Abstract
Cardiovascular disease (CVD) is the leading cause of death worldwide and is the clinical manifestation of the atherosclerosis. Elevated LDL-cholesterol levels are the first line of therapy but the increasing prevalence in type 2 diabetes mellitus (T2DM) has positioned the cardiometabolic risk as the most relevant parameter for treatment. Therefore, the control of this risk, characterized by dyslipidemia, hypertension, obesity, and insulin resistance, has become a major goal in many experimental and clinical studies in the context of CVD. In the present review, we summarized experimental studies and clinical trials of recent anti-diabetic and lipid-lowering therapies targeted to reduce CVD. Specifically, incretin-based therapies, sodium-glucose co-transporter 2 inhibitors, and proprotein convertase subtilisin kexin 9 inactivating therapies are described. Moreover, the novel molecular mechanisms explaining the CVD protection of the drugs reviewed here indicate major effects on vascular cells, inflammatory cells, and cardiomyocytes, beyond their expected anti-diabetic and lipid-lowering control. The revealed key mechanism is a prevention of acute cardiovascular events by restraining atherosclerosis at early stages, with decreased leukocyte adhesion, recruitment, and foam cell formation, and increased plaque stability and diminished necrotic core in advanced plaques. These emergent cardiometabolic therapies have a promising future to reduce CVD burden.
Keywords: cardiometabolic risk, incretin system, dipeptidyl peptidase 4, sodium-glucose-co-transporter 2 inhibitors, proprotein convertase subtilisin kexin 9
1. Introduction
Despite the existence of different cardiometabolic drugs, cardiovascular disease (CVD) remains the first cause of death worldwide [1]. A main classical risk factor is elevated blood levels of LDL-cholesterol (LDL-C) in the blood which are closely related to atherosclerosis [2], and constitute the first line of therapy [3]. However, the change in lifestyle patterns that promotes sedentarism and an aging of the population have raised the incidence of type 2 diabetes (T2DM), thus becoming a major emergent risk. T2DM features are frequently associated with hypercholesterolemia, dyslipidemia, hypertension, and obesity which altogether represent a cardiometabolic risk whose main complication is the atherosclerotic disease [4].
In recent decades, several mechanisms have been shown to contribute to aggravating atherosclerosis in T2DM patients [5]. Insulin resistance (IR) which plays a pivotal role in the onset of T2DM, increases endothelial cell (EC) dysfunction by diminishing the bioavailability of vasodilators like nitric oxide (NO) [6]. Other mechanisms include IR induced apoptosis in macrophages [4] and reduced survival in vascular smooth muscle cells (VSMC) [7] in lesions. Notably, these seem to be due to inflammatory signaling pathways that promote plaque instability and rupture [4,7,8]. Moreover, hyperglycemia contributes to glucotoxicity and exerts in the vascular bed a proatherogenic synergistic effect alongside dyslipidemia and hypertension. These and other mechanistic studies led to the hypothesis that anti-diabetic drugs might exert atheroprotection by acting directly in vascular cells, prompting many experimental studies and clinical trials to study the expanded use of these drug therapies in additional cardiovascular contexts [9,10].
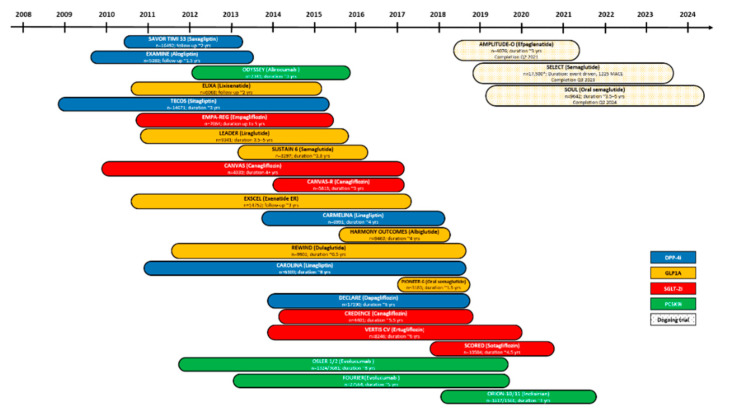
In the following sections, we will summarize the main current strategies for the management of carbohydrate and lipid metabolism and their relationship with CVD. These include incretin-based therapies, sodium-glucose co-transporter 2 inhibitors (SGLT2i), and proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors. A schematic summary of clinical trials over the years is displayed in Figure 1.
Figure 1.
Clinical trials with cardiovascular end-points for T2DM patients in trials for incretin-based and SGLT2i therapies and with LDL-C percentage change from baseline end-points for trials testing PCSK9i. Based on https://clinicaltrials.gov/ct2/home (accessed 10 December 2020).
2. Therapies Based on the Incretin System
2.1. Biology of the Incretins
Incretin hormones are small gut-derived peptides secreted by the endocrine cells of the intestine, mainly in the postprandial state, with potent insulinotropic actions. There are two main incretin hormones, the glucose-dependent insulinotropic polypeptide (GIP), secreted by the enteroendocrine K cells, and the glucagon-like peptide 1 (GLP1), secreted by the gut L cells of the distal small intestine and large bowel [11,12].
GLP1 is produced in response to nutrient intake, mainly sugars and fat, and its two active forms are the GLP1-(7–37) and the GLP1-(7–36)NH(2) generated by selective cleavage of the proglucagon molecule [13]. The GLP1 hormone exerts its effects through the GLP1 receptor (GLP1R) located in the gastrointestinal tract and pancreatic β-cells. However, it is also found in the heart, VSMCs, EC, immune cells, lung, kidney, and nervous system [13]. This protein is a G coupled receptor that mediates GLP1 actions to modulate glucose levels and body weight [13]. In β-cells GLP1 promotes the de novo synthesis and secretion of insulin by increasing cAMP and intracellular calcium levels. GLP1 also enhances β-cell number and mass through β-cell proliferation and neogenesis and decreases glucagon secretion in a glucose-dependent fashion that prevents hypoglycemia. These actions are concomitant to a reduction in food intake, gastric emptying, nutrient absorption and disposal, reduction of appetite by stimulation of the brain satiety center, and enhanced insulin sensitivity in periphery tissues [11]. GLP1 hormone is present at low concentrations in the circulation in fasting and inter-prandial state but, within minutes of food intake, its levels rise up to 2–3 times leading to postprandial insulin secretion [13]. GLP1 in the circulation has a very short half-life and, within 1.5 to 5 min, it is rapidly degraded by the dipeptidyl peptidase 4 (DPP4) into smaller peptides whose functions are not fully understood [14].
GIP production in the enteroendocrine K-cells of the proximal small intestine is mediated by the prohormone convertase (PC) one-third to a full-length peptide form. Alternatively, GIP can be processed by the PC2 to a C-terminal truncated form named GIP(1-30) [11] and it can be secreted under carbohydrate and fat regulation. In β-cells, GIP promotes insulin secretion by increasing cAMP and calcium intracellular levels and by regulating volatage-potassium Kv channel expression. Since its receptor, GIPR, is expressed in multiple tissues—such as the pancreas, gastrointestinal tract, adipose tissue, heart, testis, bone, lung, adrenal cortex, and central nervous system—other effects are under study. In the pancreas, GIP and GLP1 functions overlap, although inactivation of GIP in mice leads to impaired glucose tolerance and insulin release, hence indicating its metabolic relevance [11].
2.2. Degradation of the Incretins by Dipeptidyl Peptidase 4 (DPP4)
GLP1 and GIP incretins are cleaved by DPP4 during the passage across the hepatic bed. They are further processed in peripheral tissues into smaller metabolites to be finally eliminated by the kidney. DPP4, also known as CD26, is a transmembrane cell surface glycoprotein that cleaves N-terminal dipeptides position, mostly from GLP1 and GIP, thus holding an important antihyperglycemic effect. DPP4 is mainly expressed in exocrine glands and absorptive epithelium, hepatocytes, fibroblasts, leukocytes, and epithelial cells of the kidney and intestine [15]. It can be found membrane-bound or soluble.
DPP4 is also an adipokine produced by proteolytic cleavage from fully differentiated adipocytes [16] in some settings. Its production correlates with the degree of IR, inflammation, adipocyte size, and the amount of visceral adipose tissue (VAT) [16,17]. Both insulin and TNFα increase up to 50% the release of soluble DPP4 and it is believed to participate in the crosstalk between adipocytes, macrophages, and the inflamed stroma-vascular fraction [16]. DPP4 levels increase with insulin and leptin and inversely correlate with age and adiponectin levels [16,18].
Consistently, rats with genetic DPP4 deficiency, such as the strain F344/DuCrj or Dark Agouti rats, display improved IR, glucose tolerance, lipid profile, and enhanced GLP1 activity. Likewise, DPP4-deficent mice have enhanced glucose tolerance, glucose-stimulated insulin, and GLP1 release, and improved insulin sensitivity and liver homeostasis [19]. Dendritic cells (DC) and macrophages from VAT, in mice and humans, exhibit elevated DPP4 levels which promote T cell activation, proliferation, and inflammation [20]. In ECs, DPP4 promotes T cell transendothelial migration [21] and in human VSMCs promotes inflammation. Hepatic DPP4 secretion is elevated in obesity and IR while specific hepatic DPP4 deletion through a short hairpin RNA (shRNA) strategy diminished adipose tissue macrophage infiltration and IR [22]. In agreement with these studies, T2DM patients also display reduced levels of GLP1 hormone and enhanced DPP4 in the circulation, and IR correlates with the levels of DPP4 released by adipose tissue depots [23].
Altogether indicates that incretin-based therapies display anti-inflammatory and cardiovascular benefits by mechanisms independently from their glucose-lowering effect. Soluble DDP4 has been shown to induce VSMC proliferation by activating mitogen-activated protein kinases (MAPK)-pathway and the nuclear factor kappa B (NF-κB)-mediated inflammation [24]. Likewise, GLP1 hormone activates thymocytes and T cell proliferation and maintains regulatory T cells [25]. Because of this, they have been tested as therapeutic drugs for the treatment of CVD in T2DM patients. Currently, there are two different strategies to increase the benefits of the incretin system: (1) inhibition of the peptidase DPP4 and (2) the use of GLP1 and GIP incretin analogues [26].
2.3. DPP4 Inhibitors as a Therapeutic Target for T2DM
The gliptins are a family of DPP4 inhibitors developed as glucose-lowering agents that increase the half-life and bioavailability of the GLP1 incretin. These display fewer adverse side effects [27] and a low incidence of hypoglycemia [18]. The gliptins can be peptidomimetics and non-peptidomimetics, and both are extracellular competitive reversible inhibitors of the DPP4 substrate. These two groups differ in their half-lives, the strength of the effect, and therapeutic dose being the most currently prescribed: sitagliptin [28] saxagliptin [29], alogliptin [30], linagliptin [18,31], vildagliptin [32], anagliptin, and teneligliptin [18].
Most of them have shown a low frequency of hypoglycemia, pancreatitis, and pancreatic carcinoma, and no effects on heart failure (HF) rate or major adverse cardiovascular events (MACE) effects [27] except for alogliptin [30] and saxagliptin [29], which increase the rate of HF.
2.4. Effects of DPP4 Inhibitors in CVD
Although the initial rational for DPP4 inhibition is the subsequent increase in GLP1 effects, other independent mechanisms have been reported. Thus, beneficial effects have been reported in adipose tissue, vascular ECs, and monocyte/macrophages during atherosclerosis and myocardial injury in preclinical studies as described below, some of them summarized in [33]. In Table 1, a description of the mechanisms found in the preclinical animal model studies and the effects on atherosclerosis are included for incretin-therapies.
Table 1.
