Key Points
Question
Is there an association between metformin use and the development of age-related macular degeneration (AMD)?
Findings
In this large case-control study using a national database of patients, we found that metformin use was associated with decreased odds of developing AMD in a dose-dependent manner, with the greatest benefit at low to moderate dosages.
Meaning
The use of metformin may protect against the development of AMD and lead to a novel therapeutic strategy for the prevention of this disease.
This case-control study uses a national database to assess whether metformin use is associated with reduced odds of developing age-related macular degeneration.
Abstract
Importance
Age-related macular degeneration (AMD), the leading cause of irreversible blindness in older adults, appears to have no effective preventive measures. The common antidiabetic drug metformin has been shown to have protective outcomes in multiple age-associated diseases and may have the potential to protect against the development of AMD.
Objective
To determine whether metformin use is associated with reduced odds of developing AMD.
Design, Setting, and Participants
This case-control study of patients from a nationwide health insurance claims database included a population-based sample of patients. Those aged 55 years and older with newly diagnosed AMD from January 2008 to December 2017 were defined as cases and matched with control participants. Data analyses were completed from June 2019 to February 2020.
Exposures
Dosage of metformin and exposure to other prescribed medications, as identified from outpatient drug claims.
Main Outcomes and Measures
Risk of developing AMD.
Results
A total of 312 404 affected individuals were included (181 817 women [58.2%]). After matching, 312 376 control participants were included (172 459 women [55.2%]; age range, 55 to 107 years). The case group had a slightly higher percentage of participants with diabetes (81 262 participants [26.0%]) compared with the control group (79 497 participants [25.5%]). Metformin use was associated with reduced odds of developing AMD (odds ratio [OR], 0.94 [95% CI, 0.92-0.96]). This association was dose dependent, with low to moderate doses of metformin showing the greatest potential benefit (dosages over 2 years: 1-270 g, OR, 0.91 [95% CI, 0.88-0.94]; 271-600 g, OR, 0.90 [95% CI, 0.87-0.93]; 601-1080 g, OR, 0.95 [95% CI, 0.92-0.98]). Doses of more than 1080 g of metformin over 2 years did not have reduced odds of developing AMD. Both the reduction in odds ratio and the dose-dependent response were preserved in a cohort consisting only of patients with diabetes. Metformin use was associated with a decreased OR of AMD in patients with diabetes without coexisting diabetic retinopathy (OR, 0.93 [95% CI, 0.91-0.95]) but was a risk factor in patients with diabetic retinopathy (OR, 1.07 [95% CI, 1.01-1.15]).
Conclusion and Relevance
In this study, metformin use was associated with reduced odds of developing AMD. This association was dose dependent, with the greatest benefit at low to moderate doses. When looking only at patients with diabetes, we saw a preservation of the dose-dependent decrease in the odds of patients developing AMD. Metformin does not appear to be protective in patients with diabetes and coexisting diabetic retinopathy. This study suggests that metformin may be useful as a preventive therapy for AMD and provides the basis for potential prospective clinical trials.
Introduction
In the US, age-related macular degeneration (AMD) is the leading cause of irreversible blindness in adults older than 50 years. As the older adult population increases worldwide, there is a growing economic burden of AMD.1 Currently, there are no efficacious preventive measures for AMD development or available treatments for the nonexudative form, which accounts for 90% of cases.
Metformin, the most commonly prescribed oral antihyperglycemic drug for diabetes, has been shown to have antiaging and protective effects against many age-associated diseases.2 In epidemiology studies, metformin lowers the risk of developing cardiovascular disease, stroke, cancer, dementia, and primary open-angle glaucoma.3,4,5,6,7,8,9,10 Metformin also reduces inflammatory markers and increases life span in murine models.11,12 These exciting findings have led to the Targeting Aging With Metformin (TAME) trial, a prospective randomized clinical trial to assess metformin’s antiaging effects.13
Given the known antiaging effects of metformin and its relatively safe adverse-effect profile, we sought to determine if the use of metformin is associated with reduced odds of developing AMD in a large, nationwide sample. We replicated a case-control study that was conducted in a small health care system.14 To do this, we conducted a case-control study using a nationwide health insurance claims database. We also investigated a potential dose-dependent association in the study population, as well as in a subgroup of only patients with diabetes. Additionally, we evaluated whether the findings were dependent on the presence or absence of coexisting diabetic retinopathy (DR).
