Abstract
Context
Novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (RA) tirzepatide demonstrated substantially greater glucose control and weight loss (WL) compared with selective GLP-1RA dulaglutide.
Objective
Explore mechanisms of glucose control by tirzepatide.
Design
Post hoc analyses of fasting biomarkers and multiple linear regression analysis.
Setting
Forty-seven sites in 4 countries.
Patients or other Participants
Three hundred and sixteen subjects with type 2 diabetes.
Interventions
Tirzepatide (1, 5, 10, 15 mg), dulaglutide (1.5 mg), placebo.
Main Outcome Measures
Analyze biomarkers of beta-cell function and insulin resistance (IR) and evaluate WL contributions to IR improvements at 26 weeks.
Results
Homeostatic model assessment (HOMA) 2-B significantly increased with dulaglutide and tirzepatide 5, 10, and 15 mg compared with placebo (P ≤ .02). Proinsulin/insulin and proinsulin/C-peptide ratios significantly decreased with tirzepatide 10 and 15 mg compared with placebo and dulaglutide (P ≤ .007). Tirzepatide 10 and 15 mg significantly decreased fasting insulin (P ≤ .033) and tirzepatide 10 mg significantly decreased HOMA2-IR (P = .004) compared with placebo and dulaglutide. Markers of improved insulin sensitivity (IS) adiponectin, IGFBP-1, and IGFBP-2 significantly increased by 1 or more doses of tirzepatide (P < .05). To determine whether improvements in IR were directly attributable to WL, multiple linear regression analysis with potential confounding variables age, sex, metformin, triglycerides, and glycated hemoglobin A1c was conducted. WL significantly (P ≤ .028) explained only 13% and 21% of improvement in HOMA2-IR with tirzepatide 10 and 15 mg, respectively.
Conclusions
Tirzepatide improved markers of IS and beta-cell function to a greater extent than dulaglutide. IS effects of tirzepatide were only partly attributable to WL, suggesting dual receptor agonism confers distinct mechanisms of glycemic control.
Keywords: tirzepatide, beta-cell function, insulin sensitivity, type 2 diabetes, GLP-1, GIP
Tirzepatide is a 39 amino acid synthetic peptide with agonist activity at both the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, with a greater affinity to GIP receptors (1). Its structure, which is primarily based on the GIP amino acid sequence, includes a C20 fatty di-acid moiety that prolongs the duration of action, thus allowing once-weekly subcutaneous administration (1). In a 26-week, randomized, double-blind Phase 2b trial in subjects with type 2 diabetes (T2D), treatment with tirzepatide (1 mg, 5 mg, 10 mg, and 15 mg) resulted in significant, dose-dependent reductions in glycated hemoglobin A1c (HbA1c) (up to –2.4%) at 26 weeks compared with placebo (+0.1%) and selective GLP-1 receptor agonist, dulaglutide (–1.1%), and dose-dependent reductions in body weight (ranging from –4.8 to –11.3 kg with the 5 mg, 10 mg, and 15 mg doses) compared with –0.4 kg with placebo and –2.7 kg with dulaglutide (2). Impairment in insulin production from pancreatic beta cells and in insulin action both contribute to hyperglycemia in T2D (3), and distinct mechanisms by which dual agonism of both GLP-1 and GIP receptors may improve glycemic control warrant additional study. To explore mechanisms by which tirzepatide resulted in greater reductions of hyperglycemia than a selective GLP-1 receptor agonist, we conducted post hoc exploratory analyses of biomarkers associated with pancreatic beta-cell function and insulin sensitivity.
Materials and Methods
Trial design and participants
The clinical trial design, full inclusion and exclusion criteria, and primary results of this tirzepatide Phase 2b study in participants with T2D have been previously reported (2). The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All participants provided signed informed consent and protocols were approved by local ethics review boards. This study is registered with ClinicalTrials.gov (NCT03131687).
Key eligibility criteria included adults aged 18-75 years with T2D suboptimally controlled by diet and exercise with or without stable metformin therapy for at least 3 months before screening. All participants had a baseline HbA1c between 7.0% and 10.5% (53-91.3 mmol/mol) and a body mass index between 23 and 50 kg/m2. Subjects were randomly assigned (1:1:1:1:1:1) to receive either once-weekly subcutaneous tirzepatide (1 mg, 5 mg, 10 mg, or 15 mg), dulaglutide (1.5 mg), or placebo for 26 weeks. Subjects assigned to the tirzepatide 10 mg group received 5 mg for 2 weeks followed by 10 mg for the remainder of the treatment period, and subjects assigned to the tirzepatide 15 mg group received 5 mg for 2 weeks, then 10 mg for 4 weeks followed by 15 mg for the remainder of the treatment period. Exploratory biomarker analyses were conducted in the reported modified intention-to-treat population, defined as all subjects that received at least one dose of study drug with at least 1 postbaseline outcome measurement without rescue or discontinuation (2).
