Abstract
In this study, the cervicovaginal environment of women with reproductive failure (repetitive abortion, infertility of unknown origin) was assessed and compared to that of healthy fertile women. Subsequently, the ability of Ligilactobacillus salivarius CECT5713 to increase pregnancy rates in women with reproductive failure was evaluated. Vaginal pH and Nugent score were higher in women with reproductive failure than in fertile women. The opposite was observed regarding the immune factors TGF-β 1, TFG-β 2, and VEFG. Lactobacilli were detected at a higher frequency and concentration in fertile women than in women with repetitive abortion or infertility. The metataxonomic study revealed that vaginal samples from fertile women were characterized by the high abundance of Lactobacillus sequences, while DNA from this genus was practically absent in one third of samples from women with reproductive failure. Daily oral administration of L. salivarius CECT5713 (~9 log10 CFU/day) to women with reproductive failure for a maximum of 6 months resulted in an overall successful pregnancy rate of 56%. The probiotic intervention modified key microbiological, biochemical, and immunological parameters in women who got pregnant. In conclusion, L. salivarius CECT5713 has proved to be a good candidate to improve reproductive success in women with reproductive failure.
Keywords: infertility, repetitive abortion, implantation failure, Lactobacillus salivarius, probiotics, vaginal microbiome, TGF-β, VEGF
1. Introduction
Increasing evidence has highlighted the relevance of the microbiota of the female genital tract for human reproduction [1,2]. Under physiological conditions, and in contrast to the gut, the human vaginal microbiota is usually characterized by a low microbial diversity and the dominance of bacteria from the genus Lactobacillus [3,4]. In fact, a low diversity in the gut has been linked to a variety of gastrointestinal processes, including inflammatory bowel disease [5], while a high diversity in the vagina has been associated to vaginosis [6].
The vaginal microbiota in healthy reproductive-age women is mainly composed of one or a few Lactobacillus species, which represent more than 90% of the total microbiota [7,8]. In a seminal study, the bacterial communities of 396 asymptomatic women were classified into five distinct vaginotypes; four of them were dominated by Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, respectively; in contrast, the fifth one had lower proportions of lactobacilli and was predominantly composed of strictly anaerobic bacterial genera, such as Gardnerella, Prevotella, Megasphaera, Atopobium, or Dialister [3]. This last vaginotype was associated to high Nugent scores, a Gram-staining based technique used for the diagnosis of bacterial vaginosis (BV).
Several factors are known to contribute to interindividual and intraindividual changes in the vaginal microbiota [9]. Although shifts between different vaginotypes may occur naturally, increase of diversity and colonization by strict anaerobes and decrease or depletion of lactobacilli are considered as risk factors for BV. In fact, vaginal microbiota dysbiosis has been associated with higher rates of intra-amniotic infection, premature delivery, spontaneous abortion, and infertility [10,11,12,13,14,15].
Different studies have shown that infertile women harbor a differential vaginal microbiota when compared to fertile women [16,17,18,19]. Therefore, the composition of the vaginal microbiota (and, particularly, any deviation from the Lactobacillus-dominated, low-diversity vaginal microbiome) may play a key role in fertility and in the outcomes of assisted reproduction treatments (ARTs) [20,21,22]. Abundant isolation of enterococci, streptococci, staphylococci, and/or Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae) from the tip of the catheter used for embryo transfer has been correlated with lower implantation and pregnancy rates and increased miscarriage rates [23], while abundant isolation of lactobacilli and low density or no isolation of the aforementioned bacteria has been correlated with better reproductive outcomes [24,25,26,27,28]. Metataxonomic studies of endometrial samples have also revealed that an abnormal endometrial bacterial profile (with a low percentage of sequences of the genus Lactobacillus) is a common feature in a high percentage of infertile women subjected to ART [21,29]. Although at least a part of the bacterial DNA detected in endometrial samples may arise from vaginal contamination during sampling, these studies suggest that an abundant presence of Lactobacillus DNA in such samples may be a predictor of implantation success [29,30].
As a consequence, the assessment of the microbial communities in the reproductive tract should be considered as a relevant part of the evaluation and personalized care in cases of reproductive failure of unknown cause or origin. When this happens, the use of probiotics may be a possible strategy to modulate the reproductive tract microbiome and to increase the success rates [31]. However, such a combined strategy (assessment of vaginal communities together with use of a target-selected probiotic) has not been explored yet, and commercially available probiotics are being empirically prescribed for repopulation of the female reproductive tract with Lactobacillus strains [2], without a proper scientific evidence of their actual usefulness.
Lactobacilli may have different biological activities that contribute to fertility and to a healthy pregnancy, including, among others: (a) the inhibition of the colonization and growth of potentially harmful microbes, including viruses, bacteria, yeast, and protozoa that may compromise fertility [32,33]; (b) contribution to angiogenesis and vasculogenesis that may favor the implantation of the embryo [34]; and, (c) induction of immunomodulation activities, such as those involved in implantation and in tolerance towards the embryo, first, and the fetus, later [35,36]. However, those properties might be strain-specific and, therefore, a strain-by-strain evaluation has to be performed for this specific target.
Lactobacillus salivarius CECT5713 [37] has been shown to be a probiotic strain suitable for applications in the mother–infant dyad due to a wide repertoire of desirable phenotypic and genotypic properties [38]. This includes a high survival rate when exposed to gastrointestinal tract conditions, a high acidifying activity, and antimicrobial, anti-inflammatory, and immunomodulatory properties, which have been demonstrated in vitro, in animal models, and in human clinical trials [38,39,40,41,42,43,44]. Therefore, and after evaluating some vaginal-related properties in this study, it was selected to be administered in a clinical trial in order to assess its efficacy for the infertility target. It must be highlighted that this species has been renamed as Ligilactobacillus salivarius in the recent proposal for reclassification of the genus Lactobacillus [45].
In this context, the first objective of this study was to assess the differences in several vaginal parameters (pH, Nugent score, microbiota composition as determined through culture and metataxonomic methods, and soluble immune factor levels) between women with reproductive failure (because of repetitive abortion during the first 12 weeks of pregnancy or infertility of unknown origin) and fertile women. The second objective was to evaluate the ability of L. salivarius CECT5713 to modulate those vaginal parameters and to increase pregnancy rates (currently ~29% after IVF procedures in this setting) in the group of women with reproductive failure.
2. Materials and Methods
2.1. Characterization of Vaginal-Related Properties of L. salivarius CECT5713
An overlay method [46] was used to determine the ability of L. salivarius CECT5713 to inhibit the growth of various species of bacteria and yeasts. It was performed as described previously [37]. All indicator strains had been previously isolated from clinical cases of vaginal or cervical infections, and included five strains of G. vaginalis, three of Streptococcus agalactiae and of Candida albicans, and two of Candida glabrata, Candida parapsilosis, and Ureaplasma urealyticum (our own culture collection). All inhibitory activity assays were performed in triplicate.
The ability of L. salivarius CECT5713 to aggregate with cells of the indicator strains cited above was investigated following the procedure of Younes et al. [47]. The suspensions were observed under a phase-contrast microscope. Adherence to vaginal epithelial cells collected from healthy premenopausal women was performed and interpreted as described previously [48]. Adherence was measured as the number of lactobacilli adhered to the vaginal cells in 20 random microscopic fields. L. salivarius CECT9145 was used as a control strain because of its high adherence to vaginal cells [49]. The assay was performed in triplicate.
Initially, the α-amylase activity of L. salivarius CECT5713 was qualitatively assessed using the method described by Padmavathi et al. [50]. Briefly, the strain was inoculated into a modified MRS media containing starch (0.5% peptone, 0.7% yeast extract, 0.2% NaCl, 2% starch, and 1.5% agar). The plates were incubated at 37 °C for 48 h in anaerobiosis and, then, the zone of clearance was observed by adding Gram’s iodine as detecting agent. Quantitation of the cell-bound α-amylase activity of L. salivarius CECT5713 was done with a kit (Kikkoman Co., Tokyo, Japan) using 2-chloro-4-nitrophenyl 65-azido-65-deoxy-β-maltopentaoside as substrate and using conditions described previously [51]. One unit of activity was defined as the amount of enzyme needed to release 1 μmol 2-chloro-4-nitrophenol from 2-chloro-4-nitrophenyl 65-azido-65-deoxy-β-maltopentaoside per min at 37 °C.
2.2. Participants, Sampling, and Design of the Human Study
A total of 58 women, aged 28–45, participated in this study (Table 1). Volunteers were classified into 3 groups. All women in the RA group (n = 21) had a history of recurrent miscarriage with three or more pregnancy losses during the first 12 weeks of pregnancy. All women of the INF group (n = 23) had a history of infertility (inability to conceive) despite being the recipients of ART for at least three times, including two cycles, at least, of in vitro fertilization (IVF). Finally, the control group (n = 14) included fertile women having at least two children after uncomplicated term pregnancies. None of the women of the RA and INF groups received ART during the whole period of the study. None of the RA group components were diagnosed of antiphospholipidic syndrome and, therefore, they did not receive either heparin and/or salicylic acid during the study. None of the participants had received hormonal therapy, antibiotics or probiotics in the 4 weeks previous to sampling. Vaginal samples were taken at least 7 days after coitus to avoid or minimize the impact of the partner’s semen on the vaginal pH, microbiota composition or immunoprofile (in the latter case, particularly in relation to the concentration of the two isoforms of the transforming growth factors beta 1 and 2 (TGF-β 1 and TGF-β 2)). Women with lactose intolerance or cow’s milk protein allergy were excluded because of the excipient used to administer the strain in the subsequent pilot trial (see below). Informed consent was obtained from all subjects involved in the study.
Table 1.
Characteristics of the participants (N = 58) which included fertile women (Control group), women with a history of repetitive abortion (RA group), and women with infertility of unknown origin (INF group).
| Group | ||||
|---|---|---|---|---|
| Characteristic | Control (n = 14) | RA (n = 21) | INF (n = 23) | p-Value |
| Age (years) | ||||
| Mean (95% CI) | 34.6 (33.5–35.8) a | 39.4 (38.5–40.4) b | 38.0 (37.1–38.9) b | <0.001 2 |
| Range (min–max) | (28.0–45.0) | (36.0–44.0) | (34.0–44.0) | |
| Weight (kg) | ||||
| Mean (95% CI) | 62.4 (59.7–65.0) | 68.3 (66.1–70.4) | 66.5 (64.5–68.6) | 0.054 2 |
| Range (min–max) | (46.0–87.0) | (50.0–87.0) | (51.0–78.0) | |
| Height (cm) | ||||
| Mean (95% CI) | 166 (164–168) | 167 (165–169) | 168 (166–169) | 0.761 2 |
| Range (min–max) | (156–175) | (152–190) | (160–182) | |
| Regularity of the menstrual cycle | ||||
| Yes, n (%) | 10 (71) | 10 (48) | 11 (48) | 0.337 3 |
| No, n (%) | 4 (29) | 11 (52) | 12 (52) | |
| Duration of the menstrual cycle (days) | ||||
| Mean (95% CI) | 28.0 (27.4–28.7) | 27.4 (26.9–27.9) | 27.5 (27.0–28.0) | 0.502 2 |
| Range (min–max) | (25.0–32.5) | (24.0–30.0) | (24.0–30.0) | |
| History of infections | ||||
| Vaginal, n (%) | 2 (14) | 13 (62) | 8 (35) | 0.017 3 |
| Urinary tract, n (%) | 2 (14) | 13 (62) | 15 (65) | 0.006 3 |
| Otorhinolaryngology, n (%) | 3 (21) | 13 (62) | 12 (52) | 0.057 3 |
| Lower respiratory tract, n (%) | 2 (14) | 7 (33) | 7 (30) | 0.490 3 |
| Skin, n (%) | 1 (7) | 3 (14) | 4 (17) | 0.800 3 |
| Gastrointestinal, n (%) | 0 (0) | 1 (5) | 1 (4) | 1.000 |
| Antibiotic usage 1 | ||||
| In infancy, n (%) | 4 (29) | 19 (90) | 14 (61) | <0.001 3 |
| In adulthood, n (%) | 4 (29) | 16 (76) | 19 (83) | 0.003 3 |
| History of other conditions | ||||
| Allergies, n (%) | 2 (14) | 5 (24) | 4 (17) | 0.835 3 |
| Food intolerance, n (%) | 0 (0) | 8 (38) | 13 (57) | 0.001 3 |
| Thyroid disease, n (%) | 0 (0) | 5 (24) | 3 (13) | 0.125 3 |
1 Antibiotic usage means ≥4 annual treatments due to recurrent infections. 2 One-way ANOVA tests were used to evaluate differences in mean values of women age, weight, and height and duration of the menstrual cycle between groups. Values followed by different superscript letters within the same row indicate statistically significant differences between groups according to Scheffé post hoc comparison tests. 3 Freeman–Halton extension of the Fisher exact probability tests for a 2 × 3 contingency table were used to compute the (two-tailed) probability of obtaining a distribution of values of categorical variables (regularity of the menstrual cycle, history of infections, antibiotic usage and history of other conditions).
At recruitment (within the first three days post-ovulation; day 0), two samples were collected: A vaginal swab specimen for in fresh determination of the Nugent score, and a cervicovaginal lavage (CVL) of the cervical and the vaginal walls with 10 mL of sterile normal saline for all the other analysis. Aliquots of the CVL samples were used for culture-based analysis. Subsequently, CVL samples were clarified by centrifugation at 800× g for 10 min at 4 °C. Aliquots of CVL supernatants and cell pellets were stored at −80 °C until the immunological and metataxonomic analyses were performed. Demographic, anthropometric, and health data (including a past or present history of recurrent infections at different body locations and use of antibiotics) were recorded at recruitment (Supplementary Figure S1). High use of antibiotics was defined as receiving ≥4 antibiotic treatments per year because of recurrent infections while a range between 0 and 2 annual treatments was considered as a low use of antibiotics.
Starting at day 0, women of the RA and INF groups consumed (oral route) a daily sachet with ~50 mg of freeze-dried probiotic (~9 log10 CFU of L. salivarius CECT5713) for 6 months or until a diagnosis of pregnancy (whatever happened first). At that point, the same two samples described above were collected from each woman. After a diagnosis of pregnancy, oral administration of the probiotic strain was maintained until the 15th week of pregnancy. All the spontaneous pregnancies that occurred within the first year after day 0 were recorded in this study.
Probiotic-containing sachets were kept at 4–8 °C throughout the study. All volunteers signed a written consent and were provided with diaries to record compliance with the study product intake. Minimum compliance rate (% of the total treatment doses) was set at 86%. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and it was approved by the Ethical Committee of Biomedical Research of Consejería de Salud y Familias (Junta de Andalucía, Granada, Spain) (P050/19, Act 11/19). The study was registered in the ClinicalTrials.gov database (NCT04446572).
2.3. Measurement of Vaginal pH and Nugent Score
At each of the two study visits, the pH of the lateral vaginal wall was measured (Whatman pH paper, pH 3.8–5.5 and pH 6.0–8.1). Nugent scoring was performed as described previously [52]. Briefly, the swab material was transferred to a glass slide, heat fixed, and Gram stained. Gram-positive, Gram-negative, and Gram-variable bacterial morphotypes were quantified. A Nugent score of 0–3 was considered normal, 4–6 was considered intermediate, and 7–10 was considered consistent with bacterial vaginosis [52].