Methods
Data were derived from the IBM MarketScan Commercial and Medicare Supplemental Databases from January 2006 to December 2017. These databases represent the annual health services of approximately 30 to 50 million employees, dependents, and retirees annually in the US with primary or Medicare supplemental coverage through privately insured health plans. The databases include enrollment records and inpatient, outpatient, and outpatient prescription drug claims. The University of Chicago institutional review board approved this study and granted an exemption to requiring consent from study participants because identifiable private information had been removed from the data. Using the MarketScan databases, we identified cases as adults aged 55 years and older who were newly diagnosed with AMD from January 2008 to December 2017 and had at least 2 eye examinations during the previous 12 months. We identified AMD using International Classification of Disease, Ninth Revision and Tenth Revision (ICD-9 and ICD-10) codes, and eye examinations were identified using Current Procedural Terminology (CPT) codes (eTable 1 in the Supplement). We excluded patients who did not have continuous health insurance plan enrollment that included prescription drug coverage for 24 months prior to the diagnosis (eFigure 1 in the Supplement). Since we had the option of using more or fewer years of data, based on preliminary analyses, we chose to power the study to detect odds ratios (ORs) of 0.95 with 90% power in a subgroup of 76 000 individuals with diabetes, which required 10 years of cases. Odds ratios were calculated via logistic regression.
Age on the index date was classified into 10-year age groups (55-64, 65-74, 75-84, 85-94, and >95 years); there were very few participants older than 95 years, so they were combined with the subgroup aged 84 to 95 years for data analysis. Sex information was classified as men and women. Comorbidities associated with AMD were identified using ICD-9 and ICD-10 codes from inpatient and outpatient claims during the 12 months prior to and including the index date (eFigure 2 in the Supplement). Known risk factors for AMD and comorbidities were anemia, hypertension, diabetes, smoking, hyperlipidemia, nonproliferative DR, and proliferative DR (eTable 1 in the Supplement).15,16,17,18,19,20,21 Patients were considered to have a comorbidity if at least 1 ICD code was observed. We also computed modified Charlson Comorbidity Index (CCI) scores using inpatient and outpatient data for 12 months, ending on the index date. Charlson comorbidities were myocardial infarction, congestive heart failure, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease/rheumatic disease, peptic ulcer disease, mild liver disease, paraplegia and hemiplegia, kidney disease, cancer, moderate or severe liver disease, metastatic carcinoma, and HIV/AIDS. Patients were classified as having a weighted CCI score of 0, 1, 2 or 3 or more. Patients were classified into 4 regions (Northeast, South, North Central, and West) using the location of residence during the year of the index event.
We selected a control group from the general population represented in MarketScan data, matched 1:1 to the cohort of affected patients based on age, anemia, hypertension, region, and CCI score. The selected matching variables were based on methods used in similar studies.8,10,14,20 To identify a pool of candidates from all patients in the MarketScan databases, each year, each enrolled adult 55 years or older was screened for eye examinations. Eligible candidates had 2 or more eye examinations during the prior 12 months. An index date was randomly selected from all dates of eye examinations. Candidates also had continuous health insurance plan coverage that included prescription drug coverage during the 24 months prior to the index date. Potential control participants could be included in the pool in multiple years but were excluded from the control pool once they had an incident or previous AMD diagnosis. Such patients would initially be counted as a control participant and then become a participant with a case in later years. Since the control pool was quite large, for each year’s individuals with cases, control participants were randomly selected from among those with exact matches based on 5 criteria: age on index date, anemia, hypertension, region, and CCI score (0, 1, 2, or ≥3). We chose not to match by diabetes status because, by design, matching criteria do not vary between groups; however, we wanted to test the independent effect of diabetes in adjusted models. After selection, the remaining comorbidities were operationalized for control participants using the same criteria as for participants with cases.
To operationalize exposures, outpatient prescription drug claims for participants with cases and control participants were examined for the 24 months preceding the index date. We identified claims by National Drug Code for drugs with generic names that included names of diabetes medications (metformin, insulin, sulfonureas, glitazones, meglitinides, and others) and statins, which are listed in eTable 1 in the Supplement. Patients with exposures had to have at least 1 outpatient prescription drug claim for a medication within 2 years preceding the index date. We calculated total exposure to metformin and classified the dosage by approximate quartiles as 0, 1 to 270, 271 to 600, 601 to 1080, and more than 1080 g over 2 years. We excluded participants with cases involving ambiguous or incomplete claims data.
Statistical Analysis
Descriptive statistics for participants with cases, the control pool, and control participants were frequencies and percentages for categorical variables or means and SDs for continuous variables. To examine significant differences in categorical sample characteristics and exposures between participants with cases and control participants, we used χ2 tests. We report standard differences between the 2 groups to assess the balance of the matched samples. Univariable logistic regressions tested the bivariate associations between incident AMD and sex, diabetes, smoking, hyperlipidemia, nonproliferative and proliferative DR, metformin, insulin, sulfonureas, glitazones, meglitinides, other diabetes medications, and statins. Multivariable logistic regression models were constructed to determine whether the medications were risk factors for AMD by estimating the influence of diabetes medication and various comorbidities on incident AMD. One model tested any medication use, and a second model tested categories of 2-year cumulative dosage of metformin. We repeated these regressions using the subgroup of participants with cases and control participants with diabetes. All regressions were stratified by year, 10-year age group, anemia, hypertension, region, and CCI score. The comorbidities included in our multivariable analyses were age, sex, region, hypertension, hyperlipidemia, obesity, CCI score, diabetes, smoking, diabetic medications, statins, and DR. Two-sided tests were considered significant with an α of .05. We used SAS version 9.4 (SAS Institute) for all analyses. Analyses took place from June 2019 to February 2020.
