Abstract
Purpose of review:
Nephrologists are increasingly providing care to transgender individuals with chronic kidney disease (CKD). However, they may lack familiarity with this patient population that faces unique challenges. The purpose of this review is to discuss the care of transgender persons and what nephrologists should be aware of when providing care to their transgender patients.
Sources of information:
Original research articles were identified from MEDLINE and Google Scholar using the search terms “transgender,” “gender,” “sex,” “chronic kidney disease,” “end stage kidney disease,” “dialysis,” “transplant,” and “nephrology.”
Methods:
A focused review and critical appraisal of existing literature regarding the provision of care to transgender men and women with CKD including dialysis and transplant to identify specific issues related to gender-affirming therapy and chronic disease management in transgender persons.
Key findings:
Transgender persons are at an increased risk of adverse outcomes compared with the cisgender population including mental health, cardiovascular disease, malignancy, sexually transmitted infections, and mortality. Individuals with CKD have a degree of hypogonadotropic hypogonadism and decreased levels of endogenous sex hormones; therefore, transgender persons with CKD may require reduced exogenous sex hormone dosing. Exogenous estradiol therapy increases the risk of venous thromboembolism and cardiovascular disease which may be further increased in CKD. Exogenous testosterone therapy increases the risk of polycythemia which should be closely monitored. The impact of gender-affirming hormone therapy on glomerular filtration rate (GFR) trajectory in CKD is unclear. Gender-affirming hormone therapy with testosterone, estradiol, and anti-androgen therapies changes body composition and lean body mass which influences creatinine generation and the performance for estimated glomerular filtration rate (eGFR) equations in transgender persons. Confirmation of eGFR with measured GFR is reasonable if an accurate knowledge of GFR is needed for clinical decision-making.
Limitations:
There are limited studies regarding the intersection of transgender persons and kidney disease and those that exist are mostly case reports. Randomized controlled trials and observational studies in nephrology do not routinely differentiate between cisgender and transgender participants.
Implications:
This review highlights important considerations for providing care to transgender persons with kidney disease. Additional research is needed to evaluate the performance of eGFR equations in transgender persons, the effects of gender-affirming hormone therapy, and the impact of being transgender on outcomes in persons with kidney disease.
Keywords: transgender, transmen, transwomen, kidney disease, gender-affirming therapy
Abrégé
Motif de la revue:
Dans leur pratique, les néphrologues sont de plus en plus appelés à traiter des personnes transgenres atteintes d’insuffisance rénale chronique (IRC). Une population de patients qui fait face à des défis particuliers et avec laquelle les spécialistes ne sont pas toujours familiers. L’objectif de cette revue est de discuter des soins prodigués aux personnes transgenres et des enjeux auxquels les néphrologues qui soignent ces patients devraient être sensibilisés.
Sources:
Les articles de recherche originaux ont été répertoriés sur MEDLINE et Google Scholar à l’aide des termes transgender (transgenre), gender (genre), sex (sexe), chronic kidney disease (insuffisance rénale chronique), end stage kidney disease (insuffisance rénale terminale), dialysis (dialyse), transplant (transplantation) et nephrology (néphrologie).
Méthodologie:
On a procédé à un examen ciblé et une évaluation critique de la documentation portant sur les soins aux personnes transgenres atteintes d’IRC, notamment la dialyse et la transplantation, afin de cerner les enjeux liés spécifiquement aux thérapies d’affirmation du genre et à la prise en charge de la néphropathie chronique chez les personnes transgenres.
Principaux résultats:
Les personnes transgenres courent un risque accru d’événements défavorables comparativement à la population cisgenre, notamment en matière de santé mentale, de maladies cardiovasculaires, de tumeurs malignes, d’infections transmissibles sexuellement et de mortalité. Les individus atteints d’IRC présentent un certain degré d’hypogonadisme hypogonadotrope et des taux réduits d’hormones sexuelles endogènes; les personnes transgenres atteintes d’IRC pourraient ainsi nécessiter un dosage réduit d’hormones sexuelles exogènes. L’estradiol exogène augmente le risque de thrombo-embolie veineuse et de maladies cardiovasculaires, un risque pouvant être augmenté davantage en contexte d’IRC. La testostérone exogène augmente le risque de polycythémie et doit être surveillée étroitement. L’impact de l’hormonothérapie d’affirmation du genre sur la trajectoire du débit de filtration glomérulaire (DFG) en IRC est encore mal connu. L’hormonothérapie d’affirmation du genre avec testostérone, estradiol et anti-androgène altère la composition corporelle et la masse maigre, ce qui influe sur la production de créatinine et la performance des équations calculant le DFG estimé (DFGe) chez les personnes transgenres. La confirmation du DFGe à l’aide du DFG mesuré est raisonnable si connaître la valeur précise du DFG est nécessaire pour la prise de décision clinique.
Limites:
Peu d’études se sont penchées sur l’intersection des personnes transgenres et de la néphropathie, et les études existantes consistent principalement en des rapports de cas. Les essais contrôlés randomisés et les études observationnelles en néphrologie ne font pas systématiquement de distinction entre les participants cisgenres et transgenres.
Conclusion:
Cette revue met en lumière des considérations importantes pour la prise en charge des personnes transgenres atteintes de néphropathies. D’autres recherches sont nécessaires pour évaluer la performance des équations calculant le DFGe, les effets de l’hormonothérapie d’affirmation du genre et l’incidence d’être transgenre sur les résultats des personnes atteintes de néphropathies.
Introduction
Nephrologists are increasingly providing care to transgender patients with chronic kidney disease (CKD) including those with end-stage kidney disease (ESKD) treated with dialysis or kidney transplantation. However, clinicians may lack familiarity with this patient population that faces unique challenges. There are several issues that need to be considered when providing nephrology care to transgender patients given that sex and gender differences exist within the cisgender (non-transgender) population regarding kidney disease but it is unknown how this applies to transgender individuals with kidney disease who may be undergoing gender-affirming hormone therapy or surgery.
Scope and Purpose
The purpose of this review is to discuss the provision of kidney care to adult transgender persons and the issues nephrologists should be aware of when interacting with and treating their transgender patients.
Methods
MEDLINE and Google Scholar were searched using the terms “transgender” on its own or in combination with “chronic kidney disease,” “end stage kidney disease,” “dialysis,” “transplant” and “nephrology” by the first author (D.C.) to identify relevant literature. We also searched “sex” and “gender” in combination with the same MeSH terms. The searches were completed in May 2020. References of key articles were reviewed for additional papers relevant to the care of transgender with and without kidney disease. This was supplemented by key articles from other authors who either provide gender-affirming hormone therapy to transgender persons (N.S.) or are experts in sex and gender in kidney disease (S.A.). We prioritized the inclusion of clinical practice guidelines, systematic reviews, meta-analyses, and large observational studies given the lack of randomized controlled trials in this patient population.
