Abstract
Cellular homeostasis plays a critical role in how an organism will develop and age. Disruption of this fragile equilibrium is often associated with health degradation and ultimately, death. Reactive oxygen species (ROS) have been closely associated with health decline and neurological disorders, such as Alzheimer’s disease or Parkinson’s disease. ROS were first identified as by-products of the cellular activity, mainly mitochondrial respiration, and their high reactivity is linked to a disruption of macromolecules such as proteins, lipids and DNA. More recent research suggests more complex function of ROS, reaching far beyond the cellular dysfunction. ROS are active actors in most of the signaling cascades involved in cell development, proliferation and survival, constituting important second messengers. In the brain, their impact on neurons and astrocytes has been associated with synaptic plasticity and neuron survival. This review provides an overview of ROS function in cell signaling in the context of aging and degeneration in the brain and guarding the fragile balance between health and disease.
Keywords: Reactive species, ROS, Hormesis, Homeostasis
Introduction
The cellular functions rely on a variety of extracellular signals and intracellular signaling that function in concert to maintain cellular homeostasis. Most, if not all, cellular processes require considerable energy. Mitochondria are known to fulfill this crucial role, producing the majority of the energy supporting cell growth and homeostasis. However, the dark side of energy production is the formation of reactive oxygen species (ROS) as a by-product by the mitochondria’s electron transport chain [1]. Until recently, ROS were essentially considered to be responsible for significant cellular damages [2], causing premature aging and neurodegenerative disorders. Since the 1950s and Harman’s Free-radical theory of aging [3], a compelling amount of research has investigated how ROS and reactive nitrogen species (RNS) influence disease progression. However, this theory is now being challenged on the basis of considerable evidence suggesting that ROS can act as second messengers. Furthermore, antioxidants that purportedly should antagonize the putative oxidative damage produced ROS have largely been ineffective in preventing disorders in which ROS are the considered as being the cause [4–7]. It is clear that ROS have complex influences on the cells, depending on their concentration. While their role in macromolecular damage and cell death upon loss of redox homeostasis is still a valid model, a mild increase of reactive species triggers various cellular signaling cascades that allow cell growth and survival [8–10]. Recently the concept of hormesis (which can also be dubbed “what does not kill you makes you stronger”) has been applied to ROS. Indeed, a contained production of these reactive species promotes stress resistance and longevity in model organisms such as Caenorhabditis elegans [11–13], Drosophila melanogaster [14, 15] and rodents [16].
Nature of Reactive Species
ROS are, by definition, chemical molecules containing one oxygen atom that, through cellular and extracellular reactions become more reactive than oxygen itself. Reactive species are present in both radical, with and unpaired electron, and non-radical form. An example of ROS is the superoxide anion (O2•−) produced as a by-product of the mitochondrial respiration and NADPH oxidase activity. Other ROS include the hydroxyl radicals (OH•) and hydrogen peroxide (H2O2) a non-radical species. Another group is called RNS. Nitric oxide (NO•) is produced from l-arginine, by nitric oxide synthase (NOS) and acts a potent second messenger. NO promotes glycolytic metabolism by inhibiting mitochondrial respiration through cytochrome c oxidase and increased AMPK phosphorylation [17, 18]. In parallel, NO•− interacts with superoxide (O2•−) to form peroxynitrite (ONOO•−) a highly reactive molecule capable of protein nitrosylation and target glutathione, a critical non-enzymatic antioxidant [17, 18].
Sources of Reactive species
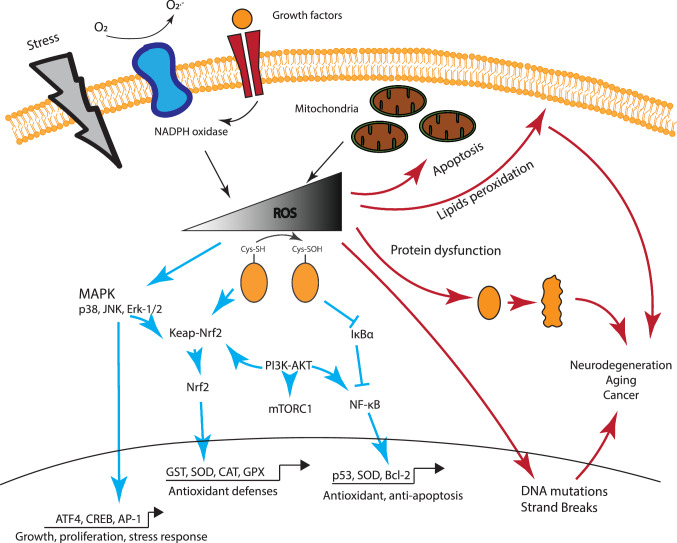
Reactive species originate from two primary sources. ROS can either be released as by-products of oxidative metabolism, mainly through mitochondrial respiration or produced during cellular response to xenobiotics or cytokines released as part of a defense mechanism [19, 20] (Fig. 1). Energy production by the mitochondrial electron transport chain accounts for the majority of ROS in the cell. This leak of protons, originating from the oxidation of NADH and FADH2, at the complexes I (NADH dehydrogenase) and III (coenzyme Q and cytochrome c oxidoreductase) [21, 22] of the electron transport chain, produce a reduced oxygen ion known as superoxide (O2•−) [1].
