Abstract
Candidaalbicans represents one of the most common fungal pathogens. Due to its increasing incidence and the poor efficacy of available antifungals, finding novel antifungal molecules is of great importance. Camphor and eucalyptol are bioactive terpenoid plant constituents and their antifungal properties have been explored previously. In this study, we examined their ability to inhibit the growth of different Candida species in suspension and biofilm, to block hyphal transition along with their impact on genes encoding for efflux pumps (CDR1 and CDR2), ergosterol biosynthesis (ERG11), and cytotoxicity to primary liver cells. Camphor showed excellent antifungal activity with a minimal inhibitory concentration of 0.125–0.35 mg/mL while eucalyptol was active in the range of 2–23 mg/mL. The results showed camphor’s potential to reduce fungal virulence traits, that is, biofilm establishment and hyphae formation. On the other hand, camphor and eucalyptol treatments upregulated CDR1; CDR2 was positively regulated after eucalyptol application while camphor downregulated it. Neither had an impact on ERG11 expression. The beneficial antifungal activities of camphor were achieved with an amount that was non-toxic to porcine liver cells, making it a promising antifungal compound for future development. The antifungal concentration of eucalyptol caused cytotoxic effects and increased expression of efflux pump genes, which suggests that it is an unsuitable antifungal candidate.
Keywords: terpenoids, camphor, eucalyptol, antifungal activity, virulence factors, efflux pumps, cytotoxicity
1. Introduction
Candida albicans resides as a part of the healthy human microbiome, however, it is also one of the most frequent human fungal pathogens [1,2]. Due to the fact that mortality rates in patients suffering from candidemia can be up to 54% [3], this fungus represents a serious risk to human health and a significant economic burden for our societies. Therefore, the search for novel alternatives for candidiasis therapy is of major interest. This has been highlighted in several recent review papers [4,5,6] that provide a contemporary overview of the current knowledge on alternative antifungal therapies.
The pathogenicity of C. albicans is directly related to the expression of various virulence factors that this fungus uses to damage the host cell. These include the transition from yeast to the hyphal growth phase, biofilm formation and the secretion of hydrolytic enzymes [7]. Virulence factors are now being extensively studied as antifungal targets since commercial antifungals are mostly ineffective against them, making the search for efficient fungal antivirulence agents an attractive and challenging mission [6,8,9]. Besides using an antivirulence approach as a novel antimicrobial strategy, several authors [10,11,12] have also suggested the inhibition of microbial efflux pumps as an alternative strategy for combating microbial pathogens. Some of the fungal efflux pumps, such as the ones belonging to ATP binding cassette transporters, Cdr1 and Cdr2, have been proven to have a role in the development of resistance to azole drugs [13]. Likewise, in the case of patients suffering from fungal infections, upregulation of CDR1 and CDR2 is an undesirable property for any therapeutic given along the way [14], while their downregulation is seen as a promising antifungal trait [15]. ERG11 is another gene whose expression is linked to antifungal resistance. This gene is involved in the biosynthesis of ergosterol, an essential lipid in the fungal kingdom; ERG11 upregulation in C. albicans leads to azole treatment insensitivity [16].
Many different compounds of natural origin have been explored so far [17,18,19] in order to shed light on the huge antifungal potential of natural products. Terpenoids and terpenes are one of the most abundant classes of compounds found in nature, with antimicrobial activity being one of the many bioactivities attributed to them [20,21]. In the current study, camphor and eucalyptol were selected as representatives of terpenoid compounds in order to examine their anticandidal potential. This study focused on the ability of the terpenoids to inhibit the growth of different Candida species in suspension and biofilm; to block hyphal transition along with their impact on genes encoding for efflux pumps (CDR1 and CDR2); their effect on ergosterol biosynthesis (ERG11); and their cytotoxicity to primary liver cells with the aim to enlighten novel antifungal strategy.
2. Results
2.1. Impact of Camphor and Eucalyptol on Candida Albicans Growing in Planktonic and Biofilm Forms
Of the two compounds that were subject to this investigation, camphor was found to have much better antifungal potential against all the examined Candida strains compared to eucalyptol (minimal inhibitory concentration (MIC) 0.125–0.35 mg/mL and 2–23 mg/mL, respectively) (Table 1). Strains showed different susceptibility to camphor with the most sensitive strains being C. albicans 475/15, C. albicans 527/14, C. albicans 10/15, C. albicans 532/15, C. albicans 16/15 and C. parapsilosis ATCC 22019 (MIC 0.125 mg/mL), while C. krusei H1/16 was most resistant to the camphor treatment (MIC 0.35 mg/mL). On the other hand, eucalyptol resulted in the strongest inhibition of C. glabrata 4/6/15 and C. parapsilosis ATCC 22019 (MIC 2 mg/mL), while much lower activity was observed in the rest of the examined microorganisms, especially C. albicans 475/15, C. albicans 527/14 and C. albicans 10/15 with MIC 23 mg/mL.
Table 1.
Anticandidal activity of camphor and eucalyptol. Results are expressed in mg/mL. Values are expressed as means ± SD of three replicates. Different letters (a, b, c) in each row indicate a significant statistical difference between the samples (p < 0.05). MIC and MFC values of the compounds are compared separately for each of the fungal strain tested.