Preclinical studies of incretin-based therapies in animal models of atherosclerosis, vascular injury, and myocardial infarction.
| Animal Model | Incretin Therapy | Mechanism of Action | Reported Effects | References |
|---|---|---|---|---|
| Apoe-/- mice | DPP4i Anagliptin |
Suppressed VSMCs proliferation and macrophages inflammatory responses | Restrained atherosclerosis | [34] |
| DPP4i Linagliptin+HFD |
Anti-inflammatory phenotype of macrophages | Improved atherosclerosis | [35] | |
| GIP active forms | Decreased VSMCs proliferation, monocyte infiltration, foam cell formation and related genes (Cd36, Acat1), and NF-kB-mediated inflammation in macrophages | Stabilization of atherosclerotic plaque | [36] | |
| GIP and GLP1 | Suppressed foam cell formation | Reduced atheroma plaque | [37] | |
| Liraglutide | Suppressed Acat1 expression and foam cell formation | Decreased atherosclerosis | [38] | |
| Anti-inflammatory macrophage polarization | Reduced atherosclerosis | [39] | ||
| Exenatide+CS | Reduced oxidative stress and inflammation | Reduced plaque | [40] | |
| Exendin-4 | Reduced monocyte adhesion and pro-inflammatory cytokines via cAMP/PKA pathway | Decreased lesion size | [41] | |
| APOE*3-Leiden.CETP mice | Exendin-4 | Decreased monocyte recruitment and adhesion and foam cell formation | Reduced atherosclerotic lesions | [42] |
| Apoe-/-Irs2+/- mice | Liraglutide Lixisenatide |
STAT3-mediated macrophage polarization to an anti-inflammatory phenotype | Decreased atherosclerosis, necrotic core | [43] |
| Apoe-/- and Ldlr-/- mice | Liraglutide Semaglutide+WD | Changes in inflammatory markers | Reduced lesion size | [44] |
| Lldr-/- mice | DPP4i | Decreased pro-inflammatory genes expression and macrophage content | Decreased plaque size | [45] |
| Arterial hypertension Angiotensin II-mouse model | Liraglutide | Reduced leukocyte rolling and neutrophils infiltration | — | [46] |
| C57Bl6 mice | Liraglutide+45% HFD | Reduced eNOS expression and ER-stress response | Reduced cardiac fibrosis, hypertrophy, and necrosis | [47] |
| Myocardial injury mouse model | Liraglutide | Enhanced GSK3β, PPARβ-δ, Nrf-2, and HO-1 genes | Reduced mortality, infarct size, and rupture | [48] |
| Ischemia-reperfusion injury rats | Lixisenatide | --- | Reduced infarct-size, improved cardiac function |
[49] |
| Restenosis mouse/rat model | Lixisenatide Exendin-4 | Reduced VSMC proliferation | Neointimal hyperplasia | [50] |
| Diabetic rats | Liraglutide | Decreased macrophage ER-stress-induced secretion of microvesicles | Diminished atherosclerosis and intima thickening | [51] |
| GLP1+adenovirus-mediated delivery | Reduced VSMC and monocyte migration and inflammation | Reduced intima thickening | [52] | |
| Rabbits | DPP4i Anagliptin+CD |
Reduced macrophage infiltration | Restrained atherosclerosis | [53] |
| WHHL rabbits | Lixisenatide | Reduced macrophage, calcium deposition, necrosis | Prevention of plaque growth and instability | [54] |
ACAT1: acetyl-CoA acetyltransferase; CD: cholesterol diet; CS: chronic stress; eNOS: endothelial nitric oxide synthase; ER: endoplasmatic reticulum; HFD: high fat diet; VSMCs: vascular smooth muscle cells; WD: western diet; WHHL: Watanabe heritable hyperlipidemic.
2.4.1. Mechanisms of Gliptins in Experimental Atherosclerosis
High levels of soluble DPP4 are found in atherosclerosis and many studies have shown that its inhibition results in restrained atherosclerosis progression (Table 1).
Combination of DPP4 inhibitors with granulocyte-colony-stimulating factor (G-CSF) have shown protection against cardiovascular injury by increasing the number of endothelial stem cell and improving cardiac function [25]. The mechanism was an inhibition of DPP4-mediated degradation of the chemokine stromal cell-derived factor-1α (SDF-1α) which promotes endothelial progenitor cells (EPC) bone marrow mobilization to sites of cellular injury [55,56]. In this sense, SDF engineered to be DPP4-resistant showed improved blood flow in an animal model of peripheral artery disease [25].
In human umbilical vein endothelial cells (HUVECs), alogliptin induces endothelial nitric oxide synthase (eNOS), and AKT phosphorylation leading to enhanced NO production and improved homeostasis [24]. Likewise, alogliptin also promoted vascular relaxation via eNOS production of NO and endothelial-derived hyperpolarizing factor-mediated mechanisms [55]. Ex vivo DPP4 inhibition of human arteries from patients undergoing coronary artery bypass surgery reduced vascular oxidative stress and improved endothelial function [57]. DPP4 inhibitor treatment of HUVECs cultured under hypoxic condition prevented apoptosis in a CXCR4/STAT3-dependent manner [58].
In VSMCs, DPP4 activates MAPK and NF-κB increasing proliferation and production of iNOS proinflammatory cytokines [24]. Consistently, in Apoe-/- mice, anagliptin restrained atherosclerosis by suppressing VSMC proliferation [34].
DPP4 inhibition also modulates immune cells and inflammation. In diabetic patients, DPP4 inhibitors treatment resulted in a reduced production of reactive oxygen species and inflammatory mediators TNFα, JNK1, TLR2, TLR4, IL1β, and SOCS3 by monocytes [25]. DPP4 is highly expressed in bone marrow-derived CD11b+ cells. In Lldr-/- mice, DPP4 inhibitors downregulated proinflammatory genes and diminished aortic plaque macrophage content and lesion size [45]. In agreement with these results, treatment with linagliptin of high-fat diet-fed Apoe-/- mice, ameliorated atherosclerosis by inducing an anti-inflammatory phenotype in macrophages [35]. Likewise, anagliptin treatment restrained atherosclerosis by reducing macrophage plaque infiltration in cholesterol-fed rabbits [53] and suppressed inflammatory responses in macrophages in Apoe-/- mice [34].
In T cells, the non-cleaved membrane-bound DPP4 interacts with the T cell receptor (TCR)/CD3, promoting the phosphorylation cascade and antigen-presenting cell interactions engaging NF-κB inflammatory pathway activation [17].
2.4.2. Clinical Studies on DPP4 Inhibitors in T2DM with CVD
Both sitagliptin and anagliptin are being evaluated in clinical trials for their potential in the regulation of lipid metabolism and CVD in T2DM patients (Table 2). Anagliptin has been shown to decrease LDL-C triglycerides, total cholesterol, and non-HDL-C levels in a mechanism independent from its hypoglycemic effects [59]. Other gliptins increase adiponectin levels and reduce intestinal cholesterol absorption [18].
Table 2.
Clinical trials of incretin-based therapies.
| Incretin-Therapy | Clinical Trial | Patients | Reported Effects | References |
|---|---|---|---|---|
| DPP4i saxagliptin | SAVOR-TIMI 53 [NCT01107866] |
T2DM patients with CV risk | -Unaffected CV risk -Increased HF hospitalization rate |
[29] |
| DPP4i alogliptin | EXAMINE [NCT00968708] |
T2DM patients with ACS | -Unaffected CV death and hospital admission for HF | [30] |
| DPP4i sitagliptin | TECOS [NCT00790205] |
T2DM patients with established CVD | -Unaffected MACE or hospitalization for HF risk | [60] |
| DPP4i Linagliptin | CAROLINA [NCT01243424] |
T2DM patients and CV risk | -Unaffected CV risk | [61] |
| CARMELINA [NCT01897532] |
T2DM patients and CV risk and kidney disease | -Unaffected HF incidence -No influence of kidney disease -Reduced albuminuria |
[31,62] | |
| Lixisenatide (exendin-4 based) | ELIXA [NCT01147250] |
T2DM patients with a recent ACS | -No effects on MACE, hospitalization for HF -Decreased SBP and heart rate |
[63] |
| Exenatide (exendin-4 synthetic) |
EXSCEL [NCT01144338] |
T2DM patients with or without CVD | -Unaffected incidence of MACE, retinopathy or renal outcomes -Modest reduction in SBP but increased heart rate |
[64] |
| -Modest reduction in CV risk | [65] | |||
| Liraglutide (human GLP1A) | LEADER [NCT01179048] |
T2DM patients at high CV risk | -Reduced rates of MACE and death -Reduced SBP and microvascular renal and retinal complications -Enhanced heart rate |
[66] |
| -Same benefits for polyvascular and single vascular disease | [67] | |||
| -Reduced CV outcomes (MI/stroke) and CVD | [68] | |||
| -Unaffected HF hospitalization and death risk after MI | [69] | |||
| -Decreased rates of diabetic kidney disease | [70] | |||
| Semaglutide (human GLP1A) | SUSTAIN-6 [NCT01720446] |
T2DM patients at high CV risk | -Reduced rates of CV death and non-fatal MI/stroke -Decreased SBP but enhanced mean pulse rate |
[71] |
| PIONEER 6 [NCT02692716] |
T2DM patients with high CV risk | -Unaffected CV risk -Decreased SBP and LDL-C -Gastrointestinal adverse events |
[72] | |
| Albiglutide (modified human GLP1) | Harmony Outcomes [NCT02465515] |
T2DM and CVD patients | -Decreased SBP but augmented heart rate -improved glomerular filtration rate -Reduced risk of MACE -Unaffected CV death |
[73] |
| Dulaglutide (modified human GLP1) |
REWIND [NCT01394952] |
T2DM patients at high CV risk | -Unaffected all-cause mortality rate -Decreased SBP, pulse pressure and arterial pressure but enhanced heart rate -Reduced risk of CV outcomes, total CV, or fatal event burden |
[74,75] |
| Dulaglutide (modified human GLP-1) and tirzepatide (LY3298176) | SURPASS-CVOT [NCT04255433] |
T2DM patients with atherosclerotic CVD | Estimated completion date: October 2024 | https://clinicaltrials.gov/NCT04255433 |
| Efpeglenatide | AMPLITUDE-O [NCT03496298] |
T2DM patients with high CDV risk | Estimated completion date: April 2021 | https://clinicaltrials.gov/NCT03496298 |
| NNC0090-2746 (RG7697) [NCT02205528] |
Phase 2 trial | T2DM patients | -Improved glycemia control -Diminished body weight, cholesterol, and leptin. |
[76] |
| Tirzepatide (LY3298176) [NCT03131687] |
Phase 2 trial | T2DM patients | -Improved glycemic and body weight control -Enhanced pulse rate -Acceptable safety and tolerability profile |
[77,78] |
ACS: acute coronary syndrome; HF: heart failure; CV: cardiovascular; CVD: cardiovascular disease; DBP: Diastolic blood pressure; LDL-C: low density lipoprotein cholesterol; MACE: major adverse cardiovascular events; MI: myocardial infarction; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus.
Over the years, the cardiosafety of DPP4 inhibitors in T2DM patients with high cardiovascular risk has been confirmed in several clinical trials (Table 2). The SAVOR-TIMI 53 trial [29], the EXAMINE trial [30], the TECOS trial [60], and the CAROLINA [61] and CARMELINA [31,62] trials, that evaluated saxagliptin, alogliptin, sitagliptin, and linagliptin treatment, respectively, are considered the most important. Nevertheless, no cardiovascular benefits were reported in comparison with the placebo group. In fact, in the SAVOR-TIMI 53 trial, an increase in the rate of HF in patients with no previous history, was detected in the treated groups. The SAVOR-TIMI 53 trial [29], the EXAMINE trial [30] and TECOS trial [60] showed very low frequencies of hypoglycemia, pancreatitis, and pancreatic carcinoma [27]. However, the CARMELINA trial did not detect any effect on kidney disease or cardiovascular and kidney events.
2.5. GLP1 Analogues as Therapeutic Strategies
2.5.1. Development of Drugs Based on GLP1 and Rational Design
Given that GLP1 stimulates insulin secretion, soon after its discovery, this incretin became useful for T2DM treatment [79]. The native peptide has no clinical applicability due to its short plasma life-time of less than 2 min [80]. Therefore, the GLP1 analogues (GLP1A) approved for clinical use [81] are either derivatives of the human native GLP1, with chemical modifications to increase its stability and half-life [82] or peptides based on exendin-4. Exendin-4 is a GLP1A peptide isolated from the saliva of the lizard Heloderma suspectum [83], whose an amino acidic identity of 53% to the mammalian GLP1 that allows it to bind to the human GLP1 receptor [11].
Exenatide was the first GLP1A approved in 2005 and it is a synthetic version of the exendin-4. It has a half-life of up to 2.4 h, 10 times longer than endogenous GLP1, because its resistance to human DPP4 degradation [81,82]. Since the first generated GLP1As have a subcutaneous administration twice daily, other compounds have been developed to extend durability [84]. The optimization approach was addressed to keep the human GLP1 backbone to avoid immunogenic problems [84] and to get DPP4 action resistance through GLP1-(7-36) region modifications [26,81]. These modifications consist in the replacement of the penultimate alanine at the N-terminal end of the peptide by a glycine, serine, D-alanine, or by the most optimal chemical group, aminoisobutyric acid (Aib) [80], since it does not interfere with GLP1 receptor binding [85]. Other modifications were a replacement of the histidine residue at the N-terminal end by a glucitol group or by performing a deamination [80]. To increase the GLP1 half-life, the addition of fatty acids to the C-terminal domain was also performed to allow binding to the albumin and protection to renal filtration [26,86].
By combining all of the above-mentioned modifications, the approved GLP1As were designed and generated progressively over time: liraglutide (2009), exenatide (2005) and exenatide once-weekly (2011), lixisenatide (2013), albiglutide (2014) dulaglutide (2014), and semaglutide (2017), whose specific modifications can be found elsewhere [79,81].
These compounds have shown favorable effects in experimental and clinical studies with the limitation of parenteral administration [26,82]. Therefore, current research is focused on developing novel small GLP1As to be administered orally [26,84]. Among these, oral semaglutide stands out, which is a small acetylated peptide [79] whose oral daily administration has recently been approved in combination with sodium N-(8-(2-hydroxybenzoyl) amino) caprylate (SNAC) [84].
2.5.2. Studies in Preclinical Models of T2DM, Atherosclerosis, and CVD
As summarized in Table 1, studies in rodents and rabbits have shown beneficial actions of GLP1As in atherosclerosis, vascular injury, and cardiac function. Liraglutide treatment in diabetic rats led to diminished atherosclerotic lesion formation and intima-media thickening through a decrease of macrophage secretion of ER-stress induced microvesicles [51]. Similarly, the expression of GLP1 by adenovirus-mediated delivery in diabetic rats reduced intima-media thickening, VSMC and monocyte migration, and inflammatory processes [52].
