Abstract
Tualang, Gelam and Kelulut honeys are tropical rainforest honeys reported to have various medicinal properties. Studies related to the medicinal properties and physicochemical characteristics of these honeys are growing extensively and receiving increased attention. This review incorporated and analysed the findings on the biological and physicochemical properties of these honeys. Tualang, Gelam and Kelulut honeys were found to possess a wide variety of biological effects attributed to their physicochemical characteristics. Findings revealed that these honeys have anti-diabetic, anti-obesity, anti-cancer, anti-oxidative, anti-microbial, anti-inflammatory and wound-healing properties and effects on the cardiovascular system, nervous system and reproductive system. The physicochemical properties of these honeys were compared and discussed and results showed that they have high-quality contents and excellent antioxidant sources.
Keywords: Tualang honey, Gelam honey, Kelulut honey, health benefits, anti-oxidative, anti-cancer
1. Introduction
Honey has been utilised since ancient times due to its various health benefits. Despite medical advances achieved today, honey remains an integral part of a particular regional demographic. Owing to recent advancements in biotechnology, honey has been revealed to possess newly discovered unique contents and medicinal properties. Stingless bee honey, especially those from Malaysia, Australia, and Brazil, has been recently discovered as a novel source of trehalulose, a biologically active disaccharide with various health benefits [1]. A recent systematic review and meta-analysis showed that honey was superior to usual care for improving upper respiratory tract infections (URTIs). It was also more effective than antibiotics when prescribed to reduce the spread of anti-microbial resistance in URTIs [2]. Honey exhibited superior effects compared with conventional treatments for acute wounds, superficial partial-thickness burns and infected post-operative wounds [3,4]. Some honeys were licensed as a medical product and used in the clinical setting for wound care in Europe and Australia [5].
Tualang honey, Gelam honey and Kelulut honey are tropical rainforest honeys that mostly originated from Malaysia. The health benefits of these honeys have recently been gaining increased attention and popularity, as evidenced by numerous documented studies. Tualang honey is a wild polyfloral honey produced by Apis dorsata. This honey is named after one of the tallest tropical rainforest trees, the Koompassia excelsa tree (known locally as the Tualang tree), where bees build their hives. The bees collect nectar from plants in the tropical rain forest in the North-eastern region of Peninsular Malaysia in Kedah. This honey possesses anti-microbial, anti-cancer, antioxidant, and anti-inflammatory effects attributed to its high antioxidant contents [6,7]. Gelam honey is also wild honey produced by the A. dorsata bees, which build hives on trees locally called Gelam, or Melaleuca cajuputi Powell. Gelam honey is mostly obtained from the East Coast of Peninsular Malaysia in Terengganu state. This honey may delay the onset of chronic diseases and infections and regulate the optimal level of enzymatic activity in the body [8]. Kelulut honey is produced by Trigona spp., a stingless bee that gathers nectar from polyfloral sources [6,9]. This honey has a slightly sour taste and is more liquid than the other honeys. It possesses antibacterial, antioxidant, and anti-osteoporotic properties due to its high antioxidant properties [10,11].
An updated study to comprehensively analyse the benefits of Tualang, Gelam and Kelulut honey is still required compared to other types of honey such as Manuka honey which has been extensively reviewed. With the recent growth in the body of literature for these kinds of honey, assessment of Tualang, Gelam, and Kelulut honeys is needed to avoid future cross-studies or unnecessary research due to missed reviews of the existing research. In this review, relevant studies related to the medicinal properties, health benefits and physicochemical properties of Tualang, Gelam, and Kelulut honeys were identified and critically analysed. The findings showed that most studies reported the health advantages of honey consumption, whereas some reported disadvantages or no significant changes upon consumption. Furthermore, analyses of physiochemical-related research revealed that Tualang, Gelam and Kelulut honeys have high-quality contents and excellent antioxidant sources.
2. Methods of Review
A literature search was undertaken to identify and map out relevant and pertinent articles related to the physicochemical and medicinal properties of Tualang, Gelam, and Kelulut honeys. Peer-reviewed and full-text English articles were gathered from a time frame as early as 1960 to May 2020 in electronic databases, including Scopus, MEDLINE via EBSCOhost, and Google Scholar. A literature search was performed by combining the following set of keywords: (1) Tualang or Kelulut or Gelam and (2) honey. The literature search was further supplemented by referencing related review articles and scientific reports found from the search results.
3. Physicochemical Characteristics of Tualang, Gelam, and Kelulut Honeys
Honey from different botanical and geographical origins has different physicochemical properties and compositions that ultimately affect their biological properties [8]. Numerous studies were conducted to investigate the physicochemical properties of Tualang, Gelam and Kelulut honeys. Twenty-two studies related to the physicochemical and content analyses on these selected honeys were found. These honeys have unique physicochemical properties mostly attributed to Malaysia’s tropical climate. Table 1; Table 2 show the physicochemical characterisation of Tualang, Gelam and Kelulut honeys extracted from various studies. Considering Kelulut honey is a polyfloral honey, this review only included studies on Kelulut honey that mainly originated from the Acacia mangium trees to obtain a fair comparison.
Table 1.
Physicochemical characteristics of Tualang, Gelam and Kelulut honeys.
| Physicochemical Characteristics | Tualang Honey | Gelam Honey | Kelulut Honey |
|---|---|---|---|
| Colour characteristic (mm Pfund) | 74.00–113.0 [15,16,29] |
122.00–139.0 [15,16,30] |
1.25 [31] |
| pH | 3.14–3.80 [15,16,29,32] |
3.38–3.83 [15,16,30,32] |
3.27–3.30 [31,33] |
| Moisture content | 17.53–26.51% [15,16,29,32] |
17.93–26.50% [15,16,30,32] |
21.52–31% [31,33,34] |
| Electrical conductivity, (mS/cm) | 0.75–1.37 [15,16,29] |
0.74–1.51 [15,16,30] |
0.33–0.69 [35] |
Table 2.
Chemical constituents of Tualang, Gelam and Kelulut honeys.
| Chemical Constituent | Tualang Honey | Gelam Honey | Kelulut Honey |
|---|---|---|---|
| Total sugar content (g/100 g) | 63.60–72.94 [29,32,36] |
64.93–72.57 [30,32,36] |
65.83–71.65 [31,33,34,36] |
| Reducing sugar (g/100 g) | 61.94 [29] |
62.17 ± 0.73 [30] |
No report found |
| Sucrose (g/100 g) | ˂0.01–1.66 [29,32,36] |
˂0.01–2.77 [30,32] | ˂0.01–32.30 [31,33,35,36] |
| Glucose (g/100 g) | 30.07–47.134 [32,36] |
32.85–50.447 [32,36] | 9.22–23.44 [31,33,35,36] |
| Fructose (g/100 g) | 41.732–44.56 [32,36] |
44.908–44.74 [32,36] |
15–22.05 [31,33,35,36] |
| Maltose (g/100 g) | 4.491 [32] |
1.291 [32] |
0–27.41 [31,33] |
| Protein content (g/kg) | 3.6–6.6 [29,32,36] |
3.14–7.0 [30,32,36] | 3.9–8.5 [33,34,36] |
| Proline content (mg/kg) | 248.53 ± 1.33 [29] |
261.33 ± 1.33 [30] | No report found |
| Mineral content (mg/kg) | |||
| Sodium | 268.23–704.5 [28,36] |
196.84–666.0 [28,36] |
589.7 [36] |
| Potassium | 976.9–1576.40 [28,36] |
1132.2–1363.40 [28,36] | 732.2 [36] |
| Calcium | 76.4–165.10 [28,36] |
177.9–275.77 [28,36] |
191.9 [36] |
| Iron | 11.17–128.13 [28,36] |
8.45–142.37 [28,36] |
6.57 [36] |
| Magnesium | 35.03–71.04 [28,36] |
31.63–49.38 [28,36] |
33.81 [36] |
| Zinc | 2.28–13.20 [28,36] |
1.45–29.23 [28,36] |
2.162 [36] |
| Arsenic | 0.051–0.062 [28,36] |
0.064–0.070 [28,36] |
0.019 [36] |
| Lead | 0.183–0.231 [28,36] |
0.145–0.777 [28,36] |
0.154 [36] |
| Cadmium | 0–0.004 [28,36] |
0.005–0.05 [28,36] |
0.002 [36] |
| Copper | 1.25–2.144 [28,36] |
2.089–2.21 [28,36] |
1.776 [36] |
| Cobalt | 0.033 ± 0.002 [28] |
0.082 ± 0.005 [28] |
No report found |
| Total phenolic content mg/kg (gallic acid) | 251.7–1103.94 [16,21,29,37,38] | 606.17–1597.43 [16,21,38] | 477.30–614.7 [31,33] |
| Total flavonoid content | 49.04–185.11 (rutin) [16,21] 65.65 (catechin) [29] 504.5 (quercetin) [38] |
183.43–328.86 (rutin) [16,21] 461.1 (quercetin) [38] |
36.3 (quercetin) |
On the one hand, Tualang and Gelam honeys mostly complied with the accepted range by the two most common legislation of honey criteria and standards referred to as the European Honey Legislation [12] and Codex Alimentarius Standards for Honey [13]. On the other hand, the Kelulut honey in various studies followed the Malaysian Standard Kelulut (Stingless Bee) [14]. According to the European Honey Legislation and the Codex Alimentarius Standards, honey moisture should be less than 20%, with glucose and fructose composition of more than 60 g/100 g, sucrose content of not more than 5 g/100 g and electrical conductivity of not more than 0.8 mS/cm. According to the Malaysian Standard Kelulut, the raw honey moisture content from stingless bee must be less than 35 g/100 g, with a pH of less than 3.8 and 5-hydroxymethylfurfural of less than 30 mg/kg.
Most of the studies reported that Tualang and Gelam honeys contained more than 20% moisture content, thus violating European Honey Legislation and Codex Alimentarius Standards. Nonetheless, honey samples from tropical countries, such as Malaysia, typically have higher moisture content, which could be due to the rainy season all over the year [15]. Therefore, Malaysia’s honey is always first treated by evaporation to reduce the water content, thereby simultaneously increasing the honey quality [16].
Meanwhile, the electrical conductivity of honey is used to determine the botanical origin and purity of honey [17]. An electrical conductivity of less than 0.8 mS/cm indicates blossom honey, whilst more than 0.8 mS/cm indicates honeydew honey [18]. According to the European Honey Legislation and the Codex Alimentarius Standards, honey’s electrical conductivity should not be more than 0.8 mS/cm. However, the Malaysian Standard Kelulut has not determined any range for an acceptable limit of electrical conductivity. The electrical conductivity of Tualang, Gelam and Kelulut honeys reported in this review is in a broad range of 0.74–1.51 mS/cm. Various factors, such as storage, time, temperature, water content and concentration of ions and minerals, were reported to contribute to the electrical conductivity of honey [16].
Most bacteria grow in a neutral and mildly alkaline environment, whereas yeasts and moulds could grow in an acidic environment (pH = 4.0–4.5). Conversely, the pH values of honey are neither those needed for bacteria nor yeast growth [15]. This is of great importance during storage, as they influence the texture, stability and shelf-life of honey [18]. The pH value of Tualang, Gelam, and Kelulut honeys were reported to be in the range of 3.14–3.83. Moreover, the sugar content of these three honeys has complied with the requirement of the European Honey Legislation and Codex Alimentarius Standards. In terms of honey colour characteristics, Tualang honey is described as light amber honey and Gelam honey belongs to dark amber honey, whilst standard Kelulut honey is amber brown [15,19].
Many studies found that the antioxidant capacity of honey is strongly correlated with the concentration of its phenolic contents [20,21]. The phenolic content of honey comes from the plant that the bees feed on [22]. Thus, botanical origin plays a significant role in determining the constituents and antioxidant activity of honey [23]. Colour intensity is also associated with antioxidant properties. Numerous studies, including those on Gelam, Tualang and Kelulut honey, associated the honey colour intensity with antioxidant properties. Darker-coloured honey showed higher antioxidant activity and higher total phenolic content [22,24,25,26]. The present review also found that these three honeys have a high content of phenolic and flavonoid. Amongst the reviewed studies comparing the antioxidant properties of Tualang, Gelam and Kelulut honey, one study showed that Kelulut honey has the highest antioxidant properties in ranking after Manuka honey amongst Gelam and Tualang honeys [25]. By contrast, another study found Tualang honey to have higher antioxidant properties than Manuka, Kelulut, and Gelam honeys [27]. As mentioned earlier, the study also revealed higher free radical scavenging and antioxidant activity in Tualang honey than in Gelam and Borneo honeys [26].
The determination of trace elements in honey is essential for quality control and monitoring of trace element composition. High levels of trace elements could be dangerous and cause toxicity. The levels of trace elements in Tualang, Gelam and Kelulut honeys were within the permissible value set by the World Health Organization (WHO), and no honey contamination with pesticide residues was found [28].
4. Medicinal Properties of Tualang, Gelam and Kelulut Honeys
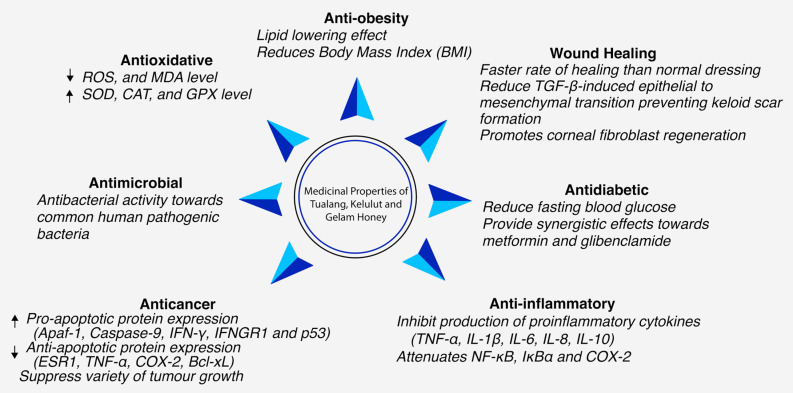
In this section, the medicinal properties of Tualang, Gelam and Kelulut honeys are discussed in accordance with different properties. A total of 99 articles regarding the medicinal properties of Tualang, Gelam and Kelulut honeys were obtained, including 59 in vivo studies, 25 in vitro studies, 2 ex vivo, one study combine in-viva and invitro technique and 12 clinical trials involving humans. Among medicinal properties discussed below, all three honey shared these major health benefits; (a) anti-oxidative; (b) anti-cancer; (c) anti-inflammatory; (d) wound-healing; (e) anti-microbial; (f) anti-diabetic and; (g) anti-obesity (as seen in Figure 1).
Figure 1.
The medicinal properties of Tualang, Gelam and Kelulut honeys. ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase; GPX: glutathione peroxidase; TGFβ: transforming growth factor beta; Apaf-1: Apoptotic protease activating factor-1; IFN-γ: interferon-gamma; IFNGR1: interferon gamma receptor-1; p53: tumor protein P53; ESR1: oestrogen receptor-1; TNF-α: tumor necrosis factor alpha; Bcl-xL: B-cell lymphoma-extra-large; IL-1β: interleukin-1 beta; IL-6: interleukin 6; IL-8: interleukin 8; IL-10: interleukin 10; NF-kB: nuclear factor kappa B; IκBα: NF-kappa-B inhibitor alpha; COX-2: cyclooxygenase-2.