| Strain | Camphor | Eucalyptol | Ketoconazole | |||
|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |
| C. albicans 475/15 | 0.125 ± 0.01 b | 0.25 ± 0.02 b | 23 ± 0.1 c | 46 ± 0.2 c | 0.0031 ± 0.0001 a | 0.0062 ± 0.0001 a |
| C. albicans 527/14 | 0.125 ± 0.01 b | 0.25 ± 0.02 b | 23 ± 0.2 c | 46 ± 0.2 c | 0.0031 ± 0.0001 a | 0.0062 ± 0.0001 a |
| C. albicans 10/15 | 0.125 ± 0.02 b | 0.25 ± 0.02 b | 23 ± 0.1 c | 46 ± 0.2 c | 0.0031 ± 0.0001 a | 0.05 ± 0.0001 a |
| C. albicans 27/15 | 0.25 ± 0.004 b | 0.5 ± 0.02 b | 6 ± 0.08 c | 12 ± 0.2 c | 0.0031 ± 0.001 a | 0.1 ± 0.01 a |
| C. albicans 532/15 | 0.125 ± 0.01 b | 0.25 ± 0.01 b | 6 ± 0.08 c | 12 ± 0.2 c | 0.0031 ± 0.0001 a | 0.0062 ± 0.0001 a |
| C. albicans 503/15 | 0.25 ± 0.008 b | 0.5 ± 0.02 b | 3 ± 0.06 c | 6 ± 0.08 c | 0.0031 ± 0.0001 a | 0.0062 ± 0.0001 a |
| C. albicans 13/15 | 0.25 ± 0.01 b | 0.5 ± 0.008 b | 6 ± 0.08 c | 12 ± 0.2 c | 0.0016 ± 0.001 a | 0.05 ± 0.002 a |
| C. albicans 16/15 | 0.125 ± 0.008 b | 0.25 ± 0.004 b | 6 ± 0.1 c | 12 ± 0.2 c | 0.0031 ± 0.001 a | 0.1 ± 0.001 a |
| C. albicans ATCC 10231 | 0.175 ± 0.02 b | 0.35 ± 0.02 b | 4 ± 0.06 c | 8 ± 0.008 c | 0.0016 ± 0.001 a | 0.0062 ± 0.001 a |
| C. tropicalis ATCC 750 | 0.175 ± 0.02 b | 0.35 ± 0.02 b | 4 ± 0.004 c | 8 ± 0.006 c | 0.0016 ± 0.002 a | 0.0062 ± 0.002 a |
| C. parapsilosis ATCC 22019 | 0.125 ± 0.003 b | 0.25 ± 0.008 b | 2 ± 0.003 c | 4 ± 0.003 c | 0.0031 ± 0.0001 a | 0.0062 ± 0.0001 a |
| C. krusei H1/16 | 0.35 ± 0.06 b | 0.7 ± 0.06 b | 4 ± 0.004 c | 8 ± 0.008 c | 0.0016 ± 0.001 a | 0.0032 ± 0.002 a |
| C. glabrata 4/6/15 | 0.175 ± 0.02 b | 0.35 ± 0.04 b | 2 ± 0.004 c | 4 ± 0.007 c | 0.0016 ± 0.001 a | 0.0062 ± 0.002 a |
MIC—minimal inhibitory concentration, MFC—minimal fungicidal concentration.
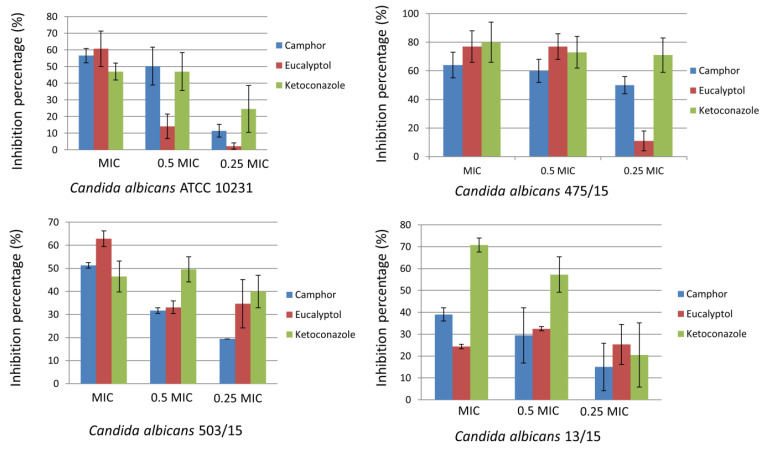
Treatment with both of the terpenoids impaired the ability of various C. albicans strains to establish biofilms in vitro (Figure 1). The application of camphor and eucalyptol reduced the formation of biofilm biomass by more than 50% in three C. albicans strains (C. albicans ATCC 10231, C. albicans 475/15 and C. albicans 503/15) at their MIC concentrations. Biofilm formation of C. albicans ATCC 10231 and C. albicans 475/15 was also inhibited (>50% inhibition) with camphor in a concentration equal to its ½ MIC (Figure 1). Since applied MIC and subMIC concentrations of camphor were lower than those for eucalyptol (Table 1), its antibiofilm potential is of higher significance.
Figure 1.
Inhibition of Candida albicans biofilm formation after treatment with camphor, eucalyptol and ketoconazole, expressed as inhibition percentage (100% means no biofilm was established), values represent means ± SD of three replicates.
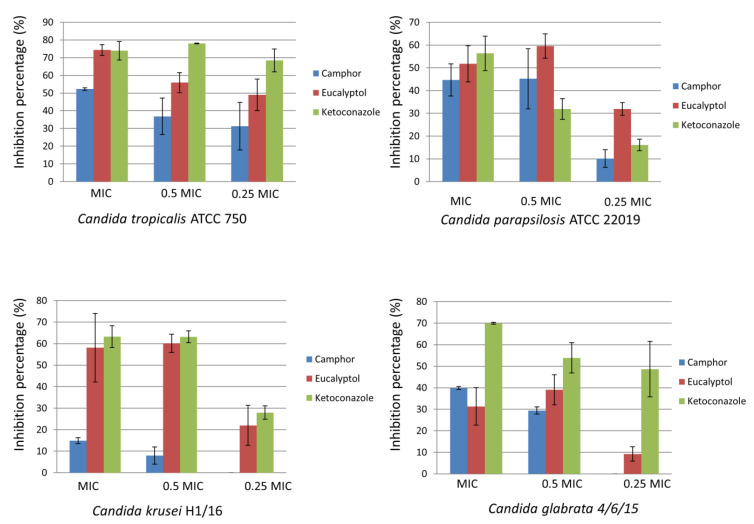
Among the examined non-albicans Candida strains, biofilm establishment was significantly disrupted for the strain C. tropicalis ATCC 750 at the MICs of both camphor and eucalyptol (>50% inhibition). On the other hand, C. krusei H1/16 and C. glabrata 4/6/15 biofilms were the strains most resistant to the application of camphor and eucalyptol, respectively (Figure 2).
Figure 2.
Inhibition of non-albicans Candida biofilm formation after treatment with camphor, eucalyptol and ketoconazole, expressed as inhibition percentage (100% means no biofilm was established), values represent means ± SD of three replicates.
2.2. Camphor and Eucalyptol as Inhibitors of Candida Albicans Hyphae Formation
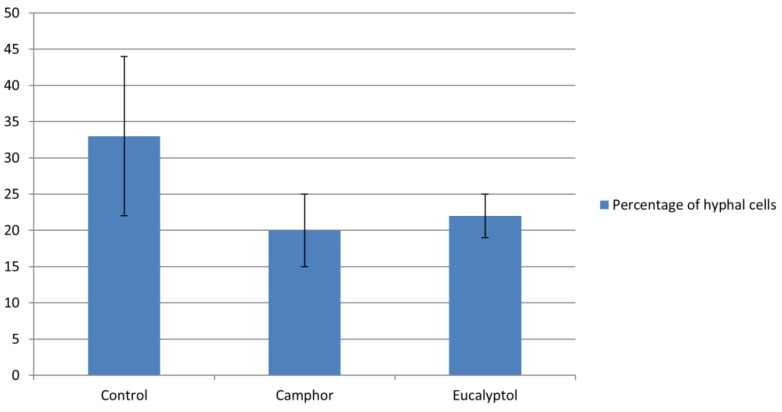
The application of camphor (0.125 mg/mL) induced a notable reduction in the number of hyphal cells as shown in Figure 3, similar to eucalyptol applied in higher concentration (23 mg/mL).
