Abstract
Objective
To investigate the association between KRT17 and the prognosis in bladder cancer patients.
Methods
The clinical data of 101 patients with bladder cancer from May 2013 to May 2015 were retrospectively analyzed. At the same time, the expression of KRT17 and its correlation with clinicopathological factors were examined by immunohistochemistry. We search the prognostic value of KRT17 in bladder cancer from the cancer genome map (TCGA) online database. To explore the possible cellular mechanism, gene set enrichment analysis (GSEA) was used. The patients were divided into two groups: high expression of KRT17 and low expression of KRT17. The patients were followed up for 5 years to observe the survival. Kaplan–Meier method and Log rank test were used for univariate survival analysis, and Cox regression analysis was used for multivariate analysis. Finally, a nomogram was constructed on this basis for internal verification.
Results
Among the 101 patients, 46 (45.5%) were in the KRT17 low expression group and 55 (54.5%) in the high KRT17 expression group. After 5 years of follow-up, 79 patients survived with a survival rate of 78.2% and 22 patients died with a mortality rate of 21.8%. Kaplan–Meier survival analysis showed that OS and PFS of patients with high expression of KRT17 were significantly higher than those of patients with low expression of KRT17 (p<0.001, p=0.005). Cox multivariate analysis showed that KRT17 expression was an independent risk factor for tumor progression (p=0.019). And tumor size, vascular tumor thrombus, and T stage also affected tumor progression (p<0.05). In the internal validation, the c-index of nomogram was 0.898 (95% CI: 0.854–0.941).
Conclusion
The decreased expression of KRT17 is associated with poor prognosis in patients with bladder cancer. KRT17 can be used as a novel predictive biomarker to provide a new therapeutic target for bladder cancer patients.
Keywords: keratin17, bladder cancer, overall survival, prognosis, progression-free survival, risk factors, nomograph
Introduction
Bladder cancer (BC) is the 10th most common cause of cancer and the 13th leading cause of cancer death in the world,1 with an estimated 549,000 new cases and 200,000 deaths in 2018.2 Although considerable progress has been made in surgical techniques, the five-year survival rate is still at a relatively low level.3 Non-muscle invasive bladder cancer (NMIBC) is the most common type of bladder cancer, accounting for about 75%.4 Postoperative tumor recurrence and NMIBC progression to MIBC are the main clinical events affecting the prognosis of such patients.5 At present, many studies have shown that tumor pathological stage, pretreatment neutrophils-lymphocyte ratio (NLR) and let-7g,6 pretreatment lymphocyte–monocyte ratio (LMR), pretreatment platelet–lymphocyte ratio (PLR),7 CENPF,8 Ki-679 and Serum Cholinesterase10 can predict the survival and prognosis of NMIBC patients, but the recurrence rate and progression rate of the disease are still very high,11 so it is particularly important to find new prognostic evaluation indicators and treatment targets.
Keratin is an intermediate fiber in epithelial cells, including 28 type I and 26 type II keratins. It is an important part of cytoskeleton formation, and is mainly used for cell protection and cell structure support.12,13 Loss or mutation of specific types of keratin can increase the sensitivity to apoptosis, further lead to decreased immunity, increased permeability of tight junctions, and misplacement of apical proteins in different epithelial cells.14,15 With the development of tumor-targeting therapy, the influence of keratin family in human tumor is also being studied and discovered. For example, keratin 1, as a target receptor for p160 peptide overexpression in breast cancer cells, plays a key role in the early detection and treatment of breast cancer.16 A recent study reported that knockdown of KRT17 may be an effective method to treat osteosarcoma by inhibiting the proliferation of osteosarcoma cells and inhibiting the Warburg effect of Akt/mTOR/HIF1-α pathway.17 KRT17 was initially studied in basal cell carcinoma of the skin, which belongs to type I keratin family with a molecular weight of about 48kd.18 Existing studies have shown that KRT17 is involved in the occurrence and development of a variety of tumors or guide the different prognosis of many tumors, such as colorectal cancer,19 gastric cancer,20 cervical cancer,21 renal cell carcinoma,22 pancreatic cancer,23 non-small cell cancer,24 etc. However, the role of KRT17 in the prognosis of bladder cancer and its possible pathogenesis are still unclear.
In this study, we used immunohistochemical staining to examine the expression of KRT17 in a large number of bladder cancer tissue samples to determine its clinicopathological significance. In addition, we further elucidated the possible pathogenesis of KRT17 through the analysis of GSEA database.
Data and Methods
The Cancer Genome Atlas (TCGA) and Oncomine database were used for computer analysis.
To investigate the expression pattern of KRT17 in bladder cancer, two data sets were used (https://tcga-data.nci.nih.gov/) (www.oncomine.com/). Data were collected and analyzed using R3.1.2software. The expression of KRT17 gene in bladder cancer tissues and normal tissues was compared according to the standard calculation method mentioned above.25
Tissue Specimen
From May 2013 to May 2015, 101 patients with bladder cancer underwent surgical resection in the Department of Urology, Nantong cancer hospital. There were 30 cases of bladder cancer with corresponding matched paracancerous tissue. None of these patients had received neoadjuvant chemoradiotherapy or other anti-tumor therapy before surgery, and these patients only had bladder cancer as a tumor diagnosis. Postoperative adjuvant treatment was performed according to normal time and dose. Follow up was performed by outpatient review or telephone follow-up. The follow-up was performed every 3 months in the first year, half a year in the second year, and once a year after the operation. The patients were followed up until May 2020. Follow-up items: abdominal and pelvic CT or color Doppler ultrasound, chest X-ray, tumor markers, routine urine test, liver function test and kidney function test, etc. (additional examinations should be conducted according to the patient’s condition).