Results
We identified 312 404 patients who met the case inclusion criteria for AMD (eFigure 2 in the Supplement). The total control pool was 31 343 467 eligible patients (Table 1). Compared with control participants, those with cases were older (eg, 55-64 years: control participants, 20 477 450 [65.3%] vs participants with cases, 65 891 [21.1%]; 75-84 years: 3 233 025 [10.3%] vs 108 734 [34.8%]; ≥85 years: 774 606 [2.5%] vs 51 955 [16.6%]), more often resided in the US Northeast region (control participants, 4 829 594 [15.4%] vs participants with cases, 60 734 [19.4%]) and the US North Central region (control participants, 8 686 649 [27.7%] vs participants with cases, 96 413 [30.9%]), were more likely to have hypertension (control participants, 16 689 204 [53.3%] vs participants with cases, 203 463 [65.1%]) and anemia (control participants, 1 209 904 [3.9%] vs participants with cases, 20 197 [6.5%]), and had a higher CCI score (eg, CCI score of 1: control participants, 5 305 055 [16.9%] vs participants with cases, 63 305 [20.3%]; CCI score of 2: 3 377 497 [10.8%] vs 47 842 [15.3%]; CCI score of ≥3: 2 327 571 [7.4%] vs 46 525 [14.9%]). After matching, 312 376 control participants were included in the analysis (Table 2). The case group included 181 817 women (58.2%), compared with 172 459 women (55.2%) in the control group (P < .001); the overall age range was 55 to 107 years. Those with cases were slightly more likely to have diabetes than control participants (participants with cases, 81 262 [26.0%] vs control participants, 79 497 [25.5%]; P < .001) and also more likely to smoke (17 841 [5.7%] vs 12 920 [4.1%]) and have hyperlipidemia (155 080 [49.6%] vs 149 627 [47.9%]), nonproliferative DR (11 400 [3.7%] vs 5281 [1.7%]), and proliferative DR (2241 [0.7%] vs 1229 [0.4%]). As expected from exact matching, the samples were balanced on age, region, year, CCI score, hypertension, and anemia (eTable 2 in the Supplement).
Table 1. Cohort Characteristics Before Matching, MarketScan Research Databases 2008-2017.
| Characteristic | Control pool, person-years (%) | Participants with cases, No. (%) |
|---|---|---|
| Total | 31 343 467 (100) | 312 404 (100) |
| Age, y | ||
| 55-64 | 20 477 450 (65.3) | 65 891 (21.1) |
| 65-74 | 6 858 386 (21.9) | 85 824 (27.5) |
| 75-84 | 3 233 025 (10.3) | 108 734 (34.8) |
| ≥85 | 774 606 (2.5) | 51 955 (16.6) |
| Mean (SD) | 64.4 (8.4) | 74.9 (10.3) |
| Region | ||
| Northeast | 4 829 594 (15.4) | 60 734 (19.4) |
| North Central | 8 686 649 (27.7) | 96 413 (30.9) |
| South | 12 154 200 (38.8) | 102 207 (32.7) |
| West | 5 673 024 (18.1) | 53 050 (17.0) |
| Hypertension | 16 689 204 (53.3) | 203 463 (65.1) |
| Anemia | 1 209 904 (3.9) | 20 197 (6.5) |
| Charlson Comorbidity Index score | ||
| 0 | 20 333 344 (64.9) | 154 732 (49.5) |
| 1 | 5 305 055 (16.9) | 63 305 (20.3) |
| 2 | 3 377 497 (10.8) | 47 842 (15.3) |
| ≥3 | 2 327 571 (7.4) | 46 525 (14.9) |
| Mean (SD) | 0.69 (1.22) | 1.10 (1.50) |
| Years in study | ||
| 2008-2011 | 11 428 666 (36.5) | 107 706 (34.5) |
| 2012-2014 | 10 930 937 (34.9) | 119 020 (38.1) |
| 2015-2017 | 8 983 864 (28.7) | 85 678 (27.4) |
Table 2. Sample Characteristics, MarketScan Research Databases, January 2008 to December 2017.
