Abstract
Patients supported with extracorporeal membrane oxygenation (ECMO) often receive renal replacement therapy (RRT). We conducted this systematic review and meta-analysis (between January 2000 and September 2020) to assess outcomes in patients who received RRT on ECMO. Random-effects meta-analyses were performed using R 3.6.1 and certainty of evidence was rated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. The primary outcome was pooled mortality. The duration of ECMO support and ICU/hospital lengths of stay were also investigated. Meta-regression analyses identified factors associated with mortality. A total of 5896 adult patients (from 24 observational studies and 1 randomised controlled trial) were included in this review. Overall pooled mortality due to concurrent use of RRT while on ECMO from observational studies was 63.0% (95% CI: 56.0–69.6%). In patients receiving RRT, mortality decreased by 20% in the last five years; the mean duration of ECMO support and ICU and hospital lengths of stay were 9.33 days (95% CI: 7.74–10.92), 15.76 days (95% CI: 12.83–18.69) and 28.47 days (95% CI: 22.13–34.81), respectively, with an 81% increased risk of death (RR: 1.81, 95% CI: 1.56–2.08, p < 0.001). RRT on ECMO was associated with higher mortality rates and a longer ICU/hospital stay compared to those without RRT. Future research should focus on minimizing renal dysfunction in ECMO patients and define the optimal timing of RRT initiation.
Keywords: extracorporeal membrane oxygenation, renal replacement therapy, acute kidney injury, mortality
1. Introduction
Almost 50% of patients on extracorporeal membrane oxygenation (ECMO) require renal replacement therapy (RRT) [1]. The indications for initiating RRT while on ECMO are similar to other critically ill patients and can be multifactorial [1,2]. Acute kidney injury (AKI) is a common indication in almost 80% of patients receiving extracorporeal membrane oxygenation (ECMO) [3]. The aetiology of AKI in the ECMO patient population can be attributed to pre-ECMO and ECMO factors such as hypoxaemia and haemodynamic perturbations around the time of initiation, low cardiac output state, severe right heart dysfunction, underlying multisystem disorders, systemic inflammation, hormonal imbalances, exposure to nephrotoxins, and ischaemic-reperfusion injury [2,3,4,5,6,7]. The mortality of critically ill patients who develop AKI is estimated to be 40–70% [8,9]. On the other hand, the reported incidence of mortality due to AKI associated with ECMO is approximately 80% [6,10,11,12].
Patients needing ECMO have fluid and electrolyte imbalances which can be regulated better using RRT. Fluid balance on day 3 of ECMO has been found to be an independent marker of mortality in some studies and the use of RRT to offset fluid overload has been shown to improve clinical outcomes [13]. RRT also helps clear dialyzable toxins where ECMO would be needed for hemodynamic stability [14,15]. Nonetheless, previous reviews have shown that the use of RRT on ECMO is associated with increased mortality in both adult and paediatric patients [16,17]. We performed a systematic review and meta-analysis to examine how the use of RRT may affect outcomes in adult patients receiving ECMO.
2. Materials and Methods
A systematic search was conducted after registering on the International Prospective Register of Systematic Reviews (PROSPERO CRD42020188331). The review of literature followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement in three major international medical bibliographical databases (PubMed, EMBASE, and Cochrane) from 1st January 2000 to 30th September 2020. The search strings included the Boolean terms “AND”, “OR” and “NOT” with the following keywords and their respective variants or derivatives in any relevant combination: renal replacement therapy, haemofiltration, haemodiafiltration, continuous renal replacement therapy, continuous venovenous haemodialysis, continuous venovenous haemodiafiltration, continuous venovenous haemofiltration, continuous arteriovenous haemodialysis, and extracorporeal membrane oxygenation. We have included randomised controlled trials, case-control studies, cohort studies and case series (sample size of minimum 10 patients). We also included studies where patients were on RRT prior to initiation of ECMO. Studies related to animals, paediatric patients (<18 years), pregnant patients, pharmacokinetics, technical aspects of ECMO and RRT, studies involving mechanical circulatory support other than ECMO, those published from the same centres and covering the same time period as well as those published in non-English languages were excluded. Publications reporting on Extracorporeal Life Support Organisation (ELSO) registry data were also excluded to avoid duplication of data. Additionally, we considered national databases rather than single centre data where applicable to avoid overlapping.
A hand search of all relevant studies and their citation lists was performed to identify articles for inclusion. The eligibility of the studies was independently assessed by two reviewers (SM and RRL) and any conflicts were resolved by consensus or by a third reviewer (KR). Included studies were reviewed using the appropriate Joanna Briggs Institute (JBI) checklists.
The following data were extracted for each trial: study design (duration of study, type of study, country of origin of study centre, year of publication), patient demographics (sample size, number of patients on RRT, number of male/female patients, mean age), pre-RRT characteristics (indications for ECMO, cannulation strategy (veno-venous [VV] or veno-arterial [VA] ECMO), pre-RRT serum lactate and creatinine), number of patients with other multi-organ failures (MOFs) apart from the primary organ failure for which ECMO was initiated (including liver failure, bowel ischaemia, acute stroke, intracranial haemorrhage and disseminated intravascular coagulation), number of patients with new-onset infections (sepsis or bacteraemia) after ECMO initiation and relevant clinical outcomes (mortality, hospital and intensive care unit (ICU) length of stay, and ECMO duration).
Statistical Analysis
Our primary outcome was overall mortality due to concurrent use of RRT while on ECMO. Overall mortality for this review was defined as in-hospital mortality, ICU mortality, 30-day mortality or 90-day mortality. Secondary outcomes included the mean duration of ECMO support and ICU and hospital lengths of stay in patients with combined therapies. Additionally, we measured the pooled incidence of other MOFs and new-onset infections in patients who were on both ECMO and RRT therapies compared to those who were treated with ECMO alone.
