Abstract
Globally, many crop production areas are threatened by drought and salinity. Potato (Solanum tuberosum L.) is susceptible to these challenging environmental conditions. In this study, an in vitro approach was employed to compare the tolerance of potato cultivars ‘BARI-401’ (red skin) and ‘Spunta’ (yellow skin). To simulate ionic and osmotic stress, MS media was supplemented with lithium chloride (LiCl 20 mM) and mannitol (150 mM). GC-MS and spectrophotometry techniques were used to determine metabolite accumulation. Other biochemical properties, such as total phenols concentration (TPC), total flavonoids concentration (TFC), antioxidant capacity (DPPH free radical scavenging capacity), polyphenol oxidase (PPO), and peroxidase (POD) activities, were also measured. The two cultivars respond differently to ionic and osmotic stress treatments, with Spunta accumulating more defensive metabolites in response, indicating a higher level of tolerance. While further investigation of the physiological and biochemical responses of these varieties to drought and salinity is required, the approach taken in this paper provides useful information prior to open field evaluation.
Keywords: drought, salinity, phenols, trehalose, antioxidant, micropropagation, screening
1. Introduction
Potato (Solanum tuberosum L.) is the leading tuber crop [1], with beneficial nutritional impact [2,3], but is very sensitive in terms of food security [4,5]. Potato is grown in relatively cool climates and is sensitive to drought [6] due to its shallow root system [7], moderately tolerant to salinity, although highly sensitive during tubers formation [8,9]. Globally, desertification and salinization are currently affecting about 10% of arable land, which decrease yield more than 50% in major crops [10,11]. Salinity and drought interrupt many physiological and biochemical processes, causing osmotic and oxidative stress, ion imbalance, mineral deficiency, and ion toxicity problems [12]. Hence, understanding of plant tolerance to drought and salinity is a critical research topic [13].
One of the major signatures of abiotic stress leading to yield reduction is the overproduction of reactive oxygen species (ROS) [14]. These ROS include superoxide anions (O2•−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radicals (OH•), which can be damaging and cause different physiological, molecular, and biochemical responses [15]. ROS are also vital signaling molecules that alert plants to adjust their metabolism [16] to adapt to adverse environments. Additionally, production of secondary metabolites like phenols, flavonoids, tannins, saponins, and anthocyanin have been reported in different plant species under abiotic stresses [17]. These alterations may be positive or negative depending on many factors, such as plant species, developmental stage, and stress duration [18].
In response to stress, plants adopt defense strategies such as: ion homeostasis, activation of antioxidant enzymes, and production of different organic compatible solutes [19,20]. Plants tolerant to abiotic stresses can be identified by applying selective agents such as NaCl (for salinity), PEG, or mannitol (for drought) [19]. Lithium (Li), an analogue of sodium, has been used elsewhere for studying ionic toxicity under saline conditions [21]. Successful in vitro selection for drought and salt tolerance using different selection agents has been applied to crops, including potato [12]. As a low tolerance to salinity and drought are major limitations to potato cultivation, development of stress-tolerant varieties is important to enhance crop productivity [19].
Metabolomics is one of the developing “omics” techniques critical to the study of abiotic stress tolerance of crops [22]. It aims to decode, by metabolite determination, the probable effect of abiotic stresses on all dynamic biochemical processes of plant cells [23]. Metabolomics techniques such as gas chromatography-mass spectrometry (GC-MS) are useful to identify, assess, and evaluate the response of important metabolites [24]. In plant abiotic stress research, an important aim is to examine particular metabolites that are critical in tolerance and metabolic variations leading to different responses [25,26]. Different metabolomics approaches have been used to investigate salinity stress in crops, including barley, tomato, maize, and wheat [27,28,29]. Similarly, for drought tolerance, metabolites profiling of various crops such as soybean, barley, wheat, and rice, have been recorded [30,31,32,33,34].
Plant tissue culture approaches can be applied to pinpoint characteristics, and to simplify abiotic stresses experiments in a controlled environment. In vitro techniques can also be used to activate genes already present in plant genome via epigenetic changes [35]. The study presented here screened two potato cultivars: ‘BARI-401’ (red skin) and ‘Spunta’ (yellow skin), for osmotic and ionic stress responses, by in vitro culture using Murashege and Skooge (MS) medium [36], supplemented with 20 mM LiCl and 150 mM mannitol. These experiments may help to identify genotypes with improved ionic and osmotic tolerance less expensively and more time effectively than open field trials [37]. Hence, this work aims to explore the performance of these cultivars by using different selective agents for ionic (LiCl) and osmotic (mannitol) stress response assessed by metabolomics finger printing and biochemical analysis.
2. Results
2.1. Biochemical Analysis under Different Levels of LiCl and Mannitol
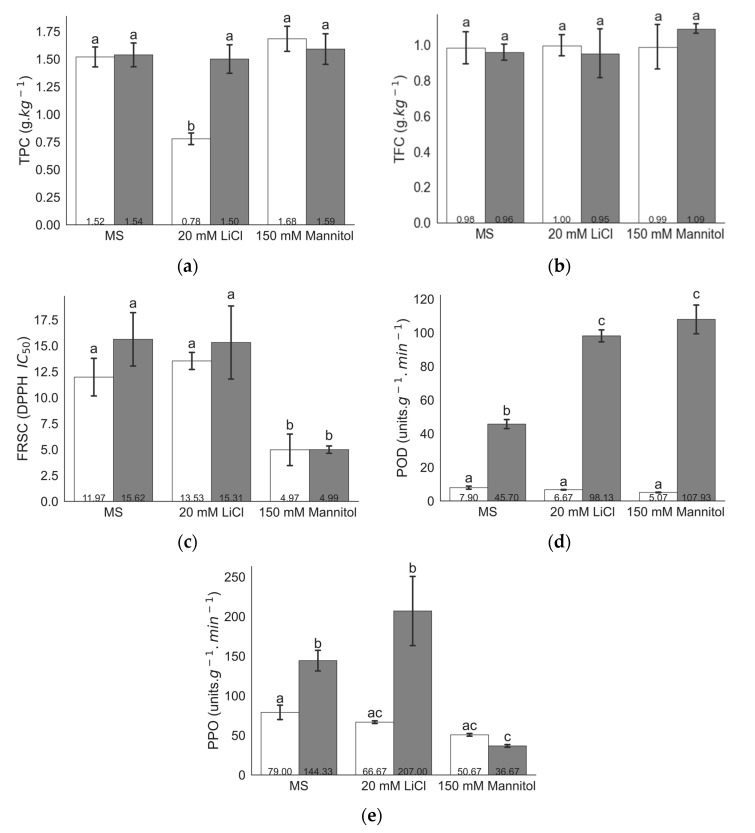
BARI-401 showed lower (p ≤ 0.05) total phenols concentration (TPC) in LiCl treatment compared to control grown plantlet, while TPC was similar to control under mannitol treatment (Figure 1a). The treatments had no significant effect on total flavonoids concentration (TFC) (Figure 1b). Antioxidant capacity measured by DPPH free radical scavenging capacity (FRSC) was higher (lower DPPH, half-maximal inhibitory concentration (IC50) value) in mannitol treatment compared to control and LiCl (Figure 1c). Similarly, activity of antioxidant enzymes POD and PPO activity were lower in mannitol compared to control and LiCl treatment (Figure 1d,e). In Spunta, TPC and TFC were not affected at tested LiCl and mannitol concentrations (Figure 1a,b), however antioxidant capacity measured by DPPH free radical scavenging capacity was higher (lower DPPH IC50 value) in mannitol treatment compared to control and LiCl (Figure 1c). Plantlets treated with either 20 mM LiCl or 150 mM mannitol had increased (p < 0.01) POD activity compared to control and all BARI-401 samples. In 150 mM mannitol, plantlets had reduced (p < 0.01) PPO activity compared with controls (Figure 1d,e).
Figure 1.
Biochemical analysis of cultivars BARI-401 (white) and Spunta (grey) grown on MS with addition of 20 mM LiCl or 150 mM mannitol. (a) Total phenols content (TPC), (b) total flavonoid content (TFC), (c) free radical scavenging capacity (FRSC DPPH IC50 value), (d) polyphenol oxidase (PPO), and (e) peroxidase (POD) activity. Values are means ± standard deviation (SD) (n = 3) with letters indicating significant differences between cultivars and treatments, determined by two-way analysis of variance (ANOVA) and Tukey-Kramer HSD (p ≤ 0.05).