| Characteristic | Participants, No (%) | ||
|---|---|---|---|
| With cases | Control | Total | |
| Total | 312 404 (100) | 312 376 (100) | 624 780 (100) |
| Age, y | |||
| 55-64 | 65 891 (21.1) | 65 903 (21.1) | 131 794 (21.1) |
| 65-74 | 85 824 (27.5) | 85 878 (27.5) | 171 702 (27.5) |
| 75-84 | 108 734 (34.8) | 108 768 (34.8) | 217 502 (34.8) |
| ≥85 | 51 955 (16.6) | 51 827 (16.6) | 103 782 (16.6) |
| Mean (SD) | 74.9 (10.3) | 74.9 (10.3) | 74.9 (10.3) |
| Sex | |||
| Men | 130 587 (41.8) | 139 917 (44.8) | 270 504 (43.3) |
| Women | 181 817 (58.2) | 172 459 (55.2) | 354 276 (56.7) |
| Region | |||
| Northeast | 60 734 (19.4) | 60 700 (19.4) | 121 434 (19.4) |
| North Central | 96 413 (30.9) | 96 384 (30.9) | 192 797 (30.9) |
| South | 102 207 (32.7) | 102 180 (32.7) | 204 387 (32.7) |
| West | 53 050 (17.0) | 53 112 (17.0) | 106 162 (17.0) |
| Years in study | |||
| 2008-2011 | 107 706 (34.5) | 107 697 (34.5) | 215 403 (34.5) |
| 2012-2014 | 119 020 (38.1) | 119 028 (38.1) | 238 048 (38.1) |
| 2015-2017 | 85 678 (27.4) | 85 651 (27.4) | 171 329 (27.4) |
| Charlson Comorbidity Index score | |||
| 0 | 154 732 (49.5) | 154 768 (49.6) | 309 500 (49.5) |
| 1 | 63 305 (20.3) | 63 279 (20.3) | 126 584 (20.3) |
| 2 | 47 842 (15.3) | 47 823 (15.3) | 95 665 (15.3) |
| ≥3 | 46 525 (14.9) | 46 506 (14.9) | 93 031 (14.9) |
| Mean (SD) | 1.1 (1.5) | 1.1 (1.5) | 1.1 (1.5) |
| Diabetes | 81 262 (26.0) | 79 497 (25.5) | 160 759 (25.7) |
| Smoking | 17 841 (5.7) | 12 920 (4.1) | 30 761 (4.9) |
| Hyperlipidemia | 155 080 (49.6) | 149 627 (47.9) | 304 707 (48.8) |
| Hypertension | 203 463 (65.1) | 203 475 (65.1) | 406 938 (65.1) |
| Anemia | 20 197 (6.5) | 20 122 (6.4) | 40 319 (6.5) |
| Nonproliferative DR | 11 400 (3.7) | 5281 (1.7) | 16 681 (2.7) |
| Proliferative DR | 2241 (0.7) | 1229 (0.4) | 3470 (0.6) |
Abbreviation: DR, diabetic retinopathy.
The exposure rate to metformin for individuals with cases was 12.8% (40 081 of 312 404), and for control participants, it was 13.0% (40 730 of 312 376; P = .01; eTable 3 in the Supplement), with very small differences in the dosages of each group. The case group had slightly more patients with no exposure (participants with cases, 272 323 [87.2%] vs control participants, 271 646 [87.0%]) and with the highest doses (9833 [3.2%] vs 9121 [2.9%]) and slightly smaller percentages of those with low to moderate doses (271-600 g/2 years: 9097 [2.9%] vs 9780 [3.1%]; 601-1080 g/2 years: 11 010 [3.5%] vs 11 075 [3.6%]: P < .001). Mean total metformin dose over 2 years was 116 g in both groups (P = .06).
Univariable analysis identified several associations in patients with AMD, including known risk factors such as smoking (OR, 1.43 [95% CI, 1.39-1.46]) and diabetes (OR, 1.03 [95% CI, 1.02-1.04]) (Table 3). This analysis also showed an association with metformin use. The use of any metformin over 2 years reduced the odds of developing AMD (OR, 0.98 [95% CI, 0.97-1.00]; P = .01). In the multivariable model adjusting for known risk factors and other medications, the reduced odds associated with metformin persisted (OR, 0.94 [95% CI, 0.92-0.96]; P < .001), and a dose-dependency was revealed, with low to moderate doses being the most protective. Metformin doses of 1 to 270 g over 2 years had reduced odds of AMD (OR, 0.91 [95% CI, 0.89-0.94]; P < .001), with a similar finding for 271 to 600 g over 2 years. Taking 601 to 1080 g of metformin over 2 years had an OR of 0.95 (95% CI, 0.93-0.98; P = .002). The highest dose of more than 1080 g over 2 years had no difference in AMD incidence.
Table 3. Univariable and Multivariable Logistic Regression Models for Incident Age-Related Macular Degeneration Cases and Matched Controls 55 Years and Older Using MarketScan Research Databases, January 2008 to December 2017 (N = 624 780)a.
