Abstract
Simple Summary
Immune-based treatment strategies, which include immune checkpoint inhibition, have recently become a new frontier for the treatment of B-cell-derived lymphoma. Whereas checkpoint inhibition has given oncologists and patients hope in specific lymphoma subtypes like Hodgkin lymphoma, other entities do not benefit from such promising agents. Understanding the factors that determine the efficacy and safety of checkpoint inhibition in different lymphoma subtypes can lead to improved therapeutic strategies, including combinations with various chemotherapies, biologics and/or different immunologic agents with manageable safety profiles.
Abstract
For years, immunotherapy has been considered a viable and attractive treatment option for patients with cancer. Among the immunotherapy arsenal, the targeting of intratumoral immune cells by immune-checkpoint inhibitory agents has recently revolutionised the treatment of several subtypes of tumours. These approaches, aimed at restoring an effective antitumour immunity, rapidly reached the market thanks to the simultaneous identification of inhibitory signals that dampen an effective antitumor response in a large variety of neoplastic cells and the clinical development of monoclonal antibodies targeting checkpoint receptors. Leading therapies in solid tumours are mainly focused on the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) pathways. These approaches have found a promising testing ground in both Hodgkin lymphoma and non-Hodgkin lymphoma, mainly because, in these diseases, the malignant cells interact with the immune system and commonly provide signals that regulate immune function. Although several trials have already demonstrated evidence of therapeutic activity with some checkpoint inhibitors in lymphoma, many of the immunologic lessons learned from solid tumours may not directly translate to lymphoid malignancies. In this sense, the mechanisms of effective antitumor responses are different between the different lymphoma subtypes, while the reasons for this substantial difference remain partially unknown. This review will discuss the current advances of immune-checkpoint blockade therapies in B-cell lymphoma and build a projection of how the field may evolve in the near future. In particular, we will analyse the current strategies being evaluated both preclinically and clinically, with the aim of fostering the use of immune-checkpoint inhibitors in lymphoma, including combination approaches with chemotherapeutics, biological agents and/or different immunologic therapies.
Keywords: immune checkpoint, lymphoid neoplasms, programmed death 1, cytotoxic T-lymphocyte antigen 4, monoclonal antibodies, combination therapies
1. Biology of B-Cell Lymphoma
The term B-cell lymphoma encompasses different neoplasms characterised by an abnormal proliferation of lymphoid cells at various stages of differentiation. B-cell lymphoma develops more frequently in older adults and immunocompromised individuals and includes both Hodgkin’s lymphomas (HLs) and most B-cell non-Hodgkin lymphomas (B-NHLs). The latter accounts for up to 4% of the globally diagnosed cancers [1] and are characterised by a malignant proliferation of mature or immature B-lymphocytes in lymphoid tissues and extranodal territories such as the gastrointestinal tract, the central nervous system (CNS), or, essentially, any other body organ [2]. Inherited events such as chromosomal translocations, oncogene activation or even certain viral infections such as the Epstein–Barr virus (EBV) may trigger lymphomagenesis [3]. B-NHLs are divided into low and high grades, typically corresponding to indolent (slow-growing) lymphomas and aggressive lymphomas, respectively. Indolent lymphomas include follicular lymphoma (FL), marginal zone lymphoma (MZL), small cell lymphocytic lymphoma (SLL)/chronic lymphocytic leukaemia (CLL) and Waldenström macroglobulinemia (WM). Early-stage indolent B-cell lymphomas can often be treated with radiation alone, with long-term nonrecurrence. Early-stage aggressive disease is treated with chemotherapy and, often, radiation, with a 70–90% curation rate. Aggressive lymphomas include both precursor lymphoid neoplasms and numerous mature B-cell neoplasms like mantle cell lymphoma (MCL), primary effusion lymphoma (PEL), Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL) and its many subtypes and variants, and unclassifiable B-cell lymphoma, with features intermediate between DLBCL and BL. These entities usually require intensive treatments, with some having a good prospect for a permanent cure [4].
1.1. Diffuse Large B-Cell Lymphoma
Diffuse large B-cell lymphoma (DLBCL) represents the most common type of B-NHL in Western countries. The 2016 World Health Organization (WHO) classification of lymphoid malignancies recognises several subtypes characterised by unique clinical and pathological features, including primary DLBCL of the central nervous system (PCNSL), primary cutaneous DLBCL, leg type, T-cell/histiocyte-rich large cell lymphoma, and EBV-positive DLBCL of the elderly. Nevertheless, most cases of DLBCL fall into the “not otherwise specified” (NOS) category [4].
DLBCL, like other cancers, develops in a complex tissue environment with a high content of malignant and nonmalignant compartments of the disease, as well as extracellular components that constitute the tumour microenvironment (TME). The cellular and molecular features of TME have a profound prognostic impact [5] and include T-cells, tumour-associated macrophages (TAMs), dendritic cells (DCs), neutrophils, natural killer (NK) cells and stromal cells [6]. DLBCL harbours a noninflamed phenotype characterised by lack of immune cell infiltration, which could explain the modest efficacy of immune checkpoint blockade therapy in relapsed/refractory (R/R) DLBCL patients [7].
1.2. Primary Mediastinal B-Cell Lymphoma
Primary mediastinal B-cell lymphoma (PMBL) is a rare but aggressive lymphoma of thymic B-cell origin, accounting for 3% of B-NHLs. Although it presents similar histology to DLBCL, the genetic profile of PMBL is distinct and shares many features with classic Hodgkin lymphoma (cHL, see below) [8]. Patients are generally not cured after first-line treatment, and, after relapse, autologous stem cell transplantation (ASCT) is usually beneficial. However, relapsed/refractory (R/R) PMBL cases have poor outcomes and are often managed like other forms of DLBCL [9].
1.3. Follicular Lymphoma
Follicular lymphoma (FL) is the second most common B-NHL, accounting for 29–35% of cases. It is a neoplasm of germinal centre B-cells, which display rearrangement of immunoglobulin (Ig) heavy and light chain genes and somatic hypermutation and express common germinal centre markers such as BCL6, AID and CD10 [10,11,12]. FL generally presents an indolent clinical course, with median overall survival (OS) of more than 15 years [10,13]. However, about 20% of patients relapse during the first 2 years after treatment, and others evolve into transformed-FL (t-FL), a much more aggressive subtype [10].
The crosstalk between malignant FL cells and the surrounding cells of their TME is driven by some recurrent genetic events [14]. FL is strongly regulated by direct interaction with a germinal centre (GC)-like microenvironment, including myeloid cells, follicular helper T-cells (TFH), and stromal cells, that may orchestrate efficient immune escape mechanisms [15]. The TME of FL also displays deregulation of the extracellular matrix proteins involved in collagen deposition and organization [16]. Cancer-associated fibroblasts (CAFs) are another important FL tumour-promoting actor, providing a niche with high levels of factors involved in B-cell activation and the activation/recruitment of some TME components such as TAMs [17]. The crosstalk between TFH cells and FL cells is orchestrated by the interaction between antigen-loaded MHC class II molecules and antigen-specific T-cell receptors.
1.4. Burkitt Lymphoma
Burkitt lymphoma (BL) includes a heterogeneous group of highly aggressive malignancies of intermediate-sized B-cells that may be found infiltrating both nodal or extranodal tissues in a diffuse pattern [18]. BL is invariably associated with chromosomal translocations that dysregulate the expression of c-MYC, and, consequently, several downstream genes involved in the control of cellular processes such as cell cycle progression and apoptosis [19]. The malignant cells usually express the B-cell-specific surface markers CD19 and CD20, as well as low-to-intermediate levels of common acute lymphoblastic leukaemia (ALL) antigen (CD10/CALLA) [20].
The complex interplay between BL cells and the TME also regulates lymphomagenesis and provides new insights for target immunotherapies. Like DLBCL, BL tumours harbour a noninflamed environment with low infiltration of immune cells and are usually resistant to immune checkpoint blockade. One of the hallmarks of the TME in BL tumours is the high content of TAMs which contribute to tumour progression through the secretion of cytokines and chemokines, and the expression of immune checkpoint proteins such as programmed death ligand 1 (PD-L1) [21] (see below). The crosstalk between tumour cells, TAMs, PD-1 signalling, viral antigens, and T-cells may result in the high prevalence of M2 macrophages in the TME and contribute to the failed immunity of BL patients [22].
1.5. Marginal Zone Lymphoma
Marginal zone lymphoma (MZL) originates from memory B-cells at the marginal zone of lymphoid follicles and account for 5–15% of all NHLs [23,24]. Three distinct entities have been described. Splenic (SMZL) and nodal marginal zone lymphoma (NMZL) arise from the follicle marginal zone of the spleen and the lymph nodes, respectively [24,25,26]. Extranodal marginal zone lymphoma (EMZL) of the mucosa-associated lymphoid tissue (MALT) is the most common subtype, accounting for about 60% of MZL cases. This entity is strongly associated with chronic inflammation derived from autoimmune disease or infection, such as Helicobacter pylori. Other tumours sites include eyes and ocular adnexa (13%), skin (9%), lungs (9%) and salivary glands (8%) [23,27,28]. MZLs mostly have indolent clinical courses, although NMZL has a poorer prognosis than other subtypes [25,26,27].
The course of MZL disease is strongly influenced by the TME, and this latter may therefore represent a promising strategy for early diagnosis and therapy choice. SMZL cells are supported by immune cells such as mast cells and macrophages, which may be recruited by tumour cells through the secretion of cytokines and chemokines [29]. The TME components of SMZL can regulate stromal cell proliferation, angiogenesis, extracellular matrix remodelling, and induction of adhesion molecule expression [29]. The chronic inflammation of MALT lymphomas not only triggers B-cell growth but also recruits T-cells, macrophages and neutrophils to the site of inflammation, which contribute to genetic aberrations, DNA damage and genetic instability of the B-cells during somatic hypermutation and class-switching recombination [30].
1.6. Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) originates from B-cells, a proportion of them being antigen-experienced B-cells, in the mantle zone of lymph nodes. MCL is usually diagnosed as a late-stage disease and may be observed in both the gastrointestinal tract and bone marrow [31]. The diagnosis of MCL is mainly performed by a microscopic evaluation of a biopsy, although the detection of chromosomal translocation t (11:14), with the consequent cyclin D1 expression, is considered the molecular hallmark [32].
The crosstalk between MCL tumour cells and its microenvironment has a central role in disease expansion [33]. MCL cells have shown constitutive expression of PD-1 and its ligand PD-L1, which converts it into an interesting candidate for immunotherapy targeting this checkpoint [34] (see below). Aggressive MCL cases are characterised by a low number of T-cells [35] and a high frequency of regulatory T-cells (Treg) [36]. Moreover, follicular dendritic cells (FDCs) have been shown to support MCL cell survival through a cell–cell interaction mechanism [37]. Autocrine and paracrine secretion of soluble factors could also have an important role within the MCL TME. Interestingly, the blood of MCL patients contains high levels of several cytokines and chemokines, such as IL-8, CCL3 and CCL4, which are correlated with poor survival [38].
1.7. Classical Hodgkin Lymphoma
Classical Hodgkin lymphoma (cHL) is a neoplasm derived from B-cells and is mainly constituted by a small number of neoplastic mononuclear cells, i.e., Hodgkin cells, and multinucleated Reed–Sternberg (HRS) cells. cHL accounts for 15–25% of all lymphomas and represents the most common lymphoma subtype in children and young adults in the Western world. The cell of origin (COO) is nowadays unequivocally considered to be a (post)germinal centre B-cell [39]. Several genetic alterations, targeting a few pathways, have been identified, but none of them can be considered “dominant”. The affected pathways include NF-κB and JAK-STAT, whose aberrant activation fuel HRS cells with proliferative and antiapoptotic stimuli [40]. Moreover, the LMP1 protein, encoded by EBV that often latently infects HRS cells, likely contributes to NF-κB signalling since LMP1 mimics constitutively active CD402 [41].
Genetic lesions of NF-κB pathway genes largely contribute to aberrant activation of this cascade in a cell-intrinsic manner and/or by amplifying signals from the microenvironment [42]. In addition, HRS cells are outnumbered by reactive cells in the TME, including T- and B-lymphocytes, eosinophils, macrophages, mast cells, plasma cells and stromal cells [43,44].
2. Immune Checkpoint Blockade in B-Cell Lymphoma
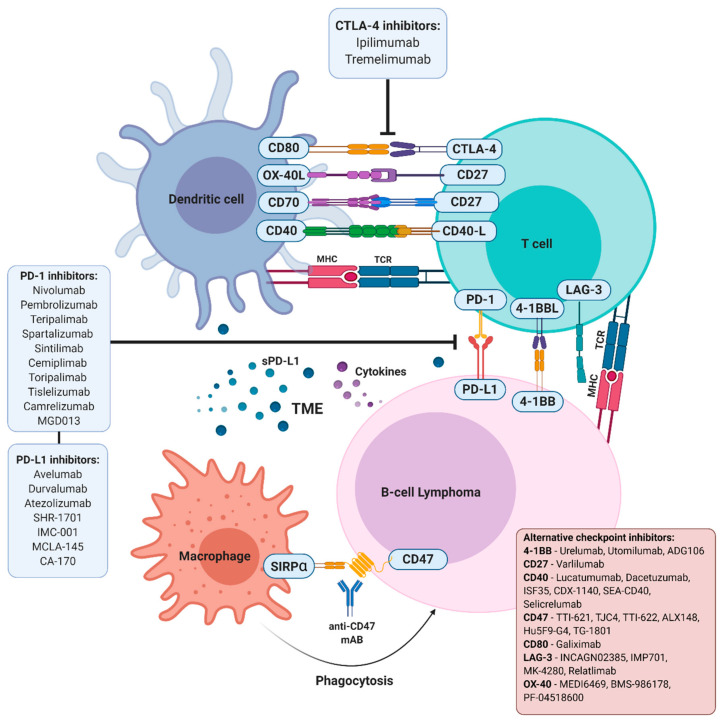
Among the armament of immunotherapies aimed at allowing the host’s own immune system to detect and eliminate malignant cells, immune checkpoints blockers are able to modulate molecules that regulate immune signalling, either positively, by promoting the activation, maturation and proliferation of T-cells, or negatively, by blocking T-cell activity, eventually leading to the programmed death of these latter (Figure 1). Most B-NHLs, including BL, DLBCL, FL and CLL, are characterised by a low infiltration of immune cells, a feature that may condition a priori the applicability of immune checkpoint blockers. Although there is no evidence of a specific genetic immune escape program that may prevent immune cells from entering the local TME to promote an effective antitumor response in a determined lymphoma subtype, recent data support the notion that oncogenic signalling can promote a “noninflamed” TME. As an example, PTEN, EZH2, and TP53 dysregulation have been associated with the downregulation of genes related to innate or adaptive immunity in DLBCL, potentially leading to immune suppression, decreased HLA expression and reduced T-cell infiltration [45,46,47,48,49,50]. The oncogene MYC, involved in the pathogenesis of BL and other lymphoma subtypes, may also be involved in the regulation of the immune environment by regulating the transcription of different immune checkpoint molecules, including CD47 and PD-L1 [51].
Figure 1.
Therapeutic approaches based on immune checkpoint blockade in B-cell lymphomas. Different therapeutic strategies to block PD-1/PD-L1 interaction are under clinical development in order to prevent PD-1-mediated attenuation of TCR signalling, allowing for activity restoration of exhausted CD8+ T-cells. CTLA-4 inhibition by monoclonal antibodies may induce tumour rejection through direct blockade of CTLA-4 competition for CD-80 (B7-1) and CD-86 (B7-2) ligands, which enhances CD28 costimulation and, thus, activation. Alternative immune checkpoint molecules expressed on tumour cells or immune cells in the TME can be simultaneously modulated to restore an effective antilymphoma immune response.
2.1. PD-1/PD-L1 Blockade
2.1.1. PD-1 Signalling Overview
Overexpression of PD-1 and its ligands, PD-L1 (CD274) and/or PD-L2 (PDCD1LG2), by malignant neoplastic cells allows the ligation of PD-1 on T-cells and the consequent induction of T-cell “exhaustion”, a phenomenon closely linked to peripheral tolerance and homeostasis. That way, the malignant cells escape from the antitumor immune response in a process known as immune evasion [52].
PD-1 is a protein encoded by the PDCD1 gene at chromosome 2q37.3, which contains an extracellular domain, a transmembrane domain, and a cytoplasmic domain with two tyrosine signalling motifs [53]. PD-1 is expressed on CD4+ and CD8+ T-cells, B-cells, NK cells, macrophages, and some DCs during immune activation and inflammation [54,55]. On B-cells, PD-1 is markedly regulated by B-cell receptor (BCR) signalling, lipopolysaccharide (LPS), CpG oligodeoxynucleotides, and several proinflammatory cytokines [56] (Figure 1).
The PD-L1 protein is encoded by the CD274 gene on chromosome 9p24.1 and harbours two extracellular domains, a transmembrane domain, and a short cytoplasmic tail that lacks signalling motifs [57]. The expression of PD-L1 is strongly affected by structural alterations such as amplifications, gains, and translocations of chromosome 9p24.1 [58]. Remarkably, 9p24.1 amplification also induces Janus kinase 2 (JAK2) expression, leading to activation of JAK/signal transducers and activators of transcription (STAT) signalling, which in turn, upregulates PD-L1 [41]. Upon engagement with PD-L1, PD-1 becomes phosphorylated by Src family kinases and transmits a negative costimulatory signal through tyrosine phosphatase proteins to attenuate the strength of T-cell receptor (TCR) signals and downstream signalling pathways such as PTEN–PI3K–AKT and RAS–MEK–ERK. The functional outcome of this regulation is the inhibition of cytotoxic T-lymphocyte function [59,60,61,62,63].
In 70–87% of cHL patients, PD-L1 is detected on the surface of both HRS cells and TAMs [64,65,66,67,68] and is associated with worse event-free survival (EFS) and shorter progression-free survival (PFS) [64]. This overexpression can be consequent to EBV infection [69]; in a large majority of cases, PDL-1 upregulation is the result of genetic alterations of chromosome 9p24.1, thereby also affecting the expression of PDL-2 and JAK2 [41,64,66,68]. Increased PDL-1 expression by TAMs following interferon (IFN)-γ signalling may be particularly relevant in cHL clinical outcomes due to the close relationship between HRS and PD-1+ CD4+ T-cells [70,71].
In DLBCL, PD-L1 has been shown to be expressed by the nonmalignant compartment in only 26% to 75% of the cases [65,72,73,74,75]. Godfrey et al. showed that 27% of DLBCL patients (especially from the nongerminal centre subgroup) presented a PD-L1 amplification associated with inferior PFS following front-line chemoimmunotherapy [58,71,72,74,76,77,78]; this was more often detected in de-novo than transformed cases [65,76]. Similar to cHL, EBV infection has been correlated with a much higher PD-L1 expression in DLBCL tumours [74]. The prognostic significance of PD-L1 expression in DLBCL patients is controversial, but most of the studies have reported a poorer outcome in cases with PD-L1+ macrophages [74]. Additionally, overexpression of PD-L1 is associated with the immune escape gene signature involving Bruton’s tyrosine kinase (BTK) and JAK/STAT signalling [79].
Genetic alterations of chromosome 9p24.1 of PD-L1 and/or PD-L2 have also been reported in PMBL, and in two other lymphoma subtypes that arise in immune-privileged extranodal sites, i.e., PCNSL, and primary testicular lymphoma (PTL) [58,71,80,81,82,83]. Accordingly, PD-L1 and PD-L2 are found to be overexpressed in a majority of PMBL patients [41,66,71,84] and about 50% of PCNSL and PTL patients harbour PD-1 ligand overexpression [80].
Regarding PD-1, receptor expression was detected in 39.5–68.6% of DLBCL cases [85], and data support the notion that a high number of PD-1+ tumour-infiltrating lymphocytes (TILs) are associated with favourable clinical features and prognosis [72,86]. In contrast to DLBCL, FL tumour cells are largely negative for PD-L1 and PD-L2, and in this disease, the TILS are characterised by high PD-1 expression and suppressed cytokine signalling [87]. Importantly, the presence of PD-1+ TILs is a favourable prognostic factor, whereas a low number of TILs is associated with increased risk of histologic transformation [88,89].
Finally, in MCL, available data on the expression of PD-L1 are often conflicting. Several studies have shown that PD-L1 expression is low or absent in MCL [65,90], whereas others have shown a variable but constitutive expression of PD-L1 on tumour cells in both cell lines and primary patient samples [34].
2.1.2. PD-1/PD-L1 Inhibition in B-Cell Lymphoma
The blockade of the PD-1/PD-L1 pathway (Figure 2) has transformed immunotherapy with a promising increase in OS rates, leading to U.S. Food and Drug Administration (FDA) approval of these immune checkpoint blockade drugs for the treatment of a broad range of tumour types over the past decade. Two anti-PD-1 antibodies (nivolumab (BMS-936558/ONO-4538, Opdivo®) and pembrolizumab (Keytruda®)) and three anti-PD-L1 antibodies (durvalumab, atezolizumab, and avelumab) have been approved for the treatment of various types of cancer, including lymphomas [68,91,92,93,94,95,96].
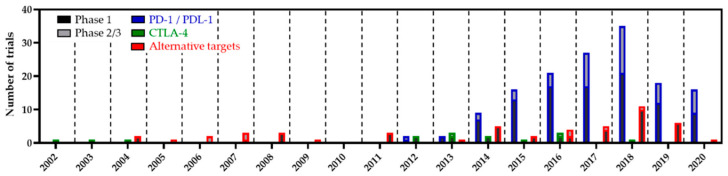
Figure 2.
Clinical evolution of immune checkpoint-based therapies in B-cell lymphoma over the last 20 years (according to https://beacon-intelligence.com/checkpoint; data actualised in September 2020).
Nivolumab and pembrolizumab, two fully humanised IgG4-kappa-blocking monoclonal antibodies, target the PD-1 receptor on human T-cells [97,98,99]. Nivolumab binds specifically to PD-1 and does not affect the related members of the CD28 family, such as CD28, CTLA-4, inducible co-stimulator, and B- or T-lymphocyte attenuator. The blockade of the PD-1 signalling pathway by nivolumab induces both the proliferation of lymphocytes and the release of IFN-γ. Pembrolizumab binds with high affinity to human PD-1, blocking receptor ligation by both PD-L1 and PD-L2 and leading to enhanced T-lymphocyte immune responses in preclinical models of cancer, with the modulation of key cytokines like interleukin (IL)-2, tumour necrosis factor (TNF)-α, and IFN-γ [100,101].