In Apoe-/- mice, treatment with both native GIP and GLP1 incretins diminished atherosclerosis by suppressing macrophage foam cell formation [37]. In Apoe-/- mice liraglutide suppressed acyl-coenzyme A cholesterol acyltransferase 1 (Acat1) expression, foam cell formation and decreased atherosclerosis [38], and inhibited progression of early-onset atherosclerosis lesions in a GLP1R-dependent fashion [87]. Liraglutide administration also improved cardiac function through reduced cardiac fibrosis, hypertrophy, and necrosis by ameliorating endothelial NOS expression and ER-stress response in 45% high-fat diet (HFD) fed C57Bl6 mice [47].
Studies conducted by us have shown diminished atherosclerosis by lixisenatide and liraglutide in a mouse model of atherosclerosis and IR, the Apoe-/-Irs2+/- mice. Moreover, lixisenatide generated more stable plaques with thicker fibrous caps, smaller necrotic cores, and reduced inflammatory cell infiltration [43]. Lixisenatide also promoted macrophage polarization toward a pro-resolving phenotype in a STAT3-dependent manner [43]. A similar mechanism was found in Apoe-/- mice where liraglutide modulated polarization of macrophages toward a pro-resolving phenotype too [39]. Likewise, exenatide exerted protective effects in cultured macrophages via STAT3 activation which promoted adiponectin secretion when co-cultured with 3T3-L1 adipocytes [88]. In another investigation, liraglutide, and semaglutide treatments reduced lesion development in both Apoe-/- and Low density lipoprotein-deficient (Ldlr-/-) mice fed western diet, through major changes in inflammatory markers in aortic tissue [44]. Similarly, exenatide beneficial effects in atherosclerosis in Apoe-/- mice were attributed to reduced plaque oxidative stress, inflammation, and proteolysis in mice under chronic stress [40]. In the same line of results, lixisenatide treatment of Watanabe heritable hyperlipidemic (WHHL) rabbits prevented plaque growth and instability by decreasing macrophage infiltration, calcium deposition, and necrosis, as well as increased fibrotic content in VSMC-rich plaques [54]. On the other hand, exendin-4 treatment also decreased lesion size in Apoe-/- mice through a reduction of monocyte adhesion and expression of pro-inflammatory cytokines in activated macrophages via cAMP/PKA pathway [41]. Exendin-4 also diminished liver inflammation and atherosclerotic lesions through monocyte/macrophage recruitment and adhesion, and foam cell formation in the atherosclerotic mouse model APOE*3-Leiden.CETP [42].
In in vitro studies, treatment of HUVECs with exendin-4 [89] or liraglutide [90] incremented the activity, phosphorylation, and protein levels of eNOS, thus protecting against proatherosclerotic factors [89,90]. Besides, liraglutide in HUVECs diminished high-glucose-induced NF-kB phosphorylation and associated endothelial dysfunction [90]. Liraglutide also alleviated TNFα and LPS-mediated monocyte adhesion in cultured human aortic endothelial cells (HAECs) by increasing Ca2+ and CAMKKβ/AMPK activities and diminishing E-selectin and VCAM1 expression [91]. On the other hand, exendin-4 reversed glucolipotoxic gene dysregulation in diabetic human coronary artery endothelial cells (HCAECs) by upregulating eNOS and proangiogenic genes, while downregulating inflammatory, prothrombotic, and apoptosis genes [92].
In ischemia-reperfusion injury rat models (Table 1), prolonged treatment with lixisenatide reduced infarct-size and improved cardiac function [49]. Furthermore, in restenosis mouse and rat models, exendin-4 and lixisenatide reduced VSMC proliferation and neointimal hyperplasia, respectively [50]. In a mouse model of myocardial injury, liraglutide pretreatment reduced mortality and infarct size by enhancing cardioprotective genes such as PPARβ-δ, Nrf-2, and HO-1. Moreover, liraglutide-treated cardiomyocytes displayed diminished caspase-3 activation and increased cAMP production [48]. In another study, which employed an arterial hypertension angiotensin II-mouse model, liraglutide reduced leukocyte rolling and infiltration of myeloid neutrophils into the vascular wall [46].
2.5.3. Clinical Studies on GLP1-Based Strategies in T2DM with CVD
Many clinical trials based on GLP1 analogues have been designed to approve their use in CVD complications in T2DM subjects (Table 2).
In the ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome [NCT01147250]) trial, T2DM patients with a recent acute coronary syndrome were enrolled to test a daily injection of lixisenatide, an exendin-4-based analogue, for 25 months. Results showed no effects on MACE or other serious adverse events in T2DM patients who had recently suffered an acute coronary event [63]. The HF hospitalization rates and death remained unchanged but systolic blood pressure (SBP) and heart rate were reduced [63].
The effect on cardiovascular events of weekly injections of exenatide, another exendin-4 based analogue, was evaluated in the EXSCEL [NCT01144338] clinical trial. The study included T2DM patients of which 70% exhibited CVD and 30% did not displayed any CVD. In this study CVD was defined as atherosclerosis peripheral artery disease, coronary artery disease or stroke. The follow-up of patients, which were randomly treated with once-weekly exenatide (2 mg) or placebo for 5 years, did not show differences in MACE. A modest decrease in SBP and LDL-C was seen but treated patients also showed enhanced heart rate. The lack of effect was related to a shorter follow-up period and lower glycated hemoglobin baseline levels in the patients that in the other trials [64]. Once-weekly exenatide did not affect retinopathy or renal outcomes and produced a modest reduction in CV risk in subjects with mild loss of kidney function (estimated glomerular filtration rate, eGFR ≥ 60 mL/min/1.73 m2) [65].
In the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [NCT01179048]) clinical trial, the human GLP1-based analogue liraglutide or placebo were administered once daily as a subcutaneous injections to T2DM patients at high cardiovascular risk with a median follow-up of 3.8 years. In the first analysis, the treated group had lower rates of cardiovascular events and death from any cause but they also suffered more episodes of gallstone disease. The liraglutide group displayed a reduced risk of MACE, defined as cardiovascular death, non-fatal stroke, and non-fatal, first or recurrent MI. Analysis of vascular function showed decreased SBP and microvascular retinal and renal complications but also augmented DBP and heart rate [66]. A second analysis of this trial indicated the same benefits for polyvascular and single vascular disease in T2DM patients, independently of a previous history of MI [67]. In a posthoc analysis which took into account T2DM patients with high cardiovascular risk—due to MI, stroke, or atherosclerosis—liraglutide reduced cariovascular outcomes [68]. In another analysis of the LEADER trial, there was no evidence of decreased cardiovascular outcomes in patients with MI treated with liraglutide [69]. In a third clinical study, liraglutide treatment in T2DM patients resulted in a lower rate of diabetic kidney disease development and progression than placebo [70].
Likewise, semaglutide, whose structure is also based on human GLP1, injected once-weekly at two doses (0.5 mg or 1.0 mg) for 104 weeks in the SUSTAIN-6 trial (NCT01720446) reduced the rate of cardiovascular death and non-fatal MI and stroke in patients with T2DM at high cardiovascular risk. Consistent with the other GLP1A, SBP was reduced but mean pulse rate enhanced [71]. However, in the PIONEER 6 (NCT02692716) trial, once-daily oral semaglutide did not alter the cardiovascular risk profile of T2DM patients at high cardiovascular risk, but decreaed SBP and LDL-C parameters. Moreover, gastrointestinal adverse events were more common in patients receiving oral semaglutide than patients with placebo [72].
Another chemical variant of human GLP1, albiglutide, which consists of two tandem copies of modified human GLP1 bound to human albumin, showed increased effectivity due to its long-acting effect. In the Harmony Outcomes (NCT02465515) clinical trial, albiglutide in subjects of 40 or more years of age with T2DM and CVD, showed reduced MACE, SBP, and improved glomerular filtration rate in a follow up of 1.6 years. However, no effect was observed in death from cardiovascular causes [73].
A long-acting GLP1A, called dulaglutide, has also been generated. This analogue is the human GLP1 peptide covalently linked to a Fc fragment of a human IgG4 that protects against DPP4 degradation. The REWIND (NCT01394952) clinical trial, which tested weekly subcutaneous injection of dulaglutide in T2DM patients with a previous cardiovascular event or cardiovascular risk factors, showed no effect in all-cause mortality rate but reduced the risk of CV outcomes, SBP, pulse and arterial pressure although increased heart rate [74]. A subsequent analysis showed that weekly subcutaneous dulaglutide injections reduced total cardiovascular or fatal event burden [75].
Ongoing clinical trials are the SURPASS-CVOT (NCT04255433) with an estimated enrollment of 12,500 participants and the AMPLITUDE-O (NCT03496298) with 4076 patients. In the first trial, the effect in MACE of two incretins, tirzepatide (LY3298176) and dulaglutide is being compared in T2DM patients and the completion date of the study is in October 2024 (https://clinicaltrials.gov/NCT04255433). The second trial studies the effect of efpeglenatide in T2DM patients at high CVD risk and its estimated completion date is April 2021 (https://clinicaltrials.gov/NCT03496298).
2.6. GIP1 Emergent Therapies
2.6.1. Development of Drugs Based on GIP and Rational Design
Currently, there are no clinical therapies using GIPR agonists [93]. Experimental results of GLP1R/GIPR dual co-agonists derived from intermixed incretin sequence have shown augmented antihyperglycemic and insulinotropic effect compared with specific GLP1 agonist in db/db mice, ZDF diabetic rats, monkeys and humans [94,95]. Moreover, these dual agonists have been shown to reduce plasma biomarkers of cardiovascular risk in diet-induced obese mice [95,96].
2.6.2. Investigations of GIP Therapies in Preclinical Models
GIP has been related to different signalling pathways in vascular cells with both anti-atherogenic via NO production, or pro-atherogenic effects through increased endothelin-1 and VSMCs osteopontin expression [93]. In HUVECs cultures GIP/GIPR and GLP1/GLP1R interactions reduce the advanced glycation end products (AGEs) receptor (RAGE) expression, blocking the signalling pathways associated with diabetes-associated vascular damage [97].
On the other hand, as shown in Table 1, GIPR levels are diminished in diabetic experimental models and consequently, administration of GIP in Apoe-/- and db/db diabetic mice reduced atherosclerotic plaque lesions and foam cell formation [37,98]. Furthermore, the administration of GIP active forms to Apoe-/- mice increased atherosclerotic collagen content [36], suppressed VSMCs proliferation and reduced aortic endothelial expression of MCP1, VCAM1, ICAM1, and PAI1 [37,93] and monocyte migration and macrophage activation in a NFkB-dependent manner [36]. Other observed actions are the blocking of proinflammatory monocyte aortic infiltration, the decrease in Cd36 and Acat1 expression, foam cell formation, LPS-induced IL-6 secretion, and MMP-9 activity [36,37,93].
2.6.3. Studies in Humans with T2DM and CVD
GIP serum levels are elevated in patients with atherosclerotic vascular disease [36]. In humans, physiological doses of GIP increased heart rate, arterial blood pressure, and blood levels of osteopontin CCL8 and CCL2, accordingly with reduced CCL2/CCR2-mediated migration of cultured human monocytes (THP-1 cells) [93].
GLP1R/GIPR co-agonist NNC0090-2746 tested in T2DM patients in a randomized, placebo-controlled, double-blind phase 2 trial, administered subcutaneously once a day, showed an important effect in glycemia control and diminished body weight, cholesterol levels, and leptin [76] (Table 1). On the other hand, the dual co-agonist LY3298176 (tirzepatide) biased towards GIPR agonism obtained promising results as antidiabetic treatments in T2DM clinical trials as it showed improvements in glycemic and body weight control although is also showed an increased pulse rate [77,78,96]. Further research is needed about their cardioprotective effects, while interest in new GLP1R/GIPR co-agonists development increases [94].
3. Therapies to Inhibit Sodium-Glucose Co-Transporter 2
3.1. Structure of Sodium-Glucose Co-Transporters and Mechanism of Action
Human sodium-glucose co-transporters (SGLTs), encoded by SLC5, are a family of 12 secondary active glucose transporters, being the SGLT1 and SGLT2 the most important. The core domain structure of this LeuT-superfamily is a five-helix inverted repeat motif [99,100] with a substrate-binding site in the middle of the protein and with an outer and an inner gate. Glucose adsorption by SGLT1 mostly takes place in the intestine and SGLT2 plays a key role in the renal glucose reabsorption with a highly similar sodium-coupled sugar transport mechanism.
SGLTs transport glucose across the brush-border membrane of the proximal renal tubule segments. SGLT2 mostly located in the S1 segment accounts for up to 90% of glucose reabsorption and SGLT1 in the S3 segment accounts for 10% of reabsorption [100,101]. The transport mechanism consists of Na+ binding to the transporter in the apical side of the epithelial cells, the opening of the external gate and glucose binding. This is followed by the closing of the external gate, the opening of the inner gate, the release of both substrates to the cytosolic side of the cell and the adoption of the ligand-free conformation [101,102]. Through the basolateral membrane of the epithelial cells, GLUT2 transporters and Na+/K+ pump alleviate the intracellular accumulation of sodium and glucose by delivering both molecules in the blood.
3.2. Development of SGLT Inhibitors
The rational for developing SGLT2 inhibitors (SGLT2i) is the existence of up to 50 human mutations of SGLT2 resulting in renal glucosuria and important urinary glucose losses. These suggested that SGLT2i could be of use for hyperglycemic states such as T2DM patients [103].