| Characteristic | Odds ratio (95% CI) | ||
|---|---|---|---|
| Univariable models | Any metformin | Metformin dose | |
| Risk factor | |||
| Women | 1.13 (1.12-1.14) | 1.14 (1.13-1.15) | 1.14 (1.13-1.15) |
| Diabetes | 1.03 (1.02-1.04) | 1.03 (1.02-1.05) | 1.03 (1.02-1.05) |
| Smoking | 1.43 (1.39-1.46) | 1.45 (1.411.48) | 1.45 (1.41-1.48) |
| Hyperlipidemia | 1.08 (1.07-1.10) | 1.10 (1.08-1.11) | 1.10 (1.08-1.11) |
| Nonproliferative diabetic retinopathy | 2.22 (2.15-2.30) | 2.33 (2.25-2.42) | 2.32 (2.24-2.41) |
| Proliferative diabetic retinopathy | 1.84 (1.71-1.97) | 1.26 (1.17-1.36) | 1.26 (1.17-1.36) |
| Medications | |||
| Any metformin | 0.98 (0.97-1.00) | 0.94 (0.92-0.96) | NA |
| Metformin dose, g/2 y | |||
| 1-270 | 0.94 (0.92-0.97) | NA | 0.91 (0.89-0.94) |
| 271-600 | 0.93 (0.90-0.96) | NA | 0.90 (0.87-0.93) |
| 601-1080 | 0.99 (0.96-1.02) | NA | 0.95 (0.92-0.98) |
| >1080 | 1.07 (1.04-1.10) | NA | 1.02 (0.99-1.05) |
| Insulin | 1.06 (1.03-1.08) | 0.91 (0.89-0.93) | 0.91 (0.89-0.93) |
| Sulfonureas | 0.96 (0.95-0.98) | 0.92 (0.90-0.94) | 0.91 (0.89-0.93) |
| Glitazones | 0.97 (0.94-0.99) | 0.96 (0.93-0.98) | 0.95 (0.92-0.98) |
| Meglitinides | 1.04 (0.98-1.11) | 1.00 (0.94-1.07) | 1.00 (0.94-1.06) |
| Other diabetes medications | 1.08 (1.05-1.11) | 1.09 (1.06-1.12) | 1.08 (1.05-1.11) |
| Statins | 0.98 (0.97-0.99) | 0.95 (0.94-0.96) | 0.95 (0.94-0.96) |
| Akaike Information Criterion | NA | 848 475 | 848 436 |
Abbreviation: NA, not applicable.
Stratified by age group, anemia, hypertension, Charlson Comorbidity Index (0, 1, 2, and ≥3), region, and year.
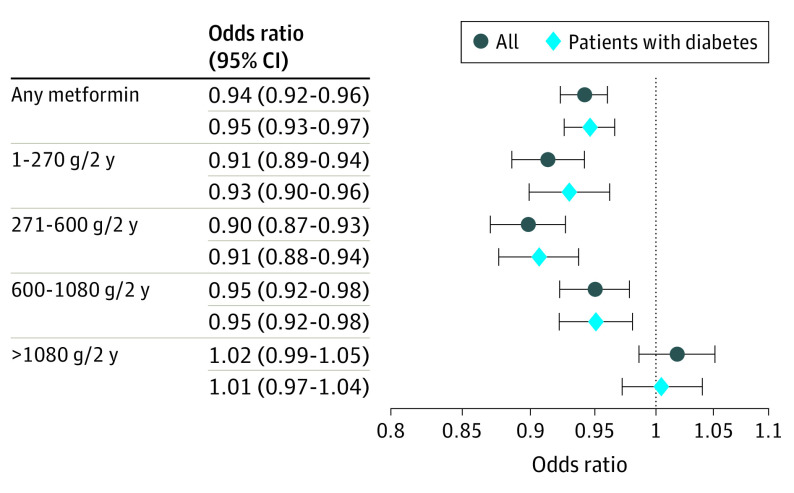
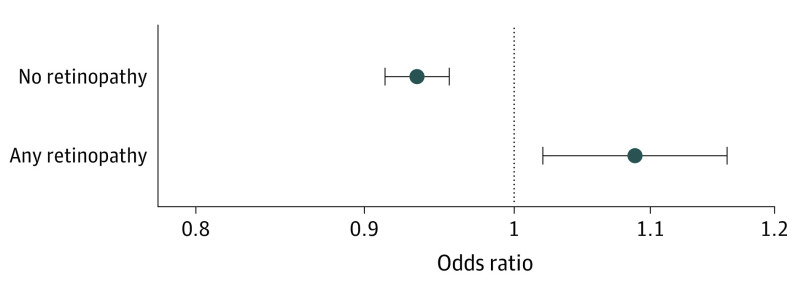
Since metformin is most commonly used as an antidiabetic drug, subgroup analyses were performed to examine associations of exposures with AMD among patients with diabetes. As seen in the full cohort of patients, patients with diabetes had a reduced odds of developing AMD when using metformin in the 2 years prior (OR, 0.95 [95% CI, 0.93-0.97]; P < .001) (Figure 1). A dose-dependent outcome was redemonstrated, with the largest benefit seen at small to moderate doses of metformin. The greatest reduction in risk was seen at metformin doses of 271 to 600 g over 2 years with an OR of 0.91 (95% CI, 0.88-0.94). Doses of 1 to 270 g over 2 years and 600 to 1080 g over 2 years were also associated with reduced odds (ORs, 0.93 [95% CI, 0.90-0.96] and 0.95 [95% CI, 0.92-0.98], respectively). As in the full cohort, the highest metformin dose of more than 1080 g over 2 years had no association with AMD incidence. Furthermore, metformin use was only reduced the odds of AMD in the absence of DR (OR, 0.93 [95% CI, 0.91-0.95]; P < .0001). In contrast, the presence of DR was associated with an increased odds of AMD in patients using metformin (OR, 1.07 [95% CI, 1.01-1.15]; P = .03) (Figure 2).
