Abstract
Stilbenes are a small family of polyphenolic secondary metabolites that can be found in several distantly related plant species. These compounds act as phytoalexins, playing a crucial role in plant defense against phytopathogens, as well as being involved in the adaptation of plants to abiotic environmental factors. Among stilbenes, trans-resveratrol is certainly the most popular and extensively studied for its health properties. In recent years, an increasing number of stilbene compounds were subjected to investigations concerning their bioactivity. This review presents the most updated knowledge of the stilbene biosynthetic pathway, also focusing on the role of several environmental factors in eliciting stilbenes biosynthesis. The effects of ultraviolet radiation, visible light, ultrasonication, mechanical stress, salt stress, drought, temperature, ozone, and biotic stress are reviewed in the context of enhancing stilbene biosynthesis, both in planta and in plant cell and organ cultures. This knowledge may shed some light on stilbene biological roles and represents a useful tool to increase the accumulation of these valuable compounds.
Keywords: secondary metabolites, polyphenols, stilbenes, phytoalexins, biosynthetic pathway, phenylpropanoid pathway, stilbene biosynthesis, stilbene synthase, resveratrol synthase, pinosylvin synthase, environmental factors
1. Introduction
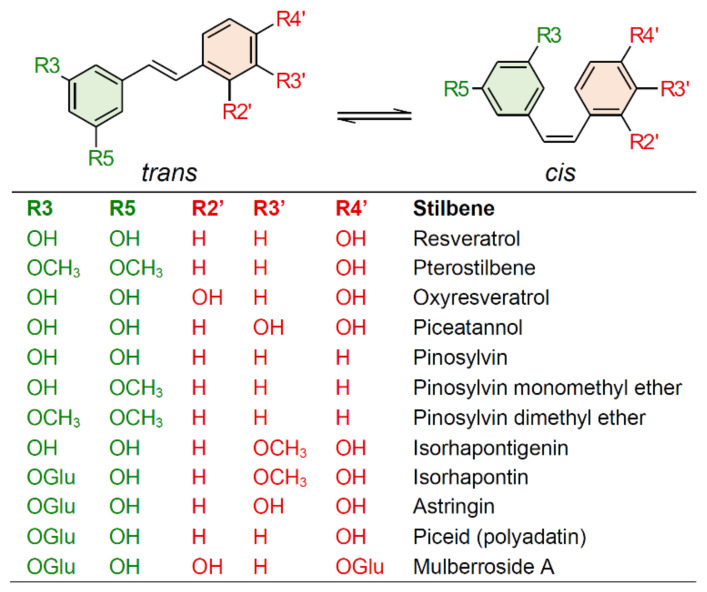
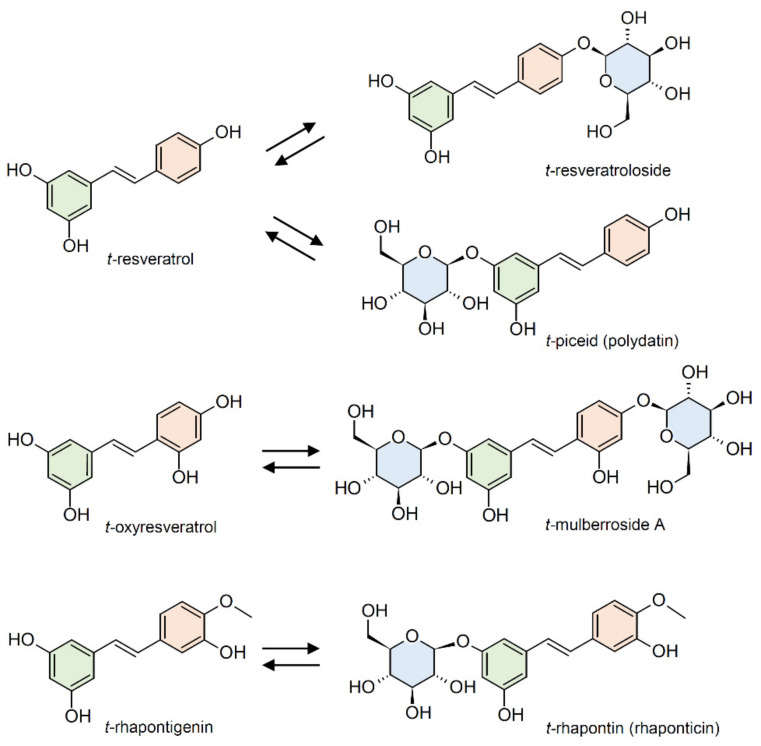
Stilbenes are a small yet important class of non-flavonoid polyphenols, sharing a common structure characterized by a 14-carbon skeleton composed of two benzene rings linked by an ethylene bridge (Figure 1). Due to the presence of the central ethylene moiety between the aromatic rings, stilbenes exist as the two possible stereoisomers cis and trans. However, the naturally occurring stilbenes are usually in the trans form [1]. Plant stilbenes, together with other polyphenols such as flavonoids, isoflavonoids, curcuminoids, and xanthones, belong to the class of polyketides. Over 400 different stilbene compounds are currently known [2], mostly derived from trans-resveratrol (3,5,4′-trihydroxy-trans-stilbene) (Figure 1), although different structures can be found in specific plant families [3].
Figure 1.
Chemical structures of common stilbene monomer derivatives. (OGlu) O-β-D-glucopyranoside.
Stilbenes have been identified in at least 72 plant species belonging to 31 genera and 12 distantly related families, including Pinaceae (e.g., Picea abies (L.) Karst. and Pinus nigra J.F. Arnold), Gnetaceae (e.g., Gnetum parvifolium (Warb.) W.C. Cheng and G. africanum Welw.), Fabaceae (Arachis hypogaea L. and Robinia pseudoacacia L.), Vitaceae (e.g., Vitis vinifera L. and V. amurensis Rupr.), Moraceae (e.g., Morus alba L. and M. macroura Miq.), and Polygonaceae (e.g., Polygonum cuspidatum Sieb. et Zucc. and P. multiflorum Thunb.) [4,5]. Given their nutraceutical value, stilbene content and composition have mainly been investigated in food plants, and the knowledge of stilbene distribution in nature is still poor. This is partially related to the complexity of the quali-quantitative analysis of stilbenes, which is in turn related to the unavailability of standards and the detection limits of analytical methods [2]. For these reasons, most of the studies carried out to date have been focused on simple stilbenes, such as resveratrol, piceid, pterostilbene, and piceatannol (Figure 1). Current knowledge on the distribution of stilbenes in the plant kingdom will not be presented in this review, as this topic is covered by excellent recent reviews [4,5].
Stilbenes are mainly involved in constitutive and inducible protection of the plant against biotic (phytopathogenic microorganisms and herbivores) and abiotic (e.g., UV radiation and tropospheric ozone) stress [3,6]. On one side they counteract the aggression exerting a direct toxic effect on the pathogen, while on the other they act as antioxidants, protecting the cells from oxidative damage [7,8,9]. Stilbenes possess several antipathogenic properties including antibacterial, antifungal [10,11], nematocidal [12], and insecticidal [13,14]. They could also act as a deterrent towards vertebrate herbivory [15], as a possible negative effect of stilbenes has been reported on snowshoe hares (Lepus americanus Erxleben) [16,17] and field voles (Microtus agrestis L.) [18]. The role of stilbenes, among other polyphenols, in counteracting oxidative stress is just as important, as the plant response to pathogen attack involves the production of reactive oxygen species (ROS), which both act as signals for the activation of stress and defense pathways and as toxic substances capable of directly damaging the pathogen. Oxidative stress may also be induced by many abiotic conditions, such as drought, thermal stress, ultraviolet radiation, mechanical stress, heavy metals, salts, and air pollutants such as ozone [19]. Unsurprisingly, many of these factors also affect stilbene production [20].
Over the past 20 years, the bioactivities of stilbenes have been intensively investigated due to their impact on human health. Among stilbenes, resveratrol is the best known and the most studied. Basic scientific research and over 240 clinical studies have demonstrated the multiplicity of trans-resveratrol pharmacological effects, including antioxidant [21], anti-inflammatory [22], anticancer [23,24], estrogenic [25], neuroprotective [26], cardioprotective [27], anti-atherosclerotic [28], anti-aging [29], anti-diabetic [30], anti-osteoporosis [25], and anti-obesity properties [31]. In recent years, considerable attention has also been paid to other monomeric stilbenes, including pterostilbene [32], pinosylvin [33], and piceatannol [34], as well as to oligomeric stilbenes such as viniferins [35,36], which have been shown to possess similar and often more pronounced health-promoting properties than resveratrol.
Due to their potential use in the nutraceutical, cosmeceutical, and pharmaceutical fields, great interest is directed at the methods for large-scale production of stilbenes. For instance, it has been estimated that the global market for trans-resveratrol will almost double in the next 6 years, from 58 million USD in 2020 to 99.4 million USD by 2026 [37]. Methods for obtaining stilbenes can be grouped into three categories: direct extraction from plants, chemical synthesis, and the use of biotechnologies. The chemical synthesis of stilbenes has been reported, but this method is not economically feasible, in addition to being difficult in terms of stereospecific synthesis [38,39]. Considerable efforts have been devoted to the development of biotechnological methods for stilbene production, which broadly include tissue culture techniques [40], biotransformation [41], and metabolic engineering [42]. Nevertheless, the major way of supplying stilbenes is the direct extraction from plants such as P. cuspidatum and V. vinifera [43].
The stilbene content and profile in stilbene-producing plants vary strongly in response to a variety of environmental factors. In recent years, a considerable body of knowledge regarding the stilbene biosynthetic pathway and the impact of environmental conditions on the production of these valuable metabolites has accumulated. This review presents the recent knowledge of the stilbene biosynthetic pathway and the impact of different environmental factors on stilbene production.
2. Biosynthesis of Stilbenes and Stilbenoids
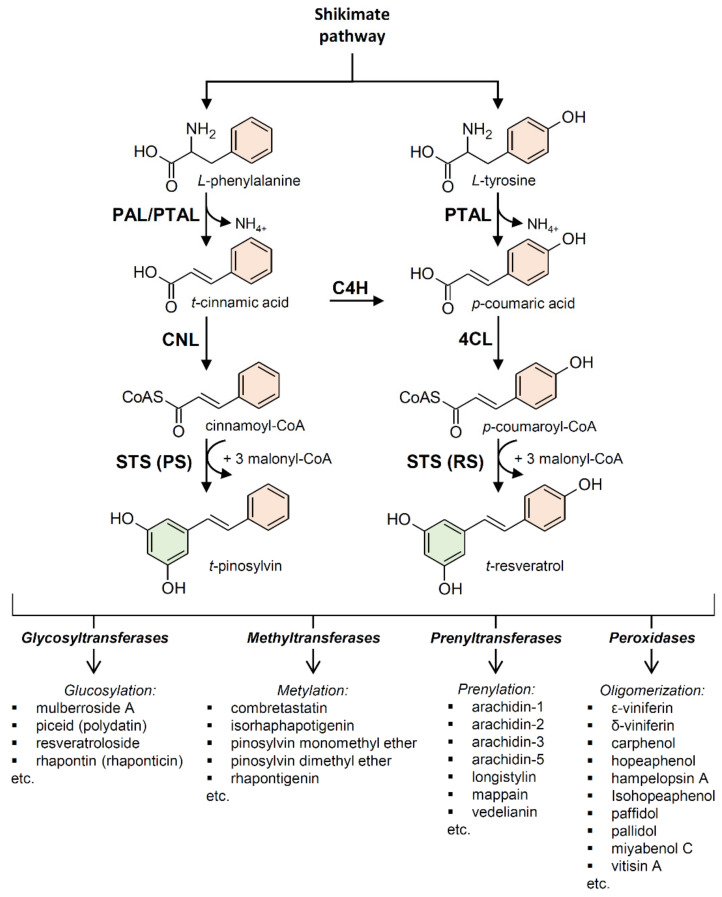
Stilbenes and stilbenoids are biosynthesized through the phenylpropanoid pathway, which is also responsible for the biosynthesis of numerous primary and secondary metabolites including flavonoids, coumarins, hydrolyzable tannins, monolignols, lignans, and lignins [44]. Generated by the shikimate pathway, the aromatic amino acid L-phenylalanine is the primary starting molecule of the phenylpropanoid pathway (Figure 2). The non-oxidative deamination of L-phenylalanine to form trans-cinnamic acid, catalyzed by phenylalanine ammonia-lyase (PAL; EC 4.3.1.24), is the entry step for the carbon channeling from primary metabolism into the phenylpropanoid secondary metabolism. PAL is ubiquitous in plants [45], and it is undoubtedly the most studied enzyme involved in plant secondary metabolism [46]. Cinnamic acid can be bound to a coenzyme A (CoA) molecule by cinnamate:CoA ligase (CNL; EC 6.2.1.-) to form cinnamoyl-CoA. Alternatively, cinnamic acid can be hydroxylated by cinnamate 4-hydroxylase (C4H), a cytochrome P450 enzyme (EC 1.14.14.91), to form p-coumaric acid. Some plants (mainly monocots but also dicots) possess a bifunctional phenylalanine/tyrosine ammonia-lyase (PTAL, EC 4.3.1.25) that efficiently deaminates both L-phenylalanine (PAL activity) and L-tyrosine (TAL activity) [47,48,49,50]. These plants can directly produce p-coumaric acid using L-tyrosine as a substrate, bypassing the requirement for L-phenylalanine and C4H. A molecule of CoA is then bound to p-coumaric acid by 4-coumarate: CoA ligase (4CL; EC 6.2.1.12), generating p-coumaroyl-CoA, which provides an active intermediate in numerous branches of the general phenylpropanoid pathway [51].
Figure 2.
Stilbene biosynthesis in plants. (PAL) phenylalanine ammonia-lyase; (PTAL) bifunctional L-phenylalanine/L-tyrosine ammonia-lyase; (C4H) cinnamate 4-hydroxylase; (4CL) 4-coumarate:CoA ligase; (CNL) cinnamate: CoA ligase; (STS) stilbene synthase; (RS) resveratrol synthase; (PS) pinosylvin synthase.
2.1. Stilbene Synthase
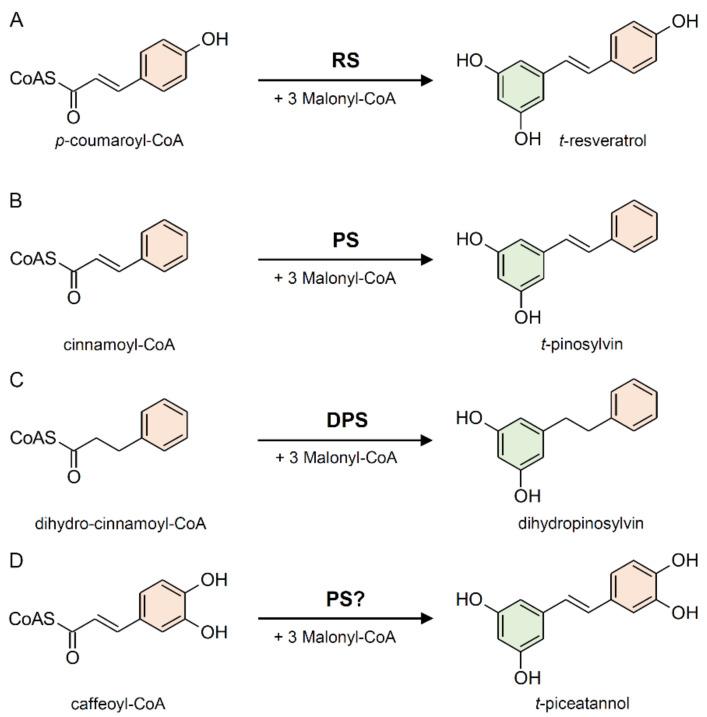
The enzyme stilbene synthases (STS) catalyze the direct formation of the stilbene skeleton through a single reaction from three units of malonyl-CoA and one CoA-ester of a cinnamic acid derivative (p-coumaroyl-CoA to form trans-resveratrol or cinnamoyl-CoA to form trans-pinosylvin) [52] (Figure 2 and Figure 3). Malonyl-CoA is generated through a carboxylation reaction between acetyl-CoA and a bicarbonate ion (HCO3−) catalyzed by acetyl-CoA carboxylase (EC 6.4.1.2) in the presence of ATP (Figure 4).
Figure 3.
Examples of reactions catalyzed by stilbene synthase enzymes. (A) Conversion of p-coumaroyl-CoA into t-resveratrol by resveratrol (RS) synthase (or trihydroxystilbene synthase I). (B) Conversion of cinnamoyl-CoA into t-pinosylvin by pinosylvin synthase (PS). (C) Conversion of dihydro-cinnamoyl-CoA into dihydropinosylvin by dihydro-pinosylvin synthase (DPS). (D) Conversion of caffeoyl-CoA into t-piceatannol, probably catalyzed by PS.
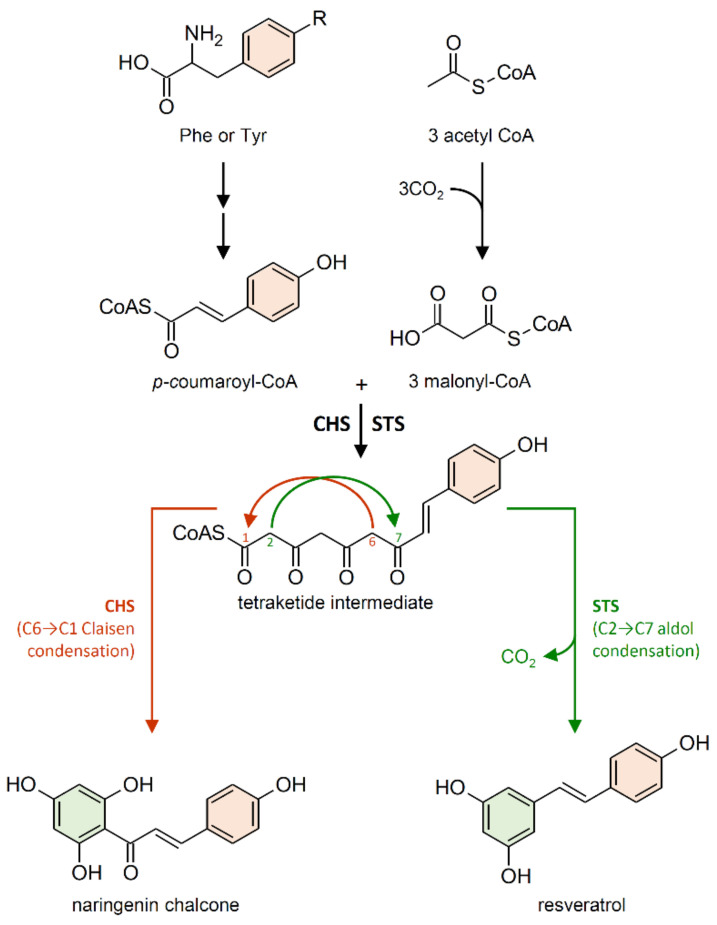
Figure 4.
Reactions catalyzed by chalcone synthase (CHS) and stilbene synthase (STS) to produce naringenin chalcone and resveratrol, respectively. R = H phenylalanine (Phe); R = OH tyrosine (Tyr). Double arrows indicate multiple steps in the biosynthetic pathway.
Based on the preferred starting substrate, STS enzymes are classified into either a p-coumaroyl-CoA-specific type, such as trihydroxystilbene synthase I (also known as resveratrol synthase, EC 2.3.1.95), or a cinnamoyl-CoA-specific type, such as pinosylvin synthase (EC 2.3.1.146) (Figure 2 and Figure 3). The former type has been mainly found in angiosperms like peanut [53], grapevine [54], and Tatar rhubarb (Rheum tataricum L.f) [55], while the latter type is typical in conifers and has been identified in several Pinus species like Scots pine (P. sylvestris L.) [56], Japanese red pine (P. densiflora Siebold & Zucc.) [57], and Eastern white pine (P. strobus L.) [58].
Pinus species can biosynthesize two types of stilbenes, i.e., pinosylvin and dihydropinosylvin, which are biosynthetically derived from cinnamoyl-CoA and dihydrocinnamoyl-CoA, respectively (Figure 3B,C). STS from P. strobus shows a clear preference for cinnamoyl-CoA and was therefore characterized as pinosylvin synthase [58]. Otherwise, STS from P. sylvestris shows an unusual preference for dihydro-cinnamoyl-CoA, identifying it as a dihydro-pinosylvin synthase [56]. STS does not exhibit absolute substrate specificity. While showing a preference for a given substrate, the same STS enzyme can accept different cinnamic acid derivatives as starting substrates catalyzing the biosynthesis of different stilbenes. For example, the enzyme responsible for the biosynthesis of piceatannol (3,5,3′,4′-tetrahydroxystilbene) has not been identified yet, however, pinosylvin synthase from P. strobus proved to be active with caffeoyl-CoA in vitro (Figure 3D), suggesting that it could be responsible for piceatannol biosynthesis in planta [58].
STS enzymes belong to the type III polyketide synthase superfamily (PKSs), which also includes chalcone synthase (CHS; EC 2.3.1.74) [59]. STS and CHS share a high degree of similarity both in their amino acid sequence identity (which reaches 75–90% depending on the species) and in their crystallographic structures [51,60]. CHS genes are present in the genome of all plants analyzed so far, while STS have been identified in a limited number of plant species, often phylogenetically unrelated. Converging lines of evidence indicate that CHS is the archetypal enzyme from which STS evolved multiple times independently in stilbene-producing plants, through gene duplication followed by functional divergence [60,61,62]. CHS and STS are the most investigated enzymes among PKSs and, due to their high sequence similarity, they are often referred to as the CHS/STS family [63,64].
Although it employs the same substrates as STS, CHS is responsible for the first committed step in the biosynthesis of flavonoid-type compounds. Both enzymes generate the same linear tetraketide intermediate. However, CHS catalyzes a C6→C1 Claisen condensation of the intermediate to produce naringenin chalcone, while STS catalyzes an alternative C2→C7 aldol condensation of the intermediate to form a stilbene backbone (Figure 4) [59,65,66].
STS was first extracted and purified from suspension cultures of peanut cells elicited with UV radiation [53]. Cloning of two peanut STS genes revealed a high sequence identity with CHS throughout the coding region and the presence of an intron at the same position as a conserved intron in CHS [67]. STS genes and cDNAs were subsequently cloned and characterized from grapevine cell suspension cultures [68] and Scots pine plantlets [56], both induced by fungal elicitors. At present, STS genes have been cloned from several plant species including mulberry (Morus notabilis C.K. Schneid and M. atropurpurea Roxb.) [42,66], Scots pine [69], white spruce (Picea glauca (Moench) Voss) [70], Norway spruce (Picea abies (L.) H. Karst.) [71], Japanese red pine [57], and sorghum (Sorghum bicolor (L.) Moench) [72]. To the best of our knowledge, sorghum is the only monocot plant in which an STS gene (SbSTS1) has been identified.
In most stilbene-producing plants, STS exists as a small family consisting of 1–10 closely related paralogs. For example, the STS multigene family is represented by two members in white spruce [70] and Norway spruce [71], three members in Japanese red pine [57], almost five members in Scots pine [69], six members in peanut [73], and ten members in mulberry [66]. Remarkable exceptions to this role are sorghum, in whose genome only one STS gene has been identified [74,75], and grapevine, which possesses an uncommonly large number of STS genes. Both grapevine and sorghum genomes have been entirely sequenced [74,76]. Early Southern-blot analysis suggested that the grapevine STS gene family consisted of 15–20 members [77]. Genome-wide analysis carried out on the V. vinifera PN40024 genome led to the identification of 48 putative STS genes, designated VvSTS1 to VvSTS48, with at least 33 potentially coding for functional STS proteins [60,62].