Review
The Nomenclature of Gender Identity
Every person goes through a process of gender identity development starting in infancy and develops their gender identity over time which refers to one’s internal, deeply held sense of gender such as being a male or a female or an alternative gender that may or may not correspond to a person’s sex assigned at birth.1-3 Gender includes gender expression (physical appearance and behavior related to gender), gender identity (a person’s perception of their gender), and gender roles (behaviors, values, and attitudes that a society considers appropriate to gender). In general, umbrella terms such as “cisgender” and “transgender” are used to classify gender identities.3 A cisgender person is an individual whose gender identity aligns with their sex assigned at birth and a transgender person1 is an individual whose gender identity does not align with their sex assigned at birth.1 Specifically, a transgender man is an individual who was assigned female at birth but who identifies and lives as a man2 and a transgender woman is an individual who was assigned male at birth but who identifies and lives as a woman.2 Importantly, gender identities and their expression are diverse beyond the binary framework; while this review focuses on transgender men and women, it also applies to other gender diverse individuals4 including those who identify as without gender or “agender.” Furthermore, it is crucial to create a safe and welcoming clinical environment for transgender patients with culturally competent care.5 In clinical practice and for research purposes, it is important to document gender identity data and to use patients’ preferred name and pronouns.5 In addition, other important aspects to consider include appropriate staff training, bathrooms, waiting areas, and having a basic understanding of transgender terminology.5 All of this is important in acknowledging and respecting individuals’ gender identity and fostering trust in the patient-physician relationship.5
The Epidemiology of Transgender Persons
It is estimated that 0.3% to 0.6% of the adult population6 is transgender and that there are more than 150 000 transgender persons in Canada, 1 million transgender persons in the United States,7 and 25 million transgender persons worldwide.6 The prevalence of transgender individuals in a population depends on the definition of transgender and its classification by self-report, the use of sex hormone therapy, or the receipt of gender-affirming surgery.8 In the Behavioral Risk Factor Surveillance System which included more than 500 000 random respondents from across the United States, 0.24% identified as male-to-female, 0.14% identified as female-to-male, and 0.10% identified as gender nonconforming.9
Transgender persons have a higher burden of chronic diseases when compared with the cisgender population10 and have worse overall health outcomes due to many different factors.11 Coexisting mental health comorbidities12 are common including mood disorders, substance abuse and dependency, and self-harm behaviors13 that all negatively impact health-related quality of life.14-16 Many of these factors can influence or contribute to underlying CKD including nonadherence, HIV, highly active antiretroviral therapy, illicit drugs, renovascular disease, and gender-affirming hormone therapy.
Gender-Affirming Care
When transgender individuals experience gender dysphoria or incongruence, they have distress or discomfort associated with their gender identity being incongruent with their sex assigned at birth and may seek treatment to alleviate this.1,2,17 The World Professional Association for Transgender Health (WPATH) clearly outlines the importance of mental health support and treatment as well as the medical necessity for providing gender-affirming hormone and/or surgical therapy in many transgender individuals with gender dysphoria.17 Gender-affirming care includes providing mental health support as well as support in transition-related care such as with a social transition, coping with transphobia, changing legal documents, and connecting patients to accessing gender services. It also involves supporting transgender individuals interested in gender-affirming hormone therapy and/or surgical therapy2 in addition to general medical care1 which includes addressing mental health issues, malignancy screening, the prevention and treatment of HIV18 and other sexually transmitted infections, cardiovascular (CV) risk reduction,19,20 and vaccinations.21 Inclusive22 and culturally competent care23 of transgender persons is typically provided by a network of physicians that includes primary care physicians, mental health professionals, endocrinologists, and surgeons but barriers to accessing care24,25 resulting in health inequities26 and unmet healthcare needs27 are common, especially outside of larger cities and urban settings.
Gender-Affirming Hormone Therapy in Transgender Persons
There are 2 main objectives of gender-affirming hormone therapy in transgender individuals. The first is to decrease the levels of endogenous sex hormones and their associated secondary sex characteristics of the sex assigned at birth.2 The second is to then replace and shift the individual’s biochemical sex hormone configuration to reflect their affirmed gender in a way that mimics physiology as best as possible using the principles from hormone replacement therapy in hypogonadal cisgender individuals.2 This is guided by the individual’s gender-affirming goals as well as providing this hormonal therapy in a risk reduction manner depending on the clinical scenario and presence of medical comorbidities. Gender-affirming hormone therapy is associated with an improvement in quality of life,28,29 psychological outcomes,30 and sexual function2 in transgender persons. Data also suggest that the overall satisfaction is high after gender-affirming treatment.31
Masculinizing Hormone Therapy in Transgender Men
For transgender men, the general goal of masculinizing hormone therapy is virilization with the development of masculine secondary sexual characteristics that match their masculine gender identity.1,31 This is accomplished by using exogenous testosterone therapy to raise testosterone levels into the male physiological range by using either injectable or transdermal testosterone formulations.1,2 This leads to masculine changes which typically include voice deepening, menstrual suppression, facial and body hair growth, and increased muscle mass.1,31 In particular, exogenous testosterone therapy leads to changes in body composition with increased body weight, decreased body fat, and increased lean body mass usually by several kilograms.31 Adverse effects of exogenous testosterone therapy include erythrocytosis32 and a risk of polycythemia especially with additional risk factors,1 acne, scalp hair loss, unexpected menstrual bleeding, hypertriglyceridemia, and infertility.31
Feminizing Hormone Therapy in Transgender Women
For transgender women, the general goal of feminizing hormone therapy is to decrease the effects of endogenous testosterone and to induce feminine secondary sex characteristics that match their feminine gender identity.31 This is achieved by adding estrogen and by reducing endogenous testosterone to attain estradiol and testosterone levels within the female physiological range. This is accomplished by using exogenous estradiol therapy which in itself can suppress endogenous testosterone production via central feedback mechanism as well as anti-androgen therapy that suppresses either the secretion or action of androgens33 such as spironolactone, cyproterone acetate, or gonadotropin-releasing hormone (GnRH) agonists.1,2,31 Guidelines recommend using oral, transdermal, or injectable exogenous estradiol therapy in this clinical setting.2 Feminizing hormone therapy generally leads to skin softening, breast development, decrease in body hair,31 as well as changes in body composition31 with increased body fat and decreased lean body mass which usually increases body weight by several kilograms without any negative effects on bone mineral density.34 Adverse effects of estrogens include venous thromboembolism (VTE),35,36 malignancy,37,38 hypertriglyceridemia, CV disease,19,20 and infertility.31 Progesterone may also be used for breast development in transgender women but may have side effects and increase the risk of breast cancer37 and vascular disease.