As a high degree of inter-study heterogeneity was expected, random effects meta-analyses (DerSimonian and Laird) [18,19] were conducted on R 3.6.1 using the meta (v4.12-0) and dmetar (v0.0.9000) packages, and confidence intervals (CI) were computed using the Clopper–Pearson method [20]. Mortality outcomes are presented as pooled proportions and 95% confidence intervals (CI), and dichotomous outcomes are presented as odds ratios (ORs) and 95% CI. Planned subgroup analyses were conducted with continuity correction to allow the inclusion of studies with zero events and included the other reported mortalities (in-hospital, ICU, 30-day or 90-day), the geographical location (Asia, Europe, America, and Australia), the presence of renal replacement therapy (RRT and no RRT), the duration ECMO (more and less than 7 days) and the year of publication (before and after 2016). Summary-level meta-regression was conducted if a minimum of 6 data points could be collected to explore potential sources of heterogeneity or prognostically-relevant study-level covariates [18]. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) guidance to assess between-study heterogeneity and rated the certainty of evidence using the GRADE approach [21,22,23]. We used the ‘GRADEpro’ app to rate the evidence [24] and presented in GRADE evidence profiles and summaries of findings tables using standardised terms [25,26].
Publication bias was assessed using Egger’s test. Leave-one-out sensitivity analysis (LOO) was performed for all analyses by omitting 1 study at a time to identify outliers or influential studies. Means and standard deviations of continuous variables were pooled using the methods proposed by Wan et al. [27].
3. Results
Our preliminary search identified 2343 articles and 404 duplicates were removed. Of the 1939 articles screened, 1769 studies were excluded after examining the abstract. We obtained 155 citations in full text and 130 of these studies did not meet our inclusion criteria (Figure S1: PRISMA diagram). In total, 25 studies detailing 5896 adult patients that reported on the use of RRT and ECMO were included (Table 1) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. Twenty-four studies were observational in nature [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51], while one study was a randomised controlled trial (RCT) [52]. For our quantitative analysis, we included 24 observational studies (5855 patients), while the findings of the RCT were reported separately. Overall, 3223 patients received combined therapy with ECMO and RRT (both observational studies and RCT). The quality assessment of the studies was performed using the JBI checklists (Table S1), which revealed that the studies were of the highest quality. Continuous renal replacement therapy (CRRT) was the most commonly used modality in these patients.
Table 1.
Summary of all studies.
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Author/Year | Study Type, Sample Size | Country | ECMO/RRT | Mortality | ICU/Hospital LOS | ECMO Duration |
| Allyn, 2018 [28] | Cohort, 145 | France | + | + | −/− | − |
| Antonucci, 2016 [29] | Cohort, 135 | Belgium | + | + | +/− | + |
| Baek, 2016 [30] | Cohort, 12 | Korea | + | + | −/− | + |
| Chen, 2019 [31] | Cohort, 3251 | Taiwan | + | + | +/+ | + |
| Combes, 2008 [32] | Cohort, 81 | France | + | + | −/− | − |
| Dado, 2020 [33] | Cohort, 90 | USA | + | + | −/− | − |
| Deatrick, 2020 [34] | Cohort, 187 | USA | + | + | −/+ | + |
| Devasagayaraj, 2018 [35] | Cohort, 54 | USA | + | + | −/− | + |
| Elsharkawy, 2010 [36] | Cohort, 233 | USA | + | + | −/− | − |
| Fong, 2020 [37] | Cohort, 123 | HK SAR | + | + | −/− | − |
| Haneya, 2015 [38] | Cohort, 262 | Germany | + | + | −/− | − |
| He, 2018 [39] | Cohort, 32 | China | + | + | +/+ | + |
| Kielstein, 2013 [40] | Cohort, 200 | Germany | + | + | −/− | − |
| Lee SY, 2020 [41] | Cohort, 91 | Korea | + | + | −/− | − |
| Luo, 2009 [42] | Cohort, 45 | China | + | + | −/− | − |
| McCanny, 2019 [43] | Cohort, 24 | Ireland | + | + | −/− | + |
| Paek, 2018 [44] | Cohort, 296 | Korea | + | + | −/+ | − |
| Panholzer, 2017 [45]. | Cohort, 46 | Germany | + | + | −/− | − |
| Schmidt, 2014 [46] | Cohort, 172 | Australia | + | + | −/− | − |
| Thajudeen, 2015 [47] | Cohort, 40 | USA | + | + | −/− | − |
| Unosawa, 2013 [48] | Cohort, 47 | Japan | + | + | −/− | − |
| Xie, 2020 [49] | Cohort, 212 | China | + | + | −/− | − |
| Yan, 2010 [50] | Cohort, 67 | China | + | + | −/− | − |
| Yap, 2003 [51] | Case-control, 10 | Taiwan | + | + | −/− | − |
| Li, 2019 [52] | RCT, 41 | China | + | + | +/+ | + |
ECMO: extracorporeal membrane oxygenation, RRT: renal replacement therapy, ICU: intensive care unit, LOS: length of stay.
3.1. Demographic Analysis
3.1.1. Observational Studies
The pooled mean age of patients (Figure S2) receiving RRT on ECMO was 50.9 years (95% CI: 46.9–54.8). The proportion of male patients across the studies (Figure S3) was 68.2% (95% CI: 64.4–71.9%). After removing the two outliers detected by LOO analysis [29,31], the proportion of males was 67.0% (95% CI: 63.0–70.8%). Pooled proportion of concurrent use of VA-ECMO with RRT from 22 studies (Figure S4) was 71.8% (95% CI: 49.8–89.6%) with a significant publication bias (Pegger = 0.007). Pooled mean serum lactate at the initiation of combined therapies from six studies was 3.79 mmol/L (95% CI: 2.41–5.17). After the removal of one outlier [43], the pooled serum lactate level was estimated to be 4.22 mmol/L (95% CI: 3.29–5.15). The pooled mean serum creatinine at the initiation of RRT from four studies was calculated to be 2.12 mg/dL (95% CI: 1.75–2.49). After removing the only outlier detected by LOO [29], the pooled serum creatinine was calculated to be 2.25 mg/dL (95% CI: 1.84–2.66).
