Abstract
Background
Catheter ablation is an effective treatment for patients with atrial fibrillation (AF) and heart failure (HF). However, little is known about how healthcare utilization and cost change after ablation in this population. We sought to determine healthcare utilization and cost patterns among patients with AF and HF undergoing ablation.
Methods
Using a large United States administrative database, we identified (n = 1568) treated with ablation with a primary and secondary diagnosis of AF and HF, respectively, were evaluated 1‐year pre‐ and postablation for outcomes including inpatient admissions (AF or HF), emergency department (ED) visits, cardioversions, length of stay (LOS), and cost. A secondary analysis was extended to 3‐years postablation.
Results
Reductions were observed in AF‐related admissions (64%), LOS (65%), cardioversions (52%), ED visits (51%, all values, p < .0001), and HF‐related admissions (22%, p = .01). There was a 40% reduction in inpatient admission cost ($4165 preablation to $2510 postablation, p < .0001). In a sensitivity analysis excluding repeat‐ablation patients, a greater reduction in overall AF management cost was observed compared to the full cohort (−43% vs. −2%). Comparing 1‐year pre‐ to 3‐years postablation, both total mean AF‐management cost ($850 per‐patient per‐month 1‐year pre‐ to $546 3‐years postablation, p < .0001) and AF‐related healthcare utilization was reduced.
Conclusions
Catheter ablation in patients with AF and HF resulted in significant reductions in healthcare utilization and cost through 3‐years of follow‐up. This reduction was observed regardless of whether repeat ablation was performed, reflecting the positive impact of ablation on longer term cost reduction.
Keywords: atrial fibrillation, catheter ablation, health policy, healthcare economics, heart failure
Abbreviations
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- CCI
Charlson comorbidity index
- CI
confidence interval
- CPT
common procedural terminology
- CRT‐D
cardiac resynchronization therapy‐defibrillator
- ED
emergency department
- HF
heart failure
- ICD
implantable cardioverter–defibrillator
- LOS
length of stay
- PPPM
per‐patient per‐month
- US
United States
1. INTRODUCTION
Atrial fibrillation (AF) and heart failure (HF) are leading cardiovascular epidemics and they are both associated with significant morbidity, mortality, and economic burden. 1 , 2 , 3 Estimates suggest that $20 613–$40 169 is spent per AF patient per year in the United States (US). 4 , 5 In 2015, AF accounted for approximately 6 million office visits and 499 000 emergency department (ED) visits. 6 , 7 , 8 Total direct costs for HF were estimated at $30.7 billion in 2012 and are projected to increase to $70 billion by 2030. 9 Due to shared risk factors, AF and HF frequently coexist, 1 , 10 and patients with both conditions have significantly worse outcomes than patients with HF alone. 11 , 12
Catheter ablation has been shown to improve outcomes in patients with AF and HF compared to medical therapy by reducing the burden of AF, improving the left ventricular ejection fraction, and potentially lowering mortality and hospitalization for worsening HF. 13 , 14 , 15 , 16 The 2019 AHA/ACC/HRS Focused Update of the 2014 Guideline for Management of Patients with Atrial Fibrillation recommends AF catheter ablation as a reasonable (class of recommendation IIb) option in selected patients with symptomatic AF and HF with reduced left ventricle (LV) ejection fraction to potentially lower mortality and reduce hospitalizations. 17
Given the high economic burden of both AF and HF, the effect of catheter ablation on healthcare expenditures in this population is an important health policy issue. However, the impact of catheter ablation on subsequent healthcare utilization and cost among patients with AF and HF has not been well studied. 18 The objective of this retrospective observational study was to examine healthcare use and cost among patients with AF and HF before and after ablation treatment in a real‐world setting using a nationally representative claims database. The primary objective was to compare 1‐year pre‐ and postablation healthcare utilization and costs among patients with AF and HF. The secondary objective was to compare these outcomes extended to the 3‐year postablation period.
2. METHODS
2.1. Data source
A retrospective analysis of the Optum® De‐Identified Clinformatics® Data Mart Database‐date of death claims database was performed. The Optum database contains de‐identified data derived from health plan members’ enrollment data and facility, physician, and pharmacy claims from approximately 13 million covered health plan members annually. The study was reviewed by the New England Institutional Review Board and determined to be exempt from broad institutional review board approval, as the study did not involve identifiable human subjects.
