Abstract
In Rhizobiales bacteria, such as Sinorhizobium meliloti, cell elongation takes place only at new cell poles, generated by cell division. Here, we show that the role of the FtsN-like protein RgsS in S. meliloti extends beyond cell division. RgsS contains a conserved SPOR domain known to bind amidase-processed peptidoglycan. This part of RgsS and peptidoglycan amidase AmiC are crucial for reliable selection of the new cell pole as cell elongation zone. Absence of these components increases mobility of RgsS molecules, as well as abnormal RgsS accumulation and positioning of the growth zone at the old cell pole in about one third of the cells. These cells with inverted growth polarity are able to complete the cell cycle but show partially impaired chromosome segregation. We propose that amidase-processed peptidoglycan provides a landmark for RgsS to generate cell polarity in unipolarly growing Rhizobiales.
Subject terms: Cell growth, Cell polarity, Cellular microbiology
In Sinorhizobium bacteria, cell elongation takes place only at new cell poles, generated by cell division. Here, Krol et al. show that an FtsN-like protein and a peptidoglycan amidase are crucial for reliable selection of the new cell pole as cell elongation zone.
Introduction
In all three kingdoms of life, cell polarization is a dynamic phenomenon characterized by active accumulation or confinement of proteins within a part of the cell, resulting in their asymmetric distribution and formation of molecule gradients1,2. Binary fission of bacterial cells itself is a source of asymmetry3. Polar positioning of a monotrichous flagellum4,5, polarized chromosome segregation6–8 and unipolar cell wall growth9 are striking examples of bacterial cell polarity.
Implicit in bacterial cell growth is an increase in cell volume and surface, involving expansion of the peptidoglycan (PG) sacculus. Insertion of new PG into the existing mesh is mediated by tightly controlled PG hydrolysis, synthesis, and maturation enzymes10. Cell elongation of most rod-shaped bacteria takes place in a dispersed manner along the sidewall, using filaments of the actin homolog MreB as scaffold for the PG biosynthesis machinery11,12. However, MreB-independent polar cell wall expansion has been reported for a considerable share of bacteria, such as Gram-positive Streptomyces, Mycobacteria and Actinobacteria, and alphaproteobacterial Rhizobiales9,13–15. The latter include the plant pathogen Agrobacterium tumefaciens, the plant symbiont Sinorhizobium meliloti and the animal pathogen Brucella abortus, which exhibit unipolar cell wall growth9,16,17.
In most of the bacteria, cell division is mediated by a complex multiprotein assembly that includes PG synthesis and remodeling enzymes, designated as divisome18. A number of core divisome components are conserved in the majority of bacteria19. PG amidases play an important role in septum splitting during cell division in γ-proteobacterial Escherichia coli20. These enzymes require activation by cognate enzymatically inactive LytM (Lysostaphin-like metalloproteases) domain proteins21. PG amidases generate glycan chains free of peptide stems, referred to as denuded PG22. The latter serves as binding substrate for the non-essential C-terminal SPOR domain of the essential divisome protein FtsN22–24. FtsN was initially identified in E. coli, followed by discovery of highly variable FtsN-like proteins in α, β, and δ-proteobacteria25,26.
Unraveling the pivotal processes of regulation and scaffolding of unipolar cell wall growth is key to understanding the molecular basis of asymmetric cellular organization that enables coordination of this growth mode with faithful replication and segregation of the genomic DNA and cell division. In S. meliloti, polar growth zones are placed at the new cell poles, generated by cell division9,16. The chromosomal origin of replication is located at the old cell pole and the newly replicated chromosomal origin migrates to the new, growing cell pole16. How stable inheritance of the PG growth zones at the new cell poles is achieved in unipolarly growing Rhizobiales is still enigmatic. A promising candidate for a polar growth scaffold protein is the A. tumefaciens growth pole ring protein GPR27. Moreover, we recently identified eleven novel essential S. meliloti Rhizobial growth and septation (Rgs) proteins with yet-unknown functions, which localized to sites of zonal cell wall synthesis16,28. They constitute a protein interaction network, including the FtsN-like cell division protein RgsS, GPR homolog RgsE, and inner membrane components of the Tol-Pal system28.
In this study, we show that in S. meliloti, polar positioning of the cell wall growth zone correlates with positioning of the FtsN-like protein RgsS. Faithful localization of RgsS to the new cell pole requires its SPOR domain, amidase AmiC and AmiC cofactor LytM domain protein AmcA. Furthermore, our data indicate that the chromosome segregation process is influenced by the polar positioning of RgsS and the cell wall growth zone.
Results
S. meliloti AmiC generates binding targets for the RgsS SPOR domain at the growth pole and septum
Previously, we observed mVenus-RgsS localization at the sites of zonal PG synthesis at the growth pole and the septum in a S. meliloti strain carrying the mVenus-rgsS gene fusion in place of the rgsS wild type allele (Rm2011 mVenus-rgsS)28. RgsS is a FtsN-like protein, indispensable for cell division28. RgsS contains a conserved SPOR domain at the periplasmic C-terminus, designated here as SPORRgsS (Supplementary Fig. 1). SPORRgsS shares sequence similarities with SPOR domains of E. coli FtsN (FtsNEc), Pseudomonas aeruginosa RlpA and Bacillus subtilis cell wall amidase CwlC29–31, including residues involved in binding of denuded PG in these proteins (Supplementary Fig. 1).
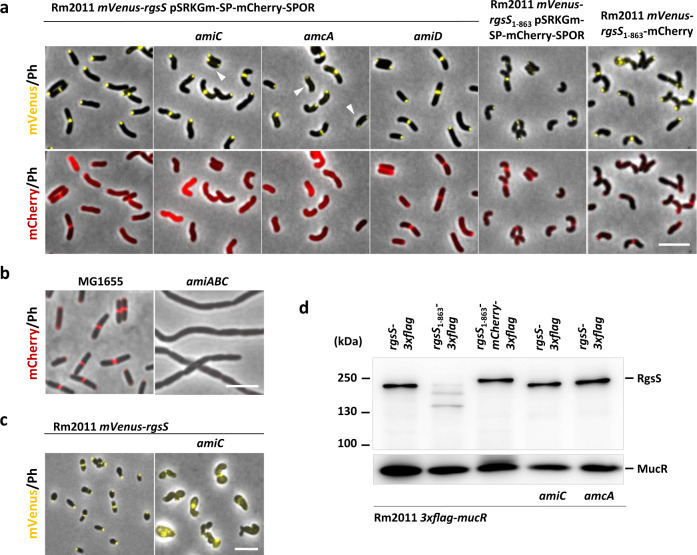
To analyze if SPORRgsS was able to accumulate at cell division sites, similar to the SPOR domain of FtsNEc32, we designed plasmid pSRKGm-SP-mCherry-SPOR for ectopic production of periplasmic mCherry-SPORRgsS. In the Rm2011 mVenus-rgsS strain, we observed robust septal mCherry-SPORRgsS colocalization with mVenus-RgsS and in a major part of the cells also polar colocalization (Fig. 1a and Supplementary Table 1), suggesting that SPORRgsS binding targets are present at both these sites. mCherry-SPORRgsS accumulated at the septum in E. coli wild-type strain MG1655, but not in a MG1655 mutant strain lacking the PG amidases AmiA, AmiB, and AmiC (Fig. 1b). Thus, SPORRgsS is likely able to bind denuded PG, generated by amidases in the E. coli septum.
Fig. 1. Cell morphology and localization of mVenus-RgsS and mCherry-SPORRgsS in strains, sufficient or deficient in AmiC, AmcA, or SPORRgsS.
a Fluorescence microscopy images of exponentially growing TY cultures of indicated S. meliloti strains. White arrowheads indicate cells with bipolar mVenus-RgsS localization. Scale bar, 5 µm; Ph, phase contrast. The images are representative of two independent cultivations and microscopy analyses. b Fluorescence microscopy images of cells from M9 cultures of indicated E. coli strains, carrying pSRKGm-SP-mCherry-SPOR. Scale bar, 5 µm; Ph phase contrast. The images are representative of two independent cultivations and microscopy analyses. c Fluorescence microscopy images of cells from exponential phase LB cultures of S. meliloti Rm2011 mVenus-rgsS and its amiC-deficient derivative. Scale bar, 5 µm; Ph phase contrast. The images are representative of three independent cultivations and microscopy analyses. d Western blot analysis with α-FLAG antibody of Rm2011 3xflag-mucR strain, expressing genes encoding C-terminal 3XFLAG tag-RgsS fusions from the rgsS promoter at the native genomic location. Strains were grown in TY supplemented with gentamicin. 3×FLAG-MucR produced from 3xflag-mucR at the native genomic location was used as a loading control. The result is representative of three biological replicates.
Next, we asked if S. meliloti SPORRgsS binding targets were produced by S. meliloti PG amidases. As deduced from genome annotation, S. meliloti possesses the putative PG amidases AmiC and AmiD, homologous to E. coli AmiC and AmiD (Supplementary Figs. 2 and 3), but no AmiA and AmiB homologs. Whereas E. coli AmiC is involved in PG splitting during cell division33, AmiD is not required for this process34. Septal and polar foci of ectopically produced mCherry-SPORRgsS were still observed in the amiD knockout mutant Rm2011 mVenus-rgsS amiD but were absent in the amiC deletion strain Rm2011 mVenus-rgsS amiC (Fig. 1a). These results suggest that S. meliloti AmiC but not AmiD activity generates denuded PG that serves as mCherry-SPORRgsS binding substrate within polar and septal cell wall growth zones.
AmiC with an intact catalytic site is required for straight rod cell shape and envelope integrity
To further characterize amiC and amiD mutant strains, we analyzed growth and cell morphology. In TY medium, strains lacking either functional amiC or amiD were not affected in growth, however the double amiC amiD mutation resulted in a minor slow-down of growth (Supplementary Fig. 4). In TY broth, the amiD mutant showed wild type-like straight rod cell morphology, whereas the amiC mutation resulted in cells with increased curvature (Fig. 1a, Supplementary Figs. 5 and 6, and Supplementary Table 2). Unlike the amidase-deficient E. coli35, S. meliloti amiC-deficient strains showed no cell chaining phenotype. Thus, AmiC is not strictly required for S. meliloti cell division.