Fasting blood samples were collected for in-protocol and exploratory biomarker measures. In-protocol biomarker analyses included blood glucose, insulin, C-peptide, glucagon, and total adiponectin. Exploratory post hoc biomarker analyses included intact proinsulin, insulin-like growth factor binding protein (IGFBP)-1, and IGFBP-2. Homeostatic model assessment indices for beta-cell function (HOMA2-B) or for insulin resistance (HOMA2-IR) were computed with fasting glucose and insulin or C-peptide levels. Intact proinsulin levels enabled intact proinsulin/insulin and intact proinsulin/C-peptide ratio calculations. Glucagon levels were adjusted for glucose change from baseline as previously reported (2). All in-protocol biomarkers were measured in fasting samples by the central laboratory (Pacific Biomarkers Inc., Seattle, WA, USA) (2). Post hoc exploratory biomarkers were measured at Lilly Research Laboratories, using validated, commercially available ELISA assays.
Statistical analyses
All analyses were performed on the modified intent-to-treat population excluding data after study drug discontinuation or rescue drug initiation (2). Overall, 316 participants were included in this analysis (tirzepatide 1 mg, n = 52; 5 mg, n = 55; 10 mg, n = 51; 15 mg, n = 53; dulaglutide, n = 54; placebo, n = 51) (2). Only subjects with nonmissing baseline values and at least 1 nonmissing postbaseline value of the response variable were included in analyses. Analyses for change from baseline as well as percent change from baseline were conducted using analyses of variance for baseline measures and mixed model repeated measures (MMRM) for postbaseline measures. For percent change from baseline analyses, percent changes with respect to baseline values were calculated at individual level before performing MMRM. There were no adjustments for multiplicity. Statistical test results were considered statistically significant at P < .05.
Linear regression analysis was conducted with change in HOMA2-IR (computed with fasting insulin) at 26 weeks as the dependent variable and body weight (BW) change from baseline at 26 weeks as the independent variable with age, sex, metformin dose, baseline fasting triglyceride levels, and baseline HbA1c as confounding variables. No transformation was conducted on covariates in the model. The independent variable, BW change from baseline, was preincluded, and an exhaustive search in the model space was performed to select confounding variables that would join BW change in the final model. The best model was selected based on the Akaike information criteria (AIC) across all tirzepatide dose groups, dulaglutide, and placebo. AIC was used to balance the trade-off between goodness-of-fit and model simplicity. This approach would therefore minimize the chance of overfitting and provide a more robust criterion than P value of the overall fit or the R2 (4, 5). None of the confounding variables were included in the final selected model with the lowest AIC, and, therefore, BW change from baseline was the sole independent variable along with the intercept.
The analysis was focused on the direct effect of BW change on insulin resistance by considering confounding variables into candidate models. The explained variability of improvement in HOMA2-IR at 26 weeks by weight loss was measured for each dose of tirzepatide separately, by performing analysis of variance on the final selected model with Type III sums of squares.
Statistical analyses were performed using R 3.6.0, SAS 9.4 software (Cary, NC, USA) and GraphPad Prism 8 (San Diego, CA, USA).
Results
Tirzepatide improved markers of beta-cell function
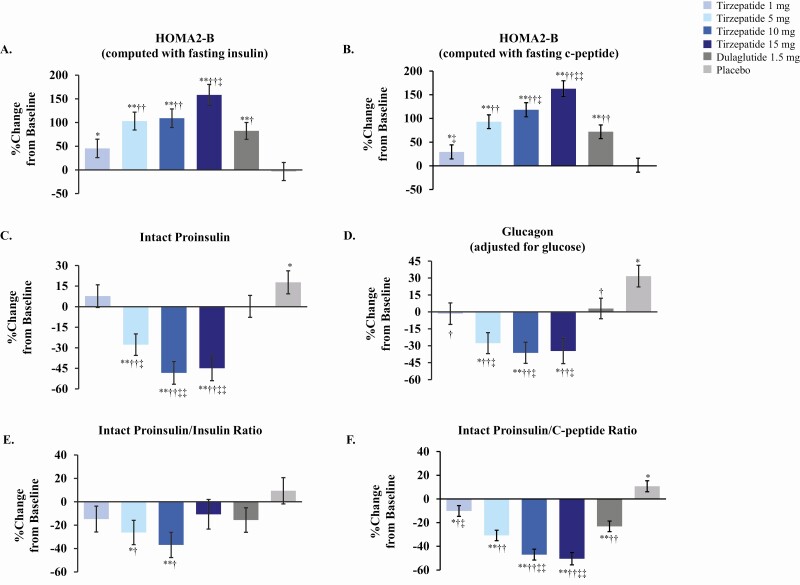
We measured HOMA2-B indices as an initial assessment of pancreatic beta-cell function, as blood samples were collected under fasting conditions. Percent change from baseline in HOMA2-B indices (computed either with fasting insulin or with fasting C-peptide) were significantly increased in all treatment groups except placebo at 26 weeks (Fig. 1A and 1B). HOMA2-B (computed with fasting C-peptide) percent change from baseline increases ranged from 93% to 163% for tirzepatide 5-, 10-, and 15-mg doses (P < .001), 72% for dulaglutide (P < .001), 29% for the tirzepatide 1-mg dose (P = .049), and 1% for placebo (P = .932), suggestive of substantial improvements in beta-cell function. A similar pattern was observed for HOMA2-B indices computed with fasting insulin. Absolute change from baseline HOMA2-B indices for tirzepatide 5-, 10-, and 15-mg doses and dulaglutide were each significantly different from placebo at 26 weeks (P < .001). HOMA2-B (computed with fasting C-peptide) absolute change from baseline for tirzepatide 10- and 15-mg doses were significantly different from dulaglutide at 26 weeks (P ≤ .004). Dose-dependent increases in HOMA2-B indices over time are shown in Supplemental Figure 1A and 1B in (6).