Phlorizin, a glucoside of phloretin found in the apple tree, was the lead of the SGLT inhibitors as studies on IR diabetic rats showed that its administration subcutaneously normalized plasma glucose profiles and insulin sensitivity [104]. Because of its poor solubility, low bioavailability, and unselective SGLT1/2 inhibition, it was set aside as a therapeutic candidate. Their synthetic derivates, T-1095A and T-1095, had better results though their clinical development did not continue because of the same limitations [103,105].
Novel molecules with a C-glycosylation modification that conferred the phlorizin resistance to hydrolysis by endogenous β-glucosidases, increasing their half-life, were developed [103]. Selective SGLT2i based on this meta-C-glycosylated diarylmethane pharmacophore led to dapagliflozin, canagliflozin, empagliflozin, and ertugliflozin. The gliflozins have a higher selectivity profiles for SGLT2 over SGLT1, except for sotagliflozin with a selectivity for SGLT1 of about 20:1. Sergliflozin is another SGLT2i based on benzylphenol glucoside that did not undergo further development after phase II (https://adisinsight.springer.com/search). Others SGLT2i are ipragliflozin, tofogliflozin, and luseogliflozin, which are approved and available in Japan [106].
Effects of gliflozins also include actions on pancreatic islet cells and modulation of endogenous glucose production in insulin-sensitive tissues. Hence, empagliflozin treatment of T2DM subjects improved β-cell function [107], while dapagliflozin affects glucagon secretion by α-cells [108]. Moreover, T2DM patients treated with dapagliflozin and empagliflozin have shown an endogenous production of glucose and improved insulin sensitivity [107,109].
3.3. Effect of Gliflozins in Preclinical Models of CVD
Tahara et al. [110] studied different gliflozins (dapagliflozin, empagliflozin, canagliflozin, luseogliflozin, ipragliflozin, tofogliflozin) in KK/Ay T2DM mice (Table 3). The treatment with these gliflozins decreased hyperglycemia, lowered plasma levels of inflammatory mediators and improved endothelial dysfunction associated with human atherosclerosis.
Table 3.
Summary of the studies about the effect of SGLT2i in preclinical animal models and in clinical trials of T2DM patients.
| Drug | Animal Model | Effect on Lipids | Effect on Atherosclerosis | References |
|---|---|---|---|---|
| Dapagliflozin | KK/Ay mice | Decreased T-Chol, TG, and NEFAs (ipragliflozin and dapagliflozin) | -Decreased ED (CAMs and E-selectin) and plasmatic inflammatory parameters | [110] |
| Ipragliflozin | ||||
| Canagliflozin | ||||
| Luseogliflozin | ||||
| Empagliflozin | ||||
| Tofogliflozin | ||||
| Empagliflozin | db/db mice | No change in TG and T-Chol | -Reduced aortic and endothelial cell stiffness | [111] |
| Empagliflozin | ZDF rats | No change in TG, T-Chol, HDL, and LDL | -Reduced oxidative stress and inflammation -ED partially prevented |
[112] |
| Dapagliflozin | db/db mice | --- | -Lower arterial stiffness -Improved ED and VSMC dysfunction |
[113] |
| Ipragliflozin Dapagliflozin |
STZ Apoe-/- mice db/db mice |
No changes in TG, HDL and T-chol | -Dapagliflozin decreased macrophage infiltration, atherosclerotic lesions and plaque size -Ipragliflozin decreased foam cell formation |
[114] |
| Dapagliflozin | Apoe-/- mice | --- | -Attenuated ED and VCAMs expression -Induced vasorelaxation |
[115] |
| Empagliflozin | Apoe-/- mice | Decreased TG and increased HDL | -Decreased atherosclerotic plaque and inflammation | [116] |
| Canagliflozin | Apoe-/- mice | Decreased TG, T-chol and LDL | -Increased plaque stability -Reduced atherosclerosis and inflammatory parameters |
[117] |
| Dapagliflozin | ob/ob mice | Decreased of TG | -Reduced expression of inflammatory parameters | [118] |
| Drug | Trial | Structural basis | -Effect on vascular and blood parameters, and MACE | References |
| Empagliflozin | EMPA-REG OUTCOME | C-glycosyl compound | -Decreased CV death (HR = 0.86), SBP and DBP -Increased HDL-C and LDL-C |
[119] |
| Canagliflozin | CANVAS/CANVAS-R | -No effect (HR = 0.86) -Increased HDL-C and LDL-C |
[120] | |
| Canagliflozin | CREDENCE | -Decreased nonfatal stroke/MI and CV death (HR = 0.80) | [121] | |
| Dapagliflozin | DECLARE-TIMI 58 | -No effect (HR = 0.93) -Decreased SBP and DBP |
[122] | |
| Dapagliflozin | DEFINE-HF | -No decrease in HF (HR = 0.84) | [123] | |
| Ertugliflozin | VERTIS-CV | -No effect (HR = 0.97) -Decreased SBP |
[124] | |
| Sotagliflozin | SCORED | -Decreased CV death (HR = 0.84) | [125] | |
| Sotagliflozin | SOLOIST-WHF | -Decreased CV death (HR = 0.72) | [126] |
CAMs: cellular adhesion molecules; CV: cardiovascular; CVD: cardiovascular disease; DBP: dyastolic blood pressure; ED: endothelial dysfunction; HF: heart failure; HR: hazard ratio for three-component; HDL cholesterol; LDL-C: LDL cholesterol; MACE; MACE: major adverse cardiovascular events; MI: myocardial infarction; SBP: systolic blood pressure; T-chol: total cholesterol; TG: triglycerides; VSM: vascular smooth muscle; VCAMs: vascular cell adhesion molecules.
Studies with empagliflozin in diabetic ZDF rats and db/db mice also showed an improvement of vascular stiffness and endothelial function by preventing oxidative stress, AGE-dependent signaling and inflammation [111,112]. Likewise, dapagliflozin treatment of C57BLKS/J-leprdb/leprdb mice, alleviated arterial stiffness and endothelial and VSMC dysfunction. Interestingly, a decrease in circulating markers of inflammation and alterations in microbial richness and diversity were reported [113].
In vascular disease, dapagliflozin decreased atherosclerosis in streptozotozin-induced diabetic Apoe-/- mice, but not in non-diabetic Apoe-/- mouse counterparts. Moreover, analysis of peritoneal macrophages from dapagliflozin-treated diabetic Apoe-/- mice and ipragliflozin-treated db/db mice showed decreased cholesterol-ester accumulation, suggesting less macrophage foam cell formation capacity compared. Both SGLT2i normalized the expression of lectin-like ox-LDL receptor1 (Lox1), Acat1, and ATP-binding cassette transporter A1 (Abca1) in peritoneal macrophages from diabetic Apoe-/- and db/db mice. These results suggested that SGLT2i exert an anti-atherogenic effect in diabetic conditions by modulating genes involved in cholesterol accumulation in macrophages [114].
Studies carried out in Apoe-/- mice showed that dapagliflozin improved EC function without variations in plasma glucose concentrations [115] and that empagliflozin reduced plaque formation in the aortic arch and ameliorated insulin sensitivity [116]. A decrease in inflammatory mediators, like VCAM and NFκB [115] and TNFα and IL6 [116] were also observed in these studies. These results point to an atheroprotection through anti-inflammatory mechanisms. Consistently, high-fat diet fed Apoe-/- mice, treated with canagliflozin, developed smaller and more stable plaques and displayed decreased Vcam and Mcp1 expression [117].
In the T2DM mouse model, the ob/ob mice, dapagliflozin improved left ventricular function, attenuated activation of the Nlrp3 inflammasome and reduced inflammatory mediators by an AMPK-dependent mechanism. These changes were observed also in cardiomyofibroblasts derived from mice and were independent of the glucose-lowering effect [118].
3.4. Clinical Studies of Gliflozins in HF and CVD
In the EMPA-REG OUTCOME (NCT01131676) study, the empagliflozin treatment of patients with T2DM and CVD reduced MACE, death and hospitalization for HF. Both SBP and DBP were reduced while HDL-C and LDL-C levels were increased. However, it did not ameliorate non-fatal MI and stroke, and patients showed an increased rate of genital infection [119] (Table 3).
In the CANVAS (NCT01032629) and CANVAS-R (NCT01989754) program, canagliflozin treatment of patients with T2DM at high CV risk resulted in diminished MACE but it did not alter the occurrence of CV death or overall mortality. Like empaglifliozin, it enhanced the levels of HDL-C and LDL-C and the treated patients displayed a greater risk of fractures and amputations [120].
Differences between EMPA-REG OUTCOME and CANVAS trial might be due to the study design. Thus, in the EMPA-REG all subjects had prior CV disease and additional treatments (i.e., statins, RAS inhibitors, and acetylsalicylic acid), while in the CANVAS study only 65% of patients had prior CV disease [127].
Canagliflozin tested in T2DM and albuminuric chronic kidney disease patients in the CREDENCE (NCT02065791) clinical trial, demonstrated that, besides a lower risk of cardiovascular death, MI or stroke, this drug also diminishes the risk of end-stage kidney disease without changes in the amputation and fracture rates [121].
In the DECLARE-TIMI 58 (NCT01730534) trial with T2DM and CVD patients, dapagliflozin treatment failed to reduce MACE, although the rates of cardiovascular death or hospitalization for HF were lower. In this study reductions in SBP and DBP were described for dapagliflozin-treated patients [122]. Dapagliflozin in the DEFINE-HF (NCT02653482) trial with patients displaying chronic HF, both patients with or without T2DM, did not reduce HF determined as B-type natriuretic peptide levels [123].
Similar to dapagliflozin in the DECLARE-TIMI 58, ertugliflozin in the VERTIS CV (NCT01986881) trial, which included T2DM and CVD patients, did not reduce MACE or neither modify the death rate attributed to CV causes. However, ertugliflozin reduced SBP [124].
Two trials were carried out with sotagliflozin, the SCORED (NCT03315143) clinical trial [125] with TD2M chronic kidney disease and CVD participants, and the SOLOIST-WHF (NCT03521934) trial [126], with TD2M patients with HF. In both trials, a decrease in MACE was observed, but patients in the treatment group of the SCORED trial suffered more genital infections and volume depletion, in addition to diarrhea and hypoglycemia in both trials.
4. Lipid-Lowering Therapies Based on the Proprotein Convertase Subtilisin Kexin 9 Inhibition
As mentioned in the introduction one main mechanism for the regulation of blood LDL-C levels relies on its clearance by LDLR located on the surface of hepatic cells. This receptor binds the apoprotein B-100 (Apo B-100) present in LDL, VLDL, and IDL particles. After LDL binding, the receptor leads to the clathrin-mediated endocytosis of the ligand-receptor complex, which finishes in LDL degradation into amino acids and cholesterol. LDLR undergoes internalization and degradation by the proprotein convertase subtilisin kexin 9 (PCSK9), thus playing a pivotal role in the regulation of lipoprotein clearance [128]. PCSK9 gene was identified in 2003 in a family with Familial hypercholesterolemia (FH), an autosomal dominant disease with elevated LDL-C in blood [129]. FH was attributed to two missense mutations in the coding region of the PCSK9 gene with “gain of function—GOF”, hence promoting LDLR degradation [129]. Two years later, lower plasmatic levels of LDL-C were associated with the existence of two “loss of function mutations—LOF” in PCSK9 that resulted in elevated LDLR concentration in hepatic cells [130,131]. All these findings pointed to PCSK9 protein inhibition as a new potential lipid-lowering therapeutic target. To reduce CVD, PSCK9 inhibition has been set as a therapy in patients with FH, in those resistant to statin treatment or in subjects that did not reach the LDL-C goal levels at maximum statin dose [132,133]. By contrary, no recommendations exist today for T2DM patients’ treatment.
4.1. Biology of PCSK9 in Lipid Metabolism and Vascular Homeostasis
PCSK9, also known as neural apoptosis-regulated convertase 1, is the ninth member of the secretory serine proteases of the subtilase family. Although it is mainly expressed by hepatocytes, PCSK9 is also present in the small intestine, kidney, pancreas, brain [128,134,135], and ischemic heart [136]. The 25-kb PCSK9 human gene on chromosome 1p32 has 12 exons and 11 introns and is under the regulation of SREBP-2 (the sterol regulatory element-binding protein) [132,135]. It encodes a 692 amino acid serine protease that is synthesized as an inactive precursor, pre-proPCSK9. The pre-proPCSK9, stored in the ER, is processed to pro-PCSK9 and this last into mature PCSK9 which is bound to a cleaved prodomain that acts as a catalytic inhibitor and chaperone until its secretion into the circulation. The prodomain-mature PCSK9 heterodimer, with a plasma half-life of 5 min, binds to high-affinity specific proteins, leading to their intracellular degradation [135].
PCSK9 function is to regulate the amount of LDLR by forming a PCSK9-LDLR complex in the hepatocyte surface which is internalized by endocytosis and engaged into endosomal-lysosomal degradation [135,137]. PCSK9 can also bind to LDLR intracellularly through a Golgi-lysosome pathway for degradation [135,138].
