Abstract
Infectious diseases constitute a problem of great importance for animal and human health, as well as the increasing bacterial resistance to antibiotics. In this context, medicinal plants emerge as an effective alternative to replace the use antibiotics. The essential oil (EO) of Minthostachys verticillata (Griseb.) Epling (Lamiaceae) has demonstrated a strong antimicrobial activity. However, its instability and hydrophobicity under normal storage conditions are limitations to its use. Nanoemulsion technology is an excellent way to solubilize, microencapsulate, and protect this compound. This study aimed to obtain a nanoemulsion based on M. verticillata EO and evaluate its antibacterial activity against Staphylococcus aureus. The EO was obtained by steam distillation. Identification and quantification of their components were determined by GC-MS revealing that the dominated chemical group was oxygenated monoterpenes. Nanoemulsions (NE) were characterized by measuring pH, transmittance, separation percentage, release profile, and morphology. The effect of NE on the growth of S. aureus and cyto-compatibility was also evaluated. The results showed that NE containing a higher percentage of tween 20 exhibited higher stability with an approximated droplet size of 10 nm. The effect of encapsulation process was evaluated by GC-MS revealing that the volatile components in EO were no affected. After 24 h, 74.24 ± 0.75% of EO was released from NE and the antibacterial activity of EO was enhanced considerably by its encapsulation. The incubation of S. aureus with the NE and pure EO, show a bacterial growth inhibition of 58.87% ± 0.99 and 46.72% ± 3.32 (p < 0.05), respectively. In addition, nanoemulsión did not cause toxicity to porcine and equine red blood cells. The results obtained showed that NE could be a potential vehicle for M. verticillata EO with promissory properties to emerge as a tool for developing advanced therapies to control and combat infections.
Keywords: Minthostachys verticillata, Essential oil, Nanoemulsion, Antibacterial activity
Minthostachys verticillata; Essential oil; Nanoemulsion; Antibacterial activity.
1. Introduction
The irrational use of antibiotics has led to a considerable decrease in their effectiveness generating resistance in certain microorganisms and a significant impact on human and animal health (Foster, 2017). Natural products of plant origin could be a viable alternative to the use of antibiotics (Lillehoj et al., 2018; Salehi et al., 2018). It has been reported that they possess significant antiseptic, antibacterial, antiviral, antioxidant, anti-parasitic, antifungal, and insecticidal effects (Salehi et al., 2019, 2020a, 2020b, 2020c, 2020d; Sharifi-Rad et al., 2018; Adorjan and Buchbauer, 2010). Minthostachys verticillata (Griseb.) Epling, also known as “peperina”, is a species of the Lamiaceae family. M. verticillata essential oil (EO) is used to alleviate respiratory and digestive disorders in folkloric medicine and as a food preservative and flavoring (Ojeda et al., 2001; Schmidt-Lebuhn, 2008).
The main components of EOs are complex mixtures of hydrocarbon terpenes and terpenoids (El Asbahani et al., 2015). The majority of the first group consists of monoterpenes and sesquiterpenes, and the second group consists of oxygenated derivatives of hydrocarbon terpenes. Studies about volatile constituents of M. verticillata from various phytogeographic areas of Argentina revealed that there is a high genetic diversity in this species, which appears to be divided into three chemotypes: carvone, thymol-carvacrol and pulegone-menthone (Zygadlo et al., 1996). Testing the EO chemical composition is fundamental because several biological activities are attributed to the EO compounds (León-Méndez et al., 2019). Recent studies on M. verticillata EO (pulegone-menthone chemotype) have revealed in vitro antimicrobial and anti-biofilm properties on Streptococcus uberis, Escherichia coli, Enterococcus faecium and Bacillus pumilus strains isolated from cows with mastitis (Montironi et al., 2016; Cerioli et al., 2018). In addition, M. verticillata EO has also demonstrated immunomodulatory effects by decreasing pro-inflammatory mediators as histamine and β-hexosaminidase or pro-inflammatory cytokines (TNF-α, IL-1β) and increasing anti-inflammatory cytokines (IL-10) both in vitro and in experimental mouse models (Cariddi et al., 2011; Montironi et al., 2019). However, the limitations in the use and application of EOs are mainly attributed to their photosensitive, volatile, and hydrophobic nature, which has stimulated the search for new conservation systems to exploit their great potential. For this purpose, a large number of encapsulation methods have been developed in recent years. These methods include film hydration (Asprea et al., 2017), coacervation (Gonçalves et al., 2017), spray drying (Herculano et al., 2015), ionic gelation (Noppakundilograt et al., 2015), complexation (Da Rocha Neto et al., 2018; Shrestha et al., 2017), liposomes (Cui et al., 2020; Lin et al., 2019), chitosan nanoparticles embedded gelatin nanofibers (Cui et al., 2018) and nanoemulsions (Lou et al., 2017; Chang et al., 2013). The encapsulation of EO in a nanoemulsion (NE) is a promising strategy to facilitate its applicability and enhance its properties. These formulations offer numerous advantages including a controlled small droplet size which are kinetically stabilized against aggregation and gravitational separation (Donsí and Ferrari, 2016; Qian and McClements, 2011). Additionally, NEs have a larger surface area of active ingredients compared to conventional emulsions and, therefore, the functionality is enhanced when they interact with biological systems, improving the diffusion and biological efficacy of dispersed bioactive agents (Salvia-trujillo et al., 2013; Bajerski et al., 2016). NEs have been extensively exploited in many fields, including the pharmaceutical field, due to their medicinal properties. Several EO formulations were tested against Gram-positive and Gram-negative bacteria (Donsí and Ferrari, 2016). However, to the best of our knowledge there are no reports about M. verticillata EO based NE and their antibacterial effect. The present study aimed to characterize M. verticillata EO nanoemulsions and evaluate its antibacterial activity against a Staphylococcus aureus strain (ATCC, 209213) when compared with that of the pure EO.
2. Materials and methods
2.1. Plant material and essential oil extraction
The plant material was obtained in a commercial herb store in February 2017. To obtain the EO, 60 g of ground leaves from the collected specimens were subjected to a steam distillation process. For this, the dry leaves of M. verticillata were placed in an extraction column. A 1 L capacity balloon, containing distilled water, was heated until boiling; the water steam, flowing through the plant material in the column, carrying the volatile components, which condensed in the glass condenser and then by decantation, separated into two phases, one oily and one aqueous. The emulsified water inside the oil was separated from it, by freezing. The EO fraction was stored at -20 °C and protected from light until use (Cariddi et al., 2011; Montironi et al., 2016).