Figure 3.
The number of hyphae and germ tubes was determined 4 h post treatment of Candida albicans cells with compounds and the percentage of hyphal cells was calculated. Values represent means ± SD of three replicates.
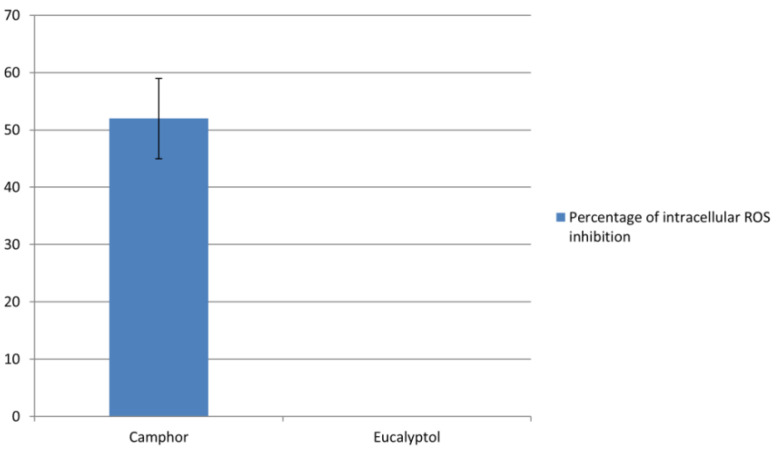
Hyphal growth and damage of the host epithelial cells is linked to the increase in reactive oxygen species (ROS) generated by C. albicans [22], thus, the ability of camphor to reduce ROS generation by 52% is of great interest in the development of antivirulent candidiasis therapy. On the other hand, eucalyptol did not reduce the generation of ROS (Figure 4).
Figure 4.
Percentage of inhibition of reactive oxygen species in Candida albicans cells treated with camphor and eucalyptol (no activity). Values represent means ± SD of three replicates.
2.3. Impact on Genes Coding Fungal Efflux Pumps and Gene Involved in Ergosterol Biosynthesis
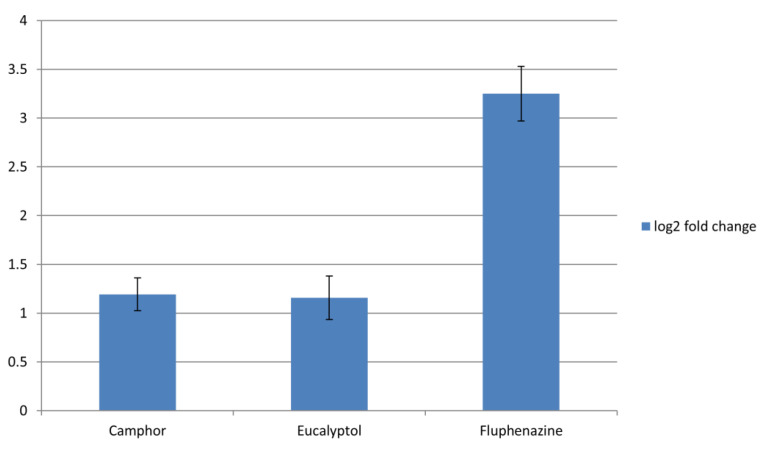
Treatment with both camphor and eucalyptol led to the increased expression of CDR1 (log2 fold change (FC) > 1) in fungal cells (Figure 5).
Figure 5.
Expression levels of CDR1 after treatment with MIC of camphor and eucalyptol; fluphenazine was used as a positive control for CDR1 expression. Values are expressed as the log2 fold change (log2 FC) of Relative Quantification (RQ) values and presented as an average of two biological replicates.
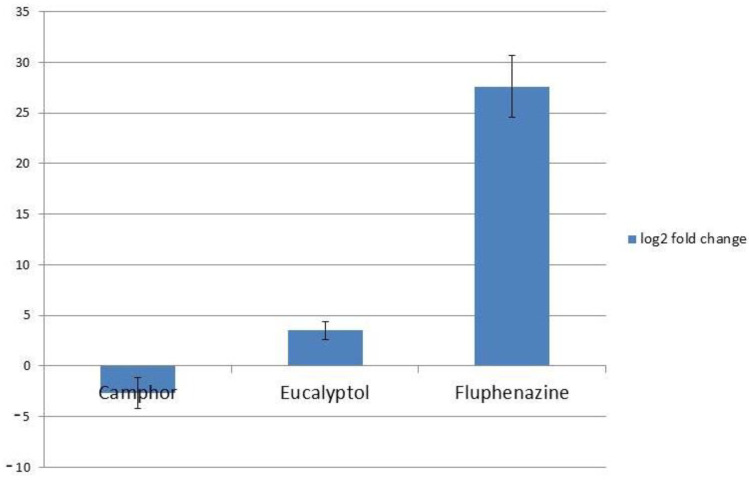
On the other hand, the two investigated compounds had a different impact on CDR2 expression—treatment with camphor reduced the level of CDR2 (log2FC < −2) while treatment with eucalyptol upregulated this gene (log2FC > 3) (Figure 6).
Figure 6.
Expression levels of CDR2 after treatment with MIC of camphor and eucalyptol; fluphenazine was used as a positive control for CDR2 expression. Values are expressed as the log2 FC of RQ values and presented as an average of two biological replicates.
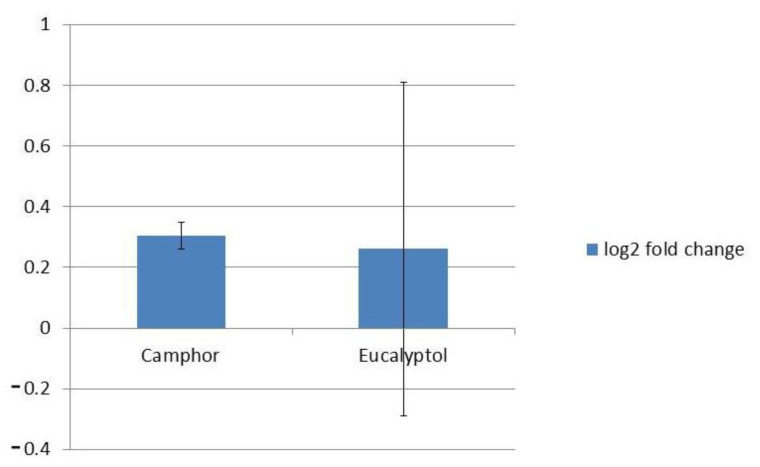
The application of the selected compounds in the current study did not interfere with the ERG11 expression (log2FC < 0.5) (Figure 7).
Figure 7.
Expression levels of ERG11 after treatment with MIC of camphor and eucalyptol. Values are expressed as the log2 FC of RQ values and presented as an average of two biological replicates.