Recent findings indicate the participation of PCSK9 in other processes beyond lipid homeostasis such as cell cycle, apoptosis, and inflammation with a potential effect on atherosclerosis [139]. Thus, LPS upregulate PCSK9 in liver and kidney in mice, in human EC and VSMCs [140]. Consistently, the injection of LPS into Pcsk9 deficient mice resulted in less synthesis of pro-inflammatory IL-6 and TNFα cytokines and reduced expression of adhesion molecules in ECs and VSMCs [141].
PCSK9 expression is promoted by TNF in liver and VSMCs and by oxidized LDL in ECs, macrophages, VSMCs, and DC [140,141]. In experimental atherosclerosis, PCSK9 is mostly found in VSMCs, although its expression is LDLR-dependent, as Ldlr-/- mice do not display Pcsk9 expression [142]. Notably, Pcsk9 expression is localized in the artery branches with low shear stress and mirrors cytokine expression of these sites indicating a proatherogenic effect [142]. On the other hand, Apoe-/- mice overexpressing human PCSK9 specifically in the bone marrow displayed augmented levels of proinflammatory Ly6Chi monocyte infiltration in atheromas. Moreover, human PCSK9-overexpresing macrophages displayed enhanced pro-inflammatory Tnf and Il1b genes and diminished anti-inflammatory Il10 and Arg genes [143]. In agreement with these, in vivo Pcsk9 gene silencing by lentiviral transduction of Apoe-/- mice decreased lesion size and macrophage infiltration and expression of Nfkb, Tnfa, Il1, and Tlr4 in atherosclerotic plaques [144]. In macrophages, PCSK9 stimulates proinflammatory cytokine expression and foam cell formation by upregulating Sra and Cd36. Other actions of PCSK9 include activation of T cells, monocyte migration, and VSMC apoptosis [142]. Altogether indicates a role of PCSK9 in atheroma lesion development by regulating inflammatory pathways. Notwithstanding, the clinical relevance of these anti-inflammatory actions remains to be established, as patients treated with PSCK9 inhibitors (PSCK9i) do not display altered levels of the hs-CRP inflammatory marker [142].
4.2. Rational for PCSK9 Inhibition as a Potential Therapy
The main rational for the development of PCSK9i as CVD therapy were the positive correlation between high PCSK9 levels and increased CVD risk and the association between different PCSK9 polymorphisms and vascular illness [145,146]. Moreover, the moderately-high prevalence and the lack of harmful effects of PCSK9 LOF mutations in healthy individuals suggested a high safety of a potential PCSK9 therapeutic inhibition [132,146].
On the other hand, experimental animal data also gave a foundation for the development of PCSK9i. Pcsk9-/- mice displayed up to 80% decrease in plasma LDL-C levels due to increased hepatic LDLR and LDL clearance [147], which resulted in dramatic reductions in aortic atherosclerosis [148].
To date, up to nine PCSK9 inhibitory strategies, acting either preventing its binding to the LDLR, or its maturation, secretion, or synthesis, have been or are being developed [149]. These therapies, described below and summarized in Table 4, include the use of monoclonal antibodies (mAb) against PCSK9 [150], antisense oligonucleotides (ASOs), small interfering RNA (siRNAs), vaccines, and small molecules [151].
Table 4.
Pre-clinical and clinical trials studying PCSK9 inhibitors in the context of hypercholesterolemia.
| Inhibition Strategy | Animal Models | Effects on Lipids | Effects on Atherosclerosis | References |
|---|---|---|---|---|
| PCSK9 mAb | Cynomolgus monkeys | Decreased LDL-C (80%), T-Chol (48%) | --- | [153] |
| PCSK9 mAb | C57BL/6 mice Cynomolgus monkeys |
Decreased LDL-C (40%) | --- | [154] |
| PCSK9 mAb: alirocumab | APOE*3Leiden.CETP mice | Decreased non-HDL-C and TGs | -Decreased inflammation and atherosclerotic lesion -Increased plaque stability |
[152] |
| siRNA PCSK9 | Cynomolgus monkeys C57BL/6 mice Sprague–Dawley rats |
Decrease of LDL-C and T-Chol (60%) | --- | [155] |
| Inhibition Strategy | Trial Name | Type of Patients | Reported Effects | References |
| PCSK9 mAb: evolocumab | FOURIER/OSLER [NCT01764633; NCT01439880] Phase III |
Patients with atherosclerotic CVD | -Increased LDLR and HDL-C -Decreased LDL-C (61%), Total-C (36.1%), TG (12.6%), and Lp(a) (25.5%). -Increased Apolipoprotein A1 and HDL-C -Reduced CV (12–19%) |
[156,157] |
| PCAK9 mAb: alirocumab | ODYSSEY [NCT01507831] Phase III |
ACS patients | -Increased hepatic LDLR and HDL-C (4%) -Decreased LDL-C (58%), Total-C (37.8%), TG (15.6%), and Lp(a) (29.3%) and MACE -Increased ApoA1 and HDL-C -Reduced CV events (48%) |
[158,159] |
| PCSK9 mAb: alirocumab, evolocumab | Meta-analysis | Alirocumab or evolocumab-treated patients | -Decreased nonfatal CV events and mortality. -Improved atherogenic events |
[150,160] |
| PCSK9 siRNA: ALN-PCS |
Phase I dose-escalation study [NCT01437059] |
Healthy adult volunteers | -Reduced LDL-C (40%) | [161] |
| PCSK9 siRNA: ALN-60212 (Inclisirian) |
ORION 1 Phase II: [NCT02597127] Phase III 10, 11: [NCT03399370; NCT03400800] |
Patients at CVD risk with elevated LDL-C and some receiving statins | -Reduced LDL-C (52.6%) relative to base-line, as well as apoB and non-HDL-C. | [162,163] |
ACS: Acute coronary syndrome; ApoA1: Apolipoprotein A1; CV: cardiovascular; CVD: cardiovascular disease; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; LDLR: LDL receptor; Lp(a): lipoprotein (a); MACE: Major adverse cardiovascular events; Total-C: total cholesterol; TG: triglycerides.
4.2.1. PCSK9 Monoclonal Antibodies
The only current clinical therapy to inhibit PCSK9 function in use is the administration of human mAb against soluble PCSK9. A single intravenous injection of the PCSK9 mAb, alirocumab, in hyperlipidemic APOE*3Leiden.CETP transgenic mice, decreased non-HDL-C, inflammatory parameters, and atherosclerosis lesion size and improved plaque stability in a dose-dependent manner [152]. Similarly, administration of a PCSK9 mAb reduced about 80% the levels of LDL-C in non-human primates [153]. An improved version of this mAb, with greater antigen affinity and resistance to degradation, achieved a reduction of around 40% in LDL-C levels at lower doses and an efficiency of 2.8 times longer [154].
The two monoclonal antibodies approved as a PCSK9-blocking therapies are alirocumab (SAR236553/REGN727 from Regeneron Pharmaceuticals/Sanofi) and evolocumab (AMG145 from Amgen). These are currently prescribed to reduce the risk of atherosclerotic CVD and lower LDL-C levels in patients with FH and resistance to statin therapy, among others [150,164]. Alirocumab is a human IgG1 mAb that strongly binds PCSK9 with a maximum effect between 8 and 15 days after its subcutaneous administration [165]. Evolocumab is a human mAb of the isotype G2 against PCSK9 with a maximum efficacy between the first and second week after subcutaneous administration [166].
Evolocumab was tested in the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk [NCT01764633]) and OSLER (Open-label Extension Study of Evolocumab [NCT01439880]) trials [156,157], and alirocumab was evaluated in the ODYSSEY trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome With Alirocumab) [158,159]. These showed increased hepatic LDLR and reduced circulating levels of LDL-C by up to 60% as well as VLDL [167]. Both alirocumab and evolocumab slightly increased HDL levels and apolipoprotein A1 and diminished plasmatic lipoprotein(a) [132]. A current meta-analysis have shown that PCSK9 antibodies reduce nonfatal cardiovascular events and all-cause mortality [156,160], improve several atherogenic events [150], and promote plaque regression [168].
Notwithstanding, the use of mAbs as PCSK9 inhibitors for CVD therapy holds several limitations, such as the high cost (about 14,500 USD per patient each year), a limited lifetime, and administration frequency of twice a month [149]. Because of this, novel approaches to pharmacologically inhibit PCSK9 are under development consisting of gene silencing either with siRNAs or by using ASOs.
4.2.2. Small Interfering RNA (siRNA)
The mechanism of action of PCSK9 gene silencing uses a duplex 20 bp RNA molecule acting as a siRNA. Within the cell, it renders an anti-sense strand that binds to the mRNA gene, generating an incomplete duplex RNA later processed by DICER [151], thus preventing PCSK9 translation and increasing LDLR protein hepatic content.
Pre-clinical trials have shown that PCSK9 gene silencing by siRNA reduced cholesterol levels in monkeys by 60% due to a 50–70% decrease in the PCSK9 mRNA levels [155]. PCSK9 siRNA suppressed the inflammatory response by diminishing the generation of ROS and LDL oxidation-induced apoptosis in ECs and macrophages, respectively [145]. In clinical trial phase I, this same PCSK9 siRNA reduced LDL-C levels by 40% [161] but the effects were not lasting and an improved version of the siRNA was developed giving rise to Inclisiran (ALN-60212 from Alnylam) [149,169]. Inclisiran has been designed for hepatic cellular internalization, does not induce immunogenic reactions, and has a greater resistance to degradation by nucleases [169]. In phase I clinical trial, intravenous injection of Inclisiran, a siRNA in a lipid nanoparticle (ALN-60212), reduced the levels of PCSK9 and LDL-C by 74.5% and 50.6% respectively, with lasting effects between 3 to 6 months and decreases in non-HDL-C and apolipoprotein B (apoB) [170]. Phase II trial ORION-1, which evaluated different Inclisiran doses in patients who had a history of atherogenic CVD or high atherosclerotic CVD-risk with elevated LDL-C levels, showed a dose-dependent decrease in PCSK9 and LDL-C levels [162]. Besides, patients who received two doses presented a reduction of 69.1% and 52.6% of PCSK9 and LDL-C [151,162], without adverse reactions [169]. Results of ORION phase III trials (ORION 10 and 11) have shown the effectivity of Inclisiran in patients with CVD, CVD risk or with elevated LDL-C levels despite statin-treatment at the maximum dose, with reductions in LDL-C, non-HDL-C, and apoB [163].
Because its potent and long-lasting effect lowering LDL-C levels, the administration frequency (once/twice per year) [149], low cost and easier long-term storage, Inclisiran holds great promise as an hypolipemiant agent.
4.3. Other Developing Approaches to PCSK9 Inhibition
Other drug strategies to inhibit PCSK9 function include the use of ASOs (antisense oligonucleotides), small-molecules inhibitors, PCSK9 vaccination or the CRISPR/Cas9 system (clustered regularly interspaced short palindromic repeat associated protein 9) to lower PCSK9 concentration [151]. These approaches, in preclinical phase or phase I [150], are expected to offer a number of several additional benefits such as prolonged effects, easier production and lower cost [149]. The administration of an ASO, which is a 15–30 pb single-strand DNA that hybridizes with PCSK9 mRNA and ensues RNase H degradation route [164], resulted in a high specific decrease of LDL-C and PCSK9 levels of 25–50% and 50–85%, respectively, in a phase I clinical trial [151]. Notably, berberine, a natural product obtained from a plant, and its derivatives have been shown to inhibit the transcription of PCSK9, and therefore it could also be useful in the management of patients with CVD risk [149,168].
5. Conclusions
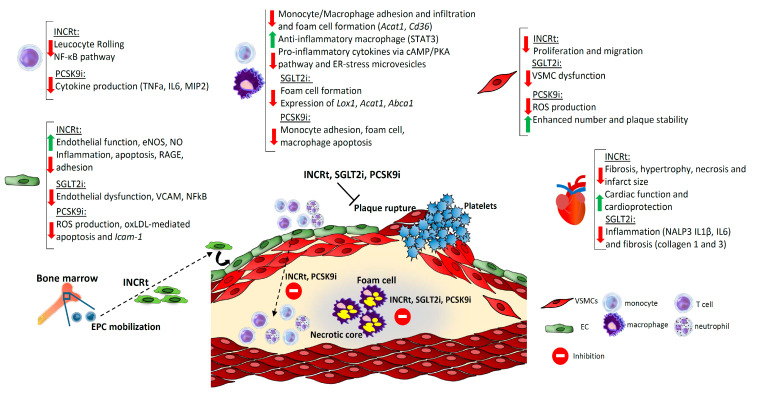
The investigations reviewed here indicate that the new emergent clinical therapies designed to restore metabolic homeostasis have major effects on the cardiovascular system beyond metabolic control. In addition to their expected anti-diabetic and lipid-lowering actions, the main common mechanisms for CVD prevention and atheroprotection of the three class drugs in the studies reviewed here are a direct modulation of vascular and inflammatory cell phenotypes and an improvement of the vascular and cardiomyocyte function. These effects of the incretin-therapies, SGLT2i and PCSK9i in vascular, immune cells and cardiomyocytes have been summarized in Figure 2. These have been shown to prevent early stages of atherosclerosis such as decreased leukocyte adhesion, endothelial dysfunction, immune cell recruitment, and foam cell formation. Moreover, several animal studies also demonstrated that these strategies stabilize atheromas and diminish necrotic cores of advanced plaques. A promising future of incretin-based therapies and SGLT2i for their use in cardiovascular prevention, and possibly, the use of small molecules as PCSK9i for more affordable therapeutics, is anticipated. Notwithstanding, completion of the ongoing clinical trials and further insight about the mechanisms of action of these drugs is needed to reduce the burden of CVD in the future.
