Abstract
BACKGROUND:
Very little direct evidence exists on use of corticosteroids in patients with coronavirus disease 2019 (COVID-19). Indirect evidence from related conditions must therefore inform inferences regarding benefits and harms. To support a guideline for managing COVID-19, we conducted systematic reviews examining the impact of corticosteroids in COVID-19 and related severe acute respiratory illnesses.
METHODS:
We searched standard international and Chinese biomedical literature databases and prepublication sources for randomized controlled trials (RCTs) and observational studies comparing corticosteroids versus no corticosteroids in patients with COVID-19, severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS). For acute respiratory distress syndrome (ARDS), influenza and community-acquired pneumonia (CAP), we updated the most recent rigorous systematic review. We conducted random-effects meta-analyses to pool relative risks and then used baseline risk in patients with COVID-19 to generate absolute effects.
RESULTS:
In ARDS, according to 1 small cohort study in patients with COVID-19 and 7 RCTs in non–COVID-19 populations (risk ratio [RR] 0.72, 95% confidence interval [CI] 0.55 to 0.93, mean difference 17.3% fewer; low-quality evidence), corticosteroids may reduce mortality. In patients with severe COVID-19 but without ARDS, direct evidence from 2 observational studies provided very low-quality evidence of an increase in mortality with corticosteroids (hazard ratio [HR] 2.30, 95% CI 1.00 to 5.29, mean difference 11.9% more), as did observational data from influenza studies. Observational data from SARS and MERS studies provided very low-quality evidence of a small or no reduction in mortality. Randomized controlled trials in CAP suggest that corticosteroids may reduce mortality (RR 0.70, 95% CI 0.50 to 0.98, 3.1% lower; very low-quality evidence), and may increase hyperglycemia.
INTERPRETATION:
Corticosteroids may reduce mortality for patients with COVID-19 and ARDS. For patients with severe COVID-19 but without ARDS, evidence regarding benefit from different bodies of evidence is inconsistent and of very low quality.
On Mar. 11, 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19) a pandemic.1 The worldwide spread of COVID-19 represents a profound threat to human health.
Clinicians frequently treat patients with COVID-19 with corticosteroids. 2 Their use is controversial: 2 commentaries published recently in The Lancet expressed opposing views based partly on original studies of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and influenza: 1 recommended against using corticosteroids, while the other recommended using corticosteroids in some patients with COVID-19.3,4
Formulating recommendations for clinicians regarding use of corticosteroids in patients with COVID-19 requires systematic summaries of the available evidence. Therefore, to support a clinical practice guideline addressing management of patients with COVID-19,5 we conducted a series of systematic reviews. Because we anticipated a paucity of direct evidence from patients with COVID-19, we included available evidence addressing corticosteroids in the treatment of acute respiratory distress syndrome (ARDS), SARS, MERS, influenza and community-acquired pneumonia (CAP), all providing indirect evidence that informs the efficacy and safety of corticosteroid use in patients with COVID-19.
Methods
For ARDS, we used definitions in eligible studies. For severe COVID-19, we used the World Health Organization definition of severity: fever or suspected respiratory infection, plus 1 of the following: respiratory rate > 30 breaths/min, severe respiratory distress, or peripheral oxygen saturation (SpO2) ≤ 93% on room air.6
For COVID-19, SARS and MERS, we conducted systematic reviews that sought all eligible primary studies. For ARDS, influenza and CAP, we chose the most recent methodologically rigorous systematic reviews and searched for recent eligible primary studies. Choice of outcomes were informed by our preliminary protocol, by guidance from the guideline panel, and from what authors of eligible studies reported.
Search strategies and selection criteria
Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200645/-/DC1) presents the protocol we developed before launching these systematic reviews, which follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).7
COVID-19, SARS and MERS
With the assistance of a medical librarian (R.J.C.), we searched MEDLINE, Embase, PubMed and the Cochrane Central Register of Controlled Trials from the date of their inception to Apr. 19, 2020, and searched medRxiv until Apr. 25, 2020. For studies of patients with COVID-19, we also searched Chinese databases, including China National Knowledge Infrastructure (CNKI), Wanfang, Chongqing VIP Information (CQVIP), and ChinaXiv. Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200645/-/DC1) presents the complete search strategy.
We included randomized controlled trials (RCTs), cohort and case–control studies comparing corticosteroids versus no corticosteroids in patients with COVID-19, SARS or MERS. For cohort studies and case–control studies, we included only studies that performed adjusted analysis unless all studies failed to conduct an adjusted analysis, in which case we included unadjusted analyses. For overlapping studies (studies that included patients from the same data sources), we included only the larger unless there was a specific additional helpful analysis in the smaller.
ARDS, influenza and CAP
We conducted separate searches for ARDS, influenza and CAP using a 2-stage process (for search strategy, see Appendix 2). First, to identify systematic reviews that examined the effect of corticosteroids on ARDS, influenza or CAP, we searched MEDLINE, Embase, the Cochrane Database of Systematic Reviews and Epistemonikos, and chose the most recent methodologically rigorous one. Second, we searched MEDLINE, Embase and ClinicalTrials.gov for ARDS and CAP, and searched MEDLINE, Embase, PubMed and the Cochrane Central Register of Controlled Trials for influenza, for studies published subsequent to the search of the chosen reviews. For ARDS and CAP, we included only RCTs. For influenza, we included RCTs and cohort studies.
For all searches, 2 reviewers independently screened titles and abstracts and, subsequently, full texts of potentially eligible studies to determine final eligibility. Disagreements were resolved by discussion or, if necessary, referral to a third reviewer. We applied no language restriction.
Data analysis
Two reviewers independently extracted study characteristics, with adjudication by a third reviewer if necessary. Outcomes included mortality, length of intensive care unit (ICU) stay, length of hospital stay, duration of mechanical ventilation, need for mechanical ventilation, viral ribonucleic acid (RNA) clearance, viral shedding time, serious hyperglycemia, superinfection, neuromuscular weakness and gastrointestinal bleeding.
We calculated summary estimates using Stata or Review Manager and calculated relative effects (odds ratios [ORs], risk ratios [RRs] or hazard ratios [HRs]) and 95% confidence intervals (95% CIs) for dichotomous outcomes, and mean differences (MDs) and 95% CIs for continuous outcomes using a random-effects model. For continuous outcomes and adjusted estimates, we used the inverse variance ( DerSimonian and Laird) method; for dichotomous outcomes from RCTs, we used the Mantel–Haenszel method. We assessed inconsistency among studies by differences in point estimates and overlap of the confidence intervals, and the I2 statistic. For dichotomous outcomes, we calculated the absolute treatment effects by applying relative effects to risk in patients not receiving corticosteroids in 2 groups: patients with severe COVID-19 and patients with COVID-19 and ARDS. We chose the baseline mortality risk of patients with COVID-19 and ARDS from an observational study of patients with COVID-19 and ARDS,8 and the baseline mortality risk of patients with severe COVID- 19 from an observational study of patients with severe COVID-19.2 For other outcomes, we relied for baseline risks on the medians of the groups not receiving corticosteroids in the included studies.