Figure 1. Odds Ratios for Metformin by Dose in the Full Cohort and Only Patients With Diabetes.
Error bars show 95% CIs.
Figure 2. Odds Ratios for Metformin in Patients With Diabetes With and Without Diabetic Retinopathy.
Error bars show 95% CIs.
Although we did not include diabetes as a matching variable by design, the matched samples had similar percentages with diabetes (81 262 [26.0%] in the case group and 79 497 [25.5%] in the control group). When looking at exposure to the subgroup of only patients with diabetes, there was an expected increase in the proportion of patients taking metformin (participants with cases, 38 580 [47.5%] vs those without cases, 38 388 [48.3%]; P = .001), as well as insulin (18 277 [22.5%] vs 17 175 [21.6%]; P < .001), sulfonureas (25 509 [31.4%] vs 25 919 [32.6%]; P < .001), glitazones (9863 [12.1%] vs 9958 [12.5%]; P = .02), and other antidiabetic medications (11 969 [14.7%] vs 10 987 [13.8%]; P < .001) (eTable 4 in the Supplement). Although exposure to medications was greater, the differences in exposure between participants with cases and control participants were the same as in the full sample. In bivariate analyses, the unadjusted risk of incident AMD was similar to the full sample, as were the adjusted models, suggesting that diabetes is not among the most important risk factors for AMD (eTable 5 in the Supplement).
In evaluation of other medications, univariable and multivariable analyses showed a small decrease in the odds of developing AMD with insulin (univariable OR, 1.06 [95% CI, 1.03-1.08]; P < .001; multivariable OR, 0.91 [95% CI, 0.89-0.93]; P < .001), sulfonureas (univariable OR, 0.96 [95% CI, 0.95-0.98]; P < .001; multivariable OR, 0.92 [95% CI, 0.90-0.94]; P < .001), glitazones (univariable OR, 0.97 [95% CI, 0.94-0.99]; P < .001; multivariable OR, 0.96 [95% CI, 0.93-0.98]; P < .001), and statins (univariable OR, 0.98 [95% CI, 0.97-0.99]; P < .001; multivariable OR, 0.95 [95% CI, 0.94-0.96]; P < .001). In the full sample, patients with AMD had higher rates of insulin use (18 257 [5.8%] in the case group and 17 383 [5.6%] in the control group; P < .001) and lower rates of metformin (40 081 [12.8%] vs 40 730 [13.0%]; P = .01), sulfonurea (25 775 [8.3%] vs 26 702 [8.6%]; P < .001), and statin use (statin (162 856 [52.1%] vs 165 393 [53.0%]; P < .001) (eTable 3 in the Supplement). Although statistically different in this large database, exposure to all medications differed by less than 1.0% between groups.
Discussion
This case-control study using a large, national sample suggests that metformin use over 2 years in adults aged 55 years and older is associated with 5% to 10% reduced odds ratio of developing AMD. Moreover, there is a dose-dependent association of this potential protective effect, with low to moderate doses of metformin being associated with the lowest odds ratio for the development of AMD, suggestive of a J-shaped or U-shaped nonlinear dose-response curve. The association of high doses of metformin was similar to no exposure. It is possible that high doses of metformin may be more commonly used in patients with poorly controlled diabetes and such patients may benefit less from metformin use. In a subgroup of only patients with diabetes, similar ORs were seen when compared with the study population as a whole. Metformin was found to decrease the odds of new AMD among patients with diabetes and without DR, but this same outcome was not seen in patients with coexisting DR. To our knowledge, this is the largest study investigating the association between metformin and the development of AMD.
Metformin has been shown to have beneficial effects on multiple age-associated diseases. The UK Prospective Diabetes Study (UKPDS), a randomized intervention trial on patients with overweight and newly diagnosed diabetes, suggested a cardioprotective outcome of metformin use.3 This was substantiated in the Hyperinsulinemia: the Outcome of its Metabolic Effects (HOME) trial, a placebo-controlled randomized clinical trial that showed that addition of metformin to the drug regimens of patients already receiving insulin treatment was associated with the prevention of macrovascular events.4 Metformin has also been associated with a decreased rate of several cancers, particularly breast, lung, and colorectal cancers.2,5,6,7,8 The Singapore Longitudinal Aging Study revealed a significant protective outcome of metformin use in cognitive impairment.9 Metformin use has also been shown to reduce the risk of ocular age-associated disease, such as primary open-angle glaucoma.10
Work in in vitro studies and animal models has demonstrated that metformin affects multiple pathways that may modulate the biology of aging. Metformin acts directly and indirectly on several targets, including 5′ adenosine monophosphate-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), and sirtuin-1 (SIRT1) to affect important cell functions, such as survival, stress defense, autophagy, oxidative stress, protein synthesis, and inflammatory response.11,22,23,24,25,26,27,28,29 The scope of this study does not determine the molecular pathways involved in the potential protective outcome of metformin use in the development of AMD. Further studies are needed to investigate the role of metformin on these pathways specifically as it pertains to the pathogenesis of AMD.