While TY is a standard medium for S. meliloti propagation, we previously observed that cultivation in LB augmented growth and cell morphology defects of strains affected in cell envelope integrity16,28. This effect was likely caused by outer membrane destabilization in the absence of divalent cations36,37 and was alleviated by addition of CaCl216,28. The amiD mutant strain grew nearly normally on LB and showed wild-type-like cell morphology in LB broth (Supplementary Figs. 4 and 5). In contrast, the mVenus-rgsS amiC strain showed a strong growth defect on LB (Supplementary Fig. 4), and in LB broth produced enlarged cells, which partially lost mVenus-RgsS localization (Fig. 1c, and Supplementary Fig. 5). These defects were alleviated by addition of 2.5 mM CaCl2 (Supplementary Figs. 4 and 5). The augmented cell morphology defect of the amiC mutant in the absence of CaCl2 implies that AmiC has a function in maintaining cell envelope integrity.
To test if the phenotypic alterations observed in the amiC mutant resulted from a lack of AmiC enzymatic activity, we generated complementation constructs on the single-copy vector pABC-Psyn. These constructs included the native amiC promoter and comprised the native gene or variants encoding AmiC with alanine replacements at the conserved catalytic histidine residues H206 and H276 (Supplementary Fig. 2). The catalytic residues were determined by similarity to E. coli AmiC38. Western blot analysis using similar constructs, additionally carrying a 3xFLAG tag, showed that H206A and H276A mutations did not affect AmiC protein stability (Supplementary Fig. 7). Complementation with native amiC, but not amiCH206A or amiCH276A restored wild type-like growth, cell morphology and septal localization of mCherry-SPORRgsS in the mVenus-rgsS amiC strain (Supplementary Figs. 4, 5, and 6). This implies that processing of PG by AmiC is required for normal cell growth and morphology as well as septal localization of mCherry-SPORRgsS.
EnvC-like LytM domain protein AmcA is required for AmiC function
PG amidases in E. coli, Xanthomonas campestris and Neisseria gonorrhoeae require allosteric activation by cognate enzymatically inactive LytM domain proteins38–41. In S. meliloti, deletion of SMc03782, encoding a LytM domain protein, phenocopied the effect of amiC deletion. It did not affect growth on TY, abolished septal localization of mCherry-SPORRgsS and resulted in a curved cell phenotype (Fig. 1a, Supplementary Figs. 4 and 5, and Supplementary Table 2). Moreover, the SMc03782 mutant grew poorly on LB and showed enlarged cells in liquid LB culture resembling the phenotype of the amiC strains (Supplementary Figs. 4 and 5). These defects were relieved upon complementation with an ectopic gene copy driven by the native promoter on single-copy plasmid pABC-Psyn (Supplementary Figs. 4, 5, and 6). Western blot analysis revealed that deletion of SMc03782 did not affect abundance of 3xFLAG-tagged AmiC (Supplementary Fig. 7), thus SMc03782 is likely required for AmiC function. Therefore, the gene was named amcA, for “amidase C cofactor A”. The LytM domain of AmcA shares similarities with the corresponding regions of E. coli PG amidase cofactors EnvC and NlpD39 (Supplementary Fig. 8). Like EnvC and NlpD, it lacks the HxH motif, conserved in enzymatically active LytM domains42. The remaining AmcA sequence is non-homologous to either EnvC or NlpD, however it contains two predicted coiled-coil-forming alpha-helical regions (Supplementary Fig. 8). This constitutes a similarity to E. coli EnvC, which contains two coiled-coil domains21. Moreover, the four residues identified as crucial for amidase activation by EnvC43 are represented by identical or similar amino acids in AmcA (Supplementary Fig. 8). These findings corroborate the assumption that AmcA is required for AmiC activation.
SPORRgsS is non-essential for cell propagation, but is required for straight rod cell morphology
Binding to denuded PG via the SPOR domain was suggested to stabilize septal positioning of FtsNEc44. To gain insight into the role of the SPOR domain for RgsS function, we generated the strain Rm2011 mVenus-rgsS1–863, producing an mVenus-RgsS variant lacking the SPOR domain. Viability of this strain suggests that the RgsS SPOR domain is non-essential for cell propagation, reminiscent of the FtsNEc SPOR domain45. This truncation of mVenus-RgsS did not result in a growth defect when the mVenus-rgsS1−863 strain was cultivated on TY agar, whereas growth on LB was substantially reduced (Supplementary Fig. 4). When grown in liquid TY and LB media, mVenus-rgsS1−863 cells appeared curved, and in LB, a proportion of enlarged cells was observed (Supplementary Fig. 5 and Supplementary Table 2). mCherry-SPORRgsS, produced from pSRKGm-SP-mCherry-SPOR, was detected at the septum in mVenus-rgsS1−863 cells (Fig. 1a), implying that the truncation of mVenus-RgsS did not affect AmiC activity, which is assumed to generate denuded PG serving as binding substrate for the SPOR domain22.
The mVenus fluorescence signal in the mVenus-rgsS1−863 strain appeared weaker than in the mVenus-rgsS wild type and its amiC- and amcA-deficient derivatives (Fig. 1a and Supplementary Fig. 5). This indicates that RgsS abundance was affected by the lack of the SPOR domain, but not by the amiC and amcA mutations. We exchanged rgsS and rgsS1−863 with rgsS-3xflag and rgsS1−863-3xflag, respectively, at the native genomic location and analyzed protein abundance in the resulting strains by Western blots. In contrast to RgsS-3xFLAG, RgsS1−863-3xFLAG was hardly detectable indicating proteolysis (Fig. 1d). A similar result was obtained when 3xFLAG-RgsS and 3xFLAG-RgsS1−863 were ectopically produced (Supplementary Fig. 9). The FtsNEc SPOR domain contains two disulfide bond-forming cysteines that are important for FtsNEc stability45,46. SPORRgsS contains cysteines at positions 931 and 941. C931A and C941A mutations in the SPOR domain of 3xFLAG-RgsS negatively affected protein abundance similarly to removal of the SPOR domain (Supplementary Fig. 9). This suggests that the intact SPOR domain plays a role in protecting RgsS from degradation, similar to the SPOR domains of FtsNEc and FtsN-like C. crescentus protein CC200726,45.
Since RgsS is an essential protein, we reasoned that its destabilization in the mVenus-rgsS1−863 strain might have caused physiological alterations, not directly related to the functional link between RgsS and AmiC. Thus, we inserted the mCherry coding sequence into the rgsS1−863-3xflag sequence, generating Rm2011 rgsS1−863-mCherry-3xflag. This modification restored the stability of RgsS lacking the SPOR domain (Fig. 1d). This data shows that although protection of RgsS from degradation required intact SPORRgsS, it is not unique to this domain, since it was also achieved by its replacement with the unrelated mCherry protein.
RgsS1−863-mCherry represents a protein, likely unable to bind amidase-processed PG, but retaining stability similar to the full-length RgsS. To gain insight into the function of SPORRgsS, not related to RgsS protein stability, we replaced mVenus-rgsS with mVenus-rgsS1−863-mCherry at the native genomic location. This modification resulted in cell morphology alterations, similar to the phenotype caused by the amiC and amcA mutations (Fig. 1e, Supplementary Fig. 5, and Supplementary Table 2). Although mVenus-rgsS1−863-mCherry cells showed a moderate slow-down of growth in LB, no cell swelling was observed (Supplementary Figs. 4 and 5). Thus, maintaining a straight rod cell morphology may require interaction between SPORRgsS and AmiC-processed PG, whereas AmiC and AmcA likely have additional functions related to maintaining cell envelope integrity.
AmiC, AmcA, and SPORRgsS facilitate cell division
In TY cultures of exponentially growing mVenus-rgsS amiC, mVenus-rgsS amcA, mVenus-rgsS1−863 and mVenus-rgsS1−863-mCherry strains, cells with septal mVenus-RgsS localization were overrepresented compared to the mVenus-rgsS wild-type strain, implying a prolonged cell division period (Fig. 2a and Supplementary Table 3). 21% of the mVenus-rgsS1−863 cells showed no distinct polar fluorescence focus, whereas septal foci were consistently detected. Time-lapse microscopy revealed that in amiC- and amcA-deficient cells the estimated average duration of the septal mVenus signal increased to 164% and in mVenus-rgsS1−863 and mVenus-rgsS1−863-mCherry cells to 121% and 118% of that of the mVenus-rgsS wild type, respectively (Supplementary Table 4). The mutant strains showed an increase in the estimated average doubling time, consistent with prolonged cell division (Supplementary Table 4). These findings point to an accessory function of AmiC and SPORRgsS in cell division and corroborate the assumed role of AmcA in AmiC activation.
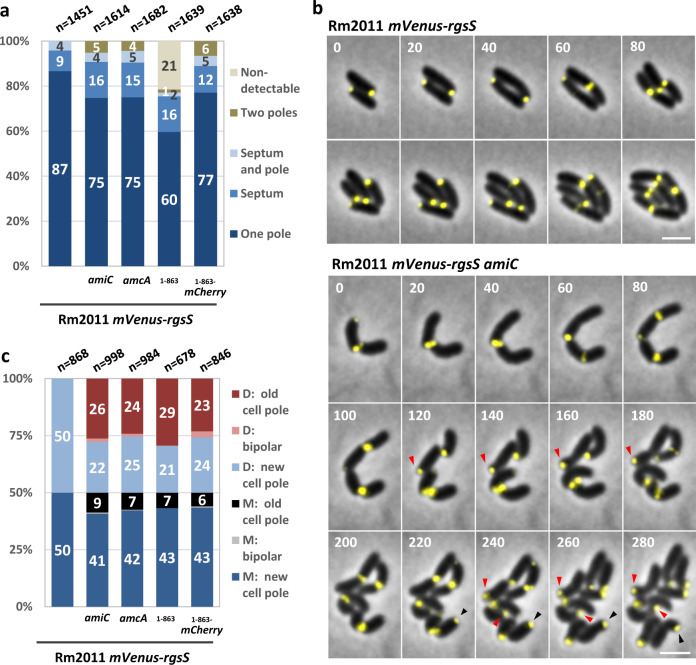
Fig. 2. AmiC, AmcA, and the SPORRgsS are required for stable inheritance of RgsS to the newly formed cell poles after cell division.
a mVenus-RgsS localization patterns in cells from exponential phase TY cultures. n, total number of analyzed cells. The values represent the mean values of three biological replicates. The standard deviation values and statistical comparison between the strains are shown in Supplementary Table 3. b Time lapse microscopy images of Rm2011 mVenus-rgsS and its amiC mutant derivative, growing on MM-agarose. Merged mVenus fluorescence and phase contrast pictures are shown. Red and black arrowheads point to cells with localization of mVenus-RgsS foci to old cell poles of the daughter and the mother cells, respectively. Time is shown in minutes. Scale bar, 2 µm. The images are representative of three independent cultivations and microscopy analyses. c mVenus-RgsS foci inheritance observed in time-lapse microscopy. D daughter cell, M mother cell. n total number of analyzed cells. The values represent the mean values of three biological replicates. The standard deviation values and statistic comparison between the strains are shown in Supplementary Table 5.