2.2. Study sample
Patients who had an inpatient ablation procedure between January 1, 2012 and June 30, 2018 were identified by an ablation procedure code as specified by the International Classification of Diseases, Ninth Edition and Tenth Edition, listed with a primary diagnosis of AF and a secondary diagnosis of HF. Patients who had an outpatient ablation procedure occurring between January 1, 2012 and December 31, 2012 were identified by common procedural terminology (CPT) code 93651 (which was then discontinued) with a primary diagnosis of AF and a secondary diagnosis of HF. Patients who underwent outpatient ablation between January 1, 2013 and June 30, 2018 were identified by CPT code 93656, with a primary or secondary diagnosis of AF and concomitant diagnosis of HF. Whether the ablation procedure was inpatient or outpatient, status was defined based on the site of care of service listed in the database. Note that the outpatient status, which is commonly used for AF ablation procedures, could include a single overnight stay in the hospital. For our secondary analysis, we identified inpatient or outpatient ablation between January 1, 2012 and June 30, 2016 and extended the duration of follow‐up to the 3‐year postindex ablation for outcomes assessment and comparison.
The first ablation procedure meeting these criteria was designated as the index date. Eligible patients were required to be at least 19 years of age at the time of index admission. Patients also needed to be continuously enrolled in the 1‐year pre‐ and postindex periods. Patients who had a catheter ablation procedure for primary or secondary diagnoses of AF performed in the 1‐year preindex period and patients who underwent surgical ablation, valvular procedure, or left atrial appendage occlusion during the 1‐year preindex period were excluded. Patients who had negative aggregated costs in the 1‐year pre‐ or postindex periods were excluded. Similar inclusion and exclusion criteria were applied to the secondary analysis (1‐year pre‐ and 3‐year postindex periods). The final study population consisted of 1568 patients. The cohort formation for the primary analysis is presented in Figure 1. The secondary outcome analysis included 378 patients (Supporting Information Figure).
Figure 1.
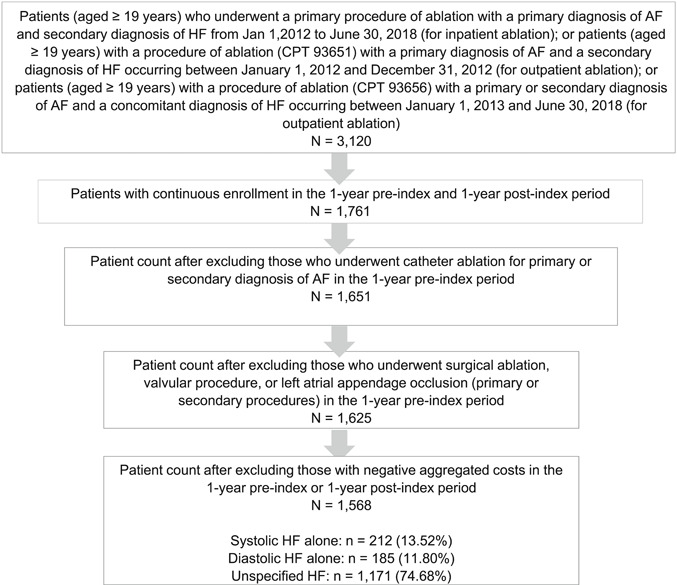
Attrition flow diagram. Inclusion and exclusion criteria used to select the analysis cohort in the primary analysis (1‐year pre‐ and 1‐year postablation). AF, atrial fibrillation; CPT, current procedural terminology; HF, heart failure
2.2.1. Covariates
Patient characteristics including age (19–49, 50–59, 60–69, and ≥70 years), gender (male and female), insurance type (exclusive provider organization, group purchasing organization, health maintenance organization, indemnity, point of service, preferred provider organization, state policy network, and other), index ablation setting (inpatient or outpatient), and admission type (elective, urgent, emergency, other, and unknown) were collected.
Comorbidity indices included the extended‐Charlson comorbidity index (CCI) score and the CHA2DS2‐VASc score. Specific comorbidities collected included: obstructive sleep apnea, obesity, diabetes, hypertension, chronic pulmonary disease, renal disease, other arrhythmias, valvular disease, cardiomyopathy, and myocardial infarction. All comorbidities were assessed in the 1‐year preindex period. Implantable cardioverter–defibrillator (ICD) or cardiac resynchronization therapy‐defibrillator (CRT‐D) use also was measured if the procedure occurred in the 1‐year preindex period. Hospital characteristics including hospital bed size (small [<100], medium [101–249], and large [≥250]) and geographic region (Northeast, Midwest, South, and West) were determined.