Fig. 1.
Schematic representation of the impact of ROS on cellular physiology. Low and Mild ROS level have a large impact on cell signaling, promoting activation of growth signals and kinases (Erk-1/2, PI3K, ATF4 and mTOR) and the transcription of pro-survival (Nrf2, PGC1α) factors. This interactive signaling culminates in the increased expression of antioxidant enzyme (SOD, CAT, GST), the effectors of the survival response. However, increased concentration of ROS disrupt cell signaling and activate pro-apoptotic signals in the mitochondria, as well as lipid peroxidation, protein oxidation and DNA damage. The accumulation of macromolecules and cell damage leads to a wide range of disorders and is associated with accelerate aging
The second primary source of ROS is the enzyme complex Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Mammals possess seven NADPH oxidases (NOX1–5 and DUOX1–2) that produce ROS in the cytoplasm in response to a variety to stimuli. Initially identified in neutrophils, NADPH oxidase is a membrane-associated enzymatic complex involved in cellular signaling and disease through ROS production in the cytoplasm. Various ligands like TNFα, angiotensin II, PDGF and EGF [23–26] have been associated to NOX-mediated ROS production in response to cellular stimuli such as pathogen invasion, inflammation, growth factors and calcium signaling [27–29]. The complexes produce superoxide radicals and hydrogen peroxide, the latter being more stable and capable of diffusing through the cell membrane [30]. Another significant source of reactive species is the NOS. Present in different isoforms, these complexes are found as a constitutive form in neurons (nNOS or NOS1), as an inducible isoform in glial cells (iNOS or NOS2) and in the endothelial tissue (eNOS or NOS3). NOS produce nitric oxide (NO) that shapes the metabolic profile of the cell by inhibiting the mitochondrial respiration via inhibition of its complex IV (cytochrome c oxidase) and promoting glycolytic activity [31]. However, as mentioned previously, NO•− also reacts with O2•− to produce peroxynitrite (ONOO•−) a reactive specie involved in protein nitration, lipid peroxidation, and DNA damage. In addition of the mitochondria, NOS and NOX, other endogenous, and exogenous, sources have been linked to ROS production, such as the xanthine oxidase, cyclooxygenase, lipoxygenase and the cytochrome P450 [32–35], summarized in Table 1.
Table 1.
Summary of the primary source of ROS
| Source of ROS | Response stimuli | Pathway complexes | Main ROS |
|---|---|---|---|
| Mitochondria | Oxidative metabolism | Electron transport chain, NADH | O2•− |
| NADPH oxidase | Inflammation | NAPDH | O2•− |
| Xanthine oxidase | Purine catabolism | O2 | O2•− |
| Nitric oxide synthase | Synaptic activity, inflammation, hypoxia | NADPH | NO, OONO |
| Peroxisomes | Lipid metabolism (β-oxidation) | NADH, NADPH, FADH2 | H2O2, O2•− |
| Cytochrome P450 | Clearance of various compounds (hormones, lipids, xenobiotics) | NADPH | O2•− |
| Lipoxygenases | Arachidonic Acid (PUFA) metabolism | O2 | O2•− |
| Exogenous stress | Direct peroxidation, increased NOS, DNA damage | UV, environmental toxins, drugs | O2•−, ONOO•−, H2O2 |
Antioxidant Mechanisms
Reactive species concentration need to be maintained at a low level to guarantee a proper cellular environment [36]; mechanisms that ensure antioxidant homeostasis are highly conserved across different species, from the simplest bacteria to humans. The endogenous antioxidant defense is composed of enzymatic and non-enzymatic factors. While the most reactive and toxic form of ROS is the superoxide radical (O2•−), its half-life is relatively short, and it does not diffuse far from the site of production. However, superoxide is quickly converted to hydrogen peroxide (H2O2), a more stable form of ROS that can diffuse through membranes. This conversion is mediated by superoxide dismutase (SOD). SODs come in three isoforms, located in different compartments. SOD1 (CuSOD) is mainly cytoplasmic, SOD2 (MnSOD) is located in the mitochondria and SOD3 (CuSOD) is an extracellular isoform. Loss of SOD is associated with an increased level of cellular damage such as lipid peroxidation and protein carbonylation. Mutations in SOD1 are also associated to familial cases of Amyotrophic Lateral Sclerosis (ALS), a devastating neurodegenerative disorder [37]. Although a high concentration of H2O2 in the cell can trigger cell death, a low concentration has been linked to several cellular processes related to cell development, growth and survival (see section “ROS Impact on Cell Signaling”). Accumulation of hydrogen peroxide is mainly limited by the activity of other types of enzymes, such as glutathione peroxidase (GPx) and catalases, active in the cytoplasm and the peroxisome respectively. The end-products of these enzymes are water and oxygen. The third ROS converted from H2O2, is the Hydroxyl radical (OH•), extremely active and oxidizing for lipids, proteins, and DNA [38–40].