2.2. Changes in Metabolite Profile Induced by LiCl and Mannitol Exposure
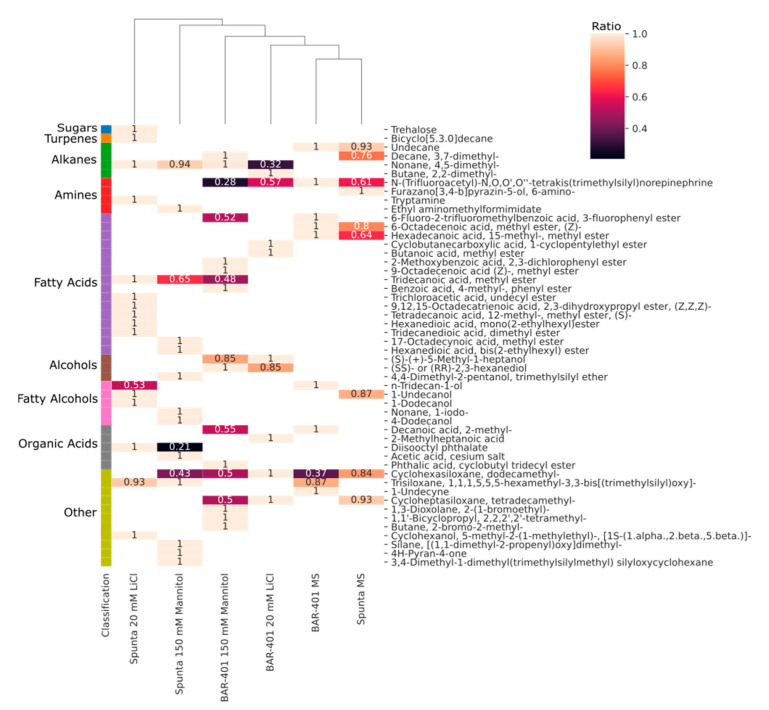
A total of 50 different compounds were identified by GC-MS in methanolic shoots extracts of both varieties (Figure 2) and were classified into 9 groups (Figure 3a,b). Both cultivars exhibited considerable changes in detected compounds and signal intensity in LiCl and mannitol treatments (Supplementary Table S5: Metabolites detected in shoots).
Figure 2.
Heat map and cluster hierarchical analysis of 50 metabolites recorded from methanolic shoots extract of BARI-401 and Spunta cultivated on MS media, with addition of 20 mM LiCl and 150 mM Mannitol. Where detected, compounds are annotated and show color variation according to ratio of signal intensity to the max intensity of each compound amongst treatments.
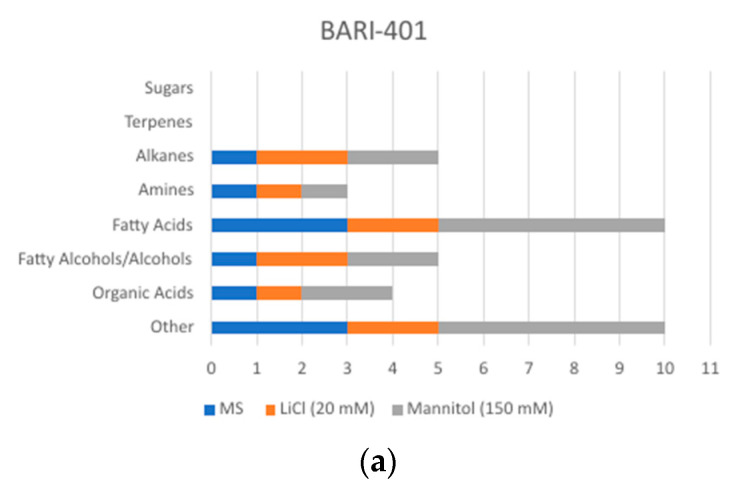
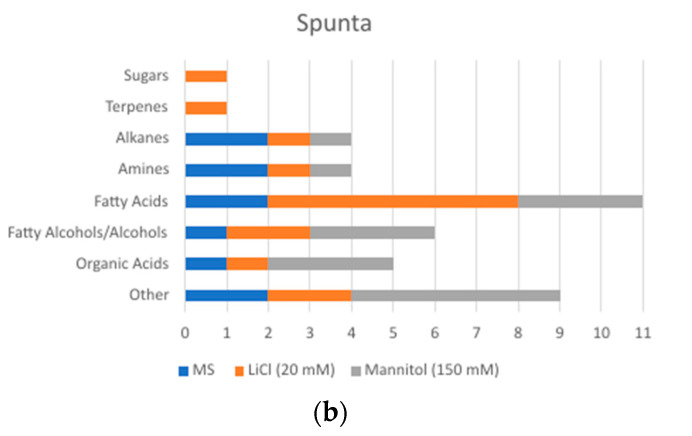
Figure 3.
Distribution of different chemical groups detected by GC-MS in (a) BARI-401 and (b) Spunta cultivated on MS medium (MS) and MS with addition of 20 mM LiCl and 150 mM mannitol.
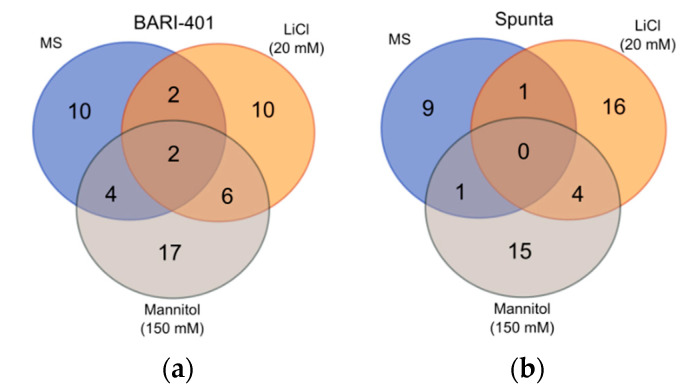
In BARI-401, a total of 30 unique compounds were identified in treated plantlets (10 control, 10 LiCl, and 17 mannitol). LiCl-treated plantlets shared two compounds with control while mannitol shared four compounds with control. Only six compounds were found common between LiCl and mannitol treatments and only two compounds were found in all treatments (Figure 4a). The distribution of different groups of compounds varied with organic acids the highest in mannitol-treated plants, and fatty alcohols/alcohols were higher in mannitol and LiCl treatments than control. Fatty acids were most abundant in mannitol treatment, followed by control and LiCl treatments. Different alkanes (two in LiCl, two in mannitol treatments, and one in control) were also detected, with the amine norepinephrine detected in all the treatments (Figure 4a, and Supplementary Table S5).
Figure 4.
Venn diagram showing number of metabolites produced by BARI-401 (a) and Spunta (b), cultivated on MS medium (MS) and MS with addition of 20 mM LiCl and 150 mM mannitol.
In Spunta, a total of 36 unique compounds were identified (9 control, 16 LiCl, 15 mannitol). LiCl- and mannitol-treated plantlets shared only one compound each with control. Only four compounds were found common between LiCl and mannitol treatments and no compounds were shared by all treatments (Figure 4b). Similar to BARI-401, the mannitol treatment had the highest number of organic acids detected, followed by LiCl. Fatty alcohols/alcohols were also higher in mannitol treatment. Among the treatments, LiCl treatment produced more fatty acids than mannitol and control treatments, respectively. Interestingly, the only sugar, Trehalose, was found in LiCl treatment, along with a terpene, Bicyclo[5.3.0]decane. Alkanes were also found in all treatments (Figure 3b and Supplementary Table S5).