4.1. Anti-Oxidative Properties
One of the most well-known benefits of honey is its anti-oxidative property. In this review, the most positive medicinal effects of Malaysian honey were attributed to its antioxidant properties. A plethora of studies regarding the antioxidative characteristic and pathways of Tualang, Gelam and Kelulut honeys are summarised and illustrated in Table 3 and Figure 2, respectively. Two studies reported that Gelam honey reduced oxidative damage by reducing DNA damage and the malondialdehyde (MDA) level of young, middle-aged and aged rats by modulating antioxidant enzyme activities [39,40]. Another study showed that the combination of Gelam honey and ginger provided a better antioxidant effect than Gelam honey or ginger alone, as evidenced by the significantly reduced superoxide dismutase (SOD) and catalase (CAT) activities, reduced MDA level, increased (reduced glutathione) GSH level and increased GSH/glutathione disulfide (GSSG) ratio in diabetic rats [41]. Another separate study reported the protective effect of Gelam honey on human diploid fibroblast (HDFs) exposed to gamma rays. Gamma irradiation to HDFs decreased SOD, CAT, and glutathione peroxidase (GPx) gene expression levels and enzyme activities, whilst treatment with Gelam honey increased both parameters [42].
Table 3.
Summary of anti-oxidative properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Tualang honey | In vivo | Tualang honey reduced elevated malondialdehyde (MDA) levels and restored superoxide dismutase (SOD) and catalase (CAT) activities in diabetic rats. | [48] |
| Tualang honey | In vivo | Tualang honey potentiated the effect of glibenclamide and metformin to protect diabetic rat pancreas against oxidative stress and damage. | [49] |
| Tualang honey | In vivo | Combination of Tualang honey with metformin or glibenclamide provided additional antioxidant effect to these drugs toward diabetic rat kidneys. | [51] |
| Kelulut honey | In vivo | Kelulut honey reduced oxidative stress by reducing lipid peroxidation and increasing SOD. It also maintained bone structure, increased osteoblast and reduced osteoclast number in glucocorticoid-induced osteoporosis rat. |
[11] |
| Gelam honey | In vivo | Gelam honey reduced oxidative damage in young and middle-aged rats by modulating antioxidant enzyme activities. | [39] |
| Gelam honey | In vivo | Gelam honey reduced oxidative damage in young and aged rats through modulation of antioxidant enzyme activities. | [40] |
| Gelam honey | In vivo | Combination of Gelam honey and ginger provided a better antioxidant effect than Gelam honey or ginger alone, as evidenced by significantly reduced SOD and CAT activities, depleted MDA level, increased glutathione (GSH) level and increased GSH/glutathione disulfide (GSSG) ratio in diabetic rats. | [41] |
| Gelam honey | In vitro | Gelam honey and quercetin attenuated the oxidative stress-induced inflammatory and insulin signalling pathways in pancreatic hamster cells. | [46] |
| Gelam honey | In vitro | Gelam honey-induced differential expression of mitogen-activated protein kinases (MAPK), nuclear factor kappa B (NF-κB), insulin receptor substrate-1 (IRS-1) (ser307), and Akt in HIT-T15 cells showed that Gelam honey exerted protective effects against diabetes and hyperglycaemia-induced oxidative stress by improving insulin content and insulin resistance. | [45] |
| Tualang honey | In vitro | Tualang honey possessed antioxidant properties and could improve cell migration and cellular resistance to oxidative stress in human corneal epithelial progenitor cells in vitro. | [52] |
| Gelam honey | In vitro | Gelam honey modulated the expression of antioxidant enzymes at gene and protein levels in irradiated human diploid fibroblasts, suggesting its potential as a radioprotectant agent. | [42] |
| Gelam honey | In vitro | Pre-treatment of cells with Gelam honey extract or its flavonoid components before culturing in hyperglycaemic condition showed a significant decrease in reactive oxygen species (ROS) production and glucose-induced lipid peroxidation and a significant increase in insulin content and the viability of pancreatic cells. | [47] |
| Tualang honey | Human study | Consumption of high (1.5 g/kg) and low (0.75 g/kg) doses of Tualang honey demonstrated a comparable response in increasing antioxidant activity and suppressing oxidative stress in female athletes. The time-course effect of Tualang honey that provided optimal antioxidant activity and oxidative stress protection was between 1 and 2 hours after its consumption. | [54] |
| Tualang honey | In vivo | No significant difference between Tualang honey treatment and conventional treatment in terms of inflammatory feature and antioxidant status in alkali injury on the eyes of rabbits. | [53] |
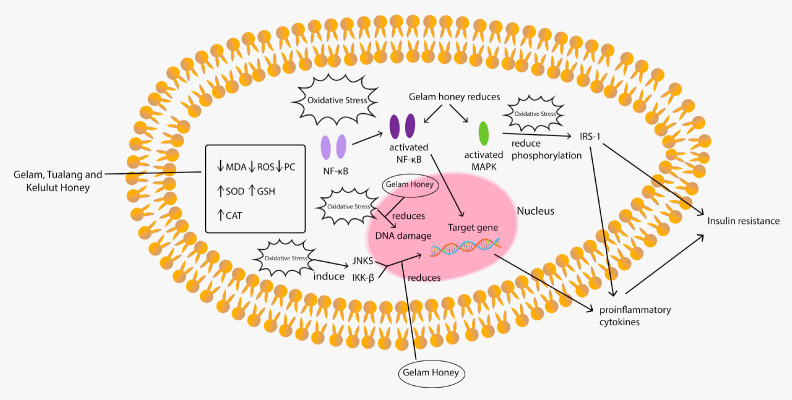
Figure 2.
Anti-oxidative mechanism of Tualang, Gelam and Kelulut honeys. ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione; PC: protein carbonyl; NF-kB: nuclear factor kappa B; MAPK: mitogen-activated protein kinases; JNKS: c-jun N-terminal kinases; IKK-β: I-kappa-B-kinase beta; IRS-1: insulin receptor substrate-1.
In diabetes, oxidative stress interrupts glucose metabolism’s normal coupling to insulin secretion by activating stress signaling nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (MAPK) pathways. The activation of NF-κB causes the production of inflammatory cytokines that promote insulin resistance whilst activating MAPK to reduce insulin receptor substrate-1 (IRS-1) serine phosphorylation, thereby causing insulin resistance [43,44]. A series of studies have proven Gelam honey’s protective effect on diabetes through this insulin resistance signaling pathway mechanism. Gelam honey exerts protective effects against diabetes and hyperglycemia-induced oxidative stress through improving insulin content and insulin resistance by producing the differential expression of insulin signaling pathways MAPK, NF-κB, IRS-1 (ser307) and Akt in HIT-T15 cells [45]. Gelam honey had been shown to attenuate the oxidative stress-induced inflammatory pathways when pretreatment with Gelam honey or quercetin reduced the expression of phosphorylated jun-N-terminal kinase, IkappaB kinase and IRS-1, thereby significantly reducing the expression of proinflammatory cytokines, such as tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β). A significant increase in phosphorylated Akt was also found, indicating the protective effects against inflammation and insulin resistance [46]. Batumalaie et al. (2014) reported that pretreatment of HIT-T15 cells with Gelam honey before culturing in hyperglycemic media yielded a significant decrease in the production of reactive oxygen species (ROS), glucose-induced lipid peroxidation and a significant increase in insulin content and the viability of cells cultured under hyperglycemic condition [47]. This series of studies demonstrated the potential anti-oxidative role of Gelam honey in managing diabetes through improving pathway-related insulin resistance and production.
Other studies have also explored the antioxidant capacity of Tualang honey. In animal study, Tualang honey was reported as having a hypoglycemic effect with reduced elevated MDA levels. It restored SOD and CAT activities in the pancreas of diabetic rats [48], suggesting the hypoglycemic effect of Tualang honey through its anti-oxidative effect on the pancreas. By contrast, another study using glibenclamide- and metformin-treated diabetic rats yielded no significant thiobarbituric acid reactive substances (TBARS) and CAT effects except that in GPx. However, the authors reported that a combination of glibenclamide, metformin and Tualang honey significantly up-regulated the CAT activity, down-regulated the GPx activity and reduced TBARS [49]. These findings suggested that Tualang honey potentiates the effect of glibenclamide and metformin to protect diabetic rat pancreas against oxidative stress and damage. Another study on diabetic rats revealed that Tualang honey ameliorated oxidative stress in kidneys of diabetic rats [50] and potentiated the effect of glibenclamide and metformin to protect diabetic rat kidneys against oxidative stress [51]. An in vitro study showed that Tualang honey improved human corneal epithelial progenitor cell migration and cellular resistance to H2O2-induced oxidative stress [52]. However, a study investigating the anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits did not find any significant difference between the Tualang honey-treated group and the group who underwent conventional treatment in terms of inflammatory feature and antioxidant status [53]. Consistent with the previous findings, Ahmad et al. (2017) reported the anti-oxidative properties of Tualang honey in a human study. The authors stated that consumption of 1.5 (high dose) and 0.75 (low dose) g/kg of Tualang honey demonstrated a comparable response in increasing antioxidant activity (ferric reducing antioxidant power) and suppressing oxidative stress (MDA and ROS) in female athletes. The time-course effect of Tualang honey that provides optimal antioxidant activity and oxidative stress protection was between 1 and 2 hours after its consumption [54].
Kelulut honey has also been demonstrated to have anti-oxidative properties when it ameliorated glucocorticoid-induced osteoporosis via a reduction in lipid peroxidation and increase in SOD [11]. Kelulut honey supplementation prevented sperm and testicular oxidative damage in streptozotocin-induced diabetic rats by increasing SOD activity and GSH level and decreasing protein carbonyl (PC) and MDA levels in the sperm and testis [55].
The antioxidant properties of Gelam honey were well demonstrated by animal and in vitro studies discussed above. However, future works should explore the anti-oxidative effect of Gelam honey on humans. The antioxidant properties of honey have been attributed to its phytochemical content, which is phenolic and flavanoid. A study reported that the antioxidant activity of several types of honey originating from different countries depends on the concentration of phenolic and flavonoid groups [56]. These phenolic and flavonoids were reported to derive from the plant that the bees feed on [57]. The proposed honey antioxidant action mechanisms include free radical sequestration, hydrogen donation, metallic ion chelation, flavonoid substrate action for hydroxyl and superoxide radical actions [58].
4.2. Anti-Cancer Properties
Most of the literature on Tualang, Gelam, and Kelulut honeys focused on their anti-cancer properties in many models and various mechanisms. Studies involved all sets of subjects, from animal to cell culture and human studies. A total of 18 studies were found: five animal studies, 11 in vitro studies and two human studies. A summary of the anti-cancer properties for Tualang, Gelam, and Kelulut honey is provided in Table 4, while their anti-cancer pathway is illustrated in Figure 3.
Table 4.
Summary of anti-cancer properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Kelulut honey | In vivo | Kelulut honey possessed chemo-preventive properties in rats induced with colorectal cancer and it was found to be not toxic to rats. | [71] |
| Tualang honey | In vivo | Tualang honey ameliorated breast cancer by increasing the susceptibility of proapoptotic proteins; apoptotic protease activating factor-1 (Apaf-1) interferon-gamma (IFN-γ) interferon gamma receptor-1 (IFNGR1) tumor protein P53 (p53) and decreased the expression of anti-apoptotic proteins; tumour necrosis factor alpha (TNF-α), cyclooxygenase-2 (COX-2) and B-cell lymphoma-extra-large (Bcl-xL). | [61] |
| Tualang honey | In vivo | Tualang Honey alleviated breast cancers in rats by reducing cancer cell growth and enhanced histological grading. | [60] |
| Tualang honey | In vivo | Tualang honey showed chemo-preventive properties in oral squamous cell carcinoma-induced rats by suppressing cancer cell proliferation and activity and preserving cellular adhesion. | [66] |
| Tualang honey | In vivo | Tualang Honey alleviated breast carcinogenesis by modulating haematologic, oestrogenic and apoptotic activities in the breast cancer animal model. | [59] |
| Tualang honey | In vitro | Tualang honey promoted apoptotic cell death induced by tamoxifen in breast cancer cell lines. | [63] |
| Gelam honey | In vitro | Gelam honey exhibited anti-proliferative activity and apoptotic induction in liver cancer cell line. | [72] |
| Tualang honey | In vitro | Tualang honey showed an anti-proliferative effect on oral squamous cell carcinoma and osteosarcoma cell lines by inducing early apoptosis. | [67] |
| Gelam honey | In vitro | Gelam honey acted as a radioprotector against gamma irradiation by attenuating radiation-induced cell death in human diploid fibroblasts. | [77] |
| Gelam honey combine with ginger | In vitro | Combined treatment of Gelam honey and ginger extract potentiated the anti-cancer effect of fluorouracil against colorectal cancer cells. | [73] |
| Gelam and Nenas honey | In vitro | Gelam and Nenas honeys are capable of suppressing HT 29 colon cancer cell growth by inducing apoptosis and suppressing inflammation. | [78] |
| Tualang honey | In vitro | Tualang honey demonstrated cytotoxic and apoptotic activities against human breast and cervical cancer cell lines with the mitochondrial apoptotic pathway’s involvement. | [62] |
| Gelam honey | In vitro | Gelam honey showed a synergistic cytotoxic effect with 5-fluorouracil against human adenocarcinoma colon cancer HT-29 cells. | [74] |
| Gelam honey combined with ginger | In vitro | Combined treatment of ginger and Gelam honey was more effective than treatments with either Gelam honey only or ginger only in inhibiting the growth of HT29 colon cancer cells by inducing early apoptosis, modulating the expression of genes involved in the KRAS/ERK/ PI3K/AKT pathways and suppressing inflammation via the NFκB pathway. | [75] |
| Tualang honey | In vitro | Tualang honey demonstrated apoptosis-inducing ability for acute and chronic myeloid leukaemia (K562 and MV4-11) cell lines. | [68] |
| Tualang honey | In vitro | Tualang honey protected keratinocytes from ultraviolet radiation-induced inflammation and DNA damage via modulation in early biomarkers of photocarcinogenesis. | [69] |
| Tualang honey | In vitro | Tualang honey was found to be cytotoxic to breast cancer cell line (MCF-7) but protected non-tumorigenic epithelial breast cell line (MCF-10A) from the toxic effects of tamoxifen active metabolite 4-hydroxytamoxifen. | [64] |
| Tualang honey | Human study | Tualang honey improved cancer-related fatigue and quality of life of patients with head and neck cancer post-chemotherapy or radiotherapy. | [70] |
| Tualang honey | Human study | Combination of Tualang honey and anastrozole showed more improvement in decreasing breast background parenchymal enhancement in patients with breast cancer than anastrozole alone. | [65] |
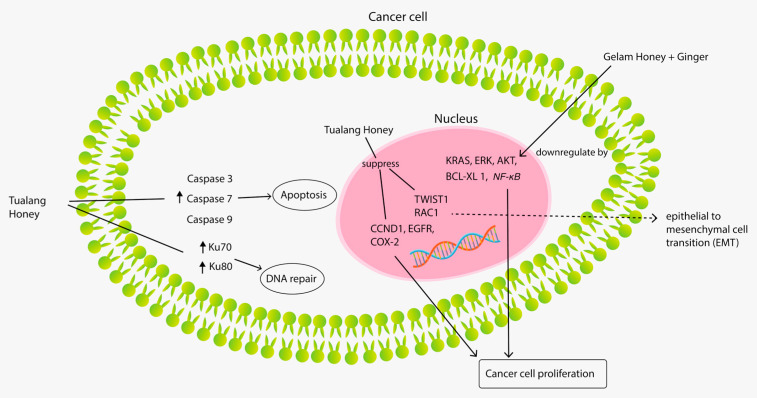
Figure 3.