Non-enzymatic antioxidants are molecules characterized by their capacity to inactivate reactive species quickly. The most common is glutathione (GSH), involved in both non-enzymatically reduction of ROS as well as being a cofactor in the glutathione peroxidase reduction of peroxides. The other primary non-enzymatic antioxidants include metal-binding proteins (albumin, ferritin, myoglobin, and transferrin) able to scavenge free radicals and metals [41–43], and coenzyme Q, a membrane-associated electron carrier involved in electron transfer capable of sustaining significant redox changes [44].
In parallel to the endogenous defense system, natural compounds like the flavonoids, polyphenols (flavonoids, phenolic acids), ascorbic acid (vitamin C) or α-tocopherol have antioxidant capacities that are important to ensure adequate protection against reactive species [45, 46].
ROS Impact on Cell Signaling
At physiological concentrations, ROS have a broad spectrum of roles in signaling as second messengers, with a significant influence on physiological responses (Fig. 1). Several growth factors have been associated with an increase of ROS. Multiple external stimuli, including tumor necrosis factor- (TNF-), growth factors (PDGF, EGF) and cytokines, stimulate the formation of ROS. The main mechanism underlying ROS signaling is the oxidation of thiol (-SH) group on cysteine residues, an amino acid with a low pKa [47]. This reversible action regulates post-translational modification, alteration of protein activity, and relocation in a different cellular compartment.
ROS have been associated with an increased mitogen-activated protein kinases (MAPK) activity [48–50], either through activation of tyrosine kinases or oxidation–reduction of cysteine residues. MAPK are composed of three kinases playing a pivotal role relaying extracellular signals with important outcomes on cell growth, differentiation, development, cell cycle, survival, and cell death [51–53] The main MAPK pathways consist of extracellular signal-related kinases (ERK1/2), the c-Jun N-terminal kinases (JNK), the p38 kinase (p38). These serine/threonine kinases are activated by external stimuli (see above) or by environmental stress [54–57]. ROS influence other tyrosine phosphatases (PTP) and kinases (PK) that are sensitive to redox changes. These include PTEN, phosphatidylinositide 3-kinase (PI3K), AKT, and mTOR [58]. The PI3K–AKT axis plays an important role in cell growth, survival, and protein synthesis. Upon its activation by growth factors (EGF, PDGF) [8], PI3K promotes, and is influenced, by ROS production through NOX and mitochondrial activity, while ROS inactivate phosphatase PTEN [59, 60], PI3K’s primary inhibitor. Recently, the emergence of proteomic approaches has allowed identification of over 500 proteins sensitive to redox state, thereby demonstrating ROS capacity to deeply modulate cell activity [61].
ROS also impact the activity of important growth and metabolism-related transcription factors, sensitive to redox changes. The list includes, but is not limited to, Hypoxia Inducible Factor 1α (HIF-1α), NF-kB, Heat Shock Factor 1 (HSF1), p53 and nuclear factor erythroid 2-related factor 2 (Nrf2) [9, 10, 62]. Nrf2 and Kelch-like ECH-associated protein 1 (Keap1) are associated in the cytosol, promoting ubiquitination of Nrf2 and its degradation by the proteasome [63, 64]. However, ROS induce the oxidation of key reactive cysteine on Keap1, promoting the dissociation of Keap1-Nrf2, allowing translocation of the latter to the nucleus. There, Nrf2 engages with antioxidant response elements (ARE) and on the promoter region of antioxidant factors such as the Glutathione S-transferase (GST), leading to increased resistance to oxidative stress [65]. Overall, at low or moderate concentrations ROS play a role in signal transduction. They influence a variety of cellular pathways with a crucial impact on cell physiology, metabolism, and survival.
Impact of Reactive Species on the Brain
The brain and, more specifically, neurons are susceptible to oxidative damage because of the high content of lipids and the heavy oxidative metabolism on which they rely [66]. Oxidative damages, through an accumulation of misfolded proteins and loss of antioxidant defenses, have been associated with the aging-mediated loss of functions [67] and neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [68].