Risk of bias assessment
We used the ROBIS risk of bias tool9 to choose the most methodologically rigorous systematic review to be updated. We used a modified version of the Cochrane risk of bias tool10 to assess risk of bias in RCTs, and a revised version of the Newcastle–Ottawa Scale11,12 for observational studies (details available at www.evidencepartners.com/resources/methodological-resources/). Two reviewers independently assessed risk of bias, resolving disagreements with a third reviewer if necessary.
Rating of evidence quality
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to rate the quality of evidence for each outcome as high, moderate, low or very low.13 The assessment included judgments addressing risk of bias,14 imprecision, 15 inconsistency,16 indirectness17 and publication bias.18 If there were serious concerns in any of these domains (for instance, in risk of bias), we rated down the quality of the evidence. Because the effect of corticosteroids in these diseases might differ from effects in the COVID-19 population, using the GRADE approach, for benefit outcomes in SARS and MERS, we rated down 1 level for indirectness, and for ARDS, influenza and CAP, we rated down 2 levels. Because we considered estimates of harm to be more likely to apply across populations than benefit outcomes, for all populations we rated down 1 level for harms.
Ethics approval
Ethics approval was not required for this systematic review.
Results
Appendix 3 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200645/-/DC1) presents the study selection process. Our search for COVID-19, SARS and MERS identified 5120 citations. After removing duplicates, screening titles and abstracts, and reviewing full texts, we ultimately included 1 cohort study8 including 84 patients with COVID-19 and ARDS, 5 cohort studies19–23 including 679 patients with COVID-19 but without ARDS, 3 studies (2 cohort studies24,25 and 1 RCT26) including 7087 patients with SARS, and 2 cohort studies27,28 including 623 patients with MERS.
Our search for systematic reviews of ARDS identified 836 citations; we ultimately chose a systematic review published in 2019 as the target for updating.29 Our search for primary studies identified 1 new eligible RCT published in 2020.30 Including 6 RCTs identified from the previous review, we included 7 RCTs30–36 with 851 patients.
Our search for systematic reviews for influenza identified 525 citations; we ultimately chose a systematic review published in 2019 as the target for updating.37 Our search for primary studies identified 1 new eligible study published in 2020.38 Including 30 studies identified from the previous review, we identified 31 eligible studies,39–69 of which 21 with 9536 patients were included in meta-analyses.41,43–47,50,52,53,55–61,63–65,68,69
Our search for systematic reviews for CAP identified 346 citations. We ultimately chose a systematic review published in 2015 as the target for updating.70 Our search for primary studies identified 1 new eligible study published in 2016.71 With 12 RCTs from the previous review, our systematic review included 13 RCTs71–83 including 2095 patients.
Appendix 4 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200645/-/DC1) presents characteristics of the included studies. Appendix 5 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200645/-/DC1) presents the risk of bias assessment for each study. Forest plots of the meta-analysis results are shown in Figures 1–5 for mortality and in Appendix 6 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200645/-/DC1) for other outcomes.
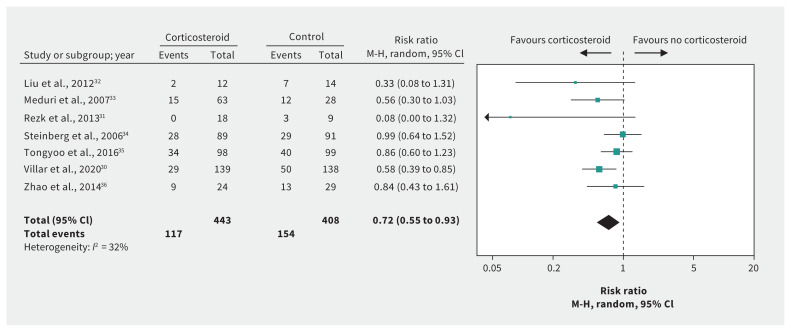
Figure 1:
Effect of corticosteroids on mortality in patients with acute respiratory distress syndrome without coronavirus disease 2019. Note: CI = confidence interval, M-H = Mantel–Haenszel.
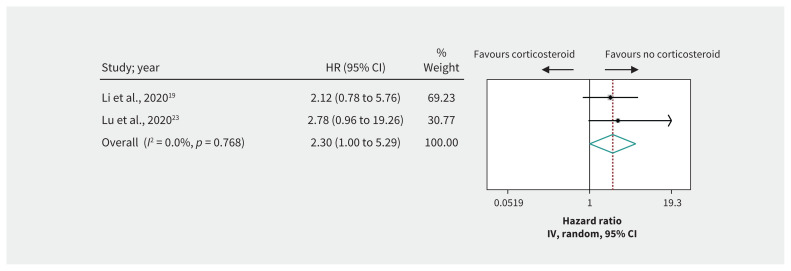
Figure 2:
Effect of corticosteroids on mortality in patients with severe coronavirus disease 2019. Weights are from random-effects analysis. Note: CI = confidence interval, HR = hazard ratio, IV = inverse variance.
Figure 3:
Effect of corticosteroids on mortality in patients with severe acute respiratory syndrome. Weights are from random-effects analysis. Note: CI = confidence interval, HR = hazard ratio, IV = inverse variance.
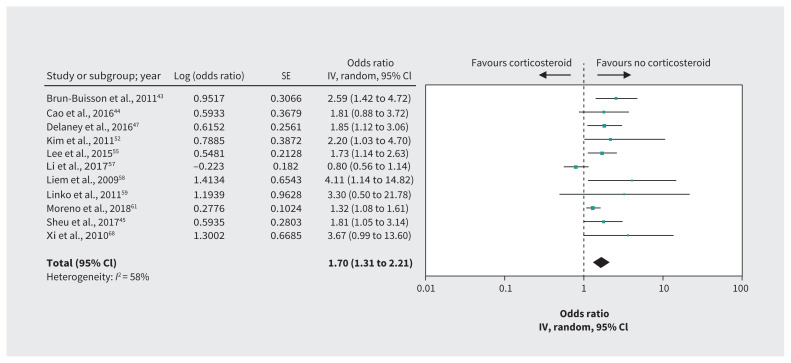
Figure 4:
Effect of corticosteroids on mortality in patients with influenza. Note: CI = confidence interval, IV = inverse variance, SE = standard error.