Figure 2.
Common mechanism of action of incretin-therapies, SGLT2i and PCSK9i in immune and vascular cells involved in atherosclerosis progression and in cardiomyocyte function. The main protective mechanisms of PCSK9i and INCRt in T cells include the impairment of leukocyte rolling and a decrease in NFkB activation and cytokine secretion. Both PCSK9i and INCRt also reduce monocyte adhesion and recruitment while in macrophages all three class drugs decrease macrophage foam cell formation. INCRt also decreases inflammatory genes in macrophages and PCSK9i impairs restrains proatherogenic apoptosis. Endothelial function is improved by all three class drugs by several mechanisms such as enhanced eNOS, reduced adhesion of leukocytes and inflammatory molecules and diminished ROS production. Notably, INCrt also promote endothelial cell repair by the mobilization of EPC from the bone marrow. In VSMCs’ INCRt decrease proliferation and hyperplasia, PCSK9i promotes plaque stability by enhancing VSMC number, and SGLT2i reduce their dysfunction. All these mechanisms lead to a decrease in leukocyte retention in the subendothelial space, less foam cell formation, reduced necrotic core and prevention of plaque rupture that, in patients, ensues the acute cardiovascular events. Lastly, among the actions observed in cardiomyocytes stands out the improved cardiac function by INCRt and SGLT2i by decreasing inflammation and fibrosis and necrosis. EPC: endothelial progenitor cell; INCRt: incretin-therapies; SGLT2i: sodium-glucose co-transporter 2 inhibitor; PCSK9i: proprotein convertase subtilisin kexin 9 inhibitor.
Author Contributions
M.A.-B., G.H.-G., and A.T.-C. reviewed all the literature helped to write the manuscript. A.H.-C. help to write some sections of the manuscript. S.M.-H. and H.G.-N. designed the review, supervised the literature, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by from Carlos III Health Institute and the European Regional Development Fund (FEDER) (CB07/08/0043, PI19/00169 to H.G.-N., S.M.-H.), and JR18/00051 from Generalitat Valenciana, (GenT CDEI-04/20-B to H.G.-N.).
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Ärnlöv J., Afshin A., et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1: Evidence from genetic, epidemiologic, and clinical studies. A consensus statement fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., Goldberg A.C., Gordon D., Levy D., Lloyd-Jones D.M., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bornfeldt K.E. Uncomplicating the macrovascular complications of diabetes: The 2014 edwin bierman award lecture. Diabetes. 2015;64:2689–2697. doi: 10.2337/db14-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeva-Andany M.M., Martínez-Rodríguez J., González-Lucán M., Fernández-Fernández C., Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13:1449–1455. doi: 10.1016/j.dsx.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Kubota T., Kubota N., Kumagai H., Yamaguchi S., Kozono H., Takahashi T., Inoue M., Itoh S., Takamoto I., Sasako T., et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Hervás S., Vinué Á., Núñez L., Andrés-Blasco I., Piqueras L., TomásReal J., Ascaso J.F., Burks D.J., Sanz M.J., González-Navarro H. Insulin resistance aggravates atherosclerosis by reducing vascular smoothmuscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovasc. Res. 2014;103:324–336. doi: 10.1093/cvr/cvu115. [DOI] [PubMed] [Google Scholar]
- 8.Van Dijk R.A., Duinisveld A.J.F., Schaapherder A.F., Mulder-Stapel A., Hamming J.F., Kuiper J., de Boer O.J., van der Wal A.C., Kolodgie F.D., Virmani R., et al. A change in inflammatory footprint precedes plaque instability: A systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lytvyn Y., Bjornstad P., Pun N., Cherney D.Z.I. New and old agents in the management of diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2016;25:232–239. doi: 10.1097/MNH.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White W.B., Baker W.L. Cardiovascular effects of incretin-based therapies. Annu. Rev. Med. 2016;67:245–260. doi: 10.1146/annurev-med-050214-013431. [DOI] [PubMed] [Google Scholar]
- 11.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Drucker D.J. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Müller T.D., Finan B., Bloom S.R., D’Alessio D., Drucker D.J., Flatt P.R., Fritsche A., Gribble F., Grill H.J., Habener J.F., et al. Glucagon-like peptide 1 (GLP-1) Mol. Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ussher J.R., Drucker D.J. Cardiovascular actions of incretin-based therapies. Circ. Res. 2014;114:1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 15.Lambeir A.M., Durinx C., Scharpé S., De Meester I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 16.Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D.M., Eckardt K., Kaufman J.M., Ryden M., Müller S., et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong J., Maiseyeu A., Davis S.N., Rajagopalan S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015;116:1491–1504. doi: 10.1161/CIRCRESAHA.116.305665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisman E.Z., Tenenbaum A. Antidiabetic treatment with gliptins: Focus on cardiovascular effects and outcomes. Cardiovasc. Diabetol. 2015;14:1–13. doi: 10.1186/s12933-015-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Röhrborn D., Wronkowitz N., Eckel J. DPP4 in diabetes. Front. Immunol. 2015;6:386. doi: 10.3389/fimmu.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong J., Rao X., Deiuliis J., Braunstein Z., Narula V., Hazey J., Mikami D., Needleman B., Satoskar A.R., Rajagopalan S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62:149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikushima H., Munakata Y., Iwata S., Ohnuma K., Kobayashi S., Dang N.H., Morimoto C. Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell. Immunol. 2002;215:106–110. doi: 10.1016/S0008-8749(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 22.Ghorpade D.S., Ozcan L., Zheng Z., Nicoloro S.M., Shen Y., Chen E., Blüher M., Czech M.P., Tabas I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance HHS Public Access. Nature. 2018;555:673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sell H., Blüher M., Klöting N., Schlich R., Willems M., Ruppe F., Knoefel W.T., Dietrich A., Fielding B.A., Arner P., et al. Adipose dipeptidyl peptidase-4 and obesity: Correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36:4083–4090. doi: 10.2337/dc13-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wronkowitz N., Görgens S.W., Romacho T., Villalobos L.A., Sánchez-Ferrer C.F., Peiró C., Sell H., Eckel J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2014;1842:1613–1621. doi: 10.1016/j.bbadis.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Alonso N., Teresa Julián M., Puig-Domingo M., Vives-Pi M. Incretin hormones as immunomodulators of atherosclerosis. Front. Endocrinol. 2012;3:112. doi: 10.3389/fendo.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X., Tavallaie M.S., Sun R., Wang J., Cai Q., Shen J., Lei S., Fu L., Jiang F. Drug discovery approaches targeting the incretin pathway. Bioorg. Chem. 2020;99 doi: 10.1016/j.bioorg.2020.103810. [DOI] [PubMed] [Google Scholar]
- 27.Standl E., Schnell O., McGuire D.K., Ceriello A., Rydén L. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5:391–402. doi: 10.1016/S2213-8587(17)30033-5. [DOI] [PubMed] [Google Scholar]
- 28.McGuire D.K., van de Werf F., Armstrong P.W., Standl E., Koglin J., Green J.B., Bethel M.A., Cornel J.H., Lopes R.D., Halvorsen S., et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: Secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126–135. doi: 10.1001/jamacardio.2016.0103. [DOI] [PubMed] [Google Scholar]
- 29.Scirica B.M., Bhatt D.L., Braunwald E., Steg P.G., Davidson J., Hirshberg B., Ohman P., Frederich R., Wiviott S.D., Hoffman E.B., et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 30.Zannad F., Cannon C.P., Cushman W.C., Bakris G.L., Menon V., Perez A.T., Fleck P.R., Mehta C.R., Kupfer S., Wilson C., et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 31.McGuire D.K., Alexander J.H., Johansen O.E., Perkovic V., Rosenstock J., Cooper M.E., Wanner C., Kahn S.E., Toto R.D., Zinman B., et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139:351–361. doi: 10.1161/CIRCULATIONAHA.118.038352. [DOI] [PubMed] [Google Scholar]
- 32.Mcinnes G., Evans M., del Prato S., Stumvoll M., Schweizer A., Lukashevich V., Shao Q., Kothny W. Cardiovascular and heart failure safety profile of vildagliptin: A meta-analysis of 17,000 patients. Diabetes Obes. Metab. 2015;17:1085–1092. doi: 10.1111/dom.12548. [DOI] [PubMed] [Google Scholar]
- 33.Remm F., Franz W.M., Brenner C. Gliptins and their target dipeptidyl peptidase 4: Implications for the treatment of vascular disease. Eur. Hear. J. Cardiovasc. Pharm. 2016;2:185–193. doi: 10.1093/ehjcvp/pvv044. [DOI] [PubMed] [Google Scholar]
- 34.Ervinna N., Mita T., Yasunari E., Azuma K., Tanaka R., Fujimura S., Sukmawati D., Nomiyama T., Kanazawa A., Kawamori R., et al. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo e-deficient mice. Endocrinology. 2013;154:1260–1270. doi: 10.1210/en.2012-1855. [DOI] [PubMed] [Google Scholar]
- 35.Nishida S., Matsumura T., Senokuchi T., Murakami-Nishida S., Ishii N., Morita Y., Yagi Y., Motoshima H., Kondo T., Araki E. Inhibition of inflammation-mediated DPP-4 expression by linagliptin increases M2 macrophages in atherosclerotic lesions. Biochem. Biophys. Res. Commun. 2020;524:8–15. doi: 10.1016/j.bbrc.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Kahles F., Liberman A., Halim C., Rau M., Möllmann J., Mertens R.W., Rückbeil M., Diepolder I., Walla B., Diebold S., et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE−/− mice by blocking monocyte/macrophage activation. Mol. Metab. 2018;14:150–157. doi: 10.1016/j.molmet.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagashima M., Watanabe T., Terasaki M., Tomoyasu M., Nohtomi K., Kim-Kaneyama J., Miyazaki A., Hirano T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein e knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tashiro Y., Sato K., Watanabe T., Nohtomi K., Terasaki M., Nagashima M., Hirano T. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides. 2014;54:19–26. doi: 10.1016/j.peptides.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Bruen R., Curley S., Kajani S., Crean D., O’Reilly M.E., Lucitt M.B., Godson C.G., McGillicuddy F.C., Belton O. Liraglutide dictates macrophage phenotype in apolipoprotein E null mice during early atherosclerosis. Cardiovasc. Diabetol. 2017;16 doi: 10.1186/s12933-017-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G., Lei Y., Inoue A., Piao L., Hu L., Jiang H., Sasaki T., Wu H., Xu W., Yu C., et al. Exenatide mitigated diet-induced vascular aging and atherosclerotic plaque growth in ApoE-deficient mice under chronic stress. Atherosclerosis. 2017;264:1–10. doi: 10.1016/j.atherosclerosis.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Arakawa M., Mita T., Azuma K., Ebato C., Goto H., Nomiyama T., Fujitani Y., Hirose T., Kawamori R., Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Parlevliet E.T., Geerling J.J., van der Tuin S.J.L., Zhang H., Bieghs V., Jawad A.H.M., Shiri-Sverdlov R., Bot I., de Jager S.C.A., et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br. J. Pharm. 2014;171:723–734. doi: 10.1111/bph.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinué Á., Navarro J., Herrero-Cervera A., García-Cubas M., Andrés-Blasco I., Martínez-Hervás S., Real J.T., Ascaso J.F., González-Navarro H. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia. 2017;60:1801–1812. doi: 10.1007/s00125-017-4330-3. [DOI] [PubMed] [Google Scholar]
- 44.Rakipovski G., Rolin B., Nøhr J., Klewe I., Frederiksen K.S., Augustin R., Hecksher-Sørensen J., Ingvorsen C., Polex-Wolf J., Knudsen L.B. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE/− and LDLr/Mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018;3:844–857. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah Z., Kampfrath T., Deiuliis J.A., Zhong J., Pineda C., Ying Z., Xu X., Lu B., Moffatt-Bruce S., Durairaj R., et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helmstädter J., Frenis K., Filippou K., Grill A., Dib M., Kalinovic S., Pawelke F., Kus K., Kröller-Schön S., Oelze M., et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arter. Thromb. Vasc. Biol. 2020;40:145–158. doi: 10.1161/atv.0000615456.97862.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noyan-Ashraf M.H., Shikatani E.A., Schuiki I., Mukovozov I., Wu J., Li R.K., Volchuk A., Robinson L.A., Billia F., Drucker D.J., et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 48.Noyan-Ashraf M.H., Abdul Momen M., Ban K., Sadi A.M., Zhou Y.Q., Riazi A.M., Baggio L.L., Henkelman R.M., Husain M., Drucker D.J. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wohlfart P., Linz W., Hübschle T., Linz D., Huber J., Hess S., Crowther D., Werner U., Ruetten H. Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies. J. Transl. Med. 2013;11:84. doi: 10.1186/1479-5876-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirano T., Mori Y. Anti-atherogenic and anti-inflammatory properties of glucagon-like peptide-1, glucose-dependent insulinotropic polypepide, and dipeptidyl peptidase-4 inhibitors in experimental animals. J. Diabetes Investig. 2016;7:80–86. doi: 10.1111/jdi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J., Liu X., Fang Q., Ding M., Li C. Liraglutide attenuates atherosclerosis via inhibiting ER-induced macrophage derived microvesicles production in T2DM rats. Diabetol. Metab. Syndr. 2017;9 doi: 10.1186/s13098-017-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim S., Lee G.Y., Park H.S., Lee D.H., Jung O.T., Min K.K., Kim Y.B., Jun H.S., Chul J.H., Park K.S. Attenuation of carotid neointimal formation after direct delivery of a recombinant adenovirus expressing glucagon-like peptide-1 in diabetic rats. Cardiovasc. Res. 2017;113:183–194. doi: 10.1093/cvr/cvw213. [DOI] [PubMed] [Google Scholar]