In the brain, the different cell types are not equal regarding their resistance to oxidative stress. Thus glial cells, like astrocytes, are more resilient to oxidative insults, compared to neurons [18]. Similarly, neurons in different anatomical regions also display variability in their capacity to scavenge reactive species. Neurons in the amygdala, the hippocampus, and cerebellar granules cells appear to be the most sensitive [69, 70]. This sensitivity, compared to astrocytes for example, is also due to a low expression of antioxidant mechanisms [71]. Astrocytes synthetize most of the GSH content in the brain, express transcription factors such as Nrf2, at higher levels than neurons [72, 73] and clear ROS more efficiently [71]. Astrocytes release GSH that is either hydrolyzed to cysteine and used as a source for new GSH molecules in neurons via the γ-glutamate-cysteine ligase catalytic (Gclc) and modifier (Gclm) subunits and build antioxidant defense of their own [74–76]. There are several evidences supporting the role of astrocytes in organizing the antioxidant response through the release of cofactors or energy substrates to support neurons metabolism and synaptic activity [77–79]. Recently, some disputed work has shown that mild oxidative stress was able to stimulate astrocytes’ antioxidant defense through translocation of Nrf2, and promote neuronal survival [80, 81] but also that astrocytic ROS influence neuronal metabolism and improve survival [80, 82].
At synapses ROS are associated to long-term potentiation (LTP), to modulate plasticity and memory [83–85]. LTP is produced through high-frequency signals (HSF) resulting in activation of glutamate-activated N-methyl-d-aspartate (NMDA) receptors (NMDAR) permeable to calcium (Ca2+). Ca2+ entry triggers ROS production by the mitochondria [86] but also promotes nNOS activity [87, 88] through its binding to calmodulin leading to the formation of nitric oxide (NO•−). NO acts as a neurotransmitter, associated with synaptic plasticity and synaptic activity regulation through protein S-nitrosylation [89–95]. In astrocytes, induction of NOS2 is Ca2+ independent and can be triggered by external stimuli such as inflammation (LPS, TNFα, cytokines, Interferon-γ). Interestingly, NOS activity differs between neurons and astrocytes. NO synthetized in glial cells stimulates glycolytic function, while it does not induce a similar effect in neurons, despite similar capacity to inhibit mitochondrial respiration [96]. Besides direct synaptic regulation, ROS modulate the activity of a variety of protein kinases such as ERK, CAMKII, PKA, PKC involved LTP through transcriptional changes and increased number of glutamate (AMPA) transporters [97]. Manipulations aiming to reduce ROS production limit or abrogate LTP, strengthening the view that ROS have a signaling role in the brain [98–100].
The Role of Reactive Species in the Periphery
Immune cells like macrophages and neutrophils release oxygen radicals upon phagocytic activity, potentially leading to tissue damage, yet these immune cells also are endowed with a high antioxidant capacity ROS are required for both innate and adaptive immune mechanisms [101, 102]. Reactive species are necessary for Lipopolysaccharide (LPS)-mediated activation of Toll-like receptor, leading to the production of pro-inflammatory cytokines [103]. Similarly, ROS can activate and maintain activation of lymphocytes (B and T) involved in the adaptive immune system, participating in its fine regulation. Furthermore, recent work has shown that the use of antioxidant can reverse these effects, leading to a deactivation of the immune system [104, 105].
In muscle cells, ROS play an essential role in contraction and adaptation to repetitive efforts [106]. As in other cell types, mitochondria are central for ROS formation; however in muscle cells, also NOX contributes significantly to reactive species formation both at rest and during exercise [107–110], resulting in particular in cell biogenesis through activation of peroxisome proliferator-activated receptor-g coactivator-1α (PGC-1α) [111]. However, excess levels of ROS induce a loss of contractile power that translates into muscle weakness and fatigue [112, 113]. The primary cellular mechanism involves the sustained activation of NF-kB and FoxO, leading to transcription of a degradation-related protein such as C/EBP homology protein (CHOP) [114–116]. Regular activity, however, can promote adaptation and increase muscle capacities (section “Beyond ROS Reactive Behavior”).
Reactive Species in Aging and Disease
The principal harmful effect of ROS is observed during aging where a disequilibrium of the redox state is observed. With aging, neuronal metabolism is impaired, mainly through mitochondrial decay, resulting in decreased ATP and NAD+ production [117, 118]. This decrease, together with a failure in antioxidant defense mechanisms [119] leads to a rise in intracellular ROS-mediated dysfunction [120, 121]. Considerable evidence has demonstrated increased ROS levels in the nervous system of animal models of Alzheimer and Parkinson diseases or Amyotrophic Lateral Sclerosis [122–124]. Upon disruption of the redox homeostasis, ROS cause protein degradation [125–127], DNA damage [128, 129] and lipid peroxidation [130] (Fig. 1).
Accumulation of damage on macromolecules leads to cellular dysfunction, including in muscles and neurons. In tumoral cells, ROS promote stabilization of hypoxia-inducible factor 1α (HIF-1α), which in turn results in tumor survival by promoting angiogenesis and support of glycolytic metabolism [131–134]. Lipid peroxidation promotes inflammation and tissue damage in the heart and cardiovascular dysfunction [135].