2.2. Identification and quantification of essential oil pure compounds
The essential oil recovered was analyzed by gas chromatography coupled to mass spectrometry (GC-MS) using a gas chromatograph Clarus 600 (Perkin Elmer) equipped with DB5 column. Gas chromatographic (GC) conditions were as follows: injector temperature, 260 °C; flame ionization detection temperature, 240 °C; carrier gas, Helium; flow rate, 1 mL/min; and split injection with split ratio 1:40. The oven temperature was initially 60 °C and was increased to 240 °C at a rate of 5ºC/min. One microliter of each sample, dissolved in hexane (1:100 mg/μL), was injected into a DB-5 column (0.25 mm I. D, 60 m in length, and 0.25 μm film thickness). The chromatogram was obtained in “scan” mode, from m/z = 30 am/z = 450 (scan time: 0.2 s, inter-scan time: 0.1s), solvent delay: 5 min. Data were acquired using TurboMass 5.4.2 software. The identification of the peaks was made by comparison with the mass spectra of the components found with that of the NIST MS Search 2.0 program libraries. To confirm the identity of the compounds, pure compounds (standard grade) were injected under the same chromatographic conditions. Retention rates were calculated by analyzing a C8–C20 alkane standard (Fluka cod 101385968) under the same conditions as the sample (Montironi et al., 2016).
2.3. Nanoemulsion preparation
Nanoemulsions (NE) consisted of M. verticillata EO, Tween 20 as a surfactant and deionized water were synthesized as Rodrigues et al. (2014) with some modifications. The essential oil and Tween 20 were stirred at 800 rpm using a magnetic stirrer for 30 min. Then, water was added dropwise at a flow rate of 2 mL/min. The mixture was stirred at 800 rpm for 30 min. Final homogenization was achieved using a Heidolph DIAX 900 homogenizer (Germany) equipped with a 10 G disperser for 5 min (8000 rpm). Formulation variable was oil/surfactant ratio (1:1, 1:2, 1:3 and 1:4). EO percentage was 6% for all formulations.
2.4. Physic-chemical characterization
2.4.1. Percentage transmittance
The turbidity of all the formulated NE was analyzed by measuring the transmittance of undiluted emulsions at a wavelength of 600 nm ultraviolet using Shimadzu UV/VIS spectrophotometer (Jasco, Mod. V-630 Bio).
2.4.2. pH measurement
The pH value of the NE was measured by immersing the electrode directly into the NE using a calibrated pH meter (Cole-Parmer pH/mV/ºC meter), at 25 °C ± 1 °C. The measurement was carried out in triplicates.
2.4.3. Stability study
Formulated NE were centrifuged at 3,500 rpm for 30 min to prove stability and observe phase separation. Separation percentage was calculated using the following equation:
| Separation percentage (%) = (separated aqueous phase weight/total weigh of nanoemulsion) ∗ 100 | (1) |
2.5. Morphological analysis by transmission electron microscopy
The morphology of the NE was visualized by transmission electron microscopy (TEM) (Siemens, Elmiskop 101). Samples (50 mL) were placed on 200 mesh formvar coated copper TEM grids, and then negatively stained with 50 mL of 1.5% (weight/volume) phosphotungstic acid for 10 min at room temperature. Excess liquid was blotted with Whatman filter paper. The measurements of droplets were performed using AxioVision software (Carl Zeiss; Oberkochen, Germany).
2.6. Effect of nanoemulsification on M. verticillata EO compounds
The effects of nanoencapsulation process on the EO volatile constituents was evaluated using GC-MS as previously was described. A sample of pure EO obtained from dry leaves of M. verticillata (collected in February 2020) and NE were analyzed. As Tween 20 and water are not compatible with the GC system, EO was separated using the non-polar solvent, n-hexane. The hexane layer was evaporated using a vacuum rotary evaporator. The residual oil was dried over anhydrous Na2SO4 and kept in a refrigerator (4 °C) until its use. The relative percentage of the identified constituents was calculated from the GC peak areas.
2.7. In vitro release of M. verticillata EO from nanoemulsion
The in vitro release experiments of the EO were carried out using the dialysis technique (Rodrigues et al., 2018). An aliquot of nanoemulsion was placed inside a dialysis bag (cellulose membrane, molecular weight cut-off 14,000 Da, Roth®), sealed, and immersed in a vessel containing 150 mL of 10 mM phosphate buffer solution (PBS, pH 7.4). The releasing system was maintained at 37 ± 1 °C, under magnetic stirring (100 rpm). Samples (1 mL) were taken out of the dissolution medium at different time intervals (1, 3, 6, 9 and 24 h). This volume was replaced with fresh PBS, and the sample was analyzed by UV spectrophotometry at 260 nm. In order to establish the linearity of the proposed method, three calibration curves were constructed at eight concentrations levels within the range of 2–10 μg/mL solubilizing a known amount of EO in ethanol. The release profile of M. verticillata EO was expressed as cumulative amount of released EO (mean ± SD) and plotted versus time. The experiments were carried out in triplicate.
2.8. In vitro evaluation of nanoemulsión
2.8.1. Antimicrobial activity
2.8.1.1. Bacterial culture
Staphylococcus aureus ATCC 29213 (wound isolated) was cultured in Trypticase Soy Broth (TSB, Britania Laboratory, Ref. B0410361, Argentina) and grown overnight at 37 °C. The concentration of the inoculum was estimated by spectrophotometric turbidity measurement at 600 nm on a microplate reader (Biolatin, Venezuela) and adjusted to an optical density (OD) of 0.07 which corresponds to a 0.5 McFarland standard (1 × 108 CFU/mL).
2.8.1.2. Antibacterial assay
In order to compare antibacterial activity, it was important to make sure that the concentrations of EO used both pure and encapsulated in the NE, were the same. Five concentrations of EO were chosen to be tested: 3, 9, 15, 21, and 27 mg/mL. Then, dilutions of the NE that would contain equal concentrations of EO mentioned above were calculated based on the density of the EO (950 mg/mL) and its ratio in the NE formulation (6%). The concentrations obtained were 50, 150, 250, 350 and 450 mg/mL of NE, which corresponded to 3, 9, 15, 21 and 27 mg/mL of EO encapsulated in the NE, respectively. In all cases, stock solutions of each concentration of EO and NE were prepared immediately before been used, diluted in phosphate buffered saline (PBS).
Antibacterial activity assay was conducted in 96-wells plates. Briefly, 10 μL of the initial inoculum standardized at 1 × 108 CFU/mL and 90 μL of the stock solutions of EO and NE (corresponding to the different concentrations) were added to each well. Then, all the wells were filled with 100 μL of fresh TSB for a final volume of 200 μL per well with a bacteria concentration of 1 × 106 CFU/mL in the wells. Growth control wells contained 10 μL of inoculum and 190 μL of TSB. Plates were incubated at 37 °C for 24 h.
The total number of bacteria was determined by plating serial dilutions (1:104 to 1:108) of the content of each well on Petri dishes containing Trypticase soy agar (TSA); the dishes were incubated at 37 °C for 24 h, and colonies counted. The number of bacteria was calculated, based on the dilution factor. To normalize the data, a positive control (0.2 % Triton x100) and negative control (PBS) were used.