Figure 1.
Percent change from baseline in markers of beta-cell function with tirzepatide treatment at 26 weeks. Doses are indicated in figure inset for tirzepatide, dulaglutide, and placebo. Data presented as LSM ± SE at 26 weeks from MMRM; modified intent-to-treat population excluding data after study drug discontinuation or rescue drug initiation. Abbreviations: LSM = least squares mean; MMRM = mixed models repeated measures; SE = standard error. *P < .05 and **P < .001 vs baseline, †P < .05 and ††P < .001 vs placebo, ‡P < .05 and ‡‡P < .001 vs dulaglutide.
Defective proinsulin processing under conditions of increased metabolic demand for insulin production has been implicated as an indicator of pancreatic beta-cell stress in T2D (7, 8). We measured intact proinsulin levels to evaluate potential changes in insulin processing in T2D subjects in response to tirzepatide treatment. Percent change from baseline in intact proinsulin significantly decreased with tirzepatide 5, 10, and 15 mg from 28% to 48% (P < .001), did not significantly change for tirzepatide 1 mg or dulaglutide (P = .350 and P = .981, respectively), and significantly increased by 18% with placebo at 26 weeks (P = .035) (Fig. 1C). Absolute change from baseline proinsulin levels at 26 weeks were significantly different for tirzepatide 5, 10, and 15 mg compared with placebo (P ≤ .021) and with dulaglutide (P ≤ .036) (Supplemental Figure 1C and Table 1 in (6)). No significant differences in absolute change from baseline in proinsulin levels were observed for tirzepatide 1 mg or dulaglutide compared with placebo or for tirzepatide 1 mg compared with dulaglutide at 26 weeks (6).
To further assess proinsulin processing changes in response to tirzepatide, we evaluated intact proinsulin/insulin and intact proinsulin/C-peptide ratios. We observed significant reductions in percent change from baseline of intact proinsulin/insulin ratios for tirzepatide 5 mg (26%, P = .012) and 10 mg (37%, P < .001) at 26 weeks (Fig. 1E). Absolute change from baseline proinsulin/insulin ratios were significantly different for all tirzepatide doses compared with placebo (P ≤ .016) and for tirzepatide 10 and 15 mg compared with dulaglutide (P ≤ .007), but not for dulaglutide compared with placebo (P = .088) at 26 weeks. We observed significant reductions in percent change from baseline of intact proinsulin/C-peptide ratios for all doses of tirzepatide ranging from 10% to 50% (P ≤ .025) at 26 weeks (Fig. 1F). Absolute change from baseline proinsulin/C-peptide ratios were significantly different for tirzepatide 5-, 10-, and 15-mg doses compared with placebo (P < .001), for dulaglutide compared with placebo (P = .003) and for tirzepatide 10- and 15-mg doses compared with dulaglutide (P < .001). Decreases in proinsulin/insulin and proinsulin/C-peptide ratios were observed over time for all tirzepatide doses and for dulaglutide (Supplemental Figure 1E and 1F (6)). Absolute and percent changes from baseline at 26 weeks in markers of beta-cell function are presented in Supplemental Tables 1 and 2 (6).
Tirzepatide treatment also significantly reduced glucose-adjusted glucagon levels at 26 weeks (2). Percent change from baseline of glucose-adjusted glucagon levels significantly decreased by 28% to 36% for tirzepatide 5, 10, and 15 mg (P ≤ .003), did not significantly change for tirzepatide 1 mg or dulaglutide, and increased by 32% for placebo (P = .001) (Fig. 1D). Absolute changes from baseline in glucose-adjusted glucagon levels were significantly lower for all tirzepatide doses and dulaglutide (P ≤ .006) compared with placebo and for tirzepatide 10 and 15 mg (P ≤ .015) compared with dulaglutide (Supplemental Table 1 (6)). Collectively, these results indicate that tirzepatide-mediated improvements in glucose control were accompanied by improvements in markers of pancreatic beta-cell function and insulin processing in addition to reductions in glucagon levels. Tirzepatide doses of 10 mg and 15 mg differentiated from dulaglutide with greater reductions of proinsulin, proinsulin/insulin ratios, and proinsulin/C-peptide ratios, suggestive of distinct benefits in beta-cell function derived from tirzepatide administration.