AmiC, AmcA, and SPORRgsS are required for faithful persistence of mVenus-RgsS at the new cell poles after cell division
In the course of the microscopy analysis of mVenus-rgsS amiC, mVenus-rgsS amcA, mVenus-rgsS1−863, and mVenus-rgsS1−863-mCherry cell populations, exponentially growing in TY medium, we noticed that one to six percent of the cells showed bipolar localization of mVenus-RgsS, which was not observed in mVenus-rgsS cells (Figs. 1a and 2a, Supplementary Fig. 5, and Supplementary Table 3). Thus, we analyzed these strains in time-lapse microscopy. Consistent with our previous finding28, in the mVenus-rgsS strain, mVenus-RgsS persisted at the growing cell pole during the cell elongation phase and in predivisional cells, mVenus-RgsS fluorescence signal accumulated at the septal site and vanished at the cell pole. After septum constriction and cell division, the mVenus-RgsS focus was retained at the new cell pole of each sibling, which became the growing pole (Fig. 2b). Hereafter we define the progeny cell that originated from the compartment that contained the growing pole as the daughter cell and the former compartment that contained the non-growing pole as the mother cell.
In the amiC-deficient strain, mVenus-RgsS was located at the growing cell pole during cell elongation and accumulated in the mid-cell area of predivisional cells in a similar manner as in the wild type. However, after cell division, only 63% of mVenus-rgsS amiC cells retained the mVenus-RgsS focus at the new cell poles. In 26% of the cells, the mVenus-RgsS fluorescence focus was observed at the former growing pole (old pole of the daughter cells), and in 9% of the cells, the mVenus-RgsS fluorescence focus was located at the former non-growing pole (old pole of the mother cell; Fig. 2b, c and Supplementary Table 5). Moreover, 0.5% of mother cells and 1.5% of daughter cells established a second mVenus-RgsS focus at the old cell pole while retaining a focus at the new cell pole, resulting in bipolar localization. mVenus-RgsS localization at the old cell pole did not detrimentally affect cell growth and division, since these cells were able to elongate and to produce viable progeny. The amcA-deficient strain showed similar alterations in mVenus-RgsS focus positioning as the amiC mutant (Fig. 2c, Supplementary Fig. 10, and Supplementary Table 5), consistent with the suggested role of AmcA in AmiC activation. Likewise, mVenus-rgsS1−863 and mVenus-rgsS1−863-mCherry cells showed aberrant mVenus-RgsS focus inheritance (Fig. 2c, Supplementary Figs. 11 and 12, and Supplementary Table 5). Taken together, this data indicates that AmiC-processed PG and SPORRgsS are crucial for faithful localization of mVenus-RgsS to the new cell poles of both progeny cells.
Polar positioning of RgsS correlates with polar positioning of other Rgs proteins and PG insertion zones
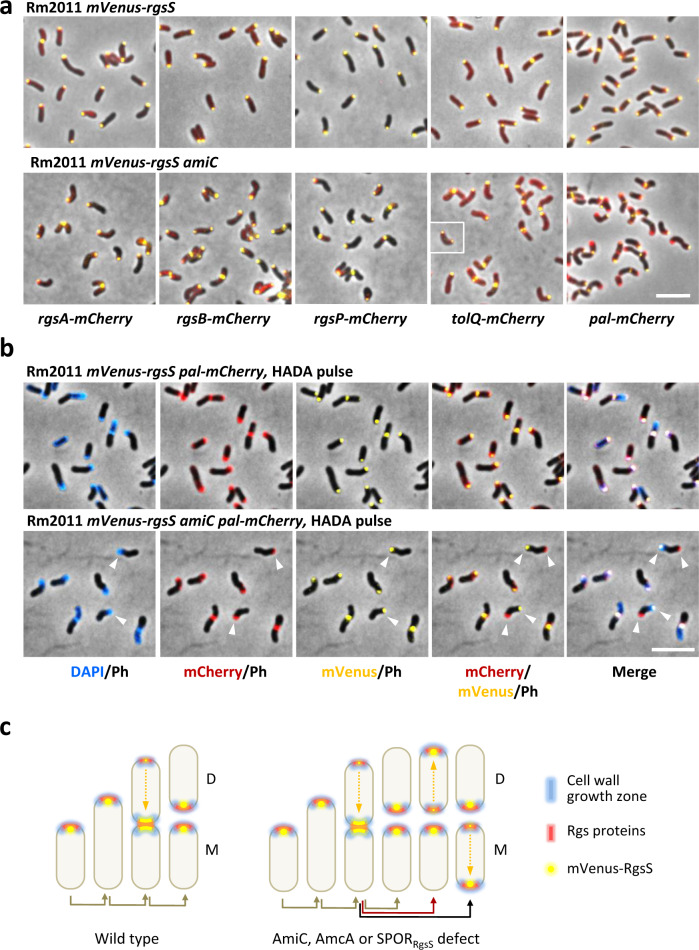
We have previously reported that RgsS, other Rgs proteins and the Tol-Pal system colocalize with RgsP28, and that RgsP was exclusively found within the cell wall growth zones at the growth pole and septum16. Since we had observed that after cell division, 36% of the amiC-deficient cells displayed the mVenus-RgsS focus at the old cell pole (Fig. 2c), we asked if localization of other Rgs and Tol-Pal proteins and polar cell wall growth zones was affected by the amiC mutation. Therefore, the corresponding genes were fused to mCherry at their native genome locations in both amiC-sufficient and -deficient mVenus-rgsS strains. In exponentially growing cells of both strains, the polar signals of mCherry-tagged RgsP, RgsA, RgsB, RgsD, RgsE, and TolQ colocalized with mVenus-RgsS (Fig. 3a and Supplementary Fig. 13). Time-lapse microscopy analysis of mCherry-tagged RgsP, RgsA and TolQ confirmed that their localization dynamics correlated with the one of mVenus-RgsS both in amiC-sufficient and amiC-deficient cells (Supplementary Figs. 14–16). This indicates coordinated conjoint positioning of Rgs proteins and TolQ after cell division, in case of both correct and aberrant polar mVenus-RgsS focus inheritance. Polar Pal-mCherry and mVenus-RgsS foci consistently colocalized only in amiC-sufficient cells, whereas in amiC-deficient cells, also divergent polar localization of mVenus-RgsS and Pal-mCherry foci was observed (Fig. 3b). Time-lapse microscopy revealed that Pal-mCherry faithfully colocalized with mVenus-RgsS in wild type cells (Supplementary Fig. 17). In amiC-deficient cells, which inherited mVenus-RgsS at the old cell pole, Pal-mCherry was retained at the new cell pole and accumulated de novo in the course of cell elongation at the old cell pole (Supplementary Fig. 17). Thus, we considered divergent mVenus-RgsS and Pal-mCherry localization as a marker of cells with mVenus-RgsS focus at the old cell pole. Pulse-labeling with HADA revealed conjoint positioning of PG incorporation zones with mVenus-RgsS in both amiC-sufficient and amiC-deficient cells, independent of Pal-mCherry focus localization (Fig. 3b, Supplementary Fig. 18, and Supplementary Table 6). Interestingly, a minor fraction of both amiC-sufficient and amiC-deficient cells exhibited diffuse HADA staining, despite presence of mVenus-RgsS and Pal-mCherry foci in either conjoint or divergent location. These may represent newborn cells that did not start the cell elongation yet. The major proportion of elongating amiC-deficient cells contained the single mVenus-RgsS focus, HADA staining zone and Pal-mCherry focus at the same cell pole, indicating cells with normal growth polarity (Supplementary Fig. 18 and Supplementary Table 6). In a minor fraction of the cells, the single mVenus-RgsS focus and a HADA staining zone were found at the cell pole opposite to the one containing the Pal-mCherry focus, indicating cells with inverted growth polarity (Fig. 3c, Supplementary Fig. 18, and Supplementary Table 6). Moreover, bipolar HADA staining was detected in cells with bipolar mVenus-RgsS localization (Supplementary Fig. 18 and Supplementary Table 6). Taken together these data and aberrant polar localization patterns of RgsS in cells lacking functional amiC or amcA or containing an rgsS variant encoding SPOR domain deficient RgsS (Fig. 2a, b, Supplementary Figs. 10–12, and Supplementary Tables 3, 5, and 6), we infer that aberrant positioning of mVenus-RgsS at the old cell pole is promoted by these genetic alterations and correlates with positioning of the PG growth zone, other Rgs proteins and TolQ at the same cell pole (Fig. 3c).