3.1.2. RCT
The mean age of the patients receiving both the therapies in the study by Li et al. [52] was 61.2 ± 8.3 years and all of them were on VA-ECMO. Around 72.2% were males who required combined therapies. Initial serum lactate and creatinine levels were 12.5 ± 8.3 mmol/L and 1.1 ± 0.4 mg/dL, respectively.
3.2. Primary Outcome
3.2.1. Observational Studies
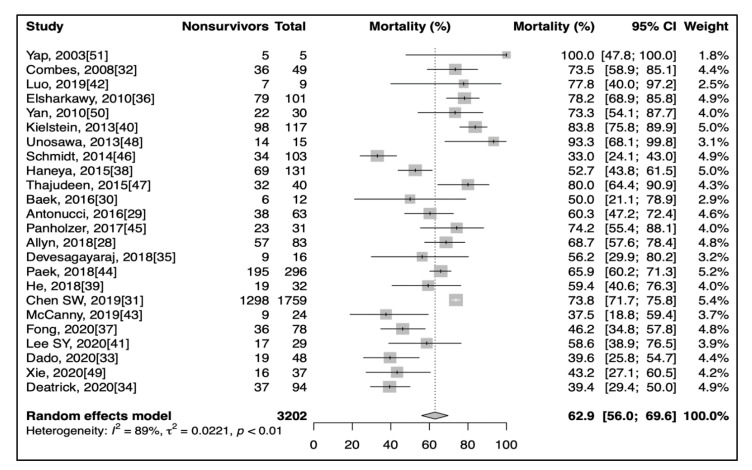
The pooled overall mortality (Figure 1) in patients due to the use of RRT on ECMO (24 studies) was 62.9% (95% CI: 56.0–69.6%). Subgroup analysis found no significant differences (p = 0.57) between in-hospital mortality (14 studies, 60.1%, 95% CI: 50.1–69.8%) [31,33,34,35,36,37,38,39,42,43,45,48,49,50], ICU mortality (3 studies, 67.3%, 95% CI: 60.1–74.1%) [28,29,32], 30-day mortality (3 studies, 68.4%, 95% CI: 58.0–77.9%) [41,44,47], and 90-day mortality (3 studies, 57.0%, 95% CI: 17.6–92.0%) [30,40,46]. Only one study mentioned overall mortality [51].
Figure 1.
Forest plot showing pooled mortality.
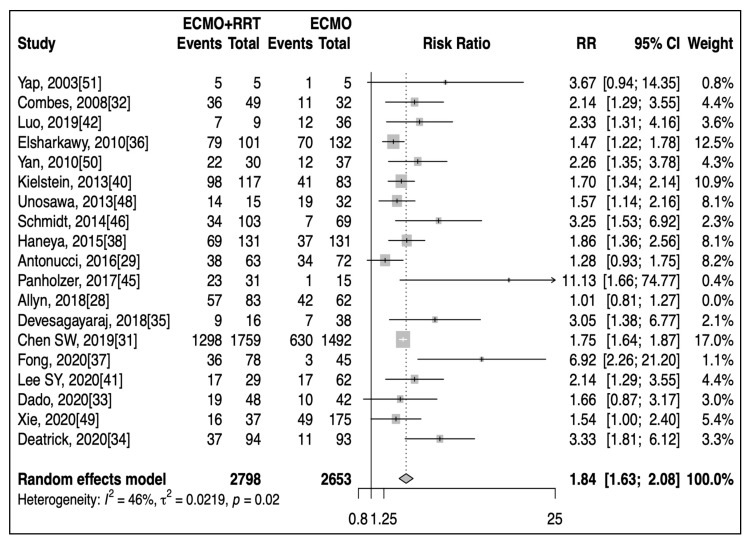
Subgroup analysis found that mortality was significantly different when considering the publication years (before and after 2016), the presence of RRT, the duration of ECMO, and geographical region. The pooled mortality (Figure S5) prior to 2016 (10 studies) was 74.1% (95% CI: 60.0–86.2%), while pooled mortality after 2016 (14 studies) was 56.1% (95% CI: 47.7–64.5%, p = 0.03). The presence of RRT was associated with a significant increase in mortality (19 studies, Relative Risk (RR): 1.81, 95% CI: 1.56–2.08, p < 0.001, Figure 2) when compared to patients on ECMO alone. After removing the only outlier detected by LOO, [28] this increased risk of mortality remained significant (RR: 1.84, 95% CI: 1.63–2.08, p < 0.001), with significant publication bias (Pegger = 0.02). Additionally, the pooled mortality among patients (Figure S6) with ECMO durations of less than 7 days (3 studies) was 66.5% (95% CI: 54.5–77.5%), compared to 41.5% (95% CI: 33.3–49.9%, p < 0.001) for those with ECMO durations of more than 7 days (4 studies). Pooled mortality reported by studies from Asia (11 studies, 65.7%, 95% CI: 54.8–74.0%), Europe (7 studies, 65.7%, 95% CI: 53.9–76.6%), and America (5 studies, 59.4%, 95% CI: 38.6–78.6%) were relatively similar, and higher than those reported from Australia (1 study, 33.0%, 95% CI: 24.2–42.4%).
Figure 2.
Forest plot showing increased risk of mortality in patients receiving combined therapies (ECMO: extracorporeal membrane oxygenation, RRT: renal replacement therapy).
3.2.2. RCT
Li et al. [52] reported that the patients who received both ECMO and RRT had 61.9% mortality at the end of 30 days.