Tirzepatide improved markers of insulin sensitivity
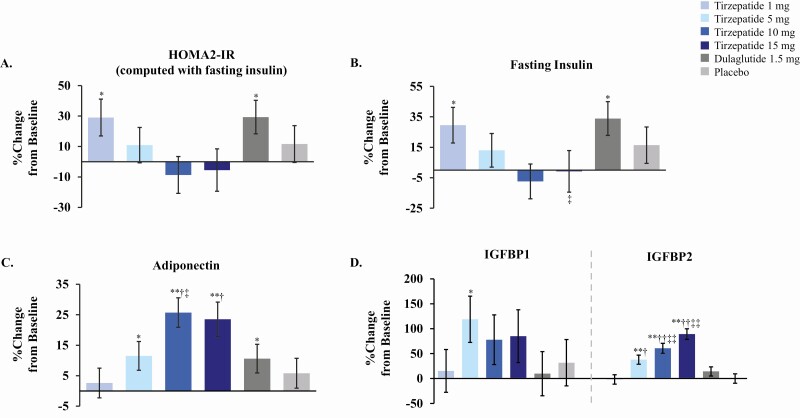
Multiple mechanisms related to dual receptor agonism may have contributed to improved glucose control in T2D subjects, in addition to potential improvements in beta-cell function. We next evaluated HOMA2-IR indices and circulating biomarkers that have been associated with improvements in insulin sensitivity (Fig. 2A). Absolute change from baseline in the insulin resistance index HOMA2-IR (computed with fasting insulin) was significantly decreased with tirzepatide 10 mg compared with placebo (P = .004) and dulaglutide (P = .004) at 26 weeks (Supplemental Table 1 (6)). HOMA2-IR decreases with tirzepatide 15 mg compared with placebo and dulaglutide approached but did not reach statistical significance (P = .063 and .067, respectively). Hyperinsulinemia is strongly correlated with insulin resistance (3), so we also evaluated changes in fasting insulin levels with tirzepatide administration (Fig. 2B and Supplemental Figure 2C (6)). Absolute change from baseline in fasting insulin levels was significantly decreased with tirzepatide 10 and 15 mg compared with placebo (P = .004) and dulaglutide (P = .014) at 26 weeks. Reductions in HOMA2-IR indices and fasting insulin levels were observed over time from 4 to 26 weeks (Supplemental Figure 2A-C (6)). Collectively, these results suggested that treatment with the higher doses of tirzepatide improved markers of insulin resistance in T2D subjects.
Figure 2.
Percent change from baseline in markers of insulin sensitivity with tirzepatide treatment at 26 weeks. Doses are indicated in figure inset for tirzepatide, dulaglutide, and placebo. Data presented as LSM ± SE at 26 weeks from MMRM; modified intent-to-treat population excluding data after study drug discontinuation or rescue drug initiation. Abbreviations: LSM, least squares mean; MMRM, mixed models repeated measures; SE, standard error. *P < .05 and **P < .001 vs baseline, †P < .05 and ††P < .001 vs placebo, ‡P < .05 and ‡‡P < .001 vs dulaglutide.
We further assessed whether treatment with tirzepatide could alter other circulating biomarkers that have been associated with improvements in insulin sensitivity in response to diet, bariatric surgery, or weight loss. Increases in circulating levels of adiponectin, an adipokine implicated in the regulation of both glucose and lipid metabolism, have been associated with multiple interventions that increase insulin sensitivity (9-12). Percent change from baseline in adiponectin levels significantly increased from 12% to 26% with tirzepatide 5, 10, and 15 mg (P ≤ .015) and by 11% with dulaglutide at 26 weeks (P = .025) (Fig. 2C). Absolute change from baseline for adiponectin was significantly different for tirzepatide 10 and 15 mg (P ≤ .004) compared with placebo (Supplemental Table 1 (6)).
Fasting levels of IGF binding proteins IGFBP-1 and IGFBP-2, members of the insulin and IGF signaling pathways and markers associated with insulin sensitivity (13, 14), increased with tirzepatide treatment (Fig. 2D). Absolute change from baseline for IGFBP-1 was significantly different for tirzepatide 5 and 15 mg (P ≤ .047) compared with dulaglutide at 26 weeks. IGFBP-2 levels also significantly increased with tirzepatide 5, 10, and 15 mg but not dulaglutide at 26 weeks, with percent increases progressively ranging from 38% to 89% (P < .001) (Fig. 2D). Absolute change from baseline of IGFBP-2 was significantly different for tirzepatide 5-, 10-, and 15-mg doses compared with placebo and significantly different for tirzepatide 10- and 15-mg doses compared with dulaglutide (P ≤ .001) (6). Both time- and dose-dependent increases in IGFBP-2 levels were observed for higher doses of tirzepatide (Supplemental Figure 2D (6)). Absolute and percent changes from baseline at 26 weeks in markers associated with insulin sensitivity are presented in Supplemental Tables 1 and 2 (6).
Tirzepatide-mediated improvements in insulin sensitivity and weight loss
Weight loss with diet, exercise, or bariatric surgery often is associated with improvements in insulin sensitivity. In the setting of the substantial weight loss that differentiated tirzepatide from the selective GLP-1 receptor agonist dulaglutide in this trial, we asked whether the insulin-sensitizing actions of tirzepatide could be attributable to weight loss. To evaluate the impact of weight loss on tirzepatide-mediated changes in insulin sensitivity as reflected by improvement in HOMA2-IR after 26 weeks, we conducted a model selection in multiple linear regression framework as described in “Materials and Methods”. The results of applying the final selected model to each of the dose arms are described in Supplemental Figure 3 and Table 3 (6). Weight loss significantly explained 21% (P = .014) and 13% (P = .028) of the variation in improvement in HOMA2-IR with tirzepatide 15 mg and 10 mg, respectively. This analysis suggested that the observed insulin-sensitizing effects of tirzepatide in the Phase 2 study were only partly attributable to weight loss.