2.4. Culture-Dependent Analysis
CVL samples collected during the trial were serially diluted and plated onto Columbia Nalidixic Acid (CNA), Gardnerella (GAR), CHROMagar StrepB (CHR), Mac Conkey (MCK), Mycoplasma (MYC), and Sabouraud Dextrose Chloramphenicol (SDC) agar plates (BioMerieux, Marcy l’Etoile, France) for selective isolation and quantification of the main cultivable non-Lactobacillus bacteria and yeasts that may be found in the vagina, including the agents most frequently involved in vaginal infections. They were also inoculated onto agar plates of MRS (Oxoid, Basingstoke, UK) supplemented with either L-cysteine (2.5 g/L) (MRS-C) or horse blood (5%) (MRS-B) for isolation of lactobacilli, including L. iners (MRS-B). All media were incubated for 48 h at 37 °C under aerobic conditions, with the exception of the MRS-C and MRS-B plates, which were incubated anaerobically (85% nitrogen, 10% hydrogen, 5% carbon dioxide) in an anaerobic workstation (DW Scientific, Shipley, UK) for up to 72 h. After incubation, the colonies were recorded and at least one representative of each colony morphology was selected from the agar plates. The isolates were identified by Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) mass spectrometry (Bruker, Bremen, Germany). When the identification by MALDI-TOF was not possible at the species level (particularly in the case of lactobacilli isolates), the identification was carried out by 16S ribosomal RNA (rRNA) gene sequencing as described by Mediano et al. [53].
2.5. DNA Extraction from the Samples
Approximately 1 mL of each CVL sample was used for DNA extraction following a method described previously [54]. Extracted DNA was eluted in 22 μL of nuclease-free water and stored at −20 °C until further analysis. Purity and concentration of each extracted DNA was initially estimated using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Inc., Rockland, DE, USA). Negative controls (blanks) were processed in parallel.
2.6. Real-Time Quantitative PCR (qPCR) Assay for the Detection and Quantification of L. salivarius DNA
Primers and conditions for quantification of L. salivarius DNA have been described previously [55]. The DNA concentration of all samples was adjusted to 5 ng µL−1. A commercial real-time PCR thermocycler (CFX96™, Biorad Laboratories, Hercules, CA, USA) was used for all experiments. Standard curves using 1∶10 DNA dilutions (ranging from 2 ng to 0.2 pg) from L. salivarius CECT5713 were used to calculate the concentrations of the unknown bacterial genomic targets. Threshold cycle (Ct) values between 15.29 and 20.07 were obtained for this range of L. salivarius DNA (R2 = 0.9915). The Ct values measured for DNA extracted from non-target species (L. reuteri MP07 and Lactobacillus plantarum MP02; our own collection) were ≥39.27 ± 0.64. These two control strains were selected because they belong to the L. salivarius taxonomically closest species [56]. All samples and standards were run in triplicate.
2.7. Metataxonomic Analysis
The V3-V4 hypervariable region of the 16S rDNA was amplified by PCR using the universal primers S-D-Bact-0341-b-S-17 (CCTACGGGNGGCWGCAG) and S-D-Bact-129 0785-a-A-21 (GACTACHVGGGTATCTAATCC) [57] and sequenced in the MiSeq system of Illumina at the facilities of Parque Científico de Madrid (Tres Cantos, Spain). Barcodes appended to 3′ and 5′ terminal ends of the PCR amplicons allowed separation of forward and reverse sequences in a second PCR-reaction. DNA concentration of the PCR products was quantified in a 2100 Bioanalyzer system (Agilent, Santa Clara, CA, USA). After pooling the PCR products at about equal molar ratios, DNA amplicons were purified by using a QIAEX II Gel Extraction Kit (Qiagen, Hilden, Germany) from the excised band having the correct size after running on an agarose gel. DNA concentration was then quantified with PicoGreen (BMG Labtech, Jena, Germany). The pooled, purified and barcoded DNA amplicons were sequenced using the Illumina MiSeq pair-end protocol (Illumina Inc., San Diego, CA, USA) following the manufacturer’s protocols.
2.8. Bioinformatic Analysis
Raw sequence data were demultiplexed and quality filtered using Illumina MiSeq Reporter analysis software. Microbiome bioinformatics was done with QIIME 2 2019.1 [58]. Denoising was performed with DADA2 [59]. Taxonomy was assigned to ASVs using the q2-feature-classifier [60] and the naïve Bayes classifier classify-sklearn against the SILVA database version 132 [61]. Posterior bioinformatic analysis was conducted using the R version 3.5.1 (https://www.R-project.org) [62]. A table of Operational Taxonomic Units (OTUs) counts per sample was generated, and bacterial taxa abundances were normalized to the total number of sequences in each sample. The relative abundance values of the different bacterial taxa in the three groups of CVL samples (control, RA and INF) were analyzed using the linear discriminant analysis (LDA) effect size (LEfSe) algorithm [63] in an online version (http://huttenhower.sph.harvard.edu/galaxy/). Alpha diversity was studied with the Shannon and Simpson diversity indexes with the R Vegan package (Version 2.5.6) (https://github.com/vegandevs/vegan/). Beta diversity was studied using principal coordinates analysis (PCoA) to visually display patterns of bacterial profiles at the genus level through a distance matrix containing a dissimilarity value for each pairwise sample comparison. The Bray–Curtis and binary Jaccard indices were used for quantitative (relative abundance) and qualitative analyses (presence/absence), respectively. Analysis of variance of the distance matrices was performed with the “nonparametric MANOVA test” Adonis with 999 or permutational multivariate ANOVA (PERMANOVA) with 999 permutations with the R Vegan package. The heatmap graph was generated by using gplots package. Dendogram linkages were based on the relative abundance of the 20 most abundant bacterial genera within the samples and on the complete linkage method for hierarchical clustering (hclust function).
2.9. Immunological Analysis
The concentrations of several soluble immune factors (IL1β, IL1ra, IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12, IL13, IL15, IL17, IL6, basic FGF, eotaxin, GCSF, GMCSF, IFNγ, MCP1, MIP1α, MIP1β, PDGF-BB, RANTES, TNFα, VEGF) were determined by magnetic bead-based multiplex immunoassays, using a Bioplex 200 instrument (Bio-Rad, Hercules, CA, USA) and the Bio-Plex Pro™ Human Cytokine 27-plex Assay (ref. M500KCAF0Y, Bio-Rad). In parallel, the levels of TGF-β 1 and TGF-β 2 were measured by ELISA with the RayBio® Human TGF-β 1 and Human TGF-β 2 ELISA kits, respectively (RayBiotech, Norcross, GA, USA). All determinations were carried out following the manufacturer’s protocols and standard curves were performed for each analyte.
2.10. Statistical Analysis
Microbiological data were recorded as CFU/mL and transformed to logarithmic values before statistical analysis. The normality of data distribution was analyzed using the Shapiro–Wilks test. Then, the quantitative variables were expressed as means and 95% confidence intervals (CI) or standard deviations (SD) when normally distributed and as medians and interquartile ranges (IQR) if they did not follow a normal distribution. The qualitative values were presented as total number of events and percentages. One-way ANOVA tests were used to compare the means of the experimental groups and Scheffé post hoc tests were used to identify which pairs of means were statistically different. The effect of the probiotic intervention on several vaginal parameters in each group of women with reproductive failure was analyzed using one-way ANOVA repeated measures tests. The Fisher’s exact probability test, or the Freeman–Halton extension of the Fisher exact probability test for a 2 × 3 contingency table, was used for comparison of proportions and frequencies. For non-parametric analyses, differences between groups were assessed using Kruskal–Wallis tests and Wilcoxon–Mann–Whitney tests to identify which pair of groups were different, with Bonferroni correction for multiple comparisons when indicated. Correlations between the 20-major relative abundant bacterial genera were visualized using R package qgraph [64]. Statistical analysis and plotting were performed either using Statgraphics Centurion XVIII version 18.1.06 (Statgraphics Technologies, Inc., The Plains, VA, USA) or in the R environment (version 3.5.1; R-project, http://www.r-project.org) and ggplot2 [Wickham, 2016]. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterization of Vaginal-Relevant Properties of L. salivarius CECT5713
L. salivarius CECT5713 showed inhibitory antimicrobial activity (inhibition zone > 2 mm around the streak) against all the G. vaginalis, S. agalactiae, C. albicans, C. glabrata, C. parapsilosis, and U. urealyticum strains used as indicators in this study. The strain was able to form large, well defined co-aggregates with all the selected vaginal and cervical pathogens. Co-aggregation was particularly intense with G. vaginalis, S. agalactiae, and C. albicans strains. In this study, the strain tested was strongly adhesive to vaginal epithelial cells, a mean (±SD) of 329 (±46) adherent lactobacilli in 20 random microscopic fields. The mean (±SD) value for L. salivarius CECT9145, a control strain with a high adherence to vaginal cells, was 336 (±52) adherent lactobacilli in 20 microscopic fields. Extracellular amylase production by L. salivarius CECT5713 was observed by the zone of clearance around the colonies (~2.0 mm) when flooded with iodine solution. Later, when the α-amylase activity was measured, this strain showed a high level of α-amylase activity (0.83 U/mL) at 16 h (concentration of L. salivarius CECT5713: ~8.6 log10 CFU/mL), and could be detected in supernatants at a similar level for up to 48 h (when the assay was finished).
3.2. Demographic, Anthropometric, and Clinical Characteristics of the Participants in the Human Study
The characteristics of the 58 women that participated in this study are presented in Table 1. The mean (95% CI) age in the control group was 34.6 years (33.5–35.8), while in those of repetitive abortions (RA) and with infertility of unknown origin (INF) was 39.4 (38.5–40.4) and 38.0 (37.1–38.9) years, respectively (Table 1). Women in the control group were significantly younger than other participants (p < 0.001; one-way ANOVA), but there were no differences in mean values of body weight and height between the three groups of women (Table 1).
About 71% of the women in the control group had a regular menstrual cycle, while in the other two (RA and INF) this percentage was 48%, although this difference was not statistically significant (p = 0.337; Fisher exact probability tests). No differences were observed in the mean duration of the menstrual cycle that was 28, 27.4, and 27.5 days for women in the control, RA, and INF groups, respectively (Table 1).
Interestingly, statistically significant differences were found between the control women and those in the other two groups regarding a history of recurrent vaginal and urinary tract infections (p = 0.017 and p = 0.006, respectively; Fisher exact probability tests) and the use of antibiotics both during infancy (p < 0.001) and adulthood (p = 0.003), which were higher in the last two groups (Table 1; Supplementary Figure S1). A trend to a higher rate of ORL infections (pharyngitis, otitis) among women with repetitive abortion or infertility was also observed but it did not reach statistical significance (p = 0.057). In contrast, no differences were observed among the three groups in relation to the rates of skin, lower respiratory tract and gastrointestinal infections (Table 1).
3.3. Baseline Vaginal Health Parameters
The vaginal pH values of the control group (4.53; range 4.38–4.68) were statistically different from those of the two study groups: 5.67 (5.55–5.79) and 5.96 (5.84–6.07) for RA and INF, respectively (p = 0.000; one-vay ANOVA). Similarly, the Nugent scores of the two study groups were significantly higher (5.95 (5.54–6.37) and 6.30 (5.91–6.70), respectively), than those from controls (1.79 (1.27–2.30); p = 0.000; one-way ANOVA) (Table 2). The CVL concentrations of the growth factors TGF-β 1, TFG-β 2 and VEFG of the control group were 4.83 (4.65–5.01) pg/mL, 3.22 (3.10–3.34) pg/mL, and 406.0 (322.0–490.0) pg/mL, respectively, while they appeared to be halved in both study groups (RA and INF), the differences being statistically significant (Table 2). No differences were observed among the three groups in relation to the remaining soluble immune factors analyzed in this work, which showed a high degree of interindividual variability (data not shown).
Table 2.
Comparison of baseline vaginal parameters (pH, Nugent score, cytokines, and microbiology) of the participants (n = 58) which included fertile women (Control group), women with a history of repetitive abortion (RA group), and women with infertility of unknown origin (INF group).
| Group | ||||
|---|---|---|---|---|
| Vaginal Parameter | Control (n = 14) | RA (n = 21) | INF (n = 23) | p-Value |
| pH | ||||
| Mean (95% CI) | 4.53 (4.38–4.68) a | 5.67 (5.55–5.79) b | 5.96 (5.84–6.07) b | 0.000 1 |
| Range (min–max) | (4.20–5.00) | (4.70–6.50) | (4.90–6.30) | |
| Nugent score | ||||
| Mean (95% CI) | 1.79 (1.27–2.30) a | 5.95 (5.54–6.37) b | 6.30 (5.91–6.70) b | 0.000 1 |
| Range (min–max) | (0.00–4.00) | (3.00–8.00) | (4.00–8.00) | |
| TGF-β 1, pg/mL | ||||
| Mean (95% CI) | 4.83 (4.65–5.01) a | 2.62 (2.47–2.76) b | 2.19 (2.05–2.33) c | 0.000 1 |
| Range (min–max) | (4.20–5.30) | (1.70–3.80) | (1.50–2.90) | |
| TGF-β 2, pg/mL | ||||
| Mean (95% CI) | 3.22 (3.10–3.34) a | 1.52 (1.43–1.62) b | 1.33 (1.24–1.43) b | 0.000 1 |
| Range (min–max) | (2.70–3.70) | (0.90–2.20) | (0.80–2.00) | |
| VEGF, pg/mL | ||||
| Mean (95% CI) | 406.0 (322.0–490.0) a | 274.8 (206.0–343.0) a,b | 181.2 (116.0–247.0) b | 0.016 1 |
| Range (min–max) | (1.4–929.0) | (95.0–562.0) | (38.0–431.0) | |
| Lactobacilli | ||||
| Positive women | 14 (100) | 12 (57) | 6 (26) | <0.001 3 |
| Viable counts, log10 CFU/mL 2 | ||||
| Mean (95% CI) | 7.24 (6.89–7.60) a | 5.04 (4.66–5.42) b | 5.78 (5.24–6.32) b | 0.000 1 |
| Range (min–max) | (6.80–7.70) | (3.60–6.70) | (3.70–7.50) | |
| L. salivarius qPCR, log10 copies/mL | ||||
| n (%) | 1 (7) | 0 | 0 | |
| Mean (95% CI) | 7.29 |
1 One-way ANOVA tests were used to evaluate differences in mean values between groups. Values followed by different superscript letters within the same row indicate statistically significant differences between groups according to Scheffé post hoc comparison tests. 2 Mean (95% CI) and range (min–max) values in lactobacilli-positive women. 3 Freeman–Halton extension of the Fisher exact probability test for a 2 × 3 contingency table were used to compute the (two-tailed) probability of obtaining a distribution of values of lactobacilli positive women. Abbreviations: TGF-β 1, transforming growth factor β 1; TGF-β 2, transforming growth factor β 2; VEGF, vascular endothelial growth factor.