To date, there is no evidence regarding the different substrate specificity and enzymatic activity of different VvSTSs. Functional characterization of nine VvSTSs confirmed that they encode for functional STS enzymes [62]. Since these nine genes were specifically chosen to represent the diversity of the VvSTS gene family, it is most likely that all grapevine VvSTSs encode enzymes with similar activity and specificity. Despite the high similarity between STS genes which makes it difficult to accurately distinguish the individual transcripts, gene expression studies revealed different transcriptional responses of distinct VvSTSs during development and in response to environmental stresses [60,78,79]. The expression of some VvSTSs was also found to be tissue-specific [60,79]. It is therefore likely that the large quantity of members in the grapevine STS gene family has evolved to allow for fine spatial and temporal regulation of stilbene biosynthesis under both normal and stress conditions.
2.2. Glucosylation/Deglucosylation
Glycosylation is one of the most common modifications of plant secondary metabolites [80,81] that can modify their physicochemical and biological properties. Water-solubility, physicochemical stability, biological half-life, compartmentalization, and biological activity of stilbenes and other phenylpropanoids can be dramatically altered by glycosylation [82,83,84].
In stilbene-producing plants, a significant fraction of stilbenes is accumulated in a glucosylated form [85]. For instance, Fallopia japonica Houtt. (formerly Polygonum cuspidatum) produces both resveratrol and resveratrol 3-O-β-glucoside (commonly known as piceid or polydatin) (Figure 5), and the glucosylated form can reach concentrations of up to six times higher than the free aglycone [86]. Morus alba L. and Rheum undulatum L. accumulate the glucosylated stilbenes mulberroside A (a diglucoside of oxyresveratrol) and rhapontin (a monoglucoside of rhapontigenin, also known as rhaponticin) [87] (Figure 5). Significant amounts of cis- and trans-piceid are accumulated in grapevine, both constitutively [40,88,89] and in response to pathogen attack [90,91] and to environmental stresses such as UV light [88,92,93,94], salinity [95], and drought [96,97].
Figure 5.
Common examples of stilbene glucosylation.
Numerous glycosyltransferases that produce glucose esters of hydroxybenzoic and hydroxycinnamic acids accept a broad spectrum of structurally similar substrates [98]. A bi-functional glycosyltransferase from Concord grape (Vitis labrusca L.) (VLRSgt) that produces stilbene glucosides and glucose esters of hydroxycinnamic acids in vitro has been characterized in 2007 by Hall and De Luca [99]. The mesocarp-specific expression of VLRSgt reflected the increased accumulation of resveratrol glucosides during berry maturation, coherent with a role for this enzyme in stilbene glucosylation in the mesocarp.
It is well known that glucosylation increases resveratrol water solubility [82] and helps to protect stilbenes and other polyphenols from enzymic oxidation [100,101], which could extend their half-life in plant cells, preserve their biological properties, and assist stilbene transportation and accumulation.
Considerable levels of trans-piceid can be found in grape derivatives such as wines and juices [102,103]. Due to the β-glucosidase activity of yeasts, a decrease in trans-piceid concentration accompanied by an increase in trans-resveratrol concentration is often observed during grape fermentation [104,105]. Since trans-resveratrol has better health properties than piceid, a great deal of interest has been paid to strategies aimed to increase trans-resveratrol concentration during winemaking. One of these consists in the selection of yeast strains with high β-glucosidase activity, capable of efficiently converting trans-piceid into the free aglycone during the alcoholic fermentation [106,107]. Bacterial or fungal β-glucosidases can also be used to obtain trans-resveratrol from plant extracts rich in piceid, such as those obtained from P. cuspidatum [108,109]. Other glycosylated stilbenes, such as mulberroside A and rhaponticin from M. alba and R. undulatum, can be enzymatically converted to their aglycones oxyresveratrol and rhapontigenin with an increase in their bioactivity [87,110,111,112].
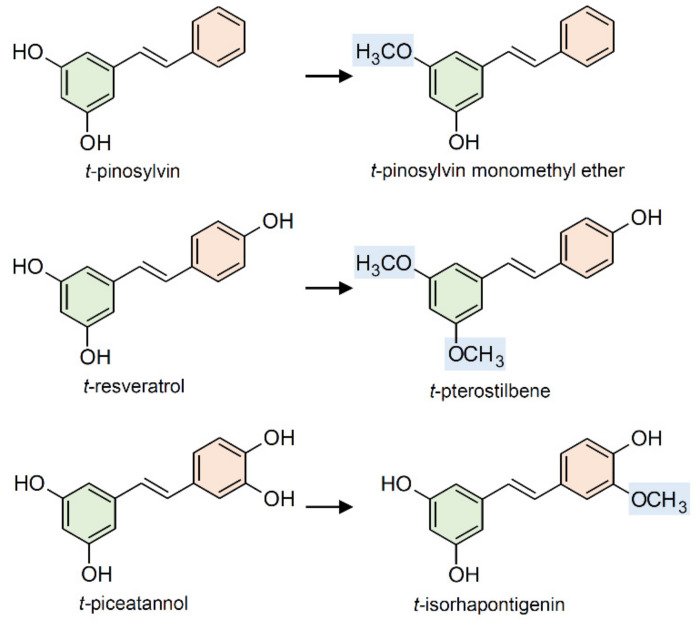
2.3. Methylation
Methylation of stilbene hydroxyl side groups leads to the formation of methoxystilbenes (Figure 6). Among the most known methoxystilbenes, there is pinosylvin monomethyl ether (3-hydroxy-5-methoxystilbene) found in several Pinus spp. [113,114] and Alnus spp. [115], and pterostilbene (3,5-dimethoxy-4′-hydroxystilbene), biosynthesized by red sandalwood (Pterocarpus santalinus Lf) [116], Indian Kino (Pterocarpus marsupium Roxb.) [117], Vaccinium spp. berries [118], and, at low levels, in grapevine leaves and berries [92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. Methylation of stilbenes, as well as of several other thousands of plant secondary metabolites, is catalyzed by S-adenosyl-L-methionine (SAM)-dependent O-methyltransferases (OMTs; EC 2.1.1).
Figure 6.
Common methylated stilbenes are biosynthetically derived from pinosylvin, resveratrol, and piceatannol.
Methylation of hydroxyl groups alters the solubility and reactivity of stilbenes and can therefore affect their biological activity. For instance, pterostilbene has been shown to be 5–10 times more active than non-methylated resveratrol in inhibiting the germination of downy mildew (Plasmopara viticola) sporangia and grey mold (Botrytis cinerea) conidia [120]. Pinosylvin monomethyl ether has been reported to have significantly lower antifungal and antibacterial activity than pinosylvin [121], although it has shown greater activity against some brown-rot fungi [122].
Investigations on the relationship between chemical structure and biological activity revealed increased cytotoxicity and anticancer activity associated with resveratrol methylation [123,124]. The substitution of hydroxy with methoxy groups enhances the lipophilicity of pterostilbene over resveratrol, which results in high bioavailability. This difference in pharmacokinetics might explain the higher bioactivity of pterostilbene over its parental compound resveratrol. Methylated resveratrol derivatives have consequently become attractive target compounds for both bioproduction and metabolic engineering [125,126].
Several plant OMTs have been characterized in the last decades. However, the majority of them have been found to be involved in the methylation of aromatic hydroxyl groups of different compounds such as benzylisoquinoline alkaloids [127], phenylpropanoids [128], and flavonoids [129], while only a few stilbene-specific OMTs have been reported to date.
V. vinifera resveratrol OMT (VvROMT) was shown to specifically catalyze the methylation of resveratrol and pinosylvin (3,5-dihydroxystilbene) at the C-3 or C-5 positions [130]. VvROMT was shown to convert resveratrol to pterostilbene both in vitro and in planta. The transient co-expression of VvROMT and VvSTS in tobacco resulted in the accumulation of pterostilbene. VvROMT gene expression in grapevine leaves was induced by different stresses, including P. viticola infection and UV radiation, accordingly with the role of pterostilbene in chemical plant defense [130]. S. bicolor resveratrol OMT (SbOMT1) has been shown to catalyze the 4′-O-methylation of resveratrol both in vitro and in planta [125,131]. In 2019, Koeduka and colleagues [132] isolated and characterized a putative aromatic O-methyltransferase gene (AcOMT1) in Acorus calamus (Araceae) using RNA-seq analysis. Recombinant AcOMT1 expressed in Escherichia coli showed high 4′-O-methylation activity toward resveratrol and its derivative, isorhapontigenin (3,4′,5-trihydroxy-3′-methoxystilbene).
In Scots pine, pinosylvin can be methylated by a pinosylvin O-methyltransferase (PsPMT1) to pinosylvin monomethyl ether, following ozone or fungal elicitation treatment [133]. However, it should be noted that PsPMT1 showed a relatively broad substrate specificity, methylating several compounds such as stilbene aglycones, flavonoids, and hydroxycinnamic acids, many of these even more efficiently than pinosylvin. In 2017, Paasela and co-workers [134] subsequently isolated and characterized an O-methyltransferase from P. sylvestris (PpPMT2), which is held responsible for the methylation of pinosylvin. Unlike the multifunctional PsPMT1, PsPMT2 preferentially methylated pinosylvin into its monomethyl ether, showing a high degree of specificity for stilbenes. The authors observed that PsPMT2 is co-expressed with STS in response to wounding of xylem and UV-C treatment of needles, suggesting that these two enzymes are under common regulation.
2.4. Prenylation
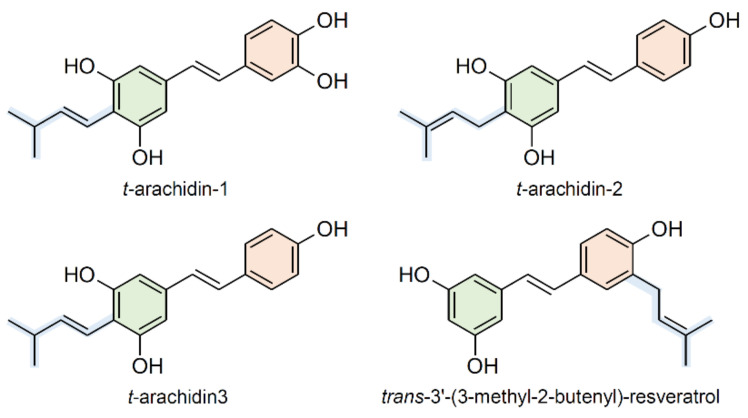
Prenylated stilbenoids have been isolated from a restricted number of stilbene-producing plants including Macaranga spp. (Euphorbiaceae) [135,136], Glycyrrhiza spp. [137,138,139,140], peanut [141,142,143,144,145,146], and mulberry [147].
Over 45 prenylated stilbenoids have been identified in A. hypogaea [148]. The major prenylated stilbenoids accumulated in peanuts are trans-arachidin-1, trans-arachidin-2, trans-arachidin-3, and trans-3′-(3-methyl-2-butenyl)-resveratrol (Figure 7) [148]. In accordance with their role as phytoalexins, peanut prenylated stilbenoids have been shown to accumulate upon challenge with microorganisms [142,143,144,145,149] and to possess remarkable antifungal activity [150,151,152]. Several recent studies have shown interesting therapeutic potential for peanut prenylated stilbenoids [135,136,153,154]. Prenylated compounds generally exhibit greater bioavailability than their non-prenylated counterparts, due to the increase in lipophilicity linked to the prenyl groups. Despite their biological and medical relevance, the biosynthetic pathways of prenylated stilbenoids have yet to be elucidated and the genes encoding stilbenoid-specific prenyltransferases have only recently been identified in plants.
Figure 7.
Major prenylated stilbenoids contained in peanuts.
In 2018, membrane-bound stilbene-specific prenyltransferases have been described in peanut and mulberry [148,155]. Combining targeted transcriptomic and metabolomic analyses, Yang et al. [148] discovered five candidate prenyltransferase genes in elicitor-treated A. hypogaea hairy root cultures. Two of these, AhR4DT-1 and AhR3DT-1, were functionally characterized in a transient expression system consisting of Agrobacterium-infiltrated leaves of Nicotiana benthamiana Domin. The authors demonstrated that AhR4DT-1 catalyzes the prenylation of resveratrol at its C-4 position leading to arachidin-2 formation, while AhR3DT-1 is responsible for resveratrol prenylation at C-3′ leading to the 3-methyl-2-butenyl-3-resveratrol formation. In 2018, Zhong and colleagues [155] identified and functionally characterized a stilbenoid-specific prenyltransferase from M. alba (MaOGT) that recognizes oxyresveratrol and geranyl diphosphate (GPP) as natural substrates and catalyzes oxyresveratrol prenylation. Both peanut and mulberry prenyltransferases have proved to be highly specific for stilbene substrates and fluorescent microscopy analysis has shown that they are localized in the chloroplast, similarly to other membrane-bound plant prenyltransferases [156].
2.5. Oligomerization
Stilbenes are often accumulated in plants as oligomers (oligostilbenes) resulting from the oxidative coupling of stilbene monomers [157,158]. Oligostilbenes have been isolated from species belonging to different plant families including Vitaceae [159], Fabaceae [160], Cyperaceae [161], Dipterocarpaceae [162], Gnetaceae [163], Paeoniaceae [164], Iridaceae [165], and Moraceae [166]. The interest addressed to oligostilbenes in recent decades is linked to their important biological role in the plant as phytoalexins [167], to their chemical diversity with more than 200 different molecules known to date [158], and to the wide spectrum of biological activities such as β-secretase inhibitory, anti-influenza virus, and anti-herpes simplex virus activities [168].
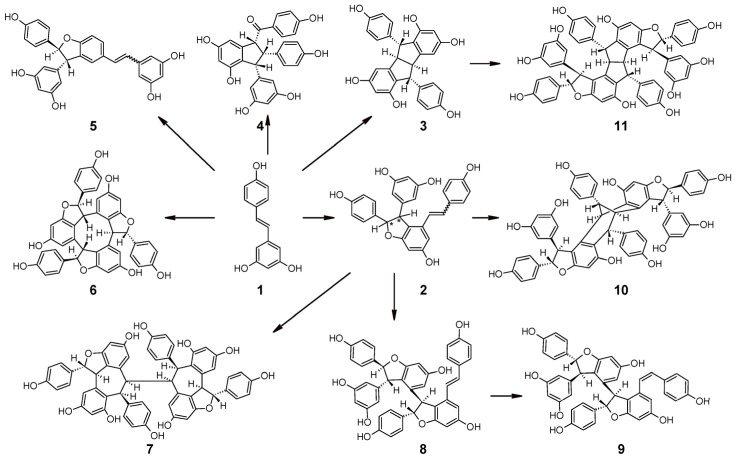
The largest group of oligostilbenes is represented by resveratrol oligomers, arising from the polymerization of two to eight resveratrol units [169,170] (Figure 8). Among these, the most investigated are viniferins, which accumulate in V. vinifera upon abiotic stress (e.g., UV irradiation) or fungal infection (e.g., Botrytis cinerea and Plasmopara viticola) [8,120,171].
Figure 8.
Of resveratrol trimers and tetramers in grapevine: (1) trans-resveratrol; (2) (E and Z) ε-viniferin/ω-viniferin; (3) pallidol; (4) caraphenol B; (5) δ-viniferin (E and Z); (6) α-viniferin; (7) isohopeaphenol; (8) E-miyabenol C; (9) Z-miyabenol C; (10) vaticanol C isomer; and (11) ampelopsin H [181,182].
Resveratrol oligomerization has been achieved in vitro by enzymatic oxidation utilizing horseradish peroxidase [172,173] or laccase-like stilbene oxidase from B. cinerea [174,175]. It has been proposed that peroxidases (POD or POX EC 1.11.1.7) located in the plant cell wall and vacuole are responsible for oxidative polymerization of resveratrol to form its natural oligomers [157,176]. POD enzymes use hydrogen peroxide to catalyze oxidative reactions and have already been exploited for the in vitro synthesis of oligostilbenes [173,177,178]. However, it should be noted that in vitro experiments with these enzymes did not lead to the formation of oligomers with the natural configuration found in plants, and to date, there is no direct evidence of the involvement of specific peroxidases in the formation of oligomeric stilbenes in planta [5].
In recent years, considerable efforts have also been made to develop non-enzymatic methods to produce oligostilbenes [179,180].
3. Impact of Environmental Factors on the Biosynthesis of Stilbenes
It is known that the biosynthesis of stilbenes in plants can be triggered by a variety of biotic and abiotic environmental factors. Some of these, like fungal infection and UV-C radiation, have been under study for many years, while others like bacterial infection and ozone stress have only recently caught the attention of the research community. In Table 1 and in the following paragraphs some of the most important results coming from studies on the influence of different environmental factors on the biosynthesis of stilbenes are reported and described.
Table 1.
Induction of stilbene biosynthetic gene expression and stilbene accumulation by environmental factors.