Most DLBCL patients were initially thought to be not amenable to PD-1 blockade since PDL-1/2 alterations are nonfrequent in this disease, and, accordingly, PD-1 blockade therapy has been disappointing to date in R/R DLBCL and FL. While several ongoing clinical trials are evaluating the use of pembrolizumab in different DLBCL subtypes, this antibody failed to improve PFS in ASCT-relapsed patients [102]. Similarly, a first phase-1b dose-escalation cohort expansion study evaluating nivolumab in R/R DLBCL patients (NCT01592370) and a subsequent larger phase-2 study (NCT02038933) in ASCT-relapsed and ASCT-ineligible DLBCL patients reported overall response rates (ORRs) <40% [78,97,100,103] (Table 1). In contrast, in CLL patients with Richter’s transformation (RT), the recent phase-2 trial, MC1485 (NCT02332980), demonstrated an ORR of 44%, including 1 complete response (CR), 2 partial responses (PR) and median PFS and OS of 5.4 months and 10.7 months, with manageable adverse events (AEs; Table 2). As expected, those patients displayed higher levels of PD-L1 expression related to the presence of chromosome 9p24.1 amplification or EBV infection [104].
Table 1.
Clinical evaluation of immune checkpoint-based therapies blockade for the treatment of B-cell lymphomas.
| Targets | Drug/Regimen | Trial ID | Phase | N | Disease | Response | DOR/PFS/OS | Ref |
|---|---|---|---|---|---|---|---|---|
| PD-L1 CD20 |
Atezolizumab + Obinutuzumab + Bendamustine/ Atezolizumab + Obinutuzumab + CHOP |
NCT02596971 | 1/2 | 40 | FL, DLBCL | ORR = 95% CR = 75% |
PFS = 74.9% OS = 86.4% (24-month) |
[121] |
| PD-L1 CD20 |
Atezolizumab + Obinutuzumab + Lenalidomide |
NCT02631577 | 1/2 | 20 | FL | ORR = 85% CR = 80% |
14.5 months | [122] |
| PD-L1 CD20 EZH2 |
Atezolizumab + Obinutuzumab/ Atezolizumab + Tazemetostat |
NCT02220842 | 1 | 43 | FL, DLBCL | ORR = 16% CR = 5% |
PFS = 1.90 months | [123] |
| PD-L1 CD20 BCL2 |
Atezolizumab + Obinutuzumab + Venetoclax |
NCT03276468 | 2 | 58 | DLBCL | ORR = 23.6% | N/A | [124] |
| PD-L1 | Mosunetuzumab/ Atezolizumab + Mosunetuzumab |
NCT02500407 | 1 | 218 | FL, DLBCL, t-FL, iNHL |
ORR = 64.1% (iNHL)/34.7% (others) CR = 42.2% (iNHL)/18.6% (others) |
92.6% (5.8 months, iNHL) 68.2% (8.8 months, aNHL) |
[125] |
| PD-L1 | Atezolizumab + CD20-TCB (RG6026) |
NCT03533283 | 1 | 36 | FL, DLBCL, MCL, PMBL, LPL, iNHL | ORR = 36% CR = 17% |
N/A | [126] |
| PD-L1 | Atezolizumab + KTE-C19 (Axi-cel) |
NCT02926833 | 1/2 | 28 | DLBCL | ORR = 75% CR = 46% |
not reached | [127] |
| PD-L1 BTK |
Durvalumab + Ibrutinib |
NCT02401048 | 1/2 | 61 | FL, DLBCL | ORR = 25% | PFS = 4.6 months OS = 18.1 months |
[120] |
| PD-L1 | Durvalumab + R-CHOP |
NCT03003520 | 2 | 46 | DLBCL | CR = 54.1% | PFS = 12 months | [128] |
| PD-L1 | Durvalumab + JCAR014 |
NCT02706405 | 1 | 13 | DLBCL LBCL HG-BCL |
ORR = 50% CR = 42% |
N/A | [129] |
| PD-1 | Pembrolizumab | NCT01953692 | 1b | 31 | DLBCL, FL, PMBL, cHL, MM | ORR = 58.1% CR = 19.4% PR = 38.7% SD = 22.6% PD = 19.4% |
DOR: not reached PFS = 11.4 months |
[130] |
| PD-1 | Pembrolizumab | NCT02650999 | 1/2 | 12 | DLBCL, FL, MCL, PMBL | ORR = 27% CR = 9% PR = 18% SD = 9% PD = 64% |
N/A | [131] |
| PD-1 | Nivolumab | NCT02038933 | 2 | 121 | DLBCL, B-NHL | ORR = 18% CR = 5% PR = 14% SD = 14% PD = 49% |
DOR = 7.4 months | [7] |
| PD-1 | Nivolumab | NCT02038946 | 2 | 116 | FL | ORR = 4% | DOR = 114 months | [68] |
| PD-1 | Pembrolizumab + ASCT |
NCT02362997 | 2 | 31 | DLBCL, PMBL, iNHL | CR = 59% | OS = 93% PFS = 58% |
[102] |
| PD-1 CD20 |
Nivolumab + Rituximab |
NCT03245021 | 1 | 19 | FL, B-NHL | ORR = 84% CR = 47% PR = 37% PR = 5% SD = 11% |
N/A | [132] |
| PD-1 | Pembrolizumab + R-CHOP |
NCT02541565 | 1 | 33 | DLBCL, FL | ORR = 90% CR = 77% |
PFS = 83% (2-year) |
[133] |
| PD-1 TLR4 CD20 |
Pembrolizumab + G100 + Rituximab |
NCT02501473 (Discontinued) |
1/2 | 18 | FL, MZL | ORR = 33.3% PR = 33.3% SD = 61.1% PD = 5.6% |
N/A | N/A |
| PD-1 | Pembrolizumab + cyclophosph. + DPX-Survivac |
NCT03349450 | 1 | 17 | DLBCL | 2 CR, 2 PR, 2 SD | N/A | [134] |
| PD-1 BTK |
Nivolumab + Ibrutinib |
NCT02329847 | 1/2 | 144 | DLBCL, FL, CLL-RT, SLL | CR = 61% PR = 14% SD = 3% |
N/A | [135] |
| PD-1 BTK |
Pembrolizumab + Acalabrutinib |
NCT02362035 | 1/2 | 61 | DLBCL, cHL, CLL, MM, WM | ORR = 26% CR = 7% PR = 20% SD = 30% PD = 36% |
PFS = 1.9 months | [136] |
| PD-1 BTK PI3K |
Pembrolizumab + Ibrutinib + Idelalisib |
NCT02332980 | 2 | 29 | FL, CLL, CLL-RT, MZL, RT, WM, SLL | ORR = 17% CR = 3% PR = 7% |
N/A | [104] |
| PD-1 CDK |
Pembrolizumab + Dinaciclib |
NCT02684617 | 1 | 128 | DLBCL, FL, CLL, MM | ORR = 18% 3 CR, 4 PR |
DOR = 4.9 months PFS = 2.1 months |
[137] |
| PD-1 HDAC |
Pembrolizumab + Vorinostat |
NCT03150329 | 1 | 30 | DLBCL, PMBL, FL, cHL | ORR = 30% CR = 30% |
DOR = 6 months PFS = 59% |
[138] |
| PD-1 HDAC |
Pembrolizumab + Entinostat |
NCT03179930 | 2 | 22 | FL, cHL | ORR = 92% | N/A | [139] |
| PD-1 | Nivolumab + Lenalidomide |
NCT03015896 | 1/2 | 10 | DLBCL, FL, MCL, MZL, WM, cHL | 1 CR, 2 PR, 3 PD | N/A | [138] |
| PD-1 CD30 |
Nivolumab + Brentuximab vedotin |
NCT02581631 | 1/2 | 30 | DLBCL, PMBL, PTCL, CTCL, MF, SS | ORR = 73% CR = 37% SD = 10% PD = 10% |
DOR = not reached PFS = 63.5% (6 months) |
[106] |
| PD-1 CD19 CD22 |
Pembrolizumab + AUTO3 |
NCT03287817 | 1/2 | 24 | DLBCL, t-FL, PMBL | ORR = 75% CR = 63% |
N/A | [140] |
| PD-1 CD19 |
Pembrolizumab + Tisagenlecleucel |
NCT03630159 | 1 | 8 | DLBCL | 1 PR 2 PD |
N/A | [141] |
| PD-1 CTLA-4 | Nivolumab + Ipilimumab |
NCT01592370 | 1/2 | 169 | cHL, B-NHL, T-NHL, MM | ORR = 20% PR = 20% SD = 7% |
DOR = not reached PFS = not reached OS = not reached |
[142] |
| CTLA-4 | Ipilimumab | NCT00089076 | 1/2 | 18 | DLBCL, FL, MCL | 1 CR, 1 PR | N/A | [143] |
| CTLA-4 CD20 |
Ipilimumab + Rituximab |
NCT01729806 | 1 | 33 | DLBCL, FL, MCL | ORR = 24% | PFS = 2.6 months, FL = 5.6 months | [144] |
| CTLA-4 PD-1 |
Ipilimumab + Nivolumab |
NCT01822509 | 1 | 28 | B-NHL | ORR = 32% | PFS = 1 year | [145] |
| CTLA-4 | Ipilimumab + Lenalidomide |
NCT01919619 | 2 | 11 | DLBCL, FL, MCL | ORR = 73% | 4.6–12 months | [146] |
| CD47 CD20 |
Hu5F9-G4 + Rituximab |
NCT02953509 | 1b/2 | 115 | DLBCL, iNHL | ORR = 36% (DLBCL)/61% (iNHL) CR = 15% (DLBCL)/24% (iNHL) SD = 12% (DLBCL)/24% (iNHL) |
N/A | [147] |
| CD47 CD20 |
TTI-621 + Rituximab |
NCT02663518 | 1 | 32 | DLBCL | ORR = 29% CR = 14% (monotherapy) ORR = 24% CR= 4% (combination) |
N/A | [148] |
| CD47 CD38 |
TTI-622 + Daratumumab |
NCT03530683 | 1 | 19 | DLBCL, MCL, FL | CR = 10% PR = 10% |
N/A | [149] |
| CD47 CD20 |
ALX148 + Rituximab |
NCT03013218 | 1 | 33 | DLCBL, MCL, FL, MZL | ORR = 41%/62.5% CR = 9%/11% |
N/A | [150,151] |
| CD40 | Dacetuzumab | NCT00103779 | 1 | 50 | DLBCL, MCL, FL, MZL | CR = 2% PR = 10% SD = 26% |
N/A | [152] |
| CD40 | Dacetuzumab | NCT00435916 | 2 | 46 | Relapsed FL, DLBCL, MZL |
ORR = 9% CR = 4% PR = 4% 28% SD |
N/A | [153] |
| CD40 | Lucatumumab | NCT00670592 | 1/2 | 111 (74) | FL, MZL, MCL, DLBCL | 33% OR 5% CR 29% PR 52% SD |
N/A | [154] |
| CD40 CD20 |
Dacetuzumab + Rituximab + chemotherapy |
NCT00655837 | 1 | 30 | DLBCL | ORR = 47% CR = 20% PR = 27% |
PFS = 25 weeks | [155] |
| CD40 CD20 |
Dacetuzumab Rituximab + chemotherapy |
NCT00529503 | 2 | 154 (101) | DLBCL, FL | 67% OR 18% SD 33% CR 33% PR |
not reached | [156] |
| CD27 | Varlilumab | NCT01460134 | 1 | 90 (18) | MCL, MZL, DLBCL, CLL, cHL, TCL | SD = 16% | DOR = 6% (14-month) | [157] |
| CD80 CD20 |
Galiximab + Rituximab | NCT00363636 | 3 | 337 | FL | 51% | 12 months | [158] |
| CD80 | Galiximab | NCT00575068 | 1/2 | 38 | FL | ORR = 63% | PFS = 11.7 months | [159] |
| CD80 CD20 |
Galiximab + Rituximab |
NCT00048555 | 1/2 | 73 | FL | ORR = 62% | 11.7 months | [160] |
| CD80 CD20 |
Galiximab + Rituximab |
NCT00117975 | 2 | 61 | FL | 72.1% | 2.9 years | [160] |
| 4-1BB | Urelumab | NCT01471210 | 1 | 60 | DLBCL, FL, B-NHL | ORR = 6% (DLBCL)/12% (FL)/17% (others) | PFS = 8.1 weeks (DLBCL)/8.9 weeks (FL)/13.4 weeks (others) | [161] |
| 4-1BB CD20 |
Urelumab + Rituximab |
NCT01775631 | 1 | 46 | DLBCL, FL |
ORR = 10% (DLBCL)/35% (FL) | PFS = 9 weeks (DLBCL)/40.4 weeks (FL) | [161] |
| 4-1BB CD20 |
Utomilumab + Rituximab |
NCT01307267 | 1 | 67 | FL, MCL, DLBCL | ORR = 21 | PFS = 4.6 months | [162] |
| CD70 | SGN-CD70A |
NCT02216890 Terminated |
1 | 38 | DLBCL, FL, MCL | PR = 15% SD = 30% |
PFS = 1.9 months | [163] |
Abbreviations: FL, follicular lymphoma; t-FL, transformed follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; iNHL, indolent B-cell non-Hodgkin lymphoma; aNHL, aggressive B-cell non-Hodgkin lymphoma; LBCL, large B-cell lymphoma; HG-BCL, high-grade B-cell lymphoma; cHL, classical Hodgkin lymphoma; CLL-RT, chronic lymphocytic leukaemia Richter transformation; PMBL, primary mediastinal B-cell lymphoma; PTCL, peripheral T-cell lymphoma; CTCL, cutaneous T-cell lymphoma; MF, myelofibrosis; SS, Sezary syndrome; MM, multiple myeloma; ORR, overall response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DOR, duration of response; PFS, progression-free survival; OS, overall survival; NA, not available.
Table 2.
Adverse events of immune-checkpoint-based therapy blockade for the treatment of B-cell lymphomas.
| Targets | Drug/Regimen | Trial ID | Phase | N | Disease | All-Grade AEs | Grade ≥3 AEs | SAEs and Discontinuation | Ref |
|---|---|---|---|---|---|---|---|---|---|
| PD-L1 CD20 |
Atezolizumab + Obinutuzumab + Bendamustine/ Atezolizumab + Obinutuzumab + CHOP |
NCT02596971 | 1/2 | 40 | FL, DLBCL | 100% Neutropenia (52%) Constipation (43%) Fatigue (40.5%) |
67% | 36% DAEs 1 TRD |
[121] |
| PD-L1 CD20 |
Atezolizumab + Obinutuzumab + Lenalidomide |
NCT02631577 | 1/2 | 20 | FL | 100% | 73.6% | 29% SAEs 23.7% DAEs |
[122] |
| PD-L1 CD20 EZH2 |
Atezolizumab + Obinutuzumab/ Atezolizumab + Tazemetostat |
NCT02220842 | 1 | 43 | FL, DLBCL | 95% Anaemia (26%) Fatigue (23%) |
47% | 35% SAEs 14% DAEs |
[123] |
| PD-L1 CD20 BCL2 |
Atezolizumab + Obinutuzumab + Venetoclax |
NCT03276468 | 2 | 58 | DLBCL | 84% Lymphopenia (35%) Neutropenia (33%) TP (17.5%) |
10.5% DAEs | [124] | |
| PD-L1 | Mosunetuzumab/ Atezolizumab + Mosunetuzumab |
NCT02500407 | 1 | 218 | FL, DLBCL, tFL, iNHL |
CRS (28.4%) Neurologic AE (44%) |
CRS (1.4%) Neurologic AE (3.2%) |
5.5% DAEs | [125] |
| PD-L1 CD20 |
Atezolizumab + CD20-TCB (RG6026) |
NCT03533283 | 1 | 36 | FL, DLBCL, MCL, PMBL, LPL | CRS (42%) Pyrexia (37%) Anaemia (29%) |
Neutropenia (18%) Anaemia (13%) No G ≥ 3 CRS |
[126] | |
| PD-L1 | Atezolizumab + KTE-C19 (Axi-cel) |
NCT02926833 | 1/2 | 28 | DLBCL | 100% | 86% Neurologic AE (29%) CRS (4%) |
[127] | |
| PD-L1 BTK |
Durvalumab + Ibrutinib |
NCT02401048 | 1/2 | 61 | FL, DLBCL | Diarrhoea (52%) Fatigue (46%) Nausea (34%) |
56% Neutropenia 13% Dyspnea (10%) |
51% SAEs 32% DAEs |
[120] |
| PD-L1 | Durvalumab + JCAR014 |
NCT02706405 | 1 | 13 | DLBCL, HG-BCL, PMBL | CRS (38%) Neurotoxicity (8%) |
[129] | ||
| PD-1 | Pembrolizumab | NCT01953692 | 1b | 31 | DLBCL, FL, PMBL, cHL, MM | 61% Hypothyroidism (11%) Diarrhoea (11%) Nausea (11%) |
11% Neutropenia Liver disease |
No DAEs No TRD |
[130] |
| PD-1 | Pembrolizumab | NCT02576990 | 2 | 53 | PBML | 57% Neutropenia (19%) Hypothyroidism (8%) |
23% Neutropenia (13%) |
2% DAEs No TRD |
[108] |
| PD-1 | Nivolumab | NCT02038933 | 2 | 121 | DLBCL, B-NHL | 98% Nausea (17%) Fatigue (17%) Diarrhoea (12%) |
62% Neutropenia (4%) TP (3%) |
12% SAEs 3% DAEs No TRD |
[7] |
| PD-1 | Pembrolizumab + ASCT |
NCT02362997 | 2 | 31 | DLBCL, PMBL, iNHL | 79% Neutropenia (26%) |
19% DAEs No TRD |
[102] | |
| PD-1 CD20 |
Nivolumab + Rituximab |
NCT03245021 | 1 | 19 | FL, B-NHL | Fatigue (74%) Infection (59%) Nausea (36%) |
Lipase increased (11%) Hyperglycemia (11%) Infection (11%) |
No DAEs | [132] |
| PD-1 | Pembrolizumab + R-CHOP |
NCT02541565 | 1 | 33 | DLBCL, FL | 43% Neutropenia (23%) Infection (10%) Syncope (10%) |
13% SAEs | [133] | |
| PD-1 TLR4 CD20 |
Pembrolizumab + G100 + Rituximab |
NCT02501473 (Discontinued) |
1/2 | 18 | FL, MZL | 100% | Abdominal pain (16%) Diarrhoea (16%) Anaemia (10%) |
10% SAEs 6% DAEs |
N/A |
| PD-1 BTK |
Nivolumab + Ibrutinib |
NCT02329847 | 1/2 | 144 | DLBCL, FL, CLL-RT, SLL | Diarrhoea (33%) Neutropenia (31%) Fatigue (26%) |
82% Neutropenia (28%) Anaemia (23%) |
77% SAEs 28% DAEs No TRD |
[135] |
| PD-1 BTK |
Pembrolizumab + Acalabrutinib |
NCT02362035 | 1/2 | 61 | DLBCL, cHL, CLL, MM, WM | Neutropenia (15%) Anaemia (11%) |
41% DAEs | [136] | |
| PD-1 CDK |
Pembrolizumab + Dinaciclib |
NCT02684617 | 1 | 128 | DLBCL, FL, CLL, MM | 63% TP (21%) Lymphopenia (16%) Anaemia (13%) |
32% Lymphopenia (13%) Neutropenia (11%) TP (8%) |
3% DAEs No TRD |
[137] |
| PD-1 HDAC |
Pembrolizumab + Entinostat |
NCT03179930 | 2 | 22 | FL, cHL | 62% Neutropenia (48%) TP (19%) Anaemia (10%) |
18% SAEs 15% DAE |
[139] | |
| PD-1 CD30 |
Nivolumab + Brentuximab Vedotin |
NCT02581631 | 1/2 | 30 | DLBCL, PMBL, PTCL, CTCL, MF, SS | 83% Neutropenia (30%) Peripheral neuropathy (27%) |
53% Neutropenia (30%) TP (10%) Peripheral neuropathy (10%) |
13% SAEs 7% DAEs No TRD |
[106] |
| PD-1 CD19 CD22 |
Pembrolizumab + AUTO3 |
NCT03287817 | 1/2 | 24 | DLBCL, tFL, PMBL | Neutropenia (89%) TP (58%) Anaemia (47%) |
[140] | ||
| PD-1 CD19 |
Pembrolizumab + Tisagenlecleucel |
NCT03630159 | 1 | 8 | DLBCL | 100% CRS (25%) Tachycardia (25%) |
50% Anaemia (25%) Pancreatitis (25%) |
No DAEs | [141] |
| PD-1 CTLA-4 | Nivolumab + Ipilimumab |
NCT01592370 | 1/2 | 65 | cHL, B-NHL, T-NHL, MM | Fatigue (26%) Pyrexia (23%) Diarrhoea (18%) |
29% | 48% SAEs 8% DAEs No TRD |
[142] |
| CTLA-4 | Ipilimumab | NCT00089076 | 1/2 | 18 | DLBCL, FL, MCL | 100% Diarrhoea (56%) Fatigue (56%) TP (28%) |
44.4% Diarrhoea (28%) Fatigue (6%) Neutropenia (6%) |
[143] | |
| CTLA-4 CD20 |
Ipilimumab + Rituximab |
NCT01729806 | 1 | 33 | DLBCL, FL, MCL | Fatigue (33%) Anaemia (30%) Diarrhoea (15%) |
Lymphopenia (18%) Diarrhea (12%) Anaemia (12%) |
[144] | |
| CTLA-4 | Ipilimumab | NCT01822509 | 1 | 28 | B-NHL | TP (27%) Chronic GVHD of liver (10%) Anaemia (7%) |
18% DAEs | [145] | |
| CTLA-4 | Ipilimumab + Lenalidomide |
NCT01919619 | 2 | 11 | DLBCL, FL, MCL, others | Neutropenia (44%) GVHD (9%) |
[146] | ||
| CD47 CD20 |
Hu5F9-G4 + Rituximab |
NCT02953509 | 1b/2 | 115 | DLBCL, iNHL | Infusion reaction (38%) Headache (34%) Fatigue (30%) |
Anaemia (15%) | 7% DAEs | [147] |
| CD47 CD20 |
TTI-621 + Rituximab |
NCT02663518 | 1 | 32 | DLBCL | Infusion reaction Transient TP |
[148] | ||
| CD47 CD38 |
TTI-622 + Daratumumab |
NCT03530683 | 1 | 19 | DLBCL, MCL, FL | Abdominal pain (10.5%) Fatigue (10.5%) Nausea (10.5%) |
Neutropenia (10.5%) No G ≥ 3 anaemia or TP |
[149] | |
| CD47 CD20 |
ALX148 + Rituximab |
NCT03013218 | 1 | 33 | DLCBL, MCL, FL, MZL | 79% Rash (18%) Fatigue (9%) |
Neutropenia (6%) | [150,151] | |
| CD40 | Dacetuzumab | NCT00103779 | 1 | 50 | DLBCL, MCL, FL, MZL | 98% Fatigue (28%) Headache (20%) Pyrexia (18%) |
30% | 26% SAEs No TRD |
[152] |
| CD40 | Dacetuzumab | NCT00435916 | 2 | 46 | FL, DLBCL, MZL | 98% Fatigue (41%) Headache (35%) Chills (33%) |
46% | 39% SAEs | [153] |
| CD40 | Lucatumumab | NCT00670592 | 1/2 | 111 (74) | FL, MZL, MCL, DLBCL | 100% Chills (39%) Pyrexia (34%) Fatigue (25%) |
65% Lipase elevation (25%) |
28% SAEs | [154] |
| CD40 CD20 |
Dacetuzumab + Rituximab + chemotherapy |
NCT00655837 | 1 | 30 | DLBCL | 100% CRS (61%) Nausea (36%) TP (36%) |
21% TP (6%) AST/ALT elevation (3%) |
45% SAEs 1 TRD |
[155] |
| CD40 CD20 |
Dacetuzumab Rituximab + chemotherapy |
NCT00529503 | 2 | 154 (101) | DLBCL, FL | 80% | 44% SAEs 8% DAEs |
[156] | |
| CD27 | Varlilumab | NCT01460134 | 1 | 90 (18) | MCL, MZL, DLBCL, CLL, cHL, TCL | 59% Fatigue (24%) Anaemia (12%) |
3% ALP elevation | [157] | |
| CD80 CD20 |
Galiximab + Rituximab |
NCT00363636 | 3 | 337 | FL | Pyrexia (18%) Anaemia (12%) |
No TRD | [158] | |
| CD80 | Galiximab | NCT00575068 | 1/2 | 38 | FL | 60% Fatigue (32%) Nausea (14%) Headache (11%) |
3% Axillary pain (3%) Venous thrombosis (3%) |
No SAEs No DAEs |
[159] |
| CD80 CD20 |
Galiximab + Rituximab |
NCT00048555 | 1/2 | 73 | FL | 96% Lymphopenia (48%) Leukopenia (36%) Fatigue (36%) |
26% Lymphopenia (14%) Leukopenia (3%) Anaemia (3%) |
13% SAEs 1 possible TRD |
[159] |
| CD80 CD20 |
Galiximab + Rituximab |
NCT00117975 | 2 | 61 | FL | 13% Lymphopenia Leukopenia Neutropenia |
13% of events Lymphopenia |
[160] | |
| 4-1BB | Urelumab | NCT01471210 | 1 | 60 | DLBCL, FL, B-NHL | 52% Fatigue (15%) Neutropenia (12%) |
15% | 3.3% DAEs | [161] |
| 4-1BB CD20 |
Urelumab + Rituximab |
NCT01775631 | 1 | 46 | DLBCL, FL |
72% Fatigue (20%) AST/ALT elevation (15/13%) |
28% | 2% TRD (CRS) | [161] |
| 4-1BB PD-1 |
Urelumab + Nivolumab | NCT02253992 | 1/2 | 22 | DLBCL | 63% Fatigue (26%) ALT/AST elevation (13/9%) |
ALT/AST elevation (3/3%) | 7% DAEs | [172] |
| 4-1BB CD20 |
Utomilumab + Rituximab |
NCT01307267 | 1 | 67 | FL, MCL, DLBCL | 95.5% Fatigue (16%) |
3% Neutropenia Diarrhoea ALT elevation |
4.5% DAEs No TRD |
[162] |
| CD70 | SGN-CD70A |
NCT02216890 Terminated |
1 | 38 | DLBCL, FL, MCL | 100% TP 75% Anaemia 50% |
90% TP 65% |
55% SAEs | [163] |
Abbreviations: FL, follicular lymphoma; tFL, transformed follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; iNHL, indolent B-cell non-Hodgkin lymphoma; aNHL, aggressive B-cell non-Hodgkin lymphoma; LBCL, large B-cell lymphoma; HG-BCL, high-grade B-cell lymphoma; cHL, classical Hodgkin lymphoma; CLL-RT, chronic lymphocytic leukaemia Richter transformation; PMBL, primary mediastinal B-cell lymphoma; PTCL, peripheral T-cell lymphoma; CTCL, cutaneous T-cell lymphoma; MF, myelofibrosis; SS, Sezary syndrome; MM, multiple myeloma; AE, adverse event; SAE, serious adverse event; TP, thrombocytopenia; AST, alanine aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CRS, cytokine release syndrome; GVHD, graft-versus-host disease; TRD, treatment-related death; DAE, discontinuation due to adverse event; NA, not available.