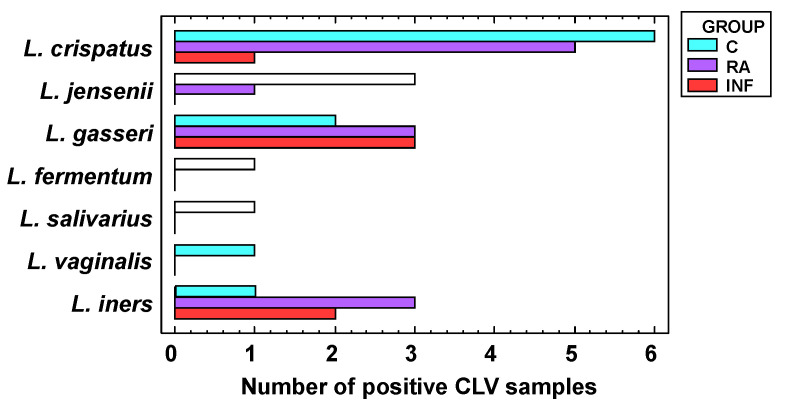
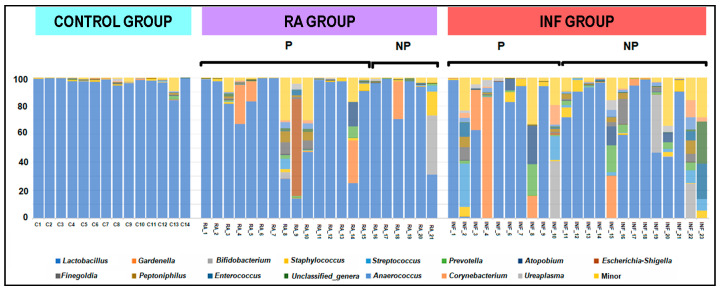
All women of the control group harbored lactobacilli in their vaginas (n = 14), the mean (95% CI) value being 7.24 (6.89–7.60) log10 CFU/mL using culture-dependent assessment. The frequency of lactobacilli detection was lower in the RA and INF groups: 57% and 26%, respectively (p < 0.001; Fisher exact probability tests). In addition, mean lactobacilli concentrations were 2.20 and 1.46 log10 units lower in CVL samples from lactobacilli-positive women in the RA and INF groups, respectively. The lactobacilli profile was also different (Figure 1). Seven species were identified in the samples from women of the control group, including L. crispatus (the dominant species), L. jensenii, L. gasseri, L. iners, Limosilactobacillus (formely Lactobacillus) fermentum, L. salivarius, and Limosilactobacillus vaginalis. However, the lactobacilli species profiles in the study groups (RA and INF) were narrower than in controls and L. fermentum, L. salivarius, and L. vaginalis were not detected. L. crispatus was the dominant species in 6 samples (43%) from fertile women, 5 samples (24%) from women with repetitive abortion and only 1 sample (4%) from infertile women. It is interesting to note that L. iners was isolated only from one CVL sample of the control group while it was isolated from about one-third (5 out of a total of 18 lactobacilli positive samples) from samples of RA and INF groups. L. salivarius was detected in the sample of a unique woman from the control group as determined by species-specific qPCR (7.29 log10 copies/mL) and culture (7.3 log10 CFU/mL) (Table 2). The strain was genetically different from L. salivarius CECT5713 (results not shown).
Figure 1.
Dominant lactobacilli species (when lactobacilli could be isolated) in cervovaginal lavage (CVL) samples of fertile women (C, bluish green), women with repetitive abortion (RA, purple) and women with infertility of unknown origin (INF, red).
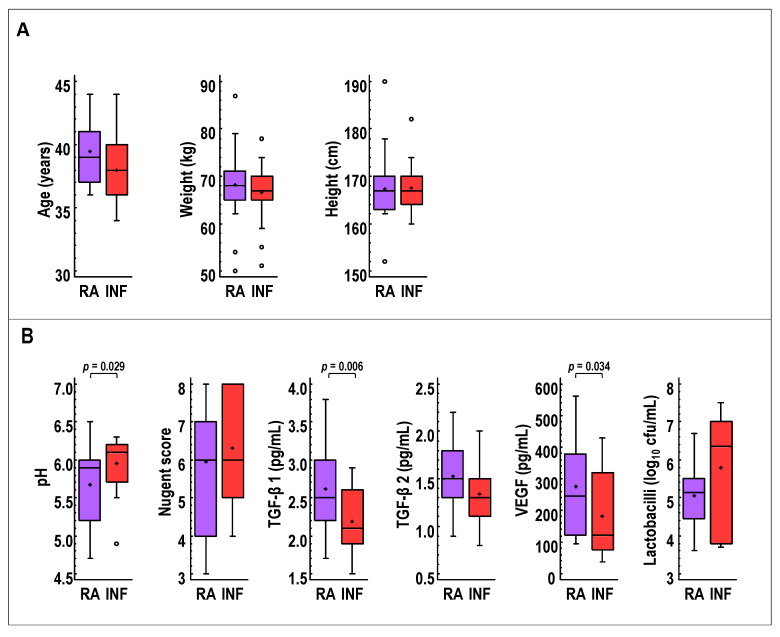
Globally, the comparison of RA and INF groups at the beginning of the study revealed some statistically relevant differences (Figure 2). The mean of the vaginal pH values was 0.29 units higher in the INF group, but the opposite was observed for TGF-β 1 and VEGF, which had mean concentrations 0.43 pg/mL and 94 pg/mL higher, respectively, in the RA group. No differences were observed regarding other characteristics, including age, weight, height, Nugent score, TGF-β 2, and lactobacilli viable counts (Figure 2).
Figure 2.
Comparison of selected baseline (A) demographic characteristics (age, weight and height) and (B) vaginal parameters (pH, Nugent score, TGF-β 1, TGF-β 2, and VEGF concentrations, and viable Lactobacillus counts) in CVL samples of women with repetitive abortion (RA, purple) and women with infertility of unknown origin (INF, red) at recruitment. For each boxplot, the line and the cross within the box represent the median and mean, respectively. The bottom and top boundaries of each box indicate the first and third quartiles (the 25th and 75th percentiles), respectively. The whiskers represent the lowest and highest values within the 1.5 interquartile range (IQR) and the dots outside the rectangles are suspected outliers (>1.5 × IQR). One-way ANOVA tests were used to compare both groups.
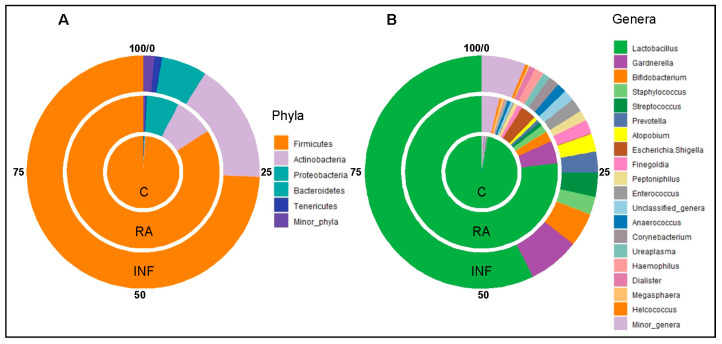
The 16S rRNA gene sequencing analysis of the CVL samples (n = 58) yielded 4,363,364 high quality filtered sequences, ranging from 33,160 to 139,044 per sample (median [IQR] = 73,383 [66,587–82,821] sequences per sample). Sequences were assigned to a total of 23 phyla and 453 genera, and Figure 3 shows the 5 most abundant phyla and the 20 most abundant genera in CVL samples from the fertile control group and from the RA and INF groups. The comparison of the relative abundance (% of total) of sequences at the phylum level from the three groups revealed statistically significant differences with regard to the 4 dominant phyla: Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes (Table 3). The most frequent (present in all samples) and abundant phylum was Firmicutes (Figure 3). The relative abundance of Firmicutes in samples provided by fertile controls (median [IQR] = 99.60% [99.18–99.80%]) was higher than in samples from women of RA and INF groups (median [IQR] = 97.29% [72.34–99.35%] and 89.96% [52.46–98.85%], respectively) (p < 0.001; Kruskal–Wallis rank test with Bonferroni correction) (Table 3). In contrast, the median (IQR) values of the relative abundance of Actinobacteria, Proteobacteria, and Bacteroidetes were higher in women of the RA and INF groups (p < 0.012, p < 0.003, and p < 0.006, respectively; Kruskal–Wallis rank tests with Bonferroni correction) (Table 3).
Figure 3.
Pie charts showing the percentages of the relative abundances of the 5 most abundant phyla (A) and the 20 most abundant genera (B) in the CVL samples from healthy fertile women (inner pie charts; C group), women with a history of repetitive abortion (middle pie charts; RA group), and women with infertility of unknown origin (outer pie charts; INF group).
Table 3.
Relative frequencies, medians and interquartile range (IQR) of the most abundant bacterial phyla and genera detected in CVL samples from fertile women (Control group), women with a history of repetitive abortion (RA group), and women with infertility of unknown origin (INF group).
| Control (n = 14) | RA (n = 21) | INF (n = 23) | |||||
|---|---|---|---|---|---|---|---|
| Phylum Genus |
n (%) 1 |
Median (IQR) |
n (%) |
Median (IQR) |
n (%) |
Median (IQR) |
p-Value 2 |
| Firmicutes | 14 (100) |
99.60 (99.18–99.80) |
21 (100) |
97.29 (72.34–99.35) |
23 (100) |
89.96 (52.46–98.85) |
0.001 |
| Lactobacillus | 14 (100) |
97.88 (96.92–99.31) |
21 (100) |
93.49 (67.18–97.53) |
23 (100) |
71.95 (0.76–94.09) |
0.001 |
| Staphylococcus | 13 (93) |
0.31 (0.11–0.66) |
19 (90) |
0.45 (0.03–1.51) |
22 (96) |
0.75 (0.14–5.40) |
0.260 |
| Streptococcus | 9 (64) |
0.02 (<0.01–0.03) |
14 (67) |
0.01 (<0.01–0.34) |
16 (70) |
0.06 (<0.01–2.04) |
0.180 |
| Finegoldia | 13 (93) |
0.17 (0.03–0.28) |
18 (86) |
0.16 (0.07–0.61) |
17 (74) |
0.12 (0.02–1.24) |
0.760 |
| Peptoniphilus | 11 (79) |
0.06 (0.01–0.21) |
16 (76) |
0.10 (0.02–0.49) |
17 (74) |
0.09 (<0.01–1.45) |
0.670 |
| Enterococcus | 2 (14) |
<0.01 (<0.01–<0.01) |
6 (29) |
<0.01 (<0.01–0.04) |
12 (52) |
0.01 (<0.01–0.19) |
0.044 |
| Anaerococcus | 11 (79) |
0.03 (0.01–0.16) |
18 (86) |
0.10 (0.05–0.30) |
18 (78) |
0.12 (0.01–1.71) |
0.220 |
| Actinobacteria | 12 (86) |
0.09 (0.02–0.20) |
21 (100) |
0.32 (0.08–7.87) |
23 (100) |
4.84 (0.1–34.36) |
0.012 |
| Gardnerella | 4 (29) |
<0.01 (<0.01–0.01) |
11 (52) |
0.01 (<0.01–0.12) |
9 (39) |
<0.01 (<0.01–0.04) |
0.300 |
| Bifidobacterium | 3 (21) |
<0.01 (<0.01–<0.01) |
9 (43) |
<0.01 (<0.01–0.07) |
9 (39) |
<0.01 (<0.01–0.03) |
0.300 |
| Atopobium | 2 (14) |
<0.01 (<0.01–<0.01) |
7 (33) |
<0.01 (<0.01–0.01) |
13 (57) |
0.02 (<0.01–0.12) |
0.015 |
| Proteobacteria | 1 (93) |
0.07 (0.02–0.10) |
21 (100) |
0.28 (0.09–0.69) |
22 (96) |
0.23 (0.09–0.64) |
0.003 |
| Escherichia/Shigella | 1 (7) |
<0.01 (<0.01–<0.01) |
9 (43) |
<0.01 (<0.01–0.02) |
8 (35) |
<0.01 (<0.01–0.01) |
0.084 |
| Bacteroidetes | 10 (71) |
0.03 (<0.01–0.08) |
18 (86) |
0.16 (0.06–1.33) |
22 (96) |
0.80 (0.05–3.19) |
0.006 |
| Prevotella | 8 (57) |
0.02 (<0.01–0.08) |
15 (71) |
0.06 (<0.01–0.45) |
19 (83) |
0.70 (0.01–2.55) |
0.660 |
| Tenericutes | 6 (43) |
<0.01 (<0.01–0.16) |
5 (24) |
<0.01 (<0.01–<0.01) |
10 (43) |
<0.01 (<0.01–0.97) |
0.290 |
| Minor phyla | 14 (100) |
0.13 (0.07–0.18) |
21 (100) |
0.16 (0.07–0.65) |
23 (100) |
0.17 (0.09–1.29) |
0.280 |
| Minor genera | 14 (100) |
0.30 (0.09–0.70) |
21 (100) |
0.91 (0.27–2.54) |
23 (100) |
2.26 (0.40–8.35) |
0.038 |
| Unclassified_genera | 14 (100) |
0.09 (0.05–0.12) |
21 (100) |
0.13 (0.07–0.66) |
23 (100) |
0.14 (0.04–0.36) |
0.170 |
1n (%): Number of samples in which the phylum/genus was detected (relative frequency of detection). 2 Kruskal–Wallis rank tests with Bonferroni correction.
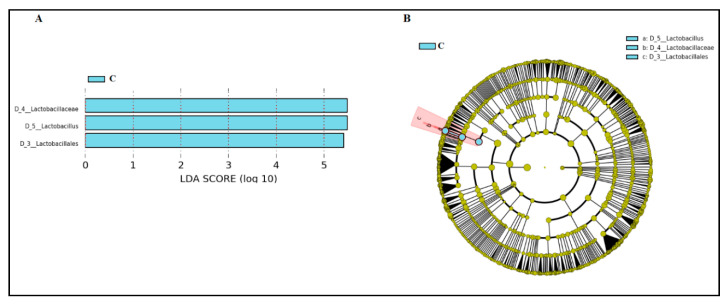
The only bacterial genus that was detected in all samples was Lactobacillus, but there were significant differences in its relative abundance in samples from the three groups (Table 3; Figure 3). The median [IQR] relative abundance of Lactobacillus in CVL samples from women of RA and INF groups (93.49% [67.18–97.53%] and 71.95% [0.76–94.09%], respectively) was lower than in samples from fertile control women (97.88% [96.92–99.31%]) (p = 0.001; Kruskal–Wallis rank test with Bonferroni correction) (Table 3). In fact, the only bacterial genus that characterized and differentially explained the greatest difference between the microbial communities in CVL samples between fertile control women and women of RA and INF groups was Lactobacillus, according to the LEfSe analysis (Figure 4).
Figure 4.
LEfSe analysis identifying taxonomic differences in the microbiota of CVL samples from healthy fertile women (C, bluish green) and women with repetitive abortion (RA) and with infertility of unknown origin (INF). Differentially abundant bacterial taxa were identified using linear discriminant analysis (LDA) and the effect size (LEfSe) algorithm. (A) Histogram of LDA scores (absolute LDA (log10) score > 2.0, p < 0.05) showing the substantial enrichment of Lactobacillus in the microbiota profile of the CVL samples from healthy fertile women. (B) Cladogram showing LEfSe comparison of differential bacterial taxa in CVL samples. The central point represents the root of the bacterial tree and each ring the next lower taxonomic level from phylum to genus (from the inner to the outer ring: phylum, class, order, family, and genus). The color node (other than yellow) indicates which taxa are significantly higher in relative abundance, and the diameter of the node is proportional to the relative abundance of the taxon.
Other genera were present in a variable number of samples, ranging from 96% (Staphylococcus in the INF group) to 7% (Escherichia/Shigella in the control group), but the median relative abundance of any of these genera was <1% (Table 3). The bacterial profile at the genus level in some individual samples from women in the RA and INF groups did not differ from that of samples from women from the fertile control group, which were highly homogenous (Figure 5). However, aberrant profiles with reduced content or even complete absence of Lactobacillus were registered in some samples from women of the RA and INF groups (Figure 5).
Figure 5.
Relative abundance of the predominant bacterial genera in CVL samples of healthy fertile women (C), women with repetitive abortion (RA) and women with infertility of unknown origin (INF). In women with a history of reproductive failure, because either of recurrent miscarriage (RA group) or infertility (INF groups), P indicates the group of women who got pregnant after the probiotic intervention with L. salivarius CECT5713 and NP those women who did not.