2.2.2. Outcomes
Study outcomes included AF‐ and HF‐related inpatient admission (where the primary diagnosis code for admission was AF or HF), AF‐related ED visits, AF‐related ambulatory care visits, cardioversion, inpatient length of stay (LOS), and cost. The cost was adjusted for inflation and reported in 2018 US dollars.
2.2.3. Sensitivity analysis
As a measure of sensitivity analysis, patients who underwent repeat ablation were excluded, and the above study objectives were assessed and compared in the 1‐year pre‐ and postindex ablation periods.
2.2.4. Statistical analysis
Means and standard deviations were reported for continuous variables and frequencies and percentages were reported for categorical variables. McNemar′s test was used to compare changes in the proportion of study outcomes pre‐ and postablation. Wilcoxon signed‐rank test was used to compare mean changes in study outcomes in the pre‐ and postablation periods. While assessing outcomes across 1‐year preablation and 3‐year postablation periods, Wilcoxon signed‐rank test also was used to compare mean changes in healthcare utilization and cost in a per‐patient per‐month (PPPM) basis.
Means with 95% confidence intervals (CIs) were used to summarize the results. Nonparametric bootstrapping (using sampling with replacement) was used to generate 95% CIs for the mean estimates of inpatient admission, ED visits, cardioversion, ambulatory care visits, and cost. 19 In all analyses, a two‐sided p‐value less than .05 was the threshold for which differences were considered to be statistically significant. All analyses were conducted using SAS for Windows, Version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Baseline characteristics
Patient demographics and characteristics and hospital characteristics for the primary analysis are presented in Table 1. The mean age was 69 ± 10 years and 61% were male. The majority of ablations occurred in the south, at large hospitals, in an outpatient setting, and were elective. Insurance other than exclusive provider organization/healthcare maintenance organization, indemnity, or point of service/preferred provider organization was the most common. An ICD/CRT‐D was present in 29.7% of patients. A majority of patients had a history of hypertension. Other common conditions among patients included valvular disease, cardiomyopathy, other arrhythmias, chronic pulmonary disease, and sleep apnea. The prevalence of comorbidities was high as indicated by a CCI score ≥ 3 in 67.2% of patients. A majority (88.97%) of patients also had a CHA2DS2‐VASc score ≥ 3. In the secondary analysis, patient demographics and characteristics and hospital characteristics were similar (Supporting Information Table).
Table 1.
Patient demographics and characteristics and hospital characteristics in the primary analysis including repeat ablation patients
| Patients, No. (%) | |
|---|---|
| Demographic/characteristic | (N = 1568) |
| Age | |
| Mean (SD), years | 69 (10) |
| Age group, n (%), years | |
| 19–49 | 49 (3.13) |
| 50–59 | 182 (11.61) |
| 60–69 | 472 (30.10) |
| ≥70 | 865 (55.17) |
| Male | 957 (61) |
| Region | |
| Midwest | 430 (27.42) |
| Northeast | 205 (13.07) |
| South | 716 (45.66) |
| West | 214 (13.65) |
| Unknown | 3 (0.19) |
| Insurance type | |
| EPO/HMO | 224 (14.29) |
| Indemnity | 22 (1.40) |
| POS/PPO | 376 (23.98) |
| Other | 946 (60.33) |
| Ablation setting | |
| Inpatient | 525 (33.48) |
| Outpatient | 1043 (66.52) |
| Hospital bed size | |
| Large (≥250) | 945 (60.27) |
| Medium (101–249) | 210 (13.39) |
| Small (<100) | 27 (1.72) |
| Unknown | 386 (24.62) |
| Admission type | |
| Elective | 1198 (76.40) |
| Emergency | 159 (10.14) |
| Urgent | 102 (6.51) |
| Unknown | 109 (6.95) |
| ICD/CRT‐D use, n (%) | 465 (29.66) |
| Admission year, n (%) | |
| 2012 | 97 (6.19) |
| 2013 | 110 (7.02) |
| 2014 | 106 (6.76) |
| 2015 | 192 (12.24) |
| 2016 | 267 (17.03) |
| 2017 | 427 (27.23) |
| 2018 | 369 (23.53) |
| History of condition | |
| Hypertension | 1460 (93.11) |
| Valvular disease | 1065 (67.92) |
| Cardiomyopathy | 876 (55.87) |
| Other arrhythmias | 905 (57.72) |
| Chronic pulmonary disease | 673 (42.92) |
| Sleep apnea | 657 (41.90) |
| Obesity | 653 (41.65) |
| Diabetes | 599 (38.20) |
| Renal disease | 486 (30.99) |
| Myocardial infarction | 376 (23.98) |
| CCI score | |
| 1–2 | 514 (32.78) |
| ≥3 | 1054 (67.22) |
| CHA2DS2‐VASc score | |
| 1–2 | 173 (11.03) |
| ≥3 | 1395 (88.97) |
Abbreviations: AF, atrial fibrillation; CCI, Charlson Comorbidity Index; CRT‐D, cardiac resynchronization therapy‐ defibrillator; EPO, exclusive provider organization; HMO, health maintenance organization; ICD, implantable cardioverter–defibrillator; POS, point of service; PPO, preferred provider organization; SD, standard deviation.