2.4. Camphor and Eucalyptol—Diverse Cytotoxicity to Porcine Liver Cells
Camphor did not influence porcine liver cell proliferation at the maximum tested concentration (400 µg/mL, Table 2). Eucalyptol exhibited cytotoxicity with GI50 56 µg/mL (Table 2), a concentration much lower than the one required to inhibit fungal growth (Table 1). These results showed that camphor was much safer than eucalyptol since it had no toxic effects on tested cells and can be used at higher concentrations without side effects on the PLP cells. Nevertheless, more studies are needed to corroborate these results, especially in vivo experiments.
Table 2.
Cytotoxicity of camphor and eucalyptol (mean ± SD). GI50 values (µg/mL) corresponding to the sample concentration achieving 50% of growth inhibition in liver primary cultured PLP2 cells.
| Compound | GI50 |
|---|---|
| Camphor | >400 |
| Eucalyptol | 56 ± 4 |
| Ellipticine | 3.22 ± 0.2 |
3. Discussion
Previous studies focused on camphor oil have determined its MIC and MFC as 0.5% and 1%, respectively, for a range of C. albicans strains [23] and as 0.5% (w/v) for C. albicans DAY185 [24]. On the other hand, a study by Zuzarte et al. [25] claimed that camphor is not efficient in reducing the growth of C. albicans ATCC 10231 and clinical isolates. Previous studies of the anticandidal potential of eucalyptol have reported MIC 8 g/L and MFC 64 g/L [26], MIC ≥ 8% (v/v) [27] and MIC 4 mg/mL [28]. In their search for C. albicans inhibitors, Bin Jantan et al. [29] found that the activity of eucalyptol was lower than camphor (MIC > 5 µg/µL and 3.75 µg/µL, respectively). Furthermore, essential oil of Salvia officinalis has shown remarkable antifungal potential against C. albicans (MIC 2.5 µL/mL) [30], suggesting that a synergistic activity between its dominant compounds, camphor and eucalyptol, rather than its single constituents contribute to this effect. Aromatic plant compounds, camphor, carvacrol and eucalyptol were tested in a study by Sokovic et al. [31] and of these, eucalyptol exhibited the lowest antifungal potential against different pathogenic fungal species; it was also less effective compound in tea tree oil [32]. In our study, the antifungal potential of eucalyptol was also proven to be much lower than the potential exhibited by camphor (Table 1). This is the first comparative study of their antifungal and antibiofilm potential in a range of oral clinical isolates. Furthermore, to the best of the authors’ knowledge, this is the first study to highlight the effect of selected compounds on the expression of genes encoding for C. albicans efflux pumps.
The antibiofilm activities of camphor (0.005 and 0.01% w/v) were previously confirmed by Manoharan et al. [24] on C. albicans strain DAY185. It has been shown that the mechanism of camphor antibiofilm action involves downregulation of HWP1 (hyphal wall protein 1), RBT1 (repressed by Tup1) and EED1 (epithelial escape and dissemination 1) [24]. A study by Sancineto et al. [33] investigated camphor diselenide and found it was efficient against both candidal and bacterial biofilms when applied in concentrations of 6.25–50 mg/L, which suggests that some modifications of this compound could significantly increase its bioactivity. The potential of camphor to interfere with fungal biofilms could be further explored in order to find novel antivirulent agents and develop them as a part of an antifungal strategy. Future directions might also include synthetizing novel camphor derivatives in order to improve its bioactivities [33].
In a study by Hendry et al. [26], eucalyptol was established as a more efficient antibiofilm agent than eucalyptus oil. The eucalyptol MIC for C. albicans (ATCC 76615) cells embedded in biofilms was two times lower than the MIC for the cells in suspension (4 g/L compared to 8 g/L) [26]. In another study, application of 1/16 MIC of eucalyptol was able to significantly interfere with MRSA (methicillin resistant Staphylococcus aureus) biofilm development [34]. Although the potency of eucalyptol to inhibit fungal [26] and bacterial [34] biofilms was determined previously, due to its high MIC (Table 1) observed in our study it is necessary to expand the microbial strains used in these assays in order to completely elucidate its antimicrobial and antibiofilm capacity.
Complete C. albicans hyphal inhibition with 0.01% camphor was noticed by Manoharan et al. [24] along with reduced expression of ECE1 (extent of cell elongation), a hypha-specific gene [24]. The application of eucalyptol (23 mg/mL) also reduced the number of hyphal cells (Figure 3), while significantly lower concentrations of this compound (1 mg/mL) provided anti-hyphal activity in a previous study against hyphal formation of the reference strain C. albicans ATCC 90028 [28].
Unlike fungal cells (Figure 4), treatment of rat thymocytes with camphor significantly induced the generation of ROS [35]. On the other hand, eucalyptol was not able to inhibit ROS production in fungal cells (Figure 4), while it inhibited ROS generation in a human astrocytoma cell line treated with hydrogen peroxide and acted as a regulator of redox balance inside cells [36]. Differences between fungal and human cells, including lack of cell wall in human cells [37], might be the reasons for these diverse consequences of eucalyptol application in human and fungal cells.
Eucalyptol was previously shown to inhibit bacterial efflux pumps in the Pseudomonas aeruginosa and Acinetobacter baumannii strains [38], so, observed stimulation of efflux pumps gene expression (Figure 5) might be specific to fungal cells. The increased expression of genes encoding for efflux pumps is not a desirable trait of potential antifungal agents because it leads to requiring higher doses of antifungals associated with harmful side effects [6,39]. Bearing that in mind, the efflux-inducing properties of camphor and eucalyptol determined in our study and their potential adverse effect on human health should be further explored.
The impact of camphor and eucalyptol on expression levels of CDR2 was explored as well. The observed effect of these compounds on CDR2 expression levels (Figure 6) implies that these two compounds might affect different regulators of CDR gene expression. TAC1 (transcriptional activator of CDR genes) is an activator of transcription included in the regulation of both CDR1 and CDR2, since both genes have Tac1 binding regulatory element DRE (drug-responsive element) [40], which suggests it as a possible eucalyptol target. Regulation of CDR1 expression also involves the CaNdt80p transcription factor [41]; there is no data on whether this factor also regulates CDR2, thus it might be one of camphor’s specific targets in C. albicans cells; however, this hypothesis should be further investigated. In a previous study, the application of thymol and carvacol at their minimal inhibitory concentrations reduced efflux in a fluconazole-resistant Candida strain by 70–90% via a mechanism involving the decreased expression of efflux pump genes CDR1 and MDR1 [42]. On the other hand, bacterial efflux pumps are efficiently inhibited with terpene geraniol [43] and terpenoid ursolic acid [44], although not much is recorded about the undesirable trait of molecules observed in this study—efflux pump induction. Given that treatment of C. albicans cells with eucalyptol leads to the upregulation of both CDR1 and CDR2, special attention should be given to the application of this compound in pharmaceuticals, especially in combination with azoles, since it might reduce their activity.