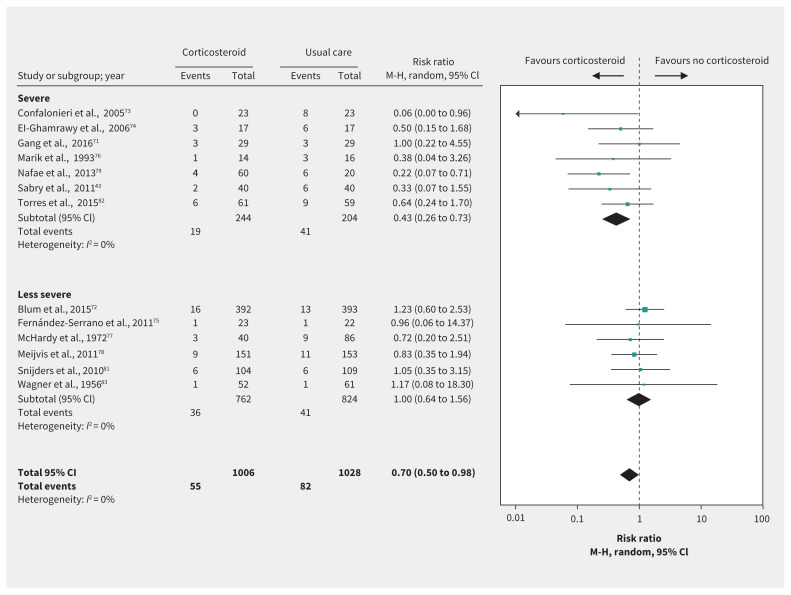
Figure 5:
Effect of corticosteroids on mortality in patients with community-acquired pneumonia. Note: CI = confidence interval, M-H = Mantel–Haenszel.
ARDS
Evidence for patients with COVID-19 and ARDS was available from a single observational study of 84 patients8 that suggested corticosteroids may result in a large mortality reduction compared with no corticosteroids (HR 0.41, 95% CI 0.20 to 0.83, MD 29.2% lower; very low-quality evidence) (Table 1).
Table 1:
GRADE summary of findings: corticosteroids in patients with COVID-19 and ARDS, based on direct evidence from observational studies of patients with COVID-19 and ARDS
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group,* % | Difference (95% CI)% | ||||
| Mortality | HR 0.41 (95% CI 0.20 to 0.83) Based on data from 84 patients with COVID-19 and ARDS in 1 observational study8 | 61.8 | −29.2 (−44.3 to −6.8) | Very low (serious imprecision†) | We are very uncertain of the effect of corticosteroids on mortality |
Note: ARDS = acute respiratory distress syndrome, CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, HR = hazard ratio.
Mortality baseline risk from patients with COVID-19 and ARDS without corticosteroid treatment.8
Observational study started at low quality of evidence. Although the CI appears narrow, the small sample size and implausibly large effect led to rating down for imprecision.
Evidence for ARDS without COVID-19 was available from 7 RCTs30–36 including 851 patients (Table 2). We considered the evidence for most outcomes to be high quality for patients with ARDS in general. After rating down 2 levels for indirectness of populations, we considered the evidence to be low quality for COVID-19. These RCTs suggest that corticosteroids may substantially reduce mortality (RR 0.72, 95% CI 0.55 to 0.93, MD 17.3% lower; low-quality evidence) (Figure 1). Very low-quality evidence raised the possibility that corticosteroids may have little or no impact on length of ICU stay32–34 (MD 0.1 days longer, 95% CI 3.0 days shorter to 3.2 days longer) but may reduce length of hospital stay33,34,36 (MD 3.6 days shorter, 95% CI 0.02 to 7.2 days shorter). Low-quality evidence shows that corticosteroids may reduce the duration of mechanical ventilation (MD −4.8 days, 95% CI −7.0 to −2.6),30,31,33–36 but increase serious hyperglycemia (risk increase 8.1%, 95% CI 0.7% to 16.2%),30,33,35 with few or no adverse effects on neuromuscular weakness, 33,34 gastrointestinal bleeding35,36 and superinfection.30,33–36
Table 2:
GRADE summary of findings: corticosteroids in patients with COVID-19 and ARDS, based on indirect evidence from randomized controlled trials of patients with ARDS but without COVID-19
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group* | Difference (95% CI) | ||||
| Mortality | RR 0.72 (95% CI 0.55 to 0.93) Based on data from 851 patients and ARDS in 7 RCTs30–36 |
61.8% | −17.3% (−27.8% to −4.3%) | Low (very serious indirectness†) | Corticosteroids may result in a large reduction in mortality |
| Length of ICU stay | Based on data from 297 patients in 3 RCTs32–34 | The median duration of length of ICU stay was 8.0 days | MD 0.1 days (−3.0 to 3.2) | Very low (serious inconsistency, very serious indirectness and serious imprecision‡) | We are very uncertain of the effect of corticosteroids on length of ICU stay |
| Length of hospital stay | Based on data from 324 patients in 3 RCTs33,34,36 | The median duration of length of hospital stay was 18.0 days | MD −3.6 days (−7.2 to −0.02) | Very low (very serious indirectness and serious imprecision§) | We are very uncertain of the effect of corticosteroids on length of hospital stay |
| Duration of mechanical ventilation | Based on data from 888 patients in 6 RCTs30,31,33–36 | The median duration of mechanical ventilation was 14.5 days | MD −4.8 days (−7.0 to −2.6) | Low (very serious indirectness†) | Corticosteroids may reduce duration of mechanical ventilation |
| Serious hyperglycemia | RR 1.12 (95% CI 1.01 to 1.24) Based on data from 565 patients in 3 RCTs30,33,35 |
67.6% | 8.1% (0.7% to 16.2%) | Low (serious indirectness and serious imprecision¶) | Corticosteroids may increase serious hyperglycemia events |
| Neuromuscular weakness | RR 0.85 (95% CI 0.62 to 1.18) Based on data from 271 patients in 2 RCTs33,34 |
26.4% | −3.9% (−10% to 4.7%) | Low (serious indirectness, serious imprecision**) | Corticosteroids may not increase neuromuscular weakness |
| Gastrointestinal bleeding | RR 0.71 (95% CI 0.30 to 1.73) Based on data from 250 patients in 2 RCTs35,36 |
14.0% | −4.0% (−9.8% to 10.2%) | Low (serious indirectness, serious imprecision**) | Corticosteroids may not increase gastrointestinal bleeding |
| Superinfection | RR 0.82 (95% CI 0.67 to 1.02) Based on data from 798 patients in 5 RCTs30,33–36 |
33.0% | −5.9% (−10.8% to 0.6%) | Moderate (serious indirectness††) | Corticosteroids probably do not increase superinfection events |
Note: ARDS = acute respiratory distress syndrome, CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, ICU = intensive care unit, MD = mean difference, RCTs = randomized controlled trials, RR = risk ratio.
Mortality baseline risk from patients with COVID-19 and ARDS who do not receive corticosteroid treatment.8 The baseline risk for the length of ICU stay, hospital stay, duration of mechanical ventilation and adverse events was obtained from the median estimate from the control group in the included RCTs.
We rated down 2 levels owing to indirectness; the cause of ARDS across the studies is inconsistent and might not represent the COVID-19 population.
We rated down 2 levels owing to indirectness; 1 for inconsistency (I2 = 73%, heterogeneity p value 0.03) and 1 for imprecision because effect estimate is consistent with benefit or harm.
We rated down 2 levels owing to indirectness and 1 for imprecision owing to the CI including a trivial reduction in hospital stay.