Gender-Affirming Surgery
Gender-affirming surgery refers to any surgery in which a transgender individual uses to align their body with their affirmed gender identity.39 Among these gender-affirming surgeries, there is a group of surgeries that do not affect fertility which include facial feminization, breast augmentation, bilateral mastectomy, and metoidioplasty.1,2 The other group of surgeries that directly affect fertility include orchiectomy, penectomy, vaginoplasty, hysterectomy, oophorectomy, vaginectomy, and phalloplasty.1,2 Gender-affirming surgeries affecting fertility are irreversible and guidelines recommend first having used gender-affirming hormone therapy continuously for 1 year and having successfully been living full-time in one’s affirmed gender role for 1 year.2 Obstructive uropathy is a complication of gender-affirming surgery and altered anatomy may result in challenges in the management of specific urological issues and kidney transplantation. After gender-affirming surgery, transgender persons remain at an increased risk of psychiatric morbidity, suicidality, and death.40
Special Considerations for Transgender Care in the Setting of Kidney Disease
CKD and Hypogonadotropic Hypogonadism
It is believed that there is proportional increase in hypothalamic-pituitary-gonadal (HPG) dysfunction as glomerular filtration rate (GFR) decreases in individuals with CKD that results in testosterone deficiency in men41 and estrogen deficiency and functional menopause in females.42 However, postmenopausal females with kidney disease have similar estrogen levels as postmenopausal females without kidney disease.43 How kidney disease suppresses the HPG axis is not entirely clear but is thought to be due to the reduction in cyclic release of GnRH leading to the loss of pulsatile luteinizing hormone and follicular stimulating hormone release with subsequent decrease in the secretion of sex hormones.44 Another potential factor is the presence of hyperprolactinemia which is also common in CKD and dialysis as a result of increased pituitary production due to resistance from dopamine inhibition and decreased excretion by the kidneys.45,46 Transgender persons with CKD may have decreased levels of endogenous sex hormones and may thus potentially require lower dosages of gender-affirming hormone therapy to meet their gender-affirming goals but there is no evidence to support this beyond the presumed hypogonadotropic hypogonadism of patients with kidney disease.
The Role of Sex Hormones in Kidney Disease
The effects of sex on kidney function are uncertain because in human studies, unlike animal studies, it is challenging to separate sex and gender as prognostic factors for the development and progression of CKD. In a meta-analysis of studies in the Chronic Kidney Disease-Prognosis Consortium, there was no difference in the progression of kidney disease between men and women even after stratifying by age to account for menopause.47 This is different from previous meta-analyses where female sex was associated with either improved or deleterious kidney outcomes,48,49 which in addition to differences in menopausal status in the included populations likely reflects dissimilarities in definitions of kidney outcomes in the different studies.
Estrogen and Kidney Disease
Estrogen has been shown to be renoprotective in animal studies with estrogen receptors located in mesangial, endothelial, and endothelial cells of the kidney where it affects the synthesis and activity of cytokines, growth factors, vasoactive mediators, renin-angiotensin aldosterone system (RAAS) activity, and transforming growth factor-β signal transduction.50 However, the renoprotective effects of estrogen in human prospective and observational studies have been inconsistent. If in fact female sex is renoprotective, is not clear whether this is because of a protective effect of endogenous estrogen or due to decreased exposure to the potentially harmful effects of testosterone. However, exogenous estrogen may also be harmful. Oral contraceptives with estrogen are associated with the development of microalbuminuria51 and macroalbuminuria52 as well as RAAS and an increase in blood pressure.53 Postmenopausal hormone therapy in the setting of kidney disease is associated with a lower low-density lipoprotein and higher high-density lipoprotein without any effects on triglycerides or blood pressure.54 Studies examining the effect of postmenopausal hormone therapy on long-term CV outcomes in CKD and ESKD are lacking with those examining the effects of exogenous estrogen on albuminuria and GFR showing conflicting results55-57 which likely reflect differences in estrogen doses, routes, concurrent use of progestins, and timing of initiation of therapy relative to menopausal onset.
Whether the potential benefits and harms of exogenous estrogen in transgender women and decreased estrogen in transgender men are similar to that of cisgender individuals are unclear. The pharmacokinetics of estrogen differs in cisgender women with CKD,58,59 in that while little estradiol and estrone are excreted in the urine and neither is dialyzed, free and total estradiol plasma concentrations are still higher in cisgender women with ESKD so it is recommended that only 50% of its dose is administered. The absolute risks of VTE from estrogen are likely increased in CKD and dialysis60 so its benefit to risk ratio should be revaluated as CKD progresses. The development of additional risk factors such as surgery and immobilization, as is in the setting of kidney transplantation, may further increase VTE risk so that it may be reasonable to temporarily discontinue estrogen to prevent the risk of VTE (and graft thrombosis) until additional risk factors have resolved but there is no evidence to guide this approach. The route of estrogen (ie, transdermal vs oral vs other) may influence the risk of VTE as transdermal estrogen may be safer in transgender61 and postmenopausal women.62 Similarly, given the already increased CV risk in the setting of CKD, the use of estrogen in transgender women may further increase the of CV events.63 In an electronic medical record–based cohort study of 2842 transwomen and 2118 transmen from Kaiser Permanente integrated health systems, the incidences of ischemic stroke and myocardial infarction were higher in transwomen than cisgender men and women controls but eGFR and eGFR/proteinuria estrogen interactions were not considered.19 Observational studies that accurately identify transgender women, estrogen exposure, and both VTE and CV events across the spectrum of CKD are needed to determine the degree of effect modification by GFR and proteinuria.
Testosterone and Kidney Outcomes
Low testosterone is common in CKD64 as a result of aging, comorbidities such as diabetes, obesity, liver disease, and medications (eg, glucocorticoids, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, opioids) with many adverse outcomes including low muscle mass,65 frailty,66 arterial stiffness,67 CV events,68 and mortality.64,69-71 It is unknown whether low testosterone levels from the effects of feminizing hormone therapy or as a result of gonadectomy in transgender women are associated with similar risks. Similarly, it is also unknown whether the goal of gender-affirming hormone therapy in transgender men with CKD should be that of mimicking normal physiology or targeting the lower testosterone levels seen in the cismale CKD population.