Since the ERG11 gene encodes the azole target, CYP51, application of any compound that might increase ERG11 expression could lead to azole resistance [45]. The antifungal activity of different essential oils with high terpenoid and terpene content has previously been linked to reduction in ergosterol content [46,47,48]. On the other hand, the study by Połeć et al. [49] found that phytosterols, rather than fungal sterols, are influenced by eucalyptol and terpinen-4-ol. In this study we did not observe any significant interference with the ERG11 gene when selected terpenoids were applied (Figure 7).
One of the crucial steps at the very beginning of the development of novel antifungals is to observe the effects that the investigated compounds might have on cultured mammalian cells [50]. A previous study showed that camphor influenced the proliferation of fetal lung fibroblasts MRC-5 with IC50 11.0 mM [51]. In the study by Nikolic et al. [51], eucalyptol showed IC50 11 mM against fetal lung fibroblasts MRC-5, while both 0.025 and 0.05% eucalyptol induced significant cytotoxicity against peritoneal macrophages in a study by Zaccaro et al. [52]. The toxicity of eucalyptol that was determined in our study once again raises questions regarding its safety for human use and puts it out of contention for potential antifungal applications.
4. Materials and Methods
4.1. Microbial Culture Conditions
Strains used in the study were clinical isolates: C. albicans 475/15, C. albicans 527/14, C. albicans 10/15, C. albicans 27/15, C. albicans 532/15, C. albicans 503/15, C. albicans 13/15, C. albicans 16/15, C. krusei H1/16 (Pichia kudriavzevii), C. glabrata 4/6/15 as well as strains obtained from the American Type Culture Collection: C. albicans ATCC 10231, C. tropicalis ATCC 750 and C. parapsilosis ATCC 22019. Clinical Candida strains were isolated from oral cavities of patients at the ENT Clinic, Clinical Hospital Centre Zvezdara, Belgrade, Serbia and determined by using CHROMagar (Biomerieux, Craponne, France) and on HiCrome™ Candida differential agar plates (HiMedia, Mumbai, India). Fungal strains are deposited at the Mycological Laboratory, Department of Plant Physiology, Institute for Biological Research ‘‘Siniša Stanković’’—National Institute of Republic of Serbia, University of Belgrade.
4.2. Microdilution Method
Microdilution assay [53] was used to determine anticandidal activity in 96-well microtiter plates, with some modification. Yeast cultures were adjusted to 1.0 × 105 CFU/well with sterile saline. The 96-well microtiter plates were incubated with serially diluted compounds at 37 °C for 24 h after which minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) were determined. The lowest concentrations without microscopically observed growth were considered as the MIC, while the MFC values were determined as the concentrations without visible growth after serial sub-cultivation of 10 µL of samples in 100 µL of broth/well at 37 °C for 24 h. Ketoconazole (SigmaAldrich, Darmstadt, Germany) was used as a positive control. Camphor and eucalyptol were obtained from SigmaAldrich, Steinheim, Germany.
4.3. Antibiofilm Activity
The influence of selected compounds on the ability of C. albicans ATCC 10231, C. albicans 475/15, C. albicans 503/15, C. albicans 13/15, C. krusei H1/16, C. glabrata 4/6/15, C. tropicalis ATCC 750 and C. parapsilosis ATCC 22,019 to form biofilms was investigated as previously described [54]. Yeasts were incubated with the compounds in their previously determined concentrations equal to MIC, 0.5 MIC and 0.25 MIC in 96-well microtiter plates with an adhesive bottom (Sarstedt, Nümbrecht, Germany), at 37 °C for 24 h. After incubation, wells were washed twice with sterile PBS (Phosphate buffered saline, pH 7.4) and methanol was added into each well. After the fixation of the fungal cells, the methanol was discarded and the plate was air dried. Formed biofilms were stained with 0.1% crystal violet (Bio-Merieux, Craponne, France) for 30 min. The plate was washed with water and air dried. Ethanol 96% (Zorka Pharma—Hemija, Sabac, Serbia) was used to dissolve the biofilm bound stain. Absorbance was read on a Multiskan™ FC Microplate Photometer, Thermo Scientific™. The percentage of inhibition of biofilm formation was calculated according to the formula: ((A620control − A620sample)/A620control) × 100.
4.4. Inhibition of Morphological Transition
C. albicans 475/15 cells were incubated with the MICs of the tested compounds in YPD + 10% FBS at 37 °C for 4 h. Fungal cells were watched under microscope (Nikon Eclipse TS2, Amsterdam, The Netherlands) and the number of cells growing in yeast or hyphal and germ tube formations was determined. The assay was performed in triplicate and the percentage of hyphal cells was determined.
4.5. Determination of Intracellular ROS Levels in C. albicans 475/15
The impact of compounds on intracellular levels of ROS was determined according to Paez et al. [55]. C. albicans 475/15 was incubated with MICs of compounds overnight at 37 °C. The suspension of C. albicans treated cells (0.4 mL) was further incubated with 0.5 mL of nitro blue tetrazolium (1 mg/mL) at 37 °C for 30 min. After the addition of 0.1 mL 0.1 M HCl, tubes were centrifuged at 2500× g for 10 min. The pellets were treated with 0.6 mL dimethyl sulfoxide and 0.8 mL phosphate saline buffer and absorbance was recorded at 575 nm (Agilent/HP 8453 UV-Visible Spectrophotometer; Agilent Technologies, Santa Clara, CA, USA).
4.6. RNA Isolation and cDNA Synthesis
Total RNA was extracted from 5 mL C. albicans 475/15 logarithmic-phase cultures grown in YEPD medium by a technique of cell mechanical disruption with glass beads according to Sanglard et al. [56] and modified as described in Ivanov et al. [57]. The concentration and purity of the RNA was determined using a NanoDrop (ND-1000, Witec AG, Sursee, Switzerland) where OD260 nm/OD280 nm of the samples ranged from 1.80 to 2.05 and the OD260 nm/OD230 nm ranged from 2.00 to 2.60. For qPCR assay, 1 µg RNA was reverse transcribed to cDNA (Transcriptor High Fidelity cDNA synthesis kit, Roche) using random hexamer as a priming method. Prior to reverse transcription reaction, the total RNA samples were treated with DNase for 30 min at 37 °C according to the manufacturer’s instructions (DNA-free™ DNA Removal Kit, Ambion, Bleiswijk, The Netherlands).
4.7. qPCR
For the qPCR assay we used primers (0.2 µM) and probes (0.2 µM) for ACT1, CDR1, CDR2 and ERG11 genes (Table 3). Assay was performed by StepOnePlusTM Real Time PCR System using the iTAQ Supermix with ROX (BioRad, Reinach, Switzerland) according to the manufacturer’s instructions. Normalization of expression was done with ACT1, and fold changes were calculated for CDR1, CDR2 and ERG11 in vitro in the absence and presence of the compounds in their previously determined minimal inhibitory concentrations for 30 min.