Considering the recurrent alteration of the PD-L1 gene in PCNSL and PTL and the poor prognosis of these rare subtypes of DLBCL [80], nivolumab was evaluated in patients with R/R PCNSL or R/R PTL, in whom it demonstrated impressive activity (NCT02857426), with clinical and radiographic response and PFS extended to 13+ to 17+ months for some patients [105]. The phase-2 CheckMate 436 clinical trial further demonstrated that nivolumab combined with brentuximab vedotin represents a promising therapy in PBML patients post-ASCT or after ≥2 prior chemotherapy regimens [106]. Similarly, pembrolizumab therapy has also yielded excellent results in PMBL patients. In the phase-1b multicohort KEYNOTE-013 study, the ORR was 41%, while the median duration of response (DOR) and OS were not reached in this subset of patients [107]. The subsequent international phase-2 KEYNOTE-170 study (NCT02576990), with 53 R/R PBML patients enrolled, reported an ORR of 45% (including 13% CR) [108]. As expected, the magnitude of chromosome 9p24.1 abnormality was associated with PD-L1 expression in responding patients [108]. Importantly, in both KEYNOTE studies, no patient who previously achieved CR relapsed during the follow-up. Altogether, these results led to the accelerated FDA approval of pembrolizumab in 2018 for the treatment of R/R PMBL [99].
Similarly, the phase-1/2 studies employing nivolumab and pembrolizumab have reported high ORRs in patients with R/R cHL; the patients that reached CR were characterised by higher PFS [67,68,109,110,111,112] (Table 1). In subsequent phase-2 studies, pembrolizumab led to 100% OS and 82% PFS at 18 months in post-ASCT consolidation settings, suggesting that pembrolizumab could be used in high-risk patients after ASCT to remodel the immune landscape [113]. Following these trials, pembrolizumab is currently being used in frontline and salvage regimens in R/R cHL patients [114].
Two ongoing and three completed clinical trials evaluated the safety and efficacy of the humanised IgG1 monoclonal anti-PD-L1 antibody atezolizumab (MPDL-3280A), in combination with the anti-CD20 antibody obinutuzumab, for the treatment of aggressive B-cell lymphoma. A phase-1/2 trial (NCT02596971) evaluated the safety and efficacy of atezolizumab, in combination with either obinutuzumab + the alkylating agent bendamustine or obinutuzumab + chemotherapy (CHOP) in FL patients, and atezolizumab + rituximab + chemotherapy in DLBCL patients. The analysis from 40 patients demonstrated high efficacy (ORR of 95%) and durable responses (24 months for 80% of patients) for the combinational approach. Another phase-1/2 study (NCT02631577) that enrolled 38 patients with R/R FL demonstrated durable clinical responses and the remarkable ORR of atezolizumab, in combination with obinutuzumab, plus the immunomodulatory drug lenalidomide. Nevertheless, a phase-1 trial (NCT02220842), including 14 patients with R/R FL and 17 patients with R/R DLBCL, showed the weak efficacy of atezolizumab in combination with obinutuzumab or the EZH2 inhibitor tazemetostat. Subsequently, a multicentre phase-2 trial (NCT03276468) assessed the antilymphoma activity of atorolimumab associated with Veneto lax (a BCL-2 inhibitor) and blinatumomab in three cohorts: R/R FL patients, R/R DLBCL patients and iNHLs, including MZL and MALT cases. The data from the 58 DLBCL patients enrolled at the time of the primary analysis demonstrated that the efficacy of combinatory therapy is comparable with currently available options for this population, with durable responses. The phase-1/2 clinical trial NCT02729896 evaluated the combination of atezolizumab with obinutuzumab and polatuzumab, an anti-CD79b, in 13 participants with R/R FL and atezolizumab with the anti-CD20 antibody rituximab and polatuzumab in 21 participants with R/R DLBCL. The percentage of participants with an objective response (CR + PR) was 33.33–57.14% (depending on the polatuzumab dose) for FL patients and 25% for DLBCL patients. The results of a large phase-1 clinical trial (NCT02500407) that enrolled 72 iNHL patients (including 69 FL) and 141 cases with aggressive B-NHL (87 DLBCL and 29 tFL) to evaluate the combination of atezolizumab with mosunetuzumab, a bispecific CD20-CD3 monoclonal antibody, demonstrated high response rates and durable complete remissions, as well as the maximum tolerated dose. The ORR and CR of the iNHL patients across all dose levels were 64% and 42%, respectively. The ORR and CR of aggressive NHL patients across all dose levels were 34.7% and 18.6%, respectively (Table 1).
According to the outcome of the NP39488 study (NCT03533283), the combination of atezolizumab with glofitamab, another bispecific antibody designed to target CD20 on the surface of B-cells and CD3 on the surface of T-cells, resulted in low ORR in 38 aggressive B-NHL patients or iNHL.
The use of CAR-modified T-cells targeting specific tumour cell antigens to enhance immune responses against tumour cells is certainly a great breakthrough in oncoimmunotherapy research. In NHLs, targeting CD19-malignant B-cells has proven highly efficacious in the refractory-disease setting, resulting in T-cell activation, proliferation and secretion of inflammatory cytokines and chemokines, with consequent tumour cell lysis [115,116]. KTE-C19 (Axi-cel) is an autologous anti-CD19 CAR T-cell that was approved by the FDA in October 2017 for the treatment of R/R aggressive B-cell lymphomas after two or more lines of systemic therapy. As PD-1/PD-L1 blockade has been shown to be upregulated after CAR T-cell infusion, the ZUMA-6 clinical trial (NCT02926833) evaluated outcomes of KTE-C19 combined with the anti-PD-L1 atezolizumab. The data suggested that PD-L1 blockade with atezolizumab after KTE-C19 has a manageable safety profile and a promising efficacy outcome (Table 1).
Durvalumab is a selective, high-affinity, humanised IgG1-kappa monoclonal antibody against PD-L1 [117]. In vitro and in vivo xenograft assays have demonstrated that durvalumab evokes a 75% tumour growth reduction in the presence of tumour-reactive human T-cells, supporting the immunological mechanism of action of this drug [118]. Currently, ten clinical trials are underway to investigate the use of durvalumab as monotherapy or in combination with other reagents or CAR T-cells to treat B-NHL patients. Data from murine lymphoma models suggest that the BTK inhibitor ibrutinib, combined with an anti–PD-L1 therapy, may have synergistic antitumor activity [119]. A phase-1b/2 study (NCT02401048) evaluating the efficacy and safety of the combination of ibrutinib and durvalumab in patients with R/R DLBCL or FL has highlighted longer PFS and OS in patients with FL compared to those with DLBCL. However, the efficacy of ibrutinib + durvalumab treatment demonstrated similar activity to single-agent ibrutinib [120] (Table 1). FUSION NHL 001 (NCT02733042) is a phase-1/2, open-label, multicentre study to assess the safety and tolerability of durvalumab as monotherapy or in combination with different regimens (lenalidomide ± rituximab; ibrutinib; rituximab ± bendamustine (an alkylating agent)) in subjects with B-NHL or CLL. From the 106 enrolled participants, 23 were FL, 37 were DLBCL, 17 were MCL, 5 were MZL, 1 was t-FL, 5 were cHL and 18 were CLL/SLL. The efficacy of the durvalumab and rituximab or durvalumab and lenalidomide + rituximab combination was evaluated initially in 3 B-NHL patients. The ORR of durvalumab and rituximab therapy was 33.3% and reached 66.7–80% with the addition of lenalidomide. A remarkable ORR was seen in ten MCL patients after durvalumab and ibrutinib combination therapy. The combination treatment of durvalumab, rituximab and bendamustine led to an ORR of 88.9% in FL patients and 30% in DLBCL patients. On the other hand, none of FL (n = 5), MCL (n = 5) or DLBCL (n = 10) patients responded to durvalumab as a monotherapy. Although these early findings are encouraging, serious AEs were commonly seen in patients treated with durvalumab when administrated either alone or in combination therapy (Table 2). Another phase-2, two-arm, open-label clinical trial (NCT03003520) is ongoing to evaluate the safety, activity, and predictive biomarkers of durvalumab in combination with chemoimmunotherapy (R-CHOP) or lenalidomide plus R-CHOP, followed by Durvalumab consolidation therapy, in previously untreated subjects with DLBCL. The ORR from the evaluable patients of the durvalumab–R-CHOP arm showed that 54.10% of the patients achieved CR but 51% of the cases presented serious AEs. Finally, NCT03310619 (PLATFORM) and NCT02706405 are two studies aimed at determining the safety, tolerability, and efficacy of CAR T-cells (JCAR017 and JCAR014, respectively) in combination with durvalumab in subjects with R/R B-cell malignancies. Among the first 11 evaluable patients, investigators reported an ORR of 91%, including 64% CR. The NCT02706405 study enrolled 15 patients, in which 12 were DLBCL, 2 were high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, and 1 was PMBL. The ORR from the 12 evaluable patients was 50%, with 42% CR and 8% PR. Only one patient who achieved CR has relapsed.
2.2. CTLA-4 Signalling and Inhibition
Cytotoxic T-lymphocyte antigen 4 (CTLA-4 or CD152) is expressed by both CD4+ and CD8+ T-cells and mediates T-cell activation together with CD28 as both receptors are homologous and share a pair of ligands, CD80 and CD86, found on the surface of APCs [164]. The interaction between CTLA-4 and both ligands is of higher affinity and avidity than CD28 and plays opposite roles. While CD28 mediates T-cell costimulation in conjunction with TCR signals, CTLA-4 and its interaction with its ligands drive the inhibition of T-cell responses, although the precise mechanisms are not fully understood [165]. Two CTLA-4 blocking antibodies have been developed: tremelimumab, the first full human CTLA-4 antibody [166], and ipilimumab, an anti-CTLA-4 IgG2 monoclonal antibody and IL-2 stimulant [167]. Both agents are able to recognise human CTLA-4 and to block its interaction with CD80 or CD86 [168], potentiating an antitumor T-cell response [169]. Though ipilimumab binds to the same epitope, with a similar affinity as tremelimumab, the higher dissociation rate of ipilimumab may indicate a dynamic binding to CTLA-4, which may provide it with an improved pharmacokinetic profile [170].
While only one ongoing trial is evaluating the efficacy of tremelimumab as a single agent and in combination with durvalumab and the JAK/STAT inhibitor AZD9150 in R/R DLBCL patients, several trials have evaluated the efficacy of ipilimumab in lymphoma patients in combination with other existing therapies (mostly rituximab and nivolumab). The first phase-1/2 trial evaluating ipilimumab in relapsed settings was launched in 2004 with 18 lymphoma patients (NCT00089076). Although only two patients could be evaluated, both had clinical responses: 1 DLBCL patient had a CR of 31+ months and 1 FL patient had a PR for up to 19 months [143]. Due to the study design of phase 1, the trial was finally terminated. Subsequently, a phase-1 clinical trial was launched in 2012 to assess the effect of ipilimumab in combination with rituximab in the same cases as the previous trial (NCT01729806). The enrolment was formed by patients with FL (n = 13), DLBCL (n = 7), MCL (n = 2), SLL (n = 2) and 9 patients with an undetermined diagnosis. At 7 weeks, toxicity was evaluated and considered as manageable (Table 2). The combination of rituximab and ipilimumab resulted in more effective B-cell depletion, together with an increase in IL-2 and TNF-α levels; both phenomenons were associated with treatment response [144]. Ipilimumab was also evaluated in patients with relapsed hematologic malignancies after an allogeneic stem cell transplant (Allo-SCT) in combination with lenalidomide (NCT01919619) or nivolumab (NCT01822509). In this last combination, only a modest antitumour activity was observed, mainly in lymphoid patients. However, substantial toxicities were also observed due to graft-vs.-host disease (GVHD) [171]. In a second trial evaluating the combination of lenalidomide and ipilimumab in 13 post-Allo-SCT patients with DLBCL, FL or MCL, a 46% CR and only one GVHD were reported, although one patient died after developing a T-cell lymph proliferative disorder after treatment [146]. The combination of ipilimumab and nivolumab is currently under study in a phase-1/2 trial in patients at high risk of recurrence after Allo-SCT (NCT02681302). Out of 31 patients, 14 have DLBCL, both primary refractory (n = 7) and relapsed (n = 7). As of 2018, 65% of patients had developed immune-related AEs of grade 2 or higher, which required treatment with systemic steroids, but no GVHD (Table 2).
2.3. CD47 Signalling and Inhibition
2.3.1. Overview of the Pathway
CD47 (cluster of differentiation 47) is an integrin-associated molecule belonging to the Ig superfamily [173] that interacts with SIRPα (signal regulatory protein-alpha), spreading the “don’t eat me” signal to macrophages, a strategy employed as an immune-mediated clearance evasion mechanism in several types of cancers [174] (Figure 1). Mechanistically, the CD47-SIRPα binding leads to tyrosine phosphorylation of SIRPα immunotyrosine inhibitory motifs and activates SHP (tyrosine phosphatases Src homology 2 (SH2)-containing protein tyrosine phosphatase)-1 and -2. The interaction of the phosphatase SH2 domains to phosphorylated SIRPα disrupts their autoinhibitory activity, triggering enzymatic activity and, ultimately, leading to the blockade of macrophage phagocytic function [175,176].
Although CD47 is widely expressed on the surface of a broad range of cell types, high levels of CD47 have also been observed in haematological cancers such as acute myeloid leukaemia (AML), ALL, CLL, multiple myeloma (MM), myelodysplastic syndrome (MDS), DLBCL, MCL, and MZL [174,177,178,179,180,181,182]. High levels of CD47 are considered to be an adverse prognostic indicator of survival [174].
2.3.2. CD47-SIRPα Axis Inhibition
One of the first attempts to target CD47 was carried out therapeutically in AML primary human xenograft models [174]. The CD47 antibody B6H12 induced phagocytosis and eliminated AML stem cells. Subsequently, it was demonstrated that this antibody synergised with anti-CD20 (which can also bind Fc-receptors), promoting a more potent prophagocytic signal in B-NHL xenograft models [179,180,183]. Based on these results, the humanised anti-CD47 antibody Hu5F9-G4 was developed [184]. Preclinical studies showed that this antibody could bind specifically to CD47, blocking CD47–SIRPα interaction and enabling macrophage-mediated phagocytosis in primary AML cells. This antibody was further shown to potently synergise with rituximab in B-NHL xenografts, supporting its evaluation in a phase-1 clinical trial (NCT02216409) that revealed its safety, pharmacokinetics, and pharmacodynamics [185]. Subsequently, a larger phase-1b/2 study was launched to evaluate the combination of Hu5F9-G4 with rituximab in 115 B-NHL patients (70 DLBCL and 45 iNHL (41 FL and 4 MZL); NCT02953509). In this trial, a Hu5F9-G4 and rituximab combination was well tolerated, with rapid and durable responses [147,186] (Table 1).
Recently, the fully human anti-CD47 IgG4 antibody TJC4 (TJ011133) was shown to specifically block the CD47-SIRPα axis, enhancing phagocytosis in a set of tumour cell lines and AML primary cells. In BL and DLBCL xenograft models, TJC4 inhibited tumour growth and extended mice OS as monotherapy. When combined with rituximab, the antibody showed superior efficacy in a DLBCL model over the single agent. In addition, single-dose or repeat-dose treatment of TJC4 minimally affected red blood cells in cynomolgus monkeys, with no impact on platelets [187].
TTI-621 is a fully human recombinant fusion protein based on the structure of SIRPα linked to the Fc region of human IgG1; it was conceived and designed as a decoy receptor. First, in vitro data showed that TTI-621 could bind CD47 and induce a potent macrophage-mediated antibody-dependent cell phagocytosis (ADCP) and apoptosis in an extensive range of hematologic and solid tumour cells [188,189,190]. In vivo data indicated that the fusion protein was able to block CD47 and impair the tumour growth in several haematological xenograft models, including AML, BL and DLBCL. Preclinical data also suggested that TTI-621 was less likely to evoke anaemia when compared to other anti-CD47, thanks to its low erythrocyte–binding profile [188]. TTI-621 was also suggested to enhance the adaptive immune response [188,191]. A phase-1, open-label, multicentre study is currently ongoing to evaluate the activity of TTI-621 as a single agent or in combination with rituximab in R/R cohorts of haematologic malignancies (NCT02663518; Table 1) [148]. Another set of preclinical data show TTI-622, a new human SIRPα linked to human IgG4, induces ADCP in a panel of haematological and solid tumour cells, with a superior affinity for tumour cells than for platelets. In vivo DLBCL xenograft models indicated that TTI-622 treatment leads to a decrease in tumour growth and improves survival [192]. Based on these data, a phase-1 dose-escalation study was initiated (NCT03530683). As of April 2020, 19 R/R lymphoma patients have been enrolled (n = 10 DLBCL, n = 5 HL, n = 1 FL, n = 1 MCL, n = 2 peripheral T-cell lymphoma (PTCL)) and objective response has been reported in 2 DLBCL patients (1 PR and 1 CR) [149].
A newly engineered high-affinity SIRPα-Fc fusion protein, ALX148, was able to trigger both innate and adaptive antitumor immune responses, characterised by an enhancement on phagocytosis. In an MCL xenograft mice model, although ALX148 was able to inhibit tumour growth, superior activity was observed by combining this agent with obinutuzumab. Similarly, in a BL xenograft mice model, the combination of ALX148 with rituximab enhanced tumour growth inhibition (TGI) and improved mice survival when compared to the control group [193]. Currently, ALX148 is being investigated in a phase-1 dose-escalation/expansion in patients with R/R B-NHL patients (NCT03013218). Preliminary data showed that ALX148 is well tolerated, with ORR ranging from 41% (9% CR) to 62.5% (11% CR) [150,151].
Assuming that CD47 is upregulated in both tumour cells and erythrocytes and platelets, it is understandable that targeting CD47 leads to side effects, including anaemia. To get through such unwanted effects, a fully human bispecific antibody, TG-1801 (NI-1701), comprising a high-affinity CD19-targeting arm combined with CD47-blocking arms, with a range of affinities on a human IgG1 Fc backbone, was developed [194]. In vitro TG-1801 specifically and strongly binds to human B-cells, avoiding hemagglutination. The specific blockade of the CD47-SIRPα axis on CD19-expressing cells mediates effective killing of primary and immortalised B-NHL cells via ADCP and antibody-dependent cell cytotoxicity (ADCC) [195,196]. Moreover, the bispecific antibody prevented the recruitment of CD19 to the BCR signalling complex, and the coligation of CD19 and CD47 by TG-1801 limited CD19 mobility at the B-cell surface by the cytoskeleton-anchored glycoprotein CD47, inhibiting B-cell proliferation and BCR-mediated gene expression [197]. While TG-1801 has demonstrated to be superior to rituximab in killing B-cells from primary leukaemia and lymphoma cells [196], its combination with the novel glycoengineered anti-CD20 mAb ublituximab or U2-regimen-associating ublituximab, with the dual PI3Kσ/CK1ε inhibitor umbralisib, allowed a synergistic effect in both ADCC and ADCP [198,199]. In vivo xenograft BL and B-ALL models showed that TG-1801 reduced tumour growth and also increased survival time [196]. Complementarily in DLBCL patient-derived xenografts (PDX), the antibody reduced tumour burden, with significantly higher efficacy than ibrutinib [200]. Lastly, the TG-1801–U2 combination has shown synergistic activity in-vivo in a BL xenograft model, associated with infiltration of effector cells (NK and macrophages) [198,199]. Based on the preclinical data, TG-1801 is currently in a phase-1 trial (NCT03804996) for histologically confirmed B-cell lymphoma, relapsed or refractory to prior standard therapy.
