Idelalisib, a specific inhibitor of the delta isoform of phosphatidylinositol 3 kinase (PI3Kδ, is approved with rituximab for the therapy of relapsed refractory patients with CLL in the United States and Europe1, 2. Idelalisib has been associated with a characteristic pattern of toxicities that include transaminase elevation, diarrhea/colitis, pneumonitis and rash3, 4. Transaminase elevation (TAE) has been observed in all CLL clinical trials involving idelalisib, with company-sponsored studies showing rates of grade 3+ elevations ranging from approximately 10%5–7 to 41%8. Cross-study comparison has suggested that these toxicities tend to be more frequent among previously untreated patients9. This trend was particularly notable in an investigator-sponsored study at Dana-Farber Cancer Institute, in which grade 3–4 transaminitis occurred early in more than 50% of untreated patients with CLL receiving single agent idelalisib prior to planned addition of ofatumumab two months later4. Risk factors for transaminitis in that small study included younger age < 65 and mutated IGHV, which together explained almost all of the risk for this event4. We undertook the present study in order to understand risk factors for grade 3 or higher TAE across all fully enrolled, Gilead sponsored phase 2 and 3 studies, involving 853 total patients.
Data were included from all patients who received at least 1 dose of idelalisib in any of 6 phase 2 or 3 studies (Supplementary Table 1), three for treatment naïve (TN) and three for relapsed/refractory (R/R) CLL. Patients with underlying liver disease or elevated liver function tests were excluded from these trials. The following variables were analyzed in univariable and multivariable analysis: age, prior therapy (Y vs N), combination with bendamustine plus rituximab (BR; Y vs N), del(17p) (Y vs N), and IGHV status (mutated vs unmutated). In addition, association of transaminase elevation with specific other drug exposures (allopurinol, acetaminophen, trimethoprim-sulfamethoxazole and acyclovir) was assessed by assuming a potential association if the first occurrence of any grade TAE that was continuous with the grade 3 event occurred within 7 days of exposure to the concomitant medication.
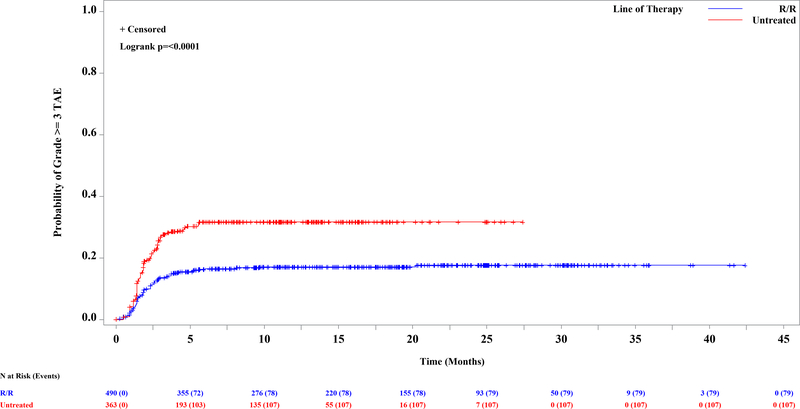
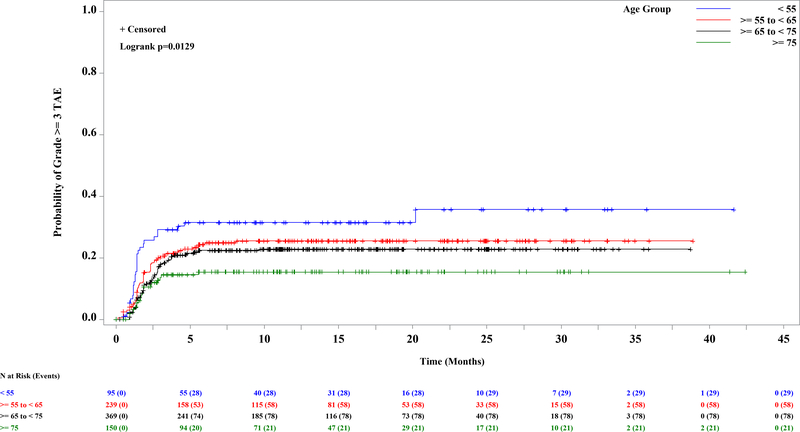
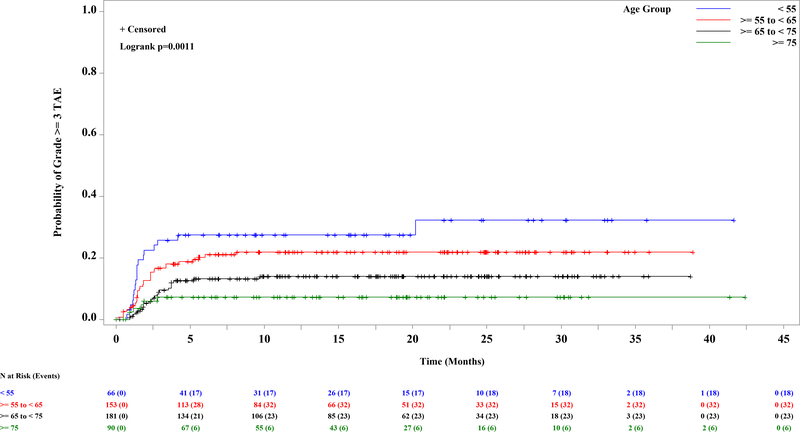