Table 3.
Sequences of TaqMan primers and probes used in qPCR.
| Primer | Sequence |
| CDR1-ORF-F | ATGACTCGAGATATTTTGATA |
| CDR1-ORF-R | TTAACAGCAATGGTCTTTA |
| CDR2-ORF-F | TAGATATTTGAGCCACATG |
| CDR2-ORF-R | TTGGCATTGAAATTTTCG |
| ERG11-ORF-F | ATTGTTGAAACTGTCATTG |
| ERG11-ORF-R | CCCCTAATAATATACTGATCTG |
| ACT-ORF-F | GCATCACACTTTTTACAAT |
| ACT-ORF-R | AAACATAATTTGAGTCATCTTT |
| Probe | Sequence |
| CDR1-P2 | CATTATGAGACCTGGTGAACTTACT |
| CDR2-P2 | TTAGTCCATTCAACGGCAACATTAG |
| ERG11-P2 | TTTGTCCCTTAGTGTTACACA |
| ACT1-P2 | TTGCTCCAGAAGAACATCCAGT |
4.8. Cytotoxicity of Compounds to Porcine Liver Primary Cells
Porcine liver was obtained from a local slaughterhouse, freshly harvested, and subsequently exploited for the preparation of PLP2 cell line [58,59]. The obtained tissue of the liver was washed in Hank′s balanced salt solution enriched with 100 U/mL penicillin and 100 μg/mL streptomycin and was separated into explants (1 × 1 mm3). Some of the explants were transferred into 25 cm2 tissue flasks in Dulbecco′s modified Eagle′s medium (DMEM) enriched with 10% FBS, 2 mM nonessential amino acids, 100 U/mL penicillin, and 100 mg/mL streptomycin. Explants were placed in a humidified atmosphere containing 5% CO2 at 37 °C with fresh medium added every 2 days. Direct observation of the cells was conducted each 2–3 days with a phase-contrast microscope. Before confluence was achieved, cells were sub-cultured and 1.0 × 104 cells per well was seeded in 96-well plates and cultivated in DMEM with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. In order to determine the cytotoxicity, we used the previously described Sulforhodamine B assay [59]. The cytotoxicity results were defined by GI50 values corresponding to the concentration of compound that inhibits 50% of the net cell growth. The positive control in this assay was the cytotoxic agent, ellipticine.
4.9. Statistical Analysis
The results are presented as the mean value of three replicates ± standard deviation (SD). The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey′s HSD test with α = 0.05 with the SPSS v. 18.0 program. QPCR analysis was performed in technical triplicates and the results are presented as the mean value of two biological replicates.
5. Conclusions
In this study, camphor and eucalyptol, terpenoids that are abundantly present in a range of medicinal plants, showed antifungal properties. Although the anticandidal potential of these terpenoids has been studied previously with a focus on the determination of MICs and MFCs, this is the first parallel study of their inhibitory activity with regard to 13 Candida strains, including 8 different C. albicans oral isolates reporting the involvement of terpenoids on the expression of efflux pumps in treated yeast. However, eucalyptol antimicrobial potential was achieved in concentrations that are toxic to liver cells and induce the expression of genes encoding fungal efflux pumps, suggesting it is not suitable for further drug development. On the other hand, the activities of camphor in terms of its antifungal potential were more promising. Camphor showed better antimicrobial, antibiofilm and antihyphal potential when compared to eucalyptol and all the above-mentioned bioactive properties could be fulfilled by quantities that were not toxic to liver cells. Camphor induced expression of the CDR1 gene, while unlike eucalyptol, it downregulated CDR2. Due to its impact on efflux pumps it could be suggested that treatment with camphor along with azole therapy would induce less side effects than the application of eucalyptol. Our results confirm the great potential of camphor as an antifungal therapeutic and raise doubts regarding the safety of eucalyptol as an anticandidal molecule. Eucalyptol is already present in a wide range of pharmaceutical products so there is an urgent need to conduct further research regarding its potential interference with antifungal azole drugs.
Abbreviations
| ROS | Reactive oxygen species |
| MIC | Minimum inhibitory concentration |
| MFC | Minimum fungicidal concentration |
| PBS | Phosphate buffered saline |
| FC | Fold change |
Author Contributions
Conceptualization, M.I., M.S., J.G., D.S. and I.C.F.R.F., methodology, M.I., A.K., D.S.S., and R.C.C., investigation, M.I., A.K., D.S.S., R.C.C., writing—original draft preparation, M.I., D.S.S., R.C.C.; writing—review and editing, J.G., M.S., A.K., D.S. and I.C.F.R.F.; supervision, I.C.F.R.F., D.S., M.S.; funding acquisition, I.C.F.R.F., D.S., M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Serbian Ministry of Education, Science and Technological Development [Contract No. 451-03-68/2020-14/200007]. The authors are grateful to the FEMS for providing FEMS Research and Training Grant [FEMS-GO-2017-015] to Mrs Marija Ivanov for her visit to the Institute of Microbiology, University Hospital Lausanne and University Hospital Center, Rue du Bugnon 48, Lausanne, Switzerland. The authors are also grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to CIMO [UIDB/00690/2020] and to the national funding by FCT, P.I., through the institutional scientific employment program-contract for R. Calhelha’s contract.