| Species/Cultivar/Variety | Treatment/s | Metabolites | Results | Reference |
|---|---|---|---|---|
| Vitis vinifera cvs. Alphonse Lavallée, Dan Ben-Hanna, Dabuki, Early Superior, Flame seedless, Kishmish, Muscat Hamburg, Perlette, Spring Blush, Superior, Thompson seedless, Zeiny, Gamay, Gamaret, Pinot, Shasla | Irradiation of grape berries with UV-C or inoculation of grape berries with Rhizopus stolonifer | Stilbenes (resveratrol and pterostilbene) | Increased stilbene accumulation, greater with UV-C compared to fungal inoculum | [183] |
| V. vinifera cv. Napoleon | Irradiation of grape berries with UV-C or UV-B | Stilbenes (resveratrol and piceid); anthocyanins; flavonoids; hydroxycinnamic acids (caffeoyltartaric acid and chlorogenic acid) | Increased stilbene accumulation, greater with UV-C compared to UV-B (3 and 2-fold, respectively) | [184] |
| V. vinifera cv. Corvina | Irradiation of grape berries with UV-B and wilting at different temperatures | Stilbenes (trans and cis-resveratrol, trans and cis-piceid); total polyphenols, flavonoids, anthocyanins, catechins, and proanthocyanidins | Enhanced stilbene accumulation and STS gene expression | [185] |
| V. vinifera cvs. Black Corinth and Flame seedless | Irradiation of grape berries with UV-C | Resveratrol; total anthocyanins | Greater resveratrol increase (4-fold) in cv. Flame Seedless. Lower increase in cv. Black Corinth. Negative relationship between resveratrol synthesis and anthocyanin concentration | [186] |
| V. vinifera cv. Flame seedless, Red Globe, Crimson seedless, Napoleon, Superior seedless, Moscatel Italica, Dominga | Irradiation of grape berries with UV-C | Trans- and cis-resveratrol, trans-piceatannol, trans-piceid, trans-astringin, α-viniferin, ε-viniferin | Increased stilbene concentration, with higher accumulation of trans-resveratrol, trans-piceatannol, and viniferins | [187] |
| V. vinifera cv. Monastrell | Irradiation of grape berries with UV-C, followed by traditional maceration | Stilbenes (trans-resveratrol, trans-piceatannol); anthocyanins; flavonols; flavanols (total catechins); hydroxycinnamic acids (p-coumaroyltartaric acid) | Increased in trans-piceatannol and trans-resveratrol content (1.5 and 2-fold, respectively) in wines without impacting standard oenological parameters | [188] |
| V. vinifera cvs. Tempranillo, Cabernet-Sauvignon, Merlot, Syrah, Monastrell, Garnacha, Cariñena | Irradiation of grape berries with UV-C | Trans-resveratrol, trans-piceatannol, α-viniferin, ε-viniferin | Increased concentrations of trans-resveratrol, trans-piceatannol, and viniferins in grape skins of all varieties, except Monastrell, in which only trans-piceatannol concentration increased | [189] |
| V. vinifera cv. Superior | Irradiation of grape berries with UV-C | Trans-resveratrol, trans-piceid, trans-piceatannol, viniferins (resveratrol dehydrodimers and dehydrotrimers) | Increased trans-resveratrol accumulation (10-fold); induction of trans-piceid, trans-piceatannol, and viniferins (not detected in control grapes) | [190] |
| V. vinifera cv. Superior | Comparison of UV-C and ozone (O3) treatments on grape berries | Trans-resveratrol, piceatannol, and viniferins (resveratrol dehydrodimers and dehydrotrimers) | Increased accumulation of stilbenes after both UV-C and O3 treatments. O3 more effective than UV-C in inducing the accumulation of viniferins | [191] |
| V. vinifera cv. Superior | Irradiation of grape berries with UV-C, followed by maceration with Na2S2O5 and enzymes | Stilbenes (trans-resveratrol; trans-piceid; trans-piceatannol, viniferins); hydroxycinnamic acids; flavonols; flavanols (catechins and procyanidins) | Increased stilbene concentration (35-fold) in grape juice under optimum conditions (maceration for 2 h at 45 °C with 0.2% Na2S2O5 using UV-C-treated grape berries) | [192] |
| V. vinifera cv. Red Globe | Irradiation of grape berries with UV-B nanosecond laser pulses | Trans-resveratrol | Increased trans-resveratrol accumulation (6-fold) in grape berries subjected to a resonant wavelength of the compound (302.1 nm) | [193] |
| V. vinifera sylvestris var. V9. V15, V16; V. vinifera sativa var. Merlot, Syrah, Graciano, Tempranillo, Palomino fino, Palomino negro, Tintilla de Rota; V. vinifera sativa hybrid Orion, Regent | Irradiation of grape berries with UV-C | Trans-resveratrol, piceatannol, ε-viniferin, δ-viniferin | Increased stilbene concentration, with differences depending on variety and campaign, but not on subspecies | [194] |
| V. vinifera × V. labrusca cv. Kyoho | Irradiation of grape berries with UV-C and storage at different temperatures (0 °C or 20 °C) | Resveratrol | Increased resveratrol concentration, especially in UV-treated grapes stored at high temperature | [195] |
| V. vinifera cv. Redglobe | Irradiation of grape berries with UV-C and storage at different temperatures (25 °C or 4 °C) | Stilbenes (trans-resveratrol, cis- and trans-piceid); flavonols; anthocyanins; flavanols (catechins) | Increased concentration of cis- and trans-piceid after UV-C treatment and cold storage | [196] |
| V. vinifera cv. Crimson | Treatment of grape berries with UV-C and chitosan, followed by storage at different temperatures | Trans-resveratrol | Increased resveratrol content in grapes and lower susceptibility to fungal decay after UV-C treatment combined with chitosan coating followed by storage at 20 °C for 24 h before refrigerated storage | [197] |
| V. amurensis cv. Tonghua-3 | Treatment of grape berries with UV-C | Trans- and cis-resveratrol | Increased accumulation of stilbene compounds, up-regulation of multiple STS genes, down-regulation of CHS genes | [198] |
| V. vinifera × V. labrusca cv. Summer Black | Treatment of grape berries with UV-B or UV-C | Stilbenes (trans-resveratrol, trans-piceid); gallic acid; hydroxycinnamic acids (caffeic acid, trans-ferulic acid); flavanols [(+)-catechin, (−)-epicatechin, epicatechin gallate] | Increased accumulation of phenolic compounds and STS gene expression, more induced by UV-C than UV-B | [199] |
| V. vinifera cv. Kyoho | Irradiation of grape berries with UV-B | Trans-resveratrol, trans-scirpusin A, trans-ε-viniferin, trans-δ-viniferin, trans-pterostilbene | Increased production of the analyzed stilbenes, up-regulation of stilbene biosynthetic genes | [200] |
| Arachis hypogaea cv. Georgia green | Treatment of peanuts with UV-C or ultrasonication | Trans-resveratrol, trans-piceid | Increased resveratrol, piceid, and total stilbene concentration, more induced by ultrasound than UV-C | [201] |
| A. hypogaea var. Jinpoong | Leaves subjected to UV-C, wounding, paraquat, H2O2, salicylic acid, jasmonic acid ethephon, abscisic acid | Resveratrol | Maximum resveratrol increases in response to UV (over 200-fold), followed by paraquat (20-fold) and wounding, H2O2, salicylic acid, jasmonic acid, and ethephon (between 2- and 9-fold) | [202] |
| A. hypogaea Georgia green | Treatment of peanuts with UV-C | Trans-resveratrol | Increased trans-resveratrol accumulation (10-fold) | [203] |
| Gnetum parvifolium | Treatment of 1-year-old seedlings with high temperature (40 °C) and UV-C treatments | Resveratrol and piceatannol | Both high temperature and UV-C strongly induce the expression of PAL, C4H-, 4CL-, and STS-like genes, but only UV-C enhance stilbene accumulation | [204] |
| Gnetum parvifolium | Treatment of 1-year-old seedlings with high temperature (40 °C) and UV-C treatments | Resveratrol and piceatannol | Both high temperature and UV-C strongly induce the expression of PAL, C4H, 4CL, STS, and CYP genes. High temperatures do not affect stilbene accumulation in stems but decrease stilbene concentration in roots at 3 h. UV-C irradiation induces total stilbene accumulation in stems but not in roots. | [205] |
| Pinus sylvestris | Treatment of needles from 5-years-old plantlets with UV-C | Pinosylvin and pinosylvin monomethylether | Induction of PMT2 expression | [134] |
| V. vinifera cv. Sangiovese | Potted vines grown in air-conditioned greenhouses under high temperature or low temperature regimes (26 and 21 °C as average and 42 and 35 °C as maximum air daily temperature, respectively) | Stilbenes | Increased expression of STS and PAL genes under low temperatures | [206] |
| V. vinifera cv. Cabernet Sauvignon | Treatment of cell suspension cultures with high temperature (38 °C) or low temperature (16 °C) and CuSO4 | Stilbenes | Downregulation of STS expression under both low and high temperature and upregulation of STS expression in response to CuSO4 | [207] |
| V. vinifera cv. Cardinal | Treatment of grape berries with low temperature (0 °C) and high CO2 levels (20%) | Trans-resveratrol; total anthocyanins | Low temperature reduces trans-resveratrol content in both treated and non-treated grapes, although the decrease is higher in CO2-treated grapes | [208] |
| V. vinifera cvs. Dominga, Superior seedless, Autumn Royal, Red Globe | Treatment of grape berries with low temperature (0 °C) and high CO2 levels (3 days) | Resveratrol, resveratrol-glucoside, trans-piceatannol, z-miyabenol, pallidol | Stilbene accumulation in response to low temperature and CO2 is cultivar dependent. High CO2 levels activate stilbene pathways in cv. Dominga. Low temperature increase stilbenes biosynthesis in cv. Red Globe. Stilbene accumulation is independent of the atmosphere storage in cvs. Superior Seedless and Autumn Royal | [209] |
| V. vinifera cv. Shiraz | Treatment of grape berries with high light (2500 μmol m−2 s−1), high temperature (40 °C), oxidative stress (120 μM menadione), 3.026 mM abscisic acid, and 200 μM jasmonic acid (JA) | Resveratrol, piceid, and viniferin | At the pre-veraison stage, an increase in anthocyanins levels is accompanied by a declining stilbene accumulation in response to JA, menadione, and high light. At the veraison stage, mild change in anthocyanin levels in response to all the treatments is accompanied by stilbene accumulation | [210] |
| V. vinifera cv. Barbera | Treatment of cell suspensions with red LED light (1.34 μE m−2 s−1, 625 ± 10 nm) and 10 μM methyl-jasmonate (MeJa) | Stilbenes (cis- and trans-piceid, cis- and trans-resveratrol, cis- and trans-resveratroloside); catechins; anthocyanins | Strong increase in total stilbenes induced by MeJa, whose effect is enhanced by a red light. Increase in total anthocyanins in response to MeJa, used alone or in combination with a red light. Decrease in catechins under red light; increase in response to MeJa alone or in combination with red light | [211] |
| V. labruscana cvs. Campbell Early and Kyoho | Treatment of grape berries and leaves with fluorescent white light and purple, blue, and red LED lights | Cis- and trans-resveratrol, cis- and trans-piceid, cis- and trans-piceatannol | Increased accumulation of stilbenes (mainly trans- and cis-piceid) and induction of stilbene biosynthetic genes in response to red and blue LED light | [212,213] |
| V. vinifera cv. Negramaro | Light-exposed and dark-maintained cell cultures | Trans-resveratrol, trans-piceid, cis-ε-viniferin, trans-ε-viniferin, trans-δ-viniferin | Higher levels of trans-resveratrol and viniferins under darkness; higher levels of trans-piceid under light | [214] |
| V. vinifera cv. Shahani | High-level white light irradiation (10,000 lux) and MeJa (25, 50, 100 and 200 μM) | Stilbenes (trans-resveratrol, trans-piceid); total phenols; total flavonoids | Inhibitory effect of high light on stilbene biosynthesis; 50 μM MeJa is optimal for efficient production of total phenols, flavonoids, and stilbenes | [215] |
| V. vinifera cv. Malvasia; V. rupestris Du Lut | Light-exposed and dark-maintained cell cultures | Trans-resveratrol, trans-piceid, trans-ε-viniferin, trans-δ-viniferin | Increase in stilbene content under light conditions | [40] |
| Arachis hypogaea | White LED light and UV-C radiation during peanut germination | Stilbenes (resveratrol, piceid, piceatannol); total phenols; total flavonoids | White light significantly induces stilbene accumulation by upregulating the expression of genes and enzymes involved in the stilbene biosynthetic pathway. UV-C is more effective than white light in promoting stilbene accumulation | [216] |
| V. vinifera cvs. Cabernet Franc, Chardonnay, Chenin, Malbec (Côt), Gamay, Grolleau, Pinot Noir, Sauvignon Blanc | Wounding (stem pruning) | Trans-resveratrol, trans-piceatannol, trans-ε-viniferin, ampelopsin A, trans-miyabenol C, cis- and trans-vitisin B, hopeaphenol, isohopeaphenol | Induction of trans-resveratrol and trans-piceatannol during the first 6 weeks of storage at 20 °C | [217] |
| V. vinifera cv. Pinot Noir | Wounding (leaf discs) | Stilbenes | Increase in transcription levels of several STS genes | [60] |
| V. vinifera cv. Pinot Noir | Wounding (leaf discs) | Stilbenes | Increased transcript level of VviSTS29, -41, and -48, coupled with the induction of WRKY and R2R3-MYB transcription factors | [218] |
| V. vinifera cv. Alphonse Lavallée | Mechanical stress (low-energy ultrasound) alone or in combination with MeJa on cell suspension cultures | Trans-resveratrol, trans-piceid, trans-ε-viniferin, trans-δ-viniferin | Increase in trans-ε-viniferin production in response to ultrasounds. Increase in trans-δ-viniferin in response to ultrasound and MeJa co-treatment | [219] |
| V. quinquangularis | Wounding, exogenous stress-associated hormones, and biotic stress in leaves of transgenic tobacco transformed with VqSTS36 promoter fused to the GUS reporter gene | Stilbenes | Induction of VqSTS36 promoter activity in response to wounding, salicylic acid, and inoculation with the phytopathogenic fungus Erysiphe cichoracearum | [220] |
| Pinus sylvestris | Wounding of stem-phloem alone or in combination with fungal infection | Stilbenes | Transient increase in STS and PMT expression in response to wounding, more pronounced with wounding in combination with fungal inoculation | [221] |
| P. sylvestris | Wounding of seedlings | Pinosylvin and pinosylvin monomethyl ether | Upregulation of stilbene biosynthetic genes including PMT2 during heartwood formation and in response to stress | [222] |
| P. sylvestris | Wounding and infection of seedlings with Heterobasidion parviporum or H. annosum | Pinosylvin and pinosylvin monomethylether | Significantly higher amounts of stilbenes 10 days after treatment. Greater increase in infected than in just wounded samples | [223] |
| P. sylvestris | Wounding 5-years-old seedlings | Pinosylvin and pinosylvin monomethylether | Induction of PMT2 | [134] |
| V. vinifera cv. Pinot Noir | Mechanical wounding on freshly pruned canes | Trans-resveratrol and trans-piceatannol | Transient expression of PAL and STS genes, followed by a rapid accumulation of stilbenes | [224] |
| Arachis hypogaea | Wounding stress (cotyledons) | Resveratrol, arachidin-3, arachidin-4 | Induction of all analyzed stilbenes | [225] |
| A. hypogaea | Wounding stress (size reduction, grinding, chopping, slicing, ultrasound) | Trans-resveratrol | Slicing produces the highest increase of trans-resveratrol accumulation | [203] |
| Pinus sylvestris | Ozone fumigation (saplings grown in phytotron) | Stilbenes | Enhanced STS and PMT transcript levels in needles but not in healthy phloem | [221] |
| V. quinquangularis (accession Shang-24; powdery mildew (PM) resistant); V. pseudoreticulata (accession Hunan-1; PM susceptible) | Infection by Uncinula necator (sin. Erysiphe necator) | Stilbenes | VqSTS36 transcript levels increase substantially following PM infection | [220] |
| V. vinifera cv. Barbera | Elicitation of cell suspension cultures with salicylic acid, Na-orthovanadate, jasmonates, chitosan, D-glucosamine, N-acetyl-D-glucosamine, ampicillin, rifampicin | Trans- and cis-resveratrol | Induction of ex-novo synthesis of stilbenes stilbene synthase protein by MeJa and chitosan | [226] |
| V. vinifera cv. Gamay Fréaux var. Teinturier | Elicitation of cell suspension cultures with MeJa in combination with sucrose | Stilbenes (trans-resveratrol and piceids); total anthocyanins | Induction of PAL, CHS, STS, UDP-glucose: flavonoid-O-glucosyltransferase, proteinase inhibitor and chitinase gene expression. Enhanced accumulation of piceids and anthocyanins in cells, and trans-resveratrol and piceids in culture medium | [227] |
| V. vinifera cv. Monastrell albino | Elicitation of cell suspension cultures with MeJa and cyclodextrin (CDs) used independently or in combination | Trans-resveratrol | Induction of stilbene biosynthetic gene expression by MeJa and CDs when used independently. Enhanced trans-resveratrol production in CDs-treated cells but not in MeJa-treated cells | [228] |
| V. vinifera cv. Barbera | Elicitation of cell suspension cultures with chitosan | Trans- and cis-resveratrol | Induction of trans-resveratrol production and STS gene expression | [229] |
| V. vinifera cvs. Red Globe and Michele Palieri | Elicitation of calli with MeJa | Trans-piceid, resveratrol glucoside, cis-piceid, resveratrol diglucoside, resveratrol dimer monoglucosides, resveratrol dimer diglucosides, resveratrol dimer triglucosides, resveratrol dimer tetraglucosides, picetannol monoglycosylated, picetannol diglycosylated | Enhanced production of stilbenes, mainly trans-piceid and ε-viniferin | [230] |
| V. vinifera cv. Isabelle | Elicitation of calli with biotic (fungal extract of Fusarium oxysporum) and abiotic (mannitol, abscisic acid, jasmonic acid) elicitors | Trans-resveratrol | Optimum accumulation of trans-resveratrol with a combined treatment of mannitol (2 mM) and jasmonic acid (40 µM) | [231] |
| V. vinifera cv. Barbera | Elicitation of cell suspension cultures with chitosan | Mono-glucosylated derivatives resveratrol (trans- and cis-piceid and trans- and cis-resveratroloside) | Increased in trans-resveratrol endogenous accumulation and decreased release into the culture medium. De-novo synthesis and/or accumulation of STS proteins. No influence on cis-resveratrol and on resveratrol mono-glucosides | [232] |
| V. vinifera cv. Italia | Elicitation of calli and cell suspension cultures with MeJa, jasmonic acid or chitosan | Trans-resveratrol, piceid trans-δ-viniferin, trans-ε-viniferin | Induction of trans-resveratrol, piceid, and viniferins by jasmonates. Jasmonic acid enhances simultaneously δ- and ε-viniferin biosynthesis, whereas MeJa stimulates preferentially δ-viniferin production. | [233] |
| V. vinifera cv. Monastrell | Elicitation of cell suspension cultures with MeJa, cyclodextrins, and UV-C used independently or in combination | Trans-resveratrol | Highest increase in trans-reveratrol production was obtained with the combined use of MeJa, cyclodextrins, and an optimal sucrose concentration. Greatest release of trans-resveratrol into the culture medium is achieved with the combined use of MeJa, cyclodextrin, and UV-C | [234] |
| V. vinifera cv. Gamay Fréaux | Elicitation of cell suspension cultures with indanoyl-isoleucine (In-Ile), N-linolenoyl-l-glutamine (Lin-Gln), and insect saliva (from Manduca sexta larvae) | 3-O-Glucosyl-resveratrol; 4-(3,5-dihydroxy-phenyl)-phenol; total anthocyanins | Increased accumulation of phenolic acids, particularly 3-O-glucosyl-resveratrol, in response to In-Ile, Lin-Gln, and saliva | [235] |
| V. vinifera cv. Hongbaladuo; V. vinifera × V. amurensis cv. Beihong | Treatment of leaves and berries with CaCl2 and UV-C used alone or in combination | Cis- and trans-resveratrol | Increased resveratrol content with single treatments, greater increase with combined treatment | [236] |
| V. vinifera cv. Gamay Fréaux | Elicitation of cell suspension cultures with jasmonic acid, salicylic acid, β-glucan, and chitosan | Stilbenes (trans-resveratrol and trans-piceid); total anthocyanins | Increased resveratrol production with co-treatment with jasmonic acid and β-glucan | [237] |
| V. vinifera cv. Negramaro | Elicitation of cell cultures with chitosan, MeJa, jasmonic acid, coronatine, and 12-oxo-phytodienoic acid | Trans-resveratrol, trans-piceid, cis-ε-viniferin, trans-ε-viniferin, trans-δ-viniferin | MeJa is the most effective in inducing trans-resveratrol the biosynthesis, while 12-oxo-phytodienoic acid, jasmonic acid, and coronatine are the most effective in inducing the biosynthesis of viniferins | [214] |
| V. vinifera cv. Monastrell | Elicitation cell suspension cultures with cyclodextrins and coronatine | Trans-resveratrol | Induction of stilbene biosynthetic genes by cyclodextrins and/or coronatine. Maximum level of trans-resveratrol production and secretion into the culture medium with co-treatment with 50 mM cyclodextrins and 1 μM coronatine | [238] |
| V. vinifera cv. Tempranillo | Foliar application of MeJa, chitosan, and yeast extract | Stilbenes (trans- and cis-piceid and trans- and cis-resveratrol); flavonols; anthocyanins; hydroxybenzoic acids; hydroxycinnamic acids | MeJa and yeast extract improve both grape and wine anthocyanin content. Stilbene content is clearly improved by yeast extract | [239] |
| Vitis vinifera L. cv. Kalecik Karası | Elicitation of grape berries with ultrasound | Trans-resveratrol | About 20-fold increase in trans-resveratrol content in grape skin | [240] |
| Arachis hypogaea cv. Tainan No. 14 | Elicitation of calli with bacteria and fungi (both viable and autoclaved) or with chitin | Trans-resveratrol and trans-piceatannol | Induction of stilbene biosynthesis by fungi (both viable and autoclaved) and chitin | [241] |
| A. hypogaea cv. Hull line 3 | Elicitation of hairy root cultures with MeJa and methyl-β-cyclodextrin | Trans-resveratrol, trans-piceatannol, trans-arachidin-1 and trans-arachidin-3 | Co-treatment with MeJa and cyclodextrin led to high levels of stilbenes in the culture medium | [242] |
| Arachis hypogaea cv. Georgia green | Treatment of peanuts with ultrasonication or UV-C | Trans-resveratrol, trans-piceid | Increased resveratrol, piceid, and total stilbene concentration, more induced by ultrasound than UV-C | [201] |
3.1. UV Radiation
Multiple lines of evidence indicate that stilbenes, as well as other polyphenols, play an important role in protecting plants from the damaging effects of ultraviolet (UV) radiation [243,244,245]. Induction of polyphenolic phytoalexin biosynthesis in response to UV exposure has been observed in numerous plants [246,247]. Non-polyphenolic phytoalexins can also be elicited by UV, e.g., labdane-related diterpenoids in rice [248] and terpenoid indole alkaloids in the Madagascar periwinkle (Catharanthus roseus (L.) G.Don) [249].
The solar UV spectrum is conventionally subdivided into three wavelength ranges: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm) [250]. UV-C is extremely harmful to organisms, but it is naturally filtered by the stratospheric ozone layer and it is consequently not relevant under natural conditions of solar irradiation. Ozone absorption coefficient drops rapidly at wavelengths longer than 280 nm, reaching zero around 330 nm, therefore UV-B and UV-A can reach Earth’s surface and interact with plants [251]. However, UV harmfulness declines in a similar way, as UV-A cannot be absorbed by DNA and it is thus far less damaging for plants [252]. As a result, for a long time, the effects of UV-A on plant physiology had been underestimated and most studies have focused primarily on UV-C and subordinately on UV-B.
UV-C significantly increases stilbene production in different stilbene-producing plants including grapevine [92,184,194,253], peanut [216,243,254], Gnetum parvifolium (Warb.) W.C. Cheng [204,205], Picea jezoensis (Siebold & Zucc.) Carr. [255], and Polygonum cuspidatum Siebold and Zucc. [256] (Table 1). Recently, Vannozzi and colleagues [60] performed a genome-wide analysis of the VvSTS multigene family expression pattern on V. vinifera cv. Pinot Noir (‘PN40024’ genotype) leaf disks subjected to different stresses (i.e., UV-C exposure, wounding, and downy mildew infection) through a whole transcriptomic (RNA-seq) and real-time qRT-PCR approach. They observed that all stress treatments led to a significant up-regulation of at least several members of the VvSTS multigene family. However, UV-C exposure resulted in the highest induction of the majority of VvSTS members. The induction of VvSTSs was accompanied by reduced expression of VvCHS genes to lower levels compared to untreated leaf discs, suggesting that the competitive relationship between VvSTS and VvCHS may play a role in the accumulation of stilbenes in response to UV-C [60,198].
Molecular mechanisms behind the induction of stilbene biosynthesis in response to UV radiation are still not completely understood. The induction is accompanied by transcriptional activation, protein accumulation, and activation of STS and other enzymes involved in stilbene biosynthesis [5]. In 2013, Höll and co-workers [257] reported that two R2R3-MYB–type transcription factors, namely MYB14 and MYB15, control the transcriptional expression of VvSTS genes under UV-C irradiation in V. vinifera cv. Shiraz leaf discs. However, these transcription factors appear not to be specifically involved in response to UV-C, but also to other stresses including fungal infections and wounds.
In a recent study, the member of the stilbene synthase family VpSTS29 derived from Chinese wild Vitis pseudoreticulata W.T. Wang was overexpressed in V. vinifera cv. Thompson Seedless and the localization of the VpSTS29-GFP protein was investigated [247]. The accumulation of stilbenes elicited by UV-C irradiation was accompanied by the translocation of the VpSTS29-GFP protein from the cytoplasm to the chloroplast. Interestingly, transgenic plants overexpressing VpSTS29-GFP exhibited much lower H2O2 content than untransformed plants and an altered expression of genes related to redox processes, stilbene biosynthesis, and light stimulus [247].
Regarding UV-B, several studies showed that these wavelengths can stimulate the biosynthesis of polyphenols, including stilbenes [184,199,200,258]. UV-B triggers a radiation-specific signaling pathway in grape skin, which activates the biosynthesis and accumulation of secondary metabolites [259]. A UV-B treatment was found to elicit a significant increase in stilbene production, although less marked than that achieved with UV-C [260]. In 2008, Berli and co-workers [258] analyzed the stilbene content in berry skins collected from plants of V. vinifera cv. Malbec cultivated under sunlight with full UV-B (+UV-B) or filtered UV-B (−UV-B) in three different locations at 500, 1000, and 1500 m above sea level (asl) and observed that different solar UV-B levels affect the accumulation of trans-resveratrol. The highest resveratrol content was detected in the berry skins from the +UV-B treatment at 1500 m asl, where the difference between +UV-B and −UV-B was statistically significant. Studies on ozone-treated Scots pine also showed a slight increase in stilbene synthase mRNA, as well as in pinosylvin and pinosylvin methyl ether contents under exposure to UV-B light [261,262].
Post-harvest treatment with UV-B and UV-C has been widely used in fruit and vegetable storage [199,263], where it delays fruit ripening and senescence [264], and activates the defenses against pathogens [265]. Post-harvest UV treatment has been exploited in grapes to increase the content in phenolic compounds including stilbenes in berries and wines [199] (Table 1).
As previously mentioned, the impact of UV-A on stilbene biosynthesis has been underestimated, however recent studies suggest that this radiation deserves more attention. An increase in stilbene content related to UV-A irradiation has been observed in Eucalyptus nitens (H. Deane and Maiden) Maiden by Close and colleagues [266]. The authors postulated that, since stilbenes have absorbance properties consistent with a function as UV-A screens, they could be part of an active UV-A response. This conclusion is consistent with the observation that adult retinal pigment epithelial cells treated with resveratrol show higher viability when exposed to UV-A [267]. A role of UV-A in stilbene biosynthesis was observed in leaves of grey alder (Alnus incana (L.) Moench) and white birch (Betula pubescens Ehrh.) trees under field conditions [268]. It was recently observed that trans-resveratrol and trans-pterostilbene biosynthesis in leaves of O’Neal high bush blueberries (Vaccinium corymbosum L.) can be enhanced by irradiation with both UV-A and UV-C [269]. The comparison of the two UV-treatments showed that UV-A is more effective in promoting trans-resveratrol production, while UV-C is better in enhancing trans-pterostilbene production.
3.2. Light
Light is vital for plants, representing the main energy source for these phototrophic organisms. Light plays a pivotal role in plant growth and development, but it also affects secondary metabolism [20,270]. Extensive literature shows the relationship between light and biosynthesis of polyphenol compounds such as anthocyanins and flavonols [271,272]. However, only a limited number of studies are available on the light-dependent regulation of the stilbene biosynthetic pathway.
Advancements in technology have recently brought light-emitting diodes (LEDs) to the scene of botanical research, allowing for the use of specific wavelengths at high irradiance levels, with realistic results on the study of plant physiological responses to them [273]. Although there is still scarce information about stilbene biosynthesis in response to LED lighting, several studies observed an increase in stilbene biosynthesis, as well as in the expression of stilbene biosynthetic genes, in grapevine exposed to specific wavelengths.