2.8.2. Hemolytic activity
Bovine and equine peripheral blood samples were freshly collected and put into a test tube containing an anticoagulant (EDTA-Na2 10%). For the quantification of hemolytic activity, same concentrations of NE used in the previous assay were tested (50, 150, 250, 350 and 450 mg/mL). Aliquots of 50 μL of blood were mixed with the different solutions of NE; the tubes were homogenized and incubated for 60 min at room temperature. The samples were centrifuged at 2000 rpm for 5 min, and the supernatant was removed to measure the absorbance (540 nm) corrected with the blank (buffered solution containing red blood cells). The results were compared with a positive control in which the cells were completely lysed by distilled water. Experiments were carried out in triplicate. The hemolytic activity of each sample was calculated by the following equation:
| Hemolysis (%) = (DO sample - DO control (-)) / (DO control (+) - DO control (-)) × 100 | (2) |
2.9. Statistical analysis
Statistical analysis was performed using Infostat software (Di Rienzo et al., 2011). Data were represented as means standard deviation (SD). Differences were considered significant at the level of p < 0.05. Levels of significance were evaluated by Kruskal-Wallis test.
3. Results
3.1. Main compounds detected in M. verticillata EO
The essential oil obtained from fresh leaves showed a yield of 4.6% (w/v). Oil density was 0.95 g/mL and pH was equal to 5.0. In total, 13 compounds were identified with the predominance of monoterpenes and sesquiterpenes (98%). Chromatography showed that the most abundant chemical group of M. verticillata essential oil was oxygenated monoterpenes, a monoterpenes group attached to an oxygen atom and followed by monoterpenes (C10H16). Among these, the main oxygenated monoterpenes reported were monocyclic ketone such as pulegone (76.96%) and menthone (20.38%), and limonene (1.54%). Sesquiterpene group (C15H24) was represented by γ-elemene and spathulenol (Table 1).
Table 1.
Chemical composition of M. verticillata EO.
| Identified compounds | Molecular formula | Relative percentage (%) |
|---|---|---|
| δ-carene | C10H16 | 0.10 |
| β-pinene | C10H16 | 0.20 |
| Limonene | C10H16 | 1.54 |
| Menthone | C10H18O | 20.38 |
| ρ-menthone | C10H18O | 0.10 |
| Isopulegone | C10H16O | 0.15 |
| 2-Isopropyl-2,5-dimethylcyclohexanone | C11H20O | 0.14 |
| Pulegone | C10H16O | 76.96 |
| Piperitone | C10H16O | 0.15 |
| bicyclo,heptan-2-ol, 2-allyl-1,7,7-trimet | C10H16O | 0.10 |
| Piperitenone | C10H14O | 0.06 |
| γ-elemene | C15H24 | 0.07 |
| Spathulenol | C15H24O | 0.05 |
| Total | 100 |
3.2. Nanoemulsion characterization
3.2.1. Physicochemical properties
A continuous increase in pH was observed when surfactant concentration increased from 1:1 to 1:4 ratio by maintaining the oil concentration constant. The turbidity of NE decreased when the surfactant concentration increased, while its stability increased (Table 2). This indicates that varying o/s ratio has a high impact on the nature of colloidal surfaces. To carry out the electron microscopy and the antibacterial properties test, it was decided to use nanoemulsion D, due to its higher stability.
Table 2.
Physicochemical parameters of nanoemulsions.
| Formulation (oil:surfactant) | pH | Transmittance percentage (%) | Separation percentage (%) |
|---|---|---|---|
| A (1:1) | 3.02 ± 0.01 | 0.12 ± 0.02 | 20.35 ± 0.12 |
| B (1:2) | 3.31 ± 0.03 | 0.37 ± 0.04 | 18.55 ± 0.11 |
| C (1:3) | 3.41 ± 0.01 | 0.65 ± 0.01 | 18.98 ± 0.20 |
| D (1:4) | 3.64 ± 0.02 | 1.04 ± 0.02∗ | 12.71 ± 0.14∗ |
Data presented as mean (n = 3) ± standard deviation (SD). ∗ Indicate statistical significance (p < 0.05). Kruskal Wallis test, Infostat, 2011.
3.2.2. Morphology study
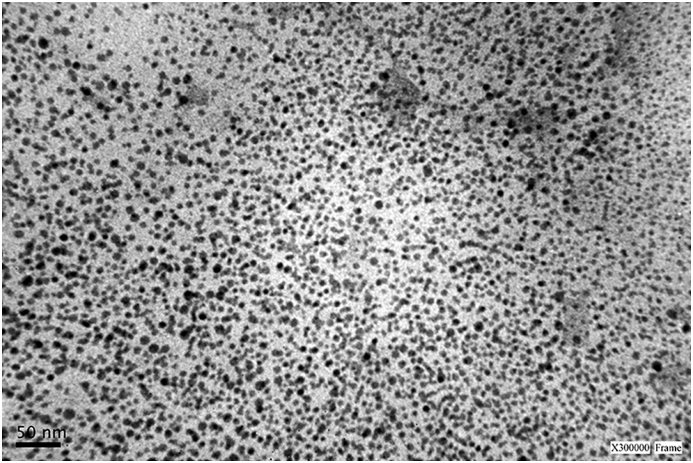
To study the NE morphology and distribution of the droplets size, transmission electron microscopy photomicrographs were taken, as shown in Figure 1. TEM showed discrete droplets of spherical shape, with an approximated diameter of 10 nm and without any aggregation.
Figure 1.
TEM image of nanoemulsion morphology (scale bar = 50nm; 300,000x Frame).
3.3. Effect of nanoemulsification on EO compounds
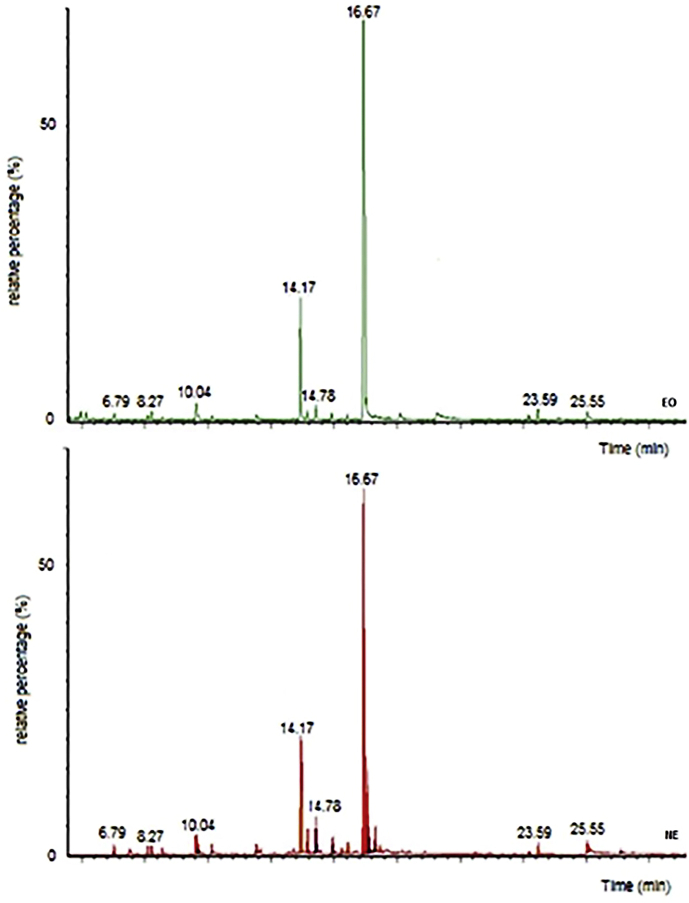
The volatiles composition of the NE was found to be similar from that of the EO. GC-MS analysis showed that the use of ultrahomogenization to prepare nanoemulsions led to no significant quantitative differences in the predominant compounds so the pulegone/menthone chemotype was maintained. Sample components of less than 1%are not reported (Table 3, Figure 2).