Institutional Review Board Statement
The study was conducted according to the guidelines of the ICH-GCP and local legislation and approved by the Ethics committee of Office for Human Research Protection, ZVEZDARA UNIVERSITY MEDICAL CENTER, Belgrade, Serbia, document issued 26 October 2016.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Janbon G., Quintin J., Lanternier F., d’Enfert C. Studying fungal pathogens of humans and fungal infections: Fungal diversity and diversity of approaches. Genes Immun. 2019;20:403–414. doi: 10.1038/s41435-019-0071-2. [DOI] [PubMed] [Google Scholar]
- 2.Nikou S.A., Kichik N., Brown R., Ponde N.O., Ho J., Naglik J.R., Richardson J.P. Candida albicans interactions with mucosal surfaces during health and disease. Pathogens. 2019;22:53. doi: 10.3390/pathogens8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Z., Wang Q., Zhu F., An Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob. Resist. Infect. Control. 2019;8:89. doi: 10.1186/s13756-019-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Oliveira Santos G.C., Vasconcelos C.C., Lopes A.J.O., de Sousa Cartágenes M., Filho A., do Nascimento F., Ramos R.M., Pires E., de Andrade M.S., Rocha F., et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018;3:1351. doi: 10.3389/fmicb.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Mambro T., Guerriero I., Aurisicchio L., Magnani M., Marra E. The Yin and Yang of current antifungal therapeutic strategies: How can we harness our natural defenses? Front. Pharmacol. 2019;5:80. doi: 10.3389/fphar.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smiljković M., Kostić M., Stojković D., Glamočlija J., Soković M. Could flavonoids compete with synthetic azoles in diminishing Candida albicans infections? A comparative review based on in vitro studies. Curr. Med. Chem. 2019;26:2536–2554. doi: 10.2174/0929867325666180629133218. [DOI] [PubMed] [Google Scholar]
- 7.Dadar M., Tiwari R., Karthik K., Chakraborty S., Shahali Y., Dhama K. Candida albicans—Biology, molecular characterization, pathogenicity, and advances in diagnosis and control—An update. Microb. Pathog. 2018;117:128–138. doi: 10.1016/j.micpath.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Zida A., Bamba S., Yacouba A., Ouedraogo-Traore R., Guiguemdé R.T. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J. Mycol. Med. 2017;27:1–19. doi: 10.1016/j.mycmed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Cavalheiro M., Teixeira M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018;13:28. doi: 10.3389/fmed.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askoura M., Mottawea W., Abujamel T., Taher I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J. Med. 2011;13:6. doi: 10.3402/ljm.v6i0.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tegos G.P., Haynes M., Strouse J.J., Khan M.M., Bologa C.G., Oprea T.I., Sklar L.A. Microbial efflux pump inhibition: Tactics and strategies. Curr. Pharm. Des. 2011;17:1291–1302. doi: 10.2174/138161211795703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Venter H., Ma S. Efflux pump inhibitors: A novel approach to combat efflux-mediated drug resistance in bacteria. Curr. Drug Targets. 2016;17:702–719. doi: 10.2174/1389450116666151001103948. [DOI] [PubMed] [Google Scholar]
- 13.Sanglard D., Coste A. Activity of isavuconazole and other azoles against Candida clinical isolates and yeast model systems with known azole resistance mechanisms. Antimicrob. Agents Chemother. 2016;60:229–238. doi: 10.1128/AAC.02157-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kofla G., Turner V., Schulz B., Storch U., Froelich D., Rognon B., Coste A.T., Sanglard D., Ruhnke M. Doxorubicin induces drug efflux pumps in Candida albicans. Med. Mycol. 2011;49:132–142. doi: 10.3109/13693786.2010.512022. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov M., Kannan A., Stojković D., Glamočlija J., Grdadolnik S.G., Sanglard D., Soković M. Revealing the astragalin mode of anticandidal action. EXCLI J. 2020;19:1436–1445. doi: 10.17179/excli2020-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohberger A., Coste A.T., Sanglard D. Distinct roles of Candida albicans drug resistance transcription factors TAC1, MRR1, and UPC2 in virulence. Eukaryot. Cell. 2014;13:127–142. doi: 10.1128/EC.00245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolić M., Smiljković M., Marković T., Ćirić A., Glamočlija J., Markovic D., Soković M. Sensitivity of clinical isolates of Candida to essential oils from Burseraceae family. EXCLI J. 2016;19:280–289. doi: 10.17179/excli2014-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smiljković M., Dias M.I., Stojković D., Barros L., Bukvički D., Ferreira I., Soković M. Characterization of phenolic compounds in tincture of edible Nepeta nuda: Development of antimicrobial mouthwash. Food Funct. 2018;9:5417–5425. doi: 10.1039/C8FO01466C. [DOI] [PubMed] [Google Scholar]
- 19.Smiljković M., Stanisavljević D., Stojković D., Petrović I., Marjanović Vicentić J., Popović J., Golic Grdadolnik S., Marković D., Sanković-Babić S., Glamočlija J., et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017;16:795–807. doi: 10.17179/excli2017-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paduch R., Trytek M., Król S.K., Kud J., Frant M., Kandefer-Szerszeń M., Fiedurek J. Biological activity of terpene compounds produced by biotechnological methods. Pharm. Biol. 2016;54:1096–1107. doi: 10.3109/13880209.2015.1103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrović J., Stojković D., Soković M. Terpene core in selected aromatic and edible plants: Natural health improving agents. Adv. Food Nutr. Res. 2019;90:423–451. doi: 10.1016/bs.afnr.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Schröter C., Hipler U.C., Wilmer A., Künkel W., Wollina U. Generation of reactive oxygen species by Candida albicans in relation to morphogenesis. Arch. Dermatol. Res. 2000;292:260–264. doi: 10.1007/s004030050484. [DOI] [PubMed] [Google Scholar]
- 23.Devkatte A.N., Zore G.B., Karuppayil S.M. Potential of plant oils as inhibitors of Candida albicans growth. FEMS Yeast Res. 2005;5:867–873. doi: 10.1016/j.femsyr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Manoharan R.K., Lee J.H., Lee J. Antibiofilm and antihyphal activities of cedar leaf essential oil, camphor, and fenchone derivatives against Candida albicans. Front. Microbiol. 2017;3:1476. doi: 10.3389/fmicb.2017.01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuzarte M., Gonçalves M.J., Cavaleiro C., Canhoto J., Vale-Silva L., Silva M.J., Pinto E., Salgueiro L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J. Med. Microbiol. 2011;60:612–618. doi: 10.1099/jmm.0.027748-0. [DOI] [PubMed] [Google Scholar]
- 26.Hendry E.R., Worthington T., Conway B.R., Lambert P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009;64:1219–1225. doi: 10.1093/jac/dkp362. [DOI] [PubMed] [Google Scholar]
- 27.Dalleau S., Cateau E., Bergès T., Berjeaud J.M., Imbert C. In vitro activity of terpenes against Candida biofilms. Int. J. Antimicrob. Agents. 2008;31:572–576. doi: 10.1016/j.ijantimicag.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Raut J.S., Shinde R.B., Chauhan N.M., Karuppayil S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29:87–96. doi: 10.1080/08927014.2012.749398. [DOI] [PubMed] [Google Scholar]
- 29.Bin Jantan I., Moharam B.A.K., Santhanam J., Jamal J.A. Correlation between chemical composition and antifungal activity of the essential oils of eight Cinnamomum species. Pharm. Biol. 2008;46:406–412. doi: 10.1080/13880200802055859. [DOI] [Google Scholar]