3.2. Outcomes
3.2.1. One‐year postablation healthcare utilization
Healthcare utilization was significantly lower following ablation in patients with AF and HF (Figure 2A). One year following ablation, the proportion of patients with AF‐related inpatient admissions was reduced by 64% (95% CI: −67.2, −62.0; p < .0001) and the proportion with ED visits was reduced by 51% (95% CI: −53.6, −48.1; p < .0001). The proportion of patients with HF‐related inpatient admissions was reduced by 22% (95% CI: −23.3, −20.1; p = .01) and the proportion requiring cardioversion was reduced by 52% (95% CI: −53.6, −49.8; p < .0001). We did observe that the proportion of patients with AF ambulatory care visits increased by 4% (95% CI: 3.7, 4.5; p < .0001). Considering the potential of misclassification of primary diagnosis during claims processing, we also assessed all‐cause inpatient admissions and the associated change in the proportion of patients with all‐cause admission in the preablation versus postablation period. We observed a 29% reduction in all‐cause inpatient admission (95% CI: −30.8, −28.3; p < .0001), with 53% of patients having an all‐cause inpatient admission in the preablation period versus 37% in the postablation period.
Figure 2.
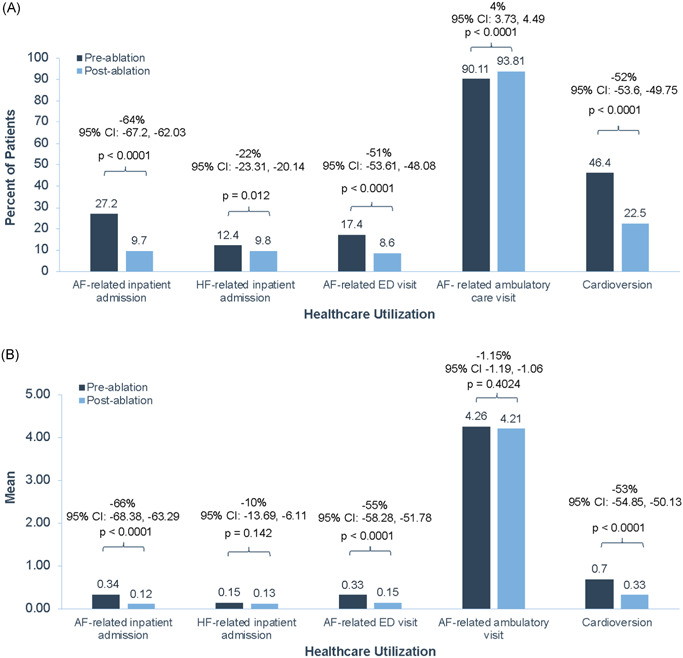
Change in AF‐ and HF‐related healthcare utilization pre‐ and post‐AF ablation in patients with AF and HF. Proportional change (A) and mean change (B) in AF‐ and HF‐related healthcare utilization in the 1‐year pre‐ and postablation periods in patients with AF and HF. AF, atrial fibrillation; CI, confidence interval; ED, emergency department; HF, heart failure
AF‐related inpatient LOS was significantly reduced by 65% (95% CI: −67.4, −62.0; p < .0001). Although the proportion of patients with HF‐related inpatient admissions was reduced, the LOS for incident admissions was overall unchanged (5% decrease; 95% CI: −24.3, 23.0).
The proportion of patients with the use of ≥1 antiarrhythmic drugs (AADs) in 0–6‐months postablation period was 55% (n = 869), and reduced to 36% (n = 563) in 6–12‐months postablation period.
Some patients included in the analysis may have had more than one admission or visit, and as a result, mean changes in study outcomes were also examined. The mean reductions in AF‐related inpatient admission, AF‐related ED visits, and cardioversion were also significant (Figure 2B). However, the mean changes in HF‐related inpatient admissions and AF‐related ambulatory care visits were not significant.