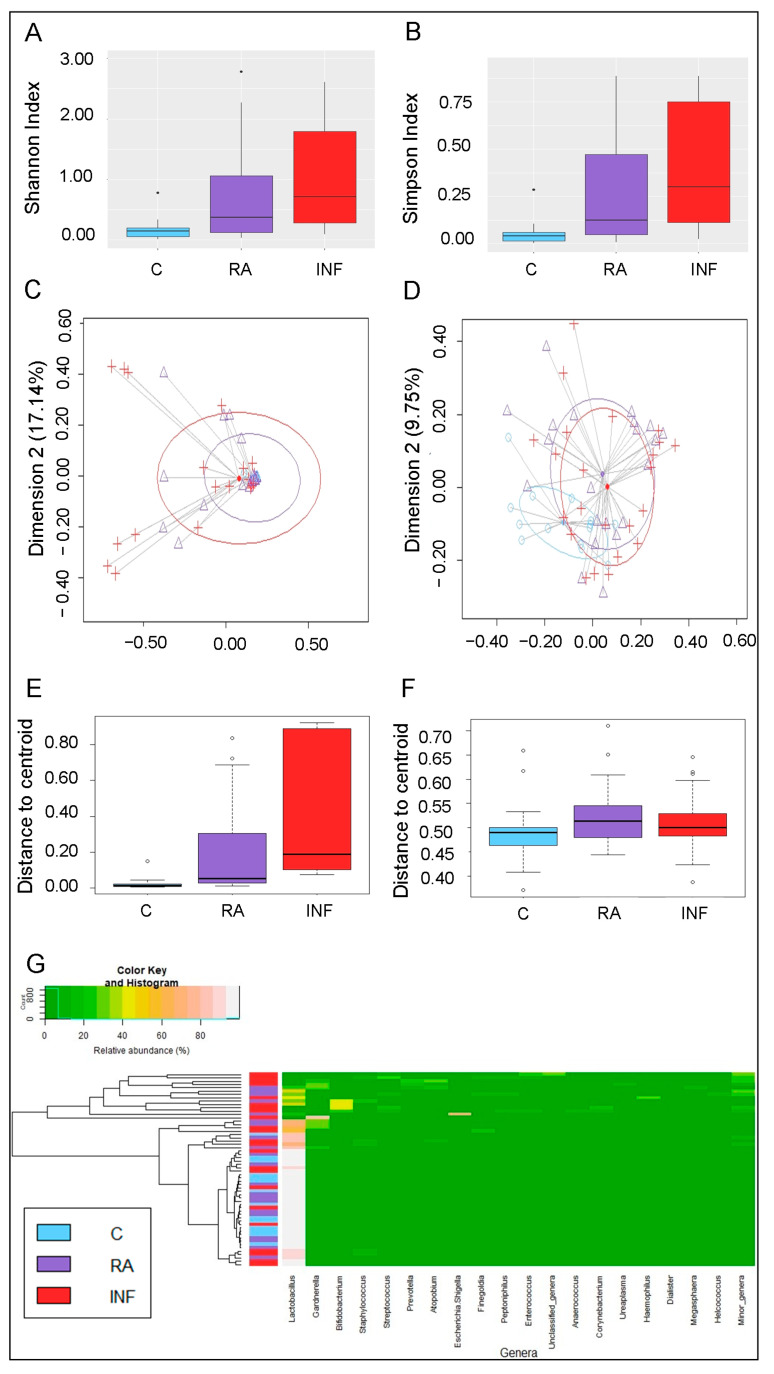
The analysis of alpha diversity at the genus level, calculated either by the Shannon or the Simpson’s indices, revealed significant differences between the vaginal microbiota of women in the fertile and INF groups (p < 0.001; Kruskal–Wallis tests with Bonferroni correction) (Figure 6A,B).
Figure 6.
Metataxonomic profiles of CVL samples of healthy fertile women (C; bluish green), women with repetitive abortion (RA; purple) and women with infertility of unknown origin (INF; red). (A) Comparison of alpha diversity at genus level calculated using the Shannon index between the three groups of women. (B) Comparison of alpha diversity at genus level calculated using the Simpson index between the three groups of women. (C) Principal coordinate analysis (PCoA) plots of bacterial profiles at the genus level based on the Bray–Curtis dissimilarity analysis (relative abundance). (D) Principal coordinate analysis (PCoA) plots of bacterial profiles at the genus level based on the Jaccard’s coefficient for binary data (presence or absence). The values on each axis label in graphs C and D represent the percentage of the total variance explained by that axis. The differences between groups of CVL samples were analyzed using the PERMANOVA test with 999 permutations. (E) Comparison of the mean distances of samples to the centroids in the PCoA plots based on the Bray–Curtis dissimilarity index in each group. (F) Comparison of the mean distances of samples to the centroids in the PCoA plots based on the Jaccard’s coefficient (graph D) in each group. (G) Heatmap showing the relative abundance of the 20 most abundant bacterial genera (x axis) detected in CVL samples. The relative abundance of each bacterial genus within each sample is indicated by the color of the scale ranging from white (high relative abundance) to green (low relative abundance) as indicated in the scale shown at the left down corner. Dendrogram linkages are based upon relative abundance of the genus within the samples and hclust was used as the clustering algorithm. The column between the dendrogram of the vaginal samples and the individual values of the relative abundance of bacterial genera indicates the study group (control fertile women: C, in bluish green; women with repetitive abortion: RA, in purple; women with infertility of unknown origin: INF, in red). The differences between groups (C, healthy fertile women; RA, women with repetitive abortion; INF, women with infertility of unknown origin) were analyzed using Kruskal–Wallis tests with Bonferroni correction for data in panels A and B, and with one-way ANOVA tests for data in panels E and F.
The analysis of the beta diversity, calculated according to the relative abundance of bacterial genera (Bray–Curtis distance) and the presence/absence of bacterial genera (Binnary Jaccard distance matrix), indicated that the profiles of bacterial genera of CVL samples of the 3 groups clustered apart (p = 0.004 and p = 0.002, respectively; PERMANOVA) (Figure 6C,D). In addition, samples from fertile controls clustered closer (shorter distance to centroid) according to the relative abundance of bacterial genera (Bray–Curtis distance) than those from RA and INF groups, indicating that the bacterial profiles in CVL samples from controls were highly uniform (Figure 6E,F).
An initial assessment of potentially dominant patterns in the bacteriological profile of the CVL samples is shown in the heatmap plot presented in Figure 6G. There was a clear separation of samples based on the presence of Lactobacillus. One cluster was characterized by the marked and almost exclusively presence of Lactobacillus in CVL samples. This cluster comprised all the samples from fertile women although not exclusively, because it included also some samples from the RA and INF groups. The second cluster was characterized by the absence or reduced presence of Lactobacillus and the presence of multiple bacterial genera, such as Gardenella and Bifidobacterium. This second cluster contained exclusively CVL samples from the RA and INF groups. Although globally there was no clear separation between the CVL samples from the three groups, it was perceived a higher similarity between samples from the fertile control group and women with a history of repetitive abortion than between the fertile control group and women with infertility of unknown origin (Figure 6G).
3.4. Main Outcome of the Clinical Trial: Pregnancies and Successful Pregnancies
Administration of L. salivarius CECT5713 (~9 log10 CFU/day) for 6 months (or until a diagnosis of pregnancy if this happened first) to the women of the RA and INF groups led to 29 pregnancies out of the 44 participating patients. This means a pregnancy effectiveness of 66% with a 95% CI of 52–80% (Table 4). Among them, there were 25 successful pregnancies and 4 abortions. This means an effectiveness for reproductive success of 57% with a 95% CI of 42–72% (Table 4). Interestingly, all successful pregnancies led to full-term singletons (gestational age ≥ 38 weeks).
Table 4.
Main outcomes after the probiotic treatment with L. salivarius CECT5713 in women with repetitive abortion (RA) and women with infertility of unknown origin (INF).
| Group | Total | Ratio (95% CI) |
||
|---|---|---|---|---|
| Outcome | RA | INF | RA + INF | (RA/INF) |
| Pregnancy (events/total events) | 17/21 | 12/23 | 29/44 | |
| Pregnancy effectiveness (95% CI) |
81% (64–98%) |
52% (32–73%) |
66% (52–80%) |
1.55 (1.00–2.42) |
| Successful pregnancy 1 (events/total events) | 15/21 | 10/23 | 25/44 | |
| Reproductive success (95% CI) |
71% (52–91%) |
43% (23–64%) |
57% (42–72%) |
1.64 (0.96–2.82) |
1 Two women in each group end up in abortion.
Women of the RA group had the highest rate of reproductive success (15 full term pregnancies and 2 abortions out of 21 participants) (Table 4). The rate in the INF group was lower although still noticeable: 12 pregnancies (10 full term and 2 abortions) out of 23 enrolled. Therefore, the pregnancy effectiveness and successful pregnancy rates (95% CI) tended to be higher in RA group that in INF group (RR [95% CI] = 1.55 [1.00–2.42] and 1.64 [0.96–2.82], respectively), although the difference between both groups did not reach statistical significance (Table 4). It must be highlighted that all women of these groups had been unsuccessfully subjected to ART interventions in previous attempts to avoid spontaneous miscarriage (RA group) or to get pregnant (INF group).
3.5. Secondary Outcomes Associated with the Probiotic Treatment: RA Group
There were no differences in age, weight, or height between women in the RA group that ended up having a successful pregnancy (n = 15) and those who did not (n = 6) after the probiotic intervention. However, differential changes in their vaginal parameters were observed (Table 5). The vaginal pH of women who delivered was about 0.9 units lower than in those who did not (p < 0.001; one-way ANOVA). Similar results were noted for the Nugent score (a mean [95% CI]) reduction of 3.33 [3.73–2.93] units in women who got pregnant after the probiotic intervention versus a mean [95% CI] reduction of 0.67 [1.29–0.04] units in those who did not complete a full-term pregnancy; p = 0.000 one-way ANOVA) (Table 5, Supplementary Figure S2). In fact, the probiotic treatment did not modify the Nugent score in those women that did not get pregnant (p = 0.102; one-way repeated measures ANOVA) (Table 5).
Table 5.
Effect of the probiotic intervention with L. salivarius CECT5713 on the vaginal parameters of women who were able to complete a full-term pregnancy (n = 15) and of those who did not (n = 6) among the women that had a history of repetitive abortion (RA group; n = 21).
| Probiotic Intervention Resulted in Pregnancy | |||
|---|---|---|---|
| Yes (n = 15) | No (n = 6) | ||
| Vaginal Parameter | Mean (95% CI) | Mean (95% CI) | p-Value 1 |
| pH | |||
| Baseline | 5.58 (5.39–5.77) | 5.88 (5.58–6.18) | 0.221 |
| Post-intervention | 4.45 (4.34–4.57) | 5.65 (0.13–5.46) | 0.000 |
| Change | −1.13 (−1.27–−0.99) | −0.23 (−0.45–−0.01) | <0.001 |
| p-value 3 | 0.000 | 0.002 | |
| Nugent score | |||
| Baseline | 5.87 (5.24–6.49) | 6.17 (5.18–7.15) | 0.708 |
| Post-intervention | 2.53 (2.13–2.94) | 5.50 (4.86–6.14) | 0.000 |
| Change | −3.33 (−3.73–−2.93) | −0.67 (−1.29–−0.04) | 0.000 |
| p-value 3 | 0.000 | 0.102 | |
| TGF-β 1, pg/mL | |||
| Baseline | 2.81 (2.62–3.00) | 2.15 (1.85–2.45) | 0.014 |
| Post-intervention | 4.21 (4.05–4.36) | 2.47 (2.22–2.71) | 0.000 |
| Change | 1.40 (1.18–1.62) | 0.32 (−0.02–0.66) | <0.001 |
| p-value 3 | 0.000 | 0.098 | |
| TGF-β 2, pg/mL | |||
| Baseline | 1.67 (1.57–1.78) | 1.15 (0.99–1.31) | <0.001 |
| Post-intervention | 2.93 (2.81–3.05) | 1.30 (1.11–1.49) | 0.000 |
| Change | 1.25 (1.12–1.38) | 0.15 (−0.05–0.35) | 0.000 |
| p-value 3 | 0.000 | 0.328 | |
| VEGF, pg/mL | |||
| Baseline | 341 (300–382) | 109 (44–173) | <0.001 |
| Post-intervention | 743 (640–846) | 138 (−25–301) | <0.001 |
| Change | 402 (319–485) | 29 (−102–160) | 0.002 |
| p-value 3 | 0.000 | 0.189 | |
| Lactobacilli presence, n (%) | |||
| Baseline | 9 (60) | 3 (50) | 0.523 2 |
| Post-intervention | 15 (100) | 4 (67) | 0.071 2 |
| Change | 6 (40) | 1 (17) | 0.613 2 |
| Lactobacilli counts, log10 CFU/mL | |||
| Initial | 4.99 (4.48–5.50) | 5.20 (4.31–6.09) | 0.752 |
| Final | 6.52 (6.22–6.81) | 4.74 (4.17–5.31) | <0.001 |
| Change | 2.44 (1.84–3.04) | 0.16 (−0.99–1.32) | 0.019 |
| p-value 3 | <0.001 | 0.697 | |
| L. salivarius qPCR, n (%) | |||
| Initial | nd | nd | |
| Final | 15 (100) | 3 (50) | 0.015 2 |
| L. salivarius qPCR, log10 copies/mL 4 | |||
| Initial | - | - | |
| Final | 6.85 (6.58–7.12) | 2.63 (0.41–3.24) | <0.000 |
1 One-way ANOVA tests were used to evaluate differences in mean values between groups, except for lactobacilli presence. 2 Fisher exact probability test for a 2 × 2 contingency table. 3 One-way repeated measures ANOVA tests were used to determine whether there was a change in each group of participants when comparing the baseline and post-intervention parameters. 4 Mean (95% CI) of L. salivarius qPCR (copies/mL) in positive samples.
The vaginal cytokine concentrations also differed in both subgroups of women (with successful pregnancy or not) in the RA group after the probiotic treatment. There was no modification in the vaginal TGF-β 1, TGF-β 2, and VEGF concentrations with respect to the baseline in the women who did not become pregnant, but there was a mean (95% CI) significant increase of 1.40 (1.18–1.62) pg/mL, 1.25 (1.12–1.38) pg/mL, and 402 (319–485) pg/mL, respectively, in those who did (p = 0.000; one-way repeated measures ANOVA) (Table 5, Supplementary Figure S2). In addition, it should be noted that there were already differences in the concentration of these cytokines even before starting the treatment between those that became and those that did not become pregnant (Table 5).
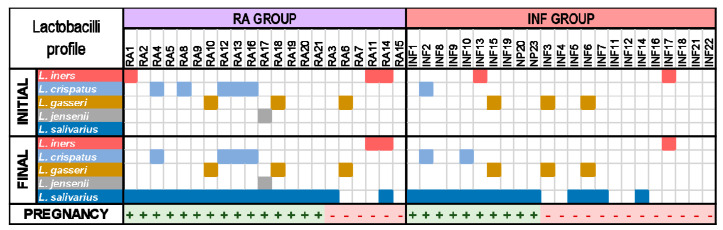
On the other hand, the probiotic treatment resulted in a mean (95% CI) increase in lactobacilli counts of 2.12 (1.66–2.59) log10 CFU/mL in women that finally got pregnant, but there was no change in those that did not (Table 5, Supplementary Figure S2). The presence of L. salivarius (mean [95% CI] = 6.85 [6.58–7.12] log10 copies/mL) was confirmed by qPCR in all women that got pregnant, but only in 50% of the women with unsuccessful pregnancies, their concentration being significantly lower (mean [95% CI] = 2.63 [0.41–3.24] copies/mL) (Table 5). The lactobacilli profile in CVL samples obtained at the beginning of the probiotic treatment and after 6 months or until a diagnosis of pregnancy is presented in Figure 7. The most noticeable difference was the presence of viable L. salivarius in most women (17/21) after the probiotic treatment. In addition, L. iners, which was present in 3 women at the beginning of the study, was isolated at the end of the treatment only from 2 women who did not end up in pregnancy. There were no differences in the metataxonomic profile at the genus level of CVL samples from women of the RA group regarding the pregnancy outcome (Figure 5; Supplementary Table S1).