3.3. Secondary Outcomes
3.3.1. Observational Studies
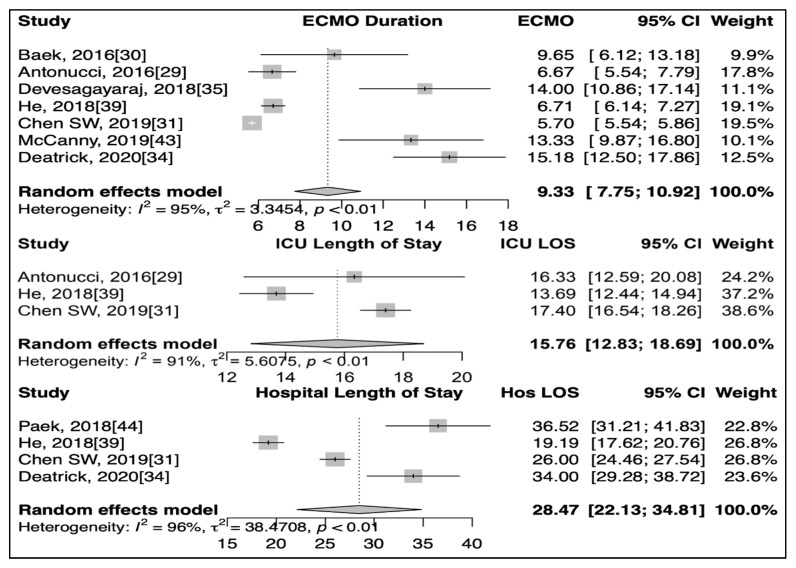
The mean ECMO duration (7 studies), ICU length of stay (3 studies) and hospital length of stay (4 studies) in patients receiving combined therapies were 9.33 days (95% CI: 7.74–10.92), 15.76 days (95% CI: 12.83–18.69) and 28.47 days (95% CI: 22.13–34.81), respectively (Figure 3).
Figure 3.
Forest plot showing mean ECMO (extra-corporeal membrane oxygenation) duration, ICU (Intensive care unit) and Hospital Length of Stay (LOS) of patients who received combined therapies.
The patients on combined therapies had a higher incidence of both other MOFs {3 studies, 31.9% (95% CI: 17.8–47.7%)} and new-onset infections {2 studies, 17.3% (95% CI: 15.5–19.2%)} in contrast to 15.5% (other MOFs: 95% CI: 10.2–21.5%) and 11.9% (New-onset infections: 95% CI: 5.87–19.5%) in patients on ECMO alone. Patients who received concurrent RRT while on ECMO had a significantly higher incidence of new-onset infections (RR: 1.65, 95% CI: 1.39–1.97, p < 0.001); this was not observed for other MOFs (RR: 2.22, 95% CI: 0.93–5.22, p = 0.072) in patients who had concurrent use of RRT while on ECMO.
3.3.2. RCT
The median durations of ECMO, ICU length of stay (LOS) and hospital LOS were 110.6 h {Interquartile Range (IQR): 94.6–144.5}, 10.5 days (IQR: 7.0–14.6) and 20.5 days (IQR: 15.8–29.3), respectively, in the combined group. The authors also noted that the incidence of stroke and infection was 9.5% and 33.3%, respectively, in patients who received concurrent RRT while on ECMO.
3.4. Meta-Regression Analysis
Meta-regression analysis of observational studies (Table 2) showed that longer ECMO durations were associated with lower odds of mortality (Odds Ratio (OR): 0.97, 95% CI: 0.95–0.98, p <0.001), while the need for RRT on VA-ECMO was associated with increased odds of mortality (OR: 1.23, 95% CI: 1.04–1.46, p = 0.02). Age, male gender, lactate level and sample size were not predictive of mortality.
Table 2.
Meta-regression analysis of covariates.
| Covariates | Studies | Odds Ratio | Lower CI | Upper CI | p Value |
|---|---|---|---|---|---|
| VA-ECMO | 24 | 1.23 | 1.04 | 1.46 | 0.02 |
| ECMO duration | 7 | 0.97 | 0.95 | 0.98 | <0.001 |
| Lactate | 7 | 1.08 | 0.95 | 1.24 | 0.22 |
| Age | 9 | 1.01 | 0.99 | 1.03 | 0.35 |
| Male | 10 | 0.82 | 0.17 | 3.94 | 0.81 |
| Sample size | 24 | 1.00 | 1.00 | 1.00 | 0.73 |
3.5. Pre-ECMO Vs. Post-ECMO RRT
Three studies reported on the timing of RRT. Deatrick et al. [34] found no significant difference (p = 0.19) in terms of survival between the patients who received RRT before ECMO (53%) and after ECMO (36%). On the other hand, Haneya et al. [38] noted that those patients who were on RRT prior to ECMO had a higher mortality rate of 43.4% compared to the survivors (21.2%). Similarly, Panholzer et al. [45] noted that those who were on RRT before ECMO initiation had a mortality of 37.5%.
3.6. Risk of Bias
We assessed the certainty of evidence for all of our primary and secondary outcome measures using the GRADE approach (Table 3). The certainty of evidence was high for the mortality of patients who received both therapies compared to those who were treated with ECMO alone, and there was a low certainty of evidence for ICU and hospital LOS.
Table 3.
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) findings.