We rated down by 1 level owing to indirectness, as we do not expect that the COVID-19 population differs as much from other populations in adverse effects as in benefits; and we rated down by 1 level for imprecision owing to the lower CI, 0.7% representing an unimportant increase in hyperglycemia.
We rated down by 1 level owing to indirectness as we do not expect that the COVID-19 population differs as much from other populations in adverse effects as in benefits; we rated down by 1 level for imprecision, effect estimate consistent with benefit or harm.
We rated down by 1 level owing to indirectness as we do not expect that the COVID-19 population differs as much from other populations in adverse effects as in benefits; we did not rate down owing to imprecision because the largest degree of harm consistent with the evidence is 7 in 1000, which we judge to be unimportant.
Severe COVID-19: direct evidence from observational studies
Very low-quality evidence from 2 cohort studies19,23 including 331 patients with severe COVID-19 raised the possibility that corticosteroids may increase mortality compared with no corticosteroids (HR 2.30, 95% CI 1.00 to 5.29, MD 11.9% more) (Table 3, Figure 2). One cohort study20 reported an increase in the composite outcome of mortality or ICU admission with steroid use. Two cohort studies21,22 suggested that corticosteroids use was associated with prolonged viral shedding (very low-quality evidence).
Table 3:
GRADE summary of findings: corticosteroids in patients with severe COVID-19, based on direct evidence from observational studies of patients with severe COVID-19
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group,* % | Difference (95% CI), % | ||||
| Mortality | HR 2.30 (95% CI 1.00 to 5.29) Based on data from 331 patients with severe COVID-19 in 2 observational studies19,23 |
10.4 | 11.9 (0 to 33.7) | Very low (serious imprecision†) | We are very uncertain of the effect of corticosteroids on mortality |
Note: CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, HR = hazard ratio.
Baseline risk from a study of the patients with severe COVID-19 without corticosteroids use.2
Observational study started at low quality of evidence. We rated down 1 level owing to serious imprecision (wide CI).
Severe COVID-19: indirect evidence from observational studies and a randomized trial of SARS
Two cohort studies24,25 including 6129 patients with SARS provide low-quality evidence for corticosteroid impact on mortality in these patients, with additional consideration of indirectness in serious COVID-19 pneumonia (HR 0.83, 95% CI 0.41 to 1.66; very low-quality evidence) (Table 4, Figure 3). An RCT26 in which 16 patients with SARS treated with ribavirin were randomized to corticosteroids or no corticosteroids raised the possibility that early (< 7 days of illness) hydrocortisone therapy may increase the median time for SARS-associated coronavirus (SARS-CoV) RNA to become undetectable in plasma (MD 4.0 days longer, 95% CI 2.0–6.0 days; very low-quality evidence for SARS with additional consideration of indirectness in COVID-19) (Table 4).
Table 4:
GRADE summary of findings: corticosteroids in patients with severe COVID-19, based on indirect evidence from randomized controlled trials and observational studies of patients admitted to hospital with SARS
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group | Difference (95% CI) | ||||
| Mortality | HR 0.83 (95% CI 0.41 to 1.66) Based on data from 6129 patients with SARS in 2 observational studies24,25 |
10.4%* | −1.7% (−6.0% to 6.3%) | Very low (serious indirectness and serious imprecision†) | We are very uncertain of the effect of corticosteroids on mortality |
| Median time for SARS-CoV RNA to become undetectable in plasma | Based on data from 16 patients with SARS in 1 RCT26 | 8.0 days‡ | MD 4.0 days (2.0 to 6.0) | Very low (serious risk of bias, serious indirectness and serious imprecision§) | We are very uncertain of the effect of corticosteroids on time for SARS-CoV RNA to become undetectable in plasma |
Note: CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, HR = hazard ratio, MD = mean difference, RCT = randomized controlled trial, RNA = ribonucleic acid, SARS = severe acute respiratory syndrome, SARS-CoV = SARS-associated coronavirus.
Baseline risk from a study of patients with severe COVID-19 without corticosteroid use.2
Observational studies start as low quality of evidence. We rated down 1 level owing to serious indirectness (we applied the results to patients with severe COVID-19, but the relative effect was derived from patients with SARS) and 1 level owing to serious imprecision (the CI includes both an important benefit and an important harm).
Baseline risk from the RCT that reported median time for SARS-CoV RNA to become undetectable in plasma for the no corticosteroids group.26
Randomized controlled trial started at high quality of evidence. We rated down owing to serious risk of bias, serious indirectness (we applied the results to patients with severe COVID-19, but the relative effect was derived from patients with SARS) and serious imprecision (because of small sample size).
Severe COVID-19: indirect evidence from observational studies of MERS
One cohort study28 that enrolled 290 patients with MERS suggests a possible reduction in mortality with administration of corticosteroids (OR 0.75, 95% CI 0.52 to 1.07; very low-quality evidence for MERS with additional consideration of indirectness in COVID- 19) (Table 5). Data from 189 patients in the same study28 suggest that corticosteroid use may be associated with a delay in Middle East respiratory syndrome coronavirus (MERS-CoV) RNA clearance (HR 0.35, 95% 0.17 to 0.72; very low-quality evidence for MERS with additional consideration of indirectness for COVID-19) (Table 5).
Table 5:
GRADE summary of findings: corticosteroids in patients with severe COVID-19, based on indirect evidence from observational studies of patients admitted to hospital with MERS
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group, % | Difference (95% CI), % | ||||
| Mortality | OR 0.75 (95% CI 0.52 to 1.07) Based on data from 290 patients with MERS in 1 observational study28 |
10.4* | −2.4 (−4.7 to 0.6) | Very low (serious indirectness and serious imprecision§) | We are very uncertain of the effect of corticosteroids on mortality |
| MERS-CoV RNA clearance | HR 0.35 (95% CI 0.17 to 0.72) Based on data from 189 patients with MERS in 1 observational study28 |
29.8† | −18.2 (−24.0 to −7.3) | Very low (serious indirectness and serious imprecision¶) | We are very uncertain of the effect of corticosteroids on MERS-CoV RNA clearance |
Note: CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, HR = hazard ratio, MERS = Middle East respiratory syndrome, MERS-CoV = Middle East respiratory syndrome coronavirus, OR = odds ratio, RNA = ribonucleic acid.
Baseline risk from a study of patients with severe COVID-19 without corticosteroid use.2
Baseline risk from the observational study that reported MERS-CoV RNA clearance for no corticosteroids group.28
Observational studies started at low quality of evidence. We rated down 1 level owing to serious indirectness (we applied the results to patients with severe COVID-19, but the relative effect was derived from patients with MERS), and 1 level owing to serious imprecision (the CI includes both a trivial and an important effect).
Observational studies started at low quality of evidence. We rated down 1 level owing to serious indirectness (we applied the results to patients with severe COVID-19, but the relative effect was derived from patients with MERS), and 1 level owing to serious imprecision because of the small sample size.