Anti-cancer mechanism of Tualang, Gelam and Kelulut honeys. KRAS: K-Ras encoding gene; ERK: extracellular signal-regulated kinase; AKT: protein kinase B; Bcl-xL: B-cell lymphoma-extra-large; NF-kB: nuclear factor kappa B; TWIST1: Twist-related protein 1 gene; RAC1: Ras-related C3 botulinum toxin substrate 1 gene; CCND1: Cyclin D1 gene; EGFR: epidermal growth factor receptor gene; COX-2: cyclooxygenase-2.
Three in-vivo studies demonstrated the anti-cancer properties of Tualang honey in a breast cancer model [59,60,61]. The anti-cancer effect of Tualang honey was demonstrated through modulation in five aspects: tumor growth, tumor grading and haematologic, oestrogenic and apoptotic activities. Lower growth rate, tumor size, weight and multiplicity of tumor in the Tualang honey-treated group than those in the untreated control were reported in a rat breast cancer model [59,60,61]. Histologically, breast cancer rats treated with Tualang honey were primarily graded I and II compared with the untreated control, in which the majority was grade III [59,60]. Furthermore, Tualang honey increased the expression of proapoptotic proteins (Apaf-1, caspase-9, IFN-γ, IFNGR1, and p53) and decreased the expression of antiapoptotic proteins (ESR1, TNF- α, COX-2 and Bcl-xL) [59,61]. Tualang honey treatment was also found to modulate haematological parameters, such as haemoglobin (Hb), red blood cells (RBCs), packed cell volume (PCV), mean corpuscular volume (MCV), red cell distribution width (RDW), mean corpuscular hemoglobin concentration MCHC, polymorphs and lymphocytes values [59]. Manuka honey was reported to have the same anti-cancer properties as Tualang honey in an animal breast cancer model [61].
The anti-cancer effect of Tualang honey was successfully demonstrated in another three in vitro studies using the human breast cancer cell line [62,63,64]. These studies have shown that Tualang honey treatment-induced caspase-3, caspase-7 and caspase-9 activation and decreased the mitochondrial membrane potential in all tested cancer cells, indicating that Tualang honey-induced apoptosis occurred least via a mitochondrial-dependent pathway [62,63]. Depolarisation of the mitochondrial membrane in breast cancer cell lines increased when Tualang honey was used in conjunction with tamoxifen compared with treatment with tamoxifen alone [63]. Interestingly, Tualang honey was found to be cytotoxic to breast cancer cell line (MCF-7); it also protected non-tumorigenic epithelial breast cell line (MCF-10A) from the toxic effects of tamoxifen active metabolite 4-hydroxytamoxifen by increasing the efficiency of the DNA repair mechanism in these cells, as evidenced by the increment in DNA repair proteins Ku70 and Ku80 [64]. Therefore, clinical trials may focus on Tualang honey as a possible adjuvant to be used with tamoxifen to minimise tamoxifen dosage and thus minimise the adverse effects. In a clinical trial, the combination of Tualang honey and anastrozole in decreasing breast background parenchymal enhancement in breast cancer patients was more successful than anastrozole alone [65]. Three-tier evidence from animal, in vitro and human studies demonstrated the benefit of Tualang honey in breast cancer, highlighting the potential use of Tualang honey as a natural supplement to chemotherapeutic agents used for breast cancer treatment.
In an animal model of oral squamous cell carcinoma, Tualang honey showed chemopreventive capabilities by suppressing cancer cell proliferation and activity, as demonstrated by the decrease in the expression of CCND1, EGFR, and COX-2, which are genes related to cancer development and cellular proliferation. In addition, Tualang honey inhibited the aggressiveness of oral squamous carcinoma by down-regulating TWIST1 and RAC1, which are the genes representing epithelial-to-mesenchymal transition (EMT), and overexpressing β-catenin and E-cadherin [66]. Meanwhile, another study proved that Tualang honey has an anti-proliferative effect on oral squamous cell carcinoma and osteosarcoma cell lines by inducing early apoptosis [67].
Other various anti-cancer potentials of Tualang honey have been discovered through in vitro studies, including anti-cancer activity against cervical cancer cell lines; this activity shares the same mechanism as in the in vitro study on breast cancer cell lines discussed above [62]. Man et al. (2018) reported the antileukemic effect of Tualang honey through its apoptosis-inducing ability for acute and chronic myeloid leukemia cell lines [68]. In another in vitro study, Tualang honey protected keratinocytes from ultraviolet radiation-induced inflammation and DNA damage by modulating the early biomarkers of photocarcinogenesis, thus providing significant protection from the adverse effects of ultraviolet B (UVB) radiation and a suggestion for its photochemopreventive potential [69]. A randomised clinical trial using Tualang honey has been conducted on cancer-related fatigue in patients with head and neck cancer. These patients received either 20 mg of Tualang honey or 100 mg of vitamin C tablet for 8 weeks, in which the level of fatigue and quality of life were measured using questionnaires with additional measurement of white cell count and C-reactive protein level. No significant changes were detected between the vitamin C and Tualang honey groups for the white cell count and C-reactive protein. However, the Tualang honey group showed a substantial improvement in fatigue and quality of life compared with the vitamin C group [70]. The author suggested that Tualang honey could be used as a supplement to improve the quality of life and reduce fatigue in patients with head and neck cancer.
Anti-cancer properties were also exhibited by Kelulut and Gelam honeys. A study by Yazan et al. (2017) showed that 1183 mg/kg of Kelulut honey treatment twice daily for 8 weeks significantly reduced the total number of aberrant crypt foci, aberrant crypts and crypt multiplicity in colorectal cancer-induced rats [71]. Several studies have demonstrated the anti-proliferative and apoptotic induction properties of Gelam honey on liver, colorectal and colon cancer cell lines [72,73,74]. A combination of Gelam honey and ginger produced synergism that provided an anti-cancer activity toward colon cancer cell through stimulation of early apoptosis (upregulation of caspase-9 and IκB genes) accompanied by downregulation of the expression of KRAS, extracellular signal-regulated kinase (ERK), protein kinase B (Akt), B-cell lymphoma-extra-large (Bcl-xL), and nuclear factor kappa B (NFkB) genes, which are related to cancer cell proliferation [75]. This combination also provided anti-cancer effects toward colorectal cancer by inhibiting mammalian target of rapamycin (mTOR) and Wnt/β catenin signaling pathways and the induction of apoptosis [76].
The combination of Gelam honey and ginger potentiated the anti-cancer effect of 5-fluorouracil against HCT 116 colorectal cancer cells by reducing proliferation and increasing apoptosis [73]. In a separate study, Gelam honey combined with 5-fluorouracil also provided a synergistic cytotoxic effect against human adenocarcinoma colon cancer HT-29 cells. The combined treatment showed a significantly higher cytotoxic effect and apoptosis induction on HT-29 cells than the stand-alone treatment with Gelam honey and 5-fluorouracil [74]. Another study showed that gamma irradiation up-regulated ATM, p53, p16ink4a and cyclin D1 genes and subsequently initiated cell cycle arrest at the G0/G1 phase and induced apoptosis. However, pre-treatment with Gelam honey caused downregulation of these genes in irradiated HDFs. Gelam honey treatment caused cells to progress to the S phase with fewer cells in the G0/G1 phase, whilst apoptosis was inhibited [77]. This finding suggested the potential of Gelam honey as a radioprotector agent against gamma irradiation. Another study showed that Gelam honey inhibited the proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis and suppressing inflammation [78]. In vitro studies have successfully revealed the anti-cancer properties of Gelam honey or in combination with ginger and their potentiating effect toward 5-fluorouracil. Thus, human studies using Gelam honey as a supplement to 5-fluorouracil should be conducted to prove the anti-cancer benefit of Gelam honey toward liver, colorectal and colon cancer cell lines as discussed above.
Honey generally contains various kinds of phytochemical with high phenolic and flavonoid content, thus contributing to its high antioxidant activity [79]. An agent with potent antioxidant properties could potentially prevent cancer, as free radicals induce oxidative stress, which causes cancer formation [80]. Moreover, a different type of polyphenol found in honey was reported to have anti-cancer properties against several types of cancer through various mechanisms. These polyphenols include caffeic acid, caffeic acid phenyl esters, chrysin, galangin, quercetin, kaempferol, acacetin, pinocembrin, pinobanksin and apigenin [81]. Honey is a natural immune booster, a natural anti-inflammatory agent, a natural anti-microbial agent, a natural promoter for chronic ulcers and wound healing and a natural antioxidant; these all serve as a basis for its anti-cancer properties.
4.3. Anti-Microbial Properties
The antibiotic resistance of bacteria is increasing nowadays; thus, the discovery of alternative antibacterial agents is urgently needed. Honey has been reported in numerous studies to possess anti-microbial activity. Although the anti-microbial activity of honey has been effectively established against an extensive spectrum of microorganisms, it differs depending on the type of honey. The anti-microbial activity of Tualang, Gelam, and Kelulut honeys has been documented in various studies. Table 5 provides a summarisation of the anti-microbial properties of these honeys. Tualang honey has been reported to possess antibacterial activity against Salmonella Typhi, Streptococcus pyogenes, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecium, Enterococcus faecalis, Escherichia coli, Salmonella enterica serovar Typhimurium, Klebsiella pneumoniae, and vancomycin-resistant enterococci [82,83].
Table 5.
Summary of anti-microbial properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Tualang honey | In vivo | Topical gentamicin, topical Tualang honey and the combination of the two all showed similar clinical and anti-microbial effects in treating Pseudomonas-induced keratitis in rabbits. | [89] |
| Tualang and Gelam honey | In vitro | Tualang and Gelam honeys showed antibacterial activity against common human pathogenic bacteria, namely Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecium, Enterococcus faecalis, Escherichia coli, Salmonella enterica serovar Typhimurium and Klebsiella pneumoniae, including vancomycin-resistant enterococci, with Gelam honey showing the highest antibacterial effect amongst the tested Malaysian honey samples. | [83] |
| Tualang honey | Human | Tualang honey demonstrated bactericidal and bacteriostatic effects for partial-thickness burn wounds. It is useful as a dressing, as it is easier to apply and is less sticky than Manuka honey. However, for Gram-positive bacteria, Tualang honey is not as effective as usual care products, such as silver-based dressing or medical-grade honey dressing. | [90] |
| Tualang honey | In vitro | Pediococcus acidilactici, a lactic acid bacteria strain, was isolated from Tualang honey; it possessed antifungal activity against pathogenic candida species. | [93] |
| Gelam, Kelulut and Tualang | In vitro | Malaysian honey, namely Gelam, Kelulut, and Tualang, exhibited high antibacterial potency derived from total and non-peroxide activities, indicating that peroxide and other constituents are mutually important as contributing factors to the antibacterial property of honey. | [9] |
| Tualang honey | In vitro | Tualang honey demonstrated variable activities against different microorganisms but within the same range as with Manuka honey. Tualang showed higher antibacterial effect on A. baumannii and S. maltophilia. | [91] |
| Tualang honey | In vitro | Tualang honey showed antibacterial activity against E. coli, S. typhi, and S. pyogenes, with the most potent activity observed against S. typhi. However, it was merely ineffective against S. sonnei, P. aeruginosa and S. aureus. | [82] |
| Gelam and Tualang honey | In vitro | Gelam and Tualang honeys have been found to contain Lactobacillus acidophilus, with anti-microbial activity against multiple antibiotic-resistant S. aureus, S. epidermis and Bacillus subtilis. The anti-microbial compounds found were stable to heating, proteolytic enzyme treatment and pH adjustments. | [88] |
| Tualang honey | Human | Tualang honey administration, especially at 40 and 60 g daily doses for 6 months in asymptomatic patients with human immunodeficiency virus (HIV), reduced viral load and improved the CD4 counts and quality of life of these patients. | [92] |
The antibacterial mechanism of honey is attributed to its high osmolarity, acidity and content of H2O2 and non-peroxide components [84]. H2O2 is derived from the hydrolysation of glucose-by-glucose oxidase, which could only occur when honey is diluted. H2O2 level is determined by relative glucose oxidase levels synthesised by the bee and catalase derived from floral [85]. In pure honey, high osmotic pressures and high acidity are responsible for the antibacterial properties of honey at this stage [84]. Some types of honey, such as Manuka honey, possess high non-peroxide antibacterial activity that could retain the antibacterial activity even after treatment with catalase [86]. They are known as active non-peroxide honey, containing various non-peroxide components that possess antibacterial actions [9]. These components include phenolic acids, flavonoids, methylglyoxal, and methyl syringate [84]. The antibacterial potencies of Tualang, Gelam, and Kelulut honeys were generally comparable to those of Manuka honey, with Tualang honey being the closest. Gelam, Kelulut, and Tualang honeys have a high antibacterial activity of total and non-peroxide activities, indicating that peroxide and non-peroxide components are mutually important antibacterial mechanism of these honeys [9]. Another study looked into the relationship between the phytochemicals and the antibacterial activity in wild honey of Sabah, Malaysia. Yap et al. (2012) found that anti-microbial activity was strongly positively correlated with the phenolic content, whilst the total flavonoid content was moderately positively correlated with antibacterial activity [87].
Gelam and Tualang honeys have been found to contain Lactobacillus acidophilus with anti-microbial activity against multiple antibiotic-resistant S. aureus, Staphylococcus epidermis and Bacillus subtilis. The anti-microbial compounds found were stable to heating, proteolytic enzyme treatment and pH adjustments [88]. This finding further strengthened the presence of anti-microbial properties in Tualang and Gelam honeys. A study comparing the anti-bacterial effects of topical gentamicin, topical Tualang honey and a combination of the two on pseudomonas-induced keratitis in rabbit eyes showed similar clinical and anti-microbial effects in all groups [89]. However, these results could not be verified because the study utilised a low sample size and no control group.
Two comparative studies on antibacterial potency revealed comparable results against Gram-negative bacteria tested with Aquacel-Manuka honey and Aquacel-Tualang honey dressing on the burn wound. However, for Gram-positive bacteria, Tualang honey was not as effective as the usual care products such as silver-based dressing or medical grade and Aquacel-Manuka honey dressings [90]. In another comparison study, Tualang honey showed equivalent or better activities than Manuka honey in some wound and enteric microorganisms, especially against Stenotrophomonas maltophilia and Acinetobacter baumannii [91]. Meanwhile, a study comparing the antibacterial potency amongst Gelam, Tualang and Durians honey showed that Gelam honey possessed the highest antibacterial effect [83].
Apart from its effective antibacterial property, honey possesses antifungal and antiviral properties. Tualang honey administration, especially at 40 and 60 g daily doses for 6 months in patients with asymptomatic human immunodeficiency virus (HIV), was shown to reduce viral load and improve their CD4 counts and quality of life [92]. Pediococcus acidilactici, a lactic acid bacteria strain, was isolated from Tualang honey and possessed antifungal activity against pathogenic Candida species [93].
The emergence of antibiotic-resistant bacteria has become a major problem among healthcare practitioners. Honey seems to be a promising alternative to solve this problem. As discussed in this section, Tualang and Gelam honeys were reported to be effective against a wide range of multi-resistant bacteria, indicating the possible utilisation of honey to combat antibiotic-resistant bacteria. Even though the current findings presented a strong case for honey’s anti-microbial properties, future works should explore anti-microbial resistance toward honey and its side effects to be safely utilised as an alternative or to provide a synergistic effect with anti-microbial therapy [9,94].