- 53.Hirano T., Yamashita S., Takahashi M., Hashimoto H., Mori Y., Goto M. Anagliptin, a dipeptidyl peptidase-4 inhibitor, decreases macrophage infiltration and suppresses atherosclerosis in aortic and coronary arteries in cholesterol-fed rabbits. Metabolism. 2016;65:893–903. doi: 10.1016/j.metabol.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Sudo M., Li Y., Hiro T., Takayama T., Mitsumata M., Shiomi M., Sugitani M., Matsumoto T., Hao H., Hirayama A. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: Serial In vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 2017;265:283–291. doi: 10.1016/j.atherosclerosis.2017.06.920. [DOI] [PubMed] [Google Scholar]
- 55.Oyama J.I., Higashi Y., Node K. Do incretins improve endothelial function? Cardiovasc. Diabetol. 2014;13:21. doi: 10.1186/1475-2840-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang C.-Y., Shih C.-M., Tsao N.-W., Lin Y.-W., Huang P.-H., Wu S.-C., Lee A.-W., Kao Y.-T., Chang N.-C., Nakagami H., et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br. J. Pharm. 2012;167:1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akoumianakis I., Badi I., Douglas G., Chuaiphichai S., Herdman L., Akawi N., Margaritis M., Antonopoulos A.S., Oikonomou E.K., Psarros C., et al. Insulin-induced vascular redox dysregulation in human atherosclerosis is ameliorated by dipeptidyl peptidase 4 inhibition. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aav8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagamine A., Hasegawa H., Hashimoto N., Yamada-Inagawa T., Hirose M., Kobara Y., Tadokoro H., Kobayashi Y., Takano H. The effects of DPP-4 inhibitor on hypoxia-induced apoptosis in human umbilical vein endothelial cells. J. Pharm. Sci. 2017;133:42–48. doi: 10.1016/j.jphs.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Chihara A., Tanaka A., Morimoto T., Sakuma M., Shimabukuro M., Nomiyama T., Arasaki O., Ueda S., Node K. Differences in lipid metabolism between anagliptin and sitagliptin in patients with type 2 diabetes on statin therapy: A secondary analysis of the REASON trial. Cardiovasc. Diabetol. 2019;18:158. doi: 10.1186/s12933-019-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green J.B., Bethel M.A., Armstrong P.W., Buse J.B., Engel S.S., Garg J., Josse R., Kaufman K.D., Koglin J., Korn S., et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 61.Rosenstock J., Kahn S.E., Johansen O.E., Zinman B., Espeland M.A., Woerle H.J., Pfarr E., Keller A., Mattheus M., Baanstra D., et al. Effect of linagliptin vs. glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The Carolina randomized clinical trial. JAMA J. Am. Med. Assoc. 2019;322:1155–1166. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perkovic V., Toto R., Cooper M.E., Mann J.F.E., Rosenstock J., McGuire D.K., Kahn S.E., Marx N., Alexander J.H., Zinman B., et al. Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: Secondary analysis of the carmelina randomized trial. Diabetes Care. 2020;43:1803–1812. doi: 10.2337/dc20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfeffer M.A., Claggett B., Diaz R., Dickstein K., Gerstein H.C., Køber L.V., Lawson F.C., Ping L., Wei X., Lewis E.F., et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 64.Holman R.R., Bethel M.A., Mentz R.J., Thompson V.P., Lokhnygina Y., Buse J.B., Chan J.C., Choi J., Gustavson S.M., Iqbal N., et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angelyn Bethel M., Mentz R.J., Merrill P., Buse J.B., Chan J.C., Goodman S.G., Iqbal N., Jakuboniene N., Katona B., Lokhnygina Y., et al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once-weekly exenatide: Insights from the EXSCEL trial. Diabetes Care. 2020;43 doi: 10.2337/dc19-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marso S.P., Daniels G.H., Frandsen K.B., Kristensen P., Mann J.F.E., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma S., Bhatt D.L., Bain S.C., Buse J.B., Mann J.F.E., Marso S.P., Nauck M.A., Poulter N.R., Pratley R.E., Zinman B., et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease results of the LEADER trial. Circulation. 2018;137:2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898. [DOI] [PubMed] [Google Scholar]
- 68.Verma S., Poulter N.R., Bhatt D.L., Bain S.C., Buse J.B., Leiter L.A., Nauck M.A., Pratley R.E., Zinman B., Ørsted D.D., et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke: Post hoc analysis from the leader trial. Circulation. 2018;138:2884–2894. doi: 10.1161/CIRCULATIONAHA.118.034516. [DOI] [PubMed] [Google Scholar]
- 69.Nauck M.A., Tornøe K., Rasmussen S., Treppendahl M.B., Marso S.P. Cardiovascular outcomes in patients who experienced a myocardial infarction while treated with liraglutide versus placebo in the LEADER trial. Diabetes Vasc. Dis. Res. 2018;15:465–468. doi: 10.1177/1479164118783935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mann J.F.E., Ørsted D.D., Brown-Frandsen K., Marso S.P., Poulter N.R., Rasmussen S., Tornøe K., Zinman B., Buse J.B. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 71.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 72.Husain M., Birkenfeld A.L., Donsmark M., Dungan K., Eliaschewitz F.G., Franco D.R., Jeppesen O.K., Lingvay I., Mosenzon O., Pedersen S.D., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 73.Hernandez A.F., Green J.B., Janmohamed S., D’Agostino R.B., Granger C.B., Jones N.P., Leiter L.A., Rosenberg A.E., Sigmon K.N., Somerville M.C., et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 74.Gerstein H.C., Colhoun H.M., Dagenais G.R., Diaz R., Lakshmanan M., Pais P., Probstfield J., Riesmeyer J.S., Riddle M.C., Rydén L., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 75.Dagenais G.R., Rydén L., Leiter L.A., Lakshmanan M., Dyal L., Probstfield J.L., Atisso C.M., Shaw J.E., Conget I., Cushman W.C., et al. Total cardiovascular or fatal events in people with type 2 diabetes and cardiovascular risk factors treated with dulaglutide in the REWIND trail: A post hoc analysis. Cardiovasc. Diabetol. 2020;19:199. doi: 10.1186/s12933-020-01179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frias J.P., Bastyr E.J., Vignati L., Tschöp M.H., Schmitt C., Owen K., Christensen R.H., DiMarchi R.D. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343–352. doi: 10.1016/j.cmet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 77.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C., Urva S., Gimeno R.E., Milicevic Z., Robins D., et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 78.Coskun T., Sloop K.W., Loghin C., Alsina-Fernandez J., Urva S., Bokvist K.B., Cui X., Briere D.A., Cabrera O., Roell W.C., et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drucker D.J. Mechanisms of Action and Therapeutic Application of Glucagon-Like Peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Baggio L.L., Drucker D.J. Harnessing the Therapeutic Potential of Glucagon-Like Peptide-1: A Critical Review. Treat Endocrinol. 2002;1:117–125. doi: 10.2165/00024677-200201020-00005. [DOI] [PubMed] [Google Scholar]
- 81.Aroda V.R. A Review of GLP-1 Receptor Agonists: Evolution and Advancement, Through the Lens of Randomised Controlled Trials. Diabetes Obes. Metab. 2018:22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 82.Knudsen L.B., Lau J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eng J., Kleinman W.A., Singh L., Singh G., Raufman J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 1992;267:7402–7405. doi: 10.1016/S0021-9258(18)42531-8. [DOI] [PubMed] [Google Scholar]
- 84.Nauck M.A., Quast D.R., Wefers J., Meier J.J. GLP-1 Receptor Agonists in the Treatment of type 2 Diabetes—State-of-the-Art. Mol. Metab. 2020:101–102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deacon C.F., Knudsen L.B., Madsen K., Wiberg F.C., Jacobsen O., Holst J.J. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia. 1998;41:271–278. doi: 10.1007/s001250050903. [DOI] [PubMed] [Google Scholar]
- 86.Knudsen J.G., Hamilton A., Ramracheya R., Tarasov A.I., Brereton M., Haythorne E., Chibalina M.V., Spégel P., Mulder H., Zhang Q., et al. Dysregulation of glucagon secretion by hyperglycemia-induced sodium-dependent reduction of ATP production. Cell Metab. 2019;29:430–442. doi: 10.1016/j.cmet.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaspari T., Welungoda I., Widdop R.E., Simpson R.W., Dear A.E. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE/mouse model. Diabetes Vasc. Dis. Res. 2013;10:353–360. doi: 10.1177/1479164113481817. [DOI] [PubMed] [Google Scholar]
- 88.Shiraishi D., Fujiwara Y., Komohara Y., Mizuta H., Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem. Biophys. Res. Commun. 2012;425:304–308. doi: 10.1016/j.bbrc.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 89.Ding L., Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharm. Sin. 2012;33:75–81. doi: 10.1038/aps.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai Y., Mehta J.L., Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc. Drugs Ther. 2013;27:371–380. doi: 10.1007/s10557-013-6463-z. [DOI] [PubMed] [Google Scholar]
- 91.Krasner N.M., Ido Y., Ruderman N.B., Cacicedo J.M. Glucagon-Like Peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Erdogdu Ö., Eriksson L., Nyström T., Sjöholm Å., Zhang Q. Exendin-4 restores glucolipotoxicity-induced gene expression in human coronary artery endothelial cells. Biochem. Biophys. Res. Commun. 2012;419:790–795. doi: 10.1016/j.bbrc.2012.02.106. [DOI] [PubMed] [Google Scholar]
- 93.Mori Y., Matsui T., Hirano T., Yamagishi S.I. Gip as a potential therapeutic target for atherosclerotic cardiovascular disease–a systematic review. Int. J. Mol. Sci. 2020;21:1509. doi: 10.3390/ijms21041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finan B., Ma T., Ottaway N., Müller T.D., Habegger K.M., Heppner K.M., Kirchner H., Holland J., Hembree J., Raver C., et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 95.Sachs S., Niu L., Geyer P., Jall S., Kleinert M., Feuchtinger A., Stemmer K., Brielmeier M., Finan B., DiMarchi R.D., et al. Plasma proteome profiles treatment efficacy of incretin dual agonism in diet-induced obese female and male mice. Diabetes Obes. Metab. 2020 doi: 10.1111/dom.14215. [DOI] [PubMed] [Google Scholar]
- 96.Khoo B., Tan T.M.M. Combination gut hormones: Prospects and questions for the future of obesity and diabetes therapy. J. Endocrinol. 2020;246:R65–R74. doi: 10.1530/JOE-20-0119. [DOI] [PubMed] [Google Scholar]
- 97.Yamagishi S.I., Fukami K., Matsui T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc. Diabetol. 2015;14:1–12. doi: 10.1186/s12933-015-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nogi Y., Nagashima M., Terasaki M., Nohtomi K., Watanabe T., Hirano T. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe A., Choe S., Chaptal V., Rosenberg J.M., Wright E.M., Grabe M., Abramson J. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature. 2010;468:988–991. doi: 10.1038/nature09580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wright E.M., Loo D.D.F.L., Hirayama B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 101.Ghezzi C., Loo D.D.F., Wright E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–2097. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bakris G.L., Fonseca V.A., Sharma K., Wright E.M. Renal sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 103.Rieg T., Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61:2079–2086. doi: 10.1007/s00125-018-4654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rossetti L., Smith D., Shulman G.I., Papachristou D., DeFronzo R.A. Correction of hyperglycemia with phlorizin normalizes tissues sensitivity to insulin in diabetic rats. J. Clin. Investig. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oku A., Ueta K., Arakawa K., Ishihara T., Nawano M., Kuronuma Y., Matsumoto M., Saito A., Tsujihara K., Anai M., et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes. 1999;48:1794–1800. doi: 10.2337/diabetes.48.9.1794. [DOI] [PubMed] [Google Scholar]
- 106.Brown E., Rajeev S.P., Cuthbertson D.J., Wilding J.P.H. A review of the mechanism of action, metabolic profile and hemodynamic effects of sodium-glucose co-transporter-2 inhibitors. Diabetes Obes. Metab. 2019;21:9–18. doi: 10.1111/dom.13650. [DOI] [PubMed] [Google Scholar]