A total of 853 patients were enrolled and treated with idelalisib (Supplementary Table 1) and comprise the safety analysis set (SAS). The median age was 67 y (range: 37–90), and the median number of prior regimens among relapsed refractory patients was 3 (range: 1–13). The median exposure to idelalisib was 10.8 mo across the entire SAS (range: <1 – 60.3 mos). The incidence of grade 3 or higher TAE ranged from 9.1% in study 116 to 41% in study 133 (IDELA+R in treatment naïve (TN) del17p; Supplementary Table 1). For comparison, the incidence of grade ≥3 TAE across the placebo groups of the R/R studies was 2.2% overall, 3.8% for BR, and 1.1% for non-BR–containing regimens (Supplementary Table 1). As previously suggested, TN patients (n=363) were at greater risk than R/R patients (n=490) (Figure 1A; HR=2; log rank p<0.0001). Of those patients experiencing grade ≥3 TAE, however, the median time to onset was similar, at 7.6 weeks among TN and 8 wks among R/R. The other risk factors were then evaluated separately in the TN and R/R groups, as well as in the entire SAS. Significantly and progressively increased risk was found in younger patients by decile of age, in the entire dataset (Figure 1B, p=0.01) and in the relapsed refractory cohort (Figure 1C; log rank p=0.001). In the entire SAS, a trend toward a significant effect of BR was also seen (p=0.08) (Supplementary Figure 1A). When the analysis was limited to R/R patients, treatment with BR was more significant (incidence rate, 23% vs 11%; Supplementary Figure 1B; log rank p=0.0005); although patients receiving BR were younger, the increased risk was seen across the age ranges. Neither del(17p) (p=0.12) nor IGHV mutation status (p=0.24) was a significant predictor of grade 3 or higher TAE in the entire SAS (Supplementary Figures 1C and 1D). When this analysis was limited to the TN population, however, del(17p) became significant (39 vs 26%; p=0.02; Supplementary Figure 1E), although this observation was driven by the very high rate of both del(17p) and TAE in study 133 and should therefore be interpreted with caution. All other univariate analyses yielded p-values greater than 0.05.
Figure 1. Kaplan-Meier Curve of First Onset of Grade 3 or Higher Transaminase Elevation Among 853 Patients Treated with Idelalisib, by Significant Risk Factor.
A. Previously untreated (31.7%) vs previously treated (17.6%) (p<=0.0001). B-C. By age, in ten year increments. B. Entire dataset (p=0.01). C. Relapsed refractory patients, N=490 (p=0.001).