Table 3.
Comparison between relative percentage of chemical compounds of pure M. verticillata EO and nanoemulsified (NE).
| Identified compounds | Retention index (min) | Molecular formula | Relative percentage (%) in EO | Relative percentage (%) in NE |
|---|---|---|---|---|
| α-pinene | 6.79 | C10H16 | 1.17 | 1.66 |
| β-pinene | 8.27 | C10H16 | 1.38 | 1.74 |
| Limonene | 10.04 | C10H16 | 3.49 | 3.83 |
| Menthone | 14.17 | C10H18O | 21.22 | 20.86 |
| Isopulegone | 14.78 | C10H16O | 2.15 | 2.75 |
| Pulegone | 16.67 | C10H16O | 67.96 | 65.49 |
| γ-elemene | 23.59 | C15H24 | 1.23 | 1.78 |
| Spathulenol | 25.55 | C15H24O | 1.40 | 1.89 |
| Total | 100 | 100 |
Figure 2.
Chromatograms for EO (green) and NE (red) GC/MS measurement.
3.4. In vitro release of M. verticillata EO from nanoemulsion
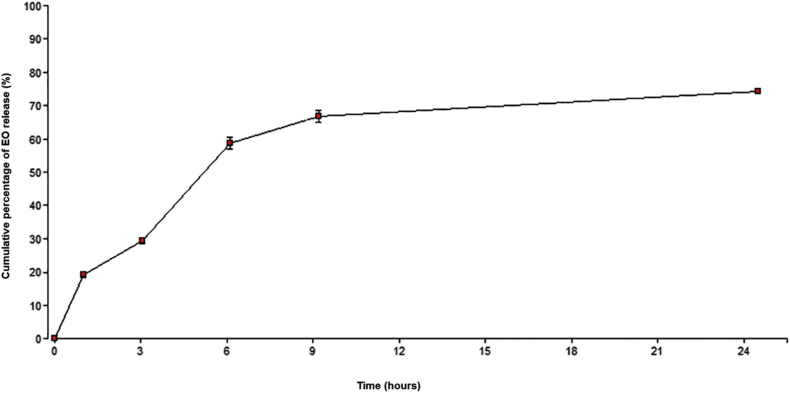
Release profile assay was carried out using in vitro dialysis experiment. As can be seen in Figure 3, after 3 h, the release of EO was 29.48 ± 0.96%. However, a sustained release of EO from the NE was observed during the first 6 h, in which more than 55% of EO was found to be released (58.75 ± 1.75). At the end of 24 h of experiment, 74.24 ± 0.75% of EO was released from NE.
Figure 3.
In vitro release profile of EO from the NE through dialysis membrane. Illustrated values correspond to the mean ± standard deviation (n = 3). Infostat, 2011.
3.5. In vitro evaluation of nanoemulsión
3.5.1. Antibacterial activity
The antibacterial activity after the incubation for 24 h of S. aureus with the different concentration of EO and NE was evaluated. The average percentage of bacterial growth inhibition of the highest concentration tested of both the EO (27 mg/mL) and the NE (450 mg/mL = 27 mg/mL of EO) was 46.72% ± 3.32 and 58.87% ± 0.99, respectively. A dose-dependent effect was not observed between all the concentrations of NE evaluated; however, a clear statistical significant difference was observed between the highest and lowest (50 mg/mL = 3 mg/mL of EO) NE concentrations. All the NE concentrations tested showed a stronger growth inhibition with statistical significant difference when compared to the same concentration of pure EO. No dose-dependent effect was observed between the concentrations of EO (Table 4).
Table 4.
Antibacterial effect after 24 h.
| EO Concentration (mg/mL) | Growth inhibition percentage (%) |
Log CFU/mL |
||
|---|---|---|---|---|
| EO | NE | EO | NE | |
| 3 | 43.67 ± 1.83 | 53.50 ± 1.36∗ | 5.66 ± 0.26a | 4.67 ± 0.18b |
| 9 | 44.52 ± 4.10 | 55.76 ± 1.89∗ | 5.57 ± 0.49a | 4.44 ± 0.24ab |
| 15 | 42.37 ± 0.45 | 54.46 ± 2.01∗ | 5.79 ± 0.13a | 4.57 ± 0.26ab |
| 21 | 45.48 ± 4.07 | 56.55 ± 0.94∗ | 5.47 ± 0.35a | 4.36 ± 0.15ab |
| 27 | 46.72 ± 3.32 | 58.87 ± 0.99∗ | 5.35 ± 0.41a | 4.13 ± 0.11a |
Data represents the mean (n = 3) ± standard deviation (SD). ∗ Indicate statistical significance between essential oil (EO) and nanoemulsión (NE) at same concentrations (p < 0.05). Different letters indicate statistical significance between concentrations of EO or NE (p < 0.05). Kruskal Wallis test, Infostat, 2011. Log CFU/mL in control group at 24 h = 10.04 ± 0.15.
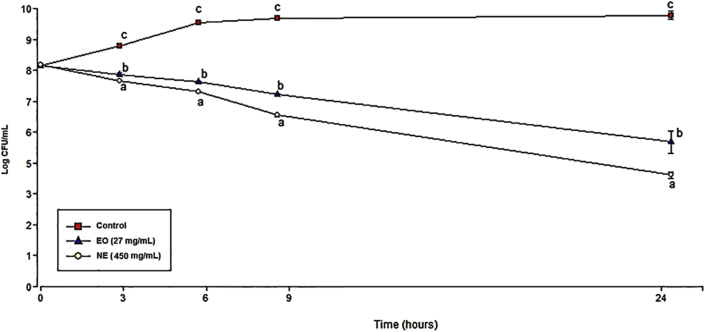
The effect of incubation with NE and EO on bacterial growth is showed in Figure 4. A significant reduction in bacterial growth was observed after 3 h (about 1.28 log UFC/mL). The greatest effect was found after 24 h of incubation with NE (450 mg/mL, equivalent to 27 mg/mL of EO) that produced a 5 log reduction approximately.
Figure 4.