2.4. CD40 Signalling and Inhibition
CD40, a member of the TNF receptor family expressed by APCs (DCs, macrophages, NK cells, and mature B-cells), interacts with its ligand CD40L (CD154), which is expressed by activated T-cells, stimulating cytokine secretion by B-cells and allowing T-cell activation [201,202]. CD40 activation promotes the conversion of DCs to APCs, the phagocytic ability of macrophages, and proliferation and antigen presentation on B-cells [203]. CD40 is expressed in a wide range of B-NHL, CLL and MM [204].
CDX-1140 is a novel agonist antibody against CD40, binding outside of the CD40L ligation site. Preclinical data showed enhanced DC and B-cell activation by CDX-1140, which synergises with recombinant CD40L to enhance agonist activity [205]. While xenograft models using CD40+ lymphoma cell lines have shown antitumour activity by CDX-1140, with attenuated tumour growth and increased survival, safety studies in cynomolgus macaques support the use of the antibody in humans [203,205,206]. A phase-1 trial (NCT03329950) is currently recruiting and will evaluate the safety and efficacy of CDX-1140 alone or in combination with the soluble recombinant Flt3 ligand CDX-301, pembrozilumab or chemotherapy (gemcitabine and nab-paclitaxel) [207].
Selicrelumab is an agonist antibody that activates both memory and naïve B-cells and triggers T-cell activation [208]. Preclinical studies, both in vivo and in vitro, resulted in antitumour activity via an immune activation; a synergy was observed in vivo when combined with chemotherapy agents or in a triple-combination with PD-L1 inhibition and the FAP-IL2v immunocytokine [209]. A phase-1 clinical trial is ongoing (NCT03892525), with an estimated enrolment of 44 patients, to assess selicrelumab’s safety profile in combination with atezolizumab in patients with R/R lymphoma.
Ad-ISF35 is a replication-defective adenovirus vector that encodes for the chimeric protein CD154. Its induction results in an antitumour response associated with macrophage infiltration and an increased proinflammatory cytokine release that will lead to a break in tumour immune tolerance and tumour regression [210,211]. Both in vitro and in vivo assays have shown safer administration and significant antitumoral activity as a single agent. In parallel, combinations of this agent with anti-PD1 or a triple-combination with an anti-PD1 and an anti-CTLA-4 have shown synergistic effects in melanoma [212].
Dacetuzumab, also known as SGN-40, provides inhibitory proliferation and apoptosis signals in high-grade B-NHL. Its signalling contributed to cell death by the degradation of BCL-6 and an increased expression of proapoptotic proteins [213,214,215]. A phase-1 clinical trial (NCT00103779) [152] was completed with 50 patients of refractory or recurrent B-cell lymphomas, and a phase-2 clinical trial (NCT00435916) [153] was completed with 46 relapsed DLBCL patients; however, due to its modest effect as a single agent, clinical trials were continued as a combination with other immune checkpoint inhibitors. A phase-1 clinical trial (NCT00655837) was completed, with 30 patients receiving dacetuzumab in combination with rituximab and chemotherapy (gemcitabine) [155]. A phase-2 clinical trial (NCT00529503) was completed with 154 DLBCL and FL patients with improved OR when combined with rituximab and chemotherapy (etoposide, carboplatin, and ifosfamide) [156].
SEA-CD40 is an agonist antibody with improved properties in vitro and in vivo when compared to dacetuzumab, as it induces more robust cytokine production and results in the activation of CD4+ and CD8+ T-cells [216,217]. A phase-1 clinical trial (NCT02376699) with an estimated enrolment of 135 patients is currently open to assess SEA-CD40’s safety profile as a single agent [218].
Lucatumumab, also known as CHIR-12.12, is an antagonist antibody that blocks CD40/CD40L interaction, thereby blocking a survival signal in B-cell lymphomas [219]. In xenograft models, the antibody reduced tumour growth and increased CD40 expression on tumour tissue [220,221]. Lucatumumab was tested in a phase-1/2 clinical trial (NCT00670592) with 74 NHL patients; nevertheless, it was discontinued in 2013 due to minimal clinical activity [154].
2.5. CD27 Signalling and Inhibition
CD27 is a transmembrane homodimeric phosphoglycoprotein and a member of the TNF superfamily; its ligand is CD70. It is constitutively expressed by most CD4+ and CD8+ T-cells, memory B-cells, and a portion of NK cells [222,223]. The CD27-CD70 activation on T-cells causes the activation, proliferation, survival, and maturation of the effector and memory capacity of those cells as in-vivo stimulation of CD27 with its ligand promotes strong cytotoxic T-cells responses. Naïve T-cells express CD27, and TCR signalling further upregulates its expression, suggesting a role during T-cell priming. Its stimulation on the B-cell subpopulation activates and promotes the generation of plasma cells, its proliferation, and the production of immunoglobulin [222,223,224]. Finally, it is also expressed in NK cells, where its activation induces cytolytic activity. Its expression is also detected in T-cell populations of different cancer subtypes, including B-cell malignancies, suggesting potential therapeutic targeting of CD27 immunomodulation [225].
Varlilumab (CDX-1127), is a monoclonal antibody that acts as an agonist of CD27-CD70 interaction. This anti-CD27 mAb provided costimulatory signals to human T-cells in a TCR-dependent manner and enhanced the number and activity of TILs [226,227]. Both in vitro assays and in vivo models have shown direct antitumor activity against CD27-positive lymphomas [228]. In-vivo assays, in combination with other immune-checkpoint-blocking antibodies such as anti-PD-L1 or anti-CD20 Abs, have demonstrated a synergistic antitumour activity [228,229]. A phase-1 clinical trial (NCT01460134) was completed with 25 DLBCL and FL patients to assess the safety and pharmacokinetic profiles of varlilumab [157]. Doses up to 10 mg/kg weekly were well tolerated, and the results obtained in this clinical trial support the hypothesis that combination therapy can enhance and improve the overall outcome. Nowadays, two clinical trials are active: a phase-1/2 (NCT03307746) study and a phase-2 (NCT03038672) study, with an estimated enrolment of 40 and 106 patients, aimed at evaluating varlilumab–rituximab and varlilumab–nivolumab combinations in R/R B-cell lymphoma patients, respectively [230,231].
2.6. CD80 Signalling and Inhibition
Cluster of differentiation 80 (CD80, B7-1) is a type I membrane protein member of the Ig superfamily that is expressed by various immune cells, from monocytes to APCs [232]. It binds to CD28 on the T-cell surface to activate the autoregulation of several functions, including CTLA-4 signalling (Figure 1). The interaction between this protein and the CD28 antigen is a costimulatory signal for the activation and proliferation of T-cells, inducing cytokine production [233]. Due to its intricate role in immune regulation, targeting CD80 for diverse B-cell lymphomas and autoimmune diseases has been attractive to both researchers and clinicians [234].
To date, only one antibody targeting CD80 has been developed; it is being evaluated in several clinical trials in B-NHL patients, specifically in FL. Galiximab (IDEC-114) is an IgG1 lambda mAb, with a high affinity to CD80. Galiximab effectively blocks CD80–CD28 interactions on T-lymphocytes but has no significant effect on CD80–CTLA-4 interactions [235]. This interaction usually leads to downregulation of T-cell activity, and it should, therefore, remain intact during galiximab therapy. Galiximab acts primarily via cross-linking of CD80 molecules and induction of ADCC, but it also inhibits cellular proliferation and upregulates apoptotic proteins [159]. In 2002, the first phase-1/2 clinical trial was launched, with the enrolment of 38 R/R FL patients (NCT00575068) [159]. In the same year, the combination of galiximab with rituximab was also evaluated in 73 patients with progressive FL that had failed at least one prior standard therapy, excluding rituximab (NCT00048555). This combination has also been evaluated as a first therapy for stages 3 and 4 or bulky FL in a 2005 clinical trial with 61 patients enrolled (NCT00117975) [160]. In 2006, a randomised phase 3 trial was initiated to evaluate if the galiximab–rituximab combination extended PFS compared to rituximab + placebo in 337 patients with grade 1–3a FL that had progressed or relapsed after at least one prior treatment (NCT00363636). One hundred seventy-five patients were given the combination and the remaining 162 were given rituximab + placebo, with 3% more incidence of side effects in the combination group [158].
2.7. 4-1BB Signalling and Inhibition
4-1BB (CD137, TNFRSF9) is another surface glycoprotein member of the TNF receptor superfamily that is expressed in a variety of immune cells, including T-lymphocytes and NK cells. A-1BB ligation by its natural ligand 4-1BBL (CD137L), expressed by DCs, macrophages and B-cells, among others, induces the activation of NF-kB and MAPK pathways [236], increasing survival, proliferation and effector function [236,237]. 4-1BB is considered a promising target for immunotherapy in B-NHL patients since microarray analyses have shown the overexpression of 4-1BB in DLBCL and FL biopsies [238]. Accordingly, treatment with agonistic anti-4-1BB antibodies in a mouse model of B-cell lymphoma eliminated the tumour in 60% of the animals, which became immune to a rechallenge after 100 days [238]. Stimulation of NK cell proliferation and function [239] and inhibition of Treg cell suppressive activity [240] could be contributing to the antitumoral effect of anti-4-1BB therapy as well; however, the role of 4-1BB signalling in these cell types is still controversial [241,242].
Urelumab (BMS-662513), the first anti-4-1BB agent to enter clinical trials, is an agonistic antibody that has shown costimulatory activities both in vitro and in primates [243,244]. In a phase-1 clinical trial (NCT01471210) with R/R B-NHL patients dosed with urelumab as a single agent, ORRs were modest in both DLBCL and FL patients (Table 1). Furthermore, half of the responses occurred in patients treated with urelumab 0.3 mg/kg, above the later-determined maximum tolerated dose (MTD) of 0.1 mg/kg, and toxicity was prominent (Table 2) [161].
The combination of urelumab plus rituximab was evaluated in a phase-1 clinical trial (NCT01775631) in relapsed B-NHL patients. The toxicity profile was similar to that of monotherapy, and ORRs were similar or lower than those previously reported for rituximab monotherapy (37% in DLBCL and 36–48% in FL), indicating no synergistic effect of the two drugs [161]. On the other hand, the combination of urelumab with nivolumab was well tolerated in a phase-1/2 clinical trial (NCT02253992) for refractory DLBCL patients. Again, no significant clinical benefit was found, as none of the patients achieved a response [172] despite the promising additive effect observed in animal models of solid cancers [245]. After these overall discouraging results in the clinical setting, there are currently no trials evaluating urelumab in B-NHL patients.
Utomilumab (PF-05082566) is an anti-4-1BB antibody with promising costimulatory activity in vitro and in vivo and antitumor efficacy in several solid cancer models [237,244,246]. Utomilumab monotherapy displayed manageable toxicity in a phase-1 clinical trial (NCT01307267) with 55 patients, including 2 relapsed B-NHL; however, these were not included in the efficacy analyses [247]. In the same trial, utomilumab in combination with rituximab achieved an ORR of 21% (n = 67 B-NHL) and presented an improved safety profile [162] that is likely due to the ability of utomilumab to block ligand binding, in contrast with urelumab [244]. Several clinical trials (NCT02951156, NCT03440567, NCT03704298) are currently evaluating this antibody in combination with other immunotherapeutic agents like avelumab, ibrutinib, CD19-CAR T-cells, or chemotherapeutic agents, but no results are available at the moment.
Two novel anti-4-1BB antibodies are being evaluated in clinical trials that include refractory B-NHL patients. The ligand-blocking agonistic antibody ADG106 has shown promising results in animal models of several cancers [248,249] and is being tested as a monotherapy in two clinical trials (NCT03707093; NCT03802955). The 4-1BB x PD-L1 bispecific antibody MCLA-145 has been developed with the specific aim of activating 4-1BB signalling in the tumour, where PD-L1 is expressed, as well as blocking immune-inhibitory signalling from the PD-1/PD-L1 axis. Antitumor efficacy has been reported in mouse models of several solid cancers [250,251], and, consequently, a phase-1 clinical trial (NCT03922204) is testing MCLA-145 as a single agent.
2.8. CD70 Signalling and Inhibition
CD70 is another transmembrane glycoprotein of the TNF superfamily that acts as a ligand for CD27. CD70 is transiently found on T-cells, B-cells, DCs, and also NK cells [222,223,252]. CD70 is controlled and induced by antigen receptor stimulation and its expression is under cytokine regulation; its expression is enhanced due to proinflammatory cytokines, such as IL-1a or IL12, or decreased due to anti-inflammatory cytokines like IL-4 or IL-10 [253]. The protein is also expressed in highly activated lymphocytes, and its expression was confirmed across different subtypes of T- and B-cell lymphomas but found absent in their normal counterparts [254,255].
SGN-CD70A is a potent antibody–drug conjugate (ADC) that consists of three functional subunits composed of an anti-CD70 antibody, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine (PBD) dimer drug. Upon binding with its target, CD70, the complex is internalised and traffics to the lysosomes, where the drug is released and will initiate cellular events when it crosslinks DNA. The drug works by activating the DNA damage pathways, in both in-vitro and in-vivo studies, causing a G2 cell cycle arrest and high levels of DNA damage in treated cells [256]. Preclinical in-vitro assays have demonstrated that the formation of double-strand breaks (DSB) is an early event that will be followed by an inhibition of proliferation and induction of apoptosis in NHL cell lines [254,257]. SGN-70A inhibited cell growth and induced higher caspase activity in CD70-positive cell lines of cutaneous T-cell lymphoma (CTCL) and patient-derived T-cell lymphoma primary cells. A phase-1 clinical trial (NCT02216890) with 38 patients of R/R MCL and DLBCL was terminated to assess the safety profile of SGN-CD70A [163]. The treatment showed antitumor activity, but no further clinical trials were conducted due to the frequency and severity of the AEs (Table 2).
2.9. LAG-3 Signalling and Inhibition
LAG-3 (lymphocyte activation gene-3), a CD4 homolog, is a member of the Ig superfamily expressed by TILs, activated CD4+ and CD8+ T-cells, regulatory T-cells, and NK, DC and B-cells [258,259,260]. LAG-3 has a high affinity for MHC class II molecules and exerts an inhibitory role on T-cell-mediated immune responses [261] and CD4+ and CD8+ memory T-cell activation [262]. Previous data has shown that LAG-3 is coexpressed with PD-1 in the development of T-cell exhaustion in viral infections [263]. In FL patients, LAG-3 expression was observed within PD-1+ functionally exhausted T-cells. Interestingly, the dual treatment with anti-PD-1 and anti-LAG-3 antibodies restored the T-cell function more efficiently. In a small cohort of 28 patients with FL, LAG-3 expression by T-cells was clinically relevant and related to patient outcome [264]. Recently, LAG-3 overexpression has been shown in a cohort of 163 DLBCL patients, mostly at the surface of CD4+ Tregs and CD8+ TILs. In this cohort, a high expression of LAG-3 and PD-1 was associated with inferior PFS and OS. In addition, the authors were able to identify a population of regulatory LAG3high B-cells that polarise tissue-resident macrophages to promote a tolerogenic TME, which could influence the response to therapy [265]. Recently, a very innovative approach was used to unravel TME architecture in cHL by spatial-resolution–based single-cell analysis. The authors were able to identify and characterised novel cellular subpopulations, including immunosuppressive LAG3+ T-cells. Interestingly, it was observed that of LAG3+ CD4+ T-cells did not coexpress PD-1. Mechanistically, an in-vitro HL coculture system revealed crosstalk between the cytokines and chemokines released by Hodgkin and HRS cells and LAG3+ T-cells, favouring the immunosuppressive activity in the cHL TME. Furthermore, the removal of the LAG3- population in primary samples of cH could restore T-cell activity [266].
IMP321 was the first LAG-3 Ig fusion protein investigated in clinical trials [267]. Relatlimab (BMS-986016), the first anti-LAG-3 antibody, is being evaluated as a single agent or in combination with nivolumab in a phase-1/2a clinical trial (NCT02061761) with 132 patients with R/R B-NHL, CLL, cHL and MM. The results of this study are expected by January 2021. In parallel, a phase-2 open-label study (NCT03365791) of the humanised IgG4 mAbs spartalizumab and ieramilimab (LAG525) was conducted in patients with solid tumours and haematological cancers (n = 7 DLBCL). The results were assessed as a clinical benefit rate after 24 weeks, and the combination therapy showed promising activity in DLBCL patients who reach the expansion criteria [268]. Recently, a high-affinity anti-LAG-3 IgG1κ antibody, INCAGN02385, was engineered. Preclinical data showed that the antibody, alone or in combination with an anti-PD1, enhanced T-cell responsiveness to TCR stimulation. These data supported the evaluation of INCAGN02385 in early phase-1 (NCT03538028) testing in patients with advanced or metastatic cancers, including DLBCL [269]. Lastly, fianlimab (previously known as REGN3767), a human-engineered IgG4 antibody, was able to rescue T-cell activation in vitro and synergise with cemiplimab. This combination was able to surpass the inhibitory effects of MHC II/LAG-3 and PD-L1 signalling. Indeed, in a PD–1xLAG-3 knock-in mice model, REGN3767 treatment was able to reduce tumour growth and enhance the antitumor efficacy of cemiplimab [270]. Accordingly, simultaneous PD-1 and LAG-3 blockades are currently being investigated as a phase-1 study in multiple tumour subtypes (NCT03005782). Finally, a radionuclide-conjugated antibody, 89Zr-REGN3767, designed for immuno-PET analysis, was useful to identify the LAG-3-expressing intratumoral T-cells in a BL xenograft model and human PBMCs [271]. Following these results, an early phase-1 study is evaluating the 89Zr-DFO-REGN3767 anti-LAG-3 antibody for PET in R/R DLBCL patients (NCT04566978).
2.10. TIM-3 Signalling and Inhibition
TIM-3 (T-cell immunoglobulin and mucin-domain containing-3) is an Ig superfamily member that is preferentially expressed in fully differentiated Th1 lymphocytes [272]. TIM-3 has recently emerged as an immune checkpoint receptor in cancer due to its selective expression in tumour tissue and the key role it plays in immunosuppression [273]. In DLBCL patients, an increased level of TIM-3 was observed in both CD4+ and CD8+ T-cells, which was positively correlated with tumour stages [274]. In addition, it was described that TIM-3 is coexpressed with PD-1 in the CD3+ T-cells of patients with DLBCL, and high levels were related to tumour stage and response to conventional chemotherapy [275]. More recently, in a set of DLBCL patients, it was shown that high levels of TIM-3 in tumour cells and TILs were associated with worse OS. The authors also suggested that the TME could be directly affected by TIM-3, which leads to decreased immune surveillance and tumour clearance [276]. Preclinical data pointed out the relevance of blocking TIM-3 together with PD-1, in particular, in several cancer models [277]. These data led to the clinical development of TIM-3 antibodies, which are being tested in combination with anti-PD-1/L1 mAbs. However, so far, no clinical trial is evaluating the effect of anti-TIM-3 alone or in combination therapy in patients with B-NHL.
2.11. OX40 Signalling and Inhibition
OX40 (CD134, TNFRSF4) is another member of the TNFR superfamily, mostly expressed in CD4+ and CD8+ T-cells. Its natural ligand, OX40L (CD252), is expressed in DCs, B-cells and macrophages, among others, and activates the NF-kB, MAPK and BCL-2/XL-dependent antiapoptotic pathways [278,279]. OX40 signalling on T-cells promotes survival and proliferation, as well as CD4+ T-cell production of IL-2, IL-5 and IFN-γ [280,281]. OX40 signalling has been shown to reduce the ability of Treg to suppress T-cell proliferation [282], setting the basis for the antitumor activity of the anti-OX40 antibody [283]. The first anti-OX40 antibody evaluated in NHL patients, MEDI-6469, is a murine antibody with demonstrated agonistic T-cell activity in animal models [284]. The combination of MEDI6469 plus rituximab was tested in a phase-1/2 clinical trial, which included 4 DLBCL cases. No responses were detected, as two patients presented stable disease and the other two patients died during the trial (NCT02205333). After early termination of this trial in 2016, MEDI-6469 has not been further evaluated in lymphoma patients.
The human OX40-agonist PF-04518600 has been reported to promote human T-cell proliferation, to increase cytokine production, to mediate Treg cell depletion ex vivo and to inhibit tumour growth in a mouse model of lymphoma [285,286]. Currently, a phase-1 clinical trial (NCT03636503) is evaluating PF-04518600 in combination with rituximab, utomilumab and avelumab in FL patients.
BMS-986178 is another human OX40-agonist with costimulatory effects observed in ex vivo human CD4+ T-cells as it enhances their T-cell effector functions and inhibits T-cell suppression by Treg cells [287,288]. After demonstrating a favourable safety profile in mice [287], BMS-986178 is currently being evaluated in a phase-1 clinical trial (NCT03410901) in combination with the TLR9 agonist SD-101 and radiotherapy in B-NHL patients, including FL, MCL and MZL, among others.
2.12. TIGIT Signalling and Inhibition
TIGIT (T-cell immunoglobulin and ITIM (immunoreceptor tyrosine-based inhibitory motif) domain) is an inhibitory receptor that is expressed in CD4+ and CD8+ T-cells, Tregs, and NK cells, among others. Its main ligand, CD155 (poliovirus receptor, PVR), is expressed on DCs, B-cells and macrophages [289,290]. Studies with mouse and human cells have revealed a variety of immunomodulatory functions of the TIGIT/CD155 axis, including polarization towards tolerogenic phenotypes of DCs and M2 macrophages, inhibition of NK cell functions, inhibition of proliferation and cytokine production by T-cells, and stimulation of Treg cells. Moreover, TIGIT blocks the costimulatory activity of DNAM1 (DNAX accessory molecule-1, CD226), which binds to CD155 with lower affinity than TIGIT [290].
Tumoral samples from B-NHL patients, including FL, DLBCL, MCL, MZL and CLL, present overexpression of TIGIT in CD4+ and CD8+ T-cells, which produce lower levels of proinflammatory cytokines as well as expression of the ligand CD155 in the TME [291]. In FL patients, high numbers of TIGIT+ T-cells have been associated with lower EFS and OS [292].
Two monoclonal antibodies targeting TIGIT are currently being evaluated in clinical trials with NHL patients. SEA-TGT (SGN-TGT) is a human anti-TIGIT antibody that blocks ligand binding and allows DNAM1 costimulatory signalling. This led to Treg depletion and CD8+ T-cell activation in in-vitro and in-vivo models, in which the drug induced a long-term antitumor response and made animals immune to a tumour rechallenge [293]. A phase-1 clinical trial (NCT04254107) recently tested SEA-TGT as a monotherapy in patients with cHL, DLBCL and PTCL, among others. The other anti-TIGIT antibody, tiragolumab (MTIG7192A, RG6058), is also being tested in a phase-1 clinical trial (NCT04045028), alone or in combination with rituximab, in B-NHL patients.