Figure 7.
Changes in the profile of dominant Lactobacillus species in CVL samples from women with a history of repetitive abortion (RA group) and women with infertility of unknown origin (INF group) after the probiotic intervention with L. salivarius CECT5713. The outcome is indicated in the last file: +, successful full-term pregnancy and -, no pregnancy. The presence of isolates from a given species is indicated by a colored square.
3.6. Secondary Outcomes Associated with the Probiotic Treatment: INF Group
The women in the INF group that got pregnant after the probiotic intervention (n = 10) and those who did not (n = 13) did not differ in age, weight and height. The CVL pH and the Nugent score decreased significantly in all members of the INF group after the probiotic treatment (p < 0.05; one-way repeated measures ANOVA), although the magnitude of the change was smaller in the women that did not get pregnant when compared to those that got pregnant (Table 6; Supplementary Figure S2). Specifically, the mean (95% CI) reductions in CVL pH and Nugent score in women that got pregnant were −1.32 (−1.43–−1.21) and −3.90 (−4.25–−3.55), respectively, and in women that did not get pregnancy these reductions were only −0.19 (−0.29–−0.09) and−0.54 (−0.85–−0.23), respectively (Table 6; Supplementary Figure S2).
Table 6.
Effect of the probiotic intervention with L. salivarius CECT5713 on the vaginal parameters of women who were able to complete a full-term pregnancy (n = 15) and of those who did not (n = 6) among the women with infertility of unknown origin (INF group; n = 23).
| Probiotic Intervention Resulted in Pregnancy | |||
|---|---|---|---|
| Yes (n = 15) | No (n = 6) | ||
| Vaginal Parameter | Mean (95% CI) | Mean (95% CI) | p-Value 1 |
| pH | |||
| Baseline | 5.85 (5.70–6.00) | 6.04 (5.58–6.17) | 0.190 |
| Post-intervention | 4.53 (4.42–4.64) | 5.85 (5.75–5.95) | 0.000 |
| Change | −1.32 (−1.43–−1.21) | −0.19 (−0.29–−0.09) | 0.000 |
| p-value 3 | 0.000 | 0.002 | |
| Nugent score | |||
| Baseline | 6.00 (5.40–6.60) | 6.54 (6.01–7.07) | 0.334 |
| Post-intervention | 2.10 (1.61–2.59) | 6.00 (5.57–6.43) | 0.000 |
| Change | −3.90 (−4.25–−3.55) | −0.54 (−0.85–−0.23) | 0.000 |
| p-value 3 | 0.000 | 0.028 | |
| TGF-β 1, pg/mL | |||
| Baseline | 2.29 (2.10–2.48) | 2.11 (1.94–2.28) | 0.308 |
| Post-intervention | 4.58 (4.41–4.75) | 2.18 (2.04–2.33) | 0.000 |
| Change | 2.29 (2.16–2.42) | 0.08 (−0.04–0.19) | 0.000 |
| p-value 3 | 0.000 | 0.281 | |
| TGF-β 2, pg/mL | |||
| Baseline | 1.56 (1.46–1.66) | 1.16 (1.07–1.25) | <0.001 |
| Post-intervention | 2.81 (2.68–2.94) | 1.26 (1.15–1.38) | 0.000 |
| Change | 1.25 (1.13–1.37) | 0.10 (<−0.01–0.20) | 0.000 |
| p-value 3 | 0.000 | 0.203 | |
| VEGF, pg/mL | |||
| Baseline | 311 (279–343) | 81 (53–109) | 0.000 |
| Post-intervention | 773 (695–850) | 87 (19–155) | 0.000 |
| Change | 462 (411–513) | 6 (−39–50) | 0.000 |
| p-value 3 | 0.000 | 0.165 | |
| Lactobacilli presence, n (%) | |||
| Baseline | 3 (30) | 3 (23) | 0.537 2 |
| Post-intervention | 10 (100) | 6 (46) | 0.007 2 |
| Change | 7 (70) | 3 (23) | 0.040 2 |
| Lactobacilli counts, log10 CFU/mL | |||
| Initial | 5.00 (3.22–6.78) | 6.57 (4.78–8.35) | 0.290 |
| Final | 6.46 (5.94–6.98) | 4.95 (4.28–5.62) | 0.017 |
| Change | 3.05 (2.45–3.64) | 0.32 (−0.46–1.09) | <0.001 |
| p-value 3 | <0.001 | 0.451 | |
| L. salivarius qPCR, n (%) | |||
| Initial | nd | nd | |
| Final | 10 (100) | 4 (31) | 0.002 2 |
| L. salivarius qPCR, log10 copies/mL 4 | |||
| Initial | - | - | |
| Final | 6.48 (6.28–6.68) | 3.55 (3.24–3.86) | 0.000 |
1 One-way ANOVA tests were used to evaluate differences in mean values between groups, except for lactobacilli presence. 2 Fisher exact probability test for a 2 × 2 contingency table. 3 One-way repeated measures ANOVA tests were used to determine whether there was a change in each group of participants when comparing the baseline and post-intervention parameters. 4 Mean (95% CI) of L. salivarius qPCR (copies/mL) in positive samples.
The change in the vaginal cytokine concentrations after the probiotic treatment was similar to that described in the RA group: There was no modification in the vaginal TGF-β 1, TGF-β 2, and VEGF levels of women who did not become pregnant, but there was a mean (95% CI) significant increase of 2.29 (2.16–2.42) pg/mL, 1.25 (1.13–1.37) pg/mL, and 462 (411–513) pg/mL, respectively, in those who did (Table 6; Supplementary Figure S2). In this INF group, there were already differences in the concentrations of TGF-β 2 and VEGF, but not in that of TGF-β 1, between those that became and those that did not became pregnant even before starting the treatment (Table 6).
The probiotic intervention resulted in a high degree of vaginal colonization by lactobacilli (6.46 [5.94–6.98] log10 CFU/mL) of all women that got pregnant, while this only happened in 46% of those that experienced a treatment failure, the density of lactobacilli reached being significantly lower (4.95 [4.28–5.62] log10 CFU/mL) (Table 6). Similarly to the RA group, the presence of L. salivarius (mean [95% CI] = 6.48 [6.28–6.68] copies/mL) was confirmed by qPCR in all women that got pregnant, but only in 31% of the women with unsuccessful pregnancies and, then, at a lower concentration (mean [95% CI] = 3.55 [3.24–3.86] copies/mL) (Table 6). The main difference in the lactobacilli profile of CVL samples of women in the INF group registered after the probiotic intervention was the detection of viable L. salivarius in all women who got pregnant, but only in 4 out of 13 of those women that failed to get pregnant. There were no differences in the metataxonomic profile at the genus level of CVL samples from women of the RA group regarding the pregnancy outcome (Figure 5; Supplementary Table S2).
3.7. Comparison of Vaginal Parameters between Women Who Became Pregnant and Those Who Did Not from Both the RA and INF Groups
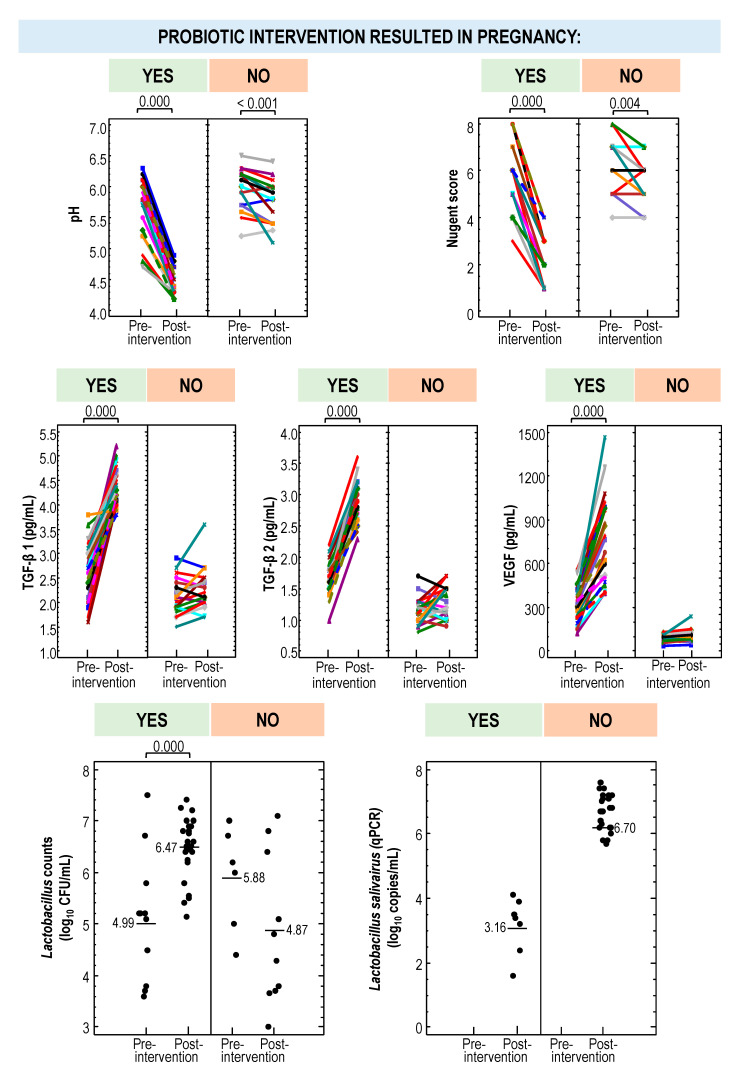
The mean [95% CI] pH value in CVL samples was slightly but significantly more acidic in the women who become pregnant (5.69 [5.57–5.81] units) than in those who did not (5.99 [5.85–6.13] units) (p = 0.024; one-way ANOVA) (Figure 8; Supplementary Table S3). There were also differences in the concentration of vaginal cytokines TGF-β 2 and VEFG at the beginning of the study according to the final pregnancy outcome, but the differences were similar to those described already separately for RA and INF groups (Figure 8; Supplementary Table S3). The only parameters that did not differed initially between both groups were the Nugent score, TGF-β 1 concentration, and the frequency of detection and counts of lactobacilli (Figure 8; Supplementary Table S3). Globally, Lactobacillus was detected in all women who became pregnant, but only in half of those that did not (p < 0.001; Fisher exact probability test).
Figure 8.
Changes in vaginal parameters (pH, Nugent score, TGF-β 1, TGF-β 2, and VEGF concentrations, viable Lactobacillus counts and L. salivarius copies in CVL samples) in women with a history of reproductive failure, because either of recurrent miscarriage (RA group) or infertility (INF groups), after the probiotic intervention with L. salivarius CECT5713 according to their outcome (pregnancy versus no pregnancy).
The probiotic intervention resulted in differential and remarkable changes in the vaginal parameters in those women who became pregnant but not in those who did not (Figure 8; Supplementary Table S3). First, the probiotic administration of L. salivarius CECT5713 resulted to be more effective regarding the change in the vaginal pH and Nugent score in women who got pregnant, which recorded mean (95% CI) decreases of −1.20 (−1.29–−1.12) and −3.56 (−3.82–−3.30) units, respectively (p = 0.000; one-way repeated measures ANOVA). In contrast, the change in these two parameters was smaller (−0.21 (−0.31–−0.10) and −0.58 (−0.88–−0.28) units, respectively) in the group of women who did not get pregnant (Figure 8; Supplementary Table S3). Second, the probiotic intervention led to a significant increase in the concentrations of vaginal cytokines TGF-β 1, TGF-β 2 and VEFG (mean [95% CI] increase of 1.76 [1.60–1.91] pg/mL, 1.25 [1.17–1.33] pg/mL, and 426 [378–473] pg/mL, respectively) in women who got pregnant but no change was registered in the group that did not (Figure 8; Supplementary Table S3). Third, regarding the lactobacilli profile of CVL samples, there was a mean (95% CI) increase of 2.67 (2.26–3.08) log10 CFU/mL units in viable Lactobacillus counts after the probiotic treatment in the group of women who became pregnant as opposed to those that did not. Differences were also noted on the L. salivarius content in CVL samples. This lactobacilli species was detected, and at a high concentration (mean [95% CI] = 6.70 [6.52–6.89] log10 copies/mL), in CVL samples from all women having a successful pregnancy unlike women who did not become pregnant (Figure 8; Supplementary Table S3). The metataxonomic profile at the genus level of CVL samples from women of the INF group was equal in women that did or did not become pregnant, except for a slightly higher relative frequency of Escherichia/Shighella in women that got pregnant (Figure 5; Supplementary Table S4).
3.8. Comparison of Vaginal Parameters between Control Women, All Women Who Became Pregnant and Those Who Did Not from Both RA and INF Groups
The analysis of post-intervention vaginal parameters (pH, Nugent score, TGF-β 1, TGF-β 2, VEGF, lactobacilli counts) revealed that the pH value of CVL samples and Nugent score in women who became pregnant after the probiotic intervention were similar to those of fertile control women (Table 7; Supplementary Figure S3). The concentrations of TGF-β 1, TGF-β 2, and VEGF in post- intervention CVL samples of women who became pregnant were closer to those found in fertile control women, although statistically significant differences were found between them (Table 7; Supplementary Figure S3). Besides, it is remarkable to note that the post-intervention concentration of VEGF in women that became pregnant was about twice that registered in fertile control women (mean [95% CI] = 755.0 [637.1–872.5] pg/mL and 406.0 [322.0–490.0] pg/mL, respectively). There was a high interindividual variation in lactobacilli counts varying from undetectable (in 57% of the women who did not become pregnant) to 7.5 log10 CFU/mL in CVL samples of women who did not become pregnant after the probiotic intervention, but the mean [95% CI] value (4.87 [3.83–5.90] log10 CFU/mL) was lower than in samples of the other participants (Table 7; Supplementary Figure S3). There was less than 1 log10 CFU/mL difference between the lactobacilli viable counts in CVL samples of women who enjoyed a full term pregnancy after the probiotic intervention and those of fertile controls (mean [95% CI] = 6.47 [6.22–6.72] log10 CFU/mL and 7.24 [6.89–7.60] log10 CFU/mL, respectively) (Table 7; Supplementary Figure S3).
Table 7.