Staphylococcus aureus growth (Log CFU/mL) after 24 h incubation with essential oil (EO, 27 mg/mL) and nanoemulsión (NE, 450 mg/mL equivalent to 27 mg/mL of EO). The illustrated values represent the mean ± standard deviation (n = 3). Different letters indicate significant differences between groups at same time (p < 0.05). Infostat, 2011. Initial log CFU/mL values at 0 h: Control (8.16 ± 0.04), EO (8.17 ± 0.03) and NE (8.20 ± 0.02). Final log CFU/mL values at 24 h: Control (10.04 ± 0.15), EO (5.35 ± 0.41) and NE (4.13 ± 0.11).
3.5.2. Hemolytic activity
The results of the hemolysis test are presented in Table 5. The absorbance of the erythrocytes suspension supernatant at 450 nm after incubation with different concentrations of nanoemulsion was studied. The hemolysis percentage values of all samples were less than 5%, suggesting no hemolytic activity by NE.
Table 5.
Percentage of hemolysis of NE in blood samples.
| Nanoemulsion concentration (mg/mL) | Porcine blood | Equine blood |
|---|---|---|
| 50 | 1.36 ± 0.58 | 0.68 ± 0,.20 |
| 150 | 1.04 ± 0.20 | 0.85 ± 0.03 |
| 250 | 0.81 ± 0.56 | 1.27 ± 0.45 |
| 350 | 1.07 ± 0.60 | 1.04 ± 0.60 |
| 450 | 0.71 ± 0.10 | 0.54 ± 0.30 |
Data represents mean ± standard deviation (SD) (n = 3). Infostat, 2011.
4. Discussion
Antimicrobial resistance has become a major medical and public health problem. New strategies to treat the infections caused by antibiotic-resistant pathogens are needed. In the present study, the development of antibacterial nanoemulsión based in natural products, was studied as an alternative method for controlling these diseases.
The extraction of M. verticillata essential oil (EO) by steam distillation of dry leaves resulted in a slightly yellow liquid with and estimated yield of 4.6% (w/v) based on dry weight. In a previous work, Cariddi et al. (2011), obtained a similar yield percentage to EO with M. verticillata species from central region of Argentina. Studies of wild peperina shows higher contents of EO in plants of the central area of Argentina than in those from northwest region, this differences were accompanied by changes in the number and size of the glandular trichomes, structures where the EO is synthesized and stored (Arteaga et al., 2016). The chromatographic analysis carried out in the present work reported that the main components identified in the EO were pulegone and menthone, in concordance with the chemotype reported by Zygadlo et al. (1996) for M. verticillata specimens from the central region of Argentina. Several studies focused on same species from this area have reported the same chemotype (Cariddi et al., 2011). It is important to highlight that although the results are coincident, there may be variations in the chemical composition of the EO. The percentage of every chemical compound not only depends on the aromatic species in question but also on its stage of development and the environmental conditions to which the plant is exposed at the time of collection (climate, soil, season) (Bakkali et al., 2005; Zygadlo et al., 1996).
Essential oils are known for their numerous biological activities (Conti et al., 2010), and the interest in their use has been renewed due to the need for innovative agents, capable of fighting bacterial resistance (Czaplewski et al., 2016; Franklyne et al., 2016; Thormar, 2010). However, its use is usually limited by its high volatility and tendency to readily degrade by oxidation and isomerization, which could be a drawback for its application as an antibacterial agent (Ghosh et al., 2013; Turek and Stintzing, 2013). In this context, nanoemulsions (NE) constitute a valid strategy to overcome this problem since they have a wide variety of advantages over pure essential oils, including better stability of the volatile compounds, and protect them against environmental factors that may cause chemical degradation (de Matos et al., 2019).
In the present study, NE was synthesized by ultrasonic emulsification, a high-energy method that rapidly and efficiently produces NEs with small droplet diameters (Manchun et al., 2014; Ghosh et al., 2013). The physical-chemical characterization of NE revealed that the different formulations presented similar pH, but the transmittance percentage and stability increased significantly with the enhancements of surfactant content. Scanning electron microscopy allowed establishing the average diameter of droplets, in around 10 nm, leading to the formation of a more homogeneous structure. Ghosh et al. (2014), obtained similar results with eugenol-loaded nanoemulsions using a non-ionic surfactant (tween 80). Previous studies revealed that nanoemulsions that minimize droplet size have a more homogeneous structure that leads to higher stability (de Oca-Ávalos et al., 2017). According to Bruxel et al. (2012), the use of nonionic surfactants from the group of poloxamer and polyoxyethylene sorbitans (tweens) has shown a favorable association with phospholipids, as they lead to the formation of compact mixed films, conferring greater stability to the formulation.
The application of encapsulated essential oils also supports their controlled and sustained release, which enhances their bioavailability and efficacy against multidrug-resistant pathogens (Prakash et al., 2018). Owing to its greater stability, the formulation with 1:4 rate (oil:surfactant) was selected for studying the antibacterial activity against S. aureus. This is a common opportunistic microorganism that is found in skin abrasions and open wounds and the most commonly isolated contagious organism from mastitis and dermatitis in cattle, sheep, goats, pigs, and horses (Peton and Le Loir, 2014; Boss et al., 2011).
Factors determining the activity of the EO are the composition functional groups present in the active components (Dorman and Deans, 2000) and the encapsulation method. The main compounds identified in M. verticillata EO were oxygenated terpenes. Previous studies state that oxygenated terpenes exhibit better antimicrobial activity than hydrocarbons (Guimarães et al., 2019) maybe through the inhibition of essential processes to microbial survival and alterations in the lipid membrane function (Mahizan et al., 2019; Cano et al., 2008).
An important requirement in the synthesis of essential oil based nanoemulsion is that the encapsulation system protects the active ingredients from chemical degradation and that the encapsulation process does not alter the bioactivity of the components. Although EOs are complex mixtures, their bioactivity is mainly dependent on a few molecules present at high concentrations. However, the compounds contained at low percentages are useful to enhance the effectiveness. Most of the actually studies that evaluate the functionality of nanoemulsions are conducted in simple model solutions, while the information regarding possible effects of the encapsulation process on the EO chemical components is scarce. For this reason, we evaluate the effect of encapsulation process on EO volatile compounds. GC-MS analysis showed no significant differences between EO and NE profile. Only few studies have evaluated changes on essential oil constituents after encapsulation. Donsì et al. (2012) reported that high-pressure homogenization (HPH) as well as high shear homogenization (HSH), results in decomposition of active constituents of EO particularly p-cymene, terpinenes, carveol, carvacrol and others. During emulsification by HPH there is an intrinsic increase in the temperature of the sample, which must be taken into account, especially when it comes to the production of nanoemulsions incorporating heat-sensitive active compounds (Salvia-Trujillo et al., 2017). Shukat and Relkin (2011) observed a substantial degradation of α-tocopherol in palm oil nanoemulsions produced with HPH. Ali et al. (2020) studied the effect of nanoencapsulation on volatile constituents of Algerian Origanum glandulosum Desf. EO that was encapsulated via HSH at 18000 rpm and via HPH. The results reported a significant decrease for carvacrol as the process intensity increased from HSH to HPH and showed that the volatiles profile of oil nanocapsules generated by HSH is still similar to that of the oil and corresponds to the thymol and/or carvacrol chemotype. In the present work, we used a lower homogenization speed than the previous reported works (approximately 8000 rpm for 5 min), only a slight percentage (~2.5%) of pulegone was affected.