| No. of Studies | Certainty Assessment | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | No. of Events | No. of Individuals | Rate (95% CI) | |||
| Mortality between patients supported with concurrent ECMO and RRT | |||||||||||
| 24 | observational studies | not serious | not serious a | not serious | not serious b | none | 2175 | 3202 | 63.0% (56.0% to 69.6%) |
⊕⊕⊕⊕
HIGH |
CRITICAL |
| ICU Length of Stay | |||||||||||
| 3 | observational studies | not serious | serious c | not serious | serious d | none | - | 1854 | 15.76 days (12.83 to 18.69) |
⊕⊕
◯◯
LOW |
IMPORTANT |
| Hospital Length of Stay | |||||||||||
| 4 | observational studies | not serious | serious c | not serious | serious d,e | none | - | 2181 | 29.00 days (21.74 to 36.26) |
⊕⊕
◯◯
LOW |
IMPORTANT |
Explanations:a There was considerable heterogeneity (I2 = 89.4%). However, subgroup analysis by geographical region, ECMO duration and time period found significant differences between subgroups among patients. Furthermore, meta-regression found that ECMO duration was significantly associated with increased survival, and VA ECMO with decreased survival. Visual inspection of the forest plots found that there was some variability in the point estimates, but the 95% CIs mostly overlapped. b The 95% CI are relatively narrow compared to the pooled estimate. In addition, there is a relatively large sample size of 3202 patients, which would reduce imprecision. c There was considerable heterogeneity. Further to this, visual inspection of the forest plot showed that the point estimates were sparsely distributed. d Very few studies reported on the outcome, yielding a small sample size that hampers precision. e The 95% CI is relatively wide in relation to the pooled estimate. High: We are very confident that the true prognosis (probability of future events) lies close to that of the estimate [23], Moderate: We are moderately confident that the true prognosis (probability of future events) is likely to be close to the estimate, but there is a possibility that it is substantially different [23], Low: Our confidence in the estimate is limited: the true prognosis (probability of future events) may be substantially different from the estimate [23], Very low: We have very little confidence in the estimate: the true prognosis (probability of future events) is likely to be substantially different from the estimate [23].
4. Discussion
This review reports pooled mortality outcomes in a heterogenous group of patients who received both ECMO and RRT. A large proportion (72%) of patients included in this review received VA-ECMO. We observed that the most commonly used RRT modality along with ECMO was CRRT; patients were predominantly middle-aged males with a pooled mortality of approximately 63%. This correlated with the mortality reported from the only RCT that reported on the use of RRT during ECMO. The mortality in patients receiving both ECMO and RRT has decreased significantly in last 5 years (20%) compared with that reported till the end of 2015. Most of the studies were observational in nature, while there was one RCT from Asia. The combined use of ECMO and RRT was associated with increased death risk by almost 81% when compared to patients receiving ECMO alone.
All patients on ECMO are at increased risk of inflammatory and haemodynamic perturbations that put them at an increased propensity of developing multiple organ dysfunction [3]. Equally, although non-pulsatile flow generated by VA-ECMO to maintain end-organ perfusion has been postulated to increase the risks of AKI [2,53], there is no robust clinical data to support this hypothesis. In addition, the higher odds of mortality seen in patients receiving both VA-ECMO and RRT in this study may also be attributed to patient selection issues and the timing of ECMO initiation. The pilot RCT conducted by Li et al. [52] in post-cardiotomy VA-ECMO patients showed that early use of RRT in these patients was associated with less mortality compared to those who received RRT late, as per conventional indications. The ELSO registry reports a mortality of 56% in a heterogeneous group of patients supported with VA-ECMO, which includes both patients who did or did not receive RRT support [54]. Given that critically ill patients needing ECMO are sicker, it is plausible that the mortality of the combined extracorporeal therapies would be higher. It can be expected that outcomes with combined VA-ECMO and RRT use may be better in potentially reversible conditions such as myocarditis and certain cardiotoxic drug ingestion (e.g., aluminium phosphide), in which VA-ECMO survival in excess of 60% has been reported [55,56]. We also noted a considerably higher incidence of other organ failures (~32%) and infections (~17%) in the combined group, which could have led to higher mortality in this group.
We observed that shorter ECMO duration was associated with higher mortality in this cohort of patients and vice-versa. Further additional analysis on mortality based on ECMO duration (more and less than 7 days) revealed that mortality was higher in patients with shorter ECMO duration (66.5% vs. 41.5%). This goes in hand with our meta-regression analysis. While there were only a few studies in this analysis, we believe that patients who had a shorter duration of ECMO were sicker and had a higher MOF needing RRT in addition to ECMO, resulting in a higher mortality. The association between longer ECMO duration and lower mortality can be attributed to immortal bias: patients must first survive long enough in order to be weaned off ECMO [57]. A proportion of ECMO patients are likely to die early, either due to progressive multiple organ failure, fatal complications, limited cardiopulmonary recovery, lack of viable bridging options such as transplantation, or simply palliation based on clinician’s judgment or patient and family wishes. There are significant differences between patients with severe respiratory failure and refractory heart failure receiving ECMO. Apart from obvious pathophysiologic differences, achieving sufficient reversibility of underlying pathology to wean from ECMO and survive to hospital discharge is an important consideration. Previous reviews also concluded high mortality in patients who received combined extracorporeal therapies [16,17,58]. Similarly, the mechanisms behind decreasing mortality trends in patients who received both ECMO and RRT over last 5 years could not be understood within the scope of this review. It is possible that better patient selection, timing and improving clinical application played a role in addition to technological advances. Additionally, the number of publications on RRT and ECMO has increased in last 5 years, which has resulted in more granularity in the overall data. Whether ECMO is a risk factor for AKI or whether early ECMO mitigates the development of AKI and other organ failures remains a much-debated entity, given the higher mortality reported in this cohort.
Our systematic review has several limitations. This analysis is based mainly on observational studies with significant heterogeneity. The random-effects model was used when conducting this meta-analysis for the anticipated heterogeneity in addition to using the GRADE approach to rate the certainty of evidence. We performed additional subgroup analysis to account for heterogeneity. Furthermore, meta-regression analyses are constrained by an inherent lack of power and increase the risk of Type II errors. The GRADE assessment showed low to high levels of certainty for the results of the analysis. An increased ECMO duration was associated with less odds of mortality in patients receiving combined supports in this review. Finally, the Egger’s test yielded non-significant results for most of our primary endpoints, except for relative risk of mortality due to combined therapies which had significant publication bias. Nonetheless, JBI appraisal of the included studies suggests that they were of high quality, limiting the possibility of publication bias. Few studies assessed the incidence of other MOFs or new-onset infections, so our results should be interpreted with caution and may be considered hypothesis-generating. We did not analyse any differences in outcomes based on timing of RRT initiation, different modalities of RRT, different ventricular unloading techniques while on ECMO, or different forms of shock because very few studies examined these data.