Fig. 3. Rgs proteins, TolQ and PG incorporation zones colocalize with RgsS in both wild type and amiC cells.
a Fluorescence microscopy images of exponentially growing Rm2011 mVenus-rgsS and Rm2011 mVenus-rgsS amiC cells, producing mCherry fusions of the indicated proteins from gene fusions at the native genome locations. Cell samples were taken from exponential phase TY cultures. The insert shows an additional cell of the same strain representative of cells with bipolar colocalization of mVenus-RgsS and TolQ-mCherry fluorescence foci. The images are representative of two independent cultivations and microscopy analyses. Scale bar, 5 µm; Ph phase contrast. b Fluorescence microscopy of Rm2011 mVenus-rgsS and Rm2011 mVenus-rgsS amiC cells, carrying pal-mCherry at the native genomic location, pulse-labeled with HADA for 3 min. Cell samples were taken from exponential phase TY cultures. Arrowheads show cells with non-colocalized mVenus-RgsS and Pal-mCherry. Scale bar, 5 µm; Ph phase contrast. The images are representative of three independent cultivations, HADA staining and microscopy analyses. c Schematic representation of cell growth polarity inheritance inferred from data shown in this figure, Fig. 2 and Supplementary Figs. 10–12 and 18. In wild-type cells, Rgs proteins and zones of PG biosynthesis are inherited to the new cell pole. In cells, lacking AmiC, AmcA, or the SPOR domain of RgsS, the Rgs proteins and PG synthesis zones are occasionally observed at the old cell pole, representing cells with inverted growth polarity. D daughter cell, M mother cell.
Inverted cell growth polarity affects positioning of the chromosomal origins of replication
In the time-lapse microscopy experiments, we observed that cells with inverted growth polarity were able to successfully complete the cell cycle and produce viable progeny. Thus, these cells were able to replicate and segregate their genomic DNA. In a newborn wild type S. meliloti cell, the chromosomal replication origin (oriC, designated here as oriC1) is located at the old, non-growing cell pole and persists there throughout the cell cycle8,16. The newly replicated oriC (designated here as oriC2) relocates from the old cell pole towards the new, growing cell pole8,16. ParB, fused to fluorescent proteins, can be used to visualize the oriC due to ParB binding at and around the oriC region47. We asked how the two oriCs were localized in the cells with inverted growth polarity. Therefore, we replaced parB with parB-cerulean at the native genome location in the mVenus-rgsS wild-type strain, in its amiC and amcA mutant derivatives as well as in the mVenus-rgsS1−863-mCherry strain.
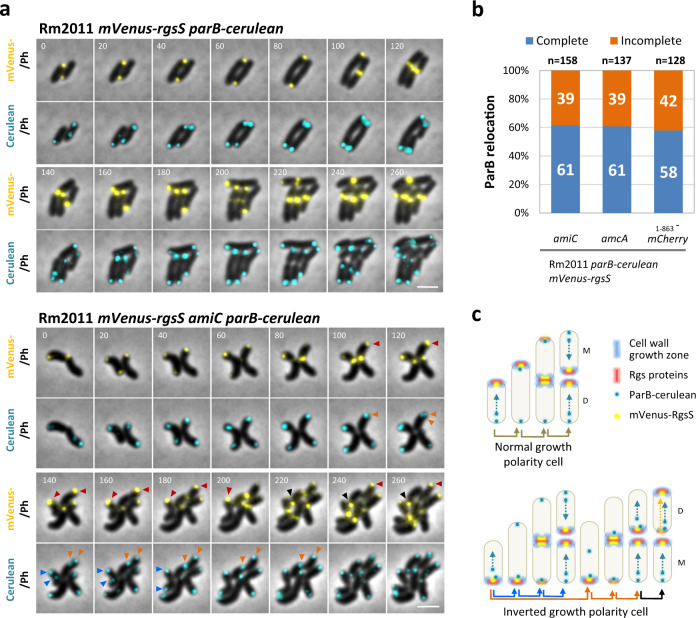
In mVenus-rgsS parB-cerulean cells, time-lapse microscopy revealed the expected spatiotemporal dynamics of ParB-cerulean (Fig. 4a), and all the considered predivisional cells with visible septum constriction contained two polar ParB foci (Fig. 4b). In cells of the mVenus-rgsS parB-cerulean amiC strain with mVenus-RgsS focus at the new cell pole (hence with normal cell polarity), wild type-like ParB-cerulean dynamics was observed (Fig. 4a). In the newborn mVenus-rgsS parB-cerulean amiC cells with inverted growth polarity, both the ParB-cerulean focus marking oriC1 and mVenus-RgsS were situated at the old, growing cell pole. This resulted in migration of the newly emerged oriC2 towards the non-growing pole. In a major part of these cells, migration of oriC2 was successfully completed (Fig. 4a, b and Supplementary Table 7), whereas in the remaining cells, it stayed partial until the end of the cell cycle (Fig. 4a, b).
Fig. 4. Effects of inverted cell growth polarity on segregation of chromosomal origins.
a Time-lapse fluorescence microscopy images of Rm2011 mVenus-rgsS wild type and its amiC mutant derivative, carrying parB-cerulean at the native genome location, growing on MM-agarose. Red and black arrowheads indicate accumulation of mVenus-RgsS at the old cell pole of the daughter cell (red) or a mother cell (black). Blue and orange arrowheads show complete (blue) or incomplete (orange) ParB-cerulean focus relocation to the opposite cell pole of a cell with inverted growth polarity. Time is shown in minutes. Scale bar, 2 µm; Ph phase contrast. The images are representative of three independent cultivations and microscopy analyses. b ParB-cerulean focus migration towards the new cell pole in cells with mVenus-RgsS focus at the old cell pole. n, total number of analyzed cells. The values represent the mean values of three biological replicates. The standard deviation values and statistic comparison between the strains are shown in Supplementary Table 7. c Schematic representation of ParB-cerulean spatiotemporal patterns observed in a. In case of cells with normal growth polarity, newborn cells contained a ParB-cerulean focus marking oriC1 at the old cell pole and the migration of a second ParB-cerulean focus marking oriC2 proceeded towards the growing cell pole. In cells with inverted growth polarity, which accumulated the Rgs proteins, which are considered markers for the PG growth zones, at the old cell pole, the migration of the second ParB-cerulean focus marking oriC2 proceeded towards the non-growing cell pole. This resulted in either complete (blue arrows) or incomplete (orange arrows) polar relocation of the second ParB-cerulean focus. A part of the cells that inherited a ParB-cerulean focus at the new cell pole accumulated mVenus-RgsS at the former non-growing pole (black arrow). The color coding is consistent with a. D daughter cell, M mother cell.
After the cell division, mVenus-rgsS parB-cerulean amiC cells with incomplete oriC2 relocation towards the non-growing cell pole typically generated a mother cell (former non-growing compartment) with both mVenus-RgsS and ParB-cerulean foci located at the new cell pole (Fig. 4a, c). In a major fraction of such cells, the mVenus-RgsS focus positioned to the old cell pole, which constituted the former non-growing pole (Fig. 4a and Supplementary Table 8). This resulted in a cell with inverted growth polarity, which restored the opposite arrangement of the polar growth zone and oriC1. This implies that not only segregation of oriC2 is affected by the position of the growing cell pole, but also positioning of the elongasome complex may be affected by the localization of oriC1. Time-lapse microscopy of mVenus-rgsS amcA and mVenus-rgsS1−863-mCherry strains revealed defects in ParB-cerulean dynamics, similar to the ones of the mVenus-rgsS amiC strain (Fig. 4b, Supplementary Figs. 19 and 20, and Supplementary Tables 7 and 8). In rare cases, the septum was formed before oriC2 relocation reached beyond mid-cell, which resulted in formation of one progeny cell containing oriC1 and oriC2, and the other oriC-free progeny cell that did not proliferate further (Supplementary Fig. 21). Thus, inverted growth polarity partially interfered with relocation of the daughter chromosome towards the non-growing cell compartment.
AmiC and SPORRgsS stabilize the positioning of mVenus-RgsS molecules at the growing cell pole
Single-molecule tracking (SMT) microscopy was applied to analyze dynamics of mVenus-tagged proteins in mVenus-rgsS wild type, mVenus-rgsS amiC, mVenus-rgsS1−863, and mVenus-rgsS1−863-mCherry cells with one polar mVenus fluorescence focus, hence undergoing cell elongation. Regarding the diffusion coefficient (D), the mVenus-RgsS molecules fell into static (D = 0.022 µm2 s−1) and mobile (D = 0.27 µm2 s−1) populations (Fig. 5a, Supplementary Fig. 22, and Supplementary Table 9). An average molecule residence time of 344 ms was estimated from the distribution of dwell events, which could be best fitted with a mixture of two single exponential decay distributions, suggesting two distinct molecule populations with two different residence times (Supplementary Fig. 23). Note that the actual residence times are longer because our analysis involves a convolution of bleaching and mobility of the molecules.
Fig. 5. AmiC and SPORRgsS facilitate stable positioning of mVenus-RgsS molecules at the cell pole.