Following the observation that TIGIT and PD-1 are coexpressed in the intratumoral T-cells of NHL patients [291] and the favourable results of the combination of anti-TIGIT and anti-PD-1/PD-L1 in preclinical models and some clinical trials for solid cancers [289,294], the combination will, most likely, be evaluated in NHL patients in future clinical trials.
3. Mechanisms Underlying B-Cell Lymphoma Refractoriness to Immune Checkpoint Blockade
Resistance to immune checkpoint blockade therapy in human cancers was extensively reviewed by Bellone M and Elia A [295]; however, the mechanisms underlying B-cell lymphoma refractoriness to immune checkpoint blockade are still poorly understood.
Although major advances have been made in the last 20 years to overcome the refractoriness of B-NHL patients to standard therapies through the introduction of immune checkpoint blockades in the clinical setting, either as single agents or in combination therapies (Table 1 and Figure 2), several parameters can impair the efficacy of these new approaches. Patient-intrinsic factors such as age, sex, HLA heterozygosity or loss of β2-microglobulin (B2M), amplification of oncogenic signalling pathways, immunosuppressive cells and molecules present in the TME may impair antigen recognition and contribute to the failure of immune checkpoint blockades [296,297] (Figure 3).
Figure 3.
Mechanisms of resistance to immune checkpoint blockade. The deregulation of MHC class I components such as the loss of β2 microglobulin (B2M) and the loss of human leukocyte antigen (HLA) heterozygosity as well as defects in IFN signalling pathways may impair antigen recognition by antitumor CD8+ T-cells. Amplification of oncogenic signalling pathways such as PI3K/AKT/mTOR, Wnt/β-catenin, and MAPK increases the production of immunosuppressive cytokines, trigger T-cell exclusion from TME and may also result in resistance to immune checkpoint blockade. Epigenetic (histone acetylation or DNA methylation) and genetic (deleterious mutations) alterations are crucial triggers of gene expression disorders related to sustained T-cell exhaustion that could eventually cause the failure of immune checkpoint therapy. Moreover, myeloid-derived suppressor cells (MDSCs), Tregs, tumour-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs) are major immunosuppressive cell types within the TME that may contribute to resistance to immune checkpoint blockade. Immunosuppressive molecules such as TGF-β and IFN-γ, secreted by tumour cells, myeloid cells and macrophages in the TME, may also suppress the functions of effector T-cells, rendering immune checkpoint blockade ineffective.
The frequent PD-L1 aberrant expression that is found among lymphoma patients results in the most responsive cancer type to anti-PD1 therapy. However, the PD-1/PD-L1 blockade can be strongly influenced by disease-specific factors, and its predictive value in clinical trials is still controversial. The inconsistent data could be attributable mainly to the variable PD-L1 resources (tumour cells, tumour microenvironment cells, peripheral blood), the differences in staining (including detecting antibodies) procedures, and positive/negative PD-L1 cut-offs. Additionally, it has been shown that PD-L1 can interact in cis with CD80 on APCs and then disrupt the binding between PD-1 and PD-L1 [298]. The functional effects of alternative binding partners also highlight the differences seen in the efficacy of PD-1/PD-L1 immunotherapy in different biological settings.
There are several mechanisms determining if a patient will respond or not to a PD-1/PD-L1 blockade. Weakly immunogenic tumours may have an insufficiently active T-cell population to respond to PD-1/PD-L1 blockade; additionally, potentially immunogenic tumours will also be resistant to PD-1/PD-L1 blockade if they develop mechanisms to suppress the activation and infiltration of T-cells after the treatment. Moreover, patients may become resistant to PD-1/PD-L1 therapy if they have insufficient reinvigoration of exhausted tumour-specific CD8+ T-cells or if they have lost target antigens or the ability to present them. Finally, some patients might initially respond to PD-1/PD-L1 blockade but become resistant if the antitumour T-cells are short-lived [299].
There are also epigenetic mechanisms of B-lymphoma cells driving the resistance to immune checkpoint blockades. The in vitro and in vivo data from Zheng et al. [300] showed that miR-155 overexpression enhances PD-L1 expression, reduces peripheral blood immune cells, induces CD8+ T-cell apoptosis and dysfunction via AKT/ERK dephosphorylation, and decreases the survival of DLBCL patients. It has also been shown that histone deacetylase 3 (HDAC3) is another important epigenetic regulator of PD-L1 in B-cell lymphoma as its inhibition increases PD-L1 transcription, resulting in a better clinical response to PD-L1 blockade [301].
The molecular mechanisms of the immune environment in regulating the efficacy of immune checkpoint blockades in B-cell lymphoma is poorly understood. It has been shown that T-cell-inflamed tumours are enriched for sensitivity to PD-1 blockade therapy [302]. Conversely, T-cell noninflamed tumours present low-infiltrating immune cells and are typically resistant to immune checkpoint blockade therapy [303]. Inflamed lymphomas are characterised by the presence of prominent T-cell infiltration [304], genetic alterations that facilitate escape from immune surveillance [78,82,305,306], and frequent mutations, resulting in hyperactivity of the NF-kB signalling pathway [78,307].
Hyperprogressive disease has been found in 9% of patients who received anti-PD-1/PD-L1 therapy [308,309]. The hyperprogression is associated with the elderly age of patients but not tumour burden or cancer type [308]. MDM2/MDM4 amplification and EGFR aberration are correlated with a higher risk of hyperprogression in solid cancers [309], although it was little known in lymphoma. Recent data showed that patients experiencing an hyperprogression have a higher prevalence of PD-L1− disease [308]. It is predicted to be because the engagement of PD-1 with anti-PD-1 mAb inhibits but does not augment T-cell activations. Therefore, anti-PD-1 mAbs might be PD-1 agonists rather than antagonists in PD-L1− status. The disease might also rapidly progress through interaction between PD-L1 and CD80 instead of PD-1 for the blocking duration by anti-PD-1 mAbs in PD-L1+ status [310]. Some polymorphisms of PD-1 could also affect the action of anti-PD-1 mAbs, and, thus, hyperprogression could be possible after PD-1 blockade [311].
4. Conclusions and Future Perspectives
Despite the remarkable implementation of immune checkpoint therapies in the last 5 to 6 years in determined subtypes of lymphoma, including cHL and PMBL, the applicability of these approaches in the management of R/R B-NHL has, so far, been mixed and predicting which lymphoma will respond to immune checkpoint blockade is currently not accurate. To date, pembrolizumab is the only FDA-approved agent for use with R/R B-NHL (i.e., PMBL) patients, illustrating the fact that, conceptually, PD-1 blockade in B-NHL appears to be promising and rarely related to severe immune-related AEs [7,97,103]. However, the efficacy of this approach is low, with no long-term durable responses except in PMBL, PCNSL, and PTL, which are due to alterations of chromosome 9p24.1 and the expression of PD-L1/PD-L2. To improve the efficacy of these agents, there have been great efforts made on combination immunotherapy with PD-1/PD-L1 and CTLA-4 checkpoint inhibitors. The superior outcomes of combined immunotherapy over single-agent regimens in preclinical studies, together with the approval of nivolumab plus ipilimumab, give hope to the therapeutic potential of CTLA-4 blockade and its possible combination with PD1/PD-L1 blockers.
Finally, understanding the complex interplay between malignant cells, lymphoid TME and immune-accompanying cells is mandatory in order to identify the specific lymphoma types that are vulnerable to a determined checkpoint and still requires improvement in the detection methods. To this aim, patient preselection based on accurate genomic and phenotypic examination of the TME will be necessary to identify the best target(s) of interest, either in monotherapy or in combination therapy, and facilitate the design of biomarker-driven trials.
Author Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
G.R. acknowledges supports from Fondo de Investigación Sanitaria PI18/01383, European Regional Development Fund (ERDF) “Una manera de hacer Europa”. J.C.S. holds a Sara Borrell research contract from Instituto de Salud Carlos III (CD19/00228).
Conflicts of Interest
G.R. received research support from TG Therapeutics. Remaining authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fisher S.G., Fisher R.I. The epidemiology of non-Hodgkin’s lymphoma. Oncogene. 2004;23:6524–6534. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 2.Goldin L.R., Landgren O. Autoimmunity and lymphomagenesis. Int. J. Cancer. 2009;124:1497–1502. doi: 10.1002/ijc.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso K., Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 2015;15:172–184. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 4.Quintanilla-Martinez L. The 2016 updated WHO classification of lymphoid neoplasias. Hematol. Oncol. 2017;35:37–45. doi: 10.1002/hon.2399. [DOI] [PubMed] [Google Scholar]
- 5.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamma R., Ranieri G., Ingravallo G., Annese T., Oranger A., Gaudio F., Musto P., Specchia G., Ribatti D. Inflammatory Cells in Diffuse Large B Cell Lymphoma. J. Clin. Med. 2020;9:2418. doi: 10.3390/jcm9082418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansell S.M., Minnema M.C., Johnson P., Timmerman J.M., Armand P., Shipp M.A., Rodig S.J., Ligon A.H., Roemer M.G.M., Reddy N., et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J. Clin. Oncol. 2019;37:481–489. doi: 10.1200/JCO.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunleavy K., Wilson W.H. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma. Blood. 2014;125:33–40. doi: 10.1182/blood-2014-05-575092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuruvilla J., Pintilie M., Tsang R., Nagy T., Keating A., Crump M. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk. Lymphoma. 2008;49:1329–1336. doi: 10.1080/10428190802108870. [DOI] [PubMed] [Google Scholar]
- 10.Carbone A., Roulland S., Gloghini A., Younes A., von Keudell G., López-Guillermo A., Fitzgibbon J. Follicular lymphoma. Nat. Rev. Dis. Prim. 2019;5:83. doi: 10.1038/s41572-019-0132-x. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe E.S., Harris N.L., Swerdlow S.H., Ott G., Nathwani B.N., de Jong D., Yoshino T., Spagnolo D., Gascoyne R.D. Follicular lymphoma. In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2017. pp. 266–277. [Google Scholar]
- 12.Freedman A., Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am. J. Hematol. 2020;95:316–327. doi: 10.1002/ajh.25696. [DOI] [PubMed] [Google Scholar]
- 13.Apostolidis J., Mokhtar N., Al Omari R., Darweesh M., Al Hashmi H. Follicular lymphoma: Update on management and emerging therapies at the dawn of the new decade. Hematol. Oncol. 2020;38:213–222. doi: 10.1002/hon.2711. [DOI] [PubMed] [Google Scholar]
- 14.Amin R., Mourcin F., Uhel F., Pangault C., Ruminy P., Dupré L., Guirriec M., Marchand T., Fest T., Lamy T., et al. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood. 2015;126:1911–1920. doi: 10.1182/blood-2015-04-640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amé-Thomas P., Tarte K. The yin and the yang of follicular lymphoma cell niches: Role of microenvironment heterogeneity and plasticity. Semin. Cancer Biol. 2014;24:23–32. doi: 10.1016/j.semcancer.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Sangaletti S., Tripodo C., Portararo P., Dugo M., Vitali C., Botti L., Guarnotta C., Cappetti B., Gulino A., Torselli I., et al. Stromal niche communalities underscore the contribution of the matricellular protein SPARC to B-cell development and lymphoid malignancies. Oncoimmunology. 2014;3:e28989. doi: 10.4161/onci.28989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamaison C., Tarte K. Impact of B cell/lymphoid stromal cell crosstalk in B-cell physiology and malignancy. Immunol. Lett. 2019;215:12–18. doi: 10.1016/j.imlet.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Giulino-Roth L., Cesarman E. Molecular biology of burkitt lymphoma. Burkitt’s Lymphoma. 2013;18:211–226. doi: 10.1007/978-1-4614-4313-1_11. [DOI] [Google Scholar]
- 19.Gerbitz A., Mautner J., Geltinger C., Hörtnagel K., Christoph B., Asenbauer H., Klobeck G., Polack A., Bornkamm G.W. Deregulation of the proto-oncogene c-myc through t(8;22) translocation in Burkitt’s lymphoma. Oncogene. 1999;18:1745–1753. doi: 10.1038/sj.onc.1202468. [DOI] [PubMed] [Google Scholar]
- 20.Sigaux F., Berger R., Bernheim A., Valensi F., Daniel M.T., Flandrin G. Malignant lymphomas with band 8q24 chromosome abnormality: A morphologic continuum extending from Burkitt’s to immunoblastic lymphoma. Br. J. Haematol. 1984;57:393–405. doi: 10.1111/j.1365-2141.1984.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 21.Pham L.V., Pogue E., Ford R.J. The role of macrophage/B-cell interactions in the pathophysiology of B-cell lymphomas. Front. Oncol. 2018;8:147. doi: 10.3389/fonc.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granai M., Mundo L., Akarca A.U., Siciliano M.C., Rizvi H., Mancini V., Onyango N., Nyagol J., Abinya N.O., Maha I., et al. Immune landscape in Burkitt lymphoma reveals M2-macrophage polarization and correlation between PD-L1 expression and non-canonical EBV latency program. Infect. Agents Cancer. 2020;15:28. doi: 10.1186/s13027-020-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sindel A., Al-Juhaishi T., Yazbeck V. Marginal Zone Lymphoma: State-of-the-Art Treatment. Curr. Treat. Options Oncol. 2019;20:90. doi: 10.1007/s11864-019-0687-5. [DOI] [PubMed] [Google Scholar]
- 24.Leslie L.A., Feldman T.A., McNeill A., Timberg M., Iida H., Goy A.H. Contemporary management of nodal and primary splenic marginal zone lymphoma. Expert Rev. Hematol. 2019;12:1011–1022. doi: 10.1080/17474086.2020.1681962. [DOI] [PubMed] [Google Scholar]
- 25.Piris M.A., Isaacson P.G., Swerdlow S.H., Thieblemont C., Pittaluga S., Rossi D., Harris N.L. Splenic marginal zone lymphoma. In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2017. pp. 223–225. [Google Scholar]
- 26.Campo E., Pileri S.A., Jaffe E.S., Nathwani B.N., Stein H., Müller-Hermelink H.K. Nodal marginal zone lymphoma. In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2017. pp. 263–265. [Google Scholar]
- 27.Cook J.R., Isaacson P.G., Chott A., Nakamura S., Müller-Hermelink H.K., Harris N.L., Swerdlow S.H. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2017. pp. 259–262. [Google Scholar]
- 28.Schreuder M.I., van den Brand M., Hebeda K.M., Groenen P.J.T.A., van Krieken J.H., Scheijen B. Novel developments in the pathogenesis and diagnosis of extranodal marginal zone lymphoma. J. Hematop. 2017;10:91–107. doi: 10.1007/s12308-017-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco G., Guarnotta C., Frossi B., Piccaluga P.P., Boveri E., Gulino A., Fuligni F., Rigoni A., Porcasi R., Buffa S., et al. Bone marrow stroma CD40 expression correlates with inflammatory mast cell infiltration and disease progression in splenic marginal zone lymphoma. Blood. 2014;123:1836–1849. doi: 10.1182/blood-2013-04-497271. [DOI] [PubMed] [Google Scholar]
- 30.Thieblemont C., Bertoni F., Copie-Bergman C., Ferreri A.J.M., Ponzoni M. Chronic inflammation and extra-nodal marginal-zone lymphomas of MALT-type. Semin. Cancer Biol. 2014;24:33–42. doi: 10.1016/j.semcancer.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Vose J.M. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2017;92:806–813. doi: 10.1002/ajh.24797. [DOI] [PubMed] [Google Scholar]
- 32.Klener P. Advances in molecular biology and targeted therapy of mantle cell lymphoma. Int. J. Mol. Sci. 2019;20:4417. doi: 10.3390/ijms20184417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiron D., Bellanger C., Papin A., Tessoulin B., Dousset C., Maiga S., Moreau A., Esbelin J., Trichet V., Chen-Kiang S., et al. Rational targeted therapies to overcome microenvironment-dependent expansion of mantle cell lymphoma. Blood. 2016;128:2808–2818. doi: 10.1182/blood-2016-06-720490. [DOI] [PubMed] [Google Scholar]
- 34.Harrington B.K., Wheeler E., Hornbuckle K., Smith L., Klamer B., Zhang X., Long M., Baiocchi R.A., Maddocks K., Johnson A.J., et al. Modulation of immune checkpoint molecule expression in mantle cell lymphoma. HHS Public Access. 2019;60:2498–2507. doi: 10.1080/10428194.2019.1569231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nygren L., Wasik A.M., Baumgartner-Wennerholm S., Jeppsson-Ahlberg Å., Klimkowska M., Andersson P., Buhrkuhl D., Christensson B., Kimby E., Wahlin B.E., et al. T-cell levels are prognostic in mantle cell lymphoma. Clin. Cancer Res. 2014;20:6096–6104. doi: 10.1158/1078-0432.CCR-14-0889. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z.Z., Novak A.J., Stenson M.J., Witzig T.E., Ansell S.M. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papin A., Le Gouill S., Chiron D. Rationale for targeting tumor cells in their microenvironment for mantle cell lymphoma treatment. Leuk. Lymphoma. 2018;59:1064–1072. doi: 10.1080/10428194.2017.1357177. [DOI] [PubMed] [Google Scholar]
- 38.Sonbol M.B., Maurer M.J., Stenson M.J., Allmer C., Laplant B.R., Weiner G.J., Macon W.R., Cerhan J.R., Witzig T.E., Gupta M. Elevated soluble IL-2Rα, IL-8, and MIP-1β levels are associated with inferior outcome and are independent of MIPI score in patients with mantle cell lymphoma. Am. J. Hematol. 2014;89:E223–E227. doi: 10.1002/ajh.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müschen M., Rajewsky K., Bräuninger A., Baur A.S., Oudejans J.J., Roers A., Hansmann M.L., Küppers R. Rare occurrence of classical Hodgkin’s disease as a T cell lymphoma. J. Exp. Med. 2000;191:387–394. doi: 10.1084/jem.191.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moy R.H., Younes A. Immune Checkpoint Inhibition in Hodgkin Lymphoma. HemaSphere. 2018;2 doi: 10.1097/HS9.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green M.R., Monti S., Rodig S.J., Juszczynski P., Currie T., O’Donnell E., Chapuy B., Takeyama K., Neuberg D., Golub T.R., et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto R., Nishikori M., Tashima M., Sakai T., Ichinohe T., Takaori-Kondo A., Ohmori K., Uchiyama T. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009;100:2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mottok A., Steidl C. Biology of classical Hodgkin lymphoma: Implications for prognosis and novel therapies. Blood. 2018;131:1654–1665. doi: 10.1182/blood-2017-09-772632. [DOI] [PubMed] [Google Scholar]
- 44.Campo E., Pileri S.A. Postgraduate Haematology: Seventh Edition. Wiley-Blackwell; Hoboken, NJ, USA: 2015. The Classification of Lymphomas: Updating the WHO Classification. [Google Scholar]
- 45.Reddy A., Zhang J., Davis N.S., Moffitt A.B., Love C.L., Waldrop A., Leppa S., Pasanen A., Meriranta L., Karjalainen-Lindsberg M.L., et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell. 2017;171:481–494.e15. doi: 10.1016/j.cell.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng W., Chen J.Q., Liu C., Malu S., Creasy C., Tetzlaff M.T., Xu C., McKenzie J.A., Zhang C., Liang X., et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bezzi M., Seitzer N., Ishikawa T., Reschke M., Chen M., Wang G., Mitchell C., Ng C., Katon J., Lunardi A., et al. Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat. Med. 2018;24:165–175. doi: 10.1038/nm.4463. [DOI] [PubMed] [Google Scholar]
- 48.Peng D., Kryczek I., Nagarsheth N., Zhao L., Wei S., Wang W., Sun Y., Zhao E., Vatan L., Szeliga W., et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ennishi D., Jiang A., Boyle M., Collinge B., Grande B.M., Ben-Neriah S., Rushton C., Tang J., Thomas N., Slack G.W., et al. Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J. Clin. Oncol. 2019;37:190–201. doi: 10.1200/JCO.18.01583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ennishi D., Takata K., Béguelin W., Duns G., Mottok A., Farinha P., Bashashati A., Saberi S., Boyle M., Meissner B., et al. Molecular and genetic characterization of MHC deficiency identifies ezh2 as therapeutic target for enhancing immune recognition. Cancer Discov. 2019;9:546–563. doi: 10.1158/2159-8290.CD-18-1090. [DOI] [PubMed] [Google Scholar]
- 51.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N., Gouw A.M., Baylot V., Gütgemann I., Eilers M., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedoeem A., Azoulay-Alfaguter I., Strazza M., Silverman G.J., Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014;153:145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrovas C., Casazza J.P., Brenchley J.M., Price D.A., Gostick E., Adams W.C., Precopio M.L., Schacker T., Roederer M., Douek D.C., et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishimura H., Agata Y., Kawasaki A., Sato M., Imamura S., Minato N., Yagita H., Nakano T., Honjo T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int. Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 56.Zhong X., Chunyan B., Gao W., Strom T.B., Rothstein T.L. Suppression of expression and function of negative immune regulator PD-1 by certain pattern recognition and cytokine receptor signals associated with immune system danger. Int. Immunol. 2004;16:1181–1188. doi: 10.1093/intimm/dxh121. [DOI] [PubMed] [Google Scholar]
- 57.Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 58.Georgiou K., Chen L., Berglund M., Ren W., De Miranda N.F.C.C., Lisboa S., Fangazio M., Zhu S., Hou Y., Wu K., et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood. 2016;127:3026–3034. doi: 10.1182/blood-2015-12-686550. [DOI] [PubMed] [Google Scholar]