Brown et al14 recently reported the protective outcomes of metformin use in AMD development in patients at the University of Florida. They found a 42% reduction in the odds of developing AMD with metformin use, in contrast with the 5% to 10% in this study.22 There are several key differences between the 2 studies, some of which are highlighted by the characteristics of the study populations. Importantly, the patients at the University of Florida have an exceptionally high mean CCI score (4.2, compared with 1.1 in this study), suggesting a substantially sicker population. Additionally, the frequency of diabetes in the University of Florida case and control groups was dissimilar (43% in the case group and 70% in the control group, compared with 26.0% and 25.5%, respectively, in this study). Although Brown et al14 included other potential drugs in their analysis, the omission of insulin, which was also shown to reduce the odds of AMD development in this study, may have inflated the outcome of metformin use. They also did not collect data on smoking, a known risk factor for the development of AMD. It is likely that our large population of patients drawn from a nationwide database is more representative of the US as a whole.
An additional finding of interest from our study is the possible association between DR and AMD. Diabetes appears to be a risk factor for AMD,17 but the presence of DR has shown an inconsistent role in the development of AMD. Two longitudinal studies have suggested that DR may increase the risk of AMD,20,21 while other studies have revealed a possible protective outcome of DR.30,31 Our study provides additional evidence that DR may be a risk factor for the development of AMD. In this large cohort of patients, those with AMD were more likely to have DR. We also found that the potential protective outcome of metformin use is seen in patients with diabetes in the absence of DR.
There are strengths of using a large cohort of patients for a case-control study. The use of diabetic medications may have small but meaningful effects on the risk of developing incident AMD; yet, by using MarketScan data, we are able to capture these outcome with a high degree of precision.
Limitations
A limitation of our study is that we are unable to determine the probability of developing AMD; rather, we are reporting the ORs of the association of metformin use with having AMD. Another limitation of this study is that it relies on diagnosis codes from billing records. It is possible that billing records underreport diagnoses or include incorrect diagnoses. For example, mild AMD may be coded as drusen, which, in the absence of an additional AMD code, was not included in our analysis, because it may often refer to peripheral retinal drusen. Using ICD coding for smoking has an excellent specificity but poor sensitivity and likely does not capture all of the individuals who smoked within our study cohort.32 The large population in this database would be expected to outweigh small numbers of misdiagnoses or incorrect coding. Additionally, the MarketScan database includes information on patients from private insurance only, which is not fully representative of the older population. Because we do not have access to medical records, we are unable to see patient characteristics, including demographic information, such as ethnicity, as well as medical information, such as the level of hemoglobin A1c. Because of this limitation, it is possible that there are ethnic differences in the patients who are taking metformin compared with the patients who do not take metformin. Knowledge of hemoglobin A1c levels may be useful in separating the antihyperglycemic effect of metformin from its anti-inflammatory or other effects. These data are insufficient to determine if metformin is acting as a director mediator of AMD or if the outcome of metformin use is, in part, through a reduction in blood glucose levels. Future studies can be aimed at identifying the pathways responsible for the potential beneficial effect of metformin, to further uncover the potential role of metformin as a novel strategy to prevent or slow AMD.
Conclusions
Knowing the beneficial associations that metformin has been shown to have with multiple diseases of aging, we asked if it could also play a role in reducing the odds of AMD. We found that not only does metformin reduce the odds of developing AMD, but that this outcome is strongest at low to moderate doses and is only seen in the absence of coexisting DR. This study highlights metformin as a possible therapeutic intervention to prevent or slow the progression of AMD. Future studies will be important to further validate and confirm this finding, in addition to determining the molecular mechanisms involved and which pathogenic pathways of AMD are affected by metformin. If a protective effect of metformin is confirmed in clinical trials, this may lead to a novel therapeutic strategy for this disease, which is the leading cause of blindness in older adults and has no previously established preventive measures.
eTable 1. ICD codes, CPT codes, and generic drugs names
eFigure 1. Sample selection flowchart.
eFigure 2. Study design.
eTable 2. Balance after matching, MarketScan Research Databases 2008-2017.
eTable 3. Exposures to metformin, other diabetes medications, and statins over 2 years, MarketScan Research Databases 2008-2017.
eTable 4. Exposures to metformin, other diabetes medications, and statins over 2 years among subgroup of diabetes patients (N=81,262 cases, 79,497 controls), MarketScan Research Databases 2008-2017.