2.3. Principal Component Analysis (PCA) of Treatment Variables
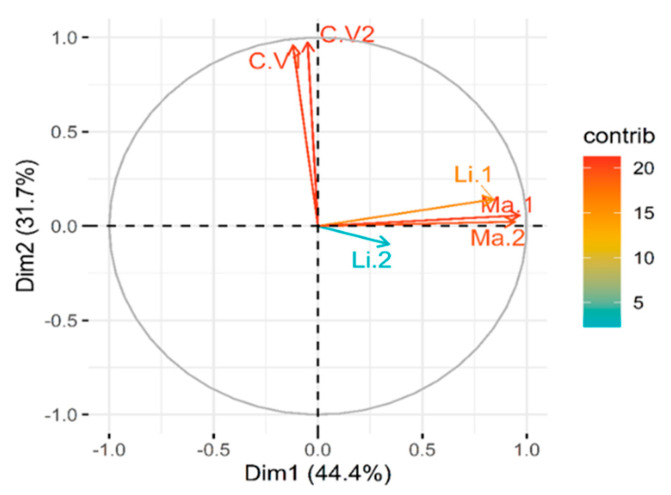
A total of 66% of variance was explained by the two principal components (Dimensions, PC) of six treatment variables: 20 mM LiCl (BARI-401 Li.1 and Spunta Li.2), 150 mM Mannitol (BARI-401 Ma.1 and Spunta Ma.2), and 2 controls (BARI-401 C.V1 and Spunta C.V2) (Figure 5). Four treatment variables: Li.1, Ma.1, Li.2, and Ma.2, are correlated positively with one another. As shown in Figure 5, the arrows of these variables are clustered together, indicating positive correlation. However, these four variables were independent from C.V1 and C.V2.
Figure 5.
Principal component analysis (PCA) for C.V1 (Control BARI-401), C.V2 (Control Spunta), Li.1,2 (LiCl 20 mM BARI-401 and Spunta) and Ma.1,2 (Mannitol 150 mM BARI-401 and Spunta). The circle has a correlation value of 1.0, and arrow lengths are proportional to the correlation coefficient for each variable.
3. Discussion
Screening of in vitro cultures of plant tissues with different selective agents like PEG, mannitol, or sorbitol for drought [19] and NaCl or LiCl for salinity [38] stresses can help to identify plants with desirable tolerance characteristics and to investigate metabolic variations that are induced by abiotic stresses [26], such as the production of reactive oxygen species (ROS) [14], organic solutes [20], and flavonoids [39]. This approach has been previously employed to increase tolerance by selecting explants that survive under these conditions [40].
In the present study, the two cultivars examined respond differently to LiCl stress, with BARI-401 having reduced TPC compared to control treatment, in contrast to Spunta which does not vary (Figure 1a). Antioxidant phenolic compounds play a role as scavengers of ROS in plants [41] and can help provide resistance against both biotic and abiotic stresses [42,43]. Previous studies have observed that high concentration of salts in media have an adverse effect on the concentration of phenolic compounds in two wild relatives of potato, S. stoloniferum and S. bulbosum [39], and in Capsicum annuum [44]. In these wild potato species, salinity stress also did not significantly increase flavonoids concentrations [39], similar to our results. Rousses [45] also observed that total phenolic compounds, flavanols, and polyamines concentrations in Jojoba (Simmondsia chinensis) explants subjected to 50–500 mM mannitol were reduced with increasing levels of stress, possibly due to reduced activity of flavonoids biosynthetic enzymes [13].
The two cultivars also show differences in PPO and POD activity (Figure 1d,e). PPO activity has been proposed to be induced under mild drought stress and declines under high drought stress, as seen in Aeluropus lagopoides [46] and in sugar beet [47]. Increase in PPO activity may also result in degradation of accumulated phenolic compounds [47].
In our experiments, the PPO activity of Spunta is significantly higher in control and LiCl treatments compared to that of BARI-401 and is decreased only under mannitol treatment, while the PPO activity of BARI-401 under both treatments is similar to control levels (Figure 1d). There does not appear to be a correlation between PPO activity and changes in TPC accumulation (Figure 1a). These results suggest that Spunta may be more responsive to stress and potentially more tolerant comparatively.
Despite the differences between PPO and POD activity between the two cultivars, overall, free radical scavenging activity was similar between cultivars, with both BARI-401 and Spunta showing increased FRSC under 150 mM mannitol. Increased antioxidant capacity of both cultivars under mannitol suggests that these cultivars have a similar response to osmotic stress. Variation in non-enzymatic antioxidant capacity has previously been seen in potato [39]. Other experiments on free radical scavenging ability of extracts of germinating grapes seeds under osmotic stress were shown to be weak but suggest that phenolic content and antioxidant capacity are positively correlated [48,49]. Differences in antioxidant activity may related to degree of stomatal closure or other responses that change the rate of CO2 fixation [50]. Additionally, this increased free radical scavenging may be contributed by other compounds such as carotenoids [51].
Drought and salinity stress may increase the accumulation of ROS and induce detoxification responses of plants, such as increased production of ROS scavenger enzymes like CAT, POX, POD, APX, PPO, and SOD [52]. It has been observed in cotton that salinity stress induces ROS scavenging enzymes in salt-tolerant cultivars but is unchanged or reduced in non-tolerant cultivars [53,54,55]. Similarly, the role of POD and CAT in ROS detoxification was found to vary in potato cultivars and suggests that the importance of ROS detoxification as a tolerance mechanism is cultivar-dependent [56]. Similarly, Demirel et al. [7] reports relatively unchanged antioxidant enzymes activities in the sensitive potato cultivar ‘Agria’ when exposed to stress.
Plant responses to abiotic stresses involve post-translational changes in proteins leading to modification and accumulation of various metabolites, resulting in specific physiological responses [57]. Osmotic adjustment by accumulation of ions and compatible solutes to combat osmotic effects [58] is a basic mechanism to protect plants under abiotic stress [59].
Although limited, the metabolomics fingerprinting by GC-MS of the two cultivars in this study revealed variations both between cultivars and between LiCl and mannitol treatments (Figure 2), potentially indicating differences in their stress tolerance ability. The metabolites of the six different variables (two varieties by three treatments) submitted to PCA separated along two principal components, showing almost 66% of the experimental variation (Figure 5). This analysis shows that control treatments are negatively correlated to the stress treatments, supporting that variation in metabolites detected between treatments exist. Based on the hierarchical clustering (Figure 2) and examining the variations in signal intensity, Spunta under LiCl and mannitol treatments varies more from both control grown plantlets and BARI-401 under all conditions. Although, without appropriate internal standardization, it is difficult to compare signal intensities, and these results indicate that Spunta responds to stress by inducing more changes in metabolite composition than BARI-401.
Several notable differences in metabolite accumulation between the two cultivars deserve mention. In Spunta, trehalose sugar was detected in 20 mM LiCl treatment. Previously, trehalose has been detected by GC-MS in soil grown potato tubers [60] and in Arabidopsis [61]. Stress-tolerant plants are known to accumulate non-reducing disaccharides like trehalose when exposed to stress [62]. Increased level of trehalose sugar has been recorded in wheat cultivars under salinity and drought [63]. Likewise, increased trehalose has been observed in maize plants’ leaves, cob, and at the silking stage [64], and in rice plants [65] under salinity treatments. Transgenic expression of microbial trehalose biosynthesis genes in tobacco [66,67,68], rice [69,70], tomato [71], and potato [72], can also improve stress tolerance. Trehalose itself is an important osmolyte and osmo-protectant [73,74] and may reduce the permeability of salt by maintaining the integrity of plasma membranes or play a role as an antioxidant [75].
Carbohydrate metabolism is directly associated with photosynthetic activities and plays an important role in stress tolerance. Plants utilize starch and fructans as an energy source in stress conditions rather than glucose [76,77]. Trehalose-6-phosphate (T6P) in plastids regulates photosynthesis and starch production [78,79]. Starch synthesis begins through activation of ADP-gluscose pyrophosphorylase (AGPase) via posttranslational redox modification by thioredoxin dependent on SNF1-related kinase (SnRK1) expression [80] (Supplementary Figure S1). Approximately 1000 genes in Arabidopsis have been shown to respond to SnRK1 control [81]. Consequently, variations in trehalose and T6P concentrations impact many biological functions, including adaptive stress responses [82]. The detection of trehalose in LiCl-treated Spunta plantlets may indicate an adaptive stress response not present in BARI-401.