- 59.Patsoukis N., Brown J., Petkova V., Liu F., Li L., Boussiotis V.A. Selective effects of PD-1 on Akt and ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., Riley J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riley J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okazaki T., Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 64.Roemer M.G.M., Advani R.H., Ligon A.H., Natkunam Y., Redd R.A., Homer H., Connelly C.F., Sun H.H., Daadi S.E., Freeman G.J., et al. PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome. J. Clin. Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menter T., Bodmer-Haecki A., Dirnhofer S., Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum. Pathol. 2016;54:17–24. doi: 10.1016/j.humpath.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Jelinek T., Mihalyova J., Kascak M., Duras J., Hajek R. PD-1/PD-L1 inhibitors in haematological malignancies: Update 2017. Immunology. 2017;152:357–371. doi: 10.1111/imm.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Younes A., Santoro A., Shipp M., Zinzani P.L., Timmerman J.M., Ansell S., Armand P., Fanale M., Ratanatharathorn V., Kuruvilla J., et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Green M.R., Rodig S., Juszczynski P., Ouyang J., Donnell E.O., Neuberg D., Shipp M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Post-transplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carey C.D., Gusenleitner D., Lipschitz M., Roemer M.G.M., Stack E.C., Gjini E., Hu X., Redd R., Freeman G.J., Neuberg D., et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. 2017;130:2420–2430. doi: 10.1182/blood-2017-03-770719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen B.J., Chapuy B., Ouyang J., Sun H.H., Roemer M.G.M., Xu M.L., Yu H., Fletcher C.D.M., Freeman G.J., Shipp M.A., et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin. Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiyasu J., Miyoshi H., Hirata A., Arakawa F., Ichikawa A., Niino D., Sugita Y., Yufu Y., Choi I., Abe Y., et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andorsky D.J., Yamada R.E., Said J., Pinkus G.S., Betting D.J., Timmerman J.M. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 74.Kwon D., Kim S., Kim P.J., Go H., Nam S.J., Paik J.H., Kim Y.A., Kim T.M., Heo D.S., Kim C.W., et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079–1089. doi: 10.1111/his.12882. [DOI] [PubMed] [Google Scholar]
- 75.Ahearne M.J., Bhuller K., Hew R., Ibrahim H., Naresh K., Wagner S.D. Expression of PD-1 (CD279) and FoxP3 in diffuse large B-cell lymphoma. Virchows Arch. 2014;465:351–358. doi: 10.1007/s00428-014-1615-5. [DOI] [PubMed] [Google Scholar]
- 76.Kwiecinska A., Tsesmetzis N., Ghaderi M., Kis L., Saft L., Rassidakis G.Z. CD274 (PD-L1)/PDCD1 (PD-1) expression in de novo and transformed diffuse large B-cell lymphoma. Br. J. Haematol. 2018;180:744–748. doi: 10.1111/bjh.14432. [DOI] [PubMed] [Google Scholar]
- 77.Xing W., Dresser K., Zhang R., Evens A.M., Yu H., Woda B.A., Chen B.J. PD-L1 expression in EBV-negative diffuse large B-cell lymphoma: Clinicopathologic features and prognostic implications. Oncotarget. 2016;7:59976–59986. doi: 10.18632/oncotarget.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Godfrey J., Tumuluru S., Bao R., Leukam M., Venkataraman G., Phillip J., Fitzpatrick C., McElherne J., MacNabb B.W., Orlowski R., et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell–inflamed phenotype. Blood. 2019;133:2279–2290. doi: 10.1182/blood-2018-10-879015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincent-Fabert C., Roland L., Zimber-Strobl U., Feuillard J., Faumont N. Pre-clinical blocking of PD-L1 molecule, which expression is down regulated by NF-κB, JAK1/JAK2 and BTK inhibitors, induces regression of activated B-cell lymphoma. Cell Commun. Signal. 2019;17:89. doi: 10.1186/s12964-019-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chapuy B., Roemer M.G.M., Stewart C., Tan Y., Abo R.P., Zhang L., Dunford A.J., Meredith D.M., Thorner A.R., Jordanova E.S., et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127:869–881. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merryman R.W., Armand P., Wright K.T., Rodig S.J. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1:2643–2654. doi: 10.1182/bloodadvances.2017012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Twa D.D.W., Steidl C. Structural genomic alterations in primary mediastinal large B-cell lymphoma. Leuk. Lymphoma. 2015;56:2239–2250. doi: 10.3109/10428194.2014.985673. [DOI] [PubMed] [Google Scholar]
- 83.Twa D.D.W., Mottok A., Chan F.C., Ben-Neriah S., Woolcock B.W., Tan K.L., Mungall A.J., McDonald H., Zhao Y., Lim R.S., et al. Recurrent genomic rearrangements in primary testicular lymphoma. J. Pathol. 2015;236:136–141. doi: 10.1002/path.4522. [DOI] [PubMed] [Google Scholar]
- 84.Shi M., Roemer M.G.M., Chapuy B., Liao X., Sun H., Pinkus G.S., Shipp M.A., Freeman G.J., Rodig S.J. Expression of Programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am. J. Surg. Pathol. 2014;38:1715–1723. doi: 10.1097/PAS.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song M.K., Park B.B., Uhm J. Understanding immune evasion and therapeutic targeting associated with PD-1/PD-L1 pathway in diffuse large B-cell lymphoma. Int. J. Mol. Sci. 2019;20:1326. doi: 10.3390/ijms20061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fang X., Xiu B., Yang Z., Qiu W., Zhang L., Zhang S., Wu Y., Zhu X., Chen X., Xie S., et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Medicine. 2017;96:e6398. doi: 10.1097/MD.0000000000006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myklebust J.H., Irish J.M., Brody J., Czerwinski D.K., Houot R., Kohrt H.E., Timmerman J., Said J., Green M.R., Delabie J., et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121:1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wahlin B.E., Aggarwal M., Montes-Moreno S., Gonzalez L.F., Roncador G., Sanchez-Verde L., Christensson B., Sander B., Kimby E. A unifying microenvironment model in follicular lymphoma: Outcome is predicted by programmed death-1-positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin. Cancer Res. 2010;16:637–650. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- 89.Lopez-Guillermo A., Carreras J., Roncador G., Villamor N., Colomo L., Martinez A., Hamoudi R., Howat W.J., Montserrat E., Campo E. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J. Clin. Oncol. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 90.Wang L., Qian J., Lu Y., Li H., Bao H., He D., Liu Z., Zheng Y., He J., Li Y., et al. Immune evasion of mantle cell lymphoma: Expression of B7-H1 leads to inhibited T-cell response to and killing of tumor cells. Haematologica. 2013;98:1458–1466. doi: 10.3324/haematol.2012.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weber J.S., D’Angelo S.P., Minor D., Hodi F.S., Gutzmer R., Neyns B., Hoeller C., Khushalani N.I., Miller W.H., Lao C.D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 92.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 93.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peters S., Gettinger S., Johnson M.L., Jänne P.A., Garassino M.C., Christoph D., Toh C.K., Rizvi N.A., Chaft J.E., Costa E.C., et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH) J. Clin. Oncol. 2017;35:2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaufman H.L., Russell J., Hamid O., Bhatia S., Terheyden P., D’Angelo S.P., Shih K.C., Lebbé C., Linette G.P., Milella M., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massard C., Gordon M.S., Sharma S., Rafii S., Wainberg Z.A., Luke J., Curiel T.J., Colon-Otero G., Hamid O., Sanborn R.E., et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016;34:3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J., Medeiros L.J., Young K.H. Cancer Immunotherapy in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2018;8:351. doi: 10.3389/fonc.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bryan L.J., Gordon L.I. Blocking tumor escape in hematologic malignancies: The anti-PD-1 strategy. Blood Rev. 2015;29:25–32. doi: 10.1016/j.blre.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Sheikh S., Kuruvilla J. Pembrolizumab for the treatment of diffuse large B-cell lymphoma. Expert Opin. Biol. Ther. 2019;19:1119–1126. doi: 10.1080/14712598.2019.1659777. [DOI] [PubMed] [Google Scholar]
- 100.Xu-Monette Z., Zhou J., Young K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hude I., Sasse S., Engert A., Bröckelmann P.J. The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica. 2017;102:30–42. doi: 10.3324/haematol.2016.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frigault M.J., Armand P., Redd R.A., Jeter E., Merryman R.W., Coleman K.C., Herrera A.F., Dahi P., Nieto Y., LaCasce A.S., et al. PD-1 blockade for diffuse large B-cell lymphoma after autologous stem cell transplantation. Blood Adv. 2020;4:122–126. doi: 10.1182/bloodadvances.2019000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lesokhin A.M., Ansell S.M., Armand P., Scott E.C., Halwani A., Gutierrez M., Millenson M.M., Cohen A.D., Schuster S.J., Lebovic D., et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase ib study. J. Clin. Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding W., LaPlant B.R., Call T.G., Parikh S.A., Leis J.F., He R., Shanafelt T.D., Sinha S., Le-Rademacher J., Feldman A.L., et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419–3427. doi: 10.1182/blood-2017-02-765685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nayak L., Iwamoto F.M., Lacasce A., Mukundan S., Roemer M.G.M., Chapuy B., Armand P., Rodig S.J., Shipp M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zinzani P.L., Santoro A., Gritti G., Brice P., Barr P.M., Kuruvilla J., Cunningham D., Kline J., Johnson N.A., Mehta-Shah N., et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: Efficacy and safety from the phase II checkmate 436 study. J. Clin. Oncol. 2019;37:3081–3089. doi: 10.1200/JCO.19.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zinzani P.L., Ribrag V., Moskowitz C.H., Michot J.M., Kuruvilla J., Balakumaran A., Zhang Y., Chlosta S., Shipp M.A., Armand P. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Armand P., Rodig S., Melnichenko V., Thieblemont C., Bouabdallah K., Tumyan G., Özcan M., Portino S., Fogliatto L., Caballero M.D., et al. Pembrolizumab in relapsed or refractory primary mediastinal large b-cell lymphoma. J. Clin. Oncol. 2019;37:3291–3299. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC Press; Lyon, France: 2008. [Google Scholar]
- 110.Chen R., Zinzani P.L., Fanale M.A., Armand P., Johnson N.A., Brice P., Radford J., Ribrag V., Molin D., Vassilakopoulos T.P., et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin Lymphoma. J. Clin. Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Armand P., Engert A., Younes A., Fanale M., Santoro A., Zinzani P.L., Timmerman J.M., Collins G.P., Ramchandren R., Cohen J.B., et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase II checkmate 205 trial. J. Clin. Oncol. 2018;36:1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Armand P., Shipp M.A., Ribrag V., Michot J.M., Zinzani P.L., Kuruvilla J., Snyder E.S., Ricart A.D., Balakumaran A., Rose S., et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Armand P., Chen Y.-B., Redd R.A., Joyce R.M., Bsat J., Jeter E., Merryman R.W., Coleman K.C., Dahi P.B., Nieto Y., et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood. 2019;134:22–29. doi: 10.1182/blood.2019000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shindiapina P., Alinari L. Pembrolizumab and its role in relapsed/refractory classical Hodgkin’s lymphoma: Evidence to date and clinical utility. Ther. Adv. Hematol. 2018;9:89–105. doi: 10.1177/2040620718761777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A.N., Lanier B.J., et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeanson A., Barlesi F. MEDI 4736 (durvalumab) in non-small cell lung cancer. Expert Opin. Biol. Ther. 2017;17:1317–1323. doi: 10.1080/14712598.2017.1351939. [DOI] [PubMed] [Google Scholar]
- 118.Stewart R., Morrow M., Hammond S.A., Mulgrew K., Marcus D., Poon E., Watkins A., Mullins S., Chodorge M., Andrews J., et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol. Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 119.Sagiv-Barfi I., Kohrt H.E.K., Czerwinski D.K., Ng P.P., Chang B.Y., Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. USA. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herrera A.F., Goy A., Mehta A., Ramchandren R., Pagel J.M., Svoboda J., Guan S., Hill J.S., Kwei K., Liu E.A., et al. Safety and activity of ibrutinib in combination with durvalumab in patients with relapsed or refractory follicular lymphoma or diffuse large B-cell lymphoma. Am. J. Hematol. 2020;95:18–27. doi: 10.1002/ajh.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Younes A., Burke J.M., Cheson B., Diefenbach C., Ferrari S., Hahn U., Hawkes E., Khan C., Lossos I.S., Musuraka G., et al. Safety and Efficacy of Atezolizumab in Combination with Rituximab Plus CHOP in Previously Untreated Patients with Diffuse Large B-Cell Lymphoma (DLBCL): Primary Analysis of a Phase I/II Study. Blood. 2018 doi: 10.1182/blood-2018-99-116678. [DOI] [Google Scholar]
- 122.Morschhauser F., Ghosh N., Lossos I., Palomba M.L., Mehta A., Casasnovas O., Stevens D., Chitra S., Knapp A., Nielsen T., et al. Efficacy and safety of obinutuzumab + lenalidomide + atezolizumab in patients with relapsed or refractory follicular lymphoma: Primary analysis of a phase 1b/2 trial; Proceedings of the 15th International Conference on Malignant Lymphoma; Lugano, Switzerland. 18–22 June 2019; p. 112. [Google Scholar]
- 123.Palomba M.L., Cartron G., Popplewell L., Ribrag V., Westin J., Chitra S., Huw L., Newberry K., Raval R., Xu J., et al. Safety and clinical activity of atezolizumab in combination with tazemetostat in relapsed or refractory diffuse large B-cell lymphoma: Primary analysis of a phase 1b study; Proceedings of the 15th International Conference on Malignant Lymphoma; Lugano, Switzerland. 18–22 June 2019. [Google Scholar]
- 124.Herbaux C., Casasnovas O., Feugier P., Damaj G., Bouabdallah R., Guidez S., Ysebaert L., Tilly H., Le Gouill S., Fornecker L.M., et al. Efficacy and safety of Atezolizumab + Obinutuzumab + Venetoclax in patients with relapsed or refractory Diffuse Large B-cell lymphomas: Primary analysis of a phase 2 trial from LYSA; Proceedings of the EHA Annual Meeting; Frankfurt, Germany. 11–14 June 2020. [Google Scholar]
- 125.Schuster S.J., Bartlett N.L., Assouline S., Yoon S.S., Bosch F., Sehn L.H., Cheah C.Y., Shadman M., Gregory G.P., Ku M., et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood. 2019 doi: 10.1182/blood-2019-123742. [DOI] [Google Scholar]
- 126.Hutchings M., Gritti G., Sureda A., Terol M.J., Dyer M.J., Iacoboni G., Townsend W., Bacac M., Bröske A.-M.E., Dimier N., et al. CD20-TCB, a Novel T-Cell-Engaging Bispecific Antibody, Can be Safely Combined with the Anti-PD-L1 Antibody Atezolizumab in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2019 doi: 10.1182/blood-2019-123978. [DOI] [Google Scholar]
- 127.Jacobson C., Westin J.R., Miklos D.B., Herrera A.F., Lee J., Seng J., Rossi J.M., Sun J., Dong J., Roberts Z.J., et al. Phase 1/2 primary analysis of ZUMA-6: Axicabtagene ciloleucel (Axi-Cel) in combination With atezolizumab (Atezo) for the treatment of patients (Pts) with refractory diffuse large B cell lymphoma (DLBCL); Proceedings of the AACR Virtual Annual Meeting II; Philadelphia, PA, USA. 27–28 April and 22–24 June 2020. [Google Scholar]
- 128.Nowakowsk G., Willenbacher W., Greil R., Larsen T.S., Patel K., Jäger U., Manges R.F., Trümper L., Haioun C., Everaus H., et al. Safety and efficacy of the PD-L1 inhibitor Durvalumab with R-CHOP or R2 -CHOP in subjects with previously untreated, high-risk DLBCL; Proceedings of the 15th International Conference on Malignant Lymphoma; Lugano, Switzerland. 18–23 June 2019; p. 132. [Google Scholar]
- 129.Hirayama A.V., Gauthier J., Hay K.A., Sheih A., Cherian S., Chen X., Pender B.S., Hawkins R.M., Vakil A., Steinmetz R.N., et al. Efficacy and Toxicity of JCAR014 in Combination with Durvalumab for the Treatment of Patients with Relapsed/Refractory Aggressive B-Cell Non-Hodgkin Lymphoma. Blood. 2018 doi: 10.1182/blood-2018-99-116745. [DOI] [Google Scholar]
- 130.Ribrag V., Armand P., Kuruvilla J., Michot J.-M., Moskowitz C.H., Marinello P., Snyder E., Balakumaran A., Shipp M.A., Zinzani P.L. An open-label, multicohort Phase Ib trial of pembrolizumab (MK-3475) for advanced hematologic malignancies: KEYNOTE-013. J. Immunother. Cancer. 2015;3:P169. doi: 10.1186/2051-1426-3-S2-P169. [DOI] [Google Scholar]
- 131.Chong E.A., Svoboda J., Dwivedy Nasta S., Landsburg D.J., Winchell N., Napier E., Mato A.R., Melenhorst J.J., Ruella M., Lacey S.F., et al. Sequential Anti-CD19 Directed Chimeric Antigen Receptor Modified T-Cell Therapy (CART19) and PD-1 Blockade with Pembrolizumab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas. Blood. 2018;132:4198. doi: 10.1182/blood-2018-99-119502. [DOI] [Google Scholar]
- 132.Barraclough A., Chong G., Gilbertson M., Grigg A., Churilov L., Fancourt T., Ritchie D., Koldej R., Agarwal R., Manos K., et al. Immune Priming with Single-Agent Nivolumab Followed By Combined Nivolumab & Rituximab Is Safe and Efficacious for First-Line Treatment of Follicular Lymphoma; Interim Analysis of the “1st FLOR” Study. Blood. 2019;134:1523. doi: 10.1182/blood-2019-123908. [DOI] [Google Scholar]
- 133.Smith S.D., Till B.G., Shadman M., Lynch R.C., Cowan A.J., Wu Q., Voutsinas J., Rasmussen H., Blue K., Ujjani C., et al. Pembrolizumab with R-CHOP in Previously Untreated Diffuse Large B-Cell Lymphoma: Potential for Biomarker Driven Therapy. SSRN Electron. J. 2020;189:1119–1126. doi: 10.1111/bjh.16494. [DOI] [PubMed] [Google Scholar]
- 134.Vanmeerbeek I., Sprooten J., De Ruysscher D., Tejpar S., Vandenberghe P., Fucikova J., Spisek R., Zitvogel L., Kroemer G., Galluzzi L., et al. Trial watch: Chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9:1703449. doi: 10.1080/2162402X.2019.1703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Younes A., Brody J., Carpio C., Lopez-Guillermo A., Ben-Yehuda D., Ferhanoglu B., Nagler A., Ozcan M., Avivi I., Bosch F., et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: A phase 1/2a study. Lancet Haematol. 2019;6:e67–e78. doi: 10.1016/S2352-3026(18)30217-5. [DOI] [PubMed] [Google Scholar]
- 136.Witzig T.E., Maddocks K.J., De Vos S., Lyons R.M., Edenfield W.J., Sharman J.P., Vose J., Yimer H.A., Wei H., Chan E.M., et al. Phase 1/2 trial of acalabrutinib plus pembrolizumab (Pem) in relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) J. Clin. Oncol. 2019;37:7519. doi: 10.1200/JCO.2019.37.15_suppl.7519. [DOI] [Google Scholar]
- 137.Gregory G., Walker P., Mahadevan D., Wang D., Chang J., Hernandez-Ilizaliturri F., Klein A., Rybka W., Wagner-Johnston N., Escobar C., et al. Antitumor Activity of Pembrolizumab Plus Dinaciclib in Patients with Diffuse Large B Cell Lymphoma: The Phase 1b KEYNOTE-155 Study. Hematol. Oncol. 2019;37:328–329. doi: 10.1002/hon.140_2630. [DOI] [Google Scholar]
- 138.Liu Y., Barta S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019;94:604–616. doi: 10.1002/ajh.25460. [DOI] [PubMed] [Google Scholar]
- 139.Sermer D.J., Vardhana S.A., Ames A., Biggar E., Moskowitz A.J., Batlevi C.L., Caron P., Hamilton A.M., Moskowitz C.H., Matasar M.J., et al. Early data from a phase II trial investigating the combination of pembrolizumab (PEM) and entinostat (ENT) in relapsed and refractory (R/R) Hodgkin lymphoma (HL) J. Clin. Oncol. 2020;38:e20018. doi: 10.1200/JCO.2020.38.15_suppl.e20018. [DOI] [Google Scholar]
- 140.Osborne W., Marzolini M., Tholouli E., Ramakrishnan A., Bachier C.R., McSweeney P.A., Irvine D., Zhang M., Al-Hajj M.A., Pule M., et al. Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR T cell therapy, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL. J. Clin. Oncol. 2020;38:8001. doi: 10.1200/JCO.2020.38.15_suppl.8001. [DOI] [Google Scholar]
- 141.Jaeger U., Worel N., McGuirk J.P., Riedell P.A., Fleury I., Borchmann P., Chu J., Abdelhady A.M., Forcina A., Bubuteishvili Pacaud L., et al. Portia: A Phase 1b Study Evaluating Safety and Efficacy of Tisagenlecleucel and Pembrolizumab in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood. 2019;134:5325. doi: 10.1182/blood-2019-129120. [DOI] [Google Scholar]
- 142.Ansell S., Gutierrez M.E., Shipp M.A., Gladstone D., Moskowitz A., Borello I., Popa-Mckiver M., Farsaci B., Zhu L., Lesokhin A.M., et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039) Blood. 2016;128:183. doi: 10.1182/blood.V128.22.183.183. [DOI] [Google Scholar]
- 143.Ansell S.M., Hurvitz S.A., Koenig P.A., LaPlant B.R., Kabat B.F., Fernando D., Habermann T.M., Inwards D.J., Verma M., Yamada R., et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuscano J.M., Maverakis E., Groshen S., Tsao-Wei D., Luxardi G., Merleev A.A., Beaven A., DiPersio J.F., Popplewell L., Chen R., et al. A phase I study of the combination of rituximab and ipilimumab in patients with relapsed/refractory B-cell lymphoma. Clin. Cancer Res. 2019;25:7004–7013. doi: 10.1158/1078-0432.CCR-19-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davids M.S., Kim H.T., Bachireddy P., Costello C., Liguori R., Savell A., Lukez A.P., Avigan D., Chen Y.-B., McSweeney P., et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016;375:143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khouri I.F., Curbelo I.F., Turturro F., Jabbour E.J., Milton D.R., Bassett R.L., Vence L.M., Allison J.P., Gulbis A.M., Sharma P. Ipilimumab plus lenalidomide after allogeneic and autologous stem cell transplantation for patients with lymphoid malignancies. Clin. Cancer Res. 2018;24:1011–1018. doi: 10.1158/1078-0432.CCR-17-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Advani R., Bartlett N.L., Smith S.M., Roschewski M., Popplewell L., Flinn I., Collins G., Ghosh N., LaCasce A., Asch A., et al. The first-in-class anti-CD47 antibody HU5F9-G4 + rituximab induces durable responses in relapsed/refractory DLBCL and indolent lymphoma: Interim phase 1b/2 results. Hematol. Oncol. 2019 doi: 10.1002/hon.57_2629. [DOI] [Google Scholar]
- 148.Trillium Therapeutics Provides Update on Its TTI-621 and TTI-622 Clinical Programs. [(accessed on 7 January 2021)]; Available online: https://www.globenewswire.com/news-release/2020/01/07/1967258/0/en/Trillium-Therapeutics-Provides-Update-on-Its-TTI-621-and-TTI-622-CD47-Programs.html.