Outcome measures: PFS was defined as an indication of disease progression from the beginning of treatment to any follow-up. At the end of follow-up, the data of survival and loss of follow-up were taken as the final cut-off time for statistical analysis. The mean age of the patients was 63.0 ± 9.5 years (range, 34–81 years). There were 82 males and 19 females. All samples were collected with the written informed consent of the participants, and the experiment was approved by the ethics committee of Nantong cancer hospital.
Construction of Tissue Microarray
Tissue microarray was constructed by the pathology department of Nantong cancer hospital. Paraffin sections of 101 cases of bladder cancer were stained with hematoxylin–eosin, and the most typical features were selected under a microscope to mark the fixed points. Each point array contains less than 160 points. Three micron thick sections were cut from the receptor block and transferred to the slide by tape transfer system for UV crosslinking.
Immunohistochemistry
IHC staining was performed according to the procedure previously described.26 Polyclonal antibody KRT17 (1:200, Proteintech, USA) was used. The tissue sections were examined by two experienced pathologists using a double-blind method. The proportion of positive cells and the intensity of cell staining were considered to score the results of immunohistochemistry. According to the proportion of positive staining cells, the scores were 0 (negative), 1 (≤25%), 2 (25–50%), 3 (51–75%) and 4 (>75%) and staining intensity: 0 (negative or no staining), 1 (weak positive), 2 (central type), 3 (strong positive). The value obtained by multiplying the two scores is the final score corresponding to each specimen. After calculating the arithmetic mean of these scores, the specimen with a score lower than 8 is defined as KRT17 low expression.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from bladder cancer and adjacent normal tissues using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using the PrimeScript RT reagent kit (Thermo Fisher Scientific). Quantitative real-time PCR was performed using SYBR Green assays (V azyme) and executed by a StepOnePlus real-time PCR system (Life Technologies, Carlsbad, CA, USA). The sequences of the primers for KRT17 were as follows: forward, 5′–CTACAGCCAGTACTACAGGACA–3′; reverse, 5′–AACTTGGTGCGGAAGTCATCA–3′. We used GAPDH as an internal control. The relative quantification values of KRT17 were calculated using the 2^−ΔΔCT method.
GSEA for KRT17