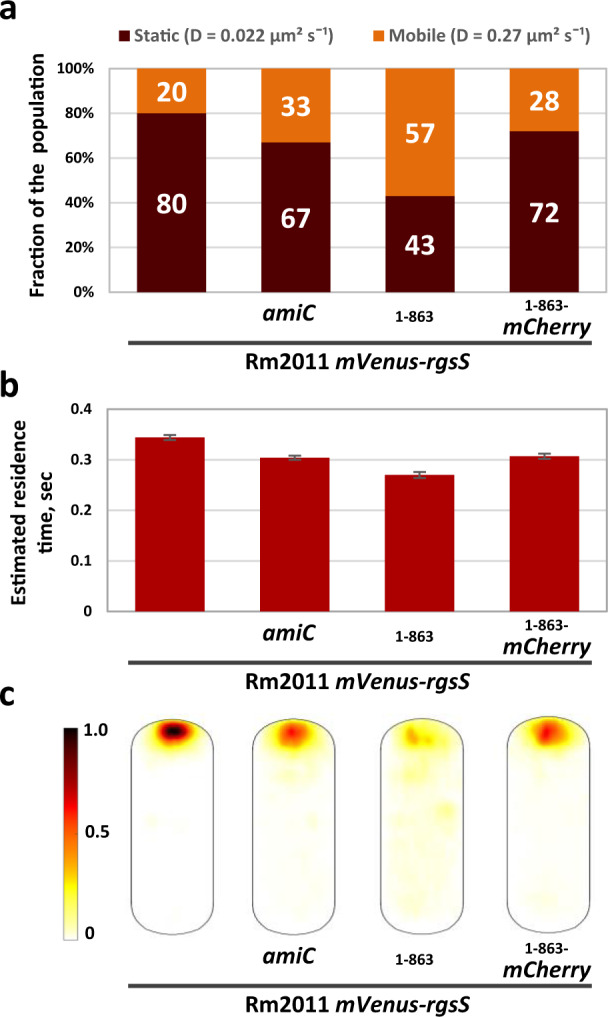
Single-molecule tracking of mVenus-RgsS, mVenus-RgsS1–863, and mVenus- RgsS1–863-mCherry molecules in cells from exponential phase TY cultures. Single molecule tracks were collected from cells, originating from three independent biological replicates. Numbers of single molecule tracks analyzed: Rm2011 mVenus-rgsS, n = 7556; Rm2011 mVenus-rgsS amiC, n = 5439; Rm2011 mVenus-rgsS1-863, n = 2296; Rm2011 mVenus-rgsS1-863-mCherry, n = 5469. a Relative fraction size of molecule populations with two different normalized diffusion constants, determined by Gaussian-mixture model (GMM) fit. Statistics analysis and numerical data are presented in Supplementary Table 9. b Estimated average residence times (time a molecule stays in a radius of 128.7 nm for more than nine 20 ms intervals), calculated from single molecule tracking data. Data presented as mean values of residence times calculated for each trajectory. Error bars indicate the standard deviation of the mean. Numerical data is presented in Supplementary Table 9. c Probability heatmaps of mVenus-RgsS, mVenus-RgsS1–863, and mVenus-RgsS1–863-mCherry molecules trajectory distribution in a normalized cell. The color code on the left indicates the probability of the molecules to be detected in the given cell area.
In the mVenus-rgsS amiC and mVenus-rgsS1−863-mCherry cells, the size of the static fraction of the tracked molecules as well as their estimated average residence time decreased relative to mVenus-rgsS wild type cells (Fig. 5a, b and Supplementary Table 9).
This implies that mVenus-RgsS binding to AmiC-processed PG via its SPOR domain reduces the overall mobility of the protein and therefore stabilizes its position. In mVenus-rgsS wild-type cells, the tracked molecules were strongly enriched at the pole, whereas in mVenus-rgsS amiC and mVenus-rgsS1−863-mCherry cells, they were more often detected in the remaining cell area (Fig. 5c). In mVenus-rgsS1−863 cells, the polar enrichment of the protein molecules, the size of the static fraction and the residence time were strongly reduced (Fig. 5a–c). This is consistent with our previous observations of weaker or even non-detectable polar mVenus-RgsS1−863 foci (Fig. 1a and Supplementary Figs. 5 and 11). Since removal of the SPOR domain destabilized RgsS (Fig. 1d), it is likely that part of the mVenus-RgsS1−863 molecules detected during tracking represented degraded protein.
To further characterize the spatial dynamics of mVenus-RgsS, mVenus-RgsS1−863 and mVenus-RgsS1−863-mCherry molecules, the tracks were classified regarding their ability to leave the defined confinement area (Supplementary Fig. 24 and Supplementary Table 9). In the mVenus-rgsS wild type strain, both confined (29%) and free (68%) tracks were almost exclusively detected at the pole (Supplementary Fig. 24). The large proportion of free tracks concentrated in the narrow polar zone may indicate high on/off rates for binding of mVenus-RgsS to its polar target sites, possibly due to a highly dynamic molecular environment in the polar cell wall elongation zone. In the mVenus-rgsS amiC and mVenus-rgsS1−863-mCherry cells, the proportion of free tracks increased to 73% and the proportion of confined tracks decreased to 22% and 23%, respectively, whereas both track types were more abundant outside the polar region (Supplementary Fig. 24). The polar area, containing confined tracks, was extended towards mid-cell (Supplementary Fig. 24). This implies that in the absence of SPORRgsS or AmiC-processed PG, other factors, which are not spatially restricted to the polar area, could bind RgsS and therefore effectuate confinement of RgsS molecules.
Discussion
Asymmetric cell growth and division is a widespread phenomenon in prokaryotes and eukaryotes48. A prerequisite for asymmetric cell growth in unipolarly growing bacteria, such as S. meliloti, is polarization of the elongating cell. We showed that in S. meliloti, faithful positioning of the polar growth zone requires the FtsN-like protein RgsS with its SPOR domain as well as peptidoglycan amidase AmiC and its EnvC-like putative cofactor AmcA.
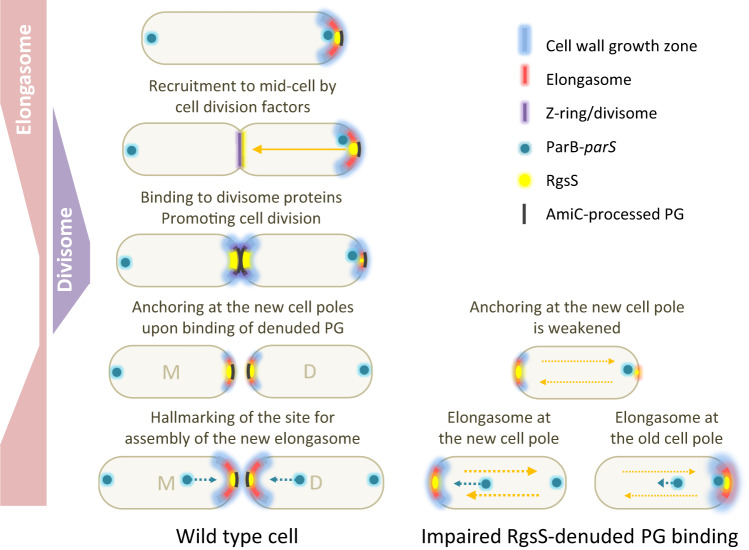
We observed that mCherry-SPORRgsS accumulated at the septum, similarly to the isolated SPOR domain of C. crescentus FtsN-like CC200726. This suggests the ability of RgsS to bind denuded PG via its SPOR domain, reminiscent of FtsNEc32,49. However, in S. meliloti, the impact of this binding extends beyond the cell division process (Fig. 6). We showed that both AmiC and an intact SPORRgsS are important for confinement of the RgsS molecules in the polar region where the elongasome can be expected to reside. AmiC-processed PG, enriched in this area, could constitute a high-affinity binding target for mVenus-RgsS molecules, whereas lower-affinity binding sites may be provided by RgsS protein interaction partners, present in- and outside the polar area. We propose a hypothetical model of RgsS function, in which during cell elongation, RgsS is localized at the growing cell pole, anchored to denuded PG generated by AmiC. It probably is embedded into the Tol-Pal-Rgs complex and possibly interacts with other elongasome components. In the course of divisome assembly, RgsS is recruited to mid-cell by a yet-unknown potent binding partner and may fulfill a FtsN-like role in cell division. AmiC-processed PG, generated during cell division, could serve to anchor RgsS at the new cell pole where accumulated RgsS is suggested to hallmark the site for assembly of a new elongasome (Fig. 6).
Fig. 6. Proposed role of RgsS-denuded PG interaction in faithful positioning of the polar cell elongation complex at the new cell pole.
During cell elongation, RgsS is situated at the growing cell pole and its position is suggested to be stabilized by interaction with AmiC-processed PG. During divisome assembly, RgsS is recruited to mid-cell possibly by one or more divisome components. During cell division, AmiC-processed PG is formed and probably provides binding sites for RgsS. This is assumed to ensure stabilization of RgsS localization at the new cell pole. We propose that RgsS hallmarks the site for assembly of the new elongasome. In the absence of AmiC-processed PG-RgsS binding, anchoring of RgsS at the new cell pole is weakened. Thus, RgsS might accumulate and promote formation of the new elongasome at the old cell pole.
In E. coli, prior to cell division, FtsNEc is recruited to the septum by FtsA in a SPOR domain-independent manner43. Likewise, decoupling of the functional link between RgsS and denuded PG in S. meliloti did not abolish septal and polar localization of RgsS. However, it resulted in increased mVenus-RgsS mobility and allowed for accumulation of the protein at the old cell pole, accompanied by positioning of the cell wall growth zone (Fig. 6). Thus, positioning of the polar assembly of the elongasome machinery appears to be flexible and hallmarked by RgsS accumulation. Anchoring of RgsS at the new cell pole, dependent on the local AmiC activity, could represent a robust mechanism of continuous propagation of the cell growth polarity, which ensures a uniform opposite arrangement of the old cell pole containing oriC1 and the polar growth zone at the newborn pole in both progeny cells.
The reasons for erroneous RgsS accumulation at the old cell pole are still an open question. S. meliloti cell division is asymmetric, with a slightly smaller daughter cell50. The onset of oriC2 migration is delayed in the daughter cell compared to the mother cell in S. meliloti as well as in the closely related A. tumefaciens8,51. After cell division, the mother cell retains its growth polarity, which may provide a head start for the new cell cycle. In contrast, the daughter cell undergoes a growth polarity switch, converting the growing pole into a non-growing pole. It cannot be excluded that this process is not completed at the time point of cell division. In cells lacking AmiC or SPORRgsS, RgsS is not confined at the new cell pole and may be recruited to the remnants of the old elongasome and landmark the old cell pole for elongasome reassembly. This could explain why aberrant growth polarity preferentially arose in the daughter cells.
Our results show that although RgsS is not strictly required for cell elongation28, its localization correlates with the localization of polar growth zone proteins and PG synthesis enzymes. Previously, we identified RgsE, a homolog of A. tumefaciens pole-forming protein GPR27, as RgsS interaction partner28. RgsE and GPR are promising candidates for cell elongation scaffold proteins. RgsE is absent from the cell division zone but is detectable at the new cell pole immediately after the cell division. It is tempting to speculate, that RgsE is recruited by RgsS and this initiates the assembly of the new elongasome.