If accurate testosterone levels are needed to guide clinical decision-making, a measurement method that takes into account sex hormone–binding globulin levels which are altered in CKD and influence total testosterone levels is recommended. This may include bioavailable testosterone, free androgen index, or free testosterone measured using a validated equilibrium dialysis assay. The treatment of low testosterone in cisgender men with hypogonadism not only results in virilization and improves sexual function, muscle strength, fat-free mass, bone density but might also increase the risk of CV events.72 Exogenous testosterone therapy increases erythropoiesis and so hemoglobin should be carefully monitored in all patients on testosterone73 given the potential risk of iatrogenic polycythemia, especially if there is the concurrent use of iron, erythropoietin-stimulating agents, or post-transplant polycythemia. It appears that exogenous testosterone is not significantly removed by hemodialysis as total and free testosterone levels do not differ before and after dialysis in men using transdermal testosterone.74
Cyproterone acetate and GnRH agonists do not need to be dose adjusted in CKD and dialysis but this has not been formally studied. However, spironolactone should be dosed reduced for eGFR < 30 mL/min/1.73m2 although lower doses may not be effective as an anti-androgen. As spironolactone may increase the risk of hyperkalemia, especially in individuals approaching or currently being treated with dialysis,75 replacement by another anti-androgen may be necessary in transgender women. Alternatively, pharmacologic (eg, diuretics, novel potassium binding resins) and nonpharmacologic treatments (strict dietary restriction, frequent dialysis) for hyperkalemia may need to be initiated or intensified. Androgen deprivation therapy for nonmetastatic prostate cancer with GnRH agonists and combination therapy with oral antiandrogens or estrogens increases the risk of acute kidney injury76 but its mechanism and whether this is the case in transgender persons are not known. In a retrospective study of weightlifters that included 57 current anabolic-androgenic steroid (AAS) users, 28 past AAS users, and 52 nonusers, adjusted eGFR was the lowest in current users, intermediate in past users, and the highest in nonusers suggesting either a direct nephrotoxic effect or an indirect effect mediated by AAS-induced CV dysfunction.77
Estimating GFR in Transgender Persons
GFR is routinely estimated78 using creatinine and equations developed from linear regression models including the Modification of Diet in Renal Disease (MDRD)79 and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)80 equations. Creatinine is a biomarker of GFR that is derived from diet and skeletal muscle metabolism so it is influenced not only by GFR but also by diet and muscle mass which is dependent on age, sex/gender, and race but the latter is controversial.81 For this reason, estimated GFR equations typically adjust for these non-GFR-related factors to improve their performance. The eGFR equations include sex as a covariate because cisgender females generate less creatinine than cisgender males due to having lower muscle mass and there may also be sex/gender differences in diet and creatinine production.82 If sex and gender factors are not considered, eGFR is systematically overestimated in cisgender females.
The adjustment in eGFR for female sex in the MDRD equation is × 0.742. The adjustment in eGFR for female sex in CKD-EPI equation83 is more complicated and is based on sex differences in the (SCr/B)C term of the equation where SCr is serum creatinine, B is 0.7 and 0.9 in cisgender females and males, respectively, and C is either −0.329 or −0.411 if SCr is ≤62 and ≤80 µmol/L in cisgender females and males (and 1.209 for both sexes above these SCr thresholds). The issue with using sex in eGFR equations in transgender persons is that it is unclear which adjustment to input into the equation for transgender men and women because their muscle mass and thus creatinine may not be the same as their respective cisgender counterparts. It should be noted that the MDRD and CKD-EPI populations used to derive and validate their eGFR formulas did not specifically report if any transgender persons were included or how sex and/or gender were captured in the initial datasets. The differences in eGFR estimation of using both sexes in the MDRD and CKD-EPI equations across ages and serum creatinine are shown in Figure 1. The absolute difference in eGFR between sexes decreases as serum creatinine increases and is lower in the elderly as compared with younger individuals. The bias between measured GFR and eGFR may be larger in women compared with men84 (ie, −14.2 [−16.5 to −10.9] in women and −3.4 [−6.3 to 0] in men using CKD-EPI) but how this compares with transgender men and women is not known.
Figure 1.
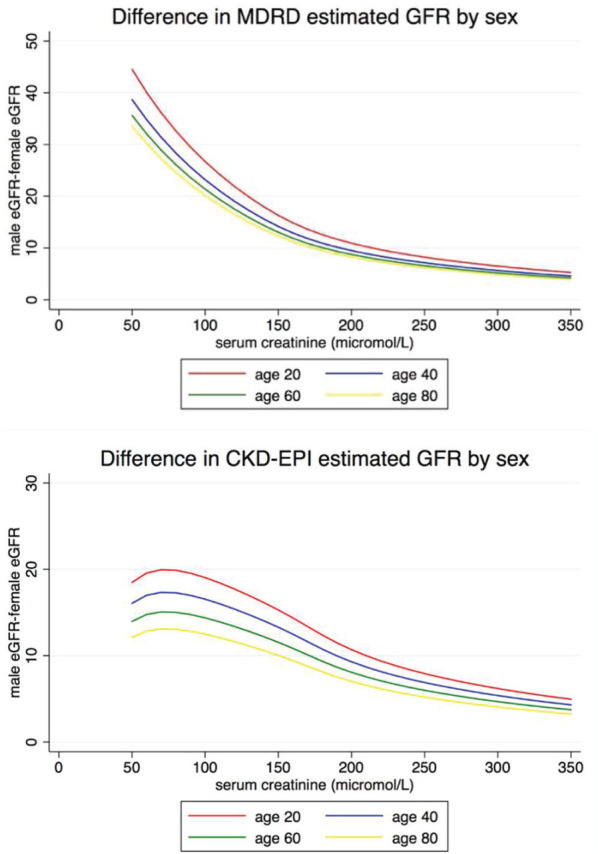
Differences in eGFR by sex and age in MDRD and CKD-EPI equations.
Note. MDRD= Modification of Diet in Renal Disease; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; eGFR = estimated glomerular filtration rate.
This potential bias (i.e., the difference in measured GFR and eGFR) in transgender persons is most problematic in the setting of gender-affirming hormone therapy, where body composition and muscle mass change as described above with a corresponding change in creatinine generation and serum creatinine85,86; whether there is any true difference in actual GFR is unknown. In a systematic review and meta-analysis, testosterone therapy increased lean mass by 3.9kg (95% confidence interval [CI]: 3.2-4.5) in transgender men and estradiol therapy with or without anti-androgen therapy decreased lean mass by 2.4 kg (95% CI: 2.8-2.1).87,88 The effects of gender-affirming hormone therapy on serum creatinine in transgender men and women before and after its initiation or between cisgender and transgender groups are shown in Table 1. On average, creatinine increases approximately 5 to 10 µmol/L in transgender men and decreases 5 to 10 µmol/L in transgender women which may or may not be clinically relevant as the minimally important difference in eGFR bias across GFR levels and clinical decision-making is unknown. The degree to which cystatin C is affected by gender-affirming hormone therapy is not known, but is presumably less than creatinine given that CKD-EPI equations that incorporate cystatin C with or creatinine are less influenced by sex (ie, the sex coefficient is .932 if female for the cystatin C only equation and the male and female values for [cystatin/B]C are identical for the combined creatinine and cystatin C equation). To our knowledge, there are no studies that have examined if there are any differences in cystatin C between cisgender and transgender persons or pre/post gender-affirming hormone therapy.
Table 1.