GSEA (gene set enrichment analysis) is a computational method to determine whether a priori defined gene sets have statistically significant and consistent differences between two biological states.27 In our study, GSEA first generated an ordered list of genes based on the correlation between all genes and KRT17 expression, and then, we used GSEA to clarify the significant survival differences observed between groups with high and low KRT17. The enriched pathways in each phenotype were determined using the nominal p value and the normalized enrichment score (NES).
Statistical Analysis
SPSS 22.0 software (IBM company) was used for statistical analysis. Chi-square test was used to compare and analyze the clinical and pathological conditions of the two groups. Kaplan–Meier method was used to evaluate the survival of patients, and Log rank statistical method was used to test the significance. Finally, Cox proportional hazards regression model was used to identify the independent prognostic factors that are significant for the prognosis of bladder cancer patients. Finally, R language was used to draw nomogram and build a prediction model. The difference was statistically significant (p< 0.05).
Results
Up-Regulation of KRT17 Expression in Bladder Cancer
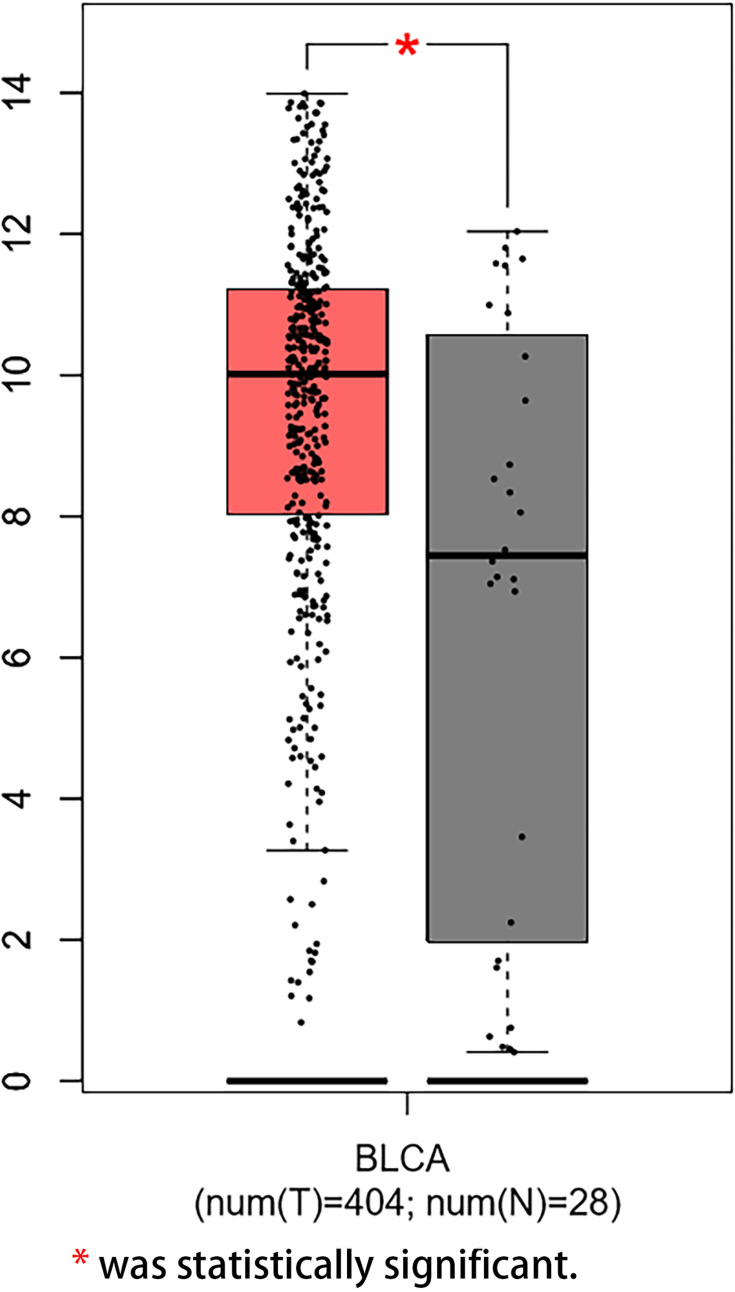
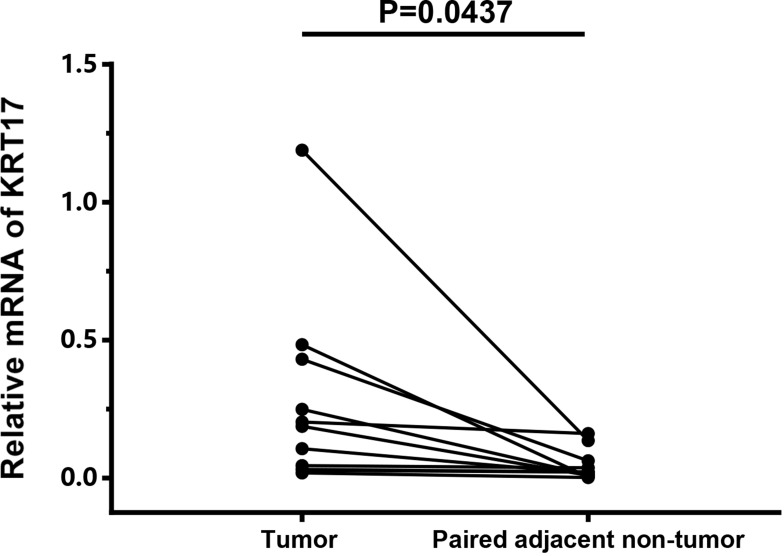
We examined the expression of KRT17 in 101 cases of bladder cancer and 30 cases of normal adjacent tissues. It was found that KRT17 was low expressed (45.5%) in 46 bladder cancer tissue samples and high expression (54.5%) in 55 bladder cancer tissue samples (Figure 1). In order to confirm the expression level of KRT17 in bladder cancer, we used the online database GEPIA (http://gepia.cancer-pku.cn/detail.php?gene=KRT17). The mRNA expression level of KRT17 was searched and analyzed. The expression of KRT17 was significantly higher than that in normal bladder tissues in the online database GEPIA (Figure 2). The mRNA expression level of KRT17 was significantly increased in 11 paired tumour tissues compared with the adjacent normal tissues using qRT-PCR in our study (Figure 3). The results of our study were consistent with those in the database.
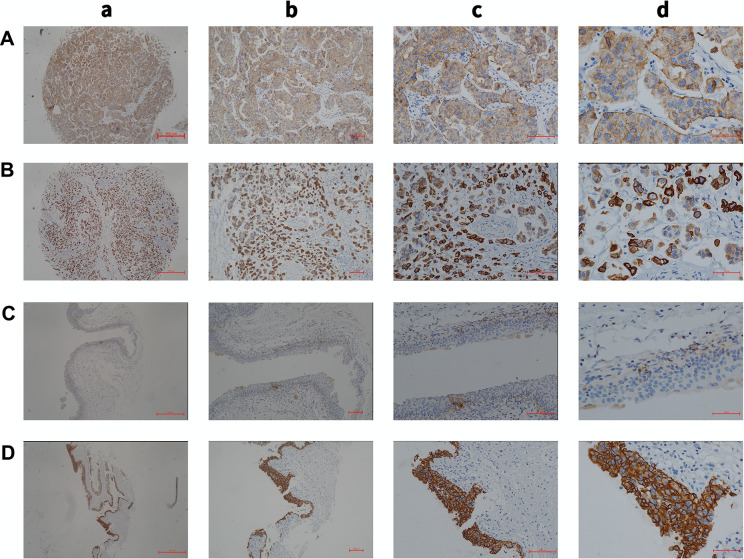
Figure 1.
(A) Low expression of KRT17 in cancer tissues (a, magnification, ×4; b, magnification, ×10; c, magnification, ×20; d, magnification, ×40). (B) High expression of KRT17 in cancer tissues (a, magnification, ×4; b, magnification, ×10; c, magnification, ×20; d, magnification, ×40). (C) Low expression of KRT17 in the adjacent tissues (a, magnification, ×4; b, magnification, ×10; c, magnification, ×20; d, magnification, ×40). (D) High expression of KRT17 in the adjacent tissues (a, magnification, ×4; b, magnification, ×10; c, magnification, ×20; d, magnification, ×40).