In many bacteria with polarly anchored oriC, such as C. crescentus, chromosome segregation relies on movement of the new oriC towards the new cell pole, enabled by interaction of oriC-bound ParB with a ParA gradient52. In C. crescentus, polar localization of the ParB-ParS complex alone determines the orientation of the ParA gradient, required for oriC migration, independent of the pole descendance53. Our observation of successful oriC2 migration in a major part of the S. meliloti cells with inverted growth polarity suggests that in S. meliloti the ParA gradient may be formed independent of the old-new pole arrangement and localization of the cell wall growth zone. However, we also observed delayed and incomplete oriC2 migration towards the non-growing cell pole. In Streptomyces coelicolor, the polar protein Scy, implicated in elongation of hyphae, recruits ParA to the hyphal tips and regulates ParA polymerization54. Our results imply that in S. meliloti, some components of the polar growth complex may influence ParA gradient formation.
PG amidases are known cell separation factors implicated in cell division in bacteria elongating by dispersed cell wall growth. Our results show that in contrast to PG amidases in E. coli, H. pylori, and C. crescentus20,55,56, S. meliloti AmiC may rather have an accessory function in septum splitting and an additional role in cell wall synthesis. The enzymatic activity of AmiC is probably required for the cell wall integrity-related function and binding targets for mCherry-SPORRgsS seem to be present at the growth pole. It is therefore likely that PG processing by AmiC takes place during cell elongation. PG synthesis processivity was suggested as an important factor in maintaining the straight rod cell morphology57, whereas spatial patterning of PG defects was predicted to define various bacterial cell shapes58. Recently, a Staphylococcus aureus PG amidase was suggested to constrain PG synthase activity during cell growth59. Its absence resulted in spatially dysregulated PG synthesis and strong cell enlargement, reminiscent of S. meliloti amiC cells growing in LB59. It could be argued that increased mobility of RgsS in the absence of denuded PG might affect the spatiotemporal dynamics of cell elongation zone proteins, leading to a disturbed pattern of PG incorporation. It is likely that in polarly growing S. meliloti, PG processing by AmiC is a part of the cell elongation process, required for spatial or functional fine-tuning of the elongasome.
Collectively, our results suggest a pivotal role for the FtsN-like protein RgsS in faithfully positioning of the cell wall growth zone at the new cell pole of S. meliloti. Rgs proteins, including RgsS, are conserved in Rhizobiales28. These proteins form an interaction network involving components of the Tol-Pal system28. We therefore assume that positioning of the growth pole in S. meliloti and other unipolarly growing members of the Rhizobiales is determined in a similar manner. The proposed model of growth pole determination involving RgsS and AmiC-processed PG is a striking example how cellular asymmetry can be established in bacteria to enable asymmetric cell elongation.
Methods
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are shown in the Supplementary Table 10.
S. meliloti was grown at 30 °C in TY medium (5 g/L tryptone, 3 g/L yeast extract, 0.4 g CaCl2×2H2O), LB medium (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl) or LB medium with added 2.5 mM CaCl2. For time-lapse microscopy, modified MOPS-buffered minimal medium (MM) (10 g/L MOPS, 10 g/L mannitol, 3.55 g/L sodium glutamate, 0.246 g/L MgSO4×7H2O, 0.25 mM CaCl2, 2 mM K2HPO4, 10 mg/L FeCl3×6H2O, 1 mg/L biotin, 3 mg/L H3BO3, 2.23 mg/L MnSO4×H2O, 0.287 mg/L ZnSO4×7H2O, 0.125 mg/L CuSO4×5H2O, 0.065 mg/L CoCl2×6H2O, 0.12 mg/L NaMoO4×2H2O, pH 7.2) was used. When required, antibiotics were added to agar media at following concentrations: streptomycin, 600 mg/L, kanamycin, 200 mg/L, gentamicin, 30 mg/L, spectinomycin, and 200 mg/L. IPTG was added to 100 µM when using pWBT-NF based constructs.
E. coli was grown at 37 °C on LB and antibiotics were added at following concentrations: kanamycin, 50 mg/L, gentamicin, 8 mg/L, spectinomycin, and 100 mg/L. IPTG was added to 100 µM. For liquid cultures, antibiotic concentrations were reduced to the half. For microscopy analysis, E. coli strains were grown in M9 medium (3 g/L KH2PO4,, 12.8 g/L Na2HPO4 ⋅7H2O, 0.5 g/L NaCl, 1.0 g/L NH4Cl, 0.05 g/L MgSO4) with 0.2% casamino acids and 0.2% maltose. Expression of mCherry-SPORRgsS was induced with 500 µM IPTG for 8 hours.
For western blot analysis, fluorescence microscopy of liquid culture samples and single molecule tracking microscopy, the S. meliloti strains were grown in glass tubes with 3 ml medium with shaking at 200 RPM and harvested at OD600 between 0.4 and 0.8.
For growth assays on agar plates, the stationary cultures were adjusted to OD600 of 2.5, serial dilutions were prepared and 10 µl of each dilution was spotted onto the agar plates. Plates were grown for 48 hours and photographed.
For growth assays in liquid cultures, the precultures were grown in TY medium in glass tubes to stationary phase. In all, 150 µl volume cultures in 96-well microtiter plate were inoculated at OD600 = 0.01 and grown with 800 RPM shaking at 30 °C. OD600 was recorded every hour in a Biotek Synergy H1 Spectrophotometer. Four independent transconjugant colonies of each strain were used as biological replicates except for the 2011 mVenus-rgsS amiC amiD pABC-Psyn strain for which three transconjugant colonies were used.
Construction of strains and plasmids
Cloning was performed using PCR, restriction digestion, ligation, and E. coli transformation. The strains and plasmids generated are listed in the Supplementary Table 5. Primers used in this study are shown in the Supplementary Table 11. The constructs were verified by sequencing.
Plasmid pSRKGm-SP-mCherry-SPOR was constructed by inserting the mCherry coding sequence, N-terminally extended with the RgsB signal peptide encoding sequence (codons 1–25), and the SPOR domain encoding sequence (codons 831–945 of rgsS) into pSRKGm under the control of the lac promoter.
To generate the strains with markerless amiC and amcA deletions, the gene flanking regions were cloned into the sucrose selection plasmid pK18mobsacB. The resulting plasmids were introduced into S. meliloti by conjugation and subsequently, the double recombinants were selected on LB agar plates containing 2.5 mM CaCl2 and 10% sucrose as described previously60.
To construct the amiC and amcA complementation plasmids, the genes including the promoter regions of 368 and 401 bp, respectively, were cloned into shuttle vector pABC-Psyn. To introduce point mutations into the amiC sequence, overlap extension PCR was applied. 3xFLAG-tagged versions of native and mutated AmiC complementation constructs were constructed by insertion of amiC including the promoter region and missing the stop codon into vector pABC-Psyn-CF.
To generate strain Rm2011 mVenus-rgsS1−863, the DNA region encoding amino acids 622-863 of RgsS, followed by a stop codon, was cloned into the non-replicative vector pK18mob2 and introduced into Rm2011 mVenus-rgsS by conjugation. Homologous recombination resulted in truncation of mVenus-rgsS after rgsS codon 863.
To generate strain Rm2011 mVenus-rgsS1−863-mCherry, the DNA region, encoding amino acids 622-863 of RgsS was cloned into non-replicative vector pK18mob2-mCherry and introduced into Rm2011 mVenus-rgsS by conjugation. Homologous recombination resulted in truncation of mVenus-rgsS after rgsS codon 863 and its fusion to mCherry.
To generate C-terminal fusions of rgsS or its derivatives to the 3xFLAG tag-encoding sequence at the native genomic location, the sequence in the range of 500 to 800 bp encoding the C-terminal portion of RgsS was cloned in frame into the non-replicative vector pG18mob-CF and resulting plasmids were introduced into S. meliloti by conjugation. Homologous recombination resulted in replacement of the native gene with a tagged gene copy. To generate the ectopic N-terminal fusions of native or mutated RgsS to the 3XFLAG-tag, corresponding coding sequences were inserted into plasmid pWBT-NF under the control of lac and T5 promoters.
To generate the strains carrying C-terminal mCherry fusions to rgsA, rgsB, rgsD, rgsE, rgsP, and pal at the native genomic location, the corresponding non-replicative constructs based on pK18mob-mCherry were introduced into S. meliloti by conjugation. Homologous recombination resulted in replacement of the native gene with the mCherry-fused version. To construct the strain carrying a 3′ mCherry fusion to tolQ at the native genomic location, the corresponding non-replicative construct was introduced by conjugation. Homologous recombination resulted in insertion of the tolQ-mCherry fusion, including the native promoter, upstream of the tolQRAB operon promoter region.
To obtain the E. coli amiABC triple deletion mutant, the amiA deletion marked with a kanamycin resistance cassette in E. coli strain JW2428, was introduced into the E. coli MG1655 strain using P1 transduction61. The kanamycin resistance cassette was removed using transformation with plasmid pCP2062. Subsequently, the amiC deletion, marked by the kanamycin resistance cassette in strain JW4127 was introduced using P1 transduction. The resulting double amiA amiC deletion mutant was cured of the kanamycin resistance marker using transformation with plasmid pCP20 and simultaneously, the amiA amiC strain was co-transformed with plasmid pSRKGm-SP-mCherry-SPOR. Finally, the amiB deletion, marked by the kanamycin resistance cassette in strain JW5449, was introduced into the amiA amiC strain carrying pSRKGm-SP-mCherry SPOR using P1 transduction.
Fluorescence microscopy
Microscopy was performed using the Nikon microscope Eclipse Ti-E equipped with a differential interference contrast (DIC) CFI Apochromat TIRF oil objective (100x; numerical aperture of 1.49) and a phase-contrast Plan Apo l oil objective (100x; numerical aperture, 1.45) with the AHF HC filter sets F36-513 DAPI (excitation band pass [ex bp] 387/11 nm, beam splitter [bs] 409 nm, and emission [em] bp 447/60 nm), F36-504 mCherry (ex bp 562/40 nm, bs 593 nm, and em bp 624/40 nm), F36-525 EGFP (ex bp 472/30 nm, bs 495 nm, and em bp 520/35 nm) and F36-528 YFP (ex bp 500/24 nm, bs 520 nm, and em bp 542/27 nm). Images were acquired with an Andor iXon3 885 electron-multiplying charge-coupled device (EMCCD) camera.