Changes in Transgender Men and Women on Sex Hormone Therapy.
| Study | Population | Duration | Prehormone therapy serum creatinine | Posthormone therapy serum creatinine |
P value (between group*) (pre/post**) |
|---|---|---|---|---|---|
| Ruestche89 | 24 transwomen 15 transmen |
12.5 y 7.6 y |
Not applicable Not applicable |
80 µmol/L (10) 90 µmol/L (6) |
Not reported |
| Lapauw90 | 23 transwomen 46 age, height Matched cismales |
>3 y | Not applicable Not applicable |
0.78 mg/dL ± 0.11 (69 µmol/L ± 10) 0.94 mg/dL ± 0.12 (83 µmol/L ± 11) |
P < .001* |
| Wierckx91 | 50 transmen 50 transwomen |
6.3 y 8.7 y |
Not applicable Not applicable |
0.9 mg/dL (0.9-1.0) (80 µmol/L) 0.8 mg/dL (0.7-0.9) (71 µmol/L) |
P < .001* |
| Sorelle85 | 133 transwomen 89 transmen |
>6 mo | 0.98 mg/dL (87 µmol/L) 0.74 mg/dL (65 µmol/L) |
0.86 mg/dL (76 µmol/L) 0.90 mg/dL (80 µmol/L) |
P < .0001** P < .0001** |
| Fernandez92 | 33 transwomen 19 transmen |
18 mo | 0.90 mg/dL (0.03) (80 µmol/L [3]) 0.73 mg/dL (0.03) (65 µmol/L [3]) |
0.83 mg/dL (0.03) (73 µmol/L [3]) 0.82 mg/dL (0.04) (73 µmol/L [4]) |
Not reported |
| Jarin93 | 72 transmen 44 transwomen |
6 mo | Not reported | Not reported | P > .05 |
| Scharff86 | 249 transwomen 278 transmen |
12 mo | 78.5 µmol/L (10.8) 66.0 µmol/L (9.0) |
73.1 µmol/L (10.6) 77.4 µmol/L (10.6) |
−5.0 (95% CI: −6.2 to −3.8)** 11.1 (95% CI: 10.1 to 12.2)** |
| Wierckx94 | 53 transmen 53 transwomen |
1 y | 0.74 mg/dL (0.1) (65 µmol/L [9]) 0.9 mg/dL (0.1) (80 µmol/L [9]) |
0.84 mg/dL (0.1) (74 µmol/L [9]) 0.8 mg/dL (0.1) (71 µmol/L [9]) |
P < .001** P < .001** |
| van Kesteren95 | 20 transwomen 19 transmen |
1 y | 81 µmol/L (7) 72 µmol/L (8) |
77 µmol/L (7) 79 µmol/L (9) |
P = .048** P < .001** |
Note. CI = confidence interval.
Gandhi et al96 report the case of a 36-year-old transgender man whose creatinine increased from 0.94 mg/dL (83 µmol/L) to 1.3 mg/dL (115 µmol/L) 2 years after the initiation of testosterone. A 24-hour urine for CrCl was 92 mL/min which, as expected, was higher than eGFR estimated by CKD-EPI due to tubular secretion Cr using either male sex (81 mL/min/1.73 m2) or female sex (61 mL/min/1.73 m2). Whitley and Greene97 report the case of a 33-year-old transgender man on testosterone therapy with CKD and eGFR of 31 mL/min/1.73 m2 or 23 mL/min/1.73 m2 using male or female sex, respectively, in the MDRD equation. The eGFR equation performance in transgender persons is limited to case reports and there are no specific data regarding transgender women.
Given that the performance of eGFR equations has never been evaluated in any transgender populations, we recommend that both male and female sexes are inputted into eGFR equations for transgender persons on gender-affirming hormone therapy. This will give a range of eGFR by sex that can be narrowed by deciding on which value likely reflects the muscle mass of the patient to which the calculations were applied. In situations in which an accurate knowledge of GFR is needed (eg, living donor suitability, dosing of medications with a narrow therapeutic index, referral for pre-emptive kidney transplantation, the initiation of dialysis with vague but potentially uremic symptoms), measured GFR can be obtained by use of non-Cr exogenous filtration markers such as iothalamate or iohexol, or radionuclide imaging with diethylenetriaminepentaacetic acid or ethylenediaminetetraacetic acid. Ideally, Cr measurements before the initiation of gender-affirming hormone therapy are available in most patients so that accurate and precise baseline eGFR levels can be referenced as well. For transgender persons not on gender-affirming hormone therapy, because their muscle mass is likely reflective of their sex assigned at birth, we recommend that their sex assigned at birth and not their affirmed gender be used in all eGFR equations. Additional research is needed to determine the role of cystatin C instead of or in addition to Cr for eGFR in transgender persons as well as the performance (and possibly modification) of current eGFR equations in transgender persons with and without gender-affirming hormone therapy.
Inaccuracy in eGFR values on an individual level in transgender persons can be harmful depending on the level of GFR, the clinical decision at hand, and the availability of confirmatory measured GFR testing. For any population with a potential non-GFR determinant of an eGFR biomarker, bias and systemic overestimation in eGFR may result in delays in accessing care (eg, referrals to nephrology, vascular access, and transplant) and systemic underestimation could lead to the overdiagnosis of CKD, earlier initiation of dialysis, the inadequate use or dosing of drugs excreted by the kidney, limit access to diagnostic imaging (contrast, gadolinium), and result in exclusion from research.98 Thus, the potential lack of precision, accuracy, and bias in any transgender eGFR calculation requires full disclosure by health care providers and shared decision-making99 not only by nephrologists but also by all clinicians who provide care to transgender persons in addition to the patient. Ultimately, if eGFR equations were validated in transgender persons on gender-affirming hormone therapy and modified as necessary to optimize their performance, this could improve clinical decision-making and potentially avoid the need for measured GFR testing which is invasive, time-consuming, impractical, and costly. Potential differences in creatinine and eGFR equations in transgender persons warrant better awareness among clinicians and researchers. For example, in the iPrEX study of preexposure prophylaxis for HIV prevention in cisgender men who have sex with men and transgender women100,101 (who comprised 14% of the trial population, 20% of whom were on gender-affirming hormone therapy), a creatinine >1.2 mg/dL (106 µmol/L) or “the upper limit of normal” and a CrCl <60 mL/min were both exclusion criteria and safety events independent of sex or gender. While this remains speculative, it is possible that thresholds of creatinine or eGFR that differed by gender may have modified the trial recruitment and population and altered its results regarding efficacy and safety.
Limitations
There is little research dedicated to the intersection of transgender persons and kidney disease unlike other priority areas for transgender persons such as HIV/AIDS, mood disorders, CV disease, malignancy, and fertility.102 However, even in these areas, there are unique methodologic and operational challenges103 to research. These barriers104 include recruitment, retention, efficiency,105 mistrust,106 a lack of individual and community engagement by researchers, and an overall concern for perceived more pressing issues such as personal safety, police violence,107 poverty, unemployment,108 and homelessness109 instead of research that may or may not directly impact transgender health. The nephrology community needs to prioritize including transgender persons in research by at minimum collecting sex and gender separately to identify transgender persons in studies as well as perform dedicated epidemiologic studies dedicated to pertinent questions such as eGFR measurement and the safety of gender-affirming hormone therapy in transgender persons with kidney disease.