A multivariable analysis was performed to assess the contributions of age, prior therapy, del(17p), IGHV mutation status, concomitant BR and their interactions, to risk of grade 3 or higher TAE across these studies (Table 1). Prior therapy was protective, with HR 0.47 (p<0.001), while younger age was associated with increased risk, with those <55 yrs having HR 2.64 compared to those 75 yrs or older (p=0.003). No other factors were significant. Furthermore, a simple comparison of frequency of exposure to key concomitant medications did not suggest an association with grade 3 TAE (Supplementary Table 2).
Table 1.
Multivariable Analysis of Risk Factors for Grade 3 or Higher Transaminase Elevation
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Prior therapy | ||
| Yes | 0.47 (0.35, 0.63) | <0.001 |
| Age, y+ | ||
| <55 | 2.64 (1.50, 4.63) | 0.003 |
| ≥55 to <65 | 1.77 (1.07, 2.91) | |
| ≥65 to <75 | 1.36 (0.84, 2.21) |
Factors considered: IGHV mutation, del(17p), age, combination with BR, prior therapy, and their interactions
Compared with ≥75 y.
CI, confidence interval; HR, hazard ratio
In this analysis of risk factors for idelalisib-related hepatotoxicity across the Gilead program of sponsored phase 2 and phase 3 trials, we again identify that absence of prior therapy as well as younger age are the two primary risk factors4. Furthermore, the risk associated with younger age is progressive with decade. This observation is striking, as it is the opposite of what is observed for most other therapeutic toxicities, where adverse events are typically worse in more heavily pretreated, older patients. This pattern is certainly consistent with an immune-mediated mechanism, as previously suggested by Lampson et al., who reported an activated CD8 T cell infiltrate in the liver and found that toxicity was associated with a decrease in regulatory T cells in the peripheral blood. Why the association with mutated IGHV is not seen in this analysis is not clear, as study 133 showed a trend toward higher risk with mutated IGHV, similar to what was seen in Lampson et al.8. This association is hypothesized to be related to the immune milieu of patents with mutated vs unmutated IGHV, and may therefore be more evident in previously untreated patients or in patient populations with high rates of TAEs, as in the two prior studies that suggested this association. Ongoing work will be required to further elucidate the validity and mechanism of this association.
In this study, we have validated clinically relevant predictors of risk for autoimmune effects of idelalisib – younger age and no prior therapy – which can be used to guide clinical decision-making at present. The effect of younger age is progressive, but in Lampson et al.4, in the untreated setting, all patients under 65 developed significant toxicity requiring steroids and drug hold. In contrast, in the phase 1 study of idelalisib1, in which most patients had had several prior courses of chemoimmunotherapy, safety was significantly better despite a median age of 62.5. Thus, the risk factors of age and prior therapy likely interact. Additionally, as patients with CLL increasingly receive only targeted agents like BTK or BCL-2 inhibitors, the impact of these therapies on risk of autoimmune side effects with PI3Kδ inhibitors remains entirely unknown and will need to be characterized in future work. Whether these risk factors apply to other PI3Kδ inhibitors or in lymphoma patients also remains to be determined in future work. As initial guidance, though, we believe our data convincingly demonstrate the impact of patient age and prior therapy on risk of transaminase elevation in CLL patients treated with idelalisib.
Supplementary Material
Acknowledgments:
JRB acknowledges funding from NCI 1R01CA213442-01A1, as well as Gilead, the National Comprehensive Cancer Network and the Melton and Rosenbach Funds.