Severe COVID-19: indirect evidence from observational studies of influenza
Evidence in patients with influenza from 11 cohort studies43–45,47,52,55,57–59,61,68 including 8530 patients with adjusted effect estimates for mortality suggests that corticosteroids may increase mortality (OR 1.70, 95% CI 1.31 to 2.21, MD 6.1% higher; low-quality evidence for influenza rated down to very low for indirectness) (Table 6, Figure 4). Very low-quality evidence for influenza with additional consideration of indirectness when applied to COVID-19 from cohort studies that failed to conduct an adjusted analysis raised the possibility that corticosteroids may increase the rate of superinfection (OR 2.74, 95% CI 1.51 to 4.95)43,44,47,52,55,57,65 and increase the number of patients requiring mechanical ventilation (OR 5.54, 95% CI 1.83 to 16.80)52,57,59,61 (Table 6).
Table 6:
GRADE summary of findings: corticosteroids in patients with severe COVID-19, based on indirect evidence from observational studies of patients admitted to hospital with influenza
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group, % | Difference (95% CI), % | ||||
| Mortality | OR 1.70 (95% CI 1.31 to 2.21) Based on data from 8530 participants from 11 observational studies43–45,47,52,55,57–59,61,68 |
10.4* | 6.1 (2.8 to 10.0) | Very low (serious indirectness‡) | We are very uncertain of the effect of corticosteroids on mortality |
| Superinfection | OR 2.74 (95% CI 1.51 to 4.95) Based on data from 6114 participants from 7 observational studies43,44,47,52,55,57,65 |
7.2† | 10.3 (3.3 to 20.5) | Very low (serious risk of bias and indirectness§) | We are very uncertain of the effect of corticosteroids on superinfections |
| Mechanical ventilation | OR 5.54 (95% CI 1.83 to 16.80) Based on data from 4364 participants from 4 observational studies52,57,59,61 |
41.8§ | 38.1 (15.0 to 50.6) | Very low (serious risk of bias and indirectness†) | We are very uncertain of the effect of corticosteroids on need for mechanical ventilation |
Note: CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, OR = odds ratio.
Baseline risk from a study of patients with severe COVID-19 without corticosteroids use.2
Baseline risk comes from the median effect of the control group in the included studies.
Observational studies started at low quality of evidence. Additional concern was indirectness (we applied the results to patients with severe COVID-19, but the relative effect was derived from patients admitted to hospital with influenza).
Observational studies started at low quality of evidence. Additional concerns included high risk of indication bias because unadjusted estimates were included, and indirectness (we applied the results to patients with severe COVID-19, but the relative effect was derived from patients admitted to hospital with influenza).
Severe COVID-19: indirect evidence from randomized trials of CAP
Thirteen RCTs71–83 including 2034 patients with CAP addressed a number of important efficacy outcomes. For patients with CAP in general, evidence varied from high to low quality. After we rated down 2 levels for indirectness, all evidence for these outcomes was of low or very low quality. Corticosteroids were associated with reductions in mortality (RR 0.70, 95% CI 0.50 to 0.98, MD 3.1% lower), need for mechanical ventilation72,75,76,79,82 (risk difference [RD] 10.4%, 95% CI 4.3% to 13.8%), duration of mechanical ventilation71,73,74,79,80 (MD 3.5 days shorter, 95% CI 1.8 to 5.2 days), length of ICU stay;72–76,78,79,82 and length of hospital stay71–76,78,79,81,82,84 (Table 7, Figure 5). Meta-analysis of 8 RCTs71,72,75,78,79,81,82,84 showed that corticosteroids may increase the rate of serious hyperglycemia (RD 5.7%, 95% CI 0.18% to 15.3%; moderate-quality evidence for CAP, low quality after rating down 1 level for indirectness).
Table 7:
GRADE summary of findings: corticosteroids in patients with severe COVID-19, based on indirect evidence from randomized controlled trials of patients admitted to hospital with community-acquired pneumonia
| Outcomes | Relative effects | Absolute effect estimates | Quality of evidence | Plain language summary | |
|---|---|---|---|---|---|
| Baseline risk for control group* | Difference (95% CI) | ||||
| Mortality | RR 0.70 (95% CI 0.50 to 0.98) Based on data from 2034 patients in 13 RCTs71–83 |
10.4% | −3.1% (−0.2% to −5.2%) | Very low (very serious indirectness† and serious inconsistency) | We are very uncertain of the effect of corticosteroids on mortality |
| Length of ICU stay | Based on data from 1376 patients in 8 RCTs72–76,78,79,82 | The median length of ICU stay was 8.3 days | MD −1.7 days (−3.4 to 0.1) | Very low (serious inconsistency, very serious indirectness and serious imprecision‡) | We are very uncertain of the effect of corticosteroids on length of ICU stay |
| Length of hospital stay | Based on data from 1636 patients in 10 RCTs71–76,78,79,81,82,84 | The median length of hospital stay was 14.3 days | MD −1.8 days (−2.8 to −0.8) | Very low (serious inconsistency, very serious indirectness and serious imprecision§) | We are very uncertain of the effect of corticosteroids on length of hospital stay |
| Need for mechanical ventilation | RR 0.42 (95% CI 0.23 to 0.76) Based on data from 1017 patients in 5 RCTs72,75,76,79,82 |
18.0% | −10.4% (−13.8% to −4.3%) | Low (very serious indirectness†) | Corticosteroids may reduce need for mechanical ventilation |
| Duration of mechanical ventilation | Based on data from 199 patients in 5 RCTs71,73,74,79,80 | The median duration of mechanical ventilation was 11.3 days | MD −3.5 days (−5.2 to −1.8) | Very low (serious risk of bias and very serious indirectness¶) | We are very uncertain of the effect of corticosteroids on duration of mechanical ventilation |
| Serious hyperglycemia | RR 1.62 (95% CI 1.02 to 2.67) Based on data from 1476 patients in 8 RCTs71,72,75,78,79,81,82,84 |
9.2% | 5.7% (0.18% to 15.3%) | Low (serious indirectness and serious imprecision**) | Corticosteroids may increase serious hyperglycemia events |
| Gastrointestinal bleeding | RR 0.99 (95% CI 0.43 to 2.24) Based on data from 1228 patients in 8 RCTs71–75,79,80,82 |
3.0% | −0.03% (−1.7% to 3.7%) | Low (serious indirectness and serious imprecision**) | Corticosteroids may have little or no impact on gastrointestinal bleeding |
| Neuropsychiatric events | RR 1.91 (95% CI 0.68 to 5.39) Based on data from 1142 patients from 4 RCTs72,81,82,84 |
1.6% | 1.4% (−0.5% to 7%) | Low (serious indirectness and serious imprecision¶) | Corticosteroids may result in a small increase in neuropsychiatric events |
| Superinfection | RR 1.31 (95% CI 0.69 to 2.50) Based on data from 1500 patients in 8 RCTs71–74,78,81,82,84 |
3.7% | 1.1% (−1.1% to 5.5%) | Low (serious indirectness and serious imprecision¶) | Corticosteroids may result in a small or no increase in superinfection events |
Note: CAP = community-acquired pneumonia, CI = confidence interval, COVID-19 = coronavirus disease 2019, GRADE = Grading of Recommendations Assessment, Development and Evaluation, ICU = intensive care unit, MD = mean difference, RCT = randomized controlled trial, RR = risk ratio.