Discussion
This post hoc exploratory biomarker study investigated the effects of the once-weekly novel dual GIP and GLP-1 receptor agonist, tirzepatide, on markers of pancreatic beta-cell function and insulin sensitivity in adult subjects with T2D. Beta-cell function was enhanced and fasting glucagon levels were reduced to a greater extent by tirzepatide than by the selective GLP-1 receptor agonist, dulaglutide. Tirzepatide improved multiple markers of pancreatic beta-cell function as shown by dose-dependently increasing HOMA2-B indices and decreasing proinsulin levels, proinsulin/C-peptide ratios, and proinsulin/insulin ratios. Proinsulin, the most abundant secretory protein in the beta cell, is a major driver of protein load to the endoplasmic reticulum under conditions of increased metabolic demand for insulin production (7). Proinsulin is prone to misfolding, and the ability for pancreatic beta cells to compensate for increased metabolic demand for insulin production in T2D may depend on their response to endoplasmic reticulum stress. Impaired conversion of proinsulin to insulin is a central component of beta-cell dysfunction in T2D (15). Elevated proinsulin levels predict both worsening of hyperglycemia and progression of nondiabetic subjects to T2D in longitudinal cohorts (16). Accordingly, tirzepatide-mediated decreases in proinsulin levels may be indicative of improvements in pancreatic beta-cell health.
Increased circulating proinsulin/C-peptide and proinsulin/insulin ratios are similarly important indicators of early and progressive pancreatic beta-cell secretory dysfunction. Proinsulin/insulin ratios are inversely correlated with maximal insulin secretory capacity in glucose clamp studies of T2D subjects (17). Proinsulin/C-peptide ratios are also strong predictors for incident diabetes in longitudinal cohorts (18) and may more closely estimate changes in beta-cell function, as levels of C-peptide are typically less variable than insulin and unaffected by changes in hepatic insulin clearance. Reductions in proinsulin levels, proinsulin/C-peptide ratios, and proinsulin/insulin ratios in response to treatment with tirzepatide collectively suggest improvements in insulin protein processing and may reflect reduced pancreatic beta-cell stress (8, 19).
Improvements in insulin sensitivity may effectively reduce metabolic demand on the pancreatic beta cell. For some individuals, reductions in metabolic demand may enable reversal of beta-cell dysfunction, as illustrated by improvements in proinsulin levels and proinsulin/insulin ratios. For example, T2D subjects who respond to bariatric surgery with diabetes remission are characterized by lower proinsulin levels and proinsulin/C-peptide ratios than nonremitters (20). In contrast, increased metabolic demand with experimental induction of insulin resistance and increased insulin secretion can increase proinsulin/insulin ratios in T2D subjects (21). Responses of T2D subjects to therapeutic interventions with insulin secretagogues or insulin sensitizers further illustrate the relationship between driving insulin secretion versus reducing pancreatic beta-cell stress. In clinical studies in T2D, administration of the sulfonylurea glyburide increased proinsulin levels and proinsulin/insulin ratios but treatment with the insulin sensitizer rosiglitazone significantly decreased proinsulin secretion (22). Tirzepatide reduced proinsulin/C-peptide ratios by up to 50%, a substantial reduction when compared with the 21% decrease reported after bariatric surgery in obese adolescents (23).
Decreases in fasting glucagon levels in response to tirzepatide treatment also may contribute to reductions in HbA1c. Fasting hyperglucagonemia is frequently noted in subjects with T2D and considered to exacerbate hyperglycemia by dysregulating hepatic glucose metabolism (24, 25).
Tirzepatide reduced fasting insulin levels and HOMA2-IR indices of insulin resistance while increasing levels of multiple markers associated with improvements in insulin sensitivity. These findings for tirzepatide are consistent with preclinical studies of dual GIP and GLP-1 receptor peptide agonists that demonstrated reductions in insulin demand, fasting insulin levels, and HOMA-IR indices compared with a selective GLP-1 receptor agonist (26). GIP overexpressing transgenic mice were reportedly more insulin sensitive than wild-type control mice on insulin tolerance tests, indicative of potential actions of GIP to reduce insulin resistance (27). Activating the GLP-1 receptor alone may not substantially reduce fasting insulin levels, as illustrated by results with dulaglutide in this study.
The increase in adiponectin levels by tirzepatide is of interest in the context of GIP receptor expression and proposed insulin-sensitizing actions of GIP on human adipocytes (28), as adiponectin is produced in adipocytes. Adiponectin levels have been inversely correlated with insulin resistance in rodent models of obesity and across multiple human cohorts (9). Potential insulin-sensitizing functions of adiponectin include enhancing Glut4-mediated glucose uptake in white adipocytes, reducing visceral fat depots, and decreasing hepatic lipid accumulation (10, 29). In this context the observations that tirzepatide reduced waist circumference in 2 independent T2D cohorts (2, 30) and reduced other biomarkers associated with nonalcoholic steatohepatitis in T2D subjects (31) are noteworthy.