Cancer cells are characterized by their “hyper-metabolism” linked to increased production of ROS [136], which is however neutralized by an equivalent increase in antioxidant defenses [137]. However, the role of oxidative stress-sensitive transcription factors such as Nrf2 is complex and depends greatly on the nature of tumors [138–140]. Altogether, it appears that cancer cells need to maintain a tight redox balance to maintain resistance to ROS. Among pro-tumorigenic factors, DNA mutations are associated to significant metabolic changes, that include reduced oxidative phosphorylation (OXPHOS) and increased glycolysis activity. Because ROS are mainly produced through OXPHOS, the diminution of ROS has been shown to promote tumorigenesis. Therefore, it appears that a minimal concentration of ROS is required for tumors to persist, and this concentration needs to be tightly regulated to prevent oxidative damage in cancer cells [141–144]. The high concentration of ROS has been at the center of attempts to develop therapeutic strategies against cancer, but the successes have been very limited or detrimental [145], suggesting that ROS are not a suitable target for therapies.
Beyond ROS Reactive Behavior
Although ROS can have a deleterious effect on cell survival and in disease, their role in cellular physiology is more complex than initially subsumed. As mentioned above, ROS have a substantial impact on cellular signaling via regulation of over 500 redox-sensitive proteins, mainly kinases, and phosphatases that have a crucial effect on cell growth, differentiation and survival (see section “ROS Impact on Cell Signaling”). For example, it has been shown in multiple models that reduction of mitochondrial respiration can has a positive effect on longevity, in part due to a mild increase in ROS production. Caloric restriction is known to promote longevity and delay neurodegeneration: several observations suggest that ROS such as H2O2 could be linked to the positive outcome on longevity by activating anti-aging pathways such as the AMPK [146–148], while we and others have revealed a link between the protective effect of l-lactate against oxidative stress and ROS production [149, 150].
During moderate and repeated exercise, the production of ROS by muscle cells has a profound positive effect. Indeed it has been shown that a low concentration of H2O2 can increase muscle contractibility [151, 152]. A mild ROS increase can stimulate the expression of antioxidant enzymes, including GSH, but also SOD, CAT, and GPX. Endurance exercise, through a ROS-dependent mechanism, also reduces DNA damage [153] and increases insulin sensitivity [154]. This dose-dependent effect also translates into a long-term growth of the muscle fibers through activation of several signaling pathways such as AMPK, p38MAPK, and PGC-1α [155–157]. Interestingly, the use of exogenous antioxidant, through diet reduces the impact of ROS on muscle adaptation to exercise [158–160].
The latter observation is consistent with the role of preconditioning to ischemia as a protective strategy. Although, re-perfusion of tissue after hypoxia results in a dramatic ROS elevation and tissues damage, a small and short period of ischemia followed by reperfusion can produce protective effects, through ROS dependent mechanisms [161, 162].
Conclusion
Reactive species are more complex than was initially thought. As of today, it appears that the equilibrium between pro-oxidant and antioxidant factors drives cellular physiology in multiple organs and organisms. While an excessive production of ROS has a dramatic negative effects on survival, a mild oxidative environment can produce a variety of positive outcomes crucial for biological organisms to survive and adapt. Therefore a better understanding of reactive species targets and effects, is necessary to target interventional strategies to improve major health-related issues.
Authors Contributions
AT and PJM wrote the review.
Funding
This work was funded by the King Abdullah University of Science and Technology.
Compliance with Ethical Standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Special issue in honor of Professor Juan Bolanos.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arnaud Tauffenberger, Email: arnaud.tauffenberger@kaust.edu.sa.
Pierre J. Magistretti, Email: pierre.magistretti@kaust.edu.sa
References
- 1.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Ristow M. Unraveling the truth about antioxidants. Nat Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 5.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox CS, McKay SE, Holmbeck MA, et al. Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell Metab. 2018;28(5):776–786.e5. doi: 10.1016/j.cmet.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bäumer AT, Freyhaus Ten H, Sauer H, et al. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem. 2008;283:7864–7876. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- 9.Chandel NS, Chandel NS, McClintock DS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 10.Ahn S-G, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tissenbaum HA. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SK. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz TJ, Zarse K, Voigt A, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155:699–712. doi: 10.1016/j.cell.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obata F, Fons CO, Gould AP. Early-life exposure to low-dose oxidants can increase longevity via microbiome remodelling in Drosophila. Nat Commun. 2018;9:975. doi: 10.1038/s41467-018-03070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem. 2008;283:26217–26227. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolaños JP, Heales SJ, Peuchen S, et al. Nitric oxide-mediated mitochondrial damage: a potential neuroprotective role for glutathione. Free Radic Biol Med. 1996;21:995–1001. doi: 10.1016/s0891-5849(96)00240-7. [DOI] [PubMed] [Google Scholar]