- 107.Ferrannini E., Muscelli E., Frascerra S., Baldi S., Mari A., Heise T., Broedl U.C., Woerle H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonner C., Kerr-Conte J., Gmyr V., Queniat G., Moerman E., Thévenet J., Beaucamps C., Delalleau N., Popescu I., Malaisse W.J., et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 109.Merovci A., Solis-Herrera C., Daniele G., Eldor R., Vanessa Fiorentino T., Tripathy D., Xiong J., Perez Z., Norton L., Abdul-Ghani M.A., et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Investig. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tahara A., Takasu T., Yokono M., Imamura M., Kurosaki E. Characterization and comparison of SGLT2 inhibitors: Part 3. Effects on diabetic complications in type 2 diabetic mice. Eur. J. Pharm. 2017;809:163–171. doi: 10.1016/j.ejphar.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 111.Aroor A.R., Das N.A., Carpenter A.J., Habibi J., Jia G., Ramirez-Perez F.I., Martinez-Lemus L., Manrique-Acevedo C.M., Hayden M.R., Duta C., et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc. Diabetol. 2018;17 doi: 10.1186/s12933-018-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steven S., Oelze M., Hanf A., Kröller-Schön S., Kashani F., Roohani S., Welschof P., Kopp M., Gödtel-Armbrust U., Xia N., et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee D.M., Battson M.L., Jarrell D.K., Hou S., Ecton K.E., Weir T.L., Gentile C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018;17:62. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Terasaki M., Hiromura M., Mori Y., Kohashi K., Nagashima M., Kushima H., Watanabe T., Hirano T. Amelioration of hyperglycemia with a sodium-glucose cotransporter 2 inhibitor prevents macrophage-driven atherosclerosis through macrophage foam cell formation suppression in type 1 and type 2 diabetic mice. PLoS ONE. 2015;10:e143396. doi: 10.1371/journal.pone.0143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaspari T., Spizzo I., Liu H., Hu Y., Simpson R.W., Widdop R.E., Dear A.E. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diabetes Vasc. Dis. Res. 2018;15:64–73. doi: 10.1177/1479164117733626. [DOI] [PubMed] [Google Scholar]
- 116.Han J.H., Oh T.J., Lee G., Maeng H.J., Lee D.H., Kim K.M., Choi S.H., Jang H.C., Lee H.S., Park K.S., et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE −/− mice fed a western diet. Diabetologia. 2017;60:364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 117.Nasiri-Ansari N., Dimitriadis G.K., Agrogiannis G., Perrea D., Kostakis I.D., Kaltsas G., Papavassiliou A.G., Randeva H.S., Kassi E., Nasiri-Ansari Ν., et al. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc. Diabetol. 2018;17:106. doi: 10.1186/s12933-018-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ye Y., Bajaj M., Yang H.C., Perez-Polo J.R., Birnbaum Y. SGLT-2 Inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC Inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc. Drugs. 2017;31:119–132. doi: 10.1007/s10557-017-6725-2. [DOI] [PubMed] [Google Scholar]
- 119.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 120.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N., Shaw W., Law G., Desai M., Matthews D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 121.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M., Edwards R., Agarwal R., Bakris G., Bull S., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 122.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., Silverman M.G., Zelniker T.A., Kuder J.F., Murphy S.A., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 123.Nassif M.E., Windsor S., Tang F., Khariton Y., Husain M., Inzucchi S., McGuire D., Pitt B., Scirica B., Austin B., et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction. Circulation. 2019;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929. [DOI] [PubMed] [Google Scholar]
- 124.Cannon C.P., Pratley R., Dagogo-Jack S., Mancuso J., Huyck S., Masiukiewicz U., Charbonnel B., Frederich R., Gallo S., Cosentino F., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 125.Bhatt D.L., Szarek M., Pitt B., Cannon C.P., Leiter L.A., McGuire D.K., Lewis J.B., Riddle M.C., Inzucchi S.E., Kosiborod M.N., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 126.Bhatt D.L., Szarek M., Steg P.G., Cannon C.P., Leiter L.A., McGuire D.K., Lewis J.B., Riddle M.C., Voors A.A., Metra M., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 127.Bailey C.J., Marx N. Cardiovascular protection in type 2 diabetes: Insights from recent outcome trials. Diabetes Obes. Metab. 2019;21:3–14. doi: 10.1111/dom.13492. [DOI] [PubMed] [Google Scholar]
- 128.Seidah N.G., Awan Z., Chrétien M., Mbikay M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014;114:1022–1036. doi: 10.1161/CIRCRESAHA.114.301621. [DOI] [PubMed] [Google Scholar]
- 129.Abifadel M., Varret M., Rabès J.P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 130.Cohen J., Pertsemlidis A., Kotowski I.K., Graham R., Garcia C.K., Hobbs H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 131.Cohen J.C., Boerwinkle E., Mosley T.H., Hobbs H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 132.Stoekenbroek R.M., Lambert G., Cariou B., Hovingh G.K. Inhibiting PCSK9—Biology beyond LDL control. Nat. Rev. Endocrinol. 2018;15:52–62. doi: 10.1038/s41574-018-0110-5. [DOI] [PubMed] [Google Scholar]
- 133.Seidah N.G., Prat A., Pirillo A., Catapano A.L., Danilo Norata G. Novel strategies to target proprotein convertase subtilisin kexin 9: Beyond monoclonal antibodies. Cardiovasc. Pathol. 2019;115:510–519. doi: 10.1093/cvr/cvz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seidah N.G., Benjannet S., Wickham L., Marcinkiewicz J., Bélanger Jasmin S., Stifani S., Basak A., Prat A., Chrétien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shapiro M.D., Tavori H., Fazio S. PCSK9 from basic science discoveries to clinical trials. Circ. Res. 2018;122:1420–1438. doi: 10.1161/CIRCRESAHA.118.311227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ding Z., Wang X., Liu S., Shahanawaz J., Theus S., Fan Y., Deng X., Zhou S., Mehta J.L. PCSK9 expression in the ischemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 2018;114:1738–1751. doi: 10.1093/cvr/cvy128. [DOI] [PubMed] [Google Scholar]
- 137.Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 138.Lagace T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014;25:387–393. doi: 10.1097/MOL.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lan H., Pang L., Smith M.M., Levitan D., Ding W., Liu L., Shan L., Shah V.V., Laverty M., Arreaza G., et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) affects gene expression pathways beyond cholesterol metabolism in liver cells. J. Cell. Physiol. 2010;224:273–281. doi: 10.1002/jcp.22130. [DOI] [PubMed] [Google Scholar]
- 140.Tang Z.H., Li T.H., Peng J., Zheng J., Li T.T., Liu L.S., Jiang Z.S., Zheng X.L. PCSK9: A novel inflammation modulator in atherosclerosis? J. Cell. Physiol. 2019;234:2345–2355. doi: 10.1002/jcp.27254. [DOI] [PubMed] [Google Scholar]
- 141.Ding Z., Liu S., Wang X., Deng X., Fan Y., Shahanawaz J., Reis R.J.S., Varughese K.I., Sawamura T., Mehta J.L. Cross-Talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 2015;107:556–567. doi: 10.1093/cvr/cvv178. [DOI] [PubMed] [Google Scholar]
- 142.Ding Z., Pothineni N.V.K., Goel A., Lüscher T.F., Mehta J.L. PCSK9 and inflammation: Role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 2020;116:908–915. doi: 10.1093/cvr/cvz313. [DOI] [PubMed] [Google Scholar]
- 143.Giunzioni I., Tavori H., Covarrubias R., Major A.S., Ding L., Zhang Y., Devay R.M., Hong L., Fan D., Predazzi I.M., et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 2016;238:52–62. doi: 10.1002/path.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang Z.H., Peng J., Ren Z., Yang J., Li T.T., Li T.H., Wang Z., Wei D.H., Liu L.S., Zheng X.L., et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis. 2017;262:113–122. doi: 10.1016/j.atherosclerosis.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 145.Guo Y., Yan B., Gui Y., Tang Z., Tai S., Zhou S., Zheng X.L. Physiology and role of PCSK9 in vascular disease: Potential impact of localized PCSK9 in vascular wall. J. Cell. Physiol. 2020 doi: 10.1002/jcp.30025. [DOI] [PubMed] [Google Scholar]
- 146.Urban D., Pöss J., Böhm M., Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 2013;62:1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 147.Rashid S., Curtis D.E., Garuti R., Anderson N.H., Bashmakov Y., Ho Y.K., Hammer R.E., Moon Y.A., Horton J.D. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Denis M., Marcinkiewicz J., Zaid A., Gauthier D., Poirier S., Lazure C., Seidah N.G., Prat A. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012;125:894–901. doi: 10.1161/CIRCULATIONAHA.111.057406. [DOI] [PubMed] [Google Scholar]
- 149.Xu S., Luo S., Zhu Z., Xu J. Small Molecules as Inhibitors of PCSK9: Current Status and Future Challenges. Eur. J. Med. Chem. 2019;162:212–233. doi: 10.1016/j.ejmech.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 150.Warden B.A., Fazio S., Shapiro M.D. The PCSK9 Revolution: Current Status, Controversies and Future Directions: The PCSK9 Revolution. Trends Cardiovasc. Med. 2020;30:179–185. doi: 10.1016/j.tcm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 151.Nishikido T., Ray K.K. Non-antibody approaches to proprotein convertase subtilisin kexin 9 inhibition: SiRNA, antisense oligonucleotides, adnectins, vaccination, and new attempts at small-molecule inhibitors based on new discoveries. Front. Cardiovasc. Med. 2019;5 doi: 10.3389/fcvm.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kühnast S., van der Hoorn J.W.A., Pieterman E.J., van den Hoek A.M., Sasiela W.J., Gusarova V., Peyman A., Schäfer H.L., Schwahn U., Jukema J.W., et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 2014;55:2103–2112. doi: 10.1194/jlr.M051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chan J.C.Y., Piper D.E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA. 2009;106:9820–9825. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chaparro-Riggers J., Liang H., deVay R.M., Bai L., Sutton J.E., Chen W., Geng T., Lindquist K., Casas M.G., Boustany L.M., et al. Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J. Biol. Chem. 2012;287:11090–11097. doi: 10.1074/jbc.M111.319764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frank-Kamenetsky M., Grefhorst A., Anderson N.N., Racie T.S., Bramlage B., Akinc A., Butler D., Charisse K., Dorkin R., Fan Y., et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sabatine M.S., Giugliano R.P., Wiviott S.D., Raal F.J., Blom D.J., Robinson J., Ballantyne C.M., Somaratne R., Legg J., Wasserman S.M., et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 157.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., Kuder J.F., Wang H., Liu T., Wasserman S.M., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., Edelberg J.M., Goodman S.G., Hanotin C., Harrington R.A., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 159.Robinson J.G., Farnier M., Krempf M., Bergeron J., Luc G., Averna M., Stroes E.S., Langslet G., Raal F.J., el Shahawy M., et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 160.Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Brégeault M.F., Dalby A.J., Diaz R., Edelberg J.M., Goodman S.G., Hanotin C., et al. Effect of alirocumab on mortality after acute coronary syndromes: An analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2019;140:103–112. doi: 10.1161/CIRCULATIONAHA.118.038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fitzgerald K., Frank-Kamenetsky M., Shulga-Morskaya S., Liebow A., Bettencourt B.R., Sutherland J.E., Hutabarat R.M., Clausen V.A., Karsten V., Cehelsky J., et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: A randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ray K.K., Landmesser U., Leiter L.A., Kallend D., Dufour R., Karakas M., Hall T., Troquay R.P.T., Turner T., Visseren F.L.J., et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 2017;376:1430–1440. doi: 10.1056/NEJMoa1615758. [DOI] [PubMed] [Google Scholar]
- 163.Ray K.K., Wright R.S., Kallend D., Koenig W., Leiter L.A., Raal F.J., Bisch J.A., Richardson T., Jaros M., Wijngaard P.L.J., et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 164.Blanchard V., Khantalin I., Ramin-Mangata S., Chémello K., Nativel B., Lambert G. PCSK9: From biology to clinical applications. Pathology. 2019;51:177–183. doi: 10.1016/j.pathol.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 165.Tomlinson B., Hu M., Zhang Y., Chan P., Liu Z.M. Alirocumab for the treatment of hypercholesterolemia. Expert Opin. Biol. 2017;17:633–643. doi: 10.1080/14712598.2017.1305354. [DOI] [PubMed] [Google Scholar]
- 166.Kasichayanula S., Grover A., Emery M.G., Gibbs M.A., Somaratne R., Wasserman S.M., Gibbs J.P. Clinical pharmacokinetics and pharmacodynamics of evolocumab, a PCSK9 inhibitor. Clin. Pharm. 2018;57:769–779. doi: 10.1007/s40262-017-0620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reyes-Soffer G., Pavlyha M., Ngai C., Thomas T., Holleran S., Ramakrishnan R., Karmally W., Nandakumar R., Fontanez N., Obunike J., et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135:352–362. doi: 10.1161/CIRCULATIONAHA.116.025253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ogura M. PCSK9 inhibition in the management of familial hypercholesterolemia. J. Cardiol. 2018;71:1–7. doi: 10.1016/j.jjcc.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 169.Stoekenbroek R.M., Kallend D., Wijngaard P.L., Kastelein J.J. Inclisiran for the treatment of cardiovascular disease: The ORION clinical development program. Future Cardiol. 2018;14:433–442. doi: 10.2217/fca-2018-0067. [DOI] [PubMed] [Google Scholar]
- 170.Fitzgerald K., White S., Borodovsky A., Bettencourt B.R., Strahs A., Clausen V., Wijngaard P., Horton J.D., Taubel J., Brooks A., et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 2017;376:41–51. doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.