IGFBP-1 and IGFBP-2 proteins are members of the larger insulin and IGF signaling pathway that have been positively correlated with improvements in insulin sensitivity in human cohorts. Low IGFBP-1 levels are associated with insulin resistance and predict the development of T2D (14, 32-34). IGFBP-1 levels increase with weight loss after bariatric surgery or calorie restriction (35, 36). Increasing IGFBP-1 levels are correlated with improvements in insulin sensitivity indices on meal tests and with reductions in HOMA-IR (13). IGFBP-2 levels are inversely associated with body mass index, fat mass, insulin levels, and fatty liver index (37-41). Relative IGFBP-2 deficiency has been associated with increased visceral and hepatic fat stores (37), and IGFBP-2 levels increase after bariatric surgery and caloric restriction (42, 43). Higher IGFBP-2 levels have been associated with decreased longitudinal risk of developing T2D in both the Nurses’ Health Study and EPIC-Potsdam cohorts (37, 44). Preclinical studies suggest that IGFBP-1 and IGFBP-2 may directly function to improve insulin sensitivity. Administration of an IGFBP-1 protein fragment improved insulin sensitivity in obese mice (45), and overexpression of IGFBP-2 reversed diabetes in several independent mouse models (46). The observed patterns of tirzepatide-dependent increases in IGFBP-1 and IGFBP-2 levels are suggestive of distinct insulin-sensitizing functions of the dual receptor agonist compared with the selective GLP-1 receptor agonist dulaglutide.
Insulin-sensitizing effects of tirzepatide were only partly attributable to weight loss based on linear regression analysis, suggesting that additional weight loss–independent mechanisms likely contribute to tirzepatide-mediated reductions in insulin resistance. Fasting triglyceride levels did not significantly influence selection of the best fit model to evaluate tirzepatide-mediated improvements in insulin resistance, despite substantial dose-dependent reductions in triglyceride levels with tirzepatide treatment (2). The findings reported here were consistent with previous results, in which a more restricted model selection process and regression analysis had been used (47). In that modeling, weight loss was found as the only covariate in the final model with no intercept, and weight loss significantly explained 28% (P = .0003) and 22% (P = .0038) of the variation in improvement in HOMA2-IR with tirzepatide 10 mg and 15 mg, respectively (47). These collective observations for tirzepatide differ from recent mediation analyses concluding that reductions in insulin resistance by the selective GLP-1 receptor agonist semaglutide were mediated primarily by weight loss (48). It is possible that differences in statistical analysis methods and study populations may have contributed to dissimilar observations. We focused our analysis on the direct effect of body weight change on improvement in insulin resistance by considering confounding variables and measuring explained variability by performing analysis of variance with type III sums of squares on the final selected model that was chosen by AIC. This analysis was different from investigating the direct and indirect (eg, drug-induced weight loss) effect of a therapeutic intervention on insulin resistance through mediation analysis that was performed in (48). Additionally, in contrast to the analysis population in (48) that was pooled from multiple Phase 3 studies, the data for our analysis were from a Phase 2b study with up to 55 patients per arm. The Phase 2b sample size could have limited the ability to perform a separate mediation analysis with a reasonable power in each arm (49). Our multiple linear regression analysis results may reflect unique insulin-sensitizing actions of tirzepatide due to the activation of both GIP and GLP-1 receptors. This hypothesis is consistent with the observed increases in multiple insulin sensitivity biomarkers in response to tirzepatide administration compared with the selective GLP-1 receptor agonist dulaglutide.
Several limitations of these mechanistic biomarker analyses should be noted. Results were generated from exploratory, post hoc studies of Phase 2b clinical trial samples. Assessments of pancreatic beta-cell function and insulin sensitivity were limited by fasting sample collection. Additional prospective clinical studies are warranted to better assess mechanistic contributions of tirzepatide to improved glycemic control, including dynamic testing with glucose and lipid challenges to extend initial observations with HOMA2 indices. The potential range of insulin-sensitizing effects of tirzepatide may have been underestimated in this study, as more than 80% of subjects in the Phase 2b trial were on concomitant metformin therapy (2).
Overall, treatment of subjects with T2D with the novel dual GIP and GLP-1 receptor agonist, tirzepatide, improved both markers of beta-cell function and insulin sensitivity compared with the selective GLP-1 receptor agonist, dulaglutide. By decreasing insulin resistance, tirzepatide likely reduced metabolic demand for insulin secretion from pancreatic beta cells, diminishing ongoing beta-cell stress. Improvements in insulin resistance with tirzepatide 10-mg and 15-mg doses were only partially attributable to weight loss, suggestive of additional mechanisms by which tirzepatide improved insulin sensitivity to achieve substantial reductions in HbA1c. By both improving pancreatic beta-cell function and reducing insulin resistance through dual activation of GLP-1 and GIP receptors, tirzepatide has the potential to confer not only glucose control and weight loss but also to reduce disease severity to improve metabolic health.