3.2.2. One‐year postablation healthcare cost
There was a significant decline in AF‐related healthcare costs among patients with AF and HF observed at 1‐year postablation (Figure 3A). Mean AF‐related inpatient cost was significantly reduced by 40%, from $4165 preablation to $2510 postablation (95% CI: −51.5, −29.1; p < .0001). In addition, the mean AF‐related ED cost was significantly reduced by 63%, from $638 preablation to $233 postablation (95% CI: −66.1, −61.5; p < .0001). Cardioversion cost was also reduced by 65%, from $4126 preablation to $1462 postablation (95% CI: −66.7, −63.1; p < .0001). The cost associated with HF‐related inpatient admissions was not significantly reduced comparing the 1‐year pre‐ and postablation periods. The mean AF‐management cost reduced significantly from $9468 in the 12‐month preindex ablation period to $9256 in the postindex ablation period (p < .0001). However, the mean total cost of all‐cause healthcare utilization including both AF‐related and non‐AF‐related factors remained unchanged between the 12‐month preindex period versus 12‐month postindex period ($42 914 vs. $46 487; p = .5177).
Figure 3.
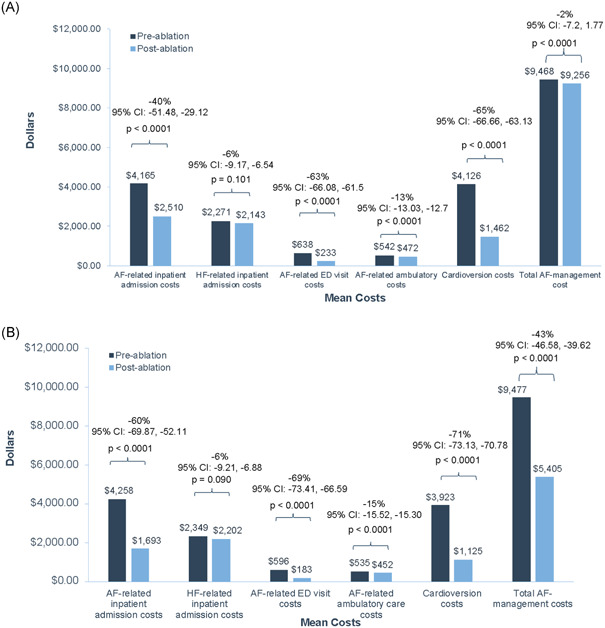
Mean change in healthcare cost per patient pre‐ and post‐AF ablation in all patients or those without repeat ablation. Mean change in healthcare cost per patient in the 1‐year pre‐ and postablation periods in all patients with AF and HF (A) or just those without repeat ablation (B). AF, atrial fibrillation; CI, confidence interval; ED, emergency department; HF, heart failure
3.2.3. Impact of repeat ablations on healthcare utilization and cost
Because repeat ablations were included in the cost analyses, we conducted a sensitivity analysis focused on the impact of ablation on cost in those individuals who did not have a repeat ablation (Figure 3B). After excluding the repeat ablation cases (n = 119), we observed a significant decline in our outcomes notably with a higher effect size compared to the full cohort in terms of AF‐related inpatient readmission (−60% vs. −40%), AF‐related ED visit (−69% vs. −63%), and cardioversion (−71% vs. −65%). However, the largest change was the overall AF management cost which was 43% lower, from $9477 preablation to $5405 postablation (95% CI: −46.6, −39.6; p < .0001), whereas for the full analysis, the cost was only 2% lower ($9468 preablation to $9256 postablation). The mean total all‐cause healthcare utilization cost was also observed to have decreased significantly (p < .0001) from $42 807 in the preindex ablation period to $41 443 in the postindex ablation period in this sensitivity analysis.
Similar to the full analysis, in the sensitivity analysis, AF‐related inpatient LOS was significantly reduced (−73%; 95% CI: −75.7, −70.1; p < .0001), but there was no significant reduction in HF‐related inpatient LOS (−3%; 95% CI: −24.3, 24.8). The proportion of patients with the use of ≥1 AAD in 0–6‐months postablation period was 55% (n = 793), and was reduced to 34% (n = 497) in 6–12‐months postablation period, similarly.
3.2.4. Secondary analysis: 3‐year postablation healthcare resource utilization
In the secondary analysis, significant reductions in mean AF‐related healthcare resource utilization (e.g., PPPM) were observed in the 1‐year preablation and 3‐year postablation periods (Figure 4A). Mean PPPM AF‐related inpatient admissions, ED visits, ambulatory care visits, and cardioversion were all significantly reduced (all values, p < .0001). Mean HF‐related inpatient cost was not significantly changed from the 1‐year preablation and 3‐year postablation periods.