4.4. Anti-Inflammatory Properties
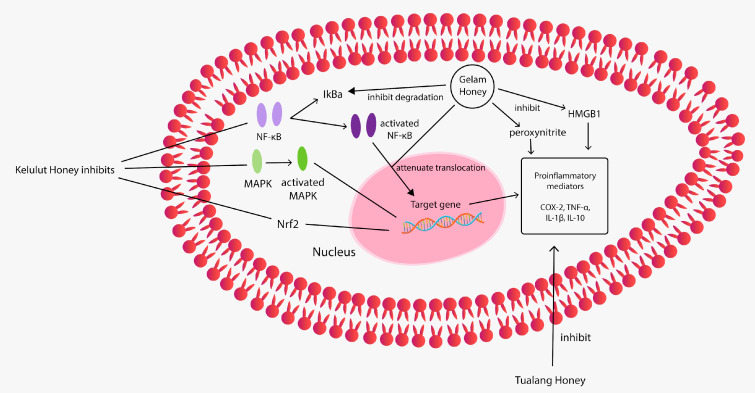
Tualang, Gelam, and Kelulut honeys demonstrated anti-inflammatory properties. Table 6 provides a summarisation of their anti-inflammatory properties and Figure 4 provides the pathway illustration. Gelam honey inhibited the production of proinflammatory mediators nitric oxide (NO), prostaglandin E2 (PGE2), TNF-α and IL-6 in carrageenan-induced acute paw oedema in rats. In this study, Gelam honey’s anti-inflammatory effect was similar to that of the anti-inflammatory drug indomethacin [95]. Another study showed that Gelam honey inhibited NO and PGE2 in inflammatory tissues of carrageenan-induced acute paw oedema in rats. The author claimed the phenolic compounds in Gelam honey to be responsible for the anti-inflammatory effects [96]. A study by Hussein et al. (2013) demonstrated Gelam honey’s inflammation inhibitory effects by attenuating NF-κB translocation to the nucleus and inhibiting IκBα degradation, which inhibits the production of proinflammatory mediators, including COX-2 and TNF-α [97]. Studies on the anti-inflammatory properties of Gelam honey were always consistent. For instance, the intravenous injection of Gelam honey inhibited lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines (TNF-α, IL-1β and IL-10), NO and high-mobility group protein B1 [98]. Besides, treatment with Gelam honey in lipopolysaccharide (LPS)-induced organ failure rat showed protective effects on organs by improving the organ blood parameters, reducing the infiltration of neutrophils and decreasing the myeloperoxidase activity and mortality compared with non-treatment. Thus, Gelam honey may have a therapeutic effect in protecting organs during inflammatory diseases [99]. Another mechanism for the anti-inflammatory properties of Gelam honey is through peroxynitrite synthesis inhibition during immune response in LPS-treated rats [100]. The anti-inflammatory properties of Gelam honey were further proven in two studies using asthma-induced mice and rabbits. Both studies reported that Gelam honey administration via oral gavage in mice and aerosolised Gelam honey in rabbits reduced airway inflammation by reducing the inflammatory cell [101,102]. Gelam honey-treated mice show similar improvement with the dexamethasone-treated group in terms of epithelium thickness, number of mast cells and mucus expression [101].
Table 6.
Summary of anti-inflammatory properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Gelam honey | In vivo | Gelam honey showed anti-inflammatory effects by reducing rat paw oedema size and inhibiting the production of proinflammatory mediators nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6) in carrageenan-induced acute paw oedema in rats. | [95] |
| Gelam honey | In vivo | Gelam honey and its extracts inhibited oedema, pain, NO and PGE(2) in rats’ paws induced with carrageenan in the non-immune inflammatory and nociceptive model and lipopolysaccharide (LPS) in the immune-inflammatory model. | [96] |
| Gelam honey | In vivo | Gelam honey exhibited its inflammation inhibitory effects in carrageenan-induced rat paw inflammation by attenuating NF-kB translocation to the nucleus and inhibiting IkBa degradation, with a subsequent decrease in inflammatory mediators COX-2 and TNF-α. | [97] |
| Kelulut honey | In vivo | Kelulut honey protected against LPS-induced chronic subclinical systemic inflammation in rats mediated via amelioration of inflammation, oxidative stress and NF-κB, p38 MAPK and Nrf2 signalling. | [106] |
| Tualang honey | In vivo | Tualang honey treatment reduced TNF-α, IL6 and IFN-γ in brain homogenates of a chronic stress rat model. | [104] |
| Tualang honey | In vivo | Pre-treatment with Tualang honey reduced neuroinflammation by reducing the elevation of TNF-α, IL-1β, glial fibrillary acidic protein, allograft inflammatory factor 1 and COX-2 in the cerebral cortex, cerebellum and brainstem of kainic acid-induced status epilepticus rat. | [103] |
| Gelam honey | In vivo | Gelam honey alleviated the histopathological changes in a mouse model of allergic asthma. | [101] |
| Gelam honey | In vivo | Treatment with aerosolised Gelam honey reduced the number of airway inflammatory cells present in bronchoalveolar lavage fluid and inhibited goblet cell hyperplasia in a rabbit model of ovalbumin-induced chronic asthma. | [102] |
| Tualang honey | Human study | Tualang honey supplementation exhibited opposite effects on TNF-α and highly sensitive C-reactive protein amongst chronic smokers. | [105] |
| Gelam honey | In vivo | Gelam honey inhibited lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide and high-mobility group protein B1. | [98] |
| Gelam honey | In vivo | LPS-induced organ failure rats treated with Gelam honey demonstrated protection on organs through improved organ blood parameters, reduced infiltration of neutrophils, decreased myeloperoxidase activity and reduced mortality compared with untreated rabbits. | [99] |
| Gelam honey | In vivo | Gelam honey showed anti-inflammatory properties through peroxynitrite synthesis inhibition during immune response in LPS-treated rats. | [100] |
Figure 4.
Anti-inflammatory mechanism of Tualang, Gelam and Kelulut honeys. TNF-α: tumor necrosis factor alpha; COX-2: cyclooxygenase-2; TNF- α: tumour necrosis factor alpha; IL-1β: interleukin-1 beta; IL-10: interleukin 10; NF-kB: nuclear factor kappa B; IκBα: NF-kappa-B inhibitor alpha; MAPK: mitogen-activated protein kinases; Nrf2: nuclear factor erythroid 2–related factor 2.
Tualang honey also demonstrated anti-inflammatory properties; pretreatment with Tualang honey reduced neuroinflammation by reducing the elevation of TNF-α, IL-1β, glial fibrillary acidic protein, allograft inflammatory factor 1 and COX-2 in the rat cerebral cortex, cerebellum and brainstem in kainic acid (KA)-induced status epilepticus rat [103]. Another study also reported a reduction in TNF-α, IL6 and IFN-γ in the brain homogenates of a Tualang honey-treated chronic stress rat model [104]. However, the anti-inflammatory properties of Tualang honey could not be translated to humans as a randomised controlled study demonstrated that Tualang honey supplementation has opposite effects on TNF-α and highly sensitive C-reactive protein, indicating the inconclusive effect of honey on inflammation amongst chronic smokers; thus, further studies are needed on other inflammatory markers or other study population [105]. Meanwhile, Kelulut honey has been reported to ameliorate the serum levels of CRP, TNF-α, IL-1β, IL-6, IL-8 and MCP-1 in LPS-induced chronic subclinical systemic inflammation in rats by modulating NF-κB, p38 MAPK and Nrf2 signaling [106].
4.5. Anti-Diabetic Properties
Only Tualang and Kelulut honeys were found to have an anti-diabetic effect in three animal studies [50,107,108] and two human studies [109,110], as summarised in Table 7. Studies using a diabetic model of rat showed that Tualang honey possessed moderate hypoglycaemic effect [50], improved liver enzyme profile [107], and possessed a synergetic benefit on glycaemic and metabolic profiles when administered together with metformin or glibenclamide [108].
Table 7.
Summary of anti-diabetic properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Tualang honey | In vivo | Tualang honey produced a moderate hypoglycaemic effect and ameliorated oxidative stress in the kidneys of streptozotocin-induced diabetic rats. | [50] |
| Tualang honey | In vivo | Tualang honey demonstrated hepatoprotective effect in diabetic rats by reducing aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities. | [107] |
| Tualang honey | In vivo | Combination of glibenclamide or metformin with Tualang honey improved glycaemic control and provided additional metabolic benefits not achieved with either glibenclamide or metformin alone. | [108] |
| Kelulut honey | Human study | Thirty g of Kelulut honey daily intake for 30 days caused no changes in fasting glucose, fasting lipid profiles, body mass index (BMI), waist circumference, blood pressure, total cholesterol, triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) in patients with impaired fasting glucose. | [109] |
| Tualang honey | Human study | Tualang honey is an intermediate glycaemic index food (GI = 65 ± 7). | [110] |
Tualang honey was found to have intermediary glycemic index values of 65 ± 7 [110]. Hussain et al. (2012) reported that supplementation of 20 g/day Tualang honey for 4 months in healthy postmenopausal women caused a significant increase in fasting blood sugar (FBS) [111]. However, by prolonging the treatment to 12 months, a decrease in FBS levels was reported in healthy and diabetic postmenopausal women [112]. Wahab et al. (2018) suggested a differential effect of honey on glucose metabolism for short- and long-term use. [112]. Recent findings by Rashid et al. (2019) supported a prior claim that the ingestion of Kelulut honey daily for 30 days has no significant effect on fasting glucose and fasting lipid profile in patients with impaired fasting blood glucose [109]. The authors suggested short-duration ingestion of Kelulut honey as the factor for the insignificant result. By contrast, one in vivo study showed that supplementation of Kelulut honey for the last 35 days in a 16-week high-carbohydrate high-fructose diet fed in rats decreased the fasting blood glucose compared with non-supplemented control [113]. However, the effect of Tualang honey as an anti-diabetic is unclear. Future studies should extend knowledge on the anti-diabetic properties of Tualang honey on patients with diabetes to reinforce the anti-diabetic ability of Tualang honey, as the animal studies discussed above [50,107,108] have successfully proven that Tualang honey possesses anti-diabetic effect in animals.
Although the anti-diabetic effect of honey may sound paradoxical as it contains a high content of sugar, several studies proved it otherwise. The honey from Nigeria has been reported to have an anti-hyperglycaemic effect in diabetic rats [114]. Moreover, the honey from Iran was reported to cause a mild reduction in FBS by 4.2% among overweight adults [115]. The fructose, oligosaccharides, antioxidants and trace minerals present in honey may contribute to its glucose-lowering effect [114]. Disaccharide trehalulose has been recently revealed as the main component of Kelulut honey with a low insulinemic and glycaemic index [1]. However, contradictory results of anti-diabetic properties of honey were also reported, as the honey from Iran increased the HbA1c level after 8 weeks of honey supplementation amongst patients with type 2 diabetes [116]. Thus, studies to discover the relation between honey and glucose metabolism in humans are needed to enlighten the natural characteristic of honey, whether it is beneficial or contraindicated in patients with diabetes.
4.6. Anti-Obesity Properties
Only Gelam and Kelulut honeys have been found to show anti-obesity properties in animal studies [117,118,119], as provided in Table 8. Supplementation of Kelulut honey yielded a higher reduction in body mass index (BMI), the percentage of body weight gain, adiposity index, and relative organ weight in a high-fat diet-induced obese rat model than that of orlistat, an anti-obesity drug [117]. Meanwhile, another study has shown that Kelulut honey could prevent changes, such as high omental fat mass, serum triglyceride, systolic blood pressure, diastolic blood pressures, adipocyte area and adipocyte perimeter, caused by metabolic syndrome-induced rats [119].
Table 8.
Summary of anti-obesity properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Kelulut honey | In vivo | Kelulut honey treatment showed improved BMI, percentage of body weight gain, adiposity index, relative organ weight, LDL, HDL and hepatoprotective effect in high-fat diet-induced obese rats. | [117] |
| Gelam honey | In vivo | Reductions in excess weight gain, adiposity index levels, plasma glucose, triglycerides, cholesterol, plasma leptin and resistin, liver enzymes, renal function test and relative organ weight were found in Gelam honey-treated groups compared with rats fed with the high-fat diet. | [118] |
| Kelulut honey | In vivo | Kelulut honey supplementation significantly prevented changes in omental fat mass, serum triglyceride, systolic blood pressure, diastolic blood pressures, adipocyte area and adipocyte perimeter induced by high-carbohydrate and high-fat diet in rat. | [119] |
Gelam honey has been shown to possess lipid-lowering and anti-oxidative capabilities in obesity-induced rats and weight-reducing ability compared with Acacia honey. However, honey showed better effects than orlistat, as the orlistat group showed hepatotoxicity effects [118].
A study conducted by Iranian researchers found that honey supplementation in humans also reduced body weight, body fat and total cholesterol [115]. As of now, Tualang honey has not been demonstrated to have an anti-obesity effect. Meanwhile, Gelam and Kelulut honeys have not been explored for their anti-obesity potential in humans.
4.7. Wound-Healing Properties
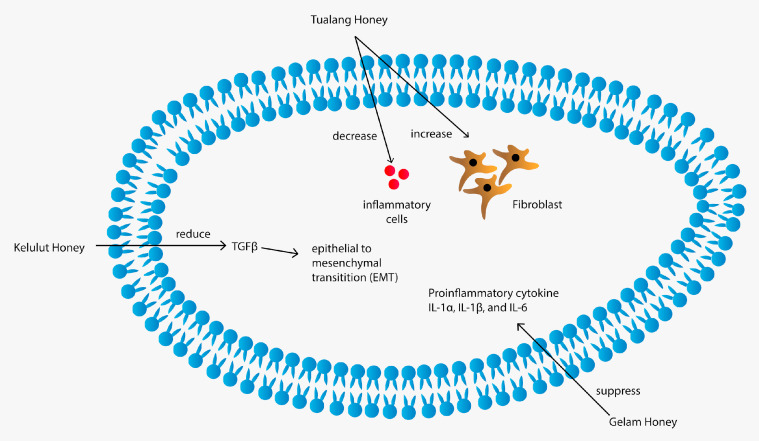
Honey has been used to treat wounds beginning from ancient times [120]. This practice was rooted primarily in tradition and folklore when investigators began to explore its medicinal potential [121]. The honey from different geographical areas has been reported to have considerable therapeutic effects on chronic wounds, ulcers and burns [122]. A study reported that honey has almost equal or slightly superior effects compared with conventional treatments for acute wounds and superficial partial-thickness burns [3]. Tualang, Gelam and Kelulut honeys have been demonstrated to have wound-healing properties, as listed in Table 9. Their wound-healing mechanism is provided in Figure 5.
Table 9.