Acknowledgments
The authors thank the clinical trial investigators and subjects for their contributions to the tirzepatide clinical trials; Lilly Research Laboratories colleagues for supporting sample collection, data acquisition, and analyses; and Chrisanthi A. Karanikas, MS (Eli Lilly and Company) for her writing and editorial assistance.
Financial Support: This study was supported by Eli Lilly and Company
Clinical Trial Information: This study is registered with Clinicaltrials.gov ID: NCT03131687 (registered April 27, 2017).
Author Contributions: D.A.R., M.K.T., Z.M., and A.H. contributed to the study design. J.W. and K.D. were responsible for exploratory biomarker assay oversight. D.A.R. provided medical oversight during the trial. A.N., R.B., and X.C. were responsible for the statistical analyses. M.K.T. and A.H. are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in interpretation of the data and critical review of the manuscript, had full access to all the data in the study, and approved of this manuscript to be submitted for publication.
Glossary
Abbreviations
- AIC
Akaike information criteria
- BW
body weight
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated hemoglobin A1c
- HOMA
homeostatic model assessment
- IGFBP
insulin-like growth factor binding protein
- IR
insulin resistance
- MMRM
mixed model repeated measures
- RA
receptor agonist
- T2D
type 2 diabetes
- WL
weight loss
Additional Information
Disclosures: M.K.T., A.N., R.B., X.C., J.W., K.D., Z.M., AH., and D.A.R. are employees and shareholders of Eli Lilly and Company.
Prior Presentation: Some of these results were previously presented at the 79th Scientific Sessions of the American Diabetes Association in San Francisco, California, on June 7-11, 2019 and encored at the 55th European Association for the Study of Diabetes Annual Meeting in Barcelona, Spain on September 16-20, 2019.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repository listed in References.
References
- 1. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180-2193. [DOI] [PubMed] [Google Scholar]
- 3. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akaike H. A new look at the statistical model identification. IEEE Trans Auto Control. 1974;19(6):716-723. [Google Scholar]
- 5. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G, eds. Selected Papers of Hirotugu Akaike. Springer Series in Statistics (Perspectives in Statistics). Springer; 1998. [Google Scholar]
- 6. Thomas MK, Nikooienejad A, Bray R, et al. Supplemental Data from: Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. Dryad Digital Repository 2020. Deposited 4 November 2020. ProMED-mail website. 10.5061/dryad.nvx0k6dqw [DOI] [PMC free article] [PubMed]
- 7. Sun J, Cui J, He Q, Chen Z, Arvan P, Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol Aspects Med. 2015;42:105-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russo GT, Giorda CB, Cercone S, De Cosmo S, Nicolucci A, Cucinotta D; BetaDecline Study Group . Beta cell stress in a 4-year follow-up of patients with type 2 diabetes: A longitudinal analysis of the BetaDecline Study. Diabetes Metab Res Rev. 2018;34(6):e3016. [DOI] [PubMed] [Google Scholar]
- 9. Putz DM, Goldner WS, Bar RS, Haynes WG, Sivitz WI. Adiponectin and C-reactive protein in obesity, type 2 diabetes, and monodrug therapy. Metabolism. 2004;53(11):1454-1461. [DOI] [PubMed] [Google Scholar]
- 10. Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim HJ, Kim SK, Shim WS, et al. Rosiglitazone improves insulin sensitivity with increased serum leptin levels in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;81(1):42-49. [DOI] [PubMed] [Google Scholar]
- 12. Sharma PK, Bhansali A, Sialy R, Malhotra S, Pandhi P. Effects of pioglitazone and metformin on plasma adiponectin in newly detected type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2006;65(6):722-728. [DOI] [PubMed] [Google Scholar]
- 13. Lee PD, Lustig RH, Lenders C, Baillargeon J, Wilson DM; Glaser Pediatric Research Network Obesity Study Group . Insulin-like growth factor binding protein 1 predicts insulin sensitivity and insulin area-under-the-curve in obese, nondiabetic adolescents. Endocr Pract. 2016;22(2):136-142. [DOI] [PubMed] [Google Scholar]
- 14. Liew CF, Wise SD, Yeo KP, Lee KO. Insulin-like growth factor binding protein-1 is independently affected by ethnicity, insulin sensitivity, and leptin in healthy, glucose-tolerant young men. J Clin Endocrinol Metab. 2005;90(3):1483-1488. [DOI] [PubMed] [Google Scholar]
- 15. Ahrén B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5(3):275-286. [DOI] [PubMed] [Google Scholar]
- 16. Vangipurapu J, Stančáková A, Kuulasmaa T, Kuusisto J, Laakso M. Both fasting and glucose-stimulated proinsulin levels predict hyperglycemia and incident type 2 diabetes: a population-based study of 9,396 Finnish men. PLoS One. 2015;10(4):e0124028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Røder ME, Porte D Jr, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(2):604-608. [DOI] [PubMed] [Google Scholar]
- 18. Loopstra-Masters RC, Haffner SM, Lorenzo C, Wagenknecht LE, Hanley AJ. Proinsulin-to-C-peptide ratio versus proinsulin-to-insulin ratio in the prediction of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetologia. 2011;54(12):3047-3054. [DOI] [PubMed] [Google Scholar]