Mortality baseline risk was obtained from patients with COVID-19 and ARDS without corticosteroid treatment.2 The baseline risk for the length of ICU stay, hospital stay, duration of mechanical ventilation and adverse events comes from the median effect of the control group in the included RCTs.
We rated down 2 levels owing to indirectness; the cause of pneumonia across the studies is inconsistent and might not represent the COVID-19 population. We also rated down for inconsistency because of a possible subgroup effect that suggests mortality benefit was restricted to those with severe pneumonia.
We rated down 2 levels owing to indirectness; 1 for inconsistency (I2 = 76%, heterogeneity p value = 0.0001); and 1 for imprecision because the effect estimates are consistent with important benefit and harm.
We rated down 2 levels owing to indirectness; 1 for inconsistency (I2 = 47%, heterogeneity p value = 0.006) and 1 for imprecision because the lower CI includes important benefit and important harm.
We rated down 1 level owing to risk of bias and 2 levels owing to indirectness. We did not rate down owing to inconsistency; the effect estimates were in the same direction, despite the I2 54% and the p value of 0.07.
We rated down by 1 level owing to indirectness, as we do not expect that the COVID-19 population differs as much from other populations in adverse effects as in benefits, and 1 for imprecision because effect estimates are not consistent with benefit or harm.
Mortality results suggested a possible subgroup effect of corticosteroids by pneumonia severity (severe pneumonia, RR 0.43, 95% CI 0.26 to 0.73; less severe pneumonia, RR 1.00, 95% CI 0.64 to 1.56; p for interaction 0.02). However, the apparent effect is based on differences between rather than within studies, is driven to a considerable extent by a small study73 that was stopped early for benefit, almost certainly represents a large overestimate of effect, and does not appear with any other outcome. Thus, the subgroup effect has low credibility.
For other adverse events (neuropsychiatric events;72,81,82,84 superinfection71–74,78,81,82,84 and gastrointestinal bleeding71–75,79,80,82), evidence was moderate quality for small, no, or uncertain harms of corticosteroids in patients with CAP, and low quality after rating down once for indirectness (Table 7).
Interpretation
This series of systematic reviews informed a guideline addressing management of patients with COVID-19.5 Direct evidence from 1 observational study8 of 84 patients with COVID-19 and ARDS was consistent with the findings of our systematic review of RCTs of patients without COVID-19 that suggested corticosteroids may reduce mortality in patients with COVID-19 and ARDS by more than 15% and reduce the duration of mechanical ventilation. The evidence suggested corticosteroids may increase the rate of serious hyperglycemia, although not of other potentially worrisome adverse effects. The evidence for these effects is mostly of low quality.
For patients who have severe COVID-19 but are not critically ill, direct evidence from observational studies provided very low-quality evidence of an increase in mortality with corticosteroids. In SARS and MERS, evidence from observational studies raises the possibility of a modest mortality reduction with corticosteroids, but also of a delay in viral clearance. In CAP, RCT evidence also raises the possibility of a mortality reduction with corticosteroids and other benefits including reduction in length of hospital and ICU stay, and need for and duration of mechanical ventilation. Low-quality evidence suggests a likely increase in hyperglycemia and possible small increases in neuropsychiatric events and superinfection, but not in gastrointestinal bleeding. Observational studies in influenza provide discrepant findings, raising the possibility of substantial increases in mortality, superinfection and mechanical ventilation with corticosteroids.
Strengths of this review include a comprehensive search, independent study selection, data abstraction and risk of bias assessment by 2 reviewers and presentation of absolute effects for dichotomous outcomes. We rated the quality of evidence with the GRADE approach, paying close attention to important methodological issues such as differences in the impact of indirectness of evidence on benefit and harm outcomes. We are more skeptical of making inferences regarding benefits in patients with COVID-19 from other patient populations than we are of making inferences on harms. For observational studies, we included, as far as possible, only those with adjusted analyses. Finally, a particular strength is the presentation of a comprehensive assessment of all the indirect evidence, including from ARDS, SARS, MERS, influenza and CAP, together in a single document.
We compared our review with another published systematic review addressing corticosteroid therapy in COVID-19.85 Apart from COVID-19, SARS and MERS, our review included 3 additional populations: ARDS, CAP and influenza. We updated our search until Apr. 19, including evidence published more recently than the previous systematic review, which searched until Mar. 15.8,19–23 Third, we included, as far as possible, only cohort and case–control studies with adjusted effect estimates. Finally, we used GRADE to rate the quality of evidence.
For ARDS, our review showed similar results to the 1 other published systematic review29 that included the latest published studies. For CAP, the results on which we focus are similar to those of other recent reviews86–89 that showed that corticosteroids may reduce mortality and length of hospital stay, and increase hyperglycemia.
The findings for influenza are consistent with other previous systematic reviews90–92 that also found increased mortality associated with corticosteroid use. One review90 focused on patients with influenza pneumonia only, excluding those with mild illness or those in the ICU. The results showed that corticosteroids were associated with higher mortality. In contrast, another review92 studied severe forms of influenza and reported that among studies with adjusted estimates, results showed no statistically significant difference between the corticosteroid and control groups.
Limitations
The limitations of this study are largely those of the underlying evidence, which is either of low or, for benefits, very low quality for the most part. One could argue that we should have broadened our consideration of indirect evidence. For instance, we could have included Pneumocystis jiroveci pneumonia, in which evidence supports corticosteroid use. Our threshold was based on patients with viral pneumonia being included in the population, which is clearly the case for SARS, MERS and influenza, but also true for ARDS and CAP.
Similarly, with respect to harms, consideration of evidence from RCTs of short-term use of corticosteroids in other conditions might have strengthened our findings. We have, however, moderate-quality evidence in patients with ARDS of no important increase in superinfection, and low-quality evidence of an increase in serious hyperglycemia. Low-quality evidence suggests a possible small increase in neuropsychiatric events. For this outcome, evidence from other conditions might have been particularly helpful.
Conclusion
Given the paucity of direct evidence and the limitations of indirect evidence, it is critical for clinicians and researchers to cooperate in conducting high-quality studies, in particular large and rigorous RCTs, to evaluate the effect of corticosteroids in both patients with COVID-19 and ARDS and patients with severe COVID-19 but who are not critically ill. Fortunately, RCTs, including those that address corticosteroid treatment, are ongoing.