Figure 2.
High mRNA expression level of KRT17 in tumor tissues in the online database GEPIA.
Note: *Was statistically significant.
Figure 3.
High mRNA expression level of KRT17 in tumour tissues using qRT-PCR.
Correlation Between Expression of KRT17 and Clinicopathological Parameters
Among the 101 bladder cancer patients, 46 (45.5%) were in the KRT17 low expression group and 55 (54.5%) in the high KRT17 expression group. There were no significant differences in gender, age, recurrence, number of tumors, operation method, adjuvant chemotherapy, involvement of triangle area, external invasion, vascular tumor thrombus, lymph node metastasis and other site metastasis between the two groups (p>0.05) (Table 1).
Table 1.
The Relationship Between the Expression of KRT17 and Clinicopathological Data
| Variables | Total | Low Expression | High Expression | p |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Gender | ||||
| Male | 82 (81.2) | 37 (45.1) | 45 (54.9) | 0.859 |
| Female | 19 (18.8) | 9 (47.4) | 10 (52.6) | |
| Age (years) | ||||
| ≤65 | 39 (38.6) | 19 (48.7) | 20 (51.3) | 0.55 |
| >65 | 62 (61.4) | 27 (43.5) | 35 (56.5) | |
| Recurrence | ||||
| Yes | 23 (22.8) | 8 (34.8) | 15 (65.2) | 0.238 |
| No | 78 (77.2) | 38 (48.7) | 40 (51.3) | |
| Number of tumors | ||||
| Single | 70 (69.3) | 30 (42.9) | 40 (57.1) | 0.415 |
| Multiple | 31 (30.7) | 16 (51.6) | 15 (48.4) | |
| Tumor size (cm) | ||||
| ≤5 | 76 (75.2) | 30 (39.5) | 46 (60.5) | 0.033* |
| >5 | 25 (24.8) | 16 (64.0) | 9 (36.0) | |
| Operation mode | ||||
| Transurethral resection | 50 (49.5) | 18 (36.0) | 32 (64.0) | 0.057 |
| Radical cystectomy | 51 (50.5) | 28 (54.9) | 23 (45.1) | |
| Adjuvant chemotherapy | ||||
| Yes | 54 (53.5) | 22 (40.7) | 32 (59.3) | 0.299 |
| No | 47 (46.5) | 24 (51.1) | 23 (48.9) | |
| Bladder triangle involvement | ||||
| Yes | 18 (17.8) | 10 (55.6) | 8 (44.4) | 0.347 |
| No | 83 (82.2) | 36 (43.4) | 47 (56.6) | |
| External invasion | ||||
| Yes | 13 (12.9) | 8 (61.5) | 5 (38.5) | 0.215 |
| No | 88 (87.1) | 38 (43.2) | 50 (56.8) | |
| Vascular tumor thrombus | ||||
| Yes | 13 (12.9) | 7 (53.8) | 6 (46.2) | 0.520 |
| No | 88 (87.1) | 39 (44.3) | 49 (55.7) | |
| T stage | ||||
| T1 | 51 (50.5) | 7 (13.7) | 44 (86.3) | <0.0001* |
| T2+T3+T4 | 50 (49.5) | 39 (78.0) | 11 (22.0) | |
| Grade | ||||
| G1+G2 | 49 (48.5) | 3 (6.1) | 46 (93.9) | <0.0001* |
| G3 | 52 (51.5) | 43 (82.7) | 9 (17.3) | |
| Lymph node metastasis | ||||
| Yes | 32 (31.7) | 14 (43.8) | 18 (56.2) | 0.805 |
| No | 69 (68.3) | 32 (46.4) | 37 (53.6) | |
| Other site metastasis | ||||
| Yes | 10 (9.9) | 5 (50.0) | 5 (50.0) | 0.766 |
| No | 91 (90.1) | 41 (45.1) | 50 (54.9) |
Note: *P < 0.05 was statistically significant.
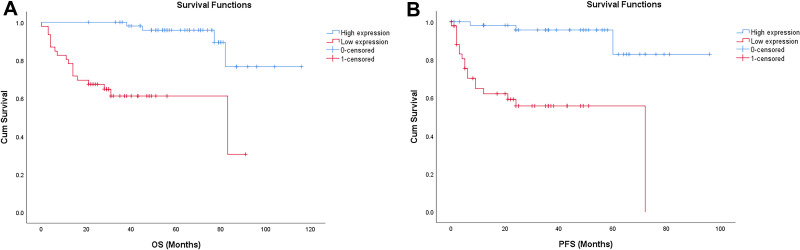
In order to determine the prognostic value of KRT17 in bladder cancer, Kaplan–Meier analysis was used to analyze the relationship between KRT17 expression and clinical follow-up data, and Log rank statistical method was used for significance test. The results showed that the high expression of KRT17 was positively correlated with overall survival (OS) and progression-free survival (PFS) (n = 61, p<0.001, Figure 4A and B), indicating that the OS and PFS of patients with low expression of KRT17 were significantly lower than those with high expression of KRT17.
Figure 4.
(A) Kaplan–Meier analysis of overall survival (OS) in patients with bladder cancer. (B) Kaplan-Meier analysis of progression-free survival (PFS) in patients with bladder cancer.