Summary of wound-healing properties.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Gelam honey | In vivo | Application of Gelam honey hydrogel dressings enhanced wound closure and accelerated the rate of re-epithelialisation, with a significant decrease in inflammatory response and expression of proinflammatory cytokines (IL-1α, IL-1β and IL-6). | [128] |
| Tualang honey | In vivo | Tualang honey demonstrated enhanced control of Pseudomonas aeruginosa and wound contraction effects on full-thickness burn wound in vivo. | [123] |
| Gelam honey | In vivo | Gelam honey dressing on excisional wounds accelerated the process of wound healing, with less scab and only thin scar formations. | [127] |
| Gelam honey | Ex vivo | Gelam honey (0.0015%) promoted ex-vivo corneal keratocyte proliferation whilst preserving phenotypical features. | [130] |
| Gelam honey | Ex vivo | Gelam honey promoted the proliferative phase of corneal reepithelialisation whilst preserving phenotypical features. | [131] |
| Gelam honey | In vitro | Gelam honey promoted ex-vivo corneal fibroblast wound healing. | [129] |
| Tualang honey | in vivo | Topical application of Tualang honey on full-thickness burn wounds contaminated with P. aeruginosa and A. baumannii provided the fastest rate of healing amongst Chitosan gel or hydrofibre silver treatments. | [124] |
| Tualang honey | In vivo | Oral treatment with Tualang honey enhanced anastomotic wound healing by increasing the number of fibroblasts and decreasing inflammatory cells, leading to increased wound strength. | [125] |
| Kelulut honey | In vivo | Rats pre-treated with Kelulut honey showed significantly reduced total area of ulcer and ulcer index compared with untreated ethanol-induced gastric ulcer rats. | [133] |
| Kelulut honey | In vitro | Kelulut honey (0.0015%) reduced TGFβ-induced EMT in human primary keratinocytes, indicating its therapeutic potential in preventing keloid scar formation. | [132] |
| Tualang honey | Human | Tualang honey enhanced the healing process in patients who underwent tonsillectomy. | [126] |
Figure 5.
Wound-healing mechanism of Tualang, Gelam and Kelulut honeys. TGFβ: transforming growth factor beta; IL-1α: interleukin-1α; IL-1β: interleukin-1β; IL-6: interleukin-6.
In two studies, Tualang honey was compared with hydrofibre, hydrofibre silver or chitosan gel on full-thickness burn wounds in rats inoculated with Pseudomonas aeruginosa, K. pneumoniae or Acinetobacter baumannii. The first study by Khoo et al. (2010) found that the Tualang honey-treated group had more improved wound contraction and control over P. aeruginosa growth than the hydrofibre- and hydrofibre silver-treated groups [123]. A study by Sukur et al. (2011) showed that topical application of Tualang honey on burn wounds contaminated with P. aeruginosa and A. baumannii provided a faster healing rate than chitosan gel or hydrofibre silver treatment [124]. Meanwhile, another study showed that the oral treatment of Tualang honey enhanced anastomotic wound healing in large bowel anastomosis in rats by increasing the number of fibroblasts and decreasing inflammatory cells, leading to increased wound strength [125]. A clinical trial looking into Tualang honey’s effect on promoting the post-tonsillectomy healing process has been conducted by comparing sultamicillin-treated group and sultamicillin + Tualang honey-treated group. Tualang honey was topically administered intraoperatively and 4 ml orally three times daily for 7 days after surgery. The results obtained showed that the healing process was much faster in the Tualang honey + sultamicillin-treated group [126]. The multiple studies discussed above found Tualang honey to yield better wound-healing improvement than the current antibiotic or topical treatment, which could suggest the use of Tualang honey as a wound dressing.
Gelam honey has also been demonstrated to have wound-healing properties. A study reported that Gelam honey-dressed wounds healed faster with less scab and only thin scar formations than the untreated, saline-treated and intrasite gel-treated full-thickness excisional wounds in rats [127]. Zohdi et al. (2012) developed a Gelam honey-based hydrogel. They found that the application of Gelam honey hydrogel dressings significantly increased wound closure and accelerated the rate of re-epithelialisation compared with control hydrogel and OpSite film dressing in deep partial-thickness burn wounds in rats [128]. The application of Gelam honey-based hydrogel decreased the inflammatory response as seen by the suppressed expression of proinflammatory cytokines IL-1α, IL-1β and IL-6 [128]. In another study, Gelam honey was shown to promote in vitro corneal fibroblast wound healing, where corneal fibroblast cultured with Gelam honey demonstrated faster wound closure than the control group [129]. Moreover, two studies have shown that Gelam honey at a concentration of 0.0015% promoted ex-vivo corneal keratocytes and corneal epithelial cell proliferation whilst preserving their phenotypical features [130,131]. These findings could suggest Gelam honey formulation gel for topical treatment in wound healing for humans.
Wound healing is associated with keloid scar formation. Keloid scar formation is due to the excessive induction of EMT by TGFβ in keratinocytes. In a study, treatment of 0.0015% Kelulut honey has been demonstrated to reduce the TGFβ-induced EMT in human primary keratinocytes, indicating Kelulut honey’s therapeutic potential in preventing keloid scar formation [132]. Rats pretreated with Kelulut honey showed significantly reduced total area of ulcer and ulcer index compared with an untreated ethanol-induced gastric ulcer rat [133]. Honey is generally reported to have remarkable effects compared with conventional treatments for acute wounds, superficial partial-thickness burns and infected post-operative wounds [3,4].
4.8. Effects on Cardiovascular System
Only Tualang honey was reported to affect the cardiovascular system in three animal studies [134,135,136], one study of combined in-vivo and in vitro techniques [137] and two human studies [111,112]. The studies are listed in Table 10. All animal studies reported a positive effect of Tualang honey on the cardiovascular system, whilst human studies reported varied results. Khalil et al. (2015) showed that the pretreatment of ischaemic rats with Tualang honey yielded significant protective effects on cardiac troponin I, triglycerides and total cholesterol. The authors contributed to improving the endogenous antioxidant enzyme activity and inhibition of lipid peroxidation by Tualang honey [134]. Two studies reported that Tualang honey supplementation considerably reduced elevated systolic blood pressure via amelioration of spontaneous hypertensive rats [135,136]. Devasvaran et al. (2019) stated that in vitro Tualang honey could inhibit H2O2-induced vascular hyperpermeability in vitro and in vivo by suppressing adherence junction protein redistribution via calcium and cAMP [137].
Table 10.
Summary of the effects on cardiovascular system.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Tualang honey | In vitro and in vivo | Tualang honey could inhibit H2O2-induced vascular hyperpermeability in vitro and in vivo by suppressing adherence junction protein redistribution via calcium and cyclic adenosine monophosphate (cAMP). | [137] |
| Tualang honey | In vivo | Pre-treatment with Tualang honey demonstrated cardioprotective effects on isoproterenol-induced myocardial infarction in rats by improving cardiac marker enzyme and ameliorating oxidative stress. | [134] |
| Tualang honey | In vivo | Tualang honey supplementation considerably reduced elevated systolic blood pressure via amelioration of oxidative stress in spontaneously hypertensive rats’ kidneys. | [135] |
| Tualang honey | In vivo | Tualang honey ameliorated oxidative stress and reduced elevated blood pressure in spontaneous hypertensive rats but not in diabetic rats. | [136] |
| Tualang honey | Human study | Tualang Honey supplementation showed a superior effect in lowering diastolic blood pressure and fasting blood sugar over honey cocktail (a mixture of honey, beebread and royal jelly) in postmenopausal women. | [112] |
| Tualang honey | Human study | Four months of Tualang honey supplementation in postmenopausal women showed no changes in blood pressure, waist circumference, BMI, lipid profile, blood sugar and bone density compared with low-dose hormone replacement therapy. | [111] |
A randomised controlled trial comparing the effects of Tualang honey and hormone replacement therapy for 4 months showed no demonstrable effects on blood pressure measurement, BMI and waist circumference. No significant difference was found in the lipid profile, blood sugar profile and bone density between the two groups. However, a statistically significant increase in total cholesterol, low-density lipoprotein cholesterol (LDL-C) and FBS levels was observed in the honey-treated group at 4 months of the study compared with the baseline value [111]. A study on humans compared the effect of 20 g Tualang honey only and honey cocktail (a mixture of Tualang honey, beebread and royal jelly) for 12 months. Supplementation of Tualang honey only showed a significant effect in lowering diastolic blood pressure and FBS compared with supplementation of honey cocktail. However, no demonstrable effect of Tualang honey on the lipid profiles and anthropometric measurements was found [112].
4.9. Effects on Reproductive System
Tualang, Gelam and Kelulut honeys demonstrated benefits toward male and female reproductive systems. All of these benefits are listed in Table 11. Findings from animal studies have shown that Tualang honey is beneficial for the postmenopausal syndrome. Amongst the benefits are preventing uterine atrophy, increasing bone density and suppressing body weight elevation [138]. Ovariectomised rats that received Tualang honey showed more trabecular bone structure improvements than those who received calcium supplements [139]. These findings may suggest the potential of Tualang honey as an alternative to hormone replacement therapy for postmenopausal women.
Table 11.
Summary of the effects on reproductive system.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Tualang honey | In vivo | The Tualang honey-treated group showed more trabecular bone structure improvements than experimental postmenopausal rats who received calcium. | [139] |
| Tualang honey | In vivo | Tualang honey prevented uterine atrophy, increased bone density and suppressed increased body weight in ovariectomised rats. | [138] |
| Kelulut honey | In vivo | Kelulut honey prevented damage of sperm and testis in diabetic rats. | [55] |
| Tualang honey | In vivo | Tualang honey protected the uterus from bisphenol-A (BPA)-induced toxicity. | [140] |
| Tualang honey | In vivo | Tualang honey demonstrated a protective effect against cigarette smoke-induced impaired sexual behaviour and fertility in male rats. | [143] |
| Tualang honey | In vivo | Tualang honey at a dose of 1.2 g kg daily increased epididymal sperm count without affecting spermatid count and reproductive hormones. | [142] |
| Tualang honey | In vivo | Tualang honey supplementation during pregnancy reduced the adverse effects of prenatal restraint stress on reproductive organ weight and sperm parameters in male rat offspring. | [144] |
| Tualang honey | In vivo | Tualang honey reduced BPA-induced ovarian toxicity by reducing the ovarian follicles’ morphological abnormalities and improving the normal oestrous cycle. | [141] |
| Tualang honey | Human study | The Tualang honey effect amongst oligospermic males was comparable with that of Tribestan in improving sperm concentration, motility and morphology. | [145] |
Moreover, Tualang honey appeared to have a protective role against bisphenol-A (BPA)-induced toxicity in rat uterus by improving the morphological abnormalities, reducing lipid peroxidation and normalising ERα, ERβ and C3 expression distribution in the rat uterus [140]. The same protective effect was also exhibited in BPA-induced ovarian toxicity, where Tualang honey reduced the morphological abnormalities of the ovarian follicles and improved the normal estrous cycle [141].
In the male reproductive system, Tualang honey at a dose of 1.2 g/kg/day is the most effective dose to alter the male reproductive parameter as it significantly increased epididymal sperm count [142]. This finding is parallel with the result of the other two studies using 1.2 g/kg/day of Tualang honey treatment that successfully demonstrated Tualang honey’s benefit on the male reproductive system [143,144]. In response to impaired sexual behavior and fertility, Tualang honey was revealed to have a protective effect against cigarette smoke-induced impaired sexual behavior and fertility in male rats by significantly increasing intromission and ejaculation and increasing mating fertility indices [143]. In a separate study, Tualang honey supplementation during prenatal restraint stress significantly increased testis and epididymis weights. It improved the percentages of abnormal spermatozoa and sperm motility in male rat offspring [144]. In humans, a randomised control study showed that the effect of Tualang honey amongst oligospermic males was comparable with that of Tribestan in improving sperm concentration, motility and morphology [145]. Apart from Tualang honey, Kelulut honey has been shown to benefit the male reproductive system. In streptozotocin-induced diabetic rats, Kelulut honey treatment prevented sperm and testicular oxidative damage by improving sperm quality and increasing the spermatozoa and spermatogenic cells [55]. One possible mechanism is the anti-oxidative properties that are mostly attributed to the phenolic and flavonoid contents of honey. For support, Budin et al. (2017) reported increased SOD and GSH activities and decreased PC and MDA levels in sperm and testis of streptozotocin-induced diabetic rats [55].
Tualang honey has been shown to have various advantages for female and male reproductive systems. However, future works should explore the chemical constituents responsible for the reproductive effect of Tualang and Kelulut honeys. Meanwhile, Gelam honey has not been explored for its effect on the reproductive system.
4.10. Effects on Nervous System
The effect of Tualang, Gelam and Kelulut honeys on the nervous system is summarised in Table 12. Tualang honey has been reported in several studies to improve memory and reduce depressive-like behavior in animals and humans. In noise stress-induced memory deficits in aged rats, Tualang honey administration was shown to protect against memory decline via enhancement of medial prefrontal cortex and hippocampal morphology, possibly through the reduction of brain oxidative stress and the up-regulation of brain-derived neurotrophic factor (BDNF) concentration and cholinergic system [146]. These findings were further corroborated by Azman et al. (2015), who showed that Tualang honey administration improved memory performance and decreased depressive-like behavior in rats exposed to loud noise stress [147].
Table 12.
Summary of the effect on nervous system.
| Type of Honey | Type of Study | Findings | References |
|---|---|---|---|
| Kelulut honey | In vivo | Kelulut honey reduced serum triglyceride and LDL and normalised blood glucose levels in metabolic disease-induced rats. Behavioural studies showed lessened anxious behaviour and enhanced memory retention. | [113] |
| Tualang honey | In vivo | Tualang honey improved memory performance and decreased depressive-like behaviour in rats exposed to loud noise stress. | [147] |
| Tualang honey | In vivo | Tualang honey or 17β-oestradiol treatment demonstrated anti-depressive-like effects, possibly via restoration of the hypothalamic-pituitary-adrenal axis and enhancement of the brain-derived neurotrophic factor (BDNF) concentration in stressed ovariectomised rats. | [149] |
| Tualang honey | In vivo | Administration of either oestrogen or Tualang honey significantly decreased anxiety-like behaviour in stressed ovariectomised rats, with improved oxidative stress status. | [151] |
| Tualang honey | In vivo | Tualang honey and oestrogen treatments improved short-term and long-term memory and enhanced the neuronal proliferation of hippocampal CA2, CA3 and DG regions in stressed ovariectomised rats. | [150] |
| Tualang honey | In vivo | Pre-treatment with Tualang honey reduced kainic acid-induced neuronal degeneration, hyperactivity and oxidative stress but failed to prevent seizures. | [153] |
| Tualang honey | In vivo | Tualang honey protected against memory decline due to noise stress exposure and ageing via enhancement of medial prefrontal cortex and hippocampal morphology, possibly secondary to a reduction in brain oxidative stress and upregulation BDNF concentration and the cholinergic system. | [146] |
| Tualang honey | In vivo | Treatment with Tualang honey ameliorated the toxic effects induced by repeated paraquat exposure observed in midbrain and lungs. Tualang honey effect was comparable to that of ubiquinol. | [156] |
| Tualang honey | In vivo | Tualang honey improved oxidative stress status and spinal cord morphology and nociceptive behaviour in the offspring of prenatally stressed rats. | [154] |
| Tualang honey | In vivo | Pre-treatment with Tualang honey (1.2 and 2.4 g/kg) and prednisolone (10 mg/kg) reduced pain responses in the rat. | [155] |
| Tualang honey | In vivo | Postmenopausal women who received Tualang honey showed improvement in their immediate memory but not in immediate memory after interference and delayed recall. This finding was comparable to the improvement observed in women receiving oestrogen plus progestin therapy. | [152] |
Several clinical studies have found that oestrogen treatment has a protective effect on aging women’s cognitive decline [148]. In a study on stressed ovariectomised rats, Tualang honey or 17β-estradiol treatment has been shown to demonstrate antidepressive-like effects, possibly via restoration of the hypothalamic-pituitary-adrenal axis and enhancement of the BDNF concentration [149]. In the same set of studies, rats administered with Tualang honey or 17β estradiol showed improved short-term and long-term memory and enhanced neuronal proliferation of hippocampal CA2, CA3 and dentate gyrus (DG) regions compared with untreated stressed ovariectomised rats [150]. In addition, Al-Rahbi et al. (2014) demonstrated the anti-anxiety effect of Tualang honey in ovariectomised rats with the improvement of its oxidative stress status [151]. A randomised controlled trial comparing Tualang honey supplementation and oestrogen plus progestin therapy demonstrated that postmenopausal women who received Tualang honey showed improvements in their immediate memory but not in immediate memory after the interference and delayed recall as oestrogen plus progestin therapy. However, this finding is comparable with the improvement seen in women receiving oestrogen plus progestin treatment [152]. These animal and human studies may serve as a basis to propose Tualang honey as a potential supplement with comparable effects as oestrogen and with no side effects in postmenopausal women.