Competing Interests: JRB has served as a consultant for Abbvie, Acerta, Astra-Zeneca, BeiGene, Catapult, Dynamo Therapeutics, Genentech/Roche, Gilead, Juno/Celgene, Kite, Loxo, MEI Pharma, Nextcea, Novartis, Octapharma, Pfizer, Pharmacyclics, Sunesis, TG Therapeutics, Verastem; received honoraria from Janssen and Teva; received research funding from Gilead, Loxo, Sun and Verastem; and served on data safety monitoring committees for Morphosys and Invectys. AZ has served as a consultant for JUNO/Celgene, Genentech/Roche, Gilead, BeiGene, Amgen, Pharmacyclics, Janssen, Astra-Zeneca, Novartis/SANDOZ, MEI Pharma; received research support from Genentech/Roche, Gilead, BeiGene, MEI Pharma; and served on scientific advisory boards for the Lymphoma Research Foundation and Adaptive Biotechnologies. RF has served as a consultant for Abbvie, Acerta, Astra-Zeneca, Beigene, Genentech, Janssen, Loxo Oncology, Oncotracker, Pharmacyclics, Sunesis, TG Therapeutics, Verastem Oncology; received speaking fees from Janssen; received research funding from TG Therapeutics and AstraZeneca; and served on the data safety and monitoring board for Incyte. NL has served as a consultant for Abbvie, Astra-Zeneca, Bei-Gene, Celgene, Gilead, Genentech, Juno, Janssen/Pharmacyclics, Octapharma; received research funding from Abbvie, Astra-Zeneca, BeiGene, Genentech, Juno, Infinity/Verastem, Octapharma, Oncternal, Ming Therapeutics, TG Therapeutics; and served on advisory boards for Abbvie, Astra Zeneca, BeiGene, Celgene, Gilead, Genentech, Juno, Janssen/Pharmacyclics, and Octapharma. ARM holds a consultancy role for TG Therapeutics, Abbvie, Pharmacyclics, Johnson & Johnson, Regeneron, Astra-Zeneca, Genentech, Loxo, Celgene, Sunesis, Adaptive; received research funding from TG Therapeutics, Abbvie, Pharmacyclics, Johnson & Johnson, Regeneron, Genentech, Loxo, Portola, DTRM, Adaptive, Acerta; and served on the data safety monitoring board for TG Therapeutics and Celgene. MM has received honoraria from Abbvie, Gilead, Janssen, Roche; and served on the advisory board for Abbvie, Acerta/Astra-Zeneca, Gilead, Janssen, Roche, and Verastem. SO has served as a consultant for Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc., Vaniam Group LLC, Abbvie, Alexion, Verastem, Eisai, Juno Therapeutics, Gilead, Pharmacyclics, TG Therapeutics, Pfizer, Sunsesis; and received research support from Kite, Regeneron, Acerta, Gilead, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis. RD has served as a consultant for Acerta Pharma; was formerly employed by Gilead; is currently employed by Juno Therapeutics (BMS); and owns stock in Gilead Sciences, Bristol-Myers-Squibb, and Forty Seven. LG has served as an independent contractor for Gilead; and is employed by Gilead. VM worked as an employee of Gilead while the work was conducted; is currently employed with Acerta Pharma; and owns stock in Astra-Zeneca and Gilead Sciences. TR has served in an advisory role for Gilead; received honoraria from Gilead; and received research funding from Gilead. PH has received honoraria from Janssen and Abbvie; received research funding from Janssen, Abbvie, Pharmacyclics, Gilead, Roche, Apellis; and has served on an advisory board for Janssen, Abbvie, and Alexion.
Footnotes
Ethics Statement: The research protocols contributing data to this project were all prospectively approved by the Human Protections Committee at each participating site. All patients provided written informed consent prior to any study procedures.
REFERENCES
- 1.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014; 123: 3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutre S, Barrientos J, Brown JR, De Vos S, Furman RR, Keating MJ, et al. Safety of idelalisib in B-cell malignancies: integrated analysis of eight clinical trials. ASCO Meeting Abstracts 2015; 33(suppl): (abstract e18030). [Google Scholar]
- 4.Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 2016; 128: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JA, Robak T, Brown JR, Awan FT, Badoux X, Coutre S, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol 2017; 4: e114–e126. [DOI] [PubMed] [Google Scholar]
- 6.Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol 2019; 37: 1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2017. March; 18: 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillmen P, Badoux X, Delgado J, Leblond V, Mato A, Simkovic M, et al. Safety results of terminated phase 2 study of idelalisib plus rituximab in treatment naïve chronic lymphocytic leukemia (CLL) with del(17p). Haemotologica 2017; 102(s1): 172 (abstract S465). [Google Scholar]
- 9.Lampson BL, Brown JR. PI3Kdelta-selective and PI3Kalpha/delta-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma. Expert Opin Investig Drugs 2017; 26: 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.