The influence of red LED light (625 nm) and methyl jasmonate (MeJa) on the production of phenylpropanoids in V. vinifera cv. Barbera cell suspension cultures were investigated by Tassoni and co-workers [211]. The combined treatment with red light and MeJa increased the biosynthesis of both anthocyanins and stilbenes, while also promoting the release of catechins into the culture medium. The treatment with red light alone produced a 50% increase in stilbene content in grapevine cells, accompanied by an average decrease in anthocyanin and catechin content of 10% and 18%, respectively. These results suggest a diversion of the phenylpropanoid pathway towards the production of stilbenes under red light [211].
A series of experiments on berries of V. labruscana Bailey cvs. Campbell Early and Kyoho irradiated with fluorescent white light or purple (380 nm), blue (440 nm), and red (660 nm) LED lights showed that red and blue light induces the upregulation of several STS genes and the accumulation of trans- and cis-resveratrol, trans- and cis-piceid, and piceatannol both in grape berry skin [212], and in detached leaves [213].
In 2015, Taurino and colleagues [214] observed an inhibitory effect of light on trans-resveratrol production and an enhancement of trans-piceid biosynthesis in cell suspension cultures of V. vinifera cv. Negramaro. Moreover, in 2018, Andi and collaborators [215] reported that high-level white light irradiation (10,000 lux) inhibits the biosynthesis of trans-piceid and trans-resveratrol in cell suspension cultures of V. vinifera cv. Shahai.
Interesting differences in the impact of light on constitutive and MeJa-induced stilbene biosynthesis emerged from the comparison of cell lines of V. vinifera cv. Malvasia and V. rupestris Du Lot [40]. In both species, the constitutive stilbene content was higher under light conditions, although V. vinifera mainly accumulated piceid, while V. rupestris accumulated trans-resveratrol, trans-δ-viniferin, and trans-ε-viniferin. Furthermore, V. vinifera cells responded to MeJa elicitation with a significant increase in stilbene production under both light and dark conditions, while V. rupestris cells were responsive to elicitation exclusively under dark conditions [40].
Research has only recently begun to investigate the relationship between light and stilbene biosynthesis in species other than grapevine. A study on A. hypogaea sprouts treated with UV-C and white LED light showed a significant response to white light in stilbene accumulation by the upregulation of genes and enzymes involved in their biosynthetic pathway, although less prominent than that observed in response to UV-C [216].
3.3. Temperature
Temperature is an environmental factor of primary importance for plants. It is among the most crucial climatic drivers of biodiversity [274,275] and significantly affects gene expression, protein synthesis, enzymatic activity, and overall primary and secondary metabolism [276,277,278]. Numerous studies have investigated the influence of temperature on the biosynthetic pathway of polyphenols, but only a small fraction of these have focused on stilbenes [204,205,206,207] (Table 1).
A recent study on Gnetum parvifolium revealed that exposure to high temperature (40 °C) enhances resveratrol and piceatannol biosynthesis in leaves of young seedlings, as well as increasing the expression of five STS-like genes in leaves of mature trees, fruit flesh, and seeds [204]. Subsequently, the same authors confirmed the increased expression of STS-like genes under high temperature; however, they did not find a significant increase in the accumulation of total stilbenes in stems and roots of one-year-old plants, suggesting an influence from post-transcriptional regulation on stilbene biosynthesis [205].
The impact of temperature on stilbene biosynthesis was also investigated in grapevines on whole plants, post-harvested fruits, and cultured cells. In 2017, Pastore and co-workers [206] analyzed the entire transcriptome of the berry skin in V. vinifera cv. Sangiovese during ripening under high temperature or low temperature regimes characterized respectively by 26 and 21 °C as average and 42 and 35 °C as maximum daily air temperature. They observed an inhibitory effect of high temperatures on stilbene biosynthesis, in contrast to low temperatures that induced the expression of several members of STS and PAL multigene families, indicating the activation of stilbene biosynthesis. A coordinated expression of STS and PAL has been often observed in grape berries, suggesting that several enzymatic steps in the stilbene biosynthetic pathway are co-regulated [279]. An inhibitory effect of high temperature on the stilbene biosynthetic pathway in grapevine was also reported by Rienth et al. [280] and Wang et al. [207].
Several studies investigated the impact of post-harvest temperature treatment on stilbene biosynthesis. Grapes stored at 0 °C showed a cultivar-dependent modulation in the expression of STS genes, as well as in the accumulation of resveratrol, resveratrol-glucoside, trans-piceatannol, z-miyabenol, and pallidol, especially in the Red Globe cultivar [208,209]. Grape berries cv. Shiraz exposed to high temperature (40 °C) showed an increase in viniferin content, which was accompanied by a decrease in the resveratrol and piceid content [210]. It was also reported that cold storage in combination with UV-C enhanced cis- and trans-piceid content in cv. Red Globe berries stored at 4 °C, while no increase of piceids was obtained by UV-C postharvest treatment alone [196].
Different wilting conditions during winemaking were also investigated by Versari and co-workers [185], revealing an increase of resveratrol and STS mRNA under traditional (ambient temperature for 100 days), low temperature (28 °C for 15 days), and mixed temperature (45 °C for 36 h, ambient temperature for 94 d) wilting, especially with the latter method. No increase in resveratrol was reported with high temperature (45 °C for 110 h) wilting. Temperature-dependent resveratrol accumulation was observed by Houillé and colleagues [217], who reported an optimal range for trans-resveratrol biosynthesis at 15–20 °C, a delayed accumulation of this stilbene at 5 °C, and inhibition at -20 °C and under heat shock (65 °C for 2 h immediately after cane harvest, followed by storage at 20 °C).
Taken together, the data available to date show that temperature has a significant impact on the biosynthesis of stilbenes, which is, however, highly variable in relation to the cultivar, the biological system investigated, and the interaction with other environmental factors.
3.4. Wounding
It is well known that the biosynthesis of stilbenes in plants can be triggered by physical stimuli such as wounding stress. This abiotic stress has been shown to affect stilbene accumulation in Vitaceae [224], Fabacee [203], and Pinaceae [221].
Freshly pruned canes of V. vinifera cv. Pinot Noir showed a transient expression of PAL and STS genes, followed by a rapid increase in trans-resveratrol and trans-piceatannol, when cut in short segments (from 0.2 to 10 cm), with the highest increase in 0.5 cm-length sections [224]. According to previous studies [217], only trans-resveratrol and trans-piceatannol biosynthesis was elicited in pruned grape canes, while no increase in the content of trans-ε-viniferin, ampelopsin A, trans-miyabenol C, cis- and trans-vitisin B, hopeaphenol, and isohopeaphenol was recorded. The sequential induction of lipoxygenase (VvLOX, involved in jasmonic acid biosynthesis) and VvSTS genes suggested that the activation of stilbenoid metabolism in response to wounding stress involves the jasmonate signaling pathway [224].
The induction of grapevine stilbene biosynthetic genes after wounding has been reported in several studies. The genome-wide analysis carried out by Vannozzi and colleagues [60] on V. vinifera cv. Pinot Noir showed an increase in the transcription level of several VvSTS gene family members. Induction of VvSTS in canes from grapevine Cabernet Franc has been recorded during the first 4 weeks of storage by Houillé and co-workers [217], indicating that grapevine wood is still transcriptionally active after pruning. In 2017, Yin et al. [220] observed a significant induction by wounding stress of VqSTS36 promoter activity in Vitis quadrangularis L. In 2018, Vannozzi and co-workers [218] reported that in response to wounding the transcript level of VviSTS29, -41 and -48 gradually increases, coupled with the induction of WRKY and R2R3-MYB transcription factors. Four WRKY genes, namely VviWRKY03, VviWRKY24, VviWRKY43, and VviWRKY53, were thus reported for being involved in the regulation of the stilbene biosynthetic pathway.
Several studies on Scots pine have shown remarkable increases in pinosylvin and pinosylvin monomethyl ether accumulation following wounding stress, associated with overexpression of PsSTS, as well as of genes coding for O-methyltransferases involved in stilbene methylation (PsMT1 and PsMT2) [134,221,223].
Increases in trans-resveratrol accumulation coupled to overexpression of stilbene genes in response to wounding stress were also observed in A. hypogaea [202,203,225].
3.5. Biotic Stress
Stilbenes are well-known to act as chemical defense compounds against pathogen attack in plants [3]. Certain stilbene-producing plants constitutively biosynthesize high levels of stilbenes, independently from pathogen infection. However, in many species, the expression of stilbene biosynthetic genes and the production of stilbenes increase rapidly and conspicuously in response to pathogenic attack. For example, in the roots of P. cuspidatum, large amounts of resveratrol and stilbene glucosides are constitutively accumulated [281,282]. In Scots pine, high levels of pinosylvin and pinosylvin 3-O-methyl ether constitutively accumulate in the heartwood where they protect the wood against decaying fungi [283], but different stress factors including herbivore and pathogen attack can also elicit the biosynthesis of both stilbenes in sapwood and needles, where they consequently act as phytoalexins [284,285].
In grapevine, both STS gene expression and de novo synthesis of stilbenes are induced upon infection with different phytopathogenic fungi like Botrytis cinerea (gray mold) [286,287,288,289], Plasmopara viticola (downy mildew) [60,92,290], Erysiphe necator (powdery mildew) [90,291,292], Rhizopus stolonifer (black bread mold) [183], Aspergillus spp. [293,294], and Phaeomoniella chlamydospora (associated with Esca and Petri diseases) [295].
To date, the impact of bacterial infection on stilbene biosynthesis in grapevine has been poorly investigated. A bacterial strain belonging to the genus Bacillus has been found to elicit trans-resveratrol biosynthesis in V. vinifera cv. Chardonnay and V. rupestris in vitro-grown plantlets [296]. In 2011, Verhagen and colleagues [297] reported that different bacterial strains originating from the vineyard such as Pantoea agglomerans (Pa-AF2), Bacillus subtilis (Bs-271), Acinetobacter lwoffii (Al-113), and Pseudomonas fluorescens (Pf-CT2) can elicit trans-resveratrol and trans-ε-viniferin biosynthesis in cells and leaves of V. vinifera cv. Chardonnay. In 2015, Gruau and co-workers [298] investigated the ability of grapevine cv. Chardonnay plants to express immune responses at both above- and below-ground after interacting with the beneficial bacterium P. fluorescens (PTA-CT2). Bacterial colonization occurred exclusively in the roots and altered the plant phenotype that exhibited multiple defense responses both locally and systemically. The interaction with bacteria-induced opposite changes in stilbene levels in leaves and roots. Significant increases in the content of trans-resveratrol, trans-piceid, and trans-ε-viniferin were observed in the leaves, while the content of all three stilbenes significantly decreased in the roots. This suggests that this interaction plays a role in the transfer of stilbene phytoalexins to the shoot, contributing to the systemic immune response [298]. Both Verhagen et al. [297] and Gruau et al. [298] showed that defense responses triggered by the interaction with beneficial bacteria can greatly improve grapevine resistance against the fungal pathogen B. cinerea.
Constitutive accumulation of resveratrol has been detected in several tissues of peanut plants, albeit at extremely low concentrations. A dramatic increase in both the quantity and diversity of stilbene phytoalexins can occur in response to fungal infection in peanuts [299]. In 2008, Sobolev [300] observed that the inoculation of peanut kernels with different fungal strains belonging to the genus Aspergillus strongly elicits the biosynthesis of trans-resveratrol, trans-arachidin-1, trans-arachidin-2, trans-arachidin-3, trans-3-isopentadienyl-4,3′,5′-trihydroxystilbene, and SB-1. All tested fungal strains of Aspergillus species infecting peanuts activated stilbenoid production in peanut kernel, with interesting variations among different kernel layers. After 24 h of incubation, the tissues closer to the infection site accumulated all the analyzed compounds, while tissues distant from the infected area almost exclusively contained trans-resveratrol. After 48 h of incubation, the six stilbenes were also accumulated in areas far from the infection site, suggesting that trans-resveratrol serves as the building block for other stilbenoids [300].
In peanuts, as well as in grapevine, data regarding the impact of bacterial infection on stilbene biosynthesis are still scarce. To compare the inductive effects of fungi and bacteria on stilbenoid biosynthesis, two phytopathogenic fungal strains (Botryodiplodia theobromae LBBT HC6-1 and B. cinerea FCBC TN1) and two Gram-negative phytopathogenic bacterial strains (Xanthomonas campestris pv. citri XW24 and Pseudochrobactrum asaccharolyticum) were used to treat peanut calluses [241]. The elicitation treatments were performed with either viable or non-viable (autoclaved) microorganisms. The results showed that fungal elicitation is much more effective in inducing biosynthesis of trans-resveratrol and trans-piceatannol than bacterial elicitation, regardless of species and viability [241].
3.6. Elicitation
Plant cell and organ cultures obtained from stilbene-producing plants represent a reliable model system, both for basic research on plant defense mechanisms and for the biotechnological stilbene production, that can be induced and/or enhanced by a wide range of elicitors. The term elicitor, which was originally referred to as molecules capable of inducing the biosynthesis of phytoalexins in plants, is currently used to designate any physical and chemical factors that can trigger any kind of defense response in plants [301]. Elicitors can be classified, according to their nature, as “abiotic elicitors” or “biotic elicitors” and, according to their origin, as “exogenous elicitors” or “endogenous elicitors” [301,302]. Elicitation has mainly been investigated as a tool to enhance the in vitro production of stilbenes [40,219,233,303,304]. However, in recent years increasing attention has been paid to elicitation in both pre- and post-harvest in vivo models, as a strategy to induce the natural plant defenses against pathogens and to improve the health properties of plant foods [188,189,239,305,306,307].
Methyl jasmonate (MeJa) has been shown to be the most effective elicitor for promoting stilbene production in grapevine cell cultures [219,227,233,234,303,308,309]. However, stilbenes are mainly accumulated intracellularly and only a small fraction of them is released into the culture medium by MeJa-treated grapevine cells. Complexation with cyclodextrins (CDs) promotes the release of stilbenes into the medium [304,310]. CDs are cyclic oligosaccharides that have been reported to activate the expression of stilbene biosynthetic genes through the induction of several transcription factors in grapevine [311]. Dimethyl-β-CDs have been shown to act as elicitors capable of stimulating the biosynthesis of stilbenes, as well as promoting their release in the medium and increasing their stability [304,308]. The efficacy of the combined use of MeJa and dimethyl-β-CDs in enhancing the biosynthesis and the extracellular secretion of stilbenes in grapevine cell cultures has been demonstrated by extensive literature [228,304,311,312,313,314]. It has been recently reported that the co-treatment with MeJa and stevioside (a diterpene glycoside extracted from leaves of Stevia rebaudiana Bertoni), elicits the production and extracellular secretion of resveratrol and viniferins in cell cultures of V. labruscana cv. Campbell Early [304].
CDs have been widely exploited to enhance the production of stilbene compounds in hairy root cultures of A. hypogaea. Co-treatment of peanut hairy roots with CDs and MeJa induced the production and secretion in the culture medium of trans-resveratrol, piceatannol, trans-arachidin-1, and trans-arachidin-3 [242]. In 2019, Somboon and colleagues [315] reported that the elicitation of A. hypogaea hairy roots with the herbicide paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride), followed by the combination of MeJa and CDs, resulted in increased amounts of stilbene compounds like trans-resveratrol, trans-arachidin-1, and trans-arachidin-3. Physical elicitors have also been widely exploited for the induction of stilbenes production in peanut, as discussed by Hasan et al. [299] and Wongshaya et al. [316].
The elicitation of white mulberry (Morus alba) callus cultures with 2-hydroxypropyl-β-CD has been shown to improve resveratrol and oxyresveratrol production [317]. It has also been shown that the treatment of cell suspension cultures of M. alba with MeJa and yeast extract (YE) increases the production of oxyresveratrol and resveratrol [318]. A recent investigation has demonstrated that root cultures of M. alba co-treated with MeJa and YE produce relatively high levels of resveratrol, oxyresveratrol, and mulberroside A [319].
In peanut, different chemical elicitors proved to be effective in inducing the biosynthesis of stilbene phytoalexins. Induction of resveratrol by treating peanut cell suspension cultures with YE was reported by Lanz and co-workers [320]. A single elicitation treatment with sodium acetate of peanut hairy root cultures resulted in a 60-fold induction and secretion of trans-resveratrol into the medium after 24 h [321].
Several studies have been conducted with various elicitors to enhance stilbene production in both pre- and post-harvest grape berries. In 2015, Guerrero and colleagues [322] investigated for the first time the effect of pre-harvest UV elicitation in grapevines. Twenty-four hours after the UV-C pre-harvest treatment, they observed a 22 to 46-folds increase in trans-resveratrol content in table grapes cv. Red Globe. They subsequently reported that pre-harvest UV-C treatment repeated for 3 consecutive days resulted in an 86-fold increase in stilbenoid content (trans-resveratrol, trans-piceatannol, isorhapontigenin, cis- and trans-piceid, ε- and ω-viniferin) in table grapes cv. Crimson seedless [323].
As previously mentioned (Section 3.1), a vast literature describes the impact of UV elicitation on stilbene accumulation in post-harvest grapes [94,324]. In 2019, Segade and co-workers [325] recently investigated the impact of post-harvest ozone elicitation on the biosynthesis of stilbenes in Moscato bianco winegrapes. They found that short-term treatment with high ozone doses (60 μL/L; 48 h exposure) not only prevented the loss of stilbenes during the dehydration process but also induced the accumulation of trans-resveratrol and trans-piceatannol. Surprisingly, long-term and continuous ozone treatment did not induce trans-resveratrol production, but it did not negatively affect the stilbene content in winegrapes. Consequently, the use of ozone could have important applications in winemaking to produce wines with high added value considering both the possible reduction of SO2 addition and, under certain conditions, increased contents of stilbenes [325].
Ultrasonication is mechanical stress that can affect stilbene biosynthesis, dramatically increasing resveratrol levels in peanut kernels, as well as in grape skin and leaves. Recent reviews [20,326] thoroughly covered this topic, reporting an increase in trans-resveratrol, trans-piceid in peanuts [201,327], and an increase of resveratrol coupled with the up-regulation of the resveratrol synthase (RS) gene in grape skins and leaves [328] treated with ultrasound. It was also reported that short-term exposure to low power ultrasonication is more effective in eliciting resveratrol accumulation. More recently, Yu and co-workers [329] obtained similar results in peanut sprouts, which responded to ultrasound treatment with an increase in trans-resveratrol, accompanied by a slight increase in total sugars and a remarkable decrease in crude fat and peanut allergenic proteins. Despite these promising results, the use of ultrasonication as a physical elicitor to increase the biotechnological stilbene production in vitro has been poorly investigated. The first published study on this topic dates to 2012 when Santamaria and collaborators [219] reported the accumulation of trans-resveratrol, trans-piceid, trans-ε-viniferin, trans-δ-viniferin, and cis-viniferin in grapevine cv. Alphonse Lavallée cell suspensions in response to low-energy ultrasounds.
3.7. Other Environmental Factors
Stilbene biosynthesis is affected by a wide array of abiotic environmental factors besides the ones mentioned above, and extensive literature has been produced regarding this subject. Past reviews identified, aside from UV, light, wounding and temperatures, drought, ultrasonication, ozone, salinity, pesticides, and soil nutrient content as important abiotic stress sources capable of modulating stilbene biosynthesis [3,5,6,20,330].
Water stress has been shown to produce conflicting results on stilbene biosynthesis in grapevine, with either an increase [96,331] or decrease [332,333] in stilbene content and different behaviors among resveratrol, piceid, and viniferin content in different cultivars, suggesting a pivotal role for genotype [334]. The diversity in polyphenol composition and its changes in response to drought has been recently investigated on a large panel of grapevine cultivars by Pinasseau et al. [335].
The effects of ozone on stilbene biosynthesis, on the other hand, have been known for several decades. Ozone was observed to increase STS activity, as well as the biosynthesis of pinosylvin and pinosylvin 3-methyl ether in Scots pine seedlings [336]. It was also reported to induce an augmented transcript level of the PMT gene in Scots pine needles [221]. Sitka spruce (Picea sitchensis (Bong.) Carr.) stem bark tissues have also been observed reacting to ozone treatment with an increase in resistance to pathogens, although no alterations in the levels of the stilbene glucosides astringin and isorhapontin were recorded [337].
Vitis spp. were also investigated through cell cultures [338] and post-harvest treatment [183,191], recording general increases in trans-resveratrol, piceatannol, pterostilbene, viniferins, and increases in STS expression in response to ozone (although only on ozone resistant phenotypes). Further investigations of STS expression in transgenic tobacco plants in combination with its promoter (Vst1) and the β-glucuronidase (GUS) reporter gene resulted in 11-fold GUS expression following a single ozone pulse and induction of the Vst1 promoter in several tissues [339]. Recent reviews also covered the elicitation of stilbenes due to ozone [5,20], a topic that was later expanded by Ghimire and colleagues [340] observations published in 2019 regarding the combined effects of elevated ozone, temperature, and nitrogen on stilbene concentrations in Scots pine. They reported that warming does suppress the induction of some stilbene compounds/derivatives in ambient ozone levels.
Another important abiotic stress is salinity. Salt stress represents a devastating constraint to plants’ development processes and physiological homeostasis, causing membrane disorganization, cytoplasm alkalinization, ROS induction, transport perturbations, tissue proliferation, and perturbations to photosynthesis, ionic and ionic-related channels [95].
Sodium chloride (NaCl) salt stress is known to strongly delay the rapid induction of STS and resveratrol production in V. rupestris (Scheele) cell cultures, with a late increase in stilbenes. V. riparia (Michx.), instead, does not seem to respond to salt stress [341].
A recent experiment on V. vinifera plantlets subjected to NaCl treatment revealed that the salt-tolerant cv. Razegui did not show important variations in stilbenes biosynthesis, while the salt-sensitive cv. Syrah showed an increase in trans-resveratrol, trans-piceid, and cis-piceid, probably to cope with the higher oxidative disturbance [95]. Resveratrol seems to possess an important role in salt adaptation, as it was found that the application of resveratrol in combination with α-tocopherol to Citrus aurantium L. seedlings reduces NaCl membrane permeability, lipid peroxidation, and pigments degradation, besides reducing H2O2 accumulation in leaves and restoring the reduction of photosynthesis induced by NaCl [342].
Different salts also affect resveratrol production in grapevine. In 2013, Cai and co-workers [343] analyzed the effects of cobalt chloride (CoCL2), silver nitrate (AgNO3), and cadmium chloride (CdCl2) on V. vinifera cv. Gamay Fréaux cell cultures. They reported that cobalt ions at 5.0, 25, and 50 μM concentrations and silver and cadmium ions at 5.0 μM concentration stimulated a 1.6-fold increase in 3-O-glucosyl-resveratrol without suppressing cell growth or compromising cell viability. In contrast, higher concentrations of silver and cadmium remarkably reduced cell viability.
To further expand this topic, we remand to a recent excellent review from Hasan and Bae [20].
4. Conclusions and Future Prospects
The study of stilbenes is of crucial importance for basic research, to understand the biological role of these metabolites in plant defense from biotic and abiotic stress. The interest towards stilbenes also comes from applied research, mainly due to the numerous bioactivities of these compounds and the consequent potentials in the nutraceutical, cosmeceutical, and pharmaceutical fields. The present review arises in this context, dealing with the most recent knowledge of the stilbene biosynthetic pathway and the environmental factors that affect their biosynthesis and accumulation in different plant species, both in planta and in in vitro systems. Given the vastness of the topic and the abundance of scientific literature available to date, this review is not intended to be comprehensive. Some issues concerning the study of stilbenes, albeit of great interest, such as their biological function, pharmacological activity, and distribution in nature and in plant foods have not been explored, because they have been the subject of recent excellent reviews [2,3,4,22,170]. Another topic of great interest, extensively covered by a recent review by Dubrovina and Kiselev [5], consists in the study of the molecular mechanisms by which environmental stimuli regulate the stilbene biosynthetic genes. In recent years, increasing efforts have been devoted by different research groups to expand knowledge on this topic, which is leading to the emergence of interesting new knowledge. Therefore, we expect to have in the near future a more detailed model of the molecular mechanisms that link the perception of environmental stimuli to the expression of genes responsible for the biosynthesis of stilbenes. Furthermore, we expect research efforts to shed light on the link between the biosynthesis of stilbenes and environmental factors that have so far been poorly studied, such as viruses, bacteria, herbivores, UV-A, and ionizing radiation.