Comparison of vaginal parameters (pH, Nugent score, TGF-β 1, TGF-β 2, and VEGF concentrations, and Lactobacillus counts) of all women who were able to complete a full-term pregnancy (n = 25) and of those who did not (n = 19) among all women with a history of repetitive abortion and with infertility of unknown origin (RA and INF groups) after the probiotic intervention with L. salivarius CECT5713 and vaginal parameters of fertile women (Control group; n = 14).
| Probiotic Intervention Resulted in Pregnancy | ||||
|---|---|---|---|---|
| Vaginal Parameter | Control (n = 14) Mean (95% CI) |
Yes (n = 25) Mean (95% CI) |
No (n = 23) Mean (95% CI) |
p-Value |
| pH | 4.53 (4.38–4.68) a | 4.48 (4.39–4.58) a | 5.78 (5.62–5.95) b | 0.000 1 |
| Nugent score | 1.79 (1.27–2.30) a | 2.36 (1.92–2.80) a | 5.84 (5.35–6.33) b | 0.000 1 |
| TGF-β 1, pg/mL | 4.83 (4.65–5.01) a | 4.36 (4.20–4.52) b | 2.27 (2.06–2.48) c | 0.000 1 |
| TGF-β 2, pg/mL | 3.22 (3.10–3.34) a | 2.88 (2.75–3.01) b | 1.27 (1.15–1.40) c | 0.000 1 |
| VEGF, pg/mL | 406.0 (322.0–490.0) a | 755.0 (637.1–872.5) b | 103.3 (82.4–124.1) c | 0.000 1 |
| Lactobacilli | ||||
| Positive women, n (%) | 14 (100) | 25 (100) | 10 (43) | <0.001 2 |
| Viable counts 3, log10 CFU/mL | 7.24 (6.89–7.60) a | 6.47 (6.22–6.72) b | 4.87 (3.83–5.90) c | 0.000 1 |
1 One-way ANOVA tests were used to evaluate differences in mean values between groups. Values followed by different superscript letters within the same row indicate statistically significant differences between groups according to Scheffé post hoc comparison tests. 2 Freeman–Halton extension of the Fisher exact probability tests for a 2 × 3 contingency table were used to compute the (two-tailed) probability of obtaining a distribution of values of lactobacilli positive women. 3 Mean (95% CI) of L. salivarius qPCR (copies/mL) in lactobacilli-positive women. TGF-β 1, transforming growth factor-β 1; TGF-β 2, transforming growth factor-β 2; VEGF, vascular endothelial growth factor.
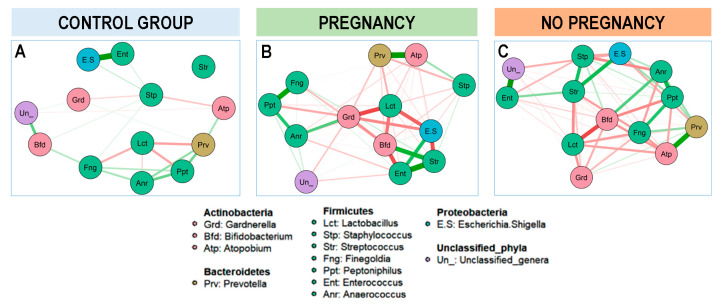
Additionally, a network structure of the baseline vaginal bacterial genera communities on the three different groups of women (fertile controls, women who got pregnant after the probiotic intervention and women who did not get pregnant after the probiotic intervention) was constructed based on the genus-genus correlations (Figure 9). In the group of fertile women, the strongest correlation was observed between two minority genera, Escherichia/Shigella and Enterococcus; the most abundant genera, Lactobacillus, established negative and weak relationship with other Firmicutes (Finegoldia and Peptoniphilus) and Prevotella. In contrast, in the group of women with either repeated abortions or infertility of unknown origin, Lactobacillus showed strong negative association with two genera of the Actinobacteria, Gardenella, and Bifidobacterium. However, in the group of women that responded to the probiotic intervention and ended up in a successful pregnancy, the strongest negative association was between Lactobacillus and Gardenella, while in those women that did not get pregnant this negative association was weaker than that registered between Lactobacillus and Bifidobacterium, indicating that indeed the bacterial profile in CVL samples may indicate different fertility problems (Figure 9).
Figure 9.
Estimated network structures based on a sample of 58 vaginal samples: 14 from healthy fertile women (A, Control group), 25 from women with a successful reproductive outcome after the probiotic intervention L. salivarius CECT5713 (B, Pregnancy) and 19 from women who did not have a successful pregnancy after the probiotic intervention with L. salivarius CECT5713 (C, No pregnancy). The 14 most abundant genera were represented. Red lines indicate negative correlation and green lines indicates positive correlation. The thickness and the intensity of the line reflects the intensity of the correlation.
4. Discussion
In this study, the comparison between the vaginal microbiota of women with a history of reproductive failure, due to recurrent miscarriage or infertility, and healthy fertile women confirmed that dominance of specific species of Lactobacillus in the vaginal microbiota plays a determinant role in the success of human reproduction. Overall, the lowest vaginal pH values and Nugent scores were associated with vaginal communities dominated by lactobacilli, while those with the highest pH values and Nugent scores were associated with a depletion of lactobacilli. Close associations between low pH, low Nugent score and a high concentration and dominance of lactobacilli in the human vagina has been repeatedly reported [3,4,65]. In this study, the frequency of detection of lactobacilli in the vaginal samples was much higher in fertile women (100%) than in women with repetitive miscarriage (57%). Interestingly, infertile women showed the lowest percentage of women from whom lactobacilli could be isolated (26%). Use of antibiotics in both infancy and adulthood was significantly higher among women of the RA and INF groups than among women of the control group. It has been long known that opportunistic vaginal infections may arise as an adverse effect to the use of antibiotics because of their negative effect on the lactobacilli population [66]. The results obtained in this study suggest, for the first time, that an antibiotic-associated depletion of vaginal lactobacilli may have long-term health consequences by impairing fertility or embryo implantation and that such effect may be contrasted reversed by microbiological modulation of the vaginal ecosystem.
The species most frequently isolated from vaginal samples in this study belonged to L. crispatus, L. gasseri, L. iners, and L. jensenii, which are particularly common and abundant in the human vagina and absent or infrequently found in other human habitats [3,32,67]. Stable codominance of multiple Lactobacillus species is rarely observed in the same vaginal community [67]. Initial presence of L. crispatus seemed to be positively correlated with a successful reproductive outcome after the intervention with the probiotic assayed in this study. In contrast, initial presence of L. iners and L. gasseri seemed to be negatively correlated with a successful reproductive outcome after the probiotic intervention unless the L. salivarius strain provided in the trial was able to become dominant in the vaginal samples. L. crispatus and L. iners are probably the most common inhabitants of the healthy human vagina and are able to perform relevant ecological functions in the vaginal environment. Transitions from a vaginal community dominated by L. iners to one dominated by L. crispatus, and viceversa, seems to be relatively frequent [68]. The relationships between these two species and their potential functions have received an increasing scientific interest in the last years [67,68,69,70,71]. However, while there is a general agreement that a L. crispatus-dominated vaginotype promotes vaginal and reproductive health [72,73,74], the role of L. iners is very controversial since this peculiar species has been associated to beneficial roles for vaginal health [8,75] but, also, to dysbiosis, vaginal infections and a variety of gynecological conditions, including adverse pregnant outcomes [69,71,76,77,78]. Functional studies are required to investigate its roles in vaginal bacterial communities and whether, under certain circumstances, it can be used as a biomarker of reproductive failure.
A characterization of some properties of L. salivarius CECT5713 that may be relevant for vaginal and reproductive health showed that this strain was able to inhibit all the clinical isolates of G. vaginalis, S. agalactiae, C. albicans, C. glabrata, C. parapsilosis, and U. urealyticum tested in this study. This antimicrobial activity is relevant since vaginal infections are associated with an increased risk of adverse urogenital and reproductive health outcomes [79]. L. salivarius CECT5713 has a high acidifying ability by producing high amounts of L-lactic acid and small amounts of acetic acid [37]. Eubiosis and dysbiosis in the vaginal communities are distinguished by the high concentration of lactic acid and the high acidity that characterize the eubiosis state [79,80,81], as a direct result of the metabolic activity of the local lactobacilli, which is enough to inactivate reproductive tract pathogens, including viruses, bacteria and yeasts [49,82,83,84,85,86,87]. The capability and rate of production of lactic acid by lactobacilli is strain-specific and only high levels of lactic acid and a concomitant very low pH can inhibit microbial growth efficiently in the local vaginal biofilm [88,89]. From this point of view, L. salivarius CECT5713 seems a suitable candidate as a probiotic for the cervicovaginal target. In addition, this strain encodes an α-amylase in its genome (GenBank: ADJ79335.1), which is fully functional as revealed in the activity assays performed in this work. This enzyme might contribute, together with host α-amylase, to degradation of vaginal glycogen and, therefore, to increase lactic acid production and to maintain the vaginal pH at ≤4.5, promoting the desired lactobacilli dominance in the vaginal ecosystem [90].
Other properties of L. salivarius CECT5713 that are interesting in relation to the control of harmful vaginal microbes include a high rate of adhesion to vaginal cells and co-aggregation with the vaginal pathogens used in this study. High adherence of L. salivarius strains to vaginal cells has been previously observed and related to the prevention of vaginal colonization by S. agalactiae [49]. Both adhesion and co-aggregation activities seem to be highly strain-specific traits [48,49,91,92]. Cell-dependent reduction of Candida spp. adhesion by Lactobacillus species has been related to co-aggregation and competition for binding sites [93,94]. Overall, L. salivarius CECT5713 seems to be a strain suitable for applications involving vaginal homeostasis. This strain was isolated from human milk and infant feces of a healthy mother–child pair [37], and has been shown to be a good probiotic strain due to its extensive repertoire of desirable properties and safety, being particularly suited for application in the mother–infant dyad [38].
In this work, oral administration of L. salivarius CECT5713 to women of the RA and INF groups led to a relevant number of pregnancies. Women of such groups who had term pregnancies experienced significant changes in some key microbiological, biochemical and immunological parameters in the vaginal samples, such as concentration of cultivable lactobacilli, concentration of L. salivarius specific DNA, pH, Nugent score, and concentrations of VEGF, TGF-β 1 and TGF-β 2. The fact that all of them had high concentrations of L. salivarius in the vaginal samples and that DNA from this species was also detected by the qPCR assay reveals that the strain was able to reach and colonize the vaginal mucosa. The significant reductions of the pH values after the treatment indicate that the strain was metabolically active and suggests a good agreement between the in vitro potential of the strain and its in vivo capabilities.
The changes induced by L. salivarius CECT5713 in the concentrations of the growth factors VEGF, TGF-β 1 and TGF-β 2 seem to be particularly relevant and can be considered as biomarkers of the efficacy of the strain for the target pursued in the clinical trial. VEGF is a 45-kDa homodimeric heparin-binding glycoprotein with angiogenic activity that plays a key role as regulator of vasculogenesis, angiogenesis and vascular function in the human endometrium [95,96]. Vasculogenesis and angiogenesis are crucial steps for embryogenesis and particularly for embryo implantation (vessel formation and trophoblastic invasion) and both processes have been correlated with an increased expression of VEGF and VEGF receptors [97,98,99,100,101]; otherwise, endometrial angiogenesis may be impaired and result in a lethal phenotype, ranging from failed implantation to first-trimester miscarriage [95,102,103,104,105].
TGF-β 1 and TGF-β 2 also promote angiogenesis in vivo [106], and participate in implantation, trophoblast differentiation, and immunoregulation at the maternal-fetal interface [100,107]. Transcription of TGF-β 1 increases notably in human uterine endometrium during the first trimester of pregnancy [108], while recurrent pregnancy is associated with a decrease in the decidual TGF-β [109,110,111]. Expression of both VEGF and TGF-β 1 is highly regulated in a temporal and spatial manner during the early stages of implantation, a fact that underlines their critical role in the evolving pregnancy [109,110,111]. In addition, TGF-β 1 increases expression of VEGF in the trophoblast [111,112,113,114,115] suggesting a link between the action of both growth factors.
TGF-β 1 and TGF-β 2 are also of particular interest in this field because of their well-known roles in regulating the inflammatory response and inducing active immune tolerance in mucosal tissues [116,117]. Interestingly, both are present at very high concentrations in human seminal fluid [118,119], acting as male-female signaling agents that regulate the female immune response to sperm after coitus and promote maternal immune tolerance for embryo implantation and subsequent pregnancy [120,121,122,123]. Although studies in mouse models have shown that exposure to the high concentrations of TGF-β present in seminal fluid is absolutely required to boost uterine Treg cells prior to embryo implantation [124,125,126,127,128,129], this fact is not taken into account in many current ARTs, including IVF techniques, where such exposure is absent. Most TGF-β present in human semen is latent and requires activation to bind to receptors on cervical cells [130,131]. Interestingly, activation after coitus is facilitated by the acid pH of the vaginal environment [123] and, in this study, administration of L. salivarius CECT5713 led to an increase of TGF-β 1 and TGF-β 2 concentrations and, concomitantly, to a significant decrease in the vaginal pH values.
Our study has some limitations. First, the microbiota of the genitourinary tract of the partner was not evaluated and some studies have shown that male microbiota may also play a fundamental role in reproductive outcomes [132,133]. In fact, the couple (when applicable) should be considered as a single entity to achieve the best reproductive outcomes [134]. This approach will be taken into account in our future studies in this field. In addition, the metataxomomic analysis included in this study was carried at the genus level since the 16S rRNA gene approach has poor discriminatory power at the species level [135,136]. Other approaches, such as shotgun sequencing, should be used in the future to solve such limitation and to have a broader view of the vaginal microbiome.
Although our knowledge of the mechanisms that these early embryo–maternal interactions has increased in recent years, implantation remains as a rate-limiting step in human ART and the currently available treatments for infertility or recurrent pregnancy loss of unknown etiology have a rather limited efficacy [137,138]. Therefore, the possibility of enhancing angiogenic and tolerance activities in the endometrium by modifying the reproductive microbiota using bacterial strains specifically tailored for these targets provides a novel strategy to improve reproductive functions and deserves future basic and clinical research efforts.
Acknowledgments
We sincerely thank all the women that participated in the assay. We also thank Evaristo Suárez (University of Oviedo, Spain) for critical reading of the manuscript and fruitful discussions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/1/162/s1, Supplementary Figure S1. History of recurrent infections and use of antibiotics in infancy and adulthood among the women recruited in this study. Supplementary Figure S2. Changes in vaginal parameters (pH, Nugent score, TGF-β 1, TGF-β 2, and VEGF concentrations, viable Lactobacillus counts in CVL samples) in women with a history of reproductive failure, because either of recurrent miscarriage (RA group) or infertility of unknown origin (INF groups), after the probiotic intervention with L. salivarius CECT5713 according to their outcome (pregnancy versus no pregnancy). Supplementary Figure S3. Comparison of vaginal parameters (pH, Nugent score, TGF-β 1, TGF-β 2, and VEGF concentrations, viable Lactobacillus counts in CVL samples) in healthy fertile women (C, control group) and those of women with a history of reproductive failure, because either of recurrent miscarriage (RA group) or infertility of unknown origin (INF groups), after the probiotic intervention with L. salivarius CECT5713. One-way ANOVA tests followed by Scheffé post hoc comparison tests were used to compare the groups; different letters above the boxplots indicate significant differences. Supplementary Table S1. Relative frequencies, medians and interquartile ranges (IQR) of the most abundant bacterial phyla (grey shadow) and genera detected in CVL samples from women who were able to complete a full-term pregnancy (n = 15) and of those who did not (n = 6) among the women that had a history of repetitive abortion (RA group; n = 21). Supplementary Table S2. Relative frequencies, medians and interquartile ranges (IQR) of the most abundant bacterial phyla (grey shadow) and genera detected in CVL samples from women who were able to complete a full-term pregnancy (n = 10) and of those who did not (n = 13) among the women with infertility of unknown origin (INF group; n = 23). Supplementary Table S3. Differences in the baseline characteristics and effect of the probiotic intervention with L. salivarius CECT5713 on the vaginal parameters of all women who were able to complete a full-term pregnancy (n = 25) and of those who did not (n = 19) among all participants from both RA and INF groups (n = 44). Supplementary Table S4. Relative frequencies, medians and interquartile ranges (IQR) of the most abundant bacterial phyla (grey shadow) and genera detected in CVL samples from women who were able to complete a full-term pregnancy (n = 25) and of those who did not (n = 19) among women with a history of reproductive failure, because either of recurrent miscarriage (RA group) or infertility of unknown origin (INF groups) (n = 44).