Our study also indicated an increased presence of saturated fatty acids like myristic acid (Tetradecanoic acid, 12-methyl-, methyl ester, (S)-) and Stearic acid (17-Octadecynoic acid, methyl ester) in LiCl and mannitol treatments, especially in Spunta (Figure 3b). Unsaturated fatty acids composition also increased with α-lenolenic acid (9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester) produced in Spunta under LiCl treatment. Fatty acids, like suberin and cutin, are important extracellular lipid polymers that safeguard against adverse environmental conditions through reshaping membrane fluidity [83]. Our results are similar to those reported by Khalid et al., in which rice cell cultures adapted to 25 mM LiCl have increased saturated fatty acids levels compared to un-adapted cultures [84]. An increase in unsaturated fatty acids has also been observed in Suaeda salsa L. [85], safflower [86], Brassica olearacea [87], and Arabidopsis [88] under salt stress. Overexpression of ω-3 desaturases, which increase C18:3 fatty acid composition, also increase salt and drought stress tolerance in tobacco [89]. The inherent level of fatty acids’ unsaturation is important in salt and drought stress tolerance [90]. Elevated concentrations of saturated fatty acids play a role in membrane fluidity reduction, which reduces the flow of ions through the membrane [91]. Conversely, unsaturated fatty acids are negative regulators of membrane fluidity [84]. The capability for adjusting membrane lipid fluidity through altering fatty acids levels is important in acclimation to stress and mainly dependent on activity of fatty acids desaturases [92].
Finally, several alkanes were detected in the two varieties in this study. The wax structure of leaf cuticle is predominated by alkanes (50–70%) [93] and plays an important role in resistance to drought [94]. The changes in alkane biosynthesis indicated by our results may reflect alteration of the wax compositions of leaf cuticles in response to stress, although this needs to be examined further.
The differences in metabolite composition, in particular the presence of saturated fatty acids and trehalose accumulation, suggests that Spunta may have a higher capacity for tolerance to LiCl and mannitol stress than BARI-401. Conversely, the fewer changes in BARI-401 metabolites compared to control grown plants may indicate a higher tolerance as tolerance mechanisms are not yet induced.
4. Materials and Methods
4.1. In Vitro Potato Plantlets Growth and Treatment with LiCl and Mannitol
Tubers of Solanum tuberosum L. cultivars BARI-401 and Spunta were sourced from Astra Food Company Ltd., Tabuk, Saudi Arabia. Plantlets were grown from healthy and homogeneous tuber sprouts. After sterilization with 70% v/v ethanol and 20% v/v commercial bleach [35], sprouts were cultured in Duran poly-carbonated tissue culture bottles (Duran® Schott, Germany) containing 50 mL autoclaved (15 min at 121 °C and 15 psi) MS medium [33] with phytagel 4 gL−1, sucrose 30 gL−1, 6-Benzylaminopurin (BAP) 2 mgL−1, Indolebutyric acid (IBA) 1 mgL−1, and 0.25 mgL−1 Gibberellic acid (GA3) [36]. The pH of the media was adjusted to 5.7–5.8 by using 0.1M HCL.
Stem nodal segments were sub-cultured every 4 weeks to obtain sufficient plantlets for experiments. For stress treatments, randomly selected uniform plantlets of each cultivar were transferred to MS medium (control) and MS with addition of 20 mM LiCl (LiCl treatment) [38] or 150 mM mannitol (mannitol treatment) [95]. Plant growth regulators BAP 2 mgL−1, IBA 1 mgL−1, and 0.25 mgL−1 GA3 [96] were supplemented in all media. Plantlets were grown for 45 days before harvest and further analysis.
4.2. Sample Preparation, Extraction, and Biochemical Analysis of Potato Plantlets
4.2.1. Extract Preparation for Total Phenols (TPC), Total Flavonoids (TFC), and Antioxidant Activity
Potato fresh shoots (2 g/sample) were selected randomly and extraction was done as per Awad et al. [51]. 20 mL of 80% methanol was mixed with sample and shaken at 150 rpm for 12 h, then was filtered with Whatman® filter paper No. 1 at room temperature.
4.2.2. Methanol Total Phenols Concentration (TPC) Estimation
The TPC was measured following Hoff and Singleton’s [97] protocol. A mixture of 50 µL methanolic extract, 100 µL of Folin-Ciocalteu reagent, and 850 µL of methanol was prepared and kept at 23 + 1 °C for 5 min. Sodium carbonate (20% w/v) was then added to the mixture and left for 30 min to react. TPC absorbency was measured at 750 nm. Results were expressed in g.kg−1 fresh weight (FW) Gallic acid equivalent after quantification from the calibration curve obtained from gallic acid absorbance at known concentrations.
4.2.3. Total Flavonoids Concentration (TFC) Estimation
The TFC was measured by a revised colorimetric method as described by Zhishen et al. [98]. 250 µL of methanolic extract was mixed with 1.25 mL of water and 75 µL of 5% w/v NaNO2. The solution was held for 6 min before mixing with 150 µL 10% w/v AlCl3, 0.5 mL NaOH (1 M), and 275 µL distilled water. Absorbance at 510 nm was recorded for total flavonoids. The calibration curve was obtained from the absorbance of known concentrations of catechin for quantification of total flavonoids and the results were given as g.kg−1 FW catechin equivalent.
4.2.4. Antioxidant Capacity by DPPH Free Radical Scavenging Capacity (FRSC) Assay
Methanolic extract of in vitro potato shoots were analyzed for free radical scavenging activity in DPPH (1,1-diphenyl-2-picrylhy-drazyl) methanol [42]. 0.1 mL of methanolic extract and 0.9 mL of fresh DPPH methanol solution (0.1 mM) were mixed. As a control, the same quantity of methanol was used. After dark incubation at room temperature for 30 min, the absorbance was noted at 517 nm. Percent scavenging activity was calculated by the following formula:
The dose response curves were used to calculate IC50 (inhibition concentration) values.
4.2.5. Enzymes Activity Evaluation
To prepare crude enzyme extract, 1 g of shoot samples was homogenized with Tris-HCl (20 mM) buffer (pH 7.2) then centrifuged at 10,000 rpm for 10 min at 4 °C [39]. The supernatant was stored at −20 °C prior to peroxidase (POD) and polyphenol oxidase (PPO) assays.
Peroxidase (POD) Assay
Peroxidase (EC 1.11.1.7) activity was examined as described by Mar’ia and Cascone [99] and Awad et al. [51]. The reaction mixture consisted of 1000:10 µL of H2O2 (0.97 M), 80 µL of guaiacol (0.5 M) respectively, 250 µL of sodium acetate buffer (pH 5.5), and 50 µL crude extract. After one minute to allow for guaiacol oxidation, absorbance at 470 nm was recorded. Per unit activity of enzyme is the quantity of enzyme required for 1.0 O.D. min−1 change under standard assay conditions.
Polyphenol Oxidase (PPO) Assay
Polyphenol oxidase (EC 1.14.18.1) activity was examined by catechol substrate following the methodology of Jiang et al. [100]. 200 µL of crude extract was mixed with 2800 µL of catechol (20 mM) solution in 0.01 M sodium phosphate buffer (pH 6.8). An absorbance increase at 400 nm was recorded over 3 min. Results are expressed as per unit activity of enzyme required for 0.1 O.D. min−1 change under standard assay conditions.
4.3. Potato Shoots Sample Preparation for GC-MS Metabolites Analysis
Shoot samples were prepared for metabolite analysis following the method of Roessner et al. [60]. Three independent biological replicates were prepared from each treatment (mannitol, LiCl, and control) for both cultivars (18 samples total). Shoots were harvested, snap-frozen in liquid nitrogen, and stored at −80 °C. 100 mg of frozen sample was ground by mortar and pestle into a fine powder in liquid nitrogen and extracted in 1.4 mL methanol.
Due to constraints, only one biological/technical replicate of each cultivar and treatment combination was examined by GC-MS. Sample methanol extracts (1 µL) were injected with an automatic sampler and separated on a QP2010 plus series GC fixed with a split/splitless injector port and determined with a FID QP 2010 plus mass selective detector. Rtx 5 ms 0.25 mm ID (column) and 0.25 µm film thickness (df) (Shimadzu, Japan) was used to perform GC. The injector temperature was 250 °C and the flow rate of helium carrier gas was at 1 mL.min−1. Ion source temperature was 210 °C and interface temperature was kept at 250 °C. Temperature of the oven was at isothermal 60 °C for five minutes followed by a stepwise rise at 5 °C.min−1 until the oven temperature touched 300 °C and held for a further minute. Before the next injection, temperature was equilibrated at 60 °C for 6 min. At 3 scans.s−1, mass spectra were recorded in the 50–500 m/z range. Peaks were identified by the automated mass spectral deconvolution and identification program (AMDIS) and the National Institute of Standards and Technology (NIST library, version v. 2.0 f).