A killing kinetics assay was performed to establish changes in the viability of S. aureus upon interaction with pure EO or NE over 24 h. A reduction of 1.28 log CFU/mL was observed after 3 h interaction of NE with the bacteria. The greatest effect was found after 24 h with NE (450 mg/mL, equivalent to 27 mg/mL of EO) reaching a reduction of approximately 5 Log CFU/mL. Sugumar et al. (2014) studied the antibacterial activity of eucalyptus oil nanoemulsion (undiluted) against S. aureus and obtained a complete loss of viability within 15 min of interaction. The antibacterial activity results founded in the present work clearly shows that the NE was more effective at inactivating the bacteria than pure EO. These results were in agreement with other studies that showed that the conversion of an EO (T. daenensis) into a NE greatly enhanced its antibacterial activity against an important food-borne pathogenic bacterium (E. coli) (Moghimi et al., 2016; Bhargava et al., 2015). The superior antimicrobial effect of NE can be explained by an increase in surface area that improves the efficacy of EO as it ensures larger contact with bacteria cells. Donsì et al. (2011) have shown that nanoemulsion-based delivery systems of d-limonene and a terpenes mixture significantly increased the antimicrobial activity of encapsulated compounds in comparison to non-encapsulated ones. This is probably because the small lipid particles inside the NE are capable of transport the EO towards the surface of the cell membranes, suggesting that the pure oil (which has little solubility) could not easily interact with the membranes (Bilia et al., 2017).
Safety is an important concern in the use of novel products such as NE. In vitro erythrocyte-induced hemolysis can be considered a simple and reliable measure for estimating the membrane damage caused in vivo (Pape and Hoppe, 1990). The behavior of M. verticillata EO based NE was studied by investigating the degree of hemolysis in vitro. No hemolytic activity on both porcine and equine blood was detected by any of NE concentrations evaluated. The NE toxicity is highly dependent on the chemical composition of the used oil (Mendanha et al., 2013). Safety assessment of M. verticillata EO has been demonstrated in vitro on Vero and Hep-2 cells (Escobar et al., 2012; Sutil et al., 2006), human lymphocytes (Cariddi et al., 2011) and bovine mammary epithelial cells (MAC-T) (Montironi et al., 2016). The administration of M. verticillata EO by different routes in experimental animals has also proven to be safe and it does not exert a cyto-genotoxic effect on the bone marrow and blood cells (Cariddi et al., 2011; Escobar et al., 2012; Montironi et al., 2019).
Our results suggest that a nanoemulsion containing M. verticillata EO may be a promising and safe formulation for S. aureus control, which also supports the idea that nanoformulations based on natural products can be considered an alternative as potential antimicrobial agents. Additional studies are necessary and ongoing to study the antimicrobial efficacy of NE against another bacterial pathogens and to reveal the mechanism of action of nanoencapsulated M. verticillata EO against these microorganisms.
5. Conclusion
M. verticillata EO nanoemulsion evidenced antibacterial activity against the S. aureus ATCC 29213 strain, improving its effect compared to the action of pure EO. Evaluating the safety of this formulation, we can conclude that M. verticillata nanoemulsión did not cause toxicity on porcine and equine red blood cells at all concentrations evaluated. The results obtained showed that nanoemulsion could be a potential vehicle for M. verticillata essential oil with promissory properties to antibacterial therapy.
Declarations
Author contribution statement
Cecchini ME: Performed the experiments; Analyzed and interpreted the data.
Soriano Perez ML; Campra N; Paoloni C: Performed the experiments.
Alustiza F; Grosso MC; Picco N: Contributed reagents, materials, analysis tools or data.
Bellingeri R; Cariddi N: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto (SECYT-UNRC).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
R. Bellingeri, L.N. Cariddi are permanent researchers of CONICET. M.E. Cecchini, N. Campra and ML Soriano thanks CONICET for a graduate fellowship.
References
- Adorjan B., Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragrance J. 2010;25(6):407–426. [Google Scholar]
- Ali H., Al-Khalifa A.R., Aouf A., Boukhebti H., Farouk A. Effect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-59686-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga M., Collado C.E., Gil A. Characterization of glandular trichomes of Minthostachys verticillata “peperina” from northwest and central Argentina: relation with essential oil content. J. Biodivers. Environ. Sci. 2016;8(4):172–181. https://repositorio.inta.gob.ar/handle/20.500.12123/2845 [Google Scholar]
- Asprea M., Leto I., Bergonzi M.C., Bilia A.R. Thyme essential oil loaded in nanocochleates: encapsulation efficiency, in vitro release study and antioxidant activity. LWT. 2017;77:497–502. [Google Scholar]
- Bajerski L., Michels L.R., Colomé L.M., Bender E.A., Freddo R.J., Bruxel F., Haas S.E. The use of Brazilian vegetable oils in nanoemulsions: an update on preparation and biological applications. Braz. J. Pharm. Sci. 2016;52(3):347–363. [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Zhiri A., Idaomar M. Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005;585(1-2):1–13. doi: 10.1016/j.mrgentox.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bhargava K., Conti D.S., da Rocha S.R., Zhang Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015;47:69–73. doi: 10.1016/j.fm.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Bilia A.R., Piazzini V., Guccione C., Risaliti L., Asprea M., Capecchi G., Bergonzi M.C. Improving on nature: the role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017;83(5):366–381. doi: 10.1055/s-0043-102949. [DOI] [PubMed] [Google Scholar]
- Boss R., Naskova J., Steiner A., Graber H.U. Mastitis diagnostics: quantitative PCR for Staphylococcus aureus genotype B in bulk tank milk. J. Dairy Sci. 2011;94(1):128–137. doi: 10.3168/jds.2010-3251. [DOI] [PubMed] [Google Scholar]
- Bruxel F., Laux M., Wild L.B., Fraga M., Koester L.S., Teixeira H.F. Nanoemulsões como sistemas de liberação parenteral de fármacos. Quím. Nova. 2012;35(9):1827–1840. [Google Scholar]
- Cano C., Bonilla P., Roque M., Ruiz J. Actividad antimicótica in vitro y metabolitos del aceite esencial de las hojas de Minthostachys mollis (muña) Rev. Peru. Med. Exp. Salud Pública. 2008;25(3):298–301. [Google Scholar]