- 30.Abu-Darwish M.S., Cabral C., Ferreira I.V., Gonçalves M.J., Cavaleiro C., Cruz M.T., Al-bdour T.H., Salgueiro L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed. Res. Int. 2013;2013:538940. doi: 10.1155/2013/538940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soković M., Tzakou O., Pitarokili D., Couladis M. Antifungal activities of selected aromatic plants growing wild in Greece. Nahrung. 2002;46:317–320. doi: 10.1002/1521-3803(20020901)46:5<317::AID-FOOD317>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 32.Yu D., Wang J., Shao X., Xu F., Wang H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015;119:1253–1262. doi: 10.1111/jam.12939. [DOI] [PubMed] [Google Scholar]
- 33.Sancineto L., Piccioni M., De Marco S., Pagiotti R., Nascimento V., Braga A.L., Santi C., Pietrella D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection. BMC Microbiol. 2016;16:220. doi: 10.1186/s12866-016-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merghni A., Noumi E., Hadded O., Dridi N., Panwar H., Ceylan O., Mastouri M., Snoussi M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018;118:74–80. doi: 10.1016/j.micpath.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Cherneva E., Pavlovic V., Smelcerovic A., Yancheva D. The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules. 2012;27:10258–10266. doi: 10.3390/molecules170910258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porres-Martínez M., González-Burgos E., Carretero M.E., Gómez-Serranillos M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015;53:921–929. doi: 10.3109/13880209.2014.950672. [DOI] [PubMed] [Google Scholar]
- 37.Lima S.L., Colombo A.L., de Almeida J.N., Jr. Fungal cell wall: Emerging antifungals and drug resistance. Front. Microbiol. 2019;10:2573. doi: 10.3389/fmicb.2019.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saviuc C., Gheorghe I., Coban S., Drumea V., Chifiriuc M., Banu O., Bezirtzoglou E., Laz V. Rosmarinus officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR strains. Rom. Biotechnol. Lett. 2016;21:11782–11790. [Google Scholar]
- 39.Scorzoni L., de Paula E Silva A.C., Marcos C.M., Assato P.A., de Melo W.C., de Oliveira H.C., Costa-Orlandi C.B., Mendes-Giannini M.J., Fusco-Almeida A.M. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017;8:36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coste A.T., Karababa M., Ischer F., Bille J., Sanglard D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell. 2004;3:1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J.S., Yang Y.L., Wu C.J., Ouyang K.J., Tseng K.Y., Chen C.G., Wang H., Lo H.J. The DNA-binding domain of CaNdt80p is required to activate CDR1 involved in drug resistance in Candida albicans. J. Med. Microbiol. 2006;55:1403–1411. doi: 10.1099/jmm.0.46650-0. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad A., Khan A., Manzoor N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 2013;23:80–86. doi: 10.1016/j.ejps.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzi V., Muselli A., Bernardini A.F., Berti L., Pagès J.M., Amaral L., Bolla J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009;53:2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dwivedi G.R., Maurya A., Yadav D.K., Khan F., Darokar M.P., Srivastava S.K. Drug resistance reversal potential of ursolic acid derivatives against nalidixic acid and multidrug-resistant Escherichia coli. Chem. Biol. Drug Des. 2015;86:272–283. doi: 10.1111/cbdd.12491. [DOI] [PubMed] [Google Scholar]
- 45.Paul S., Singh S., Sharma D., Chakrabarti A., Rudramurthy S.M., Ghosh A.K. Dynamics of in-vitro development of azole resistance in Candida tropicalis. J. Glob. Antimicrob. Resist. 2020;22:553–561. doi: 10.1016/j.jgar.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Tian J., Ban X., Zeng H., He J., Chen Y., Wang Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE. 2012;7:e30147. doi: 10.1371/journal.pone.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardoso N.N., Alviano C.S., Blank A.F., Romanos M.T., Fonseca B., Rozental S., Rodrigues I., Alviano D. Synergism effect of the essential oil from Ocimum basilicum var. Maria Bonita and its major components with fluconazole and its influence on ergosterol biosynthesis. Evid. Based Complement. Alternat. Med. 2016;2016:5647182. doi: 10.1155/2016/5647182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalagatur N.K., Nirmal Ghosh O.S., Sundararaj N., Mudili V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018;6:610. doi: 10.3389/fphar.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Połeć K., Wójcik A., Flasiński M., Wydro P., Broniatowski M., Hąc-Wydro K. The influence of terpinen-4-ol and eucalyptol—The essential oil components—On fungi and plant sterol monolayers. Biochim. Biophys. Acta Biomembr. 2019;1861:1093–1102. doi: 10.1016/j.bbamem.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Stojković D., Dias M.I., Drakulić D., Barros L., Stevanović M., Ferreira I.C.F.R., Soković M. Methanolic extract of the herb Ononis spinosa L. is an antifungal agent with no cytotoxicity to primary human cells. Pharmaceuticals. 2020;13:78. doi: 10.3390/ph13040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikolić B., Vasilijević B., Mitić-Ćulafić D., Vuković-Gačić B., Knežević-Vukčević J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015;5:263–271. doi: 10.1016/j.cbi.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Zaccaro Scelza M.F., Lima Oliveira L.R., Carvalho F.B., Côrte-Real Faria S. In vitro evaluation of macrophage viability after incubation in orange oil, eucalyptol, and chloroform. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006;102:e24–e27. doi: 10.1016/j.tripleo.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 53.EUCAST (European Committee on Antibiotic Susceptibility) Method for Determination of Minimal Inhibitory Concentration (MIC) by Broth Dilution of Fermentative Yeasts. European Society of Clinical Microbiology and Infectious Diseases; Taufkirchen, Germany: 2002. Discussion Document E. Dis. 7.1. [Google Scholar]
- 54.Smiljkovic M., Matsoukas M.T., Kritsi E., Zelenko U., Grdadolnik S.G., Calhelha R.C., Ferreira I.C.F.R., Sankovic-Babic S., Glamoclija J., Fotopoulou T., et al. Nitrate esters of heteroaromatic compounds as Candida albicans CYP51 enzyme inhibitors. ChemMedChem. 2018;6:251–258. doi: 10.1002/cmdc.201700602. [DOI] [PubMed] [Google Scholar]
- 55.Paez P.L., Becerra M.C., Albesa I. Effect of the association of reduced glutathione and ciprofloxacin on the antimicrobial activity in Staphylococcus aureus. FEMS Microbiol. Lett. 2010;303:101–105. doi: 10.1111/j.1574-6968.2009.01867.x. [DOI] [PubMed] [Google Scholar]
- 56.Sanglard D., Ischer F., Calabrese D., Majcherczyk P.A., Bille J. The ATP binding cassette transporter GeneCgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 1999;43:2753–2765. doi: 10.1128/AAC.43.11.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov M., Kannan A., Stojkovic D., Glamoclija J., Calhelha R., Ferreira I., Sanglard D., Sokovic M. Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity. Pharmaceuticals. 2021;14:27. doi: 10.3390/ph14010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abreu R.M.V., Ferreira I.C.F.R., Calhelha R.C., Lima R.T., Vasconcelos M.H., Adega F., Chaves R., Queiroz M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011;46:5800–5806. doi: 10.1016/j.ejmech.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 59.Guimaraes R., Barros L., Duenas M., Calhelha R., Carvalho A.M., Santos-Buelga C., Queiroz M.J., Ferreira I.C.F.R. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chem. 2013;136:718–725. doi: 10.1016/j.foodchem.2012.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.