- 18.Bolaños JP, Almeida A, Stewart V, et al. Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem. 1997;68:2227–2240. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 19.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–366. doi: 10.1023/A:1005427919188. [DOI] [PubMed] [Google Scholar]
- 22.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 23.Bae YS, Kang SW, Seo MS, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. doi: 10.1074/jbc.272.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Kurz S, Münzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundaresan M, Yu ZX, Ferrans VJ, et al. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 27.Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 28.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 29.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 30.Cardoso AR, Chausse B, da Cunha FM, et al. Mitochondrial compartmentalization of redox processes. Free Radic Biol Med. 2012;52:2201–2208. doi: 10.1016/j.freeradbiomed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 32.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 33.Yue Z, Zhang X, Yu Q, et al. Cytochrome P450-dependent reactive oxygen species (ROS) production contributes to Mn3O4 nanoparticle-caused liver injury. RSC Adv. 2018;8:37307–37314. doi: 10.1039/C8RA05633A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zangar R. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Rahman K. Studies on free radicals, antioxidants, and co-factors. CIA. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese V. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues Siqueira I, Fochesatto C, da Silva Torres IL, et al. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton ML, van Remmen H, Drake JA, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plantier J-L, Duretz V, Devos V, et al. Comparison of antioxidant properties of different therapeutic albumin preparations. Biologicals. 2016;44:226–233. doi: 10.1016/j.biologicals.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Kreutzer U, Jue T. Role of myoglobin as a scavenger of cellular NO in myocardium. Am J Phys Heart Circ Phys. 2004;286:H985–H991. doi: 10.1152/ajpheart.00115.2003. [DOI] [PubMed] [Google Scholar]
- 43.Guan H, Yang H, Yang M, et al. Mitochondrial ferritin protects SH-SY5Y cells against H2O2-induced oxidative stress and modulates α-synuclein expression. Exp Neurol. 2017;291:51–61. doi: 10.1016/j.expneurol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7:S34–S40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groitl B, Jakob U. Thiol-based redox switches. Biochim Biophys Acta. 2014;1844:1335–1343. doi: 10.1016/j.bbapap.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamata H, Kamata H, Honda S-I, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 49.Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol. 2004;483:163–173. doi: 10.1016/j.ejphar.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 50.Guyton KZ, Guyton KZ, Liu Y, et al. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 51.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter-Vann AM, Johnson GL. Integrated activation of MAP3Ks balances cell fate in response to stress. J Cell Biochem. 2007;102:848–858. doi: 10.1002/jcb.21522. [DOI] [PubMed] [Google Scholar]
- 53.Ravingerová T, Barancík M, Strnisková M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247:127–138. doi: 10.1023/a:1024119224033. [DOI] [PubMed] [Google Scholar]
- 54.Junttila MR, Li S-P, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 55.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 56.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Bogoyevitch MA, Ngoei KRW, Zhao TT, et al. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta Proteins Proteomics. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leslie NR, Bennett D, Lindsay YE, et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon J, Lee S-R, Yang K-S, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weerapana E, Wang C, et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paul S, Ghosh S, Mandal S, et al. NRF2 transcriptionally activates the heat shock factor 1 promoter under oxidative stress and affects survival and migration potential of MCF7 cells. J Biol Chem. 2018;293:19303–19316. doi: 10.1074/jbc.RA118.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 64.Kim KC, Kang KA, Zhang R, et al. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42:297–305. doi: 10.1016/j.biocel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 66.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 67.Castelli V, Benedetti E, Antonosante A, et al. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front Mol Neurosci. 2019;12:217–213. doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14(3):457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCormack AL, Thiruchelvam M, Manning-Bog AB, et al. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 70.Wilde GJ, Pringle AK, Wright P, Iannotti F. Differential vulnerability of the CA1 and CA3 subfields of the hippocampus to superoxide and hydroxyl radicals in vitro. J Neurochem. 1997;69:883–886. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]
- 71.Dringen R, Kussmaul L, Gutterer JM, et al. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J Neurochem. 1999;72:2523–2530. doi: 10.1046/j.1471-4159.1999.0722523.x. [DOI] [PubMed] [Google Scholar]
- 72.Bell KFS, Al-Mubarak B, Martel M-AE, et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun. 2015;6:1–15. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimenez-Blasco D, Santofimia-Castaño P, Gonzalez A, et al. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015;22:1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dringen R, Kranich O, Hamprecht B. The gamma-glutamyl transpeptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture. Neurochem Res. 1997;22:727–733. doi: 10.1023/a:1027310328310. [DOI] [PubMed] [Google Scholar]