To further identify the risk factors associated with OS in patients with bladder cancer, univariate and multivariate analyses were performed to determine whether low expression of KRT17 is an independent risk factor for poor prognosis. Univariate analysis showed that tumor size, presence or absence of vascular tumor thrombus, T stage, grade and expression of KRT17 were the factors influencing OS (p<0.05). Cox multivariate analysis showed that KRT17 expression was an independent risk factor for tumor progression (p=0.019). At the same time, tumor size, vascular tumor thrombus, and T stage also affected tumor progression (p<0.05). These data suggest that elevated KRT17 expression significantly prolongs OS and PFS in patients with bladder cancer (Tables 2 and 3).
Table 2.
Univariate Analysis of Clinical Factors on 101 Patients with OS
| Variables | HR | (95% CI) | p |
|---|---|---|---|
| Gender | |||
| Male | 1.019 | (0.344–3.021) | 0.972 |
| Female | 1 | ||
| Age (years) | |||
| ≤65 | 1 | (0.352–1.896) | 0.638 |
| >65 | 0.817 | ||
| Recurrence | |||
| Yes | 1.116 | (0.414–3.009) | 0.829 |
| No | 1 | ||
| Number of tumors | |||
| Single | 1 | (0.169–1.473) | 0.208 |
| Multiple | 0.498 | ||
| Tumor size (cm) | |||
| ≤5 | 3.342 | (1.442–7.746) | 0.005 |
| >5 | 1 | ||
| Operation mode | |||
| Transurethral resection | 1.693 | (0.704–4.075) | 0.240 |
| Radical cystectomy | 1 | ||
| Adjuvant chemotherapy | |||
| Yes | 1.105 | (0.475–2.573) | 0.817 |
| No | 1 | ||
| Bladder triangle involvement | |||
| Yes | 2.105 | (0.491–9.023) | 0.316 |
| No | 1 | ||
| External invasion | |||
| Yes | 1 | (0.229–2.027) | 0.490 |
| No | 0.681 | ||
| Vascular tumor thrombus | |||
| Yes | 1 | (0.135–0.885) | 0.027 |
| No | 0.345 | ||
| T stage | |||
| T1 | 50.404 | (5.122–496.040) | 0.001 |
| T2+T3+T4 | 1 | ||
| Grade | |||
| G1+G2 | 11.915 | (3.34–42.504) | 0.000 |
| G3 | 1 | ||
| Lymph node metastasis | |||
| Yes | 1.054 | (0.426–2.608) | 0.909 |
| No | 1 | ||
| Other site metastasis | |||
| Yes | 1 | (0.145–1.283) | 0.131 |
| No | 0.431 | ||
| Expression of KRT17 | |||
| Low expression | 1 | (0.034–0.320) | 0.000 |
| High expression | 0.105 |
Table 3.
Multivariate Analysis of Clinical Factors in 101 Patients with OS
| Variables | HR | (95% CI) | p |
|---|---|---|---|
| Tumor size (cm) | |||
| ≤5 | 1 | (0.16–0.904) | 0.029 |
| >5 | 0.380 | ||
| Vascular tumor thrombus | |||
| Yes | 3.138 | (1.18–8.343) | 0.022 |
| No | 1 | ||
| T stage | |||
| T1 | 1 | (0.001–0.400) | 0.01 |
| T2+T3+T4 | 0.022 | ||
| Expression of KRT17 | |||
| Low expression | 4.263 | (1.272–14.290) | 0.019 |
| High expression | 1 |
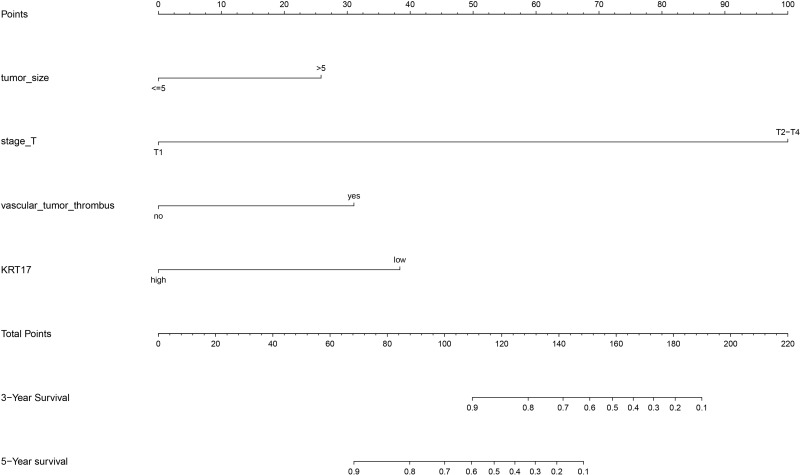
Based on Cox regression analysis, the nomogram was constructed for internal validation, and the prediction model was constructed (Figure 5). In the internal validation, the c-index of nomogram was 0.898 (95% CI: 0.854–0.941).
Figure 5.
Prediction model of nomogram construction.
