage-mediated iron recycling through receptor-mediated endocytosis (5). Hb = hemoglobin; Lu/BCAM = Lutheran blood group and basal cell adhesion molecule; RBC = red blood cell.
Importantly, the novel mechanism here proposed is groundbreaking and radically changes our current knowledge of the erythrophagocytic process as well as reshapes our understanding of iron recycling. Scavenging of hemoglobin and heme released from hemolytic senescent RBCs rather than from macrophage erythrophagolysosomes appears as the preferred and likely more efficient iron recycling mechanism. The elevated concentration of haptoglobin and hemopexin under physiologic conditions would be more than sufficient to sustain an effective iron recycling capacity and promote controlled scavenger-mediated Hb/heme uptake by macrophages.6,7 While facilitating iron internalization and reutilization, regulated heme uptake likely prevents free heme-induced cell death, reactive oxygen species formation, and inflammatory skewing of macrophages.8 These observations unravel the physiologic importance of the Hb/heme scavenging system during steady-state hemolysis, beyond its well-recognized relevance in pathologic conditions where hemolysis is accelerated (eg, sickle cell disease, thalassemia).6–8 Finally, these findings reconcile the discrepancies between in vivo and in vitro studies and explain the lack of erythrophagocytosis by cultured macrophages in vitro, where aspects of the splenic microenvironment cannot be reproduced.5 The identification of the splenic architecture as key driver of senescent erythrocyte hemolysis and ghost remnant recognition by macrophages will now pave the way to the design of novel and refined in vitro systems for the study of erythrophagocytosis and open up new directions for a better understanding of this process in pathophysiologic conditions.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005; 5:606–616 [DOI] [PubMed] [Google Scholar]
- 2.Klei TRL, Dalimot J, Nota B, et al. Hemolysis in the spleen drives erythrocyte turnover. Blood. 2020; 136:1579–1589 [DOI] [PubMed] [Google Scholar]
- 3.Loegering DJ, Commins LM, Minnear FL, et al. Effect of Kupffer cell phagocytosis of erythrocytes and erythrocyte ghosts on susceptibility to endotoxemia and bacteremia. Infect Immun. 1987; 55:2074–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellman IS, Plutner H, Steinman RM, et al. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J Cell Biol. 1983; 96:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb Y, Topaz O, Cohen LA, et al. Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro. Haematologica. 2012; 97:994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaer DJ, Vinchi F, Ingoglia G, et al. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front Physiol. 2014; 5:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiabrando D, Vinchi F, Fiorito V, et al. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol. 2014; 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinchi F, Costa da Silva M, Ingoglia G, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016; 127:473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]