Tualang honey has been reported in several studies to exert a neuroprotective effect through its antioxidant properties. In a study of kainic acid KA-treated rats, pretreatment with Tualang honey reduced the KA-induced neuronal degeneration in the piriform cortex but failed to prevent the occurrence of KA-induced seizures. In addition, KA-induced rats showed increased locomotor activity and hyperactivity, which were attenuated by Tualang honey pretreatment [103]. A recent study reported that Tualang honey pretreatment significantly attenuated an increase in lipid peroxidation level and decreased the total antioxidant status level induced by KA treatment in the rat cerebral cortex. These findings indicated that pretreatment with Tualang honey has therapeutic potential against KA-induced oxidative stress and neurodegeneration through its antioxidant effect [153]. In a separate study, Tualang honey improved the oxidative stress status, spinal cord morphology and nociceptive behavior in offspring of prenatally stressed rats [154]. The anti-nociceptive effect of Tualang honey was again demonstrated in another study that showed a similar reduction in pain behavior as the prednisolone-treated group [155]. Finally, Tualang honey pretreatment was shown to be comparable with ubiquinol in protecting the rat midbrain, lung toxicity and oxidative stress against repeated paraquat exposure [156].
Regarding Kelulut honey, a study on metabolic disease-induced rats showed that the Kelulut honey-treated group exhibited less anxious behavior and demonstrated significant memory retention than the untreated metabolic disease-induced group [113]. However, the underlying mechanism of Kelulut honey in showing this benefit has yet to be discovered by researchers.
5. Conclusions
Tualang, Gelam and Kelulut honeys were extensively demonstrated to have various health benefits to multiple diseases and systems. Clinical trials, especially in wound healing, showed the superior benefit of Tualang honey as a wound dressing over conventional dressing. However, clinical trials using Tualang, Gelam, and Kelulut honeys are still needed to add to the results because convincing effects are still yet to be finalised. In addition, the mechanism of action of these honeys was not clear, especially in human studies, thereby leaving an area to be discovered. In comparison, Tualang and Gelam honeys have been well researched compared with Kelulut honey. Thus, future studies may focus more on the potential benefit of Kelulut honey. This study also revealed that Tualang, Gelam and Kelulut honey has excellent preclinical potential in multiple diseases and physiological systems that could steer future research to thoroughly explore honey as an efficient and proven superfood to be optimised for the benefit of humanity.
Author Contributions
Conceptualization, D.A.M.K., M.H.M., S.F.I. and M.I.A.M.K.; methodology, M.H.M. and D.A.M.K.; writing—original draft preparation, D.A.M.K.; Illustration, H.K., writing—review and editing, M.H.M., H.K. and M.I.A.M.K.; supervision, M.H.M. and S.F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Kebangsaan Malaysia (Faculty of Medicine Fundamental Grant; grant number FF-2020-241).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fletcher M.T., Hungerford N.L., Webber D., De Jesus M.C., Zhang J., Stone I.S.J., Blanchfield J.T., Zawawi N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-68940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuelgasim H., Albury C., Lee J. Effectiveness of honey for symptomatic relief in upper respiratory tract infections: A systematic review and meta-analysis. BMJ Evid. Based Med. 2020 doi: 10.1136/bmjebm-2020-111336. [DOI] [PubMed] [Google Scholar]
- 3.Yaghoobi R., Kazerouni A., Kazerouni O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J. Nat. Pharm. Prod. 2013;8:100–104. doi: 10.17795/jjnpp-9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jull A., Cullum N., Dumville J.C., Westby M.J., Deshpande S., Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015:CD005083. doi: 10.1002/14651858.CD005083.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon A., Traynor K., Santos K., Blaser G., Bode U., Molan P. Medical Honey for Wound Care—Still the ‘Latest Resort’? Evid. Based Complement. Altern. Med. 2009;6:165–173. doi: 10.1093/ecam/nem175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed S., Othman N.H. Review of the Medicinal Effects of Tualang Honey and a Comparison with Manuka Honey. Malays. J. Med Sci. 2013;20:6–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Devasvaran K., Yong Y.-K. Anti-inflammatory and wound healing properties of Malaysia Tualang honey. Curr. Sci. 2016;110:47–51. [Google Scholar]
- 8.Chan B.K., Haron H. Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies. Med. Sci. 2016;4:3. doi: 10.3390/medsci4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zainol M.I., Yusoff K.M., Yusof M.Y. Antibacterial activity of selected Malaysian honey. BMC Complement. Altern. Med. 2013;13:129. doi: 10.1186/1472-6882-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaacob M., Rajab N., Shahar S., Sharif R. Stingless bee honey and its potential value: A systematic review. Food Res. 2017;2:124–133. doi: 10.26656/fr.2017.2(2).212. [DOI] [Google Scholar]
- 11.Ramli E.S., Kamaruzzaman M.A., Thanu A., Yusof M.R., Soelaiman I.N. Kelulut honey ameliorates glucocorticoid induced osteoporosis via its antioxidant activity in rats. Asian Pac. J. Trop. Biomed. 2019;9:493. doi: 10.4103/2221-1691.271722. [DOI] [Google Scholar]
- 12.EC Council directive 2001/110/EC of 20 December 2001 relating honey. Off. J. Eur. Communities. 2001;10:47–52. [Google Scholar]
- 13.Codex Alimentarius Commission . Standards and Standard Methods. WHO & FAO; Rome, Italy: 2001. Revised Codex Standard for Honey. [Google Scholar]
- 14.Standard M. MS 2683: 2017: Kelulut (Stingless Bee) Honey-Specification: Quality Requirements. Department of Standards Malaysia; Selangor, Malaysia: 2017. [Google Scholar]
- 15.Chua L.S., Abdul-Rahaman N.-L., Sarmidi M.R., Aziz R. Multi-elemental composition and physical properties of honey samples from Malaysia. Food Chem. 2012;135:880–887. doi: 10.1016/j.foodchem.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 16.A-Rahaman N.L., Chua L.S., Sarmidi M.R., Aziz R. Physicochemical and radical scavenging activities of honey samples from Malaysia. Agric. Sci. 2013;4:46–51. doi: 10.4236/as.2013.45B009. [DOI] [Google Scholar]
- 17.Baloš M.Ž., Popov N., Vidaković S., Pelić D.L., Pelić M., Mihaljev Ž., Jakšić S. Electrical conductivity and acidity of honey. Arch. Vet. Med. 2018;11:91–101. doi: 10.46784/e-avm.v11i1.20. [DOI] [Google Scholar]
- 18.Thrasyvoulou A., Tananaki C., Goras G., Karazafiris E., Dimou M., Liolios V., Kanelis D., Gounari S. Legislation of honey criteria and standards. J. Apic. Res. 2018;57:88–96. doi: 10.1080/00218839.2017.1411181. [DOI] [Google Scholar]
- 19.Amin F.A.Z., Sabri S., Mohammad S.M., Ismail M., Chan K.W., Ismail N., Norhaizan M.E., Zawawi N. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey. Adv. Pharmacol. Sci. 2018;2018:1–12. doi: 10.1155/2018/6179596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussein S.Z., Yusoff K.M., Makpol S., Yusof Y.A.M. Antioxidant Capacities and Total Phenolic Contents Increase with Gamma Irradiation in Two Types of Malaysian Honey. Molecules. 2011;16:6378–6395. doi: 10.3390/molecules16066378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua L.S., Rahaman N.L.A., Adnan N.A., Tan T.T.E. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Methods Chem. 2013;2013:1–8. doi: 10.1155/2013/313798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kek S.P., Chin N.L., Yusof Y.A., Tan S.W., Chua L.S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis spp. and Trigona spp. Bees. Agric. Agric. Sci. Procedia. 2014;2:150–155. doi: 10.1016/j.aaspro.2014.11.022. [DOI] [Google Scholar]
- 23.Tomczyk M., Tarapatskyy M., Dżugan M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019;37:232–238. doi: 10.17221/40/2019-CJFS. [DOI] [Google Scholar]
- 24.Khalafi R., Goli S.A.H., Behjatian M. Characterization and Classification of Several Monofloral Iranian Honeys Based on Physicochemical Properties and Antioxidant Activity. Int. J. Food Prop. 2016;19:1065–1079. doi: 10.1080/10942912.2015.1055360. [DOI] [Google Scholar]
- 25.Kek S.P., Chin N.L., Yusof Y.A., Tan S.W., Chua L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017;20:S2723–S2738. doi: 10.1080/10942912.2017.1359185. [DOI] [Google Scholar]
- 26.Khalil I., Mahaneem M., Jamalullail S.M.S., Alam N., Sulaiman S.A. Evaluation of radical scavenging activity and colour intensity of nine Malaysian honeys of different origin. J. ApiProd. ApiMed. Sci. 2011;3:4–11. doi: 10.3896/IBRA.4.03.1.02. [DOI] [Google Scholar]
- 27.Zae T., Azlan A., Sajak A.A.B., Hock E., Chin N.L., Noh M.M., Khalid N.M. Comparison of selected local honey with Manuka honey based on their nutritional and antioxidant properties. Food Res. 2020;4:205–213. doi: 10.26656/fr.2017.4(S1).S12. [DOI] [Google Scholar]
- 28.Moniruzzaman M., Chowdhury M.A.Z., Rahman M.A., Sulaiman S.A., Gan S.H. Determination of Mineral, Trace Element, and Pesticide Levels in Honey Samples Originating from Different Regions of Malaysia Compared to Manuka Honey. BioMed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/359890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moniruzzaman M., Khalil I., Sulaiman S.A., Gan S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013;13:43. doi: 10.1186/1472-6882-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moniruzzaman M., Sulaiman S.A., Khalil I., Gan S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013;7:138. doi: 10.1186/1752-153X-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamsudin S., Selamat J., Sanny M., Razak S.B.B.A., Jambari N.N., Khatib A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules. 2019;24:3898. doi: 10.3390/molecules24213898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua L.S., Adnan N.A. Biochemical and nutritional components of selected honey samples. Acta Sci. Pol. Technol. Aliment. 2014;13:169–179. doi: 10.17306/J.AFS.2014.2.6. [DOI] [PubMed] [Google Scholar]
- 33.Wong P., Hii S.L., Koh C.C., Moh T.S.Y., Gindi S.R.A. Chemical Analysis on the Honey of Heterotrigona itama and Tetrigona binghami from Sarawak, Malaysia. Sains Malays. 2019;48:1635–1642. doi: 10.17576/jsm-2019-4808-09. [DOI] [Google Scholar]
- 34.Lim D.C.C., Abu Bakar M.F., Majid M. Nutritional composition of stingless bee honey from different botanical origins. IOP Conf. Ser. Earth Environ. Sci. 2019;269:012025. doi: 10.1088/1755-1315/269/1/012025. [DOI] [Google Scholar]
- 35.Julika W.N., Ajit A.B., Sulaiman A.Z., Naila A. Physicochemical and Microbiological Analysis of Stingless Bees Honey Collected from Local Market in Malaysia. Indones. J. Chem. 2019;19:522–530. doi: 10.22146/ijc.40869. [DOI] [Google Scholar]
- 36.Kek S.P., Chin N.L., Tan S.W., Yusof Y.A., Chua L.S. Classification of Honey from Its Bee Origin via Chemical Profiles and Mineral Content. Food Anal. Methods. 2017;10:19–30. doi: 10.1007/s12161-016-0544-0. [DOI] [Google Scholar]
- 37.Mohamed M., Sirajudeen K., Swamy M., Yaacob N.S., Sulaiman S.A. Studies on the antioxidant properties of Tualang honey of Malaysia. Afr. J. Tradit. Complement. Altern. Med. 2010;7:59–63. doi: 10.4314/ajtcam.v7i1.57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kishore R.K., Halim A.S., Syazana M., Sirajudeen K. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011;31:322–325. doi: 10.1016/j.nutres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Yao L., Razak S., Ismail N., Fai N., Asyraf M., Sharif G., Jubri Z. Malaysian gelam honey reduces oxidative damage and modulates antioxidant enzyme activities in young and middle aged rats. J. Med. Plant Res. 2011;5:5618–5625. [Google Scholar]
- 40.Sahhugi Z., Hasenan S., Jubri Z. Research Article Protective Effects of Gelam Honey against Oxidative Damage in Young and Aged Rats. Oxidative Med. Cell. Longev. 2014;2014:1–8. doi: 10.1155/2014/673628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sani N.F.A., Belani L.K., Sin C.P., Rahman S.N.A.A., Das S., Chi T.Z., Makpol S., Yusof Y.A.M. Effect of the Combination of Gelam Honey and Ginger on Oxidative Stress and Metabolic Profile in Streptozotocin-Induced Diabetic Sprague-Dawley Rats. BioMed Res. Int. 2014;2014:1–9. doi: 10.1155/2014/160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad T.A.F.T., Jubri Z., Rajab N.F., Rahim K.A., Yusof Y.A.M., Makpol S. Gelam Honey Protects against Gamma-Irradiation Damage to Antioxidant Enzymes in Human Diploid Fibroblasts. Molecules. 2013;18:2200–2211. doi: 10.3390/molecules18022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson C.J., Koterski S., Sciotti R.J., Poccard G.B., Rondinone C.M. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes: Potential role of p38 in the downregulation of GLUT4 expression. Diabetes. 2003;52:634–641. doi: 10.2337/diabetes.52.3.634. [DOI] [PubMed] [Google Scholar]
- 44.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are Oxidative Stress-Activated Signaling Pathways Mediators of Insulin Resistance and -Cell Dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Batumalaie K., Safi S.Z., Yusof K.M., Bin Ismail I.S., Sekaran S.D., Qvist R. Effect of Gelam Honey on the Oxidative Stress-Induced Signaling Pathways in Pancreatic Hamster Cells. Int. J. Endocrinol. 2013;2013:1–10. doi: 10.1155/2013/367312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safi S.Z., Batumalaie K., Qvist R., Yusof K.M., Bin Ismail I.S. Gelam Honey Attenuates the Oxidative Stress-Induced Inflammatory Pathways in Pancreatic Hamster Cells. Evid. Based Complement. Altern. Med. 2016;2016:1–13. doi: 10.1155/2016/5843615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batumalaie K., Qvist R., Yusof K.M., Ismail I.S., Sekaran S.D. The antioxidant effect of the Malaysian Gelam honey on pancreatic hamster cells cultured under hyperglycemic conditions. Clin. Exp. Med. 2014;14:185–195. doi: 10.1007/s10238-013-0236-7. [DOI] [PubMed] [Google Scholar]