- Cariddi L., Escobar F., Moser M., Panero A., Alaniz F., Zygadlo J., Sabini, Maldonado A. Monoterpenes isolated from Minthostachys verticillata (Griseb.) Epling essential oil modulates immediate-type hypersensitivity responses in vitro and in vivo. Planta Med. 2011;77(15):1687–1694. doi: 10.1055/s-0030-1271090. [DOI] [PubMed] [Google Scholar]
- Cerioli M.F., Moliva M.V., Cariddi L.N., Reinoso E.B. Effect of the essential oil of minthostachys verticillata (Griseb.) Epling and limonene on biofilm production in pathogens causing bovine mastitis. Front. Vet. Sci. 2018;5:146. doi: 10.3389/fvets.2018.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., McLandsborough L., McClements D.J. Physicochemical properties and antimicrobial efficacy of carvacrol nanoemulsions formed by spontaneous emulsification. J. Agric. Food Chem. 2013;61(37):8906–8913. doi: 10.1021/jf402147p. [DOI] [PubMed] [Google Scholar]
- Conti B., Canale A., Bertoli A., Gozzini F., Pistelli L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol. Res. 2010;107(6):1455–1461. doi: 10.1007/s00436-010-2018-4. [DOI] [PubMed] [Google Scholar]
- Cui H., Zhang C., Li C., Lin L. Inhibition of Escherichia coli O157: H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT. 2020;117:108656. [Google Scholar]
- Cui H., Bai M., Rashed M.M., Lin L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157: H7 biofilms on cucumber. Int. J. Food Microbiol. 2018;266:69–78. doi: 10.1016/j.ijfoodmicro.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Czaplewski L., Bax R., Clokie M., Dawson M., Fairhead H., Fischetti V.A., Foster S., Gilmore B.F., Hancock R.E., Henderson I.R. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect. Dis. 2016;16(2):239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- da Rocha Neto A.C., da Rocha A.B.D.O., Maraschin M., Di Piero R.M., Almenar E. Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocolloids. 2018;77:509–523. [Google Scholar]
- de Matos S.P., Teixeira H.F., de Lima Á., Veiga-Junior V.F., Koester L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: a review. Biomolecules. 2019;9(4):138. doi: 10.3390/biom9040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oca-Ávalos J.M.M., Candal R.J., Herrera M.L. Nanoemulsions: stability and physical properties. Curr. Opin. Food Sci. 2017;16:1–6. [Google Scholar]
- Di Rienzo J.A., Casanoves F., Balzarini M.G., González L., Tablada M., Robledo Y.C. Vol. 8. Universidad Nacional de Córdoba; Argentina: 2011. InfoStat Versión 2011. Grupo InfoStat, FCA; pp. 195–199.https://www.infostat.com.ar/ URL. [Google Scholar]
- Donsì F., Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Donsì F., Annunziata M., Vincensi M., Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J. Biotechnol. 2012;159(4):342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Donsì F., Annunziata M., Sessa M., Ferrari G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44(9):1908–1914. [Google Scholar]
- Dorman H.J., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- El Asbahani A., Miladi K., Badri W., Sala M., Addi E.H.A., Casabianca H., El Mousadik A., Hartmann D., Jilale A., Renaud F.N.R., Elaissari A. Essential oils: from extraction to encapsulation. Int. J. Pharm. 2015;483:220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- Escobar F.M., Cariddi L.N., Sabini M.C., Reinoso E., Sutil S.B., Torres C.V., Sabini L.I. Lack of cytotoxic and genotoxic effects of Minthostachys verticillata essential oil: studies in vitro and in vivo. Food Chem. Toxicol. 2012;50(9):3062–3067. doi: 10.1016/j.fct.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Foster T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017;41(3):430–449. doi: 10.1093/femsre/fux007. [DOI] [PubMed] [Google Scholar]
- Franklyne J.S., Mukherjee A., Chandrasekaran N. Essential oil micro and nanoemulsions: promising roles in antimicrobial therapy targeting human pathogens. Lett. Appl. Microbiol. 2016;63(5):322–334. doi: 10.1111/lam.12631. [DOI] [PubMed] [Google Scholar]
- Ghosh V., Mukherjee A., Chandrasekaran N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B Biointerfaces. 2014;114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Ghosh V., Saranya S., Mukherjee A., Chandrasekaran N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: investigation of its bactericidal activity. J. Nanosci. Nanotechnol. 2013;13(1):114–122. doi: 10.1166/jnn.2013.6701. [DOI] [PubMed] [Google Scholar]
- Gonçalves N.D., de Lima Pena F., Sartoratto A., Derlamelina C., Duarte M.C.T., Antunes A.E.C., Prata A.S. Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Res. Int. 2017;96:154–160. doi: 10.1016/j.foodres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Guimarães A.C., Meireles L.M., Lemos M.F., Guimarães M.C.C., Endringer D.C., Fronza M., Scherer R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24(13):2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano E.D., de Paula H.C., de Figueiredo E.A., Dias F.G., Pereira V.D.A. Physicochemical and antimicrobial properties of nanoencapsulated Eucalyptus staigeriana essential oil. LWT - Food Sci. Technol. 2015;61(2):484–491. [Google Scholar]
- León-Méndez Glicerio, Pájaro-Castro, Nerlis, Pájaro-Castro, Enilson, Torrenegra-Alarcon Miladys, Herrera-Barros Adriana. Essential oils as a source of bioactive molecules. Rev. Colomb. Cienc. Quim. Farm. 2019;48(1):80–93. [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Chi F., Cravens R.L., Oh S., Gay C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018;49(1):76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Gu Y., Sun Y., Cui H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT. 2019;107:16–24. [Google Scholar]
- Lou Z., Chen J., Yu F., Wang H., Kou X., Ma C., Zhu S. The antioxidant, antibacterial, antibiofilm activity of essential oil from Citrus medica L. var. sarcodactylis and its nanoemulsion. LWT. 2017;80:371–377. [Google Scholar]
- Mahizan N.A., Yang S.K., Moo C.L., Song A.A.L., Chong C.M., Chong C.W., Abushelaibi A., Lim S.E., Lai K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019;24(14):2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchun S., Dass C.R., Sriamornsak P. Designing nanoemulsion templates for fabrication of dextrin nanoparticles via emulsion cross-linking technique. Carbohydr. Polym. 2014;101:650–655. doi: 10.1016/j.carbpol.2013.09.049. [DOI] [PubMed] [Google Scholar]
- Mendanha S.A., Moura S.S., Anjos J.L., Valadares M.C., Alonso A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. Vitro. 2013;27(1):323–329. doi: 10.1016/j.tiv.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Moghimi R., Ghaderi L., Rafati H., Aliahmadi A., McClements D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016;194:410–415. doi: 10.1016/j.foodchem.2015.07.139. [DOI] [PubMed] [Google Scholar]