Acknowledgments
Special thanks go to Alessia Massimi, who designed the graphical abstract, and Paul B. Edwards, who kindly proofread the manuscript for grammar, spelling, and punctuation.
Abbreviations
| 4CL | 4-Coumarate:CoA ligase |
| CDs | Cyclodextrins |
| CHS | Chalcone synthase |
| CNL | Cinnamate:CoA ligase |
| C4H | Cinnamate 4-hydroxylase |
| DPS | Dihydropinosylvin synthase |
| GFP | Green fluorescent protein |
| GPP | Geranyl diphosphate |
| GUS | β-Glucuronidase |
| LED | Light emitting diode |
| LOX | Lipoxygenase |
| MeJa | Methyl jasmonate |
| OGlu | O-β-D-glucopyranoside |
| OMT | O-Methyltransferase |
| PAL | Phenylalanine ammonia-lyase |
| Phe | Phenylalanine |
| PKSs | Polyketide synthase superfamily |
| PMT | Pinosylvin O-methyltransferase |
| PS | Pinosylvin synthase |
| PTAL | Bifunctional phenylalanine/tyrosine ammonia-lyase |
| ROMT | Resveratrol O-methyltransferase |
| ROS | Reactive oxygen species |
| RS | Resveratrol synthase |
| STS | Stilbene synthase |
| TAL | Tyrosine ammonia-lyase |
| Tyr | Tyrosine |
| UV | Ultraviolet radiation |
Author Contributions
Conceptualization, A.V. and L.M.I.; writing original draft preparation, A.V., L.M.I., F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roupe K.A., Remsberg C.M., Yáñez J.A., Davies N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 2.El Khawand T., Courtois A., Valls J., Richard T., Krisa S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018;17:1007–1029. doi: 10.1007/s11101-018-9578-9. [DOI] [Google Scholar]
- 3.Chong J., Poutaraud A., Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- 4.Rivière C., Pawlus A.D., Merillon J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012;29:1317–1333. doi: 10.1039/c2np20049j. [DOI] [PubMed] [Google Scholar]
- 5.Dubrovina A.S., Kiselev K.V. Regulation of stilbene biosynthesis in plants. Planta. 2017;246:597–623. doi: 10.1007/s00425-017-2730-8. [DOI] [PubMed] [Google Scholar]
- 6.Jeandet P., Delaunois B., Conreux A., Donnez D., Nuzzo V., Cordelier S., Clément C., Courot E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors. 2010;36:331–341. doi: 10.1002/biof.108. [DOI] [PubMed] [Google Scholar]
- 7.Waffo Teguo P., Fauconneau B., Deffieux G., Huguet F., Vercauteren J., Mérillon J.M. Isolation, identification, and antioxidant activity of three stilbene glucosides newly extracted from Vitis vinifera cell cultures. J. Nat. Prod. 1998;61:655–657. doi: 10.1021/np9704819. [DOI] [PubMed] [Google Scholar]
- 8.Privat C., Telo J.P., Bernardes-Genisson V., Vieira A., Souchard J.P., Nepveu F. Antioxidant properties of trans-ε-viniferin as compared to stilbene derivatives in aqueous and nonaqueous media. J. Agric. Food Chem. 2002;50:1213–1217. doi: 10.1021/jf010676t. [DOI] [PubMed] [Google Scholar]
- 9.Biais B., Krisa S., Cluzet S., Da Costa G., Waffo-Teguo P., Mérillon J.M., Richard T. Antioxidant and cytoprotective activities of grapevine stilbenes. J. Agric. Food Chem. 2017;65:4952–4960. doi: 10.1021/acs.jafc.7b01254. [DOI] [PubMed] [Google Scholar]
- 10.Albert S., Horbach R., Deising H.B., Siewert B., Csuk R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorg. Med. Chem. 2011;19:5155–5166. doi: 10.1016/j.bmc.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Chalal M., Klinguer A., Echairi A., Meunier P., Vervandier-Fasseur D., Adrian M. Antimicrobial activity of resveratrol analogues. Molecules. 2014;19:7679–7688. doi: 10.3390/molecules19067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suga T., Ohta S., Munesada K., Ide N., Kurokawa M., Shimizu M., Ohta E. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry. 1993;33:1395–1401. doi: 10.1016/0031-9422(93)85098-C. [DOI] [Google Scholar]
- 13.Torres P., Avila J.G., de Vivar A.R., García A.M., Marín J.C., Aranda E., Céspedes C.L. Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry. 2003;64:463–473. doi: 10.1016/S0031-9422(03)00348-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y.Q., Li X.J., Zhao C.Y., Lu Y., Li W.Q., Liu Z.L., Feng G., Yang L. Synthesis and insect antifeedant activity of stilbene derivatives against Brontispa longissima larvae. Med. Chem. Res. 2013;22:2196–2206. doi: 10.1007/s00044-012-0212-x. [DOI] [Google Scholar]
- 15.Hansen S.C., Stolter C., Imholt C., Jacob J. Plant secondary metabolites as rodent repellents: A systematic review. J. Chem. Ecol. 2016;42:970–983. doi: 10.1007/s10886-016-0760-5. [DOI] [PubMed] [Google Scholar]
- 16.Bryant J.P., Wieland G.D., Reichardt P.B., Lewis V.E., McCarthy M.C. Pinosylvin methyl ether deters snowshoe hare feeding on green alder. Science. 1983;222:1023–1025. doi: 10.1126/science.222.4627.1023. [DOI] [PubMed] [Google Scholar]
- 17.Clausen T.P., Reichardt P.B., Bryant J.P. Pinosylvin and pinosylvin methyl ether as feeding deterrents in green alder. J. Chem. Ecol. 1986;12:2117–2131. doi: 10.1007/BF01020314. [DOI] [PubMed] [Google Scholar]
- 18.Virjamo V., Julkunen-Tiitto R., Henttonen H., Hiltunen E., Karjalainen R., Korhonen J., Huitu O. Differences in vole preference, secondary chemistry and nutrient levels between naturally regenerated and planted Norway spruce seedlings. J. Chem. Ecol. 2013;39:1322–1334. doi: 10.1007/s10886-013-0352-6. [DOI] [PubMed] [Google Scholar]
- 19.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 20.Hasan M., Bae H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules. 2017;22:294. doi: 10.3390/molecules22020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gülçin İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010;11:210–218. doi: 10.1016/j.ifset.2009.07.002. [DOI] [Google Scholar]
- 22.Banez M.J., Geluz M.I., Chandra A., Hamdan T., Biswas O.S., Bryan N.S., Von Schwarz E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020;78:11–26. doi: 10.1016/j.nutres.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Vervandier-Fasseur D., Latruffe N. The potential use of resveratrol for cancer prevention. Molecules. 2019;24:4506. doi: 10.3390/molecules24244506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadi R., Ebrahimzadeh M.A. Resveratrol–A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020;200:112356. doi: 10.1016/j.ejmech.2020.112356. [DOI] [PubMed] [Google Scholar]
- 25.Yang M.F., Yao X., Chen L.M., Gu J.Y., Yang Z.H., Chen H.F., Zheng X., Zheng Z.T. Synthesis and biological evaluation of resveratrol derivatives with anti-breast cancer activity. Arch. Pharm. (Weinheim) 2020;353:e2000044. doi: 10.1002/ardp.202000044. [DOI] [PubMed] [Google Scholar]
- 26.Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng C.K., Luo J.Y., Lau C.W., Chen Z.Y., Tian X.Y., Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020;177:1258–1277. doi: 10.1111/bph.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo Y., Park J., Choi W., Ju Son D., Sung Kim Y., Kim M.K., Yoon B.E., Pyee J., Hong J.T., Go M.Y., et al. Antiatherogenic effect of resveratrol attributed to decreased expression of ICAM-1 (Intercellular adhesion Molecule-1) mechanistic link from focal adhesion to monocyte adhesion. Arterioscler. Thromb. Vasc. Biol. 2019;39:675–684. doi: 10.1161/ATVBAHA.118.312201. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Zhang C.X., Liu Y.M., Chen K.L., Chen G. A comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget. 2017;8:65717. doi: 10.18632/oncotarget.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X., Wu C., Qiu S., Yuan X., Li L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017;14:60. doi: 10.1186/s12986-017-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zu Y., Wang S. Resveratrol-loaded liposomes: Browning subcutaneous white adipose tissue for combating obesity in C57BL/6 J mice. Curr. Dev. Nutr. 2020;4:1709. doi: 10.1093/cdn/nzaa063_107. [DOI] [Google Scholar]
- 32.Kim H., Seo K.H., Yokoyama W. Chemistry of pterostilbene and its metabolic effects. J. Agric. Food Chem. 2020 doi: 10.1021/acs.jafc.0c00070. [DOI] [PubMed] [Google Scholar]
- 33.Xu H., Deng R., Li E.T., Shen J., Wang M. Pinosylvin provides neuroprotection against cerebral ischemia and reperfusion injury through enhancing PINK1/Parkin mediated mitophagy and Nrf2 pathway. J. Funct. Foods. 2020;71:104019. doi: 10.1016/j.jff.2020.104019. [DOI] [Google Scholar]
- 34.Piotrowska H., Kucinska M., Murias M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012;750:60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Yu B., Jiang Y., Zhang B., Yang H., Ma T. Resveratrol dimer trans-ε-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol. Res. 2018;129:453–461. doi: 10.1016/j.phrs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Nivelle L., Aires V., Rioult D., Martiny L., Tarpin M., Delmas D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018;116:323–334. doi: 10.1016/j.fct.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Industry Research; 2020. [(accessed on 1 December 2020)]. Global Resveratrol Market Research Report 2020. Available online: https://www.industryresearch.co/global-resveratrol-market-15064120. [Google Scholar]
- 38.Huang H., Liu R., Ou W. A mini review on the chemical synthesis of resveratrol. Mini Rev. Org. Chem. 2020;17:546–558. doi: 10.2174/1570193X16666190617155558. [DOI] [Google Scholar]
- 39.Lv M., Zhang Y., Wang F., Zhang S., Xu H. Non-food renewable and bioactive forest products for pest management: Valuation of agricultural properties of podophyllotoxin analogs derived from Podophyllum hexandrum as botanical pesticides. Ind. Crops Prod. 2020;153:112608. doi: 10.1016/j.indcrop.2020.112608. [DOI] [Google Scholar]
- 40.Donati L., Ferretti L., Frallicciardi J., Rosciani R., Valletta A., Pasqua G. Stilbene biosynthesis and gene expression in response to methyl jasmonate and continuous light treatment in Vitis vinifera cv. Malvasia del Lazio and Vitis rupestris Du Lot cell cultures. Physiol. Plant. 2019;166:646–662. doi: 10.1111/ppl.12813. [DOI] [PubMed] [Google Scholar]
- 41.Huber R., Marcourt L., Schnee S., Michellod E., Wolfender J.L., Gindro K., Queiroz E.F. Biotransformations with the enzymatic secretome of Botrytis cinerea combined with organic solvents for the generation of novel complex stilbene derivatives. Planta Med. 2019;85:1446–1447. [Google Scholar]
- 42.Wang C., Zhi S., Liu C., Xu F., Zhao A., Wang X., Ren Y., Li Z., Yu M. Characterization of stilbene synthase genes in mulberry (Morus atropurpurea) and metabolic engineering for the production of resveratrol in Escherichia coli. J. Agric. Food Chem. 2017;65:1659–1668. doi: 10.1021/acs.jafc.6b05212. [DOI] [PubMed] [Google Scholar]
- 43.He Q., Szczepańska P., Yuzbashev T., Lazar Z., Ledesma-Amaro R. De novo production of resveratrol from glycerol by engineering different metabolic pathways in Yarrowia lipolytica. Metab. Eng. Commun. 2020;11:e00146. doi: 10.1016/j.mec.2020.e00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma A., Shahzad B., Rehman A., Bhardwaj R., Landi M., Zheng B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24:2452. doi: 10.3390/molecules24132452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emiliani G., Fondi M., Fani R., Gribaldo S. A horizontal gene transfer at the origin of phenylpropanoid metabolism: A key adaptation of plants to land. Biol. Direct. 2009;4:1–12. doi: 10.1186/1745-6150-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv C., Zhao G., Ning Y. Interactions between plant proteins/enzymes and other food components, and their effects on food quality. Crit. Rev. Food Sci. Nutr. 2017;57:1718–1728. doi: 10.1080/10408398.2015.1023762. [DOI] [PubMed] [Google Scholar]
- 47.Havir E.A., Reid P.D., Marsh H.V. L-phenylalanine ammonia-lyase (maize) evidence for a common catalytic site for L-phenylalanine and L-tyrosine. Plant Physiol. 1971;48:130–136. doi: 10.1104/pp.48.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rösler J., Krekel F., Amrhein N., Schmid J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997;113:175–179. doi: 10.1104/pp.113.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barros J., Serrani-Yarce J.C., Chen F., Baxter D., Venables B.J., Dixon R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants. 2016;2:1–9. doi: 10.1038/nplants.2016.50. [DOI] [PubMed] [Google Scholar]
- 50.Barros J., Dixon R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020;25:66–79. doi: 10.1016/j.tplants.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Ferrer J.L., Austin M.B., Stewart C., Jr., Noel J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008;46:356–370. doi: 10.1016/j.plaphy.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupprich N., Hildebrand H., Kindl H. Substrate specificity in vivo and in vitro in the formation of stilbenes. Biosynthesis of rhaponticin. Arch. Biochem. Biophys. 1980;200:72–78. doi: 10.1016/0003-9861(80)90332-X. [DOI] [PubMed] [Google Scholar]
- 53.Schöppner A., Kindl H. Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut. J. Biol. Chem. 1984;259:6806–6811. [PubMed] [Google Scholar]
- 54.Bais A.J., Murphy P.J., Dry I.B. The molecular regulation of stilbene phytoalexin biosynthesis in Vitis vinifera during grape berry development. Funct. Plant Biol. 2000;27:425–433. doi: 10.1071/PP00007. [DOI] [Google Scholar]
- 55.Samappito S., Page J.E., Schmidt J., De-Eknamkul W., Kutchan T.M. Aromatic and pyrone polyketides synthesized by a stilbene synthase from Rheum tataricum. Phytochemistry. 2003;62:313–323. doi: 10.1016/S0031-9422(02)00545-9. [DOI] [PubMed] [Google Scholar]
- 56.Fliegmann J., Schröder G., Schanz S., Britsch L., Schröder J. Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol. Biol. 1992;18:489–503. doi: 10.1007/BF00040665. [DOI] [PubMed] [Google Scholar]
- 57.Kodan A., Kuroda H., Sakai F. A stilbene synthase from Japanese red pine (Pinus densiflora): Implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis. Proc. Natl. Acad. Sci. USA. 2002;99:3335–3339. doi: 10.1073/pnas.042698899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raiber S., Schröder G., Schröder J. Molecular and enzymatic characterization of two stilbene synthases from Eastern white pine (Pinus strobus) A single Arg/His difference determines the activity and the pH dependence of the enzymes. FEBS Lett. 1995;361:299–302. doi: 10.1016/0014-5793(95)00199-J. [DOI] [PubMed] [Google Scholar]
- 59.Austin M.B., Noel J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003;20:79–110. doi: 10.1039/b100917f. [DOI] [PubMed] [Google Scholar]
- 60.Vannozzi A., Dry I.B., Fasoli M., Zenoni S., Lucchin M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012;12:130. doi: 10.1186/1471-2229-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tropf S., Lanz T., Rensing S.A., Schröder J., Schröder G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 1994;38:610–618. doi: 10.1007/BF00175881. [DOI] [PubMed] [Google Scholar]
- 62.Parage C., Tavares R., Rety S., Baltenweck-Guyot R., Poutaraud A., Renault L., Heintz D., Lugan R., Marais G.A., Aubourg S., et al. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012;160:1407–1419. doi: 10.1104/pp.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flores-Sanchez I.J., Verpoorte R. Plant polyketide synthases: A fascinating group of enzymes. Plant Physiol. Biochem. 2009;47:167–174. doi: 10.1016/j.plaphy.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Pandith S.A., Ramazan S., Khan M.I., Reshi Z.A., Shah M.A. Chalcone synthases (CHSs): The symbolic type III polyketide synthases. Planta. 2020;251:15. doi: 10.1007/s00425-019-03307-y. [DOI] [PubMed] [Google Scholar]
- 65.Austin M.B., Bowman M.E., Ferrer J.L., Schröder J., Noel J.P. An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem. Biol. 2004;11:1179–1194. doi: 10.1016/j.chembiol.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 66.Li H., Liang J., Chen H., Ding G., Ma B., He N. Evolutionary and functional analysis of mulberry type III polyketide synthases. BMC Genom. 2016;17:540. doi: 10.1186/s12864-016-2843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schröder G., Brown J.W., Schröder J. Molecular analysis of resveratrol synthase: cDNA, genomic clones and relationship with chalcone synthase. Europ. J. Biochem. 1988;172:161–169. doi: 10.1111/j.1432-1033.1988.tb13868.x. [DOI] [PubMed] [Google Scholar]
- 68.Melchior F., Kindl H. Coordinate-and elicitor-dependent expression of stilbene synthase and phenylalanine ammonia-lyase genes in Vitis cv. Optima. Arch. Biochem. Biophys. 1991;288:552–557. doi: 10.1016/0003-9861(91)90234-A. [DOI] [PubMed] [Google Scholar]
- 69.Preisig-Müller R., Schwekendiek A., Brehm I., Reif H.J., Kindl H. Characterization of a pine multigene family containing elicitor-responsive stilbene synthase genes. Plant Mol. Biol. 1999;39:221–229. doi: 10.1023/A:1006163030646. [DOI] [PubMed] [Google Scholar]
- 70.Warren R.L., Keeling C.I., Yuen M.M., Raymond A., Taylor G.A., Vandervalk B.P., Mohamadi H., Paulino D., Chiu R., Jackman S.D., et al. Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defense metabolism. Plant J. 2015;83:189–212. doi: 10.1111/tpj.12886. [DOI] [PubMed] [Google Scholar]
- 71.Hammerbacher A., Ralph S.G., Bohlmann J., Fenning T.M., Gershenzon J., Schmidt A. Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol. 2011;157:876–890. doi: 10.1104/pp.111.181420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christine K.Y., Springob K., Schmidt J., Nicholson R.L., Chu I.K., Yip W.K., Lo C. A stilbene synthase gene (SbSTS1) is involved in host and nonhost defense responses in Sorghum. Plant Physiol. 2005;138:393–401. doi: 10.1104/pp.105.059337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu F., Han J., Liu S., Chen X., Varshney R.K., Liang X. Cloning, expression pattern analysis and subcellular localization of resveratrol synthase gene in peanut (Arachis hypogaea L.) Am. J. Plant Sci. 2014;5:3619–3631. doi: 10.4236/ajps.2014.524378. [DOI] [Google Scholar]
- 74.Paterson A.H., Bowers J.E., Bruggmann R., Dubchak I., Grimwood J., Gundlach H., Haberer G., Hellsten U., Mitros T., Poliakov A., et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 75.Sharma I., Kumari N., Sharma V. Defense gene expression in Sorghum bicolor against Macrophomina phaseolina in leaves and roots of susceptible and resistant cultivars. J. Plant Interact. 2014;9:315–323. doi: 10.1080/17429145.2013.832425. [DOI] [Google Scholar]
- 76.Lee Y.G., Choi S.C., Kang Y., Kim K.M., Kang C.S., Kim C. Constructing a reference genome in a single lab: The possibility to use oxford nanopore technology. Plants. 2019;8:270. doi: 10.3390/plants8080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sparvoli F., Martin C., Scienza A., Gavazzi G., Tonelli C. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.) Plant Mol. Biol. 1994;24:743–755. doi: 10.1007/BF00029856. [DOI] [PubMed] [Google Scholar]
- 78.Dai R., Ge H., Howard S., Qiu W. Transcriptional expression of stilbene synthase genes are regulated developmentally and differentially in response to powdery mildew in Norton and Cabernet Sauvignon grapevine. Plant Sci. 2012;197:70–76. doi: 10.1016/j.plantsci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Shi J., He M., Cao J., Wang H., Ding J., Jiao Y.T., Li R.M., He J., Wang D., Wang Y. The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera) Plant Physiol. Biochem. 2014;74:24–32. doi: 10.1016/j.plaphy.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Gachon C.M., Langlois-Meurinne M., Saindrenan P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005;10:542–549. doi: 10.1016/j.tplants.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Wang J., Hou B. Glycosyltransferases: Key players involved in the modification of plant secondary metabolites. Front. Biol. China. 2009;4:39–46. doi: 10.1007/s11515-008-0111-1. [DOI] [Google Scholar]
- 82.Lepak A., Gutmann A., Kulmer S.T., Nidetzky B. Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. ChemBioChem. 2015;16:1870–1874. doi: 10.1002/cbic.201500284. [DOI] [PubMed] [Google Scholar]
- 83.Le Roy J., Huss B., Creach A., Hawkins S., Neutelings G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016;7:735. doi: 10.3389/fpls.2016.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimoda K., Kubota N., Uesugi D., Kobayashi Y., Hamada H., Hamada H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules. 2020;25:1437. doi: 10.3390/molecules25061437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navarro G., Martínez-Pinilla E., Ortiz R., Noé V., Ciudad C.J., Franco R. Resveratrol and related stilbenoids, nutraceutical/dietary complements with health-promoting actions: Industrial production, safety, and the search for mode of action. Compr. Rev. Food Sci. Food Saf. 2018;17:808–826. doi: 10.1111/1541-4337.12359. [DOI] [PubMed] [Google Scholar]
- 86.Wang H., Liu L., Guo Y.X., Dong Y.S., Zhang D.J., Xiu Z.L. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2007;75:763. doi: 10.1007/s00253-007-0874-3. [DOI] [PubMed] [Google Scholar]
- 87.Park K.T., Kim J.K., Lim Y.H. Deglycosylation of stilbene glucoside compounds improves inhibition of 3-hydroxy-3-methylglutaryl coenzyme a reductase and squalene synthase activities. Food Sci. Biotechnol. 2014;23:647–651. doi: 10.1007/s10068-014-0088-2. [DOI] [Google Scholar]