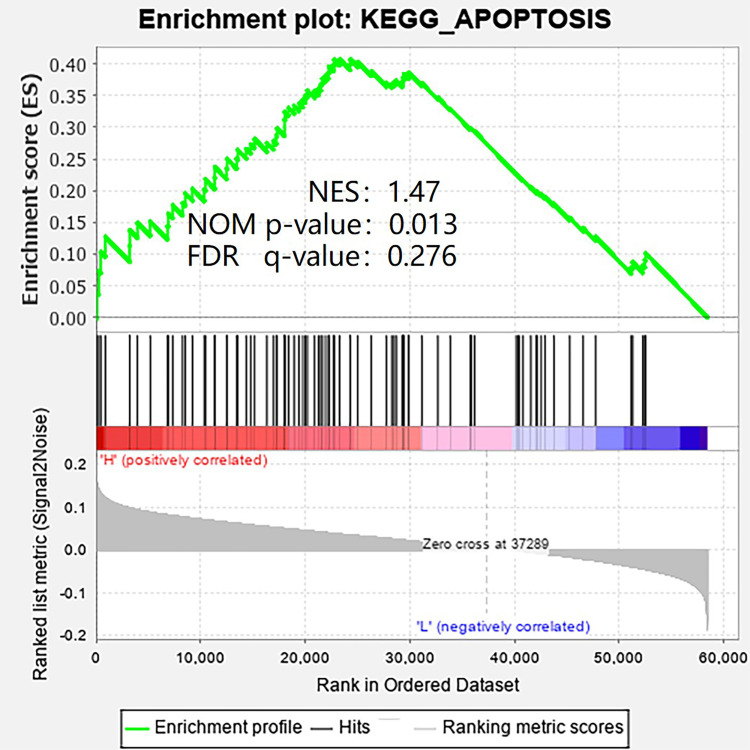
To explore the possible cellular mechanism, gene set enrichment analysis (GSEA) was used. As shown in Figure 6, apoptotic pathway is the most relevant enrichment pathway in high KRT17 group.
Figure 6.
The GSEA result showed the most relevant enrichment pathway.
Discussion
KRT17 is a member of type I keratin family with a molecular weight of about 48kd. Some members of this family have been identified as carcinogenic genes involved in many processes of tumorigenesis, such as KRT19,28 KRT1,29 KRT6c.30 However, the high expression of some members of this family is positively correlated with the good prognosis of tumor. For example, the high expression of kRT7 and kRT19 is related to the good prognosis of patients with renal clear cell carcinoma.31 Bühler et al32 also found that the high expression of KRT18 is related to the decreased invasiveness of breast cancer in vitro, that is, it is related to good prognosis.
KRT17 is also involved in the occurrence and development of a variety of tumors or is associated with poor prognosis of a variety of tumors, such as colorectal cancer,19 gastric cancer,20 cervical cancer,21 renal cell carcinoma,22 pancreatic cancer,23 non-small cell cancer,24 etc., but no related research has been conducted in bladder cancer.
The abilities to predict outcome and to identify key players in biological mechanisms that lead to poor outcome are two important objectives in cancer research.33 In our study, we first analyzed microarray data from different databases and found that KRT17 mRNA expression was significantly up-regulated in bladder cancer tissues compared with normal tissues. At the same time, our immunohistochemical results and qRT-PCR result also confirmed the high expression of KRT17 in bladder cancer. Cox regression analysis showed that KRT17 expression, maximum pathological diameter, presence or absence of vascular tumor thrombus, and T stage were independent prognostic factors for bladder cancer.34 But interestingly, we found in the Kaplan–Meier survival analysis that patients with high KRT17 expression showed higher OS and PFS than those with low KRT17 expression. The results of immunohistochemistry also showed that the patients with high expression of KRT17 had better tumor differentiation than those with low expression of KRT17, and the expression of KRT17 on the cell membrane was also higher. In contrast to the results reported in traditional studies that high expression of KRT17 is associated with poor prognosis of tumors,20 this also arouses our interest in further research and discussion. For example, Chivu-Economescu, et al20 found that KRT17 affects the prognosis of gastric cancer in two ways: AKT/mTOR, that sustains proliferation and survival, and the AMPKa1/CREB pathway, which was recently shown to induce organ protection and anti-inflammatory response. Wang, et al24 pointed out that KRT17 promotes proliferation and invasion of NSCLC cells via activation of the Wnt signaling pathway. However, bladder cancer cells are mainly transitional cells, which are different from gastric cancer cells and lung cancer cells. Bladder cancer has complex biological characteristics, such as multifocality, recurrence and heterogeneity, which is different from the biological characteristics of gastric cancer and lung cancer. Therefore, KRT17 may affect the prognosis of bladder cancer through different mechanisms.
One of the important characteristics of benign and malignant cell transformation is the loss of cell differentiation, which can lead to a series of changes in cell structure, which in turn can lead to tumor cells detachment from the epithelial layer and metastasis. In these processes, cytoskeleton and cell adhesion proteins just play a very important role.35 Cell adhesion proteins include desmosomes and adhesion junctions, which help stabilize the cytoskeleton.36 In the process of malignant transformation, the tight network of interaction between the three has undergone great changes, which leads to the reduction of cell adhesion and structural changes, and finally leads to the occurrence of migration.37 Therefore, as a member of the skeleton protein, when KRT17 is highly expressed, the cell stability is better, the cell structure is not easy to change, the cell adhesion is also better, and it is not easy to migrate. Therefore, the prognosis of patients with this type of patients is better than that of patients with low expression of KRT17. One study found that KRT17 functions as an oncoprotein by regulating the subcellular localization and degradation of p27(KIP1) in patients with cervical cancer.38 Therefore, it is necessary to further explore whether p27 and KRT17 also have such a relationship in bladder cancer in the future. This will provide better support for the effectiveness of KRT17 as a predictor.