5. Conclusions
Adult patients receiving both ECMO and RRT are at a greater risk of death. The mortality, however, has shown a decreasing trend over the last 5 years. Patients receiving RRT on VA-ECMO have greater odds of death compared with those receiving RRT on VV-ECMO. Given the higher mortality and morbidity in the group of patients who received RRT on ECMO, future research should focus on determining the optimal timing of both VA and VV ECMO initiation in order to potentially mitigate AKI. Further studies should also explore the optimal timing of RRT initiation during ECMO in patients with appropriate indications.
Acknowledgments
Kiran Shekar acknowledges research support from Metro North Hospital and Health Service. We also acknowledge Suei Nee Wong at NUS Yong Loo Lin School of Medicine medical library for helping with data collection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/2/241/s1, Figure S1. Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) flowchart for study selection, Figure S2. Forest plot showing pooled mean age, Figure S3. Forest plot showing pooled proportions of males, Figure S4. Forest plot showing pooled proportions of concurrent VA ECMO and RRT use, Figure S5. Forest plot showing pooled mortality before and after 2016, Figure S6. Forest plot showing pooled mortality based on ECMO duration (more and less than 7 days), Table S1. Joanna Briggs Institute (JBI) checklist for all studies.
Author Contributions
Conceptualization, S.M. and K.R.; methodology, S.M., R.R.L. and K.R.; software, R.R.L. and C.S.T.; validation, S.M., R.R.L. and K.R.; formal analysis, R.R.L., C.S.T., S.M., K.R.; investigation, S.M., R.R.L., K.R.; data curation, S.M., R.R.L., K.R.; writing—original draft preparation, S.M. and R.R.L.; writing—review and editing, K.S., G.M. and K.R.; visualization, R.R.L., C.S.T.; supervision, K.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest regarding this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen Y.C., Tsai F.C., Fang J.T., Yang C.W. Acute kidney injury in adults receiving extracorporeal membrane oxygenation. J. Formos. Med. Assoc. 2014;113:778–785. doi: 10.1016/j.jfma.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Villa G., Katz N., Ronco C. Extracorporeal Membrane Oxygenation and the Kidney. Cardiorenal. Med. 2015;6:50–60. doi: 10.1159/000439444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostermann M., Connor M., Jr., Kashani K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: Why, when and how? Curr. Opin. Crit. Care. 2018;24:493–503. doi: 10.1097/MCC.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 4.Millar J.E., Fanning J.P., McDonald C.I., McAuley D.F., Fraser J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care. 2016;20:387. doi: 10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hei F., Lou S., Li J., Yu K., Liu J., Feng Z., Zhao J., Hu S., Xu J., Chang Q., et al. Five-year results of 121 consecutive patients treated with extracorporeal membrane oxygenation at Fu Wai Hospital. Artif. Organs. 2011;35:572–578. doi: 10.1111/j.1525-1594.2010.01151.x. [DOI] [PubMed] [Google Scholar]
- 6.Kilburn D.J., Shekar K., Fraser J.F. The Complex Relationship of Extracorporeal Membrane Oxygenation and Acute Kidney Injury: Causation or Association? BioMed Res. Int. 2016;2016:1094296. doi: 10.1155/2016/1094296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiagarajan R.R., Barbaro R.P., Rycus P.T., Mcmullan D.M., Conrad S.A., Fortenberry J.D., Paden M.L., ELSO Member Centers Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw S.M., Laupland K.B., Doig C.J., Mortis G., Fick G.H., Mucenski M., Godinez-Luna T., Svenson L.W., Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit. Care. 2005;9:R700-9. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaara S.T., Korhonen A.M., Kaukonen K.M., Nisula S., Inkinen O., Hoppu S., Laurila J.J., Mildh L., Reinikainen M., Lund V., et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: Data from the prospective FINNAKI study. Crit. Care. 2012;16:R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll N., Kiaii B., Borger M., Bucerius J., Krämer K., Schmitt D.V., Walther T., Mohr F.W. Five-year Results of 219 Consecutive Patients Treated with Extracorporeal Membrane Oxygenation for Refractory Postoperative Cardiogenic Shock. Ann. Thorac. Surg. 2004;77:151–157; discussion 157. doi: 10.1016/S0003-4975(03)01329-8. [DOI] [PubMed] [Google Scholar]
- 11.Smedira N.G., Moazami N., Golding C.M., McCarthy P.M., Apperson-Hansen C., Blackstone E.H., Cosgrove D.M., 3rd Clinical Experience with 202 Adults Receiving Extracorporeal Membrane Oxygenation for Cardiac Failure: Survival at Five Years. J. Thorac. Cardiovasc. Surg. 2001;122:92–102. doi: 10.1067/mtc.2001.114351. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao C.C., Chang C.H., Fan P.C., Ho H.T., Jenq C.C., Kao K.C., Chiu L.C., Lee S.Y., Hsu H.H., Tian Y.C., et al. Prognosis of Patients with Acute Respiratory Distress Syndrome on Extracorporeal Membrane Oxygenation: The Impact of Urine Output on Mortality. Ann. Thorac. Surg. 2014;97:1939–1944. doi: 10.1016/j.athoracsur.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Kim H., Paek J.H., Song J.H., Lee H., Jhee J.H., Park S., Yun H.R., Kee Y.K., Han S.H., Yoo T.H., et al. Permissive fluid volume in adult patients undergoing extracorporeal membrane oxygenation treatment. Crit. Care. 2018;22:270. doi: 10.1186/s13054-018-2211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramanathan K., Tan C.S., Rycus P., MacLaren G. Extracorporeal membrane oxygenation for poisoning in adult patients: Outcomes and predictors of mortality. Intensive Care Med. 2017;43:1538–1539. doi: 10.1007/s00134-017-4842-9. [DOI] [PubMed] [Google Scholar]
- 15.De Lange D.W., Sikma M.A., Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin. Toxicol. 2013;51:385–393. doi: 10.3109/15563650.2013.800876. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Yu R.G., Yin N.N., Zhou J.X. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: A systematic review. Crit. Care. 2014;18:675. doi: 10.1186/s13054-014-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S.S., Kim H.J., Lee S.J., Kim W.J., Hong Y., Lee H.Y., Song S.Y., Jung H.H., Ahn H.S., Ahn I.M., et al. Effects of Renal Replacement Therapy in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. Ann. Thorac. Surg. 2015;100:1485–1495. doi: 10.1016/j.athoracsur.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J.J.Y., Ong J.A., Syn N.L., Lorusso R., Tan C.S., MacLaren G., Ramanathan K. Extracorporeal Membrane Oxygenation in Pregnant and Postpartum Women: A Systematic Review and Meta-Regression Analysis. J. Intensive Care Med. 2019 doi: 10.1177/0885066619892826. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Clopper C.J., Pearson E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika. 1934;26:404–413. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 21.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J., GRADE Working Group GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M., Alonso-Coello P., Glasziou P., Jaeschke R., Akl E.A., et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J. Clin. Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Iorio A., Spencer F.A., Falavigna M., Alba C., Lang E., Burnand B., McGinn T., Hayden J., Williams K., Shea B., et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 24.GRADEpro App. [(accessed on 10 October 2020)]; Available online: https://www.gradepro.org.