Figure 4.
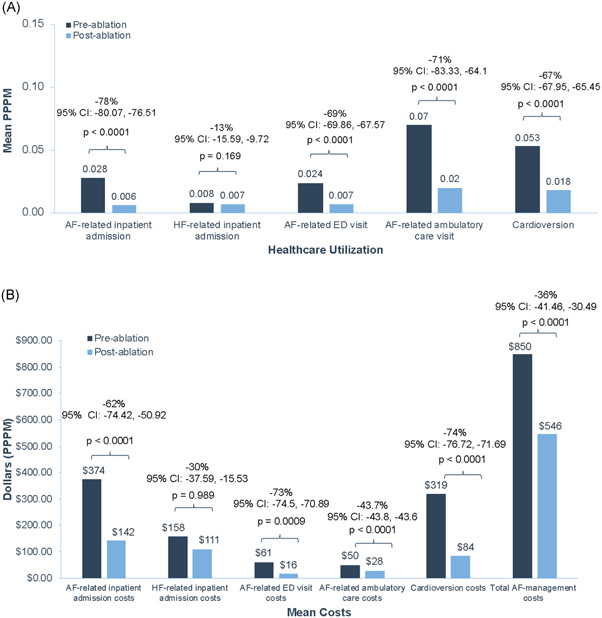
Mean changes in the AF‐ and HF‐related healthcare utilization or cost extended to the 3‐year post‐AF ablation period. Mean change in the AF‐ and HF‐related healthcare utilization (A) and cost PPPM (B) in the 1‐year preablation and 3‐year postablation periods. AF, atrial fibrillation; CI, confidence interval; ED, emergency department; HF, heart failure; PPPM, per‐patient per‐month
3.2.5. Three‐year postablation healthcare cost
Healthcare cost significantly declined from the 1‐year preablation period to the 3 years postablation period (Figure 4B). AF‐related inpatient, ED visits, and ambulatory care costs were significantly reduced. Total mean AF management cost was significantly decreased from $850 PPPM 1‐year preablation to $546 PPPM 3‐years postablation, corresponding to a 36% reduction in costs (95% CI: −41.5, −30.5; p < .0001). Cardioversion cost was also significantly reduced. HF‐related inpatient admission cost was not significantly changed from 1‐year preablation and 3‐years postablation. However, the average total all‐cause healthcare cost remained unchanged in the 1‐year pre‐ versus 3‐years postablation period ($3178 vs. $3213; p = .8296).
4. DISCUSSION
Despite the challenges in maintaining sinus rhythm in patients with AF and HF, the benefits of a rhythm control approach utilizing AF ablation in appropriately selected patients with HF include reduced AF recurrence, improvements in LV ejection fraction, and a lower risk of HF hospitalization and all‐cause mortality. 15 , 16 , 20 , 21 , 22 , 23 The current analysis extends these prior investigations by exploring the impact of AF ablation in this population on healthcare utilization and cost. There are several key findings from our analysis. First, catheter ablation leads to significantly lower AF‐related healthcare utilization in the 1‐year postablation period compared to the 1‐year period preceding ablation, including fewer inpatient admissions, shorter inpatient LOS, fewer cardioversions, and fewer ED visits. Second, extending the follow‐up duration to 3‐years postablation revealed sustained and greater reductions in healthcare utilization and total AF‐management cost. Finally, both admissions for AF as well HF were reduced with ablation.
Interventions such as catheter ablation that are associated with reduced incidence of HF admission could significantly lower the financial burden associated with the management of chronic diseases such as AF and HF, which commonly coexist. A recently published analysis of the Nationwide Readmission Database assessed rates of all‐cause hospitalization in patients with or without HF undergoing AF ablation and found a 27.5% reduction in all‐cause hospitalization in the HF group, with a greater relative reduction in the HF group, compared the group without HF. 24 The relative reduction was similar regardless of whether the HF was associated with a reduced or preserved ejection fraction. In addition, a 20% relative reduction of HF hospitalization cost for the 3‐months postcatheter ablation compared to precatheter ablation was observed. Similarly, in the CASTLE‐AF trial, which was a randomized controlled trial comparing either catheter ablation or medical therapy of AF patients with HF and reduced ejection fraction and ICD, a reduction in rates of HF hospitalization was observed. The ablation group demonstrated a decreased incidence of HF hospitalization compared to the medical therapy group (20.7% vs. 35.9%, hazard ratio, 0.56; 95% CI, 0.37–0.83; p = .004). 16 In our study, we found a significant decline in the proportion of patients experiencing HF‐related inpatient hospitalization in the 1‐year follow‐up period.