4.4. Experimental Design and Statistical Analysis
For the experiment, a completely randomized design (CRD) was used, with two varieties and three treatments: MS medium (control), MS with 20 mM LiCl, and MS with 150 mM mannitol. Three biological replications were conducted per treatment. The results were analyzed by analysis of variance (ANOVA) using Python (version 3.6.3). The Tukey-Kramer HSD test was used to separate the differences between treatments at probability level p ≤ 0.05. Moreover, to examine the relationships between the six different variables, principal component analysis (PCA) was performed by R studio (version 1.2.5033) software. Scripts used in analysis available via Github (https://github.com/gbwellman/2020PotatoGCMSanalysis). Venn diagrams were generated using the online tool provided by Ghent University, Bioinformatics and Evolutionary genomics group (http://bioinformatics.psb.ugent.be/webtools/Venn/).
5. Conclusions
This study examined the response of two potato cultivars to ionic and osmotic stress in vitro. The results discussed above indicate that Spunta shows a greater response to ionic and osmotic stress than BARI-401 by increased POD/PPO activity, reduced ROS production, trehalose accumulation, and increased saturated fatty acids composition. The interplay between TPC and free radical scavenging activity in BARI-401 and Spunta under mannitol stress needs further examination.
The GC-MS results identify multiple compounds that may be involved in stress responses or tolerance mechanisms and warrant further study. Further validation, both by in planta testing and growth under field conditions [101], is required to determine the impact of these differences on the abiotic stress tolerance of these two cultivars. The in vitro approach used in this study, with the novel insights provided by GC-MS, may be used as a preliminary trial prior to field evaluation [12,102]. Identification of traits correlated to increased ionic and osmotic tolerance, such as POD/PPO activity or trehalose accumulation, will allow this approach to rapidly screen potato genotypes to assist in selection and breeding of potato cultivars with improved tolerance.
Acknowledgments
The authors are grateful to the Deanship of Graduate Studies (DGS), king Abdulaziz University, for endowment of the Ph.D. scholarship under student ID. No 1801819. Moreover, the authors acknowledge the moral and technical support from the Department of Arid Land Agriculture.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/10/1/98/s1, Table S1: Representing different metabolites in BARI-401. Table S2: Representing different metabolites in BARI-401. Table S3: Representing different metabolites in Spunta. Table S4: Representing different metabolites in Spunta. Table S5: Metabolites detected in shoots. Figure S1: Trehalose pathway role in eukaryotes. Trehalose-6-phosphate (T6P) controls sugar metabolism and plant development. Trehalose and glucose are also responsible for many signaling and regulatory pathways and integrate external cues to adapt cells to abiotic stress, growth, and development The diagram is adapted from Reference [103]. Metabolites detected in shoots. Figure S2: GC-MS chromatography intensity readings of cv. BARI-401. Control (top), LiCl 20 mM (middle), Mannitol 150 mM (bottom). Figure S3: GC-MS chromatography absolute intensity readings of cv. Spunta. Control (top), LiCl 20 mM (middle), Mannitol 150 mM (bottom).
Author Contributions
Conceptualization, F.A.S. and M.A.A.M.; methodology, F.A.S. and M.A.A.M.; software, F.A.S. and G.W.; validation, F.A.S., M.A.A.M. and B.T.H.; formal analysis, F.A.S. and G.W.; investigation, F.A.S.; resources, M.A.A.M. and B.T.H.; data curation, F.A.S.; writing—original draft preparation, F.A.S. and M.A.A.M.; writing—review and editing, F.A.S. and G.W.; visualization, F.A.S. and G.W.; supervision, M.A.A.M. and B.T.H.; project administration, M.A.A.M. and B.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This Ph.D. research work was supported by the Deanship of Graduate studies, King Abdulaziz University, Jeddah, and Ministry of Education, Kingdom of Saudi Arabia. This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.13518899.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albiski F., Najla S., Sanoubar R., Alkabani N., Murshed R. In Vitro Screening of Potato Lines for Drought Tolerance. Physiol. Mol. Biol. Plants. 2012;18:315–321. doi: 10.1007/s12298-012-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birch P.R., Bryan G., Fenton B., Gilroy E.M., Hein I., Jones J.T., Prashar A., Taylor M.A., Torrance L., Toth I.K. Crops That Feed the World 8: Potato: Are the Trends of Increased Global Production Sustainable? Food Secur. 2012;4:477–508. doi: 10.1007/s12571-012-0220-1. [DOI] [Google Scholar]
- 3.Vaitkevičienė N., Kulaitienė J., Jarienė E., Levickienė D., Danillčenko H., Średnicka-Tober D., Rembiałkowska E., Hallmann E. Characterization of Bioactive Compounds in Colored Potato (Solanum Tuberosum L.) Cultivars Grown with Conventional, Organic, and Biodynamic Methods. Sustainability. 2020;12:2701. doi: 10.3390/su12072701. [DOI] [Google Scholar]
- 4.Brown C.R. Antioxidants in Potato. Am. J. Potato Res. 2005;82:163–172. doi: 10.1007/BF02853654. [DOI] [Google Scholar]
- 5.Kazimierczak R., Średnicka-Tober D., Hallmann E., Kopczyńska K., Zarzyńska K. The Impact of Organic vs. Conventional Agricultural Practices on Selected Quality Features of Eight Potato Cultivars. Agronomy. 2019;9:799. doi: 10.3390/agronomy9120799. [DOI] [Google Scholar]
- 6.Monneveux P., Ramírez D.A., Pino M.-T. Drought Tolerance in Potato (S. Tuberosum L.): Can We Learn from Drought Tolerance Research in Cereals? Plant Sci. 2013;205:76–86. doi: 10.1016/j.plantsci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Demirel U., Morris W.L., Ducreux L.J., Yavuz C., Asim A., Tindas I., Campbell R., Morris J.A., Verrall S.R., Hedley P.E. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020;11:169. doi: 10.3389/fpls.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katerji N., Van Hoorn J.W., Hamdy A., Mastrorilli M. Salt Tolerance Classification of Crops According to Soil Salinity and to Water Stress Day Index. Agric. Water Manag. 2000;43:99–109. doi: 10.1016/S0378-3774(99)00048-7. [DOI] [Google Scholar]
- 9.Teixeira J., Pereira S. High Salinity and Drought Act on an Organ-Dependent Manner on Potato Glutamine Synthetase Expression and Accumulation. Environ. Exp. Bot. 2007;60:121–126. doi: 10.1016/j.envexpbot.2006.09.003. [DOI] [Google Scholar]
- 10.Boyer J.S. Plant Productivity and Environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 11.Bray E.A. Plant Responses to Water Deficit. Trends Plant Sci. 1997;2:48–54. doi: 10.1016/S1360-1385(97)82562-9. [DOI] [Google Scholar]
- 12.Pérez-Clemente R.M., Gómez-Cadenas A. In Vitro Tissue Culture, a Tool for the Study and Breeding of Plants Subjected to Abiotic Stress Conditions. Recent Adv. Plant In Vitro Cult. 2012:91–108. doi: 10.5772/50671. [DOI] [Google Scholar]
- 13.Bartels D., Sunkar R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- 14.You J., Chan Z. ROS Regulation during Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noctor G., Foyer C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 16.Hancock J.T. Cell Signalling. Oxford University Press; Oxford, UK: 2017. [Google Scholar]