- 149.Patel K., Maris M.B., Cheson B.D., Zonder J.A., Lesokhin A.M., Von Keudell G., Seymour E.K., Lin G.H.Y., Catalano T., Shou Y., et al. Ongoing, first-in-human, phase I dose escalation study of the investigational CD47-blocker TTI-622 in patients with advanced relapsed or refractory lymphoma. J. Clin. Oncol. 2020;38:3030. doi: 10.1200/JCO.2020.38.15_suppl.3030. [DOI] [Google Scholar]
- 150.Kim T.M., Lakhani N., Gainor J., Kamdar M., Fanning P., Squifflet P., Jin F., Wan H., Pons J., Randolph S.S., et al. A Phase 1 Study of ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Non-Hodgkin Lymphoma. Blood. 2019;134:1953. doi: 10.1182/blood-2019-123219. [DOI] [Google Scholar]
- 151.Kim T.M., Lakhani N., Gainor J., Kamdar M., Fanning P., Squifflet P., Jin F., Wan H., Pons J., Randolph S., et al. ALX148, A CD47 blocker, in combination with Rituximab in patients with Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma (NHL); Proceedings of the EHA 2020; Frankfurt, Germany. 11–14 June 2020; p. EP1247. [Google Scholar]
- 152.Advani R., Forero-Torres A., Furman R.R., Rosenblatt J.D., Younes A., Ren H., Harrop K., Whiting N., Drachman J.G. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J. Clin. Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 153.De Vos S., Forero-Torres A., Ansell S.M., Kahl B., Cheson B.D., Bartlett N.L., Furman R.R., Winter J.N., Kaplan H., Timmerman J., et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J. Hematol. Oncol. 2014;7:44. doi: 10.1186/1756-8722-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fanale M., Assouline S., Kuruvilla J., Solal-Céligny P., Heo D.S., Verhoef G., Corradini P., Abramson J.S., Offner F., Engert A., et al. Phase IA/II, multicentre, open-label study of the CD40 antagonistic monoclonal antibody lucatumumab in adult patients with advanced non-Hodgkin or Hodgkin lymphoma. Br. J. Haematol. 2014;164:258–265. doi: 10.1111/bjh.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Forero-Torres A., Bartlett N., Beaven A., Myint H., Nasta S., Northfelt D.W., Whiting N.C., Drachman J.G., Lobuglio A.F., Moskowitz C.H. Pilot study of dacetuzumab in combination with rituximab and gemcitabine for relapsed or refractory diffuse large B-cell lymphoma. Leuk. Lymphoma. 2013;54:277–283. doi: 10.3109/10428194.2012.710328. [DOI] [PubMed] [Google Scholar]
- 156.Fayad L., Ansell S.M., Advani R., Coiffier B., Stuart R., Bartlett N.L., Forero-Torres A., Kuliczkowski K., Belada D., Ng E., et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: A randomized, double-blind, placeb. Leuk. Lymphoma. 2015;56:2569–2578. doi: 10.3109/10428194.2015.1007504. [DOI] [PubMed] [Google Scholar]
- 157.Ansell S.M., Flinn I., Taylor M.H., Sikic B.I., Brody J., Nemunaitis J., Feldman A., Hawthorne T.R., Rawls T., Keler T., et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 2020;4:1917–1926. doi: 10.1182/bloodadvances.2019001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bence-Bruckler I., Macdonald D., Stiff P.J., McKinney B., Ruffner K.L., Wilson L., Whiteley M., Kahl B. A Phase 2, Double-Blind, Placebo-Controlled Trial of Rituximab + Galiximab Vs Rituximab + Placebo In Advanced Follicular Non-Hodgkin’s Lymphoma (NHL) Blood. 2010;116:428. doi: 10.1182/blood.V116.21.428.428. [DOI] [Google Scholar]
- 159.Czuczman M.S., Thall A., Witzig T.E., Vose J.M., Younes A., Emmanouilides C., Miller T.P., Moore J.O., Leonard J.P., Gordon L.I., et al. Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma. J. Clin. Oncol. 2005;23:4390–4398. doi: 10.1200/JCO.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 160.Czuczman M.S., Leonard J.P., Jung S., Johnson J.L., Hsi E.D., Byrd J.C., Cheson B.D. Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular lymphoma international prognostic index (FLIPI) score is predictive of upfront immunotherapy responsiveness. Ann. Oncol. 2012;23:2356–2362. doi: 10.1093/annonc/mdr620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Timmerman J.M., Herbaux C., Ribrag V., Zelenetz A.D., Houot R., Neelapu S.S., Logan T.F., Lossos I.S., Urba W.J., Salles G.A., et al. Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma. Am. J. Hematol. 2020;95:510–520. doi: 10.1002/ajh.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gopal A.K., Levy R., Houot R., Patel S.P., Popplewell L., Jacobson C.A., Mu X.J., Deng S., Ching K.A., Chen Y., et al. First-in-Human Study of Utomilumab, a 4-1BB/CD137 Agonist, in Combination with Rituximab in Patients with Follicular and Other CD20+ Non-Hodgkin Lymphomas. Clin. Cancer Res. 2020;26:2524–2534. doi: 10.1158/1078-0432.CCR-19-2973. [DOI] [PubMed] [Google Scholar]
- 163.Phillips T., Barr P.M., Park S.I., Kolibaba K., Caimi P.F., Chhabra S., Kingsley E.C., Boyd T., Chen R., Carret A.S., et al. A phase 1 trial of SGN-CD70A in patients with CD70-positive diffuse large B cell lymphoma and mantle cell lymphoma. Investig. New Drugs. 2019;37:297–306. doi: 10.1007/s10637-018-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schwartz J.C.D., Zhang X., Fedorov A.A., Nathenson S.G., Almo S.C. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 165.Rowshanravan B., Halliday N., Sansom D.M. CTLA-4: A moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Camacho L.H. Novel therapies targeting the immune system: CTLA4 blockade with tremelimumab (CP-675, 206), a fully human monoclonal antibody. Expert Opin. Investig. Drugs. 2008;17:371–385. doi: 10.1517/13543784.17.3.371. [DOI] [PubMed] [Google Scholar]
- 167.Fellner C. Ipilimumab (Yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. Pharm. Ther. 2012;37:503–512. [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao Y., Yang W., Huang Y., Cui R., Li X., Li B. Evolving Roles for Targeting CTLA-4 in Cancer Immunotherapy. Cell. Physiol. Biochem. 2018;47:721–734. doi: 10.1159/000490025. [DOI] [PubMed] [Google Scholar]
- 169.Wang X.Y., Zuo D., Sarkar D., Fisher P.B. Blockade of cytotoxic T-lymphocyte antigen-4 as a new therapeutic approach for advanced melanoma. Expert Opin. Pharmacother. 2011;12:2695–2706. doi: 10.1517/14656566.2011.629187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He M., Chai Y., Qi J., Zhang C.W.H., Tong Z., Shi Y., Yan J., Tan S., Gao G.F. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 2017;8:67129–67139. doi: 10.18632/oncotarget.18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Herbaux C., Gauthier J., Brice P., Drumez E., Ysebaert L., Doyen H., Fornecker L., Bouabdallah K., Manson G., Ghesquières H., et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129:2471–2478. doi: 10.1182/blood-2016-11-749556. [DOI] [PubMed] [Google Scholar]
- 172.Massarelli E., Segal N.H., Ribrag V., Melero I., Gangadhar T.C., Urba W.J., Schadendorf D., Ferris R.L., Houot R., Morschhauser F., et al. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. J. Immunother. Cancer Natl. Harb. 2016;4:O7. [Google Scholar]
- 173.Brown E., Hooper L., Ho T., Gresham H. Integrin-associated protein: A 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D., van Rooijen N., Weissman I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lorenz U. SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 177.Zhan F., Hardin J., Kordsmeier B., Bumm K., Zheng M., Tian E., Sanderson R., Yang Y., Wilson C., Zangari M., et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–1757. doi: 10.1182/blood.V99.5.1745. [DOI] [PubMed] [Google Scholar]
- 178.Jaiswal S., Jamieson C.H.M., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chao M.P., Alizadeh A.A., Tang C., Myklebust J.H., Varghese B., Gill S., Jan M., Cha A.C., Chan C.K., Tan B.T., et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chao M.P., Alizadeh A.A., Tang C., Jan M., Weissman-Tsukamoto R., Zhao F., Park C.Y., Weissman I.L., Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim D., Wang J., Willingham S.B., Martin R., Wernig G., Weissman I.L. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 182.Pang W.W., Pluvinage J.V., Price E.A., Sridhar K., Arber D.A., Greenberg P.L., Schrier S.L., Park C.Y., Weissman I.L. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc. Natl. Acad. Sci. USA. 2013;110:3011–3016. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chao M.P., Alizadeh A.A., Tang C.Z., Myklebust J.H., Varghese B., Jan M., Levy R., Weissman I.L., Majeti R. Therapeutic Antibody Targeting of CD47 Synergizes with Rituximab to Completely Eradicate Human B-Cell Lymphoma Xenografts. Blood. 2009;114:2716. doi: 10.1182/blood.V114.22.2716.2716. [DOI] [Google Scholar]
- 184.Liu J., Wang L., Zhao F., Tseng S., Narayanan C., Shura L., Willingham S., Howard M., Prohaska S., Volkmer J., et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE. 2015;10:e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sikic B.I., Lakhani N., Patnaik A., Shah S.A., Chandana S.R., Rasco D., Colevas A.D., O’Rourke T., Narayanan S., Papadopoulos K., et al. First-in-human, first-in-class phase i trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 2019;37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., Kline J., Roschewski M., LaCasce A., Collins G.P., et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meng Z., Wang Z., Guo B., Cao W., Shen H. TJC4, a Differentiated Anti-CD47 Antibody with Novel Epitope and RBC Sparing Properties. Blood. 2019;134:4063. doi: 10.1182/blood-2019-122793. [DOI] [Google Scholar]
- 188.Petrova P.S., Viller N.N., Wong M., Pang X., Lin G.H.Y., Dodge K., Chai V., Chen H., Lee V., House V., et al. TTI-621 (SIRPαFc): A CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin. Cancer Res. 2017;23:1068–1079. doi: 10.1158/1078-0432.CCR-16-1700. [DOI] [PubMed] [Google Scholar]
- 189.Lin G.H.Y., Chai V., Lee V., Dodge K., Truong T., Wong M., Johnson L.D., Linderoth E., Pang X., Winston J., et al. TTI-621 (SIRPαFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS ONE. 2017;12:e0187262. doi: 10.1371/journal.pone.0187262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Izrailit J.B., Viller N.N., Pang X., Petrova P.S., Uger R.A., Winston J., Linderoth E. The CD47-blocking innate immune checkpoint inhibitor, TTI-621, triggers CD47-mediated tumor cell apoptosis; Proceedings of the AACR Annual Meeting 2018; Chicago, IL, USA. 14–18 April 2018; Abstract 2720. [Google Scholar]
- 191.Tseng D., Volkmer J.P., Willingham S.B., Contreras-Trujillo H., Fathman J.W., Fernhoff N.B., Seita J., Inlay M.A., Weiskopf K., Miyanishi M., et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lin G.H.Y., Viller N.N., Chabonneau M., Brinen L., Mutukura T., Dodge K., Helke S., Chai V., House V., Lee V., et al. TTI-622 (SIRPα-IgG4 Fc), a CD47-blocking innate immune checkpoint inhibitor, suppresses tumor growth and demonstrates enhanced efficacy in combination with antitumor antibodies in both hematologic and solid tumor models; Proceedings of the AACR Annual Meeting 2018; Chicago, IL, USA. 14–18 April 2018; Abstract 2709. [Google Scholar]
- 193.Kauder S.E., Kuo T.C., Harrabi O., Chen A., Sangalang E., Doyle L., Rocha S.S., Bollini S., Han B., Sim J., et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE. 2018;13:e0201832. doi: 10.1371/journal.pone.0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fischer N., Elson G., Magistrelli G., Dheilly E., Fouque N., Laurendon A., Gueneau F., Ravn U., Depoisier J.F., Moine V., et al. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat. Commun. 2015;6:6113. doi: 10.1038/ncomms7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dheilly E., Moine V., Broyer L., Salgado-Pires S., Johnson Z., Papaioannou A., Cons L., Calloud S., Majocchi S., Nelson R., et al. Selective Blockade of the Ubiquitous Checkpoint Receptor CD47 Is Enabled by Dual-Targeting Bispecific Antibodies. Mol. Ther. 2017;25:523–533. doi: 10.1016/j.ymthe.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Buatois V., Johnson Z., Salgado-Pires S., Papaioannou A., Hatterer E., Chauchet X., Richard F., Barba L., Daubeuf B., Cons L., et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol. Cancer Ther. 2018;17:1739–1751. doi: 10.1158/1535-7163.MCT-17-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hatterer E., Barba L., Noraz N., Daubeuf B., Aubry-Lachainaye J.P., von der Weid B., Richard F., Kosco-Vilbois M., Ferlin W., Shang L., et al. Co-engaging CD47 and CD19 with a bispecific antibody abrogates B-cell receptor/CD19 association leading to impaired B-cell proliferation. mAbs. 2019;11:322–334. doi: 10.1080/19420862.2018.1558698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ribeiro M.L., Normant E., Garau D.R., Miskin H.P., Sportelli P., Weiss M.S., Bosch F., Roué G. PS1310 the novel bispecific CD47-CD19 antibody TG-1801 potentiates the activity of UBLITUXIMAB-UMBRALISIB (U2) drug combination in preclinical models of B-NHL. HemaSphere. 2019;3:598. doi: 10.1097/01.HS9.0000563520.84730.09. [DOI] [Google Scholar]
- 199.Normant E., Ribeiro M.L., Reyes D., Miskin H.P., Sportelli P., Weiss M.S., Bosch F., Roue G. The novel bispecific CD47-CD19 antibody TG-1801 potentiates the activity of UBLITUXIMAB-UMBRALISIB (U2) drug combination in preclinical models of B-NHL. Hematol. Oncol. 2019 doi: 10.1002/hon.133_2630. [DOI] [Google Scholar]
- 200.Masternak K., Chauchet X., Buatois V., Salgado-Pires S., Shang L., Johnson Z., Dheilly E., Moine V., Ferlin W.G., Kosco-Vilbois M.H., et al. NI-1701, a bispecific antibody for selective neutralization of CD47 in B cell malignancies; Proceedings of the AACR Special Conference on Tumor Immunology and Immunotherapy; Boston, MA, USA. 20–23 October 2016; Abstract B37. [DOI] [Google Scholar]
- 201.Marin-Acevedo J.A., Dholaria B., Soyano A.E., Knutson K.L., Chumsri S., Lou Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018;11 doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vonderheide R.H., Glennie M.J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.He L.-Z., Testa J., Anna W., Jeffery W., Sisson C., Vitale L.A., O’Neill T., Crocker A., Widger J., Goldstein J., et al. CDX-1140, a Novel Agonist CD40 Antibody with Potent Anti-Lymphoma Activity. Blood. 2016;128:1848. doi: 10.1182/blood.V128.22.1848.1848. [DOI] [Google Scholar]
- 204.Eliopoulos A.G., Young L.S. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr. Opin. Pharmacol. 2004;4:360–367. doi: 10.1016/j.coph.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 205.Vitale L.A., Thomas L.J., He L.Z., O’Neill T., Widger J., Crocker A., Sundarapandiyan K., Storey J.R., Forsberg E.M., Weidlick J., et al. Development of CDX-1140, an agonist CD40 antibody for cancer immunotherapy. Cancer Immunol. Immunother. 2019;68:233–245. doi: 10.1007/s00262-018-2267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Thomas L.J., He L.-Z., Testa J., Wasiuk A., Weidlick J., Sisson C., Vitale L.A., O’Neill T., Forsberg E., Pilsmaker C.D., et al. Abstract 3816: Efficacy of CDX-1140, an agonist CD40 antibody, in preclinical tumor models. Am. Assoc. Cancer Res. (AACR) 2018;78:3816. doi: 10.1158/1538-7445.AM2018-3816. [DOI] [Google Scholar]
- 207.Sanborn R., Gabrail N., O’Hara M., Bhardwaj N., Gordon M., Hauke R., Bordoni R., Khalil D., Rawls T., Keler T., et al. Phase 1 study of the CD40 agonist monoclonal antibody (mAb) CDX-1140 alone and in combination with CDX-301 (rhFLT3L) in patients with advanced cancers. J. Immunother. Cancer. 2019;7:P827. [Google Scholar]
- 208.Gladue R.P., Cole S.H., Donovan C., Paradis T., Alpert R., Natoli E., Bedian V. In vivo efficacy of the CD40 agonist antibody CP-870,893 against a broad range of tumor types: Impact of tumor CD40 expression, dendritic cells, and chemotherapy. J. Clin. Oncol. 2006;24:2514. doi: 10.1200/jco.2006.24.18_suppl.2514. [DOI] [Google Scholar]
- 209.Nicolini V., Waldhauer I., Freimoser A., Corse E., Charo J., Umana P., Klein C. Abstract 2774: The triple combination of the FAP-IL2v immunocytokine with PD-L1 checkpoint inhibitory and CD40 agonistic antibodies results in long-term tumor control in the orthotopic PancO2 model. Am. Assoc. Cancer Res. (AACR) 2018;78:2774. doi: 10.1158/1538-7445.AM2018-2774. [DOI] [Google Scholar]
- 210.Urquiza M., Melo-Cardenas J., Aguillon R., Kipps T.J., Castro J.E. Intratumoral injection of Ad-ISF35 (chimeric CD154) breaks tolerance and induces lymphoma tumor regression. Hum. Gene Ther. 2015;26:14–25. doi: 10.1089/hum.2014.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Melo-Cardenas J., Urquiza M., Kipps T.J., Castro J.E. Intratumoral delivery of CD154 homolog (Ad-ISF35) induces tumor regression: Analysis of vector biodistribution, persistence and gene expression. Cancer Gene Ther. 2012;19:336–344. doi: 10.1038/cgt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Singh M., Dai Z., Khong H., Vianden C., Cantwell M., Overwijk W. Induction of potent systemic anti-melanoma immunity through intratumoral CD40 activation and checkpoint blockade. J. Immunother. Cancer. 2015;3:1. doi: 10.1186/2051-1426-3-S2-P313. [DOI] [Google Scholar]
- 213.Law C.L., Gordon K.A., Collier J., Klussman K., McEarchern J.A., Cerveny C.G., Mixan B.J., Lee W.P., Lin Z., Valdez P., et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 214.McCormick R., Lewis T., Miyamoto J., Sutherland M., Drachman J., Grewal I., Law C.-L. The humanized anti-CD40 monoclonal antibody, SGN-40, signals apoptosis in non-Hodgkin’s lymphoma by dual mechanisms. Mol. Cancer Ther. 2007;6:B63. [Google Scholar]
- 215.Lewis T.S., McCormick R.S., Stone I.J., Emmerton K., Mbow B., Miyamoto J., Drachman J.G., Grewal I.S., Law C.L. Proapoptotic signaling activity of the anti-CD40 monoclonal antibody dacetuzumab circumvents multiple oncogenic transformation events and chemosensitizes NHL cells. Leukemia. 2011;25:1007–1016. doi: 10.1038/leu.2011.21. [DOI] [PubMed] [Google Scholar]
- 216.Neff-LaFord H., Grilley-Olson J.E., Smith D.C., Curti B., Goel S., Kuzel T.M., Svetomir N., Markovic O.R., Bajor D.L., Gajewski F., et al. SEA-CD40 is a non-fucosylated anti-CD40 antibody with potent pharmacodynamic activity in preclinical models and patients with advanced solid tumors. Cancer Res. 2020;80:16. doi: 10.1158/1538-7445.AM2020-5535. [DOI] [Google Scholar]
- 217.Gardai S.J., Epp A., Linares G., Westendorf L., Sutherland M.K., Neff-LaFord H., Drachman J.G., Peng S., Law C.-L. A sugar engineered non-fucosylated anti-CD40 antibody, SEA-CD40, with enhanced immune stimulatory activity alone and in combination with immune checkpoint inhibitors. J. Clin. Oncol. 2015;33:3074. doi: 10.1200/jco.2015.33.15_suppl.3074. [DOI] [Google Scholar]
- 218.Coveler A.L., Bajor D.L., Masood A., Yilmaz E., Shields A.F., Javle M.M., Paluri R.K., Vaccaro G.M., Zalupski M., Grilley-Olson J.E., et al. Phase I study of SEA-CD40, gemcitabine, nab-paclitaxel, and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (PDAC) J. Clin. Oncol. 2020;38:TPS4671. doi: 10.1200/JCO.2020.38.15_suppl.TPS4671. [DOI] [Google Scholar]
- 219.Weng W.-K., Tong X., Luqman M., Levy R. A Fully Human Anti-CD40 Antagonistic Antibody, CHIR-12.12, Inhibit the Proliferation of Human B Cell Non-Hodgkin’s Lymphoma. Blood. 2004;104:3279. doi: 10.1182/blood.V104.11.3279.3279. [DOI] [Google Scholar]
- 220.Hsu S.J., Esposito L.A., Aukerman S.L., Kantak S., Mirza A.M. HCD122, an Antagonist Human Anti-CD40 Monoclonal Antibody, Inhibits Tumor Growth in Xenograft Models of Human Diffuse Large B-Cell Lymphoma, a Subset of Non-Hodgkins Lymphoma. Blood. 2006;108:2519. doi: 10.1182/blood.V108.11.2519.2519. [DOI] [Google Scholar]
- 221.Graf N., Li Z., Herrmann K., Aichler M., Slawska J., Walch A., Peschel C., Schwaiger M., Buck A.K., Dechow T., et al. Preclinical Evaluation of CD40-Directed Immunotherapy in B-Cell Lymphoma Using [18F]Fluorothymidine-PET. Adv. Mol. Imaging. 2015;5:17–28. doi: 10.4236/ami.2015.52002. [DOI] [Google Scholar]
- 222.Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin. Ther. Targets. 2016;20:959–973. doi: 10.1517/14728222.2016.1158812. [DOI] [PubMed] [Google Scholar]
- 223.Borst J., Hendriks J., Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 224.Han X., Vesely M.D. Stimulating T Cells Against Cancer With Agonist Immunostimulatory Monoclonal Antibodies. Int. Rev. Cell Mol. Biol. 2019;342:1–25. doi: 10.1016/bs.ircmb.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.He L.-Z., Thomas L., Weidlick J., Vitale L., O’Neill T., Prostak N., Sundarapandiyan K., Marsh H., Yellin M., Davis T.A., et al. Development of a Human Anti-CD27 Antibody with Efficacy in Lymphoma and Leukemia Models by Two Distinct Mechanisms. Blood. 2011;118:2861. doi: 10.1182/blood.V118.21.2861.2861. [DOI] [Google Scholar]
- 226.Vitale L.A., He L.Z., Thomas L.J., Widger J., Weidlick J., Crocker A., O’Neill T., Storey J., Glennie M.J., Grote D.M., et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin. Cancer Res. 2012;18:3812–3821. doi: 10.1158/1078-0432.CCR-11-3308. [DOI] [PubMed] [Google Scholar]
- 227.Ramakrishna V., Sundarapandiyan K., Zhao B., Bylesjo M., Marsh H.C., Keler T. Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab. J. Immunother. Cancer. 2015;3 doi: 10.1186/s40425-015-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Thomas L.J., He L.-Z., Wasiuk A., Gergel L.E., Boyer J.M., Round S.M., Marsh H.C., Keler T. Abstract 253: Synergistic antitumor activity of PD-1 signaling blockade and CD27 costimulation correlates with enhanced ratio of effector to regulatory T cells at the tumor site. Cancer Res. 2015;75:253. doi: 10.1158/1538-7445.AM2015-253. [DOI] [Google Scholar]