- 75.Yin B, Barrionuevo G, Weber SG. Mitochondrial GSH systems in CA1 pyramidal cells and astrocytes react differently during oxygen-glucose deprivation and reperfusion. ACS Chem Neurosci. 2017;9:738–748. doi: 10.1021/acschemneuro.7b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baxter PS, Hardingham GE. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panatier A, Vallée J, Haber M, et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 78.Pellerin L, Bouzier-Sore A-K, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 79.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bell KF, Al-Mubarak B, Fowler JH, et al. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A. 2011;108:E1–E2. doi: 10.1073/pnas.1015229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haskew-Layton RE, Payappilly JB, Smirnova NA, et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A. 2010;107:17385–17390. doi: 10.1073/pnas.1003996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vicente-Gutierrez C, Bonora NX, Bobo-Jimenez V, et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat Metab. 2019;1:201–211. doi: 10.1038/s42255-018-0031-6. [DOI] [PubMed] [Google Scholar]
- 83.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 85.Castellani GC, Quinlan EM, Cooper LN, Shouval HZ. A biophysical model of bidirectional synaptic plasticity: dependence on AMPA and NMDA receptors. Proc Natl Acad Sci U S A. 2001;98:12772–12777. doi: 10.1073/pnas.201404598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 88.Brenman JE, Chao DS, Gee SH, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 89.Hardingham N, Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J Neurosci. 2006;26:7395–7404. doi: 10.1523/JNEUROSCI.0652-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudkouskaya A, Sim V, Shah AA, et al. Long-lasting inhibition of presynaptic metabolism and neurotransmitter release by protein S-nitrosylation. Free Radic Biol Med. 2010;49:757–769. doi: 10.1016/j.freeradbiomed.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segieth J, Getting SJ, Biggs CS, Whitton PS. Nitric oxide regulates excitatory amino acid release in a biphasic manner in freely moving rats. Neurosci Lett. 1995;200:101–104. doi: 10.1016/0304-3940(95)12088-l. [DOI] [PubMed] [Google Scholar]
- 93.Steinert JR, Robinson SW, Tong H, et al. Nitric oxide is an activity-dependent regulator of target neuron intrinsic excitability. Neuron. 2011;71:291–305. doi: 10.1016/j.neuron.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi YB, Lipton SA. Redox modulation of the NMDA receptor. Cell Mol Life Sci. 2000;57:1535–1541. doi: 10.1007/PL00000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blaise G, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208:177–192. doi: 10.1016/j.tox.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 96.Almeida A, Almeida J, Bolaños JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A. 2001;98:15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elgersma Y, Silva AJ. Molecular mechanisms of synaptic plasticity and memory. Curr Opin Neurobiol. 1999;9:209–213. doi: 10.1016/S0959-4388(99)80029-4. [DOI] [PubMed] [Google Scholar]
- 98.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- 99.Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002;22:674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 101.Kamiński MM, Röth D, Krammer PH, Gülow K. Mitochondria as oxidative signaling organelles in T-cell activation: physiological role and pathological implications. Arch Immunol Ther Exp. 2013;61:367–384. doi: 10.1007/s00005-013-0235-0. [DOI] [PubMed] [Google Scholar]
- 102.West AP, Koblansky AA, Ghosh S. Recognition and signaling by Toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 103.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 104.Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laniewski NG, Grayson JM. Antioxidant treatment reduces expansion and contraction of antigen-specific CD8+ T cells during primary but not secondary viral infection. J Virol. 2004;78:11246–11257. doi: 10.1128/JVI.78.20.11246-11257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 107.Xia R, Webb JA, Gnall LLM, et al. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Phys Cell Phys. 2003;285:C215–C221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- 108.Shkryl VM, Martins AS, Ullrich ND, et al. Reciprocal amplification of ROS and Ca2+ signals in stressed mdx dystrophic skeletal muscle fibers. Pflugers Archiv. 2009;458:915–928. doi: 10.1007/s00424-009-0670-2. [DOI] [PubMed] [Google Scholar]
- 109.Sakellariou GK, Vasilaki A, Palomero J, et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao X, Bey EA, Wientjes FB, Cathcart MK. Cytosolic phospholipase A 2(cPLA 2) regulation of human monocyte NADPH oxidase activity. J Biol Chem. 2002;277:25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
- 111.Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1α. EXG. 2013;48:1343–1350. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Reid MB. Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Vollaard NBJ, Cooper CE, Shearman JP. Exercise-induced oxidative stress in overload training and tapering. Med Sci Sports Exerc. 2006;38:1335–1341. doi: 10.1249/01.mss.0000227320.23847.80. [DOI] [PubMed] [Google Scholar]