- 88.Kiselev K.V., Aleynova O.A., Grigorchuk V.P., Dubrovina A.S. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta. 2017;245:151–159. doi: 10.1007/s00425-016-2598-z. [DOI] [PubMed] [Google Scholar]
- 89.Colombo F., Di Lorenzo C., Regazzoni L., Fumagalli M., Sangiovanni E., de Sousa L.P., Bavaresco L., Tomasi D., Bosso A., Aldini G., et al. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019;10:1797–1807. doi: 10.1039/C8FO02175A. [DOI] [PubMed] [Google Scholar]
- 90.Romero-Pérez A.I., Lamuela-Raventós R.M., Andrés-Lacueva C., de la Torre-Boronat M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001;49:210–215. doi: 10.1021/jf000745o. [DOI] [PubMed] [Google Scholar]
- 91.Boubakri H., Poutaraud A., Wahab M.A., Clayeux C., Baltenweck-Guyot R., Steyer D., Marcic C., Mliki A., Soustre-Gacougnolle I. Thiamine modulates metabolism of the phenylpropanoid pathway leading to enhanced resistance to Plasmopara viticola in grapevine. BMC Plant Biol. 2013;13:1–15. doi: 10.1186/1471-2229-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adrian M., Jeandet P., Douillet-Breuil A.C., Tesson L., Bessis R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000;48:6103–6105. doi: 10.1021/jf0009910. [DOI] [PubMed] [Google Scholar]
- 93.Maurer L.H., Bersch A.M., Santos R.O., Trindade S.C., Costa E.L., Peres M.M., Malmann C.A., Schneider M., Bochi V.C., Sautter C.K., et al. Postharvest UV-C irradiation stimulates the non-enzymatic and enzymatic antioxidant system of ‘Isabel’hybrid grapes (Vitis labrusca× Vitis vinifera L.) Food Res. Int. 2017;102:738–747. doi: 10.1016/j.foodres.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 94.Mishra A.K., Choi S.J., Baek K.H. Application of ultraviolet c irradiation for the increased production of secondary metabolites in plants. J. Anim. Plant Sci. 2020;30:1082–1091. [Google Scholar]
- 95.Souid I., Toumi I., Hermosín-Gutiérrez I., Nasri S., Mliki A., Ghorbel A. The effect of salt stress on resveratrol and piceid accumulation in two Vitis vinifera L. cultivars. Physiol. Mol. Biol. Plants. 2019;25:625–635. doi: 10.1007/s12298-019-00668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deluc L.G., Decendit A., Papastamoulis Y., Mérillon J.M., Cushman J.C., Cramer G.R. Water deficit increases stilbene metabolism in Cabernet Sauvignon berries. J. Agric. Food Chem. 2011;59:289–297. doi: 10.1021/jf1024888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villangó S., Szekeres A., Bencsik O., Láposi R., Pálfi Z., Zsófi Z. The effect of postveraison water deficit on the phenolic composition and concentration of the Kékfrankos (Vitis vinifera L.) berry. Sci. Hort. 2016;209:113–116. doi: 10.1016/j.scienta.2016.06.010. [DOI] [Google Scholar]
- 98.Bowles D., Lim E.K., Poppenberger B., Vaistij F.E. Glycosyltransferases of lipophilic small molecules. Annu. Rev. Plant Biol. 2006;57:567–597. doi: 10.1146/annurev.arplant.57.032905.105429. [DOI] [PubMed] [Google Scholar]
- 99.Hall D., De Luca V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca) Plant J. 2007;49:579–591. doi: 10.1111/j.1365-313X.2006.02987.x. [DOI] [PubMed] [Google Scholar]
- 100.Regev-Shoshani G., Shoseyov O., Bilkis I., Kerem Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003;374:157–163. doi: 10.1042/bj20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Signorelli P., Ghidoni R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 102.Romero-Pérez A.I., Ibern-Gómez M., Lamuela-Raventós R.M., de la Torre-Boronat M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999;47:1533–1536. doi: 10.1021/jf981024g. [DOI] [PubMed] [Google Scholar]
- 103.Concenco F.I., Brotto G.F., Nora L. Grape wine and juice: Comparison on resveratrol levels. Int. J. Adv. Res. Sci. Eng. Technol. 2019;6:368–386. doi: 10.22161/ijaers.6.4.44. [DOI] [Google Scholar]
- 104.Vrhovsek U., Wendelin S., Eder R. Effects of various vinification techniques on the concentration of cis-and trans-resveratrol and resveratrol glucoside isomers in wine. Am. J. Enol. Vitic. 1997;48:214–219. [Google Scholar]
- 105.Kostadinović S., Wilkens A., Stefova M., Ivanova V., Vojnoski B., Mirhosseini H., Winterhalter P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem. 2012;135:3003–3009. doi: 10.1016/j.foodchem.2012.06.118. [DOI] [PubMed] [Google Scholar]
- 106.Gaensly F., Agustini B.C., da Silva G.A., Picheth G., Bonfim T.M.B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods. 2015;19:288–295. doi: 10.1016/j.jff.2015.09.041. [DOI] [Google Scholar]
- 107.Kuo H.P., Wang R., Huang C.Y., Lai J.T., Lo Y.C., Huang S.T. Characterization of an extracellular β-glucosidase from Dekkera bruxellensis for resveratrol production. J. Food Drug Anal. 2018;26:163–171. doi: 10.1016/j.jfda.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen M., Li D., Gao Z., Zhang C. Enzymatic transformation of polydatin to resveratrol by piceid-β-d-glucosidase from Aspergillus oryzae. Bioprocess Biosyst. Eng. 2014;37:1411–1416. doi: 10.1007/s00449-013-1113-1. [DOI] [PubMed] [Google Scholar]
- 109.Basholli-Salihu M., Schuster R., Mulla D., Praznik W., Viernstein H., Mueller M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess Biosyst. Eng. 2016;39:1879–1885. doi: 10.1007/s00449-016-1662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J.K., Kim M., Cho S.G., Kim M.K., Kim S.W., Lim Y.H. Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition. J. Ind. Microbiol. Biotechnol. 2010;37:631–637. doi: 10.1007/s10295-010-0722-9. [DOI] [PubMed] [Google Scholar]
- 111.Kim J.K., Kim N., Lim Y.H. Evaluation of the antibacterial activity of rhapontigenin produced from rhapontin by biotransformation against Propionibacterium acnes. J. Microbiol. Biotechnol. 2010;20:82–87. doi: 10.4014/jmb.0907.07022. [DOI] [PubMed] [Google Scholar]
- 112.Komaikul J., Kitisripanya T., Inyai C., Likhitwitayawuid K., Sritularak B., Tanaka H., Putalun W. Phytostilbenoid production in white mulberry (Morus alba L.) cell culture using bioreactors and simple deglycosylation by endogenous enzymatic hydrolysis. In Vitro Cell. Dev. Biol. Plant. 2019;55:199–208. doi: 10.1007/s11627-018-09953-3. [DOI] [Google Scholar]
- 113.Gabaston J., Richard T., Cluzet S., Palos Pinto A., Dufour M.C., Corio-Costet M.F., Mérillon J.M. Pinus pinaster Knot: A source of polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017;65:8884–8891. doi: 10.1021/acs.jafc.7b04129. [DOI] [PubMed] [Google Scholar]
- 114.Gabaston J., Leborgne C., Waffo-Téguo P., Pedrot E., Richard T., Mérillon J.M., Valls Fonayet J. Separation and isolation of major polyphenols from maritime pine (Pinus pinaster) knots by two-step centrifugal partition chromatography monitored by LC-MS and NMR spectroscopy. J. Sep. Sci. 2020;43:1080–1088. doi: 10.1002/jssc.201901066. [DOI] [PubMed] [Google Scholar]
- 115.Sati S.C., Sati N., Sati O.P. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011;5:174. doi: 10.4103/0973-7847.91115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seshadri T.R. Polyphenols of Pterocarpus and Dalbergia woods. Phytochemistry. 1972;11:881–898. doi: 10.1016/S0031-9422(00)88430-7. [DOI] [Google Scholar]
- 117.Maurya R., Ray A.B., Duah F.K., Slatkin D.J., Schiff P.L., Jr. Constituents of Pterocarpus marsupium. J. Nat. Prod. 1984;47:179–181. doi: 10.1021/np50031a029. [DOI] [Google Scholar]
- 118.Rimando A.M., Kalt W., Magee J.B., Dewey J., Ballington J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 119.Langcake P., Cornford C.A., Pryce R.J. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry. 1979;18:1025–1027. doi: 10.1016/S0031-9422(00)91470-5. [DOI] [Google Scholar]
- 120.Jeandet P., Douillet-Breuil A.C., Bessis R., Debord S., Sbaghi M., Adrian M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002;50:2731–2741. doi: 10.1021/jf011429s. [DOI] [PubMed] [Google Scholar]
- 121.Vek V., Poljanšek I., Humar M., Willför S., Oven P. In vitro inhibition of extractives from knotwood of Scots pine (Pinus sylvestris) and black pine (Pinus nigra) on growth of Schizophyllum commune, Trametes versicolor, Gloeophyllum trabeum and Fibroporia vaillantii. Wood Sci. Technol. 2020;54:1645–1662. doi: 10.1007/s00226-020-01229-7. [DOI] [Google Scholar]
- 122.Hart J.H. Role of phytostilbenes in decay and disease resistance. Annu. Rev. Phytopathol. 1981;19:437–458. doi: 10.1146/annurev.py.19.090181.002253. [DOI] [Google Scholar]
- 123.Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today. 2010;15:757–765. doi: 10.1016/j.drudis.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 124.Jeong Y.J., An C.H., Woo S.G., Jeong H.J., Kim Y.M., Park S.J., Yoon B.D., Kim C.Y. Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli. Enzyme Microb. Technol. 2014;54:8–14. doi: 10.1016/j.enzmictec.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Rimando A.M., Pan Z., Polashock J.J., Dayan F.E., Mizuno C.S., Snook M.E., Liu C.J., Baerson S.R. In planta production of the highly potent resveratrol analogue pterostilbene via stilbene synthase and O-methyltransferase co-expression. Plant Biotechnol. J. 2012;10:269–283. doi: 10.1111/j.1467-7652.2011.00657.x. [DOI] [PubMed] [Google Scholar]
- 126.Martínez-Márquez A., Morante-Carriel J.A., Palazon J., Bru-Martínez R. Rosa hybrida orcinol O-methyl transferase-mediated production of pterostilbene in metabolically engineered grapevine cell cultures. New Biotechnol. 2018;42:62–70. doi: 10.1016/j.nbt.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 127.Purwanto R., Hori K., Yamada Y., Sato F. Unraveling additional O-methylation steps in benzylisoquinoline alkaloid biosynthesis in California poppy (Eschscholzia californica) Plant Cell Physiol. 2017;58:1528–1540. doi: 10.1093/pcp/pcx093. [DOI] [PubMed] [Google Scholar]
- 128.Nakatsubo T., Kitamura Y., Sakakibara N., Mizutani M., Hattori T., Sakurai N., Shibata D., Suzuki S., Umezawa T. At5g54160 gene encodes Arabidopsis thaliana 5-hydroxyconiferaldehyde O-methyltransferase. J. Wood Sci. 2008;54:312–317. doi: 10.1007/s10086-008-0958-4. [DOI] [Google Scholar]
- 129.Shimizu T., Lin F., Hasegawa M., Okada K., Nojiri H., Yamane H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012;287:19315–19325. doi: 10.1074/jbc.M112.351270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schmidlin L., Poutaraud A., Claudel P., Mestre P., Prado E., Santos-Rosa M., Wiedemann-Merdinoglu S., Karst F., Merdinoglu D., Hugueney P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008;148:1630–1639. doi: 10.1104/pp.108.126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baerson S.R., Dayan F.E., Rimando A.M., Nanayakkara N.D., Liu C.J., Schröder J., Fishbein M., Pan Z., Kagan I.A., Pratt L.H., et al. A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J. Biol. Chem. 2008;283:3231–3247. doi: 10.1074/jbc.M706587200. [DOI] [PubMed] [Google Scholar]
- 132.Koeduka T., Hatada M., Suzuki H., Suzuki S., Matsui K. Molecular cloning and functional characterization of an O-methyltransferase catalyzing 4′-O-methylation of resveratrol in Acorus calamus. J. Biosci. Bioeng. 2019;127:539–543. doi: 10.1016/j.jbiosc.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 133.Chiron H., Drouet A., Claudot A.C., Eckerskorn C., Trost M., Heller W., Ernst D., Sandermann H. Molecular cloning and functional expression of a stress-induced multifunctional O-methyltransferase with pinosylvin methyltransferase activity from Scots pine (Pinus sylvestris L.) Plant Mol. Biol. 2000;44:733–745. doi: 10.1023/A:1026507707186. [DOI] [PubMed] [Google Scholar]
- 134.Paasela T., Lim K.J., Pietiäinen M., Teeri T.H. The O-methyltransferase PMT 2 mediates methylation of pinosylvin in Scots pine. New Phytol. 2017;214:1537–1550. doi: 10.1111/nph.14480. [DOI] [PubMed] [Google Scholar]
- 135.Pailee P., Sangpetsiripan S., Mahidol C., Ruchirawat S., Prachyawarakorn V. Cytotoxic and cancer chemopreventive properties of prenylated stilbenoids from Macaranga siamensis. Tetrahedron. 2015;71:5562–5571. doi: 10.1016/j.tet.2015.06.058. [DOI] [Google Scholar]
- 136.Leláková V., Béraud-Dufour S., Hošek J., Šmejkal K., Prachyawarakorn V., Pailee P., Widmann C., Václavík J., Coppola T., Mazella J., et al. Therapeutic potential of prenylated stilbenoid macasiamenene F through its anti-inflammatory and cytoprotective effects on LPS-challenged monocytes and microglia. J. Ethnopharmacol. 2020;263:113147. doi: 10.1016/j.jep.2020.113147. [DOI] [PubMed] [Google Scholar]
- 137.Biondi D.M., Rocco C., Ruberto G. New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. J. Nat. Prod. 2003;66:477–480. doi: 10.1021/np020365s. [DOI] [PubMed] [Google Scholar]
- 138.Biondi D.M., Rocco C., Ruberto G. Dihydrostilbene derivatives from Glycyrrhiza glabra leaves. J. Nat. Prod. 2005;68:1099–1102. doi: 10.1021/np050034q. [DOI] [PubMed] [Google Scholar]
- 139.Ye R., Fan Y.H., Ma C.M. Identification and enrichment of α-glucosidase-inhibiting dihydrostilbene and flavonoids from Glycyrrhiza uralensis leaves. J. Agric. Food Chem. 2017;65:510–515. doi: 10.1021/acs.jafc.6b04155. [DOI] [PubMed] [Google Scholar]
- 140.Meng H.C., Zhu S., Fan Y.H., Ye R., Hattori M., Komatsu K., Ma C.M. Discovery of prenylated dihydrostilbenes in Glycyrrhiza uralensis leaves by UHPLC-MS using neutral loss scan. Ind. Crops Prod. 2020;152:112557. doi: 10.1016/j.indcrop.2020.112557. [DOI] [Google Scholar]
- 141.Sobolev V.S., Potter T.L., Horn B.W. Prenylated stilbenes from peanut root mucilage. Phytochem Anal. 2006;17:312–322. doi: 10.1002/pca.920. [DOI] [PubMed] [Google Scholar]
- 142.Sobolev V.S., Neff S.A., Gloer J.B. New stilbenoids from peanut (Arachis hypogaea) seeds challenged by an Aspergillus caelatus strain. J. Agric. Food Chem. 2009;57:62–68. doi: 10.1021/jf802891v. [DOI] [PubMed] [Google Scholar]
- 143.Sobolev V.S., Neff S.A., Gloer J.B. New dimeric stilbenoids from fungal-challenged peanut (Arachis hypogaea) seeds. J. Agric. Food Chem. 2010;58:875–881. doi: 10.1021/jf903410e. [DOI] [PubMed] [Google Scholar]
- 144.Sobolev V.S. Production of phytoalexins in peanut (Arachis hypogaea) seed elicited by selected microorganisms. J. Agric. Food Chem. 2013;61:1850–1858. doi: 10.1021/jf3054752. [DOI] [PubMed] [Google Scholar]
- 145.Sobolev V.S., Krausert N.M., Gloer J.B. New monomeric stilbenoids from peanut (Arachis hypogaea) seeds challenged by an Aspergillus flavus strain. J. Agric. Food Chem. 2016;64:579–584. doi: 10.1021/acs.jafc.5b04753. [DOI] [PubMed] [Google Scholar]
- 146.Wu Z., Song L., Huang D. Food grade fungal stress on germinating peanut seeds induced phytoalexins and enhanced polyphenolic antioxidants. J. Agric. Food Chem. 2011;59:5993–6003. doi: 10.1021/jf200776w. [DOI] [PubMed] [Google Scholar]
- 147.Chan E.W., Lye P.Y., Wong S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016;14:17–30. doi: 10.3724/SP.J.1009.2016.00017. [DOI] [PubMed] [Google Scholar]
- 148.Yang T., Fang L., Sanders S., Jayanthi S., Rajan G., Podicheti R., Thallapuranam S.K., Mockaitis K., Medina-Bolivar F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018;293:28–46. doi: 10.1074/jbc.RA117.000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aisyah S., Gruppen H., Slager M., Helmink B., Vincken J.P. Modification of prenylated stilbenoids in peanut (Arachis hypogaea) seedlings by the same fungi that elicited them: The fungus strikes back. J. Agric. Food Chem. 2015;63:9260–9268. doi: 10.1021/acs.jafc.5b03570. [DOI] [PubMed] [Google Scholar]
- 150.Aguamah G.E., Langcake P., Leworthy D.P., Page J.A., Pryce R.J., Strange R.N. Two novel stilbene phytoalexins from Arachis hypogaea. Phytochemistry. 1981;20:1381–1383. doi: 10.1016/0031-9422(81)80044-1. [DOI] [Google Scholar]
- 151.Wotton H.R., Strange R.N. Circumstantial evidence for phytoalexin involvement in the resistance of peanuts to Aspergillus flavus. Microbiology. 1985;131:487–494. doi: 10.1099/00221287-131-3-487. [DOI] [PubMed] [Google Scholar]
- 152.Cooksey C.J., Garratt P.J., Richards S.E., Strange R.N. A dienyl stilbene phytoalexin from Arachis hypogaea. Phytochemistry. 1988;27:1015–1016. doi: 10.1016/0031-9422(88)80263-2. [DOI] [Google Scholar]
- 153.Péresse T., Jézéquel G., Allard P.M., Pham V.C., Huong D.T., Blanchard F., Bignon J., Lévaique H., Wolfender J.-L., Litaudon M., et al. Cytotoxic prenylated stilbenes isolated from Macaranga tanarius. J. Nat. Prod. 2017;80:2684–2691. doi: 10.1021/acs.jnatprod.7b00409. [DOI] [PubMed] [Google Scholar]
- 154.de Bruijn W.J., Araya-Cloutier C., Bijlsma J., de Swart A., Sanders M.G., de Waard P., Gruppen H., Vincken J.P. Antibacterial prenylated stilbenoids from peanut (Arachis hypogaea) Phytochem. Lett. 2018;28:13–18. doi: 10.1016/j.phytol.2018.09.004. [DOI] [Google Scholar]
- 155.Zhong Z., Zhu W., Liu S., Guan Q., Chen X., Huang W., Yang B., Tian J., Tian J. Molecular characterization of a geranyl diphosphate-specific prenyltransferase catalyzing stilbenoid prenylation from Morus alba. Plant Cell Physiol. 2018;59:2214–2227. doi: 10.1093/pcp/pcy138. [DOI] [PubMed] [Google Scholar]
- 156.Munakata R., Olry A., Karamat F., Courdavault V., Sugiyama A., Date Y., Krieger C., Silie P., Foureau E., Papon V., et al. Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 2016;211:332–344. doi: 10.1111/nph.13899. [DOI] [PubMed] [Google Scholar]
- 157.Morales M., Alcántara J., Barceló A.R. Oxidation of trans-resveratrol by a hypodermal peroxidase isoenzyme from Gamay rouge grape (Vitis vinifera) berries. Am. J. Enol. Vitic. 1997;48:33–38. [Google Scholar]
- 158.Lin M., Yao C.S. Natural oligostilbenes. Stud. Nat. Prod. Chem. 2006;33:601–644. [Google Scholar]
- 159.Chiou W.F., Shen C.C., Chen C.C., Lin C.H., Huang Y.L. Oligostilbenes from the roots of Vitis thunbergii. Planta Med. 2009;75:856–859. doi: 10.1055/s-0029-1185404. [DOI] [PubMed] [Google Scholar]
- 160.Shu N., Zhou H., Hu C. Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method. Biol. Pharm. Bull. 2006;29:608–612. doi: 10.1248/bpb.29.608. [DOI] [PubMed] [Google Scholar]
- 161.Ito T., Endo H., Oyama M., Iinuma M. Novel isolation of stilbenoids with enantiomeric and meso forms from a Cyperus rhizome. Phytochem. Lett. 2012;5:267–270. doi: 10.1016/j.phytol.2012.01.009. [DOI] [Google Scholar]
- 162.Abe N., Ito T., Oyama M., Sawa R., Takahashi Y., Iinuma M. Resveratrol derivatives from Vatica albiramis. Chem. Pharm. Bull. 2011;59:452–457. doi: 10.1248/cpb.59.452. [DOI] [PubMed] [Google Scholar]
- 163.Shimokawa Y., Hirasawa Y., Kaneda T., Hadi A.H.A., Morita H. Cuspidans A and B, two new stilbenoids from the bark of Gnetum cuspidatum. Chem. Pharm. Bull. 2012;60:790–792. doi: 10.1248/cpb.60.790. [DOI] [PubMed] [Google Scholar]
- 164.He C.N., Peng Y., Xu L.J., Liu Z.A., Gu J., Zhong A.G., Xiao P.G. Three new oligostilbenes from the seeds of Paeonia suffruticosa. Chem. Pharm. Bull. 2010;58:843–847. doi: 10.1248/cpb.58.843. [DOI] [PubMed] [Google Scholar]
- 165.Wang Y.Q., Tan J.J., Tan C.H., Jiang S.H., Zhu D.Y. Halophilols A and B, two new stilbenes from Iris halophila. Planta Med. 2003;69:779–781. doi: 10.1055/s-2003-42792. [DOI] [PubMed] [Google Scholar]
- 166.Syah Y.M., Achmad S.A., Ghisalberti E.L., Hakim E.H., Makmur L., Soekamto N.H. A stilbene dimer, andalasin B, from the root trunk of Morus macroura. J. Chem. Res. 2004;5:339–340. doi: 10.3184/0308234041639692. [DOI] [Google Scholar]
- 167.Douillet-Breuil A.C., Jeandet P., Adrian M., Bessis R. Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. J. Agric. Food Chem. 1999;47:4456–4461. doi: 10.1021/jf9900478. [DOI] [PubMed] [Google Scholar]
- 168.Wang X.F., Yao C.S. Naturally active oligostilbenes. J. Asian Nat. Prod. Res. 2016;18:376–407. doi: 10.1080/10286020.2015.1094464. [DOI] [PubMed] [Google Scholar]
- 169.Ito T., Tanaka T., Nakaya K.I., Iinuma M., Takahashi Y., Naganawa H., Ohyama M., Nakanishi Y., Bastow K.F., Lee K.H. A new resveratrol octamer, vateriaphenol A, in Vateria indica. Tetrahedron Lett. 2001;42:5909–5912. doi: 10.1016/S0040-4039(01)01137-6. [DOI] [Google Scholar]
- 170.Shen T., Wang X.N., Lou H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009;26:916–935. doi: 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]
- 171.Pezet R., Perret C., Jean-Denis J.B., Tabacchi R., Gindro K., Viret O. Delta-viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003;51:5488–5492. doi: 10.1021/jf030227o. [DOI] [PubMed] [Google Scholar]
- 172.Langcake P., Pryce R.J. Oxidative dimerization of 4-hydroxystilbenes in vitro: Production of a grapevine phytoalexin mimic. J. Chem. Soc. Chem. Commun. 1977;7:208–210. doi: 10.1039/c39770000208. [DOI] [Google Scholar]
- 173.Zhang J.Q., Li G.P., Kang Y.L., Teng B.H., Yao C.S. Biomimetic synthesis of resveratrol trimers catalyzed by horseradish peroxidase. Molecules. 2017;22:819. doi: 10.3390/molecules22050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pezet R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea Pers.: Fr. FEMS Microbiol. Lett. 1998;167:203–208. doi: 10.1111/j.1574-6968.1998.tb13229.x. [DOI] [Google Scholar]
- 175.Wu Z., Li H., Zhu X., Li S., Wang Z., Wang L., Li Z., Chen G. Using laccases in the nanoflower to synthesize viniferin. Catalysts. 2017;7:188. doi: 10.3390/catal7060188. [DOI] [Google Scholar]
- 176.Ros Barceló A., Pomar F., López-Serrano M., Pedreno M.A. Peroxidase: A multifunctional enzyme in grapevines. Funct. Plant Biol. 2003;30:577–591. doi: 10.1071/FP02096. [DOI] [PubMed] [Google Scholar]