On the basis of the results of Cox regression analysis, we drew a nomogram and conducted internal verification, so as to have a more accurate judgment on the 3-year and 5-year survival rate prediction of bladder cancer patients, which also has certain practical significance for the development of clinical treatment. In the internal validation, the c-index of nomogram is 0.898 (95% CI is 0.854–0.941). Therefore, the prediction model has relatively good accuracy.
Since patients with high KRT17 expression have better OS and PFS than patients with low KRT17 expression, we explored the possible cellular mechanism through gene set enrichment analysis (GSEA). At present, some studies have reported that the loss or mutation of certain types of keratin can increase the sensitivity to apoptosis, further lead to increased permeability of tight junctions and mispositioning of apical proteins in different epithelial cells, thus leading to the occurrence of bad results of cell migration.14,15 We also found that the apoptotic pathway was the most relevant enrichment pathway in the high KRT17 group (Figure 6). Therefore, we will further explore this pathway in future cytological research, so as to better explain this interesting phenomenon.
Conclusion
In conclusion, our study shows that KRT17 is significantly up-regulated in bladder cancer, and KRT17 expression can be used as an independent prognostic factor for bladder cancer patients. In addition, we found that patients with high expression of KRT17 have better prognosis and may regulate cell migration through apoptosis. Therefore, it is necessary to further study to elucidate the more specific molecular mechanism, which is helpful for the development of new drug candidates for targeted therapy of bladder cancer.
Funding Statement
This work was supported financially by grants from the Nantong Science and technology plan fund (MS22019010) and Clinical medicine program of Nantong University (Youth Program – 2019LQ013).
Data Sharing Statement
The datasets used or analyzed during the present study are available from the corresponding author Xiaolin Wang on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by The Ethics Committee of the Tumor Hospital Affiliated to Nantong University (Nantong, China). Written informed consent was obtained from all individual participants included in the study. And this study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). 2020;8. doi: 10.3390/medsci8010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Xie F, Zheng FX, Jiang GS, Zeng FQ, Xiao XY. Overexpression of CircRNA BCRC4 regulates cell apoptosis and MicroRNA-101/EZH2 signaling in bladder cancer. J Huazhong Univ Sci Technol Med Sci. 2017;37(6):886–890. doi: 10.1007/s11596-017-1822-9 [DOI] [PubMed] [Google Scholar]
- 4.Peng D, Gong YQ, Hao H, et al. Preoperative prognostic nutritional index is a significant predictor of survival with bladder cancer after radical cystectomy: a retrospective study. BMC Cancer. 2017;17(1):391. doi: 10.1186/s12885-017-3372-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang QH, Ji JL, Li H, et al. Preoperative lymphocyte-to-monocyte ratio predicts prognosis in patients with stage T1 non-muscle invasive bladder cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019;41(5):622–629. doi: 10.3881/j.issn.1000-503X.11227 [DOI] [PubMed] [Google Scholar]
- 6.Boubaker NS, Gurtner A, Trabelsi N, et al. Evaluating prognostic utility of preoperative neutrophil to lymphocyte ratio and hsa-let-7g/c up-regulation in patients with urinary bladder cancer. Cancer Biomark. 2020;27(1):63–73. doi: 10.3233/CBM-190483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol. 2015;36(11):8537–8543. doi: 10.1007/s13277-015-3613-x [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Zhang P, Liu L, Min X, Xiao Y. Weighted gene coexpression network analysis identifies a new biomarker of CENPF for prediction disease prognosis and progression in nonmuscle invasive bladder cancer. Mol Genet Genomic Med. 2019;7(11):e982. doi: 10.1002/mgg3.982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding W, Gou Y, Sun C, et al. Ki-67 is an independent indicator in non-muscle invasive bladder cancer (NMIBC); combination of EORTC risk scores and Ki-67 expression could improve the risk stratification of NMIBC. Urol Oncol. 2014;32(42):e13–9. doi: 10.1016/j.urolonc.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Kimura S, Soria F, D’Andrea D, et al. Prognostic value of serum cholinesterase in non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(6):e1123–e32. doi: 10.1016/j.clgc.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Afferi L, Moschini M, Cumberbatch MG, et al. European association of urology - European society of resident U. biomarkers predicting oncological outcomes of high-risk non-muscle-invasive bladder cancer. Minerva Urol Nefrol. 2020;72(3):265–278. doi: 10.23736/S0393-2249.20.03786-8 [DOI] [PubMed] [Google Scholar]
- 12.Yoon S, Leube RE, Malliri A, Caswell P, Ballestrem C, Hurlstone A. Keratin intermediate filaments: intermediaries of epithelial cell migration. Essays Biochem. 2019;63(5):521–533. doi: 10.1042/EBC20190017 [DOI] [PubMed] [Google Scholar]