- 19. Hanley AJ, Zinman B, Sheridan P, Yusuf S, Gerstein HC; Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) Investigators . Effect of Rosiglitazone and Ramipril on {beta}-cell function in people with impaired glucose tolerance or impaired fasting glucose: the DREAM trial. Diabetes Care. 2010;33(3):608-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel K, Levesque K, Mark V, et al. Proinsulin associates with poor β-cell function, glucose-dependent insulinotropic peptide, and insulin resistance in persistent type 2 diabetes after Roux-en-Y gastric bypass in humans. J Diabetes. 2020;12(1):77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D Jr. Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30(9):698-702. [DOI] [PubMed] [Google Scholar]
- 22. Smith SA, Porter LE, Biswas N, Freed MI. Rosiglitazone, but not glyburide, reduces circulating proinsulin and the proinsulin:insulin ratio in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(12):6048-6053. [DOI] [PubMed] [Google Scholar]
- 23. Inge TH, Prigeon RL, Elder DA, et al. Insulin sensitivity and β-cell function improve after gastric bypass in severely obese adolescents. J Pediatr. 2015;167(5):1042-8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilon P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. J Mol Biol. 2020;432(5):1367-1394. [DOI] [PubMed] [Google Scholar]
- 25. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5(209):209ra151. [DOI] [PubMed] [Google Scholar]
- 27. Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CH. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7(7):e40156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ceperuelo-Mallafré V, Duran X, Pachón G, et al. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J Clin Endocrinol Metab. 2014;99(5):E908-E919. [DOI] [PubMed] [Google Scholar]
- 29. Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frias JP, Nauck MA, Van J, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020;22(6):938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mogul HR, Marshall M, Frey M, et al. Insulin like growth factor-binding protein-1 as a marker for hyperinsulinemia in obese menopausal women. J Clin Endocrinol Metab. 1996;81(12):4492-4495. [DOI] [PubMed] [Google Scholar]
- 33. Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359(9319):1740-1745. [DOI] [PubMed] [Google Scholar]
- 34. Mohamed-Ali V, Pinkney JH, Panahloo A, Cwyfan-Hughes S, Holly JM, Yudkin JS. Insulin-like growth factor binding protein-1 in NIDDM: relationship with the insulin resistance syndrome. Clin Endocrinol (Oxf). 1999;50(2):221-228. [DOI] [PubMed] [Google Scholar]
- 35. Linkov F, Goughnour SL, Ma T, et al. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecol Oncol. 2017;147(1):133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fontana L, Villareal DT, Das SK, et al. ; CALERIE Study Group . Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15(1):22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wittenbecher C, Ouni M, Kuxhaus O, et al. Insulin-like growth factor binding protein 2 (IGFBP-2) and the risk of developing type 2 diabetes. Diabetes. 2019;68(1):188-197. [DOI] [PubMed] [Google Scholar]
- 38. Ballerini MG, Ropelato MG, Domené HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab. 2004;17(5):749-757. [DOI] [PubMed] [Google Scholar]
- 39. Allen NE, Appleby PN, Kaaks R, Rinaldi S, Davey GK, Key TJ. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer Causes Control. 2003;14(1):65-74. [DOI] [PubMed] [Google Scholar]
- 40. Street ME, Smerieri A, Montanini L, et al. Interactions among pro-inflammatory cytokines, IGF system and thyroid function in pre-pubertal obese subjects. J Biol Regul Homeost Agents. 2013;27(1):259-266. [PubMed] [Google Scholar]
- 41. Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB Jr. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75(3):762-767. [DOI] [PubMed] [Google Scholar]
- 42. Li Z, Martin J, Poirier P, et al. Upregulation of plasma insulin-like growth factor binding protein 2 levels after biliopancreatic diversion in humans. Obesity (Silver Spring). 2012;20(7):1469-1473. [DOI] [PubMed] [Google Scholar]
- 43. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35(5):714-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rajpathak SN, He M, Sun Q, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61(9):2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haywood NJ, Cordell PA, Tang KY, et al. Insulin-like growth factor binding protein 1 could improve glucose regulation and insulin sensitivity through its RGD domain. Diabetes. 2017;66(2):287-299. [DOI] [PubMed] [Google Scholar]
- 46. Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11(1):11-22. [DOI] [PubMed] [Google Scholar]
- 47. Thomas MK, Nikooienejad A, Bray R, et al. Tirzepatide, a dual GIP and GLP-1 receptor agonist, improves markers of beta-cell function and insulin sensitivity in type 2 diabetes patients. Paper presented at: American Diabetes Association 79th Scientific Sessions; 2019, San Francisco, CA (Abstract 980-P).
- 48. Fonseca VA, Capehorn MS, Garg SK, et al. Reductions in insulin resistance are mediated primarily via weight loss in subjects with type 2 diabetes on semaglutide. J Clin Endocrinol Metab. 2019;104(9):4078-4086. [DOI] [PubMed] [Google Scholar]
- 49. Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repository listed in References.