- 17.Jamil M., Rha E.S. NaCl Stress-Induced Reduction in Grwoth, Photosynthesis and Protein in Mustard. J. Agric. Sci. 2013;5:114. doi: 10.5539/jas.v5n9p114. [DOI] [Google Scholar]
- 18.Abdel-Farid I.B., Marghany M.R., Rowezek M.M., Sheded M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis Sativus and Solanum Lycopersicum. Plants. 2020;9:1626. doi: 10.3390/plants9111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai M.K., Kalia R.K., Singh R., Gangola M.P., Dhawan A.K. Developing Stress Tolerant Plants through in Vitro Selection—An Overview of the Recent Progress. Environ. Exp. Bot. 2011;71:89–98. doi: 10.1016/j.envexpbot.2010.10.021. [DOI] [Google Scholar]
- 20.Zhu J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama H., Horie T., Yonamine I., Shinmyo A., Yoshida K. Improving Salt Tolerance in Plant Cells. Plant Biotechnol. 2005;22:477–487. doi: 10.5511/plantbiotechnology.22.477. [DOI] [Google Scholar]
- 22.Shulaev V., Cortes D., Miller G., Mittler R. Metabolomics for Plant Stress Response. Physiol. Plant. 2008;132:199–208. doi: 10.1111/j.1399-3054.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Cela E., Kiaitsi E., Medina A., Sulyok M., Krska R., Magan N. Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. Graminearum. Toxins. 2018;10:56. doi: 10.3390/toxins10020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Che-Othman M.H., Jacoby R.P., Millar A.H., Taylor N.L. Wheat Mitochondrial Respiration Shifts from the Tricarboxylic Acid Cycle to the GABA Shunt under Salt Stress. New Phytol. 2020;225:1166–1180. doi: 10.1111/nph.15713. [DOI] [PubMed] [Google Scholar]
- 25.Khan F., Fuentes D., Threthowan R., Mohammad F., Ahmad M. Comparative Metabolite Profiling of Two Wheat Genotypes as Affected by Nitrogen Stress at Seedling Stage. J. Anim. Plant Sci. 2019;29:260–268. [Google Scholar]
- 26.Yang L., Fountain J.C., Ji P., Ni X., Chen S., Lee R.D., Kemerait R.C., Guo B. Deciphering Drought-induced Metabolic Responses and Regulation in Developing Maize Kernels. Plant Biotechnol. J. 2018;16:1616–1628. doi: 10.1111/pbi.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrelli G.M., Fragasso M., Nigro F., Platani C., Papa R., Beleggia R., Trono D. Analysis of Metabolic and Mineral Changes in Response to Salt Stress in Durum Wheat (Triticum Turgidum ssp. Durum) Genotypes, Which Differ in Salinity Tolerance. Plant Physiol. Biochem. 2018;133:57–70. doi: 10.1016/j.plaphy.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Rouphael Y., Raimondi G., Lucini L., Carillo P., Kyriacou M.C., Colla G., Cirillo V., Pannico A., El-Nakhel C., De Pascale S. Physiological and Metabolic Responses Triggered by Omeprazole Improve Tomato Plant Tolerance to NaCl Stress. Front. Plant Sci. 2018;9:249. doi: 10.3389/fpls.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zörb C., Geilfus C.-M., Mühling K.H., Ludwig-Müller J. The Influence of Salt Stress on ABA and Auxin Concentrations in Two Maize Cultivars Differing in Salt Resistance. J. Plant Physiol. 2013;170:220–224. doi: 10.1016/j.jplph.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Degenkolbe T., Do P.T., Kopka J., Zuther E., Hincha D.K., Köhl K.I. Identification of Drought Tolerance Markers in a Diverse Population of Rice Cultivars by Expression and Metabolite Profiling. PLoS ONE. 2013;8:e63637. doi: 10.1371/journal.pone.0063637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hein J.A., Sherrard M.E., Manfredi K.P., Abebe T. The Fifth Leaf and Spike Organs of Barley (Hordeum Vulgare L.) Display Different Physiological and Metabolic Responses to Drought Stress. BMC Plant Biol. 2016;16:248. doi: 10.1186/s12870-016-0922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang Z., Babar M.A., Khan N., Guo J., Khan J., Islam S., Shrestha S., Shahi D. Comparative Metabolomic Profiling in the Roots and Leaves in Contrasting Genotypes Reveals Complex Mechanisms Involved in Post-Anthesis Drought Tolerance in Wheat. PLoS ONE. 2019;14:e0213502. doi: 10.1371/journal.pone.0213502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvente S., Sobolev A.P., Lara M. Metabolite Adjustments in Drought Tolerant and Sensitive Soybean Genotypes in Response to Water Stress. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witt S., Galicia L., Lisec J., Cairns J., Tiessen A., Araus J.L., Palacios-Rojas N., Fernie A.R. Metabolic and Phenotypic Responses of Greenhouse-Grown Maize Hybrids to Experimentally Controlled Drought Stress. Mol. Plant. 2012;5:401–417. doi: 10.1093/mp/ssr102. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki M., Paszkowski J. Identification of Genes Preventing Transgenerational Transmission of Stress-Induced Epigenetic States. Proc. Natl. Acad. Sci. USA. 2014;111:8547–8552. doi: 10.1073/pnas.1402275111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murashige T., Skoog F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 37.Gopal J., Iwama K. In Vitro Screening of Potato against Water-Stress Mediated through Sorbitol and Polyethylene Glycol. Plant Cell Rep. 2007;26:693–700. doi: 10.1007/s00299-006-0275-6. [DOI] [PubMed] [Google Scholar]
- 38.Shah A.H., Shah S.H., Ahmad H., Baig A., Swati Z.A., Aiman U., Din I., Khalid Q. Co Adaptation of LiCl Tolerant Solanum Tuberosum L. Callus Cultures to NaCl Stress. Afr. J. Biotechnol. 2011;10:13444–13452. [Google Scholar]
- 39.Daneshmand F., Arvin M.J., Kalantari K.M. Physiological Responses to NaCl Stress in Three Wild Species of Potato in Vitro. Acta Physiol. Plant. 2010;32:91. doi: 10.1007/s11738-009-0384-2. [DOI] [Google Scholar]
- 40.Purohit M., Srivastava S., Srivastava P.S. Stress Tolerant Plants through Tissue Culture. In: Srivastava P.S., editor. Plant Tissue Culture and Molecular Biology: Application and Prospects. Narosa Publishing House; New Delhi, India: 1998. pp. 554–578. [Google Scholar]
- 41.Solecka D. Role of Phenylpropanoid Compounds in Plant Responses to Different Stress Factors. Acta Physiol. Plant. 1997;19:257–268. doi: 10.1007/s11738-997-0001-1. [DOI] [Google Scholar]
- 42.Boo H.-O., Hwang S.-J., Bae C.-S., Park S.-H., Heo B.-G., Gorinstein S. Extraction and Characterization of Some Natural Plant Pigments. Ind. Crops Prod. 2012;40:129–135. doi: 10.1016/j.indcrop.2012.02.042. [DOI] [Google Scholar]
- 43.Korayem A.M., El-Bassiouny H.M.S., El-Monem A.A.A., Mohamed M.M.M. Physiological and Biochemical Changes in Different Sugar Beet Genotypes Infected with Root-Knot Nematode. Acta Physiol. Plant. 2012;34:1847–1861. doi: 10.1007/s11738-012-0983-1. [DOI] [Google Scholar]
- 44.Navarro J.M., Flores P., Garrido C., Martinez V. Changes in the Contents of Antioxidant Compounds in Pepper Fruits at Different Ripening Stages, as Affected by Salinity. Food Chem. 2006;96:66–73. doi: 10.1016/j.foodchem.2005.01.057. [DOI] [Google Scholar]
- 45.Roussos P.A. Growth and Biochemical Responses of Jojoba (Simmondsia Chinensis (Link) Schneid) Explants Cultured under Mannitol-Simulated Drought Stress in vitro. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013;147:272–284. doi: 10.1080/11263504.2013.768558. [DOI] [Google Scholar]