- 13.Jacob JT, Coulombe PA, Kwan R, Omary MB, Types I, Keratin intermediate II. Filaments. Cold Spring Harb Perspect Biol. 2018;10(4):10. doi: 10.1101/cshperspect.a018275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas PJ, Forteza R, Mashukova A. Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity. Tissue Barriers. 2016;4(3):e1178368. doi: 10.1080/21688370.2016.1178368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oriolo AS, Wald FA, Ramsauer VP, Salas PJ. Intermediate filaments: a role in epithelial polarity. Exp Cell Res. 2007;313(10):2255–2264. doi: 10.1016/j.yexcr.2007.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soudy R, Etayash H, Bahadorani K, Lavasanifar A, Kaur K. Breast cancer targeting peptide binds keratin 1: a new molecular marker for targeted drug delivery to breast cancer. Mol Pharm. 2017;14(3):593–604. doi: 10.1021/acs.molpharmaceut.6b00652 [DOI] [PubMed] [Google Scholar]
- 17.Yan X, Yang C, Hu W, et al. Knockdown of KRT17 decreases osteosarcoma cell proliferation and the Warburg effect via the AKT/mTOR/HIF1alpha pathway. Oncol Rep. 2020;44(1):103–114. doi: 10.3892/or.2020.7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depianto D, Kerns ML, Dlugosz AA, Coulombe PA. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet. 2010;42(10):910–914. doi: 10.1038/ng.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CY, Jung WY, Lee HJ, Kim HK, Kim A, Shin BK. Proteomic analysis reveals overexpression of moesin and cytokeratin 17 proteins in colorectal carcinoma. Oncol Rep. 2012;27(3):608–620. doi: 10.3892/or.2011.1545 [DOI] [PubMed] [Google Scholar]
- 20.Chivu-Economescu M, Dragu DL, Necula LG, et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20(6):948–959. doi: 10.1007/s10120-017-0712-y [DOI] [PubMed] [Google Scholar]
- 21.Dong M, Dong Z, Zhu X, Zhang Y, Song L. Long non-coding RNA MIR205HG regulates KRT17 and tumor processes in cervical cancer via interaction with SRSF1. Exp Mol Pathol. 2019;111:104322. doi: 10.1016/j.yexmp.2019.104322 [DOI] [PubMed] [Google Scholar]
- 22.Sarlos DP, Yusenko MV, Peterfi L, Szanto A, Kovacs G. Dual role of KRT17: development of papillary renal cell tumor and progression of conventional renal cell carcinoma. J Cancer. 2019;10(21):5124–5129. doi: 10.7150/jca.32579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Ni XF, Tang H, et al. KRT17 functions as a tumor promoter and regulates proliferation, migration and invasion in pancreatic cancer via mTOR/S6k1 pathway. Cancer Manag Res. 2020;12:2087–2095. doi: 10.2147/CMAR.S243129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Yang MQ, Lei L, et al. Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis. Cancer Manag Res. 2019;11:7485–7497. doi: 10.2147/CMAR.S218926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zheng T. Screening of hub genes and pathways in colorectal cancer with microarray technology. Pathol Oncol Res. 2014;20(3):611–618. doi: 10.1007/s12253-013-9739-5 [DOI] [PubMed] [Google Scholar]
- 26.Laurberg JR, Jensen JB, Schepeler T, Borre M, Orntoft TF, Dyrskjot L. High expression of GEM and EDNRA is associated with metastasis and poor outcome in patients with advanced bladder cancer. BMC Cancer. 2014;14(1):638. doi: 10.1186/1471-2407-14-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabir NN, Ronnstrand L, Kazi JU. Keratin 19 expression correlates with poor prognosis in breast cancer. Mol Biol Rep. 2014;41(12):7729–7735. doi: 10.1007/s11033-014-3684-6 [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Wang Y, Zhao L, et al. Hsp74, a potential bladder cancer marker, has direct interaction with keratin 1. J Immunol Res. 2014;2014:492849. doi: 10.1155/2014/492849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu HB, Yang XP, Zhou PX, Yang XA, Yin B. High expression of keratin 6C is associated with poor prognosis and accelerates cancer proliferation and migration by modulating epithelial-mesenchymal transition in lung adenocarcinoma. Genes Genomics. 2020;42(2):179–188. doi: 10.1007/s13258-019-00889-5 [DOI] [PubMed] [Google Scholar]
- 31.Mertz KD, Demichelis F, Sboner A, et al. Association of cytokeratin 7 and 19 expression with genomic stability and favorable prognosis in clear cell renal cell cancer. Int J Cancer. 2008;123(3):569–576. doi: 10.1002/ijc.23565 [DOI] [PubMed] [Google Scholar]
- 32.Buhler H, Schaller G. Transfection of keratin 18 gene in human breast cancer cells causes induction of adhesion proteins and dramatic regression of malignancy in vitro and in vivo. Mol Cancer Res. 2005;3(7):365–371. doi: 10.1158/1541-7786.MCR-04-0117 [DOI] [PubMed] [Google Scholar]
- 33.Quan Y, Xu M, Cui P, Ye M, Zhuang B, Min Z. Grainyhead-like 2 promotes tumor growth and is associated with poor prognosis in colorectal cancer. J Cancer. 2015;6(4):342–350. doi: 10.7150/jca.10969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sejima T, Morizane S, Yao A, et al. Prognostic impact of preoperative hematological disorders and a risk stratification model in bladder cancer patients treated with radical cystectomy. Int J Urol. 2014;21(1):52–57. doi: 10.1111/iju.12161 [DOI] [PubMed] [Google Scholar]
- 35.Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31(2–3):325–335. doi: 10.1007/BF00666165 [DOI] [PubMed] [Google Scholar]
- 36.Conacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109(8):987–991. doi: 10.1172/JCI15429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith EA, Fuchs E. Defining the interactions between intermediate filaments and desmosomes. J Cell Biol. 1998;141(5):1229–1241. doi: 10.1083/jcb.141.5.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escobar-Hoyos LF, Shah R, Roa-Pena L, et al. Keratin-17 promotes p27 KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res. 2015;75(17):3650–3662. doi: 10.1158/0008-5472.CAN-15-0293 [DOI] [PubMed] [Google Scholar]