- 25.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Santesso N., Carrasco-Labra A., Langendam M., Brignardello-Petersen R., Mustafa R.A., Heus P., Lasserson T., Opiyo N., Kunnamo I., Sinclair D., et al. Improving GRADE evidence tables part 3: Detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J. Clin. Epidemiol. 2016;74:28–39. doi: 10.1016/j.jclinepi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allyn J., Ferdynus C., Lo Pinto H., Bouchet B., Persichini R., Vandroux D., Puech B., Allou N. Complication patterns in patients undergoing venoarterial extracorporeal membrane oxygenation in intensive care unit: Multiple correspondence analysis and hierarchical ascendant classification. PLoS ONE. 2018;13:e0203643. doi: 10.1371/journal.pone.0203643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonucci E., Lamanna I., Fagnoul D., Vincent J.L., De Backer D., Silvio Taccone F. The Impact of Renal Failure and Renal Replacement Therapy on Outcome During Extracorporeal Membrane Oxygenation Therapy. Artif. Organs. 2016;40:746–754. doi: 10.1111/aor.12695. [DOI] [PubMed] [Google Scholar]
- 30.Baek J.K., Lee J.S., Kim T.H., Kim Y.H., Han D.J., Hong S.K. Four-Year Experience With Extracorporeal Membrane Oxygenation for Kidney Transplant Patients With Severe Refractory Cardiopulmonary Insufficiency. Transplant. Proc. 2016;48:2080–2083. doi: 10.1016/j.transproceed.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Chen S.W., Lu Y.A., Lee C.C., Chou A.H., Wu V.C., Chang S.W., Fan P.C., Tian Y.C., Tsai F.C., Chang C.H. Long-term outcomes after extracorporeal membrane oxygenation in patients with dialysis-requiring acute kidney injury: A cohort study. PLoS ONE. 2019;14:e0212352. doi: 10.1371/journal.pone.0212352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Combes A., Leprince P., Luyt C.E., Bonnet N., Trouillet J.L., Léger P., Pavie A., Chastre J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit. Care Med. 2008;36:1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 33.Dado D.N., Ainsworth C.R., Thomas S.B., Huang B., Piper L.C., Sams V.G., Batchinsky A., Morrow B.D., Basel A.P., Walter R.J., et al. Outcomes among Patients Treated with Renal Replacement Therapy during Extracorporeal Membrane Oxygenation: A Single-Center Retrospective Study. Blood Purif. 2020;49:341–347. doi: 10.1159/000504287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deatrick K.B., Mazzeffi M.A., Galvagno S.M., Jr., Boswell K., Kaczoroswki D.J., Rabinowitz R.P., Madathil R.J., Cornachione C.R., Herr D.L., Scalea T.M., et al. Breathing Life Back Into the Kidney-Continuous Renal Replacement Therapy and Veno-Venous Extracorporeal Membrane Oxygenation. ASAIO J. 2020 doi: 10.1097/MAT.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 35.Devasagayaraj R., Cavarocchi N.C., Hirose H. Does acute kidney injury affect survival in adults with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation? Perfusion. 2018;33:375–382. doi: 10.1177/0267659118755272. [DOI] [PubMed] [Google Scholar]
- 36.Elsharkawy H.A., Li L., Esa W.A., Sessler D.I., Bashour C.A. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2010;24:946–951. doi: 10.1053/j.jvca.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Fong K.M., Au S.Y., Ng G.W.Y., Leung A.K.H. Positive fluid balance and mortality in adult patients treated with extracorporeal membrane oxygenation: A retrospective study. J. Intensive Care Soc. 2020;21:210–220. doi: 10.1177/1751143719862240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haneya A., Diez C., Philipp A., Bein T., Mueller T., Schmid C., Lubnow M. Impact of Acute Kidney Injury on Outcome in Patients With Severe Acute Respiratory Failure Receiving Extracorporeal Membrane Oxygenation. Crit. Care Med. 2015;43:1898–1906. doi: 10.1097/CCM.0000000000001141. [DOI] [PubMed] [Google Scholar]
- 39.He P., Zhang S., Hu B., Wu W. Retrospective study on the effects of the prognosis of patients treated with extracorporeal membrane oxygenation combined with continuous renal replacement therapy. Ann. Transl. Med. 2018;6:455. doi: 10.21037/atm.2018.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kielstein J.T., Heiden A.M., Beutel G., Gottlieb J., Wiesner O., Hafer C., Hadem J., Reising A., Haverich A., Kühn C., et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol. Dial. Transplant. 2013;28:86–90. doi: 10.1093/ndt/gfs398. [DOI] [PubMed] [Google Scholar]
- 41.Lee S.Y., Jeon K.H., Lee H.J., Kim J.B., Jang H.J., Kim J.S., Kim T.H., Park J.S., Choi R.K., Choi Y.J. Complications of veno-arterial extracorporeal membrane oxygenation for refractory cardiogenic shock or cardiac arrest. Int. J. Artif. Organs. 2020;43:37–44. doi: 10.1177/0391398819868483. [DOI] [PubMed] [Google Scholar]
- 42.Luo X.J., Wang W., Hu S.S., Sun H.S., Gao H.W., Long C., Song Y.H., Xu J.P. Extracorporeal membrane oxygenation for treatment of cardiac failure in adult patients. Interact. Cardiovasc. Thorac. Surg. 2009;9:296–300. doi: 10.1510/icvts.2008.197681. [DOI] [PubMed] [Google Scholar]