- 177.Takaya Y., Terashima K., Ito J., He Y.H., Tateoka M., Yamaguchi N., Niwa M. Biomimic transformation of resveratrol. Tetrahedron. 2005;61:10285–10290. doi: 10.1016/j.tet.2005.08.023. [DOI] [Google Scholar]
- 178.Wilkens A., Paulsen J., Wray V., Winterhalter P. Structures of two novel trimeric stilbenes obtained by horseradish peroxidase catalyzed biotransformation of trans-resveratrol and (−)-ε-viniferin. J. Agric. Food Chem. 2010;58:6754–6761. doi: 10.1021/jf100606p. [DOI] [PubMed] [Google Scholar]
- 179.Sako M., Hosokawa H., Ito T., Iinuma M. Regioselective oxidative coupling of 4-hydroxystilbenes: Synthesis of resveratrol and ε-viniferin (E)-dehydrodimers. J. Org. Chem. 2004;69:2598–2600. doi: 10.1021/jo035791c. [DOI] [PubMed] [Google Scholar]
- 180.Natori Y., Ito M., Anada M., Nambu H., Hashimoto S. Catalytic asymmetric synthesis of (−)-E-δ-viniferin via an intramolecular C–H insertion of diaryldiazomethane using Rh2 (S-TFPTTL) 4. Tetrahedron Lett. 2015;56:4324–4327. doi: 10.1016/j.tetlet.2015.05.072. [DOI] [Google Scholar]
- 181.Bavaresco L., Lucini L., Busconi M., Flamini R., De Rosso M. Wine resveratrol: From the ground up. Nutrients. 2016;8:222. doi: 10.3390/nu8040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Flamini R., Zanzotto A., de Rosso M., Lucchetta G., Dalla Vedova A., Bavaresco L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant Pathol. 2016;93:112–118. doi: 10.1016/j.pmpp.2016.01.011. [DOI] [Google Scholar]
- 183.Sarig P., Zutkhi Y., Monjauze A., Lisker N., Ben-Arie R. Phytoalexin elicitation in grape berries and their susceptibility to Rhizopus stolonifer. Physiol. Mol. Plant Pathol. 1997;50:337–347. doi: 10.1006/pmpp.1997.0089. [DOI] [Google Scholar]
- 184.Cantos E., García-Viguera C., de Pascual-Teresa S., Tomás-Barberán F.A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000;48:4606–4612. doi: 10.1021/jf0002948. [DOI] [PubMed] [Google Scholar]
- 185.Versari A., Parpinello G.P., Tornielli G.B., Ferrarini R., Giulivo C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agric. Food Chem. 2001;49:5531–5536. doi: 10.1021/jf010672o. [DOI] [PubMed] [Google Scholar]
- 186.Moriartry J.M., Harmon R., Weston L.A., Bessis R., Breuil A.C., Adrian M., Jeandet P. Resveratrol content of two Californian table grape cultivars. Vitis. 2001;40:43–44. [Google Scholar]
- 187.Cantos E., Espín J.C., Tomás-Barberán F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002;50:6322–6329. doi: 10.1021/jf020562x. [DOI] [PubMed] [Google Scholar]
- 188.Cantos E., Espín J.C., Fernández M.J., Oliva J., Tomás-Barberán F.A. Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J. Agric. Food Chem. 2003;51:1208–1214. doi: 10.1021/jf020939z. [DOI] [PubMed] [Google Scholar]
- 189.Cantos E., Tomás-Barberán F.A., Martínez A., Espín J.C. Differential stilbene induction susceptibility of seven red wine grape varieties upon post-harvest UV-C irradiation. Eur. Food Res. Technol. 2003;217:253–258. doi: 10.1007/s00217-003-0736-x. [DOI] [Google Scholar]
- 190.González-Barrio R., Salmenkallio-Marttila M., Tomás-Barberán F.A., Cantos E., Espín J.C. Etiology of UV-C-induced browning in var. Superior white table grapes. J. Agric. Food Chem. 2005;53:5990–5996. doi: 10.1021/jf0504115. [DOI] [PubMed] [Google Scholar]
- 191.González-Barrio R., Beltrán D., Cantos E., Gil M.I., Espín J.C., Tomás-Barberán F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. ‘Superior’ white table grapes. J. Agric. Food Chem. 2006;54:4222–4228. doi: 10.1021/jf060160f. [DOI] [PubMed] [Google Scholar]
- 192.González-Barrio R., Vidal-Guevara M.L., Tomás-Barberán F.A., Espín J.C. Preparation of a resveratrol-enriched grape juice based on ultraviolet C-treated berries. Innov. Food Sci. Emerg. Technol. 2009;10:374–382. doi: 10.1016/j.ifset.2009.01.004. [DOI] [Google Scholar]
- 193.Sánchez J.J., Corral E.C., Orea J.M., Delgado M.S., Ureña A.G. Elicitation of trans-resveratrol by laser resonant irradiation of table grapes. Appl. Phys. B. 2007;87:559–563. doi: 10.1007/s00340-007-2591-0. [DOI] [Google Scholar]
- 194.Guerrero R.F., Puertas B., Fernández M.I., Palma M., Cantos-Villar E. Induction of stilbenes in grapes by UV-C: Comparison of different subspecies of Vitis. Innov. Food. Sci. Emerg. Technol. 2010;11:231–238. doi: 10.1016/j.ifset.2009.10.005. [DOI] [Google Scholar]
- 195.Cho Y.J., Kim N., Kim C.T., Maeng J.S., Pyee J. Quantitative evaluation of resveratrol enrichment induced by UV stimulus in harvested grapes. Food Sci. Biotechnol. 2012;21:597–601. doi: 10.1007/s10068-012-0076-3. [DOI] [Google Scholar]
- 196.Crupi P., Pichierri A., Basile T., Antonacci D. Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem. 2013;141:802–808. doi: 10.1016/j.foodchem.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 197.Freitas P.M., López-Gálvez F., Tudela J.A., Gil M.I., Allende A. Postharvest treatment of table grapes with ultraviolet-C and chitosan coating preserves quality and increases stilbene content. Postharvest Biol. Technol. 2015;105:51–57. doi: 10.1016/j.postharvbio.2015.03.011. [DOI] [Google Scholar]
- 198.Yin X., Singer S.D., Qiao H., Liu Y., Jiao C., Wang H., Li Z., Fei Z., Wang Y., Fan C., et al. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr.) cv.“Tonghua-3”. Front. Plant Sci. 2016;7:503. doi: 10.3389/fpls.2016.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sheng K., Zheng H., Shui S., Yan L., Liu C., Zheng L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Techol. 2018;138:74–81. doi: 10.1016/j.postharvbio.2018.01.002. [DOI] [Google Scholar]
- 200.Kong Q., Deng R., Li X., Zeng Q., Zhang X., Yu X., Ren X. Based on RNA-Seq analysis identification and expression analysis of Trans-scripusin A synthesize-related genes of UV-treatment in postharvest grape fruit. Arch. Biochem. Biophys. 2020;690:108471. doi: 10.1016/j.abb.2020.108471. [DOI] [PubMed] [Google Scholar]
- 201.Sales J.M., Resurreccion A.V.A. Maximising resveratrol and piceid contents in UV and ultrasound treated peanuts. Food Chem. 2009;117:674–680. doi: 10.1016/j.foodchem.2009.04.075. [DOI] [Google Scholar]
- 202.Chung I.M., Park M.R., Chun J.C., Yun S.J. Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants. Plant Sci. 2003;164:103–209. doi: 10.1016/S0168-9452(02)00341-2. [DOI] [Google Scholar]
- 203.Rudolf J.R., Resurreccion A.V. Elicitation of resveratrol in peanut kernels by application of abiotic stresses. J. Agric. Food Chem. 2005;53:10186–10192. doi: 10.1021/jf0506737. [DOI] [PubMed] [Google Scholar]
- 204.Deng N., Chang E., Li M., Ji J., Yao X., Bartish I.V., Liu J., Ma J., Chen L., Jiang Z., et al. Transcriptome characterization of Gnetum parvifolium reveals candidate genes involved in important secondary metabolic pathways of flavonoids and stilbenoids. Front. Plant Sci. 2016;7:174. doi: 10.3389/fpls.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Deng N., Liu C., Chang E., Ji J., Yao X., Yue J., Bartish I.V., Chen L., Jiang Z., Shi S. High temperature and UV-C treatments affect stilbenoid accumulation and related gene expression levels in Gnetum parvifolium. Electron. J. Biotechnol. 2017;25:43–49. doi: 10.1016/j.ejbt.2016.11.001. [DOI] [Google Scholar]
- 206.Pastore C., Dal Santo S., Zenoni S., Movahed N., Allegro G., Valentini G., Filippetti I., Tornielli G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant Sci. 2017;8:929. doi: 10.3389/fpls.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang L., An M., Huang W., Zhan J. Melatonin and phenolics biosynthesis-related genes in Vitis vinifera cell suspension cultures are regulated by temperature and copper stress. Plant Cell Tiss. Org. Cult. 2019;138:475–488. doi: 10.1007/s11240-019-01643-1. [DOI] [Google Scholar]
- 208.Sanchez-Ballesta M.T., Romero I., Jiménez J.B., Orea J.M., González-Urena A., Escribano M.I., Merodio C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol. Technol. 2007;46:29–35. doi: 10.1016/j.postharvbio.2007.04.001. [DOI] [Google Scholar]
- 209.Sanchez-Ballesta M.T., Alvarez I., Escribano M.I., Merodio C., Romero I. Effect of high CO2 levels and low temperature on stilbene biosynthesis pathway gene expression and stilbenes production in white, red and black table grape cultivars during postharvest storage. Plant Physiol. Biochem. 2020;151:334–341. doi: 10.1016/j.plaphy.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 210.Degu A., Ayenew B., Cramer G.R., Fait A. Polyphenolic responses of grapevine berries to light, temperature, oxidative stress, abscisic acid and jasmonic acid show specific developmental-dependent degrees of metabolic resilience to perturbation. Food Chem. 2016;212:828–836. doi: 10.1016/j.foodchem.2016.05.164. [DOI] [PubMed] [Google Scholar]
- 211.Tassoni A., Durante L., Ferri M. Combined elicitation of methyl-jasmonate and red light on stilbene and anthocyanin biosynthesis. J. Plant Physiol. 2012;169:775–781. doi: 10.1016/j.jplph.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 212.Ahn S.Y., Kim S.A., Choi S.J., Yun H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015;56:36–43. doi: 10.1007/s13580-015-0045-x. [DOI] [Google Scholar]
- 213.Ahn S.Y., Kim S.A., Yun H.K. Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes. Eur. J. Plant Pathol. 2015;143:753–765. doi: 10.1007/s10658-015-0725-5. [DOI] [Google Scholar]
- 214.Taurino M., Ingrosso I., D’amico L., Domenico S.D., Nicoletti I., Corradini D., Santino A., Giovinazzo G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus. 2015;4:49. doi: 10.1186/s40064-015-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Andi S.A., Gholami M., Ford C.M. The effect of methyl jasmonate and light irradiation treatments on the stilbenoid biosynthetic pathway in Vitis vinifera cell suspension cultures. Nat. Prod. Res. 2018;32:909–917. doi: 10.1080/14786419.2017.1367782. [DOI] [PubMed] [Google Scholar]
- 216.Zhu T., Yang J., Zhang D., Cai Q., Zhou D., Tu S., Liu Q., Tu K. Effects of white LED light and UV-C radiation on stilbene biosynthesis and phytochemicals accumulation identified by UHPLC–MS/MS during peanut (Arachis hypogaea L.) germination. J. Agric. Food Chem. 2020;68:5900–5909. doi: 10.1021/acs.jafc.0c01178. [DOI] [PubMed] [Google Scholar]
- 217.Houillé B., Besseau S., Courdavault V., Oudin A., Glévarec G., Delanoue G., Guerin L., Simkin J.A., Papon N., Clastre M., et al. Biosynthetic origin of E-resveratrol accumulation in grape canes during postharvest storage. J. Agric. Food Chem. 2015;63:1631–1638. doi: 10.1021/jf505316a. [DOI] [PubMed] [Google Scholar]
- 218.Vannozzi A., Wong D.C.J., Höll J., Hmmam I., Matus J.T., Bogs J., Ziegler T., Dry I., Barcaccia G., Lucchin M. Combinatorial regulation of stilbene synthase genes by WRKY and MYB transcription factors in grapevine (Vitis vinifera L.) Plant Cell Physiol. 2018;59:1043–1059. doi: 10.1093/pcp/pcy045. [DOI] [PubMed] [Google Scholar]
- 219.Santamaria A.R., Innocenti M., Mulinacci N., Melani F., Valletta A., Sciandra I., Pasqua G. Enhancement of viniferin production in Vitis vinifera L. cv. Alphonse Lavallée cell suspensions by low-energy ultrasound alone and in combination with methyl jasmonate. J. Agric. Food Chem. 2012;60:11135–11142. doi: 10.1021/jf301936u. [DOI] [PubMed] [Google Scholar]
- 220.Yin X., Huang L., Zhang X., Guo C., Wang H., Li Z., Wang X. Expression patterns and promoter characteristics of the Vitis quinquangularis VqSTS36 gene involved in abiotic and biotic stress response. Protoplasma. 2017;254:2247–2261. doi: 10.1007/s00709-017-1116-x. [DOI] [PubMed] [Google Scholar]
- 221.Chiron H., Drouet A., Lieutier F., Payer H.D., Ernst D., Sandermann H. Gene induction of stilbene biosynthesis in Scots pine in response to ozone treatment, wounding, and fungal infection. Plant Physiol. 2000;124:865–872. doi: 10.1104/pp.124.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lim K. Ph.D. Thesis. University of Helsinki; Helsinki, Finland: 2017. Scots Pine (Pinus sylvestris L.) Heartwood Formation and Wounding Stress: A View from the Transcriptome. [Google Scholar]
- 223.Johansson S.M., Lundgren L.N., Asiegbu F.O. Initial reactions in sapwood of Norway spruce and Scots pine after wounding and infection by Heterobasidion parviporum and H. annosum. For. Pathol. 2004;34:197–210. doi: 10.1111/j.1439-0329.2004.00358.x. [DOI] [Google Scholar]
- 224.Billet K., Houillé B., Besseau S., Mélin C., Oudin A., Papon N., Courdavault V., Clastre M., Giglioli-Guivarc’h N., Lanoue A. Mechanical stress rapidly induces E-resveratrol and E-piceatannol biosynthesis in grape canes stored as a freshly-pruned byproduct. Food Chem. 2018;240:1022–1027. doi: 10.1016/j.foodchem.2017.07.105. [DOI] [PubMed] [Google Scholar]
- 225.Arora M.K., Strange R.N. Phytoalexin accumulation in groundnuts in response to wounding. Plant Sci. 1991;78:157–163. doi: 10.1016/0168-9452(91)90194-D. [DOI] [Google Scholar]
- 226.Righetti L., Franceschetti M., Ferri M., Tassoni A., Bagni N. Resveratrol production in Vitis vinifera cell suspensions treated with several elicitors. Caryologia. 2007;60:169–171. [Google Scholar]
- 227.Belhadj A., Telef N., Saigne C., Cluzet S., Barrieu F., Hamdi S., Mérillon J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008;46:493–499. doi: 10.1016/j.plaphy.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 228.Lijavetzky D., Almagro L., Belchi-Navarro S., Martínez-Zapater J.M., Bru R., Pedreño M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes. 2008;1:132. doi: 10.1186/1756-0500-1-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ferri M., Tassoni A., Franceschetti M., Righetti L., Naldrett M.J., Bagni N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics. 2009;9:610–624. doi: 10.1002/pmic.200800386. [DOI] [PubMed] [Google Scholar]
- 230.Santamaria A.R., Antonacci D., Caruso G., Cavaliere C., Gubbiotti R., Laganà A., Valletta A., Pasqua G. Stilbene production in cell cultures of Vitis vinifera L. cvs Red Globe and Michele Palieri elicited by methyl jasmonate. Nat. Prod. Res. 2010;24:1488–1498. doi: 10.1080/14786410903421446. [DOI] [PubMed] [Google Scholar]
- 231.Mihai R., Cristina S., Helepciuc F., Brezeanu A., Stoian G. Biotic and abiotic elicitors induce biosynthesis and accumulation of resveratrol with antitumoral activity in the long-term Vitis vinifera L. callus cultures. Rom. Biotechnol. Lett. 2011;16:6683–6689. [Google Scholar]
- 232.Ferri M., Dipalo S.C., Bagni N., Tassoni A. Chitosan elicits mono-glucosylated stilbene production and release in fed-batch bioreactor cultures of grape cells. Food Chem. 2011;124:1473–1479. doi: 10.1016/j.foodchem.2010.07.114. [DOI] [Google Scholar]
- 233.Santamaria A.R., Mulinacci N., Valletta A., Innocenti M., Pasqua G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011;59:9094–9101. doi: 10.1021/jf201181n. [DOI] [PubMed] [Google Scholar]
- 234.Belchí-Navarro S., Almagro L., Lijavetzky D., Bru R., Pedreño M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012;31:81–89. doi: 10.1007/s00299-011-1141-8. [DOI] [PubMed] [Google Scholar]
- 235.Caia Z., Knorra D., Smetanskaa I. Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culture by indanoyl-isoleucine, N-linolenoyl-L-glutamine and insect saliva. Enzym. Microb. Technol. 2012;50:29–34. doi: 10.1016/j.enzmictec.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 236.Wang L.J., Ma L., Xi H.F., Duan W., Wang J.F., Li S.H. Individual and combined effects of CaCl2 and UV-C on the biosynthesis of resveratrols in grape leaves and berry skins. J. Agric. Food Chem. 2013;61:7135–7141. doi: 10.1021/jf401220m. [DOI] [PubMed] [Google Scholar]
- 237.Vuong T.V., Franco C., Zhang W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014;1–2:15–21. doi: 10.1016/j.btre.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Almagro L., Belchí-Navarro S., Martínez-Marquez A., Bru R., Pedreño M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiol. Biochem. 2015;97:361–367. doi: 10.1016/j.plaphy.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 239.Portu J., López R., Baroja E., Santamaría P., Garde-Cerdán T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016;201:213–221. doi: 10.1016/j.foodchem.2016.01.086. [DOI] [PubMed] [Google Scholar]
- 240.Erte E., Vural N., Mehmetoğlu Ü., Güvenç A. Optimization of an abiotic elicitor (ultrasound) treatment conditions on trans-resveratrol production from Kalecik Karası (Vitis vinifera L.) grape skin. J. Food Sci. Technol. 2020 doi: 10.1007/s13197-020-04722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yang M.H., Kuo C.H., Hsieh W.C., Ku K.L. Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor. J. Agric. Food Chem. 2010;58:9537–9541. doi: 10.1021/jf1022725. [DOI] [PubMed] [Google Scholar]
- 242.Yang T., Fang L., Nopo-Olazabal C., Condori J., Nopo-Olazabal L., Balmaceda C., Medina-Bolivar F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015;63:3942–3950. doi: 10.1021/jf5050266. [DOI] [PubMed] [Google Scholar]
- 243.Tang K., Zhan J.C., Yang H.R., Huang W.D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010;167:95–102. doi: 10.1016/j.jplph.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 244.Suzuki M., Nakabayashi R., Ogata Y., Sakurai N., Tokimatsu T., Goto S., Suzuki M., Jasinski M., Martinoia E., Otagaki S., et al. Multiomics in grape berry skin revealed specific induction of the stilbene synthetic pathway by ultraviolet-C irradiation. Plant Physiol. 2015;168:47–59. doi: 10.1104/pp.114.254375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Matus J.T. Transcriptomic and metabolomic networks in the grape berry illustrate that it takes more than flavonoids to fight against ultraviolet radiation. Front. Plant Sci. 2016;7:1337. doi: 10.3389/fpls.2016.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li H., Li D., Yang Z., Zeng Q., Luo Y., He N. Flavones produced by mulberry flavone synthase Type I constitute a defense line against the ultraviolet-B stress. Plants. 2020;9:215. doi: 10.3390/plants9020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ma F., Yao W., Wang L., Wang Y. Dynamic translocation of stilbene synthase VpSTS29 from a Chinese wild Vitis species upon UV irradiation. Phytochemistry. 2019;159:137–147. doi: 10.1016/j.phytochem.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 248.Leonelli F., Valletta A., Migneco L.M., Marini Bettolo R. Stemarane diterpenes and diterpenoids. Int. J. Mol. Sci. 2019;20:2627. doi: 10.3390/ijms20112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Moon S.H., Pandurangan M., Kim D.H., Venkatesh J., Patel R.V., Mistry B.M. A rich source of potential bioactive compounds with anticancer activities by Catharanthus roseus cambium meristematic stem cell cultures. J. Ethnopharmacol. 2018;217:107–117. doi: 10.1016/j.jep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 250.Sharma S., Chatterjee S., Kataria S., Joshi J., Datta S., Vairale M.G., Veer V. UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth. Volume 75 John Wiley & Sons; West Sussex, UK: 2017. A review on responses of plants to UV-B radiation related stress. [Google Scholar]
- 251.Hollósy F. Effects of ultraviolet radiation on plant cells. Micron. 2002;33:179–197. doi: 10.1016/S0968-4328(01)00011-7. [DOI] [PubMed] [Google Scholar]
- 252.Tyunin A.P., Kiselev K.V. Alternations in VaSTS gene cytosine methylation and t-resveratrol production in response to UV-C irradiation in Vitis amurensis Rupr. cells. Plant Cell Tiss. Org. Cult. 2016;124:33–45. doi: 10.1007/s11240-015-0872-6. [DOI] [Google Scholar]
- 253.Wang W., Tang K., Yang H.R., Wen P.F., Zhang P., Wang H.L., Huang W.D. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol. Biochem. 2010;48:142–152. doi: 10.1016/j.plaphy.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 254.Fritzemeier K.H., Rolfs C.H., Pfau J., Kindl H. Action of ultraviolet-C on stilbene formation in callus of Arachis hypogaea. Planta. 1983;159:25–29. doi: 10.1007/BF00998810. [DOI] [PubMed] [Google Scholar]
- 255.Kiselev K.V., Grigorchuk V.P., Ogneva Z.V., Suprun A.R., Dubrovina A.S. The effect of ultraviolet-C and precursor feeding on stilbene biosynthesis in spruce Picea jezoensis. J. Plant Physiol. 2019;234:133–137. doi: 10.1016/j.jplph.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 256.Liu Z., Xu J., Wu X., Wang Y., Lin Y., Wu D., Zhang H., Qin J. Molecular analysis of UV-C induced resveratrol accumulation in Polygonum cuspidatum Leaves. Int. J. Mol. Sci. 2019;20:6185. doi: 10.3390/ijms20246185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Höll J., Vannozzi A., Czemmel S., D’Onofrio C., Walker A.R., Rausch T., Lucchin M., Boss P.K., Dry I.B., Bogs J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell. 2013;25:4135–4149. doi: 10.1105/tpc.113.117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Berli F., D’Angelo J., Cavagnaro B., Bottini R., Wuilloud R., Silva M.F. Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electrophoresis. J. Agric. Food Chem. 2008;56:2892–2898. doi: 10.1021/jf073421+. [DOI] [PubMed] [Google Scholar]