Conclusions
This review highlights important considerations for providing care to transgender persons with kidney disease. Additional research is needed to evaluate the performance of eGFR equations in transgender persons, the effects of gender-affirming hormone therapy on the kidney, and the impact of gender identity on outcomes of persons living with kidney disease.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: All authors provided consent for publication
Availability of Data and MaterialsNot applicable.: Not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David Collister  https://orcid.org/0000-0002-2323-6521
https://orcid.org/0000-0002-2323-6521
References
- 1. Safer JD, Tangpricha V. Care of transgender persons. N Engl J Med. 2019;381(25):2451-2460. doi: 10.1056/NEJMcp1903650. [DOI] [PubMed] [Google Scholar]
- 2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 3. Polderman TJC, Kreukels BPC, Irwig MS, et al. The biological contributions to gender identity and gender diversity: bringing data to the table. Behav Genet. 2018;48(2):95-108. doi: 10.1007/s10519-018-9889-z. [DOI] [PubMed] [Google Scholar]
- 4. Mohottige D, Lunn MR. Ensuring gender-affirming care in nephrology: improving care for transgender and gender-expansive individuals. Clin J Am Soc Nephrol. 2020;15:1195-1197. doi: 10.2215/cjn.14471119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. https://transcare.ucsf.edu/guidelines. Accessed December 21, 2020.
- 6. Reisner SL, Poteat T, Keatley J, et al. Global health burden and needs of transgender populations: a review. Lancet. 2016;388(10042):412-436. doi: 10.1016/s0140-6736(16)00684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. 2017;107(2):e1-e8. doi: 10.2105/AJPH.2016.303578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collin L, Reisner SL, Tangpricha V, Goodman M. Prevalence of transgender depends on the “case” definition: a systematic review. J Sex Med. 2016;13(4):613-626. doi: 10.1016/j.jsxm.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Downing JM, Przedworski JM. Health of transgender adults in the U.S., 2014-2016. Am J Prev Med. 2018;55(3):336-344. doi: 10.1016/j.amepre.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 10. Dragon CN, Guerino P, Ewald E, Laffan AM. Transgender Medicare beneficiaries and chronic conditions: exploring fee-for-service claims data. LGBT Health. 2017;4(6):404-411. doi: 10.1089/lgbt.2016.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Townsend M, Jaffer H, Goldman L. Adverse health outcomes in transgender people. CMAJ. 2017;32:E1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beckwith N, McDowell MJ, Reisner SL, et al. Psychiatric epidemiology of transgender and nonbinary adult patients at an urban health center. LGBT Health. 2019;6(2):51-61. doi: 10.1089/lgbt.2018.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams NJ, Vincent B. Suicidal thoughts and behaviors among transgender adults in relation to education, ethnicity, and income: a systematic review. Transgend Health. 2019;4(1):226-246. doi: 10.1089/trgh.2019.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker KE. Findings from the behavioral risk factor surveillance system on health-related quality of life among US transgender adults, 2014-2017. JAMA Intern Med. 2019;179(8):1141-1144. doi: 10.1001/jamainternmed.2018.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cicero EC, Reisner SL, Merwin EI, Humphreys JC, Silva SG. The health status of transgender and gender nonbinary adults in the United States. PLoS One. 2020;15(2):e0228765. doi: 10.1371/journal.pone.0228765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valashany BT, Janghorbani M. Quality of life of men and women with gender identity disorder. Health Qual Life Outcomes. 2018;16(1):167. doi: 10.1186/s12955-018-0995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7; 2012. World Professional Association for Transgender Health (WPATH). https://www.tandfonline.com/doi/abs/10.1080/15532739.2011.700873.
- 18. Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214-222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 19. Getahun D, Nash R, Flanders WD, et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med. 2018;169(4):205-213. doi: 10.7326/m17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Connelly PJ, Marie Freel E, Perry C, et al. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension. 2019;74(6):1266-1274. doi: 10.1161/hypertensionaha.119.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edmiston EK, Donald CA, Sattler AR, Peebles JK, Ehrenfeld JM, Eckstrand KL. Opportunities and gaps in primary care preventative health services for transgender patients: a systemic review. Transgend Health. 2016;1(1):216-230. doi: 10.1089/trgh.2016.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam JSH, Abramovich A. Transgender-inclusive care. CMAJ. 2019;191(3):E79. doi: 10.1503/cmaj.180954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnold LM. Promoting culturally competent care for the lesbian, gay, bisexual, and transgender population. Am J Public Health. 2001;91(11):1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alencar Albuquerque G, de Lima Garcia C, da Silva Quirino G, et al. Access to health services by lesbian, gay, bisexual, and transgender persons: systematic literature review. BMC Int Health Hum Rights. 2016;16:2. doi: 10.1186/s12914-015-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collier R. Addressing transgender discrimination in health. CMAJ. 2015;17:E493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006-2013. Am J Public Health. 2014;104(suppl. 4):S532-S534. doi: 10.2105/ajph.2014.302086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giblon R, Bauer GR. Health care availability, quality, and unmet need: a comparison of transgender and cisgender residents of Ontario, Canada. BMC Health Serv Res. 2017;17(1):283. doi: 10.1186/s12913-017-2226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costa R, Colizzi M. The effect of cross-sex hormonal treatment on gender dysphoria individuals’ mental health: a systematic review. Neuropsychiatr Dis Treat. 2016;12:1953-1966. doi: 10.2147/NDT.S95310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nobili A, Glazebrook C, Arcelus J. Quality of life of treatment-seeking transgender adults: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2018;19(3):199-220. doi: 10.1007/s11154-018-9459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White Hughto JM, Reisner SL. A systematic review of the effects of hormone therapy on psychological functioning and quality of life in transgender individuals. Transgend Health. 2016;1(1):21-31. doi: 10.1089/trgh.2015.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. T’Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V. Endocrinology of transgender medicine. Endocr Rev. 2019;40(1):97-117. doi: 10.1210/er.2018-00011. [DOI] [PubMed] [Google Scholar]
- 32. Defreyne J, Vantomme B, Van Caenegem E, et al. Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. Andrology. 2018;6(3):446-454. doi: 10.1111/andr.12485. [DOI] [PubMed] [Google Scholar]
- 33. Tangpricha V, den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017;5(4):291-300. doi: 10.1016/S2213-8587(16)30319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fighera TM, Ziegelmann PK, Rasia da, Silva T, Spritzer PM. Bone mass effects of cross-sex hormone therapy in transgender people: updated systematic review and meta-analysis. J Endocr Soc. 2019;5:943-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan W, Drummond A, Kelly M. Deep vein thrombosis in a transgender woman. CMAJ. 2017;189(13):e502-e504. doi: 10.1503/cmaj.160408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shatzel JJ, Connelly KJ, DeLoughery TG. Thrombotic issues in transgender medicine: a review. Am J Hematol. 2017;92(2):204-208. doi: 10.1002/ajh.24593. [DOI] [PubMed] [Google Scholar]
- 37. de Blok CJM, Wiepjes CM, Nota NM, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ. 2019;365:l1652. doi: 10.1136/bmj.l1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joint R, Chen ZE, Cameron S. Breast and reproductive cancers in the transgender population: a systematic review. BJOG. 2018;125(12):1505-1512. doi: 10.1111/1471-0528.15258. [DOI] [PubMed] [Google Scholar]