- 114.Sriram S, Subramanian S, Juvvuna PK, et al. Myostatin augments muscle-specific ring finger protein-1 expression through an NF-kB independent mechanism in SMAD3 null muscle. Mol Endocrinol. 2014;28:317–330. doi: 10.1210/me.2013-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–722. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 116.Cohen S, Brault JJ, Gygi SP, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med. 2016;100:108–122. doi: 10.1016/j.freeradbiomed.2016.04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu X-H, Lu M, Lee B-Y, et al. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 120.Suárez-Rivero J, Villanueva-Paz M, de la Cruz-Ojeda P, et al. Mitochondrial dynamics in mitochondrial diseases. Diseases. 2017;5:1–15. doi: 10.3390/diseases5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dasuri K, Zhang L, Keller JN. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol Med. 2013;62:170–185. doi: 10.1016/j.freeradbiomed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, Perry G, Smith MA, et al. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 2010;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kruman II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang H, Li Q, Graham RK, et al. Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington’s disease. Neurobiol Dis. 2008;31:80–88. doi: 10.1016/j.nbd.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aiken CT, Kaake RM, Wang X, Huang L. Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics. 2011;10(5):R110.006924. doi: 10.1074/mcp.R110.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 128.Sassa A, Kamoshita N, Matsuda T, et al. Miscoding properties of 8-chloro-2′-deoxyguanosine, a hypochlorous acid-induced DNA adduct, catalysed by human DNA polymerases. Mutagenesis. 2012;28:81–88. doi: 10.1093/mutage/ges056. [DOI] [PubMed] [Google Scholar]
- 129.Sheng Z, Oka S, Tsuchimoto D, et al. 8-Oxoguanine causes neurodegeneration during MUTYH-mediated DNA base excision repair. J Clin Invest. 2012;122:4344–4361. doi: 10.1172/JCI65053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 131.Hong B-J, Kim J, Jeong H, et al. Tumor hypoxia and reoxygenation: the yin and yang for radiotherapy. Radiat Oncol J. 2016;34:239–249. doi: 10.3857/roj.2016.02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kunz M, Ibrahim SM. Molecular responses to hypoxia in tumor cells. Mol Cancer. 2003;2:23–13. doi: 10.1186/1476-4598-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1 is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 134.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zuo L, Rose BA, Roberts WJ, et al. Molecular characterization of reactive oxygen species in systemic and pulmonary hypertension. Am J Hypertens. 2014;27:643–650. doi: 10.1093/ajh/hpt292. [DOI] [PubMed] [Google Scholar]
- 136.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 137.Gorrini C, Gorrini C, Harris IS, et al. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 138.Gonzalez-Donquiles C, Alonso-Molero J, Fernandez-Villa T, et al. The NRF2 transcription factor plays a dual role in colorectal cancer: a systematic review. PLoS One. 2017;12:e0177549. doi: 10.1371/journal.pone.0177549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ryoo I-G, Lee S-H, Kwak M-K. Redox modulating NRF2: a potential mediator of cancer stem cell resistance. Oxidative Med Cell Longev. 2016;2016:1–14. doi: 10.1155/2016/2428153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kalo E, Kogan-Sakin I, Solomon H, et al. Mutant p53 R273H attenuates the expression of phase 2 detoxifying enzymes and promotes the survival of cells with high levels of reactive oxygen species. J Cell Sci. 2013;125:5578–5586. doi: 10.1242/jcs.106815. [DOI] [PubMed] [Google Scholar]
- 141.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dakubo GD. Altered metabolism and mitochondrial genome in prostate cancer. J Clin Pathol. 2006;59:10–16. doi: 10.1136/jcp.2005.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jessie BC, Sun CQ, Irons HR, et al. Accumulation of mitochondrial DNA deletions in the malignant prostate of patients of different ages. EXG. 2001;37:169–174. doi: 10.1016/s0531-5565(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 144.Petros JA, Baumann AK, Ruiz-Pesini E, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27:156–157. doi: 10.1016/j.ccell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 146.Rabinovitch RC, Samborska B, Faubert B, et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 147.Hinchy EC, Gruszczyk AV, Willows R, et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J Biol Chem. 2018;293:17208–17217. doi: 10.1074/jbc.RA118.002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hart PC, Mao M, de Abreu ALP, et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2019;6:1–14. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tauffenberger A, Fiumelli H, Magistretti PJ. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019;10:1–16. doi: 10.1038/s41419-019-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zelenka J, Dvořák A, Alán L. L-Lactate protects skin fibroblasts against aging-associated mitochondrial dysfunction via mitohormesis. Oxidative Med Cell Longev. 2015;2015:1–14. doi: 10.1155/2015/351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 152.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Radák Z, Naito H, Kaneko T, et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Archiv. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 154.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol. 2007;192:127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 155.Dodd SL, Gagnon BJ, Senf SM, et al. Ros-mediated activation of NF-κB and Foxo during muscle disuse. Muscle Nerve. 2009;41:110–113. doi: 10.1002/mus.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Derbre F, Ferrando B, Gomez-Cabrera MC, et al. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: role of p38 MAPKinase and E3 ubiquitin ligases. PLoS One. 2012;7:e46668–e46669. doi: 10.1371/journal.pone.0046668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kröller-Schön S, Jansen T, Hauptmann F, et al. α1AMP-activated protein kinase mediates vascular protective effects of exercise. Arterioscler Thromb Vasc Biol. 2012;32:1632–1641. doi: 10.1161/ATVBAHA.111.243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 160.Gomez-Cabrera MC, Borras C, Pallardo FV, et al. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Anderson EJ (2014) The “Goldilocks Zone” from a redox perspective—adaptive vs. deleterious responses to oxidative stress in striated muscle. Front Physiol. 10.3389/fphys.2014.00358/abstract [DOI] [PMC free article] [PubMed]
- 162.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]