- Montironi I.D., Cariddi L.N., Reinoso E.B. Evaluation of the antimicrobial efficacy of Minthostachys verticillata essential oil and limonene against Streptococcus uberis strains isolated from bovine mastitis. Rev. Argent. Microbiol. 2016;48(3):210–216. doi: 10.1016/j.ram.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Montironi I.D., Reinoso E.B., Paullier V.C., Siri M.I., Pianzzola M.J., Moliva M., Campra N., Bagnis G., Ferreira LaRocque-de-Freitas I., Decote-Ricardo D., Geraldo Freire-de-Lima C., Raviolo J.M., Cariddi N. Minthostachys verticillata essential oil activates macrophage phagocytosis and modulates the innate immune response in a murine model of Enterococcus faecium mastitis. Res. Vet. Sci. 2019;125:333–344. doi: 10.1016/j.rvsc.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Noppakundilograt S., Piboon P., Graisuwan W., Nuisin R., Kiatkamjornwong S. Encapsulated eucalyptus oil in ionically cross-linked alginate microcapsules and its controlled release. Carbohydr. Polym. 2015;131:23–33. doi: 10.1016/j.carbpol.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Ojeda M., Coirini R., Cosiansi J., Zapata R., Zygadlo J. Evaluation of variability in natural populations of peperina (Minthostachys mollis (Kunth.) Griseb.), an aromatic species from Argentina. Plant Genet. Resour. Newslet. 2001;126:27–30. [Google Scholar]
- Pape W.J., Hoppe U. Standardization of an in vitro red blood cell test for evaluating the acute cytotoxic potential of tensides. Arzneim. Forsch. 1990;40(4):498–502. https://pubmed.ncbi.nlm.nih.gov/2357252/ [PubMed] [Google Scholar]
- Peton V., Le Loir Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014;21:602–615. doi: 10.1016/j.meegid.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Prakash B., Kujur A., Yadav A., Kumar A., Singh P.P., Dubey N.K. Nanoencapsulation: an efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Contr. 2018;89:1–11. [Google Scholar]
- Qian C., McClements D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocolloids. 2011;25(5):1000–1008. [Google Scholar]
- Rodrigues Fernando V.S., Diniz Laerte S., Sousa Rosa M.G., Honorato Thalita D., Simão Daniele O., Araújo Cleônia R.M., Gonçalves Talita M., Rolim Larissa A., Goto Patrícia L., Tedesco Antonio C., Siqueira-Moura, Marigilson P. Preparation and characterization of nanoemulsion containing a natural naphthoquinone. Quím. Nova. 2018;41(7):756–761. [Google Scholar]
- Rodrigues E.D.C., Ferreira A.M., Vilhena J.C., Almeida F.B., Cruz R.A., Florentino A.C., Souto R., Carvalho J., Fernandes C.P. Development of a larvicidal nanoemulsion with Copaiba (Copaifera duckei) oleoresin. Rev. Bras. Farmacogn. 2014;24(6):699–705. [Google Scholar]
- Salehi B., Konovalov D.A., Fru P., Kapewangolo P., Peron G., Ksenija M.S.…Pignata G. Areca catechu—from farm to food and biomedical applications. Phytother Res. 2020 doi: 10.1002/ptr.6665. [DOI] [PubMed] [Google Scholar]
- Salehi B., Krochmal-Marczak B., Skiba D., Patra J.K., Das S.K., Das G.…Al-Snafi A.E. Convolvulus plant—a comprehensive review from phytochemical composition to pharmacy. Phytother Res. 2020;34(2):315–328. doi: 10.1002/ptr.6540. [DOI] [PubMed] [Google Scholar]
- Salehi B., Azzini E., Zucca P., Maria Varoni E., V Anil Kumar N., Dini L.…Prakash Mishra A. Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl. Sci. 2020;10(3):947. [Google Scholar]
- Salehi B., Rescigno A., Dettori T., Calina D., Docea A.O., Singh L.…Sharifi-Rad J. Avocado–Soybean Unsaponifiables: a panoply of potentialities to be exploited. Biomolecules. 2020;10(1):130. doi: 10.3390/biom10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Upadhyay S., Erdogan Orhan I., Kumar Jugran A., LD Jayaweera S., A Dias D.…C Cho W. Therapeutic potential of α-and β-pinene: a miracle gift of nature. Biomolecules. 2019;9(11):738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Sharopov F., Martorell M., Rajkovic J., Ademiluyi A.O., Sharifi-Rad M.…Sharifi-Rad J. Phytochemicals in Helicobacter pylori infections: what are we doing now? Int. J. Mol. Sci. 2018;19(8):2361. doi: 10.3390/ijms19082361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvia-Trujillo L., Soliva-Fortuny R., Rojas-Graü M.A., McClements D.J., Martin-Belloso O. Edible nanoemulsions as carriers of active ingredients: a review. Annu. Rev. Food Sci. Technol. 2017;8:439–466. doi: 10.1146/annurev-food-030216-025908. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L., Qian C., Martín-Belloso O., McClements D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013;141(2):1472–1480. doi: 10.1016/j.foodchem.2013.03.050. [DOI] [PubMed] [Google Scholar]
- Schmidt-Lebuhn A.N. A revision of the genus Minthostachys (Labiatae) Mem. N. Y. Bot. Gard. 2008;98:1–77. [Google Scholar]
- Sharifi-Rad M., Roberts T.H., Matthews K.R., Bezerra C.F., Morais-Braga M.F.B., Coutinho H.D.…del Mar Contreras M. Ethnobotany of the genus Taraxacum—phytochemicals and antimicrobial activity. Phytother Res. 2018;32(11):2131–2145. doi: 10.1002/ptr.6157. [DOI] [PubMed] [Google Scholar]
- Shukat R., Relkin P. Lipid nanoparticles as vitamin matrix carriers in liquid food systems: on the role of high-pressure homogenisation, droplet size and adsorbed materials. Colloids Surf. B Biointerfaces. 2011;86(1):119–124. doi: 10.1016/j.colsurfb.2011.03.028. Wu, J. E., Lin, J., & Zhong, Q. (2014). Physical and antimicrobial characteristics of thyme oil emulsified with soluble soybean polysaccharide. Food Hydrocolloids., 39, 144-150. DOI. [DOI] [PubMed] [Google Scholar]
- Shrestha M., Ho T.M., Bhandari B.R. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017;221:1474–1483. doi: 10.1016/j.foodchem.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Sugumar S., Ghosh V., Nirmala M.J., Mukherjee A., Chandrasekaran N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014;21(3):1044–1049. doi: 10.1016/j.ultsonch.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Sutil S.B., Astesano A., Vogt V., Torres C.V., Zanon S.M., Sabini L.I. Minthostachys verticillata: toxicity of its essential oil and major constituents to Artemia salina and cell lines. Mol. Med. Chem. 2006;10:41–42. http://www.idecefyn.com.ar/mmcv10/13.pdf [Google Scholar]
- Thormar H. John Wiley & Sons; Chichester: 2010. Lipids and Essential Oils as Antimicrobial Agents.https://www.wiley.com/en-us/9780470976678 [Google Scholar]
- Turek C., Stintzing F.C. Stability of essential oils: a review. Compr. Rev. Food Sci. Food Saf. 2013;12(1):40–53. [Google Scholar]
- Zygadlo J.A., Maestri D.M., Lamarque A.L., Guzmán C.A., Velasco-Negueruela A., Pérez-Alonso M.J.…Grosso N.R. Essential oil variability of Minthostachys verticillata. Biochem. Systemat. Ecol. 1996;24(4):319–323. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.