For microscopy of exponentially growing cultures, 2 µl of TY cultures at OD600 of 0.4–0.8 were spotted onto 1% Molecular biology grade agarose (Eurogentec) pads, let dry for 2–3 min, closed with cover glass and microscoped. For time-lapse microscopy, bacteria from exponential growth phase TY cultures were diluted 1:20 and 2 µl were spread by gravity flow on the MM agarose pad and let dry for 14 min. The pads were closed air-tight with a cover slip and images were acquired every 20 min in an incubation chamber at 30 °C.
Staining of S. meliloti cells with fluorescently-labeled D-amino acid HADA was performed as follows: 1 μl of 36 mM HADA dissolved in dimethylsulphoxid was added to 80 μl of the exponentially growing TY culture. The culture was grown further for 3 min at 30 °C with shaking at 800 rpm. Cells were fixed with 186 μl of 100% ethanol for 10 min at room temperature, washed three times with 0.9% NaCl and microscoped.
Quantification of fluorescent protein localization patterns in snapshots and time-lapse images was performed manually using NIS software (Nikon). To evaluate the presence and localization of fluorescence foci within cells grown in liquid cultures, 5–15 images of the same sample were visually evaluated and cells with particular fluorescence foci arrangements were counted. In time-lapse microscopy analysis, tiles of images containing fluorescence signals merged with phase contrast were generated and visually evaluated for fluorescence foci dynamics, foci persistence in septum and cell cycle duration. Cell morphology analysis was performed using the MicrobeJ 5.13 l plugin to the ImageJ software (detection settings for cell length: 1–6 µm; for cell width: 0.5–1 µm, for cell curvature: 0-max).
Single molecule tracking microscopy and data analysis
The single molecule tracking (SMT) data were obtained with a customized “slim field” microscope (Nikon Eclipse Ti microscope; 100x oil-immersion objective, NA = 1.49) with a 514-nm laser diode beam line of 100 mW maximal power (~250 W/m2 were usually employed). The fluorophores were bleached to single molecule level by the laser (representative data is shown in Movie S1 and Supplementary Fig. 25) to be able to follow the single molecules that can be identified as single bleaching events. All the movies were acquired with 20 ms streams, 3000 frames) and acquired by an EMCCD camera (ImageEM X2 EM-CCD, Hamamatsu).
Movies were cropped to only include the frames containing single molecules using Fiji software63. To obtain trajectories of single molecule movements, the cropped movies were analyzed by Utrack 2.2.164. The gathered trajectories were evaluated by SMTracker65 (https://sourceforge.net/projects/singlemoleculetracker/). The Gaussian Mixture Model (GMM) was used to determine normalized diffusion coefficients and fraction sizes of molecules fitting into either mobile or static populations. For visualization of molecules and their tracks, these were projected into a normalized cell. Molecules were classified as confined if they did not leave the confinement radius of 120 nm over a period of 180 ms or longer. Molecules were classified as free if they left the confinement radius of 120 nm at each of the 20 ms steps over a period of 180 ms or longer. The confinement radius corresponds to three times the localization error.
Western blot
S. meliloti strains were grown in TY, supplemented with corresponding antibiotics and IPTG when indicated, to OD600 of 0.4–0.8. Cells were collected by centrifugation, resuspended in protein loading dye (50 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 12.5 mM EDTA, 0.02% bromophenol blue) to an OD600 of 10, frozen in liquid nitrogen and stored at −20 °C until use. Cells were lysed for 10 min at 95 °C and 10 μl of cell lysates were loaded to SDS-PAGE gel (separating gel: 0.375 M Tris-HCl pH 8.8 0.1% SDS, 8% acrylamide:bisacrylamide 37.5:1 (Fig. 1d), 12% acrylamide:bisacrylamide 37.5:1 (Supplementary Fig. 7) or 6% acrylamide:bisacrylamide 37.5:1 (Supplementary Fig. 9), ammonium persulfate 0.1% Temed, 0.001%; stacking gel: 0.125 M Tris-HCl pH 6.8 0.1% SDS, 6% acrylamide:bisacrylamide 37.5:1 (Fig. 1d and Supplementary Fig. 7) or 4% acrylamide:bisacrylamide 37.5:1 (Supplementary Fig. 9), ammonium persulfate 0.1% Temed, 0.001%) that was run in the running buffer (Tris base 3.03 g/L, Glycine 14,4 g/L, SDS 1 g/L) and separated proteins were transferred to a PVDF membrane (Thermo Fisher Scientific) using a semidry blotting procedure in transfer buffer (0.025 M Tris base, 0.192 M glycine, 20% methanol). The membranes were blocked for one hour at room temperature in PBSTM (1.44 g/L Na2HPO4*2H2O, 0.24 g/L KH2PO4, 0.2 g/L KCl, 8 g/L NaCl, 1 ml/L TWEEN-20, 2% milk powder, pH 7.2), washed one time with PBST (1.44 g/L Na2HPO4*2H2O, 0.24 g/L KH2PO4, 0.2 g/L KCl, 8 g/L NaCl, 1 ml/L TWEEN-20, pH 7.2) and hybridized overnight at 4 °C in 15 ml PBST supplemented with Monoclonal ANTI-FLAG® M2-Peroxidase (HRP) antibody produced in mouse (Sigma-Aldrich; 1:1000 dilution) in 50 ml falcon tubes on a rolling shaker. Membranes were washed 4 times for 10–15 min with PBST at 4 °C, developed with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific) according to manufacturer instructions and imaged using the luminescence image analyzer LAS-4000 (Fujifilm).
Protein sequence analysis
Protein sequence analyses were conducted using the online tools BLASTP (https://blast.ncbi.nlm.nih.gov/Blast.cgi), Phobius66 (https://phobius.sbc.su.se/), RaptorX67 (http://raptorx.uchicago.edu/), SWISS-MODEL68 (https://swissmodel.expasy.org/), and Coiled-coils69 (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_lupas.html).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank Martin Thanbichler for sharing HADA, Marcel Wagner and Justin Hawkins for sharing plasmids and Luis Oviedo Bocanegra for help with statistical analysis of SMT data. This work was supported by funding from the LOEWE program of the State of Hesse (to A.B.), and the German Research Foundation (Project 269423233—TRR 174; to A.B. and P.L.G.).
Source data
Author contributions
E.K. and J.K.S. performed genetic constructions, microscopy, and protein analyses. L.S. carried out single molecule tracking experiments, and L.S. and P.L.G. analyzed and interpreted single molecule tracking data. E.K. and A.B. analyzed and interpreted experimental data, conceived the study, and wrote the paper, with input from all other authors.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-20739-3.
References
- 1.Meiring JCM, Shneyer BI, Akhmanova A. Generation and regulation of microtubule network asymmetry to drive cell polarity. Curr. Opin. Cell Biol. 2019;62:86–95. doi: 10.1016/j.ceb.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Rappel WJ, Edelstein-Keshet L. Mechanisms of cell polarization. Curr. Opin. Syst. Biol. 2017;3:43–53. doi: 10.1016/j.coisb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woldringh CLIs. Escherichia coli getting old? Bioessays. 2005;27:770–774. doi: 10.1002/bies.20271. [DOI] [PubMed] [Google Scholar]
- 4.Echazarreta MA, Klose KE. Vibrio flagellar synthesis. Front. Cell. Infect. Microbiol. 2019;9:131. doi: 10.3389/fcimb.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundararajan K, Goley ED. Cytoskeletal proteins in Caulobacter crescentus: spatial orchestrators of cell cycle progression, development, and cell shape. Subcell. Biochem. 2017;84:103–137. doi: 10.1007/978-3-319-53047-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaichi Y, et al. A multidomain hub anchors the chromosome segregation and chemotactic machinery to the bacterial pole. Genes Dev. 2012;26:2348–2360. doi: 10.1101/gad.199869.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shebelut CW, et al. Caulobacter chromosome segregation is an ordered multistep process. Proc. Natl Acad. Sci. USA. 2010;107:14194–14198. doi: 10.1073/pnas.1005274107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frage B, et al. Spatiotemporal choreography of chromosome and megaplasmids in the Sinorhizobium meliloti cell cycle. Mol. Microbiol. 2016;100:808–823. doi: 10.1111/mmi.13351. [DOI] [PubMed] [Google Scholar]
- 9.Brown PJ, et al. Polar growth in the Alphaproteobacterial order Rhizobiales. Proc. Natl Acad. Sci. USA. 2012;109:1697–1701. doi: 10.1073/pnas.1114476109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan AJ, et al. Regulation of bacterial cell wall growth. FEBS J. 2017;284:851–867. doi: 10.1111/febs.13959. [DOI] [PubMed] [Google Scholar]
- 11.van den Ent F, et al. Bacterial actin MreB assembles in complex with cell shape protein RodZ. EMBO J. 2010;29:1081–1090. doi: 10.1038/emboj.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salje J, van den Ent F, de Boer P. Direct membrane binding by bacterial actin MreB. Mol. Cell. 2011;43:478–487. doi: 10.1016/j.molcel.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letek M, et al. DivIVA is required for polar growth in the MreB-lacking rod-shaped actinomycete Corynebacterium glutamicum. J. Bacteriol. 2008;190:3283–3292. doi: 10.1128/JB.01934-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazza P, et al. MreB of Streptomyces coelicolor is not essential for vegetative growth but is required for the integrity of aerial hyphae and spores. Mol. Microbiol. 2006;60:838–852. doi: 10.1111/j.1365-2958.2006.05134.x. [DOI] [PubMed] [Google Scholar]
- 15.Hempel AM, et al. Assemblies of DivIVA mark sites for hyphal branching and can establish new zones of cell wall growth in Streptomyces coelicolor. J. Bacteriol. 2008;190:7579–7583. doi: 10.1128/JB.00839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schäper S, et al. Seven-transmembrane receptor protein RgsP and cell wall-binding protein RgsM promote unipolar growth in Rhizobiales. PLoS Genet. 2018;14:e1007594. doi: 10.1371/journal.pgen.1007594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassen V, et al. Localized incorporation of outer membrane components in the pathogen Brucella abortus. EMBO J. 2019;38:e100323. doi: 10.15252/embj.2018100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du S, Lutkenhaus J. Assembly and activation of the Escherichia coli divisome. Mol. Microbiol. 2017;105:177–187. doi: 10.1111/mmi.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donovan C, Bramkamp M. Cell division in Corynebacterineae. Front. Microbiol. 2014;5:32. doi: 10.3389/fmicb.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidrich C, et al. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 2001;41:167–178. doi: 10.1046/j.1365-2958.2001.02499.x. [DOI] [PubMed] [Google Scholar]