- 39. Canner JK, Harfouch O, Kodadek LM, et al. Temporal trends in gender-affirming surgery among transgender patients in the United States. JAMA Surg. 2018;153(7):609-616. doi: 10.1001/jamasurg.2017.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dhejne C, Lichtenstein P, Boman M, Johansson AL, Långström N, Landén M. Long-term follow-up of transsexual persons undergoing sex reassignment surgery: cohort study in Sweden. PLoS One. 2011;6(2):e16885. doi: 10.1371/journal.pone.0016885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehtihet M, Hylander B. Semen quality in men with chronic kidney disease and its correlation with chronic kidney disease stages. Andrologia. 2015;47(10):1103-1108. doi: 10.1111/and.12388. [DOI] [PubMed] [Google Scholar]
- 42. Lim VS, Henriquez C, Sievertsen G, Frohman LA. Ovarian function in chronic renal failure: evidence suggesting hypothalamic anovulation. Ann Intern Med. 1980;93(1):21-27. doi: 10.7326/0003-4819-93-1-21. [DOI] [PubMed] [Google Scholar]
- 43. Kramer HM, Curhan G, Singh A. Hemodialysis and estrogen levels in postmenopausal (HELP) patients: the multicenter HELP study. Am J Kidney Dis. 2003;41(6):1240-1246. doi: 10.1016/s0272-6386(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 44. Ahmed SB, Ramesh S. Sex hormones in women with kidney disease. Nephrol Dial Transplant. 2016;31(11):1787-1795. doi: 10.1093/ndt/gfw084. [DOI] [PubMed] [Google Scholar]
- 45. Hou SH, Grossman S, Molitch ME. Hyperprolactinemia in patients with renal insufficiency and chronic renal failure requiring hemodialysis or chronic ambulatory peritoneal dialysis. Am J Kidney Dis. 1985;6(4):245-249. doi: 10.1016/s0272-6386(85)80181-5. [DOI] [PubMed] [Google Scholar]
- 46. Sievertsen GD, Lim VS, Nakawatase C, Frohman LA. Metabolic clearance and secretion rates of human prolactin in normal subjects and in patients with chronic renal failure. J Clin Endocrinol Metab. 1980;50(5):846-852. doi: 10.1210/jcem-50-5-846. [DOI] [PubMed] [Google Scholar]
- 47. Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant. 2003;18(10):2047-2053. doi: 10.1093/ndt/gfg317. [DOI] [PubMed] [Google Scholar]
- 49. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11(2):319-329. [DOI] [PubMed] [Google Scholar]
- 50. Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis. 2013;20(5):390-395. doi: 10.1053/j.ackd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 51. Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161(16):2000-2005. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 52. Ahmed SB, Hovind P, Parving HH, et al. Oral contraceptives, angiotensin-dependent renal vasoconstriction, and risk of diabetic nephropathy. Diabetes Care. 2005;28(8):1988-1994. doi: 10.2337/diacare.28.8.1988. [DOI] [PubMed] [Google Scholar]
- 53. Burgner A, Hladunewich MA. Contraception and CKD. Clin J Am Soc Nephrol. 2020;15(4):563-565. doi: 10.2215/cjn.09770819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramesh S, Mann MC, Holroyd-Leduc JM, et al. Hormone therapy and clinical and surrogate cardiovascular endpoints in women with chronic kidney disease: a systematic review and meta-analysis. Menopause. 2016;23(9):1028-1037. doi: 10.1097/GME.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 55. Ahmed SB, Culleton BF, Tonelli M, et al. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008;74(3):370-376. doi: 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
- 56. Kattah AG, Suarez MLG, Milic N, et al. Hormone therapy and urine protein excretion: a multiracial cohort study, systematic review, and meta-analysis. Menopause. 2018;25(6):625-634. doi: 10.1097/GME.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fung MM, Poddar S, Bettencourt R, Jassal SK, Barrett-Connor E. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: the Rancho Bernardo Study. Menopause. 2011;18(6):629-637. doi: 10.1097/gme.0b013e3181fca9c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ginsburg ES, Owen WF, Jr., Greenberg LM, Shea BF, Lazarus JM, Walsh BW. Estrogen absorption and metabolism in postmenopausal women with end-stage renal disease. J Clin Endocrinol Metab. 1996;81(12):4414-4417. doi: 10.1210/jcem.81.12.8954051. [DOI] [PubMed] [Google Scholar]
- 59. Stehman-Breen C, Anderson G, Gibson D, Kausz AT, Ott S. Pharmacokinetics of oral micronized beta-estradiol in postmenopausal women receiving maintenance hemodialysis. Kidney Int. 2003;64(1):290-294. doi: 10.1046/j.1523-1755.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 60. Molnar AO, Bota SE, McArthur E, et al. Risk and complications of venous thromboembolism in dialysis patients. Nephrol Dial Transplant. May 1. 2018;33(5):874-880. doi: 10.1093/ndt/gfx212. [DOI] [PubMed] [Google Scholar]
- 61. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642. doi: 10.1530/EJE-10-1038. [DOI] [PubMed] [Google Scholar]
- 62. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 64. Khurana KK, Navaneethan SD, Arrigain S, Schold JD, Nally JV, Jr., Shoskes DA. Serum testosterone levels and mortality in men with CKD stages 3-4. Am J Kidney Dis. 2014;64(3):367-374. doi: 10.1053/j.ajkd.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chiang JM, Kaysen GA, Segal M, Chertow GM, Delgado C, Johansen KL. Low testosterone is associated with frailty, muscle wasting and physical dysfunction among men receiving hemodialysis: a longitudinal analysis. Nephrol Dial Transplant. 2019;34(5):802-810. doi: 10.1093/ndt/gfy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yilmaz MI, Sonmez A, Qureshi AR, et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1617-1625. doi: 10.2215/CJN.10681210. [DOI] [PubMed] [Google Scholar]
- 67. Kyriazis J, Tzanakis I, Stylianou K, et al. Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant. 2011;26(9):2971-2977. doi: 10.1093/ndt/gfq847. [DOI] [PubMed] [Google Scholar]
- 68. Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant. 2011;26(1):184-190. doi: 10.1093/ndt/gfq397. [DOI] [PubMed] [Google Scholar]
- 69. Gungor O, Kircelli F, Carrero JJ, et al. Endogenous testosterone and mortality in male hemodialysis patients: is it the result of aging? Clin J Am Soc Nephrol. 2010;5(11):2018-2023. doi: 10.2215/cjn.03600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol. 2009;20(3):613-620. doi: 10.1681/ASN.2008060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bello AK, Stenvinkel P, Lin M, et al. Serum testosterone levels and clinical outcomes in male hemodialysis patients. Am J Kidney Dis. 2014;63(2):268-275. doi: 10.1053/j.ajkd.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 72. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 73. Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018;103:1745-1754. doi: 10.1210/jc.2018-00404. [DOI] [PubMed] [Google Scholar]
- 74. Singh AB, Norris K, Modi N, et al. Pharmacokinetics of a transdermal testosterone system in men with end stage renal disease receiving maintenance hemodialysis and healthy hypogonadal men. J Clin Endocrinol Metab. 2001;86(6):2437-2445. doi: 10.1210/jcem.86.6.7525. [DOI] [PubMed] [Google Scholar]
- 75. Quach K, Lvtvyn L, Baigent C, et al. The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(4):591-598. doi: 10.1053/j.ajkd.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 76. Lapi F, Azoulay L, Niazi MT, Yin H, Benayoun S, Suissa S. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. JAMA. 2013;310(3):289-296. doi: 10.1001/jama.2013.8638. [DOI] [PubMed] [Google Scholar]
- 77. Hudson JI, Kanayama G, Pope HG, Jr., et al. Glomerular filtration rate and supraphysiologic-dose anabolic-androgenic steroid use: a cross-sectional cohort study. Am J Kidney Dis. Jul. 2020;76(1):152-155. doi: 10.1053/j.ajkd.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63(5):820-834. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470. doi:199903160-00002. [DOI] [PubMed] [Google Scholar]
- 80. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. Jul 5. 2012;367(1):20-29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. National Kidney Foundation ASoN. https://www.kidney.org/news/establishing-task-force-to-reassess-inclusion-race-diagnosing-kidney-diseases. Accessed December 21, 2020.