Beyond HF admissions, this study also broadly investigated total AF management costs, including AF‐related inpatient admission, inpatient LOS, ED visits, cardioversion, and ambulatory care visits. There were relative reductions in most types of services postablation, consistent with a wide‐ranging benefit in this population. A proportional increase of 4% in the rate of AF‐related ambulatory care visits postablation was observed. We speculate that the proportional increase was the result, at least in part, to standard postprocedure follow‐up visits. The proportion of the postoperative encounters essentially were shifted from more costly inpatient and ED visits to ambulatory visits.
The effect of catheter ablation on healthcare expenditures in patients with AF and HF is an important health policy issue given the significant costs associated with either condition. 25 , 26 In addition to healthcare utilization, we analyzed cost and observed an overall decrease in the 1‐year postablation period, which was even more significant in the 3‐year postablation period. This finding indicates the potential long‐term benefit of catheter ablation in patients with AF and HF. Chang et al. 27 conducted a systematic review of the cost‐effectiveness of catheter ablation for AF. They concluded that catheter ablation could be a cost‐effective option for AF management, especially among populations with higher symptom burden. Our findings, especially with respect to the longer term cost reduction following ablation, extend these data to the HF population. Although ablation was seen to influence AF‐management cost, the total cost of all‐cause healthcare use generally remained unchanged in the 12‐month preablation versus postablation period (though we did observe a significant decline when excluding patients with repeat ablation in the postablation period) and also when considering extended follow‐up time period. The all‐cause healthcare utilization cost encompasses both AF‐related and non‐AF‐related costs, and with high comorbidity burden among patients with AF–HF, the non‐AF‐related costs are likely to have been uninfluenced by ablation. As such, the usefulness of all‐cause healthcare costs as an outcome metric in assessing the influence of ablation should be cautiously interpreted.
When the population in the primary analysis was restricted to those patients not undergoing a repeat ablation, a greater reduction in cost postablation was demonstrated as expected. Although only 7.6% of patients underwent repeat ablation, its considerable impact on cost 1‐year postablation became evident with a pronounced reduction in the overall AF management cost when these patients were excluded from the analysis. However, when the analysis was extended to a 3‐year postablation period, a significant reduction in AF‐management cost was observed, even when the repeat ablation patients were included. Therefore, the beneficial effects of ablation on longer term cost savings appear to accrue with longer term follow‐up, even accounting for the added cost of repeat ablation. Nonetheless, these findings highlight the potential cost benefits of improvements and innovations aimed at reducing the incidence of repeat ablation procedures.
4.1. Limitations
The results of this study should be considered in the context of certain limitations. As with all analyses of claims data, the results may not be generalizable beyond the population included in the database. The Optum database lacks information regarding left ventricular ejection fraction and New York Heart Association functional class, so analyses based on specific HF subtypes (systolic or diastolic) or symptom severity could not be performed. Reliance on diagnosis and procedure codes to define study variables are subject to miscoding or misclassification. Furthermore, individual patients as their own control were used to evaluate pre‐ and postablation healthcare utilization and cost, which has advantages. However, we did not compare a matched group of patients not undergoing ablation, so we cannot comment on how an ablation strategy might differ from a medical strategy with respect to healthcare utilization. Finally, we applied continuous enrollment criteria postablation, and, therefore, the results could have been influenced by immortal time bias.
5. CONCLUSION
Using a nationally representative sample, this real‐world study is the first to examine the relationship between ablation and healthcare resource utilization in patients with both AF and HF. Significant reductions in AF‐related resource use and cost were demonstrated up to 3 years postablation and the longer term cost was unaffected by repeat ablation. The impact on HF hospitalizations was comparatively more modest. The observed reductions in healthcare utilization and cost following ablation in patients with AF and HF have important implications for future guidelines, as well as for patients, providers, and payers.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This study was supported by Johnson and Johnson. The authors would like to thank Susan Bartko‐Winters, PhD, for her contributions to the analysis and review of the manuscript.