- 21.Uehara T, Parzych KR, Dinh T, Bernhardt TG. Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J. 2010;29:1412–1422. doi: 10.1038/emboj.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yahashiri A, Jorgenson MA, Weiss DS. Bacterial SPOR domains are recruited to septal peptidoglycan by binding to glycan strands that lack stem peptides. Proc. Natl Acad. Sci. USA. 2015;112:11347–11352. doi: 10.1073/pnas.1508536112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yahashiri A, Jorgenson MA, Weiss DS. The SPOR domain, a widely conserved peptidoglycan binding domain that targets proteins to the site of cell division. J. Bacteriol. 2017;199:e00118–17. doi: 10.1128/JB.00118-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ursinus A, et al. Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J. Bacteriol. 2004;186:6728–6737. doi: 10.1128/JB.186.20.6728-6737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai K, Xu Y, Lutkenhaus J. Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli isolated as a multicopy suppressor of ftsA12(Ts) J. Bacteriol. 1993;175:3790–3797. doi: 10.1128/JB.175.12.3790-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Möll A, Thanbichler M. FtsN-like proteins are conserved components of the cell division machinery in proteobacteria. Mol. Microbiol. 2009;72:1037–1053. doi: 10.1111/j.1365-2958.2009.06706.x. [DOI] [PubMed] [Google Scholar]
- 27.Zupan JR, et al. GROWTH POLE RING protein forms a 200-nm-diameter ring structure essential for polar growth and rod shape in Agrobacterium tumefaciens. Proc. Natl Acad. Sci. USA. 2019;116:10962–10967. doi: 10.1073/pnas.1905900116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krol E, et al. Tol-Pal system and Rgs proteins interact to promote unipolar growth and cell division in Sinorhizobium meliloti. mBio. 2020;11:e00306–e00320. doi: 10.1128/mBio.00306-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang JC, et al. Solution structure and domain architecture of the divisome protein FtsN. Mol. Microbiol. 2004;52:651–660. doi: 10.1111/j.1365-2958.2004.03991.x. [DOI] [PubMed] [Google Scholar]
- 30.Mishima M, et al. Solution structure of the peptidoglycan binding domain of Bacillus subtilis cell wall lytic enzyme CwlC: characterization of the sporulation-related repeats by NMR. Biochemistry. 2005;44:10153–10163. doi: 10.1021/bi050624n. [DOI] [PubMed] [Google Scholar]
- 31.Alcorlo M, et al. Structural basis of denuded glycan recognition by SPOR domains in bacterial cell division. Nat. Commun. 2019;10:5567. doi: 10.1038/s41467-019-13354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerding MA, et al. Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J. Bacteriol. 2009;191:7383–7401. doi: 10.1128/JB.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernhardt TG, de Boer PA. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 2003;48:1171–1182. doi: 10.1046/j.1365-2958.2003.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uehara T, Park JT. An anhydro-N-acetylmuramyl-L-alanine amidase with broad specificity tethered to the outer membrane of Escherichia coli. J. Bacteriol. 2007;189:5634–5641. doi: 10.1128/JB.00446-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priyadarshini R, de Pedro MA, Young KD. Role of peptidoglycan amidases in the development and morphology of the division septum in Escherichia coli. J. Bacteriol. 2007;189:5334–5347. doi: 10.1128/JB.00415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992;56:395–411. doi: 10.1128/MR.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clifton LA, et al. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir. 2015;31:404–412. doi: 10.1021/la504407v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocaboy M, et al. The crystal structure of the cell division amidase AmiC reveals the fold of the AMIN domain, a new peptidoglycan binding domain. Mol. Microbiol. 2013;90:267–277. doi: 10.1111/mmi.12361. [DOI] [PubMed] [Google Scholar]
- 39.Uehara T, Dinh T, Bernhardt TG. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J. Bacteriol. 2009;191:5094–5107. doi: 10.1128/JB.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang LC, et al. Peptidoglycan hydrolysis mediated by the amidase AmiC and its LytM activator NlpD is critical for cell separation and virulence in the phytopathogen Xanthomonas campestris. Mol. Plant Pathol. 2018;19:1705–1718. doi: 10.1111/mpp.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stohl EA, Lenz JD, Dillard JP, Seifert HS. The Gonococcal NlpD protein facilitates cell separation by activating peptidoglycan cleavage by AmiC. J. Bacteriol. 2015;198:615–622. doi: 10.1128/JB.00540-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odintsov SG, Sabala I, Marcyjaniak M, Bochtler M. Latent LytM at 1.3A resolution. J. Mol. Biol. 2004;335:775–785. doi: 10.1016/j.jmb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Peters NT, et al. Structure-function analysis of the LytM domain of EnvC, an activator of cell wall remodelling at the Escherichia coli division site. Mol. Microbiol. 2013;89:690–701. doi: 10.1111/mmi.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busiek KK, Margolin W. A role for FtsA in SPOR-independent localization of the essential Escherichia coli cell division protein FtsN. Mol. Microbiol. 2014;92:1212–1226. doi: 10.1111/mmi.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meehan BM, Landeta C, Boyd D, Beckwith J. The essential cell division protein FtsN contains a critical disulfide bond in a non-essential domain. Mol. Microbiol. 2017;103:413–422. doi: 10.1111/mmi.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan TR, et al. Identification of SPOR domain amino acids important for septal localization, peptidoglycan binding, and a disulfide bond in the cell division protein FtsN. J. Bacteriol. 2013;195:5308–5315. doi: 10.1128/JB.00911-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran NT, et al. Permissive zones for the centromere-binding protein ParB on the Caulobacter crescentus chromosome. Nucleic Acids Res. 2018;46:1196–1209. doi: 10.1093/nar/gkx1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kysela DT, Brown PJ, Huang KC, Brun YV. Biological consequences and advantages of asymmetric bacterial growth. Annu. Rev. Microbiol. 2013;67:417–435. doi: 10.1146/annurev-micro-092412-155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Addinall SG, Cao C, Lutkenhaus J. FtsN, a late recruit to the septum in Escherichia coli. Mol. Microbiol. 1997;25:303–309. doi: 10.1046/j.1365-2958.1997.4641833.x. [DOI] [PubMed] [Google Scholar]
- 50.Lam H, Matroule JY, Jacobs-Wagner C. The asymmetric spatial distribution of bacterial signal transduction proteins coordinates cell cycle events. Dev. Cell. 2003;5:149–159. doi: 10.1016/S1534-5807(03)00191-6. [DOI] [PubMed] [Google Scholar]
- 51.Ehrle HM, et al. Polar organizing protein PopZ is required for chromosome segregation in Agrobacterium tumefaciens. J. Bacteriol. 2017;199:e00111–e00117. doi: 10.1128/JB.00111-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ptacin JL, et al. A spindle-like apparatus guides bacterial chromosome segregation. Nat. Cell Biol. 2010;12:791–798. doi: 10.1038/ncb2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meléndez AB, Menikpurage IP, Mera PE. Chromosome dynamics in bacteria: triggering replication at the opposite location and segregation in the opposite direction. mBio. 2019;10:e01002–e01019. doi: 10.1128/mBio.01002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ditkowski B, et al. Dynamic interplay of ParA with the polarity protein, Scy, coordinates the growth with chromosome segregation in Streptomyces coelicolor. Open Biol. 2013;3:130006. doi: 10.1098/rsob.130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaput C, et al. Role of AmiA in the morphological transition of Helicobacter pylori and in immune escape. PLoS Pathog. 2006;2:e97. doi: 10.1371/journal.ppat.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zielińska A, et al. LytM factors affect the recruitment of autolysins to the cell division site in Caulobacter crescentus. Mol. Microbiol. 2017;106:419–438. doi: 10.1111/mmi.13775. [DOI] [PubMed] [Google Scholar]
- 57.Sliusarenko O, et al. Processivity of peptidoglycan synthesis provides a built-in mechanism for the robustness of straight-rod cell morphology. Proc. Natl Acad. Sci. USA. 2010;107:10086–10091. doi: 10.1073/pnas.1000737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang KC, et al. Cell shape and cell-wall organization in Gram-negative bacteria. Proc. Natl Acad. Sci. USA. 2008;105:19282–19287. doi: 10.1073/pnas.0805309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do T, et al. Staphylococcus aureus cell growth and division are regulated by an amidase that trims peptides from uncrosslinked peptidoglycan. Nat. Microbiol. 2020;5:291–303. doi: 10.1038/s41564-019-0632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schäfer A, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 61.Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- 62.Thomason LC, et al. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 2007;1:17. doi: 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- 63.Schindelin J, et al. Fiji. An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaqaman K, et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rösch TC, Oviedo-Bocanegra LM, Fritz G, Graumann PL. SMTracker. A tool for quantitative analysis, exploration and visualization of single-molecule tracking data reveals highly dynamic binding of B. subtilis global repressor AbrB throughout the genome. Sci. Rep. 2018;8:15747. doi: 10.1038/s41598-018-33842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Käll L, Krogh A, Sonnhammer ELL. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Källberg M, et al. RaptorX server: a resource for template-based protein structure modeling. Methods Mol. Biol. 2014;1137:17–27. doi: 10.1007/978-1-4939-0366-5_2. [DOI] [PubMed] [Google Scholar]
- 68.Waterhouse A, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Combet C, et al. Network protein sequence analysis. Trends Biochem. Sci. 2000;25:147–150. doi: 10.1016/S0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.