Acknowledgements
Quazi Ibrahim, Lehana Thabane, Diane Heels- Ansdell, Jason Busse and Li Wang provided support and suggestions for the statistical analysis. Yingqi Xiao is supported by the China Scholarship Council.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.200648
Footnotes
Competing interests: Bram Rochwerg is an investigator in a trial, supported by a Canadian Institute of Health Research grant, evaluating the effect of corticosteroids in COVID-19 patients. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Zhikang Ye and Gordon Guyatt contributed to the conception of the work. Zhikang Ye, Ying Wang, Luis Enrique Colunga-Lozano, Manya Prasad and Gordon Guyatt contributed to the design of the work. Rachel Couban, Zhikang Ye, Ying Wang, Luis Enrique Colunga-Lozano, Manya Prasad, Wimonchat Tangamornsuksan, Liang Yao, Shahrzad Motaghi, Maryam Ghadimi, Malgorzata Bala, Huda Gomaa, Fang Fang and Yingqi Xiao contributed to the acquisition, analysis and interpretation of data. Zhikang Ye, Ying Wang, Luis Enrique Colunga-Lozano and Manya Prasad drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Zhikang Ye, Ying Wang, Luis Enrique Colunga-Lozano and Manya Prasad are joint primary authors.
Funding: None.
Data sharing: Data extracted from the included studies are presented in the results; however, full extraction data tables are available upon reasonable request from the corresponding author.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. Geneva: World Health Organization; 2020. Available: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed 2020 Apr. 23). [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020;395:683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Z, Rochwerg B, Wang Y, et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. CMAJ 2020. Apr. 29 [early online release]. 10.1503/cmaj.200648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected [interim guidance]. Geneva: World Health Organization; 2020. Mar. 13. [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; Mar. 13 [Epub ahead of print]. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting P, Savovic J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt GH, Busse JW. Modification of Cochrane Tool to assess risk of bias in randomized trials. Ottawa: Evidence Partners; Available: www.evidencepartners.com/resources/methodological-resources/ (accessed 2020 Apr. 29). [Google Scholar]
- 11.Busse JW, Guyatt GH. Tool to assess risk of bias in cohort studies. Ottawa: Evidence Partners; Available: www.evidencepartners.com/resources/methodological-resources/ (accessed 2020 Apr. 29). [Google Scholar]
- 12.Busse JW, Guyatt GH. Tool to assess risk of bias in case-control studies. Ottawa: Evidence Partners; Available: www.evidencepartners.com/resources/methodological-resources/ (accessed 2020 Apr. 29). [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. rating the quality of evidence–imprecision. J Clin Epidemiol 2011;64:1283–93. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. rating the quality of evidence–inconsistency. J Clin Epidemiol 2011;64:1294–302. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. rating the quality of evidence–indirectness. J Clin Epidemiol 2011;64:1303–10. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. rating the quality of evidence–publication bias. J Clin Epidemiol 2011;64:1277–82. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; Apr. 12 [Epub ahead of print]. pii S0091-6749(20)30495-4. 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Wang J, Jiang Q, et al. No clear benefit to the use of corticosteroid as treatment in adult patients with Coronavirus disease 2019: a retrospective cohort study. medRxiv 2020. Apr. 24. 10.1101/2020.04.21.20066258. [DOI] [Google Scholar]
- 21.Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 2020; Apr. 9 [Epub ahead of print]. pii ciaa351. 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan D, Liu X, Zhu Y, et al. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in patients with SARS-CoV-2 infection. medRxiv 2020; Mar. 30. 10.1101/2020.03.22.20040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu X, Chen T, Wang Y, et al. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau EHY, Cowling BJ, Muller MP, et al. Effectiveness of Ribavirin and corticosteroids for severe acute respiratory syndrome. Am J Med 2009;122:1150.e11–.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long Y, Xu Y, Wang B, et al. Clinical recommendations from an observational study on MERS: glucocorticoids was benefit in treating SARS patients. Int J Clin Exp Med 2016;9:8865–73. [Google Scholar]
- 26.Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol 2004;31:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfaraj SH, Al-Tawfiq JA, Assiri AY, et al. Clinical predictors of mortality of Middle East respiratory syndrome Coronavirus (MERS-CoV) infection: a cohort study. Travel Med Infect Dis 2019;29:48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197:757–67. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SR, Pritchard MW, Thomas CM, et al. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev 2019;7:CD004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020;8:267–76. [DOI] [PubMed] [Google Scholar]
- 31.Abdelsalam Rezk N, Mohamed Ibrahim A. Effects of methyl prednisolone in early ARDS. Egyptian J Chest Dis Tuberc 2013;62:167–72. [Google Scholar]
- 32.Liu L, Li J, Huang YZ, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insuficiency. Zhonghua Nei Ke Za Zhi 2012;51:599–603. [PubMed] [Google Scholar]
- 33.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131:954–63. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006;354:1671–84. [DOI] [PubMed] [Google Scholar]
- 35.Tongyoo S, Permpikul C, Mongkolpun W, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care 2016;20:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao WB, Wan S, Gu DF, et al. Therapeutic effect of glucocorticoid inhalation for pulmonary fibrosis in ARDS patients. Med J Chinese People’s Liberation Army 2014;39:741–5. [Google Scholar]
- 37.Lansbury LE, Rodrigo C, Leonardi-Bee J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit Care Med 2019; Nov. 15 [Epub ahead of print]. 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 38.Tsai M-J, Yang K-Y, Chan M-C, et al. Impact of corticosteroid treatment on clinical outcomes of influenza-associated ARDS: a nationwide multicenter study. Ann Intensive Care 2020;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Busaidi M, Al Maamari K, Al’Adawi B, et al. Pandemic influenza A (H1N1) in Oman: epidemiology, clinical features, and outcome of patients admitted to Sultan Qaboos University Hospital in 2009. Oman Med J 2016;31:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annane D. Pro: the illegitimate crusade against corticosteroids for severe H1N1 pneumonia. Am J Respir Crit Care Med 2011;183:1125–6. [DOI] [PubMed] [Google Scholar]
- 41.Balaganesakumar SR, Murhekar MV, Swamy KK, et al. Risk factors associated with death among influenza A (H1N1) patients, Tamil Nadu, India, 2010. J Post-grad Med 2013;59:9–14. [DOI] [PubMed] [Google Scholar]
- 42.Boudreault AA, Xie H, Leisenring W, et al. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transplant 2011;17:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brun-Buisson C, Richard J-CM, Mercat A, et al. Group R-SAHNvR. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med 2011;183:1200–6. [DOI] [PubMed] [Google Scholar]
- 44.Cao B, Gao H, Zhou B, et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med 2016;44:e318–28. [DOI] [PubMed] [Google Scholar]
- 45.Sheu C-C, Chang W-A, Tsai M-J, et al. Early corticosteroid treatment in patients with influenza-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2017;195:A2769. [Google Scholar]