Field ME, Gold MR, Rahman M, et al. Healthcare utilization and cost in patients with atrial fibrillation and heart failure undergoing catheter ablation. J Cardiovasc Electrophysiol. 2020;31:3166–3175. 10.1111/jce.14774
Disclosure Michael E. Field received research support from Johnson and Johnson, Boston Scientific, and Medtronic. Michael R. Gold is a consultant and receives research support from Medtronic and Boston Scientific. Rahul Khanna, Laura Goldstein, Sonia Maccioni, and Motiur Rahman are Johnson and Johnson employees. Abhishek Srivastava is an employee of Mu Sigma, which has a contractual relationship with Johnson and Johnson. Daniel J. Friedman has received: research support from Boston Scientific, Biosense Webster, and Abbott; educational grants from Boston Scientific, Medtronic, Abbott, and Biotronik; and consulting fees from Abbott and AtriCure. Jonathan P. Piccini receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, Sanofi, Philips, and Up‐to‐Date.
REFERENCES
- 1. Lee Park K, Anter E. Atrial fibrillation and heart failure: a review of the intersection of two cardiac epidemics. J Atr Fibrillation. 2013;6(1):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516‐2525. [DOI] [PubMed] [Google Scholar]
- 3. Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337(19):1360‐1369. [DOI] [PubMed] [Google Scholar]
- 4. Khaykin Y, Shamiss Y. Cost of AF ablation: where do we stand? Cardiol Res Pract. 2011;2011:589781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt N, Turakhia M, Fogarty TJ. Cost‐effectiveness of cardiac radiosurgery for atrial fibrillation: implications for reducing health care morbidity, utilization, and costs. Cureus. 2016;8(8):e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56‐e528. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . National Ambulatory Medical Care Survey: 2015 State and National Summary Tables . https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2015_namcs_web_tables.pdf. Accessed July 9, 2019.
- 8. Centers for Disease Control and Prevention . National Hospital Ambulatory Medical Care Survey: 2015 Emergency Department Summary Tables . https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf. Accessed July 9, 2019.
- 9. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4(6):740‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure‐assessment of reduction in mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47(10):1997‐2004. [DOI] [PubMed] [Google Scholar]
- 12. Efremidis M, Pappas L, Sideris A, Filippatos G. Management of atrial fibrillation in patients with heart failure. J Card Fail. 2008;14(3):232‐237. [DOI] [PubMed] [Google Scholar]
- 13. Briceño DF, Markman TM, Lupercio F, et al. Catheter ablation versus conventional treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: a systematic review and meta‐analysis of randomized controlled trials. J Interv Card Electrophysiol. 2018;53(1):19‐29. [DOI] [PubMed] [Google Scholar]
- 14. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637‐1644. [DOI] [PubMed] [Google Scholar]
- 15. Packer DL, Monahan KH, Al‐Khalidi HR, et al. Ablation of atrial fibrillation in heart failure patients: additional outcomes of the CABANA trial In: 40th Annual Heart Rhythm Scientific Sessions; May 8–11, 2019; San Francisco, CA. [Google Scholar]
- 16. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492‐427. [DOI] [PubMed] [Google Scholar]
- 17. January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104‐132 [DOI] [PubMed] [Google Scholar]
- 18. Ladapo JA, David G, Gunnarsson CL, et al. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol. 2012;23(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 19. Bozic KJ, Stacey B, Berger A, Sadosky A, Oster G. Resource utilization and costs before and after total joint arthroplasty. BMC Health Serv Res. 2012;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al Halabi S, Qintar M, Hussein A, et al. Catheter ablation for atrial fibrillation in heart failure patients: a meta‐analysis of randomized controlled trials. JACC Clin Electrophysiol. 2015;1(3):200‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dagres N, Varounis C, Gaspar T, et al. Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta‐analysis. J Card Fail. 2011;17(11):964‐970. [DOI] [PubMed] [Google Scholar]
- 22. AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta‐analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noseworthy PA, Van Houten HK, Gersh BJ, et al. Generalizability of the CASTLE‐AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm. 2020;17:1057‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elkaryoni A, Al Badarin F, Spertus JA, Kennedy KF, Wimmer AP. Comparison of the effect of catheter ablation for atrial fibrillation on all‐cause hospitalization in patients with versus without heart failure (from the Nationwide Readmission Database). Am J Cardiol. 2020;125(3):392‐398. [DOI] [PubMed] [Google Scholar]
- 25. Delaney JA, Yin X, Fontes JD, et al. Hospital and clinical care costs associated with atrial fibrillation for Medicare beneficiaries in the Cardiovascular Health Study and the Framingham Heart Study. SAGE Open Med. 2018;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313‐320. [DOI] [PubMed] [Google Scholar]
- 27. Chang AY, Kaiser D, Ullal A, Perino AC, Heidenreich PA, Turakhia MP. Evaluating the cost‐effectiveness of catheter ablation of atrial fibrillation. Arrhythm Electrophysiol Rev. 2014;3(3):177‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.