Author Contributions
D.B., L.F., and J.M.R. designed and coordinated the study. D.B. recruited participants and recorded samples-associated metadata. I.C. and R.A. processed the samples and performed the microbiological and immunological analyses. C.A. executed bioinformatic analysis. L.F. and J.M.R. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by contract 291-2018 established between Complutense University of Madrid and Biosearch Life S. A. (Granada, Spain). Irma Castro is the recipient of a predoctoral contract (BES-2017-080713) from the Ministerio de Ciencia, Innovación y Universidades (Spain).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Consejería de Salud y Familias (Junta de Andalucía, Granada, Spain) (protocol code P050/19, Act 11/19, 10th December 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
Biosearch Life S.A., the company that partly funded the study, is the proprietary of the strain L. salivarius CECT 5713.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reid G., Brigidi P., Burton J.P., Contractor N., Duncan S., Fargier E., Hill C., Lebeer S., Martín R., McBain A.J., et al. Microbes Central to Human Reproduction. Am. J. Reprod. Immunol. 2015;73:1–11. doi: 10.1111/aji.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno I., Simón C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 2019;18:40–50. doi: 10.1002/rmb2.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S.K., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fredricks D., Fiedler T.L., Marrazzo J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 7.Delaney M.L. Nugent score related to vaginal culture in pregnant women. Obstet. Gynecol. 2001;98:79–84. doi: 10.1016/s0029-7844(01)01402-8. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan S., Liu C., Mitchell C.M., Fiedler T.L., Thomas K.K., Agnew K.J., Marrazzo J.M., Fredricks D.N. Temporal Variability of Human Vaginal Bacteria and Relationship with Bacterial Vaginosis. PLoS ONE. 2010;5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroon S.J., Ravel J., Huston W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018;110:327–336. doi: 10.1016/j.fertnstert.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Krohn M.A., Hillier S.L., Nugent R.P., Cotch M.F., Carey J.C., Gibbs R.S., Eschenbach D.A. Vaginal Infection and Prematurity Study Group The Genital Flora of Women with Intraamniotic Infection. J. Infect. Dis. 1995;171:1475–1480. doi: 10.1093/infdis/171.6.1475. [DOI] [PubMed] [Google Scholar]
- 11.Newton E.R., Piper J., Peairs W. Bacterial vaginosis and intraamniotic infection. Am. J. Obstet. Gynecol. 1997;176:672–677. doi: 10.1016/S0002-9378(97)70568-4. [DOI] [PubMed] [Google Scholar]
- 12.Leitich H., Bodner-Adler B., Brunbauer M., Kaider A., Egarter C., Husslein P.W. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 13.Eckert L.O., Moore D.E., Patton D.L., Agnew K.J., Eschenbach D.A. Relationship of Vaginal Bacteria and Inflammation With Conception and Early Pregnancy Loss Following In-Vitro Fertilization. Infect. Dis. Obstet. Gynecol. 2003;11:11–17. doi: 10.1155/S1064744903000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yudin M.H. Bacterial Vaginosis in Pregnancy: Diagnosis, Screening, and Management. Clin. Perinatol. 2005;32:617–627. doi: 10.1016/j.clp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Van Oostrum N., De Sutter P., Meys J., Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: A systematic review and meta-analysis. Hum. Reprod. 2013;28:1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 16.Sirota I., Zarek S.M., Segars J.H. Potential Influence of the Microbiome on Infertility and Assisted Reproductive Technology. Semin. Reprod. Med. 2014;32:035–042. doi: 10.1055/s-0033-1361821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson J.D., Ralph S.G., Rutherford A.J. Rates of bacterial vaginosis in women undergoing in vitro fertilisation for different types of infertility. BJOG Int. J. Obstet. Gynaecol. 2002;109:714–717. doi: 10.1111/j.1471-0528.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 18.Campisciano G., Florian F., D’Eustacchio A., Stanković D., Ricci G., De Seta F., Comar M. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J. Cell. Physiol. 2017;232:1681–1688. doi: 10.1002/jcp.25806. [DOI] [PubMed] [Google Scholar]
- 19.Wee B.A., Thomas M., Sweeney E.L., Frentiu F.D., Samios M., Ravel J., Gajer P., Myers G.S.A., Timms P., Allan J.A., et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. New Zealand J. Obstet. Gynaecol. 2018;58:341–348. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 20.Hyman R.W., Herndon C.N., Jiang H., Palm C., Fukushima M., Bernstein D., Vo K.C., Zelenko Z., Davis R.W., Giudice L.C. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 2012;29:105–115. doi: 10.1007/s10815-011-9694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno I., Codoñer F.M., Vilella F., Valbuena D., Martinez-Blanch J.F., Jimenez-Almazán J., Alonso R., Alamá P., Remohí J., Pellicer A., et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 22.Haahr T., Jensen J., Thomsen L., Duus L., Rygaard K., Humaidan P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016;31:795–803. doi: 10.1093/humrep/dew026. [DOI] [PubMed] [Google Scholar]
- 23.Egbase P., Al-Sharhan M., Al-Othman S., Al-Mutawa M., Udo E., Grudzinskas J. Fertilization and early embryology: Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum. Reprod. 1996;11:1687–1689. doi: 10.1093/oxfordjournals.humrep.a019470. [DOI] [PubMed] [Google Scholar]
- 24.Fanchin R., Harmas A., Benaoudia F., Lundkvist U., Olivennes F., Frydman R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil. Steril. 1998;70:866–870. doi: 10.1016/S0015-0282(98)00277-5. [DOI] [PubMed] [Google Scholar]
- 25.Egbase P.E., Udo E.E., Al-Sharhan M., Grudzinskas J.G. Prophylactic antibiotics and endocervical microbial inoculation of the endometrium at embryo transfer. Lancet. 1999;354:651–652. doi: 10.1016/S0140-6736(99)02415-0. [DOI] [PubMed] [Google Scholar]
- 26.Moore D.E., Soules M.R., Klein N.A., Fujimoto V.Y., Agnew K.J., Eschenbach D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000;74:1118–1124. doi: 10.1016/S0015-0282(00)01624-1. [DOI] [PubMed] [Google Scholar]
- 27.Salim R., Ben-Shlomo I., Colodner R., Keness Y., Shalev E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: Results of a prospective survey. Hum. Reprod. 2002;17:337–340. doi: 10.1093/humrep/17.2.337. [DOI] [PubMed] [Google Scholar]
- 28.Selman H., Mariani M., Barnocchi N., Mencacci A., Bistoni F., Arena S., Pizzasegale S., Brusco G.F., Angelini A. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. J. Assist. Reprod. Genet. 2007;24:395–399. doi: 10.1007/s10815-007-9146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riganelli L., Iebba V., Piccioni M., Illuminati I., Bonfiglio G., Neroni B., Calvo L., Gagliardi A., Levrero M., Merlino L., et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell. Infect. Microbiol. 2020;10:350. doi: 10.3389/fcimb.2020.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peric A., Weiss J., Vulliemoz N., Baud D., Stojanov M. Bacterial Colonization of the Female Upper Genital Tract. Int. J. Mol. Sci. 2019;20:3405. doi: 10.3390/ijms20143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid G., Younes J.A., Van Der Mei H.C., Gloor G.B., Knight R., Busscher H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Genet. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 32.Martín R., Soberón N., Vaneechoutte M., Flórez A.B., Vázquez F., Suárez J.E. Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates. Int. Microbiol. 2008;11:261–266. doi: 10.2436/20.1501.01.70. [DOI] [PubMed] [Google Scholar]
- 33.Amabebe E., Anumba D.O. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halper J., Leshin L., Lewis S., Li W. Wound Healing and Angiogenic Properties of Supernatants from Lactobacillus Cultures. Exp. Biol. Med. 2003;228:1329–1337. doi: 10.1177/153537020322801111. [DOI] [PubMed] [Google Scholar]
- 35.Witkin S.S., Linhares I.M. Why do lactobacilli dominate the human vaginal microbiota? BJOG Int. J. Obstet. Gynaecol. 2017;124:606–611. doi: 10.1111/1471-0528.14390. [DOI] [PubMed] [Google Scholar]
- 36.Kovachev S. Defence factors of vaginal lactobacilli. Crit. Rev. Microbiol. 2018;44:31–39. doi: 10.1080/1040841X.2017.1306688. [DOI] [PubMed] [Google Scholar]
- 37.Martín R., Jiménez E., Olivares M., Marín M., Fernández L., Xaus J., Rodríguez J. Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother–child pair. Int. J. Food Microbiol. 2006;112:35–43. doi: 10.1016/j.ijfoodmicro.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Langa S., Maldonado-Barragán A., Delgado S., Martín R., Martín V., Jiménez E., Ruíz-Barba J.L., Mayo B., Connor R.I., Suárez J.E., et al. Characterization of Lactobacillus salivarius CECT 5713, a strain isolated from human milk: From genotype to phenotype. Appl. Microbiol. Biotechnol. 2012;94:1279–1287. doi: 10.1007/s00253-012-4032-1. [DOI] [PubMed] [Google Scholar]
- 39.Díaz-Ropero M., Martin R., Sierra S., Lara-Villoslada F., Rodríguez J.M., Xaus J., Olivares M. Two Lactobacillus strains, isolated from breast milk, differently modulate the immune response. J. Appl. Microbiol. 2007;102:337–343. doi: 10.1111/j.1365-2672.2006.03102.x. [DOI] [PubMed] [Google Scholar]
- 40.Olivares M., Diaz-Ropero M., Martin R., Rodriguez J., Xaus J. Antimicrobial potential of four Lactobacillus strains isolated from breast milk. J. Appl. Microbiol. 2006;101:72–79. doi: 10.1111/j.1365-2672.2006.02981.x. [DOI] [PubMed] [Google Scholar]
- 41.Jiménez E., Fernández L., Maldonado A., Martín R., Olivares M., Xaus J., Rodríguez J.M. Oral administration of lactobacilli strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl. Environ. Microbiol. 2008;74:4650–4655. doi: 10.1128/AEM.02599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez-Cano F.J., Dong H., Yaqoob P. In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: Two probiotic strains isolated from human breast milk. Inmunobiology. 2010;12:996–1004. doi: 10.1016/j.imbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Arroyo R., Martín V., Maldonado A., Jiménez E., Fernández L., Rodríguez J.M. Treatment of Infectious Mastitis during Lactation: Antibiotics versus Oral Administration of Lactobacilli Isolated from Breast Milk. Clin. Infect. Dis. 2010;50:1551–1558. doi: 10.1086/652763. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado J., Lara-Villoslada F., Sierra S., Sempere L., Gómez M., Rodríguez J.M., Boza J., Xaus J., Olivares M. Safety and tolerance of the human milk probiotic strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutrition. 2010;26:1082–1087. doi: 10.1016/j.nut.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J., Wittouck S., Salvetti E., Franz C.M.A.P., Harris H.M.B., Mattarelli P., O’Toole P.W., Pot B., Vandamme P., Walter J., et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 46.Magnusson J., Schnürer J. Lactobacillus coryniformis subsp.coryniformis Strain Si3 Produces a Broad-Spectrum Proteinaceous Antifungal Compound. Appl. Environ. Microbiol. 2001;67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Younes J.A., Van Der Mei H.C., Heuvel E.V.D., Busscher H.J., Reid G. Adhesion Forces and Coaggregation between Vaginal Staphylococci and Lactobacilli. PLoS ONE. 2012;7:e36917. doi: 10.1371/journal.pone.0036917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boris S., Suárez J.E., Vázquez F., Barbés C. Adherence of Human Vaginal Lactobacilli to Vaginal Epithelial Cells and Interaction with Uropathogens. Infect. Immun. 1998;66:1985–1989. doi: 10.1128/IAI.66.5.1985-1989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin V.S., Cárdenas N., Ocaña S., Marín M., Arroyo R., Beltrán D., Badiola C., Fernández L., Rodríguez J.M. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients. 2019;11:810. doi: 10.3390/nu11040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padmavathi T., Bhargavi R., Priyanka P.R., Niranjan N.R., Pavitra P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechnol. 2018;16:357–362. doi: 10.1016/j.jgeb.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narita J., Okano K., Kitao T., Ishida S., Sewaki T., Sung M.-H., Fukuda H., Kondo A. Display of α-Amylase on the Surface of Lactobacillus casei Cells by Use of the PgsA Anchor Protein, and Production of Lactic Acid from Starch. Appl. Environ. Microbiol. 2006;72:269–275. doi: 10.1128/AEM.72.1.269-275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nugent R.P., Krohn M.A., Hillier S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991;29:297–301. doi: 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mediano P., Fernández L., Jiménez E., Arroyo R., Espinosa-Martos I., Rodríguez J.M., Marín M. Microbial Diversity in Milk of Women With Mastitis: Potential Role of Coagulase-Negative Staphylococci, Viridans Group Streptococci, and Corynebacteria. J. Hum. Lact. 2017;33:309–318. doi: 10.1177/0890334417692968. [DOI] [PubMed] [Google Scholar]
- 54.Lackey K.A., Williams J.E., Meehan C.L., Zachek J.A., Benda E.D., Price W.J., Foster J.A., Sellen D.W., Kamau-Mbuthia E.W., Kamundia E.W., et al. What’s Normal? Microbiomes in Human Milk and Infant Feces Are Related to Each Other but Vary Geographically: The INSPIRE Study. Front. Nutr. 2019;6:45. doi: 10.3389/fnut.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrow S.A., Ravindran V., Butler R.C., Marshall J.W., Tannock G.W. Real-Time Quantitative PCR Measurement of Ileal Lactobacillus salivarius Populations from Broiler Chickens To Determine the Influence of Farming Practices. Appl. Environ. Microbiol. 2007;73:7123–7127. doi: 10.1128/AEM.01289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvetti E., Harris H.M.B., Felis G.E., O’Toole P.W. Comparative Genomics of the Genus Lactobacillus Reveals Robust Phylogroups That Provide the Basis for Reclassification. Appl. Environ. Microbiol. 2018;84:00993-18. doi: 10.1128/aem.00993-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bokulich N.A., Kaehler B., Rideout J.R., Dillon M., Bolyen E., Knight R., Huttley G.A., Caporaso J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:1–17. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2018. [(accessed on 10 November 2020)]. Available online: https://www.R-project.org. [Google Scholar]
- 63.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Epskamp S., Cramer A.O.J., Waldorp L.J., Schmittmann V.D., Borsboom D. qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012;48:1–18. doi: 10.18637/jss.v048.i04. [DOI] [Google Scholar]
- 65.O’Hanlon D.E., Lanier B.R., Moench T.R., Cone R. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macklaim J.M., Clemente J.C., Knight R., Gloor G.B., Reid G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Heal. Dis. 2015;26:27799. doi: 10.3402/mehd.v26.27799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendes-Soares H., Suzuki H., Hickey R.J., Forney L.J. Comparative Functional Genomics of Lactobacillus spp. Reveals Possible Mechanisms for Specialization of Vaginal Lactobacilli to Their Environment. J. Bacteriol. 2014;196:1458–1470. doi: 10.1128/JB.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.France M.T., Mendes-Soares H., Forney L.J. Genomic Comparisons of Lactobacillus crispatus and Lactobacillus iners Reveal Potential Ecological Drivers of Community Composition in the Vagina. Appl. Environ. Microbiol. 2016;82:7063–7073. doi: 10.1128/aem.02385-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macklaim J.M., Gloor G.B., Anukam K.C., Cribby S., Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl. Acad. Sci. USA. 2010;108:4688–4695. doi: 10.1073/pnas.1000086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaneechoutte M. Lactobacillus iners, the unusual suspect. Res. Microbiol. 2017;168:826–836. doi: 10.1016/j.resmic.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Borgdorff H., Armstrong S.D., Tytgat H.L.P., Xia D., Ndayisaba G.F., Wastling J.M., Van De Wijgert J.H.H.M. Unique Insights in the Cervicovaginal Lactobacillus iners and L. crispatus Proteomes and Their Associations with Microbiota Dysbiosis. PLoS ONE. 2016;11:e0150767. doi: 10.1371/journal.pone.0150767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petricevic L., Domig K.J., Nierscher F.J., Sandhofer M.J., Fidesser M., Krondorfer I., Husslein P., Kneifel W., Kiss H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2015;4:5136. doi: 10.1038/srep05136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lepargneur J.-P. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann. Biol. Clin. 2016;74:421–427. doi: 10.1684/abc.2016.1169. [DOI] [PubMed] [Google Scholar]