- 259.Carbonell-Bejerano P., Diago M.P., Martínez-Abaigar J., Martínez-Zapater J.M., Tardáguila J., Núñez-Olivera E. Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. BMC Plant Biol. 2014;14:183. doi: 10.1186/1471-2229-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li X., Zheng X., Yan S., Li S. Effects of salicylic acid (SA), ultraviolet radiation (UV-B and UV-C) on trans-resveratrol inducement in the skin of harvested grape berries. Front. Agric. China. 2008;2:77–81. doi: 10.1007/s11703-008-0014-6. [DOI] [Google Scholar]
- 261.Zinser C., Ernst D., Sandermann H., Jr. Induction of stilbene synthase and cinnamyl alcohol dehydrogenase mRNAs in Scots pine (Pinus sylvestris L.) seedlings. Planta. 1998;204:169–176. doi: 10.1007/s004250050243. [DOI] [Google Scholar]
- 262.Zinser C., Jungblut T., Heller W., Seidlitz H.K., Schnitzler J.P., Ernst D., Sandermann H., Jr. The effect of ozone in Scots pine (Pinus sylvestris L.): Gene expression, biochemical changes and interactions with UV-B radiation. Plant Cell Environ. 2000;23:975–982. doi: 10.1046/j.1365-3040.2000.00613.x. [DOI] [Google Scholar]
- 263.Urban L., Charles F., de Miranda M.R.A., Aarrouf J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016;105:1–11. doi: 10.1016/j.plaphy.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 264.Imaizumi T., Yamauchi M., Sekiya M., Shimonishi Y., Tanaka F. Responses of phytonutrients and tissue condition in persimmon and cucumber to postharvest UV-C irradiation. Postharvest Biol. Technol. 2018;145:33–40. doi: 10.1016/j.postharvbio.2018.06.003. [DOI] [Google Scholar]
- 265.Zhang W., Jiang W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019;92:71–80. doi: 10.1016/j.tifs.2019.08.012. [DOI] [Google Scholar]
- 266.Close D.C., McArthur C., Hagerman A.E., Davies N.W., Beadle C.L. Phenolic acclimation to ultraviolet-A irradiation in Eucalyptus nitens seedlings raised across a nutrient environment gradient. Photosynthetica. 2007;45:36–42. doi: 10.1007/s11099-007-0006-4. [DOI] [Google Scholar]
- 267.Chan C.M., Huang C.H., Li H.J., Hsiao C.Y., Su C.C., Lee P.L., Hung C.F. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int. J. Mol. Sci. 2015;16:5789–5802. doi: 10.3390/ijms16035789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kotilainen T., Tegelberg R., Julkunen-Tiitto R., Lindfors A., Aphalo P.J. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob. Chang. Biol. 2008;14:1294–1304. doi: 10.1111/j.1365-2486.2008.01569.x. [DOI] [Google Scholar]
- 269.Kim T.E., Pyee J.H., Cho Y.J. Effect of ultraviolet irradiation on the stilbenoid content of blueberry leaves. J. Food Process Eng. 2020;43:e13546. doi: 10.1111/jfpe.13546. [DOI] [Google Scholar]
- 270.Akula R., Ravishankar G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011;6:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yang T., Ma H., Zhang J., Wu T., Song T., Tian J., Yao Y. Systematic identification of long noncoding RNA s expressed during light-induced anthocyanin accumulation in apple fruit. Plant J. 2019;100:572–590. doi: 10.1111/tpj.14470. [DOI] [PubMed] [Google Scholar]
- 272.Thoma F., Somborn-Schulz A., Schlehuber D., Keuter V., Deerberg G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020;11:497. doi: 10.3389/fpls.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lefsrud M.G., Kopsell D.A., Sams C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience. 2008;43:2243–2244. doi: 10.21273/HORTSCI.43.7.2243. [DOI] [Google Scholar]
- 274.Mayhew P.J., Jenkins G.B., Benton T.G. A long-term association between global temperature and biodiversity, origination and extinction in the fossil record. Proc. Royal Soc. B. 2008;275:47–53. doi: 10.1098/rspb.2007.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mayhew P.J., Bell M.A., Benton T.G., McGowan A.J. Biodiversity tracks temperature over time. PNAS. 2012;109:15141–15145. doi: 10.1073/pnas.1200844109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Guo J., Zhou X., Wang T., Wang G., Cao F. Regulation of flavonoid metabolism in ginkgo leaves in response to different day-night temperature combinations. Plant Physiol. Biochem. 2020;147:133–140. doi: 10.1016/j.plaphy.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 277.Lecourieux D., Kappel C., Claverol S., Pieri P., Feil R., Lunn J.E., Bonneu M., Wang L., Gomès E., Delrot S., et al. Proteomic and metabolomic profiling underlines the stage-and time-dependent effects of high temperature on grape berry metabolism. J. Integr. Plant Biol. 2020;62:1132–1158. doi: 10.1111/jipb.12894. [DOI] [PubMed] [Google Scholar]
- 278.Zandalinas S.I., Sales C., Beltrán J., Gómez-Cadenas A., Arbona V. Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 2017;7:1954. doi: 10.3389/fpls.2016.01954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zenoni S., Fasoli M., Guzzo F., Dal Santo S., Amato A., Anesi A., Commisso M., Herderich M., Ceoldo S., Avesani L., et al. Disclosing the molecular basis of the postharvest life of berry in different grapevine genotypes. Plant Physiol. 2016;172:1821–1843. doi: 10.1104/pp.16.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rienth M., Torregrosa L., Luchaire N., Chatbanyong R., Lecourieux D., Kelly M.T., Romieu C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014;14:1–18. doi: 10.1186/1471-2229-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Vastano B.C., Chen Y., Zhu N., Ho C.T., Zhou Z., Rosen R.T. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 282.Peng W., Qin R., Li X., Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc.: A review. J. Ethnopharmacol. 2013;148:729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 283.Harju A., Venalainen M. Stilbenes as constitutive and induced protection compounds in Scots pine (Pinus sylvestris L.) Gen. Tech. Rep. PSW-GTR. 2012;240:20–26. [Google Scholar]
- 284.Sullivan T.P., Crump D.R., Wieser H., Dixon E.A. Influence of the plant antifeedant, pinosylvin, on suppression of feeding by snowshoe hares. J. Chem. Ecol. 1992;18:1151–1164. doi: 10.1007/BF00980070. [DOI] [PubMed] [Google Scholar]
- 285.Gehlert R., Schöppner A., Kindl H. Stilbene synthase from seedlings of Pinus sylvestris: Purification and induction in response to fungal infection. Mol. Plant Microbe Interact. 1990;3:444–449. doi: 10.1094/MPMI-3-444. [DOI] [Google Scholar]
- 286.Langcake P., McCarthy W. The relationship of resveratrol production to infection of grapevine leaves by Botrytis cinerea. Vitis. 1979;18:244–253. [Google Scholar]
- 287.Jeandet P., Bessis R., Sbaghi M., Meunier P. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J. Phytopathol. 1995;143:135–139. doi: 10.1111/j.1439-0434.1995.tb00246.x. [DOI] [Google Scholar]
- 288.Bavaresco L., Pettegolli D., Cantü E., Fregoni M., Chiusa G., Trevisan M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis. 1997;36:77–83. [Google Scholar]
- 289.Bézier A., Lambert B., Baillieul F. Study of defense-related gene expression in grapevine leaves and berries infected with Botrytis cinerea. Eur. J. Plant Pathol. 2002;108:111–120. doi: 10.1023/A:1015061108045. [DOI] [Google Scholar]
- 290.Langcake P., Pryce R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- 291.Fung R.W., Gonzalo M., Fekete C., Kovacs L.G., He Y., Marsh E., McIntyre L.M., Schachtman D.P., Qiu W. Powdery mildew induces defense-oriented reprogramming of the transcriptome in a susceptible but not in a resistant grapevine. Plant Physiol. 2008;146:236–249. doi: 10.1104/pp.107.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schnee S., Viret O., Gindro K. Role of stilbenes in the resistance of grapevine to powdery mildew. Physiol. Mol. Plant Pathol. 2008;72:128–133. doi: 10.1016/j.pmpp.2008.07.002. [DOI] [Google Scholar]
- 293.Bavaresco L., Vezzulli S., Battilani P., Giorni P., Pietri A., Bertuzzi T. Effect of ochratoxin A-producing Aspergilli on stilbenic phytoalexin synthesis in grapes. J. Agric. Food Chem. 2003;51:6151–6157. doi: 10.1021/jf0301908. [DOI] [PubMed] [Google Scholar]
- 294.Vezzulli S., Battilani P., Bavaresco L. Stilbene-synthase gene expression after Aspergillus carbonarius infection in grapes. Am. J. Enol. Vitic. 2007;58:132–134. [Google Scholar]
- 295.Martin N., Vesentini D., Rego C., Monteiro S., Oliveira H., Ferreira R.B. Phaeomoniella chlamydospora infection induces changes in phenolic compounds content in Vitis vinifera. Phytopathol. Mediterr. 2009;48:101–116. [Google Scholar]
- 296.Paul B., Chereyathmanjiyil A., Masih I., Chapuis L., Benoît A. Biological control of Botrytis cinerea causing grey mould disease of grapevine and elicitation of stilbene phytoalexin (resveratrol) by a soil bacterium. FEMS Microbiol. Lett. 1998;165:65–70. doi: 10.1111/j.1574-6968.1998.tb13128.x. [DOI] [Google Scholar]
- 297.Verhagen B., Trotel-Aziz P., Jeandet P., Baillieul F., Aziz A. Improved resistance against Botrytis cinerea by grapevine-associated bacteria that induce a prime oxidative burst and phytoalexin production. Phytopathology. 2011;101:768–777. doi: 10.1094/PHYTO-09-10-0242. [DOI] [PubMed] [Google Scholar]
- 298.Gruau C., Trotel-Aziz P., Villaume S., Rabenoelina F., Clément C., Baillieul F., Aziz A. Pseudomonas fluorescens PTA-CT2 triggers local and systemic immune response against Botrytis cinerea in grapevine. Mol. Plant Microbe Interact. 2015;28:1117–1129. doi: 10.1094/MPMI-04-15-0092-R. [DOI] [PubMed] [Google Scholar]
- 299.Hasan M.M., Cha M., Bajpai V.K., Baek K.H. Production of a major stilbene phytoalexin, resveratrol in peanut (Arachis hypogaea) and peanut products: A mini review. Rev. Environ. Sci. Biotechnol. 2013;12:209–221. doi: 10.1007/s11157-012-9294-7. [DOI] [Google Scholar]
- 300.Sobolev V.S. Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. J. Agric. Food Chem. 2008;56:1949–1954. doi: 10.1021/jf703595w. [DOI] [PubMed] [Google Scholar]
- 301.Thakur M., Bhattacharya S., Khosla P.K., Puri S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants. 2019;12:1–12. doi: 10.1016/j.jarmap.2018.11.004. [DOI] [Google Scholar]
- 302.Namdeo A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007;1:69–79. [Google Scholar]
- 303.Tassoni A., Fornalè S., Franceschetti M., Musiani F., Michael A.J., Perry B., Bagni N. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 2005;166:895–906. doi: 10.1111/j.1469-8137.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- 304.Jeong Y.J., Park S.H., Park S.C., Kim S., Kim T.H., Lee J., Kim S.W., Ryu Y.B., Jeong C.J., Kim C.Y. Induced extracellular production of stilbenes in grapevine cell culture medium by elicitation with methyl jasmonate and stevioside. Bioresour. Bioprocess. 2020;7:1–12. doi: 10.1186/s40643-020-00329-3. [DOI] [Google Scholar]
- 305.Delaunois B., Farace G., Jeandet P., Clément C., Baillieul F., Dorey S., Cordelier S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014;21:4837–4846. doi: 10.1007/s11356-013-1841-4. [DOI] [PubMed] [Google Scholar]
- 306.Iriti M., Rossoni M., Borgo M., Faoro F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to Botrytis cinerea. J. Agric. Food Chem. 2004;52:4406–4413. doi: 10.1021/jf049487b. [DOI] [PubMed] [Google Scholar]
- 307.Gil-Muñoz R., Fernández-Fernández J.I., Crespo-Villegas O., Garde-Cerdán T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017;98:34–39. doi: 10.1016/j.foodres.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 308.Donnez D., Jeandet P., Clément C., Courot E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009;27:706–713. doi: 10.1016/j.tibtech.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 309.Almagro L., Carbonell-Bejerano P., Belchí-Navarro S., Bru R., Martínez-Zapater J.M., Lijavetzky D., Pedreño M.A. Dissecting the transcriptional response to elicitors in Vitis vinifera cells. PLoS ONE. 2014;9:e109777. doi: 10.1371/journal.pone.0109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Silva F., Figueiras A., Gallardo E., Nerín C., Domingues F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2014;145:115–125. doi: 10.1016/j.foodchem.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 311.Bru R., Sellés S., Casado-Vela J., Belchí-Navarro S., Pedreño M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006;54:65–71. doi: 10.1021/jf051485j. [DOI] [PubMed] [Google Scholar]
- 312.Martinez-Esteso M.J., Sellés-Marchart S., Vera-Urbina J.C., Pedreño M.A., Bru-Martinez R. Changes of defense proteins in the extracellular proteome of grapevine (Vitis vinifera cv. Gamay) cell cultures in response to elicitors. J. Proteom. 2009;73:331–341. doi: 10.1016/j.jprot.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 313.Martínez-Márquez A., Morante-Carriel J.A., Ramírez-Estrada K., Cusidó R.M., Palazon J., Bru-Martínez R. Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures. Plant Biotechnol. J. 2016;14:1813–1825. doi: 10.1111/pbi.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lambert C., Lemaire J., Auger H., Guilleret A., Reynaud R., Clément C., Courot E., Taidi B. Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. cell suspension from flasks to 20 L bioreactor. Plants. 2019;8:567. doi: 10.3390/plants8120567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Somboon T., Chayjarung P., Pilaisangsuree V., Keawracha P., Tonglairoum P., Kongbangkerd A., Wongkrajang K., Limmongkon A. Methyl jasmonate and cyclodextrin-mediated defense mechanism and protective effect in response to paraquat-induced stress in peanut hairy root. Phytochemistry. 2019;163:11–22. doi: 10.1016/j.phytochem.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 316.Wongshaya P., Chayjarung P., Tothong C., Pilaisangsuree V., Somboon T., Kongbangkerd A., Limmongkon A. Effect of light and mechanical stress in combination with chemical elicitors on the production of stilbene compounds and defensive responses in peanut hairy root culture. Plant Physiol. Biochem. 2020 doi: 10.1016/j.plaphy.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 317.Komaikul J., Kitisripanya T., Likhitwitayawuid K., Sritularak B., Tanaka H., Putalun W. Improvement of stilbenoid production by 2-hydroxypropyl-β-cyclodextrin in white mulberry (Morus alba L.) callus cultures. Nat. Prod. Res. 2019;33:2762–2769. doi: 10.1080/14786419.2018.1499643. [DOI] [PubMed] [Google Scholar]
- 318.Inyai C., Boonsnongcheep P., Komaikul J., Sritularak B., Tanaka H., Putalun W. Alginate immobilization of Morus alba L. cell suspension cultures improved the accumulation and secretion of stilbenoids. Bioprocess Biosyst. Eng. 2019;42:131–141. doi: 10.1007/s00449-018-2021-1. [DOI] [PubMed] [Google Scholar]
- 319.Inyai C., Yusakul G., Komaikul J., Kitisripanya T., Likhitwitayawuid K., Sritularak B., Putalun W. Improvement of stilbene production by mulberry Morus alba root culture via precursor feeding and co-elicitation. Bioprocess Biosyst. Eng. 2020 doi: 10.1007/s00449-020-02474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lanz T., Schröder G., Schröder J. Differential regulation of genes for resveratrol synthase in cell cultures of Arachis hypogaea L. Planta. 1990;181:169–175. doi: 10.1007/BF02411534. [DOI] [PubMed] [Google Scholar]
- 321.Medina-Bolivar F., Condori J., Rimando A.M., Hubstenberger J., Shelton K., O’Keefe S.F., Bennett S., Dolan M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry. 2007;68:1992–2003. doi: 10.1016/j.phytochem.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 322.Guerrero R.F., Cantos-Villar E., Fernández-Marín M.I., Puertas B., Serrano-Albarrán M.J. Optimising UV-C preharvest light for stilbene synthesis stimulation in table grape: Applications. Innov. Food Sci. Emerg. Technol. 2015;29:222–229. doi: 10.1016/j.ifset.2015.02.010. [DOI] [Google Scholar]
- 323.Guerrero R.F., Cantos-Villar E., Puertas B., Richard T. Daily preharvest UV-C light maintains the high stilbenoid concentration in grapes. J. Agric. Food Chem. 2016;64:5139–5147. doi: 10.1021/acs.jafc.6b01276. [DOI] [PubMed] [Google Scholar]
- 324.Błaszczyk A., Sady S., Sielicka M. The stilbene profile in edible berries. Phytochem. Rev. 2019;18:37–67. doi: 10.1007/s11101-018-9580-2. [DOI] [Google Scholar]
- 325.Segade S.R., Vincenzi S., Giacosa S., Rolle L. Changes in stilbene composition during postharvest ozone treatment of ‘Moscato bianco’ winegrapes. Food Res. Int. 2019;123:251–257. doi: 10.1016/j.foodres.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 326.Hasan M., Bashir T., Bae H. Use of ultrasonication technology for the increased production of plant secondary metabolites. Molecules. 2017;22:1046. doi: 10.3390/molecules22071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Potrebko I., Resurreccion A.V. Effect of ultraviolet doses in combined ultraviolet−ultrasound treatments on trans-resveratrol and trans-piceid contents in sliced peanut kernels. J. Agric. Food Chem. 2009;57:7750–7756. doi: 10.1021/jf900667d. [DOI] [PubMed] [Google Scholar]
- 328.Hasan M.M., Baek K.H. Induction of resveratrol biosynthesis in grape skins and leaves by ultrasonication treatment. Korean J. Hort. Sci. Technol. 2013;31:496–502. doi: 10.7235/hort.2013.12229. [DOI] [Google Scholar]
- 329.Yu M., Liu H., Shi A., Liu L., Wang Q. Preparation of resveratrol-enriched and poor allergic protein peanut sprout from ultrasound treated peanut seeds. Ultrason. Sonochem. 2016;28:334–340. doi: 10.1016/j.ultsonch.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 330.Dixon R.A., Paiva N.L. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085. doi: 10.2307/3870059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Herrera J.C., Hochberg U., Degu A., Sabbatini P., Lazarovitch N., Castellarin S.D., Fait A., Alberti G., Peterlunger E. Grape metabolic response to postveraison water deficit is affected by interseason weather variability. J. Agric. Food Chem. 2017;65:5868–5878. doi: 10.1021/acs.jafc.7b01466. [DOI] [PubMed] [Google Scholar]
- 332.Hochberg U., Degu A., Cramer G.R., Rachmilevitch S., Fait A. Cultivar specific metabolic changes in grapevines berry skins in relation to deficit irrigation and hydraulic behavior. Plant Physiol. Biochem. 2015;88:42–52. doi: 10.1016/j.plaphy.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 333.Savoi S., Wong D.C., Degu A., Herrera J.C., Bucchetti B., Peterlunger E., Fait A., Mattivi F., Castellarin S.D. Multi-omics and integrated network analyses reveal new insights into the systems relationships between metabolites, structural genes, and transcriptional regulators in developing grape berries (Vitis vinifera L.) exposed to water deficit. Front. Plant Sci. 2017;8:1124. doi: 10.3389/fpls.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Corso M., Vannozzi A., Maza E., Vitulo N., Meggio F., Pitacco A., Telatin A., D’Angelo M., Feltrin E., Negri A.S., et al. Comprehensive transcript profiling of two grapevine rootstock genotypes contrasting in drought susceptibility links the phenylpropanoid pathway to enhanced tolerance. J. Exp. Bot. 2015;66:5739–5752. doi: 10.1093/jxb/erv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pinasseau L., Vallverdú-Queralt A., Verbaere A., Roques M., Meudec E., Le Cunff L., Péros J.-P., Ageorges A., Sommerer N., Boulet J.-C., et al. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant Sci. 2017;8:1826. doi: 10.3389/fpls.2017.01826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rosemann D., Heller W., Sandermann H. Biochemical plant responses to ozone: II. Induction of stilbene biosynthesis in Scots pine (Pinus sylvestris L.) seedlings. Plant Physiol. 1991;97:1280–1286. doi: 10.1104/pp.97.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Pearce R.B. Effects of exposure to high ozone concentrations on stilbenes in Sitka spruce (Picea sitchensis (Bong.) Carr.) bark and on its lignification response to infection with Heterobasidion annosum (Fr.) Bref. Physiol. Mol. Plant Pathol. 1996;48:117–129. doi: 10.1006/pmpp.1996.0011. [DOI] [Google Scholar]
- 338.Sgarbi E., Fornasiero R.B., Lins A.P., Bonatti P.M. Phenol metabolism is differentially affected by ozone in two cell lines from grape (Vitis vinifera L.) leaf. Plant Sci. 2003;165:951–957. doi: 10.1016/S0168-9452(03)00219-X. [DOI] [Google Scholar]
- 339.Schubert R., Fischer R., Hain R., Schreier P.H., Bahnweg G., Ernst D., Sandermann H., Jr. An ozone-responsive region of the grapevine resveratrol synthase promoter differs from the basal pathogen-responsive sequence. Plant Mol. Biol. 1997;34:417–426. doi: 10.1023/A:1005830714852. [DOI] [PubMed] [Google Scholar]
- 340.Ghimire R.P., Kasurinen A., Häikiö E., Holopainen J.K., Julkunen-Tiitto R., Holopainen T., Kivimäenpää M. Combined effects of elevated ozone, temperature, and nitrogen on stem phenolic concentrations of Scots pine (Pinus sylvestris) seedlings. Can. J. For. Res. 2019;49:246–255. doi: 10.1139/cjfr-2018-0201. [DOI] [Google Scholar]
- 341.Ismail A., Riemann M., Nick P. The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 2012;63:2127–2139. doi: 10.1093/jxb/err426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kostopoulou Z., Therios I., Molassiotis A. Resveratrol and its combination with α-tocopherol mediate salt adaptation in Citrus seedlings. Plant Physiol. Biochem. 2014;78:1–9. doi: 10.1016/j.plaphy.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 343.Cai Z., Kastell A., Speiser C., Smetanska I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl. Biochem. Biotech. 2013;171:330–340. doi: 10.1007/s12010-013-0354-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.