- 82. Nitsch D. Is there a difference in metabolic burden between men and women? Nephrol Dial Transpl. 2014;29(6):1110-1112. doi: 10.1093/ndt/gft518. [DOI] [PubMed] [Google Scholar]
- 83. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Inker LA, Levey AS, Tighiouart H, et al. Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: the multiethnic study of atherosclerosis. Nephrol Dial Transplant. 2018;33(3):417-425. doi: 10.1093/ndt/gfx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. SoRelle JA, Jiao R, Gao E, et al. Impact of hormone therapy on laboratory values in transgender patients. Clin Chem. Jan. 2019;65(1):170-179. doi: 10.1373/clinchem.2018.292730. [DOI] [PubMed] [Google Scholar]
- 86. Scharff M, Wiepjes CM, Klaver M, Schreiner T, T’Sjoen G, den Heijer M. Change in grip strength in trans people and its association with lean body mass and bone density. Endocr Connect. 2019;8(7):1020-1028. doi: 10.1530/EC-19-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Spanos C, Bretherton I, Zajac JD, Cheung AS. Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: a systematic review. World J Diabetes. 2020;11(3):66-77. doi: 10.4239/wjd.v11.i3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Klaver M, Dekker M, de Mutsert R, Twisk JWR, den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017;49(5). doi: 10.1111/and.12660. [DOI] [PubMed] [Google Scholar]
- 89. Ruetsche AG, Kneubuehl R, Birkhaeuser MH, Lippuner K. Cortical and trabecular bone mineral density in transsexuals after long-term cross-sex hormonal treatment: a cross-sectional study. Osteoporos Int. 2005;16(7):791-798. doi: 10.1007/s00198-004-1754-7. [DOI] [PubMed] [Google Scholar]
- 90. Lapauw B, Taes Y, Simoens S, et al. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone. 2008;43(6):1016-1021. doi: 10.1016/j.bone.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 91. Wierckx K, Mueller S, Weyers S, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641-2651. doi: 10.1111/j.1743-6109.2012.02876.x. [DOI] [PubMed] [Google Scholar]
- 92. Fernandez JD, Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract. 2016;22(4):383-388. doi: 10.4158/EP15950.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jarin J, Pine-Twaddell E, Trotman G, et al. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics. 2017;139(5). doi: 10.1542/peds.2016-3173. [DOI] [PubMed] [Google Scholar]
- 94. Wierckx K, Van Caenegem E, Schreiner T, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11(8):1999-2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 95. van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf). 1998;48(3):347-354. doi: 10.1046/j.1365-2265.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 96. Gandhi P, Medeiros E, Shah AD. Physiology or pathology? elevated serum creatinine in a female-to-male transgender patient. Am J Kidney Dis. Apr. 2020;75(4):A13-A14. doi: 10.1053/j.ajkd.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 97. Whitley CT, Greene DN. Transgender man being evaluated for a kidney transplant. Clin Chem. 2017;63(11):1680-1683. doi: 10.1373/clinchem.2016.268839. [DOI] [PubMed] [Google Scholar]
- 98. Grubbs V. Precision in GFR reporting: let’s stop playing the race card. Clin J Am Soc Nephrol. 2020;15:1201-1202. doi: 10.2215/cjn.00690120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15:1203-1212. doi: 10.2215/cjn.12791019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14(6):468-475. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2(12):e512-e519. doi: 10.1016/S2352-3018(15)00206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Feldman J, Brown GR, Deutsch MB, et al. Priorities for transgender medical and healthcare research. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):180-187. doi: 10.1097/MED.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Matthews AK, Rak K, Anderson E, et al. White paper from a CTSA workshop series on special and underserved populations: enhancing investigator readiness to conduct research involving LGBT populations. J Clin Transl Sci. 2018;2(4):193-200. doi: 10.1017/cts.2018.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reisner SL, Deutsch MB, Bhasin S, et al. Advancing methods for US transgender health research. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):198-207. doi: 10.1097/MED.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hughes JP, Emel L, Hanscom B, Zangeneh S. Design issues in transgender studies. J Acquir Immune Defic Syndr. 2016;72 (suppl 3):S248-S251. doi: 10.1097/qai.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Owen-Smith AA, Woodyatt C, Sineath RC, et al. Perceptions of barriers to and facilitators of participation in health research among transgender people. Transgend Health. 2016;1(1):187-196. doi: 10.1089/trgh.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. DeVylder JE, Jun HJ, Fedina L, et al. Association of exposure to police violence with prevalence of mental health symptoms among urban residents in the United States. JAMA Netw Open. 2018;1(7):e184945. doi: 10.1001/jamanetworkopen.2018.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Conron KJ, Scott G, Stowell GS, Landers SJ. Transgender health in Massachusetts: results from a household probability sample of adults. Am J Public Health. 2012;102(1):118-122. doi: 10.2105/AJPH.2011.300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fraser B, Pierse N, Chisholm E, Cook H. LGBTIQ+ homelessness: a review of the literature. Int J Environ Res Public Health. 2019;16(15). doi: 10.3390/ijerph16152677. [DOI] [PMC free article] [PubMed] [Google Scholar]