- 46.Chawla R, Kansal S, Chauhan M, et al. Predictors of mortality and length of stay in hospitalized cases of 2009 influenza A (H1N1): experiences of a tertiary care center. Indian J Crit Care Med 2013;17:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delaney JW, Pinto R, Long J, et al. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care 2016;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delgado-Rodríguez M, Castilla J, Godoy P, et al. Prognosis of hospitalized patients with 2009 H1N1 influenza in Spain: influence of neuraminidase inhibitors. J Antimicrob Chemother 2012;67:1739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han K, Ma H, An X, et al. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis 2011;53:326–33. [DOI] [PubMed] [Google Scholar]
- 50.Huang S-F, Fung C-P, Perng D-W, Wang F-D. Effects of corticosteroid and neuraminidase inhibitors on survival in patients with respiratory distress induced by influenza virus. J Microbiol Immunol Infect 2017;50:586–94. [DOI] [PubMed] [Google Scholar]
- 51.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009;361:1935–44. [DOI] [PubMed] [Google Scholar]
- 52.Kim S-H, Hong S-B, Yun S-C, et al. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med 2011;183:1207–14. [DOI] [PubMed] [Google Scholar]
- 53.Kinikar AA, Kulkarni RK, Valvi CT, et al. Predictors of mortality in hospitalized children with pandemic H1N1 influenza 2009 in Pune, India. Indian J Pediatr 2012;79:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kudo K, Takasaki J, Manabe T, et al. Systemic corticosteroids and early administration of antiviral agents for pneumonia with acute wheezing due to influenza A(H1N1)pdm09 in Japan. PLoS One 2012;7:e32280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee N, Leo Y-S, Cao B, et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J 2015;45:1642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Chen G, Wang J, et al. A case-control study on risk factors associated with death in pregnant women with severe pandemic H1N1 infection. BMJ Open 2012;2. pii: e000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Yang S-G, Gu L, et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses 2017;11:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liem NT, Tung CV, Hien ND, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis 2009;48:1639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linko R, Pettilä V, Ruokonen E, et al. Corticosteroid therapy in intensive care unit patients with PCR-confirmed influenza A(H1N1) infection in Finland. Acta Anaesthesiol Scand 2011;55:971–9. [DOI] [PubMed] [Google Scholar]
- 60.Mady A, Ramadan OS, Yousef A, et al. Clinical experience with severe 2009 H1N1 influenza in the intensive care unit at King Saud Medical City, Saudi Arabia. J Infect Public Health 2012;5:52–6. [DOI] [PubMed] [Google Scholar]
- 61.Moreno G, Rodríguez A, Reyes LF, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 2018;44:1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ono S, Ono Y, Matsui H, et al. Factors associated with hospitalization for seasonal influenza in a Japanese nonelderly cohort. BMC Public Health 2016;16:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel KK, Patel AK, Mehta PM, et al. Clinical outcome of novel H1N1 (swine flu)- infected patients during 2009 pandemic at tertiary referral hospital in Western India. J Glob Infect Dis 2013;5:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sertogullarindan B, Ozbay B, Gunini H, et al. Clinical and prognostic features of patients with pandemic 2009 influenza A (H1N1) virus in the intensive care unit. Afr Health Sci 2011;11:163–70. [PMC free article] [PubMed] [Google Scholar]
- 65.Viasus D, Paño-Pardo JR, Cordero E, et al. Effect of immunomodulatory therapies in patients with pandemic influenza A (H1N1) 2009 complicated by pneumonia. J Infect 2011;62:193–9. [DOI] [PubMed] [Google Scholar]
- 66.Wirz SA, Blum CA, Schuetz P, et al. Pathogen- and antibiotic-specific effects of prednisone in community-acquired pneumonia. Eur Respir J 2016;48:1150–9. [DOI] [PubMed] [Google Scholar]
- 67.Wu U-I, Wang J-T, Ho Y-C, et al. Factors associated with development of complications among adults with influenza: a 3-year prospective analysis. J Formos Med Assoc 2012;111:364–9. [DOI] [PubMed] [Google Scholar]
- 68.Xi X, Xu Y, Jiang L, et al. Hospitalized adult patients with 2009 influenza A(H1N1) in Beijing, China: risk factors for hospital mortality. BMC Infect Dis 2010;10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Yang Y, Zhang Q. Clinical characteristics and risk factors of severe patients with novel pandemic influenza A H1N1. Chinese J Integr Tradit West Med Intentive Crit Care 2011;18:142–5. [Google Scholar]
- 70.Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med 2015;163:519–28. [DOI] [PubMed] [Google Scholar]
- 71.Gang LI, Chengdong GU, Zhang S, et al. Value of glucocorticoid steroids in the treatment of patients with severe community-acquired pneumonia complicated with septic shock. Chinese Crit Care Med 2016;28:780–4. [Google Scholar]
- 72.Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015;385:1511–8. [DOI] [PubMed] [Google Scholar]
- 73.Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005;171:242–8. [DOI] [PubMed] [Google Scholar]
- 74.El-Ghamrawy AH, Shokeir MH, Esmat AA. Effects of low-dose hydrocortisone in ICU patients with severe community-acquired pneumonia. Egyptian J Chest Dis Tuberc 2006;55:91–9. [Google Scholar]
- 75.Fernández-Serrano S, Dorca J, Garcia-Vidal C, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care 2011;15:R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marik P, Kraus P, Sribante J, et al. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia: a randomized controlled study. Chest 1993;104:389–92. [DOI] [PubMed] [Google Scholar]
- 77.McHardy VUSM. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co-operative controlled trial. BMJ 1972;4:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meijvis SC, Hardeman H, Remmelts HH, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet 2011;377:2023–30. [DOI] [PubMed] [Google Scholar]
- 79.Nafae RM, Ragab MI, Amany FM, et al. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egyptian J Chest Dis Tuberc 2013;62:439–45. [Google Scholar]
- 80.Sabry NA, Omar E-D. Corticosteroids and ICU course of community-acquired pneumonia in Egyptian settings. Pharmacol Pharmacy 2011;2:73–81. [Google Scholar]
- 81.Snijders D, Daniels JM, de Graaff CS, et al. Efficacy of corticosteroids in community- acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010;181:975–82. [DOI] [PubMed] [Google Scholar]
- 82.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86. [DOI] [PubMed] [Google Scholar]
- 83.Wagner HN, Jr, Bennett I, Jr, Lasagna L, et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp 1956;98:197–215. [PubMed] [Google Scholar]
- 84.Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007;185:249–55. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z, Liu J, Zhou Y, et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 2020; Apr. 10 [Epub ahead of print]. pii S0163-4453(20)30191-2. 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang J, Guo J, Li H, et al. Efficacy and safety of adjunctive corticosteroids therapy for patients with severe community-acquired pneumonia: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang S, Liu T, Hu Y, et al. Efficacy and safety of glucocorticoids in the treatment of severe community-acquired pneumonia: a meta-analysis. Medicine (Baltimore) 2019;98:e16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stern A, Skalsky K, Avni T, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev 2017;(12):CD007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu WF, Fang Q, He GJ. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: a meta-analysis. Am J Emerg Med 2018;36:179–84. [DOI] [PubMed] [Google Scholar]
- 90.Ni Y-N, Chen G, Sun J, et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 2019;23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Sun W, Svendsen ER, et al. Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit Care 2015;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 2020;10:3044. [DOI] [PMC free article] [PubMed] [Google Scholar]