- 74.Anton L., Sierra L.-J., Devine A., Barila G., Heiser L., Brown A.G., Elovitz M.A. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health. Front. Microbiol. 2018;9:2181. doi: 10.3389/fmicb.2018.02181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng Y., Yao Z., Klionsky D.J. How to control self-digestion: Transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015;25:354–363. doi: 10.1016/j.tcb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petrova M.I., Reid G., Vaneechoutte M., Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017;25:182–191. doi: 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Kindinger L.M., Bennett P.R., Lee Y.S., Marchesi J.R., Smith A., Cacciatore S., Holmes E., Nicholson J.K., Teoh T.G., MacIntyre D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5:1–14. doi: 10.1186/s40168-016-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng N., Guo R., Yao Y., Jin M., Cheng Y., Ling Z. Lactobacillus iners Is Associated with Vaginal Dysbiosis in Healthy Pregnant Women: A Preliminary Study. BioMed Res. Int. 2019;2019:6079734. doi: 10.1155/2019/6079734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tachedjian G., Aldunate M., Bradshaw C.S., Cone R. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Ealdunate M., Esrbinovski D., Hearps A.C., Latham C.F., Ramsland P.A., Egugasyan R., Cone R.A., Tachedjian G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Hanlon D.E., Cone R., Moench T.R. Vaginal pH measured in vivo: Lactobacilli determine pH and lactic acid concentration. BMC Microbiol. 2019;19:1–8. doi: 10.1186/s12866-019-1388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boskey E., Cone R., Whaley K., Moench T. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001;16:1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 83.O’Hanlon D.E., Moench T.R., Cone R. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Hanlon D.E., Moench T.R., Cone R.A. Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE. 2013;8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruíz F.O., Gerbaldo G., Garcia M.J., Giordano W., Pascual L., Barberis I.L. Synergistic Effect Between Two Bacteriocin-like Inhibitory Substances Produced by Lactobacilli Strains with Inhibitory Activity for Streptococcus agalactiae. Curr. Microbiol. 2012;64:349–356. doi: 10.1007/s00284-011-0077-0. [DOI] [PubMed] [Google Scholar]
- 86.Aldunate M., Tyssen D., Johnson A., Zakir T., Sonza S., Moench T., Cone R., Tachedjian G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013;68:2015–2025. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tyssen D., Wang Y.-Y., Hayward J.A., Agius P.A., Delong K., Aldunate M., Ravel J., Moench T.R., Cone R.A., Tachedjian G. Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid. mSphere. 2018;3:e00055-18. doi: 10.1128/mSphere.00055-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chew S.Y., Cheah Y.K., Seow H.F., Sandai D., Than L.T.L. In vitro modulation of probiotic bacteria on the biofilm of Candida glabrata. Anaerobe. 2015;34:132–138. doi: 10.1016/j.anaerobe.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 89.Matsubara V.H., Wang Y., Bandara H.M.H.N., Mayer M.P.A., Samaranayake L. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016;100:6415–6426. doi: 10.1007/s00253-016-7527-3. [DOI] [PubMed] [Google Scholar]
- 90.Nasioudis D., Beghini J., Bongiovanni A.M., Giraldo P.C., Linhares I.M., Witkin S.S. α-Amylase in vaginal fluid: Association with conditions favorable to dominance of Lactobacillus. Reprod. Sci. 2015;22:1393–1398. doi: 10.1177/1933719115581000. [DOI] [PubMed] [Google Scholar]
- 91.Hütt P., Lapp E., Štšepetova J., Smidt I., Taelma H., Borovkova N., Oopkaup H., Ahelik A., Rööp T., Hoidmets D., et al. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb. Ecol. Heal. Dis. 2016;27:30484. doi: 10.3402/mehd.v27.30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cárdenas N., Martin V.S., Arroyo R., López M., Carrera M., Badiola C., Jiménez E., Rodríguez J.M. Prevention of Recurrent Acute Otitis Media in Children Through the Use of Lactobacillus salivarius PS7, a Target-Specific Probiotic Strain. Nutrients. 2019;11:376. doi: 10.3390/nu11020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chew S.Y., Cheah Y.K., Seow H.F., Sandai D., Than L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015;118:1180–1190. doi: 10.1111/jam.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aarti C., Khusro A., Varghese R., Arasu M.V., Agastian P., Al-Dhabi N.A., Ilavenil S., Choi K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018;89:99–106. doi: 10.1016/j.archoralbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 95.Nardo L.G. Vascular endothelial growth factor expression in the endometrium during the menstrual cycle, implantation window and early pregnancy. Curr. Opin. Obstet. Gynecol. 2005;17:419–423. doi: 10.1097/01.gco.0000175362.12470.e0. [DOI] [PubMed] [Google Scholar]
- 96.Demir R., Yaba A., Huppertz B. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem. 2010;112:203–214. doi: 10.1016/j.acthis.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 97.Gordon J.D., Shifren J.L., Foulk R.A., Taylor R.N., Jaffe R.B. Angiogenesis in the Human Female Reproductive Tract. Obstet. Gynecol. Surv. 1995;50:688–697. doi: 10.1097/00006254-199509000-00024. [DOI] [PubMed] [Google Scholar]
- 98.Licht P., Russu V., Lehmeyer S., Wissentheit T., Siebzehnrübl E., Wildt L. Cycle dependency of intrauterine vascular endothelial growth factor levels is correlated with decidualization and corpus luteum function. Fertil. Steril. 2003;80:1228–1233. doi: 10.1016/S0015-0282(03)02165-4. [DOI] [PubMed] [Google Scholar]
- 99.Malamitsi-Puchner A., Sarandakou A., Tziotis J., Stavreus-Evers A., Tzonou A., Landgren B.-M. Circulating angiogenic factors during periovulation and the luteal phase of normal menstrual cycles. Fertil. Steril. 2004;81:1322–1327. doi: 10.1016/j.fertnstert.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 100.Torry D.S., Leavenworth J., Chang M., Maheshwari V., Groesch K., Ball E.R., Torry R.J. Angiogenesis in implantation. J. Assist. Reprod. Genet. 2007;24:303–315. doi: 10.1007/s10815-007-9152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaczmarek M.M., Waclawik A., Blitek A., Kowalczyk A.E., Schams D., Ziecik A.J. Expression of the vascular endothelial growth factor-receptors ystem in the porcine endometrium throughout the estrous cycle and early pregnancy. Mol. Reprod. Dev. 2008;75:362–372. doi: 10.1002/mrd.20815. [DOI] [PubMed] [Google Scholar]
- 102.Meegdes B.H., Ingenhoes R., Peeters L.L., Exalto N. Early pregnancy wastage: Relationship between chorionic vascularization and embryonic development. Fertil. Steril. 1988;49:216–220. doi: 10.1016/S0015-0282(16)59704-0. [DOI] [PubMed] [Google Scholar]
- 103.Fong G.-H., Rossant J., Gertsenstein M., Breitman M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nat. Cell Biol. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 104.Vuorela P., Carpén O., Tulppala M., Halmesmäki E. VEGF, its receptors and the Tie receptors in recurrent miscarriage. Mol. Hum. Reprod. 2000;6:276–282. doi: 10.1093/molehr/6.3.276. [DOI] [PubMed] [Google Scholar]
- 105.Reynolds L.P., Caton J.S., Redmer D.A., Grazul-Bilska A.T., Vonnahme K.A., Borowicz P.P., Luther J.S., Wallace J.M., Wu G., Spencer T.E. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J. Physiol. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Relf M., Lejeune S., Scott P.A., Fox S., Smith K., Leek R., Moghaddam A., Whitehouse R., Bicknell R., Harris A.L. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 107.Ingman W.V., Robertson S.A. Defining the actions of transforming growth factor beta in reproduction. BioEssays. 2002;24:904–914. doi: 10.1002/bies.10155. [DOI] [PubMed] [Google Scholar]
- 108.Giudice L.C. Growth factors and growth modulators in human uterine endometrium: Their potential relevance to reproductive medicine. Fertil. Steril. 1994;61:1–17. doi: 10.1016/S0015-0282(16)56447-4. [DOI] [PubMed] [Google Scholar]
- 109.Bao S.H., Wang X.P., De Lin Q., Wang W.J., Yin G.J., Qiu L.H. Decidual CD4+CD25+CD127dim/- regulatory T cells in patients with unexplained recurrent spontaneous miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:94–98. doi: 10.1016/j.ejogrb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Xu L., Qiu T., Wang Y., Chen Y., Cheng W. Expression of C-type lectin receptors and Toll-like receptors in decidua of patients with unexplained recurrent spontaneous abortion. Reprod. Fertil. Dev. 2017;29:1613–1624. doi: 10.1071/RD15489. [DOI] [PubMed] [Google Scholar]
- 111.Qian J., Zhang N., Lin J., Wang C., Pan X., Chen L., Li D., Wang L. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci. Trends. 2018;12:157–167. doi: 10.5582/bst.2018.01012. [DOI] [PubMed] [Google Scholar]
- 112.Chung I.-B., Yelian F., Zaher F., Gonik B., Evans M., Diamond M.P., Svinarich D. Expression and Regulation of Vascular Endothelial Growth Factor in a First Trimester Trophoblast Cell Line. Placenta. 2000;21:320–324. doi: 10.1053/plac.1999.0481. [DOI] [PubMed] [Google Scholar]
- 113.Poole T.J., Finkelstein E.B., Cox C.M. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev. Dyn. 2001;220:1–17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 114.Sherer D., Abulafia O. Angiogenesis during Implantation, and Placental and Early Embryonic Development. Placenta. 2001;22:1–13. doi: 10.1053/plac.2000.0588. [DOI] [PubMed] [Google Scholar]
- 115.Qian D., Lin H.-Y., Wang H.-M., Zhang X., Liu D.-L., Li Q.-L., Zhu C. Involvement of ERK1/2 pathway in TGF-beta1-induced VEGF secretion in normal human cytotrophoblast cells. Mol. Reprod. Dev. 2004;68:198–204. doi: 10.1002/mrd.20061. [DOI] [PubMed] [Google Scholar]
- 116.Robertson S.A., Ingman W.V., O’Leary S., Sharkey D.J., Tremellen K.P. Transforming growth factor β—A mediator of immune deviation in seminal plasma. J. Reprod. Immunol. 2002;57:109–128. doi: 10.1016/S0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 117.Wahl S.M., Wen J., Moutsopoulos N. TGF-beta: A mobile purveyor of immune privilege. Immunol. Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 118.Nocera M., Chu T.M. Characterization of Latent Transforming Growth Factor-β From Human Seminal Plasma. Am. J. Reprod. Immunol. 1995;33:282–291. doi: 10.1111/j.1600-0897.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 119.Loras B., Vételé F., El Malki A., Rollet J., Soufir J.-C., Benahmed M. Seminal transforming growth factor-β in normal and infertile men. Hum. Reprod. 1999;14:1534–1539. doi: 10.1093/humrep/14.6.1534. [DOI] [PubMed] [Google Scholar]
- 120.Robertson S.A., Sharkey D.J. The role of semen in induction of maternal immune tolerance to pregnancy. Semin. Immunol. 2001;13:243–254. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 121.Robertson S.A., Guerin L.R., Moldenhauer L.M., Hayball J.D. Activating T regulatory cells for tolerance in early pregnancy-the contribution of seminal fluid. J. Reprod. Immunol. 2009;83:109–116. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Robertson S.A. Immune regulation of conception and embryo implantation—All about quality control? J. Reprod. Immunol. 2010;85:51–57. doi: 10.1016/j.jri.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Sharkey D.J., MacPherson A.M., Tremellen K.P., Mottershead D.G., Gilchrist R.B., Robertson S.A. TGF-β Mediates Proinflammatory Seminal Fluid Signaling in Human Cervical Epithelial Cells. J. Immunol. 2012;189:1024–1035. doi: 10.4049/jimmunol.1200005. [DOI] [PubMed] [Google Scholar]
- 124.Sakaguchi S. Regulatory T Cells. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 125.Shevach E.M. CD4+CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 126.Chen W., Jin W., Hardegen N.J., Lei K.-J., Li J., Marinos N.J., McGrady G., Wahl S.M. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clark D.A., Fernandez J., Banwatt D. ORIGINAL ARTICLE: Prevention of Spontaneous Abortion in the CBA × DBA/2 Mouse Model by Intravaginal TGF-β and Local Recruitment of CD4+ 8+ FOXP3+ Cells. Am. J. Reprod. Immunol. 2008;59:525–534. doi: 10.1111/j.1600-0897.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- 128.Robertson S.A., Guerin L.R., Bromfield J.J., Branson K.M., Ahlström A.C., Care A.S. Seminal Fluid Drives Expansion of the CD4+CD25+ T Regulatory Cell Pool and Induces Tolerance to Paternal Alloantigens in Mice1. Biol. Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guerin L.R., Moldenhauer L.M., Prins J.R., Bromfield J., Hayball J.D., Robertson S.A. Seminal Fluid Regulates Accumulation of FOXP3+ Regulatory T Cells in the Preimplantation Mouse Uterus Through Expanding the FOXP3+ Cell Pool and CCL19-Mediated Recruitment. Biol. Reprod. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 130.Chu T., Kawinski E. Plasmin, Substilisin-like Endoproteases, Tissue Plasminogen Activator, and Urokinase Plasminogen Activator Are Involved in Activation of Latent TGF-β1 in Human Seminal Plasma. Biochem. Biophys. Res. Commun. 1998;253:128–134. doi: 10.1006/bbrc.1998.9760. [DOI] [PubMed] [Google Scholar]
- 131.Emami N., Diamandis E.P. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGFβ1 in semen. Biol. Chem. 2010;391:85–95. doi: 10.1515/bc.2010.007. [DOI] [PubMed] [Google Scholar]
- 132.Tomaiuolo R., Veneruso I., Cariati F., D’Argenio V. Microbiota and Human Reproduction: The Case of Female Infertility. High Throughput. 2020;9:12. doi: 10.3390/ht9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomaiuolo R., Veneruso I., Cariati F., D’Argenio V. Microbiota and Human Reproduction: The Case of Male Infertility. High Throughput. 2020;9:10. doi: 10.3390/ht9020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cariati F., D’Argenio V., Tomaiuolo R. The evolving role of genetic tests in reproductive medicine. J. Transl. Med. 2019;17:1–33. doi: 10.1186/s12967-019-2019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mignard S., Flandrois J. 16S rRNA sequencing in routine bacterial identification: A 30-month experiment. J. Microbiol. Methods. 2006;67:574–581. doi: 10.1016/j.mimet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 136.Bukin Y.S., Galachyants Y.P., Morozov I.V., Bukin S.V., Zakharenko A.S., Zemskaya T.I. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci. Data. 2019;6:190007. doi: 10.1038/sdata.2019.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koot Y.E., Teklenburg G., Salker M., Brosens J., Macklon N. Molecular aspects of implantation failure. Biochim. Biophys. Acta Mol. Basis Dis. 2012;1822:1943–1950. doi: 10.1016/j.bbadis.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 138.Ticconi C., Pietropolli A., Di Simone N., Piccione E., Fazleabas A.T. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019;20:5332. doi: 10.3390/ijms20215332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.