- 46.Vaziri A., Motamed N., Naghavi M.R., Niknam V.N. Physiological and Biochemical Responses of Aeluropus Lagopoides and Aeluropus Littoralis to Drought Stress. Open Access J. Med. Aromat. Plants. 2011;2:5. [Google Scholar]
- 47.Sen A., Alikamanoglu S. Antioxidant Enzyme Activities, Malondialdehyde, and Total Phenolic Content of PEG-Induced Hyperhydric Leaves in Sugar Beet Tissue Culture. Vitro Cell. Dev. Biol. Plant. 2013;49:396–404. doi: 10.1007/s11627-013-9511-2. [DOI] [Google Scholar]
- 48.Weidner S., Karamać M., Amarowicz R., Szypulska E., Gołgowska A. Changes in Composition of Phenolic Compounds and Antioxidant Properties of Vitis Amurensis Seeds Germinated under Osmotic Stress. Acta Physiol. Plant. 2007;29:283–290. doi: 10.1007/s11738-007-0035-4. [DOI] [Google Scholar]
- 49.Weidner S., Karolak M., Karamac M., Kosinska A., Amarowicz R. Phenolic Compounds and Properties of Antioxidants in Grapevine Roots [Vitis Vinifera L.] under Drought Stress Followed by Recovery. Acta Soc. Bot. Pol. 2009;78:97–103. doi: 10.5586/asbp.2009.013. [DOI] [Google Scholar]
- 50.Munns R., Tester M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 51.Awad M.A., Al-Qurashi A.D., Mohamed S.A., El-Shishtawy R.M., Ali M.A. Postharvest Chitosan, Gallic Acid and Chitosan Gallate Treatments Effects on Shelf Life Quality, Antioxidant Compounds, Free Radical Scavenging Capacity and Enzymes Activities of ‘Sukkari’Bananas. J. Food Sci. Technol. 2017;54:447–457. doi: 10.1007/s13197-016-2481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowler C., van Montagu M., Inze D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Biol. 1992;43:83–116. doi: 10.1146/annurev.pp.43.060192.000503. [DOI] [Google Scholar]
- 53.Gossett D.R., Millhollon E.P., Lucas M. Antioxidant Response to NaCl Stress in Salt-Tolerant and Salt-Sensitive Cultivars of Cotton. Crop Sci. 1994;34:706–714. doi: 10.2135/cropsci1994.0011183X003400030020x. [DOI] [Google Scholar]
- 54.Gossett D.R., Banks S.W., Millhollon E.P., Lucas M.C. Antioxidant Response to NaCl Stress in a Control and an NaCl-Tolerant Cotton Cell Line Grown in the Presence of Paraquat, Buthionine Sulfoximine, and Exogenous Glutathione. Plant Physiol. 1996;112:803–809. doi: 10.1104/pp.112.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meloni D.A., Oliva M.A., Martinez C.A., Cambraia J. Photosynthesis and Activity of Superoxide Dismutase, Peroxidase and Glutathione Reductase in Cotton under Salt Stress. Environ. Exp. Bot. 2003;49:69–76. doi: 10.1016/S0098-8472(02)00058-8. [DOI] [Google Scholar]
- 56.Rahnama H., Ebrahimzadeh H. The Effect of NaCl on Antioxidant Enzyme Activities in Potato Seedlings. Biol. Plant. 2005;49:93–97. doi: 10.1007/s10535-005-3097-4. [DOI] [Google Scholar]
- 57.Verslues P.E., Agarwal M., Katiyar-Agarwal S., Zhu J., Zhu J.-K. Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses That Affect Plant Water Status. Plant J. 2006;45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 58.Visser E.J., Voesenek L.A. Root Physiology: From Gene to Function. Springer; Dordrech, The Netherlands: 2005. Acclimation to soil flooding—sensing and signal-transduction; pp. 197–214. [Google Scholar]
- 59.Bohnert H.J., Jensen R.G. Strategies for Engineering Water-Stress Tolerance in Plants. Trends Biotechnol. 1996;14:89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- 60.Roessner U., Wagner C., Kopka J., Trethewey R.N., Willmitzer L. Simultaneous Analysis of Metabolites in Potato Tuber by Gas Chromatography–Mass Spectrometry. Plant J. 2000;23:131–142. doi: 10.1046/j.1365-313x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- 61.Vogel G., Fiehn O., Jean-Richard-dit-Bressel L., Boller T., Wiemken A., Aeschbacher R.A., Wingler A. Trehalose Metabolism in Arabidopsis: Occurrence of Trehalose and Molecular Cloning and Characterization of Trehalose-6-phosphate Synthase Homologues. J. Exp. Bot. 2001;52:1817–1826. doi: 10.1093/jexbot/52.362.1817. [DOI] [PubMed] [Google Scholar]
- 62.Xu G., Liu D., Chen J., Ye X., Shi J. Composition of Major Flavanone Glycosides and Antioxidant Capacity of Three Citrus Varieties. J. Food Biochem. 2009;33:453–469. doi: 10.1111/j.1745-4514.2009.00230.x. [DOI] [Google Scholar]
- 63.El-Bashiti T., Hamamcı H., Öktem H.A., Yücel M. Biochemical Analysis of Trehalose and Its Metabolizing Enzymes in Wheat under Abiotic Stress Conditions. Plant Sci. 2005;169:47–54. doi: 10.1016/j.plantsci.2005.02.024. [DOI] [Google Scholar]
- 64.Henry C., Bledsoe S.W., Griffiths C.A., Kollman A., Paul M.J., Sakr S., Lagrimini L.M. Differential Role for Trehalose Metabolism in Salt-Stressed Maize. Plant Physiol. 2015;169:1072–1089. doi: 10.1104/pp.15.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia A.B., Engler J.D.A., Iyer S., Gerats T., Van Montagu M., Caplan A.B. Effects of Osmoprotectants upon NaCl Stress in Rice. Plant Physiol. 1997;115:159–169. doi: 10.1104/pp.115.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han S.-E., Park S.-R., Kwon H.-B., Yi B.-Y., Lee G.-B., Byun M.-O. Genetic Engineering of Drought-Resistant Tobacco Plants by Introducing the Trehalose Phosphorylase (TP) Gene from Pleurotus Sajor-Caju. Plant Cell Tissue Organ Cult. 2005;82:151–158. doi: 10.1007/s11240-004-8124-1. [DOI] [Google Scholar]
- 67.Karim S., Aronsson H., Ericson H., Pirhonen M., Leyman B., Welin B., Mäntylä E., Palva E.T., Van Dijck P., Holmström K.-O. Improved Drought Tolerance without Undesired Side Effects in Transgenic Plants Producing Trehalose. Plant Mol. Biol. 2007;64:371–386. doi: 10.1007/s11103-007-9159-6. [DOI] [PubMed] [Google Scholar]
- 68.Lee S.-B., Kwon H.-B., Kwon S.-J., Park S.-C., Jeong M.-J., Han S.-E., Byun M.-O., Daniell H. Accumulation of Trehalose within Transgenic Chloroplasts Confers Drought Tolerance. Mol. Breed. 2003;11:1–13. doi: 10.1023/A:1022100404542. [DOI] [Google Scholar]
- 69.Garg A.K., Kim J.-K., Owens T.G., Ranwala A.P., Do Choi Y., Kochian L.V., Wu R.J. Trehalose Accumulation in Rice Plants Confers High Tolerance Levels to Different Abiotic Stresses. Proc. Natl. Acad. Sci. USA. 2002;99:15898–15903. doi: 10.1073/pnas.252637799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jang I.C., JS O.S.S., Choi W.B., Song S.I., Kim C.H., Kim Y.S., Seo H.S., Choi Y.D., Nahm B.H., Kim J.K. Expression of a Bifunctional Fusion of the E. Coli Genes for Trehalose-6-Phosphate Phosphatase in Transgenic Rice Plants Increase Trehalose Accumulation and Abiotic Stress Tolerance without Stunting Growth. Plant Physiol. 2003;131:516–524. doi: 10.1104/pp.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cortina C., Culiáñez-Macià F.A. Tomato Abiotic Stress Enhanced Tolerance by Trehalose Biosynthesis. Plant Sci. 2005;169:75–82. doi: 10.1016/j.plantsci.2005.02.026. [DOI] [Google Scholar]
- 72.Goddijn O.J., Verwoerd T.C., Voogd E., Krutwagen R.W., De Graff P., Poels J., van Dun K., Ponstein A.S., Damm B., Pen J. Inhibition of Trehalase Activity Enhances Trehalose Accumulation in Transgenic Plants. Plant Physiol. 1997;113:181–190. doi: 10.1104/pp.113.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Govind S.R., Jogaiah S., Abdelrahman M., Shetty H.S., Tran L.-S.P. Exogenous Trehalose Treatment Enhances the Activities of Defense-Related Enzymes and Triggers Resistance against Downy Mildew Disease of Pearl Millet. Front. Plant Sci. 2016;7:1593. doi: 10.3389/fpls.2016.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeid I.M. Trehalose as Osmoprotectant for Maize under Salinity-Induced Stress. Res. J. Agric. Biol. Sci. 2009;5:613–622. [Google Scholar]