eTable 5. Models for diabetes patients (N=81,262 cases, 79,497 controls), MarketScan Research Databases 2006-2017.*
References
- 1.Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;3:34. doi: 10.1186/s40662-016-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44. doi: 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865. doi: 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 4.Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169(6):616-625. doi: 10.1001/archinternmed.2009.20 [DOI] [PubMed] [Google Scholar]
- 5.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33(6):1304-1308. doi: 10.2337/dc09-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosco JL, Antonsen S, Sørensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2011;20(1):101-111. doi: 10.1158/1055-9965.EPI-10-0817 [DOI] [PubMed] [Google Scholar]
- 7.Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, Sasidhar M. The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer. 2012;12:410. doi: 10.1186/1471-2407-12-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167(3):409-416. doi: 10.1530/EJE-12-0369 [DOI] [PubMed] [Google Scholar]
- 9.Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41(1):61-68. doi: 10.3233/JAD-131901 [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Stein JD, Nan B, et al. Association of geroprotective effects of metformin and risk of open-angle glaucoma in persons with diabetes mellitus. JAMA Ophthalmol. 2015;133(8):915-923. doi: 10.1001/jamaophthalmol.2015.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15(3):196-205. doi: 10.2174/1871530315666150316124019 [DOI] [PubMed] [Google Scholar]
- 12.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060-1065. doi: 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EE, Ball JD, Chen Z, Khurshid GS, Prosperi M, Ash JD. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60(5):1470-1477. doi: 10.1167/iovs.18-26422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Lee JH, Woo SJ, et al. ; Epidemiologic Survey Committee of the Korean Ophthalmologic Society . Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014;121(9):1756-1765. doi: 10.1016/j.ophtha.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 16.Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Rong SS, Xu Q, et al. Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108196. doi: 10.1371/journal.pone.0108196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velilla S, García-Medina JJ, García-Layana A, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:895147. doi: 10.1155/2013/895147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo SJ, Ahn J, Morrison MA, et al. Analysis of genetic and environmental risk factors and their interactions in korean patients with age-related macular degeneration. PLoS One. 2015;10(7):e0132771. doi: 10.1371/journal.pone.0132771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn P, Acquah K, Cousins SW, Lee PP, Sloan FA. Ten-year incidence of age-related macular degeneration according to diabetic retinopathy classification among medicare beneficiaries. Retina. 2013;33(5):911-919. doi: 10.1097/IAE.0b013e3182831248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He MS, Chang FL, Lin HZ, Wu JL, Hsieh TC, Lee YC. The association between diabetes and age-related macular degeneration among the elderly in taiwan. Diabetes Care. 2018;41(10):2202-2211. doi: 10.2337/dc18-0707 [DOI] [PubMed] [Google Scholar]
- 22.Prattichizzo F, Giuliani A, Mensà E, et al. Pleiotropic effects of metformin: shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018;48:87-98. doi: 10.1016/j.arr.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 23.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858-876. doi: 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinninella E, Mele MC, Merendino N, et al. The role of diet, micronutrients and the gut microbiota in age-related macular degeneration: new perspectives from the gut−retina axis. Nutrients. 2018;10(11):E1677. doi: 10.3390/nu10111677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898-1906. doi: 10.1007/s00125-013-2991-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin-Hsiao T. Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis. 2019;10(1):37-48. doi: 10.14336/AD.2017.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Chen H, Li J, et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61(1):217-228. doi: 10.2337/db11-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muraleva NA, Kozhevnikova OS, Zhdankina AA, et al. The mitochondria-targeted antioxidant SkQ1 restores αB-crystallin expression and protects against AMD-like retinopathy in OXYS rats. Cell Cycle. 2014;13(22):3499-3505. doi: 10.4161/15384101.2014.958393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell JJ, Hellberg K, Turner M, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463-471. doi: 10.1016/j.cmet.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borrone R, Saravia M, Bar D. Age related maculopathy and diabetes. Eur J Ophthalmol. 2008;18(6):949-954. doi: 10.1177/112067210801800615 [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan S, Swaminathan G, Kulothungan V, Ganesan S, Sharma T, Raman R. Age-related macular degeneration in a South Indian population, with and without diabetes. Eye (Lond). 2017;31(8):1176-1183. doi: 10.1038/eye.2017.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Ruan X, Yang P, Liu H. Comparison of three information sources for smoking information in electronic health records. Cancer Inform. 2016;15:237-242. doi: 10.4137/CIN.S40604 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD codes, CPT codes, and generic drugs names
eFigure 1. Sample selection flowchart.
eFigure 2. Study design.
eTable 2. Balance after matching, MarketScan Research Databases 2008-2017.
eTable 3. Exposures to metformin, other diabetes medications, and statins over 2 years, MarketScan Research Databases 2008-2017.
eTable 4. Exposures to metformin, other diabetes medications, and statins over 2 years among subgroup of diabetes patients (N=81,262 cases, 79,497 controls), MarketScan Research Databases 2008-2017.
eTable 5. Models for diabetes patients (N=81,262 cases, 79,497 controls), MarketScan Research Databases 2006-2017.*