- 229.He L.-Z., Thomas L.J., Testa J., Weidlick J., Sisson C., Hammond R., Vitale L., Marsh H., Keler T. Combination therapies augment the anti-tumor activity of agonist CD27 mAb in human CD27 transgenic mouse models. J. Immunother. Cancer. 2013;1:P76. doi: 10.1186/2051-1426-1-S1-P76. [DOI] [Google Scholar]
- 230.Lim S.H., Linton K.M., Collins G.P., Dhondt J., Caddy J., Rossiter L., Vadher K., Fines K., Rogers L.E., Fernando D., et al. RIVA—A phase IIa study of rituximab and varlilumab in relapsed or refractory B-cell malignancies: Study protocol for a randomized controlled trial. Trials. 2018;19:619. doi: 10.1186/s13063-018-2996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Villasboas J.C., Reeder C.B., Tun H.W., Bartlett N.L., Sharon E., Laplant B., Adjei A.A., Kline J., Hernandez-Ilizaliturri F.J., Awan F.T., et al. The DIAL Study (Dual Immunomodulation in Aggressive Lymphoma): A Randomized Phase 2 Study of CDX-1127 (Varlilumab) in Combination with Nivolumab in Patients with Relapsed or Refractory Aggressive B-Cell Lymphomas (NCI 10089/ NCT03038672) Blood. 2019;134:1591. doi: 10.1182/blood-2019-130449. [DOI] [Google Scholar]
- 232.Fuse S., Obar J.J., Bellfy S., Leung E.K., Zhang W., Usherwood E.J. CD80 and CD86 Control Antiviral CD8+ T-Cell Function and Immune Surveillance of Murine Gammaherpesvirus 68. J. Virol. 2006;80:9159–9170. doi: 10.1128/JVI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Walker L.S.K. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J. Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mir M.A., Agrewala J.N. Signaling through CD80: An approach for treating lymphomas. Expert Opin. Ther. Targets. 2008;2:969–980. doi: 10.1517/14728222.12.8.969. [DOI] [PubMed] [Google Scholar]
- 235.Vinjamaram S., Czuczman M.S., Hernandez-Ilizaliturri F.J. The use of galiximab in non-hodgkin lymphoma. Clin. Lymphoma Myeloma. 2008;8:277–282. doi: 10.3816/CLM.2008.n.038. [DOI] [PubMed] [Google Scholar]
- 236.Etxeberria I., Glez-Vaz J., Teijeira Á., Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020;4:e000733. doi: 10.1136/esmoopen-2020-000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fisher T.S., Kamperschroer C., Oliphant T., Love V.A., Lira P.D., Doyonnas R., Bergqvist S., Baxi S.M., Rohner A., Shen A.C., et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol. Immunother. 2012;61:1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Houot R., Goldstein M.J., Kohrt H.E., Myklebust J.H., Alizadeh A.A., Lin J.T., Irish J.M., Torchia J.A., Kolstad A., Chen L., et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wilcox R.A., Tamada K., Strome S.E., Chen L. Signaling Through NK Cell-Associated CD137 Promotes Both Helper Function for CD8 + Cytolytic T Cells and Responsiveness to IL-2 But Not Cytolytic Activity. J. Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 240.Smith S.E., Hoelzinger D.B., Dominguez A.L., Van Snick J., Lustgarten J. Signals through 4-1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol. Immunother. 2011;60:1775–1787. doi: 10.1007/s00262-011-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang P., Gao F., Wang Q., Wang X., Zhu F., Ma C., Sun W., Zhang L. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand. J. Immunol. 2007;66:435–440. doi: 10.1111/j.1365-3083.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 242.Choi B.K., Kim Y.H., Kim C.H., Kim M.S., Kim K.H., Oh H.S., Lee M.J., Lee D.K., Vinay D.S., Kwon B.S. Peripheral 4-1BB Signaling Negatively Regulates NK Cell Development through IFN-γ. J. Immunol. 2010;185:1401–1411. doi: 10.4049/jimmunol.1000850. [DOI] [PubMed] [Google Scholar]
- 243.Jure-Kunkel M.N., Calarota S., Girit E., Abraham R., Balimane P., Price K., Weiner D., Hefta L. Functional characterization of fully human anti-CD137 antibodies. Am. Assoc. Cancer Res. (AACR) Annu. Meet. Cancer Res. 2006;66:8. [Google Scholar]
- 244.Chin S.M., Kimberlin C.R., Roe-Zurz Z., Zhang P., Xu A., Liao-Chan S., Sen D., Nager A.R., Oakdale N.S., Brown C., et al. Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-07136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sanmamed M.F., Rodriguez I., Schalper K.A., Oñate C., Azpilikueta A., Rodriguez-Ruiz M.E., Morales-Kastresana A., Labiano S., Pérez-Gracia J.L., Martín-Algarra S., et al. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rγnull immunodeficient mice. Cancer Res. 2015;75:3466–3478. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 246.Li Y., Tan S., Zhang C., Chai Y., He M., Zhang C.W.H., Wang Q., Tong Z., Liu K., Lei Y., et al. Limited Cross-Linking of 4-1BB by 4-1BB Ligand and the Agonist Monoclonal Antibody Utomilumab. Cell Rep. 2018;25:909–920. doi: 10.1016/j.celrep.2018.09.073. [DOI] [PubMed] [Google Scholar]
- 247.Segal N.H., He A.R., Doi T., Levy R., Bhatia S., Pishvaian M.J., Cesari R., Chen Y., Davis C.B., Huang B., et al. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin. Cancer Res. 2018;24:1816–1823. doi: 10.1158/1078-0432.CCR-17-1922. [DOI] [PubMed] [Google Scholar]
- 248.Tolcher A.W., Karim R., Rosas C., Liu G., Zhu Y., Liu L., Du F.F., Luo F.R., Luo P. A Phase 1, First-In-Human, dose-escalation study of ADG106, a fully human anti-CD137 agonistic antibody, in subjects with advanced or metastatic solid tumors and/or relapsed/refractory non-Hodgkin lymphoma. Am. Assoc. Cancer Res. (AACR) Annu. Meet. Cancer Res. 2019;18:12. doi: 10.1158/1535-7163.TARG-19-A082. [DOI] [Google Scholar]
- 249.Liu G., Du F.F., She X., Zhu Y., Tolcher A.W., Luo P. A safe and potent agonist ADG106 targeting a unique epitope of CD137 with novel mechanism of actions. Am. Assoc. Cancer Res. (AACR) Annu. Meet. Cancer Res. 2020;80:16. doi: 10.1158/1538-7445.AM2020-4538. [DOI] [Google Scholar]
- 250.Mayes P., Tacken P., Wang P., Van Loo P.-F., Condamine T., Van Der Maaden H., Rovers E., Engels S., Fransen F., Kulkarni A., et al. A bispecific Fc-silenced IgG1 antibody (MCLA-145) requires PD-L1 binding to activate CD137. Am. Assoc. Cancer Res. (AACR) Annu. Meet. Cancer Res. 2019;79:13. doi: 10.1158/1538-7445.AM2019-539. [DOI] [Google Scholar]
- 251.Plyte S., Geuijen C.A., de Kruif J., Van Loo P.-F., Tacken P., Zondag-van de Zande V., Klooster R., Van Der Maaden H., Rovers E., Engels S., et al. Identification and characterization of MCLA-145 (CD137 x PD-L1): A bispecific antibody that requires PD-L1 binding to activate CD137. J. Immunother. Cancer Natl. Harb. 2019;7 [Google Scholar]
- 252.O’Neill R.E., Du W., Mohammadpour H., Alqassim E., Qiu J., Chen G., McCarthy P.L., Lee K.P., Cao X. T Cell–Derived CD70 Delivers an Immune Checkpoint Function in Inflammatory T Cell Responses. J. Immunol. 2017;199:3700–3710. doi: 10.4049/jimmunol.1700380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Arens R., Tesselaar K., Baars P.A., Van Schijndel G.M.W., Hendriks J., Pals S.T., Krimpenfort P., Borst J., Van Oers M.H.J., Van Lier R.A.W. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNγ-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/S1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 254.Yang C.-Y., Wang L., Pincus L., McCormick F., Gill R., Ai W. Abstract 4589: Preclinical investigation of SGN-CD70A antibody-drug conjugate in T cell lymphomas. Cancer Res. 2017;77:4589. doi: 10.1158/1538-7445.AM2017-4589. [DOI] [Google Scholar]
- 255.Yang Z.Z., Novak A.J., Ziesmer S.C., Witzig T.E., Ansell S.M. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25-T cells. Blood. 2007;110:2537–2544. doi: 10.1182/blood-2007-03-082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sandall S., Anderson M., Jonas M., Nesterova A., Miyamoto J., Stone I.J., Zeng W., Law C.-L., Lewis T.S. Abstract 2647: SGN-CD70A, a novel and highly potent anti-CD70 ADC, induces double-strand DNA breaks and is active in models of MDR+ renal cell carcinoma (RCC) and non-Hodgkin lymphoma (NHL) Cancer Res. 2014;74:2647. doi: 10.1158/1538-7445.AM2014-2647. [DOI] [Google Scholar]
- 257.Sandall S.L., McCormick R., Miyamoto J., Biechele T., Law C.-L., Lewis T.S. Abstract 946: SGN-CD70A, a pyrrolobenzodiazepine (PBD) dimer linked ADC, mediates DNA damage pathway activation and G2 cell cycle arrest leading to cell death. Cancer Res. 2015;75:946. doi: 10.1158/1538-7445.AM2015-946. [DOI] [Google Scholar]
- 258.Huard B., Mastrangeli R., Prigent P., Bruniquel D., Donini S., El-Tayar N., Maigret B., Dréano M., Triebel F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl. Acad. Sci. USA. 1997;94:5744–5749. doi: 10.1073/pnas.94.11.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gagliani N., Magnani C.F., Huber S., Gianolini M.E., Pala M., Licona-Limon P., Guo B., Herbert D.R., Bulfone A., Trentini F., et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 260.Lino A.C., Dang V.D., Lampropoulou V., Welle A., Joedicke J., Pohar J., Simon Q., Thalmensi J., Baures A., Flühler V., et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity. 2018;49:120–133.e9. doi: 10.1016/j.immuni.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hannier S., Tournier M., Bismuth G., Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J. Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 262.Maçon-Lemaître L., Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology. 2005;115:170–178. doi: 10.1111/j.1365-2567.2005.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Blackburn S.D., Crawford A., Shin H., Polley A., Freeman G.J., Wherry E.J. Tissue-Specific Differences in PD-1 and PD-L1 Expression during Chronic Viral Infection: Implications for CD8 T-Cell Exhaustion. J. Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yang Z.Z., Kim H.J., Villasboas J.C., Chen Y.P., Price-Troska T.P., Jalali S., Wilson M., Novak A.J., Ansell S.M. Expression of LAG-3 defines exhaustion of intratumoral PD-1+ T cells and correlates with poor outcome in follicular lymphoma. Oncotarget. 2017;8:61425–61439. doi: 10.18632/oncotarget.18251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Keane C., Law S.C., Gould C., Birch S., Sabdia M.B., de Long L.M., Thillaiyampalam G., Abro E., Tobin J.W., Tan X., et al. LAG3: A novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020;4:1367–1377. doi: 10.1182/bloodadvances.2019001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Aoki T., Chong L.C., Takata K., Milne K., Hav M., Colombo A., Chavez E.A., Nissen M., Wang X., Miyata-Takata T., et al. Single-cell transcriptome analysis reveals disease-defining t-cell subsets in the tumor microenvironment of classic hodgkin lymphoma. Cancer Discov. 2020;10:406–421. doi: 10.1158/2159-8290.CD-19-0680. [DOI] [PubMed] [Google Scholar]
- 267.Brignone C., Escudier B., Grygar C., Marcu M., Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin. Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 268.Uboha N.V., Milhem M.M., Kovacs C., Amin A., Magley A., Purkayastha D.D., Piha-Paul S.A. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019;37:2553. doi: 10.1200/JCO.2019.37.15_suppl.2553. [DOI] [Google Scholar]
- 269.Savitsky D., Ward R., Riordan C., Mundt C., Jennings S., Connolly J., Findeis M., Sanicola M., Underwood D., Nastri H., et al. Abstract 3819: INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. Cancer Res. 2018;78:13. doi: 10.1158/1538-7445.AM2018-3819. [DOI] [Google Scholar]
- 270.Burova E., Hermann A., Dai J., Ullman E., Halasz G., Potocky T., Hong S., Liu M., Allbritton O., Woodruff A., et al. Preclinical development of the anti-LAG-3 antibody REGN3767: Characterization and activity in combination with the anti-PD-1 antibody cemiplimab in human PD-1xLAG-3–knockin mice. Mol. Cancer Ther. 2019;18:2051–2062. doi: 10.1158/1535-7163.MCT-18-1376. [DOI] [PubMed] [Google Scholar]
- 271.Kelly M.P., Tavare R., Giurleo J.T., Makonnen S., Hickey C., Danton M.A., Arnold T.C., Ma D., Dai J., Pei J., et al. Abstract 3033: Immuno-PET detection of LAG-3 expressing intratumoral lymphocytes using the zirconium-89 radiolabeled fully human anti-LAG-3 antibody REGN3767. Cancer Res. 2018;78 doi: 10.1158/1538-7445.AM2018-3033. [DOI] [Google Scholar]
- 272.Anderson A.C. Tim-3, a negative regulator of anti-tumor immunity. Curr. Opin. Immunol. 2012;24:213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 273.Anderson A.C. Tim-3: An emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2014;2:393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 274.Xiao T., Zhang L., Chen L., Liu G., Feng Z., Gao L. Tim-3 expression is increased on peripheral T cells from diffuse large B cell lymphoma. Tumor Biol. 2014;35:7951–7956. doi: 10.1007/s13277-014-2080-0. [DOI] [PubMed] [Google Scholar]
- 275.Zhang L., Du H., Xiao T.W., Liu J.Z., Liu G.Z., Wang J.X., Li G.Y., Wang L.X. Prognostic value of PD-1 and TIM-3 on CD3+ T cells from diffuse large B-cell lymphoma. Biomed. Pharmacother. 2015;75:83–87. doi: 10.1016/j.biopha.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 276.Chen B.J., Dashnamoorthy R., Galera P., Makarenko V., Chang H., Ghosh S., Evens A.M. The immune checkpoint molecules PD-1, PD-L1, TIM-3 and LAG-3 in diffuse large B-cell lymphoma. Oncotarget. 2019;10:2030–2040. doi: 10.18632/oncotarget.26771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Acharya N., Sabatos-Peyton C., Anderson A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer. 2020;8:e000911. doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Linch S.N., McNamara M.J., Redmond W.L. OX40 agonists and combination immunotherapy: Putting the pedal to the metal. Front. Oncol. 2015;5:1–14. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Fu Y., Lin Q., Zhang Z., Zhang L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sin. B. 2020;10:414–433. doi: 10.1016/j.apsb.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gramaglia I., Jember A., Pippig S.D., Weinberg A.D., Killeen N., Croft M. The OX40 Costimulatory Receptor Determines the Development of CD4 Memory by Regulating Primary Clonal Expansion. J. Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 281.Bansal-Pakala P., Halteman B.S., Cheng M.H.-Y., Croft M. Costimulation of CD8 T Cell Responses by OX40. J. Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 282.Valzasina B., Guiducci C., Dislich H., Killeen N., Weinberg A.D., Colombo M.P. Triggering of OX40 (CD134) on CD4 CD25 T cells blocks their inhibitory activity: A novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 283.Piconese S., Valzasina B., Colombo M.P. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lambert S.L., Heeke D., Patton K., Lin R., Shambaugh C., Aslam S., Yu L., Zuo F., Pierce A., Paliard X. Immunomodulatory effects of OX40 agonists in a defined antigen challenge in cynomolgus macaques. J. Clin. Oncol. 2015;33:3086. doi: 10.1200/jco.2015.33.15_suppl.3086. [DOI] [Google Scholar]
- 285.Long H., White A.L., Jiang B.B., Feldman R., Pappas D.C., Al-Shamkhani A., Wang C., Lin J.C. Targeting OX40 by monoclonal antibody PF-04518600 to enhance T cell functions and inhibit tumor growth. J. Clin. Oncol. 2016;34:15. doi: 10.1200/JCO.2016.34.15_suppl.e14518. [DOI] [Google Scholar]
- 286.Long H., White A.L., Wei J., Jiang B.B., Feldman R., Pappas D.C., Al-Shamkhani A., Lin J.C. Triggering of OX40 on T cells by a novel monoclonal antibody elicits robust antitumor immunity. Cancer Res. 2017;77 doi: 10.1158/1538-7445.AM2017-4598. [DOI] [Google Scholar]
- 287.Huang C., Feng Y., Barnhart B., Quigley M., Huber J., Bello A., Marathe P., Aanur P., Reilly T., Yang Z. Selection of first-in-human starting dose of anti-OX40 agonist monoclonal antibody BMS-986178 using a pharmacokinetic/pharmacodynamic-based approach. J. Immunother. Cancer. 2017;5 [Google Scholar]
- 288.Gaudreau M.-C., Milburn C., Gao C., Pritsker A., Fereshteh M., Yang Z., Barnhart B., Korman A.J., Quigley M. Examining the dynamic regulation of OX40 following receptor agonism and T-cell activation: Implications for antibody-mediated enhancement of T-cell function. Cancer Res. 2018;78:13. doi: 10.1158/1538-7445.AM2018-2782. [DOI] [Google Scholar]
- 289.Solomon B.L., Garrido-Laguna I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018;67:1659–1667. doi: 10.1007/s00262-018-2246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Harjunpää H., Guillerey C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020;200:108–119. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Josefsson S.E., Beiske K., Blaker Y.N., Kimby E., Smeland E.B., Levy R., Kolstad A., Huse K., June H., Institutet K. TIGIT and PD-1 Mark Intratumoral T Cells with Reduced Effector Function in B-cell Non-Hodgkin Lymphoma. Cancer Immunol. Res. 2019;7:355–362. doi: 10.1158/2326-6066.CIR-18-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yang Z.-Z., Kim H.J., Wu H., Jalali S., Tang X., Krull J.E., Ding W., Novak A.J., Ansell S.M. TIGIT expression is associated with T-cell suppression and exhaustion and predicts clinical outcome and anti-PD-1 response in follicular lymphoma. Clin. Cancer Res. 2020;26:5217–5231. doi: 10.1158/1078-0432.CCR-20-0558. [DOI] [PubMed] [Google Scholar]
- 293.Smith A., Zeng W., Siegall W., Grogan B., Haass J., Blackmarr A., Thurman R., Peterson S., Gardai S. SEA-TGT is a nonfucosylated antibody with distinct and amplified effector function activity that leverages the dependencies of anti-TIGIT anti-tumor activity upon Fc(gamma)R engagement. J. Immunother. Cancer. 2020;8:A151. doi: 10.1136/jitc-2020-SITC2020.0250. [DOI] [Google Scholar]
- 294.Josefsson S.E., Huse K., Kolstad A., Beiske K., Pende D., Inderberg E.M., Smeland E.B., Levy R., Irish J.M., Myklebust J.H., et al. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T-cell receptor signaling. Clin. Cancer Res. 2018;24:870–881. doi: 10.1158/1078-0432.CCR-17-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bellone M., Elia A.R. Constitutive and acquired mechanisms of resistance to immune checkpoint blockade in human cancer. Cytokine Growth Factor Rev. 2017;36:17–24. doi: 10.1016/j.cytogfr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 296.Chowell D., Morris L.G.T., Grigg C.M., Weber J.K., Samstein R.M., Makarov V., Kuo F., Kendall S.M., Requena D., Riaz N., et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pitt J.M., Vétizou M., Daillère R., Roberti M.P., Yamazaki T., Routy B., Lepage P., Boneca I.G., Chamaillard M., Kroemer G., et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 298.Sugiura D., Maruhashi T., Okazaki I.M., Shimizu K., Maeda T.K., Takemoto T., Okazaki T. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–566. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 299.Shergold A.L., Millar R., Nibbs R.J.B. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol. Res. 2019;145:104258. doi: 10.1016/j.phrs.2019.104258. [DOI] [PubMed] [Google Scholar]
- 300.Zheng Z., Sun R., Zhao H.J., Fu D., Zhong H.J., Weng X.Q., Qu B., Zhao Y., Wang L., Zhao W.L. MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+T cells. Mol. Cancer. 2019;18:54. doi: 10.1186/s12943-019-0977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Deng S., Hu Q., Zhang H., Yang F., Peng C., Huang C. HDAC3Inhibition upregulates PD-L1 expression in B-Cell lymphomas and augments the efficacy of Anti-PD-L1 therapy. Mol. Cancer Ther. 2019;18:900–908. doi: 10.1158/1535-7163.MCT-18-1068. [DOI] [PubMed] [Google Scholar]
- 302.Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R., Albright A., Cheng J.D., Kang S.P., Shankaran V., et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 304.Scott D.W., Gascoyne R.D. The tumour microenvironment in B cell lymphomas. Nat. Rev. Cancer. 2014;14:517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 305.Chong L.C., Twa D.D.W., Mottok A., Ben-Neriah S., Woolcock B.W., Zhao Y., Savage K.J., Marra M.A., Scott D.W., Gascoyne R.D., et al. Comprehensive characterization of programmed death ligand structural rearrangements in B-cell non-Hodgkin lymphomas. Blood. 2016;128:1206–1213. doi: 10.1182/blood-2015-11-683003. [DOI] [PubMed] [Google Scholar]
- 306.Challa-Malladi M., Lieu Y.K., Califano O., Holmes A.B., Bhagat G., Murty V.V., Dominguez-Sola D., Pasqualucci L., Dalla-Favera R. Combined Genetic Inactivation of β2-Microglobulin and CD58 Reveals Frequent Escape from Immune Recognition in Diffuse Large B Cell Lymphoma. Cancer Cell. 2011;20:728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Spina V., Bruscaggin A., Cuccaro A., Martini M., Di Trani M., Forestieri G., Manzoni M., Condoluci A., Arribas A., Terzi-Di-Bergamo L., et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131:2413–2425. doi: 10.1182/blood-2017-11-812073. [DOI] [PubMed] [Google Scholar]
- 308.Kato S., Goodman A., Walavalkar V., Barkauskas D.A., Sharabi A., Kurzrock R. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin. Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Champiat S., Dercle L., Ammari S., Massard C., Hollebecque A., Postel-Vinay S., Chaput N., Eggermont A., Marabelle A., Soria J.C., et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 310.Nguyen L.T., Ohashi P.S. Clinical blockade of PD1 and LAG3-potential mechanisms of action. Nat. Rev. Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 311.Ghiotto M., Gauthier L., Serriari N., Pastor S., Truneh A., Nunès J.A., Olive D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 2010;22:651–660. doi: 10.1093/intimm/dxq049. [DOI] [PMC free article] [PubMed] [Google Scholar]