- 75.Akram N.A., Waseem M., Ameen R., Ashraf M. Trehalose Pretreatment Induces Drought Tolerance in Radish (Raphanus Sativus L.) Plants: Some Key Physio-Biochemical Traits. Acta Physiol. Plant. 2016;38:3. [Google Scholar]
- 76.Kaplan F., Guy C.L. β-Amylase Induction and the Protective Role of Maltose during Temperature Shock. Plant Physiol. 2004;135:1674–1684. doi: 10.1104/pp.104.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabados L., Savoure A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 78.Lunn J.E., Feil R., Hendriks J.H., Gibon Y., Morcuende R., Osuna D., Scheible W.-R., Carillo P., Hajirezaei M.-R., Stitt M. Sugar-Induced Increases in Trehalose 6-Phosphate Are Correlated with Redox Activation of ADPglucose Pyrophosphorylase and Higher Rates of Starch Synthesis in Arabidopsis Thaliana. Biochem. J. 2006;397:139–148. doi: 10.1042/BJ20060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wingler A., Fritzius T., Wiemken A., Boller T., Aeschbacher R.A. Trehalose Induces the ADP-Glucose Pyrophosphorylase Gene, ApL3, and Starch Synthesis in Arabidopsis. Plant Physiol. 2000;124:105–114. doi: 10.1104/pp.124.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kolbe A., Tiessen A., Schluepmann H., Paul M., Ulrich S., Geigenberger P. Trehalose 6-Phosphate Regulates Starch Synthesis via Posttranslational Redox Activation of ADP-Glucose Pyrophosphorylase. Proc. Natl. Acad. Sci. USA. 2005;102:11118–11123. doi: 10.1073/pnas.0503410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baena-González E., Rolland F., Thevelein J.M., Sheen J. A Central Integrator of Transcription Networks in Plant Stress and Energy Signalling. Nature. 2007;448:938–942. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 82.Avonce N., Leyman B., Mascorro-Gallardo J.O., Van Dijck P., Thevelein J.M., Iturriaga G. The Arabidopsis Trehalose-6-P Synthase AtTPS1 Gene Is a Regulator of Glucose, Abscisic Acid, and Stress Signaling. Plant Physiol. 2004;136:3649–3659. doi: 10.1104/pp.104.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iba K. Acclimative Response to Temperature Stress in Higher Plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 84.Khalid Q., Shah S.H., Zaeem F., Shah S.H. Assessment of Adaptation, Regeneration Capability and Fatty Acid Profiles of LiCl Adapted and Unadapted Cell Lines of Oryza Sativa L. Cv. Swat-1. Sarhad J. Agric. 2017;33:549–555. [Google Scholar]
- 85.Sui N., Li M., Li K., Song J., Wang B.-S. Increase in Unsaturated Fatty Acids in Membrane Lipids of Suaeda Salsa L. Enhances Protection of Photosystem II under High Salinity. Photosynthetica. 2010;48:623–629. doi: 10.1007/s11099-010-0080-x. [DOI] [Google Scholar]
- 86.Harrathi J., Hosni K., Karray-Bouraoui N., Attia H., Marzouk B., Magné C., Lachaâl M. Effect of Salt Stress on Growth, Fatty Acids and Essential Oils in Safflower (Carthamus Tinctorius L.) Acta Physiol. Plant. 2012;34:129–137. doi: 10.1007/s11738-011-0811-z. [DOI] [Google Scholar]
- 87.López-Pérez L., del Carmen Martínez-Ballesta M., Maurel C., Carvajal M. Changes in Plasma Membrane Lipids, Aquaporins and Proton Pump of Broccoli Roots, as an Adaptation Mechanism to Salinity. Phytochemistry. 2009;70:492–500. doi: 10.1016/j.phytochem.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Gigon A., Matos A.-R., Laffray D., Zuily-Fodil Y., Pham-Thi A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis Thaliana (Ecotype Columbia) Ann. Bot. 2004;94:345–351. doi: 10.1093/aob/mch150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang M., Barg R., Yin M., Gueta-Dahan Y., Leikin-Frenkel A., Salts Y., Shabtai S., Ben-Hayyim G. Modulated Fatty Acid Desaturation via Overexpression of Two Distinct Ω-3 Desaturases Differentially Alters Tolerance to Various Abiotic Stresses in Transgenic Tobacco Cells and Plants. Plant J. 2005;44:361–371. doi: 10.1111/j.1365-313X.2005.02536.x. [DOI] [PubMed] [Google Scholar]
- 90.Beisson F., Li Y., Bonaventure G., Pollard M., Ohlrogge J.B. The Acyltransferase GPAT5 Is Required for the Synthesis of Suberin in Seed Coat and Root of Arabidopsis. Plant Cell. 2007;19:351–368. doi: 10.1105/tpc.106.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mikami K., Murata N. Membrane Fluidity and the Perception of Environmental Signals in Cyanobacteria and Plants. Prog. Lipid Res. 2003;42:527–543. doi: 10.1016/S0163-7827(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 92.Upchurch R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- 93.Kosma D.K., Bourdenx B., Bernard A., Parsons E.P., Lü S., Joubès J., Jenks M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009;151:1918–1929. doi: 10.1104/pp.109.141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bourdenx B., Bernard A., Domergue F., Pascal S., Léger A., Roby D., Pervent M., Vile D., Haslam R.P., Napier J.A. Overexpression of Arabidopsis ECERIFERUM1 Promotes Wax Very-Long-Chain Alkane Biosynthesis and Influences Plant Response to Biotic and Abiotic Stresses. Plant Physiol. 2011;156:29–45. doi: 10.1104/pp.111.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sabbah S., Tal M. Development of Callus and Suspension Cultures of Potato Resistant to NaCl and Mannitol and Their Response to Stress. Plant Cell Tissue Organ Cult. 1990;21:119–128. doi: 10.1007/BF00033430. [DOI] [Google Scholar]
- 96.Kumlay A.M., Ercisli S. Callus Induction, Shoot Proliferation and Root Regeneration of Potato (Solanum Tuberosum L.) Stem Node and Leaf Explants under Long-Day Conditions. Biotechnol. Biotechnol. Equip. 2015;29:1075–1084. doi: 10.1080/13102818.2015.1077685. [DOI] [Google Scholar]
- 97.Hoff J.E., Singleton K.I. A Method for Determination of Tannins in Foods by Means of Immobilized Protein. J. Food Sci. 1977;42:1566–1569. doi: 10.1111/j.1365-2621.1977.tb08427.x. [DOI] [Google Scholar]
- 98.Zhishen J., Mengcheng T., Jianming W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 99.Mar’ia V.M., Cascone O. Horseradish Peroxidase Extraction and Purification by Aqueous Two-Phase Partition. Appl. Biochem. Biotechnol. 1995;53:147–154. [Google Scholar]
- 100.Jiang Y., Zhang Z., Joyce D.C., Ketsa S. Postharvest Biology and Handling of Longan Fruit (Dimocarpus Longan Lour.) Postharvest Biol. Technol. 2002;26:241–252. doi: 10.1016/S0925-5214(02)00047-9. [DOI] [Google Scholar]
- 101.Gelmesa D., Dechassa N., Mohammed W., Gebre E., Monneveux P., Bündig C., Winkelmann T. In vitro Screening of Potato Genotypes for Osmotic Stress Tolerance. Open Agric. 2017;2:308–316. doi: 10.1515/opag-2017-0035. [DOI] [Google Scholar]
- 102.Jain S.M. Tissue Culture-Derived Variation in Crop Improvement. Euphytica. 2001;118:153–166. doi: 10.1023/A:1004124519479. [DOI] [Google Scholar]
- 103.Iturriaga G., Suárez R., Nova-Franco B. Trehalose Metabolism: From Osmoprotection to Signaling. Int. J. Mol. Sci. 2009;10:3793–3810. doi: 10.3390/ijms10093793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.13518899.