- 48.Erejuwa O.O., Sulaiman S.A., Wahab M.S., Sirajudeen K.N.S., Salleh M.S.M.D., Gurtu S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann. D’Endocrinol. 2010;71:291–296. doi: 10.1016/j.ando.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S., Sirajudeen K.N.S., Salleh S.M., Gurtu S. Antioxidant Protective Effect of Glibenclamide and Metformin in Combination with Honey in Pancreas of Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2010;11:2056–2066. doi: 10.3390/ijms11052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omotayo E.O., Gurtu S., Sulaiman S.A., Ab Wahab M.S., Salam S.K.N., Salleh S.M. Hypoglycemic and Antioxidant Effects of Honey Supplementation in Streptozotocin-induced Diabetic Rats. Int. J. Vitam. Nutr. Res. 2010;80:74–82. doi: 10.1024/0300-9831/a000008. [DOI] [PubMed] [Google Scholar]
- 51.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S., Sirajudeen K.N.S., Salleh S.M., Gurtu S. Comparison of Antioxidant Effects of Honey, Glibenclamide, Metformin, and Their Combinations in the Kidneys of Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2011;12:829–843. doi: 10.3390/ijms12010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan J.J., Azmi S.M., Yong Y.K., Cheah H.L., Lim V., Sandai D., Shaharuddin B. Tualang Honey Improves Human Corneal Epithelial Progenitor Cell Migration and Cellular Resistance to Oxidative Stress In Vitro. PLoS ONE. 2014;9:e96800. doi: 10.1371/journal.pone.0096800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bashkaran K., Zunaina E., Bakiah S., Sulaiman S.A., Sirajudeen K., Naik V.R. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: Experimental animal study. BMC Complement. Altern. Med. 2011;11:90. doi: 10.1186/1472-6882-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmad N.S., Aziz A.A., Kong K.W., Hamid M.S.A., Cheong J.P.G., Hamzah S.H. Dose–Response Effect of Tualang Honey on Postprandial Antioxidant Activity and Oxidative Stress in Female Athletes: A Pilot Study. J. Altern. Complement. Med. 2017;23:989–995. doi: 10.1089/acm.2017.0129. [DOI] [PubMed] [Google Scholar]
- 55.Budin S., Jubaidi F., Azam S., Mohammed Yusof N., Taib I.S., Mohamed J. Kelulut honey supplementation prevents sperm and testicular oxidative damage in streptozotocin-induced diabetic rats. J. Teknol. 2017;79 doi: 10.11113/jt.v79.9674. [DOI] [Google Scholar]
- 56.Socha R., Juszczak L., Pietrzyk S., Fortuna T. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009;113:568–574. doi: 10.1016/j.foodchem.2008.08.029. [DOI] [Google Scholar]
- 57.Jibril F.I., Hilmi A.B.M., Manivannan L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019;43:4. doi: 10.1186/s42269-019-0044-7. [DOI] [Google Scholar]
- 58.Ahmed S., Sulaiman S.A., Baig A.A., Ibrahim M., Liaqat S., Fatima S., Jabeen S., Shamim N., Othman N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018;2018:1–19. doi: 10.1155/2018/8367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed S., Othman N.H. The anti-cancer effects of Tualang honey in modulating breast carcinogenesis: An experimental animal study. BMC Complement. Altern. Med. 2017;17:208. doi: 10.1186/s12906-017-1721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadir E.A., Sulaiman S.A., Yahya N.K., Othman N.H. Inhibitory Effects of Tualang Honey on Experimental Breast Cancer in Rats: A Preliminary Study. Asian Pac. J. Cancer Prev. 2013;14:2249–2254. doi: 10.7314/APJCP.2013.14.4.2249. [DOI] [PubMed] [Google Scholar]
- 61.Ahmed S., Sulaiman S.A., Othman N.H. Oral Administration of Tualang and Manuka Honeys Modulates Breast Cancer Progression in Sprague-Dawley Rats Model. Evid. Based Complement. Altern. Med. 2017;2017:1–15. doi: 10.1155/2017/5904361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fauzi A.N., Norazmi M.N., Yaacob N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011;49:871–878. doi: 10.1016/j.fct.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Yaacob N.S., Nengsih A., Norazmi M.N. Tualang Honey Promotes Apoptotic Cell Death Induced by Tamoxifen in Breast Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2013;2013:1–9. doi: 10.1155/2013/989841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaacob N.S., Ismail N.F. Comparison of cytotoxicity and genotoxicity of 4-hydroxytamoxifen in combination with Tualang honey in MCF-7 and MCF-10A cells. BMC Complement. Altern. Med. 2014;14:106. doi: 10.1186/1472-6882-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hizan N.S., Hassan N.H.M., Haron J., Abubakar M.B., Mahdi N.M.N., Gan S.H. Tualang honey adjunct with anastrozole improve parenchyma enhancement of breast tissue in breast cancer patients: A randomized controlled trial. Integr. Med. Res. 2018;7:322–327. doi: 10.1016/j.imr.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Koshab M., AlAbsi A.M., Bakri M.M., Naicker M.S., Seyedan A. Chemopreventive activity of Tualang honey against oral squamous cell carcinoma—In vivo. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020;129:484–492. doi: 10.1016/j.oooo.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Ghashm A.A., Othman N.H., Khattak M.N., Ismail N.M., Saini R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010;10:49. doi: 10.1186/1472-6882-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Man N.M.K.N., Hassan R., Ang C.Y., Abdullah A.D., Radzi M.A.R.M., Sulaiman S.A. Antileukemic Effect of Tualang Honey on Acute and Chronic Leukemia Cell Lines. BioMed Res. Int. 2015;2015:1–7. doi: 10.1155/2015/307094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad I., Jimenez H., Yaacob N.S., Yusuf N. Tualang Honey Protects Keratinocytes from Ultraviolet Radiation-Induced Inflammation and DNA Damage. Photochem. Photobiol. 2012;88:1198–1204. doi: 10.1111/j.1751-1097.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramasamy V., Binti Mat Lazim N., Abdullah B., Singh A. Effects of Tualang Honey on Cancer Related Fatigue: A Multicenter Open-label Trial of H&N Cancer Patients. Gulf J. Oncol. 2019;1:43–51. [PubMed] [Google Scholar]
- 71.Yazan L.S., Zali M.F.S.M., Ali R.M., Zainal N.A., Esa N., Sapuan S., Ong Y.S., Tor Y.S., Gopalsamy B., Voon F.L., et al. Chemopreventive Properties and Toxicity of Kelulut Honey in Sprague Dawley Rats Induced with Azoxymethane. BioMed Res. Int. 2016;2016:1–6. doi: 10.1155/2016/4036926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jubri Z., Narayanan N., Abdul Karim N., Wan Ngah W.Z. Antiproliferative activity and apoptosis induction by gelam honey on liver cancer cell line. Int. J. Appl. Sci. Technol. 2012;2:135–141. [Google Scholar]
- 73.Hakim L., Alias E., Makpol S., Ngah W.Z.W., Morad N.A., Yusof Y.A.M. Gelam Honey and Ginger Potentiate the Anti Cancer Effect of 5-FU against HCT 116 Colorectal Cancer Cells. Asian Pac. J. Cancer Prev. 2014;15:4651–4657. doi: 10.7314/APJCP.2014.15.11.4651. [DOI] [PubMed] [Google Scholar]
- 74.Johari S.A., Fatimah H., Ismail W.I.W., Ali A.M. Corrigendum to “Combinatorial Cytotoxic Effects of Gelam Honey and 5-Fluorouracil against Human Adenocarcinoma Colon Cancer HT-29 Cells In Vitro”. Int. J. Cell Biol. 2019;2019:9050626. doi: 10.1155/2019/9050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tahir A.A., Sani N.F.A., Murad N.A., Makpol S., Ngah W.Z.W., Yusof Y.A.M. Combined ginger extract & Gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr. J. 2015;14:1–10. doi: 10.1186/s12937-015-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wee L.H., Morad N.A., Aan G.J., Makpol S., Ngah W.Z.W., Yusof Y.A.M. Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/β-catenin Pathways. Asian Pac. J. Cancer Prev. 2015;16:6549–6556. doi: 10.7314/APJCP.2015.16.15.6549. [DOI] [PubMed] [Google Scholar]
- 77.Ahmad T.A.F.T., Jaafar F., Jubri Z., Rahim K.A., Rajab N.F., Makpol S. Gelam honey attenuated radiation-induced cell death in human diploid fibroblasts by promoting cell cycle progression and inhibiting apoptosis. BMC Complement. Altern. Med. 2014;14:108. doi: 10.1186/1472-6882-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wen C.T.P., Hussein S.Z., Abdullah S., Karim N.A., Makpol S., Yusof Y.A.M. Gelam and Nenas honeys inhibit proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pac. J. Cancer Prev. 2012;13:1605–1610. doi: 10.7314/APJCP.2012.13.4.1605. [DOI] [PubMed] [Google Scholar]
- 79.Cianciosi D., Forbes-Hernández T.Y., Afrin S., Gasparrini M., Reboredo-Rodríguez P., Manna P.P., Zhang J., Lamas L.B., Flórez S.M., Toyos P.A., et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules. 2018;23:2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Jaganathan S.K., Mandal M. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J. Biomed. Biotechnol. 2009;2009:1–13. doi: 10.1155/2009/830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tumin N., Halim N., Shahjahan M., Noor Izani N., Sattar M.A., Khan A.H., Mohsin S. Antibacterial activity of local Malaysian honey. Malays. J. Pharm. Sci. 2005;3:1–10. [Google Scholar]
- 83.Ng W.-J., Ken K.-W., Kumar R.-V., Gunasagaran H., Chandramogan V., Lee Y.-Y. In-Vitro Screening of Malaysian Honey from Different Floral Sources for Antibacterial Activity on Human Pathogenic Bacteria. Afr. J. Tradit. Complement. Altern. Med. 2014;11:315–318. doi: 10.4314/ajtcam.v11i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albaridi N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019;2019:1–10. doi: 10.1155/2019/2464507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weston R. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000;71:235–239. doi: 10.1016/S0308-8146(00)00162-X. [DOI] [Google Scholar]
- 86.Allen K.L., Molan P.C., Reid G.M. A Survey of the Antibacterial Activity of Some New Zealand Honeys. J. Pharm. Pharmacol. 1991;43:817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 87.Yap P., Bakar M., Lim H., Carrier D. Antibacterial activity of polyphenol-rich extract of selected wild honey collected in Sabah, Malaysia. J. Apic. Res. 2015;54:163–172. doi: 10.1080/00218839.2016.1151633. [DOI] [Google Scholar]
- 88.Aween M.M., Hassan Z., Muhialdin B.J., Eljamel Y.A., Al-Mabrok A.S.W., Lani M.N. Antibacterial Activity of Lactobacillus acidophilus Strains Isolated from Honey Marketed in Malaysia against Selected Multiple Antibiotic Resistant (MAR) Gram-Positive Bacteria. J. Food Sci. 2012;77:M364–M371. doi: 10.1111/j.1750-3841.2012.02776.x. [DOI] [PubMed] [Google Scholar]
- 89.Punitan R., Sulaiman S.A., Hasan H.B., Shatriah I. Clinical and Antibacterial Effects of Tualang Honey on Pseudomonas-induced Keratitis in Rabbit Eyes. Cureus. 2019;11:e4332. doi: 10.7759/cureus.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nasir N.A., Halim A.S., Singh K.K., Dorai A.A., Haneef M.N. Antibacterial properties of tualang honey and its effect in burn wound management: A comparative study. BMC Complement. Altern. Med. 2010;10:31. doi: 10.1186/1472-6882-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang M.L., Rahman R.A., Gan S.H., Halim A.S., Hassan S.A., Sulaiman S.A., Singh K.-K.B. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009;9:34. doi: 10.1186/1472-6882-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yusuf W.N.W., Mohammad W.M.Z.W., Gan S.H., Mustafa M., Aziz C.B.A., Sulaiman S.A. Tualang honey ameliorates viral load, CD4 counts and improves quality of life in asymptomatic human immunodeficiency virus infected patients. J. Tradit. Complement. Med. 2019;9:249–256. doi: 10.1016/j.jtcme.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bulgasem B.Y., Lani M.N., Hassan Z., Yusoff W.M.W., Fnaish S.G. Antifungal Activity of Lactic Acid Bacteria Strains Isolated from Natural Honey against Pathogenic Candida Species. Mycobiology. 2016;44:302–309. doi: 10.5941/MYCO.2016.44.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dixon B. Bacteria can’t resist honey. Lancet Infect. Dis. 2003;3:116. doi: 10.1016/S1473-3099(03)00524-3. [DOI] [PubMed] [Google Scholar]
- 95.Hussein S.Z., Yusoff K.M., Makpol S., Yusof Y.A.M. Gelam Honey Inhibits the Production of Proinflammatory, Mediators NO, PGE2, TNF-α, and IL-6 in Carrageenan-Induced Acute Paw Edema in Rats. Evid. Based Complement. Altern. Med. 2012;2012:1–13. doi: 10.1155/2012/109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kassim M., Achoui M., Mansor M., Yusoff K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia. 2010;81:1196–1201. doi: 10.1016/j.fitote.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 97.Hussein S.Z., Yusoff K.M., Makpol S., Yusof Y.A.M. Gelam Honey Attenuates Carrageenan-Induced Rat Paw Inflammation via NF-κB Pathway. PLoS ONE. 2013;8:e72365. doi: 10.1371/journal.pone.0072365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kassim M., Yusoff K.M., Ong G., Sekaran S., Yusof M.Y.B.M., Mansor M. Gelam honey inhibits lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide, and high-mobility group protein B1. Fitoterapia. 2012;83:1054–1059. doi: 10.1016/j.fitote.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Kassim M.K.A., Mansor M., Al-Abd N.M., Yusoff K.M. Gelam Honey Has a Protective Effect against Lipopolysaccharide (LPS)-Induced Organ Failure. Int. J. Mol. Sci. 2012;13:6370–6381. doi: 10.3390/ijms13056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kassim M.K.A., Mansor M., Suhaimi A., Ong G., Yusoff K.M. Gelam Honey Scavenges Peroxynitrite during the Immune Response. Int. J. Mol. Sci. 2012;13:12113–12129. doi: 10.3390/ijms130912113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shamshuddin N.S.S., Zohdi R.M. Gelam honey attenuates ovalbumin-induced airway inflammation in a mice model of allergic asthma. J. Tradit. Complement. Med. 2018;8:39–45. doi: 10.1016/j.jtcme.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamaruzaman N.A., Sulaiman S.A., Kaur G., Yahaya B.H. Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC Complement. Altern. Med. 2014;14:176. doi: 10.1186/1472-6882-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sairazi N.S.M., Sirajudeen K.N.S., Mustapha M., Mummedy S., Asari M.A., Sulaiman S.A. Tualang Honey Reduced Neuroinflammation and Caspase-3 Activity in Rat Brain after Kainic Acid-Induced Status Epilepticus. Evid. Based Complement. Altern. Med. 2018;2018:1–16. doi: 10.1155/2018/7287820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asari M.A., Zulkaflee M.H., Sirajudeen K., Yusof N.A.M., Sairazi N.S.M. Tualang honey and DHA-rich fish oil reduce the production of pro-inflammatory cytokines in the rat brain following exposure to chronic stress. J. Taibah Univ. Med. Sci. 2019;14:317–323. doi: 10.1016/j.jtumed.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghazali W.S.W., Romli A.C., Mohamed M. Effects of honey supplementation on inflammatory markers among chronic smokers: A randomized controlled trial. BMC Complement. Altern. Med. 2017;17:175. doi: 10.1186/s12906-017-1703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ranneh Y., Akim A.M., Ab Hamid H., Khaza’Ai H., Fadel A., Mahmoud A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019;16:1–17. doi: 10.1186/s12986-019-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Erejuwa O., Sulaiman S.A., Wahab M., Sirajudeen K.N.S., Md Salleh S., Gurtu S. Hepatoprotective effect of Tualang honey supplementation in streptozotocin-induced diabetic rats. Int. J. Appl. Res. Nat. Prod. 2012;4:37–41. [Google Scholar]