- 43.McCanny P., Smith M.W., O’Brien S.G., Buscher H., Carton E.G. Fluid Balance and Recovery of Native Lung Function in Adult Patients Supported by Venovenous Extracorporeal Membrane Oxygenation and Continuous Renal Replacement Therapy. ASAIO J. 2019;65:614–619. doi: 10.1097/MAT.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 44.Paek J.H., Park S., Lee A., Park S., Chin H.J., Na K.Y., Lee H., Park J.T., Kim S. Timing for initiation of sequential continuous renal replacement therapy in patients on extracorporeal membrane oxygenation. Kidney Res. Clin. Pract. 2018;37:239–247. doi: 10.23876/j.krcp.2018.37.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panholzer B., Meckelburg K., Huenges K., Hoffmann G., von der Brelie M., Haake N., Pilarczyk K., Cremer J., Haneya A. Extracorporeal membrane oxygenation for acute respiratory distress syndrome in adults: An analysis of differences between survivors and non-survivors. Perfusion. 2017;32:495–500. doi: 10.1177/0267659117693075. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt M., Bailey M., Kelly J., Hodgson C., Cooper D.J., Scheinkestel C., Pellegrino V., Bellomo R., Pilcher D. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40:1256–1266. doi: 10.1007/s00134-014-3360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thajudeen B., Kamel M., Arumugam C., Ali S.A., John S.G., Meister E.E., Mosier J.M., Raz Y., Madhrira M., Thompson J., et al. Outcome of patients on combined extracorporeal membrane oxygenation and continuous renal replacement therapy: A retrospective study. Int. J. Artif. Organs. 2015;38:133–137. doi: 10.5301/ijao.5000381. [DOI] [PubMed] [Google Scholar]
- 48.Unosawa S., Sezai A., Hata M., Nakata K., Yoshitake I., Wakui S., Kimura H., Takahashi K., Hata H., Shiono M. Long-term outcomes of patients undergoing extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. Surg. Today. 2013;43:264–270. doi: 10.1007/s00595-012-0322-6. [DOI] [PubMed] [Google Scholar]
- 49.Xie H., Yang F., Hou D., Wang X., Wang L., Wang H., Hou X. Risk factors of in-hospital mortality in adult postcardiotomy cardiogenic shock patients successfully weaned from venoarterial extracorporeal membrane oxygenation. Perfusion. 2020;35:417–426. doi: 10.1177/0267659119890214. [DOI] [PubMed] [Google Scholar]
- 50.Yan X., Jia S., Meng X., Dong P., Jia M., Wan J., Hou X. Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: Evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria. Eur. J. Cardiothorac. Surg. 2010;37:334–338. doi: 10.1016/j.ejcts.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Yap H.J., Chen Y.C., Fang J.T., Huang C.C. Combination of continuous renal replacement therapies (CRRT) and extracorporeal membrane oxygenation (ECMO) for advanced cardiac patients. Ren. Fail. 2003;25:183–193. doi: 10.1081/JDI-120018719. [DOI] [PubMed] [Google Scholar]
- 52.Li C., Wang H., Liu N., Jia M., Hou X. The Effect of Simultaneous Renal Replacement Therapy on Extracorporeal Membrane Oxygenation Support for Postcardiotomy Patients with Cardiogenic Shock: A Pilot Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2019;33:3063–3072. doi: 10.1053/j.jvca.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 53.Pappalardo F., Montisci A. Veno-arterial extracorporeal membrane oxygenation (VA ECMO) in postcardiotomy cardiogenic shock: How much pump flow is enough? J. Thorac. Dis. 2016;8:E1444–E1448. doi: 10.21037/jtd.2016.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.International Summary, ECLS Registry Report. [(accessed on 15 November 2020)];2020 Jul; Available online: https://www.elso.org/registry/statistics/InternationalSummary.aspx.
- 55.Mohan B., Singh B., Gupta V., Ralhan S., Gupta D., Puri S., Goyal A., Aslam N., Tandon R., Wander G.S. Outcome of patients supported by extracorporeal membrane oxygenation for aluminum phosphide poisoning: An observational study. Indian Heart J. 2016;68:295–301. doi: 10.1016/j.ihj.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorusso R., Centofanti P., Gelsomino S., Barili F., Di Mauro M., Orlando P., Botta L., Milazzo F., Actis Dato G., Casabona R. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. Ann. Thorac. Surg. 2016;101:919–926. doi: 10.1016/j.athoracsur.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Shintani A.K., Girard T.D., Eden S.K., Arbogast P.G., Moons K.G., Ely E.W. Immortal time bias in critical care research: Application of time-varying Cox regression for observational cohort studies. Crit. Care Med. 2009;37:2939–2945. doi: 10.1097/CCM.0b013e3181b7fbbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thongprayoon C., Cheungpasitporn W., Lertjitbanjong P., Aeddula N.R., Bathini T., Watthanasuntorn K., Srivali N., Mao M.A., Kashani K. Incidence and Impact of Acute Kidney Injury in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. J. Clin. Med. 2019;8:981. doi: 10.3390/jcm8070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.