- 108.Erejuwa O.O. Glibenclamide or Metformin Combined with Honey Improves Glycemic Control in Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Sci. 2011;7:244–252. doi: 10.7150/ijbs.7.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rashid M.R., Aripin K.N.N., Mohideen F.B.S., Baharom N., Omar K., Taujuddin N.M.S.M., Yusof H.H.M., Addnan F.H. The Effect of Kelulut Honey on Fasting Blood Glucose and Metabolic Parameters in Patients with Impaired Fasting Glucose. J. Nutr. Metab. 2019;2019:1–7. doi: 10.1155/2019/3176018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robert S.D., Ismail A.A. Two varieties of honey that are available in Malaysia gave intermediate glycemic index values when tested among healthy individuals. Biomed. Pap. 2009;153:145–147. doi: 10.5507/bp.2009.024. [DOI] [PubMed] [Google Scholar]
- 111.Hussain N.H.N., Sulaiman S.A., Hassan I.I., Kadir A.A., Nor N.M., Ismail S.B., Yaacob L.H., Zakaria R., Shafie N.S., Haron J., et al. Randomized Controlled Trial on the Effects of Tualang Honey and Hormonal Replacement Therapy (HRT) on Cardiovascular Risk Factors, Hormonal Profiles and Bone Density Among Postmenopausal Women: A Pilot Study. J. Food Res. 2012;1:171–188. doi: 10.5539/jfr.v1n2p171. [DOI] [Google Scholar]
- 112.Ab Wahab S.Z., Hussain N.H.N., Zakaria R., Kadir A.A., Mohamed N., Tohit N.M., Norhayati M.N., Hassan I.I. Long-term effects of honey on cardiovascular parameters and anthropometric measurements of postmenopausal women. Complement. Ther. Med. 2018;41:154–160. doi: 10.1016/j.ctim.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 113.Lin T.S., Yahaya M.F., Arshad N. Ain Stingless Bee Honey Reduces Anxiety and Improves Memory of the Metabolic Disease-induced Rats. CNS Neurol. Disord. Drug Targets. 2020;19:115–126. doi: 10.2174/1871527319666200117105133. [DOI] [PubMed] [Google Scholar]
- 114.Erejuwa O.O., Nwobodo N.N., Akpan J.L., Okorie U.A., Ezeonu C.T., Ezeokpo B.C., Nwadike K.I., Erhiano E., Abdul Wahab M.S., Sulaiman S.A. Nigerian Honey Ameliorates Hyperglycemia and Dyslipidemia in Alloxan-Induced Diabetic Rats. Nutrients. 2016;8:95. doi: 10.3390/nu8030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yaghoobi N., Al-Waili N., Ghayour-Mobarhan M., Parizadeh S.M.R., Abasalti Z., Esmaeili H., Kazemi-Bajestani S.M.R., Aghasizadeh R., Saloom K.Y., Ferns G.A.A. Natural Honey and Cardiovascular Risk Factors; Effects on Blood Glucose, Cholesterol, Triacylglycerole, CRP, and Body Weight Compared with Sucrose. Sci. World J. 2008;8:463–469. doi: 10.1100/tsw.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bahrami M., Ataie-Jafari A., Hosseini S., Foruzanfar M.H., Rahmani M., Pajouhi M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009;60:618–626. doi: 10.3109/09637480801990389. [DOI] [PubMed] [Google Scholar]
- 117.Rafie A.Z.M., Syahir A., Ahmad W.A.N.W., Mustafa M.Z., Mariatulqabtiah A.R. Supplementation of Stingless Bee Honey from Heterotrigona itama Improves Antiobesity Parameters in High-Fat Diet Induced Obese Rat Model. Evid. Based Complement. Altern. Med. 2018;2018:6371582. doi: 10.1155/2018/6371582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Samat S., Enchang F.K., Hussein F.N., Ismail W.I.W. Four-Week Consumption of Malaysian Honey Reduces Excess Weight Gain and Improves Obesity-Related Parameters in High Fat Diet Induced Obese Rats. Evid. Based Complement. Altern. Med. 2017;2017:1342150. doi: 10.1155/2017/1342150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ramli N.Z., Chin K.-Y., Zarkasi K.A., Ahmad F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. Int. J. Environ. Res. Public Health. 2019;16:4987. doi: 10.3390/ijerph16244987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuropatnicki A.K., Kłósek M., Kucharzewski M. Honey as medicine: Historical perspectives. J. Apic. Res. 2018;57:113–118. doi: 10.1080/00218839.2017.1411182. [DOI] [Google Scholar]
- 121.Saikaly S.K., Litaiem N. Honey and Wound Healing: An Update. Am. J. Clin. Dermatol. 2017;18:237–251. doi: 10.1007/s40257-016-0247-8. [DOI] [PubMed] [Google Scholar]
- 122.Al-Waili N.S., Salom K., Al-Ghamdi A.A. Honey for Wound Healing, Ulcers, and Burns; Data Supporting Its Use in Clinical Practice. Sci. World J. 2011;11:766–787. doi: 10.1100/tsw.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khoo Y.-T., Halim A.S., Singh K.-K.B., Mohamad N.-A. Wound contraction effects and antibacterial properties of Tualang honey on full-thickness burn wounds in rats in comparison to hydrofibre. BMC Complement. Altern. Med. 2010;10:48. doi: 10.1186/1472-6882-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halim A.S., Singh K.K.B., Sukur S.M. Evaluations of bacterial contaminated full thickness burn wound healing in Sprague Dawley rats Treated with Tualang honey. Indian J. Plast. Surg. 2011;44:112–117. doi: 10.4103/0970-0358.81459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aznan M.I., Khan O.H., Unar A.O., Sharif S.E.T., Khan A.H., Aziz S.H.S.A., Zakaria A.D. Effect of Tualang honey on the anastomotic wound healing in large bowel anastomosis in rats-A randomized controlled trial. BMC Complement. Altern. Med. 2016;16:28. doi: 10.1186/s12906-016-1003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lazim N.M., Abdullah B., Salim R. The effect of Tualang honey in enhancing post tonsillectomy healing process. An open labelled prospective clinical trial. Int. J. Pediatr. Otorhinolaryngol. 2013;77:457–461. doi: 10.1016/j.ijporl.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 127.Tan M.K., Adli D.S.H., Tumiran M.A., Abdulla M.A., Yusoff K.M. The Efficacy of Gelam Honey Dressing towards Excisional Wound Healing. Evid. Based Complement. Altern. Med. 2012;2012:1–6. doi: 10.1155/2012/805932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zohdi R.M., Zuki A.B., Yusof N., Noordin M.M., Abdullah M.N.H. Gelam (Melaleuca spp.) Honey-Based Hydrogel as Burn Wound Dressing. Evid. Based Complement. Altern. Med. 2011;2012:1–7. doi: 10.1155/2012/843025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yusof A.M., Ghafar N.A., Kamarudin T.A., Chua K.-H., Azmi M.F., Ng S.-L., Yusof Y.A.M. Gelam honey promotes ex vivo corneal fibroblasts wound healing. Cytotechnology. 2019;71:1121–1135. doi: 10.1007/s10616-019-00349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yusof A.M., Ghafar N.A., Kamarudin T.A., Chua K.H., Yusof Y.A.M. Gelam honey potentiates ex vivo corneal keratocytes proliferation with desirable phenotype expression. BMC Complement. Altern. Med. 2016;16:76. doi: 10.1186/s12906-016-1055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Azmi M.F., Ghafar N.A., Hamzah J.C., Luan N.S., Hui C.K. The Potential of Gelam Honey in Promoting the Proliferative Phase of Corneal Reepithelialization. Wounds. 2017;29:327–332. [PubMed] [Google Scholar]
- 132.Nordin A., Chowdhury S.R., Bin Saim A., Idrus R.B.H. Effect of Kelulut Honey on the Cellular Dynamics of TGFβ-Induced Epithelial to Mesenchymal Transition in Primary Human Keratinocytes. Int. J. Environ. Res. Public Health. 2020;17:3229. doi: 10.3390/ijerph17093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yazan L.S., Zainal N.A., Ali R.M., Zali M., Shyfiq M.F., Sze O.Y., Sim T.Y., Gopalsamy B., Ling V.F., Sapuan S. Antiulcer Properties of Kelulut Honey against Ethanol-Induced Gastric Ulcer. Pertanika J. Sci. Technol. 2018;26:121–132. [Google Scholar]
- 134.Khalil I., Tanvir E.M., Afroz R., Sulaiman S.A., Gan S.H. Cardioprotective Effects of Tualang Honey: Amelioration of Cholesterol and Cardiac Enzymes Levels. BioMed Res. Int. 2015;2015:1–8. doi: 10.1155/2015/286051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S., Sirajudeen K.N.S., Salleh S., Gurtu S. Honey Supplementation in Spontaneously Hypertensive Rats Elicits Antihypertensive Effect via Amelioration of Renal Oxidative Stress. Oxidative Med. Cell. Longev. 2012;2012:1–14. doi: 10.1155/2012/374037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S., Sirajudeen K.N.S., Salleh S.M., Gurtu S. Differential Responses to Blood Pressure and Oxidative Stress in Streptozotocin-Induced Diabetic Wistar-Kyoto Rats and Spontaneously Hypertensive Rats: Effects of Antioxidant (Honey) Treatment. Int. J. Mol. Sci. 2011;12:1888–1907. doi: 10.3390/ijms12031888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Devasvaran K., Tan J.J., Ng C.T., Fong L.Y., Yong Y.K. Malaysian Tualang Honey Inhibits Hydrogen Peroxide-Induced Endothelial Hyperpermeability. Oxidative Med. Cell. Longev. 2019;2019:1202676. doi: 10.1155/2019/1202676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zaid S.S.M., Sulaiman S.A., Sirajudeen K.N.M., Othman N.H. The effects of tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats—Animal model for menopause. BMC Complement. Altern. Med. 2010;10:82. doi: 10.1186/1472-6882-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zaid S.S.M., Sulaiman S.A., Othman N.H., Soelaiman I.-N., Shuid A.N., Mohamad N., Muhamad N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics. 2012;67:779–784. doi: 10.6061/clinics/2012(07)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zaid S.S.M., Kassim N.M., Shatrah O. Tualang Honey Protects against BPA-Induced Morphological Abnormalities and Disruption of ERα, ERβ, and C3 mRNA and Protein Expressions in the Uterus of Rats. Evid.-Based Complement. Altern. Med. 2015;2015:1–18. doi: 10.1155/2015/469762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zaid S.S.M., Shatrah O., Kassim N.M. Potential protective effect of Tualang honey on BPA-induced ovarian toxicity in prepubertal rat. BMC Complement. Altern. Med. 2014;14:509. doi: 10.1186/1472-6882-14-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mohamed M., Sulaiman S.A., Jaafar H., Sirajudeen K.N.S. Effect of different doses of Malaysian honey on reproductive parameters in adult male rats. Andrologia. 2012;44:182–186. doi: 10.1111/j.1439-0272.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 143.Mohamed M., Sulaiman S.A., Sirajudeen K.N.S. Protective effect of honey against cigarette smoke induced-impaired sexual behavior and fertility of male rats. Toxicol. Ind. Health. 2013;29:264–271. doi: 10.1177/0748233711432568. [DOI] [PubMed] [Google Scholar]
- 144.Haron M.N., Mohamed M. Effect of honey on the reproductive system of male rat offspring exposed to prenatal restraint stress. Andrologia. 2016;48:525–531. doi: 10.1111/and.12473. [DOI] [PubMed] [Google Scholar]
- 145.Ismail S.B., Bakar M.B., Hussain N.H.N., Norhayati M.N., Sulaiman S.A., Jaafar H., Draman S., Ramli R., Yusoff W.Z.W. Comparison on the Effects and Safety of Tualang Honey and Tribestan in Sperm Parameters, Erectile Function, and Hormonal Profiles among Oligospermic Males. Evid. Based Complement. Altern. Med. 2014;2014:1–10. doi: 10.1155/2014/126138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Azman K.F., Zakaria R., Ab Aziz C.B., Othman Z. Tualang Honey Attenuates Noise Stress-Induced Memory Deficits in Aged Rats. Oxidative Med. Cell. Longev. 2016;2016:1–11. doi: 10.1155/2016/1549158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Azman K.F., Zakaria R., Abdaziz C., Othman Z., Al-Rahbi B. Tualang honey improves memory performance and decreases depressive-like behavior in rats exposed to loud noise stress. Noise Health. 2015;17:83–89. doi: 10.4103/1463-1741.153388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sherwin B.B. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 149.Al-Rahbi B., Zakaria R., Othman Z., Hassan A., Ahmad A.H. Enhancement of BDNF Concentration and Restoration of the Hypothalamic-Pituitary-Adrenal Axis Accompany Reduced Depressive-Like Behaviour in Stressed Ovariectomised Rats Treated with Either Tualang Honey or Estrogen. Sci. World J. 2014;2014:1–8. doi: 10.1155/2014/310821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Al-Rahbi B., Zakaria R., Othman Z., Hassan A., Ismail Z.I.M., Muthuraju S. Tualang honey supplement improves memory performance and hippocampal morphology in stressed ovariectomized rats. Acta Histochem. 2014;116:79–88. doi: 10.1016/j.acthis.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Al-Rahbi B., Zakaria R., Othman Z., Hassan A., Ahmad A.H. Protective Effects of Tualang Honey against Oxidative Stress and Anxiety-Like Behaviour in Stressed Ovariectomized Rats. Int. Sch. Res. Not. 2014;2014:1–10. doi: 10.1155/2014/521065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Othman Z., Shafin N., Zakaria R., Hussain N.H.N., Mohammad W.M.Z.W. Improvement in immediate memory after 16 weeks of tualang honey (Agro Mas) supplement in healthy postmenopausal women. Menopause. 2011;18:1219–1224. doi: 10.1097/gme.0b013e31821e2044. [DOI] [PubMed] [Google Scholar]
- 153.Sairazi N.S.M., Sirajudeen K.N.S., Asari M.A., Mummedy S., Muzaimi M., Sulaiman S.A. Effect of tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complement. Altern. Med. 2017;17:1–12. doi: 10.1186/s12906-016-1534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ab Aziz C.B., Suhaimi S.Q.A., Hasim H., Ahmad A.H., Long I., Zakaria R. Effects of Tualang honey in modulating nociceptive responses at the spinal cord in offspring of prenatally stressed rats. J. Integr. Med. 2019;17:66–70. doi: 10.1016/j.joim.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 155.Ab Aziz C.B., Ismail C.A.N., Hussin C.M.C., Mohamed M. The Antinociceptive Effects of Tualang Honey in Male Sprague-Dawley Rats: A Preliminary Study. J. Tradit. Complement. Med. 2014;4:298–302. doi: 10.4103/2225-4110.139115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang S.P., Salam S.K.N., Jaafar H., Gan S.H., Mustapha M., Sulaiman S.A. Tualang Honey Protects the Rat Midbrain and Lung against Repeated Paraquat Exposure. Oxidative Med. Cell. Longev. 2017;2017:1–12. doi: 10.1155/2017/4605782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article.